Abstract
Background
MicroRNA 194 is involved in the differentiation of various types of cells, such as adipose derived stem cells, human embryonic stem cells, and bone marrow mesenchymal stem cells. Previously, we found that miR-194 was highly expressed in the inner ear sensory patch and neurons in mice embryos. However, the role of miR-194 in the development of the inner ear and its underlying mechanism have not been elucidated yet.
Material/Methods
The expression level of miR-194 has been altered by using antisense morpholino oligonucleotides (MO) and synthesized miRNAs in zebrafish.
Results
We found that miR-194 was vastly expressed in the inner ear and central nervous system (CNS) in zebrafish. Loss of function of miR-194 could strongly affected the development of zebrafish embryos, including delayed embryonic development, edema of the pericardium, small head, axial deviation, delayed development of inner ear, closer location of two otoliths, delayed fusion of the semicircular canals, and abnormal otolith number in some cases. In addition, the behavior of zebrafish was also adversely affected with impaired balance and biased swimming route. Misexpression of miR-194 could strongly affected the development and differentiation of spiral ganglion neuron (SGN) in inner ear through Fgf4 in vitro. Similar results have also been observed that the overexpression and knockdown of miR-194 strongly disturbed the development and differentiation of the sensory patches and Statoacoustic ganglion (SAG) through Fgf4 in zebrafish in vivo. Our results indicated that miR-194 may regulate the development and differentiation of sensory patches and SAG through Fgf4.
Conclusions
Our data revealed a vital role of miR-194 in regulating the development and differentiation of the inner ear.
MeSH Keywords: Cell Dedifferentiation; Developmental Biology; Ear, Inner; MicroRNAs; Zebrafish
Background
The development of the inner ear is a complex process with multiple stages and steps, from primitive otic neuroepithelium to functionally mature hair cells afferent neurons, and supporting cells [1]. It requires precise and robust spatiotemporal patterns of gene expression [2]. A wide range of genes and signaling pathways have been identified to be crucial during this process, such as Notch [3], Wnt [4,5], Sox2 [6], Fgf [7], Hedgehog [8], and Atoh1 [6,9]. Although mechanisms controlling the precise and spatiotemporal patterns of gene expression are not well understood, microRNA (miRNA) gradually becomes one strong candidate [2].
MiRNAs are endogenous noncoding RNAs with 21–22 nucleotides. MiRNAs can restrict protein translation to regulate the behavior of cell differentiation, proliferation, and death in biological system by combining the 3′ untranslated region (3′-untranslated regions, 3-UTRs) with the target mRNAs [10]. MiRNAs are conserved between species, and nearly 1/3 of miRNAs have been detected in the inner ear [11]. It has been confirmed that miR-183 family [12,13], miR-18a, miR-15a, miR-34 have essential roles for the development and differentiation of inner ear [14]. In our previous study, we preliminarily found that miR-194 was highly expressed in the inner ear sensory patch and neurons in mice embryos [2]. However, the roles of miR-194 in the development of the inner ear and its underlying mechanism have not yet been elucidated. In the present research, we studied the miR-194 expression pattern in the zebrafish inner ear, as well as the regulation of sensory neuronal precursors in zebrafish in vivo and spiral neural cells from natal mice in vitro, to explore the mechanism of miR-194 in inner ear development and differentiation. Our study may provide potential direction for the treatment of sensorineural hearing loss.
Material and Methods
Zebrafish husbandry
Adult wild-type (AB) zebrafish were obtained from the Neurobiology Laboratory of the First Affiliated Hospital of Sun Yat-sen University.
One-cell step injection
We used 2 nL reagent morpholino oligonucleotide (MO) or miRNA mimics delivered into the cytoplasm of one-cell zebrafish embryos with MO (1 mM) or mimics (10 uM).
The sequences of the MO antisense oligos complementary to miR-194 was: TTC CAC ATG GAG TTG CTG TTA CAAG (GeneTools). A synthesized miRNA (mimics) was used for miR-194 overexpression, with the sequence: UGU AAC AGC AAC UCC AUG UGG (GenePharma). The embryos were divided into three groups: the MO group (injected with morpholino oligonucleotide), the NC (negative control, injected with Danieau’s buffer) group, and the MIMICS group (injected with miR-194 mimics).
Whole-mount in situ hybridization
Embryos were fixed in 4% paraformaldehyde (PFA), followed by dehydration in methanol. After rehydration with a graded methanol series and treatment with proteinase K (10 μg/mL), embryos were re-fixed. Embryos were then pre-incubated in hybridization buffer, followed by hybridization with locked nucleic acid-modified DNA probes for miRNA detection (10 nM; Exiqon) at 51°C overnight. After hybridization and high-stringency washing, embryos were blocked in 2% goat serum and 2 mg/mL bovine serum albumin and incubated with anti-digoxigenin conjugated to alkaline phosphatase antibody (Roche). Embryos were incubated with BCIP (5-bromo-4-chloro-3-indolyl phosphate, toluidine salt; 188 μg/mL) and NBT (nitro-blue tetrazolium chloride; 373 μg/mL; Roche) for visualization. The embryos could be observed with optical microscope.
Isolation and culture of spiral ganglion neurons in neonatal mice
Animal care and euthanization were conducted according to methods approved by the University of Sun Yat-sen Animal Care and Use Committee, following the Guidelines for the Care and Use of Laboratory Animals set forth by the National Institutes of Health. Cut down the head of neonatal C57BL/6 mice after sterilizing and anesthetizing. Isolate spiral ganglion neurons from the organ of Corti with dissecting microscope. Neurons were digested by adenosine deaminase (ADA) for 16 minutes at 37°C. After centrifuging, neurons were cultured at 37°C under 5% CO2 in DMEM/F12 supplemented with N2 supplement (1/100), B27 supplement (1/50), EGF (2 ug/mL), IFG-1 (2 ug/mL) and bFGF (2 ug/mL) in the absence of serum. All growth factors and supplements were obtained from Invitrogen.
