Abstract
Background
Cachexia is a metabolic syndrome that affects up to 50–80% of cancer patients. The pathophysiology is characterized by a variable combination of reduced food intake and abnormal metabolism, including systemic inflammation and negative protein and energy balance. Despite its high clinical significance, defined diagnostic criteria and established therapeutic strategies are lacking. The ‘omics’ technologies provide a global view of biological systems. We hypothesize that blood‐based metabolomics might identify findings in cachectic patients that could provide clues to gain knowledge on its pathophysiology, and eventually postulate new therapeutic strategies.
Methods
This is a cross‐sectional observational study in two cohorts of cancer patients, with and without cachexia. Patients were consecutively recruited from routine clinical practice of a General Oncology Department at ‘12 de Octubre’ University Hospital. Selected clinical and biochemical features were collected. Blood metabolite fingerprinting was performed using three analytical platforms, gas chromatography coupled to mass spectrometry (GC–MS), capillary electrophoresis coupled to mass spectrometry (CE–MS), and liquid chromatography coupled to mass spectrometry (LC–MS). Besides, we performed pathway‐based metabolite analyses to obtain more information on biological functions.
Results
A total of 15 subjects were included in this study, 8 cachectic and 7 non‐cachectic patients. Metabolomic analyses were able to correctly classify their samples in 80% (GC–MS), 97% (CE–MS), 96% [LC–MS (positive mode)], and 89% [LC–MS (negative mode)] of the cases. The most prominent metabolic alteration in plasma of cachectic patients was the decrease of amino acids and derivatives [especially arginine, tryptophan, indolelactic acid, and threonine, with 0.4‐fold change (FC) compared with non‐cachectic patients], along with the reduction of glycerophospholipids [mainly lysophosphatidylcholines(O‐16:0) and lysophosphatidylcholines(20:3) sn‐1, FC = 0.1] and sphingolipids [SM(d30:0), FC = 0.5]. The metabolite with the highest increase was cortisol (FC = 1.6). Such alterations suggest a role of the following metabolic pathways in the pathophysiology of cancer cachexia: arginine and proline metabolism; alanine, aspartate, and glutamate metabolism; phenylalanine metabolism; lysine degradation; aminoacyl‐tRNA biosynthesis; fatty acid elongation in mitochondria; tricarboxylic acids cycle; among others.
Conclusions
These findings suggest that plasma amino acids and lipids profiling has great potential to find the mechanisms involved in the pathogenesis of cachexia. Metabolic profiling of plasma from cancer patients show differences between cachexia and non‐cachexia in amino acids and lipids that might be related to mechanisms involved in its pathophysiology. A better understanding of these mechanisms might identify novel therapeutic approaches to palliate this unmet medical condition.
Keywords: Cancer, Cachexia, Metabolomics, Biomarkers
Introduction
Cachexia is a metabolic multifactorial syndrome that affects a large amount of cancer patients, especially in advanced stages of the disease. It is caused by a variable combination of reduced food intake and abnormal metabolism that result in negative balances of energy and protein. By international consensus, cancer cachexia is defined as an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support and that leads to progressive functional impairment.1, 2 A variety of tumours (pancreas, stomach, lung, oesophagus, colon, etc.) are associated with a high incidence of cachexia, but not all patients with these tumour types develop cachexia. This disorder may affect to 50–80% of patients with advanced cancer.3, 4 In addition, cachexia might reduce patients' ability to receive, tolerate, and respond to therapy and predict poor outcome independently of other risk factors.5
The pathophysiology of cachexia is characterized by a negative energy balance in the body resulting from a combination of inadequate energy intake (anorexia), increased energy expenditure,6 and systemic inflammation,7 but abnormal metabolism is the major component.8, 9, 10, 11 All together result in a clinical phenotype characterized by muscle wasting and body weight loss.12, 13, 14, 15 Cancer cells not only produce tumour‐specific pro‐cachectic factors but also interact with host cells to produce cytokines, which lead to an acute phase response, neuro‐endocrine activation, and a chronic state in which catabolism dominates anabolism. Changes in metabolism may be accompanied by anorexia, fatigue, and nausea that, in turn, exacerbate weight loss. This energy imbalance depletes the body energy reserves, especially muscle and adipose tissue.16, 17
The understanding of the molecular mechanisms of cachexia has increased in the last 10 years.2, 18, 19 Conventional management of cachexia is often focused on the use of oral nutritional supplements, and sometimes on the control of a systemic inflammatory response. However, although oral nutritional interventions improve some aspects of quality of life, they do not have an impact mortality.20 Indeed, cancer cachexia is an unmet medical condition21, 22 and new therapeutic targets or strategies are therefore needed.
High throughput approaches (‘‐omics’) to study diseases are being used to find markers to predict prognosis, diagnosis, and reveal targets for future therapies. Among these, metabolomics has acquired a great interest because the metabolites are the end points of biological processes, and therefore it is assumed that they can reflect all the variables that contribute to the phenotype,23 thus reflecting upstream biological events such as genetic, transcriptomic, or proteomic alterations, and environmental influences.24 Metabolomics has quickly risen as a new methodology in the cancer biomarker field.25
Despite the powerful possibilities of metabolomics, in a 2012 review about novel findings in cachexia,26 only one metabolomics Nuclear Magnetic resonance(NMR)‐based study in mice was cited.27 In another study reported in 2011, 63 metabolites were analysed by 1H‐NMR in random urine samples from cancer patients (different types and stages) to examine sample classification performance according to the cachexia, using eight different standard statistical and machine‐learning approaches, differences were shown in the excretion of amino acids and their metabolites.28 Another study showed the possibilities of metabolomics in humans with pancreatic cancer and cachexia29 with gas chromatography coupled to mass spectrometry (GC–MS). They found intraday differences in some metabolites such as lactate, different at night but not in the morning. Paraxanthine (caffeine metabolite) appeared associated with cachexia, although the authors recognized that there could be a bias in their results because coffee consumption was not controlled, and some of the patients were strong coffee drinkers.
The hypothesis of the present study is that blood‐based metabolomics using a pilot multiplatform approach might identify findings in cachectic patients that could provide clues to gain knowledge on its pathophysiology, and eventually postulate new therapeutic strategies.
Methods
Patient cohorts and samples
From April 2013 to March 2015, 15 subjects were enrolled in an exploratory, observational, cross‐sectional, case‐control study, at the Medical Oncology Department of the ‘12 de Octubre’ University Hospital (Madrid, Spain). Eligible subjects were 18 years or older patients with advanced or incurable histologically confirmed malignancy and cachexia (defined as an involuntary weight loss of 5% or more within the previous 6 months).2 Control subjects were defined as patients with cancer without significant weight loss. Non‐eligible patients for this study included those with active infection, uncontrolled diabetes, receiving glucocorticoid therapy, or having esophageal‐gastrointestinal obstructions responsible for feeding limitations. Patient characteristics are shown in Table 1.
Table 1.
