Abstract
Although the human tail is completely absent at birth, the embryonic tail is formed just as in other tailed amniotes. Since all morphological variations are created from variations in developmental processes, elucidation of the tail reduction process during embryonic development may be necessary to clarify the human evolutionary process. The tail has also been of great interest to the medical community. The congenital anomaly referred to as ‘human tail’, i.e. the occurrence of a tail‐like structure, has been reported and was thought to represent a vestige of the embryonic tail; however, this hypothesis has not been verified. Accordingly, in this study, we aimed to establish a new method to visualize all somites in an embryo. We used sagittal‐sectioned embryos from Carnegie Stage (CS) 13 to CS23. All samples were obtained from the Congenital Anomaly Research Center, Kyoto University, Japan. Combining photomicroscopy and three‐dimensional reconstruction, we clearly visualized and labeled all somites. We found that the number of somites peaked at CS16 and dramatically decreased by approximately five somites. Tail reduction with a decrease in somites has also been observed in other short‐tailed amniotes; thus, this result suggested the possibility that there is a common mechanism for morphogenesis of short tails in amniote species. Additionally, our findings provided important insights into the cause of the congenital anomaly known as ‘human tail’.
Keywords: amniotes, evolution, somite, tail, three‐dimensional reconstruction
Introduction
In primates, tail length varies greatly among species and even among conspecific local populations, reflecting the climate, locomotion, and other environmental and phylogenetic factors. Tail length is considered an important feature with respect to primate adaptation and phylogeny. Humans, however, have completely lost their tails. During the evolutionary process of humans, ancestral species with a long tail [Aegyptopitecus zeuxis, approximately 33 million years ago (Ma)] (Fleagle, 1998) and without a tail (Proconsul heseloni, approximately 18 Ma, and Nacholapithecus kerioi, approximately 15.5 Ma) (Ward et al. 1991; Nakatsukasa et al. 2003, 2004) have been found in the fossil records; however, no fossils of species connecting these animals have been found. Therefore, it is unclear how humans lost their tails.
Generally, all morphological variations observed in adults are created from variations in developmental processes. Thus, elucidation of the process and mechanism of tail reduction during human embryonic development may clarify the human evolutionary process. Although the human tail is completely eliminated at birth, human embryos have a distinct tail during development. Moreover, the human tail is at first relatively long, but the length is then reduced during embryonic development and disappears at the end of the embryonic phase (Gasser, 1975). However, the actual process through which embryonic tail reduction occurs has not yet been clarified.
The human tail is interesting not only to biologists but also to the medical community. The rare congenital anomaly referred to as ‘human tail’ has been reported in infants, manifesting as the occurrence of a tail‐like structure from the lumbar to anus region. Based on its morphology and content, many classifications have been proposed (Virchow, 1884; Harrison, 1901; Dao & Netsky, 1984; Kansal et al., 2010; Katsuno & Horisawa, 2008). Most classifications, however, focus on the presence of vertebrae within the tail and have regarded this feature as one of the most important morphological aspects. According to these studies, the ‘human tail’ can roughly be divided into two types: ‘true tail’ with some vertebrae or bony elements inside, and ‘caudal appendage’ or ‘pseudotail’ without any bones. Notably, Harrison (1901) explained this as a vestige of the embryonic tail and stated that the human embryonic tail consisted of the ‘vertebral portion’ (proximal region of the tail with some vertebrae) and ‘caudal filament’ (distal region of the tail with only mesenchymal tissue inside). Additionally, the ‘true tail’ would occur only when the entire of embryonic tail failed to disappear, whereas the ‘pseudotail’ would be produced when the ‘caudal filament’ failed to disappear. This hypothesis has been described for over 100 years (Dao & Netsky, 1984; Yoshioka et al. 2001; Shad & Biswas, 2012; Tubbs et al. 2016) but has not yet been confirmed.
