Abstract
Background
Nutrition screening on admission to hospital is mandated in many countries, but to date, there is no consensus on which tool is optimal in the oncology setting. Wasting conditions such as cancer cachexia (CC) and sarcopenia are common in cancer patients and negatively impact on outcomes; however, they are often masked by excessive adiposity. This study aimed to inform the application of screening in cancer populations by investigating whether commonly used nutritional screening tools are adequately capturing nutritionally vulnerable patients, including those with abnormal body composition phenotypes (CC, sarcopenia, and myosteatosis).
Methods
A prospective study of ambulatory oncology outpatients presenting for chemotherapy was performed. A detailed survey incorporating clinical, nutritional, biochemical, and quality of life data was administered. Participants were screened for malnutrition using the Malnutrition Universal Screening Tool (MUST), Malnutrition Screening Tool (MST), and the Nutritional Risk Index (NRI). Computed tomography (CT) assessment of body composition was performed to diagnose CC, sarcopenia, and myosteatosis according to consensus criteria.
Results
A total of 725 patients (60% male, median age 64 years) with solid tumours participated (45% metastatic disease). The majority were overweight/obese (57%). However, 67% were losing weight, and CT analysis revealed CC in 42%, sarcopenia in 41%, and myosteatosis in 46%. Among patients with CT‐identified CC, the MUST, MST, and NRI tools categorized 27%, 35%, and 7% of them as ‘low nutritional risk’, respectively. The percentage of patients with CT‐identified sarcopenia and myosteatosis that were categorised as ‘low nutritional risk’ by MUST, MST and NRI were 55%, 61%, and 14% and 52%, 50%, and 11%, respectively. Among these tools, the NRI was most sensitive, with scores <97.5 detecting 85.8%, 88.6%, and 92.9% of sarcopenia, myosteatosis, and CC cases, respectively. Using multivariate Cox proportional hazards models, NRI score < 97.5 predicted greater mortality risk (hazard ratio 1.8, confidence interval: 1.2–2.8, P = 0.007).
Conclusions
High numbers of nutritionally vulnerable patients, with demonstrated abnormal body composition phenotypes on CT analysis, were misclassified by MUST and MST. Caution should be exercised when categorizing the nutritional risk of oncology patients using these tools. NRI detected the majority of abnormal body composition phenotypes and independently predicted survival. Of the tools examined, the NRI yielded the most valuable information from screening and demonstrated usefulness as an initial nutritional risk grading system in ambulatory oncology patients.
Keywords: Cancer, Malnutrition, Cachexia, Sarcopenia, Myosteatosis, Nutrition screening tools
Introduction
Cancer‐related malnutrition is a term that encompasses a range of altered nutritional states including cancer‐induced weight loss, sarcopenia, myosteatosis, pre‐cachexia, and cancer cachexia (CC). The rate of malnutrition can vary greatly depending on tumour site and stage; however, it is estimated to affect between 8% and 85% of cancer patients.1, 2, 3, 4 Identification of malnutrition is critical as it negatively impacts on prognosis and has been associated with a range of adverse clinical outcomes.5, 6, 7, 8, 9, 10, 11, 12, 13 Severe malnutrition is independently associated with mortality, with an estimated 20–30% of cancer deaths attributable to malnutrition.14, 15 Abnormal body composition phenotypes such as sarcopenia, myosteatosis, and cachexia are of particular importance in the oncology setting as poor muscularity is strongly associated with negative clinical outcomes, however can often be masked by the excessive adiposity that is so common among newly diagnosed cancer patients.16, 17, 18 Timely detection is essential to ensure that tailored nutritional support can be implemented early when intervention is most effective, before an irreversible, refractory state takes hold.9
Computed tomography (CT) assessment is the gold standard method of body composition analysis and diagnosis of abnormal body composition phenotypes.16, 19 CT analysis is particularly useful in this setting, as CT scans are routinely performed during diagnosis and staging of the disease. CT assessment of body composition takes approximately 20 min per scan in trained personnel. Unless automation of CT analysis becomes widely available in the clinical setting, routine analysis for all oncology patients is currently not feasible. As a result, it is essential that time‐efficient, accurate, and effective methods of screening the nutritional risk of patients are available. Effective screening would allow clinicians to triage patients, referring those that are nutritionally vulnerable for urgent nutritional assessment, including assessment of body composition, and ensure timely, targeted interventions are implemented.
Despite the critical importance of early diagnosis and intervention, malnutrition is thought to be widely underdiagnosed in the oncology setting.20, 21, 22 In the USA, the Joint Commission has mandated universal screening and assessment of malnutrition in hospitalized patients within 24 h of admission since 1995.23, 24 However, no consensus exists on the optimal nutritional screening tool for the detection of malnutrition, particularly the screening of oncology patients in either the inpatient or outpatient setting.
To our knowledge, no studies have been performed in oncology patients to assess whether existing nutritional screening tools are correctly capturing forms of cancer‐related malnutrition that would otherwise require CT scan to detect, or whether these tools can be used to predict adverse clinical outcomes. Therefore, the aim of this study was to assess whether Malnutrition Universal Screening Tool (MUST), Malnutrition Screening Tool (MST), and Nutritional Risk Index (NRI) successfully detect nutritionally vulnerable oncology patients, including those with abnormal body composition phenotypes such as sarcopenia, myosteatosis, and cachexia.
Materials and methods
Ethical approval for this study was granted by the Clinical Research Ethics Committee of the Cork Teaching Hospitals. This study was performed as part of a large prospective investigation conducted at two university teaching hospitals in Cork, Ireland, between 2012 and 2016. All adult (≥18 years) ambulatory patients, diagnosed with malignant solid tumours, presenting for chemotherapy were eligible for inclusion. Signed, informed consent was obtained from all participants, and patients were assessed during the first cycle of chemotherapy (or other systemic anti‐cancer therapy).
Collection of clinical data
A detailed questionnaire was disseminated by the research team and included questions relating to demographic data, oncologic data, smoking and alcohol intake, weight loss history, and appetite. Medical history, histopathological diagnosis, performance status, details of oncologic therapy, dates of death, and any missing data were obtained through a chart review.
Collection of nutritional data
Nutritional parameters were obtained from both the study questionnaire and through a chart review. Body weight and height were measured to within 0.1 kg and 0.5 cm, respectively. Body mass index (BMI) was calculated and categorized according to the World Health Organization's classification of BMI.25 Patient‐reported pre‐illness weight and the time frame of any weight loss were recorded. The degree of weight loss was classified according the Blackburn criteria for weight loss.26 CC, a multifactorial syndrome defined by an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional therapy and leads to progressive functional impairment, was diagnosed using the international consensus definition.9 Pre‐cachexia was defined using the diagnostic criteria outlined by the European Society of Clinical Nutrition and Metabolism Special Interest Group for ‘cachexia–anorexia in chronic wasting diseases’.27 Pre‐cachexia includes patients with a chronic disease, small weight loss, a chronic or recurrent systemic inflammatory response, and anorexia. Anorexia was assessed using responses from the anorexia question within the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ) C30. ‘Have you lacked appetite’, which has a four choice response; 1 = not at all, 2 = a little, 3 = a lot, and 4 = very much. If patients give a response of 2, 3, or 4, they were classified as demonstrating some degree of anorexia. Systemic inflammation was categorized as a serum C‐reactive protein level of ≥5 mg/L, which is the upper limit for serum C‐reactive protein in the institution where the study was performed.
