Abstract
Purpose
To compare the postoperative higher-order-aberrations (HOAs) after hyperopic small incision lenticule extraction (SMILE), hyperopic laser-assisted in situ keratomileusis (LASIK), and lenticule implantation for correction of hyperopia.
Methods
Eighteen monkeys were divided to six groups: +2.00 D and +4.00 D hyperopic SMILE, +2.00 D and +4.00 D hyperopic LASIK (n = 6 eyes for each), and lenticule implantation with a −2.00 D and −4.00 D lenticule (n = 3 eyes for each). The corneal HOAs were evaluated preoperatively and 3-month postoperatively.
Results
At 3-month postoperatively, the spherical aberrations significantly increased toward negative direction in all +4.00 D groups (all P < 0.05). There was a significant change toward more negative values in the third-order vertical coma in the SMILE +4.00 D and LASIK +4.00 D groups (P = 0.026 and P = 0.036, respectively). There were also significant changes in the third-order horizontal trefoil (P = 0.034) and oblique secondary astigmatism (P = 0.012) in the LASIK +4.00 D group. In the eyes that underwent +4.00 D lenticule implantation, the fourth-order horizontal quatrefoil significantly increased (P = 0.029). In low hyperopia correction (+2.00 D), treatment with lenticule implantation tended to have less changes in HOAs, compared to the other two groups.
Conclusions
In hyperopic SMILE, hyperopic LASIK or lenticule implantation surgery, significant induction of third- and fourth-order HOAs were seen in moderate hyperopia correction but not in low hyperopia correction. In low hyperopia treatment, lenticule implantation might offer a favorable trend in the aspect of HOAs.
Translational Relevance
The results provided the knowledge of surgically induced HOAs and understanding of the effects of surgery in different types of hyperopic correction.
Keywords: higher-order- aberrations, hyperopia, SMILE, LASIK, lenticule implantation
Introduction
Hyperopia is a common refractive error with a reported prevalence of 25.2% to 31.8% in adults.1,2 There are a variety of surgical treatments available for hyperopia, aiming to modify either the lens or cornea. These surgical options include laser-assisted in situ keratomileusis (LASIK), photorefractive keratectomy, or phakic intraocular lens implantation.3 Femtosecond lenticule extraction (FLEx) is a relatively new refractive surgery procedure in which a femtosecond laser is used to create an intrastromal lenticule that is removed after lifting the flap, as first described in 2006.4,5 The FLEx procedure was further modified by eliminating the need for a flap by dissecting and extracting the lenticule through a small incision, known as a small incision lenticule extraction (SMILE).4,5 In 2016, we reported the feasibility and effects of hyperopic SMILE in an animal model, showing that hyperopic SMILE effectively steepened the central cornea, and it had less postoperative wound healing response and stromal interface reaction compared to hyperopic LASIK, especially in higher refractive correction.6 Sekundo et al.5 also reported acceptable 9-month refractive and visual outcomes in their first pilot study on the use of FLEx for the treatment of spherical hyperopia. Hence, refractive lenticule extraction (ReLEx), either FLEx or SMILE, provides new treatment options for hyperopia.
Higher-order-aberrations (HOAs) following refractive surgery, both naturally existing and surgically induced, affect postoperative visual optical quality. Postoperative HOAs have been examined following myopic-SMILE. Studies have shown that most third-order and fourth-order HOAs, mainly coma and spherical aberrations, increased after myopic SMILE.4,7–9 However, compared to femtosecond-LASIK, the induction of HOAs was significantly lower in SMILE patients.10,11 Hyperopic SMILE has several differences in the lenticule profiles from myopic-SMILE and therefore the postoperative HOAs changes are expected to be different. In hyperopic SMILE, the lenticule is thinnest in the central area, and there is the presence of a transition zone of at least 2 mm,5,6 in the mid periphery outside the optical zone (OZ), to reduce the curvature gradient of the stromal surface in the region of the maximum tissue removal and therefore reduce the amount of epithelial thickness compensation, one of the major drivers of regression in hyperopic corrections.12 Kohnen et al.13 conducted a retrospective comparative study comparing the corneal HOAs induced by myopic and hyperopic LASIK. All surgeries were performed under similar environmental and surgical conditions, and the authors reported that myopic LASIK induced more positive spherical aberrations and positive secondary astigmatism, whereas hyperopic LASIK induced more negative spherical aberrations and negative secondary astigmatism. Hyperopic LASIK also induced more third- and fifth-order coma-like aberrations than myopic LASIK.13 More recently, Plaza-Puche et al.14 also reported that a significant increase of root mean square (RMS) spherical-like, coma-like, and higher-order aberration was observed after hyperopic LASIK using an Amaris excimer. However, the data on the changes in HOAs following hyperopic SMILE are very limited.
The extracted stromal lenticule from a SMILE procedure can be used for other purposes based on the concept of intrastromal tissue addition. It has been described to be used as a corneal patch graft for the management of corneal micro-perforation or partial-thickness corneal defect,15 and for the treatment of keratoconus, by transplanting the lenticule into stroma.16,17 The lenticule implantation can also be used for the correction of hyperopia as implanting a convex-shaped lenticule obtained from a myopic SMILE procedure results in steeper central cornea.18,19
In the present study, we aimed to evaluate and compare the postoperative HOA profiles after hyperopic SMILE, hyperopic LASIK, and lenticule implantation for correction of hyperopia, by using a nonhuman primate monkey model.