As is described above, tail reduction in humans has attracted interest from researchers in various fields. To understand the tail reduction process during human embryonic development, analysis of the transition in the number of the somites, i.e. vertebral primordia, is essential. However, few studies have examined this issue. Kunitomo (1918) focused on tail reduction during human embryonic development, evaluating total somite numbers in 44 sagittal‐sectioned samples from the Carnegie Collection. Unfortunately, the Carnegie Stage (CS) had not been defined at that time, and thus, the developmental stage was expressed as the embryonic crown‐rump lengths (CRLs). The CRLs of the samples varied from 4.0 to 125.0 mm, and the longest tail was observed in a 7.5‐mm embryo. Moreover, the human tail was found to consist of two parts: a proximal longer part (the vertebrate tail) and a caudal shorter part containing only a mesodermal remnant. The tail‐like appendage occasionally found in adults may be explained as a persistence of the caudal parts. The method, however, was not accurate with regard to counting the number of somites, and median sections were collected for observation of somites, despite the observation that the embryonic tail generally excurses left or right in amniotes, including humans. Thus, it is almost impossible to observe and count all somites in the tail, even by observation of median sagittal sections. O'Rahilly & Meyer (1979) reported the transition of the number of pairs of somites during human embryonic development from CS7 to CS23. They combined previous findings and data and reported that the number of pairs of somites tended to increase from CS7 and peaked between CS15 and CS17. The numbers of somites at each CS, however, were not necessarily specified, and most of the data were cited from previous studies published in the early 1900s. In particular, descriptions of the numbers of somite pairs from CS13 to CS23 are scarce. Specific data were available only for CS13 and CS15, but these data were from references in 1905 and 1901, respectively (Barden & Lewis, 1901; Gage, 1905). These previous studies counted the number of somites by superficial observation or observation of sectioned samples, which was not suitable for counting all somites, particularly in the tail.
Thus, in this study we established a new method to visualize all somites in an embryo. Using real human embryonic specimens, we examined how humans lost their tails during development through investigating the transition in the number of somites during every developmental stage.
Methods
Human embryonic specimens
All samples used in this study were a part of the Kyoto Collection housed at the Congenital Anomaly Research Center of Kyoto University in Japan (Nishimura et al. 1968; Shiota, 1991; Yamada et al. 2004). This collection comprises about 44 000 human embryos. Most of these samples were obtained by termination of the pregnancy during the first trimester for social or economic reasons under the Maternity Protection Law of Japan. Approximately 20% of the specimens were intact, and some were stored as serial‐sectioned and stained specimens. In this study, we used 42 human embryonic specimens from CS13 to CS23 (Table 1). All samples were sagittal‐sectioned and stained with hematoxylin and eosin.
Table 1.
Human embryonic specimens used in this study
| Stage | n |
|---|---|
| CS13 | 1 |
| CS14 | 7 |
| CS15 | 1 |
| CS16 | 6 |
| CS17 | 6 |
| CS18 | 4 |
| CS19 | 3 |
| CS20 | 5 |
| CS21 | 3 |
| CS22 | 5 |
| CS23 | 1 |
Ethical considerations
The use of human embryonic specimens for research purposes was approved by the Ethics Committee of the Graduate School of Medicine and the Faculty of Medicine, Kyoto University (approval number: R0316, R0347).
Visualization and counting of somites
Serial‐sagittal sections (10 μm thick) of whole‐body embryos were imaged using photomicroscopes (Keyence All‐in‐One Fluorescence Microscope BZ‐9000; Olympus virtual slide system VZ120) at 2× magnification. The two‐dimensional images were trimmed digitally using imagej64 (ver. 1.48; National Institutes of Health, Bethesda, MD, USA). Trimmed images were saved as bitmap (.bmp) files, aligned manually, and reconstructed three‐dimensionally in amira software (ver. 6.0.1; Visualization Sciences, Berlin, Germany). After labeling and alignment of all somites, all somites in embryos were successfully visualized, as shown in Figs 1 and 2.
Figure 1.
