Abstract
Accumulating research in rodents and humans indicates that exercise benefits brain function and may prevent or delay onset of neurodegenerative conditions. In particular, exercise modifies the structure and function of the hippocampus, a brain area important for learning and memory. This review addresses the central and peripheral mechanisms underlying the beneficial effects of exercise on the hippocampus. We focus on running-induced changes in adult hippocampal neurogenesis, neural circuitry, neurotrophins, synaptic plasticity, neurotransmitters, and vasculature. The role of peripheral factors in hippocampal plasticity is also highlighted. We discuss recent evidence that systemic factors released from peripheral organs such as muscle (myokines), liver (hepatokines), and adipose tissue (adipokines) during exercise contribute to hippocampal neurotrophin and neurogenesis levels, and memory function. A comprehensive understanding of the body–brain axis is needed to elucidate how exercise improves hippocampal plasticity and cognition.
There is a global increase in physical inactivity (Guthold et al. 2008; Dumith et al. 2011; Hallal et al. 2012; Kohl et al. 2012). Sedentary behavior is associated with increased risk of cognitive decline, whereas exercise boosts brain function (Duzel et al. 2016). Moreover, multiple neurological and neurodegenerative diseases, and conditions such as stroke, traumatic brain injury, and substance addiction, which lack effective medications, are ameliorated by exercise (Table 1). Research into the underlying cellular mechanisms has shown that in rodents running results in structural, neurochemical, mitochondrial, and vascular changes in the brain (Neeper et al. 1996; Eadie et al. 2005; Stranahan et al. 2007; Dietrich et al. 2008; van Praag, 2008; Duzel et al. 2016). In this review, we will focus on the hippocampus, a brain area that is essential for learning and memory and exhibits extensive functional plasticity in response to exercise. In the dentate gyrus (DG) of the hippocampus, the production of new neurons is increased by voluntary wheel running in rodents in association with enhanced synaptic plasticity and memory function (van Praag et al. 1999a,b; Vivar et al. 2013; Voss et al. 2013). We will discuss local factors in the hippocampus such as neurotrophins and neurotransmitters as well as distal peripheral components (myokines, hepatokines, and adipokines) that may mediate changes in neural plasticity as a result of exercise (Fig. 1).
Table 1.
Exercise and neurological disorders across humans and animal model studies, with focus on the hippocampus
| Disease | Humans | Animals | Hippocampus | References |
|---|---|---|---|---|
| Alzheimer’s disease | ↑ Global cognition | ↑ Learning and memory ↓ Anxiety ↑ Sensorimotor function ↑ Exploratory behavior |
↓ Aβ load, APP ↓ or ↔Tau AT 100 epitope ↓ IL-1β, TNF-α ↑ LTP ↑ Neurogenesis |
Heyn et al. 2004; Adlard et al. 2005; Lautenschlager et al. 2008; Baker et al. 2010; Belarbi et al. 2011; Garcia-Mesa et al. 2011; Liu et al. 2011; Rodríguez et al. 2011; Marlatt et al. 2013; Zhao et al. 2015 |
| Anxiety | ↓ State anxiety ↑ Sleep quality |
↓ Anxiety-like behaviors ↓ Fear relapse ↓ Corticosterone response ↓ Social avoidance behavior ↓ Learning impairments ↓ Sympathetic nervous system activation Improved diurnal rhythms |
↑ Glucocorticoid receptors ↑ BDNF ↑ Inhibitory interneuron mechanisms (ventral hippocampus) ↓ Melanin-concentrating hormone ↓ Serotonin responsiveness to stressors |
