Abstract
Epothilones are a new class of natural and potent antineoplastic agents that stabilize microtubules. Although 12,13-epoxide derivatives are potent antiproliferative agents, the activities of the corresponding 12,13-olefin analogs are significantly decreased. These data were confirmed for two new analogs, 6-propyl-EpoB (pEB) and 6-propyl-EpoD (pED), in comparison with the natural compounds EpoB/EpoD, by using human A431, MCF7, and MDR1-overexpressing NCI/Adr cells. By using tritiated pEB/pED, compound uptake, release, and nuclear accumulation were investigated in A431 and NCI/Adr cells. In these cells, epothilones can principally be recognized and exported by Verapamil-sensitive efflux pumps, which are not identical to MDR1. The degree of export depends on the structure, olefin vs. epoxide-analog, and also on the intracellular drug concentration. The accumulation of pED used at 3.5 or 70 nM, respectively, was increased in the presence of 10 μM Verapamil in both cell lines 2- to 8-fold. In contrast, the intracellular levels of pEB were affected by Verapamil only at 3.5 nM pEB in NCI/Adr (2-fold) and not in A431 cells. In addition, strong nuclear accumulation was observed for pEB (40–50%) but not paclitaxel or pED (5–15%) in both cell lines. Our study suggests that differences in growth inhibitory efficacy between epoxide and olefin analogs may be based on different mechanisms of drug accumulation and subcellular distribution.
Epothilones are a new class of natural products and potential antineoplastic agents. Although they have no structural similarities to taxanes, they exert cellular effects similarly to paclitaxel (Taxol). Thus, epothilones bind to tubulin and cause hyperstabilization of microtubules with subsequent mitotic arrest and apoptotic cell death (1–3). The molecular mechanisms by which epothilones and paclitaxel induce apoptosis remain to be elucidated.
It has been demonstrated that epothilones are generally superior to paclitaxel in their ability to inhibit the proliferation of human cancer cell lines that are resistant to commonly used anticancer agents, including paclitaxel. The best understood mechanism of resistance to cytotoxic drugs, including antimicrotubule agents is drug export by multidrug-resistant p-glycoprotein (MDR1) (4). Although taxanes are substrates for MDR1, epothilones are not, and thus MDR1-positive tumor cells remain sensitive to epothilones (1, 2, 5–9).
The 12,13-epoxide moiety of epothilone A (EpoA) and B (EpoB) is dispensable for tubulin/microtubule-related effects in vitro, because the corresponding olefin analogs (EpoC and EpoD), as inducers of tubulin polymerization, are equally potent to EpoA and EpoB (7, 8, 10). Formal removal of epoxide oxygen in EpoB, thus leading to EpoD, does cause a significant decrease (∼10- to 30-fold) in antiproliferative activity (9). Furthermore, exposure of tumor cells to EpoB for a 4-h period produces virtually the same growth inhibitory effect as a continuous 3-day exposure, whereas for EpoD, exposure times more than 4 h were required to produce effects comparable to 3-day treatment (9). These data, as well as our own results, have led to the hypothesis that epoxides may be retained inside cells more effectively than the respective olefin analogs.
Total synthesis of EpoA to EpoD was accomplished in various laboratories including ours (5, 7, 10–14). To get more insight into the differential effects of epoxide- and olefin-bearing epothilone analogs, we have synthesized the natural compounds EpoB/EpoD and the new compounds 6-propyl-EpoB (pEB) and 6-propyl-EpoD (pED) and characterized their effects on tumor cell growth inhibition. The latter compounds were also labeled with tritium, and their uptake, release, and subcellular distribution were studied in human A431 and NCI/Adr (formerly MCF7/Adr) tumor cell lines. NCI/Adr cells were chosen, because they overexpress the p-glycoprotein and are resistant to paclitaxel (15).
Materials and Methods
Compounds.
Paclitaxel, human insulin, doxorubicin, Verapamil, and DNase were purchased from Sigma and the DNeasy Tissue Kit from Qiagen (Hilden, Germany). Estradiol was synthesized in the laboratories of Schering AG. 3H-paclitaxel (10.1 Ci/mmol) was obtained from Hartmann Analytic (Braunschweig, Germany). The epothilones were obtained in our laboratories through total syntheses, which will be described elsewhere. All stock solutions were prepared at 1 mM by using ethanol as a solvent and stored at −20°C. Stock solutions were further freshly diluted with medium containing serum. The final concentration of ethanol was 0.01%. Higher ethanol concentrations (1%) resulted in changed potencies of some epothilone derivatives (not shown).
Radiolabeling of Compounds.
The tritium label was introduced by reduction of unsaturated precursors by using tritium gas and rhodium catalyst. The crude products were purified by HPLC. The specific activity of the tritiated compounds was 29 Ci/mmol for pED and pEB, respectively. The compounds were dissolved in ethanol at 1 mCi/ml, the stock solutions stored at −20°C, and drug dilutions in growth medium were always freshly made up.
Cell Lines and Culture Conditions.
