Abstract
We provide a systematic review and meta‐analysis on the efficacy, tolerability, and safety of cannabinoids in palliative medicine. The Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, PsycINFO, PubMed, Scopus, and http://clinicaltrials.gov, and a selection of cancer journals were searched up until 15th of March 2017. Of the 108 screened studies, nine studies with a total of 1561 participants were included. Overall, the nine studies were at moderate risk of bias. The quality of evidence comparing cannabinoids with placebo was rated according to Grading of Recommendations Assessment, Development, and Evaluation as low or very low because of indirectness, imprecision, and potential reporting bias. In cancer patients, there were no significant differences between cannabinoids and placebo for improving caloric intake (standardized mean differences [SMD]: 0.2 95% confidence interval [CI]: [−0.66, 1.06] P = 0.65), appetite (SMD: 0.81 95% CI: [−1.14, 2.75]; P = 0.42), nausea/vomiting (SMD: 0.21 [−0.10, 0.52] P = 0.19), >30% decrease in pain (risk differences [RD]: 0.07 95% CI: [−0.01, 0.16]; P = 0.07), or sleep problems (SMD: −0.09 95% CI: [−0.62, 0.43] P = 0.72). In human immunodeficiency virus (HIV) patients, cannabinoids were superior to placebo for weight gain (SMD: 0.57 [0.22; 0.92]; P = 0.001) and appetite (SMD: 0.57 [0.11; 1.03]; P = 0.02) but not for nausea/vomiting (SMD: 0.20 [−0.15, 0.54]; P = 0.26). Regarding side effects in cancer patients, there were no differences between cannabinoids and placebo in symptoms of dizziness (RD: 0.03 [−0.02; 0.08]; P = 0.23) or poor mental health (RD: −0.01 [−0.04; 0.03]; P = 0.69), whereas in HIV patients, there was a significant increase in mental health symptoms (RD: 0.05 [0.00; 0.11]; P = 0.05). Tolerability (measured by the number of withdrawals because of adverse events) did not differ significantly in cancer (RD: 1.15 [0.80; 1.66]; P = 0.46) and HIV patients (RD: 1.87 [0.60; 5.84]; P = 0.28). Safety did not differ in cancer (RD: 1.12 [0.86; 1.46]; P = 0.39) or HIV patients (4.51 [0.54; 37.45]; P = 0.32) although there was large uncertainty about the latter reflected in the width of the CI. In one moderate quality study of 469 cancer patients with cancer‐associated anorexia, megestrol was superior to cannabinoids in improving appetite, producing >10% weight gain and tolerability. In another study comparing megestrol to dronabinol in HIV patients, megestrol treatment led to higher weight gain without any differences in tolerability and safety. We found no convincing, unbiased, high quality evidence suggesting that cannabinoids are of value for anorexia or cachexia in cancer or HIV patients.
Keywords: cannabinoids, marijuana, palliation, cancer, HIV, weight gain, systematic review
Introduction
Palliative care is the active, extensive approach to improve the quality of life of patients with life‐threatening illnesses in which there is no possibility of remission.1, 2 The most important target of therapy is to optimize health‐related quality of life by reducing a wide spectrum of symptoms and addressing social, psychological, and spiritual problems. Palliative care can be a useful therapy for many different basic diseases such as cancer, advanced heart, lung, and kidney failure, amyotrophic lateral sclerosis and other neurological diseases, and advanced dementia or cognitive impairment.2
The most common and troublesome symptoms for patients in palliative care are pain, nausea, poor appetite, weight loss, and anxiety.3, 4 The effectiveness of cannabinoids in treating these symptoms has not yet been established by comparison to other therapies, a task complicated by the lack of specific criteria and indications for treatment.5, 6, 7 The situation is further complicated by the active cannabis legalization advocates effectively lobbying to change political and public opinions that cannabinoids of various formulations are highly effective in palliative care and a range of other conditions.
In Germany, for example, tetrahydrocannabinol (THC) was added to Supporting Information of the German narcotics law (Betäubungsmittelverordung) in 1998. Up to 2017, the cannabis extract mixture Nabiximols (Sativex®), composed equal quantities of THC and cannabidiol (CBD), was only licensed to treat intermediate to serious, therapy‐resistant spasticity in multiple sclerosis. It was also possible to treat patients off label with cannabis extract or THC on an individual basis through the Special Access Scheme. In March 2017, new laws came into effect in Germany that legalized the use of cannabis for medicinal purposes. This law will enable physicians to prescribe cannabis to patients for whom the drug could alleviate symptoms, such as chronic pain or nausea, or who may see a positive effect on their disease progression. In other countries, such as the USA, synthetic THC (e.g. dronabinol and nabilone) is licensed to treat nausea and vomiting because of chemotherapy treatment and appetite loss in patients with anorexia because of a human immunodeficiency virus (HIV)‐infection.8 In many US states, medical practitioners can certify the purchase of various cannabis products sold in retail premises with minimal regulation. In Australia, new regulations for patients to access cannabinoids for therapeutic purposes through the Special Access Scheme are also being enacted. In 2016, the Australian state New South Wales announced that it would undertake a clinical trial to assess the effectiveness of medicinal cannabis in enhancing quality of life for terminal cancer patients.9 Another Australian state, Queensland, has approved the use of medicinal cannabis for palliative care under a compassionate access scheme.10
As more jurisdictions across different countries begin to consider using cannabis and cannabinoids as a therapy during end of life care, it is important to evaluate the current evidence for the effectiveness, tolerability, and safety of cannabinoids. The efficacy and side effects of cannabis treatment within palliative care are the focus of this systematic review.
Objectives
The aim of this study is to evaluate the efficacy, tolerability, and safety of cannabinoids as an adjunct or complementary therapy in palliative medicine.
Methods
This review was performed as per the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement11 and recommendations of the Cochrane Collaboration.12 Analytical methods and inclusion criteria were defined beforehand.
Double‐blind or open label randomized controlled trials with parallel or crossover design and a duration of ≥2 weeks and ≥10 patients per study arm were included. At least one of the primary outcomes (see subsequent section ‘Types of outcome measures’), which were defined based on clinical experience, had to be addressed in the study.13, 14, 15 Non‐randomized studies, short abstracts, case reports, and studies without focus on palliative care aspects were excluded. We included patients with HIV except for those studies on neuropathic pain in patients with HIV, as this indication is the focus of two other systematic reviews16, 17
Participants
Studies included participants of any age, diagnosed with any advanced or end‐stage medical disease (e.g. cancer, dementia, HIV/Acquired Immune Deficiency Syndrome (AIDS), heart disease, lung disease, and liver disease).
Types of intervention
Herbal cannabis, plant based or synthetic cannabinoids in every form of application and dose, were considered in comparison to a placebo or active control.
Types of outcome measures
The study outcomes are summarized in the following section and in Table 1:
Efficacy: responder (pain reduction ≥30%27), body weight, appetite, caloric intake, and nausea/vomiting (primary endpoints); sleeping dysfunction, fatigue, mood disorders, and health‐related quality of life (secondary endpoints) at the end of each medication phase.
Tolerability: Number of patients, who discontinued the study because of adverse events; dizziness, mental health symptoms, and cognitive dysfunction.
Safety: Number of serious adverse; deaths during medication.
Table 1.
