Abstract
Background
Recent data pose the question whether conservative management of chronic kidney disease (CKD) by means of a low‐protein diet can be a safe and effective means to avoid or defer transition to dialysis therapy without causing protein‐energy wasting or cachexia. We aimed to systematically review and meta‐analyse the controlled clinical trials with adequate participants in each trial, providing rigorous contemporary evidence of the impact of a low‐protein diet in the management of uraemia and its complications in patients with CKD.
Methods
We searched MEDLINE (PubMed) and other sources for controlled trials on CKD to compare clinical management of CKD patients under various levels of dietary protein intake or to compare restricted protein intake with other interventions. Studies with similar patients, interventions, and outcomes were included in the meta‐analyses.
Results
We identified 16 controlled trials of low‐protein diet in CKD that met the stringent qualification criteria including having 30 or more participants. Compared with diets with protein intake of >0.8 g/kg/day, diets with restricted protein intake (<0.8 g/kg/day) were associated with higher serum bicarbonate levels, lower phosphorus levels, lower azotemia, lower rates of progression to end‐stage renal disease, and a trend towards lower rates of all‐cause death. In addition, very‐low‐protein diets (protein intake <0.4 g/kg/day) were associated with greater preservation of kidney function and reduction in the rate of progression to end‐stage renal disease. Safety and adherence to a low‐protein diet was not inferior to a normal protein diet, and there was no difference in the rate of malnutrition or protein‐energy wasting.
Conclusions
In this pooled analysis of moderate‐size controlled trials, a low‐protein diet appears to enhance the conservative management of non‐dialysis‐dependent CKD and may be considered as a potential option for CKD patients who wish to avoid or defer dialysis initiation and to slow down the progression of CKD, while the risk of protein‐energy wasting and cachexia remains minimal.
Keywords: Low‐protein diet, Chronic kidney disease, Glomerular filtration rate, End‐stage renal disease, All‐cause death, Conservative management, Cachexia, Protein‐energy wasting
Introduction
Chronic kidney disease (CKD) is among the leading causes of death worldwide including emerging giant economies such as India and China.1 Upon its development, kidney function deteriorates over time until it permanently fails. Management strategies have largely focused on slowing down progression to end‐stage renal disease (ESRD), at which time, patients are invariably expected to transition to renal replacement therapy, mostly in the form of maintenance dialysis treatment.2 Nevertheless, recent data suggest that attempts to delay or even prevent transition to dialysis therapy may not be inappropriate,3 including a 2009 study that showed that the initiation of dialysis was associated with a substantial and sustained decline in functional status of the elderly nursing home patients.4 Many patients with kidney disease prefer to opt to exhaust all conservative management options for CKD, including nutritional strategies, prior to considering dialysis therapy.5
Century‐old evidence suggests that lower dietary protein intake may help with CKD management including slowing its progression, improving albuminuria, and controlling uraemia.6, 7, 8, 9 However, results from the Modification of Diet in Renal Disease study in 1994 were inconclusive with regards to the efficacy of a low‐protein diet (LPD) in slowing the rate of CKD progression.10 Several meta‐analyses that focused on the rate of CKD progression showed favourable but modest effects of an LPD.11, 12 Nevertheless, no single study has examined all clinically relevant outcomes, and fewer studies have focused on the role of an LPD in managing uraemia or other CKD complications such as mineral and bone disorders and metabolic acidosis without causing protein‐energy wasting or cachexia.13
Protein‐energy wasting characterized by a decline in body protein mass and energy reserves, including muscle and fat wasting and visceral protein pool contraction, is an underappreciated condition in early to moderate stages of CKD and a strong predictor of adverse outcomes.14 The applicability of many nutritional interventions and their effects on outcomes in patients with moderate to advanced CKD, including those with protein‐energy wasting or at high risk of its development, has not been well studied. The challenge remains as to how to reconcile low dietary protein intake—to avoid or delay dialysis initiation—with adequate nutrient intake and nutritional therapy while insuring favourable nutritional status and to avoid or correct protein‐energy wasting.13 The field lacks an up‐to‐date systematic review and meta‐analysis study on the subject with a focus on the conservative management of CKD. There is an urgency to revisit all traditional and novel options for the non‐dialytic management of patients with advanced CKD. Given these considerations and given commonalities and distinctions of the old and emerging controlled trials over the past two decades following the Modification of Diet in Renal Disease study, we aimed to conduct a comprehensive systematic review and meta‐analysis study examining the effect of an LPD on the clinical management of patients with CKD.
Materials and methods
K.K.‐Z., supported by other coauthors, searched MEDLINE (PubMed) and other relevant sources with no limitation in study type, language, and geographical area using the search terms including ‘low protein diet’, ‘CKD’, and ‘clinical trial’ as well as additional records identified through other sources including prior reviews. A field expert (K. K.‐Z) identified any additional relevant studies. The studies were included if they described data from controlled trials (including randomized, self‐controlled, parallel, and crossover trials) on CKD patients (excluding prevalent ESRD patients and those receiving dialysis treatment) to compare clinical outcomes across various protein intake levels (i.e. protein‐free, very‐low‐protein, low‐protein, moderate‐protein, high‐protein, very‐high‐protein, or unrestricted protein diets) or to compare a restricted protein intake with another intervention. An LPD was defined as a diet with a protein content of <0.8 g/kg/day. To ensure meaningful sample size in each study given our focus on the conservative management of CKD, we selected only controlled trials that included at least 30 participants to ensure selection of studies with adequate sample size and higher level of robustness15 (Figure 1). The aforementioned endeavour was undertaken to both provide a comprehensive roster of relevant randomized controlled trials of LPD for CKD management, which has become Supporting Information, Table S2 of a recently published New England Journal of Medicine review article, titled, ‘Nutritional Management of Chronic Kidney Disease’ by Kalantar‐Zadeh and Fouque,16 and for an additional meta‐analysis project that is presented in this manuscript.
Figure 1.
