Abstract
Octocorals are one of the most ubiquitous benthic organisms in marine ecosystems from the shallow tropics to the Antarctic deep sea, providing habitat for numerous organisms as well as ecosystem services for humans. In contrast to the holobionts of reef-building scleractinian corals, the holobionts of octocorals have received relatively little attention, despite the devastating effects of disease outbreaks on many populations. Recent advances have shown that octocorals possess remarkably stable bacterial communities on geographical and temporal scales as well as under environmental stress. This may be the result of their high capacity to regulate their microbiome through the production of antimicrobial and quorum-sensing interfering compounds. Despite decades of research relating to octocoral-microbe interactions, a synthesis of this expanding field has not been conducted to date. We therefore provide an urgently needed review on our current knowledge about octocoral holobionts. Specifically, we briefly introduce the ecological role of octocorals and the concept of holobiont before providing detailed overviews of (I) the symbiosis between octocorals and the algal symbiont Symbiodinium; (II) the main fungal, viral, and bacterial taxa associated with octocorals; (III) the dominance of the microbial assemblages by a few microbial species, the stability of these associations, and their evolutionary history with the host organism; (IV) octocoral diseases; (V) how octocorals use their immune system to fight pathogens; (VI) microbiome regulation by the octocoral and its associated microbes; and (VII) the discovery of natural products with microbiome regulatory activities. Finally, we present our perspectives on how the field of octocoral research should move forward, and the recognition that these organisms may be suitable model organisms to study coral-microbe symbioses.
Keywords: Microbiome, Holobiont, Symbiodinium, Immunity, Bacteria, Fungi, Gorgonians, Octocoral, Soft coral
Background
The Octocorallia (Haeckel, 1866) is a subclass within the Anthozoans (Ehrenberg, 1834, phylum Cnidaria (Verrill, 1865)) and is comprised of soft corals, including sea fans and sea whips (order Alcyonacea (Lamouroux, 1812)), sea pens (order Pennatulacea (Verrill, 1865)), and blue corals (order Helioporacea (Bock, 1938)). The main characteristic of Octocorallia that distinguishes them from the Hexacorallia (Haeckel, 1896), such as the reef-building Scleractinia (Bourne, 1900), is the eightfold symmetry of their polyps (Fig. 1), compared to the sixfold symmetry in their relatives. To date, over 3500 species belonging to approximately 378 octocoral genera from 55 families [1, 2] have been described worldwide. Some of those have been famous since the Classical Antiquity: for example, the beautifully red skeleton of the precious red coral Corallium rubrum (Fig. 1) has been extensively used for jewelry and other art crafts [3]. Octocorals are ubiquitous organisms of the sea, having been recorded at all depths, from littoral waters down to the deep-sea abyss, from the tropics to the arctic regions, and in all the world’s oceans, although the highest diversity of octocorals is observed in the Indo-Pacific (reviewed in [4]). While octocoral distribution is significantly influenced by various environmental factors [5], the presence of octocorals in nearly all benthic marine habitats indicates the adaptive nature of this taxonomic group compared to other cnidarian taxa. In some geographical areas, reef ecosystems have even undergone a phase shift from a hard coral-dominated state towards a higher abundance of soft corals (Table 1).
Fig. 1.

Octocorals as habitat providers. a–c Gorgonians form three-dimensional structures in a range of environments, such as a Leptogorgia sarmentosa on sandy bottoms, b Corallium rubrum on the walls and ceilings of caves and overhangs, and c Paramuricea clavata forming “marine animal forests” on rocky substrates. Close-up of the gorgonian colonies of d Paramuricea clavata with open (front) and retracted (back) polyps and e C. rubrum, showing their eightfold body symmetry. f Colonies can consist of thousands of polyps forming large three-dimensional structures. Together colonies can form vast “forests” providing refuge and habitat for numerous marine organisms (photos a and f by Eric Béraud and photos b–e by Sergio Rossi)
Table 1.
Reported coral community shifts from reef-building scleractinian corals to soft corals and their causes
| Location | Cause | Shift in octocoral cover | References | ||
|---|---|---|---|---|---|
| From (%) | Up to (%) | ||||
| Red Sea | North and Center | Crown of thorns starfish Anthropogenic pollution |
5–10 | 30–50 | [245–247] |
| South | Storms, bleaching Anthropogenic pollution |
4 | 30 | [248, 249] | |
| Caribbean | Florida Keys | Bleaching | 6 | 14 | [159] |
| US Virgin Islands | Diseases | [250] | |||
| Indian Ocean | Madagascar/Seychelles | Bleaching | 1.3 | 2 | [251] |
| Seychelles | Bleaching and anthropogenic pollution | [245] | |||
| Pacific Ocean | Malaysia | Bleaching Dynamite fishing Crown of thorns starfish |
1 | 30 | [252] |
| Indonesia | Anthropogenic pollution Dynamite fishing |
7 | 13 | [253, 254] | |
| Australia | Bleaching Crown of thorns starfish |
10 | 16 | [255] | |
| Fiji | Anthropogenic pollution | N/A | N/A | [256] | |
Octocorals are important foundational members of the benthic community. Through the formation of three-dimensional structures, they provide structural complexity to ecosystems and thereby refuge and habitats to a rich fauna (Fig. 1). While all octocorals are suspension feeders, relying upon currents to have access to food (reviewed in [6]), some octocorals also live in a mutualistic association with phototrophic zooxanthellae (dinoflagellates from the genus Symbiodinium). Although these zooxanthellate octocorals are restricted to the euphotic zone, they significantly contribute to the primary productivity of the shallow coastal ecosystems [7]. Azooxanthellate octocorals, however, rely solely on heterotrophic feeding and generally populate dark and deep environments, where they can develop very dense populations providing biomass and structural complexity [8, 9]. Because of their abundance, octocorals play a major role in the benthic-pelagic coupling and the energy transfer between plankton and benthos as they capture large quantities of plankton and thereby regulate the primary and secondary productions of the coastal food chains [6].
As all multicellular organisms, corals (encompassing both the Octocorallia and Hexacorallia) are holobiont entities, forming intricate and complex interactions with a range of microbes, including dinoflagellates, fungi, bacteria, archaea, and viruses [10]. These microbial symbionts play active roles in the health (e.g., nutrient supply, protection against pathogens) and adaptive response (e.g., toxin degradation) of the host to environmental changes [11, 12]. In zooxanthellate corals, endosymbiotic photosynthetic dinoflagellates (Symbiodinium) are the main food providers to their coral host, via the transfer of carbon rich compounds acquired through photosynthesis, as well as the recycling of nitrogen and phosphorus through the host catabolic wastes [13]. In corals, bacterial symbionts have been implicated in several other services, such as nitrogen fixation [14], sulfur cycling [15], or antibiotic production to exclude pathogens [16]. Maintaining a multi-functional microbial community is therefore essential to holobiont fitness. Recent studies have shown that corals have a “core microbiome” [17], composed of microbes that are consistently associated with a host species, as well as transient microbes whose presence depends on local conditions [18–20]. In case of environmental stress, such as rising sea water temperatures, changes in the resident microbial community composition and function may occur and lead to the occurrence of transient pathogens and to the emergence of disease [21]. Although the composition of the coral microbiota has been studied extensively under a range of environmental and experimental conditions, the diverse functions of the bacteria within the coral holobiont are still largely unknown.
The majority of scientific studies and reviews on the subject has focused on the reef-building scleractinian corals [21–23] and has shown that the holobiont is a community of Dinoflagellata, bacteria, fungi, Archaea, and viruses. Octocoral-microbe interactions have comparatively received relatively little attention, with only a limited number of studies having addressed their associated fungal and bacterial communities. In addition, most studies have used culture-based methods, which are relatively limited as only few microbes are cultivable, before next-generation sequencing techniques became more affordable in recent years, allowing higher resolution of the composition of octocoral-associated microbial community. Because of the significant advances made in the octocoral microbiome field in recent years and the relevance of these findings for our general understanding on the structure, function, and evolution of coral-microbe symbioses, a comprehensive assessment is warranted.
In this review, we outline the recent discoveries and current knowledge regarding (I) the octocoral-Symbiodinium mutualism, (II) the diversity and function of microbes (including fungi, viruses and bacteria) associated with tropical, temperate, and cold-water octocorals, and (III) the structure and stability of the microbial assemblages and remarkable dominance of a few bacterial species that suggest a close evolutionary history. We will also address (IV) the potential for microbiome regulation by the host and (V) the octocoral immune system in case of (VI) the occurrence of infections and diseases. Lastly, we will discuss (VII) the potential application of natural products derived from octocoral holobionts. The aims of this review were to summarize the latest achievements and to highlight future research directions to build a mechanistic understanding of how coral health is connected through microbial processes to its surrounding environment.
The algal symbiont Symbiodinium
Many octocoral species live in mutualistic association with unicellular algae (dinoflagellate of the genus Symbiodinium), also commonly called zooxanthellae. It is well-known now that these coral symbionts translocate carbon-based photosynthates to satisfy their host’s nutritional needs [13] as well as other nutrients such as nitrogen and phosphorus acquired from seawater or recycled from the host catabolic wastes [24]. By coupling this autotrophic nutrition, with the opportunistic heterotrophic feeding of the host (prey capture), many corals can thrive in nutrient-poor environments, also called oceanic deserts [25].
In octocorals, Symbiodinium can be acquired via vertical transmission (maternal inheritance) or horizontal transmission (environmental acquisition) [26], although the incidence of vertical transmission seems to be higher than for scleractinians [27]. Octocorals harbor five of the nine distinct phylogenetic Symbiodinium clades (called A to I) known to live in symbiosis with different organisms [28] (Fig. 2). Generally, the diversity of octocoral-associated Symbiodinium is higher in the tropics compared to temperate regions [29, 30] and the highest diversity is found on the Great Barrier Reef [29, 31]. The majority of octocorals investigated so far, however, harbored only a single algal clade, showing geographical clustering patterns based on the dominant Symbiodinium types (Fig. 2). For example, Clade C is dominant in the Pacific Ocean and the Red Sea [29, 30], whereas Caribbean octocorals are dominated by Clade B [30, 32] and Mediterranean octocorals by the temperate Clade A [26]. Interestingly, these associations are also rather stable over time and space compared to scleractinian corals, even after thermal stress and bleaching [28, 33–35]. The stability of these interactions may be due to parental effects in the establishment of this mutualistic symbiosis. For example, offspring of Briareum asbestinum has been found to contain several symbiont types early on in the symbiosis, but ultimately engaged in a mutualistic relationship with the Symbiodinium phylotype that was also dominant in the parental colonies [36]. As this specificity was observed regardless of the environmental conditions, it brings into question the adaptability of the octocoral-Symbiodinium mutualism under changing climate conditions [36]. The prominent zooxanthella genotype may thus also exclude other genotypes attempting to enter the association, possibly through faster growth rates [37], or higher services provided to their host. Multiple strains of each clade of Symbiodinium were, however, found within a single octocoral host [29, 38, 39], with a high specificity between host and symbiont lineages [39] and a high degree of connectivity between Symbiodinium populations [40].
Fig. 2.

Relative abundance of octocorals harboring specific clades of Symbiodinium in different geographical areas. A large number of octocoral species is azooxanthellate and does not possess algal symbionts
The octocoral-Symbiodinium association has received far less attention than those established with scleractinian corals. Therefore, there are critical gaps in our understanding of the functional and ecological significance of these symbioses. For example, the nutritional exchanges between the two partners, the trophic contribution of the symbionts to the energetic requirements of their host, and the stability of the symbiosis during environmental stress are still poorly understood in octocorals and deserve future attention.
Due to the paucity of zooxanthellate octocorals (compared to the azooxanthellate) both in the Indo-Pacific Ocean and temperate seas [29, 41] (Fig. 2), there is an impression that octocorals are heterotrophic species, relying on plankton and detrital material for their basic metabolism, growth and reproduction. However, there are more than 51 zooxanthellate octocoral species in the Caribbean [42], and zooxanthellate species also dominate the octocorals of the Southern Red Sea [43], suggesting that, in some locations at least, the association of octocorals with Symbiodinium can be mutualistic, providing a nutritional advantage. Productivity of zooxanthellate corals is usually estimated via the photosynthesis:respiration (P:R) ratio calculated over a daily cycle. A P:R > 1 indicates that the holobiont acquires more photosynthetic organic material than it consumes and can therefore rely on autotrophy for its energetic needs. The first few studies which have assessed the rates of photosynthesis and respiration of zooxanthellate octocorals measured very low rates of primary productivity compared to scleractinian corals, both in the temperate [44] and tropical [7, 45] areas. It was also observed that the presence of Symbiodinium increased metabolic costs, and thereby respiration rates [46], indeed suggesting that these octocorals have to rely on both auto-and heterotrophy to sustain their metabolic needs. Subsequent studies, performed on a greater number of species, however, showed that octocoral primary productivity depends on the environment, the polyp activity, the Symbiodinium clade identity, and the host morphology. For example, the P:R ratio tends to be the lowest in summer for tropical species (due to photoinhibition of the symbionts’ activity) [47], while it is the highest in summer for temperate areas, which are light limited during the other seasons [44, 48, 49]. For the same environment, the P:R ratio is also linked to the surface area:volume ratio (SA:V ratio). For example, sea fans (e.g., Gorgonia ventalina) are the most autotrophic octocorals due to their broad leaf-like morphology and small polyps (i.e., a high SA:V ratio), allowing efficient light exposure to the zooxanthellae and therefore maximized photosynthesis [50]. On the contrary, massive octocorals, with big polyps (e.g., Plexaurella fusifera and other sea rod species), are more suited to capturing plankton and particulate organic matter. Isotopic experiments with 13C-labeled inorganic carbon indeed showed that such big polyp hosts do not benefit from Symbiodinium autotrophy, due to low photosynthate translocation rates by these symbionts. As such, the host-Symbiodinium relationship in these octocorals is more commensal than mutualistic, at least for carbon, as previously observed in other host-microbe symbioses [51]. Overall, the negative phenotypic correlation observed between polyp size and carbon translocated from zooxanthellae to host suggests that there is an evolutionary trade-off between heterotrophic and autotrophic modes of nutrition. Finally, Baker et al. [50] found evidence that Symbiodinium specificity increases with holobiont productivity: generalist hosts (host with different symbionts) had lower productivities than specialist hosts (host with a specific symbiont type). Even if symbionts do not supply carbon compounds to their octocoral host, they can still be important for the acquisition of other essential nutrients such as nitrogen and phosphorus [45, 52].
As for all corals hosting algal symbionts, thermal stress (abnormally cold or warm temperatures) may lead to bleaching, i.e., the expulsion of the zooxanthellae, often due to an overproduction of reactive oxygen species (ROS) and increased oxidative stress [53, 54]. For example, high thermal anomalies induced extensive bleaching and mortalities of octocorals in the Pacific and the Caribbean in 1998, but also in 2005 and 2010 in the Florida Keys and wider Caribbean [53, 55]. The loss of zooxanthellae induces host starvation, when the host actively relies on photosynthates to sustain its metabolism. As such, it has been observed that species with large polyps, and/or a facultative symbiosis, whose nutrition is not derived exclusively from the symbionts, will bleach more easily than species forming an obligate association with their symbionts and receiving a large amount of photosynthates [50]. In the Caribbean, as many species have an obligate association with their symbionts, they were shown to be more resistant to temperature-induced bleaching than their scleractinian counterparts (reviewed in [56]). Instead of bleaching, symbiont migration into the stolon has been observed in some octocoral species, however, with a significant increase in ROS and an impairment of the photosynthesis [57–59]. Nevertheless, despite these physiological perturbations, symbionts retained some capacity for photosynthesis even after completing migration into the stolons.
Fungi
Despite the impacts of fungal disease on gorgonian populations [60–62], relatively few studies have investigated the fungal community associated with soft corals. Identification and characterization of fungal isolates have shown the consistent associations of various fungal species and genera with octocorals around the world. Particularly, Aspergillus spp. and Penicillium spp. have been commonly isolated from Gorgonia ventalina in the Caribbean [63, 64], Leptogorgia spp. in the Eastern Pacific [65], and numerous octocorals in Singapore [66] and the South China Sea [67]. Other common fungal associates of octocorals belong to genera Cladosporium [63–66], Tritirachium [63–66], Nigrospora [65, 67], and Fusarium [65–67]. Local environmental conditions, however, appeared to affect fungal community compositions [63, 67], primarly showing differences in the abundances of the most common fungal associates. While the functional ecological roles of these fungi are unknown, some possess potent antibacterial and/or antifungal activity and have been suggested to play a role in holobiont health and microbiome regulation [67]. One of the most notorious fungi, the putative aspergillosis pathogen Aspergillus sydowii, was found on both healthy and diseased gorgonians, although it was absent in some diseased colonies [63, 64]. As such, this fungus may in fact be an opportunist rather than a primary disease-causing pathogen. A. sydowii as well as nearly all other fungal isolates were initially considered terrestrial microbes, and some data suggests that these fungi could be derived from terrestrial run-off as (1) the host-associated fungal community does not differ from the seawater community and (2) the offshore fungal seawater communities resemble a diluted nearshore community [63]. One of the limitations in studies on octocoral-associated fungi has been the use of culture-based techniques, as the culture media used have been shown to be a major factor in the isolation of fungal associates (e.g., whereas some media yield the highest number of isolates, other media recover the highest number of species [66, 67]). Therefore, it is of utmost importance to employ culture-independent techniques, such as next-generation internal transcribed spacer (ITS) amplicon sequencing, to further characterize the entire diversity of fungi associated with octocorals. The lack of comprehensive fungal reference databases, however, currently limits these efforts.
Viruses
Research on coral-associated viruses is still in its early stages [68] and only two studies on the viromes of the Gorgonia ventalina have been published to date [69, 70]. The main viral groups found on gorgonians are phages of heterotrophic bacteria and cyanobacteria, but also double stranded DNA-viruses from the Phycodnaviridae family [69]. A more recent study found that this viral family was, however, not very abundant, in contrast to the phages, but also revealed members of the Parvoviridae, Totiviridae, and Circoviridae families [70]. While phages infect the bacteria and may be important regulators of the coral-associated bacterial communities, the role of the other viruses in the holobiont is unclear. Phycodnaviruses are known to infect eukaryotic algae and have been found in both healthy and bleached corals [71], as well as Symbiodinium cultures [71, 72]. However, their increased abundance in bleached corals [73] implicates them in the destruction of the algal symbiont Symbiodinium. Totiviridae use fungi (or protozoans) as their host and may be important in fungal diseases that have significantly affected G. ventalina populations, potentially by impacting the virulence of the fungal pathogen. Circoviridae are also commonly present in corals, particularly in diseased colonies [74], but their role is still unknown. Parvoviridae are known to infect numerous marine animals and infections can be asymptomatic, but also cause significant mortality. Their role in G. ventalina, however, remains to be elucidated. While two studies have identified some of the main viral groups within the octocoral virome, nothing is known about their role in holobiont functioning. As the role of viruses in coral holobiont health is becoming increasingly recognized, further studies on octocoral viromes and the role of viruses and phages in microbiome regulation and disease is warranted. One of the main challenges in virome research, however, is that the amount of viral nucleic acids present in samples is generally too low for sequencing library construction, requiring prior amplification of the viral genomic material. Although nucleic acid extraction protocols and whole genome amplification (WGA) may introduce biases (e.g., exclusion or overrepresentation of certain viral families), recent technological advancements have resulted in coral virome generation protocols that minimize such biases [75]. Optimizing and applying these protocols to octocorals will be the next phase, to better understand the role that viruses play in the holobionts of octocorals.
Bacteria
Bacterial communities associated with octocorals have received significantly more attention than fungi and viruses, particularly in the Caribbean and the temperate waters around Europe, where octocoral populations have been significantly impacted by disease outbreaks (discussed in the next section). Generally, the bacterial richness and diversity in octocorals is lower when compared with those in scleractinian corals [76–78], which could make them more suitable model organisms for studying the function and evolution of coral–microbe symbioses. Most studies on tropical and deep-sea octocorals, however, have each focused on different hosts, and the most comprehensive datasets currently available focus on temperate gorgonians residing in the Mediterranean Sea. Therefore, we will summarize the findings from studies on these temperate gorgonians and discuss the commonalities and differences with their tropical and deep-sea relatives when information is available.
