Abstract
Although electrical stimulation is therapeutically applied for neural regeneration in patients, it remains unclear how electrical stimulation exerts its effects at the molecular level on spinal cord injury (SCI). To identify the signaling pathway involved in electrical stimulation improving the function of injured spinal cord, 21 female Sprague-Dawley rats were randomly assigned to three groups: control (no surgical intervention, n = 6), SCI (SCI only, n = 5), and electrical simulation (ES; SCI induction followed by ES treatment, n = 10). A complete spinal cord transection was performed at the 10th thoracic level. Electrical stimulation of the injured spinal cord region was applied for 4 hours per day for 7 days. On days 2 and 7 post SCI, the Touch-Test Sensory Evaluators and the Basso-Beattie-Bresnahan locomotor scale were used to evaluate rat sensory and motor function. Somatosensory-evoked potentials of the tibial nerve of a hind paw of the rat were measured to evaluate the electrophysiological function of injured spinal cord. Western blot analysis was performed to measure p38-RhoA and ERK1/2-Bcl-2 pathways related protein levels in the injured spinal cord. Rat sensory and motor functions were similar between SCI and ES groups. Compared with the SCI group, in the ES group, the latencies of the somatosensory-evoked potential of the tibial nerve of rats were significantly shortened, the amplitudes were significantly increased, RhoA protein level was significantly decreased, protein gene product 9.5 expression, ERK1/2, p38, and Bcl-2 protein levels in the spinal cord were significantly increased. These data suggest that ES can promote the recovery of electrophysiological function of the injured spinal cord through regulating p38-RhoA and ERK1/2-Bcl-2 pathway-related protein levels in the injured spinal cord.
Keywords: Bcl-2, ERK1/2, p38, PGP9.5, RhoA, spinal cord injury, somatosensory evoked potential, muscle contraction, electrical impulses, neural regeneration
Introduction
Unlike the regenerative activity in the peripheral nervous system, axonal regeneration in the central nervous system (CNS) is a rare phenomenon (Huebner et al., 2009) in mammals, including humans, due to internal and external factors such as glial scars (Cregg et al., 2014). Despite this intrinsic deficit, CNS neurons in adult mammals, however, have demonstrated regenerative potential when they were provided with appropriate environmental conditions (Horner et al., 2000).
Failure of axonal regeneration in the spinal cord occurs commonly after traumatic injury. Traumatic spinal cord injury (SCI), which can be categorized into primary and secondary injuries, gives rise to a chain of complex cellular and molecular cascades (Oyinbo, 2011; Anwar et al., 2016) and frequently causes significant long-term neurological deficits (Oyinbo, 2011; Anwar et al., 2016). Initial SCI leads to swelling of the cells, release of neurotoxins, and necrosis, and then results in the secondary injury, which is associated with many obstacles to regeneration (Anwar et al., 2016) as well as with extensive apoptosis of neurons and oligodendroglia (Mekhail et al., 2012; Uchida et al., 2012). A decrease in apoptosis in both oligodendrocytes and neurons has been shown to parallel the improvements in neurological functions after SCI (Casha et al., 2012).
As the cellular and molecular cascades following SCI have become clearer, a variety of therapeutic approaches have also been attempted, including electrical stimulation (ES) (Varma et al., 2013; Ho et al., 2014). ES to the injured spinal cord has been applied for several decades. After creating a weak electric field in the damaged area by inserting a pair of electrodes into the injured spinal cord, behavioral recovery, with enhanced axonal growth, has been reported (Haan et al., 2014). In particular, application of voltage changes can promote neurite growth toward the cathode electrode, thus leading to functional improvement by stimulating axonal regeneration. Biphasic ES has also been reported to prevent growth factor-deprived apoptosis through BDNF-PI3K/Akt signaling (Wang et al., 2013). In addition, oscillating field stimulation (OFS) shortened motor evoked potentials and promoted myelin regeneration with motor function recovery (Tian et al, 2016). Recently, when OFS was applied in humans with the approval of the US Food and Drug Administration, the safety of the procedure and significant sensory recovery of spinal cord injuries were reported (Zhang et al., 2014).
Although ES is currently applied therapeutically in human patients, it remains unknown how the intervention exerts its beneficial effect at the molecular level and which signaling pathways are involved in its mechanisms of action on the injured spinal cord. In this study, we investigated the role of p38-RhoA and ERK1/2-Bcl-2 pathways in the neuroprotection of ES on injured spinal cord using behavioral, electrophysiological and western blotting techniques.
Materials and Methods
Experimental animals
All experiments were approved by the Laboratory Animals Ethics Committee of Wonkwang University (approval number: WKU11-22). Twenty-one female Sprague-Dawley rats, aged 9 weeks and weighing 200−250 g were included in this study and randomly assigned to three experimental groups: control (no surgical intervention; n = 6), SCI (SCI only, n = 5). and SCI + ES (SCI followed by ES therapy, n = 10) groups.
