Abstract
Background
Intramuscular fatty infiltration is generally associated with the accumulation of white adipocytes in skeletal muscle and unfavourable metabolic outcomes. It is, however, still unclear whether intramuscular adipocytes could also acquire a brown‐like phenotype. Here, we detected intramuscular expression of brown adipocyte markers during fatty infiltration in an obesity‐resistant mouse strain and extensively compared the potential of two different stem cell populations residing in skeletal muscle to differentiate into brown‐like adipocytes.
Methods
Fatty infiltration was induced using intramuscular glycerol or cardiotoxin injection in the tibialis anterior muscles of young or aged 129S6/SvEvTac (Sv/129) mice or interleukin‐6 (IL‐6) knockout mice, and the expression of general and brown adipocyte markers was assessed after 4 weeks. Fibro/adipogenic progenitors (FAPs) and myogenic progenitors were prospectively isolated using fluorescence‐activated cell sorting from skeletal muscle of male and female C57Bl6/6J and Sv/129 mice, and monoclonal and polyclonal cultures were treated with brown adipogenic medium. Additionally, FAPs were differentiated with medium supplemented or not with triiodothyronine.
Results
Although skeletal muscle expression of uncoupling protein 1 (Ucp1) was barely detectable in uninjected tibialis anterior muscle, it was drastically induced following intramuscular adipogenesis in Sv/129 mice and further increased in response to beta 3‐adrenergic stimulation. Intramuscular Ucp1 expression did not depend on IL‐6 and was preserved in aged skeletal muscle. Myogenic progenitors did not form adipocytes neither in polyclonal nor monoclonal cultures. Fibro/adipogenic progenitors, on the other hand, readily differentiated into brown‐like, UCP1+ adipocytes. Uncoupling protein 1 expression in differentiated FAPs was regulated by genetic background, sex, and triiodothyronine treatment independently of adipogenic differentiation levels.
Conclusions
Intramuscular adipogenesis is associated with increased Ucp1 expression in skeletal muscle from obesity‐resistant mice. Fibro/adipogenic progenitors provide a likely source for intramuscular adipocytes expressing UCP1 under control of both genetic and hormonal factors. Therefore, FAPs constitute a possible target for therapies aiming at the browning of intramuscular adipose tissue and the metabolic improvement of skeletal muscle affected by fatty infiltration.
Keywords: Intramuscular adipose tissue, UCP1, Fibro/adipogenic progenitors, Myogenic progenitors, Thyroid hormone, Aged muscle, Interleukin 6
1. Introduction
The current obesity epidemic has increased the interest in the development and function of adipose tissue, as well as in mechanisms that could elevate whole‐body energy expenditure. While white adipocytes store excess energy intake as intracellular lipid droplets, brown adipocytes dissipate energy as heat in response to cold exposure or a high‐fat diet, thereby decreasing metabolic efficiency. The ability of brown adipocytes to dissipate energy as heat results from the activity of uncoupling protein 1 (UCP1), which is located in the inner mitochondrial membrane and uncouples the electron transport chain from ATP production (extensively reviewed in Cannon and Nedergaard1). In addition to classical brown adipose tissue (BAT) depots, pockets of UCP1‐positive, multilocular adipocytes have also been described within white adipose tissue (WAT) from rodents following cold exposure,2, 3, 4 treatment with a beta 3‐adrenergic agonist,5 or exercise training.6 These adipocytes are commonly referred to as beige, brite (from brown in white), inducible, or recruitable, reviewed in Chechi et al. 7 and seem to result from the transdifferentiation of previously unilocular, white adipocytes4, 5 and from the de novo differentiation of precursor cells.8 It has been proposed that beige adipocytes can derive from a distinct precursor and display a gene expression pattern different from that of brown adipocytes.9 Recent studies also reported the presence of BAT and the formation of UCP1‐expressing adipocytes within WAT depots in adult humans.10, 11 In humans, UCP1‐positive adipocytes exhibiting both brown12, 13, 14 and beige13, 14, 15, 16 molecular signatures have been described.
In consequence, strategies to increase the amount or the activation of these adipocytes have been investigated as ways to prevent or counteract obesity and its associated disorders. In fact, BAT amount and activity are negatively correlated with body mass index and body fat percentage in adult humans.11 In rodents, ectopic BAT interspersed between skeletal muscle groups in the thigh and popliteal regions of Sv129 mice protects against high‐fat‐diet‐induced obesity as compared with C57Bl/6J (C57Bl/6) mice, in which expression of UCP1 is not detected in the same area.17 The origin of ectopic BAT, however, is currently much less understood than the formation of classical BAT.
In addition to classical white and BAT depots, adipose tissue can also accumulate in ectopic locations, including skeletal muscle. Intramuscular adipose tissue—here defined as clusters of adipocytes interspersed between skeletal muscle fibres—is generally associated with systemic insulin resistance,18, 19 decreased muscle strength20 and, in older adults, impaired mobility.21, 22, 23 In vitro, skeletal myotubes treated with conditioned media from intramuscular progenitor‐derived adipocytes display decreased glucose uptake and glycogen synthesis in response to insulin.24 Because brown adipocytes function as a sink for excess glucose in the circulation, we hypothesize that shifting intramuscular adipocytes towards a brown phenotype could attenuate the negative impact of these cells on the surrounding myofibers. Furthermore, given that skeletal muscle accounts for more than 30% of body mass in humans,25 increases in energy uncoupling within this tissue could have an important impact on whole‐body energy expenditure.
The origin of ectopic UCP1‐expressing adipocytes in skeletal muscle is still under debate. Seale et al.26 have shown that primary myoblasts isolated from newborn mice are able to differentiate into brown adipocytes when expression of the transcription factor PRDM16 is induced. Consistent with this, another study reported the differentiation of small subpopulations of Pax7+ satellite cells (less than 10%) into adipocytes.27 Importantly, the protocols used in these studies rely on the exclusive labelling of myogenic progenitors, but not other cell lineages, by Pax7 and Myf5. However, it has recently been described that Myf5+ progenitors in WAT depots can give rise to white adipocytes,28 indicating that the spectrum of cell lineages is broader than previously appreciated. In addition, there is evidence that skeletal muscle mesenchymal cells distinct from myogenic progenitors (MPs) differentiate into adipocytes. A lineage tracing study reported that most adipocytes in skeletal muscle derive from a Pax3− lineage and that adipocytes formed in aged mice during muscle regeneration derive from Myf5− cells.29 Moreover, it has been shown that Sca1+/PDGFRα+ fibro/adipogenic progenitors (FAPs) from skeletal muscle, but not alpha 7‐integrin+ myogenic progenitors, are able to differentiate into adipocytes.30, 31 Interestingly, FAPs isolated from skeletal muscle of adult 129S6/SvEvTac (Sv/129) mice are able to differentiate into UCP1‐expressing adipocytes when pre‐treated with bone morphogenetic protein 7 (BMP7) before differentiation in vitro.32 In vivo, intramuscular adipocytes have been characterized as white in B6D2 mice,33 but it is unclear whether Ucp1 expression is induced during muscle regeneration and intramuscular fat formation in other mouse strains, particularly in Sv/129 mice, which are known to express higher levels of Ucp1 in WAT compared with other mouse strains.17
Here, we show induction of Ucp1 expression in skeletal muscle during fatty infiltration in the obesity‐resistant mouse strain Sv129. Using monoclonal and polyclonal cell cultures of skeletal muscle MPs and FAPs under different media conditions, we identified FAPs as the stem cell population able to form brown‐like adipocytes. Uncoupling protein 1 expression in differentiated FAPs depends on the genetic background, sex, and thyroid hormone. The identification of brown‐like adipocyte precursors and of the factors driving their differentiation towards a brown adipogenic phenotype could contribute to the development of new strategies to treat obesity, insulin resistance, and associated disorders, such as sarcopenia and skeletal muscle fatty infiltration.
2. Materials and methods
2.1. Animals
All mice were maintained in a 12 h light/dark cycle in a pathogen‐free animal facility. C57Bl6/6J and Sv/129 mice were purchased from Harlan Laboratories (Horst, The Netherlands) and Taconic (Rensselaer, NY, USA), respectively. Interleukin‐6 knockout mice (B6.129S2‐IL‐6tm1Kopf/J) and C57Bl/6 controls were purchased from Jackson Laboratory (Bar Harbor, ME, USA). To induce the proliferation and differentiation of FAPs in vivo, mice were anaesthetized with isoflurane (Forane, Abbott, Wiesbaden, Germany), and one of the tibialis anterior (TA) muscles was injected with cardiotoxin (CTX, Sigma, Buchs, Switzerland, , 50 μL, 10 mM in phosphate buffered saline) or glycerol [GLY, Sigma, 50 μL, 50% v/v in Hank's balanced salt solution (HBSS)]. Injections of HBSS into TA muscles were previously shown to not elicit skeletal muscle regeneration or intramuscular adipocyte deposition33; therefore, we used the uninjected contralateral muscles as control as described before.30, 31, 34 To promote Ucp1 expression in GLY‐induced intramuscular adipocytes, mice received intraperitoneal injections of CL316,243 hydrate (CL316, 0.5 mg/kg body mass in saline) for five consecutive days 4 weeks after intramuscular injection of GLY. To access the effect of cold exposure on Ucp1 expression in uninjected skeletal muscle, mice were kept for 1 week at 8°C as previously described.4 All animal studies were approved by the Ethics Committee of the Cantonal Veterinary Office Zürich, and the principles of laboratory animal care were followed.