In the Mediterranean Sea, studies have focused on the iconic precious red coral Corallium rubrum (family Coralliidae (Lamouroux, 1812), sub-order Scleraxonia (Studer, 1887)) and the soft gorgonians from sub-order Holaxonia (Studer, 1887) belonging to the genera Paramuricea (family Plexauridae (Gray, 1859)), Leptogorgia, and Eunicella (family Gorgoniidae (Lamouroux, 1812)). The assessment of spatial and temporal differences in the gorgonian-associated bacterial communities has greatly facilitated our understanding on which bacteria compose the “core microbiome” and are likely essential to the holobiont. In-depth analyses of all Holaxonia species studied to date have revealed that their bacterial assemblages are highly dominated by Proteobacteria. For example, bacteria belonging to the Oceanospirillales genus Endozoicomonas can make up to over 96% of an octocoral’s bacterial assemblage [19, 20, 76, 78–82]. In addition, bacterial associates consistently found on various temperate Gorgoniidae are (in order of relative abundance) Cellvibrionales BD1-7 (previously Alteromonadales), Mycoplasma, Aquimarina, Granulosicoccus, and Vibrio species [19, 20, 76], while Paramuricea clavata was found to harbor a significant number of bacteria belonging to the candidate phylum NPL-UPA2 [19]. Interestingly, the bacterial assemblages of C. rubrum are quite unique within the phylum Cnidaria, being primarily composed of Spirochaetales, Oceanospirilalles family ME2 and Parcubacteria, and only a minor contribution of Endozoicomonas [20, 83]. While our knowledge on the composition of the octocoral microbiota has steadily increased, the exact role of these bacteria within the holobiont is currently unknown. Based on the functions of related bacteria and the recent whole genome sequencing of a few species, it has, however, been suggested that they are involved in (1) the acquisition and provision of nutrients, for example, through nitrogen fixation, carbon, nitrogen, and sulfur cycling, the synthesis of amino acids as well as aiding in food digestion, and (2) the regulation of the composition of the microbiota through the secretion of antibiotics and occupying functional niches to prevent the entry of pathogens. Below, we will describe the various bacterial taxa commonly found in the microbiota of healthy octocorals (Table 2).
Table 2.
Overview of the bacteria most commonly found within octocoral holobionts. Taxonomy of the bacteria and which octocorals they associate with and their potential function are listed
| Phylum | Class | Order | Family | Genus | Octocoral host | Potential function | Ref. |
|---|---|---|---|---|---|---|---|
| Actinobacteria | Actinobacteria | Propionibacteriales | Propionibacteriaceae | Propionibacterium |
Anthothela spp. Corallium rubrum Paramuricea spp. |
Unknown –Zooxanthellate corals: coral–Symbiodinium symbiosis |
[20, 82, 93, 94, 121] |
| Bacteroidetes | Flavobacteriia | Flavobacteriales | Flavobacteriaceae | Aquimarina |
Antillogorgia elisabethae
Eunicella spp. Leptogorgia sarmentosa |
Nutrient cycling Nitrogen cycling (denitrification) Carbon cycling (chitin degradation) Sulfur cycling (sulphate reduction) Microbiome regulation |
[20, 89, 101] |
| Chlamydiae | Chlamydiae | Chlamydiales | Simkaniaceae | Candidatus Fritschea |
Eunicella spp. Leptogorgia sarmentosa |
Unknown | [20, 76] |
| Parcubacteria | – | – | – | – | Corallium rubrum | Unknown | [20, 83] |
| Proteobacteria | Alphaproteobacteria | Rickettsiales | Rickettsiales incertae sedis |
Candidatus Lariskella |
Eunicella spp. Leptogorgia sarmentosa |
Unknown | [20, 76] |
| Gammaproteobacteria | Cellvibrionales | Spongiibacteraceae | BD1-7 clade |
Corallium rubrum
Eunicella spp. Leptogorgia sarmentosa |
Unknown | [20, 76] | |
| Oceanospirillales | Hahellaceae | Endozoicomonas |
Antillogorgia elisabethae
Corallium rubrum Erythropodium caribaeorum Eunicella spp. Eunicea fusca Gorgonia ventalina Leptogorgia sarmentosa Lobophytum pauciflorum Paramuricea clavata Plexaura sp. Sarcophyton sp. Sinularia flexibilis |
Nutrient acquisition Nitrogen cycling Carbon cycling Sulfur cycling Amino acid synthesis Microbiome regulation |
[19, 20, 76, 77, 83, 89–93, 257] | ||
| ME2 | – | Corallium rubrum | Unknown | [20, 83] | |||
| Vibrionales | Vibrionaceae | Vibrio |
Corallium rubrum
Eunicella sp. |
Putative pathogen (V. shiloi) Food digestion (V. gigantis-related) |
[19, 20, 77, 105, 113–115] | ||
| Chromatiales | Granulosicoccaceae | Granulosicoccus |
Eunicella spp. Leptogorgia sarmentosa |
Unknown | [20, 76] | ||
| Spirochaetae | Spirochaetes | Spirochaetales | Spirochaetaceae | Borrelia | Corallium rubrum | Unknown | [20, 83] |
| Spirochaeta |
Anthothela spp. Corallium rubrum Lobophytum pauciflorum Muricea spp. |
Unknown | [20, 83, 91, 103, 121] | ||||
| Leptospiraceae | Leptospira | Corallium rubrum | Unknown | [20, 83] | |||
| Tenericutes | Mollicutes | Mycoplasmatales | Mycoplasmataceae | Mycoplasma |
Cryogorgia koolsae
Eunicella spp. Leptogorgia sarmentosa Muricea spp. Pennatula phosphorea Plumarella superba Pseudoplexaura porosa Pteroeides spinosum |
Unknown Commensal feeding on captured prey |
[19, 20, 102–106] |
| Entomoplasmatales | Entomoplasmatales incertae sedis | Candidatus Hepatoplasma |
Eunicella spp. Leptogorgia sarmentosa Muricea spp. |
Unknown –Potentially copepod prey symbionts |
[20, 103] |
Endozoicomonas (order Oceanospirillales, family Hahellaceae) is commonly associated with a diverse range of marine organisms [84–88] and appears to be also one of the main constituents of the holobionts of gorgonians [19, 20, 77, 89, 90] and other octocorals [91–93] in the tropics, as well as in Antarctic waters, but is absent in some [94]. Because of its common association and the observed localization of these bacteria in aggregates within the tissues of corals [95] and possibly gorgonians [96], there appears to be an intimate biological integration between Endozoicomonas and corals. Many studies have tried to understand the role of Endozoicomonas in holobiont health (reviewed in [97]), providing indications that it may be involved in essential processes for holobiont functioning, such as nutrient acquisition (nitrogen and carbon recycling, methane and sulfur cycling, synthesis of amino acids) and bacterial community regulation via secondary metabolite production and competitive exclusion. In contrast to most other octocorals, the dominant Oceanospirillales members in the red coral Corallium rubrum microbiome belong to the family ME2 (up to 20%). Although their function is still unknown, their taxonomic relationship may indicate a similar role as Endozoicomonas [20, 83].
Cellvibrionales BD1-7 [98] are believed to be oligotrophs that may use light to generate ATP via proteorhodopsin proton pumps as an alternative energy source for mixotrophic growth [99, 100], but as they are the second most abundant bacterial taxon in Mediterranean gorgonians, they likely provide significant benefits to the holobiont. Another genus specialized for survival in oligotrophic conditions and commonly associated with a tropical [89] and various temperate gorgonians [20] is Aquimarina. Genome analysis of an Aquimarina symbiont isolated from Eunicella labiata [101] has revealed that it possesses a remarkable capacity to cycle nutrients: nitrogen (denitrification), sulfur (assimilatory sulphate reduction), and carbon (chitin degradation). In addition, it has a large arsenal of genes related to defense as well as for the production of antimicrobial compounds. Overall, this indicates that Aquimarina may play a role in nutrient acquisition and cycling, and microbiome structuring. However, the importance of these generally low abundant bacteria [20] for holobiont health remains to be investigated.
While Mycoplasma has generally been considered an intracellular parasite, it has been suggested that they are mutualists or commensals in temperate and deep-sea gorgonians [19, 20, 102–104] and sea pens [105], where they can be found in high abundance. Mycoplasma spp. may not be exclusive to soft corals from these environments, as they were recently also found in two tropical species [106]. In the cold-water scleractinian coral Lophelia pertussa, detailed studies on its Mycoplasma associates showed that they were in fact located extracellularly next to the spirocysts, suggesting that they opportunistically benefit from hemolymph leaking from prey captured by the animal, without affecting host health [107]. However, whether the octocoral-associated Mycoplasma have a similar role remains to be seen, as phylogenetic analysis showed that they form a different cluster from those associated with L. pertusa [102], and even closely related species may not perform the same functional roles. The origin and function of Hepatoplasma, a candidate genus within the Tenericutes, is also unclear. Although present in the microbiota of a number of temperate gorgonian species [20, 103], it may originate from planktonic arthropod prey as members of this genus are ectosymbionts of isopods [108, 109]. Granulosicoccus and members of the candidate genera Lariskella and Fritschea are also commonly found on temperate gorgonians [20, 76] and other cnidarians [110–112], but no functions have been identified yet.
One of the more striking findings across various studies has been the isolation and consistent presence of Vibrio bacteria in the microbiome of octocorals, including gorgonians [19, 20, 77, 113–115] and sea pens [105]. Although some Vibrio spp. are mutualistic, many have been implicated in disease, including outbreaks affecting gorgonians in the Mediterranean as well as tropical reef-building corals. Indeed, sequences matching the coral pathogen V. shiloi were present year-round in healthy specimens of various Mediterranean gorgonians [20], suggesting that it may be an opportunist rather than a specialized pathogen. However, not all gorgonian-associated Vibrio may be pathogens. For example, a Vibrio sp. that is a relatively low abundant but common (and in some cases core) member of the bacterial assemblages of nearly all investigated Mediterranean gorgonians and the red coral [20] is most closely related to Vibrio gigantis. This putative symbiont in Mediterranean clams [116] and sea cucumbers [117] likely aids its host in food digestion and belongs to the “Splendidus” clade that harbors both pathogenic and non-pathogenic Vibrio spp. Analysis of the genome of a V. gigantis-related bacterium (99.8% identity) isolated from Eunicella verrucosa suggested that it is indeed likely a generalist and opportunistic commensal symbiont [113].
Spirochaetes have received relatively little attention in the field of coral microbial ecology, probably due to their low abundance in tropical hard corals [17, 118, 119], cold-water corals [120], sea pens [105], and deep-sea soft corals [102, 104]. Recently, their potential relevance in coral holobiont health was, however, recognized when the bacterial communities of the red coral Corallium rubrum were found to be consistently composed of up to 70% Spirochaetales, taxonomically assigned to the genera Spirochaeta, Borrelia, and Leptospira [20, 83]. Since then, high abundance of Spirochaeta has been observed in the temperate gorgonian Muricea californica (up to 64%) [103], deep-sea Anthothela spp. [121], and the tropical soft coral Lobophytum pauciflorum (~ 43%) [91], while Leptospira-related sequences are commonly found in most Mediterranean gorgonians [20]. Despite their ubiquity and high abundance in at least a few soft coral species, the importance of these bacteria in holobiont functioning is still unknown. The order Spirochaetales contains many pathogens, but various species are known mutualists aiding in food digestion and fixation of nitrogen [122] and carbon [123] into bioavailable nutrients for the host. Even so, the role of Spirochaetales in octocoral holobionts remains unclear.
Another interesting feature of the microbiota of C. rubrum, is the presence of members of the phylum Parcubacteria. These bacteria represent up to 10% of the bacterial assemblages of the red coral [20, 83], but have thus far not been described as a symbiont of macro-organisms. In fact, members of this largely unknown phylum have been found primarily in anoxic conditions [124]. Genomic studies have indicated that Parcubacteria have a severely limited metabolic capacity [125] and likely rely for most of their nutrients on their host. Despite this reliance, their specialized lifestyle appears to be of a non-parasitic symbiotic nature, but the benefits to the holobiont are far from clear.
In contrast, Actinobacteria have been found in numerous studies on gorgonians, particularly from the deep sea [20, 82, 93, 94, 102, 121, 126, 127]. Recently, Actinobacteria from the Propionibacterium genus were implicated in the scleractinian coral-Symbiodinium symbiosis [22], but the presence of Propionibacterium in the absence of Symbiodinium in these gorgonians suggests their role may be different. Bacteroidetes, particularly Cytophaga and Flavobacteriia, [20, 91, 93, 104, 126, 128] may be important in the carbon cycling. These generally low abundant, but ubiquitous, gorgonian bacterial symbionts may aid in the degradation of complex organic molecules, such as the chitin from the exoskeleton of zooplankton [127].
While many studies have investigated the microbes living in association with octocorals and found numerous different taxa, our speculation on their function is based on their phylogeny and extrapolation of their role in the environment or other host organisms to the coral holobiont. While initially used, culture-based techniques have often provided significantly different assessments of bacterial community composition compared with culture-independent techniques, rarely picking up the dominant species and often overestimating the number of Vibrio spp. [77, 114, 115, 126]. However, recent culture and isolation efforts, and subsequent whole genome analysis of coral-associated bacteria has greatly facilitated our understanding of some potentially important octocoral symbionts, such as Endozoicomonas euniceicola and E. gorgoniicola [129], Aquimarina [101], Pseudobacteriovorax antillogorgiicola [130], and a Vibrio sp. [113]. Besides, certain characteristics are commonly assigned to certain taxa that may not always be true, for example, members of the genus Vibrio are often considered pathogens, but many Vibrio spp. may in fact be commensals or even mutualists. Resolving the function of the microbes within the coral holobiont should be our current priority, because only then will we truly increase our understanding on coral-microbe symbioses.
Dominance in microbial assemblages
An interesting aspect in the octocoral microbiota is that it is often dominated by a few core microbiome operational taxonomic units (OTUs). This was observed initially in the temperate gorgonian Paramuricea clavata, whose bacterial communities were dominated (up to 91%) by one Endozoicomonas OTU [82], while other OTUs of this genus were present at low abundance. These results were confirmed later with similar observations made in the tropical octocorals Lobophytum pauciflorum [91] and Erythopodium caribaeorum [93] and several other Mediterranean gorgonians [19, 20]. In fact, this was not only true for Endozoicomonas, but also for NPL-UPA2 in P. clavata, the Spirochaeta in L. pauciflorum, and all main taxa in Corallium rubrum (Spirochaetales genera, Parcubacteria, and Oceanospirillales ME2) [20, 83]. Overall, this shows that octocoral hosts appear to have a preference for particular species from different taxa, but still harbor a large pool of very low abundant species (e.g., in Mediterranean gorgonians 669 of the 1512 OTUs were Endozoicomonas, but over 99% were very low abundant). These highly structured microbiota compositions suggest strong host-driven control of its microbial partners. While the relevance of maintaining such a high diversity of bacteria that are closely related to the main representative of a taxon at a low abundance is unclear, it may allow the host to change its main symbiont to a related species/strain that performs better under certain conditions to maintain holobiont physiological functions. There may be some indications of this principal of “symbiont shuffling” in the microbiota of Eunicella verrucosa and Leptogorgia sarmentosa at disturbed and undisturbed locations [20]. However, this has thus far only been shown to occur in hard corals, which changed their Symbiodinium endosymbionts in response to thermal stress to more heat-tolerant types [131].
Spatial and temporal variability in associated microbiota
Currently, it is still difficult to assess the stability of the healthy octocoral microbiota and identify the most important core microbial symbionts, because most studies to date have focused on a limited number of species, sampled at one time point and/or from one geographical location. Spatial and temporal surveys of gorgonians from the Mediterranean have shown very little variation between relatively undisturbed locations and over time [19, 20, 81–83], even at the 97% OTU level. In fact, most of the variation observed could be attributed to changes in the abundance of core microbes [19, 20, 83]. In one study, however, Endozoicomonas transiently disappeared from the Paramuricea clavata-associated microbiota and was replaced by Paenibacillus and other bacteria from various taxa over a large geographical area at one time point [82]. It is unclear what may have caused this major shift as neither environmental anomalies nor any adverse health effects were observed. Interestingly, the microbial assemblage returned to an Endozoicomonas-dominated state again the next season, showing the selection for Endozoicomonas by the host. In contrast, significant changes in the microbiota of Gorgonia ventalina were observed during and following a thermal anomaly in 2010 [132]. Although the main taxa remained dominant, clear patterns were difficult to discern. Whether this shift adversely affected holobiont functioning or is a case of acclimation to thermal stress is unknown.
Studies on spatial microbiome variability are crucial to investigate the core microbiome. In addition, they have shown some interesting patterns in both gorgonians [19, 20, 83] as well as reef-building scleractinian corals [18], revealing that in addition to (1) the core microbiome, there are (2) locally stable microbial associates (LSMA; microbes consistently associated with a coral at a given location in addition to the core microbes) and (3) transient microbes. While the core microbiome is stable at all times, the composition of the LSMA consortium is different at each location, suggesting that adjustments in the octocoral microbiota could be a form of phenotypic plasticity that allows acclimation to local conditions. The relative stability of the bacterial communities in most octocorals suggests they are under strong host control, but the potential of microbiota plasticity in octocorals is unknown. In the Mediterranean, it appears that L. sarmentosa has a more flexible microbiome, while it may be more strictly defined in Eunicella species [19, 20]. To what extend microbiota plasticity allows a species to inhabit a larger range of environmental conditions remains to be investigated.
Disturbances and acclimation
Analyses of the bacterial community have also revealed potential local impacts of anthropogenic origin (e.g., pollution, sedimentation, and mechanical damage) on gorgonian microbiome composition [19, 20, 78, 79]. In P. clavata, human impacts were found to result in a decrease in abundance of the main Endozoicomonas OTU, which may have opened up niches to be colonized by pathogenic Vibrio spp. [78]. Reduced Endozoicomonas abundances have generally been considered characteristic of stress in corals [112, 133–136]. Disturbances in the microbial assemblages of other Mediterranean species, showing a reduced contribution of the core microbiome but higher diversity including potential pathogens, were recently also attributed to polluted freshwater influxes from rivers and municipal sewage or submarine ground water discharges [19]. However, a more recent study nuanced these results finding that the local impacts are highly host species-dependent and raising questions about what may constitute a “healthy” microbiota [20]. Specifically, they found that the abundance of the LSMA and core microbes, in particular the most abundant Endozoicomonas, was significantly reduced in E. verrucosa at a site with high anthropogenic and terrestrial impacts compared to a relatively undisturbed site, but the exact opposite pattern was found in L. sarmentosa [20]. The fact that the abundance of this Endozoicomonas was differentially affected between two sympatric host species at the “disturbed” location shows that its viability or competitive potential was not affected by the environmental conditions, but was rather likely under host control. As such, care should be taken when linking the composition of coral-associated microbial assemblages to stress as differences may also represent acclimation and not all bacteria belonging to a taxa (e.g., Vibrio) harboring some pathogens are pathogenic.
Although changes in the octocoral microbiota have been linked to environmental and anthropogenic stressors, only one study has tried to establish causal links and came up short. They found that the bacterial communities of Lobophytum pauciflorum (Spirochaetes- and Endozoicomonas-dominated) were unaffected by temperature (31 °C) and acidification (pH 7.9) stress [91], confirming the relative stability of octocoral microbial assemblages. However, one point that should be considered in the design of experimental studies focused on linking microbiome function to particular stressors is that the microbiome in aquaria may be different than in the natural environment [91], an observation also made for scleractinian corals and other marine invertebrates. In contrast to scleractinian corals [137], physical injury did cause a change in the microbiota of the Caribbean gorgonians Eunicea flexuosa and Pseudoplexaura porosa. Notably, a decrease in Endozoicomonas near the lesion site was observed [106], which might be linked to a delay of colonization of the recovering tissues by bacteria from this genus.