Oldridge et al. 1991, 1995; Dishman 1997; Broocks et al. 1998; Russo-Neustadt et al. 1999; Bandelow et al. 2000; Greenwood et al. 2003, 2008, 2013; Fulk et al. 2004; Broman-Fulks and Storey 2008; Smits et al. 2008; Carmeli et al. 2009; Wedekind et al. 2010; Herring et al. 2011, 2015; Goldin et al. 2012; Jazaieri et al. 2012; Goldin et al. 2013; Hovland et al. 2013; Schoenfeld et al. 2013; Patki et al. 2014; Mika et al. 2015; Otsuka et al. 2015; Pan-Vazquez et al. 2015; Kim and Han 2016 |
| Autism | ↓ Stereotypic behavior ↑ Cognition and attention ↑ or ↔ Social–emotional functioning |
↓ Aggressive tendencies ↑ Spatial learning ↑ Motor coordination and balance |
↑ Neurogenesis ↑ Reelin ↑ BDNF ↑ LTP |
Kern et al. 1982; Levinson and Reid 1993; Rosenthal-Malek and Mitchell 1997; Prupas and Reid 2001; Bass et al. 2009; Pan 2010; Nicholson et al. 2011; Oriel et al. 2011; Rosenblatt et al. 2011; Bahrami et al. 2012; Gabriels et al. 2012; Kim et al. 2013; Movahedi et al. 2013; Seo et al. 2013; Ward et al. 2013 |
| Alcoholism | ↓ Drinking days and heavy drinking days ↑ Days abstinent ↓ and ↔ Depression, anxiety, and abstinence self-efficacy ↓ Blood cortisol and ACTH compared with controls |
↓ Alcohol preference and consumption ↓ Alcohol withdrawal score ↓ Blood cortisol |
↑ BDNF ↑ Neurogenesis |
Sinyor et al. 1982; Crews et al. 2004; Vedamurthachar et al. 2006; Coiro et al. 2007; Brown et al. 2009, 2014b; Ehringer et al. 2009; Klintsova et al. 2012; Motaghinejad et al. 2014, 2015; Gallego et al. 2015 |
| Dementia | ↑ Balance ↓ Agitation/aggression/disinhibition ↓ Apathy ↑ or ↔ Cognitive function ↑ Ability to perform activities of daily living ↓ or ↔ Depressive symptoms |
↓ Cognitive impairment | ↑ Neurogenesis ↑ BDNF |
Van de Winckel et al. 2004; Christofoletti et al. 2008; Eggermont et al. 2009a,b; Conradsson et al. 2010; Hwang and Choi 2010; Kemoun et al. 2010; Venturelli et al. 2011; Volkers and Scherder 2011; Forbes et al. 2015; Telenius et al. 2015a,b; Choi et al. 2016 |
| Depression | ↓ Depression symptoms ↑ Coping strategies ↑ Episodic memory |
↓ Depressive symptoms (↓ immobility time in forced swim test; ↑ sucrose preference) | ↓ Toll-like receptor 4 ↓ IL-1β and IL-18 ↑ Neurogenesis ↑ VEGF, BDNF ↔ 5-HT ↑ Noradrenaline |
Craft and Landers 1998; Stathopoulou et al. 2006; Daley 2008; Foley et al. 2008; Rethorst et al. 2009; De Zeeuw et al. 2010; Forsman et al. 2011; Krogh et al. 2011; Kiuchi et al. 2012; Cooney et al. 2013; Danielsson et al. 2013; Lee et al. 2013; Silveira et al. 2013; Josefsson et al. 2014; Lu et al. 2014; Sadeghi et al. 2016a,b |
| Epilepsy | ↑ Seizure threshold ↓ or ↔ Seizure frequency ↓ EEG epileptiform discharges ↓ Seizures during mental and physical activity ↓ Comorbidities (depression, anxiety) ↑ Vigilance and attention |
↓ Seizure frequency ↓ Seizure intensity ↓ Susceptibility to kindling and chemically induced seizures |
↑ PV+ interneurons ↓ CA1 hyper-responsiveness ↑ LTP |
Gotze et al. 1967; Nakken et al. 1990; Eriksen et al. 1994; Arida et al. 1999; McAuley et al. 2001; Heise et al. 2002; Arida et al. 2004, 2007, 2008, 2009, 2010; Rambo et al. 2009; Reiss et al. 2009; Tutkun et al. 2010; Eom et al. 2016 |
| Huntington’s disease | ∼↑ Motor function (gait speed and balance) and coordination ↑ Cognitive measures |
↑ Corticostriatal connectivity ↑ or ↓ Gait function and motor coordination ↑ or ↓ Cognitive dysfunction Delays circadian dysfunction |
↓ Neurogenesis | Pang et al. 2006; van Dellen et al. 2008; Cepeda et al. 2010; Potter et al. 2010; Renoir et al. 2012; Busse et al. 2013; Harrison et al. 2013; Khalil et al. 2013; Kloos et al. 2013 |
| Multiple sclerosis | ↔ or Moderately ↓ relapse rates ↔ Markers of immune function ↓ Physical disability progression ↓ MRI brain lesion volumes ↑ Neuroperformance (walking speed and endurance) |
Delay disease onset ↓ Demyelination ↓ Duration of relapse ↓ Physical disability ↔ or ↓ Inflammatory response ↓ Dendritic spine loss ↓ Synaptic deficits ↑ NGF, BDNF ↓ Pain hypersensitivity ↓ Oxidative stress |
