Abstract
Changes in extracellular zinc concentration participate in modulating fundamental cellular processes such as proliferation, secretion, and ion transport in a mechanism that is not well understood. Here, we show that a micromolar concentration of extracellular zinc triggers a massive release of calcium from thapsigargin-sensitive intracellular pools in the colonocytic cell line HT29. Calcium release was blocked by a phospholipase-C inhibitor, indicating that formation of inositol 1,4,5-triphosphate is required for zinc-dependent calcium release. Zinc influx was not observed, indicating that extracellular zinc triggered the release. The Cai2+ release was zinc specific and could not be triggered by other heavy metals. Furthermore, zinc failed to activate the Ca2+-sensing receptor heterologously expressed in HEK293 cells. The zinc-induced Cai2+ rise stimulated the activity of the Na+/H+ exchanger in HT29 cells. Our results indicate that a previously uncharacterized extracellular, G protein-coupled, Zn2+-sensing receptor is functional in colonocytes. Because Cai2+ rise is known to regulate key cellular and signal-transduction processes, the zinc-sensing receptor may provide the missing link between extracellular zinc concentration changes and the regulation of cellular processes.
Zinc is an essential micronutrient involved in structural and regulatory cellular functions. Zinc interacts with zinc-finger domains and acts as a cofactor of numerous enzymes (1, 2). Zinc ions also specifically bind to many membrane receptors, transporters, and channels, thereby modulating their activity (3). Therefore, it is not surprising that zinc deficiency affects multiple organs, including the digestive (4), immune (5), and neuronal (2) systems. A severe lack of zinc also is linked to the attenuation of growth and sexual development (2). Conversely, an excess of extracellular zinc is considered toxic. Indeed, brain ischemia is accompanied by a massive release of synaptic zinc permeating into neurons, leading to neuronal cell death (6–8). Furthermore, striking changes in plasma-zinc concentration occur during diverse pathophysiological syndromes including myocardial infarction, hepatic renal failure, and neoplastic processes (9). Despite the large fluctuations in extracellular zinc concentrations and their subsequent clinical importance, little is known about cellular signaling mechanisms that sense changes in extracellular-zinc concentration.
The calcium-sensing receptor serves as an example for the importance of an ion-sensing mechanism. It is a G protein-coupled receptor that senses changes in extracellular calcium and regulates diverse cellular functions (10, 11). Although the existence of other ion-sensing mechanisms have been suggested (12), none have been characterized fully yet.
Gastrointestinal pathology, manifested in severe diarrhea, is an important hallmark of zinc deficiency. Indeed, zinc has a major role in the duration and severity of diarrheal diseases (4). The morbidity and mortality caused by diarrheal diseases are particularly severe in young infants and result in up to 4 million deaths per year (13). Lack of nutritional zinc not only lowers the epithelial-cell proliferation rate and thus the renewal of gastrointestinal mucosa (14), but also leads to impaired vectorial transport of Na+, Cl−, and water (2, 15). Despite the importance of zinc in controlling these cellular processes, the link between extracellular zinc and cellular signaling pathways is not understood well.
In the present work, we describe a previously uncharacterized G protein-coupled receptor that specifically senses changes in extracellular zinc concentration and subsequently triggers a release of intracellular calcium, thereby modulating ion transport in colonocytes. These findings provide evidence for a specific zinc-sensing mechanism involving cellular signaling, and hence, control of key cellular processes.
Materials and Methods
Cell Culture.
HT29-Cl (human colon carcinoma cell line), HSY (human salivary gland cell line), HEK293 (human embryonic kidney cell line) were grown in DMEM as described (16–18). The spontaneously immortalized human keratinocyte cell line (HaCat; ref. 19) and primary normal human epidermal keratinocytes (NHK; ref. 20) isolated and propagated from young foreskins were applied in this study. Growth media and growth conditions were as described. All cells were seeded on glass cover slides for 1–2 days before measurements. Measurements for the HSY cells were performed in a fluorometric cuvette as described (18). Transient transfection of the HEK293 cells was performed with 3 μg of the Ca-sensing receptor plasmid (CaR; kindly provided by K. D. Rodland, Oregon Health Science Univ., Portland, OR) by using the calcium phosphate precipitation method as described (17). Control cells were transfected with the pcDNA3 vector alone. Measurements were performed 36–48 hr after transfection.
Fluorescent Imaging.
The imaging system consisted of an Axiovert 100 inverted microscope (Zeiss), Polychrome II monochromator (TILL Photonics, Planegg, Germany) and a SensiCam cooled charge-coupled device (PCO, Kelheim, Germany). Fluorescent imaging measurements were acquired with IMAGING WORKBENCH 2 (Axon Instruments, Foster City, CA). All results shown are the means of four to six independent experiments, with averaged responses of 30–50 cells in each experiment.
Calcium Imaging.
Cells were incubated for 30 min with 5 μM Fura-2 acetoxymethyl ester (AM; TEF-Lab, Austin, TX) in 0.1% BSA in Na+ Ringer's solution. After dye loading, the cells were washed in Ringer's solution, and the cover slides were mounted in a chamber that allowed the superfusion of cells. Fura-2 was excited at 340 nm and 380 nm and imaged with a 510-nm long-pass filter. Cai was calibrated by using the calcium ionophore Bromo-A23187 in the presence of 5 mM Ca2+ or 2 mM EGTA, as described (21).
pH Imaging.
