Abstract
Tissue factor pathway inhibitor-alpha (TFPI-α) is a Kunitz-type serine protease inhibitor, which suppresses coagulation by inhibiting the tissue factor (TF)/factor VIIa complex as well as factor Xa. In static plasma-phospholipid systems, TFPI-α thus suppresses both factor Xa and thrombin generation. In this article, we used a microfluidics approach to investigate how TFPI-α regulates fibrin clot formation in platelet thrombi at low wall shear rate. We therefore hypothesized that the anticoagulant effect of TFPI-α in plasma is a function of the local procoagulant strength—defined as the magnitude of thrombin generation under flow, due to local activities of TF/factor VIIa and factor Xa. To test this hypothesis, we modulated local coagulation by microspot coating of flow channels with 0 to 100 pM TF/collagen, or by using blood from patients with haemophilia A or B. For blood or plasma from healthy subjects, blocking of TFPI-α enhanced fibrin formation, extending from a platelet thrombus, under flow only at <2 pM coated TF. This enhancement was paralleled by an increased thrombin generation. For mouse plasma, genetic deficiency in TFPI enhanced fibrin formation under flow also at 0 pM TF microspots. On the other hand, using blood from haemophilia A or B patients, TFPI-α antagonism markedly enhanced fibrin formation at microspots with up to 100 pM coated TF. We conclude that, under flow, TFPI-α is capable to antagonize fibrin formation in a manner dependent on and restricted by local TF/factor VIIa and factor Xa activities.
Keywords: fibrin clot formation, haemophilia, mouse, platelets, TFPI, thrombin generation
Introduction
Tissue factor pathway inhibitor-α (TFPI-α) is a Kunitz-type serine protease antagonist, known to downregulate the extrinsic coagulation pathway by inhibiting the complexes of tissue factor (TF), factor VIIa and factor Xa.1–4 In blood, about 15% (0.25–0.5 nM) of the TFPI circulates in an active, full-length form, designated as TFPI-α, while the remainder is carboxy-terminally truncated and bound to lipoproteins.4,5 In addition, platelets store a pool of full-length TFPI-α (~1,200 copies/cell),6 which is slowly released upon activation of the cells. The alternatively spliced form TFPI-β remains immobilized at the surface of endothelial cells via a glycophosphatidylinositol anchor.7–9 The three Kunitz domains of TFPI-α have complementary roles in coagulation suppression. The first domain binds and inhibits TF/factor VIIa; the second domain inhibits factor Xa, while the third domain regulates the binding of cofactor protein S.10–13 Furthermore, TFPI-α can interact with other components of the coagulation system, that is, factor V and phosphatidylserine (PS)-containing membranes via its positively charged C-terminus.14 From its ability to interfere with both TF and factor Xa activity (tenase), TFPI-α is considered to be a central player in the natural anticoagulant pathway.
The formation of a fibrin-containing thrombus involves complex interactions between platelets and the coagulation system.15 Exposed collagen fibres can act as a prothrombotic surface and stimulate platelet deposition, phosphatidylserine (PS) exposure, intrinsic coagulation pathway activation and thrombin generation.16,17 Together with TF, collagen furthermore triggers the extrinsic coagulation pathway.18 The coagulant process is further modulated by the coagulant capacity of plasma, the local procoagulant activity provided by platelets and the blood flow rate, jointly indicated here as procoagulant strength (defined as the magnitude of thrombin generation under flow). Earlier work has shown that, at high blood flow and shear rate, the formation of fibrin is confined to the immediate environment of a thrombus with PS-exposing platelets, whereas at low shear/flow rate such as in veins the formed fibrin fibres progressively extend into the lumen.19–21 Anchoring of the fibrin fibres at ‘fibrin-coated’ platelets appeared to rely on PS exposure, integrin αIIbβ3 epitopes, and factor XIIIa-mediated transglutaminase activity.22,23
At a low procoagulant strength, such as with low factor VIII or IX levels (haemophilia A or B), and concordant low intrinsic tenase activity, TF/collagen-induced platelet activation and fibrin formation under flow are greatly impaired,24 thus pointing to potent interactions of TF-triggered and intrinsic tenase-enhanced thrombin generation via PS-exposing platelets. Given the critical role of both TF/factor VIIa and factor Xa activities in providing the thrombin-generating activity required for fibrin clot formation, a relevant question now is how the anticoagulant protein TFPI-α interferes in this process. So far, the role of TFPI has not been investigated in this setting.
Mice with homozygous deficiency in TFPI (Tfpi−/−) die in utero from disseminated intravascular coagulation, but part of the offspring survives when combined with low TF expression or absence of the platelet receptor PAR4 (F2rl3−/−).25,26 This phenotype suggests the existence of a balanced procoagulant–anticoagulant triggering, for example, by TF and TFPI, in normal clot formation. Under static conditions in vitro, this indeed has been examined.27 However, how TPFI exerts control at physiological flow conditions in these mouse models is largely unclear.
In this article, we investigated the suppressive role of plasma TFPI-α on fibrin clot formation under conditions of high and low procoagulant strength under flow by changing the local TF concentration or lowering the intrinsic tenase activity using blood from haemophiliac patients. Collectively, our data indicate that in normal blood or plasma TFPI-α, effects are confined to low TF levels. However, at reduced factor VIII or IX levels, it markedly extends to much higher TF levels.
Materials and Methods
Materials
Bovine serum albumin (BSA) was purchased from MP Biomedicals (Illkirch, France). Fluorogenic thrombin substrate Z-Gly-Gly-Arg-7-amino-4-methylcoumarin HCl (Z-GGR-AMC) was obtained from Bachem (Bubendorf, Switzerland). Recombinant human full length TFPI-α (TFPIfl, amino acids 1-276) was a kind gift from Dr. W. Buurman (Maastricht, the Netherlands). Monoclonal antibodies against the Kunitz-1, Kunitz-2, Kunitz-3, or C-terminus domains of human TFPI-α were purchased from Sanquin (Amsterdam, the Netherlands), and were mixed in a 1:1:1:1 ratio. Recombinant TF (Innovin) was from Siemens (Marburg, Germany). Corn trypsin inhibitor was from Haematologic Technologies (Essex Junction, Vermont, United States). Monoclonal antibody against factor VIIa (200 μg/mL), polyclonal antibodies against TF (CD142) (1 mg/mL) and corresponding irrelevant antibodies of same Ig type were purchased from American Diagnostica (Stamford, Connecticut, United States). DiOC6 was from AnaSpec (Waddinxveen, the Netherlands); Alexa Fluor (AF) 647-labeled human fibrinogen from Invitrogen (Carlsbad, California, United States); Horm type I collagen from Nycomed Pharma (Munich, Germany). Congenital factor VIII–deficient plasma (citrate-anticoagulated) was obtained from George King Bio-Medical (Overland Park, Kansas, United States). Other materials were from Sigma Aldrich (Zwijndrecht, the Netherlands).