Co-diversification and inheritance
The consistent associations of specific OTUs with octocorals through space and time show the intricate relationships between these microbes and their hosts. Indeed, phylogenetic analyses at the OTU level across multiple gorgonian species from the Mediterranean Sea have shown that each host species harbors Endozoicomonas phylotypes belonging to different monophyletic clades [19, 20, 80, 81], but that species from the same family or genus share the same phylotypes in their microbiota [19, 20, 81]. The finding that the phylogenetic tree of the Endozoicomonas symbionts corresponds with the systematic classification of gorgonians suggests that co-diversification between these microbial symbionts and their hosts may have taken place [80, 81]. In fact, similar observations were made when the core microbiome as a whole was considered [19, 20]. These host-microbiota associations are therefore probably ancient and have been conserved through evolutionary times. However, these phylosymbiotic signals appear to end at the family/genus level, as there is significant overlap in OTUs between gorgonian species within the same family, although Leptogorgia sarmentosa’s microbiota showed some compositional difference with Eunicella species [20]. Interestingly, a recent study showed that there is in fact an incomplete phylogenetic separation of the Eunicella species and that there is potential for hybridization [138]. As such, the lack of divergence in the microbial assemblages between Eunicella spp. may be linked to the yet limited evolutionary divergence between these gorgonian species, and it would be interesting to observe how these associations will develop over time, what the hybrid holobiont composition is, and whether differences on the bacterial strain level may already exist. One discrepancy, however, is the difference in Endozoicomonas species found associated with Gorgonia ventalina and Eunicella spp., which both belong to the Gorgoniidae family [80]. However, these octocoral species are geographically separated by the Atlantic Ocean, while L. sarmentosa and Eunicella spp. are not, and this taxonomic family has been found to be polyphyletic [139–141], providing potential explanations for these observations.
The consistent, but host-specific, octocoral-microbe associations also raise questions concerning the mode of transmission. As most Mediterranean gorgonians are brooding (i.e., larval development occurs internally), vertical transmission of the bacterial symbionts to offspring is likely and has been shown to generate species-specific associations and co-diversification of the partners in a symbiosis [142]. Vertical transmission of symbionts is known to occur in the brooding scleractinian coral Porites astreoides [143], while horizontal transmission (i.e., uptake from the environment) is more likely to occur in broadcast spawning corals [144, 145]. Detailed studies into the transmission mode of microbial symbionts in octocorals would significantly increase our understanding on their inheritance and the evolution of coral symbioses.
Octocoral diseases
Disease outbreaks have affected many marine organisms worldwide in recent decades [146–148] and can produce major changes to ecosystem composition, structure, and function as observed on coral reefs [149–151]. The main drivers of the increased incidence, prevalence, virulence, and emergence of new marine diseases are likely related to changes in the environment linked to climate change [152]; however, local anthropogenic factors are known to exacerbate the effects [60, 153–155]. Disease is an interaction between a host organism, pathogen, and the environment. Changing environmental conditions, such as higher than normal seawater temperatures, may compromise the host (physiology and immunity), making it more susceptible to pathogens and causing shifts in the associated microbiota [156]. Currently, 19 different diseases have been identified that are known to affect octocoral populations worldwide. Octocoral diseases were recently expertly reviewed in detail by Weil et al. [56], and we will therefore only provide a brief overview of this subject focused on the main microbial diseases (Fig. 3; Table 3). A commonality among all octocoral diseases is that their prevalence seems to increase with higher seawater temperatures.
Fig. 3.
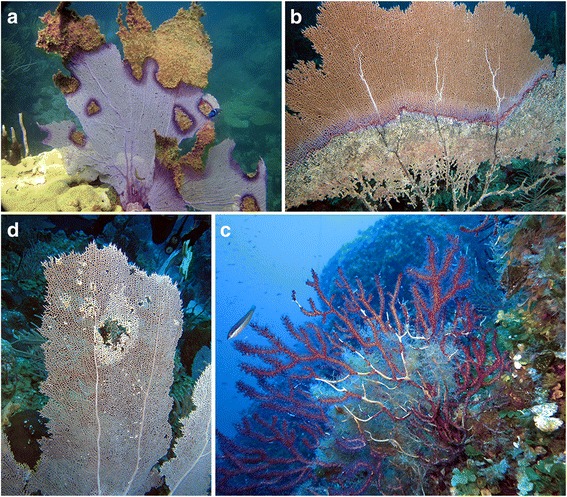
Octocoral diseases from the Caribbean and the Mediterranean Sea. a Aspergillosis, b Red Band Disease, c Octocoral Vibrio syndrome, and d Gorgonia Wasting Syndrome (photos a, b, and d by Ernesto Weil and photo c by Carlo Cerrano)
Table 3.
Overview of the main octocoral diseases, the putative pathogens and the octocoral genera/species affected. If multiple octocoral species from the same genus were affected, only the genus name is provided
| Region | Pathogen | Octocoral affected | |
|---|---|---|---|
| Microbial diseases | |||
| Aspergillosis | Caribbean |
Aspergillus sydowii
Aspergillus spp. |
Gorgonia spp. Pseudopterogorgia spp. Plexaura spp. Pseudoplexaura porosa Plexaurella spp. |
| Pacific | Pacifigorgia spp. | ||
| Red Band Disease | Caribbean | Polymicrobial mat – primarily cyanobacteria Ocillatoria sp. |
Gorgonia spp. Plexaurella nutans |
| Black Band Disease | Caribbean | Polymicrobial mat – cyanobacteria Phormidium corallyticum, sulphate-reducing Desulfovibrio, sulfide-oxidizing Beggiatoa |
Gorgonia spp. Pseudopterogorgia spp. Plexaurella spp. Erythropodium asbestinum |
| Octocoral Vibrio Syndrome (OVS) | Mediterranean | Vibrio coralliilyticus |
Eunicella spp. Paramuricea clavata |
| Fungal-Protozoan Syndrome *Possibly secondary infection following OVS |
Mediterranean | Fungus genera Trichoderma, Clodosporium, Penicillium Unidentified protozoa |
Eunicella spp. Leptogorgia sarmentosa Paramuricea clavata Corallium rubrum |
| Black Necrotic Syndrome | Pacific | Possibly Penicullium fungus | Isis hippuris |
| Multifocal Purple Spots (previously labyrinthulomycosis) | Caribbean |
Aplanochytrium protozoan Sphaerippe copepod |
Gorgonia spp. |
| Wasting Syndromes (WS) | Caribbean | Unknown pathogens | |
| Gorgonian WS |
Gorgonia spp. Plexaura nutans Erythropodium asbestinum |
||
| Briareum WS | Briareum spp. | ||
| Phyllogorgia WS | Phyllogorgia dilatata | ||
| Erythropodium WS | Erythropodium asbestinum | ||
| Bleaching Necrosis (BN) | Caribbean | Unknown pathogens | |
| Briareum BN | Briareum spp. | ||
| Erythropodium BN | Erythropodium caribaeorum | ||
| Other diseases | |||
| Growth anomalies Hyperplasia, hypoplasia No documented mortality |
Caribbean | Endolithic algae – Entocladia endozoica |
Gorgonia spp. Pseudopterogorgia spp. Plexaura spp. Pseudoplexaura spp. Plexaurella anceps |
| Bleaching Loss of zooxanthellae |
Caribbean | High seawater temperatures |
Gorgonia spp. Pseudopterogorgia spp. Plexaura spp. Pseudoplexaura spp. Plexaurella spp. Briareum spp. Muricea spp. Eunicea spp. Erythropodium asbestinum Pterogorgia citrina Muriceopsis flavida |
| Pacific |
Isis hippuris
Lobophyton spp. Sarcophyton spp. Sinularia spp. |
||
The octocoral disease aspergillosis (Fig. 3a) has been the most devastating in the Caribbean and Eastern Pacific [60, 157, 158] and is currently considered a chronic disease in the wider Caribbean region [62]. Aspergillosis disease dynamics vary across locations and reefs, indicating that local biotic and abiotic factors influence disease prevalence [60, 61]. Differential disease susceptibility (e.g., host resistance) among octocoral species has also resulted in changes in octocoral communities [159, 160]. The fungus Aspergillus sydowii was identified as the pathogen, but various other Aspergillus species have been shown to cause similar disease signs [64]. Infiltration of the fungal hyphae into the coenenchyme tissue results in degradation of the tissue exposing the skeleton, which is then rapidly colonized by other micro- and macro-organisms [60]. Characteristic for aspergillosis is the purpling of the tissue surrounding the lesions due to activation of the melanisation cascade, a component of the immune response (discussed in the next section) exhibited by the coral to prevent further progression of the fungal infection. Contrastingly, growth anomalies rarely cause mortality in octocorals [56]. Although detected throughout the Caribbean, the cause of these abnormal tissue structures (e.g., tumors, hyper- or hypoplasia) is unclear, but may be part of a general response against parasites, fungal, or algal infiltrations, competition, and damage/injury [161–164].
Black Band Disease (BBD) and Red Band Disease (RBD) (Fig. 3b) are two diseases that affect both hard [165] and soft corals [166–168] and seem to be temperature driven as they are more prevalent in warm summer months. The characteristic “band” is a polymicrobial mat that in scleractinian corals consists primarily of cyanobacteria [169, 170], combined with sulfur-reducing and sulfide-oxidizing bacteria in the BBD consortium [169]. The composition of the bacterial mats in the octocoral BBD and RBD has yet to be identified.
In the Mediterranean, two mass mortality events related to high seawater temperatures took place over large geographical areas in 1999 and 2003, affecting 60–100% of the gorgonian populations as well as many other benthic marine invertebrates [171–174]. The bacterium Vibrio coralliilyticus, which is known to cause disease in scleractinian corals in the Indo-Pacific, was identified as the putative pathogen in the 2003 outbreak, and the disease has been termed Octocoral Vibrio Syndrome (Fig. 3c) [172]. The disease manifests itself by mucus production by the gorgonian, followed by a loss of pigmentation and subsequently the coenenchyme tissue. Despite the lack of conclusive evidence, it is believed that this bacterium was also responsible for the mortality of octocorals in 1999. The fungal hyphae and protozoan ciliates found on diseased gorgonians [171] (responsible for the name Fungal-Protozoan Syndrome [175]) were likely secondary opportunistic parasites. Eunicella verrucosa colonies were also impacted by a disease with similar signs in southwest England between 2002 and 2006 [176].
Black Necrotic Syndrome is affecting gorgonians in the Pacific and is characterized by black necrotic areas along the branches, followed by rapid tissue and skeleton loss, leading to the fragmentation of the entire colony [177]. Although Penicillium fungi were isolated from lesions that contained high numbers of hyphae, it could not be proven that these microbes were indeed the disease-causing pathogens [177].
The effect of two parasitic diseases affecting the gorgonian G. ventalina classified as Multifocal Purple Spots (MFPS) on the physiological functioning of the coral holobiont is unknown, but the indication of an active immune response based on purpling of the tissues suggests the infection is indeed harmful. MFPS can be caused by ovoid protozoans form the genus Aplanochytrium [178, 179] and appears as small purple galls with the protozoans located inside. Larger MFPS galls containing one or two Sphaerippe copepods have recently also been described [180]. Parasitism of other octocorals (sea pens, deep-sea gorgonians) by endoparasitic copepods is, however, quite common [181, 182], but to what extend it affects host survival remains unclear.
Wasting Syndrome is another class of disease that has severely impacted various gorgonians, including species belonging to the genera Phyllogorgia [183], Erythropodium [56], Gorgonia (Fig. 3d) [184], and Briareum [55, 185, 186]. The disease is characterized by discoloration and disorganization of the tissues, ultimately resulting in decomposition with necrotic appearance. However, no putative pathogens have thus far been identified. In addition to these characterized diseases, other disease-resembling conditions have been observed on octocoral colonies. As little is known about the etiology and pathogens of octocoral diseases, there is an urgent need to rapidly investigate the disease-causing microbial consortia, isolate the suspected pathogens to fulfill Koch’s postulates, and develop diagnostic and management tools for the protection of the ecologically important octocoral assemblages.
Octocoral immune responses
Although diseases have had a major impact on octocoral populations, these organisms have a remarkable immune defense capacity (Fig. 4). The first evidence of self and non-self recognition in cnidarians was presented in 1893 by Metchnikoff, the pioneer of cellular immunology, following his observation of an accumulation of amoebocytes around a splinter inserted in a scyphozoan [187]. This finding gave rise to extensive graft rejection studies using the gorgonian Swiftia exserta as a model. While autografts readily fused with the colony, allografts were rejected exhibiting tissue necrosis at the graft site and thereby showing the principle of histocompatibility in octocorals [188]. This response was likely mediated by the “granular amoebocytes,” which infiltrated the graft rejection areas and are also known to accumulate in tissue wounds in soft corals [189]. In addition, some populations of these cells have been shown to possess phagocytic capabilities [190] and are thus considered putative immune cells, similar to macrophages and neutrophils in vertebrates. Recent biochemical characterizations support this notion and indicate that S. exserta possesses at least three distinct immune cell types [191]. The authors hypothesize that invading microorganisms first encounter the immediate-responders, consisting of (1) “initial-encounter cells” in the ectoderm epithelium, which fight off the microbes with chemical oxidation and (2) “focal response cells” located directly under the epithelium, which are equipped with acid phosphatase and esterases. In addition, there is a population of secondary responders, called (3) DOPA-oxidase-containing cells, that migrate within the mesoglea towards lesions and also possess the potent microbicidal enzyme phenoloxidase and peroxidase. These secondary response immunocytes are likely the large population of specialized phagocytic immune cells observed infiltrating lesions 24 h post-injury, while the immediate responders arrive on the scene within 2 h [190].
Fig. 4.
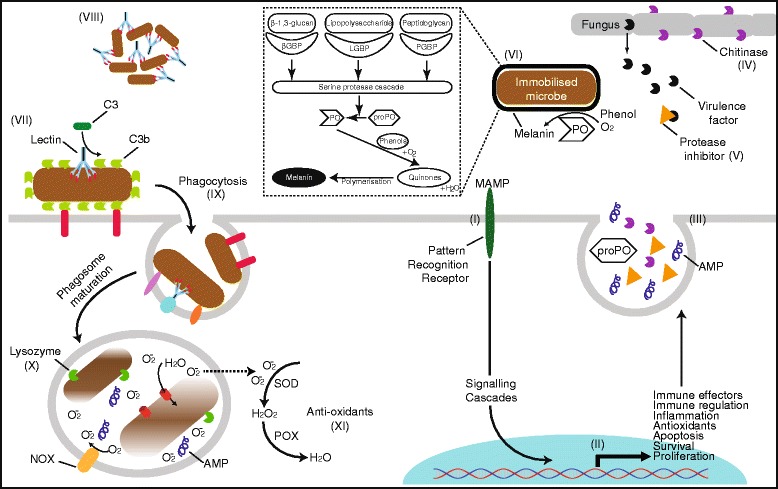
Current knowledge on the immune system of octocorals. (I) Microbe-associated molecular patterns (MAMP) are recognized by pattern recognition receptors (PRR), which subsequently activate signaling cascades that induce (II) expression of genes involved in the immune system. (III) Immune effector molecules are produced and secreted, including antimicrobial peptides (AMP). (IV) Chitinases degrade chitin, an important component of the cell wall of fungi. (V) The host also uses protease inhibitors to neutralize protease virulence factors secreted by pathogenic microbes. (VI) One of the main immune system components is the prophenoloxidase (proPO)-activating pathway. It is activated following the binding of MAMPs to their respective binding proteins (BP), leading to the activation of a protease cascade that ultimately cleaves proPO into PO. Subsequently, PO oxidizes phenolic compounds (e.g., dihydroxidephenylalanine) that undergo further non-enzymatic reactions to form a microbe-immobilizing barrier of melanin. Cytotoxic molecules are also formed during this process. Octocorals are also known to possess lectins, which can be used in (VII) the lectin-complement system that leads to the deposition of complement C3 on the target microbe, and/or to (VIII) aggregate microbes into large aggregates. Both systems facilitate (IX) the rapid phagocytosis of microbes following binding to lectin, C3-receptors or various scavenger PRRs. Once internalized, the phagosome matures and becomes microbicidal with (X) bacterial cell wall degrading lysozyme as well as AMPs and oxidative burst of reactive oxygen species (ROS). (XI) The ROS may be also damaging to the host cell and antioxidant enzymes, such as superoxide dismutase (SOD) and peroxidase (POX), are used to neutralize it
These “granular amoebocytes” were, however, only truly known to be implicated in the antimicrobial immune response of octocorals following studies into the major aspergillosis outbreaks on G. ventalina. Dense aggregations of amoebocytes were observed in tissues infected by Aspergillus sydowii, with concurrent increased phenoloxidase activity and melanin deposition [192]. The melanin was found to form a thick band around the lesion sites and surround the fungal hyphae [193] to form a protective barrier and prevent further tissue infiltration by the fungus. Surprisingly, however, the amoebocytes did not appear to migrate towards the lesion in this gorgonian, but likely originated from stem cell-like undifferentiated amoebocytes that underwent significant proliferation near the site of infection [192].
Other inducible enzymatic defense mechanisms have also been described in octocorals. For example, the activities of two classes of antioxidant enzymes have been implicated in the coral immunity: peroxidase (POX) and superoxide dismutase (SOD). While SOD scavenges superoxide radicals (O2−) and converts it to O2 or hydrogen peroxide (H2O2) depending on the SOD enzyme type, POX neutralizes H2O2. Regulation of the oxidative stress during the antimicrobial oxidative burst is crucial to prevent tissue damage and can be used as a proxy to assess the organism’s immune system’s activity. Peroxidases are present in the secondary response cells in S. exserta [191], and their activity has been shown to be induced in response to injury and heat stress [194] as well as A. sydowii infections [195]. Both SOD and POX activity have been related to potent antifungal activity [195, 196]. Particularly relevant in antifungal defense in gorgonians may also be the destruction of the fungal cell wall through chitin degradation by chitinases [197], while the digestion of peptidoglycan by lysozyme-like enzymes may be important to fight off bacteria [196]. While attacking structural components of pathogens is an efficient defense mechanism, pathogens use virulence factors, such as proteases, to damage and subsequently infiltrate the host tissues. Inhibition of virulence factors may be another defense strategy employed by octocorals as protease inhibitors that inhibit the activity of such fungal protease virulence factors were recently described [198].
The majority of immune system studies in octocorals have focused on enzymatic defense systems. However, the availability of genomic and transcriptomic approaches have provided additional insights into the octocoral immune repertoire. Characterization of a lectin [199] and a C3-like molecule [200] suggests that the lectin-complement system, which facilitates efficient phagocytosis of microbes, may also be present in these organisms. Challenges of G. ventalina with an Aplanochytrium parasite further revealed that this gorgonian upregulates the expression of various pattern recognition receptors, whose signaling may be responsible for the increased levels of antimicrobial peptides (AMP) [178] that may play a role in the regulation of the associated microbial communities as has been demonstrated in Hydra [201].
The large inducible immune repertoire of octocorals suggests that these organisms possess significant capabilities to fight off infections. Nonetheless, as diseases have particularly impacted octocoral populations worldwide in recent decades, differences in environmental conditions and locations may also affect the immunocompetence of octocorals [196]. Although little is known about the relationship between the environment and the octocoral immune system, current studies have only detected increases in immune parameters under potential stress conditions. For example, increased levels of dissolved inorganic nitrogen positively correlated with chitinase and lysozyme-like enzyme activities [196], while increases in amoebocytes [192], protease inhibitor [198], and antifungal [202] activity have been observed under elevated seawater temperatures. However, under these conditions, microbial growth may be stimulated [202], requiring the host to invest in immunity. Prolonged microbial stress and resource allocation towards immunity may significantly reduce the energetic resources available and ultimately lead to an (immuno)compromised health state and disease. A link between reduced investment in the immune system and higher disease prevalence has been demonstrated, even within colonies. In gorgonians, growing tissues possess significantly higher levels of immunity compared with older tissues [196, 203], and disease modeling studies have demonstrated that this spatial within-host difference may explain the higher prevalence of disease in larger colonies and found that new infections are more likely to occur when hosts direct their immune responses to lesions at the expense of other healthy parts in the colony [204].