SCI induction
All surgical procedures, including SCI, were performed by the same investigator. Under inhalation anesthesia (Isoflurane, Hana Pharma Co., Seoul, South Korea), the posterior part of the spinal cord was exposed by laminectomy at the 10th thoracic vertebral region. The exposed spinal cord was completely transected using No. 11 mess by the same operator. Rat body temperature was maintained at 36.5 ± 0.5°C by a thermo-regulated heating pad (DAESHIN Electric Co., Bucheon-si, Gyeonggi-do, South Korea) during the operation. After completion of all surgeries, the surgical area was sterilized and gentamicin (100 mg/kg) was injected intramuscularly for 5 days to prevent infection.
ES
In the SCI + ES group, immediately after inducing an SCI, electrodes were positioned at the transection level on both sides of the spinal cord, and were subsequently fixed by suturing to the muscle. The wires connected to the electrode were secured under the skin from the injured area to the posterior of the neck and exposed to the outside. The wires were fixed to the skin to ensure that the electrode position remained fixed (Figure 1). Walking-Man II (Cybermedic Co., Iksan, South Korea) was used for administering ES to the spinal cord. The intensity of the ES was set to half of the motion threshold to start showing contraction of the tail or lower limbs (0.5–1.0 V), a square wave of 300 µs was used as the width of stimulation, and the stimulation frequency was set at 10 Hz. The ES was performed for 4 hours per day (2 hours in the morning and 2 hours in the afternoon) for 7 days, starting from the afternoon of the day of surgery, after the anesthesia wore off.
Figure 1.
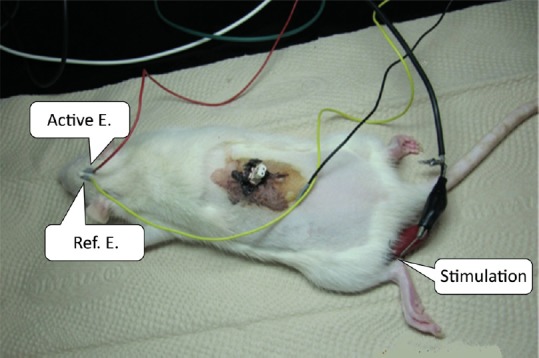
Photograph of the electrical field stimulation at the injured spinal cord and somatosensory evoked potential by tibial nerve stimulation.
Active electrode (Active E.): 3 mm posterior and 2 mm lateral to bregma; reference electrode (Ref. E.): 6 mm lateral to bregma.
Behavioral evaluation
To evaluate the rats' sensory and motor functions, Touch-Test Sensory Evaluation (TTSE, Stoelting Co., Wood Dale, IL, USA)(Chan et al., 1992) and the Basso, Beattie and Bresnahan (BBB) locomotor scale (Basso et al., 1996) were assessed on days 2 and 7 after surgery. TTSE was used for the evaluation of the sensory function, in which the soles of the feet were stimulated with a sharp needle, and the threshold of more than 50% avoidance reactions was recorded as N (= g × m/s2). The motor function was evaluated using the BBB locomotor scale, with a score ranging from 0 to 21 points, higher scores represent better locomotor function. All evaluations were measured twice repeatedly by the same investigator.
Electrophysiological function of injured spinal cord
For the electrophysiological measurements, the Grass Square Pulse stimulator (Grass Technology, Carelow, Ireland) was used for tibial nerve stimulation. Somatosensory-evoked potentials (SSEP) were recorded using the Powerlab data acquisition system and were analyzed using LabChart software (AD Instrument, Sydney, Australia). Room temperature was maintained constant at 25°C and a thermal pad was used to maintain the animal's body temperature during the procedure. For SSEP, the stimulating electrode was positioned at the tibial nerve of a hind paw of the rat. The active recording electrodes were placed at 3 mm posterior to bregma and at 2 mm lateral to the sagittal suture, while the reference electrodes were positioned at 6 mm below bregma and lateral to the sagittal suture (Figure 1). The intensity of the stimulation was set to the approximate point when the minimum movement of the ankle joint or to ebecomes visually noticeable. The stimulation frequency was 1.5 times per second with stimulus duration of 0.1 ms and a frequency width ranging from 3 Hz to 3 kHz, and the averaged wave form of 200 runs was recorded twice. The latency of the positive wave peak of the SSEP (P1) was set to the time to reach the peak of the wave that was formed toward the anode for the first time at baseline, and the latency of the negative wave peak (N1) was set to the time to reach the peak of the wave that was formed toward the cathode for the first time at baseline. The amplitude of P1-N1 was determined by the voltage difference measured from P1 to N1, and the amplitude of N1-P2 was determined by the voltage difference measured from N1 to P2. SSEP measurements were performed 1 day before and 2 and 7 days after SCI.