Human Blood Collection and Plasma Preparation
Human blood was obtained after medical ethical approval from healthy volunteers and patients, after full informed consent, according to the declaration of Helsinki. Blood donors were free from antithrombotic medication for at least 2 weeks. Two patients were included with haemophilia A (factor VIII activity 2% and <1%, the latter with 3.8 BU factor VIII inhibitor), and two patients with haemophilia B (factor IX activity 5 and 7%). Patients had not obtained treatment with coagulation factors for at least 2 weeks.
Collection of blood was by venipuncture of the median cubital vein into 1/10 volume of 3.2% trisodium citrate, with 40μg/mL corn trypsin inhibitor (CTI) added as appropriate. To prepare pooled normal platelet-free plasma, citrate anticoagulated blood was centrifuged twice for 10 minutes at 250 g. Platelet-free plasma from 23 healthy donors was pooled, aliquoted, snap frozen and stored at −80°C, as described.28 Plasma samples were treated with antibodies or isotype-matched IgG to inhibit specified coagulation factors (typically 30 μg/mL, incubated at 37°C for 5 minutes). Considering that TFPI-α is the main or only active form of TFPI in human plasma,4,5 we termed blood or plasma samples treated with antibody mixture (anti Kunitz-1, Kunitz-2, Kunitz-3 and C-terminal domains of TFPI) as TFPI-α inhibited.
Mouse Blood Collection and Plasma Preparation
Animal experiments were approved by local animal ethics committees. Mice heterozygous for the TFPI-null allele (back-crossed on C57Bl/6 background for ≥10 generations) were mated with PAR4-deficient mice (F2rl3−/−) to produce doubly heterozygous mice, as described before.26 Subsequent cross-breeding resulted in Tfpi+/+ F2rl3−/−, Tfpi+/− F2rl3−/− and Tfpi−/−F2rl3−/−mice at C57Bl/6 background.26 Blood from wild-type (C57Bl/6) or genetically transformed mice was collected into 3.2% citrate (10:1 ratio) and 50 μg/mL CTI; blood samples were immediately used to prepare platelet-free plasma by double centrifugation, and then frozen at −80°C until use.26 For thrombus formation, wild-type C57Bl/ 6 mouse blood was collected into 3.2% citrate, and used for whole blood flow perfusion measurements, as described.24
Preparation of Thrombogenic Surfaces for Flow Perfusion
Cleaned glass coverslips were coated with three consecutive microspots of Horm type I collagen, by applying 2 μL of 50 μg/ mL collagen type I (centre-to-centre distance: 3 mm).29 After rinsing with saline and drying under nitrogen gas, aliquots of 2.0 μL TF (Innovin, concentrations as indicated) or control solution were applied on each microspot. For experiments with human blood, collagen/TF-coated coverslips were blocked for 1 hour with 5% BSA in Hepes buffer pH 7.45 (136 mM NaCl, 10 mM Hepes, 2.7 mM KCl, 2 mM MgCl2, 1 mg/mL glucose). Assuming homogeneous TF distribution, a coating with 2.0 μL of 1, 2, 10, 20 or 100 pM (microspots of 6.75 mm2) gave a calculated applied TF density of 0.2, 0.4, 1.8, 3.6 or 17.8 × 106 molecules TF per mm2, respectively. Collagen was coated at a density of 14.8 ng/mm2. For experiments with mouse blood, coverslips were coated similarly, but blocked with BSA-containing modified Tyrode’s buffer pH 7.45 (136 mM NaCl, 5 mM Hepes, 2.7 mM KCl, 0.42 mM NaH2PO4, 2 mM MgCl2, 1 mg/mL glucose).
Assessment of Thrombus and Fibrin Formation under Flow
To allow human thrombus formation, citrate anticoagulated blood was perfused through a parallel-plate flow chamber with collagen/TF microspots at a wall shear rate of 1,000 s−1 for 4.0 minutes, similarly as described.19,30 The microspot with lowest TF amount was proximal in the flow chamber. In the two-step procedure, platelet thrombi were formed on the microspots, after which plasma samples (pretreated as required) were perfused over the thrombi at indicated shear rate.19 Plasma samples were recalcified immediately prior to flow. Fibrin formation was assessed by brightfield microscopy using a 40 × /1.4 numerical aperture oil immersion objective and 2× post-magnification.30 Fluorescent labels (DiOC6 and AF647-fibrinogen) were added to blood and plasma, respectively, as appropriate.
From representative parts of the microspots, brightfield and fluorescence images were recorded during flow in real time at a frequency of 0.2 to 1.0 Hz. Digital brightfield images were assessed off-line for the presence of multi-pixel fibrin fibres, radially extending from preformed platelet thrombi. For precise determination of the time to first fibrin formation,31 series of subtraction images were created using scripts written in Fiji/ImageJ (version 1.48g: Rasband, NIH, Bethesda, Maryland, United States). For measurement of fluorescent fibrin formation, fluorescence images were analysed for fibrillar structures.21 An off-set was applied for the presence of platelet-bound fibrinogen, using reference images with blocked coagulation. Supplementary Fig. S1 (online only) shows image overlays for the detection of fibrin fibres obtained with both methods.
For experiments with mouse blood and plasma, a similar two-step procedure was followed. In brief, platelet thrombi were formed on 0 to 10 pM TF/collagen microspots by perfusion of wild-type C57Bl/6 blood supplemented with DiOC6 (0.5 μg/mL) at 1,000 s−1, followed by a 1-minute rinse with modified Tyrode’s buffer (5 mM Hepes, 136 mM NaCl, 2.7 mM KCl, 0.42 mM, NaH2PO4, 2 mM MgCl2, 1 mg/mL glucose and 0.1% BSA, pH 7.45). Subsequently, plasma samples (40 μL) from indicated mice were supplemented with AF647-fibrinogen (17.3 μg/mL), recalcified and immediately perfused over the preformed thrombi at 150 s−1 to allow clotting. Image capturing in real time and analysis were performed as described previously.
For the one-step procedure with human blood, coagulation was introduced by co-perfusion of 0.1 vol. recalcification buffer (Hepes buffer pH 7.45 containing 63.2 mM CaCl2 and 31.5 mM MgCl2) at 1,000 s−1 and, after 4.0 minutes, switch to the desired wall shear rate. Blood samples were pre-labeled with AF647-fibrinogen (17.3 μg/mL) and DiOC6 (0.5 μg/mL, staining platelets). Brightfield contrast and confocal fluorescence images were recorded with a camera-containing line-scanning Live-7 microscopic system (Carl Zeiss, Oberkochen, Germany) and a 63 × /1.4 numerical aperture oil immersion objective.32 Time series of images were recorded at a frequency of 0.2 to 1.0 Hz, and analysed off-line, as described earlier.
Real-Time Thrombin Generation in Flow Chambers
To measure thrombin generation at thrombus-containing microspots, fluorogenic thrombin substrate Z-GGR-AMC (0.5 mM) was added to blood samples, and fluorescence accumulation was measured in real time.33 In short, thrombi were generated as described earlier, after which blood flow was stopped and fluorescence images were recorded at 0.03 Hz at excitation and emission wavelengths of 357 ± 44 nm and 447 ± 60 nm, respectively. Sample illumination was discontinued between captures of images to prevent bleaching.