Included studies
| Reference | Disease | Duration | n | Intervention | Addressed endpoints | Tolerability | Safety |
|---|---|---|---|---|---|---|---|
| Cancer—studies | — | Median 8 weeks (16 days–11 weeks) | 758 | — | — | — | — |
| Brisbois18 | Cancer | 22 days | 46 | Dronabinol (synthetic) oral, at start 2, 5 mg/d, from fourth day 2 × 2,5mg/d; possibility to raise the dose to 20 mg/d; Placebo | 3a; 4b; 5c; 6d; 8e | 9d; 10d; 11h | 12h |
| Jatoi19 | Cancer‐related anorexia | 57, 74, and 80 days | 469 |
Megestrol acetate oral 800 mg/d; Dronabinol (synthetic) oral, 2 × 2,5 mg/d; Combination Megestrol: Dronabinol 800 mg/d: 2 × 2,5 mg/d Placebo |
2f; 4f; 8e,g | 9h; 10h; 11h | 12h |
| Johnson20 | Cancer—resistant pain | 16 days | 157 |
Oromucosal spray THC:CB (2,7 mg):(2,5 mg) extract per 100 μl equals one pump action, max 48× per 24 h; Oromucosal spray THC Extract (herbal) 2,7 mg/100 μl pump action, max 48× per 24 h; Placebo |
1ij; 4i; 5i; 6i; 8b | 9h; 10h; 11h | 12h |
| Portenoy21 | Cancer—opioid refractive pain | 9 weeks | 360 |
Oromucosal spray THC:CB (2,7 mg):(2,5 mg) extract per 100 μl equals one pump action; low dose group 1–4 x pump actions/d; middle dose group 6–10 × pump actions/d; high dose group 11–16 × pump actions/d; Placebo 1–16 × pump actions/d |
1ij; 6i; 7k; 8l | 10h; 11h | 12h |
| Strasser22 | Cancer‐related anorexia‐cachexia syndrome | 6 weeks | 243 |
THC:CB (2,5 mg) (1 mg) (herbal) oral, 2/d; THC (2,5 mg) (herbal) oral, 2/d; Placebo |
5b; 7b; 8l | 9h; 11h | 12h |
| HIV/AIDS—studies | — | Median 6 weeks (3–12 weeks) | 258 | — | — | — | — |
| Abrams23 | HIV | 3 weeks | 67 |
Herbal Cannabis Marijuana cigarettes with average weight of 0.9 g and 3,95% Delta‐9‐THC up to 3/d; Dronabinol (synthetic) oral 3× 2,5 mg/d; Placebo |
2o | 10h; 11h | 12h |
| Beal24 | AIDS‐related anorexia | 6 weeks | 139 | Dronabinol (synthetic) oral, 2 × 2,5 mg/d; Placebo | 2o; 4b; 5h; 7b; 8m | 10h; 11h | 12h |
| Timpone25 | HIV‐related cachexia | 12 weeks | 48 |
Megestrol 750 mg 1/d, oral; Megestrol 750 mg 1/d and Dronabinol 2 × 2,5 mg/d, oral; Megestrol 250 mg 1/d and Dronabinol 2 × 2,5 mg/d, oral; Dronabinol 2 × 2,5 mg/d, oral |
2o; 5b; 7b; 8n | 10h; 11h | 12h |
| Alzheimer's disease—study | — | 2 × 6 weeks | 15 | — | — | — | — |
| Volicer26 | Alzheimer's disease | 2 × 6 weeks | 15 |
Dronabinol 2 × 2,5 mg/d, oral; Placebo |
2o; 3q; 7p; | 11h |
1, pain; 2, change in weight; 3, caloric intake; 4, appetite; 5, nausea/vomiting; 6, sleeping disorders; 7, mood disorders; 8, health‐related quality of life; 9, dizziness; 10, mental health symptoms; 11, dropouts because of adverse effects; 12, serious adverse events; a, average Kcal/day; b, visual analogue scale; c, Edmonton Symptom Assessment System; d, side effect survey; e, Functional Assessment of Anorexia/Cachexia Therapy; f, North Central Cancer Treatment Group questionnaires; g, UNISCALE (tool for measuring overall quality of life in patients with advanced cancer); h, patient reports; i, numerical rating scale; j, Brief Pain Inventory‐Short Form; k, Montgomery Asberg Depression Rating Scale; l, The European Organization for Research and Treatment of Cancer quality of life questionnaire—QLQ 30; m, Karnofsky Index; n, Functional Assessment of HIV Infection questionnaire; o, bodyweight; p, Lawton Observed Affect Scale; q, calculated from the fraction of prescribed diet; HIV, human immunodeficiency virus; AIDS, acquired immune deficiency syndrome.
Search methods for identification of studies
A systematic search for literature up until 15th of March 2017 was conducted by four authors of this review (M. M., M. W., R. C., and C. C.). The detailed search algorithm can be seen in Supporting Information. Search terms addressed relevant palliative symptoms (e.g. pain and nausea), palliative medicine, cannabis related terms (e.g. marijuana), and relevant terms describing study methodology (e.g. randomized controlled). A previous systematic review based on a literature search up to February 2015 was published in German language.28 Primary studies were identified by specific search strategies within the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, PsycINFO, PubMed, and Scopus databases. Furthermore, relevant studies, listed in bibliographies of the identified randomized studies and review papers, were hand searched and included if they met the inclusion criteria. The databases http://clinicaltrials.gov and the International Association for Cannabinoid Medicines were searched to identify additional published data, unpublished data, and ongoing trials. In instances of disagreement for inclusion, two independent review authors (L. R. and H. C.) were involved to reach a consensus.
Data extraction and management
Five review authors (C. C., M. M., R. C., W. M., and W. H.) independently extracted data using a standard form. Information on the patients, the study setting, the active treatment and control groups, cointerventions, potential conflicts of interests for the authors, and the study's funding were extracted.
Where means or standard deviations (SD) were missing, attempts were made to estimate the treatment effect via reported statistical factors (e.g. t‐values, F‐values, and P‐values). Means and SD were also extracted if they were not addressed in the full‐text but provided in supplementary material.12 If the data were not available for extraction in a suitable way, an inquiry was made directly to the corresponding authors. We calculated numbers needed to treat for an additional beneficial outcome (NNT) as the reciprocal of the absolute risk reduction.29 For unwanted effects, the NNT becomes the number needed to treat for an additional harmful outcome (NNTH) and is calculated in the same manner. We used dichotomous data to calculate risk differences (RD) with 95% confidence interval (CI) using a fixed‐effect model unless we found significant statistical or clinical heterogeneity (see subsequent discussion).
We calculated standardized mean differences (SMD) with 95% CI for continuous variables using a fixed‐effect model unless we found significant statistical or clinical heterogeneity. We calculated NNTs for continuous variables (psychological distress and health‐related quality of life) using the Wells calculator software available at the Cochrane Musculoskeletal Group editorial office, which estimates, from the SMD, the proportion of participants who will benefit from treatment.30 We used a minimal clinically important difference of 15% for the calculation of the NNT from SMDs for all continuous outcomes. We set the threshold for a clinically relevant benefit or a clinically relevant harm for categorical variables by an NNT or NNTH less than 10.31
We used Cohen's categories to evaluate the magnitude of the effect size, calculated by SMD, with Hedges' g value of 0.2 = small, 0.5 = medium, and 0.8 = large.32 We labelled a g value less than 0.2 to be a ‘not substantial’ effect size. We assumed a minimally important difference if the Hedges' g value was 0.2 or greater.33
Five authors (C. C., M. M., R. C., W. M., and W. H.) independently assessed the risk of bias for each study, using seven aspects of bias recommended by the Cochrane Collaboration. These were selection bias (randomization and allocation concealment), performance bias, detection bias, attrition bias due to incomplete outcome data, and reporting bias.12 Each study was classified into high, low, and unknown risk for each type of bias. In the case of disagreement, a solution was mutually agreed on after discussion, and involving a fourth review author (L. R.) to reach a consensus if necessary. Studies were defined qualitatively as being high quality if they had six to seven factors with low risk of bias, as moderate quality if they had three to five factors with low risk of bias, and as low quality if only zero to two factors of the seven were classified as low risk of bias. The statistical analysis was performed with RevMan Analysis (RevMan 5.3) of the Cochrane Collaboration.
Quality of evidence and strength of recommendations were assessed on the basis of Grading of Recommendations Assessment, Development and Evaluation (GRADE)‐methodology. It was developed by the GRADE Working Group, and it is now widely seen as the most effective method of linking evidence‐quality evaluations to clinical recommendations. The GRADE approach specifically assesses:
Methodological flaws within the component studies;
Consistency of results across different studies;
Generalizability of research results to the wider patient base; and
How effective the treatments have been shown to be.
Treatment comparisons are given one of four GRADE scores reflecting the quality of the evidence—high‐quality, moderate‐quality, low‐quality, or very low‐quality evidences.34
The data input (W. H. and C. C.) was checked by six authors (M. M., H. C., R. C., M. W., L. D., and J. C.). Disagreements were settled by consensus. Standardized mean value differences of continuous variables were calculated for each intervention using MW and SD. A risk difference was determined for dichotomous variables. A random‐effect model (inverse variance method) was used to examine the combined results because it is more conservative than the fixed‐effects model and still accounts for both intra‐ and inter‐study variance. The pooled estimates of event rates of categorical data, such as dropout rates because of serious adverse events, were calculated using a random effects model. Ninety‐five percent confidence intervals were determined for all aggregated data. Heterogeneity was determined by the I2‐test. We dealt with clinical heterogeneity by combining studies that examined similar conditions. We assessed statistical heterogeneity visually35 and using the I2 statistic. When the I2 value was greater than 50%, we considered possible reasons for this. Probability value of <0.05 was determined to be the significance level. Significance levels between >0.05 and <0.10 were evaluated as a statistical trend.
Subgroup analysis
Subgroup analysis for the treatment with medical cannabis and cannabinoids in different dosages and routes of administration was planned beforehand. Within each underlying condition, an analysis was conducted for every endpoint on which there were at least two studies for each condition.