Cell transfection
Spiral ganglion neurons were harvested, centrifuged and digested with 0.25% trypsin for 3 minutes at 37°C. The 5×105 single cell suspensions were plated in 6-well culture plates with cover slips on the bottom in DMEM/F12 containing N2 supplement (1/100), B27 supplement (1/50) in the absence of serum for 24 hours at 37°C. Spiral ganglion neurons were transfected with mimics (32 nM) or inhibitor (64 nM). Inhibitor or mimics was dissolved in water to make stock concentrations (20 uM) and diluted to working solution in transfection buffer (1 mL DMEM/F12, 100 uL opti-MEM, 5 uL RNAiMAX Reagent, 5 uL inhibitor or 2.5 uL mimics, Invitrogen) before injection.
Inhibitor and mimics targeting miR-194 were designed and synthesized by GenePharma. The sequence of the inhibitor antisense oligo complementary to miR-194 was: UCC ACA UGG AGU UGC UGU UACA. The sequence of the mimics was: UGU AAC AGC AAC UCC AUG CGGA. The sequence of the negative control was: UUC UCC GAA CGU GUC ACG UTT. The embryos were divided into three groups, the MO group, the NC group, and the MIMICS group, according to what they were injected with.
Cell climbing Immunofluorescence staining
Neurons on coverslips were fixed in 4% PFA and soaked in 0.5% Triton X-100 for 20 minutes. Samples were then incubated in blocking solution (5% bovine serum albumin). After that, samples were incubated in primary and secondary antibodies at 4°C overnight. Primary antibodies were as follows: anti-Sox2 (1: 400, Abcam), anti-Tuj1 (1: 400, Abcam). Secondary antibodies (1: 400, Invitrogen) were Alexa-488 or Alexa-594 donkey anti-mouse IgG and goat anti-rabbit IgG. Hochest 33342 (1: 800, Invitrogen) was used to mark nucleus.
RNA extraction and qRT-PCR
Total RNA was extracted from cultured cells using TRIzol reagent (Invitrogen). Reverse transcription was performed according to manufacture recommendation of M-MLV reverse transcriptase (Invitrogen). The real-time PCR cycle was 95°C for 10 minutes; 40 cycles at 95°C for 15 seconds, 60°C for 30 seconds, 72°C for 45 seconds. Primer sequences used for amplification are as follows:
Fgf4: Forward: TGG TGA GCA TCT TCG GAG TG,
Reverse: CGT AGG CGT TGT AGT TGT TGG;
Bmp4: Forward: CGT TAC CTC AAG GGA GTGG,
Reverse: GCG ACG GCA GTT CTT ATTC;
Sox2: Forward: TCC ATG ACC AGC TCG CAG AC,
Reverse: GCC TCG GAC TTG ACC ACAG;
Id3: Forward: TGT GCT GCC TGT CGG AACG,
Reverse: CCA CCT GGC TAA GCT GAG TG;
Prox1: Forward: CAG ATC ACG TTA CGG GAGT,
Reverse: GGA GTT CTT GTA GGC AGT TAG;
Cdkn1: Forward: TGG GTC TCA GGC AAA CTCT,
Reverse: CAG GTC GCT TCC TCA TCC;
Fzd1: Forward: TGA CGG CAC CAA GAC AGAG,
Reverse: GGC AAG GGA TGG CAT AAC TC.
Whole-mount Immunofluorescence staining
The procedure was almost the same with the Cell climbing immunofluorescence staining except that embryos were soaked in 0.5% or 1% Triton X-100 for one hour to four hours until otoliths were completely dissolved. Primary antibodies were as follows: anti-Sox2 (1: 400, Abcam), anti-HuC (1: 400, Invitrogen), anti-Islet1 (1: 200, Developmental Studies Hybridoma Bank), anti-α-tubulin (1: 400, Invitrogen), Hochest 33342 (1: 800, Invitrogen). Secondary antibodies (1: 400, Invitrogen) were Alexa-488 or Alexa-594 donkey anti-mouse IgG and goat anti-rabbit IgG. Embryos could be observed after washing in PBS with confocal microscopy (ZEISS, LSM710).
Synthesis of mRNA in vitro
Total RNA was extracted from 24 hour post fertilization (hpf) zebrafish embryos by TRIzol reagent (Invitrogen). Reverse transcription was performed according to manufacturer’s recommendation of M-MLV reverse transcriptase (Invitrogen). Polymerase chain reaction aiming to amplifying Fgf4 cDNA fragment was performed according to manufacture recommendation of PCR buffer (Roche). The primers specifically for Fgf4 were designed and synthesis by Invitrogen. The sequences were as follows: F: CGC GGA TCC GCC ACC ATG AGT GTC CAG TCG GCC CT, R: CCG GAA TTC TCA AAT TCT AGG CAA GAA AT. The PCR production was tested and purified by UV electrophoresis. The sequence was connected to plasmid. The Fgf4+ plasmid was amplification by E. coli. The Fgf4+ plasmid was lined by Bst1107I (Thermo). The Fgf4 mRNA was synthesized by the template of the linearized Fgf4+ plasmid with mMESSAGE mMACHINE® Kit (Invitrogen).