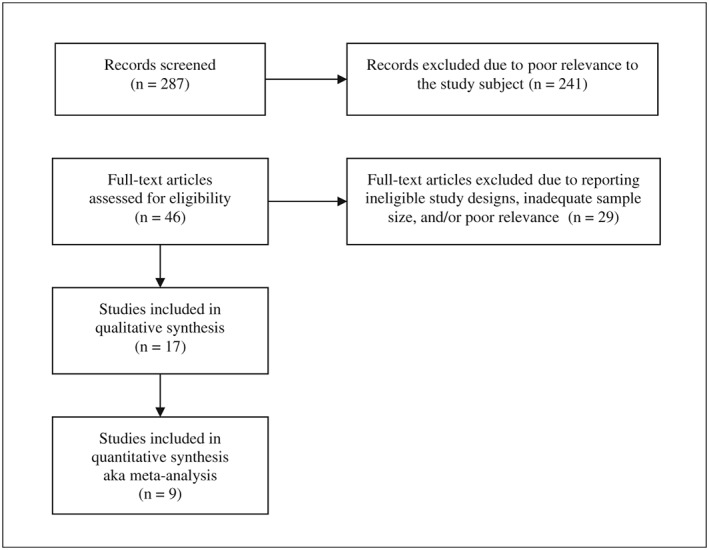
Flow diagram of the study selection. See also Supporting Information, Table S2 of the New England Journal of Medicine review article, titled, ‘Nutritional Management of Chronic Kidney Disease’ by Kalantar‐Zadeh and Fouque.16
We extracted and tabulated the main characteristics and findings of the included studies as well as comments on their methodological quality and clinical significance (Table 1). Also, we evaluated the risk of bias using the Cochrane Collaboration's tool for assessing risk of bias (Table 2). We examined the effects of an LPD or very‐low‐protein diet (VLPD) (with or without supplementation with ketoacids or amino acids) on various outcome measures in CKD patients. The corresponding authors of the studies with incomplete results were contacted in order to request further data.
Table 1.
Selected controlled trials (with >30 participants) that have examined the effects of an LPD or VLPD, with or without supplementation with ketoacids or amino acids, on various outcome measures in chronic kidney disease patients
| Study (year) | Participants | Dietary intervention | Outcomes | Follow‐Up time | Results | Comment |
|---|---|---|---|---|---|---|
| LPD vs. HPD | ||||||
| Jiang et al.17, 18 | 60 new ESRD pts on PD with RKF | LPD vs. sLPD (LPD + ketoacids) vs. HPD | RKF and nutritional markers on PD | 12 months | RKF stable in sLPD group but decreased in the LPD and HPD groups. | No change from baseline on nutritional status in any of the groups during follow‐up. |
| Cianciaruso et al.19 | 423 pts with CKD 4–5 | Two different DPI levels 0.55 (n = 212) vs. 0.80 g/kg/day (n = 211) | CKD progression and changes in blood and urinary biomarkers | 18 months | Reduced urinary excretion of urea, Na, phos in LPD. No differences in phos, albumin, PTH, bicarbonate. No changes in body composition. | Estimated DPI in low vs. high groups was 0.72 vs. 0.92 g/kg/day (P < 0.05). 9 vs. 13 pts in LPD vs. higher DPI stated dialysis. |
| MDRD study 1 Klahr et al.10 | 585 pts with CKD 3–4 (GFR 25–55 mL/min/1.73m2) | Usual protein diet (DPI 1.3 g/kg/day) vs. LPD (0.6 g/kg/day) | CKD progression, blood pressure, proteinuria, nutrition | 27 months (mean follow‐up) | Projected mean GFR decline at 3 years did not differ significantly between the diet groups. Faster GFR decline in the first 4 months in the LPD group. | Two concurrent randomized controlled trials. Serum albumin increased in both sVLPD and LPD groups and did not differ between groups. |
| Locatelli et al.20 | 456 pts with CKD 3–4 | LPD (0.78 g/kg/day) vs. normal DPI (0.9 g/kg/day), both DEI > 30 cal/kg/day | Renal survival defined as dialysis start or doubling of serum creatinine | 2 years | Borderline difference, slightly fewer pts assigned to LPD group reached the endpoint (P = 0.059). | Substantial overlap in DPI between two groups. |
| Williams et al.21 | 95 pts with CKD 4–5 | LPD (0.7 g/kg/day) vs. normal diet (DPI 1.02 and 1.14 g/kg/day) and varied phos content | CKD progression rates across three groups | 18 months | No differences in the reduction in creatinine clearance, dialysis initiation, or mortality among three groups. | Minor weight loss in LPD. |
| Ihle et al.22 | 72 pts with CKD 4–5 | LPD (0.6 g/kg/day) vs.higher DPI (0.8 g/kg/day) | GFR every 6 months | 18 months | Stable GFR in LPD vs. loss of GFR in control group (P < 0.05). | LPD pts lost weight but no change in anthropometric measures or serum albumin. |
| Rosman et al.23, 24 | 247 pts with CKD 3–5 | 0.90–0.95 (CKD 3) vs. 0.70–0.80 g/kg/day (CKD 4–5) vs. unrestricted DPI | GFR after 2 or 4 years | 4 years | After 2 years significant slowing of CKD progression in LPD but only in male pts. | 4 year renal survival improvement in LPD (60 vs. 30%, P < 0.025). PKD pts did not respond to LPD. |
| VLPD vs. LPD | ||||||
| Garneata et al.25 | 207 non‐diabetic pts with CKD 4–5 (eGFR <30 mL/min/1.73m2) and proteinuria <1 g/day | LPD (0.6 g/kg/day) vs. sVLPD (vegetarian VLPD 0.3 g/kg/day with KA) | Dialysis initiation or 50% reduction in initial eGFR | 15 months | Adjusted NNT (95% CI) to avoid dialysis was 22.4 (21.5–25.1) for pts with eGFR < 30 mL/min/1.73m2 but decreased to 2.7 (2.6–3.1) for pts with eGFR < 20 mL/min/1.73m2 in ITT analysis. | Correction of metabolic abnormalities occurred only with sVLPD. Compliance to diet was good, with no changes in nutritional measure. |
| Mircescu et al.26 | 53 non‐diabetic CKD 4–5 pts (eGFR < 30 mL/min/1.73m2) | sVLPD (0.3 g/kg/day vegetable proteins) suppl. with KA vs. LPD | Transition to dialysis, eGFR, and laboratory markers | 48 weeks | Less dialysis initiation with sVLPD (4 vs. 27%). Stable eGFR in sVLPD but decreased eGFR in controls. | Open‐label randomized, controlled trial. Higher bicarbonate and lower phos in sVLPD group. |