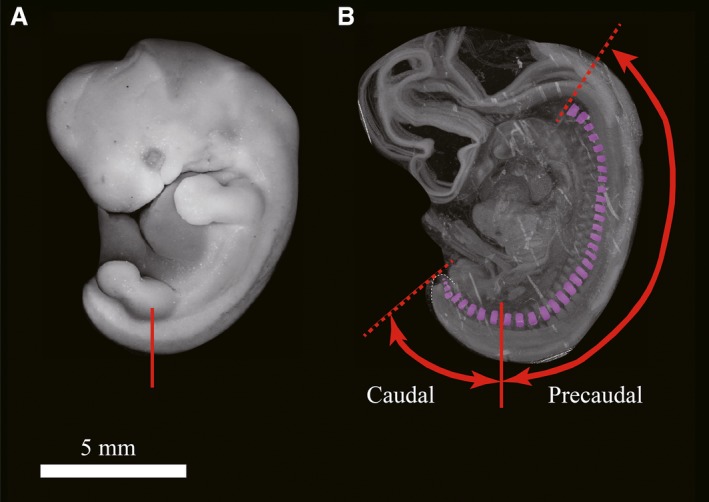
Definitions of caudal and precaudal region in this study. Representative view of a whole‐mount human embryonic sample (CS17, no. 13042). The tail tip is concealed behind the left hind limb (A) and in a 3D reconstructed sample, the tail tip is shown with a white dashed line (B). The red line indicates the midpoint of the base of the hind limb bud (after CS18, this point corresponded to the head of the femur). We defined the caudal region as the posterior area with regard to the red line, and the precaudal region was defined as the anterior area with regard to the red line.
Figure 2.

Three‐dimensional reconstructed human embryonic specimens from CS13 to CS23 with all somites visible. To make it easier to visualize the somites, all specimens are shown as the same size.
Definition of the ‘tail’ in this study
Previously, the human tail had been defined as the portion of the embryo that projects freely beyond the trunk (Harrison, 1901); however, this definition did not define the trunk and was not specific. In this study, we defined ‘tail’ as an elongated trunk posterior to the cloaca, as in other amniotes. The cloaca or anus is generally positioned at the base of the hind limbs in humans. Thus, we defined the ‘caudal region’ as the region posterior to the midpoint of the base of hind limb buds (Fig. 1). After CS18, when the chondrogenesis starts, this point corresponds to the femoral head. Our definition is more specific and nearly consistent with the previous definition described by Harrison (1901). Through three‐dimensional (3D) reconstruction of all somites in an embryo, we counted the number of somite pairs both in the caudal and precaudal regions.
Results
The numbers of pairs of somites during development are shown in Table 2, Figs 2 and 3. The total number of somite pairs increased linearly from CS13, at which time there were 30 pairs of somites, and peaked at CS16 (39–41 pairs). The number of pairs was then reduced by four or five between CS16 and CS17. After CS17, the number of pairs of total somites did not change dramatically. Within each developmental stage, the number of pairs of total somites varied by approximately two or three pairs, although CS14 tended to have more variation (typically seven pairs).
Table 2.
Number of total, precaudal, and caudal somite pairs
| Stage | n | CR length (mm) | No. of somites | No. of precaudal somites | No. of caudal somites |
|---|---|---|---|---|---|
| 13 | 10 756 | 4.2 | 30 | 21 | 9 |
| 14 | 542 | 7.4 | 35 | 23 | 12 |
| 14 | 677 | 7 | 34 | 21 | 13 |
| 14 | 764 | 7.3 | 38 | 25 | 13 |
| 14 | 1070 | 7.5 | 32 | 21 | 11 |
| 14 | 2271 | 7.2 | 31 | 24 | 7 |
| 14 | 10 774 | 8.2 | 35 | 24 | 11 |
| 14 | 14 654 | NA | 34 | 21 | 13 |
| 15 | 1298 | 8.7 | 35 | 23 | 12 |
| 16 | 845 | 9.2 | 41 | 26 | 15 |
| 16 | 10 974 | 8.9 | 39 | 23 | 16 |
| 16 | 12 760 | 9.7 | 39 | 26 | 13 |
| 16 | 13 640 | 8.4 | 40 | 24 | 16 |
| 16 | 14 114 | 11.2 | 39 | 23 | 16 |
| 16 | 14 394 | 9.9 | 40 | 24 | 16 |
| 17 | 659 | 12.8 | 35 | 25 | 10 |
| 17 | 1008 | 10.8 | 36 | 24 | 12 |
| 17 | 11 858 | 10.1 | 38 | 27 | 11 |
| 17 | 12 366 | 12.1 | 39 | 27 | 12 |
| 17 | 13 042 | 11.2 | 35 | 26 | 9 |
| 17 | 14 398 | 11.1 | 35 | 27 | 8 |
| 18 | 895 | 9.9 | 35 | 25 | 10 |
| 18 | 7277 | 12.4 | 35 | 24 | 11 |
| 18 | 10 309 | 13.7 | 37 | 26 | 11 |
| 18 | 17 418 | 15.2 | 36 | 26 | 10 |
| 19 | 923 | NA | 34 | 27 | 7 |
| 19 | 12 172 | 18.1 | 36 | 26 | 10 |
| 19 | 17292 | 15.7 | 34 | 24 | 10 |
| 20 | 567 | 20.8 | 35 | 27 | 8 |
| 20 | 806 | NA | 37 | 26 | 11 |
| 20 | 1219 | 19.3 | 35 | 26 | 9 |
| 20 | 10 344 | 19.1 | 34 | 25 | 9 |
| 20 | 17 268 | 16.8 | 37 | 26 | 11 |
| 21 | 2021 | 21.4 | 34 | 25 | 9 |
| 21 | 7316 | 22.2 | 34 | 25 | 9 |
| 21 | 14 831 | 20.5 | 31 | 25 | 6 |
| 22 | 3147 | 24.2 | 35 | 25 | 10 |
| 22 | 3332 | 24.1 | 34 | 25 | 9 |
| 22 | 6225 | 21.4 | 34 | 24 | 10 |
| 22 | 10 642 | 18.9 | 34 | 26 | 8 |
| 22 | 15 768 | 22.8 | 35 | 25 | 10 |
| 23 | 4381 | 26.3 | 31 | 24 | 7 |
Figure 3.