Human epidermoid A431 and mammary MCF7 carcinoma cells were obtained from American Type Culture Collection, whereas NCI/Adr cells were kindly provided by I. Fichtner, Max Delbrück Center, Berlin–Buch, Germany. A431 cells were cultured in a 1:1 mixture of DMEM and F12 (Biochrom, Berlin, Germany) supplemented with 10% FCS. MCF7 cells were cultivated in phenol-free RPMI medium 1640 supplemented with 10% FCS, 200 milliunits/ml of insulin, and 0.1 nM estradiol. NCI/Adr cells were routinely grown in phenol-free RPMI medium 1640 supplemented with 10% FCS and 0.5 μg/ml of doxorubicin, which was omitted when cells were used for experiments. At 60–80% confluency, the tumor cells were harvested by using either 0.125% trypsin plus 2 mM EDTA in PBS (A431) or 0.05% trypsin plus 0.02% EDTA in PBS (MCF7 and NCI/Adr). The media supplemented with all ingredients listed were referred to as growth medium for the respective tumor cell lines.
Growth Studies.
Tumor cells were detached with trypsin/EDTA and seeded in 200 μl at 1,500 (A431), 3,000 (MCF7), or 4,000 (NCI/Adr) cells/well in 96-well plates. Cells were allowed to adhere for 24 h, and then fresh growth medium plus compounds was added. Compounds were either left continuously for 3 days or alternatively for 4 h, with two subsequent washes and addition of fresh growth medium. After 3 days, cells were fixed with glutaraldehyde, stained with crystal violet, and the absorbance recorded (16). Values were normalized to the absorbance of untreated cells. For determination of the drug concentration needed for half-maximal growth inhibition (IC50), absorbance of control cells was taken as 100% and absorbance of cells at the highest epothilone B concentration as 0%, respectively. Experiments were performed at least twice, and one representative experiment with mean ± SD from six wells is shown in Results.
Cellular Uptake and Release of Radiolabeled Compounds.
Tumor cells were seeded in 0.5 ml of growth medium at 30,000/well in 24-well plates in triplicates. After 2 days, the medium was changed, and either 1 μCi (corresponding to 70 nM) or 0.05 μCi (corresponding to 3.5 nM) of radiolabeled compounds was added in fresh growth medium. At the indicated time points, cell monolayers were washed twice with growth medium, lysed in 0.3 ml of PBS/0.2% SDS/0.25 units/ml DNAse, and the radioactivity quantified in a β-counter. On parallel wells, the cell numbers were estimated, and the radioactive compound bound expressed as pmol/1 × 106 cells. For measurement of compound release, the tumor cells were incubated with the radiolabeled compounds for 2 h, washed twice with growth medium, and subsequently 0.5 ml of fresh growth medium was added. At the indicated time points, the supernatant was aspirated and cellular radioactivity estimated, as described above. Experiments were performed at least twice and one representative experiment with mean ± SD from three wells is shown in Results.
Subcellular Distribution of Radiolabeled Epothilones.
A431 or NCI/Adr cells were seeded in 10 ml of growth medium at 2 × 106 cells in 10-cm plates. After 2 days, the medium was aspirated, and 5 μCi (corresponding to 35 nM) of radiolabeled compounds was added in 5 ml of growth medium on parallel plates, and the cell numbers were estimated and found to be 4–7 × 106 cells/plate. After 2 h, monolayers were washed once with growth medium and twice with PBS plus Ca2+ and Mg2+. The plates were transferred onto ice and 2 ml of lysis buffer (10 mM Tris⋅HCl, pH 7.0/10 mM NaCl/1.5 mM MgCl2/0.2% NP 40) was added to each plate and incubated for 10 min at 4°C. Subsequently, cells were removed with the aid of a rubber policeman, transferred to a Potter vessel, and homogenized by using 30 strokes at 500 rpm. At this point, an aliquot was taken to use as whole cell extract (total cpm) and the remaining material centrifuged at 1,000 × g for 20 min at 4°C. Radioactivity was determined in aliquots of the supernatant (cytosol) and the pellet (nucleus). The remaining supernatant was centrifuged at 100,000 × g for 2 h at 4°C. Thereafter, radioactivity was determined in aliquots of the supernatant (protein) and the pellet (pellet and membranes). Crude separation of nuclear proteins and genomic DNA was done by several steps of phenol–chloroform–isoamylalcohol extraction of the resuspended nuclei. The DNA-containing aqueous phase was reextracted several times, and radioactivity of the combined extracts was measured (DNA). Radioactivity of the remaining organic phase containing denatured nuclear proteins was determined as well (nuclear proteins). Additional estimation of radioactivity bound to DNA was done by measurement of genomic DNA prepared by the DNeasy Tissue Kit (Qiagen, Hilden, Germany). The indicated cpm were corrected for volume. Experiments were performed at least twice, and one representative experiment is shown in Results.
Results
Proliferation Studies.