Le Page et al. 1994, 1996; Motl and Snook 2008; Motl et al. 2008; Rossi et al. 2009; Motl and Pilutti 2012; Patel and White 2013; Benson et al. 2015; Pryor et al. 2015; Alvarez-Saavedra et al. 2016; Souza et al. 2016 | |
| Parkinson’s disease | ↑ Motor function (gait, balance, strength) Improved mood ↓ or ↔ Depressive symptoms ↑ Spatial and verbal working memory ↑ Attention and processing speed ↓ Sleep disturbance (insomnia + daytime sleepiness) |
↓ Spatial learning deficits ↔ Spatial memory ↑ Motor function |
↑ Neurogenesis | Cruise et al. 2011; Shulman et al. 2013; Tomlinson et al. 2013; Nascimento et al. 2014; Park 2014; Uc et al. 2014; Canning et al. 2015; Dashtipour et al. 2015; David et al. 2015; Klein et al. 2016 |
| Posttraumatic stress disorder (PTSD) | ↓ PTSD symptoms ↓ Depressive symptoms |
↓ Acoustic startle response ↑ Stress resilience ↑ Elevated plus maze activity ↑ Spatial learning ↓ Plasma corticosterone ↓ Depressive-like behavior ↓ Anxiety-like behavior |
↑ BDNF ↑ Neuropeptide Y ↑ Phosphorylated δ-opioid receptor ↑ Neurogenesis |
Kim and Seo 2013; Mitchell et al. 2014; Patki et al. 2014; Van Der Kolk et al. 2014; Hoffman et al. 2015; Powers et al. 2015; Rosenbaum et al. 2015a,b |
| Schizophrenia | ↑ Neurocognitive function ↑ Serum BDNF ↑ Short-term memory ↓ Positive and negative symptoms ↓ Anxiety, ↓ or ↔ depression ↑ Mental and physical QoL ↓ Psychotic symptom severity |
↓ Behavioral abnormalities and deficits ↑ NMDA receptor and BDNF expression |
↑ or ↔ hippocampal volume ↑ N-acetylaspartate to creatine ratio ↑ NMDA receptor expression |
Beebe et al. 2005; Acil et al. 2008; Pajonk et al. 2010; Scheewe et al. 2012, 2013a,b; Battaglia et al. 2013; Falkai et al. 2013; Kim et al. 2014a,b; Park et al. 2014; Vancampfort et al. 2014; Kimhy et al. 2015, 2016 |
| Stroke | ↑ QoL and ability to perform activities of daily living ↓ Comorbid cardiovascular disease risk ↓ Depressive symptoms ↑ Executive function and memory ↓ Poststroke fatigue ↑ Cognition |
↑ Spatial memory recovery ↓ Ischemia-induced cell death |
↑ Blood flow ↑ Neurogenesis ↑ BDNF ↓ Myelin damage ↓ Microvessel damage |
Stummer et al. 1994; Lee et al. 2003; Ding et al. 2004; Sim et al. 2004, 2005; Ploughman et al. 2005; Quaney et al. 2009; Rand et al. 2010; Chen and Rimmer 2011; Graven et al. 2011; Cumming et al. 2012; Dean et al. 2012; Zedlitz et al. 2012; Billinger et al. 2014; Moore et al. 2014; Ahn et al. 2016; Himi et al. 2016 |
| Substance abuse | ↓ Risk of daily smoking ↓ Risk of illicit drug use |
↓ Drug (amphetamine, cocaine, nicotine) intake ↓ Drug-seeking behavior during extinction and reinstatement ↑ Cognitive function ↓ Withdrawal signs |
↑ Bcl-2 ↑ LTP |
Kanarek et al. 1995; Smith et al. 2008; Korhonen et al. 2009; Zlebnik et al. 2010, 2012; Terry-McElrath and O’Malley 2011; Miladi-Gorji et al. 2014; Mokhtari-Zaer et al. 2014; Wang et al. 2014; Sobieraj et al. 2016 |
| Traumatic brain injury | ↑ Mood ↑ Gait ↑ Ambulatory status ↑ Aerobic endurance |
↓ Susceptibility to seizures ↑ Motor function ↑ Cognitive function |
↑ BDNF | Charrette et al. 2016; Mychasiuk et al. 2016; Setkowicz et al. 2016; Weinstein et al. 2016 |
Aβ, Amyloid β; APP, amyloid precursor protein; IL, interleukin; TNF-α, tumor necrosis factor α; LTP, long-term potentiation; BDNF, brain-derived neurotrophic factor; ACTH, adrenocorticotropic hormone; VEGF, vascular endothelial growth factor; 5-TH, 5-hydroxytryptamine (serotonin) receptors; PV, parvalbumin; CA1, Cornu Ammonis 1; NGF, nerve growth factor; MRI, magnetic resonance imaging; PTSD, posttraumatic stress disorder; NMDA, N-methyl-d-aspartate; QoL, quality of life; Bcl-2, B-cell lymphoma 2.