By using the same experimental setup, cells were loaded for 20 min with 5 μM 2,7-bis-(carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF)-AM (TEF-Lab) in 0.1% BSA in Ringer's solution. The excitation was at 440 nm and 490 nm, and emission was monitored through a 510-nm long-pass filter. pHi was calibrated at the end of each experiment, as described (22).
Spectrofluorometric Measurements.
Some experiments were conducted with the Ratiomaster spectrofluorometer (PTI, South Brunswick, NJ). Cells were trypsinized and loaded with Fura-2 AM, as described above. After loading, the cells were washed twice and resuspended in a stirring cuvette for measurements.
Results
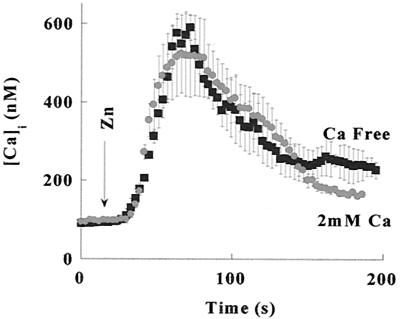
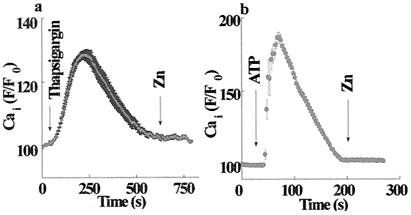
Calcium ions are known to regulate many cellular processes. To determine what role zinc plays in intracellular calcium fluxes, we tested whether changes in extracellular Zn2+ concentration trigger a rise in Cai2+. HT29 cells loaded with Fura-2 were superfused with Ringer's solution, and Fura-2 fluorescence was monitored. The addition of 100 μM Zn2+ triggered a rapid increase in fluorescence (Fig. 1). Such increases in Fura-2 fluorescence may be the result of either calcium influx from the extracellular medium or calcium release from intracellular pools. To distinguish between these mechanisms, HT29 cells were superfused with either Ca2+-containing or Ca2+-free Ringer's solution (Fig. 1). The increase in fluorescence triggered by 100 μM Zn2+ was independent of the extracellular calcium; hence, Cai2+ is released from intracellular pools. The lack of an elevated plateau in the calcium response when the experiment was performed in calcium containing Ringer's solution may be attributed to the inhibitory effect of zinc on the store-operated calcium influx (23, 24). Fura-2 is also a highly sensitive probe for changes in intracellular Zn2+ concentration (25). To distinguish whether Zn2+ influx or zinc-induced Ca2+ release caused the rise in Fura-2 fluorescence, Zn2+ was applied after the depletion of intracellular Ca2+ stores. As shown in Fig. 2, Zn2+ (200 μM) applied after pretreatment with either the Ca2+ pump inhibitor, thapsigargin (200 nM), or with ATP (100 μM) in Ca2+-free Ringer's solution failed to elicit an increase in Fura-2 fluorescence. Thus, the rise in Fura-2 fluorescence was triggered by extracellular zinc rather than Zn2+ influx. These findings suggest that a metabotropic pathway triggering Cai2+ release from thapsigargin-sensitive intracellular calcium pools was initiated by an extracellular zinc-sensing receptor (ZnR).
Figure 1.
Cai2+ increase in response to application of zinc. Changes in Cai2+ in HT29 cells loaded with Fura-2 in response to application of Zn2+, as described under Materials and Methods. Zinc (100 μM) was added at the indicated time to HT29 cells superfused with 2 mM Ca2+-containing Ringer's solution (●) or Ca2+-free Ringer's solution (■). In both experiments, application of Zn2+ resulted in a similar increase of Cai2+, indicating that Ca2+ is released from intracellular pools.
Figure 2.
Zinc triggers the release of calcium from thapsigargin-sensitive intracellular stores. (a) HT29 cells were superfused with Ca2+-free Ringer's solution and treated with thapsigargin (200 nM), thus inducing a transient Cai2+ rise caused by the emptying of intracellular calcium stores. Zn2+ (200 μM) was added after Cai2+ returned to a resting level. (b) ATP (100 μM) was applied to HT29 cells superfused with Ca2+-free Ringer's solution, resulting in intracellular calcium release; then, Zn2+ (200 μM) was applied (indicated by Zn arrow). No Zn2+-dependent Cai2+ release was observed after the emptying of intracellular stores, thus indicating that extracellular Zn2+ induces the release of Ca2+ from thapsigargin-sensitive intracellular stores.
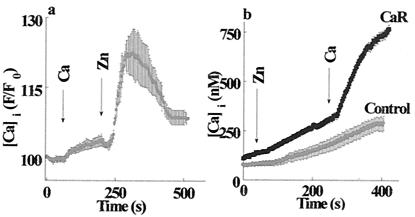
The expression and activity of a CaR has been documented (10, 11, 26). To determine whether the Zn2+-dependent Cai2+ release is related to the CaR, we performed the following experiment. A solution containing 2 mM calcium (a concentration well above the reported Km for the CaR; ref. 26) was applied, followed by a solution containing 100 μM Zn2+. As shown in Fig. 3a, administration of extracellular calcium elicited an increase in Cai2+, similar to that reported (26). The subsequent superfusion of the cells with Zn2+ elicited a much greater calcium response. These findings suggest that HT29 cells express not only the CaR but also the putative ZnR.