Nutritional data gathered as part of the study questionnaire were used to assess patients' nutritional risk using three commonly used malnutrition screening tools: MUST,28 MST,29 and NRI.30 All three tools can be completed in 2–3 min and incorporate information that is readily available in patients receiving chemotherapy. MUST generates scores on the basis of BMI, unplanned weight loss, and acute disease effect, with the overall score then allowing categorization into three ‘at risk’ levels: low risk (MUST score 0), medium risk (MUST score 1), and high risk (MUST score 2) groups. MST investigates risk of malnutrition on the basis of unintentional weight loss, the extent of weight loss that has occurred and reduced oral intake secondary to anorexia. A total score of 0–7 is generated, with a score of 0 or 1 indicating a patient that is not at risk of malnutrition, while a score of ≥2 represents a patient at risk of malnutrition. NRI is a screening tool based on serum albumin and weight loss. A score is calculated for each patient using the formula: 1.519 (serum albumin; g/dL) + 41.7 (current weight/usual weight).30 An NRI score of >100 indicates that the patient is not malnourished. An NRI score of 97.5–100 denotes mild malnourishment, 83.5–97.5 indicates moderate malnourishment, while <83.5 indicates severe malnourishment.
Body composition analysis by computed tomography
Computed tomography scans, performed routinely as part of diagnostic investigations, were used to analyse body composition at baseline. Cross‐sectional area of muscle was analysed in contrast‐enhanced CT scans at the level of the third lumbar vertebra (L3), using OsiriX software (Version 5.0) (Pixmeo, Geneva, Switzerland), as previously described.31, 32 Muscle area was normalized for stature by dividing by height in metres squared and was then reported as skeletal muscle index (cm2/m2). Skeletal muscle density was assessed by computing the mean radiation attenuation of the entire muscle area at L3 in contrast enhanced CT scans, expressed in Hounsfield units. Sarcopenia is defined as appendicular skeletal muscle mass greater than 2 SD below the mean of a healthy, reference population, while myosteatosis is characterized by low radiation attenuation secondary to increased levels of intermuscular and intramuscular fat. Sarcopenia and myosteatosis were diagnosed using previously defined sex‐specific and BMI‐specific cut‐off points, devised from a cancer population.18
Statistical analysis
Statistical analysis was performed by SPSS (version 22) for Windows (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.). Preliminary analyses were carried out to ensure that the assumptions of normality, linearity, or homoscedasticity were not violated. χ2 tests were used to compare categorical variables. Receiver operating characteristic curves were generated to assess the diagnostic performance of screening tools, with the area under the curve (AUC) indicating the accuracy of the tool. The Hosmer and Lemeshow's classification of AUC values was used to classify AUC scores.33 Multivariate Cox proportional hazards models were used to estimate covariate adjusted hazard ratios (HRs). All statistical tests were two‐sided, and exact P‐values are reported. Significance was taken at the level of P < 0.05.
Results
Demographic, clinical, and nutritional characteristics of study cohort
In total, 725 oncology outpatients, of mixed cancer types, receiving chemotherapy provided signed informed consent. The demographic, clinical, and nutritional characteristics of the cohort, according to sex, are displayed in Table 1. Colorectal cancer was most common, representing close to one‐third of the cohort (32.3%), followed by gastro‐oesophageal (15.9%) and respiratory malignancies (11.4%). Metastatic disease was present in 45% of the cohort, with the liver being the most common site of metastases. The cohort had a good level of functioning, with 79.2% having an Eastern Cooperative Oncology Group (ECOG) score of 0–1. The majority had a BMI in the overweight/obese range (55.6%), while 4.4% had a BMI in the underweight category. At screening, CC was identified in 41.5% of the cohort. CC occurred most frequently in hepatobillary (64%), oesophageal (60%), and gynae cancers (58%). Of patients that were diagnosed with CC, 137 (37.2% of cachectic patients) had either no weight loss or limited weight loss and were diagnosed by ‘Step 3’ or by CT analysis of muscle mass alone. Overall, sarcopenia was present in 41% of the cohort and was most prevalent in patients with hepatobillary (53%) and breast cancer (52%). Myosteatosis was identified in 45.5% of participants and was most prevalent in those with respiratory (60.7%) and gynaecological (53.7%) malignancies.
Table 1.
Demographic, clinical, and nutritional characteristics according to sex
| Male n = 433 (59.7%) | Female n = 292 (40.3%) | Overall n = 725 | |
|---|---|---|---|
| Age, median (IQR) | 65.3 (57.4–71.9) | 62.2 (52.9–70.0) | 64.3 (55.9–71.0) |
| Age > 65 years, n (%) | 221 (51) | 128 (43.8) | 349 (48.1) |
| Treatment plan, n (%) | |||
| Chemotherapy only | 191 (44.1) | 157 (53.8) | 348 (48) |
| Chemotherapy and surgery | 132 (30.5) | 76 (26) | 208 (28.7) |
| Chemo‐radiotherapy and surgery | 49 (11.3) | 39 (13.4) | 88 (12.1) |
| Chemo‐radiotherapy | 38 (8.8) | 11 (3.8) | 49 (6.8) |
| Unknown | 23 (5.3) | 9 (3.0) | 32 (4.4) |
| Metastasis present, n (%) | 192 (44.3) | 134 (45.9) | 326 (45) |
| Weight change since diagnosis n (%) | |||
| Weight loss | 297 (68.6) | 190 (65.1) | 487 (67.2) |
| Weight stable | 52 (12.0) | 38 (13.0) | 90 (12.4) |
| Weight gain | 84 (19.4) | 64 (21.9) | 148 (20.4) |
| Weight loss (%) [mean (±SD)] | 6.3 (±7.3) | 7.3 (±8.5) | 6.7 (±7.8) |
| >10% weight loss in past 6 months n (%) | 75 (17.3) | 61 (20.9) | 136 (18.8) |
| BMI (kg/m2) n (%) | |||
| Underweight (BMI <18.5 kg/m2) | 10 (2.3) | 22 (7.5) | 32 (4.4) |
| Healthy weight (BMI 18.5–24.9 kg/m2) | 156 (36.0) | 134 (45.9) | 290 (40.0) |
| Overweight (BMI 25–29.9 kg/m2) | 185 (42.7) | 83 (28.4) | 268 (37.0) |
| Obese (BMI ≥30 kg/m2) | 82 (19) | 53 (18.2) | 135 (18.6) |
| BMI (kg/m2) mean (±SD) | 26.3 (±4.3) | 25.1 (±5.1) | 25.8 (±4.6) |
| Cancer cachexia n (%) | 192 (44.3) | 109 (37.3) | 301 (41.5) |
| Pre‐cachexiaa n (%) | 18 (5.7) | 12 (6.0) | 30 (5.8) |
| Sarcopeniab n (%) | 144 (35.8) | 130 (48.9) | 274 (41) |
| Myosteatosisc n (%) | 149 (40.8) | 132 (52.2) | 281 (45.5) |
BMI, body mass index; IQR, interquartile range; SD, standard deviation.