Methods
Study Animals and Experimental Groups
A total of 18 Macaca fascicularis monkeys, aged 2 to 5, were used. Among the 36 eyes, 24 eyes were randomly divided to four groups: +2.00 dioptres (D) hyperopic SMILE, +2.00 D hyperopic LASIK, +4.00 D hyperopic SMILE, and +4.00 D hyperopic LASIK (n = 6 eyes for each group). The other 12 eyes were used for the lenticule implantation experiments, with the right eyes having −2.00 D (n = 3 eyes) and −4.00 D (n = 3 eyes) SMILE performed to obtain lenticules, and the left eyes having corresponding −2.00 D (n = 3 eyes) and −4.00 D (n = 3 eyes) lenticule implantation. The study was approved by the Institutional Animal Care and Use Committee (IACUC) of SingHealth, Singapore. All animals are treated according to the guidelines of the Association for Research in Vision and Ophthalmology's Statement for the Use of Animals in Ophthalmic and Vision Research. During surgical and examination procedures, the animals were tranquilized intramuscularly with ketamine hydrochloride 10 mg/kg or medetomidine 0.02 mg/kg. Additionally, anesthesia was induced with 2% to 3% inhaled isoflurane and maintained with 1% to 2% inhaled isoflurane.
Hyperopic SMILE Procedure
Hyperopic SMILE procedures were performed as described previously.6 Correction of +2.00 D or +4.00 D was performed using a 500-kHz femtosecond laser (Visumax; Carl Zeiss Meditec, Germany). The eye was docked on a small curved interface suction cone. Once suction was applied, laser incisions were made in the following automated sequence: (1) a spiral-in pattern on the posterior surface of the lenticule with a 5.5-mm diameter, equating the OZ, (2) a spiral-out pattern on the posterior surface of the lenticule, from the edge of the OZ for 2.0 mm, corresponding to the transition zone, (3) a vertical 90° lenticule side cuts, (4) a spiral-out anterior surface of the lenticule, cutting 7.9 mm diameter cap (5) followed by a superiorly placed 2.5-mm wide incision at 120°. The femtosecond laser parameters were as follows: 120-μm cap thickness, 7.9-mm cap diameter, 170 nJ power, and side cut angles at 90°. The spot distance and tracking spacing were set respectively at 3 μm for the lenticule, 1 μm for the lenticule side, 3 μm for the cap, and 2 μm for the cap side cut. The diameter of the lenticule was 7.5 mm, which is equal to the 5.5-mm OZ plus the 2-mm transition zone. After completion of the laser firing, the anterior surface of the lenticule was bluntly dissected with a SMILE dissector, followed by the posterior surface. The lenticule was then grasped and removed by a Tan DSAEK forceps (ASICO, Westmont, IL).
Hyperopic LASIK Procedure
LASIK flaps were created with the Visumax femtosecond laser. The laser parameters were as follows: 120-μm flap thickness, 7.9-mm flap diameter, 170 nJ power, spot distance and tracking spacing of 4.8 μm for lamellar flap and 2 μm for flap side cut, flap side cut at 90°, hinge position at 90°, hinge angle of 50°, and spiral in scanning pattern direction. After the flap was lifted, the underlying stroma underwent a hyperopic ablation of +2.00 D or +4.00 D with a 10.0 × 10.0 mm and 10.1 × 10.1 mm treated zone, respectively, and with a 7.5-mm OZ using an excimer laser (Technolas; Bausch & Lomb, Rochester, NY). The excimer laser parameters were as follows: spot size of 2.0 μm diameter, fluence of 120 mJ/cm2, and repetition rate of 100 Hz. The flaps were subsequently repositioned, a bandaged contact lens was immediately applied, and temporary tarsorrhaphy was used to close the eyelids.
Lenticule Implantation
One eye of each animal was randomly selected for myopic SMILE procedure. Refraction correction of −2.00 D (n = 3) and −4.00 D (n = 3) were performed using a previously described technique20,21 with the use of the Visumax femtosecond laser. The laser parameters were as follows: 120-μm cap thickness, 7.5-mm cap diameter, and 6.5-mm lenticule diameter, with the laser energy at 170 nJ. The lenticule was grasped and removed by Tan DSAEK forceps and then implanted into the contralateral eye, in a 7.9-mm intrastromal pocket created by the Visumax femtosecond laser, at the depth of 160 μm and over the pupillary center.
All the procedures were performed by an experienced corneal surgeon (J.S.M). A subconjunctival injection of dexamethasone and gentamicin was given immediately after surgery. After removal of sutures of tarsorrhaphy on day 3, 0.3% tobramycin and 0.1% dexamethasone eye drops were administrated four times daily for 1 week.
Assessment of Corneal Thickness and Higher-Order Aberrations
The preoperative and postoperative corneal HOAs at 3 months were evaluated by the ATLAS 9000 Corneal Topography System with corneal wavefront Zernike analysis (Carl Zeiss Meditec) with a 4.5-mm pupil without topical pharmacological intervention. Slit lamp examination was also performed with the instillation of 1% mydriacyl eye drops for the lenticule implantation group to better visualize the margin of implanted lenticule. The preoperative and 3-month central corneal thickness (CCT) were measured using the in-built ruler of the anterior segment optical coherence tomography (ASOCT; RTVue; Optovue, Inc, Fremont, CA). For each eye at each time point, three high-resolution corneal cross-sectional scans (8-mm scan length, single scan mode) were obtained, and the average was taken. All examinations were performed by an experienced technician (N.L.C).
Statistical Analysis
All data were expressed as mean ± standard deviation. The Wilcoxon signed rank test was used for the comparison of preoperative and postoperative HOAs. The Mann-Whitney U test was applied to compare the HOAs between +2.00 D and +4.00 D. Kruskal-Wallis rank test with pairwise Mann-Whitney U tests and Bonferroni corrections were used to compare the HOAs across the three or six groups. P values less than 0.05 were considered statistically significant. All the statistical analyses were performed using STATA software (version 13; StataCorp, College Station, TX).