Figure 3.
ZnR is distinct from the CaR. (a) HT29 cells were superfused with 2 mM Ca2+-containing Ringer's solution to activate the CaR. At the time indicated by the arrow, Zn2+ (100 μM) was added to the superfusing solution. The response to Zn2+ was ≈7-fold larger than the response to Ca2+. (b) HEK293 cells transfected with the CaR or the vector alone (Control) were superfused with Ringer's solution containing 0.3 mM Ca2+ and 250 μM Zn2+; then, 2 mM Ca2+ was added to activate the CaR. Cells expressing the CaR did not respond to the application of Zn2+.
Experiments performed on HEK293 cells that heterologously express the CaR (27) provide further evidence that the ZnR is distinct from the CaR. Whereas the addition of extracellular Ca2+ to control cells did not trigger any Cai2+ response, an increase of Cai2+ was apparent in the cells heterologously expressing the CaR (Fig. 3b). However, application of zinc failed to evoke any Cai2+ release in cells expressing the Ca2+-sensing receptor (Fig. 3b). These findings strongly indicate that the CaR is not activated by Zn2+ and is in agreement with previous reports (28, 29). The slow rise in Fura-2 fluorescence observed in the control and CaR-transfected cells represents a slow zinc permeation that, according to our preliminary results, occurs by means of endogenous Ca2+ channels in HEK293 cells (D. Segal, M.H., A.M., I.S., unpublished work).
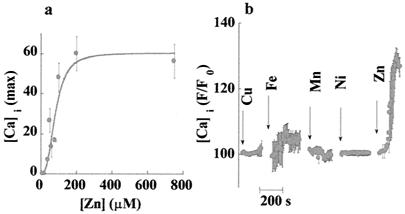
Further, we asked whether the ZnR is activated by physiological concentrations of Zn2+, and whether other heavy metals are able to activate the receptor. The amplitude of the Cai2+ response as a function of increasing Zn2+ concentration is shown in Fig. 4a. The data were fitted to a Michaelis–Menten equation and yielded an apparent Km of 80 ± 15 μM with a Hill coefficient of ≈3. This result is consistent with the cooperative action of zinc ions with the ZnR, as shown for numerous G protein-coupled receptors (27, 30). ZnR specificity was tested through the application of heavy metals to HT29 cells. In Fig. 4b, the Cai2+ responses following the administration of Cu (250 μM), Ni (250 μM), Mn (500 μM), and Fe (500 μM) are shown in comparison to the response to Zn. None of the other heavy metals tested triggered significant changes in Cai2+ concentration, indicating that the response to Zn was specific.
Figure 4.
Affinity and specificity of the ZnR. (a) Maximal responses to zinc at various concentrations are shown. Each data point represents the means ± SE of five independent experiments. Data were fitted by using the Michaelis–Menten equation, yielding an apparent Km of 80 ± 15 μM. The curve is sigmoidal with a Hill coefficient of 3, which is consistent with the cooperative interaction of zinc ions with ZnR. (b) Cells were superfused with Ca2+-containing Ringer's solution containing 500 μM Mn or Fe; other experiments were done with Ca2+-free Ringer's solution containing 250 μM Cu or Ni (the beginning of each experiment is marked by an arrow). Also, no response was observed when Ni or Cu were introduced in Ca2+-containing Ringer's solution (data not shown). The lack of response to any of the other heavy metals measured indicates that the ZnR was specifically activated by Zn2+.
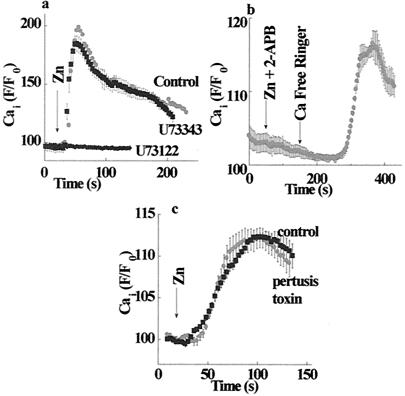
Many metabotropic receptors trigger intracellular Ca2+ release through activation of Gq or G11, as well as by activation of the Gi and Go proteins. The α-subunits of these G proteins activate phospholipase-C (PLC) (31). Subsequent generation of inositol 1,4,5 triphosphate (IP3) leads to the release of calcium from intracellular pools. Experiments designed to study the signal-transduction pathway activated by the putative ZnR were carried out in the absence of calcium in the experimental solutions and are shown in Fig. 5. Application of the PLC inhibitor U73122 (1 μM) completely blocked the Zn2+-induced increase in Cai2+ (Fig. 5a). In contrast, treatment with the inactive analogue of the PLC inhibitor U73343 had no effect on the activity of the ZnR (Fig. 5a). We next applied 2-APB, an inhibitor of the IP3 receptor (32). Cells were preincubated for 5 min with 300 μM 2-APB and then perfused with 100 μM 2-APB in calcium-free Ringer's solution. Fig. 5b shows that 2-APB totally blocked the zinc-dependent calcium release and did not cause calcium release, as suggested for another cell line (33). Subsequent washing with calcium-free Ringer's solution without the inhibitor of the IP3 receptor triggered a massive intracellular Ca2+ release. It has been suggested recently that, in some cell types, 2-APB also inhibits Ca2+ release-activated channels (CRAC) (33–35). Because the experiment described in Fig. 5b was performed in calcium-free solutions, the apparent calcium rise followed by the inhibitor wash is caused by the activation of the IP3 receptors rather than CRAC activity. The reversible inhibition of the IP3 receptor by 2-APB suggests that the ZnR triggers the release of IP3, which, upon the removal of the 2-APB, binds to the receptor and releases calcium from intracellular stores. Therefore, our results indicate that ZnR triggers activation of PLC, followed by the release and the binding of IP3 to its receptor, with a subsequent release of calcium from intracellular stores. The activity of ZnR was not affected by the pretreatment of HT29 cells with 50 ng/ml of pertussis toxin, an inhibitor of Go and Gi (Fig. 5c). Thus, the pathway activated by the ZnR is likely to be mediated by Gq.