Transitions in the number of somite pairs during development. Three plots show the transition in the total number of somite pairs (A), the number of caudal somite pairs (B), and the number of precaudal somite pairs (C). In all graphs, black dots indicate the mean number of somite pairs in each stage. The bars above and below the black dots show the standard deviations.
In the precaudal region, the number of pairs of somites tended to increase from CS13 to CS17 and peaked at CS17, with 24–27 pairs, after which there was little reduction in the number of somites. Variations of two or three pairs of somites were observed within each stage.
The transition in the number of pairs of caudal somites showed a tendency similar to that of the total number of somite pairs. Specifically, there was a gradual increase from CS13, with a peak at CS16 of 13–16 pairs of caudal somites. However, there was then a dramatic decrease between CS16 and CS17 of about five pairs of caudal somites, after which the number of caudal somite pairs did not change much, remaining at 7–11 pairs in the caudal region. The number of pairs of caudal somites also varied by two or three pairs within the each developmental stage.
Discussion
This is the first study that has succeeded in visualizing all somites in the human embryo using three‐dimensional (3D) reconstruction methods. We also reported variations in the number of pairs of somites within each developmental stage. Our findings provided important insights into human development that are expected to be useful for researchers in biological and medical fields.
Mechanism of tail reduction during embryonic development
Our findings provided important information for clarifying the short‐tail morphogenetic mechanism, which may be commonly preserved among amniote species, including humans.
In this study, we found that the number of pairs of caudal somites tended to increase gradually with developmental progress, peaking at CS16. Consistent with these findings, a previous study reported that the number of somites peaked between C15 and CS17 (O'Rahilly & Meyer, 1979). Moreover, previous studies have shown that the transition in the number of somite pairs is related to the presence or loss of occipital somites. Sensenig (1957) reported that there were four or five occipital somites that disappeared at CS17. Gasser (1975) showed that one of four occipital pairs of somites disappeared at 5 weeks, and the rest of somites then fused together at 6 weeks. O'Rahilly & Meyer (1979) reported the transition in the number of pairs of total somites and stated that the number of somite pairs increased because of the presence of occipital somites or decreased because of the loss of these somites. However, our findings revealed that both precaudal and caudal somite pairs increased during development. In contrast, a decrease in the number of somite pairs was observed only in the caudal region. The loss of occipital somites should have occurred during CS15 and CS17, but no reduction in somites was observed during these stages because the number of somites, which increased during somitogenesis, was larger than the number of lost occipital somites. Although the number of precaudal somites did not change after the peak, the number of caudal pairs of somites decreased substantially between CS16 and CS17. This result suggested that the number of somites decreased not in the precaudal region but in the caudal region.