Results
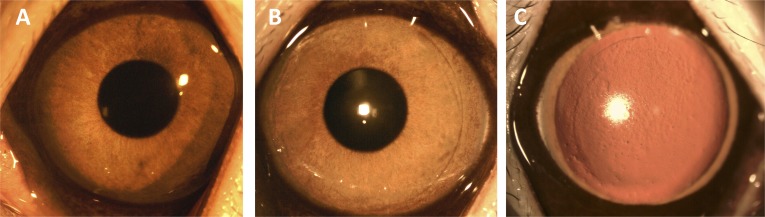
On the slit lamp evaluation, all corneas were clear throughout the study period. There was no haze formation, flap dislocation, wound tear, or other complications. The implanted lenticule was visible and well-centered, and had integrated with the surrounding stroma at 3 months (Fig. 1). The preoperative CCT was 438.8 ± 34.4, 458.2 ± 28.4, 464.7 ± 30.5, 428.5 ± 32.9, 442.9 ± 19.2, and 452.2 ± 24.8 μm (P = 0.62), and 3-month CCT was 420.2 ± 28.9, 444.0 ± 21.8, 480.3 ± 31.6, 398.9 ± 25.8, 417.2 ± 23.7, and 471.5 ± 30.0 μm (P = 0.54), for the SMILE +2.00 D, LASIK +2.00 D, lenticule +2.00 D implantation, SMILE +4.00 D, LASIK +4.00 D, and lenticule +4.00 D implantation groups. The CCT was 458.0 and 449.7 μm for the control group preoperatively and at 3 months.
Figure 1.
Slit-lamp biomicroscopy of corneas at 3 months after hyperopic SMILE (+4.00 D; A), hyperopic LASIK (+4.00 D; B), and lenticule implantation (+4.00 D; C). All corneas remained clear. The arrow indicated the margin of implanted lenticule.
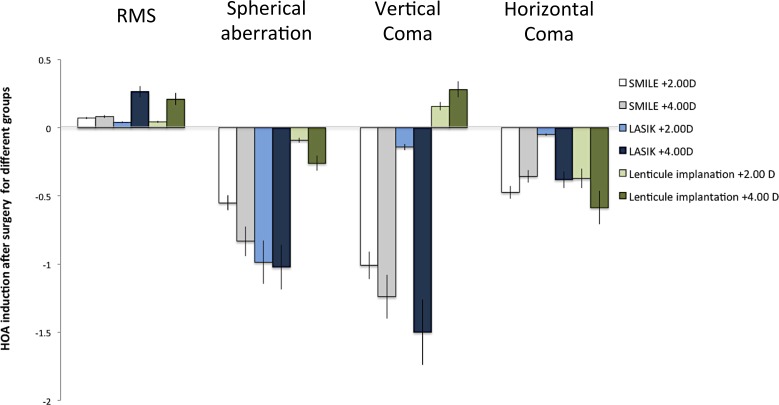
The preoperative and postoperative values of HOAs for all the groups are summarized in Table 1, and the changes of HOAs 3 months after surgery for different groups are summarized in Table 2 and Figure 2. The values of all parameters across the six groups preoperatively were comparable (P > 0.05 for all parameters). At 3 months' postoperatively, the RMS of total HOAs increased after surgery in all eyes, and the amount of increase was greater in the +4.00 D than in +2.00 D treatment in all types of surgery. The spherical aberrations significantly increased toward negative direction for the SMILE +4.00 D, LASIK +4.00 D, and lenticule +4.00 D implantation eyes (P = 0.046, P = 0.022, and P = 0.048, respectively). There was a significant change toward more negative values in the third-order vertical coma for the SMILE +4.00 D and LASIK +4.00 D groups (P = 0.026 and P = 0.036, respectively). There were also significant changes in the third-order horizontal trefoil (toward more positive values; P = 0.034) and oblique secondary astigmatism (toward more negative values; P = 0.012) in the eyes treated with LASIK +4.00 D. In the eyes that underwent +4.00 D lenticule implantation, the fourth-order horizontal quatrefoil significantly increased (P = 0.029; Table 2). Of note, in the eyes with a lower refractive correction (SMILE +2.00 D, LASIK +2.00 D, and lenticule implantation +2.00 D groups), there were no significant changes in the HOAs parameters after surgery. The eyes treated with +2.00 D lenticule implantation tended to have less changes in the majority of HOA parameters, compared to the SMILE +2.00 D and LASIK +2.00 D groups.
Table 1.