| Prakash et al.27 | 34 CKD pts (mean eGFR 28 mL/min/1.73m2) | LPD (0.6 g/kg/day) with placebo vs. sVLPD (0.3 g/kg/day with KA) | Changes in GFR and nutritional markers | 9 months | Stable GFR in the sVLPD vs. worsening nutritional measures and faster GFR decline in LPD group. | Prospective, randomized, double‐blind, placebo‐controlled single centre trial. |
| Malvy et al.28 | 50 pts with CKD 4–5 (eGFR < 20 mL/min/1.73m2) | sVLPD (0.3 g/kg/day) with KA vs. LPD (0.65 g/kg/day) | 3 mo to eGFR >5 mL/min/1.73m2 or need for dialysis | 3 years | SUN, lean body mass, and fat mass decreased in sVLPD group. | Randomized trial. No difference in renal survival, sVLPD pts lost 2.7 kg (both fat and lean body mass). |
| Montes‐Delgado et al.29 | 33 pts with CKD 3–5 | LPD vs. LPD suppl. with a low‐protein and hypercaloric supplement | Renal function and nutritional status | 6 months | Slower CKD progression in the supplemented group, with better nutritional status and higher adherence. | 22 patients completed the full 6 month study. |
| MDRD study 2 Klahr et al.10 | 255 pts with CKD 4–5 (GFR 13–24 mL/min/1.73m2) | LPD (0.6 g/kg/day) vs. sVLPD (0.3 g/kg/day with KA) | CKD progression, blood pressure, proteinuria, nutrition | 27 months (mean follow‐up) | sVLPD group had a marginally slower decline in GFR than LPD group (P = 0.067). Higher Ca, lower phos, alkaline phosphorus, and PTH levels in sVLPD group. | Two concurrent randomized controlled trials. Serum albumin increased in both sVLPD and LPD groups and did not differ between groups. |
| Lindenau et al.30 | 40 pts with CKD 5 (GFR < 15 mL/min/1.73m2) | LPD with calcium suppl. (n = 18) vs. sVLPD (0.4 g/kg) with KA (n = 22) | Bone and mineral markers including via bone biopsies | 12 months | Decreased serum phosphorus with sVLPD, improved markers of bone breakdown in bone biopsies in sVLPD group. | CKD progression and other outcomes not assessed. |
| VLPD or LPD vs. other interventions | ||||||
| Brunori et al.31 | 56 non‐diabetic pts (>70 yrs old) CKD 5 (GFR 5–7 mL/min/1.73m2) | sVLPD (DPI: 0.3 g/kg/day, DEI: 35 Cal/kg/day) with KA, vs. dialysis initiation | Survival, hospitalization, and metabolic markers. | Median time 26.5 months | Similar survival in both groups. Patients assigned to dialysis had a 50% higher degree of hospitalization. | There was a continuous benefit of LPD over time. |
| Teplan et al.32 | 105 CKD pts (GFR 22–36 mL/min/1.73m2) | LPD with KA and EPO vs. LPD without KA (with/without EPO) | CKD progression rate and nutritional measures | 3 years | sLPD with KA/EPO showed slower CKD progression and increased leucine, isoleucine, valine and mild decrease in proteinuria (P < 0.01). | Role of EPO remained unclear. |
AA, amino acid; AGE, advanced glycation end products; CKD, chronic kidney disease; DEI, dietary energy intake; DPI, dietary protein intake; eGFR, estimated glomerular filtration rate; EPO, recombinant human erythropoietin; EAA, essential amino acids; ESRD, end‐stage renal disease; HPD, high‐protein diet; ITT, intention to treat; KA, ketoacids supplement; LPD, low‐protein diet; NNT, number needed to treat; PEW, protein‐energy wasting; phos, phosphorus; PKD, polycystic kidney disease; pt, patient; pts, patients; sLPD, supplemented low‐protein diet; SUN, serum urea nitrogen; sVLPD, supplemented very‐low‐protein diet; VLPD, very‐low‐protein diet.
Table 2.
Risk of bias assessment in included studies
| Random sequence generation? | Allocation concealment? | Blinding of participants?a | Blinding of outcome assessors? | Complete outcome data? | No selective reporting? | |
|---|---|---|---|---|---|---|
| LPD vs. higher PD | ||||||
| Jiang et al.17, 18 | Unclear | Unclear | Unclear | Unclear | Yes | Yes |
| Cianciaruso et al.19 | Yes | Yes | Unclear | Unclear | Yes | Yes |
|
MDRD study 1 Klahr et al.10 |
Yes | Unclear | Unclear | Unclear | Yes | Yes |
| Locatelli et al.20 | Yes | Yes | Unclear | Unclear | Yes | Yes |
| Williams et al.21 | Yes | Unclear | Unclear | Unclear | Yes | Yes |
| Ihle et al.22 | Yes | Unclear | Unclear | Unclear | Yes | Yes |
| Rosman et al.23, 24 | Unclear | Unclear | No | No | Yes | Yes |
| VLPD vs. LPD | ||||||
| Garneata et al.25 | Yes | Unclear | Unclear | Unclear | Yes | Yes |
| Mircescu et al.26 | Yes | Unclear | Unclear | Unclear | Yes | Yes |
| Prakash et al.27 | Yes | Unclear | Yes | Yes | Yes | Yes |
| Malvy et al.28 | Unclear | Unclear | Unclear | Unclear | Yes | Yes |
| Montes‐Delgado et al.29 | Unclear | Unclear | Unclear | Unclear | Unclear | Yes |
|
MDRD study 2 Klahr et al.10 |
Yes | Unclear | Unclear | Unclear | Yes | Yes |
| Lindenau et al.30 | Unclear | Unclear | Unclear | Unclear | No | Unclear |
| V/LPD vs. other | ||||||
| Brunori et al.31 | Yes | Yes | No | No | Yes | Yes |
| Teplan et al.32 | Unclear | Unclear | Unclear | Unclear | Unclear | Yes |
Blinding of participants in diet‐based interventions is very difficult and almost unattainable.