Apoptosis may be related to caudal somite reduction. For example, Fallon & Simandl (1978) performed supravital staining of human embryos with neutral red or brilliant cresyl blue and visualized phagocytized vacuoles by macrophages. They found active apoptosis at the tail after CS16 and concluded that the human embryonic tail was reduced by apoptosis. Indeed, a rapid and substantial decrease in caudal somites also occurs between CS16 and CS17, and this event may be caused by apoptosis. Moreover, Tojima (unpublished data, 2017) observed the transition in the number of somite pairs in chicken embryos as well. Among amniotes, the mechanism of axis elongation and somitogenesis is highly preserved. In chicken embryos, a similar event, i.e. a rapid and substantial decrease in caudal somites almost by five pairs, was observed as well (Tojima, S., unpublished data, 2017). Tojima also examined whether the apoptotic signal increased when the caudal somites decreased. Both immunohistostaining of active caspase3 and supravital staining of macrophages by Nile blue were performed, and the apoptotic signal was found to increase when the caudal somites decreased, similar to the results in chicken embryos.
Many genes related to axis elongation and somitogenesis may be involved in tail reduction during human embryonic development. In particular, Wnt‐3a and Hoxb13 may be important molecules for determining the number of caudal somites and tail length. Greco et al. (1996) showed that Wnt‐3a +/− mice had an extremely shortened tail. Additionally, Economides et al. (2003) showed that Hoxb13‐knockout mice had longer tails and two additional caudal vertebrae. Other genetic studies of short‐tailed cat breeds have also revealed that T‐box genes (Brachyury) are related to the short‐tail phenotype in the ‘tail‐less’ Manx breed (Buckingham et al. 2013) and that HES7 contributes to the phenotype of Asian bobtail cats (Xu et al. 2016). Other genes that are important for somitogenesis and axis elongation, such as the genes encoding fibroblast growth factor (FGF; Olivera‐Martinez et al. 2012; Bénazéraf & Pourquié, 2013), Notch, and retinoic acid receptor (Bénazéraf & Pourquié, 2013), may also affect tail morphology.
Is the ‘human tail’ really a vestige of the embryonic tail?
In this study, we also demonstrated that the previous hypothesis regarding the cause of the ‘human tail’ is partly incorrect. Previous studies have suggested that this anomaly may be caused by failure of the disappearance of the embryonic tail (Harrison, 1901). In particular, the ‘pseudotail’ without vertebrae inside has been explained as a vestige of the ‘caudal filament’, a vertebra‐less region at the tip of the tail. However, we found that caudal somites were distributed throughout the tail to the tip during development; thus, we showed that the ‘caudal filament’ without bony elements (somites) was not actually present. Accordingly, we concluded that Harrison's hypothesis regarding the cause of the ‘pseudotail’ as a vestige of the embryonic tail was not correct. Additionally, to determine whether the ‘true tail’ was a vestige of the embryonic tail, more information regarding the number of total vertebrae and presence of skeletal muscle fibers homologous to caudal musculature is needed. Unfortunately, most previous reports have lacked such information; thus, it is still unclear whether the ‘true tail’ is derived from the embryonic tail.
Author contributions
Dr. Sayaka Tojima, who is the first and corresponding author, designed this research project, acquired data, and drafted the manuscript. Dr. Haruyuki Makishima supported the establishment of the 3D reconstruction method. Professor Tetsuya Takakuwa provided constructive advice during the study and helped with data acquisition. Professor Shigehito Yamada allowed the first author to use the human embryonic specimens and provided important comments during revision of the manuscript.
Acknowledgements
We thank Ms Aoi Ishikawa (Human Health Science, Graduate School of Medicine, Kyoto University, Japan) for assistance with photomicroscopy. Professor Yoshiko Takahashi (Laboratory of Developmental Biology, Graduate School of Science, Kyoto University, Japan) allowed the first author to conduct this research when the author was hired as a postdoctoral researcher in her laboratory. We thank her for providing us with the opportunity to perform the research.