Western blotting
Rats were sacrificed on day 7after SCI, and the spinal cords were subsequently isolated. The expression levels of protein gene product 9.5 (PGP 9.5), RhoA, p38, Bcl-2, total extracellular signal-regulated kinase1/2 (ERK1/2), and phosphorylated extracellular signal-regulated kinase (pERK1/2) were examined by western blot analysis. Spinal cord tissues were preserved frozen. Tissues were washed with phosphate-buffered saline (PBS), and then homogenized in an approximately 500 μL of lysis buffer. Subsequently, the samples were centrifuged at 12,000 r/min for 30 minutes to obtain the cytosol. For protein quantification, the absorbance of each sample was measured in duplicate and applied to the interaction formula to calculate the amount of proteins using bovine serum albumin (BSA) as a standard solution, and the amount of proteins in all samples was adjusted to be equal. Sample buffer was added to each isolated sample, followed by shaking at 100°C for 5 minutes, and the samples were subsequently subjected to electrophoresis using a minigel electrophoresis apparatus (mini-PROTEIN Tetra cell, Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 100 V for 2 hours. After electrophoresis, the gel was stained with Coomassie brilliant blue R-250 (Sigma-Aldrich, Saint Louis, MO, USA) for 1 hour and subsequently destained in 10% acetic acid and 10% methanol to detect proteins in the gel. These proteins were transferred to 0.45-μm polyvinylidene difluoride (PVDF) membrane (Roche Diagnostics GmbH, Mannheim, Germany) using a protein transfer device (mini Trans-blot cell, Bio-Rad Laboratories, Inc.) at 100 V for 1 hour and 30 minutes. To block nonspecific binding of the primary antibody to the PVDF membrane, the membrane was incubated in a blocking buffer in which 5% skim milk was dissolved in 0.05% Tween 20-Tris buffer saline (TBST, pH 7.6). After washing for three times with TBST, the primary antibodies against PGP9.5 (1:1,000; goat anti-rabbit, Abcam, Cambridge, MA, USA), RhoA (1:1,000; goat anti-rabbit, Cell Signaling, Danvers, MA, USA), p38 (1:1,000; goat anti-rabbit, Cell Signaling), ERK1/2 (1:1,000; goat anti-rabbit, R&D Systems, Minneapolis, MN, USA), and pERK1/2 (1:2,000; goat anti-rabbit, R&D Systems), Bcl-2 (1:1,000; goat anti-rabbit, Cell Signaling), GAPDH (1:1,250; goat anti-rabbit, Cell Signaling) were added to the membrane at a dilution of 1:1,000 in 3% bovine serum albumin-containing TBS and were incubated overnight at 4°C, followed by four washes with TBST. Subsequently, the membrane was incubated for 1 hour at 25°C room temperature with the secondary antibody, namely goat anti-rabbit IgG conjugated to horseradish peroxidase (HRP; 1:5,000; Enzo Life Science International Inc., Plymouth Meeting, PN, USA). After four washes with TBST, Immobilon Western Chemiluminescent HRP Substrate (Millipore, Billerica, MA, USA) was added to the membrane, which was subsequently exposed to a film in the dark room to visualize and compare the protein expression levels using ImageJ (Wayne Rasband National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All values are represented as the mean ± SE. Behavioral and electrophysiological data and western blot quantitative results were compared between control, SCI, and SCI + ES groups using SPSS 24.0 software (IBM Corp., Armonk, NY, USA). We compared the behavioral and electrophysiological evaluations data by SSEP between the SCI and SCI + ES groups using the two-sample Student's t-test. For comparing western blot quantitative results, independent sample t-test or one-way analysis of variance (ANOVA) with Bonferroni post-hoc tests was performed to analyze the difference of the expression intensity between groups. Null hypotheses of no differences were rejected if P values were less than 0.05.
Results
Behavioral changes
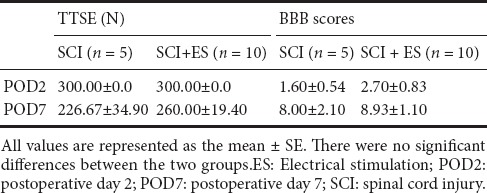
On days 2 and 7 after SCI, the TTSE scores were respectively 300 ± 0 N and 226.7 ± 34.90 N in the SCI group, and 300 ± 0 N and 260 ± 19.40 N in the SCI + ES group. On days 2 and 7 after SCI, the BBB locomotor scale scores were respectively 1.60 ± 0.54 and 8.00 ± 2.10 in the SCI group, and 2.70 ± 0.83 and 8.93 ± 1.10 in the SCI + ES group. There was no statistical significance observed between SCI and SCI + ES groups (Table 1).
Table 1.