Results
MiR-194 intensely expressed in brain and inner ear of zebrafish
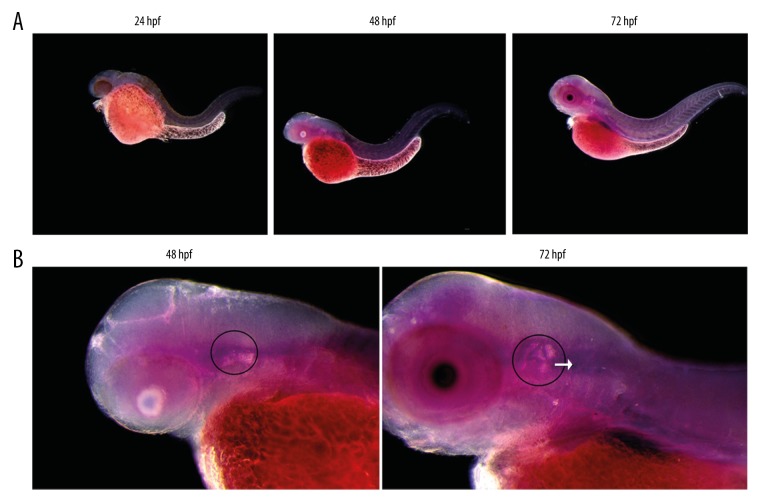
The expression of miR-194 was found in the embryos of the wild type zebrafish in 24 hpf, 48 hpf, 72 hpf by in situ hybridization. The expression of miR-194 mainly concentrated in brain tissue, sensory patches, and vestibulocochlear ganglion (VCG) of inner ear and the other parts were weak. The expression of miR-194 increased gradually from 24 hpf until 72 hpf (Figure 1).
Figure 1.
Expression of miR-194 in the embryos and inner ear of zebrafish. (A) Expression of miR-194 in the embryos of zebrafish from 24 to 72 hpf. (B) Expression of miR-194 in the inner ear of zebrafish. Inner ear was indicated by a black circle; the VCG was indicated by a white arrow. VCG – vestibulocochlear ganglion; hpf – hour post fertilization.
Altered level of miR-194 affected the development of embryo and inner ear
The effects of microinjection with MO or miRNA-194 mimics were confirmed by in situ hybridization. MO injection could successfully downregulate the level of miR-194 in MO group; while miR-194 mimics injection could elevate the level of miRNA-194 in mimics group (data not shown). The alteration of miRNA-194 lasted up to 72 hpf when the development of the inner ear was nearly mature.
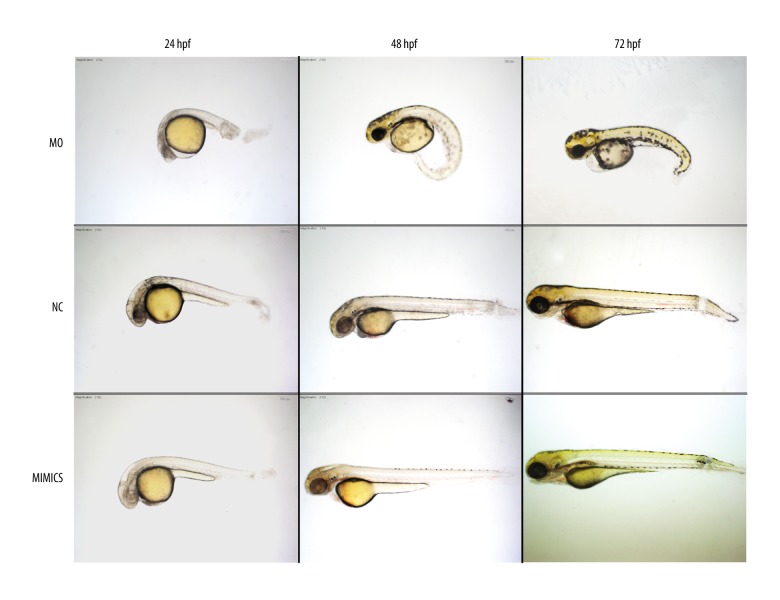
Decreased miR-194 in MO group could change the morphology of the embryos and slow down the embryonic development, causing edema of the pericardium, small head and axial deviation (Figure 2). It also led to delayed development of inner ear, closer location of two otoliths, delayed fusion of the semicircular canals, and abnormal otolith number in some cases (Figures 2 and 3). Moreover, the behavior was changed with impaired balance indicating by the biased swimming route.
Figure 2.
Altered expression of miR-194 affected the development of embryos from 24 to 72 hpf. In the MO group, the whole development of embryo slowed down, the edema of the pericardium, the small head, and the axis of the body were skewed. But there was no obvious change in the whole development and inner ear development pattern in the mimics group. MO – microinjection with morpholino oligonucleotide; NC – negative control; Mimics – microinjection with miR-194 mimics; hpf – hour post fertilization.
Figure 3.
Effect of altered expression of miR-194 on the development of inner ear and otolith. (A) Effect of altered expression of miR-194 on the development of inner ear from 24 to 72 hpf. A black circle was used to indicate inner ear. (B) Changing the expression of miR-194 affected the otolith. MO – microinjection with morpholino oligonucleotide; NC – negative control; Mimics – microinjection with miR-194 mimics; hpf – hour post fertilization.
Increased miR-194 had no obvious change in the development of the whole embryo and inner ear, except that the inner ear was larger and the number of otoliths was abnormal in some cases (Figures 2 and 3).