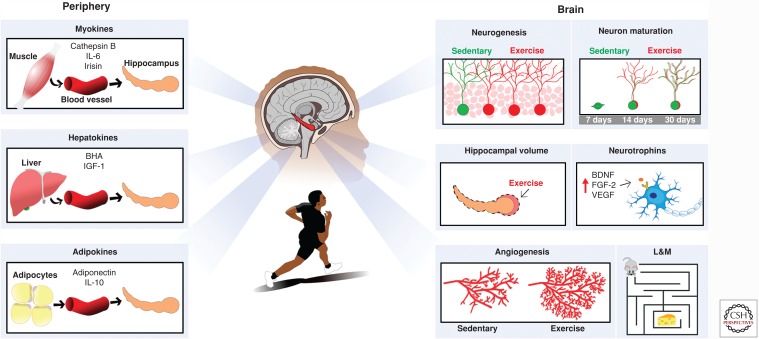
Figure 1.
Running induces structural and functional plasticity in the hippocampus. Illustration summarizing how running enhances neurogenesis, accelerates new neuron maturation, augments hippocampal volume (in humans), and promotes angiogenesis. Enhanced neural plasticity and improved memory function may be supported by central and peripheral factors. Increased levels of growth factors in the brain may result, in part, from systemic factors secreted by muscle (myokines), liver (hepatokines), and fat cells (adipokines). BHA, β-Hydroxybutyrate; BDNF, brain-derived neurotrophic factor; FGF-2, fibroblast growth factor 2; IGF-1, insulin-like growth factor 1; IL-6, interleukin 6; IL-10, interleukin 10; L&M, learning and memory; VEGF, vascular endothelial growth factor.
EXERCISE AND THE HIPPOCAMPUS
The hippocampus is critical for the acquisition of new memories (Squire 1992; Riedel et al. 1999; Scoville and Milner 2000) and is affected by aging and age-related cognitive disorders, including progressive atrophy of hippocampal volume in humans (Hackert et al. 2002; Raji et al. 2009; Duzel et al., 2016), a process that may be attenuated or ameliorated by exercise (Erickson et al. 2009, 2011, 2014). Higher fitness levels are associated with larger hippocampal volume, and better performance on memory tests (Erickson et al. 2009, 2011; Voss et al. 2013). Intervention studies show that aerobic training 3 days per week for at least 3 months to 1 year can prevent and/or reverse the age-related decline in hippocampal volume (Erickson et al. 2011; Maass et al. 2015; ten Brinke et al. 2015). Interestingly, the exercise-induced increase in grey matter volume seems to occur in the hippocampus (Maass et al. 2015; Duzel et al. 2016), entorhinal (Whiteman et al. 2016), and prefrontal cortex (Erickson et al. 2014), with no change in the thalamus or caudate nucleus (Erickson et al. 2011).
The hippocampus consists of three subfields: area CA1, area CA3, and the DG; each play a role in memory function. Area CA1 is considered to encode memories (Nakazawa et al. 2004), whereas area CA3 is thought to mediate retrieval of complete memories from partial information (pattern completion) (Nakazawa et al. 2002, 2003). The DG is deemed important for spatial pattern separation, the process by which similar incoming information or stimuli is transformed into distinct nonoverlapping experiences (Leutgeb et al. 2007; McHugh et al. 2007; Kesner and Rolls 2015). The DG is unique because it can generate new neurons in the adult brain (Altman and Das 1965), which are considered to play a functional role in spatial memory and pattern separation (Fig. 2) (Vivar et al. 2013). Residual stem cells located in the DG inner granule cell layer continue to proliferate and differentiate in mammals, including humans (Kuhn et al. 1996; Eriksson et al. 1998; Spalding et al. 2013). The stem/progenitor cells consist of quiescent type-1 radial glia-like cells that express glial fibrillary acidic protein (GFAP), nestin and Sox2, and type-2 cells expressing Sox-2 that can generate both astrocytes as well as rapidly proliferating neuronal progenitor cells (NPCs) that differentiate into mature granule cells over several weeks (Kronenberg et al. 2003; Kempermann et al. 2004; Bonaguidi et al. 2011; Encinas et al. 2011; Gebara et al. 2016). The proliferation, survival, and integration of new neurons can be up- or down-regulated by intrinsic factors, pathological events, and activity (for review, see Zhao et al. 2008; Hsieh and Zhao 2016).