Changes in the Touch Test Sensory Evaluator (TTSE) and motor function assessed by the Basso-Beattie-Bresnahan (BBB) locomotor scale in the SCI and SCI + ES groups
Electrophysiological function of injured spinal cord
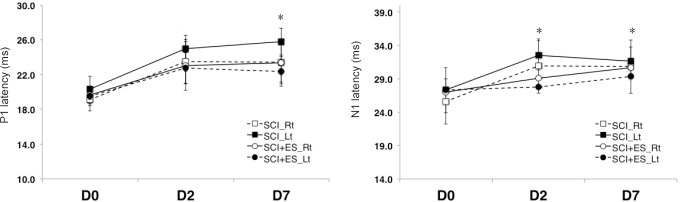
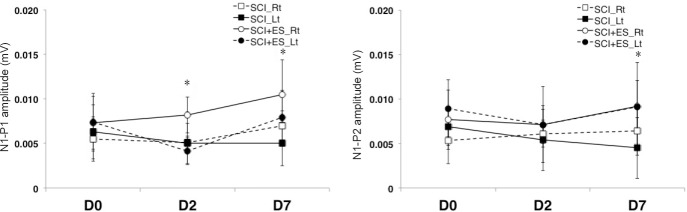
There was no difference in the latency or amplitude in the right tibial nerve. On days 2 and 7, there was a significant difference in the P1 and N1 latency of the left tibial nerve between the SCI and SCI + ES groups (P < 0.05; Figure 2). The P1-N1 amplitudes of the right and left tibial nerves were significantly increased in the SCI + ES group than in the SCI group on days 2 and 7 (P < 0.05; Figure 3). On day 7, the N1-P2 amplitude of the left tibial nerve in the SCI + ES group was significantly increased than that in the SCI group (P < 0.05; Figure 3).
Figure 2.
P1 and N1 latencies recorded by somatosensory evoked potentials on days 2 and 7 after SCI (n = 5) or SCI + ES (n = 10).
For recording somatosensory evoked potentials, the stimulating electrode was positioned at the tibial nerve of a hind paw of the rat. The active recording electrodes were placed at 3 mm posterior to bregma and at 2 mm lateral to the sagittal suture, while the reference electrodes were positioned at 6 mm below bregma and lateral to the sagittal suture. All values are represented as the mean ± SE. There were significant differences in P1 and N1 latency between the SCI and the SCI + ES groups on days 2 and 7 (*P < 0.05). Two-sample Student's t-test was used. ES: Electrical stimulation; Lt: left; Rt: right; SCI: spinal cord injury.
Figure 3.
P1-N1 and N1-P2 amplitudes recorded by somatosensory evoked potentials on days 2 and 7 after SCI (n = 5) or SCI + ES (n = 10).
For recording somatosensory evoked potentials, the stimulating electrode was positioned at the tibial nerve of a hind paw of the rat. The active recording electrodes were placed at 3 mm posterior to bregma and at 2 mm lateral to the sagittal suture, while the reference electrodes were positioned at 6 mm below bregma and lateral to the sagittal suture. All values are represented as the mean ± SE. There were significant differences in the P1-N1 amplitude on days 2 and 7 and in the N1-P2 amplitude on day 7 between SCI and SCI + ES groups (*P < 0.05). Two-sample Student's t-test was used. ES: Electrical stimulation; Lt: left; Rt: right; SCI: spinal cord injury.
Protein expression
PGP9.5
PGP9.5 expression was significantly decreased in the SCI group than that in the control group. In the SCI + ES group, however, the expression of PGP9.5 was almost completely recovered to the control level (Figure 4, P < 0.05).
Figure 4.
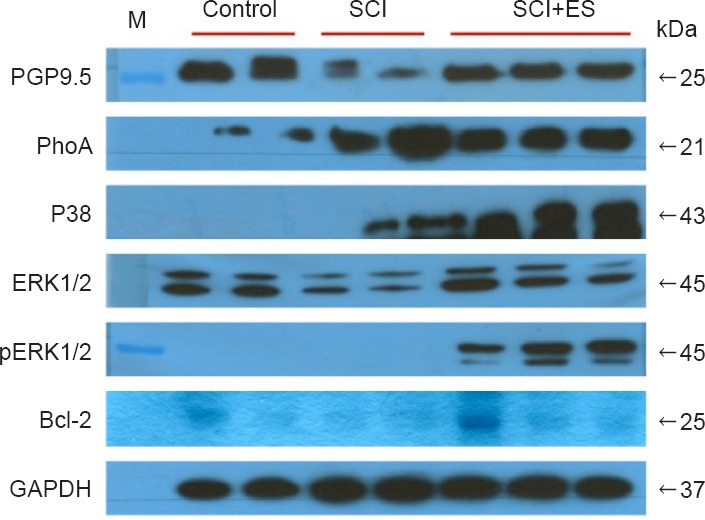
Anti-apoptosis and neurite outgrowth related protein expression in the spinal cord at 7 days post SCI.