Microbiome regulation
While most inducible cellular immune mechanisms found to date are likely used by the host in response to microbial infections, constitutive expression of compounds with antimicrobial properties may be used by the host organism to regulate its microbiome and keep pathogens out. Gorgonian tissue extracts have been tested extensively for antibacterial [114, 196, 205–207] and antifungal activities [196, 202, 203, 208, 209]. However, it is unclear which compounds are responsible for the bioactivity observed in these studies and which member within the holobiont produces these compounds. One of the main microbiome regulatory mechanisms in octocorals may be the host’s immune system (see the previous section). For example, the expression of antimicrobial molecules is modulated via pattern recognition receptors that monitor the microbiome and thereby regulate the composition of the associated microbiota, as has been demonstrated in other cnidarians [111, 201, 210].
Another strategy for microbiome regulation is interference with quorum-sensing (QS). QS is a microbial communication process using signaling molecules to mediate cooperative behaviors between related microbes (Fig. 5; expertly reviewed by Asfahl et al. [211]). However, a host organism may regulate its microbiome through QS interference, thereby stimulating or inhibiting the growth and functions of beneficial and potential damaging microorganisms, respectively (Fig. 5). A recent study in Hydra showed the importance of QS interference for microbiome regulation in cnidarians [212]. Through an enzymatic modification of N-acylhomoserine lactone (AHL) QS molecules, the host was found to manipulate bacterial QS, thereby changing gene expression patterns and inducing a phenotypic switch in the bacteria, which ultimately lead to reduced colonization of the host by specific bacterial symbionts. Octocoral extracts also contain compounds with QS regulatory properties [213–215], particularly diterpenes [213]. Several cembranoid diterpenes have been isolated from soft corals and were implicated in the inhibition of N-acylhomoserine lactone (AHL)-mediated QS, the best studied QS system in gram-negative bacteria, resulting in reduced biofouling [213, 216–218]. Interestingly, however, other cembranoid diterpenes and furanoditerpenes appeared to be QS mimics and possessed stimulatory properties [213]. While diterpenes contain lactone-rings used to bind the AHL receptors, it was demonstrated recently that a sterol abundantly present in the octocoral Nephthea chabroli may also efficiently stimulate AHL-type QS [213]. Using a range of QS inhibitory and stimulatory compounds specific for various microbes may allow octocorals to tightly regulate the composition of their microbiota and could explain the observed relative stability of soft coral-associated bacterial communities. However, some bacteria associated with octocorals, such as Endozoicomonas, have also been found to exhibit potent QS activity [213], providing another potential explanation why these bacteria are highly dominant in some gorgonians. In contrast, Vibrio species generally inhibited QS in biosensor species [213]. This potentially disruptive effect on QS in other members of the microbiota may be a competitive strategy of these potential pathogens to establish themselves in the microbiota of the host. As most QS interfering molecules have been characterized as part of natural product discovery efforts focused on finding antimicrobial compounds, QS stimulatory molecules may have been largely overlooked. More research into these compounds, their ecological role, and the interplay between inhibitory and stimulatory QS signaling will be required to fully understand the importance of QS interference by soft corals in their microbiome regulation.
Fig. 5.
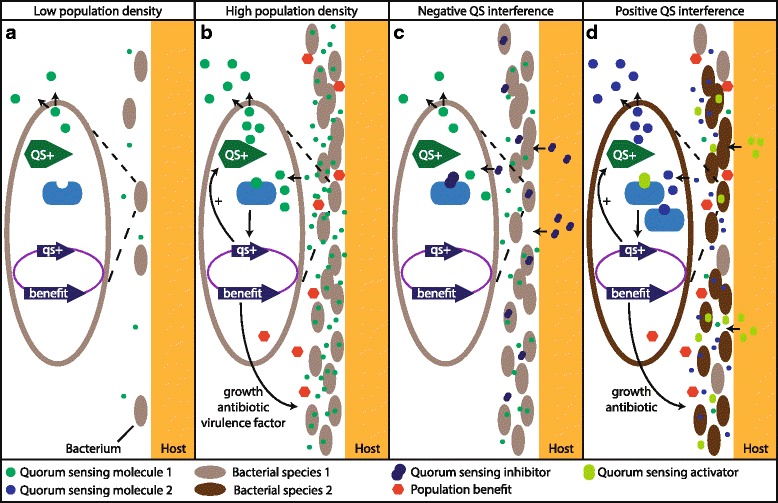
Quorum sensing and interference. a Bacteria produce quorum-sensing stimulating compounds (QS+), but due to low bacterial density and environmental conditions (e.g., diffusion, advection, degradation) the concentration does not reach levels sufficient to bind to the receptor. b At high population densities, the QS compounds reach sufficient levels to bind to its receptor leading to gene transcription and subsequently (1) increased production of QS+ signaling molecules and (2) population beneficial processes, such as cooperative growth and migration, secretion of antibiotics to reach effective concentrations for competition or in case of pathogens the production of virulence factors. c–d Host organisms have the capacity to interfere with QS, possibly species specific. c By secreting QS inhibiting compounds, bacterial population benefits can be counteracted, thereby reducing bacterial growth and potentially inhibiting pathogen virulence. d Host-induced QS activation in specific bacterial species may provide a growth advantage, selecting for those species. A balance of negative and positive QS interference may allow the host to regulate its associated microbiota. Other than intended bacterial species may, however, cheat and benefit from QS by other species without investing in QS themselves
In addition to QS interference, numerous secondary metabolites with antimicrobial, antiviral and antifouling activity have been isolated from soft corals (overview provided in [219]). Although these compounds are likely used by the host to eliminate unwanted microbes, prevent overgrowth by other benthic organisms, and maintain colony health, their ecological relevance for holobiont functioning remains to be elucidated. Interestingly, however, microbes are increasingly recognized to be involved in regulation of holobiont composition and defense as well. Various compounds have recently been extracted from microorganisms that were isolated from octocorals. For example, several phenyl ethers, anthraquinones and alkaloids with anti-fouling properties [220], and merosesquitepenoids, macrolides and alternariol derivatives with antibacterial [221, 222] and potentially antiviral activities [222], were derived from symbiotic fungi associated with soft corals. Various bacteria belonging to the Firmicutes, Actinobacteria, and Gammaproteobacteria isolated from tropical and temperate octocorals have also been shown to possess potent antibacterial and antifungal activities [223–225]; however, only the antimicrobial cyclic tetrapeptide Cereustatin A and two esters of p-hydroxybenzoic acid have so far been extracted and characterized [226]. Interestingly, the algal symbiont Symbiodinium was found to be a rich source of diterpenes in the gorgonian Antillogorgia elisabethae and A. bipinnata (previously belonging to the genus Pseudopterogorgia [227]), called pseudopterosins [228, 229]. While those molecules possess anti-inflammatory and antimicrobial activities, one study has implicated them in the regulation of a damage-inducible oxidative burst in cultured algal cells [230].
Taken together, soft corals and their microbial associates possess a range of molecules that affect the growth and survival of microorganisms. As such, the role of the microbial community in holobiont physiology and immunity should also be considered. The composition of a healthy symbiotic microbiome may be largely regulated via positive or negative quorum sensing interference, while antimicrobial compounds may be used to prevent pathogen infiltration, supporting the “coral probiotic hypothesis” [231]. As the majority of studies has been conducted on isolated microbes or extracted compounds, their ecological importance in coral microbiome regulation remains an avenue of research.
Natural product discovery and challenges
Since the 1950s, when research into marine natural products started, sponges have been considered to have the highest potential for drug discovery. However, new technologies and increased efforts identified soft corals as a rich source of potentially bioactive secondary metabolites. Despite an initial surge in the testing and characterization of compounds extracted from octocorals [219], relatively few new compounds have been described in recent years [232]. Overall, more than 3500 bioactive molecules from octocorals have now been described and tested, with some promising drug leads (Table 4; reviewed in [219]). The vast majority of such compounds belong to the highly diverse classes of terpenes and terpenoids, particularly diterpenoids and cembranoids, as well as steroids and prostanoids. For example, Plexaura homomalla contains very high levels of prostaglandin A2, which has a predator-deterring effect [233]. Testing for medically relevant bioactivity revealed that many of those compounds possess anticancer, anti-inflammatory, or antimicrobial (e.g., antiviral, anti-ulcer, anti-malaria, anti-tuberculosis, or more general antibacterial and/or antifungal) properties. Especially from a pharmaceutical perspective, it is interesting to note that non-marine bacterial pathogens were generally more sensitive to gorgonian tissue extracts than marine bacteria [206, 214]. However, another consideration that should be further investigated are the ecologically and pharmaceutically relevant doses, particularly as some compounds with antibacterial activity have been shown to induce QS at low concentrations [213]. Although octocoral-derived compounds are not being used in the clinic yet, pseudopterosins derived from A. elisabethae are the main components of some cosmetic skincare products, such as Resilience by Estée Lauder, because of potential anti-aging effects due to their anti-inflammatory properties [234]. Other compounds have been found to have potent anti-fouling activity (Table 4), making them potentially suitable natural alternatives to tributyltin for fouling prevention on ships.
Table 4.
List of 25 promising natural product compounds isolated from octocorals, showing the diversity of chemical compounds with various pharmaceutically relevant activities produced by a range of different octocoral species
| Activity | Compound | Chemical | Origin | Region | Ref |
|---|---|---|---|---|---|
| Anti-inflammatory | Austrasulfone | Sulfone | Cladiella australis | Taiwan | [258] |
| Simplexin E | Diterpenoid | Klyxum simplex | Taiwan | [259] | |
| Crassumolides A and C | Terpenoid | Lobophytum crassum | Taiwan | [260] | |
| Ergostanoids 1 and 3 | Ergostanoid | Nephthea erecta | Taiwan | [261] | |
| Paralemnolin Q and S | Sesquiterpenoid | Paralemnalia thyrsoides | Taiwan | [262] | |
| Lobocrassin B | Cembranoid | Lobophytum crassum | Taiwan | [263] | |
| Antitumor | Polyoxygenated gorgosterol (2–4) | Steroid | Isis hippuris | Japan | [264] |
| Clavulones | Prostanoid | Clavularia viridis | Taiwan | [265] | |
| Bis(pseudopterane) amine | Dialkylamine | Antillogorgia acerosa | Bahamas | [266] | |
| Klysimplexin B and H | Diterpenoid | Klyxum simplex | Taiwan | [267] | |
| 13-acetoxysarcophytoxide | Cembranoid | Lobophytum crassum | Taiwan | [268] | |
| Capilloquinol | Farnesyl quinoid | Sinularia capillosa | Taiwan | [269] | |
| Antimicrobial | Curcuphenol | Terpenoid | Antillogorgia rigida | USA | [270] |
| Lipids | Polyketide | Sinularia sp. | Russia | [271] | |
| Pseudopterosin X | Diterpenoid | Antillogorgia elisabethae | USA | [272] | |
| Antiviral | Durumolide Q | Cembranoid | Lobophytum durum | Taiwan | [273] |
| Lobohedleolide | Diterpenoid | Lobophytum sp. | Philippines | [274] | |
| Antituberculosis | Bipinnapterolide | Terpenoid | Antillogorgia bipinnata | USA | [275] |
| Homopseudopteroxazole | Diterpenoid | Antillogorgia elisabethae | USA | [276] | |
| Antimalaria | Aberrarone | Diterpenoid | Antillogorgia elisabethae | Colombia | [277] |
| Dolabellane | Diterpenoid | Eunicea sp. | Colombia | [278] | |
| Anti-fouling | Homarine | Pyridine | Leptogorgia setacea | USA | [279] |
| 11-episinulariolide | Diterpenoid | Sinularia flexibilis | Australia | [280] | |
| Isogosterones A–D | Steroid | Dendronephthya sp. | Japan | [281] | |
| 3β-methoxyguaian-10(14)-en-2β-ol | Sesquiterpenoid | Echinogorgia pseudosassapo | Taiwan | [282] |
As secondary metabolites are generally present in the holobiont at relatively low concentrations, large quantities of organisms will be required. Such unsustainable harvesting is unwanted because of the potentially severe impacts on ecosystems. Due to the complex structures of soft coral-derived natural products, it is very difficult to produce these molecules synthetically in the laboratory. While aquaculture has been suggested as a viable alternative to natural harvesting as it has limited environmental impact, it is a relatively slow and intensive process. Culturing the microbes that produce natural product (NPs) of interest has also proven challenging as the production of the secondary metabolites depends significantly on the culture conditions and many of these microbes are yet uncultivable under laboratory conditions. Given these challenges in supply, the identification of the exact metabolic pathways and cloning of the genes involved into resistant prokaryotic or eukaryotic expression vectors for constitutive production would be ideal.
Overall, soft corals and their microbial associates are recognized as excellent sources for potentially interesting drugs and anti-fouling compounds. While small quantities are sufficient for initial screens and pre-clinical studies, major challenges in the supply of these molecules still need to be overcome, before they become feasible drug candidates for clinical applications.
Future directions
Although octocorals function as ecosystem engineers in a wide variety of environments, they have received significantly less attention than scleractinian corals, whose physiology and holobiont composition have been extensively studied. Octocorals are, however, severely affected by pollution, disease, and global climate change threats, such as rising seawater temperatures [53], and therefore deserve further research, particularly at the holobiont level. Microbes are emerging as very diverse and flexible symbionts of corals and microbial processes are important for coral health and resilience to stress, but the functional role of these microbes within the coral holobiont is poorly understood. There are thus many questions at the forefront of discovery, some of those being the same as for scleractinian corals.
Concerning the octocoral-dinoflagellate symbiosis, one fundamental but still unknown aspect in this relationship is the importance of autotrophy versus heterotrophy in the energetic budget of octocoral species at the different seasons and under different environmental conditions. Such knowledge will be essential to understand how octocorals acquire energy to face stressful conditions occurring at the global and local scales. The contribution of Symbiodinium to the reproductive effort of octocorals under different stress scenarios will also be crucial for understanding the potential of these species to spread and colonize new environments. The increased frequency in bleaching events and seawater eutrophication cause coral mortality in tropical reefs worldwide [235]. Octocorals appear to have a lower bleaching susceptibility as well as a higher resilience to eutrophication compared to scleractinians, and phase shifts towards soft coral dominance has already occurred in some regions (Table 1). This higher resistance of octocorals compared to other coral groups has been attributed to their lower dependency on the autotrophic input of the dinoflagellate symbionts, replaced by a higher degree of heterotrophy [7]. However, not all octocorals can afford a reduction in autotrophic input [50]. More studies are thus necessary to unravel the energetic needs of octocorals and to estimate the cost of this symbiosis for mixotrophic species. For example, the stability of the symbiosis suggests that octocorals are more resilient to global warming than predicted, or on the contrary, that the host is unable to switch its symbionts towards more resistant ones [131]. To answer this question, there is a need to increase our knowledge on the resilience and recovery of octocoral species following bleaching events.
The microbial diversity associated with octocorals needs to be better characterized to identify those microbes that are essential to holobiont health and those that may impair holobiont functioning and cause disease. Knowing which microbes are (opportunistic) pathogens could be used to develop diagnostic tools to monitor soft coral populations and inform management strategies when changes in the octocoral microbiota towards a pathogenic state occur. Many soft corals harbor a microbiota of lower diversity and present a more defined and stable core microbiome than their scleractinian relatives [19, 20, 83, 91]. Such stable associations are particularly useful to study the functional role of the associated bacteria and show that octocorals may be a good model organism to study coral-microbe interactions. For example, Endozoicomonas symbionts are dominant in some octocoral species and the fact that multiple genotypes can be present in a single host suggests that the host may be able to alter its Endozoicomonas population to the environmental conditions. However, environmental stress tends to correlate with a decreased Endozoicomonas abundance, indicating that this bacterium likely plays a role in host fitness [19, 110, 134, 135]. The exact functions of this bacterial genus in coral holobiont symbiosis remain to be identified though. While Endozoicomonas has received significant attention due to its wide global distribution and associations with many marine invertebrates, the apparent equally important role of Spirochaetes and Mycoplasma in some octocorals, for example, should also be given substantial consideration. Metagenomic and metatranscriptomic approaches will allow us to reconstruct the genomes of those difficult-to-culture symbionts and assess the impacts of stressors on their functioning. However, technical challenges regarding the low recovery of microbial reads due to host contamination need to be resolved to allow this technique to be used cost-effectively. Another avenue of research that is vastly underexplored is the role of fungi, archaea, and viruses in octocorals. Research on these taxa in scleractinian corals is only in its early stages and, as a potential model, the associations between octocorals and those microbial taxa may provide important insights applicable to reef coral biology.
Progress can also be made on experimental and technological fronts. As the field of octocoral microbiome research is still in its infancy, we have the opportunity to benefit from the knowledge gained from other fields. For example, each 16S rRNA gene-targeting primer set is known to have an inherent bias towards certain taxa. We also observed significant differences in bacterial community composition associated with octocorals when using primers targeting the V5–V6 regions of the 16S rRNA gene or the V1–V2 regions, which was used in the Human Microbiome Project (personal communication). As such, comparisons between studies that used different primers are difficult to make. Recent efforts by the Earth Microbiome Project (EMP) have resulted in the generation of primer sets that detect the highest diversity and are currently being used to elucidate the microbiomes of numerous organisms and environments on planet Earth. Consistent use of the same primer set across studies, particularly the use of EMP primers, will allow us to accurately compare microbiome compositions across species (as well as time and locations), conduct broad scale phylogenetic studies to investigate the evolution of symbiosis and draw more meaningful conclusions. In addition, it may help us to more readily solve the issues faced regarding unclassified bacterial sequences that may constitute a large portion of an organism’s microbiome [106] or may be responsible for differences observed [91]. As it is also easy to implement, requiring only a change in amplicon library construction with no effect on bioinformatic or computational analyses, this minor change in laboratory protocols may significantly benefit the field of octocoral microbiome research and microbial ecology in general.
New and emerging methods in microbiology are also becoming available (described in detail in [97]) and will allow a better understanding of the localization and potential functions of bacterial symbionts. Using these methods, it will be possible to highlight the different holobiont compartments where microbial processes are taking place and the mechanisms which mediate these processes. Briefly, such techniques include halogen in situ hybridization secondary ion mass spectrometry (HISH-SIMS) [236], to precisely locate microbes within-host tissues. Metagenome, whole genome and single cell genomic sequencing [237] and RNA-Seq on isolated single cells [238] will be useful to shed light on the potential functional role and life cycle of bacterial symbionts. Pulse chase isotope labelling coupled with Nanoscale SIMS (NanoSIMS), can be used to image and quantify the transfer of specific metabolites from microbial symbionts to host cells [239]. Finally, molecules within a given cell or tissue can be identified by high resolution mass spectrometry techniques, such as time-of-flight SIMS (TOF-SIMS) [240].
Once the functions of coral-associated microbes have been established, it will be important to assess how environmental and anthropogenic stressors affect the host-microbe symbioses and eventually promote microbial disease development. The goal is to better understand how microbes are related to coral health and to enable accurate predictions of resilience and responses of corals to climate change perturbations. We can then use this knowledge to identify microbes that may provide a coral with enhanced resistance to environmental stress, which may ultimately allow us to engineer the coral-associated microbiota to culture stress-tolerant corals for coral reef restoration [241, 242].
Octocorals in holobiont research
Holobiont research has taken huge steps in recent years. Discussions on the hologenome concept have contributed significantly to this progress and identified some of the most pressing issues in this field [243]. For example, does the response to selection occur at the level of the host or microbiota? Is vertical inheritance of complex microbiomes common? And is phylosymbiosis taxonomically widespread among hosts? Research on octocorals may provide new insights to answer these questions.
Phylosymbiosis has been observed in a diverse range of organisms, including insects, rodents, and hominids [244]. Evidence of phylosymbiosis is also present within the octocoral holobionts, showing a parallel between host phylogeny and its microbial community. However, the observation that there is a significant overlap in the core microbiome between various Mediterranean gorgonian species belonging to the same genera [20], as well as an incomplete phylogenetic separation of those species [138], provides a unique opportunity to study the principle of phylosymbiosis and how phylosymbiotic signals may arise in complex holobionts and potentially shed some light on the drivers of speciation and holobiont assembly.