Thrombin Generation in Plasma
In citrated human (patient) plasmas, thrombin generation was measured by calibrated automated thrombinography. Reaction mixtures (125 μL) contained 80 μL plasma, to which were added indicated amounts of TF (0–15 pM, f.c.), 16 mM CaCl2, 30 μM phospholipids and 0.32 mM fluorogenic thrombin substrate. Plasma samples were pre-treated with anti-TFPI antibodies, as indicated. Thrombin peak height (in nM) was plotted against the TF concentration. Assays were run in triplicate. In constructed TF dose–response curves, the thrombin peak height is known to saturate at lower levels when using pooled plasmas from male donors than those from female donors.34 Hence, for an adequate comparison with the male haemophilia patients, in this study we used controlled reference plasmas obtained from male donors.
Statistical Analysis
Data are presented as means ± SEM. Significance of difference between conditions was determined with a nonparametric Mann–Whitney U-test, using the statistical package for social sciences (SPSS 11.0). p-Values <0.05 were considered statistically significant. The program GraphPad Prism 6.0 (San Diego, California, United States) was used for graphics.
Results
Moderate Anticoagulant Role of Human Plasma TFPI-α in Fibrin Formation from Platelet Thrombi under Flow
To study the anticoagulant potential of TFPI-α under physiological flow conditions as a function of the local TF density, microfluidic chambers were used, containing three microspots of collagen with increasing amounts of TF in the flow direction. Initial experiments were performed using microspots (2 μL applied, spreading over about 6.75 mm2) coated with 50 μg/mL collagen-I and 0, 2 or 10 pM TF (corresponding to coated densities of 14.8 ng collagen/ mm2 and 0, 0.4 or 1.8 × 106 molecules TF/mm2). In a two-step approach, initially platelet thrombi were formed on the microspots by 4-minute perfusion of citrate-anticoagulated whole blood (from healthy donors, collected in the presence of corn trypsin inhibitor to prevent activation of the contact system). Brightfield microscopy confirmed the presence of stable thrombi consisting of multilayered (20–25% surface area coverage) platelets on all microspots (Fig. 1A). No platelet deposition was observed outside of TF/collagen-coated areas, such as described earlier.21,35 In step 2, coagulation was induced by superfusion of the thrombi with recalcified normal plasma at defined, low wall shear rate (150 s− 1).
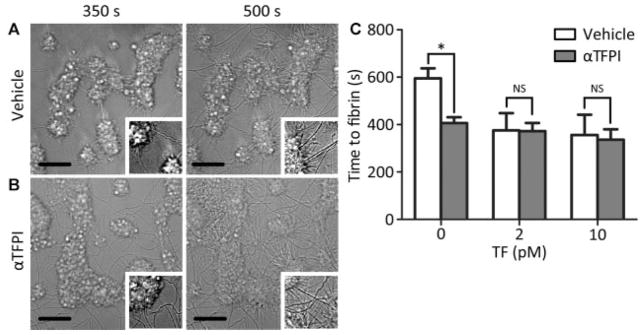
Fig. 1.
TFPI-α inhibition enhances fibrin formation on platelet thrombi under flow at low TF density. Platelet thrombi were generated by perfusion of human blood over three consecutive microspots of collagen with increasing amounts of 0, 2 and 10 pM coated TF (4 minutes at 1,000 s−1). Platelet thrombi were superfused at shear rate of 150 s−1 with recalcified normal plasma (vehicle or TFPIα inhibited); fibrin formation from the platelet thrombi was determined from recorded images. Shown are representative brightfield images, captured at indicated times after start of perfusion with vehicle-treated (A) or TFPI-α-inhibited (B) plasma (no TF, bar = 25 μm). (C) Time to fibrin formation at microspots with consecutively 0, 2 and 10 pM TF. Means ± SEM, n = 4–5; *p < 0.05 versus vehicle; NS, not significant.
For microspots with 0 pM TF/collagen, this resulted in the appearance of fibrin fibres starting after 595 ± 73 seconds (mean ± SEM, n = 8). Real-time image recording indicated that the fibres were formed by radially extending from the platelet thrombi, similarly as earlier observed for fibrin on individual platelets. Time to first fibrin formation could be precisely assessed by image subtraction.31 For the downstream microspots with 2 or 10 pM TF/collagen, fibrin clots formed at earlier time points, that is, with first appearance of fibres after 376 ± 73 and 356 ± 101 seconds, respectively (Fig. 1A–C).
Control experiments indicated that on microspots coated with only 10 pM TF (no collagen), platelet deposition and fibrin formation were absent during >10 minutes of flow, which is in agreement with our earlier observation that platelets scaffold the formation of fibrin in this flow model.21 Supplementation of blood and plasma with blocking antibodies against TF/factor VIIa resulted in a marked delay in start of fibrin formation to >600 seconds (n = 3) at all microspots. This confirmed overall activity of the TF/factor VIIa pathway.
To investigate the role of TFPI-α in this process, plasma (from healthy blood donors) was preincubated with a cocktail of antibodies inhibiting all forms of TFPI, including the active TFPI-α. Thrombin generation measurements con-firmed that TFPI-α was fully blocked in the plasma samples (see later). Flow perfusion of TFPI-inhibited plasma over preformed thrombi (shear rate of 150 s−1) resulted in a significant shortening of the onset of fibrin formation on microspots with 0 pM TF/collagen, that is, starting at 413 ± 27 seconds (mean ± SEM, n = 4), when compared to the lag time of 595 ± 73 seconds for vehicle-treated plasma (Fig. 1B, C). Markedly, TFPI-α inhibition did not significantly shorten the time to fibrin formation on microspots with 2 or 10 pM TF/collagen (p > 0.50). This suggested that the procoagulant strength provided by even low amounts of immobilized TF overruled the anticoagulant effect of TFPI-α.
Dual-colour confocal fluorescence microscopy was then applied for precise assessment of the localization and kinetics of fibrin formation. Platelets in the blood were stained with a vital membrane label DiOC6 (green), while AF647-fibrinogen (red) was added to the plasma, as a label that effectively incorporates into fibrin clots.21 Blood perfusion resulted in thrombi with DiOC6-labeled platelets, which were similarly sized on all collagen microspots with or without TF (Fig. 2A). Low-shear flow with plasma did not change the fluorescence from platelets (Fig. 2D), while labeled fibrin fibres started to accumulate after about 600 seconds (0 pM TF) or about 360 seconds (2 and 10 pM TF; Fig. 2A, C). Dual recording of brightfield and fluorescence images indicated a precise overlap of fibrin fibres, radially extending from the thrombi, in the two sets of images (Supplementary Fig. S1 [online only]).