Baseline demographic and clinical characteristics
| Cachexia | No‐cachexia | P | |
|---|---|---|---|
| Overall, N (%) | 8 (53.3) | 7 (46.7) | |
| Gender | |||
| Female | 1 | 1 | |
| Male | 7 | 6 | 0.919 |
| Age, years | |||
| Median (range) | 61.5 (36‐81) | 63.86 (48‐80) | 0.737 |
| Up to 64 | 5 | 5 | |
| 65 or higher | 2 | 3 | |
| Relative weight loss in the last 3 months, % | 17.59 ± 14.09 | 1.02 ± 2.7 | 0.011 |
| BMI, kg/m2 | 20.80 ± 3.37 | 24.54 ± 2.07 | 0.025 |
| BSA (DuBois), m2 | 1.67 ± 1.45 | 1.81 ± 1.21 | 0.068 |
| CRP, mg/L | 5.72 ± 5.63 | 0.74 ± 0.52 | 0.041 |
| Serum albumin, g/dL | 3.30 ± 0.58 | 4.07 ± 0.55 | 0.020 |
| Cortisol, μg/dL | 25.34 ± 10.57 | 13.90 ± 3.14 | 0.017 |
| Cholesterol (mg/dL) | 137.75 ± 42.57 | 180.29 ± 47.83 | 0.091 |
| cLDL, mg/dL | 77.50 ± 41.41 | 99.0 ± 43.21 | 0.343 |
| cHDL, mg/dL | 40.77 ± 14.57 | 58.89 ± 17.75 | 0.049 |
| Triglycerides, mg/dL | 97.13 ± 34.47 | 111.57 ± 41.79 | 0.476 |
| Haemoglobin, g/dL | 11.50 ± 1.34 | 11.34 ± 1.67 | 0.843 |
| Neutrophils | 6412.5 ± 3158.9 | 3685.7 ± 1528.0 | 0.058 |
| Lymphocytes, No/μL | 1262.5 ± 785.5 | 1400.0 ± 668.3 | 0.723 |
| NLR | 7.45 ± 6.61 | 3.24 ± 2.09 | 0.131 |
| Leukocytes, No/ μL | 8512.5 ± 3659.6 | 5428.57 ± 1913.74 | 0.067 |
| ECOG | |||
| 0 | 0 | 2(28.6) | |
| 1 | 6 (75.0) | 5 (71.4) | |
| 2 | 1 (12.5) | 0 | |
| 3 | 1 (12.5) | 0 | 0.257 |
| Tumour type, N (%) | |||
| Pancreas | 3 (37.5) | 1 (14.3) | |
| Ampulloma | 1 (12.5) | ||
| Melanoma | 3 (37.5) | 1 (14.3) | |
| Cholangiocarcinoma | 1 (12.5) | ||
| Stomach | 1 (14.3) | ||
| Oesophageal | 2 (28.6) | ||
| Colon | 1 (14.3) | ||
| Sarcoma | 1 (14.3) | ||
| Tumour spread | |||
| Locoregional | 3 (37.5) | 2 (28.6) | |
| Metastatic | 5 (62.5) | 5 (71.4) |
BMI, Body mass index; BSA, Body surface area; CRP, C‐reactive protein; NLR, Neutrophil‐to‐lymphocyte ratio. Data are mean ± standard deviation.
Blood samples were collected from all the participants, and plasma was separated and immediately stored within 2 h of collection at −80 °C until analysis.
This study was approved by the Ethics Committee of the ‘12 de Octubre’ University Hospital, and it was done in accordance with the Declaration of Helsinki, International Conference on Harmonization, and Good Clinical Practice. Written informed consent was provided by all participating patients.
Multiplatform metabolic fingerprinting
To increase the metabolite coverage, samples were analysed by three platforms commonly employed in metabolomics studies: GC–MS, liquid chromatography–mass spectrometry (LC–MS), and capillary electrophoresis–mass spectrometry (CE–MS), using protocols previously described (Appendix S1). Quality control (QC) samples were prepared by pooling surrogate plasma provided by an external source. QCs were analysed throughout the runs, at the beginning and the end of each analysis and every five sample injections, to check the stability and performance of the system.30
Data treatment
Liquid chromatography–mass spectrometry and CE–MS data processing were performed in MassHunter Profinder software version B.06.00 using Molecular Feature Extraction and Recursive Feature Extraction tools. For GC–MS data treatment, deconvolution and identification were carried out using Agilent MassHunter Unknowns Analysis version B.07.00. Data obtained in the Unknowns Analysis Tool were aligned in Agilent Mass Profiler Professional version B.12.1 and exported to Agilent MassHunter Quantitative Analysis version B.07.00 to obtain the abundance of the compounds. Data matrix obtained from Profinder (CE–MS and LC–MS data) and Quantitative analysis (GC–MS data) were exported to Excel Software (Microsoft Office 2013) for data filtration. As the QC surrogate was used, features were filtered by selecting the data present in at least 80% of the samples in 1 of the 2 groups: cachexia and controls (Appendix S1).
Compound identification
Putative identification of features from LC–MS and CE–MS representing significant differences in class separation was performed by searching accurate masses against different databases available online as METLIN (http://metlin.scripps.edu), KEGG (http://www.genome.jp/kegg/genome.html), and LIPID MAPS (http://www.lipidmaps.com) by the CEU Mass Mediator tool (http://ceumass.eps.uspceu.es/mediator). HMDB (http://hmdb.ca) was also considered for additional information. Some compounds putatively identified by LC–MS were confirmed by LC–MS/MS (Appendix S2, Table S1).
Statistical analysis
Differences between profiles from cachexia and control groups, obtained within each technique, were evaluated with univariate and multivariate (MVDA) data analysis. For univariate data analysis, differences between the two groups were evaluated for each individual metabolite by applying the Mann–Whitney U test (p‐value ≤ 0.05), using Matlab (R2015a, MathWorks). For MVDA, principal component analysis and orthogonal partial least squares regression (OPLS‐DA) were applied using SIMCA‐P+ 12.0 (Umetrics, Umea, Sweden). Significant variables in MVDA were selected according to the Jack‐knife confident interval and variance important in projection from OPLS‐DA model. Furthermore, in order to avoid the risk of overfitting, the model was validated by cross‐validation tool. The percentage of correctly classified samples for each model is described in the Results section.
Pathway mapping of metabolites
Given that metabolites and enzymes involved in the same biological processes are often dysregulated together in cancer, we believe that pathways‐based metabolomics analyses provide more information on biological functions than the metabolites alone. For this purpose, we used MetaboAnalyst 3.0 (http://www.metaboanalyst.ca/).31, 32 The Metabolomics Pathway Analysis module combines results from powerful pathway enrichment analysis along with the pathway topology analysis to identify the most relevant pathways involved in our study. Metabolites mapped to pathways are included in Appendix S2, Table S2.
Results
The diagnosis of cachexia in this study was based on weight loss. Patients with cachexia had significantly lower values in body mass index and body surface area than control patients (Table 1). Clinical biochemical analyses detected higher values of C‐reactive protein (CRP) and cortisol in cachectic patients, as well as a significantly lower concentration of plasma albumin and a non‐significant decrease in circulating lipids (total cholesterol, triglycerides) (Table 1).