Studies with clinical homogeneity (e.g. similar patients, interventions, and outcomes) were included in meta‐analyses. Statistical heterogeneity was assessed using the I 2 statistic. Summary estimates with a corresponding I 2 ≤ 50% were pooled using fixed‐effects meta‐analysis while those with a corresponding I 2 > 50% were pooled using the random‐effects model. In addition, in order to ascertain that our results were not dependent on the selected summary estimate or meta‐analysis model, we completed sensitivity analyses. Statistical significance was defined as a 95% confidence interval with no overlap with the null effect value (risk difference/mean difference = 0). For statistical procedures, we used Stata 12 (StataCorp., College Station, TX, USA).
Results
Sixteen randomized controlled trials, reported in 17 articles, each with at least 30 participants, were included in our review (Table 1). Based on the interventions and comparisons, the included studies were divided into the following groups: (i) those comparing LPD with higher‐protein diets10, 17, 18, 19, 20, 21, 22, 23, 24; (ii) those comparing VLPD with LPD10, 25, 26, 27, 28, 29, 30; and (iii) those involving other comparisons31, 32 (Table 1). As all studies were not similar in their recruited patients and/or outcome measures, not all studies in each category were meta‐analysed (Figures 2 and 3).
Figure 2.
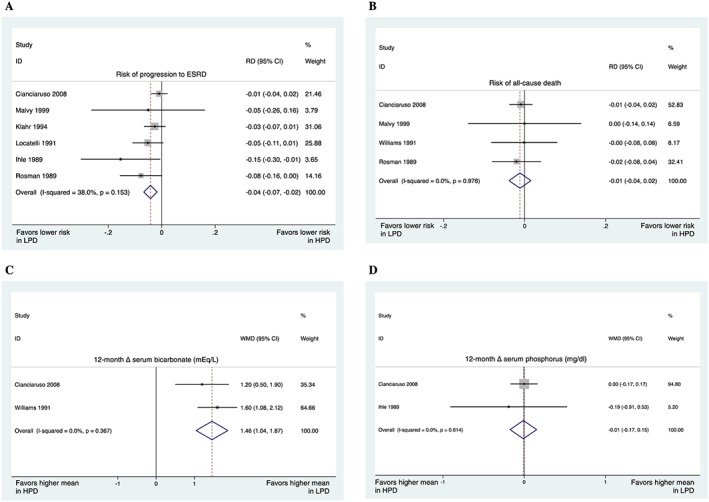
Low‐protein diets (LPD: <0.8 g/kg/day) vs. higher‐protein diets (HPD: >0.8 g/kg/day). (A) Risk of progression to end‐stage renal disease was 4% lower in those who received low‐protein diets. (B) The pooled results showed a trend towards a lower risk of all‐cause death in those who received low‐protein diets; however, the trend was not significant. (C) On average, 12 month serum bicarbonate was 1.46 mEq/L higher in those who received low‐protein diets. (D) The pooled results indicated that 12 month serum phosphorus (in mg/dL) was comparable. The ‘Overall’ shows the results of the heterogeneity test (in all forest plots). RD, risk difference aka absolute risk reduction. WMD, weighted mean difference.
Figure 3.
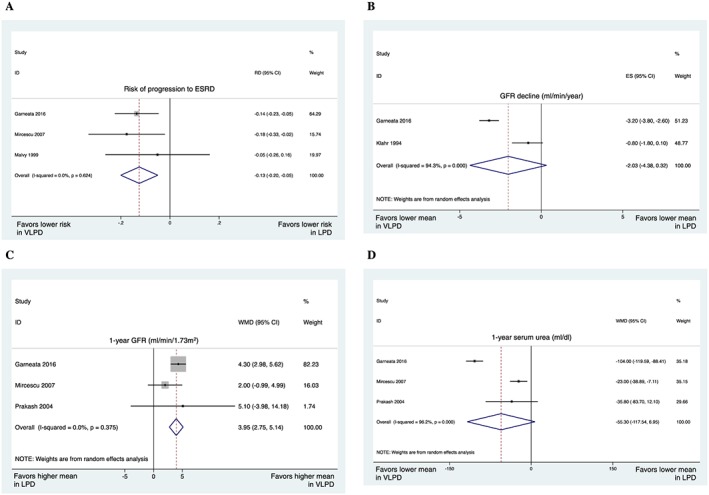
Very‐low‐protein diets (VLPD: <0.4 mg/kg/day) vs. low‐protein diets (LPD: 0.4–0.8 mg/kg/day). (A) Risk of progression to end‐stage renal disease was 13% lower in those who received very‐low‐protein diets. (B) The pooled results showed a trend towards lower glomerular filtration rate (GFR) decline (in mL/min/year) in those who received very‐low‐protein diets; however, the difference was not significant. The results were pooled using the random‐effects model because of the observed statistical heterogeneity. (C) On average, 1 year GFR was 3.95 mL/min/1.73m2 higher in those who received very‐low‐protein diets. (D) The pooled results indicated a trend towards lower 1 year serum urea (in mg/dL) in those who received very‐low‐protein diets. The results of Prakash27 were based on a follow‐up of 9 months. ES, estimate of effect (in this case: GFR decline).
Low‐protein diet vs. higher‐protein diets
For this comparison, LPD was defined as a protein intake of <0.8 mg/kg/day; therefore, VLPD was also considered a subgroup of LPD. Our pooled results showed that the risk of progression to ESRD was significantly lower in those who received LPD compared with those who received higher‐protein diets (Figure 2A). In addition, the pooled results indicated a trend towards lower all‐cause death in those who received LPD (Figure 2B).
We also meta‐analysed other metabolic factors, which showed that 1 year serum bicarbonate was significantly higher in those who received LPD (Figure 2C). However, 1 year serum phosphorus was comparable in the two groups (Figure 2D). We could not meta‐analyse results representing parathyroid hormone (PTH), calcium, and other metabolic factors because of their clinical and methodological heterogeneity.