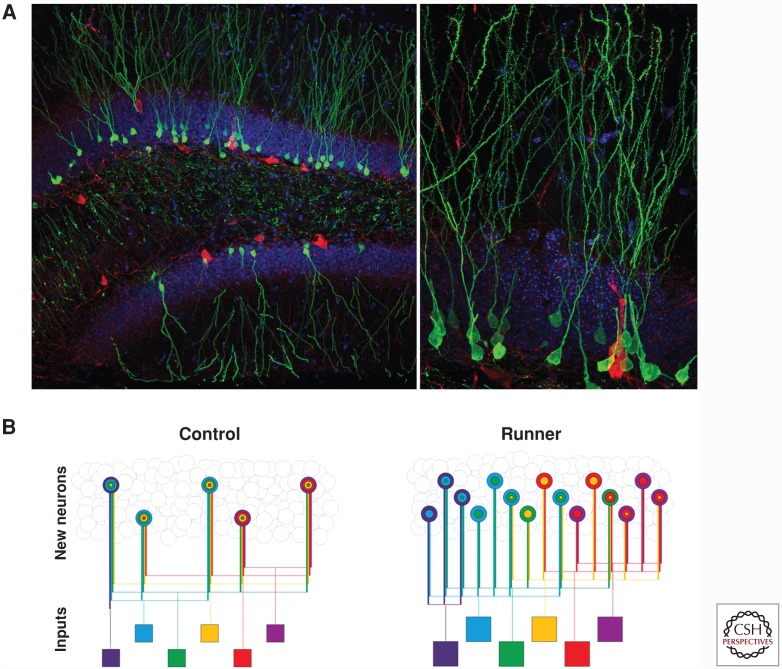
Figure 2.
Neurogenesis in the adult mouse dentate gyrus (DG) of the hippocampus and a diagram of modification of new neuron network by running. (A) Photomicrographs of new neurons (green) in a coronal mouse brain section; a mouse 2 months after injection with retrovirus-expressing green fluorescent protein (GFP) in the DG. Section was stained for GFP (green) and GABAergic inhibitory interneuron marker parvalbumin (red), and nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI, blue). (B) Diagram illustrating how running reorganizes the network of new hippocampal neurons (Vivar et al. 2016). One month of running in male C57Bl/6 mice enhanced DG neurogenesis (threefold). Afferent input (squares) was also increased, but less so (twofold). The resulting change in new neuron connectivity may promote sparse encoding of information and result in a more robust memory-processing system. The expansion of the neural network and enhanced distribution of information over new DG cells may provide more structural redundancy in which failure of one pathway can be compensated for by another.
Running increases neurogenesis in the DG of the hippocampus (for review, see van Praag 2008; Vivar et al. 2013; Voss et al. 2013; Patten et al. 2015). The neurogenic response to running results in an ∼2–3-fold increase in new neurons, depending on genetic background (Clark et al. 2011; Gregoire et al. 2014), age (van Praag et al. 2005; Kronenberg et al. 2006; Kannangara et al. 2011; Marlatt et al. 2012), running wheel type (Creer et al. 2010), labeling method used (bromodeoxyuridine [BrdU], retroviral vector [Vivar et al. 2016], or doublecortin [DCX] [Kuhn et al. 2016]), and distance run (Clark et al. 2011). Running-induced neurogenesis is localized to the dorsal rather than the ventral DG (Bolz et al. 2015; Vivar et al. 2016). The dorsal aspect of the hippocampus is considered important for spatial navigation (Moser et al. 1995), whereas the ventral hippocampus seems to be more attuned to changes in mood (Henke 1990; Kjelstrup et al. 2002). Consistently, running is associated with improved spatial memory function (van Praag et al. 1999b; Anderson et al. 2000; van der Borght et al. 2007; Clark et al. 2008; Patten et al. 2015). New neurons are also considered to play a role in DG-mediated pattern separation (Clelland et al. 2009; Sahay et al. 2011), the ability to differentiate similar information (Aimone et al. 2011). Running improves fine discrimination (Creer et al. 2010; Bolz et al. 2015) as does enhancement of adult neurogenesis in a mouse model with conditional ablation of BAX-dependent programmed cell death in neural progenitors (Sahay et al. 2011). It has also been suggested that increasing neurogenesis by running may cause forgetting by weakening preexisting memories in mice (Akers et al. 2014; Epp et al. 2016). A recent study in rats, however, could not replicate these results, casting doubt on this hypothesis (Kodali et al. 2016).