Results
After excluding duplicates, the literature search returned 108 publications. Of these, 89 were excluded during title and abstract screening. The remaining 19 full‐text publications were reviewed for suitability with 10 studies not matching the inclusion criteria, five studies not matching the specified study duration,36, 37, 38, 39, 40 four investigated too few patients,41, 42, 43, 44 and one focused on patients without an advanced or end stage disease.45 In total, nine studies were included in the analysis (Figure 1). The study settings are summarized in Table 1, demonstrating that six of the included studies were conducted as multicentre studies, four in North America,19, 21, 24, 25 one in Great Britain,20 and one in Europe.22 Another three studies were performed in North America. One of these was split up to two study centres,18 and another two studies were each conducted at a single centre.23, 26
Figure 1.
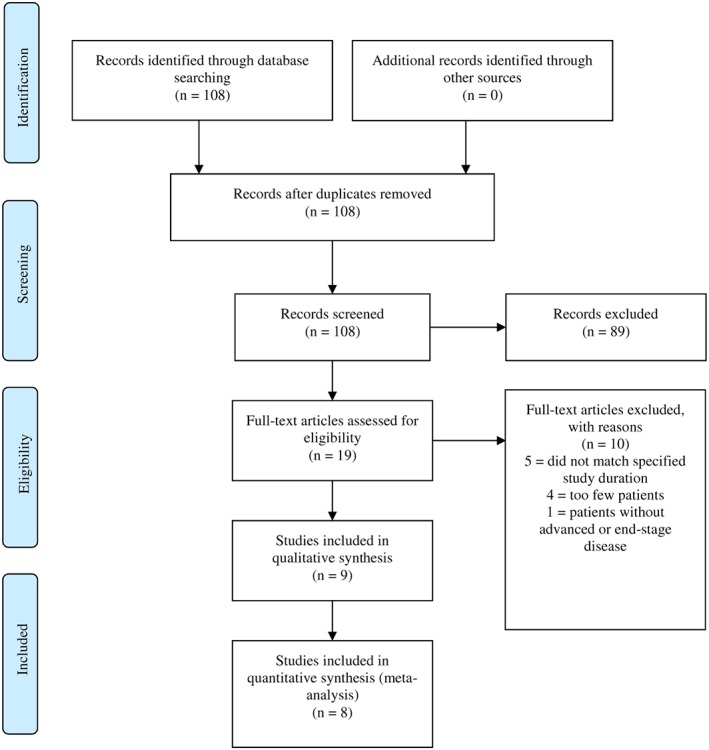
Study flow diagram (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses diagram).
Participants
The meta‐analysis included a total of 1561 adult patients. In five studies, patients were diagnosed with terminal advanced cancer, defined as an incurable malignant cancer, and in some cases, with an estimated life expectancy of less than 2 or 3 months18, 19, 20, 21, 22 (N = 758, age range 58–66). Three other studies focused on advanced HIV‐infection23, 24, 25 (N = 251, age range 39–43), and the last involved patients with a diagnosis of Alzheimer's disease26 (N = 15, age range 65–82). Within these groups, male patients (90.8%) substantially outweighed females. Patients with documented substance abuse were excluded in all studies. Only one of the studies did not explicitly exclude patients with current or lifetime psychiatric illnesses and dysfunctions.19
Intervention
The duration of the studies concentrating on cancer lasted a median of 8 weeks (range 16 days–11 weeks). Studies considering HIV infections lasted a median of 6 weeks (range 3–12 weeks). The Alzheimer's disease study was implemented in two phases of 6 weeks but did not give any information about the wash out phase. Synthetic THC (dronabinol) was tested in six studies18, 19, 23, 24, 25, 26; three tested a combination of THC and CBD,20, 21, 22 and one study tested herbal cannabis (Cannabis sativa).23
Two studies focusing on cancer‐related anorexia compared orally administered THC 5 mg/d and 5–20 mg/d with placebo.19, 20 One study compared the orally administered combination of THC/CBD 5 mg:2 mg/d to THC 5 mg/d with placebo in the treatment of cancer‐related anorexia.22 One study compared an oromucosal spray containing THC/CBD (up to 20 mg:10 mg/d) with THC (up to 20 mg/d) and placebo in the treatment of cancer‐related pain.21 One other study compared three different dosages of an oromucosal application of THC/CBD (10 mg:5 mg; 20 mg:10 mg; 40 mg:20 mg each per day) with each other and with placebo in the treatment of cancer‐related pain.20 Two studies focusing on HIV‐related cachexia compared orally administered THC 5 mg/d with placebo.23, 24 Another study compared the treatment of HIV‐related wasting with the oral administration of THC 5 mg/d with megestrol 750 mg/d, as well as to two dosage combinations of megestrol and THC (750 mg:5 mg/d; 250 mg:5 mg/d).25 The final study analysed the difference between the efficacy of dronabinol 5 mg/d and placebo in treating loss of appetite in Alzheimer's disease.26
Quality of evidence
When studies were evaluated against the seven Cochrane criteria for possible methodical flaws,12 five studies were judged to be at high risk of an attrition bias, one was at high risk of a performance bias, and another one was at high risk of a selection bias (Figure 2). Overall, three of the studies were judged to be of moderate quality, and six were judged to be of low methodological quality (Figure 3).
Figure 2.
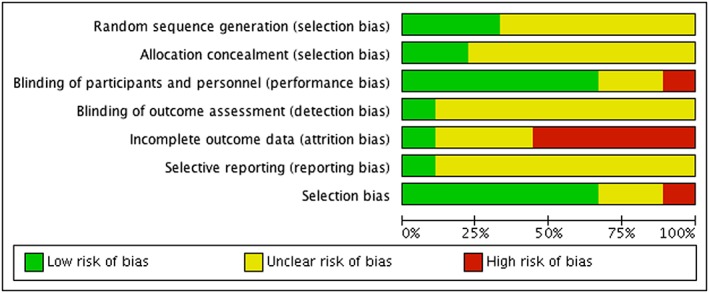
‘Risk of bias’ graph: review of authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
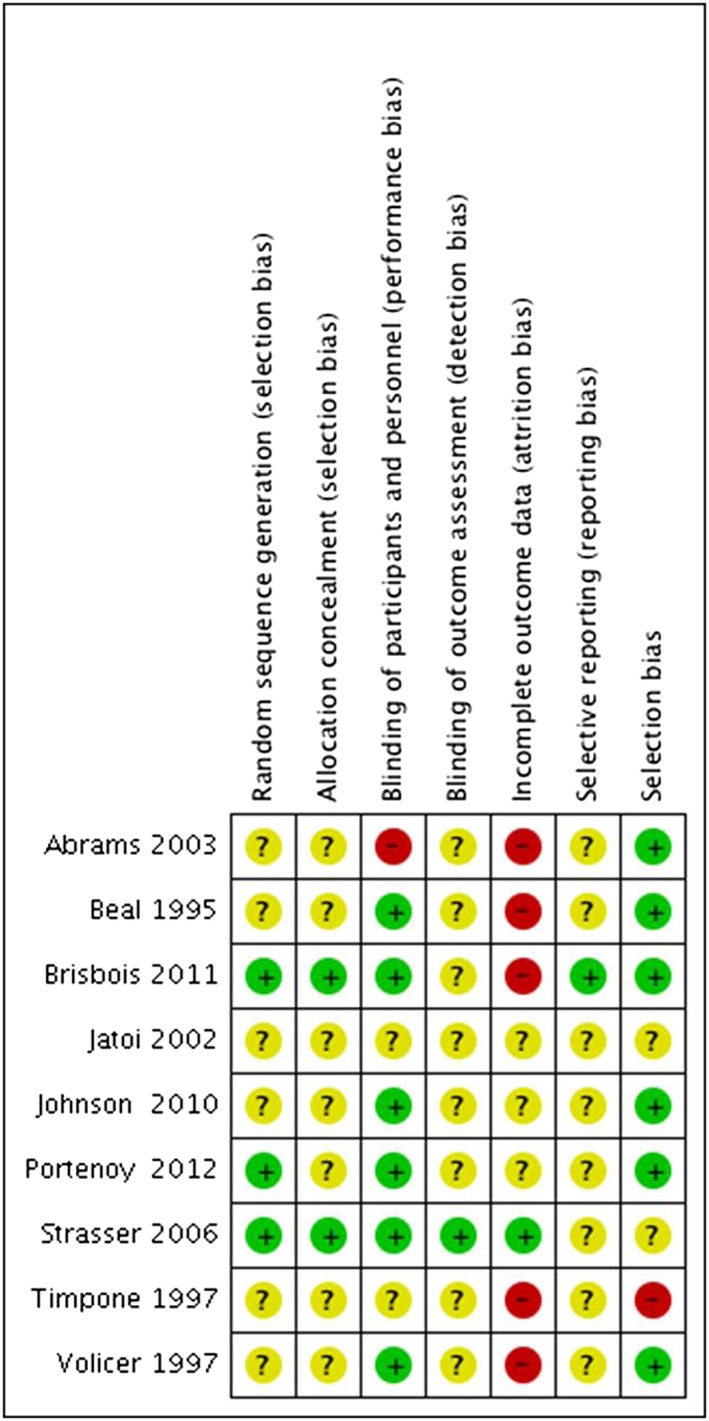
‘Risk of bias’ summary: review of authors' judgements about risk of bias items for each included study.