MiR-194 promoted differentiation of SGN precursor in vitro
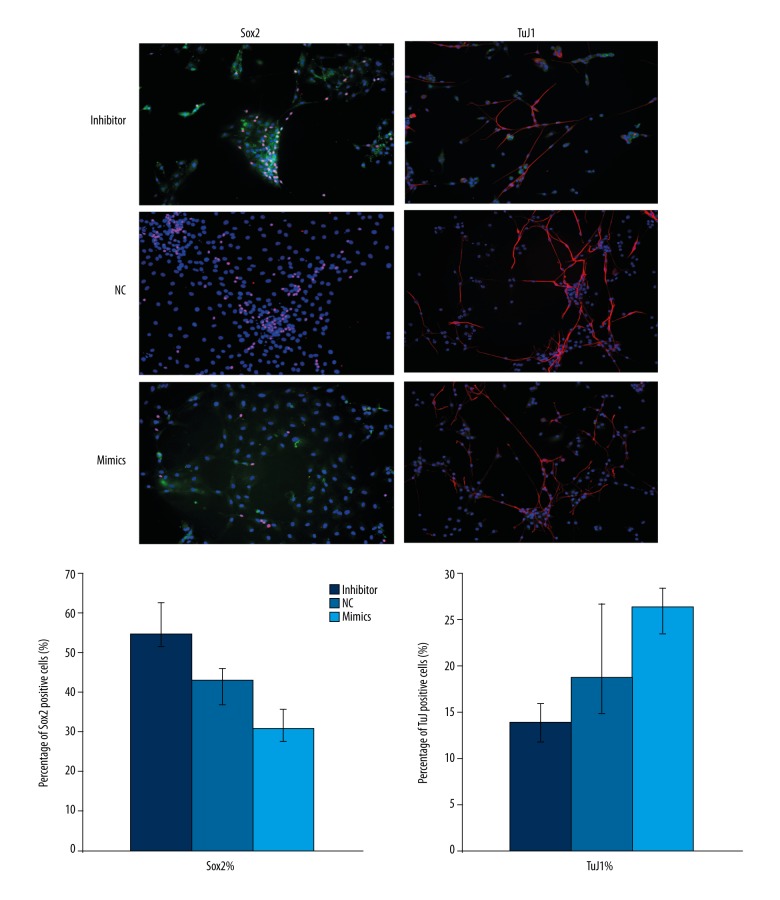
Sox2 was highly expressed in early sensory areas after specialization. Cells highly expressed Sox2 still had the characteristic of embryonic cells [8]. Some of SGN precursor in vitro still had pluripotency which could express Sox2. SGN precursors losing pluripotency and differentiating into mature neuron cells gradually formed synaptic connections and expressed mature neuronal marker TuJ1. Our results showed that the percentage of Sox2 positive cells was lower in the mimics group; while the percentage of TuJ1 positive cells was higher in the mimics group as compared with that in NC group (Figure 4). The results indicate that increased level of miR-194 could promote differentiation of SGN precursor in vitro.
Figure 4.
MiR-194 promoted differentiation of SGN precursor in vitro. The proportion of Sox2 and TuJ1 positive cells were measured using immunofluorescence staining. Red staining indicated Sox2 or TuJ1 expression; Green indicated successful transfection NC, inhibitor or miR-194 mimics; Blue indicated cell nucleus. NC – negative control.
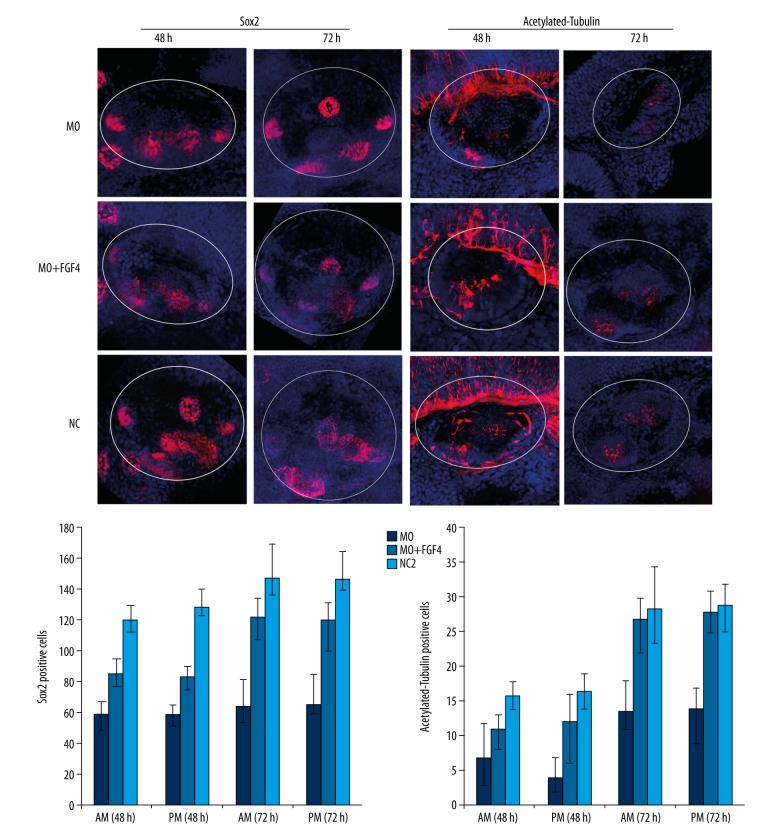
Development of sensory patches in the inner ear of zebrafish was regulated by miR-194
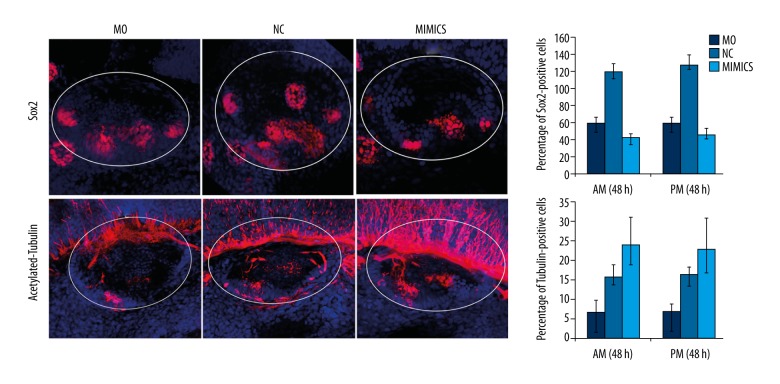
In the MO group, the relative spatial structure of the sensory patches of inner ear in zebrafish was abnormal. The posterior macula was ectopic to anterior cristae. The distance between the anterior and posterior macula was shortened. In the MIMICS group, significantly changes were not observed in the spatial structure of sensory patches in the inner ear as compared with the NC group (Figure 5). These results suggested that miR-194 was involved in the regulation the normal spatial structure of zebrafish sensory patches.