In addition to the results of our meta‐analysis, some results by individual studies were also informative: serum PTH was not significantly different in the two intervention groups according to the results by Cianciaruso et al.19 and Ihle et al.22 However, Malvy et al.28 completed a longer follow‐up (up to 40 months) and revealed a significantly lower PTH in those who received a lower protein intake (mean PTH: 2.71 vs. 5.91 ng/mL; P < 0.001). Similarly, serum calcium was not significantly different as reported by Ihle et al.22 and Rosman et al.23 However, it was significantly higher in the study by Malvy et al.28 with a longer follow‐up duration (serum calcium: 2.42 vs. 2.25 mmol/L; P < 0.01).
Also, Jiang et al.17 showed that in peritoneal dialysis patients, those who received an LPD had better preservation of glomerular filtration rate (GFR) and residual kidney function. In addition, PTH was significantly lower in those who received ketoacid‐supplemented LPD. However, serum phosphorus and calcium were not significantly different between the two intervention groups.
Very‐low‐protein diet vs. low‐protein diet
Although the primary aim of this review was to compare LPD with higher‐protein diets, we also performed meta‐analyses of studies comparing VLPD with LPD. The two dietary groups were respectively defined as those with protein intakes of <0.4 and 0.4–0.8 mg/kg/day. The pooled results showed that the progression to ESRD was significantly lower (Figure 3A) and 1 year GFR was significantly higher (Figure 3C) in those who received VLPD compared with LPD. In addition, the pooled results revealed trends towards lower GFR decline (Figure 3B) and lower 1 year serum urea (Figure 3D) in those who received VLPD; however, the trends were not significant. The results representing bicarbonate, phosphorus, PTH, and calcium could not be meta‐analysed because of their heterogeneity.
Again, in addition to the results of our meta‐analysis, we summarize some key results from the individual studies: Garneata et al.25 observed higher serum bicarbonate (22.9 vs. 16.2 mEq/L, P < 0.01), higher serum calcium (4.4 vs. 3.9 mmol/L, P < 0.01), and lower serum phosphorus levels (4.4 vs. 6.2 mg/dL, P < 0.01) in those who received VLPD vs. LPD. Similarly, Mircescu et al.26 showed higher serum bicarbonate (23.4 vs. 17.6 mg/dL), higher serum calcium (4.4 vs. 3.9 mmol/L), and lower phosphorus (4.5 vs. 6 mg/dL) in those who received VLPD compared with LPD.
Also, Lindenau et al.30 reported that in patients with advanced CKD, those who received VLPD showed better control of renal osteodystrophy markers including PTH (0.6 vs. 1.53 ng/mL), osteoid surface (34.3 vs. 51.9), and bone volume (27.9 vs. 25.2); however, most differences did not reach statistical significance.
Sensitivity analyses
The results of our sensitivity analyses were comparable with the main meta‐analyses, indicating that our results were not dependent upon the selected meta‐analysis methods (see Supporting Information, Figures S1 and S2).
Other comparisons
Two included studies31, 32 compared LPD/VLPD with other interventions: Brunori et al.31 completed a non‐inferiority trial of supplemented VLPD vs. dialysis in elderly ESRD patients without diabetes, and they observed that the survival was not higher in those who received VLPD. In the other study, Teplan et al.32 compared three interventions in CKD patients: (i) LPD supplemented with ketoacids plus human recombinant erythropoietin (EPO); (ii) non‐supplemented LPD plus EPO; and (iii) non‐supplemented LPD alone. They observed that patients receiving supplemented LPD plus EPO had lower GFR decline and proteinuria and better metabolic profile. None of the studied trials reported increased risk of protein‐energy wasting or cachexia despite lower protein intake. There was no safety issue noted in any of the trials.
Discussion
Upon meta‐analysing contemporary clinical trials of large sample size (>30 participants in each trial) that have examined an LPD for the management of CKD, we found that in comparison with diets with protein intake of >0.8 g/kg/day, diets with restricted protein intake (<0.8 g/kg/day) were associated with higher serum bicarbonate levels, lower phosphorus levels, lower rates of progression to ESRD, and a trend towards lower rates of all‐cause death. In addition, VLPD (protein intake: <0.4 g/kg/day) was associated with even greater preservation of kidney function and reduction in the rate of progression to ESRD. These data may have important clinical and public health implications upon the conservative management of CKD.
For over half a century, we have regarded dialysis therapy as a life‐saving intervention among patients with advanced kidney failure, in whom survival would otherwise be impossible. To that end, more dialysis and earlier dialysis initiation have been considered to more favourable patient care strategies, whereas delayed initiation or less frequent haemodialysis treatment (i.e. less than thrice‐weekly haemodialysis) has been discouraged. However, emerging studies in recent years have cast doubt on the universal superiority of earlier dialysis initiation and more frequent dialysis treatments in the management of advanced CKD. A study in 2009 suggested that among nursing home residents with advanced kidney disease, initiation of dialysis was associated with a substantial and sustained decline in functional status.4 A provocative clinical trial from Australia and New Zealand in 2010 suggested that planned early initiation of dialysis in patients with Stage 5 CKD was not associated with improved survival or other clinical outcomes.3 These data have been supported by an increasing number of observational studies across different dialysis populations.33 To that end, it is important to revisit the old adage of the nutritional management of CKD and re‐examine whether the use of LPD can be leveraged to conservatively manage uraemic symptoms without the need for dialysis initiation. This potential application of an LPD is beyond and above its traditional use in studies that have employed it to slow progression of kidney disease. Even though the latter is of immense clinical importance, many clinicians often encounter patients in very late stages of CKD, for example, estimated GFR < 25 mL/min/1.73m2, who wish to avoid or defer dialysis therapy by any means possible. Several individual trials17, 18, 22, 23, 24, 25, 26, 27, 28, 29, 30, 34 as well as meta‐analysis studies12, 35, 36, 37 have shown the benefits of protein restriction for such conservative management of CKD patients.38
We found that the risk of progression to ESRD was significantly lower in the LPD compared with higher‐protein diets and also found a trend towards greater survival in the former group. The 1 year serum bicarbonate level was significantly higher in those who received an LPD, which is an important metric in the treatment of CKD‐associated acidosis and its deleterious effects.39 We also found lower phosphorus levels and lower azotemia with an LPD, which are important targets for the conservative management of CKD without dialysis. The safety and adherence to an LPD was not inferior to a normal protein diet in individual studies. However, we could not meta‐analyse results representing PTH, calcium, and other selected metabolic factors because of the clinical and methodological heterogeneity of the clinical trials. However, these collective results suggest that an LPD may have a potential role in the conservative management of CKD.