Merging the results
The results are provided with a 95%CI where possible. Where possible, studies were grouped according to the form of therapy, underlying disease, and the identified study outcomes. The overall assessment of quality of evidence according to GRADE methodology for cancer, HIV, and both diseases combined was summed up in Table 2, and Supporting Information, respectively.
Table 2.
Cannabinoids compared with placebo in cancer patients receiving palliative treatment
| Quality assessment | № of patients | Effect | Quality | Importance and NNT(H) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Cannabinoids | Placebo | Relative (95% CI) | Absolute (95% CI) | ||
| Weight loss/gain—Cancer—(follow up: mean 6 weeks) | ||||||||||||
| 1 a | randomized trials | not serious | not serious | not serious | very serious b | none | 196 | 48 | — | 0 (0 to 0) | ⨁⨁◯◯LOW | CRITICAL NNT = not determinable |
| Caloric intake—Cancer (follow up: median 22 days) | ||||||||||||
| 1 | randomized trials | serious c | not serious | not serious | very serious b , d , e | none | 11 | 10 | — | SMD 0.2 higher (0.66 lower to 1.06 higher) | ⨁◯◯◯VERY LOW | IMPORTANT NNT = not determinable |
| Appetite—Cancer (follow up: range 16 days to 6 weeks) | ||||||||||||
| 3 | randomized trials | serious c | not serious | not serious | very serious b , d , e | none | 324 | 117 | — | SMD 0.81 SD higher (1.14 lower to 2.75 higher) | ⨁◯◯◯VERY LOW | CRITICAL NNT = 72 |
| Nausea and Vomiting—Cancer (follow up: median 16 days) | ||||||||||||
| 1 | randomized trials | not serious | not serious | not serious | very serious b , d , e | none | 118 | 59 | — | SMD 0.21 SD higher (0.1 lower to 0.52 higher) | ⨁⨁◯◯LOW | IMPORTANT NNT = 10 |
| Pain reduction ≥30%—Cancer (follow up: range 16 days to 9 weeks) | ||||||||||||
| 2 | randomized trials | not serious | not serious | not serious | very serious b , d , e | none | 118/387 (30.5%) | 34/150 (22.7%) | RR 1.33 (0.95 to 1.85) | 75 more per 1.000 (from 11 fewer to 193 more) | ⨁⨁◯◯LOW | CRITICAL NNT = 13 |
| Sleeping disorders—Cancer (follow up: range 16 to 22 days) | ||||||||||||
| 2 | randomized trials | serious c | not serious | not serious | very serious b , d , e | none | 129 | 69 | — | SMD 0.09 lower (0.62 lower to 0.43 higher) | ⨁◯◯◯VERY LOW | IMPORTANT NNT = 55 |
| Dizziness—Cancer (follow up: range 16 days to 9 weeks) | ||||||||||||
| 4 | randomized trials | serious c | not serious | not serious | very serious b , d , e | none | 86/605 (14.2%) | 24/218 (11.0%) | RR 1.17 (0.76 to 1.80) | 19 more per 1.000 (from 26 fewer to 88 more) | ⨁◯◯◯VERY LOW | IMPORTANT NNTH = 32 |
| Mental health symptoms—Cancer (follow up: range 16 days to 9 weeks) | ||||||||||||
| 3 | randomized trials | serious c | not serious | not serious | very serious b , d , e | none | 13/410 (3.2%) | 7/172 (4.1%) | RR 0.72 (0.28 to 1.82) | 11 fewer per 1.000 (from 29 fewer to 33 more) | ⨁◯◯◯VERY LOW | CRITICAL NNTH = 112 |
| Health‐related quality of life—Cancer (follow up: range 16 days to 6 weeks) | ||||||||||||
| 3 | randomized trials | serious c | not serious | not serious | very serious b , d , e | none | 324 | 117 | — | SMD 0.09 SD higher (0.13 lower to 0.3 higher) | ⨁◯◯◯VERY LOW | CRITICAL NNT = 19 |
| Tolerability: Dropout because of adverse events—Cancer (follow up: range 16 days to 9 weeks) | ||||||||||||
| 4 | randomized trials | serious c | not serious | not serious | very serious b , d , e | none | 98/605 (16.2%) | 32/220 (14.5%) | RR 1.15 (0.80 to 1.66) | 22 more per 1.000 (from 29 fewer to 96 more) | ⨁◯◯◯VERY LOW | CRITICAL NNTH = 59 |
| Safety: serious adverse events—Cancer (follow up: range 16 days to 9 weeks) | ||||||||||||
| 4 | randomized trials | serious c | not serious | not serious | very serious b , d , e | none | 178/605 (29.4%) | 53/220 (24.1%) | RR 1.12 (0.86 to 1.46) | 29 more per 1.000 (from 34 fewer to 111 more) | ⨁◯◯◯VERY LOW | CRITICAL NNTH = 19 |
CI, confidence interval; SMD, standardized mean difference; RR, risk ratio; NNT, number needed to treat; NNTH, number needed to harm.
Explanations
Strasser 2006 also addresses the outcome weight loss/gain. The supplied data, however, is not suitable for meta‐analysis.
Very low sample size <1000.
Incomplete outcome data.
Total number of patients included is less than the number of patients generated by a conventional sample size calculation for a single adequately powered trial.
The boundaries of the CI are not on the same side of the threshold.
Cannabis and cannabinoids compared with placebo
The efficacy, tolerability, and safety of Cannabis and cannabinoids in relation to the study outcomes are summarized in Table 1.
Cancer—weight loss/gain
In a study with 243 patients, there were no differences in observed weight gain.22 Body weight after 6 weeks of therapy did not significantly differ from initial body weight in either group. There were also no significant differences in the average loss of bodyweight between groups (600 g over 6 weeks). The quality of evidence on this outcome measure according to GRADE methodology was low.
Cancer—caloric intake
One small study with 21 participants reported no statistically significant difference in the caloric intake of patients treated with THC or placebo (THC 1726 ± 378 kcal; placebo 1647 ± 379 kcal) (SMD: 0.20; 95% CI: [−0.66, 1.06]; P = 0.5).18 As shown in the risk of bias graph in Supporting Information, the study quality is seriously affected by incomplete outcome data. The quality of evidence was very low.
Cancer—appetite
Three studies involving 441 cancer patients18, 20, 22 did not find that cannabinoids were statistically significantly superior to placebo. (SMD: 0.81; 95% CI: [−1.14, 2.75]; P = 0.42; I2 = 98). The quality of evidence was very low.
Cancer—nausea and vomiting
A study of nausea and vomiting in 177 cancer patients20 found that cannabinoids were not superior to placebo (SMD: 0.21; 95% CI: [−0.10, 0.53]; P = 0.19; I2 = 0%). Nausea and vomiting, in this instance, were assessed through a self‐report quality of life questionnaire however did not distinguish between chemotherapy‐induced nausea and vomiting and non‐chemotherapy‐related nausea and vomiting. Another study with 243 patients comparing cannabis extract (THC/CBD) with THC or placebo found suggestive evidence of an antiemetic effect of the cannabis extract (61% reported a decrease in vomiting compared with 50% of the THC‐recipients and 40% of those treated with placebo), but these differences were not statistically significant (P = 0.37).22 The quality of evidence on nausea and vomiting was low according to GRADE methodology.
Cancer—pain reduction
Two studies20, 21 that measured the outcome of at least 30% pain reduction in 537 patients were entered in an analysis of response. Most patients reported experiencing mixed pain (between 42% and 51%); between 11% and 20% experience neuropathic pain. One hundred eighteen over three hundred eighty‐seven patients (30.5%) in the cannabinoid groups and 34/150 (22.7%) in placebo groups reported a pain reduction of at least 30% (RD 0.07; 95% CI: [−0.01, 0.16]; P = 0.07; I2 = 0). There was a statistical trend towards a greater pain reduction with cannabinoids. The quality of evidence was assessed as low according to GRADE methodology.