As explained, the metabolite fingerprint was assessed using three analytical platforms, GC–MS, CE–MS, and LC–MS. In GC–MS analysis, data set showed 115 identified compounds after alignment and 70 compounds after grouping the different derivatives of the same metabolites. For CE and LC analysis, data set from molecular feature extraction analysis showed 1140 features in positive CE–MS, 1860 and 1062 in positive and negative LC–MS. After recursive feature extraction analysis, feature inspection, and filtering, the data set was reduced to 232, 149, and 95, respectively.
In order to evaluate the performance of analytical platforms reducing data dimensionality, we generated different models by means of principal component analyses (Figure 1). The results show good grouping of QC samples, indicating appropriate performance of the bioanalyses and data quality, and therefore, supporting that the separation between groups are related to biological and not analytical variations. As can be seen, biological samples were slightly separated by the two techniques and one biological outlier was detected in positive and negative LC–MS using Hotelling's T 2 Range. Supervised OPLS‐DA were employed for modelling differences between the groups. The applied models showed clear separation of cachexia and control group in all analytical techniques used (Figure 2) with high quality, proven by good values of variance explained (R2) and variance predicted (Q2). To estimate the predictive ability of the OPLS‐DA models, cross‐validation was performed. Samples were classified correctly in 80% in GC–MS, 97% in CE–MS, 96% in LC–MS (positive mode), and 89% in LC–MS (negative mode). Additionally, the univariate statistical interpretation revealed individual significant metabolites, 7 statistically significant features in GC–MS, 24 in LC–MS electrospray ionization (ESI)+, and 58 in LC–MS ESI−.
Figure 1.
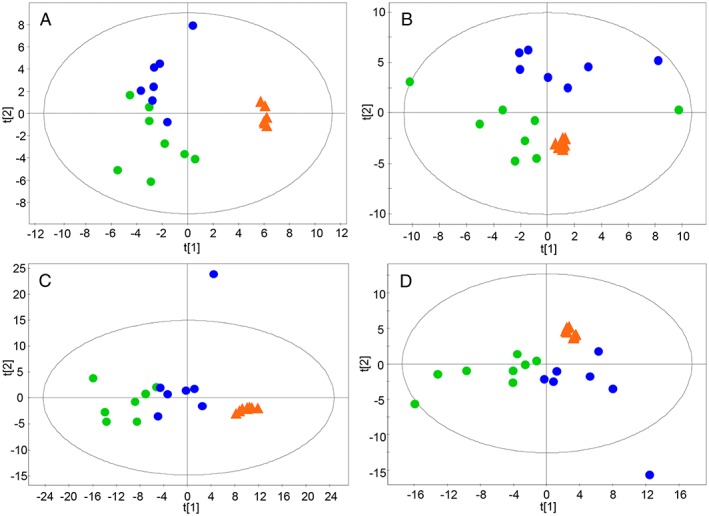
Principal component analysis‐X score plots for all samples in the study. (A) Gas chromatography–mass spectrometry, (B) capillary electrophoresis–mass spectrometry ESI(+), (C) liquid chromatography–mass spectrometry ESI(+), (D) liquid chromatography–mass spectrometry ESI(−). Blue dots, control; green dots, cachexia; orange triangles, quality control.
Figure 2.
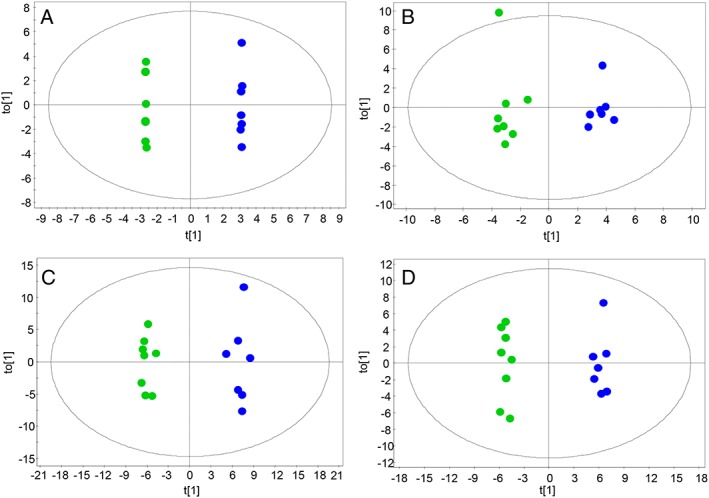
Orthogonal partial least squares regression models. (A) Gas chromatography–mass spectrometry (R 2 = 0.998, Q 2 = 0.678), (B) capillary electrophoresis–mass spectrometry ESI(+) (R 2 = 0.929, Q 2 = 0.816), (C) liquid chromatography–mass spectrometry ESI(+) (R 2 = 0.827, Q 2 = 0.699), (D) liquid chromatography–mass spectrometry ESI(−) (R 2 = 0.827, Q 2 = 0.703). Blue dots, control; green dots, cachexia.
The results of statistically significant compounds identified and confirmed identification by LC–MS/MS are summarized in Table 2 and Appendix S2. In GC–MS, amino acids were the majority of significant compounds. For CE–MS, the largest group belong to amino acids and amino acid derivatives groups. Finally, in LC–MS, the most populated category corresponded to lysophospholipids, followed by sterol lipids.
Table 2.
List of statistically significant compounds identified by a gas chromatography–mass spectrometry (GC–MS), capillary electrophoresis–mass spectrometry (CE–MS), and liquid chromatography–mass spectrometry (LC–MS) with regulation in the cachexia group
| Compound name | Molecular formula | Mass (DB) | RT (min) | Mass error (ppm) | p‐value | VIP | Jack‐knifeb | FCc | Analytical technique | Confirmation | DET |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Carboxylic acids and derivatives | |||||||||||
| Citric acid | C6H8O7 | 192.0270 | 0.81 | 4 | 0.014a | 1.5 | JK | 0.52 | LC | MS/MS | ESI± |
| Amino Acids and derivatives | |||||||||||
| Glutamine | C5H10N2O3 | 146.0691 | 14.97 | 1 | 0.013 | 1.1 | JK | 0.66 | CE/GC | Putative | ESI+ |
| Asparagine | C4H8N2O3 | 132.0535 | 15.86 | 3 | 0.0092 | 1.2 | JK | 0.68 | CE | Putative | ESI+ |
| Arginine | C6H14N4O2 | 174.1117 | 11.52 | 1 | 0.0087a | 1.8 | JK | 0.44 | CE | Putative | ESI+ |
| Citrulline | C6H13N3O3 | 175.0957 | 16.65 | 1 | 0.021 | 1.3 | JK | 0.54 | CE | Putative | ESI+ |
| Ornithine | C5H12N2O2 | 132.0899 | 11.09 | 0 | 0.0087a | 1.7 | JK | 0.50 | CE/GC | Putative | ESI+ |
| Lysine | C6H14N2O2 | 146.1055 | 11.17 | 2 | 0.0044a | 1.9 | JK | 0.51 | CE/GC | Putative | ESI+ |