Figure 5.
MiR-194 promoted the differentiation of the sensory patches in zebrafish inner ear. The percentage of Sox2 (upper portion) and acetylated-tubulin (lower portion) positive cells were measured by in situ hybridization at 48 hpf. The white circle area indicated zebrafish inner ear. Red staining indicated Sox2 expression (upper portion) or acetylated-tubulin expression (lower portion); blue indicated cell nucleus. MO – microinjection with morpholino oligonucleotide; NC – negative control; Mimics – microinjection with miR-194 mimics; hpf – hour post fertilization.
In the MO group, the number of Sox2 positive cells decreased in the sensory patches, and the expression of Sox2 was prolonged in sensory cristae. Acetylated-tubulin has been reported to be mainly expressed in mature hair cells. Our results showed that the number of acetylated-tubulin positive cells was decreased in sensory patches in the MO group. In the MIMICS group, the number of Sox2 positive cells in both the sensory patches and sensory cristae was decreased, and the expression of Sox2 was shortened in both areas. The number of acetylated-tubulin positive cells was increased in the sensory patches (Figure 5). These results indicated that miR-194 promoted the differentiation of sensory patches in the zebrafish inner ear.
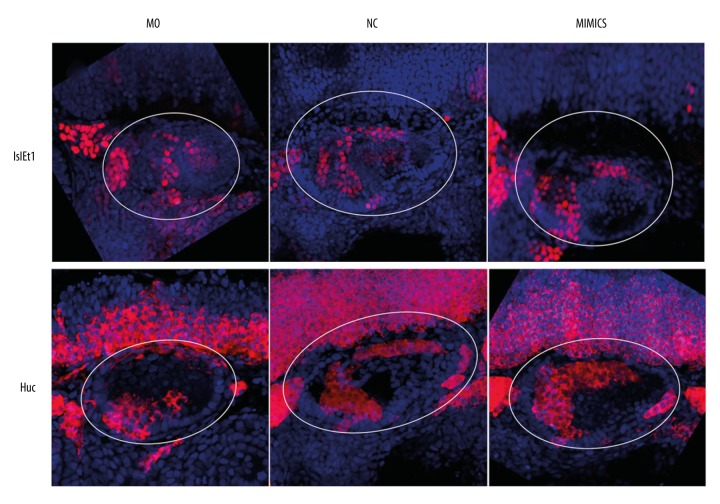
Differentiation of statoacoustic ganglion (SAG) in zebrafish was regulated by miR-194
In the MO group, the relative spatial structure of the statoacoustic ganglion (SAG) in zebrafish was abnormal. The SAG was located in the anteroventral without migrating to posteromedial area. In the MIMICS group, no significant changes were observed in the spatial structure of SAG in the zebrafish inner ear (Figure 6). Our results suggest that miR-194 participated in the regulation of the normal spatial structure of SAG in the zebrafish inner ear.
Figure 6.
MiR-194 promoted the development and differentiation and regulation of the normal spatial structure of SAG in zebrafish inner ear. The percentage of Islet1 (upper portion) and HuC (lower portion) were measured by in situ hybridization at 48 hpf. The white circle area indicated zebrafish inner ear. Red staining indicated Islet1 expression (upper portion) or HuC expression (lower portion); blue indicated cell nucleus. MO – microinjection with morpholino oligonucleotide; NC – negative control; Mimics – microinjection with miR-194 mimics; hpf – hour post fertilization; SAG – statoacoustic ganglion.
Islet1 was expressed in the SAG precursor; while HuC could be detected in the mature neuron cells. In the MO group, although the number of Islet1 positive cells decreased in SAG, the percentage of Islet1 positive cells was increased. The percentage of HuC positive cells was significantly decreased. In the MIMICS group, the proportion of Islet1 positive cells was decreased in SAG; while the proportion of HuC positive cells was increased (Figure 6). Collaboratively, these results indicated that miR-194 promoted the development and differentiation of SAG in the zebrafish inner ear.
miR-194 upregulated the level of Fgf4 in vitro
A variety of genes and signaling pathways are involved in the differentiation of SGN precursor. Here we selected seven genes including Fgf4, Prox1, Bmp4, Id3, P27, Sox2, and Fzd1. We found that the levels of Fgf4, Bmp4, and Fzd1 were increased after the overexpression of miR-194; while the levels of Sox2 and Id3 were decreased. Among all the seven genes, we found that the expression of Fgf4 was the most sensitive to the alteration of miR-194. The expression of Fgf4 was decreased by more than 80% in the inhibitor group; while Fgf4 was increased by more than nine times after overexpression of miR-194 in the MIMICS group, indicating that miR-194 could significantly upregulate the level of Fgf4 in vitro (Figure 7).
Figure 7.
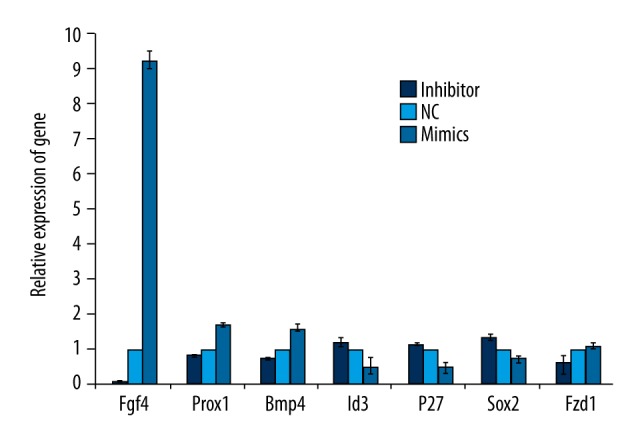
MiR-194 affects neuronal development related gene expression. Genes which promoted neuronal differentiation increased with the overexpression of miR-194. And genes which inhibit neuronal differentiation decreased with the overexpression of miR-194. Fgf4 gene expression was most sensitive to miR-194.