Cancer—sleep
Two studies with 203 cancer patients18, 20 did not find cannabinoids to be superior at promoting sleep than placebo (SMD: −0.09; 95% CI: [−0.62, 0.43]; P = 0.72; I2 = 63%). The quality of evidence was very low.
Cancer—dizziness
Four studies with 823 patients18, 20, 21, 22 did not find any statistically significant difference between cannabinoids and placebo in the percentage of patients who reported new symptoms of dizziness [14.2% in the cannabinoid group and 11.0% of patients in the placebo group (RD: 0.03; 95% CI: [−0.02, 0.08]; P = 0.23; I2 = 0)]. The quality of evidence was very low.
Cancer—mental health symptoms
Three studies with 582 patients were included into analysis of the development of mental health symptoms, such as depression21 or a change in emotional function.18, 20 The percentages of patients in cannabinoid and placebo groups were 3.2% and 4.1%, respectively, which showed signs of worsening mental health symptoms, but these were not statistically significant differences (RD: −0.01; 95% CI: [−0.04, 0.03]; P = 0.69; I2 = 0). One study with 243 cancer patients reported an improvement in depressed mood among 60% of the patients receiving cannabis extract (THC/CBD), compared with 46% of the patients treated with THC solely and 64% of those who received placebo, but these differences were not significant (P = 0.461).22 One study with 360 participants showed no significant difference between the effects of three different dosages of Nabiximols and placebo (P = 0.48; P = 0.15; P = 0.08).21 The quality of evidence was very low.
Cancer—quality of life
Two studies that included 420 cancer patients whose results were combined into analysis of health‐related quality of life20, 22 did not find any statistically significant differences between cannabinoids and placebo (SMD: 0.10; 95% CI: [−0.122, 0.33]; P = 0.30; I2 = 0). The quality of evidence was very low.
Cancer—tolerability
Four studies with 825 patients were included in an analysis of the tolerability of cannabinoids.18, 20, 21, 22 Ninety‐eight over six hundred five (16.2%) of patients treated with cannabinoids and 32/220 (14.5%) of patients who received placebo dropped out of the studies because of adverse events. There was no statistically significant difference in treatment tolerability between the two groups (RD: 1.15; 95% CI: [0.80, 1.6]; P = 0.46; I2 = 0). The quality of evidence was very low.
Cancer—safety
Four studies with 825 patients were analysed on the safety of cannabinoids.18, 20, 21, 22 Serious adverse events were reported by 178/605 (29.4%) of the patients in the cannabinoid groups and by 53/220 (24.1%) of patients in the placebo groups. This difference was non‐significant (RD: 1.12; 95% CI: [0.86, 1.46]; P = 0.39; I2 = 0). The quality of evidence was very low.
HIV—weight gain
Two studies with 192 participants were analysed for effects on weight.23, 24 Cannabinoids were statistically significantly better than placebo in increasing body weight (SMD: 0.57; 95% CI: [0.22, 0.92]; P = 0.001; I2 = 15). The quality of evidence was very low.
HIV—appetite
One study including 139 participants reported a significantly greater increase in appetite among patients who received cannabinoids.24 More than a quarter (27%) of patients who received dronabinol reported an increase in appetite, compared with 17% of patients treated with placebo (SMD: 0.57; 95% CI: [0.11, 1.03]; P = 0.02). The quality of evidence was very low.
HIV—nausea
One study with 139 participants reported a larger reduction in nausea (22%) in patients receiving dronabinol compared with placebo (4%), but this difference was not significant. (SMD: 0.20; 95% CI: [−0.15, 0.54]; P = 0.26).24 The quality of evidence was very low.
HIV—development of mental health symptoms
Two studies with 206 patients23, 24 compared the development of mental health symptoms in patients receiving cannabinoids (5.1%) with that in patients receiving placebo (0%). The difference was statistically significant (RD: 0.05; 95% CI: [0.00, 0.10]; P ≤ 0.05; I2 = 0). The quality of evidence was very low.
HIV—quality of life
One study with 139 participants reported of a reduction of the Karnofsky‐score (measuring health‐related quality of life) by 2.5 points in the dronabinol group.24 In comparison, the score of the placebo group remained the same as before, but this difference was not significant (SMD: −0.24; 95% CI: [−0.58, 0.11]; P = 0.18). The quality of evidence was very low.
HIV—tolerability
Two studies with 206 patients were included in the analysis of tolerability of cannabinoids as measured by the number who dropped out of treatment.23, 24 There were no significant differences in dropout rates between patients receiving cannabinoids (9/118 or 7.6%) and those receiving placebo (3/88 or 3.4%) (RD: 1.87; 95% CI: [0.60, 5.84]; P = 0.28; I2 = 0). The quality of evidence was very low.
HIV—safety
Safety, quantified as the number of serious adverse events experienced, was measured in two studies with a total of 206 participants.23, 24 Serious adverse events were reported by 7/118 (5.9%) patients in the cannabinoid groups and by 0/88 (0%) of patients in the placebo groups. This difference was not statistically significant (RD: 4.51; 95% CI: [0.54, 37.45]; P = 0.16; I2 = 1). The quality of evidence was very low.
Alzheimer's disease
One crossover study with 15 participants with Alzheimer's disease examined the effect of dronabinol on weight gain and caloric intake, mood disorders, tolerability and safety.26 The weight gain was higher in the group receiving dronabinol prior to placebo (P = 0.017). Average weight increased in both groups, but patients in the dronabinol–placebo group gained 3.95 kg, while those in the placebo–dronabinol group gained 3.13 kg. Caloric intake did not change during the study period. Negative affect (anger, anxiety, and sadness) decreased in both therapy phases (P = 0.045), but the decrease was significantly larger, while patients were in the dronabinol phase than the placebo phase (P = 0.004). Three patients dropped out, one because of adverse effects, and two because of serious infections. One patient died of heart attack in the placebo phase. The study has a serious risk of bias due to incomplete outcome data and a very serious imprecision because the study has an extremely small sample size, and the total number of patients included is less than the number generated by a conventional sample size calculation for a single adequately powered trial. Therefore, the quality of evidence on previously mentioned outcome criteria in Alzheimer's disease is graded very low.
Cannabis and cannabinoids vs. megestrol acetate
Megestrol acetate is used to improve appetite and to increase weight in cancer‐associated anorexia. It was approved in 1993 by the US Food and Drug Administration for the treatment of anorexia and cachexia in patients with AIDS.46
Cancer
One study with 469 participants compared dronabinol with megestrol acetate in increasing appetite in patients suffering from advanced cancer.19 Megestrol was superior to dronabinol in increasing appetite (49% to 75%; P = 0.0001), producing weight gain greater than 10% of baseline (3% to 11%; P = 0.02) and improving health‐related quality of life (P = 0.003). The number of dropouts because of adverse events in the megestrol treatment group was significantly lower compared to the cannabinoid treatment group (58% to 45%; χ2 = 4.9; P = 0.03), which showed a better tolerability for megestrol. The safety of the treatments, assessed by the number of serious adverse events, did not differ significantly between treatment groups (15% to 22%; χ2 = 2.4; P = 0.12). Regarding study quality, there is a serious imprecision because the study has a small sample size and provides no data to assess a total number of patients generated by a conventional sample size calculation for a single adequately powered trial. In summary, the quality of evidence is graded very low.
HIV
One study with 48 participants compared dronabinol to megestrol in treating HIV‐related cachexia.25 The average change in weight during megestrol treatment (6.5 ± 1.1 kg) was significantly better than that in patients receiving dronabinol (−2 ± 1.3 kg) (P = 0.0001). No differences were found in health‐related quality of life, nausea and vomiting, depressive mood, or tolerability and safety. The study has a serious risk of bias due to incomplete outcome data and a serious imprecision because of a very small sample size. Furthermore, the total number of patients included is less than the number of patients generated by a conventional sample size calculation for a single adequately powered trial. In summary, the overall quality of evidence is graded very low.
Herbal cannabis vs. plant‐derived THC in HIV
One study with 45 patients with HIV compared standardized Herbal Cannabis Marijuana cigarettes with dronabinol.23 Participants in the marijuana group gained 3.0 kg (0.75–8.6 kg) on average and those in the dronabinol group gained 3.2 kg (−1.4–7.6 kg). Small numbers of patients in the smoked marijuana (2/21, 9.5%) and dronabinol group (2/24, 8.3%) dropped out because of adverse events. These rates were not significantly different, and there were no serious adverse events in this study. The overall quality of evidence is graded very low.