Figure 5.
Signal transduction pathway activated by ZnR. (a) Cells were treated (as marked) with 1 μM PLC inhibitor U73122 (active form), or U73343 (inactive form), or the equivalent volume of DMSO (control), and the calcium response was determined. Zinc-dependent calcium release was inhibited by the active but not by the inactive compound, indicating that ZnR activates the PLC. (b) Cells were pretreated with the IP3 receptor–inhibitor diphenylboric acid 2-aminoethyl ester (2-ApB), and the calcium response was monitored in Ca2+-free Ringer's solution. The zinc-dependent calcium release was blocked in the presence of 2-APB. Washing out the inhibitor with Ca2+-free Ringer's solution resulted in a calcium rise. This fact indicates that ZnR activated the release of IP3, which only was apparent once the inhibition of its receptor was released. The binding of the IP3 to the unblocked receptor resulted in intracellular Ca2+ rise. (c) Controls and cells treated with 50 ng/ml pertussis toxin were superfused with Zn2+ (200 μM). No significant change in ZnR activity was apparent, suggesting that the Go and Gi proteins are not part of the ZnR signal-transduction pathway.
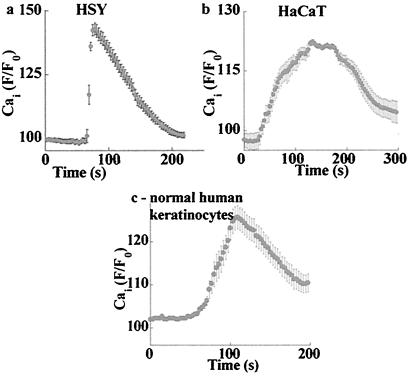
Further, we tested whether ZnR is functional only in colonocytes or whether it also is expressed in other cells originating from tissues either susceptible to zinc deficiency or involved in zinc homeostasis. As shown in Fig. 6, application of zinc (100 μM) triggered intracellular Ca2+ release in HSY (Fig. 6a), HaCat (Fig. 6b), and also in a primary culture of normal human keratinocytes (Fig. 6c). In the latter case, the calcium response was observed in about 20% of the cells. The response was apparent after the application of zinc, which was preceded by a 1-min superfusion with the same zinc-containing Ringer's solution. By using the same experimental procedures described for the HT29 cells, we demonstrated that the Ca2+-dependent zinc release occurred in the absence of calcium, was inhibited by the PLC inhibitor U73122 (1 μM), and was specific to zinc ions (data not shown). These findings indicate that the expression of ZnR is not limited to colonocytes but may play a physiological role in other tissues.
Figure 6.
ZnR activity is observed in various cell types. The indicated cells were superfused with calcium-free Ringer's solution followed by the application of 100 μM zinc; changes in Cai2+ were monitored. (a) HSY, human salivary-gland cell line. (b) HaCat, human keratinocytic cell line. (c) For the primary culture of normal human keratinocytes, the graph shows the averaged fluorescence signal of the zinc-responding cells (as described in Results).
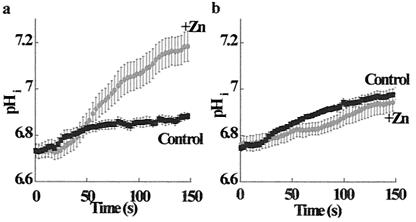
The sodium–hydrogen exchanger (Na+/H+) plays a key role in colonocyte recovery from acid load and transepithelial reabsorption of sodium and water (36, 37). Therefore, it was of interest to determine whether this physiological process is regulated by the ZnR. The Na+/H+ exchanger in HT29 cells was activated by an ammonium prepulse in sodium-free medium; the recovery of intracellular pH was monitored by BCECF fluorescence after the readministration of sodium-containing Ringer's solution (38). As shown in Fig. 7a, the application of 100 μM Zn2+ accelerated the activity of the Na+/H+ exchanger ≈5-fold. The rate of pHi recovery was 15.7 ± 0.1 × 10−3 pHi units per s in the presence of Zn2+ as compared with 3.0 ± 0.1 × 10−3 pHi units per s in the control.