RhoA was significantly increased in the SCI and SCI + ES groups than that in the control group. Compared to the SCI group, however, RhoA was obviously reduced in the SCI + ES group. Additionally, there was an obvious decrease in the density of PGP9.5, ERK1/2, p38, and Bcl-2 in the SCI group. In particular, pERK1/2 was only expressed in the SCI + ES group and the expression of pERK1 was markedly greater than that of pERK2. Western blotting was performed more than twice for each group of sample. The right side numbers indicate the molecular weight of each protein (kDa). CON: Control (n = 2); SCI: spinal cord injury (n = 2); SCI + ES: spinal cord injury with electrical stimulation (n = 3).
RhoA
The expression of RhoA was significantly increased in both the SCI and SCI + ES groups as compared to the control group. The expression of RhoA in the SCI + ES group, however, was significantly decreased as compared to the SCI group (Figure 4, P < 0.05).
p38
p38 expression was absent in the control group. In contrast, p38 was well expressed in both SCI and SCI + ES groups. In particular, the expression of p38-MAPKwas significantly increased in the SCI + ES group than that in the SCI group (Figure 4, P < 0.05).
ERK1/2 and pERK1/2
ERK1/2 expression was significantly lower in the SCI group than that in the CON group. In the SCI + ES group, however, the expression of ERK1/2 recovered almost completely to the control level. Additionally, the phosphorylated form of ERK1/2 was significantly expressed in the SCI + ES group, but was absent in the control and SCI groups. Expression levels of the phosphorylated form of ERK1 (pERK1) were markedly higher than those of the phosphorylated form of ERK2 (pERK2) in the SCI + ES group (Figure 4, P < 0.05).
Bcl-2
Bcl-2 was expressed in the control group, but it was not expressed in the SCI group. Even though BCl-2 was absent in the SCI group, it was highly expressed in the SCI + ES group (Figure 4, P < 0.05).
Discussion
Recovery from neural damage by applying an electrical field to the damaged nerve has been attributed to inhibition of dieback and development of astrocytic scars, which consequently promotes axonal growth toward the glial scars within the damaged spinal cord (McCaig et al., 1991). The application of a direct current electric field to the neural tissue is known to promote neurite outgrowth (Haan et al., 2014) and it was reported that the growth on the cathode side is several times faster than that on the anode side (Tian et al., 2016). Therefore, the direction of the stimulation in this study was set such that the current flowed between the cathode, positioned at the ninth thoracic vertebral region, and the anode, positioned at the eleventh thoracic vertebral region, creating an electrical field. The intensity of the ES was set to half of the motion threshold, where visible contraction of the tailor lower limbs starts.
In the present study, no significant differences were observed in the TTSE score and BBB locomotor scale in terms of behavioral evaluation after 1 week of ES. However, considering previously reported results (Zhang et al., 2014), achieving a significant difference in the BBB locomotor scale at 6 weeks of ES after SCI, may require additional long-term ES and follow-up observations. Although no significant improvement was observed in behavioral evaluation, the results of the electrophysiological evaluation by SSEPs showed a significant improvement in the prolonged latency of P1 and N1 in the SCI + ES group as compared with the SCI group. In addition, considering that the amplitudes of P1-N1 and N1-P2 were also significantly increased, ES in the injured spinal cord is believed to be partly involved in the complex recovery mechanisms of myelinor axons.
According to the western blot analysis reported in a previous study, PGP9.5 is expressed abundantly, specifically in differentiated neurons and neuroendocrine cells of all animals with a spinal cord injury, including humans. It is observed in the central and peripheral nerves at all developmental stages and has been used as an axonal regeneration marker (Cheng et al., 2013). Considering that expression of PGP9.5 is used as a marker of neural cells and that the amount of PGP9.5 may decrease in the tissue after neural damage, the quantitative difference in the expression of PGP9.5 in this study is likely to reflect the difference in the number of live neural cells between groups. Thus, the significantly reduced expression of PGP9.5 in the SCI group, but not in the SCI + ES group, as compared with the control group, in this study suggests that more neural cells were damaged or dead in the SCI group, and that electrical stimulation in the SCI + ES group was effective in suppressing neural cell death or promoting neural cell regeneration.
Successful axonal regeneration requires not only deactivation of growth-inhibitory proteins, but also activation of growth-promoting signaling pathways, including several MAPKs and downstream proteins, including RhoA. RhoA is one of many important growth-inhibitory proteins in the CNS. After trauma in the spinal cord, RhoA is activated and plays a critical role in inhibiting axonal regeneration by means of glial apoptosis (Dill et al, 2010). Thus, the deactivation of RhoA is an important therapeutic target for improving recovery from injury and may help to promote neurite outgrowth. In this study, the expression of RhoA was significantly increased in both the SCI and SCI + ES groups, as compared with the control group, which is consistent with previous findings that demonstrated a three- to five-fold higher than normal expression of RhoA after SCI, which lasted for several months (Wu et al., 2016). Furthermore, considering that the expression of RhoA was significantly decreased in the SCI + ES group as compared with the SCI group, it is thought that electrical stimulation in the damaged region of the spinal cord acts on the Rho signaling pathway, inhibiting its expression, and leading to neurophysiological and functional recovery.