Vertical inheritance of a microbiome may also occur in octocorals, particularly as many species are brooding (i.e., fertilization and larval development happen within the mother colony and fully developed larvae are released). While it is likely that the microbes are transferred from parent to offspring, current investigations endeavor to address this question as well as whether core microbiome members are already present within the larval tissues prior to release. Heredity of the microbiota may also explain, in part, the spatial stability of the host-microbe associations observed in octocorals. However, there is likely also a strong selection for a specific microbiota by the host and potentially some microbes (see the “Microbiome regulation” section), which would be required for such stability, especially for life in a “microbial soup,” like the ocean. Given their selection potential and microbiota stability, as well as their associations with microbes commonly found on a range of marine invertebrates, octocorals are likely good model systems to study complex marine invertebrate-microbe symbioses. Taken together, octocorals may provide a good system to not only study coral-microbe symbioses, but also address basic questions in our understanding on holobiont assembly, functioning, and ecological evolution.
Conclusions
Since the recognition that corals are holobionts through their intricate relationships with microbial symbionts, significant research efforts have investigated the coral microbiome composition and are beginning to focus on its functional role. Currently, we know that the microbial assemblages associated with soft corals are relatively stable and that the holobiont possesses various mechanisms to regulate its composition depending on the environmental conditions. This regulatory capacity may be one of the reasons why octocorals are so successful and inhabit many marine habitats. Connecting the functional links between host and microbial symbionts and elucidating the microbiome dynamics under various conditions will be one of the main challenges. The use of novel approaches, such as metagenomics and metatranscriptomics, combined with specialized mass spectrometry techniques will help to unravel the functions of the octocoral-associated microbes and highlight their importance for host fitness and may further reveal the potential of the octocoral holobiont as a source of new natural products and drugs. Understanding octocoral microbiome dynamics and the functional roles of all microbial symbionts within the holobiont will assist the development of strategies to help build resilience in corals under environmental change.
Acknowledgements
The authors thank Eric Béraud (Scientific Centre of Monaco, Monaco), Carlo Cerrano (Polytechnic University of Marche, Italy), Sergio Rossi (University of Salento, Italy), and Ernesto Weil (University of Puerto Rico at Mayagüez, Puerto Rico) for sharing their photos for inclusion in this work. We also thank the Fondation Paul Hamel for the funding that made this work possible.
Funding
The funding was provided by the Fondation Paul Hamel.
Availability of data and materials
All data discussed in this review have been previously published and are available.
Abbreviations
- AHL
N-acylhomoserine lactone
- AMP
Antimicrobial peptide
- BBD
Black band disease
- DOPA
Dihydroxyphenylalanine
- EMP
Earth Microbiome Project
- HISH
Halogen in situ hybridization
- ITS
Internal transcribed spacer
- LSMA
Locally stable microbial associates
- MFPS
Multifocal purple spots
- NP
Natural product
- OTU
Operational taxonomic unit
- P:R ratio
Photosynthesis:respiration ratio
- POX
Peroxidase
- QS
Quorum sensing
- RBD
Red band disease
- ROS
Reactive oxygen species
- SA:V ratio
Surface area:volume ratio
- SIMS
Secondary ion mass spectrometry
- SOD
Superoxide dismutase
- TOF
Time-of-flight
Authors’ contributions
JvdW and CFP wrote the manuscript. DA guided and assisted in writing the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McFadden CS, Sánchez JA, France SC. Molecular phylogenetic insights into the evolution of Octocorallia: a review. Integr Comp Biol. 2010;50(3):389–410. doi: 10.1093/icb/icq056. [DOI] [PubMed] [Google Scholar]
- 2.Systematic list of valid octocoral genera. http://researcharchive.calacademy.org/research/izg/OCTOCLASS.htm. Accessed 12 Feb 2018.
- 3.Ascione C. The art of coral: myth, history and manufacture from ancient times to the present. In: Cicogna F, Cattaneo-Vietti R, editors. Red coral in the Mediterranean sea: art, history and science. Rome: Ministero delle Risorse Agricole, Alimentari e Forestali; 1993. pp. 11–36. [Google Scholar]
- 4.Pérez CD, de Moura Neves B, Cordeiro RT, Williams GC, Diversity CSD. Distribution of Octocorallia. In: Goffredo S, Dubinsky Z, editors. The Cnidaria, past, present and future: the world of Medusa and her sisters. Cham: Springer International Publishing; 2016. pp. 109–123. [Google Scholar]
- 5.Yesson C, Taylor ML, Tittensor DP, Davies AJ, Guinotte J, Baco A, Black J, Hall-Spencer JM, Rogers AD. Global habitat suitability of cold-water octocorals. J Biogeogr. 2012;39(7):1278–1292. [Google Scholar]
- 6.Gili J-M, Coma R. Benthic suspension feeders: their paramount role in littoral marine food webs. Trends Ecol Evol. 1998;13(8):316–321. doi: 10.1016/s0169-5347(98)01365-2. [DOI] [PubMed] [Google Scholar]
- 7.Fabricius KE, Klumpp DW. Widespread mixotrophy in reef-inhabiting soft corals: the influence of depth, and colony expansion and contraction on photosynthesis. Mar Ecol Prog Ser. 1995;125:195–204. [Google Scholar]
- 8.Ballesteros E. Mediterranean Coralligenous assemblages. In: Oceanography and marine biology. Boca Raton: CRC Press; 2006. p. 123–95.
- 9.Roberts JM, Wheeler AJ, Freiwald A. Reefs of the deep: the biology and geology of cold-water coral ecosystems. Science. 2006;312(5773):543–547. doi: 10.1126/science.1119861. [DOI] [PubMed] [Google Scholar]
- 10.Knowlton N, Rohwer F. Multispecies microbial mutualisms on coral reefs: the host as a habitat. Am Nat. 2003;162(4 Suppl):S51–S62. doi: 10.1086/378684. [DOI] [PubMed] [Google Scholar]
- 11.Bourne DG, Webster NS. Coral reef bacterial communities. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F, editors. The prokaryotes: prokaryotic communities and ecophysiology. Berlin, Heidelberg: Springer Berlin Heidelberg; 2013. pp. 163–187. [Google Scholar]
- 12.McFall-Ngai M, Hadfield MG, Bosch TCG, Carey HV, Domazet-Lošo T, Douglas AE, Dubilier N, Eberl G, Fukami T, Gilbert SF, et al. Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci. 2013;110(9):3229–3236. doi: 10.1073/pnas.1218525110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muscatine L, R McCloskey L, E Marian R. Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration1. Limnol Oceanogr. 1981;26(4):601–611. [Google Scholar]
- 14.Bednarz VN, Grover R, Maguer J-F, Fine M, Ferrier-Pagès C. The assimilation of Diazotroph-derived nitrogen by Scleractinian corals depends on their metabolic status. MBio. 2017;8(1):e02058–e02016. doi: 10.1128/mBio.02058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raina J-B, Tapiolas D, Willis BL, Bourne DG. Coral-associated bacteria and their role in the biogeochemical cycling of sulfur. Appl Environ Microbiol. 2009;75(11):3492–3501. doi: 10.1128/AEM.02567-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kvennefors EC, Sampayo E, Kerr C, Vieira G, Roff G, Barnes AC. Regulation of bacterial communities through antimicrobial activity by the coral holobiont. Microb Ecol. 2012;63(3):605–618. doi: 10.1007/s00248-011-9946-0. [DOI] [PubMed] [Google Scholar]
- 17.Ainsworth TD, Krause L, Bridge T, Torda G, Raina J-B, Zakrzewski M, Gates RD, Padilla-Gamino JL, Spalding HL, Smith C, et al. The coral core microbiome identifies rare bacterial taxa as ubiquitous endosymbionts. ISME J. 2015;9(10):2261–2274. doi: 10.1038/ismej.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez-Agreda A, Leggat W, Bongaerts P, Ainsworth TD. The microbial signature provides insight into the mechanistic basis of coral success across reef habitats. MBio. 2016;7(4):e00560–e00516. doi: 10.1128/mBio.00560-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van de Water JAJM, Melkonian R, Voolstra CR, Junca H, Beraud E, Allemand D, Ferrier-Pagès C. Comparative assessment of Mediterranean gorgonian-associated microbial communities reveals conserved core and locally variant bacteria. Microb Ecol. 2017;73(2):466–478. doi: 10.1007/s00248-016-0858-x. [DOI] [PubMed] [Google Scholar]
- 20.van de Water JAJM, Voolstra CR, Rottier C, Cocito S, Peirano A, Allemand D, Ferrier-Pagès C. Seasonal stability in the microbiomes of temperate gorgonians and the red coral Corallium rubrum across the Mediterranean Sea. Microb Ecol. 2018;75(1):1–15. doi: 10.1007/s00248-017-1006-y. [DOI] [PubMed] [Google Scholar]
- 21.Bourne DG, Garren M, Work TM, Rosenberg E, Smith GW, Harvell CD. Microbial disease and the coral holobiont. Trends Microbiol. 2009;17(12):554–62. doi: 10.1016/j.tim.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Ainsworth TD, Thurber RV, Gates RD. The future of coral reefs: a microbial perspective. Trends Ecol Evol. 2010;25(4):233–240. doi: 10.1016/j.tree.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Mouchka ME, Hewson I, Harvell CD. Coral-associated bacterial assemblages: current knowledge and the potential for climate-driven impacts. Integr Comp Biol. 2010;50(4):662–674. doi: 10.1093/icb/icq061. [DOI] [PubMed] [Google Scholar]
- 24.Grover R, Maguer J-F, Reynaud-Vaganay S, Ferrier-Pagès C. Uptake of ammonium by the scleractinian coral Stylophora pistillata: effect of feeding, light, and ammonium concentrations. Limnol Oceanogr. 2002;47(3):782–790. [Google Scholar]
- 25.Houlbrèque F, Ferrier-Pagès C. Heterotrophy in tropical Scleractinian corals. Biol Rev. 2009;84(1):1–17. doi: 10.1111/j.1469-185X.2008.00058.x. [DOI] [PubMed] [Google Scholar]
- 26.Forcioli D, Merle PL, Caligara C, Ciosi M, Muti C, Francour P, Cerrano C, Allemand D. Symbiont diversity is not involved in depth acclimation in the Mediterranean Sea whip Eunicella singularis. Mar Ecol Prog Ser. 2011;439:57–71. [Google Scholar]
- 27.Benayahu Y, Achituv Y, Berner T. Embryogenesis and acquisition of algal symbionts by planulae of Xenia umbellata (Octocorallia: Alcyonacea) Mar Biol. 1988;100(1):93–101. [Google Scholar]
- 28.Franklin EC, Stat M, Pochon X, Putnam HM, Gates RD. GeoSymbio: a hybrid, cloud-based web application of global geospatial bioinformatics and ecoinformatics for Symbiodinium–host symbioses. Mol Ecol Resour. 2012;12(2):369–373. doi: 10.1111/j.1755-0998.2011.03081.x. [DOI] [PubMed] [Google Scholar]
- 29.Van Oppen MJH, Mieog JC, SÁNchez CA, Fabricius KE. Diversity of algal endosymbionts (zooxanthellae) in octocorals: the roles of geography and host relationships. Mol Ecol. 2005;14(8):2403–2417. doi: 10.1111/j.1365-294X.2005.02545.x. [DOI] [PubMed] [Google Scholar]
- 30.Goulet TL, Simmons C, Goulet D. Worldwide biogeography of Symbiodinium in tropical octocorals. Mar Ecol Prog Ser. 2008;355:45–58. [Google Scholar]
- 31.FitzPatrick SK, Liberatore KL, Garcia JR, Burghardt I, Colman DR, Moquin SA, Takacs-Vesbach CD, Shepherd UL. Symbiodinium diversity in the soft coral Heteroxenia sp. and its nudibranch predator Phyllodesmium lizardensis. Coral Reefs. 2012;31(3):895–905. [Google Scholar]
- 32.Goulet TL, Coffroth MA. The genetic identity of dinoflagellate symbionts in Caribbean octocorals. Coral Reefs. 2004;23(4):465–472. [Google Scholar]
- 33.Tamar LG, Mary Alice C. Stability of an octocoral-algal symbiosis over time and space. Mar Ecol Prog Ser. 2003;250:117–124. [Google Scholar]
- 34.Hannes AR, Barbeitos M, Coffroth MA. Stability of symbiotic dinoflagellate type in the octocoral Briareum asbestinum. Mar Ecol Prog Ser. 2009;391:65–72. [Google Scholar]
- 35.Kirk NL, Ward JR, Coffroth MA. Stable Symbiodinium composition in the sea fan Gorgonia ventalina during temperature and disease stress. Biol Bull. 2005;209(3):227–234. doi: 10.2307/3593112. [DOI] [PubMed] [Google Scholar]
- 36.Poland DM, Coffroth MA. Trans-generational specificity within a cnidarian–algal symbiosis. Coral Reefs. 2017;36(1):119–129. [Google Scholar]
- 37.Fitt WK. Effect of different strains of the zooxanthella Symbiodinium microadriaticum on growth and survival of their coelenterate and molluscan hosts. Proc 5th Int Coral Reef Congress. 1985;6:131–136. [Google Scholar]
- 38.Andras JP, Kirk NL, Drew Harvell C. Range-wide population genetic structure of Symbiodinium associated with the Caribbean sea fan coral, Gorgonia ventalina. Mol Ecol. 2011;20(12):2525–2542. doi: 10.1111/j.1365-294X.2011.05115.x. [DOI] [PubMed] [Google Scholar]
- 39.Prada C, McIlroy SE, Beltrán DM, Valint DJ, Ford SA, Hellberg ME, Coffroth MA. Cryptic diversity hides host and habitat specialization in a gorgonian-algal symbiosis. Mol Ecol. 2014;23(13):3330–3340. doi: 10.1111/mec.12808. [DOI] [PubMed] [Google Scholar]
- 40.Kirk NL, Andras JP, Harvell CD, Santos SR, Coffroth MA. Population structure of Symbiodinium sp. associated with the common sea fan, Gorgonia ventalina, in the Florida Keys across distance, depth, and time. Mar Biol. 2009;156(8):1609–1623. [Google Scholar]
- 41.Vafidis D, Koukouras A, Voultsiadou-Koukoura E. Octocoral fauna of the Aegean Sea with a check list of the Mediterranean species: new information, faunal comparisons. Ann Inst Oceanogr. 1994;70(2):217–230. [Google Scholar]
- 42.Sanchez JA, Wirshing HH. A field key to the identification of tropical western Atlantic Zooxanthellate Octocorals (Octocorallia: Cnidaria) Caribb J Sci. 2005;41(3):508–522. [Google Scholar]
- 43.Benayahu Y, Yosief T, Schleyer MH, Schleyerc MH, Yosief T, Schleyerc MH. Soft corals (Octocorallia, Alcyonacea) of the southern Red Sea. Isr J Zool. 2002;48(4):273–283. [Google Scholar]
- 44.Ferrier-Pagès C, Reynaud S, Béraud E, Rottier C, Menu D, Duong G, Gévaert F. Photophysiology and daily primary production of a temperate symbiotic gorgonian. Photosynth Res. 2015;123(1):95–104. doi: 10.1007/s11120-014-0042-4. [DOI] [PubMed] [Google Scholar]
- 45.Ribes M, Coma R, Gili J-M. Heterotrophic feeding by gorgonian corals with symbiotic zooxanthella. Limnol Oceanogr. 1998;43(6):1170–1179. [Google Scholar]
- 46.Coma R. Seasonality of in situ respiration rate in three temperate benthic suspension feeders. Limnol Oceanogr. 2002;47(1):324–331. [Google Scholar]
- 47.Bednarz VN, Cardini U, van Hoytema N, Al-Rshaidat MMD, Wild C. Seasonal variation in dinitrogen fixation and oxygen fluxes associated with two dominant zooxanthellate soft corals from the northern Red Sea. Mar Ecol Prog Ser. 2015;519:141–152. [Google Scholar]
- 48.Farrant PA. Population dynamics of the temperate Australian soft coral Capnella gaboensis. Mar Biol. 1987;96(3):401–407. [Google Scholar]
- 49.Cocito S, Ferrier-Pagès C, Cupido R, Rottier C, Meier-Augenstein W, Kemp H, Reynaud S, Peirano A. Nutrient acquisition in four Mediterranean gorgonian species. Mar Ecol Prog Ser. 2013;473:179–188. [Google Scholar]
- 50.Baker DM, Freeman CJ, Knowlton N, Thacker RW, Kim K, Fogel ML. Productivity links morphology, symbiont specificity and bleaching in the evolution of Caribbean octocoral symbioses. ISME J. 2015;9(12):2620–2629. doi: 10.1038/ismej.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Porter SS, Simms EL. Selection for cheating across disparate environments in the legume-rhizobium mutualism. Ecol Lett. 2014;17(9):1121–1129. doi: 10.1111/ele.12318. [DOI] [PubMed] [Google Scholar]
- 52.Ferrier-Pagès C, Peirano A, Abbate M, Cocito S, Negri A, Rottier C, Riera P, Rodolfo-Metalpa R, Reynaud S. Summer autotrophy and winter heterotrophy in the temperate symbiotic coral Cladocora caespitosa. Limnol Oceanogr. 2011;56(4):1429–1438. [Google Scholar]
- 53.Prada C, Weil E, Yoshioka PM. Octocoral bleaching during unusual thermal stress. Coral Reefs. 2010;29(1):41–45. [Google Scholar]
- 54.Sammarco PW, Strychar KB. Responses to high seawater temperatures in Zooxanthellate Octocorals. PLoS One. 2013;8(2):e54989. doi: 10.1371/journal.pone.0054989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harvell D, Kim K, Quirolo C, Weir J, Smith G. Coral bleaching and disease: contributors to 1998 mass mortality in Briareum asbestinum (Octocorallia, Gorgonacea) Hydrobiologia. 2001;460(1):97–104. [Google Scholar]
- 56.Weil E, Rogers CS, Croquer A. Octocoral diseases in a changing ocean. In: Rossi S, Bramanti L, Gori A, Orejas Saco del Valle C, editors. Marine animal forests: the ecology of benthic biodiversity hotspots. Cham: Springer International Publishing; 2015. pp. 1–55. [Google Scholar]
- 57.Netherton SE, Scheer DM, Morrison PR, Parrin AP, Blackstone NW. Physiological correlates of symbiont migration during bleaching of two octocoral species. J Exp Biol. 2014;217(9):1469–1477. doi: 10.1242/jeb.095414. [DOI] [PubMed] [Google Scholar]
- 58.Parrin AP, Goulet TL, Yaeger MA, Bross LS, McFadden CS, Blackstone NW. Symbiodinium migration mitigates bleaching in three octocoral species. J Exp Mar Biol Ecol. 2016;474:73–80. [Google Scholar]
- 59.Goulet TL, Shirur KP, Ramsby BD, Iglesias-Prieto R. The effects of elevated seawater temperatures on Caribbean gorgonian corals and their algal symbionts, Symbiodinium spp. PLoS One. 2017;12(2):e0171032. doi: 10.1371/journal.pone.0171032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim K, Rypien K. Aspergillosis of Caribbean sea fan corals, Gorgonia spp. In: Diseases of coral. Hoboken: Wiley; 2015. p. 236–41.
- 61.Kiho K, Harvell CD. The rise and fall of a six-year coral-fungal epizootic. Am Nat. 2004;164(S5):S52–S63. doi: 10.1086/424609. [DOI] [PubMed] [Google Scholar]
- 62.Smith GW, Weil E. Aspergillosis of gorgonians. In: Rosenberg E, Loya Y, editors. Coral health and disease. Berlin, Heidelberg: Springer Berlin Heidelberg; 2004. pp. 279–287. [Google Scholar]
- 63.Zuluaga-Montero A, Toledo-Hernández C, Rodríguez JA, Sabat AM, Bayman P. Spatial variation in fungal communities isolated from healthy and diseased sea fans Gorgonia ventalina and seawater. Aquat Biol. 2010;8(2):151–160. [Google Scholar]
- 64.Toledo-Hernández C, Zuluaga-Montero A, Bones-González A, Rodríguez JA, Sabat AM, Bayman P. Fungi in healthy and diseased sea fans (Gorgonia ventalina): is Aspergillus sydowii always the pathogen? Coral Reefs. 2008;27(3):707–714. [Google Scholar]
- 65.Soler-Hurtado MM, Sandoval-Sierra JV, Machordom A, Diéguez-Uribeondo J. Aspergillus sydowii and other potential fungal pathogens in gorgonian octocorals of the Ecuadorian Pacific. PLoS One. 2016;11(11):e0165992. doi: 10.1371/journal.pone.0165992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koh LL, Tan TK, Chou LM, Gob NKC. Fungi associated with gorgonians in Singapore. In: Proceedings of the 9th international coral reef symposium 23–27 October 2000. Ministry of Environment: Bali; 2002;1:521–526.