Figure 7.
Cai2+ rise mediated by ZnR stimulates Na+/H+ exchanger activity. The Na+/H+ exchanger was stimulated by acidifying HT29 cells using an ammonium prepulse protocol. The rate of pHi recovery was determined by monitoring changes in BCECF fluorescence in the presence or absence of 200 μM zinc (as marked). (a) The rate of pHi recovery was accelerated ≈5-fold in the presence of Zn2+. The rate of pHi recovery was 15.7 ± 0.1 × 10−3 pHi units per s in the presence of Zn2+, as compared with 3.0 ± 0.1 × 10−3 pHi units per s in the control. (b) Cells treated with thapsigargin (200 nM) were superfused with calcium-free Ringer's solution. The rate of pHi recovery after thapsigargin treatment was 3.5 ± 0.4 × 10−3 pHi units per s in the presence of Zn2+, as compared with 2.7 ± 0.4 × 10−3 pHi units per s in the control. Eliminating the changes in Cai2+ inhibited the zinc-stimulated pHi recovery. These results indicate that the stimulation of the Na+/H+ exchanger by the ZnR is mediated by the zinc-induced rise in Cai2+.
We further examined whether the stimulatory effect of Zn2+ on the Na+/H+ exchanger is mediated through the ZnR-induced Cai2+ release. This possibility was determined by two independent methods: emptying intracellular Ca2+ stores with thapsigargin (see Fig. 2) or clamping intracellular Ca2+ by using 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA)-AM (25 μM; data not shown). As shown in Fig. 7b, when intracellular calcium changes were inhibited, the rate of pHi recovery was not stimulated by the application of Zn2+. The rate of pHi recovery after thapsigargin treatment was 3.5 ± 0.4 × 10−3 pHi units per s in the presence of Zn2+, as compared with 2.7 ± 0.4 × 10−3 pHi units per s in the control; in BAPTA, the rates were 4.0 ± 0.4 × 10−3 and 4.5 ± 0.4 × 10−3 pHi units per s in zinc-treated and control cells, respectively. These results indicate that, indeed, the stimulation of Na+/H+ exchanger activity is caused by the ZnR-mediated Cai2+ release.
Discussion
Changes in ion concentration have a fundamental effect on numerous physiological processes. The sensing and signaling of these changes may have an important homeostatic role. Indeed, a CaR has been identified (10), and receptors to other ions such as K+, Na+, and Cl− have been proposed (12, 39). A nonselective heavy-metal receptor also has been described in hepatocytes. This receptor is activated by Ni2+, Cu2+, La3+, and Zn2+ (40). In fibroblasts and endothelial cells, a nonselective heavy-metal receptor is inhibited strongly by zinc (41, 42). We now provide evidence for the existence of a specific ZnR that is distinct from the calcium and the heavy-metal receptors and is functional in HT29 cells.
The activity of the ZnR is demonstrated by the robust increase in cytosolic Ca2+ concentrations triggered by the application of extracellular Zn2+ (Fig. 1). Calcium is released from thapsigargin-sensitive intracellular stores, as indicated by the failure of Zn2+ to trigger such a release after the emptying of stores by thapsigargin or ATP (Fig. 2). The lack of any apparent increase in Fura-2 fluorescence after the emptying of intracellular stores implies that ZnR activity is mediated by extracellular Zn2+ rather than by cellular Zn2+ permeation. The apparent affinity of the receptor (80 μM) is within the physiological range of Zn2+ concentration in the lumen of the gastrointestinal tract (43–46). Metal specificity of the ZnR is demonstrated by the inability of other heavy metals to trigger Cai2+ release (Fig. 4b). This fact distinguishes ZnR from the nonselective metal receptors described previously. The functional demonstration of the ZnR in several cell types suggests that ZnR may have a general physiological role.
Release of Cai2+ by the ZnR is mediated by PLC, as it can be inhibited completely by the PLC inhibitor U73122 (Fig. 5a). The reversible inhibition of the calcium response by the IP3 receptor–inhibitor, 2-APB, suggests that the ZnR-dependent calcium release requires the binding of the IP3 to its receptor (Fig. 5b). Insensitivity of the ZnR to pertussis toxin (Fig. 5c) indicates that Gq or G11 rather than Gi or Go is involved in the signal-transduction pathway leading to Cai2+ release.
Our results further suggest that the ZnR is distinct from the CaR because of the following reasons. (i) Although the CaR was present in HT29 cells and could be activated by Ca2+ (26), application of Zn2+ in the presence of 2 mM calcium triggered a much larger Cai2+ release (Fig. 3a). (ii) HEK293 cells heterologously expressing a functional CaR failed to show Zn2+-dependent Ca2+ release (Fig. 3b). The failure of zinc to activate the CaR in this work is in agreement with previous reports (28, 29).
Notwithstanding the distinction between the two receptors, the ZnR shares several features with the CaR, such as activation by a metal ligand and a similar signal-transduction pathway, indicating that ZnR may be a previously uncharacterized member of the mGlu receptor family. Indeed, several CaR homologues have been identified but have not been functionally characterized yet (47).