One of the representative MAPKs related to RhoA is p38, which has been associated with inconsistent results related to neuronal survival. It has been demonstrated that RhoA expression can be suppressed by the activation of p38-MAPK (Temporin et al., 2008). Moreover, a strong correlation between p38 activation and nerve growth factor-induced neurite outgrowth has previously been reported (Morooka et al., 1998), whereas it has also been suggested that p38 plays important roles in neuronal apoptosis (Takeda et al., 2002), via activation of a RhoA-p38alpha MAPK pro-apoptotic pathway (Stankiewicz et al., 2014).
In this study, p38 was not expressed at all in the control group. In contrast, its expression was marked in both the SCI and SCI + ES groups. More specifically, the expression of p38-MAPK was significantly increased in the SCI + ES group as compared with the SCI group.
These results implied that, although p38 may act as a mediator in both anti-apoptotic cell survival or stress-activated neuronal apoptosis (Takeda et al., 2002; Cai et al., 2006), enhanced levels of activated p38, induced by ES, in the SCI + ES group may lead to the inhibition of RhoA expression to overcome the pro-apoptotic effect of the secondary injury.
Another important MAPK for neuronal survival or neuroprotection in the injured spinal cord or CNS neurons is ERK1/2 (Liu et al., 2015). It has also been reported that the ERK pathway is essentially involved in promoting neuronal regeneration by ES through increasing brain-derived neurotrophic factor (Wenjin et al., 2011). Several contradictory results, however, were also reported, in terms of secondary damage mechanisms in a number of neurodegenerative diseases, such as stroke, CNS injury, and autoimmune diseases of the CNS (Subramaniam et al., 2010; Yu, 2012).
Recently, it has been emphasized that ERK1 and ERK2 played different roles in the physiology and pathology of the CNS (Yu, 2012), even though they share about 83% of conserved amino acid sequences (Cargnello et al., 2011). Thus, in contrast to its essential role in normal CNS development, ERK2 reduction is important for preventing a secondary injury mechanism under pathological conditions. Moreover, enhancement of ERK1 is also critical, because it antagonizes the expression of ERK2 (Yu, 2012). Accordingly, the reduction of ERK2 and enhancement of ERK1 activation has been suggested as a novel therapeutic approach (Yu, 2012).
In this study, ERK1/2 expression in the SCI group was significantly lower than that in the control group. In the SCI + ES group, however, the expression of ERK1/2 recovered almost fully to the control level. Additionally, the phosphorylated form of ERK1/2 was significantly expressed in the SCI + ES group, although it was absent from the control and SCI groups. In particular, the expression of pERK1 was markedly higher than that of pERK2, which implies that the protective effect of electrical stimulation exceeded the influence of secondary injury. In contrast to Rho signaling pathways, ERK-dependent pathways stimulate Bcl-2 family proteins, such as Bcl-2, as a pro-survival protein (Stankiewicz et al., 2014) or as an anti-apoptotic protein working through several pathways, including CREB (Hetman et al., 2004). The expression pattern of Bcl-2 in this study revealed that it was expressed in the control group, but was not expressed at all in the SCI group. Interestingly, even though Bcl-2 was absent in the SCI group, it was highly expressed in the SCI + ES group. Considering the relationship between the activation of ERK1/2 and Bcl-2, the increase in pERK1 levels over those of pERK2 might be related to the activation of the pro-survival protein Bcl-2 and may, as a result, promote neuronal survival against secondary injury.
This study has the following limitations. The optimal time and duration of electrical stimulation have not yet been established. In future, the total time and amount of electrical stimulation required to achieve neural regeneration should be investigated. Additionally, to compare the correlation between electrophysiological test and western blot analysis results, samples should be obtained at several time points during the experimental period. Unfortunately in this experiment, we obtained spinal cord samples only at the end of the experiment.
In summary, we propose that electrical stimulation for treatment of SCI prevents secondary injury and improves neuronal regeneration/recovery through two complementary pathways (Figure 5). In the induced pathway, electrical stimulation promotes the growth and survival of neuronal cells by preventing apoptosis via (p)ERK1/2 and Bcl-2. In the inhibited pathway, electrical stimulation inhibits the outgrowth of neuronal cells via p38 and RhoA, thereby inducing differentiation. Neural regeneration after SCI may be facilitated by electrical stimulation via both pathways, leading to the recovery of electrophysiological function of injured spinal cord.
Figure 5.