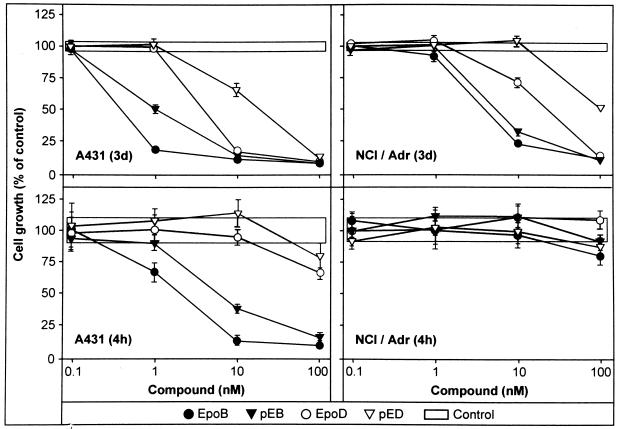
The effect of exposure times on the ability of epothilones to inhibit cancer cell growth in vitro was tested by using pED and pEB in comparison with the corresponding natural EpoB and EpoD (Fig. 1) and paclitaxel on a panel of human tumor cell lines. Tumor cells were exposed to different concentrations of compounds for 3 days or 4 h, respectively, and the extent of growth inhibition was measured 3 days after initiation of drug treatment (Fig. 2 and Table 1). The IC50 values were then determined for each experimental condition and compared with the corresponding IC50 for the 3-day exposure time. Table 1 indicates that for the 3-day exposure period, EpoB is five to six times more potent than paclitaxel on A431 and MCF7 tumor cells, whereas the new epoxide pEB is comparable to paclitaxel. NCI/Adr cells were not inhibited by paclitaxel up to 1 μM (data not shown), indicating very efficient resistance mechanisms in these cells, whereas all epothilones tested inhibited NCI/Adr cells growth, albeit most of them with diminished potencies compared with A431 cells. The olefins EpoD and pED exhibited similar inhibitory potencies on A431 and MCF7 cells, whereas NCI/Adr were less sensitive. Exposure of A431 and MCF7 carcinoma cells to EpoB and pEB for a 4-h period produced slightly diminished growth inhibitory effects compared with a continuous 3-day exposure (factor of 6 or 3, respectively), whereas for EpoD and pED, exposure times significantly above 4 h would be required to obtain significant growth inhibition with the compound concentrations used (Fig. 2). Furthermore, A431 cells responded to a 4-h treatment with paclitaxel with a 6- to 7-fold increase in the IC50. In contrast, the NCI/Adr carcinoma cell line requires exposure times above 4 h for all four compounds tested to reach inhibitory activities, indicating effective efflux mechanisms.
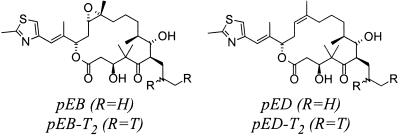
Figure 1.
Chemical structures of pEB and pED. Structures of epothilone analogs pED, pEB, and position of tritium labeling.
Figure 2.
Antiproliferative effects of epothilones. Compounds were either left continuously for 3 days or alternatively for 4 h, with two subsequent washes and addition of fresh growth medium. After 3 days, cell growth was determined by staining with crystal violet.
Table 1.
Antiproliferative effects of epothilones
| Cell type | Effects of epothilones on a 3-day
incubation period
|
Cell type | Effects of
epothilones on a 4-hr incubation
period
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Paclitaxel | EpoB | pEB | EpoD | pED | Paclitaxel | EpoB | pEB | EpoD | pED | ||
| A431 | 3.6 ± 0.6* (6†) | 0.5 ± 0.3 (11) | 1.5 ± 1.3 (3) | 12 ± 13 (3) | 24 ± 12 (3) | A431 | 24 ± 3.2 (3) | 3.0 ± 0.2 (3) | 4.0 ± 2 (3) | 64 ± 30 (2), >100 | >100 (3) |
| MCF7 | 3.3 ± 0.6 (3) | 0.6 ± 0.4 (22) | 3.4 ± 1.2 (3) | 19 ± 12 (4) | 38 ± 9 (3) | MCF7 | 16 ± 13 (3) | 1.9 ± 0.6 (3) | 6.2 ± 2 (3) | ni (2) | >100, ni (2) |
| NCI/Adr | ni‡ (2) | 3.5 ± 0.8 (13) | 4.0 ± 0.4 (3) | 48 ± 26 (3) | 77 ± 14 (3) | NCI/Adr | nd§ | >100 (2) | >100, ni (2) | >100 (2) | ni (2) |
Compounds were either left continuously for 3 days or, alternatively, for 4 h, with two subsequent washes and addition of fresh growth medium. After 3 days, cell growth was determined by staining with crystal violet.
IC50, nM (mean ± SD).
Number of experiments.
No inhibition measured up to 1,000 nM.
Not determined.
These data might lead to the conclusion that epoxides are more potent, because they may be retained inside cells more effectively compared to their corresponding olefin analogs and/or are removed less efficiently by efflux mechanisms. These results prompted us to perform the following experiments by using tritium-labeled pED, pEB, and, in some experiments, paclitaxel to investigate level, rate of uptake, release, and subcellular distribution of the compounds in human tumor cell lines.
Kinetics of Compound Uptake.
The kinetics of cellular uptake at two compound concentrations (70 and 3.5 nM) into two human tumor cell lines (A431 and NCI/Adr) was measured over a 0.5–4 h period (Table 2). In NCI/Adr cells, saturation levels were reached within 1 h for both compounds (pED and pEB) at 70 nM and in A431 cells, after 2 h. At saturation, there was a 5-fold difference in total uptake for pEB vs. pED in A431 and 8-fold in NCI/Adr cells, respectively.
