Abstract
Background
Cancer‐associated wasting, termed cancer cachexia, has a profound effect on the morbidity and mortality of cancer patients but remains difficult to recognize and diagnose. While increases in circulating levels of a number of inflammatory cytokines have been associated with cancer cachexia, these associations were generally made in patients with advanced disease and thus may be associated with disease progression rather than directly with the cachexia syndrome. Thus, we sought to assess potential biomarkers of cancer‐induced cachexia in patients with earlier stages of disease.
Methods
A custom multiplex array was used to measure circulating levels of 25 soluble factors from 70 pancreatic cancer patients undergoing attempted tumour resections. A high‐sensitivity multiplex was used for increased sensitivity for nine cytokines.
Results
Resectable pancreatic cancer patients with cachexia had low levels of canonical pro‐inflammatory cytokines including interleukin‐6 (IL‐6), interleukin‐1β (IL‐1β), interferon‐γ (IFN‐γ), and tumour necrosis factor (TNF). Even in our more sensitive analysis, these cytokines were not associated with cancer cachexia. Of the 25 circulating factors tested, only monocyte chemoattractant protein‐1 (MCP‐1) was increased in treatment‐naïve cachectic patients compared with weight stable patients and identified as a potential biomarker for cancer cachexia. Although circulating levels of leptin and granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) were found to be decreased in the same cohort of treatment‐naïve cachectic patients, these factors were closely associated with body mass index, limiting their utility as cancer cachexia biomarkers.
Conclusions
Unlike in advanced disease, it is possible that cachexia in patients with resectable pancreatic cancer is not associated with high levels of classical markers of systemic inflammation. However, cachectic, treatment‐naïve patients have higher levels of MCP‐1, suggesting that MCP‐1 may be useful as a biomarker of cancer cachexia.
Keywords: Wasting, Weight loss, Biomarker
Introduction
Profound wasting of skeletal muscle and adipose tissue is perhaps the most obvious side effect of advanced cancer. This wasting, which is a part of the cancer cachexia syndrome, affects approximately 50% of all cancer patients, and nearly all patients with advanced disease.1, 2 While it is difficult to quantify the number of patient deaths that result directly from cachexia, it is clear that cancer cachexia is a significant contributor to both morbidity and mortality.3, 4
Pancreatic cancer patients have among the highest rates of cachexia, with an estimated 80–90% of patients with end‐stage disease affected.4, 5 Additionally, cachexia in pancreatic cancer patients is not only associated with end‐stage disease as in other cancers, as our own analysis demonstrated that over 70% of newly diagnosed pancreatic cancer patients meet cachexia criteria.6 Although nearly 70% of pancreas cancers are diagnosed after they have become unresectable, even pancreatic cancer patients undergoing resection have cachexia, and estimates indicate that 40% of pancreatic cancer patients considered candidates for resection already exhibit a 10% body weight loss.7, 8, 9, 10 Even in patients with resectable disease, cachexia is associated with poor outcomes and decreased survival.11
While the significance of cancer cachexia is clear, this syndrome remains under‐recognized due to the high reliance on self‐reported weight loss, which has poor reliability.12, 13, 14 Further, the levels of weight loss associated with poor outcomes are often not considered clinically meaningful.5, 15 Thus, a biomarker of cancer cachexia would be a valuable tool to improve the recognition of this syndrome.
A number of pro‐inflammatory cytokines are associated with cancer cachexia and thus considered potential biomarkers.16, 17, 18, 19 However, published data demonstrate that such associations were largely made in patients with advanced disease, including many in patients whom were undergoing treatment, often second‐line or third‐line chemotherapy. Further, patients with advanced disease commonly have multiple causes of high systemic inflammation, making it difficult to differentiate cytokines associated with cachexia from cytokines associated with cancer progression.
In an effort to establish a more direct relationship between specific circulating factors and cancer cachexia, we set out to profile the landscape of a number of soluble factors in pancreatic cancer patients undergoing an attempted resection of their tumour. Using a multiplex array platform, we were surprised to find that cytokines traditionally associated with cachexia, including tumour necrosis factor (TNF), interleukin‐6 (IL‐6), interleukin‐1β (IL‐1β), and Interferon‐γ (IFN‐γ), were present in the circulation at low levels and did not associate with cachexia. However, using this same platform and validated by enzyme‐linked immunosorbent assay (ELISA), we found that the cytokine monocyte chemoattractant protein‐1 (MCP‐1), also referred to as C‐C Motif Chemokine Ligand 2 (CCL2), was associated with cancer cachexia in treatment‐naïve patients.
Materials and methods
Generation of the patient cohort
Patients undergoing attempted pancreatic resections at The Ohio State University Wexner Medical Center were asked to participate in a tissue banking protocol under which blood was deposited into a tissue bank run by The Ohio State University Comprehensive Cancer Center Pancreatic Cancer Cachexia Program. The Ohio State Cancer Institutional Review Board approved all experimental procedures (IRB 2010C0051). All study participants provided informed consent. Between November 2013 and November 2015, intraoperative peripheral blood was collected from 84 patients diagnosed with pancreatic adenocarcinoma who were undergoing surgical evaluation. From this total, patients who were undergoing operations other than attempted resection of their tumour (n = 3), patients with a final pathological diagnosis of adenosquamous carcinoma (n = 4), and patients with an ampullary tumour (n = 3) were excluded. Two patients were excluded because of unclear documentation of weight loss. Patients were classified as either weight stable or cachectic, defined as weight loss of >5% of their pre‐illness weight, which is consistent with the diagnostic criteria of the international consensus definition of cancer cachexia.20 Although patients were asked to estimate the time period over which they had lost weight, patients were considered cachectic based upon losses from their pre‐illness weight, regardless of the time course over which the weight was lost. Two patients who reported weight loss of <5% were excluded from the study, as neither of these patients met the secondary criteria of low body mass index (BMI) or low appendicular muscle mass. Patients were considered weight stable if they denied weight loss in their preoperative surgical consultation and no documentation of weight loss could be located within his or her existing Ohio State Medical Center medical record. These exclusions left a final total of 70 patients for analysis. Patients were considered treatment‐naïve if they had not received chemotherapy or radiation prior to surgical exploration for this incidence of cancer. A previous history of a cancer other than pancreatic cancer was not an exclusionary criterion. Further, we did not exclude patients based upon any medications. Although we did not track their use, at Ohio State University, a standard protocol is used prior to surgery, including withholding statins 1 day prior to surgery and NSAIDS 5 days prior to surgery. Clinical characteristics of the patients included in our study appear in Table 1.