- 67.Zhang X-Y, Bao J, Wang G-H, He F, Xu X-Y, Qi S-H. Diversity and antimicrobial activity of Culturable fungi isolated from six species of the South China Sea gorgonians. Microb Ecol. 2012;64(3):617–627. doi: 10.1007/s00248-012-0050-x. [DOI] [PubMed] [Google Scholar]
- 68.Thurber RV, Payet JP, Thurber AR, Correa AMS. Virus-host interactions and their roles in coral reef health and disease. Nat Rev Microbiol. 2017;15(4):205–216. doi: 10.1038/nrmicro.2016.176. [DOI] [PubMed] [Google Scholar]
- 69.Hewson I, Brown JM, Burge CA, Couch CS, LaBarre BA, Mouchka ME, Naito M, Harvell CD. Description of viral assemblages associated with the Gorgonia ventalina holobiont. Coral Reefs. 2012;31(2):487–491. doi: 10.1007/s00338-011-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gudenkauf BM, Hewson I. Comparative metagenomics of viral assemblages inhabiting four phyla of marine invertebrates. Front Mar Sci. 2016;3(23):1–12. [Google Scholar]
- 71.Correa AMS, Welsh RM, Vega Thurber RL. Unique nucleocytoplasmic dsDNA and +ssRNA viruses are associated with the dinoflagellate endosymbionts of corals. ISME J. 2013;7(1):13–27. doi: 10.1038/ismej.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lawrence SA, Wilson WH, Davy JE, Davy SK. Latent virus-like infections are present in a diverse range of Symbiodinium spp. (Dinophyta) J Phycol. 2014;50(6):984–997. doi: 10.1111/jpy.12242. [DOI] [PubMed] [Google Scholar]
- 73.Marhaver KL, Edwards RA, Rohwer F. Viral communities associated with healthy and bleaching corals. Environ Microbiol. 2008;10(9):2277–2286. doi: 10.1111/j.1462-2920.2008.01652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soffer N, Brandt ME, Correa AMS, Smith TB, Thurber RV. Potential role of viruses in white plague coral disease. ISME J. 2014;8(2):271–283. doi: 10.1038/ismej.2013.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weynberg KD, Wood-Charslon EM, Suttle C, van Oppen MJ. Generating viral metagenomes from the coral holobiont. Front Microbiol. 2014;5:206. doi: 10.3389/fmicb.2014.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bayer T, Arif C, Ferrier-Pagès C, Zoccola D, Aranda M, Voolstra C. Bacteria of the genus Endozoicomonas dominate the microbiome of the Mediterranean gorgonian coral Eunicella cavolini. Mar Ecol Prog Ser. 2013;479:75–84. [Google Scholar]
- 77.Correa H, Haltli B, Duque C, Kerr R. Bacterial communities of the gorgonian octocoral Pseudopterogorgia elisabethae. Microb Ecol. 2013;66(4):972–985. doi: 10.1007/s00248-013-0267-3. [DOI] [PubMed] [Google Scholar]
- 78.Vezzulli L, Pezzati E, Huete-Stauffer C, Pruzzo C, Cerrano C. 16SrDNA pyrosequencing of the Mediterranean gorgonian Paramuricea clavata reveals a link among alterations in bacterial holobiont members, anthropogenic influence and disease outbreaks. PLoS One. 2013;8(6):e67745. doi: 10.1371/journal.pone.0067745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ransome E, Rowley SJ, Thomas S, Tait K, Munn CB. Disturbance to conserved bacterial communities in the cold-water gorgonian coral Eunicella verrucosa. FEMS Microbiol Ecol. 2014;90(2):404–416. doi: 10.1111/1574-6941.12398. [DOI] [PubMed] [Google Scholar]
- 80.La Rivière M, Garrabou J, Bally M. Interspecific comparisons of host- associated bacterial diversity support coevolution of Hahellaceae and gorgonian corals. Rapp Comm Int Mer Médit. 2013;40:388.
- 81.La Rivière M, Garrabou J, Bally M. Evidence for host specificity among dominant bacterial symbionts in temperate gorgonian corals. Coral Reefs. 2015;34(4):1087–1098. [Google Scholar]
- 82.La Rivière M, Roumagnac M, Garrabou J, Bally M. Transient shifts in bacterial communities associated with the temperate gorgonian Paramuricea clavata in the northwestern Mediterranean Sea. PLoS One. 2013;8(2):e57385. doi: 10.1371/journal.pone.0057385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van de Water JAJM, Melkonian R, Junca H, Voolstra CR, Reynaud S, Allemand D, Ferrier-Pagès C. Spirochaetes dominate the microbial community associated with the red coral Corallium rubrum on a broad geographic scale. Sci Rep. 2016;6:27277. doi: 10.1038/srep27277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jensen S, Duperron S, Birkeland N-K, Hovland M. Intracellular Oceanospirillales bacteria inhabit gills of Acesta bivalves. FEMS Microbiol Ecol. 2010;74(3):523–533. doi: 10.1111/j.1574-6941.2010.00981.x. [DOI] [PubMed] [Google Scholar]
- 85.Morrow KM, Moss AG, Chadwick NE, Liles MR. Bacterial associates of two Caribbean coral species reveal species-specific distribution and geographic variability. Appl Environ Microbiol. 2012;78(18):6438–6449. doi: 10.1128/AEM.01162-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Katharios P, Seth-Smith HMB, Fehr A, Mateos JM, Qi W, Richter D, Nufer L, Ruetten M, Guevara Soto M, Ziegler U, et al. Environmental marine pathogen isolation using mesocosm culture of sharpsnout seabream: striking genomic and morphological features of novel Endozoicomonas sp. Sci Rep. 2015;5:17609. doi: 10.1038/srep17609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Forget NL, Kim Juniper S. Free-living bacterial communities associated with tubeworm (Ridgeia piscesae) aggregations in contrasting diffuse flow hydrothermal vent habitats at the main Endeavour field, Juan de Fuca Ridge. MicrobiologyOpen. 2013;2(2):259–275. doi: 10.1002/mbo3.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fiore CL, Labrie M, Jarett JK, Lesser MP. Transcriptional activity of the giant barrel sponge, Xestospongia muta Holobiont: molecular evidence for metabolic interchange. Front Microbiol. 2015;6(364):1–18. [DOI] [PMC free article] [PubMed]
- 89.Robertson V, Haltli B, McCauley E, Overy D, Kerr R. Highly variable bacterial communities associated with the Octocoral Antillogorgia elisabethae. Microorganisms. 2016;4(3):23. doi: 10.3390/microorganisms4030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sunagawa S, Woodley CM, Medina M. Threatened corals provide underexplored microbial habitats. PLoS One. 2010;5(3):e9554. doi: 10.1371/journal.pone.0009554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wessels W, Sprungala S, Watson S-A, Miller DJ, Bourne DG. The microbiome of the octocoral Lobophytum pauciflorum: minor differences between sexes and resilience to short-term stress. FEMS Microbiol Ecol. 2017;93(5):fix013. doi: 10.1093/femsec/fix013. [DOI] [PubMed] [Google Scholar]
- 92.Bourne DG, Dennis PG, Uthicke S, Soo RM, Tyson GW, Webster N. Coral reef invertebrate microbiomes correlate with the presence of photosymbionts. ISME J. 2013;7(7):1452–1458. doi: 10.1038/ismej.2012.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCauley EP, Haltli B, Correa H, Kerr RG. Spatial and temporal investigation of the microbiome of the Caribbean octocoral Erythropodium caribaeorum. FEMS Microbiol Ecol. 2016;92(9):fiw147. doi: 10.1093/femsec/fiw147. [DOI] [PubMed] [Google Scholar]
- 94.Kellogg CA, Ross SW, Brooke SD. Bacterial community diversity of the deep-sea octocoral Paramuricea placomus. PeerJ. 2016;4:e2529. doi: 10.7717/peerj.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bayer T, Neave MJ, Alsheikh-Hussain A, Aranda M, Yum LK, Mincer T, Hughen K, Apprill A, Voolstra CR. The microbiome of the Red Sea coral Stylophora pistillata is dominated by tissue-associated Endozoicomonas bacteria. Appl Environ Microbiol. 2013;79(15):4759–4762. doi: 10.1128/AEM.00695-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.La Rivière M, Garel M, Bally M. Localization of endobacteria in the gastrodermis of a Mediterranean gorgonian coral, Paramuricea clavata, using fluorescence in situ hybridization. Mar Biol. 2016;163(10):206. [Google Scholar]
- 97.Neave MJ, Apprill A, Ferrier-Pagès C, Voolstra CR. Diversity and function of prevalent symbiotic marine bacteria in the genus Endozoicomonas. Appl Microbiol Biotechnol. 2016;100(19):8315–8324. doi: 10.1007/s00253-016-7777-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Spring S, Scheuner C, Goker M, Klenk HP. A taxonomic framework for emerging groups of ecologically important marine gammaproteobacteria based on the reconstruction of evolutionary relationships using genome-scale data. Front Microbiol. 2015;6:281. doi: 10.3389/fmicb.2015.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oh H-M, Kang I, Ferriera S, Giovannoni SJ, Cho J-C. Genome sequence of the oligotrophic marine Gammaproteobacterium HTCC2143, isolated from the Oregon coast. J Bacteriol. 2010;192(17):4530–4531. doi: 10.1128/JB.00683-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spring S, Riedel T. Mixotrophic growth of bacteriochlorophyll a-containing members of the OM60/NOR5 clade of marine gammaproteobacteria is carbon-starvation independent and correlates with the type of carbon source and oxygen availability. BMC Microbiol. 2013;13(1):117. doi: 10.1186/1471-2180-13-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Keller-Costa T, Silva R, Lago-Lestón A, Costa R. Genomic insights into Aquimarina sp. strain EL33, a bacterial symbiont of the gorgonian coral Eunicella labiata. Genome Announc. 2016;4(4):e00855–e00816. doi: 10.1128/genomeA.00855-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gray MA, Stone RP, McLaughlin MR, Kellogg CA. Microbial consortia of gorgonian corals from the Aleutian islands. FEMS Microbiol Ecol. 2011;76(1):109–120. doi: 10.1111/j.1574-6941.2010.01033.x. [DOI] [PubMed] [Google Scholar]
- 103.Holm JB, Heidelberg KB. Microbiomes of Muricea californica and M. fruticosa: comparative analyses of two co-occurring eastern Pacific Octocorals. Front Microbiol. 2016;7:917. doi: 10.3389/fmicb.2016.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Penn K, Wu D, Eisen JA, Ward N. Characterization of bacterial communities associated with deep-sea corals on gulf of Alaska Seamounts. Appl Environ Microbiol. 2006;72(2):1680–1683. doi: 10.1128/AEM.72.2.1680-1683.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Porporato EMD, Lo Giudice A, Michaud L, De Domenico E, Spanò N. Diversity and antibacterial activity of the bacterial communities associated with two Mediterranean sea pens, Pennatula phosphorea and Pteroeides spinosum (Anthozoa: Octocorallia) Microb Ecol. 2013;66(3):701–714. doi: 10.1007/s00248-013-0260-x. [DOI] [PubMed] [Google Scholar]
- 106.Shirur KP, Jackson CR, Goulet TL. Lesion recovery and the bacterial microbiome in two Caribbean gorgonian corals. Mar Biol. 2016;163(12):238. [Google Scholar]
- 107.Neulinger SC, Gärtner A, Järnegren J, Ludvigsen M, Lochte K, Dullo W-C. Tissue-associated “Candidatus Mycoplasma corallicola” and filamentous bacteria on the cold-water coral Lophelia pertusa (Scleractinia) Appl Environ Microbiol. 2009;75(5):1437–1444. doi: 10.1128/AEM.01781-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fraune S, Zimmer M. Host-specificity of environmentally transmitted Mycoplasma-like isopod symbionts. Environ Microbiol. 2008;10(10):2497–2504. doi: 10.1111/j.1462-2920.2008.01672.x. [DOI] [PubMed] [Google Scholar]
- 109.Wang Y, Stingl U, Anton-Erxleben F, Geisler S, Brune A, Zimmer M. “Candidatus Hepatoplasma crinochetorum,” a new, stalk-forming lineage of Mollicutes colonizing the midgut glands of a terrestrial isopod. Appl Environ Microbiol. 2004;70(10):6166–6172. doi: 10.1128/AEM.70.10.6166-6172.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ziegler M, Roik A, Porter A, Zubier K, Mudarris MS, Ormond R, Voolstra CR. Coral microbial community dynamics in response to anthropogenic impacts near a major city in the central Red Sea. Mar Pollut Bull. 2015;105(2):629–640. doi: 10.1016/j.marpolbul.2015.12.045. [DOI] [PubMed] [Google Scholar]
- 111.Fraune S, Bosch TCG. Long-term maintenance of species-specific bacterial microbiota in the basal metazoan Hydra. Proc Natl Acad Sci U S A. 2007;104(32):13146–13151. doi: 10.1073/pnas.0703375104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ziegler M, Seneca FO, Yum LK, Palumbi SR, Voolstra CR. Bacterial community dynamics are linked to patterns of coral heat tolerance. Nat Commun. 2017;8:14213. doi: 10.1038/ncomms14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Franco T, Califano G, Gonçalves ACS, Cúcio C, Costa R. Draft genome sequence of Vibrio sp. strain Evh12, a bacterium retrieved from the gorgonian coral Eunicella verrucosa. Genome Announc. 2016;4(1):e01729–e01715. doi: 10.1128/genomeA.01729-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Harder T, Lau SCK, Dobretsov S, Fang TK, Qian P-Y. A distinctive epibiotic bacterial community on the soft coral Dendronephthya sp. and antibacterial activity of coral tissue extracts suggest a chemical mechanism against bacterial epibiosis. FEMS Microbiol Ecol. 2003;43(3):337–347. doi: 10.1111/j.1574-6941.2003.tb01074.x. [DOI] [PubMed] [Google Scholar]
- 115.Pasquale V, Guida M, Cennamo P, Mastascusa V, Greco M, Sandulli R. Cultivable heterotrophic bacteria associated to Corallium rubrum. Biol Mar Mediterr. 2011;18(1):274–275. [Google Scholar]
- 116.Le Roux F, Goubet A, Thompson FL, Faury N, Gay M, Swings J, Saulnier D. Vibrio gigantis sp. nov., isolated from the haemolymph of cultured oysters (Crassostrea gigas) Int J Syst Evol Microbiol. 2005;55(6):2251–2255. doi: 10.1099/ijs.0.63666-0. [DOI] [PubMed] [Google Scholar]
- 117.Beleneva IA, Kukhlevskii AD. Characterization of Vibrio gigantis and Vibrio pomeroyi isolated from invertebrates of Peter the Great Bay, Sea of Japan. Microbiology. 2010;79(3):402–407. [Google Scholar]
- 118.Frias-Lopez J, Zerkle AL, Bonheyo GT, Fouke BW. Partitioning of bacterial communities between seawater and healthy, black band diseased, and dead coral surfaces. Appl Environ Microbiol. 2002;68(5):2214–2228. doi: 10.1128/AEM.68.5.2214-2228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sekar R, Kaczmarsky L, Richardson L. Microbial community composition of black band disease on the coral host Siderastrea siderea from three regions of the wider Caribbean. Mar Ecol Prog Ser. 2008;362:85–98. [Google Scholar]
- 120.Kellogg CA, Lisle JT, Galkiewicz JP. Culture-independent characterization of bacterial communities associated with the cold-water coral Lophelia pertusa in the northeastern Gulf of Mexico. Appl Environ Microbiol. 2009;75(8):2294–2303. doi: 10.1128/AEM.02357-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lawler SN, Kellogg CA, France SC, Clostio RW, Brooke SD, Ross SW. Coral-associated bacterial diversity is conserved across two deep-sea Anthothela species. Front Microbiol. 2016;7:458. doi: 10.3389/fmicb.2016.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lilburn TG, Kim KS, Ostrom NE, Byzek KR, Leadbetter JR, Breznak JA. Nitrogen fixation by symbiotic and free-living spirochetes. Science. 2001;292(5526):2495–2498. doi: 10.1126/science.1060281. [DOI] [PubMed] [Google Scholar]
- 123.Leadbetter JR, Schmidt TM, Graber JR, Breznak JA. Acetogenesis from H2 plus CO2 by spirochetes from termite guts. Science. 1999;283(5402):686–689. doi: 10.1126/science.283.5402.686. [DOI] [PubMed] [Google Scholar]
- 124.Harris JK, Kelley ST, Pace NR. New perspective on uncultured bacterial phylogenetic division OP11. Appl Environ Microbiol. 2004;70(2):845–849. doi: 10.1128/AEM.70.2.845-849.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nelson W, Stegen J. The reduced genomes of Parcubacteria (OD1) contain signatures of a symbiotic lifestyle. Front Microbiol. 2015;6:713. doi: 10.3389/fmicb.2015.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brück TB, Brück WM, Santiago-Vázquez LZ, McCarthy PJ, Kerr RG. Diversity of the bacterial communities associated with the Azooxanthellate deep water octocorals Leptogorgia minimata, Iciligorgia schrammi, and Swiftia exertia. Mar Biotechnol. 2007;9(5):561–576. doi: 10.1007/s10126-007-9009-1. [DOI] [PubMed] [Google Scholar]
- 127.Kirchman DL. The ecology of Cytophaga–Flavobacteria in aquatic environments. FEMS Microbiol Ecol. 2002;39(2):91–100. doi: 10.1111/j.1574-6941.2002.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 128.Duque-Alarcón A, Santiago-Vásquez LZ, Kerr RG. A microbial community analysis of the octocoral Eunicea fusca. Electron J Biotechnol. 2012;15(5):1–9.