2.2. Skeletal muscle histology
For histological analysis, skeletal muscle was dissected and flash frozen in isopentane cooled in liquid nitrogen. Frozen cross sections of 30 μm were prepared from different areas of the TA muscle and stained with haematoxylin and eosin. Representative images were obtained with a digital camera (Zeiss AxioCam HRc) connected with a microscope (Zeiss Axioplan2).
2.3. Skeletal muscle primary cell cultures
For the isolation of primary skeletal muscle cells, murine skeletal muscle tissue was excised from the hind limbs (and when indicated also from the paraspinal muscle) and processed as previously described.35 In brief, muscle tissue was digested with 2 mg/mL collagenase type II (Thermo Fisher Scientific, Waltham, MA, USA) in collagenase buffer [2% bovine serum albumin (Sigma) in HBSS] for 1 h (3 × 20 min) at 37°C, followed by erythrocyte lysis (erythrocyte lysis buffer: 154 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA) and filtration using 40 μM cell strainers. After incubation with fluorescent antibodies, pure populations of MPs and FAPs cells, respectively, were obtained using fluorescence‐activated cell sorting (FACS, BD FACSAria III, BD Biosciences, San Diego, CA, USA). Fibro/adipogenic progenitors were sorted based on positive staining for Sca1 and absence of staining for alpha 7‐integrin, CD31, and CD45,30 while MPs were sorted based on positive staining for alpha 7‐integrin and absence of Sca1, CD31, and CD45.35 For analysis of monoclonal cell colonies, single FAPs and MPs were sorted directly into 96‐well cell culture plates containing growth medium. Allophycocyanin (APC)‐conjugated anti‐mouse Ly‐6A/E (Sca‐1), Alexa Fluor® 488‐conjugated anti‐mouse CD31, and Alexa Fluor® 488‐conjugated anti‐mouse CD45 antibodies were purchased from BioLegend (London, UK); PE‐conjugated anti‐mouse alpha 7‐integrin antibody was purchased from R&D Systems (Minneapolis, MN, USA).
Fibro/adipogenic progenitors were grown in low‐glucose Dulbecco's modified Eagle's medium (DMEM, Invitrogen, Carlsbad, CA, USA) containing 20% foetal bovine serum (FBS, Invitrogen), 1% penicillin/streptomycin (P/S, Invitrogen), and 5 ng/mL basic fibroblast growth factor (Invitrogen). Myogenic progenitors were grown on collagen‐coated plates in culture medium containing 40% F10 nutrient mixture, 40% low‐glucose DMEM, 20% FBS, 1% P/S, and 5 ng/mL basic fibroblast growth factor. Myogenic differentiation medium contained 2% horse serum (Invitrogen) and 1% P/S in low glucose DMEM. To stimulate adipogenic differentiation, cells were treated for 2 days with high‐glucose DMEM supplemented with 10% FBS, 1% P/S, 5 μg/mL insulin, 125 μM indomethacin, 5 μM dexamethasone, 0.5 mM isobutylmethylxanthine, 1 nM triiodothyronine (T3), and 1 μM rosiglitazone (brown adipogenic induction medium); subsequently, cells were kept in high‐glucose DMEM containing 10% FBS, 1% P/S, 5 μg/mL insulin, 1 nM T3, and 1 μM rosiglitazone (brown adipogenic differentiation medium) for up to 8 days. Where indicated, T3 was not added to the brown adipogenic induction and differentiation media. All cells were cultured in a 5% CO2 atmosphere at 37°C.
2.4. White adipose tissue primary cultures
For isolation of primary cells from WAT, inguinal WAT (ingWAT) was excised and digested with 2 mg/mL collagenase in collagenase buffer (25 mM NaHCO3, 12 mM KH2PO4, 1.2 mM MgSO4·7H2O, 4.8 mM KCl, 120 mM NaCl, 1.4 mM CaCl2·2H2O, 5 mM glucose, 2.5% bovine serum albumin, and 1% P/S) for 1 h at 37°C; after centrifugation, the resulting pellet containing the stromal vascular fraction was washed, erythrocytes were lyzed using erythrocyte lysis buffer, and cells were filtered through 40 μm and plated in collagen‐coated cell culture plates containing growth medium (10% FBS and 1% P/S in high‐glucose DMEM). Two days after reaching confluence, cells were differentiated with the same brown adipogenic media used for skeletal muscle MPs and FAPs.
2.5. RNA isolation and quantitative reverse transcription PCR
Total RNA was isolated using the TRIzol reagent (Invitrogen). After DNAse digestion of total RNA (DNA‐free DNA Removal kit, Ambion, Thermo Fisher Scientific, Waltham, MA, USA), complementary DNA was synthesized with random hexamer primers using Super Script III Reverse Transcriptase (SuperScript® III First‐Strand Synthesis System, Invitrogen). mRNA was analysed by quantitative reverse transcription PCR with a 7500 Fast Start Real‐Time PCR system (Applied Biosystems Foster City, CA, USA) using the FastStart Universal SYBR Green Master Mix (Roche, Basel, Switzerland). Transcript levels were normalized to 18S ribosomal RNA levels. Primer sequences are available upon request.
2.6. Protein extraction and western blotting
Cells and tissues were lyzed in radioimmunoprecipitation assay buffer. For western blotting, cell lysates were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. Proteins were detected using the following antibodies: UCP1 (Merck Millipore, Darmstadt, Germany, 662045, 1:1000 dilution), fatty acid‐binding protein 4 (FABP4, Cell Signaling, Beverly, MA, USA, 3544, 1:1000 dilution), myosin heavy chain (MyHC) (Developmental Studies Hybridoma Bank, MF 20, 1:100 dilution), beta‐actin (ACTB, Proteintech, Manchester, UK, 20536‐1‐AP, 1:2000 dilution), mouse immunoglobulin (IgG) (Merck Millipore, 401253, 1:8000 dilution), and rabbit IgG (Merck Millipore, 401393, 1:8000 dilution).
2.7. Immunofluorescence and image analysis
Fibro/adipogenic progenitors were grown and differentiated on chamber slides (Sigma). At Day 10 of differentiation, cells were fixed, permeabilized, and incubated overnight with rabbit anti‐UCP1 (Abcam, Cambridge, UK, 1:500 dilution), followed by incubation with Alexa 488 anti‐rabbit IgG and DAPI. Slides were posteriorly analysed with a confocal laser scanning microscope (SP5 Resonant APD, Leica, Wetzlar, Germany).
For quantification of differentiated and UCP1+ cells, FAPs were seeded and differentiated into a 96‐well plate and posteriorly fixed and stained with LD540 (lipid droplets), Hoechst (nuclei), and rabbit anti‐UCP1 (Abcam, 1:500 dilution) followed by incubation with Alexa 488 anti‐rabbit IgG; 17 pictures were taken per well using an automated microscope imaging system (PerkinElmer Operetta High Content Imaging System, Perkin Elmer, Waltham, MA, USA).
2.8. Immunohistochemistry
IngWAT and TA muscles were excised, fixed in 4% formalin, and embedded in paraffin using a benchtop tissue processor (TP1020, Leica Biosystems). Sections of 4 μm thickness were prepared using a microtome (RM2255, Leica Biosystems) and deparaffinized. For immunostaining, endogenous peroxidase activity was blocked by incubation in 3% H2O2, followed by heat‐induced epitope retrieval in 0.01 M sodium citrate. Tissue sections were then incubated with primary antibodies against UCP1 (Thermo Fisher Waltham, MA, USA, PA1‐24894, 1:200) or cytochrome c oxidase subunit IV (Abcam, ab16056, 1:250), and signals were visualized using SignalStain® Boost IHC Detection Reagent (Cell Signaling, 8114) and the SignalStain® DAB Substrate kit (Cell Signaling, 8059).
2.9. Statistical analyses
Student's t‐tests were used for pairwise comparisons and one‐way or two‐way analyses of variance with Bonferroni post hoc tests were used for comparisons between more than two groups. A significance level of 0.05 was considered for all tests. Results are shown as means ± standard error of the mean. All statistical analyses were performed using GraphPad Prism. When gene expression values did not show a normal distribution, data were log transformed before statistical analysis. For experiments in which at least one sample had an undetermined Ct value for a given gene, half of the lowest detectable value was added to all samples, allowing for logarithmic transformation.
3. Results
3.1. Intramuscular adipogenesis induces uncoupling protein 1 expression in skeletal muscle of obesity‐resistant mice
Intramuscular adipocyte formation in mice is rare compared with humans but can be stimulated by intramuscular injection of either GLY or CTX.33, 34 To determine whether adipocyte formation can induce Ucp1 expression in skeletal muscle, we injected GLY or CTX into the TA muscle of mice, a skeletal muscle that contains no significant amounts of adipocytes under basal conditions (Figure 1A). We used Sv/129 mice, which express detectable Ucp1 levels in subcutaneous WAT even under non‐stimulated conditions.17 In line with a previous report,34 adipocyte formation was observed in the TA muscles 4 weeks after the intramuscular injections but was more pronounced following GLY injection (Figure 1A). Accordingly, we observed that the mRNA levels of the general adipocyte markers Pparg2 and Fabp4 were significantly increased in both regeneration models (Figure 1B). Importantly, mRNA levels of the brown adipocyte markers Ucp1 and Cidea, barely detectable in the non‐injected control legs, were markedly elevated in response to the GLY injections (Figure 1B). No significant differences in the response to GLY injections were observed between male and female mice (Figure 1B). Contrasting with the increase in intramuscular Ucp1 expression following GLY injection, Ucp1 levels in TA muscles of uninjected mice remained low even after cold exposure for 1 week (Figure 1C). These results point to newly formed intramuscular adipocytes as the source of Ucp1 expression in skeletal muscle.