Preoperative and Postoperative Values of HOAs
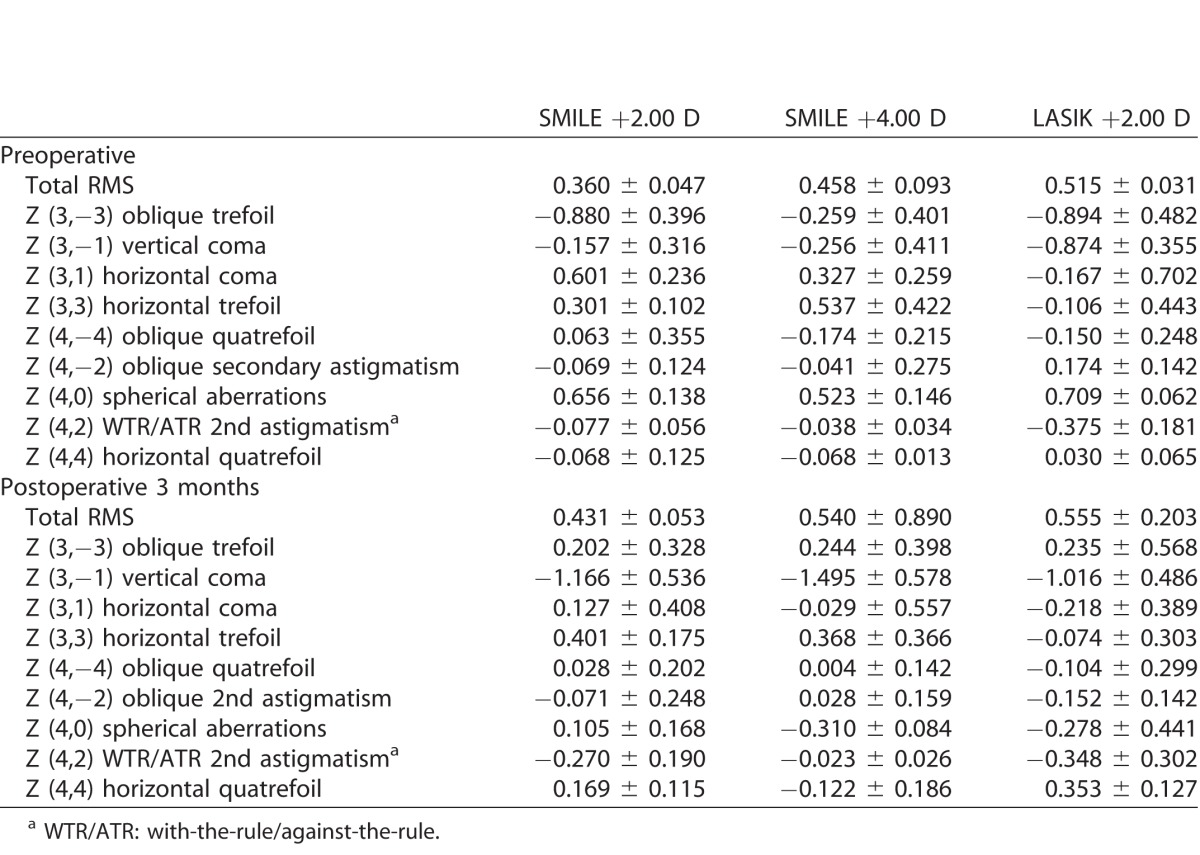
Table 2.
Mean Changes of HOAs at 3 Months Postoperatively

Figure 2.
Bar graph showing the induction of RMS, spherical aberrations, vertical coma, and horizontal coma 3 months after different types of surgery. The HOA induction was more significant in the +4.00 D correction than +2.00 D correction, regardless of the surgical types. In lower hyperopic treatment, the lenticule implantation group had less HOA induction than the other two groups. Error bars: standard errors.
Table 1.
Extended
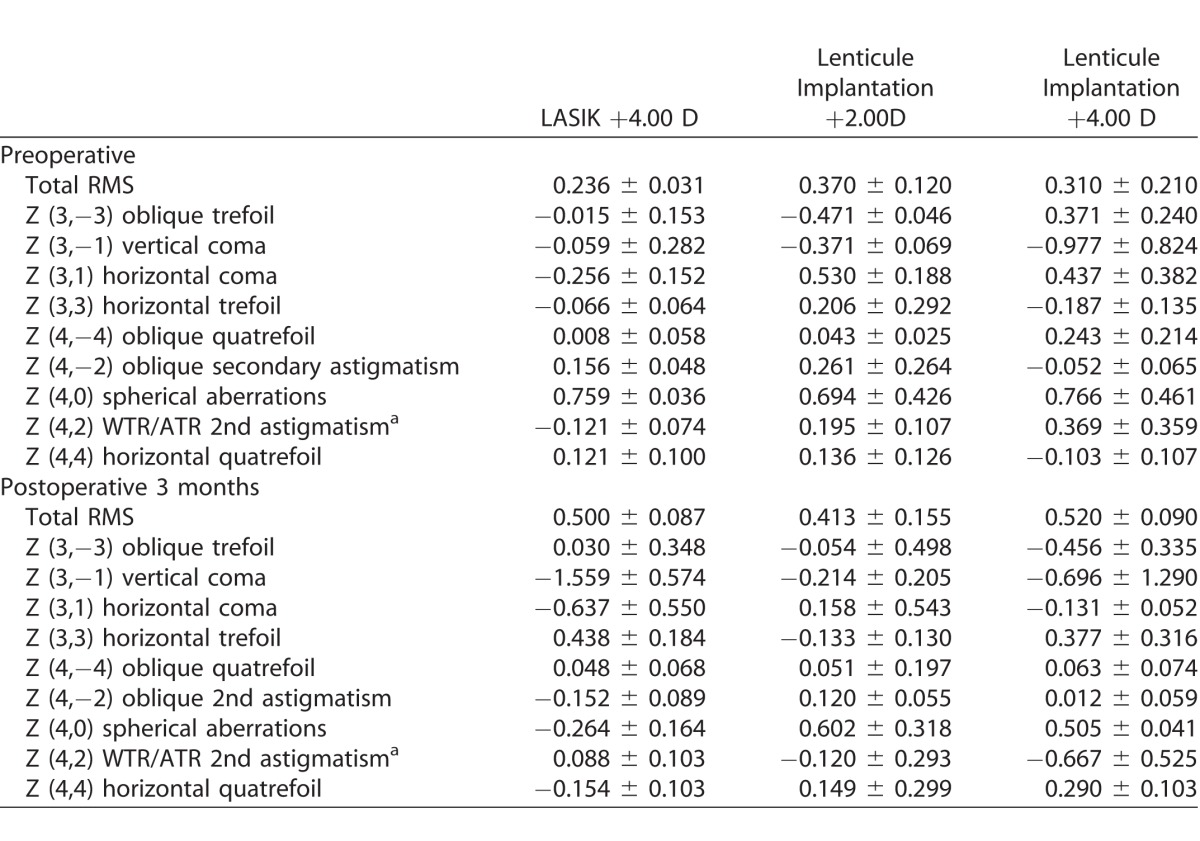
Table 3 compares the postoperative HOA induction between any two groups. At 3 months, there was no significant difference in the HOA induction between any two of the three groups.