Pre‐cachexia assessed in n = 514 (male n = 315, female n = 199),
Sarcopenia measurable in n = 661 (male n = 402, female n = 266),
Muscle density measureable in n = 618 (male n = 365, female n = 253). All percentages given for total available.
Nutrition risk assessed by Malnutrition Universal Screening Tool, Malnutrition Screening Tool, and Nutritional Risk Index
Table 2 outlines the nutritional risk of patients when assessed using nutritional screening tools. MUST identified 40.8% of the cohort as being at a moderate or high risk of malnutrition (MUST score ≥ 1). MST deemed 41.2% of the cohort to be at risk of malnutrition (MST score ≥ 2), while 90% of patients were classified as having some degree of malnutrition, according to NRI (NRI score of <100).
Table 2.
Nutritional risk scores from Malnutrition Universal Screening Tool, Malnutrition Screening Tool, and Nutritional Risk Index
| Male n = 433 (59.7%) | Female n = 292 (40.3%) | Overall n = 792 | |
|---|---|---|---|
| MUST | |||
| Score 0 (low risk) | 272 (62.8) | 157 (53.8) | 429 (59.2) |
| Score 1 (moderate risk) | 78 (18.0) | 54 (18.5) | 132 (18.2) |
| Score ≥ 2 (high risk) | 83 (19.2) | 81 (27.7) | 164 (22.6) |
| MST | |||
| Score 0–1 (not at risk of malnutrition) | 255 (58.9) | 171 (58.6) | 426 (58.8) |
| Score 2–5 (at risk of malnutrition) | 178 (41.1) | 121 (41.4) | 299 (41.2) |
| NRIa | |||
| Score > 100 (not malnourished) | 35 (9.9) | 21 (10.3) | 56 (10) |
| Score 97.5–100 (mild malnourishment) | 37 (10.5) | 23 (11.2) | 60 (10.7) |
| Score 83.5–97.5 (moderate malnourishment) | 220 (62.3) | 119 (58.0) | 339 (60.8) |
| Score < 83.5 (severe malnourishment) | 61 (17.3) | 42 (20.5) | 103 (18.5) |
MST, Malnutrition Screening Tool; MUST, Malnutrition Universal Screening Tool; NRI, Nutrition Risk Index.
NRI data available in n = 558 (male n = 353; female n = 205).
Comparing body composition analysis by computed tomography to nutrition risk assessment by nutrition screening tools
Table 3 displays the proportion of each of the abnormal body composition phenotypes, diagnosed by gold standard methods that were given a score indicative of no/low nutritional risk by the screening tool. MUST classified 55% of true sarcopenia cases, as diagnosed using gold standard methods, as being at a low risk of malnutrition. MUST misclassified the highest number of both myosteatosis (52%) and pre‐cachexia (57%), while MST misclassified the highest proportion of sarcopenia (61%), CC (35%), and the presence of at least one of the conditions (52%). Figure 1 illustrates the relationship between sarcopenia diagnosed by gold standard body composition and ‘at risk’ scores from MUST, MST, and NRI. Of the 41% of the cohort with sarcopenia, 18.6% were categorized as moderate to high risk by MUST and 16.2% as ‘at risk’ by MST. NRI captured a large proportion of sarcopenic patients, highlighting 36.7% of those with sarcopenia as being at nutritional risk. Figure 2 illustrates the relationships between CC and ‘at risk’ scores from MUST, MST, and NRI. Of the proportion of the cohort with CC (41.5%), 30.2% were classified as being at moderate to high risk by MUST and 26.3% as ‘at risk’ by MST. NRI detected the highest proportion of cachectic patients, flagging 42.1% of the cohort as at nutritional risk.
Table 3.
Prevalence of abnormal muscle mass and cancer cachexia in patients screened as ‘low risk’ using screening tools
| Low risk scoring | Sarcopenia | Myosteatosis | Pre‐cachexia | Cancer cachexia | Any one of these conditions |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| MUST (score 0) | 150 (55) | 146 (52) | 17 (57) | 82 (27) | 244 (49) |
| MST (score 0–1) | 166 (61) | 141 (50) | 7 (23) | 129 (35) | 259 (52) |
| NRI (score > 97.5) | 32 (14) | 26 (11) | 1 (3) | 18 (7) | 54 (13) |
MST, Malnutrition Screening Tool; MUST, Malnutrition Universal Screening Tool; NRI, Nutrition Risk Index.
Figure 1.
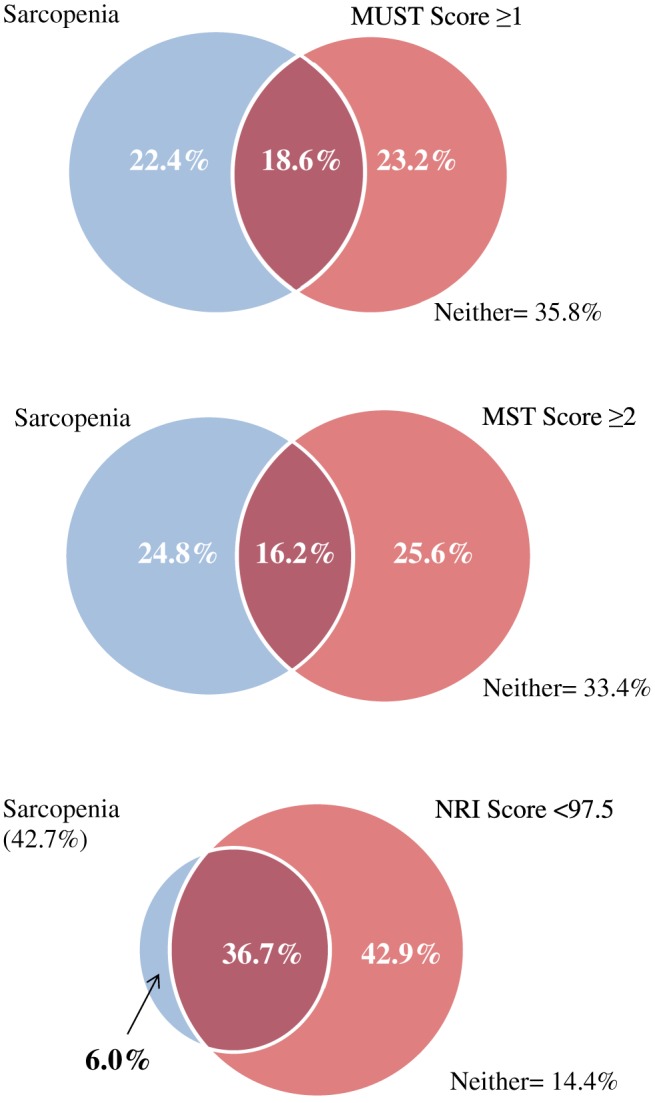
The relationship between sarcopenia diagnosed by gold standard body composition analysis and Malnutrition Universal Screening Tool (MUST) ‘high risk’ scores, Malnutrition Screening Tool (MST) ‘at risk’ scores, and Nutritional Risk Index (NRI) ‘at risk’ scores.