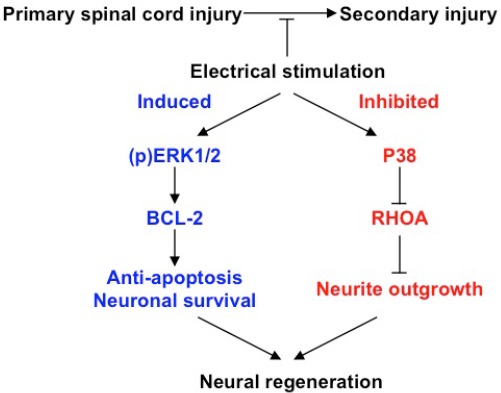
A molecular mechanism for improving neural regeneration in spinal cord injuries (SCI) through the use of electrical stimulation (ES).
In the absence of treatment, primary SCI can progressively lead to the development of secondary injuries. ES treatment improves neural growth, differentiation, and regeneration through the induced and inhibited expression of specific molecular pathways. In the induced pathway, ES increases the expression of ERK1/2 and the phosphorylation (p) of ERK1/2, which in turn upregulates the expression of the anti-apoptotic protein BCL-2, thus preventing apoptosis and prompting neuronal cell survival. Conversely, in the inhibited pathway, ES upregulates the expression of P38, which in turn downregulates the expression of RhoA, preventing neurite outgrowth and improving neuronal differentiation. The induced and inhibited pathways together lead to the promotion of neuronal regeneration.
Additional file (187KB, pdf) : Open peer reviewer reports 1 and 2.
Footnotes
Funding: This study was supported by a grant from Wonkwang Institute of Clinical Medicine in 2011.
Conflicts of interest: Walking-Man II was applied for administering electrical stimulation. All authors stated that they did not accept the relevant financial support, and no competing interests existed.
Financial support: This study was supported by Wonkwang Institute of Clinical Medicine in 2011. The funder had no role in study conception and design, literature retrieval, experimental studies, data acquisition and analysis, statistical analysis, paper writing and decision to submit the paper for publication.
Research ethics: This study was performed in accordance with the guidelines and requirements of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985) and approved by the Laboratory Animals Ethics Committee of Wonkwang University (approval number: WKU11-22).
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Hojjat Allah Abbaszadeh, Shaheed Beheshti University of Medical Sciences, Iran; Masaaki Hori, Juntendo University School of Medicine, Japan.
(Copyedited by Li CH, Song LP, Zhao M)
References
- 1.Anwar MA, Al Shehabi TS, Eid AH. Inflammogenesis of econdary spinal cord injury. Front Cell Neurosci. 2016;10:98. doi: 10.3389/fncel.2016.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 3.Cai B, Chang SH, Becker EB, Bonni A, Xia Z. p38 MAP kinase mediates apoptosis through phosphorylation of BimEL at Ser-65. J Biol Chem. 2006;281:25215–25222. doi: 10.1074/jbc.M512627200. [DOI] [PubMed] [Google Scholar]
- 4.Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casha S, Zygun D, McGowan MD, Bains I, Yong VW, Hurlbert RJ. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain. 2012;135:1224–1236. doi: 10.1093/brain/aws072. [DOI] [PubMed] [Google Scholar]
- 6.Chan AW, MacFarlane IA, Bowsher D, Campbell JA. Weighted needle pinprick sensory thresholds: a simple test of sensory function in diabetic peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1992;55:56–59. doi: 10.1136/jnnp.55.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng HT, Dauch JR, Porzio MT, Yanik BM, Hsieh W, Smith AG, Singleton JR, Feldman EL. Increased axonal regeneration and swellings in intraepidermal nerve fibers characterize painful phenotypes of diabetic neuropathy. J Pain. 2013;14:941–947. doi: 10.1016/j.jpain.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dill J, Patel AR, Yang XL, Bachoo R, Powell CM, Li S. A molecular mechanism for ibuprofen-mediated RhoA inhibition in neurons. J Neurosci. 2010;30:963–972. doi: 10.1523/JNEUROSCI.5045-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haan N, Song B. Therapeutic application of electric fields in the injured nervous system. Adv Wound Care. 2014;3:156–165. doi: 10.1089/wound.2013.0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hetman M, Gozdz A. Role of extracellular signal regulated kinases 1 and 2 in neuronal survival. Eur J Biochem. 2004;271:2050–2055. doi: 10.1111/j.1432-1033.2004.04133.x. [DOI] [PubMed] [Google Scholar]