Table 1.
Patient characteristics
| Weight stable (n = 22) | Cachectic (n = 48) | t‐test | |
|---|---|---|---|
| Mean age (±SD) | 64.6 ± 10.8 | 67.6 ± 10.2 | P = 0.27 |
| Male (%) | 10 (45) | 23 (48) | P = 1.00 |
| Mean pre‐illness BMI (±SD) | 27.6 ± 5.3 | 30.1 ± 7.4 | P = 0.15 |
| Mean BMI at time of surgery (±SD) | 27.6 ± 5.3 | 26.2 ± 6.1 | P = 0.36 |
| Median weight loss (%) | 0 | 10% | P < 0.05 |
| History of hypertension | 11 (50) | 32 (67) | P = 0.20 |
| History of diabetes | 4 (18) | 21 (44) | P = 0.06 |
| Clinical tumour stage | |||
| 1A (%) | 1 (5) | 0 (0) | P = 0.998 |
| 2B (%) | 12 (54) | 31 (64) | |
| 3 (%) | 6 (27) | 8 (17) | |
| 4 (%) | 3 (14) | 9 (19) | |
| Neoadjuvant‐treated (%) | 11 (50) | 14 (29) | P = 0.11 |
| Treatment: | |||
| Chemotherapy: folfirinox | 7 (64) | 9 (64) | |
| Chemotherapy: gemcitabine‐abraxane | 3 (27) | 5 (36) | |
| Gemcitabine‐cisplatin | 1 (9) | 0 (0) | |
| Chemo/radiation | 9 (82) | 12 (86) | P = 0.79 |
| Resected (%) | 19 (86) | 37 (77) | P = 0.59 |
| Treatment‐naïve, resected (%) | 11 (50) | 27 (56) | P = 0.80 |
BMI, body mass index; SD, standard deviation.
Blood collection
Peripheral blood was collected intraoperatively in heparinized tubes from consented patients under anaesthesia. The final concentration of heparin was approximately 15 USP per mL of blood. Plasma was produced by centrifugation at 500 g for 10 min, then aliquoted and stored at −80°C until batched analysis.
Multiplex analyses
Our initial screen of 25 cytokines and growth factors was conducted using a custom ProcartaPlex (eBioscience) Luminex Multiplex Panel. Our custom platform contained 25 factors (25plex) that we identified as either previously associated with cachexia in patients with advanced disease or that we hypothesized may be associated with cancer cachexia, based on the literature. Our screen included 18 cytokines, 5 growth factors, 1 enzyme, and 1 hormone. A list of the soluble factors assessed appears in Table 2. For increased sensitivity, we also used a High Sensitivity 9‐Plex Human ProcartaPlex Panel (eBioscience). Both panels were assayed in accordance with the manufacturer's instructions.
Table 2.
Soluble factors analysed
| CD40L | IL‐1β | PDGF‐BB |
| FGF‐2 | IL‐4 | RANKL |
| G‐CSF | IL‐6 | SDF‐1α |
| GM‐CSF | IL‐8 | TGFα |
| HGF | Leptin | TNF |
| IFN‐γ | LIF | TRAIL |
| IL‐10 | M‐CSF | VEGF‐A |
| IL‐15 | MCP‐1 | |
| IL‐17A | MMP‐13 |
ELISA analysis
Differences in MCP‐1 between weight stable and cachectic patients were confirmed using an eBioscience ELISA according the manufacturer's instructions using a Biotek Synergy HT multiplate reader and Gen5 software. Samples were run on two plates of the same lot, and data were fit to a standard curve using four‐parameter logistic regression.
Data analysis and statistics
Differences in continuous clinical variables between cachectic and weight stable patients were assessed using Student's t‐tests, and differences in categorical variables were assessed using Fisher's exact tests. Differences in tumour stage between groups was assessed using a Mann–Whitney U test.21 For cytokine data, our analysis strategy using both continuous and categorical variables was modified from Lerner et al.17 For soluble factors where <25% of patients reached the lower limit of quantitation, no analysis was performed. Factors for which >75% of patients tested exceeded the lowest standard were analysed as continuous variables. Remaining factors detected in >25% of patients but exceeding the lowest standard in <75% of patients were analysed as categorical variables based upon if a given patient exceeded the lowest standard or not. For continuous variables, all statistical tests were performed on log‐transformed cytokine values to reduce the effect of skew. Two‐tailed, unpaired t‐tests were used when making comparisons between weight stable and cachectic patients. For categorical variables, because of the relatively small sample sizes, Fisher's exact tests were used. For MCP‐1 ELISA data with more than two groups, one‐way ANOVA was used. Relationships between weight loss or BMI and plasma cytokine concentrations were determined using simple linear regressions. A priori, alpha was set at P < 0.05. The relationship between circulating MCP‐1 and survival was assessed by dividing patients into high and low MCP‐1 groups using the median MCP‐1 level, as determined by ELISA. One patient who died within 30 days of surgery was excluded from the analysis, as this death was presumed to result from operative complications. Patients were censored from analysis at the date of their last known visit to The Ohio State Wexner Medical Center through April 2017. Differences in overall survival between groups were assessed using a log‐rank test. Differences in survival at 1 year were assessed using a Fisher's exact test. Statistical analysis was conducted with GraphPad Prism 6.0.
Results
Resectable pancreatic cancer patients have low levels of canonical pro‐inflammatory cytokines
In an effort to identify a biomarker associated with cancer cachexia, we used a multiplex assay to compare levels of 25 soluble factors in plasma of 70 pancreatic adenocarcinoma patients undergoing an attempted resection of their tumour. Patients were classified either as weight stable or cachectic, with cachexia defined as weight loss of >5% of their pre‐illness weight, consistent with the diagnostic criteria of the international consensus definition of cancer cachexia.20
Of the 25 tested soluble factors, 13 reached the lower limit of quantitation in <25% of patients (see Supporting Information, Table S1). Because of the low number of patients with detectable values of these cytokines, no analyses were performed on these cytokines. Surprisingly, this group of soluble factors included IL‐6, IFN‐γ, and IL‐1β, which have traditionally been associated with cachexia.