Figure 1.
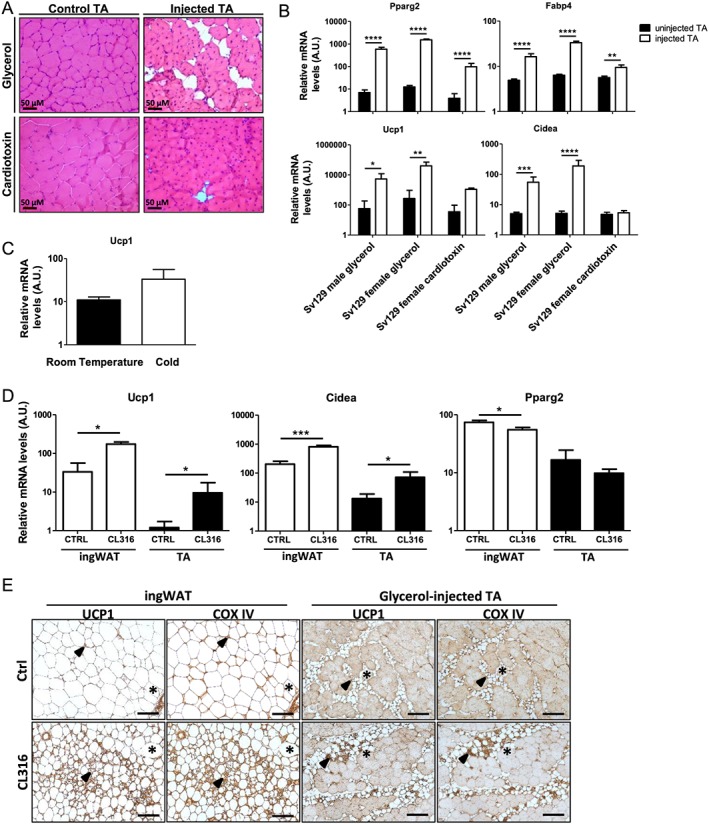
Glycerol (GLY), but not cardiotoxin (CTX), induces intramuscular adipogenesis and uncoupling protein 1 (Ucp1) expression in skeletal muscle, which responds to treatment with a beta 3‐adrenergic agonist. (A) Haematoxylin and eosin staining of tibialis anterior (TA) cross sections 4 weeks after intramuscular injection of CTX or GLY. (B) mRNA levels of general (Pparg2 and Fabp4) and brown (Ucp1 and Cidea) adipocyte markers in TA 4 weeks after intramuscular GLY injection in 129S6/SvEvTac (Sv/129) male and Sv/129 female mice and 4 weeks after intramuscular CTX injection in Sv/129 female mice, n = 5 mice at 15 weeks of age per group. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, repeated measures two‐way analysis of variance. (C) Ucp1 mRNA levels in TA muscles of uninjected Sv/129 male mice kept at room temperature or exposed to cold (8°C) for 1 week. n = 5, unpaired Student's t‐test. (D, E) Four weeks after intramuscular GLY injection in the TA muscles, mice were treated with daily injections of CL316,243 hydrate (CL316) for 5 days, and TA muscles were excised for quantification of Ucp1, Cidea, and Pparg2 mRNA levels and immunohistochemical analysis of paraffin sections; inguinal white adipose tissue (ingWAT) was used as a control. n = 5 Sv/129 female mice at 17 weeks of age per group. *P < 0.05 and ***P < 0.001, unpaired Student's t‐test. For (B–D), mRNA levels were measured by quantitative reverse transcription PCR. Results are shown as mean and standard error of the mean of mRNA levels normalized to 18S rRNA levels, in arbitrary units (A.U.). In (E), ‘*’ indicates a corresponding area on consecutive sections, and arrows indicate UCP1+/cytochrome c oxidase subunit IV (COX IV)+ adipocytes. Scale bar = 100 μm. CL316, CL316,243 hydrate.
Uncoupling protein 1 up‐regulation in response to cold exposure via the stimulation of beta 3‐adrenergic receptors is a remarkable feature of brown36 and beige3 adipocytes. Ectopic Ucp1 expression in ingWAT, for example, increases almost five‐fold following treatment with the beta 3‐adrenergic agonist CL316.17 To test the response of intramuscular Ucp1 levels to beta 3‐adrenergic stimulation in vivo, Sv/129 female mice were treated with CL316 for five consecutive days 4 weeks after intramuscular injection of GLY into the TA muscles. Notably, intramuscular mRNA levels of Ucp1 and Cidea were markedly elevated in CL316‐treated compared with control mice, similarly to ingWAT, and independent from adipocyte differentiation levels as measured by Pparg2 expression (Figure 1D). Histological analysis of CL316‐stimulated GLY‐injected TA muscles revealed pockets of intramuscular adipocytes expressing both UCP1 protein and the mitochondrial marker cytochrome c oxidase subunit IV, similarly to ingWAT (Figure 1E). Together, we conclude that UCP1 expression in intramuscular adipocytes can be regulated through a classical brown adipocyte pathway.
3.2. Fibro/adipogenic progenitors, but not myogenic progenitors, differentiate into uncoupling protein 1‐expressing adipocytes
Our next aim was to determine the potential source for UCP1‐expressing intramuscular adipocytes. At least two stem cell populations have been proposed as the precursors for intramuscular Ucp1‐expressing adipocytes: satellite cells/MPs27 and FAPs.32 To define the potential of MPs and FAPs to differentiate into UCP1+ adipocytes, we used FACS to isolate both populations from skeletal muscle based on expression of the surface markers alpha 7‐integrin and Sca1, respectively.
First, we addressed the potential of MPs to form adipocytes and expanded them as myoblasts under cell culture conditions. Nearly confluent myoblasts were treated with myogenic or brown adipogenic medium for 8 days. As expected, myoblasts treated with myogenic medium differentiated into MyHC‐expressing myotubes (Figure 2A). Remarkably, myoblasts were still able to form MyHC+ myotubes and did not differentiate into adipocytes even when treated with brown adipogenic medium (Figure 2A). In contrast, stromal vascular fraction cells isolated from ingWAT, used as a control, readily differentiated into FABP4+ adipocytes upon treatment with the same medium (Figure 2A). Adipocyte formation was also not observed when myoblasts from Sv/129 or C57Bl/6 mice were treated with brown adipogenic medium further supplemented with different compounds reported to promote brown adipogenic differentiation (Figure 2B). Because polyclonal cell culture conditions could be disadvantageous for adipogenic subclones, we next sorted single MPs directly into 96‐well plates and, after expansion, treated the monoclonal colonies for 10 days with brown adipogenic medium. Similarly to our initial results, adipogenesis was again not observed and myotube formation occurred to different extents, confirming the commitment of MPs to the myogenic fate (Figure 3). Importantly, more than 30% of monoclonal colonies derived from single‐sorted FAPs contained adipocytes following the same treatment (Figure 3). These results demonstrate that the lack of adipocyte formation in monoclonal colonies of MPs cannot simply be explained by the cell culture conditions (Figure 3). Together, these data suggest that MPs are strictly committed to the myogenic lineage and do not have the potential to differentiate into adipocytes even under adipogenic conditions.
Figure 2.
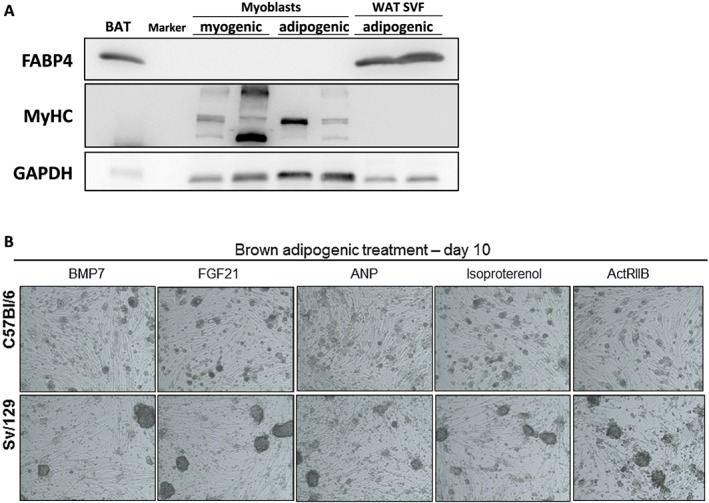
Myoblasts are committed to the myogenic fate under treatment with brown adipogenic medium. (A) Expression of the adipocyte marker fatty acid‐binding protein 4 (FABP4), different isoforms of the myotube marker myosin heavy chain (MyHC), and glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) (loading control) in primary mouse myoblasts following 8 days of differentiation in myogenic or brown adipogenic medium (n = 2 cultures from individual 129S6/SvEvTac (Sv/129) male mice per condition). Cells from the stromal vascular fraction of inguinal white adipose tissue (WAT SVF) differentiated for 8 days with adipogenic medium were used as a control [n = 1 culture from a C57Bl6/6J (C57Bl/6) female mouse and 1 culture from a C57Bl/6 male mouse]. Brown adipose tissue (BAT) was used as a positive control. (B) Representative light microscopy images of myoblasts isolated from C57Bl/6 and Sv/129 mice following 10 days of differentiation in brown adipogenic medium supplemented with the indicated compounds (BMP7, FGF21, ANP, and ActRIIB), ×50 magnification. ActRIIB, activin receptor IIB inhibitor; ANP, atrial natriuretic peptide; BMP7, bone morphogenetic protein 7; FGF21, fibroblast growth factor 21.