Table 3.
Comparisons of the HOA Induction at Postoperative 3 Months Between Any Two Groups
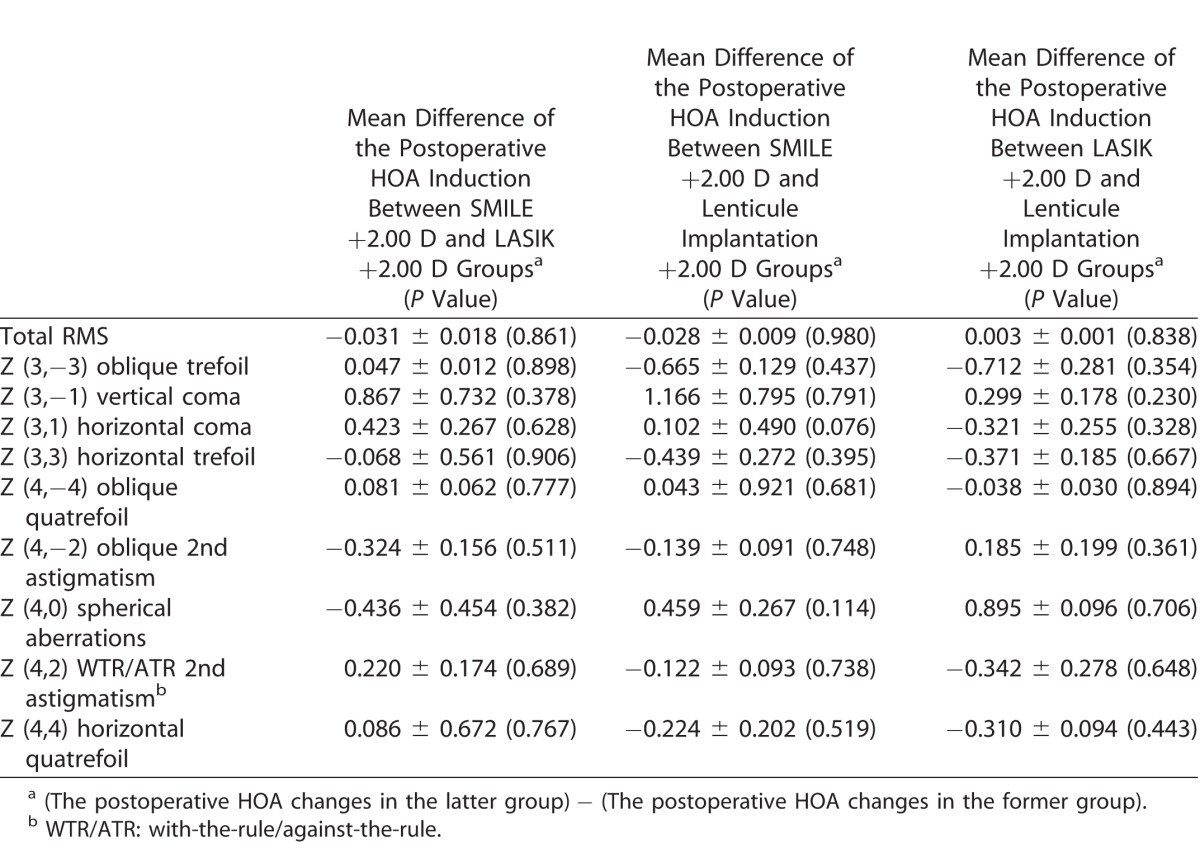
Table 3.