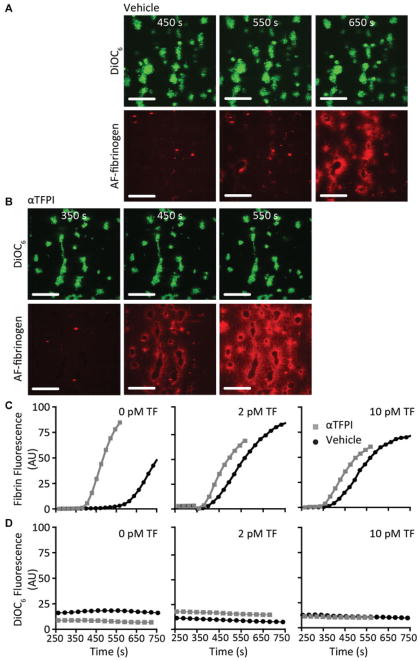
Fig. 2.
Effect of TFPI-α inhibition on kinetics of platelet-dependent fibrin formation under flow. Platelet thrombi were generated by perfusion of DiOC6-labeled human blood over microspots with collagen and indicated amounts of TF, as for Fig. 1. Platelet thrombi were superfused with recalcified normal plasma containing AF647 fibrinogen. Shown are representative confocal fluorescence images (no TF, time points indicated) after perfusion with vehicle-treated plasma (A) or TFPI-α-inhibited plasma (B). Bars = 25 μm. Time traces of fibrin formation (C) and platelet adhesion (D) (fluorescence image analysis) at platelet thrombi for microspots with 0, 2 and 10 pM TF (n = 3).
The fibrin(ogen) labeling experiments confirmed that inhibition of plasma TFPI-α shortened the onset of fibrin formation on 0 pM TF/collagen microspots under flow with 200 seconds (Fig. 2B). Time traces for vehicle-treated plasma samples also showed a marked shortening of fibrin accumulation at 2 or 10 pM TF/collagen microspots, when compared to 0 pM TF/collagen microspots, although rates of fibrin accumulation were similar for all microspots (Fig. 2C). Upon TFPI-α inhibition, a no more than small shortening of the fibrin accumulation was observed at 2 to 10 pM TF/ collagen spots, which was statistically not significant (p = 0.25). The appearance of fluorescent fibrin fibres, extending from platelet thrombi, was not affected by inhibition of TFPI (Supplementary Fig. S2A [online only]).
Reducing the wall shear rate during plasma perfusion from 150 to 50 s−1 gave similar results, in that for 0 TF/ collagen microspots TFPI-α inhibition prolonged the time to fibrin formation by 63 ± 15% with borderline significance (n = 4, p = 0.06). Taken together, these experiments pointed to an inhibiting effect of plasma TFPI-α on platelet-dependent fibrin formation which, however, could be overruled by local immobilized TF.
Moderate Anticoagulant Role of Mouse Plasma TFPI-α in Fibrin Formation from Platelet Thrombi under Flow
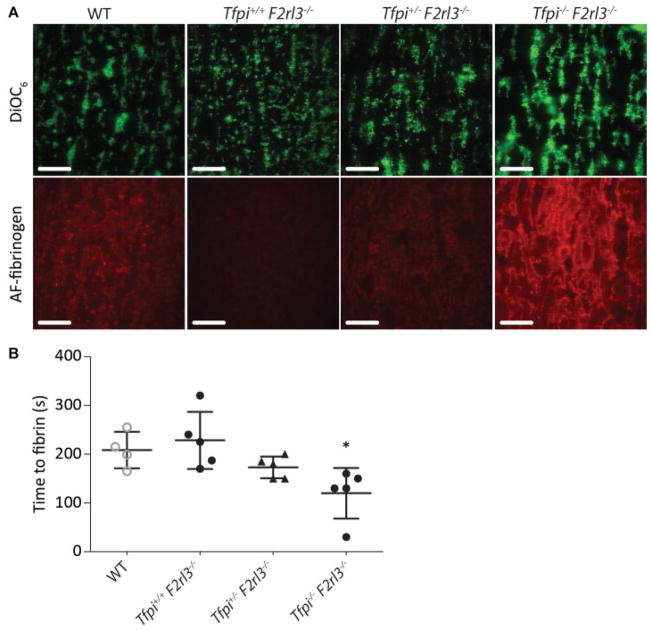
To further underscore the role of TFPI in fibrin formation at TF/ collagen surfaces, TFPI-deficient mouse plasma was used. Since TFPI deficiency as such is lethal in mouse,36 plasmas were used from Tfpi+/− or Tfpi−/− mice, which also lacked the PAR4 thrombin receptor (F2rl3−/−) and thereby survived.26 First, platelet thrombi were generated with wild-type mouse blood and then post-perfused at 150 s−1 with plasma from wild type or TFPI-deficient mice. For collagen microspots and wild-type plasma, we observed a relatively fast onset of fibrin formation (in comparison with human plasma) of 208 ± 19 seconds (Fig. 3A, B). Markedly, the plasmas from Tfpi−/− F2rl3−/− mice, in comparison to those from Tfpi+/− F2rl3−/− and Tfpi+/+ F2rl3−/− mice, gave a further shortening and enhancement of fibrin formation (Fig. 3A, B). Also in this mouse system, fibrin fibres were exclusively produced from the platelet thrombi. Thrombin generation measurements in the knockout plasmas confirmed the absence of TFPI activity.26 We concluded that, in mouse, the absence of plasma TFPI led to an increased platelet-dependent fibrin formation under flow at limited coagulant strength (noTF), thus in agreement with the human data.
Fig. 3.
Deficiency in murine TFPI enhances fibrin formation on platelet thrombi under flow. Wild-type murine platelet thrombi were generated whole blood (DiOC6-labeled) perfusion over collagen microspots. Recalcified plasmas containing AF647-fibrinogen from wild type, Tfpi+/+ F2rl3−/−, Tfp+;/− F2rl3−/− or Tfpi−/− F2rl3−/− mice were post-perfused at low shear rate (150 s−1), while DiOC6 and AF647 fluorescence was monitored by confocal microscopy. (A) Representative DiOC6 and AF647-fibrin images after 200 s of flow (bars = 25 μm). (B) Times to first fibrin formation per genotype, measured by image subtraction analysis. Means ± SEM, n = 4–5; *p < 0.05 versus wild type.
Increased Anticoagulant Role of Plasma TFPI-α in Fibrin Formation in Haemophilic Blood
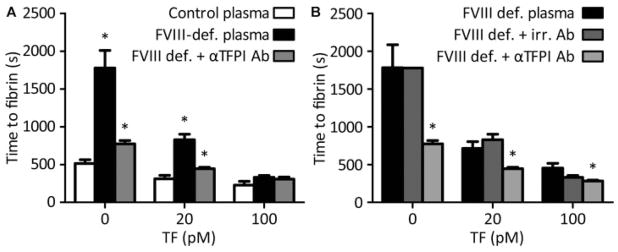
Earlier findings using static assay systems or isolated factors have indicated that the anticoagulant effect of TFPI can increase under conditions of factor VIII or IX deficiency.37–39 To investigate this further, at first commercial haemophilia plasmas were used to assess fibrin formation under flow. Since pilot results indicated that higher TF concentrations could be used with such plasmas, microspots were prepared of collagen with 0, 20 or 100 pM coated TF (corresponding to 0, 3.6 and 18 × 106 molecules TF/mm2) in the flow direction. For the 0 and 20 pM TF microspots, perfusion with factor VIII-deficient plasma resulted in a greatly delayed fibrin formation, which was nearly normalized with blocking antibodies against TFPI-α but not with irrelevant control antibodies (Fig. 4A, B). On spots with the highest concentration of TF (100 pM), the blocking of TFPI-α was only limitedly effective (Fig. 4A, B).