| Carnitine | C7H15NO3 | 161.1052 | 0.75 | 1 | 0.041 | 0.64 | — | 0.72 | LC | MS/MS | ESI+ |
| Methionine | C5H11NO2S | 149.0510 | 16.14 | 0 | 0.0044a | 1.8 | JK | 0.52 | CE/GC | Putative | ESI+ |
| Phenylalanine | C9H11NO2 | 165.0790 | 16.61 | 0 | 0.028a | 1.9 | JK | 0.56 | CE/GC/LC | Putative | ESI+ |
| Tyrosine | C9H11NO3 | 181.0739 | 17.02 | 0 | 0.028 | 1.4 | JK | 0.66 | CE | Putative | ESI+ |
| Tryptophan | C11H12N2O2 | 204.0899 | 16.54 | 0 | 0.0065a | 1.8 | JK | 0.43 | CE/GC/LC | Putative | ESI+ |
| Indolelactic acid | C11H11NO3 | 205.0739 | 1.01 | 0 | 0.024a | 1.8 | JK | 0.41 | LC | MS/MS | ESI+ |
| Histidine | C6H9N3O2 | 155.0695 | 11.75 | 2 | 0.013 | 0.92 | JK | 0.71 | CE | Putative | ESI+ |
| Serine | C3H7NO3 | 105.0426 | 15.22 | 3 | 0.040 | 1.0 | JK | 0.74 | CE | Putative | ESI+ |
| Threonine | C4H9NO3 | 119.0582 | 15.91 | 5 | 0.044a | 1.8 | JK | 0.41 | CE/GC | Putative | ESI+ |
| Alanine | C3H7NO2 | 89.0477 | 13.95 | 2 | 0.013 | 0.75 | JK | 0.77 | CE | Putative | ESI+ |
| Valine | C5H11NO2 | 117.0790 | 0.79 | 0 | 0.021 | 1.1 | JK | 0.55 | LC | Putative | ESI+ |
| Glycerophospholipids | |||||||||||
| LPC(14:0) sn‐2 | C22H46NO7P | 467.3012 | 15.14 | 1 | 0.040 | 1.2 | JK | 0.50 | LC | MS/MS | ESI± |
| LPC(14:0) sn‐1 | C22H46NO7P | 467.3012 | 15.90 | 0 | 0.028 | 1.2 | JK | 0.46 | LC | MS/MS | ESI± |
| LPC(16:0) sn‐2 | C24H50NO7P | 495.3325 | 19.18 | 2 | 0.028a | 1.8 | JK | 0.57 | LC | MS/MS | ESI± |
| LPC(16:0) sn‐1 | C24H50NO7P | 495.3325 | 19.95 | 2 | 0.028a | 1.8 | JK | 0.65 | LC | MS/MS | ESI± |
| LPC(O‐16:0) | C24H52NO6P | 481.3532 | 21.16 | 4 | 0.038a | 1.5 | JK | 0.11 | LC | Putative | ESI± |
| LPC(O‐16:1)/LPC(P‐16:0) | C24H50NO6P | 479.3376 | 21.17 | 1 | 0.045a | 1.6 | JK | 0.70 | LC | Putative | ESI± |
| LPC(18:0) sn‐2 | C26H54NO7P | 523.3638 | 23.46 | 0 | 0.028a | 1.8 | JK | 0.52 | LC | MS/MS | ESI+ |
| LPC(18:0) sn‐1 | C26H54NO7P | 523.3638 | 24.25 | 2 | 0.014a | 1.6 | JK | 0.53 | LC | MS/MS | ESI+ |
| LPC(O‐18:1)/LPC(P‐18:0) | C26H54NO6P | 507.3689 | 22.00 | 0 | 0.040 | 1.5 | JK | 0.69 | LC | Putative | ESI± |
| LPC(18:2) sn‐2 | C26H50NO7P | 519.3325 | 17.68 | 0 | 0.031a | 1.5 | JK | 0.60 | LC | MS/MS | ESI± |
| LPC(18:2) sn‐1 | C26H50NO7P | 519.3325 | 18.28 | 1 | 0.024a | 1.4 | JK | 0.58 | LC | MS/MS | ESI− |
| LPC(20:1) sn‐2 | C28H56NO7P | 549.3794 | 21.31 | 4 | 0.045a | 1.5 | JK | 0.52 | LC | Putative | ESI+ |
| LPC(20:1) sn‐1 | C28H56NO7P | 549.3794 | 24.85 | 0 | 0.028a | 1.7 | JK | 0.70 | LC | MS/MS | ESI+ |
| LPC(20:2) | C28H54NO7P | 547.3638 | 21.08 | 0 | 0.038a | 1.5 | JK | 0.60 | LC | Putative | ESI+ |
| LPC(20:3) sn‐2 | C28H52NO7P | 545.3481 | 19.17 | 0 | 0.028a | 1.6 | JK | 0.38 | LC | MS/MS | ESI± |
| LPC(20:3) sn‐1 | C28H52NO7P | 545.3481 | 20.73 | 4 | 0.045a | 0.77 | JK | 0.12 | LC | MS/MS | ESI± |
| LPC(20:4) | C28H50NO7P | 543.3325 | 17.74 | 4 | 0.040 | 1.3 | JK | 0.63 | LC | MS/MS | ESI− |
| LPC(22:4) | C30H54NO7P | 571.3638 | 21.31 | 0 | 0.045a | 1.5 | JK | 0.52 | LC | Putative | ESI+ |
| LPC(22:5) | C30H52NO7P | 569.3481 | 20.26 | 0 | 0.013 | 1.2 | JK | 0.44 | LC | MS/MS | ESI+ |
| LPE(16:0) sn‐2 | C21H44NO7P | 453.2855 | 18.81 | 0 | 0.020 | 1.2 | JK | 0.67 | LC | MS/MS | ESI± |
| LPE(16:0) sn‐1 | C21H44NO7P | 453.2855 | 19.59 | 0 | 0.028 | 1.2 | JK | 0.65 | LC | MS/MS | ESI± |
| LPE(18:0) sn‐2 | C23H48NO7P | 481.3168 | 19.89 | 3 | 0.014a | 1.6 | JK | 0.68 | LC | MS/MS | ESI+ |
| LPE(18:0) sn‐1 | C23H48NO7P | 481.3168 | 22.94 | 6 | 0.038a | 1.3 | JK | 0.63 | LC | MS/MS | ESI+ |
| LPE(20:0) | C25H52NO7P | 509.3481 | 22.09 | 1 | 0.028a | 1.8 | JK | 0.62 | LC | Putative | ESI± |
| LPE(20:3) | C25H46NO7P | 503.3012 | 19.49 | 7 | 0.020 | 1.0 | — | 0.40 | LC | MS/MS | ESI+ |
| PE(24:0) | C29H58NO8P | 579.3900 | 19.89 | 8 | 0.014a | 1.5 | JK | 0.68 | LC | Putative | ESI− |
| PE(26:0) | C31H62NO8P | 607.4213 | 24.24 | 9 | 0.011a | 1.6 | JK | 0.55 | LC | Putative | ESI− |
| Sphingolipids | |||||||||||
| SM(d30:0) | C35H75N2O6P | 650.5362 | 31.23 | 3 | 0.028 | 1.3 | JK | 0.59 | LC | Putative | ESI+ |
| SM(d34:1) | C39H79N2O6P | 702.5676 | 33.40 | 0 | 0.013 | 1.4 | JK | 0.78 | LC | MS/MS | ESI+ |
| Sterol Lipids | |||||||||||
| Cortisol | C21H30O5 | 362.2093 | 4.27 | 1 | 0.030a | 1.3 | JK | 1.67 | LC | Putative | ESI− |
VIP, variable importance in the projection; FC, fold change; LPC, lysophosphatidylcholines; LPE, lysophosphatidylethanolamine; PE: phosphatidylethanolamine; LPC: lysophosphatidylcholine; SM: sphingomyelin.
p‐value corrected by Benjamini Hochberg (FDR correction).