As a systematic review and meta‐analysis study, our findings were limited by the available data regarding the role of restricted protein intake in the management of CKD patients. The included studies were heterogeneous in their interventions, and they did not report all relevant clinical outcomes. Also, because of our limited resources, we were unable to search other electronic databases beyond PubMed. Nevertheless, we were inclusive in our data synthesis and reported on the meta‐analyses of various clinical outcomes using the most up‐to‐date evidence, while we excluded studies with <30 participants as our key a priori selection criterion to ensure that only well‐designed studies with adequate statistical powers are included. In addition, the external validity of our findings may be less certain, as the efficacy of the LPD in the controlled conditions of RCTs does not necessarily translate into its effectiveness if CKD patients are not adequately compliant to the LPD. Furthermore, we did not assess the risk of publication bias across our included studies because of the few studies pooled together in each meta‐analysis. In fact, tests of publication bias, for example, Egger or Begg & Mazumdarand may generate inaccurate or misleading results if their ‘sample size’, that is, the number of pooled studies, is small.
In conclusion, the current evidence confirms the effect of restricted dietary protein intake on favourable metabolic surrogates of kidney function including azotemia, bone and mineral disorder, and acidosis, as well as slower kidney function loss, slower progression of CKD, and lower rates of ESRD and death. None of the studied trials reported increased risk of protein‐energy wasting or cachexia despite lower protein intake, nor was there any safety issue in any of the trials. Future studies may focus on further examining the selection of appropriate patients for nutritional interventions and other approaches in the conservative management of patients with advanced CKD and investigating the role of adherence to such therapies.
Conflict of interest statement and disclosure
K.K.‐Z. has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, American Society of Nephrology, Astra‐Zeneca, AVEO Oncology, Chugai, DaVita, Fresenius, Genentech, Haymarket Media, Hofstra Medical School, International Federation of Kidney Foundations, International Society of Hemodialysis, International Society of Renal Nutrition & Metabolism, Japanese Society of Dialysis Therapy, Hospira, Kabi, Keryx, Novartis, National Institutes of Health, National Kidney Foundation, Pfizer, Relypsa, Resverlogix, Sandoz, Sanofi, Shire, Vifor, UpToDate, and ZS‐Pharma. S.‐F.A., C.P.K., and C.M.R. declare that they have no conflict of interest.
Supporting information
Supplemental Figure S1A. Risk of progression to end‐stage renal disease in low‐protein diet (≤0.6 g/kg/day) vs. higher‐protein diets (>0.6 g/kg/day): Using Random‐effects model.
Supplemental Figure S1B. Risk of progression to end‐stage renal disease in low‐protein diet (≤0.6 g/kg/day) vs. higher‐protein diets (>0.6 g/kg/day): Using Relative Risk.
Supplemental Figure S1C. Risk of all‐cause death in low‐protein diet (≤0.6 g/kg/day) vs. higher‐protein diets (>0.6 g/kg/day): Using Relative Risk.
Supplemental Figure S1D. 12‐month serum bicarbonate (mEq/L) in low‐protein diet (≤0.6 g/kg/day) vs. higher‐protein diets (>0.6 g/kg/day): Using Standardized Mean Difference.
Supplemental Figure S1E. 12‐month serum phosphorus (in mg/dl) in low‐protein diet (≤0.6 g/kg/day) vs. higher‐protein diets (>0.6 g/kg/day): Using Standardized Mean Difference.
Supplemental Figure S2A. Risk of progression to end‐stage renal disease in very low protein diet (<0.4 g/kg/day) vs. low protein diet (0.4–0.6 g/kg/day): Using Relative Risk.
Supplemental Figure S2B. 1‐year glomerular filtration rate decline (ml/min/1.73m2) in very low protein diet (<0.4 g/kg/day) vs. LPD (0.4–0.6 g/kg/day): Using Standardized Mean Difference (SMD).
Supplemental Figure S2C. 1‐year serum urea (mg/dl) in VLPD (<0.4 g/kg/day) vs. LPD (0.4–0.6 g/kg/day): Using Standardized Mean Difference (SMD).
Supplementary Table S2. Selected controlled trials (with greater than 30 participants) that have examined the effects of low‐protein or very low‐protein diets (with or without supplementation with ketoacids or amino‐acids) on various outcome measures in patients with chronic kidney disease.
Acknowledgements
K.K.‐Z. has been supported by the National Institute of Health/National Institute of Diabetes and Digestive and Kidney Diseases mid‐career award K24‐DK091419. K.K.‐Z and C.P.K. have been supported by the National Institute of Health/National Institute of Diabetes and Digestive and Kidney Diseases grant R01‐DK096920. C.M.R. has been supported by the National Institute of Health/National Institute of Diabetes and Digestive and Kidney Diseases early career award K23‐DK102903.
The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015.40
Rhee, C. M. , Ahmadi, S.‐F. , Kovesdy, C. P. , and Kalantar‐Zadeh, K. (2018) Low‐protein diet for conservative management of chronic kidney disease: a systematic review and meta‐analysis of controlled trials. Journal of Cachexia, Sarcopenia and Muscle, 9: 235–245. doi: 10.1002/jcsm.12264.