Although a running-induced increase in new neuron number may underlie, in part, the benefits for cognitive function, qualitative changes in maturation, morphology, and connectivity are likely also important. Running may shorten the cell cycle of rapidly amplifying progenitor cells (Farioli-Vecchioli et al. 2014; see, however, Fischer et al. 2014) and accelerate neuronal maturation of adult-born DG neurons (Zhao et al. 2006; Piatti et al. 2011; Steib et al. 2014). In particular, retroviral labeling studies (van Praag et al. 2002; Zhao et al. 2006) have shown that running promotes spine formation in the outer molecular layer of adult-born dentate granule cell dendrites (Zhao et al. 2014). In addition, dendritic spine motility, total dendritic length, branch points, dendritic complexity, and mitochondria density are significantly higher in runners’ adult-born DG neurons for up to 3 weeks post-retroviral injection (Zhao et al. 2006; Dietrich et al. 2008; Steib et al. 2014). However, by the fourth week, dendritic morphology and mitochondria content are indistinguishable from adult-born DG neurons in the sedentary brain (Zhao et al. 2006, 2014; Steib et al. 2014). Similar observations were made in a live imaging study in mice housed in an enriched environment containing running wheels (Gonçalves et al. 2016). Accelerated maturation in runners may vary along the temporal axis, with dorsal aspect exhibiting the fastest maturation (Piatti et al. 2011).
Running also alters the circuitry into which new neurons integrate (Vivar et al. 2016). Analysis of the direct afferent inputs to 1-month-old newly born neurons using a dual-virus selective neuroanatomical tracing approach (Vivar et al. 2012) showed that running expanded the number of afferent cells synapsing onto newborn neurons. Running recruited presynaptic inputs from the entorhinal cortex, mammillary nuclei, and medial septum (Vivar et al. 2016). These brain regions are important for relaying content and context of experiences (Knierim et al. 2014; Knierim 2015; Kropff et al. 2015), spatial–temporal information processing (Vann 2010; Dillingham et al. 2015), and initiating hippocampal theta rhythms (Thinschmidt et al. 1995; Vertes et al. 2004). Within the hippocampus, running reduced the ratio of inhibitory interneurons and glutamatergic mossy cell innervation to new neurons (Vivar et al. 2016). However, inhibitory synaptic transmission onto newborn neurons was not affected, and excitatory synaptic transmission showed only a small decrease in amplitude but not frequency (Vivar et al. 2016). Achieving the same excitatory and inhibitory drive with less afferent input per neuron may result in more efficient integration of new neurons. Moreover, the overall ratio of afferent cells to newborn neurons was decreased (Vivar et al. 2016). A reduction in inputs converging onto individual neurons may be conducive to pattern separation by facilitating sparse activation (Fig. 2).
NEUROTRANSMITTERS
The elaborate network connectivity of newly born neurons with structures throughout the brain is consistent with involvement of multiple neurotransmitters in the development and integration of newly born neurons (for review, see Suh et al. 2009). Glutamate and γ-aminobutyric acid (GABA) are the primary excitatory and inhibitory neurotransmitters, respectively, in the brain. Both regulate the integration and survival of newly born neurons (Ge et al. 2006; Tashiro et al. 2006). Glutamate is also important for exercise-induced changes in DG synaptic plasticity. Running enhances DG long-term potentiation (LTP), a form of synaptic plasticity that is considered a cellular model for learning and memory (Bliss and Collingridge 1993), in vivo and in vitro (van Praag et al. 1999a; Farmer et al. 2004; Vasuta et al. 2007; Bruel-Jungerman et al. 2009; O’Callaghan et al. 2009; Liu et al. 2011), potentially by lowering the LTP induction threshold. Indeed, weak theta-patterned stimulation that did not produce LTP in the DG of controls, elicited long-lasting LTP in rats housed with a running wheel (Farmer et al. 2004). Acutely, voluntary movement and sensory stimulation can elicit theta pattern activity in the hippocampus (Bland 1986; Czurko 1999; Bland and Oddie 2001), specifically in the CA1 and DG. Thus, exercise may “prime” the network to learn by initiating oscillations that promote plasticity (Greenstein et al. 1988; Pavlides et al. 1988; Christie and Abraham 1992; Abraham et al. 2001; Orr et al. 2001). The contribution of new neurons to running-induced DG plasticity is supported by recordings from individual newly born neurons in slices derived from mice housed under running enrichment conditions, which exhibited increased LTP (Schmidt-Hieber et al. 2004). Running elevates DG gene expression of glutamate receptor subunits NR2A, NR2B, and glutamate receptor 5 (Farmer et al. 2004). The NR2B N-methyl-d-aspartate (NMDA) receptor subunit is highly expressed in new neurons (Ge et al. 2007; Kheirbek et al. 2012) and NR2B overexpression facilitates LTP induction (Tang et al. 1999, 2001).