Extended
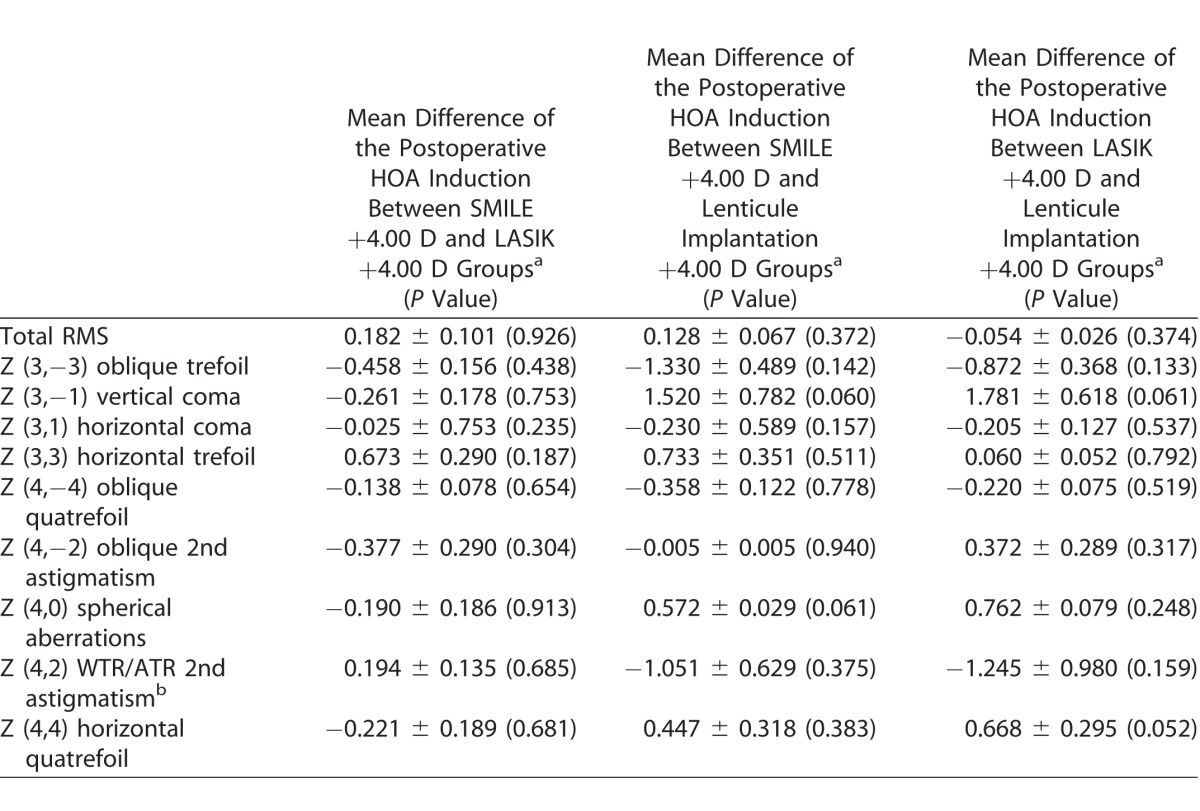
Discussion
In the present study, we reported that the RMS of total HOAs increased, and the spherical aberrations became more negative after hyperopic treatments, regardless of the treatment options (SMILE, LASIK, or lenticule implantation) and with no significant differences among the treatment options. In lower refractive correction, there were no significant changes in the HOA parameters after surgery in all groups, and the eyes treated with +2.00 D lenticule implantation tended to have less changes in postoperative HOAs, compared to the SMILE +2.00 D and LASIK +2.00 D groups. Significant induction of third- and fourth-order HOAs, including that of spherical aberrations, was seen in higher refractive correction (+4.00 D) in all three groups.
The change in corneal shape following refractive surgery, and consequently the induction of HOAs, may affect visual quality despite good visual outcomes.22 The laser ablation profiles on corneas for hyperopic treatment are different from those for myopic correction. In hyperopic LASIK, the excimer laser ablates a ring of tissue in the midperipheral zone, generating central corneal steepening, making the cornea more prolate and decreasing corneal asphericity. It is known that after hyperopic laser vision correction, HOAs increase even more than they do in myopic eyes but in the opposite direction (i.e., toward negative values).22 Hyperopic LASIK has been reported to induce negative spherical aberrations and negative secondary astigmatism, and it induced more third- and fifth-order coma-like aberrations compared to myopic LASIK.14 Hyperopic SMILE is a new treatment option for hyperopia, and there is limited published literature.6,23 In hyperopic SMILE, the lenticule to be extracted is thinnest in the central area (preset to 25–30 microns), and there is the presence of a transition zone, in the mid periphery outside the OZ. These lenticule profiles are different from those in a myopic SMILE procedure. A myopic SMILE procedure has been reported to have less induction of postoperative HOAs than femtosecond-LASIK.23 In the present study, we did not observe significant difference in the postoperative HOAs induced between the hyperopic SMILE (OZ = 5.5 mm) and hyperopic LASIK groups (OZ = 7.5 mm). In a recent study reporting the postoperative spherical aberrations on 60 hyperopic SMILE procedures, the authors reported that spherical aberrations changed in the SMILE procedures with an OZ of 6.37 mm was significantly less than that in the LASIK procedures with a similar OZ (6.5 mm).23 The smaller OZ used in the hyperopic SMILE groups than that in the hyperopic LASIK groups in the present study may contribute to why no significant differences were observed between these two groups.
The use of refractive lenticule extracted from SMILE for the treatment of hyperopia has been shown as a safe and effective procedure in patients as well as animal studies.18,24–26 It offers an alternative to excimer laser ablation, such as hyperopic photorefractive keratectomy (PRK) or hyperopic LASIK, but also offers advantages of possible low risks of refractive regression, postoperative dry eye, and elimination of flap-related complications. The changes in the HOAs following lenticule implantation for hyperopic treatment have yet to be investigated. Ganesh et al.18 reported in a pilot study, on nine hyperopic eyes that underwent implantation of a cryopreserved allogeneic lenticule, with a mean preoperative spherical equivalent of +4.5 ± 1.1 D. The RMS did not significantly increase postoperatively. Hence lenticule implantation may be an attractive alternative to hyperopic PRK and hyperopic LASIK, with respect to induction of HOAs because the shape of the cornea becomes more natural after tissue addition compared with tissue subtraction, although in their study there were no comparison groups and the induced RMS was not consistent in those who received similar refractive power of lenticule.18 We also observed that in low hyperopic correction, the lenticule implantation group tended to have less induction of postoperative HOAs than the other two groups, in the majority of parameters. In view of postoperative HOAs, lenticule implantation might be a favorable option for low hyperopic correction, but the relationship between the depth of implantation and HOA induction warrants further investigation.