Figure 3.
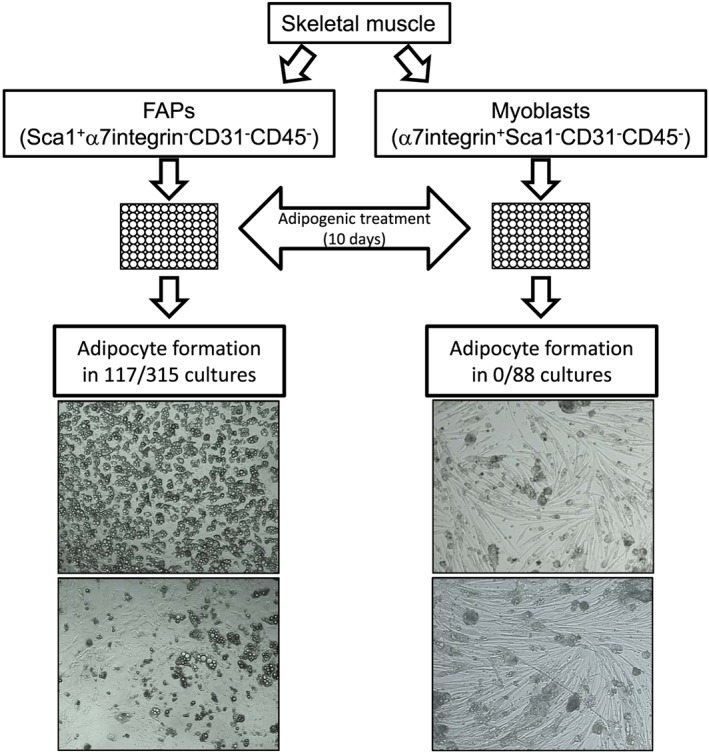
Monoclonal cultures of fibro/adipogenic progenitors (FAPs), but not myoblasts, have the potential to differentiate into adipocytes. FAPs and myoblasts were isolated from skeletal muscle (hindlimbs and paraspinal), and single cells were fluorescence‐activated cell sorting sorted directly into 96‐well plates. Nearly confluent cells were treated for 10 days with brown adipogenic medium. Adipocyte formation was assessed through microscopy in 315 FAP and 88 myoblast monoclonal cultures from C57Bl6/6J (C57Bl/6) (46 myoblast and 143 FAP colonies) and 129S6/SvEvTac (42 myoblast and 172 FAP colonies) male mice. Shown are representative light microscopy images of monoclonal colonies of FAPs (×100 magnification) and myoblasts (×50 magnification).
In contrast to MPs, FAPs readily differentiated into multilocular adipocytes (Figure 4C) expressing UCP1 and FABP4 in response to the brown adipogenic treatment (Figure 4A). In addition to Ucp1, other genes, which are highly expressed in brown adipocytes, were also significantly up‐regulated in differentiated FAPs, including Pgc1a, Cidea, Prdm16, Elovl3, and Eva1 (Figure 4B). Therefore, our results show that FAPs, but not MPs, are the likely source for UCP1‐expressing intramuscular adipocytes.
Figure 4.
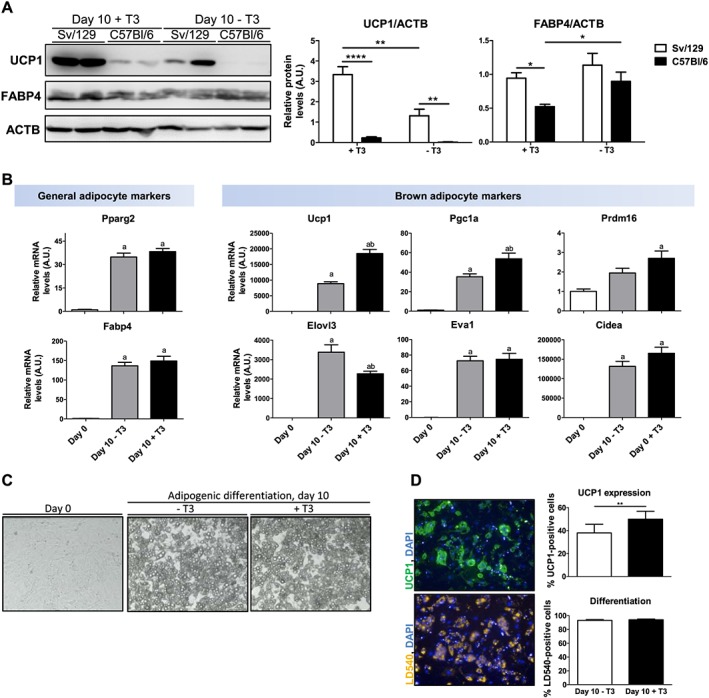
Fibro/adipogenic progenitors (FAPs) differentiate into brown‐like adipocytes in a strain‐dependent and T3‐dependent manner. FAPs were isolated from skeletal muscle (hindlimbs) of 129S6/SvEvTac (Sv/129) and C57Bl6 mice and, upon confluence, differentiated for 10 days with brown adipogenic medium supplemented (+T3) or not (−T3) with triiodothyronine. (A) Uncoupling protein 1 (UCP1), fatty acid‐binding protein 4 (FABP4), and beta‐actin (ACTB) (loading control) protein expression in differentiated FAPs. Results are shown as mean and standard error of the mean (SEM) of relative protein expression levels normalized to ACTB relative levels in arbitrary units (A.U.), n = 6 mice; *P < 0.05, **P < 0.01, and ****P < 0.0001, repeated measures two‐way analysis of variance. (B) mRNA levels of general and brown adipocyte markers in FAPs at Day 0 and at Day 10 of differentiation in medium +T3 or medium −T3. Results are shown as mean ±SEM of gene expression levels normalized to 18S rRNA levels, in A.U., n = 6 Sv/129 female mice; a: significantly different from undifferentiated cells and b: significantly different from cells differentiated with medium −T3, repeated measures one‐way analysis of variance. (C) Representative light microscopy images (×100 magnification) of FAPs at Day 0 and at Day 10 of differentiation with medium +T3 or −T3. (D) FAPs at Day 10 of differentiation (+T3 or −T3) were stained for nuclei (DAPI) and lipid droplets (LD540) and immunostained for UCP1. Automated image acquisition was followed by automated quantification of cells positive for UCP1 and of differentiated cells (i.e. cells containing lipid droplets). Results are shown as mean and SEM of percentage of cells positive for UCP1 or percentage of differentiated cells; n = 10 Sv129 female mice, **P < 0.01, paired Student's t‐test.
3.3. Uncoupling protein 1 levels in fibro/adipogenic progenitor‐derived adipocytes depend on mouse strain
Previous studies have shown that adipocyte precursors residing in WAT and skeletal muscle from C57Bl/6 and Sv/129 mice differ in their potential to express UCP1 following differentiation.32, 37 In line with these findings, we observed higher levels of UCP1 in skeletal muscle FAPs isolated from Sv/129 mice than in those isolated from C57Bl/6 mice after the brown adipogenic treatment (Figure 4A). This confirms that FAPs isolated from skeletal muscle of different mouse strains retain genetic differences, which affect UCP1 expression under cell culture conditions.
3.4. Uncoupling protein 1 expression in fibro/adipogenic progenitor‐derived adipocytes responds to the thyroid hormone triiodothyronine
The thyroid hormone T3 plays an important role in the activation of brown adipocytes, and thyroidectomized rats exhibit impaired BAT activation and thermoregulation in response to cold.38, 39 In vitro, it has been shown that T3 up‐regulates UCP1 expression in foetal rat brown adipocytes40 and human multipotent adipose‐derived stem cells under adipogenic conditions.41 Similarly, we observed higher UCP1 expression levels in FAPs differentiated with medium supplemented with T3 (+T3) compared with cells differentiated with medium lacking addition of T3 (−T3). The expression of the adipocyte marker FABP4 was not decreased in cells treated with medium –T3 (Figure 4A), indicating that the increased UCP1 expression was not a mere consequence of enhanced differentiation. Uncoupling protein 1 was also up‐regulated in response to T3 at the mRNA level together with the inducer of mitochondrial biogenesis Pgc1a (Figure 4B). Up‐regulation of the transcriptional co‐regulator of Pgc1a, Prdm16, during differentiation also depended on addition of T3 to the medium, while other brown adipocyte markers remained unchanged (Eva1 and Cidea) or showed a slightly lower expression (Elovl3) (Figure 4B). mRNA levels of the general adipocyte markers Pparg2 and Fabp4 did not differ between cells differentiated with medium +T3 and −T3, confirming that T3 supplementation does not enhance adipocyte differentiation (Figure 4B). To further define the mechanisms for the T3‐dependent increase in UCP1 expression, differentiated FAPs were stained for lipid droplets (LD540 dye), nuclei (DAPI), and immunostained for UCP1; UCP1+ cells and differentiated adipocytes were then quantified using an automated microscope imaging system. We observed a slight increase in the percentage of UCP1+ adipocytes in cultures differentiated in medium +T3 compared with cells differentiated with medium −T3 (Figure 4D), while the percentage of differentiated cells was similar in both conditions. This supports the notion that the increased UCP1 expression in response to T3 is independent of cell differentiation and reflects both an increase in the number of cells expressing UCP1 and higher UCP1 levels per cell.