Zinc deficiency directly reduces the uptake of amino acids in the intestine (48). In addition, zinc ions in physiological concentrations decrease the membrane potential in oxyntic cells, thereby reducing the transport of K+, Cl−, and NO2 (4, 48–51). The Na+/H+ exchanger plays a central role in vectorial Na+ transport and pH homeostasis in the colon (52–54). Activation by Cai2+ has been demonstrated for the Na+/H+ exchanger in several cell lines (55–57). We show that the zinc-triggered Cai2+ rise stimulates Na+/H+ exchanger activity (Fig. 7); this increase may enhance the vectorial Na+ transport and the subsequent transport of water across the colon epithelia.
Zinc ions have a well recognized mitogenic effect that is essential for the development and functioning of numerous tissues. Yet, the mechanism linking zinc to cell proliferation remains unknown. The proliferative effect of zinc is not likely to be mediated by direct interaction with intracellular metalloproteins, because symptoms of zinc deficiency precede changes in intracellular Zn2+ content (14, 58). Zn2+ also activates major signal-transduction pathways involved in cell proliferation, such as the mitogen-activated protein (MAP) kinase (14). However, chelating the intracellular zinc does not change the activation state of MAP kinase (59). Altogether, these findings suggest the presence of a mechanism capable of sensing changes in extracellular zinc and triggering cell signaling even before changes in intracellular Zn2+ are manifested. We propose that ZnR, described in the present work, may be responsible for sensing extracellular Zn2+ and for triggering Cai2+ release. Such a release of intracellular calcium may lead to the opening of the calcium release activated channel (CRAC), thus prolonging the period of elevated intracellular calcium levels. Hence, activation of the ZnR leading to calcium rise, from intracellular pools and the subsequent activation of the CRAC, may regulate cell proliferation.
Zinc is essential for the healing of wounds, promoting cell proliferation, and growth (60, 61). In fibroblasts, zinc enhances cell proliferation synergistically with calcium (62). Keratinocyte proliferation is activated by changes in Cai2+ concentration (63, 64) and is enhanced by extracellular zinc. Our results suggest that ZnR activation provides the mechanism linking the proliferation of keratinocytes, Cai2+ changes, and zinc. Indeed, our results show that the ZnR is functional in keratinocytes. Zinc is excreted by the pancreas, salivary, and sweat glands (2). It is conceivable that the activity of the ZnR in these tissues may play a role in regulating their excretory functions. Furthermore, our finding that the ZnR regulates the Na+/H+ exchanger in colonocytes may suggest the importance of the ZnR in modulating the intense proton transport mediated by these tissues. In the brain, chelatable zinc is present in glutamatergic synaptic vesicles. Zinc is released into glutamatergic synapses during neuronal activity and modulates the activity of postsynaptic receptors such as N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) (3, 7, 8, 65). The presence of a ZnR may provide the link between extracellular Zn2+ and the diverse responses that occur in neurons.
In conclusion, our results suggest that a specific Zn2+-sensing receptor triggers massive Ca2+ release from intracellular stores. This receptor is capable of sensing extracellular Zn2+, thereby activating diverse signal-transduction pathways. Thus, the ZnR may be the missing link between changes in extracellular Zn2+ and fundamental cell processes.
Acknowledgments
We thank Karin D. Rodland for the CaR plasmid and Nathan Daskal, Nava Moran, Zofia Shreiber, and Rachel Teitelbaum for critical reading. The work was partially supported by National Institutes of Health Grant TW01134–01 (to A.M.), and Israel Science Foundation Grant 9023198 (to I.S.).
Abbreviations
- CaR
calcium-sensing receptor
- ZnR
zinc-sensing receptor
- PLC
phospholipase-C
- AM
acetoxymethyl ester
- a-ApB
diphenyl boric acid 2-aminboethyl ester
- IP3
insitol 1,4,5 triphosphate
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Berg J M, Shi Y. Science. 1996;271:1081–1085. doi: 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]