JK, selected by Jack‐Knife confidence intervals estimative, 95 % confidence level.
FC, fold change in the comparison (average in cachexia/average control).
The most prominent metabolic alteration in plasma of cachectic patients was the decrease of amino acids and derivatives [especially arginine, tryptophan, indolelactic acid, and threonine, with 0.4‐fold change (FC) compared with non‐cachectic patients], along with the reduction of glycerophospholipids [mainly lysophosphatidylcholines (LPC(O‐16:0) and LPC(20:3) sn‐1, FC = 0.1] and sphingolipids [SM(d30:0), FC = 0.5]. The metabolite with the highest increase was cortisol (FC = 1.67).
Additionally, and according to the pathway‐based metabolite sets analysis, we identified several pathways with differential features between cachectic and non‐cachectic patients in our cohort (Appendix S2, Table S2). Aminoacyl‐tRNA biosynthesis is the top‐ranked pathway according to enrichment criteria. From a pathway topology analysis perspective, arginine and proline metabolism; alanine, aspartate, and glutamine metabolism; and glycine, serine, and threonine metabolism are the pathways with the highest impact. Amino acids implicated in these pathways showed decreased level ranked between 0.41‐fold and 0.77‐fold in plasma from cachectic patients (Table 2).
Moreover, cortisol is present at higher concentrations in samples from cachectic patients. The increase in cortisol concentrations in samples from cachectic patients was observed with two independent methodologies: the fold change in cortisol found with our blind approach was 1.67, whereas the respective fold change for cortisol determined with a routine immunoassay method was very similar, 1.82. This adds reliability to the methodology used to find differences in the metabolic profile.
Discussion
Several studies have tried to correlate different biomarkers with cachexia. Especially oesophageal, pancreatic, and gastrointestinal cancers have shown a positive correlation between high levels of plasma glycerol and free fatty acids, relative weight loss,33, 34, 35 and low serum levels of carnitine.36 Also, several plasma markers of inflammation have been identified as potential biomarkers of cachexia, including haemoglobin, albumin, CRP, ghrelin, adiponectin, leptin, and IGF‐1.37 Cytokines including IL‐1, IL‐6, and TNF‐alpha have been associated with cachexia, and clinical trials with antagonists of these molecules are currently under evaluation.38, 39, 40 Most of the evidence about inflammation and cytokines in cachexia has led to the assumption that the major cause for cachexia is a cytokine overproduction.41
In our study, we found higher molecular surrogates of inflammation in cachectic patients (CRP, cortisol). Lipid‐mobilizing and protein‐mobilizing factors produced by the tumour also contribute to activate the inflammatory cascade,42 which in turn stimulate the release of cortisol and catecholamines from the adrenal glands,41 subsequently leading to changes in the metabolism of proteins (together with direct cytokine activation of the ubiquitin‐mediated proteolytic system), carbohydrates, and lipids.
One of the most relevant results shown in Tables 1 and 2 is the increased concentration of cortisol in cachectic patients, found with two different methodologies. This correlation was already described in 1999, although treatment with an antagonist of glucocorticoid receptors could not abrogate detrimental wasting of muscle.43 Nevertheless, higher cortisol levels in cachectic patients has not always been detected, because Chauhan et al.44 in a pilot study with nine cachectic patients found only non‐significant differences versus nine healthy controls. The type of cancer, the number of patients, the type of treatment, and the sampling hour can be the reasons for this lack of consistency in the elevation of cortisol. This glucocorticoid has a highly relevant role in the complications of age‐related degenerative diseases,45 and the regulation of muscle mass mediated by cortisol.46 In fact, most of the variations seen in our metabolomics study can be directly related to the increase in cortisol and its relationship with insulin resistance,47 which is strongly related to cancer cachexia.48 Noteworthy, given that glucocorticoids might increase appetite,49 these are frequently offered in routine clinical practice to cancer patients with cachexia.50 However, this clinical practice cannot be supported by our findings.
In our study, the most prominent metabolic alteration in plasma of cachectic patients was the decrease of amino acids and derivatives, along with the reduction of glycerolysophospholipids.
Although the studies differ in the change of some amino acids (such as glutamine, histidine, asparagine, arginine, or phenylalanine), our work coincides in the decrease levels of derivatives of histidine, lysine, and tryptophan described by Ubhi et al.51 in patients with cancer related cachexia. Similar to Nezami Ranjbar et al.52 in plasma samples from hepatocellular carcinoma patients vs. patients with liver cirrhosis, we show decreased levels of citric acid (p‐value = 0.014) in plasma from cachectic cancer versus non‐cachectic cancer patients.
Protein and amino acid metabolism has been recognized as a key process in cancer cachexia more than 20 years ago, focusing efforts to understand the molecular basis of the problem and identify potential biomarkers.53, 54 The decrease of amino acids showed additional metabolic disorders involving either protein synthesis or amino acid metabolism pathways (Appendix S2, Table S2). Dysregulation of amino acid metabolism and metabolites are early events associated with cancer tumorigenesis. Protein turnover seems to be increased in about 32–35% in cancer patients as a consequence of muscle catabolism; as a result, increased nitrogen excretion is present. In fact, plasma free amino acid profile has been recently proposed as a diagnostic tool for lung cancer.55 Our multiplatform metabolomics approach has allowed us to see differences (lower signal in cachectic patients) in 13 of the 20 proteinogenic amino acids. Twelve out of the 13 (all but lysine) are glucogenic and could act as substrates for glucose synthesis. Despite the differences in the metabolism for each of them, it is out of discussion that such common trend must be due to alterations in major metabolic pathways that affect simultaneously to all of them, what has been called ‘hypermetabolism’. In cachexia, alterations of lactate metabolism (the Cori cycle, i.e. glycolysis in muscle and gluconeogenesis in liver) have been described since the 1970s,56, 57 and increased gluconeogenesis from alanine was found in lung cancer patients with weight‐loss vs. weight‐stable or healthy controls.58 In 2006, Bongaerts et al.59 postulated that ‘the Cori‐cycle, especially the gluconeogenesis, may completely elucidate the cause of cancer cachexia’. Paradoxically, even though there is a dramatic weight loss in these patients, increased proteolysis and increased gluconeogenesis from amino acids are common to diabetes/insulin resistance and metabolic syndrome. This is because insulin resistance and/or metabolic syndrome can be also originated by disturbances in glucocorticoid metabolism, as shown in cases of adrenocortical tumours.60
Lower values of triglycerides in plasma of cachectic patients has been shown in other studies,29, 36 but our study has not been able to detect it. Several studies suggest that an increase in catabolism rather than a defect in anabolism may be more important in triglyceride hydrolysis in these patients.6 It has been described that cancer patients who are losing weight show an increased turnover of both glycerol and fatty acids compared with normal subjects or cancer patients without weight loss. However, there was no evidence for a decreased level of lipoprotein lipase in the adipose tissue of cancer patients, but there was a two‐fold increase in the relative level of mRNA for hormone‐sensitive lipase, suggesting an upregulation of triacylglycerol hydrolysis.6, 61 Also, in response to glucose limitation, fatty acid can also be consumed through β‐oxidation to provide key substitute energy for cancer cell survival. In our study, there were no big differences in total triglycerides, and the metabolomics approach did not show up differences neither in individual monoglycerides, diglycerides, or triglycerides, nor in free fatty acids. Nevertheless, 19 LPC and 6 lysophosphatidylethanolamines were found lower in cachexia group. To our knowledge, this difference in circulating lysophospholipids has not been previously described associated with cachexia, although Sasagawa et al.62 described changes in the circulating phospholipids associated with the evolution of multiple myeloma, and Muqaku et al.63 showed decreased levels of phosphatidylcholine concentration in blood from metastatic melanoma patients with cachexia.63 In addition, one of the interesting features of our methodology is that we have been able to find differences in different sn‐1 and sn‐2 lysophospholipids, and therefore, not only phospholipase A2 is involved in these changes. It is noteworthy to mention that among the putatively identified LPC, three have been identified as monoalkylglycerophospholipids [LPC(O‐16:0), LPC(O‐16:1)/LPC(P‐16:0), LPC(O‐18:1)/LPC(P‐18:0)]. Given that pharmacologically, some monoalkylphospholipids have shown antineoplastic potential,64 the mechanisms resulting in reduction of such compounds might contribute to cancer progression.