References
- 1. Kovesdy CP, Kalantar‐Zadeh K. Enter the dragon: a Chinese epidemic of chronic kidney disease? Lancet (London, England) 2012;379:783–785. [DOI] [PubMed] [Google Scholar]
- 2. Saggi SJ, Allon M, Bernardini J, Kalantar‐Zadeh K, Shaffer R, Mehrotra R. Considerations in the optimal preparation of patients for dialysis. Nat Rev Nephrol 2012;8:381–389. [DOI] [PubMed] [Google Scholar]
- 3. Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, Harris A, Johnson DW, Kesselhut J, Li JJ, Luxton G, Pilmore A, Tiller DJ, Harris DC, Pollock CA, Study I. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 2010;363:609–619. [DOI] [PubMed] [Google Scholar]
- 4. Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009;361:1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bolasco P, Kalantar‐Zadeh K, Rhee C. Conservative management of chronic kidney disease: how to avoid or defer dialysis. Panminerva Med 2017;59:115. [DOI] [PubMed] [Google Scholar]
- 6. Kovesdy CP, Kalantar‐Zadeh K. Back to the future: restricted protein intake for conservative management of CKD, triple goals of renoprotection, uremia mitigation, and nutritional health. Int Urol Nephrol 2016;48:725–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang M, Chou J, Chang Y, Lau WL, Reddy U, Rhee CM, Chen J, Hao C, Kalantar‐Zadeh K. The role of low protein diet in ameliorating proteinuria and deferring dialysis initiation: what is old and what is new. Panminerva Med 2017;59:157–165. [DOI] [PubMed] [Google Scholar]
- 8. Ko GJ, Obi Y, Tortorici AR, Kalantar‐Zadeh K. Dietary protein intake and chronic kidney disease. Curr Opin Clin Nutr Metab Care 2017;20:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalantar‐Zadeh K, Moore LW, Tortorici AR, Chou JA, St‐Jules DE, Aoun A, Rojas‐Bautista V, Tschida AK, Rhee CM, Shah AA, Crowley S, Vassalotti JA, Kovesdy CP. North American experience with low protein diet for non‐dialysis‐dependent chronic kidney disease. BMC Nephrol 2016;17:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G. The effects of dietary protein restriction and blood‐pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 1994;330:877–884. [DOI] [PubMed] [Google Scholar]
- 11. Kasiske BL, Lakatua JD, Ma JZ, Louis TA. A meta‐analysis of the effects of dietary protein restriction on the rate of decline in renal function. Am J Kidney Dis 1998;31:954–961. [DOI] [PubMed] [Google Scholar]
- 12. Fouque D, Laville M. Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database Syst Rev 2009:CD001892. [DOI] [PubMed] [Google Scholar]
- 13. Kovesdy CP, Kopple JD, Kalantar‐Zadeh K. Management of protein‐energy wasting in non‐dialysis‐dependent chronic kidney disease: reconciling low protein intake with nutritional therapy. Am J Clin Nutr 2013;97:1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Obi Y, Qader H, Kovesdy CP, Kalantar‐Zadeh K. Latest consensus and update on protein‐energy wasting in chronic kidney disease. Curr Opin Clin Nutr Metab Care 2015;18:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Billingham SA, Whitehead AL, Julious SA. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom Clinical Research Network database. BMC Med Res Methodol 2013;13:104, PubMed PMID: 23961782;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kalantar‐Zadeh K, Fouque D. Nutritional management of chronic kidney disease. N Engl J Med 2017; [in press] [October 30, 2017]. [DOI] [PubMed] [Google Scholar]
- 17. Jiang N, Qian J, Sun W, Lin A, Cao L, Wang Q, Ni Z, Wan Y, Linholm B, Axelsson J, Yao Q. Better preservation of residual renal function in peritoneal dialysis patients treated with a low‐protein diet supplemented with keto acids: a prospective, randomized trial. Nephrol Dial Transplant : Official Publication Eur Dial Transpl Assoc ‐ Eur Ren Assoc 2009;24:2551–2558. [DOI] [PubMed] [Google Scholar]
- 18. Jiang N, Qian J, Lin A, Fang W, Zhang W, Cao L, Wang Q, Ni Z, Yao Q. Low‐protein diet supplemented with keto acids is associated with suppression of small‐solute peritoneal transport rate in peritoneal dialysis patients. Int J Nephrol 2011;2011:542704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cianciaruso B, Pota A, Pisani A, Torraca S, Annecchini R, Lombardi P, Capuano A, Nazzaro P, Bellizzi V, Sabbatini M. Metabolic effects of two low protein diets in chronic kidney disease stage 4‐5—a randomized controlled trial. Nephrol Dial Transplant : Official Publication Eur Dial Transpl Assoc ‐ Eur Ren Assoc 2008;23:636–644. [DOI] [PubMed] [Google Scholar]
- 20. Locatelli F, Alberti D, Graziani G, Buccianti G, Redaelli B, Giangrande A. Prospective, randomised, multicentre trial of effect of protein restriction on progression of chronic renal insufficiency. Northern Italian Cooperative Study Group. Lancet (London, England) 1991;337:1299–1304. [DOI] [PubMed] [Google Scholar]
- 21. Williams PS, Stevens ME, Fass G, Irons L, Bone JM. Failure of dietary protein and phosphate restriction to retard the rate of progression of chronic renal failure: a prospective, randomized, controlled trial. Q J Med 1991;81:837–855. [PubMed] [Google Scholar]
- 22. Ihle BU, Becker GJ, Whitworth JA, Charlwood RA, Kincaid‐Smith PS. The effect of protein restriction on the progression of renal insufficiency. N Engl J Med 1989;321:1773–1777. [DOI] [PubMed] [Google Scholar]
- 23. Rosman JB, ter Wee PM, Meijer S, Piers‐Becht TP, Sluiter WJ, Donker AJ. Prospective randomised trial of early dietary protein restriction in chronic renal failure. Lancet (London, England) 1984;2:1291–1296. [DOI] [PubMed] [Google Scholar]