Our remaining 12 cytokines were analysed as either continuous or categorical variables, based upon the proportion of patients with plasma levels that exceeded the lowest standard for each cytokine (see Supporting Information, Table S1). For the eight cytokines with >75% of patients exceeding the lowest standard, differences in cytokine levels between cachectic and weight stable patients were analysed as continuous variables (Figure 1). To reduce skew, all statistical analysis was conducted on log‐transformed values. Of these eight soluble factors, only leptin was significantly decreased in cachectic patients compared with weight stable ones. This finding is consistent with a number of other reports.19, 22, 23 TNF, another cytokine commonly associated with cancer cachexia,17 was not significantly different between groups in this patient population.
Figure 1.
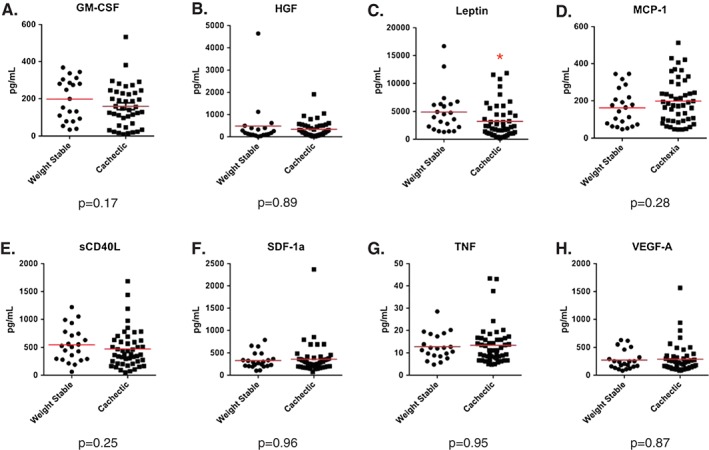
Soluble factors analysed as continuous variables. Differences between weight stable and cachectic patients were assessed using a Student's t‐test on log‐transformed values to eliminate skew. N = 70 patients with pancreatic cancer. Solid line indicates mean. * indicates P < 0.05.
For our final four soluble factors, for which plasma levels were detectable in >25% of patients yet >25% of patients were below the lowest standard, data were converted to categorical variables of above or below the lowest standard and assessed using a Fisher's exact test. No significant differences existed in the proportion of patients with measureable TNF‐related apoptosis‐inducing ligand (TRAIL), matrix metalloproteinase‐13 (MMP‐13), fibroblast growth factor 2 (FGF2), or interleukin‐8 (IL‐8) (Table 3).
Table 3.
Soluble factors analysed as categorical variables
| Proportion of patients reaching lowest standard (%) | |||
|---|---|---|---|
| Soluble factor | Weight stable | Cachectic | Fisher's exact test |
| TRAIL | 17/22 (77) | 33/48 (69) | P = 0.57 |
| MMP‐13 | 12/22 (55) | 34/48 (71) | P = 0.28 |
| FGF‐2 | 10/22 (45) | 29/48 (60) | P = 0.30 |
| IL‐8 | 4/22 (18) | 17/48 (35) | P = 0.17 |
FGF‐2, fibroblast growth factor 2; IL‐8, interleukin‐8; MMP‐13, matrix metalloproteinase‐13; TRAIL, apoptosis inducing ligand.
Our original intention was to profile all patients undergoing an attempted resection of their pancreatic tumour, as this would be the data available to a clinician when the decision to attempt a resection is made. However, as noted above, in a number of cachexia biomarker studies, cachectic patients appeared to have more advanced disease.17 While we attempted to control for disease progression by only enrolling patients into this study with potentially resectable tumours, 14 of 70 patients (3 weight stable, 11 cachectic) did not have their tumours resected either due to distant metastases or the finding of an anatomically unresectable tumour. When we analysed the remaining 56 resected patients, we found similar results to our 70‐patient cohort, with the sole difference being leptin that only tended to decline in cachectic patients (see Supporting Information, Figure S1; Table S2).
From these results, we were struck by the lack of differences in so many pro‐inflammatory cytokines that had been previously associated with cancer cachexia. We considered the possibility that our inability to detect such differences was due to a lack in sensitivity in the multiplex platform. To address this concern, we performed a high‐sensitivity multiplex in our population of 56 resected patients for nine cytokines, which included IL‐6, IFN‐γ, IL‐1β, and TNF. This assay has quantitation limits that are approximately 10‐fold lower than the custom 25plex. Using a similar analysis strategy as for our previous multiplex platform, four cytokines (IL‐4, IL‐6, IL‐10, and TNF) were analysed as continuous variables, as >75% of samples exceeded the lowest standard. We identified that IL‐10 was increased in cachectic patients (Figure 2), which is consistent with previous reports.17, 18 However, no significant differences in IL‐4, IL‐6, or TNF were present between cachectic and weight stable pancreatic cancer patients with resectable disease (Figure 2). Similarly, the remaining five cytokines (IL‐1β, IL‐2, IL‐12p70, IL‐17a, and IFN‐γ) were analysed as categorical variables, and there were no significant differences in proportion of patients with detectable levels (Table 4). These data strongly suggest that in patients with resectable pancreatic cancer, cachexia is not associated with high levels of canonical pro‐inflammatory cytokines.
Figure 2.
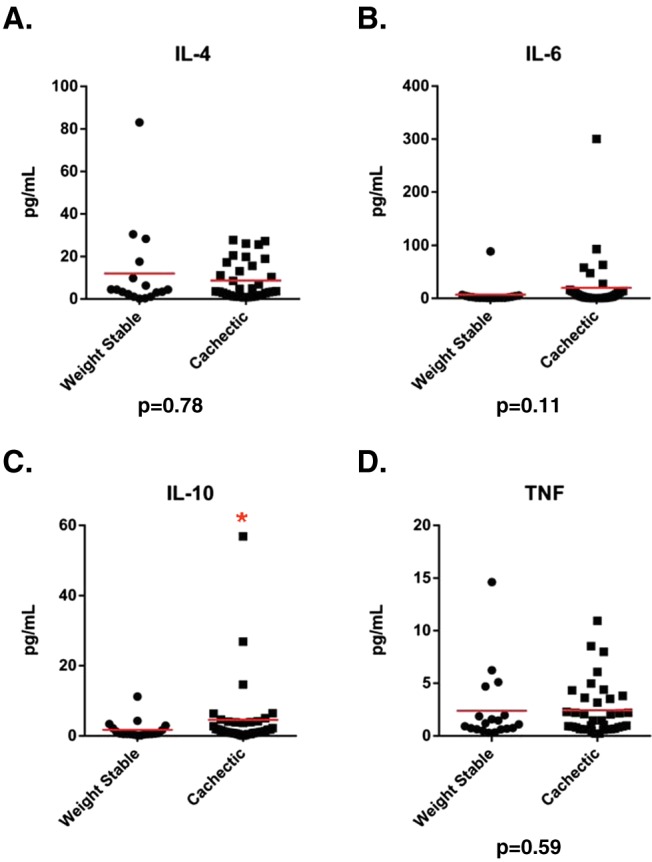
Soluble factors analysed as continuous variables from the high‐sensitivity multiplex. Differences between weight stable and cachectic patients were assessed using a Student's t‐test on log‐transformed values to eliminate skew. N = 56 resected patients. Solid line indicates mean. * indicates P < 0.05.