Fig. 4.
TFPI-α inhibition restores fibrin formation of haemophiliac plasma on platelet thrombi at higher TF density. Platelet thrombi were generated by blood perfusion over microspots with collagen and increasing amounts of 0, 20 and 100 pM coated TF. Thrombi were superfused with indicated recalcified plasmas, and time to fibrin formation was recorded as for Fig. 1. (A) Perfusion with control or factor (F)VIII-deficient plasma, and depletion of TFPI-α as indicated. (B) Perfusion with factor VIII–deficient plasma, treated with irrelevant (irr.) control antibody or anti-TFPI antibodies. Means ± SEM, n = 4–7; *p < 0.05 versus control plasma (A) or FVIII-deficient plasma (B).
Anticoagulant Role of Human TFPI-α in Single-Step, Whole Blood Flow Tests
To substantiate these findings, we also monitored fibrin formation in a one-step, whole blood procedure, in which recalcified blood was directly flowed over TF/collagen microspots (0, 1 or 10 pM coated) initially at high shear rate (1,000 s−1) to allow platelet binding, and subsequently at low shear rate (150 s−1) to stimulate fibrin formation. With blood from control subjects, this shear rate reduction shortened the time to fibrin formation on 0 pM TF/collagen spots from >1,500 seconds to 880 ± 110 seconds (n = 4, p < 0.05). Treatment of blood with anti-TFPI antibodies further shortened this time to <600 seconds on both 0 and 1 pM TF microspots (Fig. 5A). On the contrary, addition of recombinant TFPI (3.75 nM) to the blood delayed the time to fibrin formation on all TF/collagen microspots by about threefold (n = 3, p < 0.05).
Fig. 5.
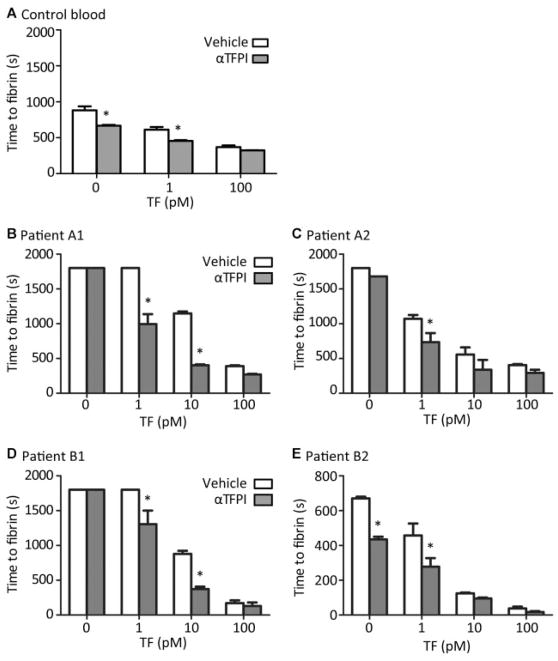
TFPI-α inhibition normalizes platelet-dependent fibrin formation in haemophiliac blood. Platelet thrombi were generated by blood perfusion over microspots of collagen with indicated concentrations of TF; blood samples were treated with vehicle or anti-TFPI antibodies. Time to first fibrin formation was recorded per microspot during blood perfusion at 150 s−1. Presented are times to fibrin formation. (A) Blood from control subjects. (B, C) Blood from haemophilia A patients A1 (2% factor VIII) and A2 (<1% with 3.8 BU factor VIII inhibitor). (D, E) Blood from haemophilia B patients B1 (5% factor IX) and B2 (7% factor IX). Means ± SEM, n = 3–4; *p < 0.05 versus vehicle.
Similar studies were then performed with blood from two patients with haemophilia A (2 and <1% of normal factor VIII) and two patients with haemophilia B (5 and 7% of normal factor IX). In comparison to blood from controls, in blood from all patients, anti-TFPI antibody treatment markedly shortened the prolonged fibrin formation on 0, 1 and 10 pM TF microspots (Fig. 5B–E). However, on 100 pM TF spots, the already fast onset of fibrin formation was not further affected by antibody treatment. Interestingly, fibrin formation curves (assessed from confocal images of fibrin fluorescence) indicated that, in spite of the earlier onset, anti-TFPI treatment did not affect the slope of fibrin formation, in case of haemophilia A (Supplementary Fig. S3A, B [online only]) or haemophilia B (Supplementary Fig. S3C–D [online only]), blood or with coatings of 1 or 10 pM TF. Taken together, these results indicate that, for these patients with relatively low intrinsic tenase capacity, blockage of TFPI-α shortened the lag time to fibrin formation, as long as this was not maximally shortened by the highest (100 pM) TF coating density.
Anticoagulant Role of Plasma TFPI-α in Platelet-Dependent Thrombin Generation Measurement
Previous thrombin generation tests with phospholipid-containing plasma demonstrated that the thrombin generation–inhibiting effect of TFPI-α can extend to TF concentrations up to 10 pM.13,40,41 This was confirmed in the current study (Supplementary Fig. S4A, B [online only]). Blocking of factor XIa was without effect on the thrombin generation profile, which pointed to a no more than small feedback role of thrombin-activated factor XI. Thrombin generation measurements with plasmas from the two haemophilia A and two haemophilia B patients showed an almost annulled thrombin generation peak with 1 pM TF (Supplementary Fig. S4B [online only]). This can be explained by the absence of the intrinsic tenase amplification loop of thrombin generation. Using a higher concentration of 10 pM TF, thrombin generation in the patients’ plasmas was less severely impaired, indicating that the amplification loop is partly circumvented. For all patient samples, TFPI-α inhibition partly antagonized the annulation of thrombin generation at 1 pM TF, while it (nearly) normalized this process at 10 pM TF. This supports the idea that plasma TFPI-α acts as a more potent anticoagulant at a lower magnitude of thrombin generation (resulting in a reduced procoagulant strength), that is, at low intrinsic tenase activity.
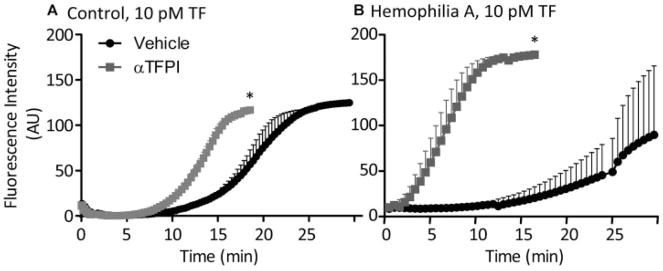
In addition, we examined the effect of TFPI inhibition on thrombin generation at thrombi formed on TF/collagen microspots in flow chambers. Therefore, fluorescent thrombin substrate was added to blood and, after thrombus formation, stasis was applied to allow fluorescence accumulation.33 With control blood, inhibition of TFPI-α moderately enhanced the cleavage of thrombin substrate, with initial starts of thrombin generation in the same time interval (Fig. 6A). However, with haemophilia A blood, TFPI-α inhibition had a stronger accelerating effect on substrate cleavage (Fig. 6B). This is in good agreement with the enhancement of thrombin generation seen in platelet-free haemophilia plasma samples.