- 129.Pike RE, Haltli B, Kerr RG. Description of Endozoicomonas euniceicola sp. nov. and Endozoicomonas gorgoniicola sp. nov., bacteria isolated from the octocorals Eunicea fusca and Plexaura sp., and an emended description of the genus Endozoicomonas. Int J Syst Evol Microbiol. 2013;63(11):4294–4302. doi: 10.1099/ijs.0.051490-0. [DOI] [PubMed] [Google Scholar]
- 130.McCauley EP, Haltli B, Kerr RG. Description of Pseudobacteriovorax antillogorgiicola gen. nov., sp. nov., a bacterium isolated from the gorgonian octocoral Antillogorgia elisabethae, belonging to the family Pseudobacteriovoracaceae fam. nov., within the order Bdellovibrionales. Int J Syst Evol Microbiol. 2015;65(2):522–530. doi: 10.1099/ijs.0.066266-0. [DOI] [PubMed] [Google Scholar]
- 131.Berkelmans R, van Oppen MJH. The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc R Soc Lond B Biol Sci. 2006;273(1599):2305–2312. doi: 10.1098/rspb.2006.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tracy AM, Koren O, Douglas N, Weil E, Harvell CD. Persistent shifts in Caribbean coral microbiota are linked to the 2010 warm thermal anomaly. Environ Microbiol Rep. 2015;7(3):471–479. doi: 10.1111/1758-2229.12274. [DOI] [PubMed] [Google Scholar]
- 133.Meyer JL, Paul VJ, Teplitski M. Community shifts in the surface microbiomes of the coral Porites astreoides with unusual lesions. PLoS One. 2014;9(6):e100316. doi: 10.1371/journal.pone.0100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Morrow KM, Bourne DG, Humphrey C, Botte ES, Laffy P, Zaneveld J, Uthicke S, Fabricius KE, Webster NS. Natural volcanic CO2 seeps reveal future trajectories for host-microbial associations in corals and sponges. ISME J. 2015;9(4):894–908. doi: 10.1038/ismej.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Roder C, Bayer T, Aranda M, Kruse M, Voolstra CR. Microbiome structure of the fungid coral Ctenactis echinata aligns with environmental differences. Mol Ecol. 2015;24(13):3501–3511. doi: 10.1111/mec.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bourne D, Iida Y, Uthicke S, Smith-Keune C. Changes in coral-associated microbial communities during a bleaching event. ISME J. 2008;2(4):350–363. doi: 10.1038/ismej.2007.112. [DOI] [PubMed] [Google Scholar]
- 137.van de Water JAJM, Ainsworth TD, Leggat W, Bourne DG, Willis BL, van Oppen MJH. The coral immune response facilitates protection against microbes during tissue regeneration. Mol Ecol. 2015;24(13):3390–3404. doi: 10.1111/mec.13257. [DOI] [PubMed] [Google Scholar]
- 138.Aurelle D, Pivotto ID, Malfant M, Topçu NE, Masmoudi MB, Chaoui L, Kara HM, Coelho MAG, Castilho R, Haguenauer A. Fuzzy species limits in Mediterranean gorgonians (Cnidaria, Octocorallia): inferences on speciation processes. Zool Scr. 2017;46(6):767–778. [Google Scholar]
- 139.McFadden CS, France SC, Sanchez JA, Alderslade P. A molecular phylogenetic analysis of the Octocorallia (Cnidaria: Anthozoa) based on mitochondrial protein-coding sequences. Mol Phylogenet Evol. 2006;41(3):513–527. doi: 10.1016/j.ympev.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 140.Wirshing HH, Messing CG, Douady CJ, Reed J, Stanhope MJ, Shivji MS. Molecular evidence for multiple lineages in the gorgonian family Plexauridae (Anthozoa: Octocorallia) Mar Biol. 2005;147(2):497–508. [Google Scholar]
- 141.Sánchez JA, McFadden CS, France SC, Lasker HR. Molecular phylogenetic analyses of shallow-water Caribbean octocorals. Mar Biol. 2003;142(5):975–987. [Google Scholar]
- 142.Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet. 2008;42(1):165–190. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- 143.Sharp KH, Distel D, Paul VJ. Diversity and dynamics of bacterial communities in early life stages of the Caribbean coral Porites astreoides. ISME J. 2012;6(4):790–801. doi: 10.1038/ismej.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Apprill A, Marlow HQ, Martindale MQ, Rappe MS. The onset of microbial associations in the coral Pocillopora meandrina. ISME J. 2009;3(6):685–699. doi: 10.1038/ismej.2009.3. [DOI] [PubMed] [Google Scholar]
- 145.Sharp KH, Ritchie KB, Schupp PJ, Ritson-Williams R, Paul VJ. Bacterial acquisition in juveniles of several broadcast spawning coral species. PLoS One. 2010;5(5):e10898. doi: 10.1371/journal.pone.0010898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lafferty KD, Hofmann EE. Marine disease impacts, diagnosis, forecasting, management and policy. Philos Trans R Soc Lond B Biol Sci. 2016;371(1689):20150200. doi: 10.1098/rstb.2015.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Harvell D, Aronson R, Baron N, Connell J, Dobson A, Ellner S, Gerber L, Kim K, Kuris A, McCallum H, et al. The rising tide of ocean diseases: unsolved problems and research priorities. Front Ecol Environ. 2004;2(7):375–382. [Google Scholar]
- 148.Harvell D, Jordán-Dahlgren E, Merkel S, Rosenberg E, Raymundo L, Smith G, Weil E, Willis B. Coral disease, environmental drivers, and the balance between coral and microbial associates. Oceanography. 2007;20:172–195. [Google Scholar]
- 149.Aronson RB, Precht WF. Evolutionary paleoecology of Caribbean coral reefs. In: Evolutionary paleoecology. New York: Columbia University Press; 2001. p. 171–234.
- 150.Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301(5635):929–933. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- 151.Hughes TP. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science. 1994;265(5178):1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- 152.Maynard J, van Hooidonk R, Eakin CM, Puotinen M, Garren M, Williams G, Heron SF, Lamb J, Weil E, Willis B, et al. Projections of climate conditions that increase coral disease susceptibility and pathogen abundance and virulence. Nat Clim Chang. 2015;5(7):688–694. [Google Scholar]
- 153.Weil E, Rogers CS. Coral reef diseases in the Atlantic-Caribbean. In: Dubinsky Z, Stambler N, editors. Coral reefs: an ecosystem in transition. Dordrecht: Springer Netherlands; 2011. pp. 465–491. [Google Scholar]
- 154.Weil E, Hernandez EA, Bruckner AW, Ortiz AL, Nemeth M, Ruiz H. Distribution and status of acroporid (scleractinia) coral populations in Puerto Rico. In: Proceedings of the Caribbean workshop: potential application of the US Endangered Species Act (ESA) as a conservation strategy. Silverspring: NOAA Technical Memorandum NMFS-OPR-24. 2003; p. 71–92.
- 155.Aronson RB, Precht WF. White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia. 2001;460(1):25–38. [Google Scholar]
- 156.Rosenberg E, Ben-Haim Y. Microbial diseases of corals and global warming. Environ Microbiol. 2002;4(6):318–326. doi: 10.1046/j.1462-2920.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- 157.Smith GW, Ives LD, Nagelkerken IA, Ritchie KB. Caribbean sea-fan mortalities. Nature. 1996;383(6600):487. [Google Scholar]
- 158.Sánchez JA, Ardila NE, Andrade J, Dueñas LF, Navas R, Ballesteros D. Octocoral densities and mortalities in Gorgona Island, Colombia, Tropical Eastern Pacific. Rev Biol Trop. 2014;62:209–219. [Google Scholar]
- 159.Ruzicka RR, Colella MA, Porter JW, Morrison JM, Kidney JA, Brinkhuis V, Lunz KS, Macaulay KA, Bartlett LA, Meyers MK, et al. Temporal changes in benthic assemblages on Florida Keys reefs 11 years after the 1997/1998 El Niño. Mar Ecol Prog Ser. 2013;489:125–141. [Google Scholar]
- 160.Schärer MT, Nemeth MI. Mass mortality of gorgonians due to a Cyphoma gibbosum (Linnaeus) population outbreak at Mona Island, Puerto Rico. Coral Reefs. 2010;29(2):533. [Google Scholar]
- 161.Botero L. Observations on the size predators and tumor like outgrowths of gorgonian octocoral colonies in the area of Santa Marta Caribbean coast of Colombia. Northeast Gulf Sci. 1990;11(1):1–10. [Google Scholar]
- 162.Goldberg WM, Makemson JC, Colley SB. Entocladia endozoica sp. nov., a pathogenic chlorophyte: structure, life history, physiology, and effect on its coral host. Biol Bull-Us. 1984;166(2):368–383. [Google Scholar]
- 163.Kim K. Diseases of Octocorals. In: Diseases of coral. Hoboken: Wiley; 2015. p. 410–5.
- 164.Morse DE, Morse A, Duncan H, Trench RK. Algal tumors in the Caribbean Octocorallian, Gorgonia Ventalina: II. Biochemical characterization of the algae, and first epidemiological observations. B Mar Sci. 1981;31(2):399–409. [Google Scholar]
- 165.Willis B, Page C, Dinsdale E. Coral disease on the great barrier reef. In: Rosenberg E, Loya Y, editors. Coral health and disease. Berlin: Springer Berlin Heidelberg; 2004. p. 69–104.
- 166.Antonius A. Tenth meeting of the Association of Island Marine Laboratories of the Caribbean. Mayaguez, Puerto Rico: University of Puerto Rico; 1973. New observations on coral destruction in reefs; p. 3. [Google Scholar]
- 167.Antonius A. The ‘band’ diseases in coral reefs. In: Proceedings of the fourth international coral reef symposium in 1981. Quezon City: Marine Science Center; 1982;2:7–14.
- 168.Weil E. Coral reef diseases in the wider Caribbean. In: Rosenberg E, Loya Y, editors. Coral health and disease. Berlin, Heidelberg: Springer Berlin Heidelberg; 2004. pp. 35–68. [Google Scholar]
- 169.Sato Y, Ling EYS, Turaev D, Laffy P, Weynberg KD, Rattei T, Willis BL, Bourne DG. Unraveling the microbial processes of black band disease in corals through integrated genomics. Sci Rep. 2017;7:40455. [DOI] [PMC free article] [PubMed]
- 170.Richardson LL. Coral diseases: what is really known? Trends Ecol Evol. 1998;13(11):438–43. [DOI] [PubMed]
- 171.Cerrano C, Bavestrello G, Bianchi CN, Cattaneo-vietti R, Bava S, Morganti C, Morri C, Picco P, Sara G, Schiaparelli S, et al. A catastrophic mass-mortality episode of gorgonians and other organisms in the Ligurian Sea (North-Western Mediterranean), summer 1999. Ecol Lett. 2000;3(4):284–293. [Google Scholar]
- 172.Bally M, Garrabou J. Thermodependent bacterial pathogens and mass mortalities in temperate benthic communities: a new case of emerging disease linked to climate change. Glob Chang Biol. 2007;13(10):2078–2088. [Google Scholar]
- 173.Rafel C, Cristina L, Marta R, David D, Joaquim G, Enric B. Consequences of a mass mortality in populations of Eunicella singularis (Cnidaria: Octocorallia) in Menorca (NW Mediterranean) Mar Ecol Prog Ser. 2006;327:51–60. [Google Scholar]
- 174.Garrabou J, Coma R, Bensoussan N, Bally M, ChevaldonnÉ P, Cigliano M, Diaz D, Harmelin JG, Gambi MC, Kersting DK, et al. Mass mortality in Northwestern Mediterranean rocky benthic communities: effects of the 2003 heat wave. Glob Chang Biol. 2009;15(5):1090–1103. [Google Scholar]
- 175.Sutherland KP, Porter JW, Torres C. Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar Ecol Prog Ser. 2004;266:273–302. [Google Scholar]
- 176.Hall-Spencer JM, Pike J, Munn CB. Diseases affect cold-water corals too: Eunicella verrucosa (Cnidaria: Gorgonacea) necrosis in SW England. Dis Aquat Org. 2007;76(2):87–97. doi: 10.3354/dao076087. [DOI] [PubMed] [Google Scholar]
- 177.Morrison-Gardiner S. Studies on the morphology and ecology of fungi associated with the Australian marine environment. Townsville: James Cook University; 2001. [Google Scholar]
- 178.Burge CA, Mouchka ME, Harvell CD, Roberts S. Immune response of the Caribbean sea fan, Gorgonia ventalina, exposed to an Aplanochytrium parasite as revealed by transcriptome sequencing. Front Physiol. 2013;4:180. doi: 10.3389/fphys.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Burge CA, Douglas N, Conti-Jerpe I, Weil E, Roberts S, Friedman CS, Harvell CD. Friend or foe: the association of Labyrinthulomycetes with the Caribbean sea fan Gorgonia ventalina. Dis Aquat Org. 2012;101(1):1–12. doi: 10.3354/dao02487. [DOI] [PubMed] [Google Scholar]
- 180.Ivanenko VN, Nikitin MA, Hoeksema BW. Multiple purple spots in the Caribbean sea fan Gorgonia ventalina caused by parasitic copepods at St. Eustatius, Dutch Caribbean. Mar Biodivers. 2017;47(1):79–80. [Google Scholar]
- 181.Buhl-Mortensen L, Mortensen PB. Gorgonophilus canadensis n. gen., n. sp. (Copepoda: Lamippidae), a gall forming endoparasite in the octocoral Paragorgia arborea (L., 1758) from the Northwest Atlantic. Symbiosis. 2004;37:33–62. [Google Scholar]
- 182.Williams JD, Anchaluisa B, Boyko CB, McDaniel N. Description of a new endoparasitic copepod genus and species (Lamippidae) that induces gall formation in leaves of the sea pen Ptilosarcus gurneyi (Octocorallia) from British Columbia. Mar Biodivers. 2016. 10.1007/s12526-016-0593-z.
- 183.Erni Cassola G, Pacheco MSC, Barbosa MC, Hansen DM, Ferreira CEL. Decline in abundance and health state of an Atlantic subtropical gorgonian population. Mar Pollut Bull. 2016;104(1):329–334. doi: 10.1016/j.marpolbul.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 184.Weil E, Croquer A, Flynn K, Lucas M, Soto D, Lucas M, Rodriguez L, Sanabria D. Spatial and temporal dynamics of diseases affecting the sea-fan Gorgonia ventalina in La Parguera, southwest coast of Puerto Rico. In: Indo-Pacific coral reef symposium. Taiwan: International Society for Reef Studies; 2014.
- 185.Weil E, Croquer A, Urreiztieta I. Temporal variability and impact of coral diseases and bleaching in La Parguera, Puerto Rico from 2003–2007. Caribb J Sci. 2009;45(2–3):221–246. [Google Scholar]
- 186.Weil E, Cróquer A. Spatial variability in distribution and prevalence of Caribbean scleractinian coral and octocoral diseases. I. Community-level analysis. Dis Aquat Org. 2009;83(3):195–208. doi: 10.3354/dao02011. [DOI] [PubMed] [Google Scholar]
- 187.Metchnikoff E. Lectures on the comparative pathology of inflammation: delivered at the Pasteur institute in 1891. London: Kegan Paul Trench, Trubner & Co; 1893. [Google Scholar]
- 188.Salter-Cid L, Bigger CH. Alloimmunity in the gorgonian coral Swiftia exserta. Biol Bull. 1991;181(1):127–134. doi: 10.2307/1542495. [DOI] [PubMed] [Google Scholar]
- 189.Meszaros A, Bigger C. Qualitative and quantitative study of wound healing processes in the coelenterate, Plexaurella fusifera: spatial, temporal, and environmental (light attenuation) influences. J Invertebr Pathol. 1999;73(3):321–331. doi: 10.1006/jipa.1999.4851. [DOI] [PubMed] [Google Scholar]
- 190.Olano CT, Bigger CH. Phagocytic activities of the gorgonian coral Swiftia exserta. J Invertebr Pathol. 2000;76(3):176–184. doi: 10.1006/jipa.2000.4974. [DOI] [PubMed] [Google Scholar]
- 191.Menzel LP, Bigger CH. Identification of unstimulated constitutive immunocytes, by enzyme histochemistry, in the Coenenchyme of the octocoral Swiftia exserta. Biol Bull. 2015;229(2):199–208. doi: 10.1086/BBLv229n2p199. [DOI] [PubMed] [Google Scholar]
- 192.Mydlarz LD, Holthouse SF, Peters EC, Harvell CD. Cellular responses in sea fan corals: granular Amoebocytes react to pathogen and climate stressors. PLoS One. 2008;3(3):e1811. doi: 10.1371/journal.pone.0001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Petes LE, Harvell CD, Peters EC, Webb MAH, Mullen KM. Pathogens compromise reproduction and induce melanization in Caribbean sea fans. Mar Ecol Prog Ser. 2003;264:167–171. [Google Scholar]
- 194.Mydlarz LD, Jacobs RS. An inducible release of reactive oxygen radicals in four species of gorgonian corals. Mar Freshw Behav Phy. 2006;39(2):143–152. [Google Scholar]
- 195.Mydlarz LD, Harvell CD. Peroxidase activity and inducibility in the sea fan coral exposed to a fungal pathogen. Comp Biochem Physiol A Mol Integr Physiol. 2007;146(1):54–62. doi: 10.1016/j.cbpa.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 196.Couch CS, Mydlarz LD, Harvell CD, Douglas NL. Variation in measures of immunocompetence of sea fan coral, Gorgonia ventalina, in the Florida Keys. Mar Biol. 2008;155(3):281. [Google Scholar]
- 197.Douglas NL, Mullen KM, Talmage SC, Harvell CD. Exploring the role of chitinolytic enzymes in the sea fan coral, Gorgonia ventalina. Mar Biol. 2007;150(6):1137–1144. [Google Scholar]
- 198.Mann WT, Beach-Letendre J, Mydlarz LD. Interplay between proteases and protease inhibitors in the sea fan—aspergillus pathosystem. Mar Biol. 2014;161(10):2213–2220. [Google Scholar]
- 199.Jimbo M, Koike K, Sakai R, Muramoto K, Kamiya H. Cloning and characterization of a lectin from the octocoral Sinularia lochmodes. Biochem Biophys Res Commun. 2005;330(1):157–162. doi: 10.1016/j.bbrc.2005.02.137. [DOI] [PubMed] [Google Scholar]
- 200.Dishaw LJ, Smith SL, Bigger CH. Characterization of a C3-like cDNA in a coral: phylogenetic implications. Immunogenetics. 2005;57(7):535–548. doi: 10.1007/s00251-005-0005-1. [DOI] [PubMed] [Google Scholar]
- 201.Franzenburg S, Walter J, Kunzel S, Wang J, Baines JF, Bosch TC, Fraune S. Distinct antimicrobial peptide expression determines host species-specific bacterial associations. Proc Natl Acad Sci U S A. 2013;110(39):E3730–E3738. doi: 10.1073/pnas.1304960110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ward JR, Kiho K, Harvell CD. Temperature affects coral disease resistance and pathogen growth. Mar Ecol Prog Ser. 2007;329:115–121. [Google Scholar]
- 203.Dube D, Kiho K, Alker AP, Harvell CD. Size structure and geographic variation in chemical resistance of sea fan corals Gorgonia ventalina to a fungal pathogen. Mar Ecol Prog Ser. 2002;231:139–150. [Google Scholar]
- 204.Ellner SP, Jones LE, Mydlarz LD, Harvell CD. Within-host disease ecology in the sea fan Gorgonia ventalina: modeling the spatial immunodynamics of a coral-pathogen interaction. Am Nat. 2007;170(6):E143–E161. doi: 10.1086/522841. [DOI] [PubMed] [Google Scholar]
- 205.Jensen PR, Harvell CD, Wirtz K, Fenical W. Antimicrobial activity of extracts of Caribbean gorgonian corals. Mar Biol. 1996;125(2):411–419. [Google Scholar]
- 206.Kim K. Antimicrobial activity in gorgonian corals (Coelenterata, Octocorallia) Coral Reefs. 1994;13(2):75–80. [Google Scholar]
- 207.Slattery M, McClintock JB, Heine JN. Chemical defenses in Antarctic soft corals: evidence for antifouling compounds. J Exp Mar Biol Ecol. 1995;190(1):61–77. [Google Scholar]
- 208.Kim K, Kim PD, Alker AP, Harvell CD. Chemical resistance of gorgonian corals against fungal infections. Mar Biol. 2000;137(3):393–401. [Google Scholar]
- 209.Kim K, Harvell CD, Kim PD, Smith GW, Merkel SM. Fungal disease resistance of Caribbean sea fan corals (Gorgonia spp.) Mar Biol. 2000;136(2):259–267. [Google Scholar]
- 210.Franzenburg S, Fraune S, Kunzel S, Baines JF, Domazet-Loso T, Bosch TC. MyD88-deficient Hydra reveal an ancient function of TLR signaling in sensing bacterial colonizers. Proc Natl Acad Sci U S A. 2012;109(47):19374–19379. doi: 10.1073/pnas.1213110109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Asfahl KL, Schuster M, Gibbs K. Social interactions in bacterial cell–cell signaling. FEMS Microbiol Rev. 2017;41(1):92–107. doi: 10.1093/femsre/fuw038. [DOI] [PubMed] [Google Scholar]
- 212.Pietschke C, Treitz C, Forêt S, Schultze A, Künzel S, Tholey A, Bosch TCG, Fraune S. Host modification of a bacterial quorum-sensing signal induces a phenotypic switch in bacterial symbionts. Proc Natl Acad Sci. 2017;114(40):E8488–E8497. doi: 10.1073/pnas.1706879114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Freckelton ML. Quorum sensing in Australian soft corals. Townsville: PhD thesis, James Cook University; 2015.