- 24. Rosman JB, Langer K, Brandl M, Piers‐Becht TP, van der Hem GK, ter Wee PM, Donker AJ. Protein‐restricted diets in chronic renal failure: a four year follow‐up shows limited indications. Kidney Int Suppl 1989;27:S96–102. [PubMed] [Google Scholar]
- 25. Garneata L, Stancu A, Dragomir D, Stefan G, Mircescu G. Ketoanalogue‐supplemented vegetarian very low‐protein diet and CKD progression. J Am Soc Nephrol 2016; [in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mircescu G, Garneata L, Stancu SH, Capusa C. Effects of a supplemented hypoproteic diet in chronic kidney disease. J Ren Nutr 2007;17:179–188. [DOI] [PubMed] [Google Scholar]
- 27. Prakash S, Pande DP, Sharma S, Sharma D, Bal CS, Kulkarni H. Randomized, double‐blind, placebo‐controlled trial to evaluate efficacy of ketodiet in predialytic chronic renal failure. J Ren Nutr 2004;14:89–96. [DOI] [PubMed] [Google Scholar]
- 28. Malvy D, Maingourd C, Pengloan J, Bagros P, Nivet H. Effects of severe protein restriction with ketoanalogues in advanced renal failure. J Am Coll Nutr 1999;18:481–486. [DOI] [PubMed] [Google Scholar]
- 29. Montes‐Delgado R, Guerrero Riscos MA, Garcia‐Luna PP, Martin Herrera C, Pereira Cunill JL, Garrido Vazquez M, Lopez Munoz I, Suarez Garcia MJ, Martin‐Espejo JL, Soler Junco ML, Barbosa Martin F. Treatment with low‐protein diet and caloric supplements in patients with chronic kidney failure in predialysis. Comparative study. Rev Clin Esp 1998;198:580–586. [PubMed] [Google Scholar]
- 30. Lindenau K, Abendroth K, Kokot F, Vetter K, Rehse C, Frohling PT. Therapeutic effect of keto acids on renal osteodystrophy. A prospective controlled study. Nephron 1990;55:133–135. [DOI] [PubMed] [Google Scholar]
- 31. Brunori G, Viola BF, Parrinello G, De Biase V, Como G, Franco V, Garibotto G, Zubani R, Cancarini GC. Efficacy and safety of a very‐low‐protein diet when postponing dialysis in the elderly: a prospective randomized multicenter controlled study. Am J Kidney Dis 2007;49:569–580. [DOI] [PubMed] [Google Scholar]
- 32. Teplan V, Schuck O, Knotek A, Hajny J, Horackova M, Skibova J, Maly J. Effects of low‐protein diet supplemented with ketoacids and erythropoietin in chronic renal failure: a long‐term metabolic study. Ann Transplant 2001;6:47–53. [PubMed] [Google Scholar]
- 33. Rosansky SJ, Cancarini G, Clark WF, Eggers P, Germaine M, Glassock R, Goldfarb DS, Harris D, Hwang SJ, Imperial EB, Johansen KL, Kalantar‐Zadeh K, Moist LM, Rayner B, Steiner R, Zuo L. Dialysis Initiation: what's the rush? Semin Dial 2013;26:650–657. [DOI] [PubMed] [Google Scholar]
- 34. Di Iorio BR, Cucciniello E, Martino R, Frallicciardi A, Tortoriello R, Struzziero G. Acute and persistent antiproteinuric effect of a low‐protein diet in chronic kidney disease. G Ital Nefrol 2009;26:608–615. [PubMed] [Google Scholar]
- 35. Fouque D, Laville M, Boissel JP, Chifflet R, Labeeuw M, Zech PY. Controlled low protein diets in chronic renal insufficiency: meta‐analysis. BMJ 1992;304:216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pedrini MT, Levey AS, Lau J, Chalmers TC and Wang PH. The effect of dietary protein restriction on the progression of diabetic and nondiabetic renal diseases: a meta‐analysis. Ann Intern Med. 1996;124(7):627–632. [DOI] [PubMed] [Google Scholar]
- 37. Schwingshackl L, Hoffmann G. Comparison of high vs. normal/low protein diets on renal function in subjects without chronic kidney disease: a systematic review and meta‐analysis. PLoS One 2014;9:e97656. Epub 2014/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kovesdy CP, Kalantar‐Zadeh K. DASH‐ing toward improved renal outcomes: when healthy nutrition prevents incident chronic kidney disease. Nephrol Dial Transplant : Official Publication Eur Dial Transpl Assoc ‐ Eur Ren Assoc 2017;32:ii231–ii233. [DOI] [PubMed] [Google Scholar]
- 39. Kovesdy CP, Anderson JE, Kalantar‐Zadeh K. Association of serum bicarbonate levels with mortality in patients with non‐dialysis‐dependent CKD. Nephrol Dial Transplant : Official Publication Eur Dial Transpl Assoc ‐ Eur Ren Assoc 2009;24:1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1A. Risk of progression to end‐stage renal disease in low‐protein diet (≤0.6 g/kg/day) vs. higher‐protein diets (>0.6 g/kg/day): Using Random‐effects model.
Supplemental Figure S1B. Risk of progression to end‐stage renal disease in low‐protein diet (≤0.6 g/kg/day) vs. higher‐protein diets (>0.6 g/kg/day): Using Relative Risk.
Supplemental Figure S1C. Risk of all‐cause death in low‐protein diet (≤0.6 g/kg/day) vs. higher‐protein diets (>0.6 g/kg/day): Using Relative Risk.
Supplemental Figure S1D. 12‐month serum bicarbonate (mEq/L) in low‐protein diet (≤0.6 g/kg/day) vs. higher‐protein diets (>0.6 g/kg/day): Using Standardized Mean Difference.
Supplemental Figure S1E. 12‐month serum phosphorus (in mg/dl) in low‐protein diet (≤0.6 g/kg/day) vs. higher‐protein diets (>0.6 g/kg/day): Using Standardized Mean Difference.
Supplemental Figure S2A. Risk of progression to end‐stage renal disease in very low protein diet (<0.4 g/kg/day) vs. low protein diet (0.4–0.6 g/kg/day): Using Relative Risk.
Supplemental Figure S2B. 1‐year glomerular filtration rate decline (ml/min/1.73m2) in very low protein diet (<0.4 g/kg/day) vs. LPD (0.4–0.6 g/kg/day): Using Standardized Mean Difference (SMD).
Supplemental Figure S2C. 1‐year serum urea (mg/dl) in VLPD (<0.4 g/kg/day) vs. LPD (0.4–0.6 g/kg/day): Using Standardized Mean Difference (SMD).
Supplementary Table S2. Selected controlled trials (with greater than 30 participants) that have examined the effects of low‐protein or very low‐protein diets (with or without supplementation with ketoacids or amino‐acids) on various outcome measures in patients with chronic kidney disease.