Table 4.
High sensitivity cytokines analysed as categorical variables
| Proportion of patients reaching lowest standard (%) | |||
|---|---|---|---|
| Soluble factor | Weight stable | Cachectic | Fisher's exact test |
| IL‐1β | 7/19 (37) | 15/37 (41) | P = 1.00 |
| IL‐2 | 11/19 (58) | 21/37 (57) | P = 1.00 |
| IL‐12p70 | 11/19 (58) | 22/37 (59) | P = 1.00 |
| IL‐17A | 12/19 (63) | 21/37 (57) | P = 0.78 |
| IFN‐γ | 7/19 (37) | 15/37 (41) | P = 1.00 |
IFN, interferon; IL, interleukin.
Effect of neoadjuvant therapy on circulating factors
Given that developing tumours are associated with increases in many of the same soluble factors that have been associated with cachexia, we considered that neoadjuvant chemotherapy and/or radiation therapy of tumours prior to surgery could alter the circulating levels of these factors. Indeed, when we assessed levels of soluble factors only in resected, treatment‐naïve patients (n = 38), we observed that both leptin and granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) were significantly decreased in cachectic patients compared with weight stable patients (Figure 3A and D). Further, MCP‐1, tended to be increased in cachectic patients (P = 0.05, Figure 3G). No significant differences between cachectic and weight stable treatment‐naive patients were identified in any other analysed cytokine (see Supporting Information, Table S3). Further, analysis of the high‐sensitivity multiplex data for resected, treatment‐naïve patients also did not identify any significant differences between cachectic and weight stable patients (see Supporting Information, Table S4).
Figure 3.

Leptin, granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), and monocyte chemoattractant protein‐1 (MCP‐1) are associated with pancreatic cancer‐induced cachexia in resected treatment‐naïve pancreatic cancer patients. Differences in (A) leptin, (D) GM‐CSF, and (G) MCP‐1 between weight stable and cachectic patients were assessed using a Student's t‐test on log‐transformed values to eliminate skew. An inverse relationship exists between increasing weight loss and both (B) leptin and (E) GM‐CSF, while MCP‐1 tends to have a positive relationship with increasing (H) weight loss. Finally, while circulating (C) leptin and (F) GM‐CSF levels are associated with BMI, (I) MCP‐1 levels did not. N = 38 resected, treatment‐naïve patients. Solid line indicates mean. * indicates P < 0.05.
Upon closer examination, we identified a strong relationship between leptin and weight loss in our population of successfully resected, treatment‐naïve patients (Figure 3B). These data are consistent with many other reports demonstrating a strong inverse relationship between circulating leptin and weight loss, which includes cancer patients with cachexia.23 However, although associated with weight loss, the ability of leptin to be used as a biomarker in cancer cachexia is limited by its strong positive association with BMI,24, 25, 26 which was also identified in our patient population (Figure 3C). Thus, a cachectic patient with a high BMI may still have a high leptin level and likely would not meet the criteria to be diagnosed as cachectic based on leptin alone, limiting the utility of leptin as a biomarker of cancer cachexia.
Our analysis also revealed a novel association between GM‐CSF and cancer cachexia, including a linear relationship between plasma GM‐CSF levels and weight loss (Figure 3E). However, similar to leptin, GM‐CSF was also tightly associated with BMI (Figure 3F). Thus, GM‐CSF is also unlikely to serve as a useful biomarker of cancer cachexia, as it also serves as an indicator of BMI.
In contrast, while circulating MCP‐1 levels only exhibited a modest trend towards a relationship with weight loss (P = 0.11, Figure 3H), no relationship existed between plasma MCP‐1 and BMI (Figure 3I). Thus, unlike leptin and GM‐CSF, MCP‐1 appears not to simply serve as a surrogate marker of a patient's BMI but may instead be considered as a potential biomarker for the diagnosis of cancer cachexia in treatment‐naïve pancreatic cancer patients.
MCP‐1 as a potential biomarker of cancer cachexia
Although circulating MCP‐1 has been shown to be elevated in patients with pancreatic cancer,27, 28 as far as we are aware, increased circulating MCP‐1 has never been associated with cancer cachexia. To confirm our findings, we used a commercially available ELISA. Similar to our multiplex data, MCP‐1 was higher in cachectic, treatment‐naïve patients vs. weight stable treatment‐naïve patients (Figure 4A). When we stratified cachectic patients by weight loss, MCP‐1 was found to be higher in patients having lost 5–10% of their body weight compared with weight stable patients, whereas patients with more severe weight loss (>10%) were not different from either weight stable or moderately cachectic (5–10% weight loss) patients (Figure 4B).
Figure 4.

Monocyte chemoattractant protein‐1 (MCP‐1) as a biomarker for pancreatic cancer‐induced cachexia. Increased (A) MCP‐1 in cachectic pancreatic cancer patients was confirmed by ELISA. (B) MCP‐1 levels are significantly increased in patients who have lost 5–10% of their body weight compared with weight stable patients but not in those who had lost >10%. (C) Circulating MCP‐1 is not associated with survival in treatment‐naïve patients with R0 resections. N = 38 successfully resected, treatment‐naïve patients. Solid line indicates mean. * indicates P < 0.05 vs. weight stable.
In some studies, increased circulating MCP‐1 has been associated with increased BMI.29, 30, 31 However, in our cohort of 38 chemotherapy‐naïve, successfully resected patients, neither BMI at time of surgery nor pre‐illness BMI associated with plasma MCP‐1 as determined by ELISA (see Supporting Information, Figure S2A and B). Metabolic syndrome, and specifically diabetes, has also been associated with increased circulating MCP‐1.32 When we subdivided our patients based upon their diabetes status, we still identified a significant main effect for cachexia, but no main effect for diabetes or interaction effect for diabetes and cachexia (see Supporting Information, Figure S2C).