Fig. 6.
Effect of TFPI-α inhibition on whole blood thrombin generation on platelet thrombi under stasis. Thrombi with procoagulant platelets were generated by whole blood perfusion over 10 pM TF/collagen microspots. Blood samples were pretreated with vehicle or anti-TFPI antibodies, and supplemented with Z-GGR-AMC (0.5 mM). After 4 minutes, the fluorescence accumulation of cleaved thrombin substrate was recorded under stasis to assess for thrombin generation. Shown are representative thrombin generation curves with blood from control subjects (A) or haemophilia A patients (B). Means ± SEM (n = 3), *p < 0.05 versus vehicle.
Discussion
This study describes how plasmatic TFPI-α antagonizes the process of fibrin formation on platelet thrombi in coagulating blood/plasma under conditions of low shear flow. With blood or plasma from control subjects, the anticoagulant role TFPI-α appeared to be restricted to surfaces with no more that low TF density (i.e., coating of <2 pM TF, corresponding to <0.4 × 106 molecules/mm2). This was confirmed in a mouse system by using plasmas from mice with a genetic deficiency in TFPI. Furthermore, we found that the anticoagulant role of TFPI-α was markedly enhanced in haemophilia patients with a deficiency in factor VIII or IX and, hence, an impaired intrinsic tenase activity and lower thrombin generation potential. This anticoagulant effect detected with haemophilia blood now extended surfaces with higher TF densities (up to 40 × 106 molecules/mm2). Together, these data indicate that a prohaemostatic effect of human TFPI-α inhibition is not restricted to static systems of thrombin generation in plasma, but extends to conditions of flowing whole blood and fibrin clot production from platelet thrombi, provided that the local procoagulant strength (TF density) is low. Our data furthermore underscore the proposal that inhibition of TFPI activity can be an effective way to normalize haemostasis in factor VIII-deficient patients.42
Different mechanisms for the action of TFPI have been proposed beyond that of TF/factor VIIa inhibition. These include protein S–dependent inhibition of factor Xa,10,13,41–43 and inhibition of prothrombinase activity.12 Regardless of the relative contribution of these mechanisms, it appears that in flowing blood the anticoagulant action of TFPI is confined to conditions where limited amounts of TF are present and/or the amount of factor Xa generated is reduced. One explanation for this restricted effect of TFPI might be ongoing accumulation of PS-exposing platelets with high coagulant capacity.19,30 Earlier, we have shown that these procoagulant platelets provide an active surface for the assembly of factors VIIIa and IXa, thereby greatly promoting intrinsic tenase activity.24 Hence, in normal blood under flow, the procoagulant strength provided by platelets may restrict the anticoagulant actions of plasma pool of TFPI-α. From another point of view, it can be considered that, under conditions of flow, the enhancement of fibrin formation caused by higher densities of coated TF is already near maximal, and thus so strong that it cannot further be accelerated by TFPI inhibition.
Strikingly, in blood or plasma with low levels of factor VIII (haemophilia A patients) or factor IX (haemophilia B patients), TFPI inhibition caused a more marked improvement of fibrin formation, even at higher concentrations of coated TF (10–100 pM). TFPI belongs to the category of slow-tight binding coagulation inhibitors that inactivate factor Xa molecules in a biphasic fast/slow fashion, but can be overruled by high factor Xa generation.11 This may explain why its activity is limited under conditions of high factor Xa and thrombin generation. On the other hand, static thrombin generation measurements indicate that, also at high TF levels, TFPI blockage has an additional increasing effect on thrombin peak levels by 50 to 100 nM (Supplementary Fig. S4 [online only]). From this perspective, it is likely that under conditions of high thrombin generation, the anticoagulant activity of TFPI is insufficient to suppress thrombin levels so much that they become limiting for the control of fibrin formation. Taken together, this suggests that, in an additive way, the local activities of both TF/factor VIIa and intrinsic tenase determine the procoagulant strength of fibrin formation at a thrombus under flow, and accordingly the ability of TFPI-α to antagonize fibrin formation relies on both the TF/factor VIIa and tenase activities. In support of this suggestion, others have also proposed that blood flow can control coagulation onset via a positive feedback loop of TF/factor VIIa and factor Xa activities.44
Already in 1991, it was proposed that inhibition of TFPI activity can reverse the low clotting potential of blood from haemophilic patients.37–39 Later studies have supported this effect in whole blood or plasma assays with a variety of TFPI-α inhibitors, albeit under stasis. Using thromboelastography and thrombin generation assays, it was shown that the anti-TFPI aptamer BAX499 can partly restore coagulation in case of factor VIII deficiency.45 Under stasis, the same aptamer was found to improve fibrin formation in the setting of low coagulation.27 Also in rabbits, TFPI inhibition has been shown to shorten bleeding times.46,47
In addition to TFPI, also the Kunitz domain containing amyloid-beta A4 protein (protease nexin II)48 has been identified in the human platelet proteome.6 In the experiments, however, we did not obtain evidence for a consistent role of platelet-derived anticoagulants on the kinetics of fibrin formation under the present flow conditions. In future studies, however, it is worth to reinvestigate this in more detail.
Taken together, our data indicate that, under venous, low-shear flow conditions, TFPI-α suppresses platelet-dependent fibrin formation most actively under conditions of low TF density and intrinsic tenase activity, that is, at low procoagulant strength. These findings thereby provide novel insight into the possibilities and limitations of TFPI antagonism to normalize haemostasis in haemophilia patients.
Supplementary Material
What is known about this topic?
Tissue factor pathway inhibitor-α (TFPI-α) in plasma inhibits TF and factor Xa.
Antagonism of TFPI-α effectively normalizes impaired thrombin generation in haemophiliac plasmas.
Both TF and intrinsic tenase activity (factors VIII and IX) support fibrin generation at thrombi under flow conditions.
What does this paper add?
In flowed normal blood or plasma, TFPI-α antagonism enhances platelet-dependent fibrin formation only at low TF density.
In flowed haemophilia A or B blood, providing reduced coagulant strength, TFPI-α is a stronger anticoagulant even at high TF density.
Deficiency of mouse TFPI enhances fibrin formation under flow at low TF density.
Acknowledgments
Funding
S.T., J.M.E.M.C., T.M.H. and J.W.M.H. were funded by the Center for Translational Molecular Medicine (INCOAG). T. G.M. and J.M.E.M.C. were funded by Dutch Heart Foundation (2015T79). J.M.E.M.C. was funded by the Netherlands Organization for Scientific Research (NWO Vidi 91716421). A.E.M. was funded by the National Heart, Lung, and Blood Institute grant HL068835. F.S. was funded by Alexander von Humboldt Foundation.