Metabolic regulation is elaborately linked to cancer progression, as proliferating cells demand nutrients for energy production as well as synthesis of genetic materials, proteins, and lipids. Moreover, amino acid, glucose, and phospholipid metabolism can be interconnected through glutaminolysis, a process that supplies carbon and nitrogen resources to the growing and proliferating cancer cells.65
In summary, cachexia is multifaceted, and its management will require a multi‐modality approach. The complexity of cancer‐associated weight loss, coupled with the fact that cancer treatment in general utilizes the paradigm of multi‐agent therapy, suggests that cachexia treatment will necessitate more than one concurrent intervention.66 Also, it is important to recognize the importance of chemotherapy or radiotherapy‐induced muscle loss.67 Intervention is more likely to be effective when given early, before pronounced metabolic abnormalities produce resistance to nutritional intervention.68 The earlier phase of active anti‐cancer therapy, which frequently achieves good control of tumour, offers a window of opportunity for intervention against malnutrition and, by reducing catabolic drive, against cachexia.
We have described a new model to study metabolomics data for disease identification. Also, pathway‐based analysis has significantly enhanced our capacity to explore large‐scale omic data, providing an invaluable tool for identifying the modified functionalities involved in this complex disease.
Blood‐based metabolomics is a promising method for cachexia research. However, similar to other types of biomarkers, metabolomics biomarker results are difficult to replicate among different studies for a combination of reasons, such as the heterogeneity of the populations and study sizes, variability of the experimental protocols, noise in the metabolomics data, and biological variations in the turnover rates of metabolites.69 A community effort needs to be made to improve data sharing in order to accumulate statistically well‐powered data sets.
A relevant point in cachexia cancer research is its translation to a therapeutic strategy. The lack of complete association between experimental and clinical data likely relates to the fact that not all tumours impinge their effects on the host in the same manner. Finally, patients generally receive systemic antineoplastic therapy, and the interaction of these treatments with the development of cachexia is unknown. Our study was the consequence of the premise that achieving a better understanding of the integrative physiopathology of this complex syndrome may yield yet further novel therapeutic approaches. Development of a successful treatment method will likely require a better understanding of the pathogenesis of cancer cachexia and identification of a dynamic metabolic surrogate marker of pharmacological intervention.
Limitations of the study
One obvious limitation of this study is the number of samples, but this was an exploratory study in patients in very late stage of the disease. The lack of homogeneity in the types of cancer included has been similarly distributed between both groups. This study design may have resulted in a loss in the identification of tumour type‐specific compounds, but this huge data‐noise has been able to identify trans‐tumoral differences associated with cancer cachexia. Other studies have shown gender differences in metabolic regulation, treatment‐related mortality, and progression of cachexia, being the female sex a favourable prognostic factor.7, 70 Our study was not designed to evaluate the effect of gender in the genesis nor the evolution of cancer‐related cachexia.
Ethical standards
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia, and Muscle.71 This study was approved by the Ethics Committee of the ‘12 de Octubre’ University Hospital, and it was done in accordance with the Declaration of Helsinki, International Conference on Harmonization, and Good Clinical Practice. Written informed consent was provided by all participating patients.
Conflict of interest
None declared.
Supporting information
Data S1. Supporting info item
Table S1. Statistically Significant Compounds identified by LC–MS/MS and their characteristic fragments.
Table S2. Results from Pathway Analysis.
Acknowledgements
We would like to thank the patients who participated in this study. This work was supported by Instituto de Salud Carlos III, Fondo de Investigación Sanitaria, and co‐financed by the European Regional Development Fund (FEDER‐"A way to make Europe") (grant number PI10/02072); Fundación Médica Mutua Madrileña (grant number MMA10/0018); and the Spanish Ministerio de Economía y Competitividad (grant number CTQ2014‐55279‐R). The sponsors had no role in study design, data collection, data analysis, data interpretation, writing of the paper, and in the decision to submit the article for publication.
Cala, M. P. , Agulló‐Ortuño, M. T. , Prieto‐García, E. , González‐Riano, C. , Parrilla‐Rubio, L. , Barbas, C. , Díaz‐García, C. V. , García, A. , Pernaut, C. , Adeva, J. , Riesco, M. C. , Rupérez, F. J. , and Lopez‐Martin, J. A. (2018) Multiplatform plasma fingerprinting in cancer cachexia: a pilot observational and translational study. Journal of Cachexia, Sarcopenia and Muscle, 9: 348–357. doi: 10.1002/jcsm.12270.