Finally, because MCP‐1 has been positively associated with survival in pancreatic cancer patients, we assessed if there was a relationship between MCP‐1 and survival in our patient population.27, 33 When we divided patients into high and low MCP‐1 groups using the ELISA MCP‐1 values, we did not find a difference in survival between groups (low MCP‐1 median survival 522 days, high MCP‐1 median survival 447 days, Figure 4C). To ensure that disease burden was not a contributing factor to this finding, we repeated our analysis including only patients that had R0 resections with negative margins, meaning that these patients had no known disease following surgery (n = 24). We again found no significant difference in survival between groups (low MCP‐1 median survival 745 days, high MCP‐1 median survival 640 days). However, for patients in this group with at least 1 year of follow‐up, the proportion of patients surviving 1 year did tend to be higher in patients with low MCP‐1 (low MCP‐1, 10 out of 11 alive; high MCP‐1, 6 out of 10 alive; P = 0.14).
Discussion
Cancer cachexia is commonly associated with increased levels of circulating inflammatory cytokines such as TNF, IL‐1β, IL‐6, and IFN‐γ.16, 17, 18, 19 However, these associations were made in patients with late‐stage cancer, which is influenced by a number of potential confounders that may contribute to systemic inflammation. Thus, one cannot exclude the possibility that these elevated inflammatory cytokines in cachectic patients result from advanced disease, rather than directly from the cachexia syndrome. Our data using a cohort of both potentially resectable and resected pancreatic cancer patients demonstrate that circulating levels of inflammatory cytokines such as TNF, IL‐6, IL‐1β, and IFN‐γ, which have previously been considered markers of cancer cachexia, are actually quite low, and furthermore, did not associate with cachexia in this tumour type. However, differences in leptin, GM‐CSF, and MCP‐1 were associated with cachexia. While circulating levels of leptin and GM‐CSF are closely related to a patient's BMI, MCP‐1 levels do not simply report BMI, revealing MCP‐1 as a potential biomarker of cancer cachexia.
Decreased leptin and GM‐CSF are associated with cachexia in pancreatic cancer
Leptin has been previously identified to be elevated in cancer patients with a number of tumour types, including pancreatic cancer, compared with healthy controls.23, 25 However, leptin levels of cachectic patients are generally lower than weight stable cancer patients or healthy controls.19, 22, 23, 24 While large cohort studies repeatedly demonstrate that leptin levels correlate with weight loss,24, 25, 26 leptin levels in the same studies are also highly correlated with the subject's BMI. Thus, on the basis of diagnosing an individual patient with cachexia using a single blood draw, leptin is not without challenges, particularly in cancers like pancreatic cancer, where patients often have high BMIs, even after developing cachexia. One study did identify that leptin levels were lower in cachectic patients than would be predicted by their BMI,26 but this additional analysis complicates the use of leptin as an indicator of cachexia.
Although GM‐CSF is an area of intense interest in pancreatic cancer patients, as GM‐CSF appears to contribute to the immune suppression that allows pancreatic cancer to develop and progress,34, 35, 36 we were unable to find any study reporting changes in plasma GM‐CSF in pancreatic cancer patients. Further, we were unable to identify any reports demonstrating differences in circulating GM‐CSF between cachectic and weight stable patients in any cancer type. Thus, to the best of our knowledge, this is the first study to associate circulating GM‐CSF to cancer cachexia. Importantly, while associated with weight loss, similar to leptin, GM‐CSF was also tightly associated with BMI in our cohort of patients. Thus, we can conclude that similar to leptin, GM‐CSF is unlikely to be useful as a biomarker of cancer cachexia.
MCP‐1 as a potential biomarker of cancer cachexia
MCP‐1 is a ubiquitously expressed gene, including expression in adipose tissue, skeletal muscle, and pancreatic tumours. Increased MCP‐1 gene expression in both tumours and adipose tissue has been previously associated with cancer cachexia, either preoperatively or post‐operatively, although contrasting data exist for adipose messenger RNA.37, 38, 39
Our data are the first to associate increased circulating MCP‐1 with cancer cachexia. Previously, MCP‐1 was found not to associate with cachexia either in a large cohort of mixed tumour types17, 18 or lung or pancreatic cancer patients.40 The lack of differences in these studies between cachectic and weight stable patients could be due to the inclusion of patients who had previously received therapy for their disease. This would be consistent with our own results, as inclusion of neoadjuvantly‐treated patients masked differences in MCP‐1 between cachectic and weight stable patients. Thus, chemotherapy and/or radiation treatment may decrease circulating levels of this factor.
A second possible explanation for the difference between our findings and previous results is that circulating MCP‐1 could be an indicator of initiating cachexia. Data from our institution demonstrate that higher MCP‐1 is actually predictive of increased, not decreased survival in untreated metastatic pancreatic cancer patients.33 These findings suggest that there may be differences in the biology of MCP‐1 throughout the progression of PDAC.
Obesity and diabetes are common in cancer patients and particularly common in pancreatic cancer patients.41 Both obesity and diabetes are important considerations in the utility of MCP‐1 as a biomarker of cancer cachexia, as both these conditions have been linked to increased circulating levels of MCP‐1, although a significant body of literature exists where an association between obesity and diabetes and high MCP‐1 were not demonstrated.29, 30, 31, 32, 42, 43, 44, 45, 46, 47 Specifically in pancreatic cancer patients undergoing resection, an association was found between high circulating MCP‐1 and a BMI ≥37.5 kg/m2.28 Because this study did not assess weight loss, it is not possible to determine if patients with high BMIs were more likely to be cachectic. Specifically within our dataset, we had three patients with a BMI over 37.5 kg/m2 at the time of resection. Each of these patients was classified as cachectic, and circulating MCP‐1 levels for each of these patients were below the mean of the cachectic group.
Similar to the controversy surrounding increased circulating MCP‐1 in obesity, conflicting animal studies exist surrounding the role of MCP‐1 in adiposity and metabolic syndrome, with some studies suggesting MCP‐1 exacerbates the effect of high‐fat feeding and others suggesting a protective effect of MCP‐1.48, 49, 50, 51, 52, 53 While the increase in circulating MCP‐1 in cachectic patients would suggest that MCP‐1 might prevent obesity, clearly, much remains to be understood about the role of MCP‐1 in body weight control.