Figure 2.
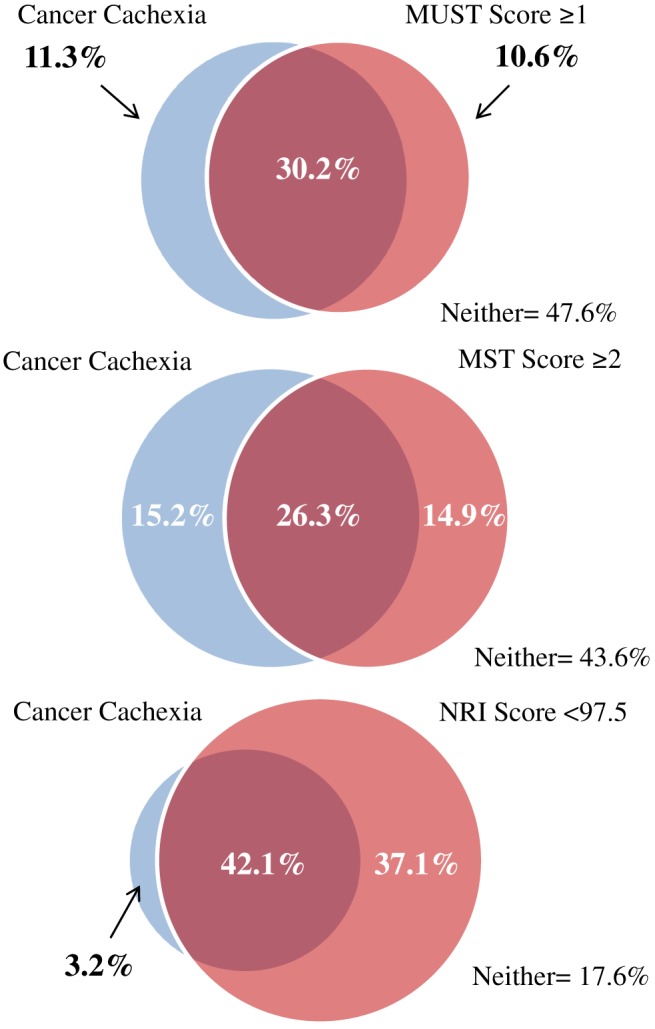
The relationship between cancer cachexia diagnosed by gold standard body composition analysis and Malnutrition Universal Screening Tool (MUST) ‘high risk’ scores, Malnutrition Screening Tool (MST) ‘at risk’ scores, and Nutritional Risk Index (NRI) ‘at risk’ scores.
Sensitivity and specificity of Malnutrition Universal Screening Tool, Malnutrition Screening Tool, and Nutritional Risk Index in detecting abnormal body composition
The sensitivity, specificity, and total accuracy of the screening tools in detecting abnormal body composition phenotypes diagnosed by gold standard methods is displayed in Table 4. Receiver operating characteristic curves were also generated to compare the tools' ability to detect the conditions. The AUC for all three tools when determining both sarcopenia and myosteatosis did not exceed 0.6. Total MST score demonstrated the largest AUC for pre‐cachexia (AUC = 0.677, P = 0.002), while MUST yielded the largest for CC (AUC = 0.816, P < 0.001); however, it did not demonstrate diagnostic reliability for the detection of the cases of CC diagnosed by CT scan alone. Of those diagnosed with CC by CT alone, 58% were given a MUST score of 0, 40% were given a score of 1, and 2% were given a score of 2. In terms of the ability of tools to detect the presence of any of the malnourished states of interest, MUST demonstrated the largest AUC (0.71, P < 0.001).
Table 4.
Sensitivity and specificity of Malnutrition Universal Screening Tool, Malnutrition Screening Tool, and Nutritional Risk Index in detecting sarcopenia, myosteatosis, and cancer cachexia
| Sarcopenia | Myosteatosis | Pre‐cachexia | Cancer cachexia | Any of these four conditions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sen (%) | Spec (%) | TA (%) | Sen (%) | Spec (%) | TA (%) | Sen (%) | Spec (%) | TA (%) | Sen (%) | Spec (%) | TA (%) | Sen (%) | Spec (%) | TA (%) | |
| MUST score ≥ 1 | 45.3 | 60.7 | 54.3 | 48.0 | 62.9 | 56.1 | 45.8 | 56.5 | 56.0 | 72.8 | 81.8 | 78.1 | 51.3 | 82.6 | 61.0 |
| MUST score ≥ 2 | 26.6 | 79.4 | 57.8 | 27.8 | 80.4 | 56.5 | 20.8 | 73.5 | 71.0 | 52.2 | 98.3 | 79.2 | 32.5 | 99.6 | 53.9 |
| MST score ≥ 1 | 52.6 | 47.0 | 49.3 | 59.8 | 52.8 | 56.0 | 100.0 | 49.0 | 51.4 | 73.1 | 63.2 | 67.3 | 59.3 | 64.7 | 61.0 |
| MST score ≥ 2 | 39.4 | 56.6 | 49.6 | 49.8 | 65.9 | 58.6 | 75.0 | 59.6 | 60.3 | 63.5 | 74.5 | 69.9 | 48.3 | 74.6 | 56.4 |
| MST score ≥ 3 | 17.2 | 81.2 | 54.9 | 21.7 | 84.9 | 56.1 | 16.7 | 81.2 | 78.2 | 35.9 | 94.6 | 70.2 | 23.0 | 92.9 | 44.6 |
| NRI score < 100 | 95.1 | 13.5 | 48.4 | 93.4 | 11.3 | 49.3 | 95.0 | 10.1 | 13.6 | 97.6 | 16.4 | 53.2 | 94.3 | 21.9 | 74.7 |
| NRI score < 97.5 | 85.8 | 25.1 | 51.0 | 88.6 | 27.5 | 55.8 | 95.0 | 21.2 | 24.3 | 92.9 | 32.1 | 59.7 | 86.7 | 41.1 | 74.4 |
| NRI score < 83.5 | 21.2 | 83.2 | 56.7 | 22.4 | 85.3 | 56.2 | 25.0 | 81.4 | 79.0 | 31.2 | 92.1 | 64.5 | 22.4 | 92.1 | 41.2 |
MST, Malnutrition Screening Tool; MUST, Malnutrition Universal Screening Tool; NRI, Nutritional Risk Index; Sen, sensitivity; spec, specificity; TA, total accuracy. The figures for highest total accuracy are denoted in bold.