3.5. Uncoupling protein 1 expression in fibro/adipogenic progenitor‐derived adipocytes is sex dependent
Ucp1 expression in adipocytes can be sex dependent as previously shown for rat BAT42 and in differentiated stem cells from human perirenal adipose tissue.43 We also observed a tendency for higher Ucp1 expression levels in GLY‐injected TA muscles from female compared with male Sv/129 mice in vivo (Figure 1B). Therefore, we compared the potential of skeletal muscle FAPs from male and female mice to express UCP1 following brown adipogenic differentiation in vitro. Interestingly, FAPs isolated from Sv/129 female mice reached higher UCP1 levels after differentiation compared with FAPs isolated from Sv/129 male mice (Figure 5A and 5B). The expression levels of the adipocyte marker FABP4 were not different between groups, indicating that the differences in UCP1 levels were not dependent on differentiation. (Figure 5B). The difference in UCP1 expression after differentiation between FAPs isolated from male and female mice was also observed in C57Bl/6 mice (data not shown).
Figure 5.
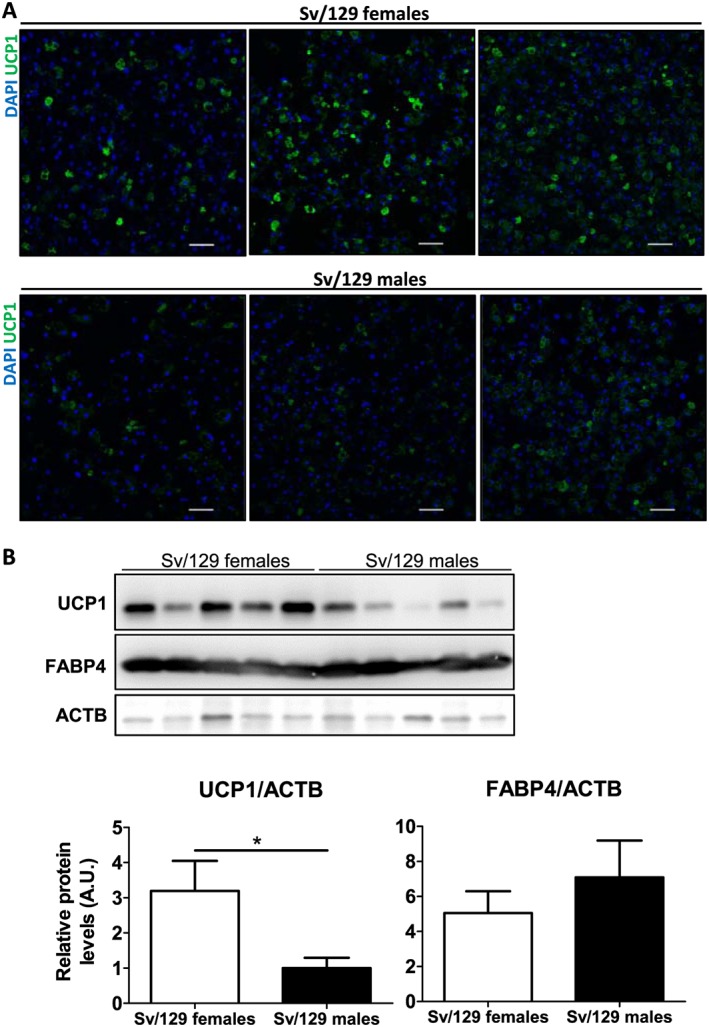
Sex‐dependent uncoupling protein 1 (UCP1) expression in differentiated fibro/adipogenic progenitors (FAPs). (A) FAPs isolated from skeletal muscle (hindlimbs) of male and female 129S6/SvEvTac (Sv/129) mice were differentiated for 10 days, stained for nuclei (DAPI), and immunostained for UCP1, n = 3 mice per group. Scale bar = 50 μM. (B) UCP1, fatty acid‐binding protein 4 (FABP4), and beta‐actin (ACTB) (loading control) protein expression in FAPs isolated from Sv/129 male and female mice at Day 10 of differentiation. Results are shown as mean and standard error of the mean of relative protein expression levels normalized to ACTB relative levels in arbitrary units (A.U.), n= 5 mice per group. *P < 0.05, unpaired Student's t‐test.
Together, our results demonstrate that FAPs isolated from skeletal muscle of not only different mouse strains but also different sexes retain genetic differences, which influence UCP1 expression following differentiation.
3.6. Intramuscular uncoupling protein 1 expression after glycerol‐induced fatty infiltration is preserved in interleukin‐6 knockout and aged mice
Fibro/adipogenic progenitor fate is strongly determined by the surrounding muscle environment.30, 31 Infiltration by neutrophils, macrophages, and eosinophils and inflammation are hallmarks of muscle regeneration.34, 44 Moreover, FAPs have been shown to be an inducible source of IL‐6, increasing IL‐6 expression for at least 5 days following muscle damage.30 To understand the role of IL‐6 in Ucp1 expression upon experimental fatty infiltration, we examined IL‐6 knockout mice 4 weeks following intramuscular GLY injections (followed or not by CL316 treatment). Intriguingly, neither adipocyte deposition as determined by the expression levels of Pparg2 nor GLY‐induced Ucp1 expression was altered in the mutant mice (Figure 6). These results demonstrate that IL‐6 is dispensable for intramuscular fatty infiltration and Ucp1 expression, at least in this animal model.
Figure 6.
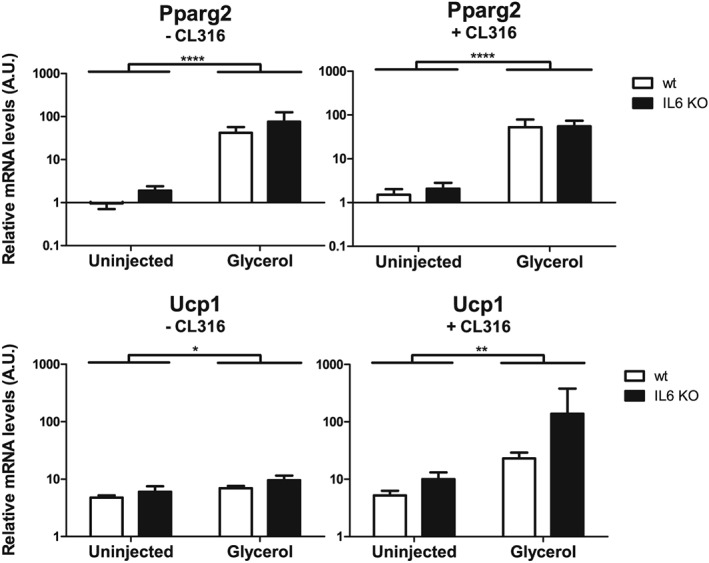
Intramuscular uncoupling protein 1 (Ucp1) expression after glycerol‐induced fatty infiltration does not depend on interleukin 6 (IL‐6). Tibialis anterior muscles from 12 weeks old female IL‐6 knockout (KO) or wild‐type C57Bl6/6J control mice were injected with glycerol. The uninjected contralateral muscles served as control. After 4 weeks, mice were treated with five daily intraperitoneal injections of CL316,243 (+CL316) or saline (−CL316). Pparg2 and Ucp1 mRNA levels were determined using quantitative reverse transcription PCR and normalized to 18S rRNA, n = 5 mice per group. Data are shown as mean and standard error of the mean. *P < 0.05, **P < 0.01, and ****P < 0.0001 for group effect of glycerol injection, two‐way analysis of variance and Bonferroni post hoc test.
It is tempting to speculate that browning of intramuscular adipocytes could improve skeletal muscle function in conditions such as ageing/sarcopenia, in which skeletal muscle fatty infiltration is associated with poor outcomes.22 However, whether the ability of FAPs to differentiate into Ucp1‐expressing adipocytes is preserved in older/sarcopenic individuals remains unclear. Thus, we assessed the presence of Ucp1 expression in an experimental condition of sarcopenia. To this end, 18 months old Sv/129 female mice received intramuscular injections of GLY into the TA and were compared with younger mice (3 months old). While TA muscle weight was not significantly affected by age (data not shown), aged muscle expressed higher levels of the two muscle‐specific E3 ubiquitin ligases muscle RING finger 1 and muscle atrophy F‐box (Atrogin‐1), which are reportedly increased during muscle atrophy at the transcriptional level45 (Figure 7). Importantly, intramuscular GLY injections induced an up‐regulation of Pparg2 and Ucp1 mRNA levels to the same extent in TA muscles of young and aged mice (Figure 7). This suggests that even in aged skeletal muscle, in which a molecular signature of sarcopenia is already present, the ability of GLY‐induced adipocytes to acquire a brown‐like phenotype is preserved.