Discussion
This systematic review and meta‐analysis evaluated the therapeutic use of cannabinoids in palliative care. The aim was to compare the available evidence on the efficacy, tolerability, and safety of cannabinoids (primarily dronabinol, nabiximols, and herbal cannabis in a range of conditions with placebo or active comparators).
A systematic literature search and screening process identified nine randomized controlled and crossover trials of palliative care. Five studies focused on advanced stage cancer, three on HIV infection, and one on late‐stage Alzheimer's disease. Meta‐analyses were conducted where possible, and results were interpreted based on study quality of evidence based on GRADE methodology.
Patients with cancer who were studied were evaluated for changes in weight gain, food intake, and nausea, and cannabinoids did not produce any significant changes. Appetite was measured in three studies of cancer patients,18, 19, 20 and a small benefit was found. The quality of evidence was assessed as very low. Results indicated a large SMD of 0.81. Taking the small number of participants and the high heterogeneity (I2 = 98) into account, further studies on this outcome are warranted. The same recommendation applies to cannabis extract (THC/CBD), which in one study was marginally better in reducing vomiting than placebo.22 Overall, the low quality of evidence does not allow for a convincing assessment of the possible risks or benefits of cannabinoids on alleviating cancer‐related anorexia and cachexia. On the other hand, in a large study involving 469 cancer patients providing moderate quality of evidence megestrol was clearly superior to dronabinol in increasing appetite and facilitating weight gain.19 Furthermore, the megestrol group showed a lower number of dropouts because of adverse events and a larger increase in health‐related quality of life than dronabinol.
Pain is a particularly debilitating symptom in cancer patients. Meta‐analysis of two studies that measured a pain reduction of at least 30% revealed a trend, albeit not significant, favouring patients receiving cannabis experiencing greater pain relief20, 21 with a NNT of 13. However, the low quality of evidence renders it impossible to draw meaningful conclusions. Further high quality studies are needed on this topic to further investigate the use for cannabinoids for pain in the context of clinical trials in palliative medicine. No statistically significant benefit from cannabinoids was found in treating sleeping problems.
As cannabinoids may have psychoactive and hallucinogenic effects, the assessment of dizziness and mental health symptoms due to cannabinoid use is of particular importance. There was very low quality of evidence to suggest patients receiving cannabinoids did not experience either of these symptoms more than patients receiving placebo.
Arguably, the most important target of palliative cancer therapy is patients' health‐related quality of life. Despite this importance, our search identified only three relevant studies. The overall quality of evidence was rated as very low. Study findings did not provide any evidence that cannabinoids produced larger improvements in quality of life than either placebo or active comparators.20, 22 This is in line with other recent meta‐analyses which found the current evidence for the impact of medical cannabis on quality of life is inconclusive.7 In view of the ongoing debate concerning the use of cannabinoids in palliative practice, high quality studies on quality of life are urgently needed. Very low quality evidence suggested that patients receiving cannabinoids were not significantly more likely than patients receiving placebo to drop out of the study or experience serious adverse events.
Among HIV patients, there was very low quality evidence that cannabinoids were superior to placebo at increasing appetite and weight gain. The NNT for appetite was 5. There was a medium effect size, however, this evidence was based on two studies with under 200 participants.23, 24 In contrast, a single study providing very low quality showed that dronabinol was significantly inferior to megestrol in weight gain.25 In this study, HIV patients on dronabinol lost over the course of 12 weeks on average 2 kg, whereas patients on megestrol gained on average 6.5 kg weight. There was no difference in tolerability or safety between both therapies. Adverse events of megestrol may have been underestimated because the authors did not assess the highly relevant side effect of newly developed impotence, however, it is unclear how relevant this side effect is to the management of AIDS related wasting at the stage of palliative care. In a further comparison of herbal cannabis and synthetic cannabinoids in 62 HIV patients,23 there were no significant differences in weight gain, tolerability, or safety. However, the current quality of evidence does not allow for a sound assessment of the benefits of cannabinoids in HIV‐related anorexia and cachexia.
With regard to the development of mental health symptoms for patients receiving cannabinoids, there was very low quality evidence to suggest patients receiving dronabinol were at a significantly increased risk of developing symptoms. The RD of 0.05 corresponds to a number needed to harm of 20.23, 24 Analysis of the outcome measures quality of life over the course of 6 weeks revealed no statistically significant benefits of cannabinoid treatment. Tolerability as measured by the number of dropouts in two studies with a total of 206 participants did not show any statistically significant difference between dronabinol and placebo, however, there were considerably more dropouts in the dronabinol than the placebo group, as reflected in the RD of 0.05.23, 24 The safety of cannabinoid treatments, as measured by the number of serious adverse events, did not differ significantly compared with placebo, despite the underlying RD of 0.06 and a NNTH of 17. The evidence on tolerability and safety of cannabinoids in HIV was graded as very low. The lack of power due to the small number of participants may explain the lack of significant differences regarding tolerability and safety.
Our search and screening process identified only one study providing very low quality of evidence on the treatment of Alzheimer's disease.26 In this crossover study, the effect of cannabinoids vs. placebo was investigated in only 15 patients. There was a reported advantage in favour of cannabinoids for weight gain and reduction of negative affect, and there were no significant differences in the tolerability or the safety of the treatments. However, the very small number of participants (15) in this single study greatly limits validity of these results.
It is important to note that the analysed studies found improvement in outcomes regarding gastrointestinal symptoms or pain with placebo, even though all patients were at stages of advanced disease. This highlights the importance of the placebo response in palliative care,47 which has been extensively shown in the context of analgesia.48, 49 Recent studies could show that placebo analgesic effects are mediated through the same spinal pathways as drug‐induced ones.50
The informative value of the present review and meta‐analysis is limited by the small number of participants in many of the studies. This particularly applies to Alzheimer's disease where only one study was extracted and to a single study comparing the efficacy of megestrol with THC in patients with HIV‐related cachexia. More generally, the studies were not long enough to fully assess long‐term efficacy, tolerability, and safety.
The studies largely provided biologically plausible arguments for using particular cannabinoids; all studies used a herbal or synthetic form of THC. Only one study specifically hypothesized about the effect that cannabinoids would have on the patient's quality of life20; other studies included and reported quality of life scales, but the main aims of the study were to target specific symptoms. It was less clear how the objective of obtaining ‘the best palliative care’ was defined across these studies, as most approaches made pharmacological comparisons only, rather than considering interdisciplinary or holistic treatments in which the cannabinoid or comparator treatment form part of a wider treatment regimen.
As cannabinoids are increasingly investigated for their potential therapeutic effect, there is an ongoing debate regarding whether ‘natural’ cannabinoids (such as herbal C. sativa) should be used in therapeutic settings, in comparison to cannabis derivatives (such as pharmaceutically controlled products dronabinol and nabiximols). In countries such as Israel and the Netherlands, herbal cannabis products are being produced for medicinal use. A primary argument for the use of herbal cannabis is to harness what is known as the ‘entourage effect’ by engaging a number of cannabinoid receptors.51, 52 However, while herbal cannabis can be grown in controlled environments to prevent the amount of pesticides or metals that the cannabis is exposed to, there is less control over the specific compounds that are desired for treatment effects. It may instead be considered beneficial to have control over which cannabinoid compounds the patient is receiving, particularly in the context of conducting clinical trials to determine efficacy. The use of specific cannabinoid compounds also allows for targeted treatment approaches. For example, emerging evidence suggests CBD may have a therapeutic effect for children with Dravet Syndrome.53 Using specific cannabinoid compounds may also help avoid experiencing some undesirable side effects, such as feeling ‘high’ or sedated.
To meet the appropriate standard underlying evidence‐based medicine, the included studies were chosen and classified according to Cochrane criteria.12 In the context of palliative medicine, however, collection for high quality evidence is difficult. According to the Cochrane criteria, none of the included studies was at the highest level of evidence (>400 patients, >8 weeks duration, and >50% pain reduction). Such studies are difficult to achieve in palliative medicine and research. Relevant reasons for recruitment difficulties and high dropout rates have been described elsewhere.54, 55, 56 It should be borne in mind that in palliative medicine findings classified as lower level of evidence by Cochrane criteria may for practical reasons be the only evidence available.