Survival analysis
In total, 287 deaths occurred. Overall, the median follow‐up time was 12.2 months (interquartile range 6.6–26.1 months), with a median follow‐up for censored cases of 19.9 months (interquartile range 8.2–32.2 months). The predictive value of sarcopenia, myosteatosis, pre‐cachexia, CC, MUST score, MST score, and NRI score on survival were all individually assessed; in each case, controlling for factors that were either significant on a univariate level or known to impact upon prognosis, specifically age, sex, performance status, BMI, disease stage, and disease site. Controlling for these factors, myosteatosis (HR 1.6, 95% confidence interval: 1.2–2.1, P < 0.001) and NRI score remained significant predictors of survival on a multivariate level. NRI was the strongest significant predictor, with those having a score < 97.5 found to be at an increased risk of mortality (HR 1.8, 95% confidence interval: 1.2–2.8, P = 0.007) (median survival of 10 months for those with a NRI score of <97.5 vs. 30 months for those with a NRI score of >100, P < 0.001). A Kaplan–Meier curve comparing the survival (in days) of those with an NRI ≥97.5 vs. those with an NRI < 97.5 is displayed in Figure 3.
Figure 3.
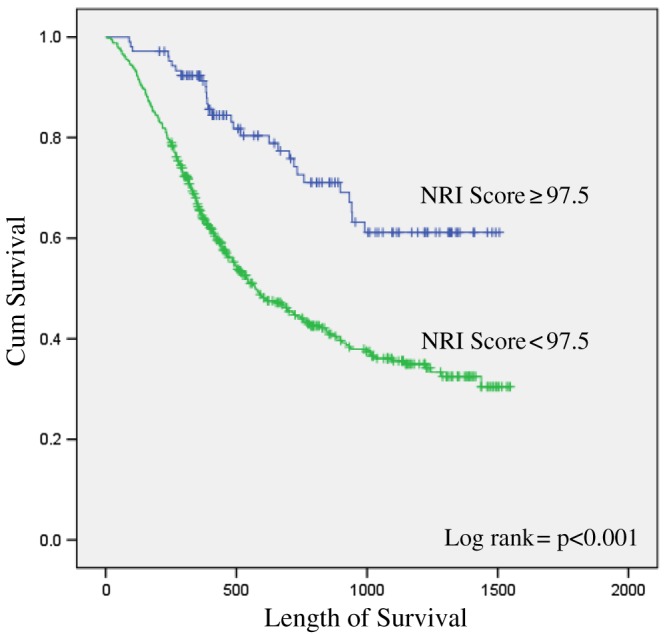
Kaplan–Meier curve comparing the survival of those with a Nutritional Risk Index (NRI) ≥ 97.5 vs. those with a NRI < 97.5.
Discussion
Cancer‐related malnutrition is an umbrella term used to describe the range of adverse nutritional conditions that occur in the oncology setting and can encompass a number of nutritional or body composition disorders. Involuntary weight loss can occur in isolation, as a result of a negative energy balance, or can be a symptom or manifestation of a cachexia–anorexia syndrome.9 The definition of CC is complex, as there are a number of definitions available to characterize the wasting syndrome in general disease. The primary concepts that form diagnostic criterion include involuntary weight loss, reduced nutritional intake, presence of systemic inflammation, anorexia, diminished physical function, reduced muscle mass, and reduced muscle strength.34, 35, 36 CC represents a spectrum, from pre‐cachexia to refractory cachexia, and the success of a nutritional intervention depends upon the point at which an intervention is implemented.9
Although starvation and CC both yield weight loss, the proportion of muscle and fat mass lost is different depending on the primary driver of the weight loss, i.e. negative energy balance or systemic inflammation, with muscle degradation promoted in states of inflammation.37 Sarcopenia refers to a low relative muscle mass and is defined as appendicular skeletal muscle mass (kg)/height2 (m2) less than 2 SD below the mean of a healthy, young reference group.38 Muscle quantity cannot be considered in isolation and also needs to be assessed in the context of muscle quality. Myosteatosis, a condition characterized by low muscle density secondary to increased levels of intermuscular and intramuscular fat, has come to the fore in recent years as an area of interest in the oncology setting.39
Assessment of malnutrition should no longer focus primarily on weight loss or BMI, as muscle quality and quantity continue to emerge as important predictors of treatment tolerance and clinical outcome. Sarcopenia and myosteatosis have been significantly associated with a range of adverse outcomes including increased rate of post‐operative complications,10, 11, 40, 41, 42 longer length of hospital stay,41, 43 30‐day post‐operative mortality and in‐hospital mortality,44 and dose‐limiting toxicities.5, 6, 7, 8, 45, 46, 47, 48, 49, 50 In addition, the conditions have been demonstrated as independent predictors of reduced overall survival.18, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64 The situation is further complicated by the obesity epidemic that has been progressively worsening in recent times. Adiposity is an established risk factor for cancer, and recent studies have reported that between 40% and 60% of cancer patients are overweight or obese, even in the setting of metastatic disease.4, 17, 18, 65, 66 Irrespective of BMI, hidden malnutrition can be present, yields adverse clinical consequences, and needs to be identified and addressed.18
Although treatment of cancer‐related malnutrition is often treated with a degree of nihilism; early, targeted, multimodal nutritional treatment is essential and yields tangible clinical benefits.67, 68 Lack of nutritional treatment not only impacts on clinical outcomes and prognosis but it also causes significant distress for patients69 and their family members and carers.70
Although CT assessment is the gold standard method of body composition analysis in oncology patients, widespread application in the clinical setting is not feasible because of resource and staffing constraints. Nutritional screening is critically important in order to identify patients in greatest need of nutritional therapy. This is further emphasized by recent findings that highlight the strong association between nutritional screening and survival prediction in oncology populations.71 In the USA, universal screening and assessment of malnutrition in hospitalized patients is mandatory within 24 h of admission.23, 24
While an abundance of screening tools are available, a few have been developed to capture abnormal body composition phenotypes, and to date, there is no consensus on the optimal tool to be employed in the oncology setting. A recent study constructed a five step questionnaire to predict patients at risk of sarcopenia specifically72; however, little research has been performed to assess whether tools currently in use are adequately capturing patients with nutrition‐related syndromes that place them at a higher risk of adverse clinical outcomes. It has been previously suggested that the latter should be the priority for further research, rather than the development of new screening tools in an already saturated setting.73
To our knowledge, this is the first study of its kind to explore what proportion of patients with abnormal body composition phenotypes are being detected by commonly used nutritional screening tools. As these tools were not devised specifically for the detection of sarcopenia, myosteatosis, or CC, validation is most appropriately performed against a full nutritional assessment. These tools are widely employed in oncology patients; however, and therefore, it is important to examine their capacity in this setting specifically and to assess their ability to detect conditions that are so influential in this patient population.