Table 2.
Kinetics of uptake at two concentrations of radiolabeled compounds
| Kinetics
of radiolabeled compound uptake at 70 nM
|
Kinetics of
radiolabeled compound uptake at 3.5 nM
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time, h | pED
|
pEB
|
Time, h | pED
|
pEB
|
||||
| A431 | NCI/Adr | A431 | NCI/Adr | A431 | NCI/Adr | A431 | NCI/Adr | ||
| 0.5 | 7.5 ± 0.5* | 4.1 ± 0.3 | 23.9 ± 0.7 | 34.4 ± 1.0 | 0.5 | 116.4 ± 4.0 | 20.7 ± 1.1 | 0.7 ± 0.0 | 0.4 ± 0.0 |
| 1 | 9.7 ± 0.2 | 5.6 ± 0.5 | 30.8 ± 6.0 | 40.4 ± 2.3 | 1 | 114.1 ± 13.2 | 18.3 ± 0.5 | 0.9 ± 0.1 | 0.5 ± 0.0 |
| 2 | 11.8 ± 1.2 | 4.7 ± 0.1 | 47.0 ± 1.2 | 41.8 ± 2.9 | 2 | 157.1 ± 19.8 | 19.3 ± 0.3 | 1.3 ± 0.1 | 0.6 ± 0.0 |
| 3 | 11.3 ± 0.5 | 4.6 ± 0.4 | 48.6 ± 4.1 | 41.3 ± 2.4 | 3 | 130.5 ± 19.9 | 18.0 ± 0.6 | 1.3 ± 0.2 | 0.6 ± 0.0 |
| 4 | 12.3 ± 1.4 | 4.5 ± 0.3 | 50.8 ± 2.0 | 40.5 ± 1.0 | 4 | 170.9 ± 18.0 | 19.6 ± 1.2 | 1.4 ± 0.1 | 0.6 ± 0.0 |
Tumor cells were incubated for the indicated times with the indicated concentrations of tritiated compounds. After washing, cells were solubilized and the radioactivity measured and normalized to the cell numbers.
Mean ± SD (pmol/106 tumor cells).
When uptake of radiolabeled compounds was determined at a lower drug concentration (3.5 nM), saturation was reached for pED in A431 and pEB in both cell lines after 2 h. In contrast, NCI/Adr cells accumulated only very low levels of pED, and saturation was reached at the earliest time point measured (0.5 h). There was a 7-fold difference in uptake of both compounds by A431 cells, whereas in NCI/Adr cells, the difference was 28-fold.
From these data, we concluded that, irrespective of the drug concentration used, saturation of uptake was reached within 2 h, and this time point was chosen for subsequent experiments.
Uptake as a Function of Compound Concentration.
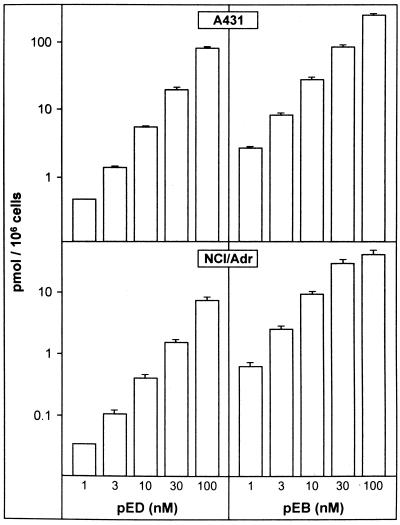
The dependency of uptake on compound concentration was tested. Radioactive compounds were used at 1, 3, 10, 30, or 100 nM and cell-bound radioactivity in A431 and NCI/Adr cells determined after a 2-h incubation period. Fig. 3 shows that no saturation can be achieved by these concentrations for pED in either cell line. Similarly, for pEB, no saturation was achieved over all concentrations in A431, whereas in NCI/Adr cells, it was reached between 30 and 100 nM of pEB. Therefore, different uptake or release mechanisms for epothilones are operative in NCI/Adr vs. A431 cells.
Figure 3.
Uptake of epothilones as function of compound concentrations. A431 and NCI/Adr tumor cells were exposed to compounds for 2 h.
In the following experiments, the labeled compounds were used at two concentrations, with 1 μCi, corresponding to 70 nM, or 0.05 μCi, corresponding to 3.5 nM of compounds, respectively.
Kinetics of Compound Release.