Figure 7.
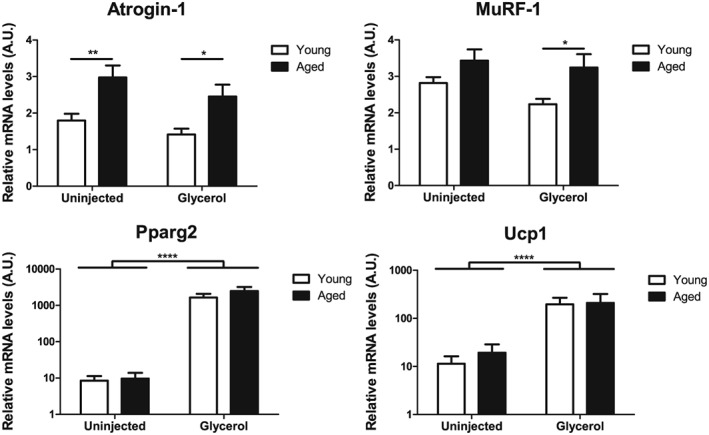
Ageing does not affect intramuscular uncoupling protein 1 (Ucp1) expression after glycerol‐induced fatty infiltration. Tibialis anterior muscles from young (3 months) or aged (18 months) female 129S6/SvEvTac mice were injected with glycerol. The uninjected contralateral muscles served as control. After 4 weeks, muscles were excised and atrogin‐1, MuRF‐1, Pparg2, and Ucp1 mRNA levels were determined using quantitative reverse transcription PCR and normalized to 18S rRNA, n = 8 mice per group. Data are shown as mean and standard error of the mean. *P < 0.05 and **P < 0.01 for young vs. aged, ****P < 0.0001 for group effect of glycerol injection, two‐way analysis of variance and Bonferroni post hoc test.
4. Discussion
Our study provides a comprehensive analysis of UCP1 expression in skeletal muscle based on two in vivo models of intramuscular adipogenesis and the prospective isolation of two different skeletal muscle progenitor cell populations. We show that Ucp1 expression is increased in skeletal muscle following GLY‐induced intramuscular adipogenesis in an obesity‐resistant mouse strain and that intramuscular Ucp1 expression is responsive to beta 3‐adrenergic stimulation. Furthermore, we observed that skeletal muscle FAPs, but not MPs, are able to differentiate into UCP1+ adipocytes ex vivo, with UCP1 levels being affected by both genetic factors (background and sex) and hormonal signalling (T3).
The intramuscular injections of either CTX or GLY are widely established protocols to induce muscle regeneration and the formation of intramuscular adipocytes.29, 30, 33, 34 Both models involve the proliferation of satellite cells and FAPs followed by satellite cell fusion to damaged myofibers and intramuscular adipogenesis. In line with our results, intramuscular adipogenesis was reported to be stronger and more persistent following intramuscular GLY injections.34 Additionally, we show that these two skeletal muscle regeneration models also differ regarding the induction of brown adipocyte markers: significant increases in the mRNA levels of Ucp1 and Cidea were only observed in response to GLY injections. Thus, intramuscular GLY injection provides a suitable model to study intramuscular formation of adipocytes expressing Ucp1 in obesity‐resistant mice.
In humans, intramuscular adipose tissue is generally associated with impaired myofiber function, both from a mechanical20 and a metabolic point of view.18, 19 Although this adipose tissue depot is not yet fully characterized, studies analysing CTX‐induced and GLY‐induced adipogenesis in murine skeletal muscle describe the intramuscular adipocytes as white rather than brown.29, 33, 34 In contrast, the increase in Ucp1 expression in skeletal muscle that we observed during GLY‐induced adipogenesis suggests that intramuscular adipocyte precursors can also adopt a brown‐like phenotype in an obesity‐resistant mouse strain. The further increase in intramuscular Ucp1 and Cidea expression in response to beta 3‐adrenergic stimulation resembles the browning process of WAT depots and points to intramuscular adipocytes as a possible and accessible target for therapies promoting the brown adipocyte‐like phenotype. It remains to be shown whether brown‐like intramuscular adipocytes could improve skeletal muscle metabolism and insulin sensitivity in skeletal muscle affected by fatty infiltration.
The identity of the skeletal muscle progenitor population able to differentiate into brown‐like adipocytes is currently under debate. Previous studies claimed that MPs are able to differentiate into white46, 47, 48 or brown‐like adipocytes26, 27 under certain conditions. However, most of these studies relied on single muscle fibre isolations46, 47, 48 or on pre‐plating of collagenase‐digested tissue47 to obtain the progenitor populations, which could potentially result in the isolation of non‐myogenic, interstitial cells surrounding myofibers. Indeed, Starkey et al.49 reported that most adipogenic clones that derived from single myofibers could be removed by adding two myofiber washes to the isolation protocol before plating the cells. Moreover, studies that observed adipogenic differentiation of MPs identified the myogenic progenitors by labelling for Pax7 or the myogenic regulatory factor Myf5. However, recent studies showed that Myf5 can be more widely distributed than previously appreciated and also labels white adipocytes.28 Moreover, a subpopulation of Myf5+ cells from extraocular muscle expresses Sca1 and is adipogenic.50 In our study, we relied on a prospective isolation strategy to obtain MPs from skeletal muscle by FACS based on the absence of CD31, CD45, and Sca1 and the presence of alpha 7‐integrin, which results in highly pure myogenic cultures.35 Analysing both polyclonal and monoclonal cell cultures, we could not identify any MP subpopulation with adipogenic potential. Our results further support previous work showing that MPs are committed to the myogenic fate30, 31, 49, 51 and that FAPs are the adipocyte precursor population residing in murine30, 31, 32, 52 and human24, 51, 53, 54 adult skeletal muscle. Although we cannot reject the hypothesis that a small subpopulation of satellite cells could form brown adipocytes—as shown by Yin et al.27 relying on the expression of Pax7 exclusively in muscle satellite cells—our results suggest that FAPs are a more likely source for intramuscular adipocytes and bear the necessary machinery to express UCP1.
Notably, we show that FAPs differentiate into brown‐like adipocytes more readily than previously appreciated, that is, without any pre‐differentiation exposure to adipocyte browning agents such as BMP7.55 The differences in UCP1 expression following FAP differentiation between previous studies and ours could be explained at least partially by differences in cell culture conditions. Firstly, Ucp1 expression in response to BMP7 in FAPs differentiated in the absence of a PPARg agonist has been reported to be highly dependent on the serum batch used.55 This could explain the low Ucp1 expression in protocols that do not include the PPARg agonist rosiglitazone in the differentiation medium.29, 31, 32 Moreover, we show that the thyroid hormone T3 is critical for UCP1 expression in differentiated FAPs, and this hormone was not always included in the differentiation medium.30, 31 Lastly, studies using cells that have been passaged or frozen thawed prior to differentiation report low Ucp1 levels in FAP‐derived adipocytes.24 Similarly, a decrease in differentiation and UCP1 expression following cell passaging has been previously reported for CD34+ cells isolated from human foetal skeletal muscle.56 We have also noticed a decrease in the proliferation and differentiation capacity of FAPs after trypsinization and re‐seeding and therefore used only freshly isolated cells expanded for a maximum of 10 days in our experiments.
The up‐regulation of UCP1 mRNA and protein levels in response to T3 treatment in differentiated FAPs underscores the importance of T3 for UCP1 expression in adipocytes, already observed in studies with rat BAT cells40 and human multipotent adipose‐derived stem cells.41 Importantly, in our study, this effect was dependent on a direct effect of T3 on UCP1 levels rather than on FAP differentiation, as indicated by similar mRNA and protein levels of adipocyte markers and automated microscopy analysis of differentiation. We speculate that the increase in UCP1 gene and protein expression in FAPs differentiated in medium supplemented with T3 could result at least partially from the stimulation of thyroid hormone response elements in the Ucp1 gene promoter,57 increased stability of Ucp1 mRNA,40 and/or enhanced mitochondriogenesis (extensively reviewed in Wrutniak‐Cabello et al.58). In addition to intramuscular adipocytes being a target for T3‐induced thermogenesis, our results also point to FAPs as a possible tool to further dissect the effects of T3 on thermogenesis and UCP1 expression.
We observed two genetic factors affecting UCP1 levels in differentiated FAPs: background and sex. The differences in UCP1 expression after differentiation between FAPs isolated from C57Bl/6 and Sv/129 mice are in line with previous results obtained in adipogenic progenitors isolated from skeletal muscle,32 subcutaneous WAT, and epididymal WAT32, 37 of these strains and highlight the importance of genetic background on the ectopic expression of UCP1.17, 59, 60 Regarding differences in brown and brown‐like adipocytes between sexes, evidence is scarcer and mostly derives from human studies. For example, previous studies have reported that functionally active BAT11 and UCP1+ adipocytes in perirenal adipose tissue43 are more frequently found in women than in men. Additionally, perirenal mesenchymal stem cells isolated from women express higher mRNA (but not protein) levels of Ucp1 after differentiation than those isolated from men.43 Further analysis of the differences between FAPs isolated from male and female mice or from mice of different genetic backgrounds could identify factors leading to enhanced UCP1 expression in intramuscular adipose tissue and potentially also in WAT depots. Finally, our observation that genetic differences affect the potential of skeletal muscle FAPs to express UCP1 following differentiation could have implications for the development of interventions to promote a brown phenotype in intramuscular adipocytes in specific populations.