Malnutrition was found to be very high in this large cohort, with abnormal body composition phenotypes highly prevalent, despite the high level of overweight and obesity recorded. The majority of patients had a BMI in the overweight/obese category at the time of screening. Although high rates of muscle wasting was detected using CT analysis, excessive adiposity appeared to mask this wasting and undernutrition. A large portion of patients that met the criteria for CC (37.2%) maintained a stable overall body weight yet had changes in muscle and fat mass that was only detectable using CT analysis. None of the screening tools assessed demonstrated a complimentary balance of sensitivity and specificity for the detection of the abnormal body composition phenotypes; however, a tool that generates a low AUC can still be useful in certain clinical settings. MUST was the most effective in detecting CC; however, this is unsurprising, as the diagnosis of CC is based upon the same parameters included in the MUST tool, i.e. weight loss and low BMI. Interestingly, 38% of CC cases in this cohort were diagnosed using CT scan alone and were not identifiable using the BMI or weight loss elements of the diagnostic criteria. No screening tool had the ability to overcome this deficit. Because of the increasing rates of overweight/obesity in this patient population, it could be proposed that less importance should be placed on BMI, because as shown, patients with a healthy, overweight, or obese BMI can suffer from severe levels of malnutrition. Incorporating a BMI score may ultimately place too much emphasis on body weight and allow hidden malnutrition to go undetected.
The NRI tool was highly sensitive; however, a lack of specificity reduced its overall accuracy. It could be argued that the cost incurred by referring patients for a secondary nutrition assessment would be far less than the cost of undetected malnutrition in the oncology setting or that the tool may have potential use as the first stage of a nutritional screening programme. Another vital issue that needs consideration is that each malnourished state is not necessarily mutually exclusive; therefore, it cannot be fully determined whether the ‘false positives’ produced when assessing one form of malnutrition are being generated on the basis of another form of malnutrition and therefore in reality are not actually ‘false’. We explored this theory by investigating the ability of the screening tools to detect the presence of any of the following conditions: sarcopenia, myosteatosis, pre‐cachexia, and CC. In this assessment, we found the NRI to have the best overall accuracy (74.7%) in highlighting patients who have at least one of the conditions in question.
Most notably, myosteatosis and NRI score ≤ 97.5 were established as prognostic indicators, independent of confounding factors. NRI was the strongest predictor, with patients categorized as being at moderate to severe nutritional risk by the tool, almost twice as likely to die (HR 1.8, confidence interval: 1.2–2.8, P = 0.007) and died on average three times earlier than nutritionally well counterparts (median survival of 10 months vs. 30 months, P < 0.001). NRI may have the ability to directly predict a primary endpoint, survival, while cutting out the intermediary stages of identifying nutritional conditions, which have previously been shown to predict survival. The tool therefore could potentially prove useful for the identification of nutritionally vulnerable patients at risk of reduced survival, at an early stage, before a refractory state manifests, and when nutritional intervention is most effective.9, 74 Effective, timely screening is essential in order to allow for appropriate referral to a dietitian for a thorough nutritional assessment and targeted treatment plan to address patients' specific nutritional inadequacies.
Conclusions
Malnutrition is highly prevalent in the Irish ambulatory oncology setting. Excessive adiposity appears to mask the muscle wasting and undernutrition that was present during screening, with vast misclassification of nutritionally vulnerable patients by commonly used nutritional screening tools. No tool assessed in the study demonstrated a complimentary balance of sensitivity and specificity for the detection of the abnormal body composition phenotypes identifiable by CT analysis. NRI was the strongest independent predictor of shorter survival and demonstrated an ability to directly predict the primary endpoint of interest, reduced survival. CT scans play a unique role because of their availability; however, automation of body composition analysis is required to truly exploit the use of CTs in the clinical oncology setting. Until that is available, an effective method of grading patient's nutritional risk is critically required in order to ensure the most nutritionally vulnerable patients, including those with hidden muscle wasting, are referred for extensive nutritional assessment and targeted nutritional treatment.
Conflicts of interest
None to disclose.
Acknowledgements
We would like to acknowledge the helpful comments on the manuscript given by Dr Darren Dahly (Principal Statistician, Health Research Board Clinical Research Facility, Cork) and the Health Research Board's Cork Clinical Research Facility for the use of their facility. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle: update 2015.75
This research was supported by Cork Cancer Research Centre/ Breakthrough Cancer Research.
Ní Bhuachalla, É. B. , Daly, L. E. , Power, D. G. , Cushen, S. J. , MacEneaney, P. , and Ryan, A. M. (2018) Computed tomography diagnosed cachexia and sarcopenia in 725 oncology patients: is nutritional screening capturing hidden malnutrition?. Journal of Cachexia, Sarcopenia and Muscle, 9: 295–305. doi: 10.1002/jcsm.12258.