Running also modulates inhibitory neurotransmission. Recent research shows increased expression levels of GABA receptor subunits and GAD67 in hippocampal subfields, including the DG (Hill et al. 2010). In addition, exercise elevated ventral DG extracellular GABA release and vesicular GABA transporter expression. Enhanced local inhibition reduced DG expression of immediate early genes (Schoenfeld et al. 2013). Increased inhibition may attenuate anxiety and improve cognition. Indeed, reduced inhibitory tone in the DG–CA3 area has been associated with aging-related memory deficits (Bakker et al. 2012). Running has also been shown to alter various other neurotransmitters and neuromodulators (for a review, see Basso and Suzuki 2017), such as monoamines (dopamine, serotonin, norepinephrine) (Chaouloff 1989; Dishman 1997), endocannabinoids (Dietrich and McDaniel 2004; Fuss and Gass 2010; Tantimonaco et al. 2014), and opioids (Sforzo 1989), which can contribute to changes in hippocampal synaptic plasticity. The serotonergic (5-HT) system plays an important role in the exercise-induced increase in adult neurogenesis (Klempin et al. 2013). Recent research has identified 5-HT3 receptor subunit signaling as an underlying mechanism. Ablation of the 5-HT3 receptor subunit abolishes running-induced neurogenesis and reduces antidepressant effects (Kondo et al. 2015). Learning, measured by contextual fear conditioning, was intact, consistent with a study in which adult neurogenesis was ablated by X-irradiation in runners (Clark et al. 2008).
BRAIN-DERIVED NEUROTROPHIC FACTOR
Neurotrophins play a significant role in brain plasticity (Intlekofer and Cotman 2013). One of the first growth factors associated with exercise was brain-derived neurotrophic factor (BDNF). In animal models, running increases BDNF expression levels in the hippocampus (Neeper et al. 1995; Kobilo et al. 2011a; Marlatt et al. 2012; Abel and Rissman 2013), in association with improvements in hippocampal plasticity, spatial memory, and object recognition (Vaynman et al. 2004; Griffin et al. 2009; Cassilhas et al. 2012; Gomes da Silva et al. 2012). In humans, exercise-induced increases in BDNF serum levels are associated with changes in hippocampal volume (Erickson et al. 2011). Conversely, reduced BDNF serum levels are observed with age-related decline in hippocampal volume (Erickson et al. 2010). BDNF promotes synaptic plasticity through downstream targets, cAMP-response element-binding (CREB) protein, synapsin I, and synaptophysin, while simultaneously increasing its own messenger RNA (mRNA) and its receptor tyrosine kinase B (TrkB) (Vaynman et al. 2003, 2006). Blocking hippocampal BDNF in rats precludes exercise-induced cognitive enhancement and hippocampal plasticity (Vaynman et al. 2004). In addition, ablation of the TrkB receptor in neural progenitor cells abolishes the neurogenic response to running (Li et al. 2008). Analysis of the hippocampal subfields shows that exercise increases BDNF mRNA levels in the DG rather than in area CA1 (Farmer et al. 2004).
Exercise-induced changes in neurotrophin levels are likely mediated by several neural cell types. Astrocytes are closely associated with new neuron dendrites (Vivar et al. 2012) and support their development (Sultan et al. 2015). Astrocytes synthesize BDNF and contain TrkB receptors (Zafra et al. 1992; Miklič et al. 2004). Running may up-regulate hippocampal astrocyte number (Li et al. 2005; Saur et al. 2014), lengthen their processes and increase cell body size (Saur et al. 2014; Brockett et al. 2015), and elevate TrkB expression levels (Fahimi et al. 2016). Other nonneuronal cell types may also play a role. Macrophage migration inhibitory factor is a cytokine that is up-regulated by exercise and can induce BDNF expression (Moon et al. 2012). Microglia number is down-regulated by running (Gebara et al. 2013). However, ablation of hippocampal microglia impairs exercise-induced neurogenesis (Vukovic et al. 2012), indicating that cytokines produced by microglia may be required to maintain neurogenic processes (Walton et al. 2006; Ziv et al. 2006; Speisman et al. 2013).