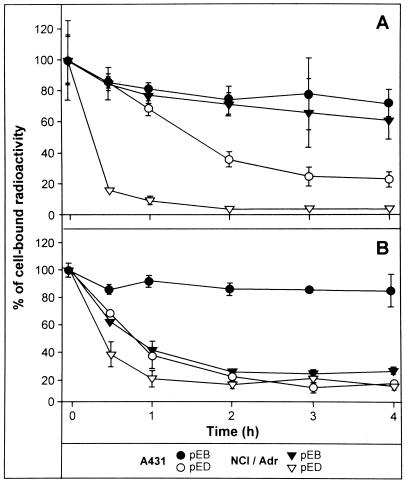
The reduced content of epothilones in NCI/Adr cells prompted us to test the kinetics of epothilone release from tumor cells in detail. A431 and NCI/Adr cells were labeled for 2 h with 70 nM (Fig. 4A) or 3.5 nM (Fig. 4B) of radiolabeled epothilones, washed, and the uptake determined (time point 0). Subsequently, the remaining radioactivity in cells was determined at several time intervals after removal of compounds and incubation of cells in compound-free culture medium. As shown in Fig. 4A, the efflux of radiolabeled compounds from both cell lines was more rapid for pED vs. pEB. In addition, there was a dramatic faster release of the olefin pED from NCI/Adr cells when compared with A431 already at early time points (0.5 h). In contrast, the efflux of the epoxide pEB was comparable in both cell lines, with 60–70% of compound still retained 4 h after drug removal. When A431 and NCI/Adr cells were loaded with 20 times lower epothilone concentrations, pED was again rapidly released from both cell types, and surprisingly, also pEB from NCI/Adr cells (Fig. 4B). In contrast, the epoxide pEB was retained within A431 cells to a similar extent as shown in Fig. 4A when higher compound concentrations were used.
Figure 4.
Kinetics of radiolabeled compound efflux from tumor cells. Human A431 and NCI/Adr tumor cells were exposed to 70 nM (A) or 3.5 nM (B) of compounds for 2 h, then washed, and remaining radioactivity in the cells estimated at the indicated time points.
Uptake of Compounds in the Presence of Verapamil.
The action of Verapamil on drug accumulation in cells is often used as evidence that efflux is mediated by the MDR1 p-glycoprotein. Thus, Verapamil was added at 10 μM together with the epothilones or paclitaxel, respectively, to A431 and NCI/Adr cells. Table 3 indicates that cellular uptake of paclitaxel, as expected, could be increased effectively in NCI/Adr cells at both compound concentrations used (70 and 3.5 nM), whereas there was no influence on A431 cells. These data indicate that different plasma membrane uptake or efflux mechanisms for paclitaxel are operative in NCI/Adr vs. A431 cells. Accumulation of pED was increased in both cell lines by Verapamil. In contrast, uptake of pEB was unaffected in both cell lines by Verapamil when 70 nM of compounds were used, whereas at 3.5 nM, uptake of pEB was unchanged in A431 and increased 2-fold in NCI/Adr cells.
Table 3.
Uptake of radiolabeled compounds in the presence of 10 μM Verapamil
| A431
|
NCI/Adr
|
|||
|---|---|---|---|---|
| − Verapamil | + Verapamil* | − Verapamil | + Verapamil | |
| Paclitaxel (70 nM) | 34.2 ± 0.8† | 7‡ | 2.2 ± 0.2 | 404 |
| pED (70 nM) | 19.0 ± 0.6 | 82 | 8.3 ± 0.8 | 306 |
| pEB (70 nM) | 58.7 ± 2.7 | 2 | 49.0 ± 1.1 | 5 |
| Paclitaxel (3.5 nM) | 3.3 ± 0.1 | −14 | 0.1 ± 0.0 | 188 |
| pED (3.5 nM) | 0.6 ± 0.0 | 174 | 0.2 ± 0.0 | 795 |
| pEB (3.5 nM) | 3.9 ± 0.1 | 22 | 4.7 ± 0.2 | 122 |
Tumor cells were incubated for 2 h with the indicated concentrations of tritiated compounds in the presence or absence, respectively, of 10 μM Verapamil. After washing, cells were solubilized and the radioactivity measured and normalized to the cell numbers.
10 μM Verapamil.
Mean ± SD (pmol/106 tumor cells).
Percent of additional uptake in the presence of Verapamil.
Subcellular Distribution of Compounds.
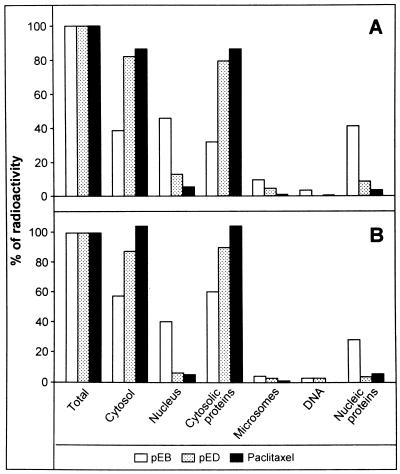
To investigate the enormous difference in total compound uptake of pED vs. pEB, we analyzed the subcellular distribution of both epothilones in A431 and NCI/Adr tumor cells in comparison to tritiated paclitaxel. Uptake of total radioactivity into the cell, into cytosol vs. nucleus, into protein vs. pellet plus membranes (subfractions of the cytosol), and into DNA vs. nuclear proteins (subfractions of the nucleus) was measured in tumor cells exposed to the compounds for 2 h. Fig. 5 indicates the relative distribution of radioactivity as percent of the total radioactivity taken up by A431 (Fig. 5A) and NCI/Adr cells (Fig. 5B) for each compound. Total uptake was comparable to previous experiments (Table 3) and is not shown here. In both cell lines, most of the labeled pED and paclitaxel (80%) were found in the cytosolic fraction and in the soluble part, whereas 5–15% was determined in the nucleus preparation and less than 5% in the microsomes. In contrast, only 40–60% of pEB was detected in the cytosolic fraction, whereas a comparable proportion (40–50%) of compound was identified in the nucleus preparation of both cell lines. Because the epoxide moiety could be chemically very reactive, we speculated that pEB might bind covalently to DNA. Thus, genomic DNA was prepared from radiolabeled compound-treated cells and found to be devoid of radioactivity, which instead was detected in the fraction of nucleic proteins in both cell lines. The nuclear uptake experiment was repeated by using lower pEB concentrations (3.5 nM), indicating accumulation in the nuclear fractions of A431 and NCI/Adr cells also at low intracellular compound concentrations (data not shown).