- 2.Vallee B L, Falchuk K H. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 3.Huang E P. Proc Natl Acad Sci USA. 1997;94:13386–13387. doi: 10.1073/pnas.94.25.13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wapnir R A. J Nutr. 2000;130:1388S–1392S. doi: 10.1093/jn/130.5.1388S. [DOI] [PubMed] [Google Scholar]
- 5.Prasad A S. Nutrition. 1995;11:93–99. [PubMed] [Google Scholar]
- 6.Koh J Y, Suh S W, Gwag B J, He Y Y, Hsu C Y, Choi D W. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- 7.Assaf S Y, Chung S H. Nature (London) 1984;308:734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- 8.Choi D W, Koh J Y. Annu Rev Neurosci. 1998;21:347–375. doi: 10.1146/annurev.neuro.21.1.347. [DOI] [PubMed] [Google Scholar]
- 9.Vikbladh I. Scand J Clin Lab Invest. 1950;2:143–148. doi: 10.3109/00365515009051850. [DOI] [PubMed] [Google Scholar]
- 10.Chattopadhyay N. Int J Biochem Cell Biol. 2000;32:789–804. doi: 10.1016/s1357-2725(00)00031-5. [DOI] [PubMed] [Google Scholar]
- 11.Brown E M, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger M A, Lytton J, Hebert S C. Nature (London) 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 12.Riccardi D. Cell Calcium. 1999;26:77–83. doi: 10.1054/ceca.1999.0066. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs G J. Am J Clin Nutr. 1998;68:480S–483S. doi: 10.1093/ajcn/68.2.480S. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald R S. J Nutr. 2000;130:1500S–1508S. doi: 10.1093/jn/130.5.1500S. [DOI] [PubMed] [Google Scholar]
- 15.Danblot N, Closs K. Acta Derm Venereol. 1942;23:127–169. [Google Scholar]
- 16.Fine D M, Lo C F, Aguillar L, Blackmon D L, Montrose M H. J Membr Biol. 1995;145:129–141. doi: 10.1007/BF00237371. [DOI] [PubMed] [Google Scholar]
- 17.Sekler I, Lo R S, Kopito R R. J Biol Chem. 1995;270:28751–28758. doi: 10.1074/jbc.270.48.28751. [DOI] [PubMed] [Google Scholar]
- 18.Moran A, Turner R J. Am J Physiol. 1993;265:C1405–C1411. doi: 10.1152/ajpcell.1993.265.5.C1405. [DOI] [PubMed] [Google Scholar]
- 19.Boukamp P, Petrussevska R, Breitkreutz D, Hornung J, Markham A, Fusenig N. J. Cell Biol. 1988. 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rheinwald J G. In: Cell Growth and Division: A Practical Approach. Baserga R, editor. Oxford: IRL; 1989. pp. 81–94. [Google Scholar]
- 21.Grynkiewicz G, Poinie M, Tsien R Y. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 22.Chu S, Montrose M H. J Gen Physiol. 1995;105:589–615. doi: 10.1085/jgp.105.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bischof G, Brenman J, Bredt D S, Machen T E. Cell Calcium. 1995;17:250–262. doi: 10.1016/0143-4160(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 24.Camello C, Pariente J A, Salido G M, Camello P J. J Physiol. 1999;516:399–408. doi: 10.1111/j.1469-7793.1999.0399v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atar D, Backx P H, Appel M M, Gao W D, Marban E. J Biol Chem. 1995;270:2473–2477. doi: 10.1074/jbc.270.6.2473. [DOI] [PubMed] [Google Scholar]
- 26.Gama L, Baxendale-Cox L M, Breitwieser G E. Am J Physiol. 1997;273:C1168–C1175. doi: 10.1152/ajpcell.1997.273.4.C1168. [DOI] [PubMed] [Google Scholar]
- 27.Gama L, Breitwieser G E. J Biol Chem. 1998;273:29712–29718. doi: 10.1074/jbc.273.45.29712. [DOI] [PubMed] [Google Scholar]
- 28.Quarles L D, Hartle J E, II, Siddhanti S R, Guo R, Hinson T K. J Bone Miner Res. 1997;12:393–402. doi: 10.1359/jbmr.1997.12.3.393. [DOI] [PubMed] [Google Scholar]
- 29.Ruat M, Snowman A M, Hester L D, Snyder S H. J Biol Chem. 1996;271:5972–5975. doi: 10.1074/jbc.271.11.5972. [DOI] [PubMed] [Google Scholar]
- 30.Christopoulos A, Lanzafame A, Mitchelson F. Clin Exp Pharmacol Physiol. 1998;25:185–194. doi: 10.1111/j.1440-1681.1998.t01-4-.x. [DOI] [PubMed] [Google Scholar]
- 31.Berridge M J. Am J Nephrol. 1997;17:1–11. doi: 10.1159/000169064. [DOI] [PubMed] [Google Scholar]
- 32.Chorna-Ornan I, Joel-Almagor T, Ben-Ami H C, Frechter S, Gillo B, Selinger Z, Gill D L, Minke B. J Neurosci. 2001;21:2622–2629. doi: 10.1523/JNEUROSCI.21-08-02622.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregory R B, Rychkov G, Barritt G J. Biochem J. 2001;354:285–290. doi: 10.1042/0264-6021:3540285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bakowski D, Glitsch M D, Parekh A B. J Physiol. 2001;532:55–71. doi: 10.1111/j.1469-7793.2001.0055g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kukkonen J P, Lund P, Akerman K E. Cell Calcium. 2001;30:117–129. doi: 10.1054/ceca.2001.0219. [DOI] [PubMed] [Google Scholar]