Whether our results are also applicable for humans is currently not clear. Previous studies have shown adipogenic potential for CD56−/CD15+, 24, 61 CD56−/PDGFRα+, 54 or CD56−/CD15+/PDGFRα+ 51 progenitors isolated from human skeletal muscle. These progenitors have been described to give rise to bona fide white adipocytes that are insensitive to insulin‐induced glucose uptake compared with adipocytes derived from progenitors isolated from subcutanous adipose tissue.51 In contrast, Uezumi et al.54 showed that skeletal muscle PDGFRα+ progenitor‐derived adipocytes from humans express both white (Leptin) and brown (UCP1) adipocyte‐specific genes. Future experiments should clarify whether human intramuscular adipocytes have the potential to differentiate into brown‐like adipocytes.
Interleukin‐6 expression is required for macrophage infiltration and muscle regeneration,62 and IL‐6 knockout mice display smaller myofibers than wild‐type mice 15 days after CTX‐induced muscle injury.62 Furthermore, increases in UCP1 protein levels in WAT after exercise training or cold exposure are much less pronounced in IL‐6 knockout mice,63 while cold‐induced UCP1 levels in intrascapular BAT seem to be unaffected.64 In mice, cancer cachexia‐induced UCP1 expression in WAT is largely reduced in IL‐6‐receptor knockout mice.65 In contrast, our results demonstrate that IL‐6 is not required for intramuscular Ucp1 expression after GLY‐induced fatty infiltration followed or not by beta 3‐adrenergic agonist treatment. Further studies will be needed to dissect the roles played by other cytokines and inflammatory signals in the regulation of Ucp1 expression in intramuscular adipocytes.
Increased Ucp1 expression in skeletal muscle could provide a new target for the treatment of fatty infiltration in muscle atrophy and sarcopenia. Indeed, we demonstrate that the capacity to induce intramuscular Ucp1 expression by GLY injections is maintained in aged mice. It would be also important to assess intramuscular Ucp1 expression in other models of atrophy. This will, however, likely require experiments with additional animal species because fatty infiltration is generally not observed during denervation atrophy66, 67, 68, 69 or cancer cachexia65, 70, 71, 72 in mice.
In summary, we show that intramuscular Ucp1 expression can be triggered during skeletal muscle fatty infiltration in an obesity‐resistant mouse strain and that FAPs, the adipocyte precursors residing in skeletal muscle, are able to differentiate into brown‐like adipocytes. Therefore, FAPs could be a target for therapies promoting a brown phenotype in intramuscular adipocytes, with potential to improve the micro‐environment of skeletal muscle affected by fatty infiltration.
Funding
This study was supported by the SNF grant ProDoc—PDFMP3_141761 to J.K., an unrestricted grant from the Uniscientia Foundation, Zürich, and by the Clinical Research Priority Program ‘small RNAs’ of the University of Zürich.
Conflict of interest
None declared.
Acknowledgements
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.73 We thank Dr Salvatore Modica and Vissarion Efthymiou, ETH Zürich, for their technical assistance with adipocyte cultures and analysis and Professor Christian Wolfrum, ETH Zürich, for his help with the animal work. We thank the Flow Cytometry Facility at the University of Zürich for their technical advice, Heidi Seiler for her help with the paraffin sections, and Dr Edlira Luca for critical reading of the manuscript.
Gorski, T. , Mathes, S. , and Krützfeldt, J. (2018) Uncoupling protein 1 expression in adipocytes derived from skeletal muscle fibro/adipogenic progenitors is under genetic and hormonal control. Journal of Cachexia, Sarcopenia and Muscle, 9: 384–399. doi: 10.1002/jcsm.12277.
References
- 1. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004;84:277–359. [DOI] [PubMed] [Google Scholar]
- 2. Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L, et al. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci 1992;103:931–942. [DOI] [PubMed] [Google Scholar]
- 3. Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, et al. The emergence of cold‐induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 2010;298:E1244–E1253. [DOI] [PubMed] [Google Scholar]
- 4. Rosenwald M, Perdikari A, Rulicke T, Wolfrum C. Bi‐directional interconversion of brite and white adipocytes. Nat Cell Biol 2013;15:659–667. [DOI] [PubMed] [Google Scholar]
- 5. Himms‐Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL‐316243‐treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol 2000;279:C670–C681. [DOI] [PubMed] [Google Scholar]
- 6. Ringholm S, Grunnet Knudsen J, Leick L, Lundgaard A, Munk Nielsen M, Pilegaard H. PGC‐1alpha is required for exercise‐ and exercise training‐induced UCP1 up‐regulation in mouse white adipose tissue. PLoS One 2013;8:e64123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chechi K, van Marken Lichtenbelt WD, Richard D. Brown and Beige Adipose Tissues: Phenotype and Metabolic Potential in Mice and Men. J Appl Physiol (1985) 2017; jap 00021 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 2013;19:1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, et al. A smooth muscle‐like origin for beige adipocytes. Cell Metab 2014;19:810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2007;293:E444–E452. [DOI] [PubMed] [Google Scholar]
- 11. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009;360:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med 2013;19:635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homoe P, Loft A, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab 2013;17:798–805. [DOI] [PubMed] [Google Scholar]
- 14. Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, et al. Evidence for two types of brown adipose tissue in humans. Nat Med 2013;19:631–634. [DOI] [PubMed] [Google Scholar]
- 15. Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One 2012;7:e49452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012;150:366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Almind K, Manieri M, Sivitz WI, Cinti S, Kahn CR. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc Natl Acad Sci U S A 2007;104:2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boettcher M, Machann J, Stefan N, Thamer C, Haring HU, Claussen CD, et al. Intermuscular adipose tissue (IMAT): association with other adipose tissue compartments and insulin sensitivity. J Magn Reson Imaging 2009;29:1340–1345. [DOI] [PubMed] [Google Scholar]
- 19. Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 2000;71:885–892. [DOI] [PubMed] [Google Scholar]
- 20. Baum T, Inhuber S, Dieckmeyer M, Cordes C, Ruschke S, Klupp E, et al. Association of Quadriceps Muscle Fat With Isometric Strength Measurements in Healthy Males Using Chemical Shift Encoding‐Based Water‐Fat Magnetic Resonance Imaging. J Comput Assist Tomogr 2016;40:447–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beavers KM, Beavers DP, Houston DK, Harris TB, Hue TF, Koster A, et al. Associations between body composition and gait‐speed decline: results from the Health, Aging, and Body Composition study. Am J Clin Nutr 2013;97:552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marcus RL, Addison O, Dibble LE, Foreman KB, Morrell G, Lastayo P. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J Aging Res 2012;2012:629637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murphy RA, Reinders I, Register TC, Ayonayon HN, Newman AB, Satterfield S, et al. Associations of BMI and adipose tissue area and density with incident mobility limitation and poor performance in older adults. Am J Clin Nutr 2014;99:1059–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laurens C, Louche K, Sengenes C, Coue M, Langin D, Moro C, et al. Adipogenic progenitors from obese human skeletal muscle give rise to functional white adipocytes that contribute to insulin resistance. Int J Obes (Lond) 2016;40:497–506. [DOI] [PubMed] [Google Scholar]
- 25. Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18‐88 yr. J Appl Physiol (1985) 2000;89:81–88. [DOI] [PubMed] [Google Scholar]
- 26. Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008;454:961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yin H, Pasut A, Soleimani VD, Bentzinger CF, Antoun G, Thorn S, et al. MicroRNA‐133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell Metab 2013;17:210–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanchez‐Gurmaches J, Guertin DA. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun 2014;5:4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu W, Liu Y, Lai X, Kuang S. Intramuscular adipose is derived from a non‐Pax3 lineage and required for efficient regeneration of skeletal muscles. Dev Biol 2012;361:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 2010;12:153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 2010;12:143–152. [DOI] [PubMed] [Google Scholar]
- 32. Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A 2011;108:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pisani DF, Bottema CD, Butori C, Dani C, Dechesne CA. Mouse model of skeletal muscle adiposity: a glycerol treatment approach. Biochem Biophys Res Commun 2010;396:767–773. [DOI] [PubMed] [Google Scholar]