MiR-194 regulated the development and differentiation of sensory patches in the inner ear by regulating the expression of Fgf4
To further evaluate whether miR-194 regulates the development and differentiation of sensory patches in the inner ear through Fgf4, we injected Fgf4 mRNA together with MO by one-cell step into zebrafish fertilized eggs, named as the MO+FGF4 group. We found that the spatial structure of the sensory patches in the inner ear was partially corrected in the MO+FGF4 group as compared with the MO group. The posterior patch moved to the posteromedial position in the MO+FGF4 group. In addition, the distance between the anterior and posterior patches was also increased in the MO+FGF4 group (Figure 8).
Figure 8.
MiR-194 regulated the development and differentiation of inner ear sensory patches by modulating the expression of Fgf4. The percentage of Sox2 (left panel) and acetylated-tubulin (right panel) were measured by in situ hybridization at 48 and 72 hpf. The white circle area indicated zebrafish inner ear. Red staining indicated Sox2 expression (left panel) or acetylated-tubulin expression (right panel); blue indicated cell nucleus. MO – microinjection with morpholino oligonucleotide; NC – negative control; MO+FGF4 – microinjection with morpholino oligonucleotide and Fgf4; hpf – hour post fertilization.
The percentage of Sox2 and acetylated-tubulin positive cells was partially increased by injection of MO and Fgf4 mRNA in the MO+FGF4 group, compared to the MO group. Compared to the NC group, there were less Sox2 positive cells in the sensory patches and sensory cristae in the MO+FGF4 group. But the expression of Sox2 was prolonged in sensory cristae. There were less acetylated-tubulin positive cells in the sensory patches in the MO+FGF4 group. This gap lasted until 72 hpf. With the passage of time, the differences between the three groups became shortened.
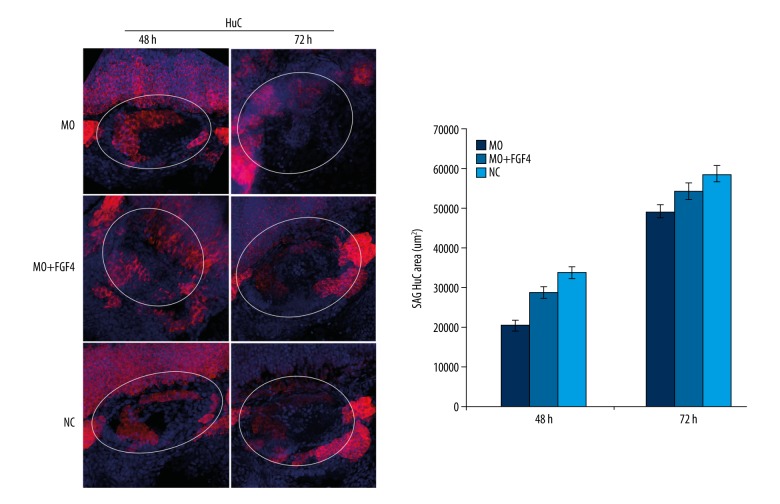
miR-194 regulated the development and differentiation of SAG in the inner ear by regulating the expression of Fgf4
To further evaluate whether miR-194 regulates the development and differentiation of SAG in the inner ear through Fgf4, we injected Fgf4 mRNA together with MO by one-cell step into zebrafish fertilized eggs, named as the MO+FGF4 group. As compared with the MO group, the spatial structure of the zebrafish inner ear SAG was partly corrected in the MO+FGF4 group, reflecting that SAG migrated to the posteromedial and began to form a comma shaped structure (Figure 9). Moreover, the percentage of HuC positive cells was partially increased in SAG after injection of Fgf4 mRNA and MO in the MO+FGF4 group, as compared with the MO group.
Figure 9.
MiR-194 regulated the development and differentiation of SAG in inner ear by regulating the expression of Fgf4. The percentage of HuC were measured by in situ hybridization at 48 and 72 hpf. The white circle area indicated zebrafish inner ear. Red staining indicated HuC expression; blue indicated cell nucleus. MO – microinjection with morpholino oligonucleotide; NC – negative control; MO+FGF4 – microinjection with morpholino oligonucleotide and Fgf4; hpf – hour post fertilization.
Discussion
In the present study, we demonstrated that miR-194 was vastly expressed in the inner ear and CNS in zebrafish, and played an important role in the development of zebrafish. Gain and loss of function of miR-194 had significantly influence on the development of zebrafish embryos and the inner ear. Loss of function of miR-194 could have strongly affected the development of zebrafish embryos, including delayed embryonic development, edema of the pericardium, small head, axial deviation, delayed development of inner ear, closer location of two otoliths, delayed fusion of the semicircular canals, and abnormal otolith number in some cases. In addition, the behavior of zebrafish was also adversely affected with impaired balance and biased swimming route after knockdown of miR-194. Moreover, we revealed for the first time that misexpression of miR-194 could strongly affected the development and differentiation of spiral ganglion neuron (SGN) in inner ear through Fgf4 in vitro. Similar results have also been observed that the overexpression and knockdown of miR-194 strongly disturbed the development and differentiation of the sensory patches and SAG through Fgf4 in zebrafish in vivo.