- 36.Chu S, Montrose M H. Proc Natl Acad Sci USA. 1995;92:3303–3307. doi: 10.1073/pnas.92.8.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janecki A J, Montrose M H, Tse C M, de Medina F S, Zweibaum A, Donowitz M. Am J Physiol. 1999;277:G292–G305. doi: 10.1152/ajpgi.1999.277.2.G292. [DOI] [PubMed] [Google Scholar]
- 38.Boyarsky G, Ganz M B, Sterzel R B, Boron W F. Am J Physiol. 1988;255:C844–C856. doi: 10.1152/ajpcell.1988.255.6.C844. [DOI] [PubMed] [Google Scholar]
- 39.Smith J B, Dwyer S D, Smith L. J Biol Chem. 1989;264:831–837. [PubMed] [Google Scholar]
- 40.McNulty T J, Taylor C W. Biochem J. 1999;339:555–561. [PMC free article] [PubMed] [Google Scholar]
- 41.Smith L, Pijuan V, Zhuang Y, Smith J B. Exp Cell Res. 1992;202:174–182. doi: 10.1016/0014-4827(92)90417-7. [DOI] [PubMed] [Google Scholar]
- 42.Dwyer S D, Zhuang Y, Smith J B. Exp Cell Res. 1991;192:22–31. doi: 10.1016/0014-4827(91)90152-k. [DOI] [PubMed] [Google Scholar]
- 43.Sandstrom B. Analyst. 1995;120:913–915. doi: 10.1039/an9952000913. [DOI] [PubMed] [Google Scholar]
- 44.Burkhart K K, Kulig K W, Rumack B. Ann Emerg Med. 1990;19:1167–1170. doi: 10.1016/s0196-0644(05)81523-9. [DOI] [PubMed] [Google Scholar]
- 45.Turnlund J R, Durkin N, Costa F, Margen S. J Nutr. 1986;116:1239–1247. doi: 10.1093/jn/116.7.1239. [DOI] [PubMed] [Google Scholar]
- 46.Knudsen E, Jensen M, Solgaard P, Sorensen S S, Sandstrom B. J Nutr. 1995;125:1274–1282. doi: 10.1093/jn/125.5.1274. [DOI] [PubMed] [Google Scholar]
- 47.Hinson T K, Damodaran T V, Chen J, Zhang X, Qumsiyeh M B, Seldin M F, Quarles L D. Genomics. 1997;45:279–289. doi: 10.1006/geno.1997.4943. [DOI] [PubMed] [Google Scholar]
- 48.Moran J R, Lyerly A. Life Sci. 1985;36:2515–2521. doi: 10.1016/0024-3205(85)90148-1. [DOI] [PubMed] [Google Scholar]
- 49.Wolosin J M, Forte J G. J Membr Biol. 1985;83:261–272. doi: 10.1007/BF01868700. [DOI] [PubMed] [Google Scholar]
- 50.Endo T, Kimura O, Hatakeyama M, Takada M, Sakata M. Toxicol Lett. 1997;91:111–120. doi: 10.1016/s0378-4274(97)03878-2. [DOI] [PubMed] [Google Scholar]
- 51.Kanno Y, Yamami T, Muneoka Y, Shiba Y. J Toxicol Sci. 1978;3:39–50. doi: 10.2131/jts.3.39. [DOI] [PubMed] [Google Scholar]
- 52.Rowe W A, Lesho M J, Montrose M H. Proc Natl Acad Sci USA. 1994;91:6166–6170. doi: 10.1073/pnas.91.13.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramakrishna B S, Nance S H, Roberts-Thomson I C, Roediger W E W. Digestion. 1990;45:93–101. doi: 10.1159/000200229. [DOI] [PubMed] [Google Scholar]
- 54.Bugaut M. Comp Biochem Physiol. 1987;86:439–472. doi: 10.1016/0305-0491(87)90433-0. [DOI] [PubMed] [Google Scholar]
- 55.Martin-Requero A, Daza F J, Hermida O G, Butta N, Parrilla R. Biochem J. 1997;325:631–636. doi: 10.1042/bj3250631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lijnen P, Fagard R, Petrov V. J Hypertens. 1998;16:305–310. doi: 10.1097/00004872-199816030-00007. [DOI] [PubMed] [Google Scholar]
- 57.Huang C L, Cogan M G, Cragoe E J, Jr, Ives H E. J Biol Chem. 1987;262:14134–14140. [PubMed] [Google Scholar]
- 58.Chesters J K. Proc Nutr Soc. 1991;50:123–129. doi: 10.1079/pns19910023. [DOI] [PubMed] [Google Scholar]
- 59.Hansson A. Arch Biochem Biophys. 1996;328:233–238. doi: 10.1006/abbi.1996.0168. [DOI] [PubMed] [Google Scholar]
- 60.Andrews M, Gallagher-Allred C. Adv Wound Care. 1999;12:137–138. [PubMed] [Google Scholar]
- 61.Reed B R, Clark R A. J Am Acad Dermatol. 1985;13:919–941. doi: 10.1016/s0190-9622(85)70242-3. [DOI] [PubMed] [Google Scholar]
- 62.Huang J S, Mukherjee J J, Chung T, Crilly K S, Kiss Z. Eur J Biochem. 1999;266:943–951. doi: 10.1046/j.1432-1327.1999.00932.x. [DOI] [PubMed] [Google Scholar]
- 63.McNeil S E, Hobson S A, Nipper V, Rodland K D. J Biol Chem. 1998;273:1114–1120. doi: 10.1074/jbc.273.2.1114. [DOI] [PubMed] [Google Scholar]
- 64.Oda Y, Tu C L, Chang W, Crumrine D, Komuves L, Mauro T, Elias P M, Bikle D D. J Biol Chem. 2000;275:1183–1190. doi: 10.1074/jbc.275.2.1183. [DOI] [PubMed] [Google Scholar]
- 65.Vogt K, Mellor J, Tong G, Nicoll R. Neuron. 2000;26:187–196. doi: 10.1016/s0896-6273(00)81149-6. [DOI] [PubMed] [Google Scholar]