- 34. Lukjanenko L, Brachat S, Pierrel E, Lach‐Trifilieff E, Feige JN. Genomic profiling reveals that transient adipogenic activation is a hallmark of mouse models of skeletal muscle regeneration. PLoS One 2013;8:e71084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Galimov A, Merry TL, Luca E, Rushing EJ, Mizbani A, Turcekova K, et al. MicroRNA‐29a in Adult Muscle Stem Cells Controls Skeletal Muscle Regeneration During Injury and Exercise Downstream of Fibroblast Growth Factor‐2. Stem Cells 2016;34:768–780. [DOI] [PubMed] [Google Scholar]
- 36. Ricquier D, Bouillaud F, Toumelin P, Mory G, Bazin R, Arch J, et al. Expression of uncoupling protein mRNA in thermogenic or weakly thermogenic brown adipose tissue. Evidence for a rapid beta‐adrenoreceptor‐mediated and transcriptionally regulated step during activation of thermogenesis. J Biol Chem 1986;261:13905–13910. [PubMed] [Google Scholar]
- 37. Li Y, Bolze F, Fromme T, Klingenspor M. Intrinsic differences in BRITE adipogenesis of primary adipocytes from two different mouse strains. Biochim Biophys Acta 2014;1841:1345–1352. [DOI] [PubMed] [Google Scholar]
- 38. Bianco AC, Silva JE. Intracellular conversion of thyroxine to triiodothyronine is required for the optimal thermogenic function of brown adipose tissue. J Clin Invest 1987;79:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Christoffolete MA, Linardi CC, de Jesus L, Ebina KN, Carvalho SD, Ribeiro MO, et al. Mice with targeted disruption of the Dio2 gene have cold‐induced overexpression of the uncoupling protein 1 gene but fail to increase brown adipose tissue lipogenesis and adaptive thermogenesis. Diabetes 2004;53:577–584. [DOI] [PubMed] [Google Scholar]
- 40. Guerra C, Roncero C, Porras A, Fernandez M, Benito M. Triiodothyronine induces the transcription of the uncoupling protein gene and stabilizes its mRNA in fetal rat brown adipocyte primary cultures. J Biol Chem 1996;271:2076–2081. [DOI] [PubMed] [Google Scholar]
- 41. Lee JY, Takahashi N, Yasubuchi M, Kim YI, Hashizaki H, Kim MJ, et al. Triiodothyronine induces UCP‐1 expression and mitochondrial biogenesis in human adipocytes. Am J Physiol Cell Physiol 2012;302:C463–C472. [DOI] [PubMed] [Google Scholar]
- 42. Rodriguez‐Cuenca S, Pujol E, Justo R, Frontera M, Oliver J, Gianotti M, et al. Sex‐dependent thermogenesis, differences in mitochondrial morphology and function, and adrenergic response in brown adipose tissue. J Biol Chem 2002;277:42958–42963. [DOI] [PubMed] [Google Scholar]
- 43. van den Beukel JC, Grefhorst A, Hoogduijn MJ, Steenbergen J, Mastroberardino PG, Dor FJ, et al. Women have more potential to induce browning of perirenal adipose tissue than men. Obesity (Silver Spring) 2015;23:1671–1679. [DOI] [PubMed] [Google Scholar]
- 44. Hardy D, Besnard A, Latil M, Jouvion G, Briand D, Thepenier C, et al. Comparative Study of Injury Models for Studying Muscle Regeneration in Mice. PLoS One 2016;11:e0147198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin‐1. American Journal of Physiology‐Endocrinology and Metabolism 2014;307:E469–E484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shefer G, Wleklinski‐Lee M, Yablonka‐Reuveni Z. Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. J Cell Sci 2004;117:5393–5404. [DOI] [PubMed] [Google Scholar]
- 47. Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation 2001;68:245–253. [DOI] [PubMed] [Google Scholar]
- 48. De Coppi P, Milan G, Scarda A, Boldrin L, Centobene C, Piccoli M, et al. Rosiglitazone modifies the adipogenic potential of human muscle satellite cells. Diabetologia 2006;49:1962–1973. [DOI] [PubMed] [Google Scholar]
- 49. Starkey JD, Yamamoto M, Yamamoto S, Goldhamer DJ. Skeletal muscle satellite cells are committed to myogenesis and do not spontaneously adopt nonmyogenic fates. J Histochem Cytochem 2011;59:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stuelsatz P, Shearer A, Yablonka‐Reuveni Z. Ancestral Myf5 gene activity in periocular connective tissue identifies a subset of fibro/adipogenic progenitors but does not connote a myogenic origin. Dev Biol 2014;385:366–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Arrighi N, Moratal C, Clement N, Giorgetti‐Peraldi S, Peraldi P, Loubat A, et al. Characterization of adipocytes derived from fibro/adipogenic progenitors resident in human skeletal muscle. Cell Death Dis 2015;6:e1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci 2011;124:3654–3664. [DOI] [PubMed] [Google Scholar]
- 53. Agley CC, Rowlerson AM, Velloso CP, Lazarus NR, Harridge SD. Human skeletal muscle fibroblasts, but not myogenic cells, readily undergo adipogenic differentiation. J Cell Sci 2013;126:5610–5625. [DOI] [PubMed] [Google Scholar]
- 54. Uezumi A, Fukada S, Yamamoto N, Ikemoto‐Uezumi M, Nakatani M, Morita M, et al. Identification and characterization of PDGFRalpha+ mesenchymal progenitors in human skeletal muscle. Cell Death Dis 2014;5:e1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lau AM, Tseng YH, Schulz TJ. Adipogenic fate commitment of muscle‐derived progenitor cells: isolation, culture, and differentiation. Methods Mol Biol 2014;1213:229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Crisan M, Casteilla L, Lehr L, Carmona M, Paoloni‐Giacobino A, Yap S, et al. A reservoir of brown adipocyte progenitors in human skeletal muscle. Stem Cells 2008;26:2425–2433. [DOI] [PubMed] [Google Scholar]
- 57. Rabelo R, Schifman A, Rubio A, Sheng X, Silva JE. Delineation of thyroid hormone‐responsive sequences within a critical enhancer in the rat uncoupling protein gene. Endocrinology 1995;136:1003–1013. [DOI] [PubMed] [Google Scholar]
- 58. Wrutniak‐Cabello C, Casas F, Cabello G. Thyroid hormone action in mitochondria. J Mol Endocrinol 2001;26:67–77. [DOI] [PubMed] [Google Scholar]
- 59. Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest 1998;102:412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Collins S, Daniel KW, Petro AE, Surwit RS. Strain‐specific response to beta 3‐adrenergic receptor agonist treatment of diet‐induced obesity in mice. Endocrinology 1997;138:405–413. [DOI] [PubMed] [Google Scholar]
- 61. Pisani DF, Clement N, Loubat A, Plaisant M, Sacconi S, Kurzenne JY, et al. Hierarchization of myogenic and adipogenic progenitors within human skeletal muscle. Stem Cells 2010;28:2182–2194. [DOI] [PubMed] [Google Scholar]
- 62. Zhang C, Li Y, Wu Y, Wang L, Wang X, Du J. Interleukin‐6/signal transducer and activator of transcription 3 (STAT3) pathway is essential for macrophage infiltration and myoblast proliferation during muscle regeneration. J Biol Chem 2013;288:1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Knudsen JG, Murholm M, Carey AL, Bienso RS, Basse AL, Allen TL, et al. Role of IL‐6 in exercise training‐ and cold‐induced UCP1 expression in subcutaneous white adipose tissue. PLoS One 2014;9:e84910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wernstedt I, Edgley A, Berndtsson A, Faldt J, Bergstrom G, Wallenius V, et al. Reduced stress‐ and cold‐induced increase in energy expenditure in interleukin‐6‐deficient mice. Am J Physiol Regul Integr Comp Physiol 2006;291:R551–R557. [DOI] [PubMed] [Google Scholar]
- 65. Petruzzelli M, Schweiger M, Schreiber R, Campos‐Olivas R, Tsoli M, Allen J, et al. A switch from white to brown fat increases energy expenditure in cancer‐associated cachexia. Cell Metab 2014;20:433–447. [DOI] [PubMed] [Google Scholar]
- 66. Sepulveda PV, Lamon S, Hagg A, Thomson RE, Winbanks CE, Qian H, et al. Evaluation of follistatin as a therapeutic in models of skeletal muscle atrophy associated with denervation and tenotomy. Sci Rep 2015;5:17535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Guo Y, Meng J, Tang Y, Wang T, Wei B, Feng R, et al. AMP‐activated kinase alpha2 deficiency protects mice from denervation‐induced skeletal muscle atrophy. Arch Biochem Biophys 2016;600:56–60. [DOI] [PubMed] [Google Scholar]
- 68. Baumann CW, Liu HM, Thompson LV. Denervation‐Induced Activation of the Ubiquitin‐Proteasome System Reduces Skeletal Muscle Quantity Not Quality. PLoS One 2016;11:e0160839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kunkel SD, Suneja M, Ebert SM, Bongers KS, Fox DK, Malmberg SE, et al. mRNA expression signatures of human skeletal muscle atrophy identify a natural compound that increases muscle mass. Cell Metab 2011;13:627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang G, Liu Z, Ding H, Miao H, Garcia JM, Li YP. Toll‐like receptor 4 mediates Lewis lung carcinoma‐induced muscle wasting via coordinate activation of protein degradation pathways. Sci Rep 2017;7:2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lee DE, Brown JL, Rosa‐Caldwell ME, Blackwell TA, Perry RA, Jr., Brown LA, et al. Cancer cachexia‐induced muscle atrophy: evidence for alterations in microRNAs important for muscle size. Physiol Genomics 2017;49:253–260. [DOI] [PubMed] [Google Scholar]
- 72. Liu S, Wu HJ, Zhang ZQ, Chen Q, Liu B, Wu JP, et al. L‐carnitine ameliorates cancer cachexia in mice by regulating the expression and activity of carnitine palmityl transferase. Cancer Biol Ther 2011;12:125–130. [DOI] [PubMed] [Google Scholar]
- 73. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]