- 12.Ho CH, Triolo RJ, Elias AL, Kilgore KL, DiMarco AF, Bogie K, Vette AH, Audu ML, Kobetic R, Chang SR, Chan KM, Dukelow S, Bourbeau DJ, Brose SW, Gustafson KJ, Kiss ZH, Mushahwar VK. Functional electrical stimulation and spinal cord injury. Phys Med Rehabil Clin N Am. 2014;25:631–654. doi: 10.1016/j.pmr.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horner PJ, Gage FH. Regenerating the damaged centralnervous system. Nature. 2000;407:963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- 14.Huebner EA, Strittmatter SM. Axon regeneration in the peripheral and central nervous systems. Results Probl Cell Differ. 2009;48:339–351. doi: 10.1007/400_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu T, Cao FJ, Xu DD, Xu YQ, Feng SQ. Upregulated Ras/Raf/ERK1/2 signaling pathway: a new hope in the repair of spinal cord injury. Neural Regen Res. 2015;10:792–796. doi: 10.4103/1673-5374.156984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCaig CD, Rajnicek AM. Electrical fields, nerve growth and nerve regeneration. Exp Physiol. 1991;76:473–494. doi: 10.1113/expphysiol.1991.sp003514. [DOI] [PubMed] [Google Scholar]
- 17.Mekhail M, Almazan G, Tabrizian M. Oligodendrocyte-protection and remyelination post-spinal cord injuries: a review. Prog Neurobiol. 2012;96:322–339. doi: 10.1016/j.pneurobio.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Morooka T, Nishida E. Requirement of p38 mitogen activated protein kinase for neuronal differentiation in PC12 cells. J Biol Chem. 1998;273:24285–24288. doi: 10.1074/jbc.273.38.24285. [DOI] [PubMed] [Google Scholar]
- 19.Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp. 2011;71:281–299. doi: 10.55782/ane-2011-1848. [DOI] [PubMed] [Google Scholar]
- 20.Stankiewicz TR, Linseman DA. Rho family GTPases: key players in neuronal development, neuronal survival, and neurodegeneration. Front Cell Neurosci. 2014;8:1–14. doi: 10.3389/fncel.2014.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramaniam S, Unsicker K. ERK and cell death: ERK1/2 in neuronal death. FEBS J. 2010;277:22–29. doi: 10.1111/j.1742-4658.2009.07367.x. [DOI] [PubMed] [Google Scholar]
- 22.Takeda K, Ichijo H. Neuronal p38 MAPK signaling: an emerging regulator of cell fate and function in the nervoussystem. Genes Cells. 2002;7:1099–1111. doi: 10.1046/j.1365-2443.2002.00591.x. [DOI] [PubMed] [Google Scholar]
- 23.Temporin K, Tanaka H, Kuroda Y, Okada K, Yachi K, Moritomo H, Murase T, Yoshikawa H. IL-1beta promotes neurite outgrowth by deactivating RhoA via p38 MAPK pathway. Biochem Biophys Res Commun. 2008;365:375–380. doi: 10.1016/j.bbrc.2007.10.198. [DOI] [PubMed] [Google Scholar]
- 24.Tian DS, Jing JH, Qian J, Chen L, Zhu B. Effect of oscillating electrical field stimulation on motor function recovery and myelin regeneration after spinal cord injury in rats. Phys Ther Sci. 2016;28:1465–1471. doi: 10.1589/jpts.28.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchida K, Nakajima H, Watanabe S, Yayama T, Guerrero AR, Inukai T, Hirai T, Sugita D, Johnson WE, Baba H. Apoptosis of neurons and oligodendrocytes in the spinal cord of spinal hyperostotic mouse (twy/twy): possible pathomechanism of human cervical compressive myelopathy. Eur Spine J. 2012;21:490–497. doi: 10.1007/s00586-011-2025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varma AK, Das A, Wallace G 4th, Barry J, Vertegel AA, Ray SK, Banik NL. Spinal cord injury: a review of current therapy, future treatments, and basic science frontiers. Neurochem Res. 2013;38:895–905. doi: 10.1007/s11064-013-0991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M, Li P, Liu M, Song W, Wu Q, Fan Y. Potential protective effect of biphasic electrical stimulation against growth factor-derived apoptosis on olfactory bulb neural progenitor cells through the brain-derived neurotrophic factor-phosphatidylinositol 3-kinase/Akt pathway. Exp Biol Med. 2013;238:951–959. doi: 10.1177/1535370213494635. [DOI] [PubMed] [Google Scholar]
- 28.Wenjin W, Wenchao L, Hao Z, Feng L, Yan W, Wodong S, Xianqun F, Wenlong D. Electrical stimulation promotes BDNF expression in spinal cord neurons through Ca2+- and Erk-dependent signaling pathways. Mol Neurobiol. 2011;31:459–467. doi: 10.1007/s10571-010-9639-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu X, Xu XM. RhoA/Rho kinase in spinal cord injury. Neural Regen Res. 2016;11:23–27. doi: 10.4103/1673-5374.169601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu CG. Distinct roles for ERK1 and ERK2 in pathophysiology of CNS. Front Biol. 2012;7:267–276. [Google Scholar]
- 31.Zhang C, Zhang G, Rong W, Wang A, Wu C, Huo X. Oscillating field stimulation promotes spinal cord remyelination by inducing differentiation of oligodendrocyte precursor cells after spinal cord injury. Biomed Mater Eng. 2014;24:3629–3636. doi: 10.3233/BME-141190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.