References
- 1. Kruizenga H, van Keeken S, Weijs P, Bastiaanse L, Beijer S, Jager‐Wittenaar H, et al. Undernutrition screening survey in 564,063 patients: patients with a positive undernutrition screening score stay in hospital 1.4 d longer. Am J Clin Nutr 2016;103:1026–1032. [DOI] [PubMed] [Google Scholar]
- 2. Stratton RJ, Green CJ, Elia M. Disease‐related Malnutrition: An Evidence‐based Approach to Treatment. Cabi; 2003. [Google Scholar]
- 3. Bozzetti F, Group SW. Screening the nutritional status in oncology: a preliminary report on 1,000 outpatients. Support Care Cancer 2009;17:279–284. [DOI] [PubMed] [Google Scholar]
- 4. Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, et al. Diagnostic criteria for the classification of cancer‐associated weight loss. J Clin Oncol 2015;33:90–99. [DOI] [PubMed] [Google Scholar]
- 5. Barret M, Antoun S, Dalban C, Malka D, Mansourbakht T, Zaanan A, et al. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer 2014;66:583–589. [DOI] [PubMed] [Google Scholar]
- 6. Cushen S, Power D, Teo M, Maceneaney P, Maher M, McDermott R, et al. Body composition by computed tomography as a predictor of toxicity in patients with renal cell carcinoma treated with sunitinib. Am J Clin Oncol 2014;40:47–52. [DOI] [PubMed] [Google Scholar]
- 7. Tan BH, Brammer K, Randhawa N, Welch NT, Parsons SL, James EJ, et al. Sarcopenia is associated with toxicity in patients undergoing neo‐adjuvant chemotherapy for oesophago‐gastric cancer. Eur J Surg Oncol 2015;41:333–338. [DOI] [PubMed] [Google Scholar]
- 8. Cousin S, Hollebecque A, Koscielny S, Mir O, Varga A, Baracos V, et al. Low skeletal muscle is associated with toxicity in patients included in phase I trials. Invest New Drugs 2014;32:382–387. [DOI] [PubMed] [Google Scholar]
- 9. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 10. Sharma P, Zargar‐Shoshtari K, Caracciolo JT, Fishman M, Poch MA, Pow‐Sang J, et al. Sarcopenia as a predictor of overall survival after cytoreductive nephrectomy for metastatic renal cell carcinoma. Urol Oncol 2015;33:e17–e23. [DOI] [PubMed] [Google Scholar]
- 11. Voron T, Tselikas L, Pietrasz D, Pigneur F, Laurent A, Compagnon P, et al. Sarcopenia impacts on short‐and long‐term results of hepatectomy for hepatocellular carcinoma. Ann Surg 2015;261:1173–1183. [DOI] [PubMed] [Google Scholar]
- 12. Pressoir M, Desne S, Berchery D, Rossignol G, Poiree B, Meslier M, et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br J Cancer 2010;102:966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lis CG, Gupta D, Lammersfeld CA, Markman M, Vashi PG. Role of nutritional status in predicting quality of life outcomes in cancer – a systematic review of the epidemiological literature. Nutr J 2012;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Argilés JM, Busquets S, Stemmler B, López‐Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 2014;14:754–762. [DOI] [PubMed] [Google Scholar]
- 15. Gupta D, Vashi PG, Lammersfeld CA, Braun DP. Role of nutritional status in predicting the length of stay in cancer: a systematic review of the epidemiological literature. Ann Nutr Metab 2011;59:96–106. [DOI] [PubMed] [Google Scholar]
- 16. Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff S, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr 2017;36:49–64. [DOI] [PubMed] [Google Scholar]
- 17. Gioulbasanis I, Martin L, Baracos V, Thézénas S, Koinis F, Senesse P. Nutritional assessment in overweight and obese patients with metastatic cancer: does it make sense? Ann Oncol 2015;26:217–221. [DOI] [PubMed] [Google Scholar]
- 18. Martin L, Birdsell L, MacDonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 19. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Attar A, Malka D, Sabaté J, Bonnetain F, Lecomte T, Aparicio T, et al. Malnutrition is high and underestimated during chemotherapy in gastrointestinal cancer: an AGEO prospective cross‐sectional multicenter study. Nutr Cancer 2012;64:535–542. [DOI] [PubMed] [Google Scholar]
- 21. Schnadig ID, Fromme EK, Loprinzi CL, Sloan JA, Mori M, Li H, et al. Patient‐physician disagreement regarding performance status is associated with worse survivorship in patients with advanced cancer. Cancer 2008;113:2205–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laviano A, Meguid MM, Inui A, Muscaritoli M, Rossi‐Fanelli F. Therapy insight: cancer anorexia–cachexia syndrome—when all you can eat is yourself. Nat Clin Pract Oncol 2005;2:158–165. [DOI] [PubMed] [Google Scholar]
- 23. Dougherty D, Bankhead R, Kushner R, Mirtallo J, Winkler M. Nutrition care given new importance in JCAHO standards. Nutr Clin Pract 1995;10:26–31. [DOI] [PubMed] [Google Scholar]
- 24. Joint Commission on Accreditation of Healthcare Organizations . Comprehensive Accreditation Manual for Hospitals: The Official Handbook: 2006 CAMH. Illinois, Chicago: Joint Commission on Accreditation of Healthcare Organizations; 2006. [PubMed] [Google Scholar]
- 25. World Health Organisation . BMI classification. 2017. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html (Accessed January 2017)
- 26. Blackburn G, Gibbons G, Bothe A, Benotti P, Harken D, McEnany T. Nutritional support in cardiac cachexia. J Thorac Cardiovasc Surg 1977;73:489–496. [PubMed] [Google Scholar]
- 27. Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer J, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre‐cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia‐anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 2010;29:154–159. [DOI] [PubMed] [Google Scholar]
- 28. Elia M. The 'MUST' Report. Nutritional Screening for Adults: A Multidisciplinary Responsibility. Development and Use of the'Malnutrition Universal Screening Tool'(MUST) for Adults. British Association for Parenteral and Enteral Nutrition (BAPEN); 2003. [Google Scholar]
- 29. Ferguson M, Capra S, Bauer J, Banks M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 1999;15:458–464. [DOI] [PubMed] [Google Scholar]
- 30. Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg 1980;139:160–167. [DOI] [PubMed] [Google Scholar]
- 31. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 32. Shen W, Punyanitya M, Wang Z, Gallagher D, Onge M‐PS‐, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross‐sectional image. J Appl Physiol 2004;97:2333–2338. [DOI] [PubMed] [Google Scholar]
- 33. Hosmer DW Jr, Lemeshow S. Applied Logistic Regression. New York, USA: John Wiley & Sons; 2004. [Google Scholar]
- 34. Fearon KC, Voss AC, Hustead DS, Group CCS . Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr 2006;83:1345–1350. [DOI] [PubMed] [Google Scholar]
- 35. Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr 2008;27:793–799. [DOI] [PubMed] [Google Scholar]
- 36. Bozzetti F, Mariani L. Defining and classifying cancer cachexia: a proposal by the SCRINIO Working Group. J Parenter Enteral Nutr 2009;33:361–367. [DOI] [PubMed] [Google Scholar]
- 37. Laviano A, Koverech A, Mari A. Cachexia: clinical features when inflammation drives malnutrition. Proc Nutr Soc 2015;74:348–354. [DOI] [PubMed] [Google Scholar]
- 38. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 39. Sabel MS, Lee J, Cai S, Englesbe MJ, Holcombe S, Wang S. Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol 2011;18:3579–3585. [DOI] [PubMed] [Google Scholar]
- 40. Lieffers J, Bathe O, Fassbender K, Winget M, Baracos V. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer 2012;107:931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Joglekar S, Asghar A, Mott SL, Johnson BE, Button AM, Clark E, et al. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J Surg Oncol 2015;111:771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Joglekar S, Nau PN, Mezhir JJ. The impact of sarcopenia on survival and complications in surgical oncology: a review of the current literature. J Surg Oncol 2015;112:503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Torres ML, Hartmann LC, Cliby WA, Kalli KR, Young PM, Weaver AL, et al. Nutritional status, CT body composition measures and survival in ovarian cancer. Gynecol Oncol 2013;129:548–553. [DOI] [PubMed] [Google Scholar]