Roles of miR-194 in differentiation of various types of cells have been widely reported. MiR-194 has been reported to promote the differentiation of adipose derived stem cells into chondrocytes via Sox5 [15]. Increased miR-194 has also been revealed during the differentiation of human embryonic stem cells into hepatocytes, which may be through regulating the expression of YAP1 [16]. In mice, overexpression of miR-194 could promote the differentiation of bone marrow mesenchymal stem cells into osteoblasts via STAT1 [17,18]. However, the role of miR-194 in embryonic development has been less elucidated. In the present study, we revealed for the first time that the loss of function of miR-194 could strongly affected the development of zebrafish embryos, causing delayed embryonic development, edema of the pericardium, small head, axial deviation, delayed development of inner ear, delayed fusion of the semicircular canals, and abnormal otolith number and location, indicating the important role of miR-194 in embryonic development, especially in inner ear development in zebrafish.
Mammalian SGN could transmit the signal of hair cell to the auditory center, playing a vital role in the transmission of auditory information. Severe damage to the SGN could cause failure of signal transmission from hair cell to the brain auditory center, which results in the irreversible disease: sensorineural hearing loss. Mammalian SGN is difficult to regenerate after injury of hair cells. It has been reported that multiple genes have been involved in the development and differentiation of SGN. It has been reported that the bHLH family is able to promote the differentiation of SGN into mature neurons [19]. Sox10 participates in the migration of the auditory ganglion neurons [20] and Sox2 functions in maintaining the proliferation ability of neuron precursor cells [21]. In addition, the role of miRNAs is gradually revealed in the development and differentiation of SGN. miR-9 could maintain the characteristics of neural stem cells by inhibiting phosphorylation of signal transduction and activator of transcription 3 (STAT3) [22]. Zhao et al. reported that the miR-200 family could promote the differentiation of neuronal precursor cells by regulating Sox2 and Klf4 [23]. Previously, we have found that miR-124 promotes the directional differentiation of cultured inner ear neural stem cells, and promote the growth of neuronal axons [24]. In the present study, our data revealed that knockdown of miR-194 could cause a significant increase of Sox2-positive SGN precursor cells and decrease of TuJ1-positive cells. These results suggest that miR-194 could promote the directional differentiation of SGN precursors into neurons, and its absence may lead to the defect of neuronal precursor differentiation in vitro.
Neurons of the SAG originate from the otic placode, along with other cell types such as sensory hair cells and supporting cells in zebrafish. Like mammalian SGN, SAG is involved in the transmission of electrical signals from the basolateral surface of zebrafish hair cells to the auditory center. In this study, we found that miR-194 participated in the regulation of the normal spatial structure of SAG, and played an important role in promoting the development and differentiation of SAG. Considering the sensory patches, the posterior macula was ectopic to anterior cristae and the distance between the anterior and posterior sensory macula was shortened after knockdown of miR-194. This indicated that miR-194 was involved in the migration of sensory patches and controlled the establishment of the normal spatial structure. In addition, miR-194 could promote the differentiation of sensory patches in zebrafish inner ear.
The Fgf family plays an important role in the development and differentiation of the inner ear. It has been reported that Fgf family has been expressed in the otic placode [25], and regulats the differentiation of the otic placode together with the Wnt signaling pathway [26]. Abnormal expression causes deletion of the otic placode. The loss of Fgfr1 expression in the late stages of hair cell development and formation in the inner ear leads to a decrease in the number of sensory hair cells and supporting cells [27,28]. The Fgf family has played a role in inhibiting proliferation in the regeneration process of damaged utricle hair cells [29,30]. Fgf4 could promote the differentiation of embryonic stem cells into neurons after KDM7A activation [31]. In the present study, increased level of Fgf4 could partially rescue the abnormality caused by the knockdown of miR-194, with the accelerated inner ear development, accelerated differentiation of sensory patches and SAG, and improved spatial structure of inner ear.
Conclusions
The precise levels of miR-194 are essential in the development of zebrafish. Gain and loss of function of miR-194 have significant influence on the development of zebrafish embryos and the inner ear. Moreover, we revealed for the first time that misexpression of miR-194 could strongly affected the development and differentiation of mammalian SGN in inner ear through Fgf4 in vitro, and sensory patches and SAG through Fgf4 in zebrafish in vivo. Our results suggest that miR-194 might be a potential therapeutic candidate to promote mammalian SGN regeneration.
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China (award No. 81500802 and 81271076); the Natural Science Fund of Guangdong Province (award No. 015A030310072 and 2016A030310286); College Science Fund of the Second Affiliated Hospital, Guangzhou Medical University (award No. 2014c32)
Conflict of interest
None.