- 214.Hunt LR, Smith SM, Downum KR, Mydlarz LD. Microbial regulation in gorgonian corals. Mar Drugs. 2012;10(6):1225. doi: 10.3390/md10061225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Skindersoe ME, Ettinger-Epstein P, Rasmussen TB, Bjarnsholt T, de Nys R, Givskov M. Quorum sensing antagonism from marine organisms. Mar Biotechnol. 2008;10(1):56–63. doi: 10.1007/s10126-007-9036-y. [DOI] [PubMed] [Google Scholar]
- 216.Tello E, Castellanos L, Arevalo-Ferro C, Rodríguez J, Jiménez C, Duque C. Absolute stereochemistry of antifouling cembranoid epimers at C-8 from the Caribbean octocoral Pseudoplexaura flagellosa. Revised structures of plexaurolones. Tetrahedron. 2011;67(47):9112–9121. [Google Scholar]
- 217.Tello E, Castellanos L, Arevalo-Ferro C, Duque C. Cembranoid diterpenes from the Caribbean sea whip Eunicea knighti. J Nat Prod. 2009;72(9):1595–1602. doi: 10.1021/np9002492. [DOI] [PubMed] [Google Scholar]
- 218.Tello E, Castellanos L, Arévalo-Ferro C, Duque C. Disruption in quorum-sensing systems and bacterial biofilm inhibition by cembranoid diterpenes isolated from the octocoral Eunicea knighti. J Nat Prod. 2012;75(9):1637–1642. doi: 10.1021/np300313k. [DOI] [PubMed] [Google Scholar]
- 219.Rocha J, Calado R, Leal M. Marine bioactive compounds from Cnidarians. In: Berlin KS-K, editor. Springer handbook of marine biotechnology. Heidelberg: Springer Berlin Heidelberg; 2015. pp. 823–849. [Google Scholar]
- 220.Wang C-Y, Wang K-L, Qian P-Y, Xu Y, Chen M, Zheng J-J, Liu M, Shao C-L, Wang C-Y. Antifouling phenyl ethers and other compounds from the invertebrates and their symbiotic fungi collected from the South China Sea. AMB Express. 2016;6(1):102. doi: 10.1186/s13568-016-0272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zheng C-J, Shao C-L, Chen M, Niu Z-G, Zhao D-L, Wang C-Y. Merosesquiterpenoids and ten-membered macrolides from a soft coral-derived Lophiostoma sp. fungus. Chem Biodivers. 2015;12(9):1407–1414. doi: 10.1002/cbdv.201400331. [DOI] [PubMed] [Google Scholar]
- 222.Hawas UW, El-Desouky S, Abou El-Kassem L, Elkhateeb W. Alternariol derivatives from Alternaria alternata, an endophytic fungus residing in red sea soft coral, inhibit HCV NS3/4A protease. Appl Biochem Micro+ 2015;51(5):579–584. [Google Scholar]
- 223.ElAhwany AMD, Ghozlan HA, ElSharif HA, Sabry SA. Phylogenetic diversity and antimicrobial activity of marine bacteria associated with the soft coral Sarcophyton glaucum. J Basic Microbiol. 2015;55(1):2–10. doi: 10.1002/jobm.201300195. [DOI] [PubMed] [Google Scholar]
- 224.Pham TM, Wiese J, Wenzel-Storjohann A, Imhoff JF. Diversity and antimicrobial potential of bacterial isolates associated with the soft coral Alcyonium digitatum from the Baltic Sea. Antonie Van Leeuwenhoek. 2016;109(1):105–119. doi: 10.1007/s10482-015-0613-1. [DOI] [PubMed] [Google Scholar]
- 225.Vizcaino MI, Johnson WR, Kimes NE, Williams K, Torralba M, Nelson KE, Smith GW, Weil E, Moeller PDR, Morris PJ. Antimicrobial resistance of the coral pathogen Vibrio coralliilyticus and Caribbean sister Phylotypes isolated from a diseased octocoral. Microb Ecol. 2010;59(4):646–657. doi: 10.1007/s00248-010-9644-3. [DOI] [PubMed] [Google Scholar]
- 226.Pinzón-Espinosa A, Martinez-Matamoros D, Castellanos L, Duque C, Rodríguez J, Jiménez C, Ramos FA. Cereusitin A, a cyclic tetrapeptide from a Bacillus cereus strain isolated from the soft coral Antillogorgia (syn. Pseudopterogorgia) elisabethae. Tetrahedron Lett. 2017;58(7):634–637. [Google Scholar]
- 227.Williams GC, Chen J-Y. Resurrection of the octocorallian genus Antillogorgia for Caribbean species previously assigned to Pseudopterogorgia, and a taxonomic assessment of the relationship of these genera with Leptogorgia (Cnidaria, Anthozoa, Gorgoniidae) Zootaxa. 2012;3505(1):39–52. [Google Scholar]
- 228.Boehnlein JM, Santiago-Vazquez LZ, Kerr RG. Diterpene biosynthesis by the dinoflagellate symbiont of the Caribbean gorgonian Pseudopterogorgia bipinnata. Mar Ecol Prog Ser. 2005;303:105–111. [Google Scholar]
- 229.Mydlarz LD, Jacobs RS, Boehnlein J, Kerr RG. Pseudopterosin biosynthesis in Symbiodinium sp., the dinoflagellate symbiont of Pseudopterogorgia elisabethae. Chem Biol. 2003;10(11):1051–1056. doi: 10.1016/j.chembiol.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 230.Mydlarz LD, Jacobs RS. Comparison of an inducible oxidative burst in free-living and symbiotic dinoflagellates reveals properties of the pseudopterosins. Phytochemistry. 2004;65(24):3231–3241. doi: 10.1016/j.phytochem.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 231.Reshef L, Koren O, Loya Y, Zilber-Rosenberg I, Rosenberg E. The coral probiotic hypothesis. Environ Microbiol. 2006;8(12):2068–2073. doi: 10.1111/j.1462-2920.2006.01148.x. [DOI] [PubMed] [Google Scholar]
- 232.Blunt JW, Copp BR, Keyzers RA, Munro MHG, Prinsep MR. Marine natural products. Nat Prod Rep. 2017;34(3):235–294. doi: 10.1039/c6np00124f. [DOI] [PubMed] [Google Scholar]
- 233.Gerhart DJ. Prostaglandin A2: an agent of chemical defense in the Caribbean gorgonian Plexaura homomalla. Mar Ecol Prog Ser. 1984;19:181–187. [Google Scholar]
- 234.Look SA, Fenical W, Jacobs RS, Clardy J. The pseudopterosins: anti-inflammatory and analgesic natural products from the sea whip Pseudopterogorgia elisabethae. Proc Natl Acad Sci. 1986;83(17):6238–6240. doi: 10.1073/pnas.83.17.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R, et al. Global warming and recurrent mass bleaching of corals. Nature. 2017;543(7645):373–377. doi: 10.1038/nature21707. [DOI] [PubMed] [Google Scholar]
- 236.Alonso C, Musat N, Adam B, Kuypers M, Amann R. HISH-SIMS analysis of bacterial uptake of algal-derived carbon in the Río de la Plata estuary. Syst Appl Microbiol. 2012;35(8):541–548. doi: 10.1016/j.syapm.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 237.Neave MJ, Rachmawati R, Xun L, Michell CT, Bourne DG, Apprill A, Voolstra CR. Differential specificity between closely related corals and abundant Endozoicomonas endosymbionts across global scales. ISME J. 2016;11(1):186–200. doi: 10.1038/ismej.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wang Y, Navin NE. Advances and applications of single-cell sequencing technologies. Mol Cell. 2015;58(4):598–609. [DOI] [PMC free article] [PubMed]
- 239.Pernice M, Levy O. Novel tools integrating metabolic and gene function to study the impact of the environment on coral symbiosis. Front Microbiol. 2014;5:448. doi: 10.3389/fmicb.2014.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Colliver TL, Brummel CL, Pacholski ML, Swanek FD, Ewing AG, Winograd N. Atomic and molecular imaging at the single-cell level with TOF-SIMS. Anal Chem. 1997;69(13):2225–2231. doi: 10.1021/ac9701748. [DOI] [PubMed] [Google Scholar]
- 241.Peixoto RS, Rosado PM, Leite DCDA, Rosado AS, Bourne DG. Beneficial microorganisms for corals (BMC): proposed mechanisms for coral health and resilience. Front Microbiol. 2017;8:341. doi: 10.3389/fmicb.2017.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Torda G, Donelson JM, Aranda M, Barshis DJ, Bay L, Berumen ML, Bourne DG, Cantin N, Foret S, Matz M, et al. Rapid adaptive responses to climate change in corals. Nat Clim Chang. 2017;7:627. [Google Scholar]
- 243.Theis KR, Dheilly NM, Klassen JL, Brucker RM, Baines JF, Bosch TCG, Cryan JF, Gilbert SF, Goodnight CJ, Lloyd EA, et al. Getting the hologenome concept right: an eco-evolutionary framework for hosts and their microbiomes. mSystems. 2016;1(2):e00028–e00016. doi: 10.1128/mSystems.00028-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Brooks AW, Kohl KD, Brucker RM, van Opstal EJ, Bordenstein SR. Phylosymbiosis: relationships and functional effects of microbial communities across host evolutionary history. PLoS Biol. 2016;14(11):e2000225. doi: 10.1371/journal.pbio.2000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wilkinson C. Status of coral reefs of the world: 2008. Townsville: Global Coral Reef Monitoring Network and Reef and Rainforest Research Center; 2008. p. 296. [Google Scholar]
- 246.Tilot V, Leujak W, Ormond RFG, Ashworth JA, Mabrouk A. Monitoring of South Sinai coral reefs: influence of natural and anthropogenic factors. Aquat Conserv Mar Freshwat Ecosyst. 2008;18(7):1109–1126. [Google Scholar]
- 247.Al-Zibdah MK, Damhoureyeh SA, Badran MI. Temporal variations in coral reef health at a coastal industrial site on the Gulf of Aqaba, Red Sea. Oceanologia. 2007;49(4):565–578. [Google Scholar]
- 248.Reinicke GB, Kroll DK, Schuhmacher H. Patterns and changes of reef-coral communities at the Sanganeb-Atoll (Sudan, central Red Sea): 1980 to 1991. Facies. 2003;49(1):271–297. [Google Scholar]
- 249.Bruckner AW, Dempsey AC, Stewart ICF. The status, threats, and resilience of reef-building corals of the Saudi Arabian Red Sea. In: Rasul NMA, editor. The Red Sea: the formation, morphology, oceanography and environment of a Young Ocean basin. Berlin, Heidelberg: Springer Berlin Heidelberg; 2015. pp. 471–486. [Google Scholar]
- 250.Lenz EA, Bramanti L, Lasker HR, Edmunds PJ. Long-term variation of octocoral populations in St. John, US Virgin Islands. Coral Reefs. 2015;34(4):1099–1109. [Google Scholar]
- 251.Stobart B, Teleki K, Buckley R, Downing N, Callow M. Coral recovery at Aldabra Atoll, Seychelles: five years after the 1998 bleaching event. Philos Trans R Soc A Math Phys Eng Sci. 2005;363(1826):251–255. doi: 10.1098/rsta.2004.1490. [DOI] [PubMed] [Google Scholar]
- 252.Wood E, Dipper F. What is the future for extensive areas of reef impacted by fish blasting and coral bleaching and now dominated by soft corals? A case study from Malaysia. In: Proceedings of the 11th international coral reef symposium. Fort Lauderdale: Nova Southeastern University National Coral Reef Institute; 2008.
- 253.Baum G, Januar I, Ferse SCA, Wild C, Kunzmann A. Abundance and physiology of dominant soft corals linked to water quality in Jakarta Bay, Indonesia. PeerJ. 2016;4:e2625. doi: 10.7717/peerj.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Fox HE, Pet JS, Dahuri R, Caldwell RL. Recovery in rubble fields: long-term impacts of blast fishing. Mar Pollut Bull. 2003;46(8):1024–1031. doi: 10.1016/S0025-326X(03)00246-7. [DOI] [PubMed] [Google Scholar]
- 255.Wakeford M, Done TJ, Johnson CR. Decadal trends in a coral community and evidence of changed disturbance regime. Coral Reefs. 2008;27(1):1–13. [Google Scholar]
- 256.Hoffmann TC. Coral reef health and effects of socio-economic factors in Fiji and Cook Islands. Mar Pollut Bull. 2002;44(11):1281–1293. doi: 10.1016/s0025-326x(02)00260-6. [DOI] [PubMed] [Google Scholar]
- 257.Webster NS, Bourne D. Bacterial community structure associated with the Antarctic soft coral, Alcyonium antarcticum. FEMS Microbiol Ecol. 2007;59(1):81–94. doi: 10.1111/j.1574-6941.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- 258.Wen ZH, Chao CH, Wu MH, Sheu JH. A neuroprotective sulfone of marine origin and the in vivo anti-inflammatory activity of an analogue. Eur J Med Chem. 2010;45(12):5998–6004. doi: 10.1016/j.ejmech.2010.09.067. [DOI] [PubMed] [Google Scholar]
- 259.Wu S-L, Su J-H, Wen Z-H, Hsu C-H, Chen B-W, Dai C-F, Kuo Y-H, Sheu J-H. Simplexins A−I, eunicellin-based diterpenoids from the soft coral Klyxum simplex. J Nat Prod. 2009;72(6):994–1000. doi: 10.1021/np900064a. [DOI] [PubMed] [Google Scholar]
- 260.Chao C-H, Wen Z-H, Wu Y-C, Yeh H-C, Sheu J-H. Cytotoxic and anti-inflammatory cembranoids from the soft coral Lobophytum crassum. J Nat Prod. 2008;71(11):1819–1824. doi: 10.1021/np8004584. [DOI] [PubMed] [Google Scholar]
- 261.Cheng SY, Wen ZH, Wang SK, Chiang MY, El-Gamal AA, Dai CF, Duh CY. Revision of the absolute configuration at C(23) of lanostanoids and isolation of secondary metabolites from formosan soft coral Nephthea erecta. Chem Biodivers. 2009;6(1):86–95. doi: 10.1002/cbdv.200800015. [DOI] [PubMed] [Google Scholar]
- 262.Huang C-Y, Su J-H, Chen B-W, Wen Z-H, Hsu C-H, Dai C-F, Sheu J-H, Sung P-J. Nardosinane-type sesquiterpenoids from the Formosan soft coral Paralemnalia thyrsoides. Mar Drugs. 2011;9(9):1543–1553. doi: 10.3390/md9091543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kao C-Y, Su J-H, Lu M-C, Hwang T-L, Wang W-H, Chen J-J, Sheu J-H, Kuo Y-H, Weng C-F, Fang L-S, et al. Lobocrassins A–E: new cembrane-type diterpenoids from the sof coral Lobophytum crassum. Mar Drugs. 2011;9:1319–1331. doi: 10.3390/md9081319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Uddin MH, Hanif N, Trianto A, Agarie Y, Higa T, Tanaka J. Four new polyoxygenated gorgosterols from the gorgonian Isis hippuris. Nat Prod Res. 2011;25(6):585–591. doi: 10.1080/14786419.2010.485303. [DOI] [PubMed] [Google Scholar]
- 265.Lin Y-S, Taha Khalil A, Chiou S-H, Kuo Y-C, Cheng Y-B, Liaw C-C, Shen Y-C. Bioactive marine prostanoids from octocoral Clavularia viridis. Chem Biodivers. 2008;5(5):784–792. doi: 10.1002/cbdv.200890075. [DOI] [PubMed] [Google Scholar]
- 266.Kate AS, Pearson JK, Ramanathan B, Richard K, Kerr RG. Isolation, biomimetic synthesis, and cytotoxic activity of Bis(pseudopterane) amines. J Nat Prod. 2009;72(7):1331–1334. doi: 10.1021/np8008144. [DOI] [PubMed] [Google Scholar]
- 267.Chen BW, Wu YC, Chiang MY, Su JH, Wang WH, Fan TY, Sheu JH. Eunicellin-based diterpenoids from the cultured sof coral Klyxum simplex. Tetrahedron. 2009;65:7016–7022. [Google Scholar]
- 268.Lin ST, Wang SK, Duh CY. Cembranoids from the Dongsha Atoll soft coral Lobophytum crassum. Mar Drugs. 2011;9(12):2705–2716. doi: 10.3390/md9122705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Cheng S-Y, Huang K-J, Wang S-K, Duh C-Y. Capilloquinol: a novel farnesyl quinol from the Dongsha atoll soft coral Sinularia capillosa. Mar Drugs. 2011;9(9):1469–1476. doi: 10.3390/md9091469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.McEnroe FJ, Fenical W. Structures and synthesis of some new antibacterial sesquiterpenoids from the gorgonian coral Pseudopterogorgia rigida. Tetrahedron. 1978;34:1661–1664. [Google Scholar]
- 271.Dmitrenok AS, Radhika P, Anjaneyulu V, Subrahmanyam C, Subba Rao PV, Dmitrenok PS, Boguslavsky VM. New lipids from the soft corals of the Andaman Islands. Russ Chem Bull. 2003;52(8):1868–1872. [Google Scholar]
- 272.Ata A, Win HY, Holt D, Holloway P, Segstro EP, Jayatilake GS. New antibacterial diterpenes from Pseudopterogorgia elisabethae. Helv Chim Acta. 2004;87(5):1090–1098. [Google Scholar]
- 273.Cheng S-Y, Chen P-W, Chen H-P, Wang S-K, Duh C-Y. New cembranolides from the Dongsha Atoll soft coral Lobophytum durum. Mar Drugs. 2011;9(8):1307–1318. doi: 10.3390/md9081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Rashid MA, Gustafson KR, Boyd MR. HIV-inhibitory cembrane derivatives from a Philippines collection of the soft coral Lobophytum species. J Nat Prod. 2000;63(4):531–533. doi: 10.1021/np990372p. [DOI] [PubMed] [Google Scholar]
- 275.Ospina CA, Rodríguez AD, Zhao H, Raptis RG. Bipinnapterolide B, a bioactive oxapolycyclic diterpene from the Colombian gorgonian coral Pseudopterogorgia bipinnata. Tetrahedron Lett. 2007;48:7520–7523. [Google Scholar]
- 276.Rodríguez II, Rodríguez AD. Homopseudopteroxazole, a new antimycobacterial diterpene alkaloid from Pseudopterogorgia elisabethae. J Nat Prod. 2003;66(6):855–857. doi: 10.1021/np030052c. [DOI] [PubMed] [Google Scholar]
- 277.Rodríguez II, Rodríguez AD, Zhao H. Aberrarone: a gorgonian-derived diterpene from Pseudopterogorgia elisabethae. J Org Chem. 2009;74(19):7581–7584. doi: 10.1021/jo901578r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Wei X, Rodríguez AD, Baran P, Raptis RG. Dolabellane-type diterpenoids with antiprotozoan activity from a southwestern Caribbean gorgonian octocoral of the genus Eunicea. J Nat Prod. 2010;73(5):925–934. doi: 10.1021/np100074r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Targett NM, Bishop SS, McConnell OJ, Yoder JA. Antifouling agents against the benthic marine diatom, Navicula salinicola Homarine from the gorgonians Leptogorgia virgulata and L. setacea and analogs. J Chem Ecol. 1983;9(7):817–829. doi: 10.1007/BF00987807. [DOI] [PubMed] [Google Scholar]
- 280.Michalek K, Bowden BF. A natural algacide from soft coral Sinularia flexibilis (Coelenterata, Octocorallia, Alcyonacea) J Chem Ecol. 1997;23(2):259–273. [Google Scholar]
- 281.Tomono Y, Hirota H, Fusetani N. Isogosterones A−D, antifouling 13,17-secosteroids from an Octocoral Dendronephthya sp. J Org Chem. 1999;64(7):2272–2275. [Google Scholar]
- 282.Gao C-H, Wang Y-F, Li S, Qian P-Y, Qi S-H. Alkaloids and sesquiterpenes from the South China Sea gorgonian Echinogorgia pseudossapo. Mar Drugs. 2011;9(11):2479–2487. doi: 10.3390/md9112479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data discussed in this review have been previously published and are available.