Figure 5.
Subcellular distribution of radiolabeled compounds. Human A431 (A) and NCI/Adr (B) tumor cells were exposed to 35 nM of radiolabeled pED, pEB, and paclitaxel for 2 h, then subcellular fractions were prepared and measured. Data are given as percent of total radioactivity incorporated per cell type.
Thus, the epoxide pEB strongly accumulates in the nuclear fraction, whereas neither pED nor paclitaxel exhibits significant nuclear accumulation.
Discussion
Among the natural epothilones, EpoB is the most potent antiproliferative agent, but it might not be the optimal candidate for cancer therapy in terms of therapeutic index (8). Thus, on the basis of their promising data demonstrating cure of paclitaxel-resistant human tumor xenografts by the olefin analog of EpoB (EpoD), Chou et al. (17) regard EpoD as their lead compound for potential development. In contrast, Novartis has chosen the natural product EpoB (9) and Bristol–Myers Squibb the lactame of EpoB (18) for Phase I clinical trials.
Our study suggests that the differences in growth inhibitory potency between epoxide- and olefin-bearing epothilone analogs may be based on different mechanisms of drug accumulation and subcellular distribution.
The concentrations of epothilones required for microtubule polymerization are in the micromolar range, with apparent Ki values of 0.4 μM (2), whereas profound inhibition of cell proliferation is already obtained in the lower nanomolar range. Epothilones, as well as paclitaxel, accumulate several hundred-fold inside cells compared to external medium concentrations (19). These data were taken as the explanation for the observed prolonged mitotic arrest of cells, even after short drug exposure periods.
This study shows that uptake of both classes of epothilones in tumor cells is rapid and reaches maximum levels within 2 h of drug exposure. However, withdrawal of compounds after 4 h results in different effects on tumor cell growth inhibition, which can be correlated with the presence or absence of the epoxide moiety in the epothilones.
We show here that epothilones can be principally recognized and exported by Verapamil-sensitive plasma membrane efflux pumps in A431 and NCI/Adr cells. The degree of export depends on the structure, olefin vs. epoxide analog, and also on the drug concentration accumulated in the cell. Thus, uptake of pED at 3.5 and 70 nM can be greatly enhanced in A431 and NCI/Adr cells in the presence of Verapamil. Moreover, release of pED from tumor cells loaded by exposure to 70 or 3.5 nM was rapid and nearly complete 60 min after withdrawal of the compound. In contrast, pEB was not released from A431 cells loaded at either 70 or 3.5 nM, whereas NCI/Adr cells retained the compound when loaded at 70 nM and exhibited rapid release when loading was performed at 3.5 nM. These data indicate that in NCI/Adr cells, high intracellular concentrations of pEB may block a plasma membrane efflux pump. This conclusion is further corroborated by the saturation kinetics of high pEB concentrations in NCI/Adr cells. Thus, different but Verapamil-sensitive transport mechanisms must be operative in the two cell lines. Furthermore, accumulation of paclitaxel was unaffected in A431 and dramatically increased in NCI/Adr cells in the presence of Verapamil.
In addition, we observed strong accumulation of the epoxide pEB but not the olefin pED or paclitaxel in the cell nucleus independent of the intracellular concentrations. First, we assumed that the more chemically reactive epoxide pEB could bind covalently to components in the nucleus not present in the cytoplasm. However, no significant radioactivity was associated with genomic DNA; it was found mainly in the fraction of nuclear proteins. The nuclear accumulation may contribute to the slow release of pEB from A431 and NCI/Adr cells. The mechanism of nuclear accumulation is not elucidated in detail. However, it is known for other cytotoxic drugs like doxorubicin (20) or daunorubicin (21) that prominent localization of these fluorescent compounds was observed in the nucleus of drug-sensitive tumor cells with a shift into the cytoplasm of drug-resistant counterpart cells.
In the present study, we used radiolabeled compounds, not allowing direct visualization of subcellular localization. Accumulation of tubulin in the perinuclear region has been described in paclitaxel- or epothilone-treated tumor cells (ref. 1; data not shown). In the immunohistochemical analysis, we had observed perinuclear accumulation with both EpoB and paclitaxel, whereas significant levels of radioactivity were found only in the nuclear fractions of pEB and not in paclitaxel-treated tumor cells. Therefore, we exclude the possibility that our nucleus preparations might be contaminated with microtubules radiolabeled with compounds.