References
- 1.Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7:837–49. doi: 10.1038/nrn1987. [DOI] [PubMed] [Google Scholar]
- 2.Wang XR, Zhang XM, Zhen J, et al. MicroRNA expression in the embryonic mouse inner ear. Neuroreport. 2010;21:611–17. doi: 10.1097/WNR.0b013e328338864b. [DOI] [PubMed] [Google Scholar]
- 3.Wang GP, Chatterjee I, Batts SA, et al. Notch signaling and Atoh1 expression during hair cell regeneration in the mouse utricle. Hear Res. 2010;267:61–70. doi: 10.1016/j.heares.2010.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montcouquiol M, Crenshaw EB, 3rd, Kelley MW. Noncanonical Wnt signaling and neural polarity. Ann Rev Neurosci. 2006;29:363–86. doi: 10.1146/annurev.neuro.29.051605.112933. [DOI] [PubMed] [Google Scholar]
- 5.Dabdoub A, Kelley MW. Planar cell polarity and a potential role for a Wnt morphogen gradient in stereociliary bundle orientation in the mammalian inner ear. J Neurobiol. 2005;64:446–57. doi: 10.1002/neu.20171. [DOI] [PubMed] [Google Scholar]
- 6.Bermingham-McDonogh O, Rubel EW. Hair cell regeneration: Winging our way towards a sound future. Curr Opin Neurobiol. 2003;13:119–26. doi: 10.1016/s0959-4388(03)00018-7. [DOI] [PubMed] [Google Scholar]
- 7.Chapman SC, Cai Q, Bleyl SB, Schoenwolf GC. Restricted expression of Fgf16 within the developing chick inner ear. Dev Dyn. 2006;235:2276–81. doi: 10.1002/dvdy.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu X, Huang J, Feng L, et al. Sonic hedgehog (SHH) promotes the differentiation of mouse cochlear neural progenitors via the Math1-Brn3.1 signaling pathway in vitro. J Neurosci Res. 2010;88:927–35. doi: 10.1002/jnr.22286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bermingham NA, Hassan BA, Price SD, et al. Math1: An essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–41. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 11.Rudnicki A, Avraham KB. microRNAs: The art of silencing in the ear. EMBO Mol Med. 2012;4:849–59. doi: 10.1002/emmm.201100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weston MD, Pierce ML, Rocha-Sanchez S, et al. MicroRNA gene expression in the mouse inner ear. Brain Res. 2006;1111:95–104. doi: 10.1016/j.brainres.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Weston MD, Pierce ML, Jensen-Smith HC, et al. MicroRNA-183 family expression in hair cell development and requirement of microRNAs for hair cell maintenance and survival. Dev Dyn. 2011;240:808–19. doi: 10.1002/dvdy.22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman LM, Dror AA, Mor E, et al. MicroRNAs are essential for development and function of inner ear hair cells in vertebrates. Proc Natl Acad Sci USA. 2009;106:7915–20. doi: 10.1073/pnas.0812446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, Kang Y, Liao WM, Yu L. MiR-194 regulates chondrogenic differentiation of human adipose-derived stem cells by targeting Sox5. PLoS One. 2012;7:e31861. doi: 10.1371/journal.pone.0031861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung KH, McCarthy RL, Zhou C, et al. MicroRNA regulates hepatocytic differentiation of progenitor cells by targeting YAP1. Stem Cells. 2016;34:1284–96. doi: 10.1002/stem.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeong BC, Kang IH, Hwang YC, et al. MicroRNA-194 reciprocally stimulates osteogenesis and inhibits adipogenesis via regulating COUP-TFII expression. Cell Death Dis. 2014;5:e1532. doi: 10.1038/cddis.2014.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, He X, Wei W, Zhou X. MicroRNA-194 promotes osteoblast differentiation via downregulating STAT1. Biochem Biophys Res Commun. 2015;460:482–88. doi: 10.1016/j.bbrc.2015.03.059. [DOI] [PubMed] [Google Scholar]
- 19.Taylor SM, Alvarez-Delfin K, Saade CJ, et al. The bHLH transcription factor NeuroD governs photoreceptor genesis and regeneration through delta-notch signaling. Invest Ophthalmol Vis Sci. 2015;56:7496–515. doi: 10.1167/iovs.15-17616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao Y, Reiprich S, Wegner M, Fritzsch B. Targeted deletion of Sox10 by Wnt1-cre defects neuronal migration and projection in the mouse inner ear. PLoS One. 2014;9:e94580. doi: 10.1371/journal.pone.0094580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou C, Yang X, Sun Y, et al. Comprehensive profiling reveals mechanisms of SOX2-mediated cell fate specification in human ESCs and NPCs. Cell Res. 2016;26:171–89. doi: 10.1038/cr.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Z, Ma X, Hsiao TH, et al. A high-content morphological screen identifies novel microRNAs that regulate neuroblastoma cell differentiation. Oncotarget. 2014;5:2499–512. doi: 10.18632/oncotarget.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandey A, Singh P, Jauhari A, et al. Critical role of the miR-200 family in regulating differentiation and proliferation of neurons. J Neurochem. 2015;133:640–52. doi: 10.1111/jnc.13089. [DOI] [PubMed] [Google Scholar]
- 24.Jiang D, Du J, Zhang X, et al. miR-124 promotes the neuronal differentiation of mouse inner ear neural stem cells. Int J Mol Med. 2016;38:1367–76. doi: 10.3892/ijmm.2016.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haddon C, Lewis J. Early ear development in the embryo of the zebrafish, Danio rerio. J Comp Neurol. 1996;365:113–28. doi: 10.1002/(SICI)1096-9861(19960129)365:1<113::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 26.Wright KD, Mahoney Rogers AA, Zhang J, Shim K. Cooperative and independent functions of FGF and Wnt signaling during early inner ear development. BMC Dev Biol. 2015;15:33. doi: 10.1186/s12861-015-0083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ono K, Kita T, Sato S, et al. FGFR1-Frs2/3 signalling maintains sensory progenitors during inner ear hair cell formation. PLoS Genet. 2014;10:e1004118. doi: 10.1371/journal.pgen.1004118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pirvola U, Ylikoski J, Trokovic R, et al. FGFR1 is required for the development of the auditory sensory epithelium. Neuron. 2002;35:671–80. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 29.Ku YC, Renaud NA, Veile RA, et al. The transcriptome of utricle hair cell regeneration in the avian inner ear. J Neurosci. 2014;34:3523–35. doi: 10.1523/JNEUROSCI.2606-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkinson PJ, Huarcaya Najarro E, Sayyid ZN, Cheng AG. Sensory hair cell development and regeneration: Similarities and differences. Development. 2015;142:1561–71. doi: 10.1242/dev.114926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang C, Xiang Y, Wang Y, et al. Dual-specificity histone demethylase KIAA1718 (KDM7A) regulates neural differentiation through FGF4. Cell Res. 2010;20:154–65. doi: 10.1038/cr.2010.5. [DOI] [PubMed] [Google Scholar]