Footnotes
Conflict of Interest
A.E.M. receives research grant support from Novo Nordisk. The remaining authors state that they have no conflict of interest.
References
- 1.Salemink I, Franssen J, Willems GM, Hemker HC, Lindhout T. Inhibition of tissue factor-factor VIIa-catalyzed factor X activation by factor Xa-tissue factor pathway inhibitor. A rotating disc study on the effect of phospholipid membrane composition. J Biol Chem. 1999;274(40):28225–28232. doi: 10.1074/jbc.274.40.28225. [DOI] [PubMed] [Google Scholar]
- 2.Adams M. Tissue factor pathway inhibitor: new insights into an old inhibitor. Semin Thromb Hemost. 2012;38(02):129–134. doi: 10.1055/s-0032-1301410. [DOI] [PubMed] [Google Scholar]
- 3.Broze GJ, Jr, Girard TJ. Tissue factor pathway inhibitor: structure-function. Front Biosci (Landmark Ed) 2012;17:262–280. doi: 10.2741/3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winckers K, ten Cate H, Hackeng TM. The role of tissue factor pathway inhibitor in atherosclerosis and arterial thrombosis. Blood Rev. 2013;27(03):119–132. doi: 10.1016/j.blre.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Maroney SA, Ellery PE, Wood JP, Ferrel JP, Martinez ND, Mast AE. Comparison of the inhibitory activities of human tissue factor pathway inhibitor (TFPI)α and TFPIβ. J Thromb Haemost. 2013;11(05):911–918. doi: 10.1111/jth.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkhart JM, Vaudel M, Gambaryan S, et al. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood. 2012;120(15):e73–e82. doi: 10.1182/blood-2012-04-416594. [DOI] [PubMed] [Google Scholar]
- 7.Maroney SA, Ferrel JP, Pan S, et al. Temporal expression of alternatively spliced forms of tissue factor pathway inhibitor in mice. J Thromb Haemost. 2009;7(07):1106–1113. doi: 10.1111/j.1538-7836.2009.03454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girard TJ, Tuley E, Broze GJ., Jr TFPIβ is the GPI-anchored TFPI isoform on human endothelial cells and placental microsomes. Blood. 2012;119(05):1256–1262. doi: 10.1182/blood-2011-10-388512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maroney SA, Ellery PE, Wood JP, Ferrel JP, Bonesho CE, Mast AE. Caveolae optimize tissue factor-Factor VIIa inhibitory activity of cell-surface-associated tissue factor pathway inhibitor. Biochem J. 2012;443(01):259–266. doi: 10.1042/BJ20111994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahnström J, Andersson HM, Hockey V, et al. Identification of functionally important residues in TFPI Kunitz domain 3 required for the enhancement of its activity by protein S. Blood. 2012;120(25):5059–5062. doi: 10.1182/blood-2012-05-432005. [DOI] [PubMed] [Google Scholar]
- 11.Peraramelli S, Suylen DP, Rosing J, Hackeng TM. The Kunitz 1 and Kunitz 3 domains of tissue factor pathway inhibitor are required for efficient inhibition of factor Xa. Thromb Haemost. 2012;108(02):266–276. doi: 10.1160/TH11-12-0902. [DOI] [PubMed] [Google Scholar]
- 12.Wood JP, Bunce MW, Maroney SA, Tracy PB, Camire RM, Mast AE. Tissue factor pathway inhibitor-alpha inhibits prothrombinase during the initiation of blood coagulation. Proc Natl Acad Sci U S A. 2013;110(44):17838–17843. doi: 10.1073/pnas.1310444110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reglińska-Matveyev N, Andersson HM, Rezende SM, et al. TFPI cofactor function of protein S: essential role of the protein S SHBG-like domain. Blood. 2014;123(25):3979–3987. doi: 10.1182/blood-2014-01-551812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincent LM, Tran S, Livaja R, Bensend TA, Milewicz DM, Dahlbäck B. Coagulation factor V(A2440G) causes east Texas bleeding disorder via TFPIα. J Clin Invest. 2013;123(09):3777–3787. doi: 10.1172/JCI69091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. 2013;93(01):327–358. doi: 10.1152/physrev.00016.2011. [DOI] [PubMed] [Google Scholar]
- 16.Siljander PR, Munnix IC, Smethurst PA, et al. Platelet receptor interplay regulates collagen-induced thrombus formation in flowing human blood. Blood. 2004;103(04):1333–1341. doi: 10.1182/blood-2003-03-0889. [DOI] [PubMed] [Google Scholar]
- 17.van der Meijden PE, Munnix IC, Auger JM, et al. Dual role of collagen in factor XII-dependent thrombus formation. Blood. 2009;114(04):881–890. doi: 10.1182/blood-2008-07-171066. [DOI] [PubMed] [Google Scholar]
- 18.Okorie UM, Denney WS, Chatterjee MS, Neeves KB, Diamond SL. Determination of surface tissue factor thresholds that trigger coagulation at venous and arterial shear rates: amplification of 100 fM circulating tissue factor requires flow. Blood. 2008;111(07):3507–3513. doi: 10.1182/blood-2007-08-106229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berny MA, Munnix IC, Auger JM, et al. Spatial distribution of factor Xa, thrombin, and fibrin(ogen) on thrombi at venous shear. PLoS One. 2010;5(04):e10415. doi: 10.1371/journal.pone.0010415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colace TV, Muthard RW, Diamond SL. Thrombus growth and embolism on tissue factor-bearing collagen surfaces under flow: role of thrombin with and without fibrin. Arterioscler Thromb Vasc Biol. 2012;32(06):1466–1476. doi: 10.1161/ATVBAHA.112.249789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swieringa F, Baaten CC, Verdoold R, et al. Platelet control of fibrin distribution and microelasticity in thrombus formation under flow. Arterioscler Thromb Vasc Biol. 2016;36(04):692–699. doi: 10.1161/ATVBAHA.115.306537. [DOI] [PubMed] [Google Scholar]
- 22.Cosemans JM, Angelillo-Scherrer A, Mattheij NJ, Heemskerk JW. The effects of arterial flow on platelet activation, thrombus growth, and stabilization. Cardiovasc Res. 2013;99(02):342–352. doi: 10.1093/cvr/cvt110. [DOI] [PubMed] [Google Scholar]
- 23.Mattheij NJ, Swieringa F, Mastenbroek TG, et al. Coated platelets function in platelet-dependent fibrin formation via integrin αIIbβ3 and transglutaminase factor XIII. Haematologica. 2016;101(04):427–436. doi: 10.3324/haematol.2015.131441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swieringa F, Kuijpers MJ, Lamers MM, van der Meijden PE, Heemskerk JW. Rate-limiting roles of the tenase complex of factors VIII and IX in platelet procoagulant activity and formation of platelet-fibrin thrombi under flow. Haematologica. 2015;100(06):748–756. doi: 10.3324/haematol.2014.116863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen B, Holscher T, Sato Y, Pawlinski R, Mackman N. A balance between tissue factor and tissue factor pathway inhibitor is required for embryonic development and hemostasis in adult mice. Blood. 2005;105(07):2777–2782. doi: 10.1182/blood-2004-09-3724. [DOI] [PubMed] [Google Scholar]