Like most other antimicrotubule agents, including colchicine and vinblastine, paclitaxel binds to the β-subunit in the αβ-tubulin heterodimer. Paclitaxel-binding sites on β-tubulin were identified at the N-terminal 31 amino acids, at residues 217–231, and at Arg282 (22) of the protein. EpoA and EpoB are able to displace 3[H] paclitaxel from microtubules, implying that the target for these agents is tubulin itself (1). A classical competitive pattern was found for the inhibition of 3[H] paclitaxel binding to polymers formed with purified tubulin by using EpoA and EpoB (2). These data were interpreted as binding of EpoB and paclitaxel at the same site, although binding in overlapping sites or allosteric phenomena could not be excluded. In an extension of these studies by using cell sublines resistant to EpoB due to acquired β-tubulin mutations (β274Thr→Ile and β282Arg→Glu), a common pharmacophore shared by taxans and epothilones was demonstrated (23). Both map near the taxane-binding site in the atomic model of tubulin (24). However, strong binding to tubulin does not necessarily cause efficient inhibition of tumor cell growth, as has been demonstrated for a new class of microtubule-stabilizing substances (25).
The α and β subunits of tubulin consist of six isotypes of α and seven of β, each encoded by different genes (26). These isotypes have significant homology among them (83%) and differ mainly at the carboxy termini. However, they show some preferential expression for specific cell types. They are suggested to have functional specialization due to their great differences of assembly, dynamics, conformation, and ligand binding (27, 28).
Preferential binding of paclitaxel to the αβII-tubulin isotype was shown, and αβIII- or αβIV-purified tubulins were several-fold less sensitive to paclitaxel compared with unfractionated tubulin (29). Furthermore, removal of the αβIII isotype enhanced sensitivity of tubulins to paclitaxel (30). Currently we are looking for the preferential β-tubulin isotype partner, which is not known for epothilones.
Interestingly, subcellular distribution of tubulin isoforms seems to be different. A variety of studies had reported the presence of tubulin in the cell nuclei of 3T3 (31) and Chinese hamster ovary cells (32). In a recent study by Walss et al. (33), αβII-tubulin was identified as the major isotype in the nucleus of rat kidney mesangial cells. Furthermore, preferential localization in the nucleoli could be demonstrated, whereas the αβIII and αβIV isoforms were mainly detected in the cytoplasm. Thus, Walss et al. (33) hypothesized that, in addition to cytoplasmic tubulins, the nuclear αβII isoform may be a target for antitubulin compounds.
We cannot exclude the possibility that the epoxide compound pEB exerts effects unrelated to tubulin. However, our data lead us to conclude that the stronger antiproliferative effects of the epoxide compound pEB in comparison with pED may be due to more efficient accumulation in the tumor cell and the inhibition of sensitive specific functions of the αβII-tubulin isotype localized in the nucleus.
Acknowledgments
We thank Dr. Jürgen Gay for the tritiation experiments. Furthermore, the technical assistance of Stefan Barm, Klaus Burmeister, Klaus Cornelius, Frank Halwaß, Frank Kuczynski, Hue-Quan Lao, Carmen Wegner, Doreen Mitschke, Gerhard Neumann, Bodo Röhr, Katica Schwarz, Marion Slopianka, and Henk Zimmermann is warmly acknowledged.
Abbreviations
- EpoA
epothilone A
- EpoB
epothilone B, EpoD, epothilone D
- pEB
6-propyl-EpoB
- pED
6-propyl-EpoD
- MDR1
multidrug-resistant p-glycoprotein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bollag D M, McQueney P A, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Wood C A. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- 2.Kowalski R J, Giannakakou P, Hamel E. J Biol Chem. 1997;272:2534–2541. doi: 10.1074/jbc.272.4.2534. [DOI] [PubMed] [Google Scholar]
- 3.Sepp-Lorenzino L, Balog A, Su D-S, Meng D, Timaul N, Scher H I, Danishefsky S J, Rosen N. Prostate Cancer Prostatic Dis. 1999;2:41–52. doi: 10.1038/sj.pcan.4500282. [DOI] [PubMed] [Google Scholar]
- 4.Horwitz S B, Cohen D, Rao S, Ringel I, Shen H J, Yang C P. J Natl Cancer Inst Monogr. 1993;15:55–61. [PubMed] [Google Scholar]
- 5.Balog A, Meng D, Kamenecka T, Bertinato P, Su D-S, Sorensen E J, Danishefsky S J. Angew Chem Int Ed Engl. 1996;35:2801–2803. [Google Scholar]