In the present study, the spherical aberrations changed toward more negative values in all eyes, and the changes were more significant in the moderate hyperopia groups (+4.00 D), regardless of the treatment. The increase of RMS was also greater in the moderate hyperopia groups than in low hyperopia groups in all three treatment types. These are consistent with previous studies showing that the higher the degree of hyperopia to be corrected, the greater the induction of negative spherical aberrations,27,28 and the postoperative RMS was linearly correlated to the refractive correction.29
This study was the first comprehensive evaluation comparing all modalities for hyperopia. The limitation of the present study was the small sample size. As no data were published on the comparisons of HOAs following hyperopic SMILE, hyperopic LASIK and lenticule implantation, the sample size (i.e., n = 6) was calculated based on the results of previous literature18 to tell a significant change in the HOAs after lenticule implantation. Due to the strict ethical regulation on the use of nonhuman primates, larger sample size >6 was not allowed, and this might account for the lack of significant difference among the groups, although the lenticule implantation group showed a favorable trend in some of HOA parameters in lower power of correction (Table 2). A nonhuman primate is the most appropriate animal model to study lenticule implantation as primates share the highest ocular similarities and genetic homologies with human,30,31 compared to other animal models such as rabbits or rats. M. fascicularis monkeys, the animal model used in this study, have been used in various eye research.32–34 They have a similar corneal diameter as well as corneal corneal vertical/horizontal ratio to human eyes.32
In conclusion, we demonstrated that in hyperopic SMILE, hyperopic LASIK, or lenticule implantation surgery, the negative spherical aberrations increased postoperatively, and this change was greater in the moderate hyperopic treatment. Significant induction of third- and fourth-order HOAs was also seen in higher refractive correction (+4.00 D) but not in lower refractive correction (+2.00 D), with comparable amount of increase across the three groups. In low hyperopia treatment, lenticule implantation appeared to have less induction of HOAs than hyperopic SMILE and hyperopic LASIK. Our results provide new information on the induction of HOAs with these new treatment modalities.
Acknowledgments
This research was supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Programme (NMRC/TCR/1021-SERI/2013) and administered by the Singapore Ministry of Health's National Medical Research Council. Geraint P. Williams' SERI fellowship is supported by a Dowager Eleanor Peel Trust Travelling Grant and a Royal College of Ophthalmologists/Pfizer Ophthalmic Fellowship.
Disclosure: Y.-C. Liu, None; J. Wen, None; E.P.W. Teo, None; G.P. Williams, None; N.C. Lwin, None; J.S. Mehta, None
References
- 1. Wolfram C, Höhn R, Kottler U,et al. Prevalence of refractive errors in the European adult population: the Gutenberg Health Study (GHS). Br J Ophthalmol. 2014; 98: 857– 861. [DOI] [PubMed] [Google Scholar]
- 2. Williams KM, Verhoeven VJ, Cumberland P,et al. Prevalence of refractive error in Europe: the European Eye Epidemiology (E(3)) Consortium. Eur J Epidemiol. 2015; 30: 305– 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Settas G, Settas C, Minos E, Yeung IY. . Photorefractive keratectomy (PRK) versus laser assisted in situ keratomileusis (LASIK) for hyperopia correction. Cochrane Database Syst Rev. 2012; 6: CD007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu YC, Riau AK, Mehta JS. . Cornea. 4th ed. Krachmer JH, Mannis MJ, Holland EJ, . Philadelphia, PA: Elsevier; 2016: 1317– 1327. [Google Scholar]
- 5. Sekundo W, Reinstein DZ, Blum M. . Improved lenticule shape for hyperopic femtosecond lenticule extraction (ReLEx FLEx): a pilot study. Lasers Med Sci. 2016; 314: 659– 664. [DOI] [PubMed] [Google Scholar]
- 6. Liu YC, Ang HP, Teo EP, Lwin NC, Yam GH, Mehta JS. . Wound healing profiles of hyperopic-small incision lenticule extraction (SMILE). Sci Rep. 2016; 6: 29802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vestergaard AH, Grauslund J, Ivarsen AR, Hjortdal JØ. . Efficacy, safety, predictability, contrast sensitivity, and aberrations after femtosecond laser lenticule extraction. J Cataract Refract Surg. 2014; 40: 403– 411. [DOI] [PubMed] [Google Scholar]
- 8. Sekundo W, Gertnere J, Bertelmann T, Solomatin I. . One-year refractive results, contrast sensitivity, high-order aberrations and complications after myopic small-incision lenticule extraction (ReLEx SMILE). Graefes Arch Clin Exp Ophthalmol. 2014; 252: 837– 843. [DOI] [PubMed] [Google Scholar]
- 9. Shah R, Shah S, Sengupta S. . Results of small incision lenticule extraction: all-in-one femtosecond laser refractive surgery. J Cataract Refract Surg. 2011; 37: 127– 137. [DOI] [PubMed] [Google Scholar]
- 10. Ganesh S, Gupta R. . Comparison of visual and refractive outcomes following femtosecond laser-assisted LASIK with smile in patients with myopia or myopic astigmatism. J Refract Surg. 2014; 30: 590– 596. [DOI] [PubMed] [Google Scholar]