A previous Cochrane review13 of the medical benefits of cannabis in reducing morbidity and mortality in patients with HIV/AIDS included seven studies, two of which were also included in this review.23, 24 This review concluded that cannabinoids do not significantly increase weight or improve appetite or mood. Our finding based on two studies also provides very low quality of evidence that patients receiving dronabinol may be more likely to report an increase in mental health symptoms. This suggests that there is an urgent need for high quality studies on possible risks of dronabinol use and to further evaluate the potential benefit‐to‐harm ratio of the therapy. Despite dronabinol being approved in the USA to treat AIDS‐associated anorexia, our findings underline the lack of convincing data on the efficacy and safety of its use for this indication. A narrative review of the safety and efficacy of marijuana in the treatment of common physical and mental disorders by Belendiuk et al.57 concluded that medical marijuana was not recommended for symptom treatment and relief in HIV/AIDS. The authors extended this recommendation to Alzheimer's disease, amyotrophic lateral sclerosis, cachexia/wasting syndrome, hepatitis C, and cancer.
A systematic review by Whiting et al.58 examined randomized controlled trials of cannabinoids in the treatment of chemotherapy‐induced nausea and vomiting, poor appetite in patients with HIV/AIDS, chronic pain, multiple sclerosis or paraplegia‐associated spasticities, depression, anxiety disorders, sleep disorders, psychoses, glaucoma, or Tourette's syndrome. Five of the nine studies in our review were also included in Whiting et al.20, 21, 23, 24, 25 As our study specifically focused on the application of cannabinoids in palliative medicine, the outcomes of interest and the conclusions of the review differ in scope and perspective. The conclusions on the effectiveness of cannabinoids in palliative medicine must cautiously weigh adverse events on the one hand against specific symptom relief on the other hand. Whiting et al. report low quality evidence for the use of cannabinoids for weight gain in HIV patients, which is in keeping with our findings. The systematic review found moderate evidence for the use of cannabinoids for the treatment of chronic pain, but this assessment was based on a wide range of varying pain conditions. With regard to cancer pain, we found only low quality evidence for a clinical benefit of cannabinoids. Whiting et al. highlight the increased risk of (short‐term) adverse events associated with THC. The critical evaluation of our findings underlines the relevance of adverse events, particularly in HIV patients. The occurrence of mental health symptoms potentially affects a feeling of self‐efficacy, which is highly important to quality of life. Overall, there was no indication that cannabinoids had a positive impact on quality of life in patients with cancer or HIV–AIDS.
Conclusion
Following the GRADE methodology, no recommendations can be made for the use of cannabinoids in palliative care treatment for cancer, HIV–AIDS, or dementia. In view of this finding, further research is urgently needed to identify the efficacy and safety of cannabinoids as adjunctive or complementary therapies and to provide evidence‐based recommendations on their clinical utility in palliative care.
Funding
“Funding for MW, JC and LD was received from the Commonwealth Department of Health, the NSW Government Centre for Medicinal Cannabis Research and Innovation, the Victorian Department of Health and Human Services and the Queensland Department of Health. LD is supported by NHMRC research fellowship #1041472. The National Drug and Alcohol Research Centre at the University of NSW is supported by funding from the Australian Government under the Substance Misuse Prevention and Service Improvements Grant Fund”. [Correction added on 19 February 2018, after first online publication: The funding section was not included and has been added in this version.]
Conflict of interest
The authors declare that there is no conflict of interest.
Supporting information
Appendix 1 CENTRAL search strategy
Appendix 2. Forest Plots ‐ Cannabinoids vs. placebo
Appendix 3. Question: Cannabinoids compared to placebo in patients receiving palliative treatment
Acknowledgements
We thank Professor Wayne Hall and Professor Meera Agar for their comments and critical feedback on later drafts of the manuscript.
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia, and Muscle.59
Mücke, M. , Weier, M. , Carter, C. , Copeland, J. , Degenhardt, L. , Cuhls, H. , Radbruch, L. , Häuser, W. , and Conrad, R. (2018) Systematic review and meta‐analysis of cannabinoids in palliative medicine. Journal of Cachexia, Sarcopenia and Muscle, 9: 220–234. doi: 10.1002/jcsm.12273.
References
- 1. World Health Organisation . Palliative Care Fact Sheet No. 402 2015 [cited 2017. February 8]; Available from: http://www.who.int/mediacentre/factsheets/fs402/en/.
- 2. Van Mechelen W, Aertgeerts B, De Ceulaer K, Thoonsen B, Vermandere M, Warmenhoven F, et al. Defining the palliative care patient: a systematic review. Palliat Med 2013;27:197–208. [DOI] [PubMed] [Google Scholar]
- 3. Sigurdardottir KR, Kaasa S, Rosland JH, Bausewein C, Radbruch L, Haugen DF, et al. The European Association for palliative care basic dataset to describe a palliative care cancer population: results from an international Delphi process. Palliat Med 2014;28:463–473. [DOI] [PubMed] [Google Scholar]
- 4. Van Lancker A, Velghe A, Van Hecke A, Verbrugghe M, Van Den Noortgate N, Grypdonck M, et al. Prevalence of symptoms in older cancer patients receiving palliative care: a systematic review and meta‐analysis. J Pain Symptom Manage 2014;47:90–104. [DOI] [PubMed] [Google Scholar]
- 5. Bar‐Sela G, Vorobeichik M, Drawsheh S, Omer A, Goldberg V, Muller E. The medical necessity for medicinal cannabis: prospective, observational study evaluating the treatment in cancer patients on supportive or palliative care. Evid Based Complement Alternat Med 2013;2013:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aggarwal S. Use of cannabinoids in cancer care: palliative care. Curr Oncol 2016;23:S33–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldenberg M, Reid MW, IsHak WW, Danovitch I. The impact of cannabis and cannabinoids for medical conditions on health‐related quality of life: a systematic review and meta‐analysis. Drug Alcohol Depend 2017;174:80–90. [DOI] [PubMed] [Google Scholar]
- 8. Grotenhermen F, Müller‐Vahl K. The therapeutic potential of cannabis and cannabinoids. Dtsch Arztebl Int 2012;109:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. NSW Government . Clinical trials: palliative care. 2016 [cited 2017. February 8]; Available from: https://www.medicinalcannabis.nsw.gov.au/clinical-trials/terminal-illness-trial.
- 10. Queensland Government . Access medicinal cannabis. 2017. [cited 2017 February 8]; Available from: https://www.qld.gov.au/health/conditions/all/medicinal-cannabis/.