In terms of the source of circuiting MCP‐1 in cachectic pancreatic cancer patients, adipose tissue produces MCP‐1, including in cachectic cancer patients.37, 39 Thus, it is tempting to speculate that the increase in circulating MCP‐1 in cachectic patients results from breakdown of adipose tissue. However, our current dataset does not allow us to associate changes in adipose tissue mass with MCP‐1. More in‐depth analysis will be required to properly address this question.
Limitations and lessons learned in attempting to identify a biomarker of cancer cachexia
Like any study, ours is not without limitations. Because we have chosen to focus on only patients undergoing an attempted resection with a single tumour type, our study cohort is relatively small, particularly after excluding patients who failed to be resected or received neoadjuvant therapy. Future work will be required to validate MCP‐1 as a potential biomarker in a much larger cohort, including careful assessments of sex, age, and other conditions. Additional work will also be required to determine if MCP‐1 is a biomarker of cancer cachexia in other cancers or is specific to pancreatic cancer.
Through the course of this study, we gained an appreciation for several important factors that need to be considered when attempting to identify a biomarker of cancer cachexia. In addition to taking into account disease progression, treatment status is an important variable of any biomarker study, which traditionally has been underappreciated in the search for a biomarker of cancer cachexia. A potential limitation to any biomarker of cachexia is how it is altered by systemic therapy, as the development of cachexia could be masked by anticancer treatment. However, it is important to remember that cachexia can be assessed in a number of ways in patients undergoing treatment for their disease, including with regularly collected weight data and via analysis of muscle and adipose tissue volumes by radiographic imaging.54, 55 The true utility of a biomarker of cancer cachexia is at the time of initial diagnosis, when patients often lack weight data or imaging to provide a baseline from which to assess cachexia. For this reason, it will be important for future studies when attempting to identify a cachexia biomarker to do so in a patient population most likely to benefit from such a discovery—those that are treatment‐naïve. Future studies need to be conducted in patients across the cancer spectrum, so long as disease burden is carefully controlled for between weight stable and cachectic groups. This is particularly important in cancers such as pancreatic cancer, where 70% of patients already have unresectable disease at the time of diagnosis.9, 10
Another important unexpected finding in our study was the overall low levels of our tested soluble factors in resectable pancreatic cancer patients that were obtained using a multiplex array platform. Thus, caution should be exercised when undertaking future studies using similar soluble factor multiplexing methodologies, as the limits of detection of this technology can be quite high.56, 57 This is particularly relevant when including healthy control patients in study designs, as levels of soluble factors are likely to be even lower in these patients. Thus, we strongly suggest that results using such platforms undergo rigorous validation.
Conflict of interest
E.T., H.L., M.F., M.R., P.R., E.H., A.S., M.B., G.L., T.P., P.H., M.D., C.S., and D.G. declare that they have no conflicts of interest.
Supporting information
Table S1. Complete Table of Analysed Soluble Factors
Table S2. Soluble Factors in Resected Patients Analysed as Categorical Variables
Table S3. Soluble Factors in Resected Chemotherapy‐naïve Patients
Table S4. High‐sensitivity Bioplex In Resected, Chemotherapy‐naïve patients
Figure S1. Soluble Factors Analysed as Continuous Variables in Resected Patients. Differences between weight stable and cachectic patients were assessed using a Student's t‐test on log‐transformed values to eliminate skew. N = 56 patients with resected pancreatic cancer. Solid line indicates mean. * indicates p < 0.05
Figure S2. Additional Information Pertaining to MCP‐1 as a Biomarker for Pancreatic Cancer‐induced Cachexia. MCP‐1 levels measured by ELISA do not associate with (A) BMI at the time of surgery or (B) pre‐illness BMI. (C) Diabetes does not have an effect on the elevation of MCP‐1 in cachectic pancreatic cancer patients. N = 38 successfully resected, treatment‐naïve patients. Solid line indicates mean.
Acknowledgements
Support for this study was provided through The National Institutes of Health R01 CA180057 (D.C.G.), T32CA106196 (E.E.T.), and T32CA090223 (M.R.F.). Additional support was provided by the Ohio State University Comprehensive Cancer Center Cachexia Group. E.E.T. was supported both through a Weiss Postdoctoral Fellowship and an American Cancer Society Postdoctoral Fellowship (PF‐15‐156‐01‐CSM). The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia, and Muscle.58
Talbert, E. E. , Lewis, H. L. , Farren, M. R. , Ramsey, M. L. , Chakedis, J. M. , Rajasekera, P. , Haverick, E. , Sarna, A. , Bloomston, M. , Pawlik, T. M. , Zimmers, T. A. , Lesinski, G. B. , Hart, P. A. , Dillhoff, M. E. , Schmidt, C. R. , and Guttridge, D. C. (2018) Circulating monocyte chemoattractant protein‐1 (MCP‐1) is associated with cachexia in treatment‐naïve pancreatic cancer patients. Journal of Cachexia, Sarcopenia and Muscle, 9: 358–368. doi: 10.1002/jcsm.12251.