- 11. Lin F, Xu Y, Yang Y. . Comparison of the visual results after SMILE and femtosecond laser-assisted LASIK for myopia. J Refract Surg. 2014; 30: 248– 254. [DOI] [PubMed] [Google Scholar]
- 12. Reinstein DZ, Archer TJ, Gobbe M, Silverman RH, Coleman DJ. . Epithelial thickness after hyperopic LASIK: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. 2010; 26: 555– 564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kohnen T, Mahmoud K, Bühren J. . Comparison of corneal higher-order aberrations induced by myopic and hyperopic LASIK. Ophthalmology. 2005; 1121: 1692. [DOI] [PubMed] [Google Scholar]
- 14. Plaza-Puche AB, Yebana P, Arba-Mosquera S, Alió JL. . Three-year follow-up of hyperopic LASIK using a 500-Hz excimer laser system. J Refract Surg. 2015; 31: 674– 682. [DOI] [PubMed] [Google Scholar]
- 15. Bhandari, V, Ganesh S, Brar S, Pandey R. . Application of the SMILE-derived glued lenticule patch graft in microperforations and partial-thickness corneal defects. Cornea. 2016; 35: 408– 412. [DOI] [PubMed] [Google Scholar]
- 16. Ganesh S, Brar S. . Femtosecond intrastromal lenticular implantation combined with accelerated collagen cross-linking for the treatment of keratoconus—initial clinical result in 6 eyes. Cornea. 2015; 34: 1331– 1339. [DOI] [PubMed] [Google Scholar]
- 17. Sachdev GS, Gupta D, Sachdev G, Sachdev R. . Tailored stromal expansion with a refractive lenticule for crosslinking the ultrathin cornea. J Cataract Refract Surg. 2015; 41: 918– 923. [DOI] [PubMed] [Google Scholar]
- 18. Ganesh S, Brar S, Rao PA. . Cryopreservation of extracted corneal lenticules after small incision lenticule extraction for potential use in human subjects. Cornea. 2014; 33: 1355– 1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun L, Yao P, Li M, Shen Y, Zhao J, Zhou X. . The safety and predictability of implanting autologous lenticule obtained by SMILE for hyperopia. J Refract Surg. 2015; 31: 374– 379. [DOI] [PubMed] [Google Scholar]
- 20. Liu YC, Teo PW, Lwin NC, Yam GH, Mehta JS. . Early corneal wound healing and inflammatory response after small incision lenticule extraction (SMILE): comparison of the effects of effects of different refractive correction and surgical experiences. J Refract Surg. 2016; 32: 346– 353. [DOI] [PubMed] [Google Scholar]
- 21. Liu YC, Rosman M, Mehta JS. . Enhancement following small incision lenticule extraction (SMILE): incidence, risk factors, and outcomes. Ophthalmology. 2017; 124: 813– 821. [DOI] [PubMed] [Google Scholar]
- 22. Hamill MB, Berdy GJ, Davidson RS,et al. Refractive Surgery: Section 13, Basic and Clinical Science Course. 2015–2016 ed. San Francisco, CA: American Academy of Ophthalmology; 2016: 11– 12. [Google Scholar]
- 23. Reinstein DZ, Pradhan KR, Carp GI,et al. Small Incision Lenticule Extraction (SMILE) for hyperopia: optical zone diameter and spherical aberration induction. J Refract Surg. 2017; 33: 370– 376. [DOI] [PubMed] [Google Scholar]
- 24. Sun L, Yao P, Li M, Shen Y, Zhao J, Zhou X. . The safety and predictability of implanting autologous lenticule obtained by SMILE for hyperopia. J Refract Surg. 2015; 31: 374– 379. [DOI] [PubMed] [Google Scholar]
- 25. Liu R, Zhao J, Xu Y,et al. Femtosecond laser-assisted corneal small incision allogenic intrastromal lenticule implantation in monkeys: a pilot study. Invest Ophthalmol Vis Sci. 2015; 56: 3715– 3720. [DOI] [PubMed] [Google Scholar]
- 26. Williams GP, Wu B, Liu YC,et al. Hyperopic refractive correction by LASIK, SMILE or lenticule reimplantation in a non-human primate model. PLoS One. 2017; In press. [DOI] [PMC free article] [PubMed]
- 27. Gatinel D, Malet J, Hoang-Xuan T, Azar D. . Corneal asphericity change after excimer laser hyperopic surgery: theoretical effects on corneal profiles and corresponding Zernike expansions. Invest Ophthalmol Vis Sci. 2004; 45: 1349– 1359. [DOI] [PubMed] [Google Scholar]
- 28. Llorente L, Barbero S, Merayo J, Total Marcos S. . and corneal optical aberrations induced by laser in situ keratomileusis for hyperopia. J Refract Surg. 2004; 20: 203– 216. [DOI] [PubMed] [Google Scholar]
- 29. Pesudovs K. . Wavefront aberration outcomes of LASIK for high myopia and high hyperopia. J Refract Surg. 2005; 21: S508– S512. [DOI] [PubMed] [Google Scholar]
- 30. Jonas JB, Hayreh SS, Tao Y. . Central corneal thickness and thickness of the lamina cribrosa and peripapillary sclera in monkeys. Arch Ophthalmol. 2009; 127: 1395– 1396. [DOI] [PubMed] [Google Scholar]
- 31. Hayashi S, Osawa T, Tohyama K. . Comparative observations on corneas, with special reference to Bowman's layer and Descemet's membrane in mammals and amphibians. J Morphol. 2002; 254: 247– 258. [DOI] [PubMed] [Google Scholar]
- 32. Augusteyn RC, Maceo Heilman B, Ho A, Parel JM. . Nonhuman primate ocular biometry. Invest Ophthalmol Vis Sci. 2016; 57: 105– 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Elsmo EJ, Kiland JA, Kaufman PL, McLellan GJ. . Evaluation of rebound tonometry in non-human primates. Exp Eye Res. 2011; 92: 268– 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koizumi N, Sakamoto Y, Okumura N,et al. Cultivated corneal endothelial cell sheet transplantation in a primate model. Invest Ophthalmol Vis Sci. 2007; 48: 4519– 4526. [DOI] [PubMed] [Google Scholar]