- 44. Reisinger KW, van Vugt JL, Tegels JJ, Snijders C, Hulsewé KW, Hoofwijk AG, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg 2015;261:345–352. [DOI] [PubMed] [Google Scholar]
- 45. Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, et al. Body composition as an independent determinant of 5‐fluorouracil‐based chemotherapy toxicity. Clin Cancer Res 2007;13:3264–3268. [DOI] [PubMed] [Google Scholar]
- 46. Prado CM, Lima IS, Baracos VE, Bies RR, McCargar LJ, Reiman T, et al. An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer Chemother Pharmacol 2011;67:93–101. [DOI] [PubMed] [Google Scholar]
- 47. Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 2009;15:2920–2926. [DOI] [PubMed] [Google Scholar]
- 48. Mir O, Coriat R, Blanchet B, Durand J‐P, Boudou‐Rouquette P, Michels J, et al. Sarcopenia predicts early dose‐limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS One 2012;7: e37563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Antoun S, Baracos V, Birdsell L, Escudier B, Sawyer M. Low body mass index and sarcopenia associated with dose‐limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol 2010;21:1594–1598. [DOI] [PubMed] [Google Scholar]
- 50. Huillard O, Mir O, Peyromaure M, Tlemsani C, Giroux J, Boudou‐Rouquette P, et al. Sarcopenia and body mass index predict sunitinib‐induced early dose‐limiting toxicities in renal cancer patients. Br J Cancer 2013;108:1034–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Camus V, Lanic H, Kraut J, Modzelewski R, Clatot F, Picquenot JM, et al. Prognostic impact of fat tissue loss and cachexia assessed by computed tomography scan in elderly patients with diffuse large B‐cell lymphoma treated with immunochemotherapy. Eur J Haematol 2014;93:9–18. [DOI] [PubMed] [Google Scholar]
- 52. Lanic H, Kraut‐Tauzia J, Modzelewski R, Clatot F, Mareschal S, Picquenot JM, et al. Sarcopenia is an independent prognostic factor in elderly patients with diffuse large B‐cell lymphoma treated with immunochemotherapy. Leuk Lymphoma 2014;55:817–823. [DOI] [PubMed] [Google Scholar]
- 53. Iritani S, Imai K, Takai K, Hanai T, Ideta T, Miyazaki T, et al. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J Gastroenterol 2015;50:323–332. [DOI] [PubMed] [Google Scholar]
- 54. Meza‐Junco J, Montano‐Loza AJ, Baracos VE, Prado CM, Bain VG, Beaumont C, et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol 2013;47:861–870. [DOI] [PubMed] [Google Scholar]
- 55. Harimoto N, Shirabe K, Yamashita YI, Ikegami T, Yoshizumi T, Soejima Y, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg 2013;100:1523–1530. [DOI] [PubMed] [Google Scholar]
- 56. Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, et al. Negative impact of skeletal muscle loss after systemic chemotherapy in patients with unresectable colorectal cancer. PLoS One 2015;10: e0129742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Psutka SP, Boorjian SA, Moynagh MR, Schmit GD, Costello BA, Thompson RH, et al. Decreased skeletal muscle mass is associated with an increased risk of mortality after radical nephrectomy for localized renal cell cancer. J Urol 2016;195:270–276. [DOI] [PubMed] [Google Scholar]
- 58. Psutka SP, Carrasco A, Schmit GD, Moynagh MR, Boorjian SA, Frank I, et al. Sarcopenia in patients with bladder cancer undergoing radical cystectomy: impact on cancer‐specific and all‐cause mortality. Cancer 2014;120:2910–2918. [DOI] [PubMed] [Google Scholar]
- 59. Cooper AB, Slack R, Fogelman D, Holmes HM, Petzel M, Parker N, et al. Characterization of anthropometric changes that occur during neoadjuvant therapy for potentially resectable pancreatic cancer. Ann Surg Oncol 2015;22:2416–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fogelman DR, Holmes H, Mohammed K, Katz MH, Prado CM, Lieffers J, et al. Does IGFR1 inhibition result in increased muscle mass loss in patients undergoing treatment for pancreatic cancer? J Cachexia Sarcopenia Muscle 2014;5:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stene GB, Helbostad JL, Amundsen T, Sørhaug S, Hjelde H, Kaasa S, et al. Changes in skeletal muscle mass during palliative chemotherapy in patients with advanced lung cancer. Acta Oncol 2015;54:340–348. [DOI] [PubMed] [Google Scholar]
- 62. Rodrigues HV, Baracos V, Wheler J, Parsons H, Hong D, Naing A, et al. Body composition and survival in the early clinical trials setting. Eur J Cancer 2013;49:3068–3075. [DOI] [PubMed] [Google Scholar]
- 63. Antoun S, Lanoy E, Iacovelli R, Albiges‐Sauvin L, Loriot Y, Merad‐Taoufik M, et al. Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer 2013;119:3377–3384. [DOI] [PubMed] [Google Scholar]
- 64. Rollins KE, Tewari N, Ackner A, Awwad A, Madhusudan S, Macdonald IA, et al. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr 2015;35:1103–1109. [DOI] [PubMed] [Google Scholar]
- 65. Chaves MR, Boléo‐Tomé C, Monteiro‐Grillo I, Camilo M, Ravasco P. The diversity of nutritional status in cancer: new insights. Oncologist 2010;15:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sundaram S, Johnson AR, Makowski L. Obesity, metabolism and the microenvironment: links to cancer. Journal of Carcinogenesis 2013;12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Faber J, Uitdehaag MJ, Spaander M, Steenbergen‐Langeveld S, Vos P, Berkhout M, et al. Improved body weight and performance status and reduced serum PGE2 levels after nutritional intervention with a specific medical food in newly diagnosed patients with esophageal cancer or adenocarcinoma of the gastro‐esophageal junction. J Cachexia Sarcopenia Muscle 2015;6:32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Konishi M, Ishida J, von Haehling S, Anker SD, Springer J. Nutrition in cachexia: from bench to bedside. J Cachexia Sarcopenia Muscle 2016;7:107–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cooper C, Burden S, Molassiotis A. An explorative study of the views and experiences of food and weight loss in patients with operable pancreatic cancer perioperatively and following surgical intervention. Support Care Cancer 2015;23:1025–1033. [DOI] [PubMed] [Google Scholar]
- 70. Amano K, Maeda I, Morita T, Okajima Y, Hama T, Aoyama M, et al. Eating‐related distress and need for nutritional support of families of advanced cancer patients: a nationwide survey of bereaved family members. J Cachexia Sarcopenia Muscle 2016;7:527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gu W, Zhang G, Sun L, Ma Q, Cheng Y, Zhang H, et al. Nutritional screening is strongly associated with overall survival in patients treated with targeted agents for metastatic renal cell carcinoma. J Cachexia Sarcopenia Muscle 2015;6:222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC‐F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 2016;7:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Guaitoli PR, Jansma EP, de Vet HC. Nutrition screening tools: does one size fit all? A systematic review of screening tools for the hospital setting. Clin Nutr 2014;33:39–58. [DOI] [PubMed] [Google Scholar]
- 74. Prado CM, Sawyer MB, Ghosh S, Lieffers JR, Esfandiari N, Antoun S, et al. Central tenet of cancer cachexia therapy: do patients with advanced cancer have exploitable anabolic potential? Am J Clin Nutr 2013;98:1012–1019. [DOI] [PubMed] [Google Scholar]
- 75. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]