References
- 1. Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 2009;89:381–410. [DOI] [PubMed] [Google Scholar]
- 2. Stewart GD, Skipworth RJ, Fearon KC. Cancer cachexia and fatigue. Clin Med 2006;6:140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern cooperative oncology group. Am J Med 1980;69:491–497. [DOI] [PubMed] [Google Scholar]
- 4. von Haehling S, Anker MS, Anker SD. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: facts and numbers update 2016. J Cachexia Sarcopenia Muscle 2016;7:507–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun L, Quan XQ, Yu S. An epidemiological survey of cachexia in advanced cancer patients and analysis on its diagnostic and treatment status. Nutr Cancer 2015;67:1056–1062. [DOI] [PubMed] [Google Scholar]
- 6. Nemer L, Krishna S, Shah Z, Conwell D, Cruz‐Monserrate Z, Dillhoff M, et al. Predictors of pancreatic cancer‐associated weight loss and nutritional interventions. Pancreas 2017;46:1152–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bachmann J, Heiligensetzer M, Krakowski‐Roosen H, Buchler MW, Friess H, Martignoni ME. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J Gastrointest Surg 2008;12:1193–1201. [DOI] [PubMed] [Google Scholar]
- 8. Bachmann J, Ketterer K, Marsch C, Fechtner K, Krakowski‐Roosen H, Buchler MW, et al. Pancreatic cancer related cachexia: influence on metabolism and correlation to weight loss and pulmonary function. BMC Cancer 2009;9:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ansari D, Bauden M, Bergstrom S, Rylance R, Marko‐Varga G, Andersson R. Relationship between tumour size and outcome in pancreatic ductal adenocarcinoma. Br J Surg 2017;104:600–607. [DOI] [PubMed] [Google Scholar]
- 10. Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, et al. Validation of the 6th edition AJCC pancreatic cancer staging system: report from the national cancer database. Cancer 2007;110:738–744. [DOI] [PubMed] [Google Scholar]
- 11. Pausch T, Hartwig W, Hinz U, Swolana T, Bundy BD, Hackert T, et al. Cachexia but not obesity worsens the postoperative outcome after pancreatoduodenectomy in pancreatic cancer. Surgery 2012;152:S81–S88. [DOI] [PubMed] [Google Scholar]
- 12. Niedhammer I, Bugel I, Bonenfant S, Goldberg M, Leclerc A. Validity of self‐reported weight and height in the French GAZEL cohort. Int J Obes Relat Metab Disord 2000;24:1111–1118. [DOI] [PubMed] [Google Scholar]
- 13. Lin CJ, DeRoo LA, Jacobs SR, Sandler DP. Accuracy and reliability of self‐reported weight and height in the sister study. Public Health Nutr 2012;15:989–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Villanueva EV. The validity of self‐reported weight in US adults: a population based cross‐sectional study. BMC Public Health 2001;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muscaritoli M, Rossi Fanelli F, Molfino A. Perspectives of health care professionals on cancer cachexia: results from three global surveys. Ann Oncol 2016;27:2230–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Argilés J, Busquets S, López‐Soriano F. Anti‐inflammatory therapies in cancer cachexia. Eur J Pharmacol 2011;668:6. [DOI] [PubMed] [Google Scholar]
- 17. Lerner L, Hayes TG, Tao N, Krieger B, Feng B, Wu Z, et al. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. J Cachexia Sarcopenia Muscle 2015;6:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lerner L, Tao J, Liu Q, Nicoletti R, Feng B, Krieger B, et al. MAP3K11/GDF15 axis is a critical driver of cancer cachexia. J Cachexia Sarcopenia Muscle 2016;7:467–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fujiwara Y, Kobayashi T, Chayahara N, Imamura Y, Toyoda M, Kiyota N, et al. Metabolomics evaluation of serum markers for cachexia and their intra‐day variation in patients with advanced pancreatic cancer. PLoS One 2014;9:e113259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 21. Hu ZD, Zhou ZR, Qian S. How to analyze tumor stage data in clinical research. J Thorac Dis 2015;7:566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diakowska D, Krzystek‐Korpacka M, Markocka‐Maczka K, Diakowski W, Matusiewicz M, Grabowski K. Circulating leptin and inflammatory response in esophageal cancer, esophageal cancer‐related cachexia‐anorexia syndrome (CAS) and non‐malignant CAS of the alimentary tract. Cytokine 2010;51:132–137. [DOI] [PubMed] [Google Scholar]
- 23. Smiechowska J, Utech A, Taffet G, Hayes T, Marcelli M, Garcia JM. Adipokines in patients with cancer anorexia and cachexia. J Invest Med 2010;58:554–559. [DOI] [PubMed] [Google Scholar]
- 24. Takahashi M, Terashima M, Takagane A, Oyama K, Fujiwara H, Wakabayashi G. Ghrelin and leptin levels in cachectic patients with cancer of the digestive organs. Int J Clin Oncol 2009;14:315–320. [DOI] [PubMed] [Google Scholar]
- 25. Kemik O, Kemik AS, Begenik H, Erdur FM, Emre H, Sumer A, et al. The relationship among acute‐phase responce proteins, cytokines, and hormones in various gastrointestinal cancer types patients with cachectic. Hum Exp Toxicol 2012;31:117–125. [DOI] [PubMed] [Google Scholar]
- 26. Brown DR, Berkowitz DE, Breslow MJ. Weight loss is not associated with hyperleptinemia in humans with pancreatic cancer. J Clin Endocrinol Metab 2001;86:162–166. [DOI] [PubMed] [Google Scholar]
- 27. Monti P, Leone BE, Marchesi F, Balzano G, Zerbi A, Scaltrini F, et al. The CC chemokine MCP‐1/CCL2 in pancreatic cancer progression: regulation of expression and potential mechanisms of antimalignant activity. Cancer Res 2003;63:7451–7461. [PubMed] [Google Scholar]
- 28. Sullivan J, Gong Q, Hyslop T, Lavu H, Chipitsyna G, Yeo CJ, et al. Serum monocyte chemoattractant protein‐1 in pancreatic cancer. J Oncol 2011;2011:518394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kitahara CM, Trabert B, Katki HA, Chaturvedi AK, Kemp TJ, Pinto LA, et al. Body mass index, physical activity, and serum markers of inflammation, immunity, and insulin resistance. Cancer Epidemiol Biomarkers Prev 2014;23:2840–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Catalan V, Gomez‐Ambrosi J, Ramirez B, Rotellar F, Pastor C, Silva C, et al. Proinflammatory cytokines in obesity: impact of type 2 diabetes mellitus and gastric bypass. Obes Surg 2007;17:1464–1474. [DOI] [PubMed] [Google Scholar]
- 31. Pahwa R, Adams‐Huet B, Jialal I. The effect of increasing body mass index on cardio‐metabolic risk and biomarkers of oxidative stress and inflammation in nascent metabolic syndrome. J Diabetes Complications 2017;31:810–813. [DOI] [PubMed] [Google Scholar]
- 32. Daniele G, Guardado Mendoza R, Winnier D, Fiorentino TV, Pengou Z, Cornell J, et al. The inflammatory status score including IL‐6, TNF‐alpha, osteopontin, fractalkine, MCP‐1 and adiponectin underlies whole‐body insulin resistance and hyperglycemia in type 2 diabetes mellitus. Acta Diabetol 2014;51:123–131. [DOI] [PubMed] [Google Scholar]