- 11. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions, Vol. 4 John Wiley & Sons; 2011. [Google Scholar]
- 13. Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. The Cochrane Library, 2013. [DOI] [PMC free article] [PubMed]
- 14. Tait RJ, Caldicott D, Mountain D, Hill SL, Lenton S. A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin Toxicol 2016;54:1–13. [DOI] [PubMed] [Google Scholar]
- 15. Zhang MW, Ho R. The cannabis dilemma: a review of its associated risks and clinical efficacy. J Addict 2015;2015:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Petzke F, Enax‐Krumova E, Häuser W. Efficacy, tolerability and safety of cannabinoids for chronic neuropathic pain: a systematic review of randomized controlled studies. Schmerz (Berlin, Germany) 2016;30:62–88. [DOI] [PubMed] [Google Scholar]
- 17. Simms VM, Higginson IJ, Harding R. What palliative care‐related problems do patients experience at HIV diagnosis? A systematic review of the evidence. J Pain Symptom Manage 2011;42:734–753. [DOI] [PubMed] [Google Scholar]
- 18. Brisbois T, de Kock IH, Watanabe SM, Mirhosseini M, Lamoureux DC, Chasen M et al. Delta‐9‐tetrahydrocannabinol may palliate altered chemosensory perception in cancer patients: results of a randomized, double‐blind, placebo‐controlled pilot trial. Ann Oncol 2011: p. mdq727;22:2086–2093. [DOI] [PubMed] [Google Scholar]
- 19. Jatoi A, Windschitl HE, Loprinzi CL, Sloan JA, Dakhil SR, Mailliard JA, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer‐associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol 2002;20:567–573. [DOI] [PubMed] [Google Scholar]
- 20. Johnson JR, Burnell‐Nugent M, Lossignol D, Ganae‐Motan ED, Potts R, Fallon MT. Multicenter, double‐blind, randomized, placebo‐controlled, parallel‐group study of the efficacy, safety, and tolerability of THC: CBD extract and THC extract in patients with intractable cancer‐related pain. J Pain Symptom Manage 2010;39:167–179. [DOI] [PubMed] [Google Scholar]
- 21. Portenoy RK, Ganae‐Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S, et al. Nabiximols for opioid‐treated cancer patients with poorly‐controlled chronic pain: a randomized, placebo‐controlled, graded‐dose trial. J Pain 2012;13:438–449. [DOI] [PubMed] [Google Scholar]
- 22. Strasser F, Luftner D, Possinger K, Ernst G, Ruhstaller T, Meissner W, et al. Comparison of orally administered cannabis extract and delta‐9‐tetrahydrocannabinol in treating patients with cancer‐related anorexia‐cachexia syndrome: a multicenter, phase III, randomized, double‐blind, placebo‐controlled clinical trial from the Cannabis‐In‐Cachexia‐Study‐Group. J Clin Oncol 2006;24:3394–3400. [DOI] [PubMed] [Google Scholar]
- 23. Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, et al. Short‐term effects of cannabinoids in patients with HIV‐1 infection: a randomized, placebo‐controlled clinical trial. Ann Intern Med 2003;139:258–266. [DOI] [PubMed] [Google Scholar]
- 24. Beal JE, Olson R, Laubenstein L, Morales JO, Bellman P, Yangco B, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage 1995;10:89–97. [DOI] [PubMed] [Google Scholar]
- 25. Timpone JG, Wright DJ, Li N, Egorin MJ, Enama ME, Mayers J, et al. The safety and pharmacokinetics of single‐agent and combination therapy with megestrol acetate and dronabinol for the treatment of HIV wasting syndrome. AIDS Res Hum Retroviruses 1997;13:305–315. [DOI] [PubMed] [Google Scholar]
- 26. Volicer L, Stelly M, Morris J, McLaughlin J, Volicer BJ. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer's disease. Int J Geriatr Psychiatry 1997;12:913–919. [PubMed] [Google Scholar]
- 27. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11‐point numerical pain rating scale. Pain 2001;94:149–158. [DOI] [PubMed] [Google Scholar]
- 28. Mücke M, Carter C, Cuhls H, Prüß M, Radbruch L, Häuser W. Cannabinoids in palliative care: systematic review and meta‐analysis of efficacy, tolerability and safety. Schmerz (Berlin, Germany) 2016;30:25–36. [DOI] [PubMed] [Google Scholar]
- 29. McQuay HJ, Moore RA. An evidence‐based resource for pain relief. USA: Oxford University Press; 1998. [Google Scholar]
- 30. Norman GR, Sridhar FG, Guyatt GH, Walter SD. Relation of distribution‐and anchor‐based approaches in interpretation of changes in health‐related quality of life. Med Care 2001;39:1039–1047. [DOI] [PubMed] [Google Scholar]
- 31. Moore R et al. Managing potential publication bias, in systematic reviews in pain research: methodology refined. Seattle: IASP Press; 2008. p 15–23. [Google Scholar]
- 32. Cohen J. Statistical power analysis for the behavioral sciences. Hilsdale. NJ: Lawrence Earlbaum Associates, 1988. 2. [Google Scholar]
- 33. Fayers PM, Hays RD. Don't middle your MIDs: regression to the mean shrinks estimates of minimally important differences. Qual Life Res 2014;23:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Interpreting results and drawing conclusions. Cochrane handbook for systematic reviews of interventions version, 2008. 5(0).
- 35. L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Ann Intern Med 1987;107:224–233. [DOI] [PubMed] [Google Scholar]
- 36. Haney M, Gunderson EW, Rabkin J, Hart CL, Vosburg SK, Comer SD, et al. Dronabinol and marijuana in HIV‐positive marijuana smokers: caloric intake, mood, and sleep. JAIDS J Acquir Immune Defic Syndr 2007;45:545–554. [DOI] [PubMed] [Google Scholar]
- 37. Haney M, Rabkin J, Gunderson E, Foltin RW. Dronabinol and marijuana in HIV+ marijuana smokers: acute effects on caloric intake and mood. Psychopharmacology (Berl) 2005;181:170–178. [DOI] [PubMed] [Google Scholar]
- 38. Noyes R, Brunk SF, Avery DA, Canter AC. The analgesic properties of delta‐9‐tetrahydrocannabinol and codeine. Clin Pharmacol Ther 1975;18:84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Regelson W et al. In Braude MC, Szara S, eds. Delta‐9‐THC as an effective antidepressant and appetite‐stimulating agent in advanced cancer patients”. The Pharmacology of Marihuana; 1976. p 763–776. [Google Scholar]
- 40. Staquet M, Gantt C, Machin D. Effect of a nitrogen analog of tetrahydrocannabinol on cancer pain. Clin Pharmacol Ther 1978;23:397–401. [DOI] [PubMed] [Google Scholar]
- 41. Bedi G, Foltin RW, Gunderson EW, Rabkin J, Hart CL, Comer SD, et al. Efficacy and tolerability of high‐dose dronabinol maintenance in HIV‐positive marijuana smokers: a controlled laboratory study. Psychopharmacology (Berl) 2010;212:675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Struwe M, Kaempfer SH, Geiger CJ, Pavia AT, Plasse TF, Shepard KV, et al. Effect of dronabinol on nutritional status in HIV infection. Los Angeles, CA: SAGE Publications Sage CA; 1993. [DOI] [PubMed] [Google Scholar]
- 43. Zadikoff C, Wadia PM, Miyasaki J, Chen R, Lang AE, So J, et al. Cannabinoid, CB1 agonists in cervical dystonia: failure in a phase IIa randomized controlled trial. Basal Ganglia 2011;1:91–95. [Google Scholar]
- 44. Zutt M, Hänßle H, Emmert S, Neumann C, Kretschmer L. Dronabinol zur supportiven Therapie metastasierter maligner Melanome mit Lebermetastasen. Hautarzt 2006;57:423–427. [DOI] [PubMed] [Google Scholar]
- 45. Gross H, Ebert MH, Faden VB, Goldberg SC, Kaye WH, Caine ED, et al. A double‐blind trial of [DELTA] 9‐tetrahydrocannabinol in primary anorexia nervosa. J Clin Psychopharmacol 1983;3:165–171. [PubMed] [Google Scholar]
- 46. Berenstein G, Ortiz Z. Megestrol acetate for treatment of anorexia‐cachexia syndrome. The Cochrane Library, 2005. [DOI] [PubMed]
- 47. Lucas V, Booth S. The importance of placebo effects in enhancing palliative care interventions. BMJ Support Palliat Care 2014: p. bmjspcare‐2013‐000571;4:212–216. [DOI] [PubMed] [Google Scholar]
- 48. Melzack R, Ofiesh J, Mount B. The Brompton mixture: effects on pain in cancer patients. Can Med Assoc J 1976;115:125–129. [PMC free article] [PubMed] [Google Scholar]
- 49. Conrad R. the hardest thing to see is what is in front of your eyes–quo vadis placebo analgesia? J Pain Res 2016;9:819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 2009;63:533–543. [DOI] [PubMed] [Google Scholar]
- 51. Ben‐Shabat S, Fride E, Sheskin T, Tamiri T, Rhee MH, Vogel Z, et al. An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2‐arachidonoyl‐glycerol cannabinoid activity. Eur J Pharmacol 1998;353:23–31. [DOI] [PubMed] [Google Scholar]
- 52. Mechoulam R. Cannabis–the Israeli perspective. J Basic Clin Physiol Pharmacol 2016;27:181–187. [DOI] [PubMed] [Google Scholar]
- 53. Devinsky O, Cross JH, Wright S. Trial of cannabidiol for drug‐resistant seizures in the Dravet Syndrome. N Engl J Med 2017;376:2011–2020. [DOI] [PubMed] [Google Scholar]
- 54. Jordhøy MS, Kaasa S, Fayers P, Ovreness T, Underland G, Ahlner‐Elmqvist M. Challenges in palliative care research; recruitment, attrition and compliance: experience from a randomized controlled trial. Palliat Med 1999;13:299–310. [DOI] [PubMed] [Google Scholar]
- 55. Kaasa S, Hjermstad MJ. and J.H.v. Loge, Methodological and structural challenges in palliative care research: how have we fared in the last decades? Palliat Med 2006;20:727–734. [DOI] [PubMed] [Google Scholar]
- 56. Sigurdardottir KR, Haugen DF, van der Rijt CC, Sjøgren P, Harding R, Higginson IJ, et al. Clinical priorities, barriers and solutions in end‐of‐life cancer care research across Europe. Report from a workshop. Eur J Cancer 2010;46:1815–1822. [DOI] [PubMed] [Google Scholar]
- 57. Belendiuk KA, Baldini LL, Bonn‐Miller MO. Narrative review of the safety and efficacy of marijuana for the treatment of commonly state‐approved medical and psychiatric disorders. Addict Sci Clin Pract 2015;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: a systematic review and meta‐analysis. JAMA 2015;313:2456–2473. [DOI] [PubMed] [Google Scholar]
- 59. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1 CENTRAL search strategy
Appendix 2. Forest Plots ‐ Cannabinoids vs. placebo
Appendix 3. Question: Cannabinoids compared to placebo in patients receiving palliative treatment