- 6.Giannakakou P, Sackett D L, Kang Y K, Zhan Z, Buters J T, Fojo T, Poruchynsky M S. J Biol Chem. 1997;272:17118–17125. doi: 10.1074/jbc.272.27.17118. [DOI] [PubMed] [Google Scholar]
- 7.Su D-S, Meng D, Bertinato P, Balog A, Sorensen E J, Danishefsky S J, Zheng Y-H, Chou T-C, He L, Horwitz S B. Angew Chem Int Ed Engl. 1997;36:757–759. [Google Scholar]
- 8.Chou T-C, Zhang X-G, Balog A, Su D-S, Meng D, Savin K A, Bertino J R, Danishefsky S J. Proc Natl Acad Sci USA. 1998;95:9642–9647. doi: 10.1073/pnas.95.16.9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altmann K-H, Wartmann M, O'Reilly T. Biochim Biophys Acta. 2000;1470:M79–M91. doi: 10.1016/s0304-419x(00)00009-3. [DOI] [PubMed] [Google Scholar]
- 10.Nicolaou K C, Winssinger N, Pastor J A, Ninkovic S, Sarabia F, He Y, Vourloumis D, Yang Z, Li T, Giannakakou P, Hamel E. Nature (London) 1997;387:268–272. doi: 10.1038/387268a0. , and erratum (1997) 390, 100. [DOI] [PubMed] [Google Scholar]
- 11.Schinzer D, Limberg A, Bauer A, Böhm O M, Cordes M. Angew Chem Int Ed Engl. 1997;36:523–524. [Google Scholar]
- 12.Yang Z, He Y, Vourloumis D, Vallberg H, Nicolaou K C. Angew Chem Int Ed Engl. 1997;36:166–168. [Google Scholar]
- 13.Klar U, Skuballa W, Buchmann B, Schwede W, Hoffmann J, Lichtner R B. New Prospects in Anticancer Agents for the 21st Century. Am. Chem. Soc.; 2001. , in press. [Google Scholar]
- 14.Stachel S J, Chappell M D, Lee C B, Danishefsky S J, Chou T-C, He L, Horwitz S B. Organic Lett. 2000;2:1637–1639. doi: 10.1021/ol005932m. [DOI] [PubMed] [Google Scholar]
- 15.Stein U, Walther W, Lemm M, Naundorf H, Fichtner I. Int J Cancer. 1997;72:885–891. doi: 10.1002/(sici)1097-0215(19970904)72:5<885::aid-ijc28>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Kueng W, Silber E, Eppenberger U. Anal Biochem. 1989;182:6–19. doi: 10.1016/0003-2697(89)90710-0. [DOI] [PubMed] [Google Scholar]
- 17.Chou T-C, Zhang X-G, Harris C R, Kuduk S D, Balog A, Savin K A, Bertino J R, Danishefsky S J. Proc Natl Acad Sci USA. 1998;95:15798–15802. doi: 10.1073/pnas.95.26.15798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borzilleri R M, Zheng X, Schmidt R J, Johnson J A, Kim S-H, DiMarco J D, Fairchild C R, Gougoutas J Z, Lee F Y F, Long B H, Vite G D J. J Am Chem Soc. 2000;122:8890–8897. [Google Scholar]
- 19.Jordan M A, Wendell K, Gardiner S, Derry W B, Copp H, Wilson L. Cancer Res. 1996;56:816–825. [PubMed] [Google Scholar]
- 20.Coley H M, Amos W B, Twentyman P R, Workman P. Br J Cancer. 1993;67:1316–1323. doi: 10.1038/bjc.1993.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gervasoni J E, Fields S Z, Krishna S, Baker M A, Rosado M, Thuraisamy K, Hindenburg A A, Taub R N. Cancer Res. 1991;51:4955–4963. [PubMed] [Google Scholar]
- 22.Rao S, He L, Chakravarty S, Ojima I, Orr G A, Horwitz S B. J Biol Chem. 1999;274:37990–37994. doi: 10.1074/jbc.274.53.37990. [DOI] [PubMed] [Google Scholar]
- 23.Giannakakou P, Gussio R, Nogales E, Downing K H, Zaharevitz D, Bollbuck B, Poy G, Sackett D, Nicolaou K C, Fojo T. Proc Natl Acad Sci USA. 2000;97:2904–2909. doi: 10.1073/pnas.040546297. . (First Published February 25, 2000; 10.1073/pnas.040546297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nogales E, Wolf S G, Downing K H. Nature (London) 1998;391:199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 25.Klar U, Graf H, Schenk O, Röhr B, Schulz H. Bioorg Med Chem Lett. 1998;8:1397–1402. doi: 10.1016/s0960-894x(98)00226-1. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan K F. Annu Rev Cell Biol. 1988;4:687–716. doi: 10.1146/annurev.cb.04.110188.003351. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee A, Luduena R F. J Biol Chem. 1992;267:13335–13339. [PubMed] [Google Scholar]
- 28.Schwarz P M, Liggins J R, Luduena R F. Biochemistry. 1998;37:4687–4692. doi: 10.1021/bi972763d. [DOI] [PubMed] [Google Scholar]
- 29.Derry W B, Wilson L, Khan I A, Luduena R F, Jordan M A. Biochemistry. 1997;36:3554–3562. doi: 10.1021/bi962724m. [DOI] [PubMed] [Google Scholar]
- 30.Lu Q, Luduena R F. Cell Struct Funct. 1993;18:173–182. doi: 10.1247/csf.18.173. [DOI] [PubMed] [Google Scholar]
- 31.Menko A S, Tan K B. Biochem Biophys Acta. 1980;888:49–61. [Google Scholar]
- 32.Armbruster B L, Wunderli H, Turner B M, Reska I, Kellenberger E. J Histochem Cytochem. 1983;31:1385–1393. doi: 10.1177/31.12.6355287. [DOI] [PubMed] [Google Scholar]
- 33.Walss C, Kreisberg J I, Luduena R F. Cell Mot Cytosk. 1999;42:274–284. doi: 10.1002/(SICI)1097-0169(1999)42:4<274::AID-CM2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]