- 33. Farren MR, Mace TA, Geyer S, Mikhail S, Wu C, Ciombor K, et al. Systemic immune activity predicts overall survival in treatment‐naive patients with metastatic pancreatic cancer. Clin Cancer Res 2016;22:2565–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Waghray M, Yalamanchili M, Dziubinski M, Zeinali M, Erkkinen M, Yang H, et al. GM‐CSF mediates mesenchymal‐epithelial cross‐talk in pancreatic cancer. Cancer Discov 2016;6:886–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pylayeva‐Gupta Y, Lee KE, Hajdu CH, Miller G, Bar‐Sagi D. Oncogenic Kras‐induced GM‐CSF production promotes the development of pancreatic neoplasia. Cancer Cell 2012;21:836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, et al. Tumor‐derived granulocyte‐macrophage colony‐stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell 2012;21:822–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haugen F, Labori KJ, Noreng HJ, Buanes T, Iversen PO, Drevon CA. Altered expression of genes in adipose tissues associated with reduced fat mass in patients with pancreatic cancer. Arch Physiol Biochem 2011;117:78–87. [DOI] [PubMed] [Google Scholar]
- 38. de Matos‐Neto EM, Lima JD, de Pereira WO, Figueredo RG, Riccardi DM, Radloff K, et al. Systemic inflammation in cachexia—is tumor cytokine expression profile the culprit? Front Immunol 2015;6:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Batista ML Jr, Henriques FS, Neves RX, Olivan MR, Matos‐Neto EM, Alcantara PS, et al. Cachexia‐associated adipose tissue morphological rearrangement in gastrointestinal cancer patients. J Cachexia Sarcopenia Muscle 2016;7:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lerner L, Gyuris J, Nicoletti R, Gifford J, Krieger B, Jatoi A. Growth differentiating factor‐15 (GDF‐15): a potential biomarker and therapeutic target for cancer‐associated weight loss. Oncol Lett 2016;12:4219–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA 2001;286:921–929. [DOI] [PubMed] [Google Scholar]
- 42. Parish RC, Todman S, Jain SK. Resting heart rate variability, inflammation, and insulin resistance in overweight and obese adolescents. Metab Syndr Relat Disord 2016;14:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim CS, Park HS, Kawada T, Kim JH, Lim D, Hubbard NE, et al. Circulating levels of MCP‐1 and IL‐8 are elevated in human obese subjects and associated with obesity‐related parameters. Int J Obes (Lond) 2006;30:1347–1355. [DOI] [PubMed] [Google Scholar]
- 44. Chacon MR, Fernandez‐Real JM, Richart C, Megia A, Gomez JM, Miranda M, et al. Monocyte chemoattractant protein‐1 in obesity and type 2 diabetes. Insulin sensitivity study. Obesity 2007;15:664–672. [DOI] [PubMed] [Google Scholar]
- 45. Sekikawa A, Kadowaki T, Curb JD, Evans RW, Maegawa H, Abbott RD, et al. Circulating levels of 8 cytokines and marine n‐3 fatty acids and indices of obesity in Japanese, white, and Japanese American middle‐aged men. J Interferon Cytokine Res 2010;30:541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Browning LM, Krebs JD, Magee EC, Fruhbeck G, Jebb SA. Circulating markers of inflammation and their link to indices of adiposity. Obes Facts 2008;1:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Accattato F, Greco M, Pullano SA, Care I, Fiorillo AS, Pujia A, et al. Effects of acute physical exercise on oxidative stress and inflammatory status in young, sedentary obese subjects. PLoS One 2017;12:e0178900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cranford TL, Enos RT, Velazquez KT, McClellan JL, Davis JM, Singh UP, et al. Role of MCP‐1 on inflammatory processes and metabolic dysfunction following high‐fat feedings in the FVB/N strain. Int J Obes (Lond) 2016;40:844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Takahashi K, Mizuarai S, Araki H, Mashiko S, Ishihara A, Kanatani A, et al. Adiposity elevates plasma MCP‐1 levels leading to the increased CD11b‐positive monocytes in mice. J Biol Chem 2003;278:46654–46660. [DOI] [PubMed] [Google Scholar]
- 50. Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP‐1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 2006;116:1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, et al. Overexpression of monocyte chemoattractant protein‐1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem 2006;281:26602–26614. [DOI] [PubMed] [Google Scholar]
- 52. Kirk EA, Sagawa ZK, McDonald TO, O'Brien KD, Heinecke JW. Monocyte chemoattractant protein deficiency fails to restrain macrophage infiltration into adipose tissue [corrected]. Diabetes 2008;57:1254–1261. [DOI] [PubMed] [Google Scholar]
- 53. Inouye KE, Shi H, Howard JK, Daly CH, Lord GM, Rollins BJ, et al. Absence of CC chemokine ligand 2 does not limit obesity‐associated infiltration of macrophages into adipose tissue. Diabetes 2007;56:2242–2250. [DOI] [PubMed] [Google Scholar]
- 54. Prado CM, Bekaii‐Saab T, Doyle LA, Shrestha S, Ghosh S, Baracos VE, et al. Skeletal muscle anabolism is a side effect of therapy with the MEK inhibitor: selumetinib in patients with cholangiocarcinoma. Br J Cancer 2012;106:1583–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 56. Breen EC, Reynolds SM, Cox C, Jacobson LP, Magpantay L, Mulder CB, et al. Multisite comparison of high‐sensitivity multiplex cytokine assays. Clin Vaccine Immunol 2011;18:1229–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Browne RW, Kantarci A, LaMonte MJ, Andrews CA, Hovey KM, Falkner KL, et al. Performance of multiplex cytokine assays in serum and saliva among community‐dwelling postmenopausal women. PLoS One 2013;8:e59498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. von Haehling S, Morley JE, Coats AJ, Anker SD. Ethical guidelines for publishing in the journal of cachexia, sarcopenia and muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Complete Table of Analysed Soluble Factors
Table S2. Soluble Factors in Resected Patients Analysed as Categorical Variables
Table S3. Soluble Factors in Resected Chemotherapy‐naïve Patients
Table S4. High‐sensitivity Bioplex In Resected, Chemotherapy‐naïve patients
Figure S1. Soluble Factors Analysed as Continuous Variables in Resected Patients. Differences between weight stable and cachectic patients were assessed using a Student's t‐test on log‐transformed values to eliminate skew. N = 56 patients with resected pancreatic cancer. Solid line indicates mean. * indicates p < 0.05
Figure S2. Additional Information Pertaining to MCP‐1 as a Biomarker for Pancreatic Cancer‐induced Cachexia. MCP‐1 levels measured by ELISA do not associate with (A) BMI at the time of surgery or (B) pre‐illness BMI. (C) Diabetes does not have an effect on the elevation of MCP‐1 in cachectic pancreatic cancer patients. N = 38 successfully resected, treatment‐naïve patients. Solid line indicates mean.