References
- 1. Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr 2008;27:793–799. [DOI] [PubMed] [Google Scholar]
- 2. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 3. von Haehling S, Anker SD. Prevalence, incidence and clinical impact of cachexia: facts and numbers‐update 2014. J Cachexia Sarcopenia Muscle 2014;5:261–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Argiles JM, Busquets S, Stemmler B, Lopez‐Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 2014;14:754–762. [DOI] [PubMed] [Google Scholar]
- 5. Wheelwright S, Darlington AS, Hopkinson JB, Fitzsimmons D, White A, Johnson CD. A systematic review of health‐related quality of life instruments in patients with cancer cachexia. Support Care Cancer 2013;21:2625–2636. [DOI] [PubMed] [Google Scholar]
- 6. Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 2009;89:381–410. [DOI] [PubMed] [Google Scholar]
- 7. Kurishima K, Watanabe H, Ishikawa H, Satoh H, Hizawa N. Modified glasgow prognostic score in patients with small‐cell lung cancer. Mol Clin Oncol 2017;7:121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fearon KC. The 2011 ESPEN Arvid Wretlind lecture: cancer cachexia: the potential impact of translational research on patient‐focused outcomes. Clin Nutr 2012;31:577–582. [DOI] [PubMed] [Google Scholar]
- 9. Mariani L, Lo Vullo S, Bozzetti F. Weight loss in cancer patients: a plea for a better awareness of the issue. Support Care Cancer 2012;20:301–309. [DOI] [PubMed] [Google Scholar]
- 10. Vazeille C, Jouinot A, Durand JP, Neveux N, Boudou‐Rouquette P, Huillard O, et al. Relation between hypermetabolism, cachexia, and survival in cancer patients: a prospective study in 390 cancer patients before initiation of anticancer therapy. Am J Clin Nutr 2017;105:1139–1147. [DOI] [PubMed] [Google Scholar]
- 11. Jouinot A, Vazeille C, Durand JP, Huillard O, Boudou‐Rouquette P, Coriat R, et al. Resting energy expenditure in the risk assessment of anticancer treatments. Clin Nutr 2017. available at: https://doi.org/10.1016/j.clnu.2017.01.007 (article in press). [DOI] [PubMed] [Google Scholar]
- 12. Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol 2013;10:90–99. [DOI] [PubMed] [Google Scholar]
- 13. Suzuki H, Asakawa A, Amitani H, Nakamura N, Inui A. Cancer cachexia‐‐pathophysiology and management. J Gastroenterol 2013;48:574–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 2012;16:153–166. [DOI] [PubMed] [Google Scholar]
- 15. Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, et al. Diagnostic criteria for the classification of cancer‐associated weight loss. Diagnostic criteria for the classification of cancer‐associated weight loss. J Clin Oncol 2015;33:90–99. [DOI] [PubMed] [Google Scholar]
- 16. Dumas JF, Peyta L, Couet C, Servais S. Implication of liver cardiolipins in mitochondrial energy metabolism disorder in cancer cachexia. Biochimie 2013;95:27–32. [DOI] [PubMed] [Google Scholar]
- 17. Aoyagi T, Terracina KP, Raza A, Matsubara H, Takabe K. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol 2015;7:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gallagher IJ, Jacobi C, Tardif N, Rooyackers O, Fearon K. Omics/systems biology and cancer cachexia. Semin Cell Dev Biol 2016;54:92–103. [DOI] [PubMed] [Google Scholar]
- 19. Aapro M, Arends J, Bozzetti F, Fearon K, Grunberg SM, Herrstedt J, et al. Early recognition of malnutrition and cachexia in the cancer patient: a position paper of a European School of Oncology Task Force. Ann Oncol 2014;25:1492–1499. [DOI] [PubMed] [Google Scholar]
- 20. Baldwin C, Spiro A, Ahern R, Emery PW. Oral nutritional interventions in malnourished patients with cancer: a systematic review and meta‐analysis. J Natl Cancer Inst 2012;104:371–385. [DOI] [PubMed] [Google Scholar]
- 21.von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle 2010;1(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kanat O. B.H. ON. Cancer cachexia: an unmet need in cancer treatment. Clin Investig 2014;4:601–604. [Google Scholar]
- 23. Mastrangelo A, Armitage EG, Garcia A, Barbas C. Metabolomics as a tool for drug discovery and personalised medicine. A review Curr Top Med Chem 2014;14:2627–2636. [DOI] [PubMed] [Google Scholar]
- 24. Huang S, Chong N, Lewis NE, Jia W, Xie G, Garmire LX. Novel personalized pathway‐based metabolomics models reveal key metabolic pathways for breast cancer diagnosis. Genome Med 2016;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wishart D. Metabolism, Metabolomics and Cancer. FASEB J 2015;29(1 Supplement). [Google Scholar]
- 26. Der‐Torossian H, Gourin CG, Couch ME. Translational implications of novel findings in cancer cachexia: the use of metabolomics and the potential of cardiac malfunction. Curr Opin Support Palliat Care 2012;6:446–450. [DOI] [PubMed] [Google Scholar]
- 27. O'Connell TM, Ardeshirpour F, Asher SA, Winnike JH, Yin X, George J, et al. Metabolomic analysis of cancer cachexia reveals distinct lipid and glucose alterations. Metabolomics 2008;4:216. [Google Scholar]
- 28. Eisner R, Stretch C, Eastman T, Xia J, Hau D, Damaraju S, et al. Learning to predict cancer‐associated skeletal muscle wasting from 1H‐NMR profiles of urinary metabolites. Metabolomics 2011;7:25–34. [Google Scholar]
- 29. Fujiwara Y, Kobayashi T, Chayahara N, Imamura Y, Toyoda M, Kiyota N, et al. Metabolomics Evaluation of Serum Markers for Cachexia and Their Intra‐Day Variation in Patients with Advanced Pancreatic Cancer. PLoS One 2014;9:e113259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gika HG, Macpherson E, Theodoridis GA, Wilson ID. Evaluation of the repeatability of ultra‐performance liquid chromatography‐TOF‐MS for global metabolic profiling of human urine samples. J Chromatogr B Analyt Technol Biomed Life Sci 2008;871:299–305. [DOI] [PubMed] [Google Scholar]
- 31. Xia J, Wishart DS. MetPA: a web‐based metabolomics tool for pathway analysis and visualization. Bioinformatics 2010;26:2342–2344. [DOI] [PubMed] [Google Scholar]
- 32. Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0‐‐making metabolomics more meaningful. Nucleic Acids Res 2015;43:W251–W257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Agustsson T, Wikrantz P, Ryden M, Brismar T, Isaksson B. Adipose tissue volume is decreased in recently diagnosed cancer patients with cachexia. Nutrition 2012. Sep;28:851–855. [DOI] [PubMed] [Google Scholar]
- 34. Zuijdgeest‐van Leeuwen SD, van den Berg JW, Wattimena JL, van der Gaast A, Swart GR, Wilson JH, et al. Lipolysis and lipid oxidation in weight‐losing cancer patients and healthy subjects. Metabolism 2000;49:931–936. [DOI] [PubMed] [Google Scholar]
- 35. Ebadi M, Mazurak VC. Potential Biomarkers of Fat Loss as a Feature of Cancer Cachexia. Mediat Inflamm 2015;2015:820934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Malaguarnera M, Risino C, Gargante MP, Oreste G, Barone G, Tomasello AV, et al. Decrease of serum carnitine levels in patients with or without gastrointestinal cancer cachexia. World J Gastroenterol 2006;12:4541–4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gioulbasanis I, Georgoulias P, Vlachostergios PJ, Baracos V, Ghosh S, Giannousi Z, et al. Mini Nutritional Assessment (MNA) and biochemical markers of cachexia in metastatic lung cancer patients: interrelations and associations with prognosis. Lung Cancer 2011;74:516–520. [DOI] [PubMed] [Google Scholar]