- 26.Ellery PE, Maroney SA, Cooley BC, et al. A balance between TFPI and thrombin-mediated platelet activation is required for murine embryonic development. Blood. 2015;125(26):4078–4084. doi: 10.1182/blood-2015-03-633958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parunov LA, Fadeeva OA, Balandina AN, et al. Improvement of spatial fibrin formation by the anti-TFPI aptamer BAX499: changing clot size by targeting extrinsic pathway initiation. J Thromb Haemost. 2011;9(09):1825–1834. doi: 10.1111/j.1538-7836.2011.04412.x. [DOI] [PubMed] [Google Scholar]
- 28.Schols SE, Lancé MD, Feijge MA, et al. Impaired thrombin generation and fibrin clot formation in patients with dilutional coagulopathy during major surgery. Thromb Haemost. 2010;103(02):318–328. doi: 10.1160/TH09-06-0396. [DOI] [PubMed] [Google Scholar]
- 29.Van Kruchten R, Cosemans JM, Heemskerk JW. Measurement of whole blood thrombus formation using parallel-plate flow chambers - a practical guide. Platelets. 2012;23(03):229–242. doi: 10.3109/09537104.2011.630848. [DOI] [PubMed] [Google Scholar]
- 30.Munnix IC, Kuijpers MJ, Auger J, et al. Segregation of platelet aggregatory and procoagulant microdomains in thrombus formation: regulation by transient integrin activation. Arterioscler Thromb Vasc Biol. 2007;27(11):2484–2490. doi: 10.1161/ATVBAHA.107.151100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cosemans JM, Schols SE, Stefanini L, et al. Key role of glycoprotein Ib/V/IX and von Willebrand factor in platelet activation-dependent fibrin formation at low shear flow. Blood. 2011;117(02):651–660. doi: 10.1182/blood-2010-01-262683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westein E, van der Meer AD, Kuijpers MJ, Frimat JP, van den Berg A, Heemskerk JW. Atherosclerotic geometries exacerbate pathological thrombus formation poststenosis in a von Willebrand factor-dependent manner. Proc Natl Acad Sci U S A. 2013;110(04):1357–1362. doi: 10.1073/pnas.1209905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Meijden PE, Feijge MA, Swieringa F, et al. Key role of integrin α(IIb)β (3) signaling to Syk kinase in tissue factor-induced thrombin generation. Cell Mol Life Sci. 2012;69(20):3481–3492. doi: 10.1007/s00018-012-1033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winckers K, Thomassen S, Ten Cate H, Hackeng TM. Platelet full length TFPI-α in healthy volunteers is not affected by sex or hormonal use. PLoS One. 2017;12(02):e0168273. doi: 10.1371/journal.pone.0168273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Witt SM, Swieringa F, Cavill R, et al. Identification of platelet function defects by multi-parameter assessment of thrombus formation. Nat Commun. 2014;5:4257. doi: 10.1038/ncomms5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan JC, Carmeliet P, Moons L, et al. Factor VII deficiency rescues the intrauterine lethality in mice associated with a tissue factor pathway inhibitor deficit. J Clin Invest. 1999;103(04):475–482. doi: 10.1172/JCI5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Repke D, Gemmell CH, Guha A, Turitto VT, Broze GJ, Jr, Nemerson Y. Hemophilia as a defect of the tissue factor pathway of blood coagulation: effect of factors VIII and IX on factor X activation in a continuous-flow reactor. Proc Natl Acad Sci U S A. 1990;87(19):7623–7627. doi: 10.1073/pnas.87.19.7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nordfang O, Valentin S, Beck TC, Hedner U. Inhibition of extrinsic pathway inhibitor shortens the coagulation time of normal plasma and of hemophilia plasma. Thromb Haemost. 1991;66(04):464–467. [PubMed] [Google Scholar]
- 39.Welsch DJ, Novotny WF, Wun TC. Effect of lipoprotein-associated coagulation inhibitor (LACI) on thromboplastin-induced coagulation of normal and hemophiliac plasmas. Thromb Res. 1991;64(02):213–222. doi: 10.1016/0049-3848(91)90120-l. [DOI] [PubMed] [Google Scholar]
- 40.Peraramelli S, Thomassen S, Heinzmann A, et al. Direct inhibition of factor VIIa by TFPI and TFPI constructs. J Thromb Haemost. 2013;11(04):704–714. doi: 10.1111/jth.12152. [DOI] [PubMed] [Google Scholar]
- 41.Wood JP, Ellery PE, Maroney SA, Mast AE. Protein S is a cofactor for platelet and endothelial tissue factor pathway inhibitor-α but not for cell surface-associated tissue factor pathway inhibitor. Arterioscler Thromb Vasc Biol. 2014;34(01):169–176. doi: 10.1161/ATVBAHA.113.302655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van ’t Veer C, Hackeng TM, Delahaye C, Sixma JJ, Bouma BN. Activated factor X and thrombin formation triggered by tissue factor on endothelial cell matrix in a flow model: effect of the tissue factor pathway inhibitor. Blood. 1994;84(04):1132–1142. [PubMed] [Google Scholar]
- 43.Hackeng TM, Seré KM, Tans G, Rosing J. Protein S stimulates inhibition of the tissue factor pathway by tissue factor pathway inhibitor. Proc Natl Acad Sci U S A. 2006;103(09):3106–3111. doi: 10.1073/pnas.0504240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shibeko AM, Lobanova ES, Panteleev MA, Ataullakhanov FI. Blood flow controls coagulation onset via the positive feedback of factor VII activation by factor Xa. BMC Syst Biol. 2010;4:5. doi: 10.1186/1752-0509-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorczyca ME, Nair SC, Jilma B, et al. Inhibition of tissue factor pathway inhibitor by the aptamer BAX499 improves clotting of hemophilic blood and plasma. J Thromb Haemost. 2012;10(08):1581–1590. doi: 10.1111/j.1538-7836.2012.04790.x. [DOI] [PubMed] [Google Scholar]
- 46.Erhardtsen E, Ezban M, Madsen MT, et al. Blocking of tissue factor pathway inhibitor (TFPI) shortens the bleeding time in rabbits with antibody induced haemophilia A. Blood Coagul Fibrinolysis. 1995;6(05):388–394. doi: 10.1097/00001721-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Hilden I, Lauritzen B, Sørensen BB, et al. Hemostatic effect of a monoclonal antibody mAb 2021 blocking the interaction between FXa and TFPI in a rabbit hemophilia model. Blood. 2012;119(24):5871–5878. doi: 10.1182/blood-2012-01-401620. [DOI] [PubMed] [Google Scholar]
- 48.Badellino KO, Walsh PN. Protease nexin II interactions with coagulation factor XIa are contained within the Kunitz protease inhibitor domain of protease nexin II and the factor XIa catalytic domain. Biochemistry. 2000;39(16):4769–4777. doi: 10.1021/bi9925468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.