References
- Barden CR, Lewis WH (1901) The development of the limbs, body‐wall and back in man. Am J Anat 1, 1–36. [Google Scholar]
- Bénazéraf B, Pourquié O (2013) Formation and segmentation of the vertebrate body axis. Annu Rev Cell Dev Biol 29, 1–26. [DOI] [PubMed] [Google Scholar]
- Buckingham KJ, McMillin MJ, Brassil MM, et al. (2013) Multiple mutant T alleles cause haploinsufficiency of Brachyury and short tails in Manx cats. Mamm Genome 24, 400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao AH, Netsky MG (1984) Human tails and pseudotails. Hum Pathol 15, 449–453. [DOI] [PubMed] [Google Scholar]
- Economides KD, Zeltser L, Capecchi MR (2003) Hoxb13 mutations cause overgrowth of caudal spinal cord and tail vertebrae. Dev Biol 256, 317–330. [DOI] [PubMed] [Google Scholar]
- Fallon JF, Simandl BK (1978) Evidence of a role for cell death in the disappearance of the embryonic human tail. Am J Anat 152, 111–130. [DOI] [PubMed] [Google Scholar]
- Fleagle J (1998) Primate Adaptation and Evolution. 2nd edn, p. 596 San Diego: Academic Press. [Google Scholar]
- Gage SP (1905) A three weeks’ human embryo, with special reference to the brain and nephric system. Am J Anat 4, 409–443. [Google Scholar]
- Gasser RF (1975) Atlas of Human Embryos, p. 318 New York: Medical Department, Harper & Row Publishers. [Google Scholar]
- Greco TL, Takada S, Newhouse MM, et al. (1996) Analysis of the vestigial tail mutation demonstrates that Wnt‐3a gene dosage regulates mouse axial development. Genes Dev 10, 313–324. [DOI] [PubMed] [Google Scholar]
- Harrison RG (1901) On the occurrence of tails in man, with a description of the case reported by Dr. Watson. Johns Hopkins Hosp Bull 12, 96–101. [Google Scholar]
- Kansal R, Agrawal N, Khare S, et al. (2010) Newborn with a tail – a genetic throwback. People's J Sci Res 3, 15–17. [Google Scholar]
- Katsuno S, Horisawa M (2008) A case of perianal human tail (in Japanese). J Jpn Soc Pediatr Surg 44, 808–813. [Google Scholar]
- Kunitomo K (1918) The development and reduction of the tail and of the caudal end of the spinal cord. Contr Embryol 8, 161–198. [Google Scholar]
- Nakatsukasa M, Tsujikawa H, Shimizu D, et al. (2003) Definitive evidence for tail loss in Nacholapithecus, an East African Miocene hominoid. J Hum Evol 45, 179–186. [DOI] [PubMed] [Google Scholar]
- Nakatsukasa M, Ward CV, Walker A, et al. (2004) Tail loss in Proconsul heseloni . J Hum Evol 46, 777–784. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Takano K, Tanimura T, et al. (1968) Normal and abnormal development of human embryos: first report of the analysis of 1,213 intact embryos. Teratology 1, 281–290. [DOI] [PubMed] [Google Scholar]
- Olivera‐Martinez I, Harada H, Halley PA, et al. (2012) Loss of FGF‐dependent mesoderm identity and rise of endogenous retinoic signaling determine cessation of body axis elongation. PLoS Biol 10, e1001415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rahilly R, Meyer DB (1979) The timing and sequence of events in the development of the human vertebral column during the embryonic period proper. Anat Embryol 157, 167–176. [DOI] [PubMed] [Google Scholar]
- Sensenig EC (1957) The development of the occipital and cervical segments and their associated structures in human embryos. Contr Embryol 36, 141–152. [Google Scholar]
- Shad J, Biswas R (2012) An infant with caudal appendage. BMJ Case Rep 2012, bcr1120115160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota K (1991) Development and intrauterine fate of normal and abnormal human conceptuses. Congenit Anom (Kyoto) 31, 67–80. [Google Scholar]
- Tubbs RS, Malefant J, Loukas M, et al. (2016) Enigmatic human tails: a review of their history, embryology, classification, and clinical manifestations. Clin Anat 29, 430–438. [DOI] [PubMed] [Google Scholar]
- Virchow R (1884) Tailed man. NY Med J 40, 684. [Google Scholar]
- Ward CV, Walker A, Teaford MF (1991) Proconsul did not have a tail. J Hum Evol 21, 215–220. [Google Scholar]
- Xu X, Sun X, Hu XS, et al. (2016) Whole genome sequencing identifies a missense mutation in HES7 associated with short tails in Asian domestic cats. Sci Rep 6, 31583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Uwabe C, Fujii S, et al. (2004) Phenotypic variability in human embryonic holoprosencephaly in the Kyoto Collection. Birth Defect Res A Clin Mol Teratol 70, 495–508. [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Goto T, Akiyama T (2001) A case of human tail in a 12‐year‐old boy: review of 49 Japanese human tail cases (in Japanese). J Jpn Soc Pediatr Surg 37, 831–836. [Google Scholar]