- 38. Mueller TC, Bachmann J, Prokopchuk O, Friess H, Martignoni ME. Molecular pathways leading to loss of skeletal muscle mass in cancer cachexia‐‐can findings from animal models be translated to humans? BMC Cancer 2016;16:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Porporato PE. Understanding cachexia as a cancer metabolism syndrome. Oncogene 2016;5:e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miyamoto Y, Hanna DL, Zhang W, Baba H, Lenz HJ. Molecular Pathways: Cachexia Signaling‐A Targeted Approach to Cancer Treatment. Clin Cancer Res 2016;22:3999–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morley JE, Thomas DR, Wilson MM. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr 2006;83:735–743. [DOI] [PubMed] [Google Scholar]
- 42. Nikolova‐Karakashian MN, Reid MB. Sphingolipid metabolism, oxidant signaling, and contractile function of skeletal muscle. Antioxid Redox Signal 2011;15:2501–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rivadeneira DE, Naama HA, McCarter MD, Fujita J, Evoy D, Mackrell P, et al. Glucocorticoid blockade does not abrogate tumor‐induced cachexia. Nutr Cancer 1999;35:202–206. [DOI] [PubMed] [Google Scholar]
- 44. Chauhan A, Sequeria A, Manderson C, Maddocks M, Wasley D, Wilcock A. Exploring autonomic nervous system dysfunction in patients with cancer cachexia: a pilot study. Auton Neurosci 2012;166:93–95. [DOI] [PubMed] [Google Scholar]
- 45. Martocchia A, Stefanelli M, Falaschi GM, Toussan L, Ferri C, Falaschi P. Recent advances in the role of cortisol and metabolic syndrome in age‐related degenerative diseases. Aging Clin Exp Res 2016;28:17–23. [DOI] [PubMed] [Google Scholar]
- 46. Braun TP, Marks DL. The regulation of muscle mass by endogenous glucocorticoids. Front Physiol 2015;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Paredes S, Ribeiro L. Cortisol: the villain in metabolic syndrome? Revista da Associacao Rev Assoc Med Bras 2014;60:84–92. [DOI] [PubMed] [Google Scholar]
- 48. Honors MA, Kinzig KP. The role of insulin resistance in the development of muscle wasting during cancer cachexia. J Cachexia Sarcopenia Muscle 2012;3:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dallman MF, la Fleur SE, Pecoraro NC, Gomez F, Houshyar H, Akana SF. Minireview: glucocorticoids‐‐food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology 2004;145:2633–2638. [DOI] [PubMed] [Google Scholar]
- 50. Dev R, Wong A, Hui D, Bruera E. The Evolving Approach to Management of Cancer Cachexia. Oncology 2017;31:23–32. [PubMed] [Google Scholar]
- 51. Ubhi BK, Cheng KK, Dong J, Janowitz T, Jodrell D, Tal‐Singer R, et al. Targeted metabolomics identifies perturbations in amino acid metabolism that sub‐classify patients with COPD. Mol BioSyst 2012;8:3125–3133. [DOI] [PubMed] [Google Scholar]
- 52. Nezami Ranjbar MR, Luo Y, Di Poto C, Varghese RS, Ferrarini A, Zhang C, et al. GC‐MS Based Plasma Metabolomics for Identification of Candidate Biomarkers for Hepatocellular Carcinoma in Egyptian Cohort. PLoS One 2015;10:e0127299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pisters PW, Pearlstone DB. Protein and amino acid metabolism in cancer cachexia: investigative techniques and therapeutic interventions. Crit Rev Clin Lab Sci 1993;30:223–272. [DOI] [PubMed] [Google Scholar]
- 54. Pisters PWT, Brennan MF. In Pisters PWT, Brennan MF, eds. Protein and Amino Acid Metabolism in Cancer Cachexia, Vol. XV Springer‐Verlag Berlin Heidelberg; 1996. p 123–132. [Google Scholar]
- 55. Shingyoji M, Iizasa T, Higashiyama M, Imamura F, Saruki N, Imaizumi A, et al. The significance and robustness of a plasma free amino acid (PFAA) profile‐based multiplex function for detecting lung cancer. BMC Cancer 2013;13:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Waterhouse C. Lactate metabolism in patients with cancer. Cancer 1974;33:66–71. [DOI] [PubMed] [Google Scholar]
- 57. Waterhouse C, Jeanpretre N, Keilson J. Gluconeogenesis from alanine in patients with progressive malignant disease. Cancer Res 1979;39:1968–1972. [PubMed] [Google Scholar]
- 58. Leij‐Halfwerk S, Dagnelie PC, van Den Berg JW, Wattimena JD, Hordijk‐Luijk CH, Wilson JP. Weight loss and elevated gluconeogenesis from alanine in lung cancer patients. Am J Clin Nutr 2000;71:583–589. [DOI] [PubMed] [Google Scholar]
- 59. Bongaerts GP, van Halteren HK, Verhagen CA, Wagener DJ. Cancer cachexia demonstrates the energetic impact of gluconeogenesis in human metabolism. Med Hypotheses 2006;67:1213–1222. [DOI] [PubMed] [Google Scholar]
- 60. Altieri B, Tirabassi G, Della Casa S, Ronchi CL, Balercia G, Orio F, et al. Adrenocortical tumors and insulin resistance: What is the first step? Int J Cancer 2016;138:2785–2794. [DOI] [PubMed] [Google Scholar]
- 61. Zhang F, Du G. Dysregulated lipid metabolism in cancer. World J Biol Chem 2012;3:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sasagawa T, Okita M, Murakami J, Kato T, Watanabe A. Abnormal serum lysophospholipids in multiple myeloma patients. Lipids 1999;34:17–21. [DOI] [PubMed] [Google Scholar]
- 63. Muqaku B, Eisinger M, Meier SM, Tahir A, Pukrop T, Haferkamp S, et al. Multi‐omics Analysis of Serum Samples Demonstrates Reprogramming of Organ Functions Via Systemic Calcium Mobilization and Platelet Activation in Metastatic Melanoma. Mol Cell Proteomics 2017;16:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. van Blitterswijk WJ, Verheij M. Anticancer mechanisms and clinical application of alkylphospholipids. Biochim Biophys Acta 2013;1831:663–674. [DOI] [PubMed] [Google Scholar]
- 65. Dang CV. Glutaminolysis: supplying carbon or nitrogen or both for cancer cells? Cell Cycle 2010;9:3884–3886. [DOI] [PubMed] [Google Scholar]
- 66. Solheim TS, Laird BJ. Evidence base for multimodal therapy in cachexia. Curr Opin Support Palliat Care 2012;6:424–431. [DOI] [PubMed] [Google Scholar]
- 67. Awad S, Tan BH, Cui H, Bhalla A, Fearon KC, Parsons SL, et al. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin Nutr 2012;31:74–77. [DOI] [PubMed] [Google Scholar]
- 68. Muscaritoli M, Molfino A, Gioia G, Laviano A, Rossi FF. The “parallel pathway”: a novel nutritional and metabolic approach to cancer patients. Intern Emerg Med 2011;6:105–112. [DOI] [PubMed] [Google Scholar]
- 69. Johnson SR, Lange BM. Open‐access metabolomics databases for natural product research: present capabilities and future potential. Front Bioeng Biotechnol 2015;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Arthur ST, Van Doren BA, Roy D, Noone JM, Zacherle E, Blanchette CM. Cachexia among US cancer patients. J Cachexia Sarcopenia Muscle 2015;6:315–316.26672494 [Google Scholar]
- 71. von Haehling S, Morley JE, Coats AJ, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting info item
Table S1. Statistically Significant Compounds identified by LC–MS/MS and their characteristic fragments.
Table S2. Results from Pathway Analysis.