(Epi)genetic factors also play an important role in the function of this neurotrophin. A single-nucleotide polymorphism of the BDNF gene (Val/Met polymorphism) that occurs in 20% to 30% of Caucasians decreases activity-dependent BDNF secretion (Egan et al. 2003; Chen et al. 2004) and is associated with increased susceptibility to depression and anxiety-related disorders (Sen et al. 2003; Verhagen et al. 2010), reduced memory function (Egan et al. 2003; Soliman et al. 2010), and impaired neural plasticity that is resistant to antidepressants (Bath et al. 2012). In addition, the effect of exercise on hippocampal volume in human subjects is attenuated in Met carriers as compared with Val/Val subjects (Brown et al. 2014a). More recent evidence shows that in adult mice with this polymorphism the running-induced increase in BDNF and neurogenesis are attenuated (Ieraci et al. 2016). These results suggest that exercise may support cognition, in part, through BDNF. The exercise-induced increase in BDNF may also be mediated through epigenetic changes (Gomez-Pinilla et al. 2011; Abel and Rissman 2013). Histone H3 acetylation is increased by exercise and associated with enhanced gene transcription, and can specifically up-regulate BDNF gene expression (Gomez-Pinilla et al. 2011; Ieraci et al. 2015). Hippocampal histone deacetylase (HDAC), on the other hand, can be down-regulated by exercise (Abel and Rissman 2013), and thereby up-regulate BDNF expression (Intlekofer and Cotman 2013; Sleiman et al. 2016).
CEREBROVASCULAR PLASTICITY
Exercise modulates the cerebrovasculature, which may allow for better perfusion, delivery of oxygen, nutrients, neurotrophins, and other factors that may promote brain function. Acute bouts of walking and running increase cerebral blood flow (CBF) in several regions within the animal brain, including the hippocampus (Osborne 1997; Nakajima et al. 2003; Nishijima and Soya 2006; Nishijima et al. 2012, 2016), cortex (Delp et al. 2001; Gu et al. 2003), and striatum (Osborne 1997), but not the olfactory bulb (Nishijima et al. 2012) or hypothalamus (Delp et al. 2001), suggesting region-specific control of CBF by exercise. In the rat hippocampus, CBF is increased soon after walking initiation and returns to baseline levels right afterward (Nakajima et al. 2003). Longer bouts resulted in prolonged CBF elevation after cessation of running (10–20 min) (Nishijima and Soya 2006). Thus, exercise duration and intensity differentially modulate CBF. Neuronal activity may drive regional hippocampal CBF increased by exercise. Infusion of tetrodotoxin (TTX), NMDA receptor antagonist (MK-801), and NO synthase inhibitor (L-NAME) suppressed the increase in hippocampal CBF induced by walking (Nishijima et al. 2012). In humans, exercise also increases CBF (Querido and Sheel 2007; Secher et al. 2008; Ogoh and Ainslie 2009) and counteracts age-related decline in CBF (Ainslie et al. 2008; Viboolvorakul and Patumraj 2014) and cerebrovascular reactivity (Barnes et al. 2013; Murrell et al. 2013). In addition, gadolinium contrast imaging in humans revealed hippocampal perfusion changes after long-term exercise in young (Pereira et al. 2007) and older adults (Maass et al. 2015).
Prolonged exercise training improves cerebrovascular plasticity in rodents (Lange-Asschenfeldt and Kojda 2008; Tarumi and Zhang 2014; Barnes 2015), likely by increasing angiogenesis in several brain regions, including the hippocampus (van Praag et al. 2005; Clark et al. 2009; van der Borght et al. 2009), striatum (Clark et al. 2009), cerebellum (Black et al. 1990; Lopez-Lopez et al. 2004), and cortex (Swain et al. 2003; Viboolvorakul and Patumraj 2014). The increase in angiogenesis is preserved in aged animals (Ding et al. 2006). Vascular endothelial growth factor (VEGF) may mediate exercise-induced vascular plasticity, adult neurogenesis, and communication between peripheral tissues and brain (Carmeliet 2003; Fabel et al. 2003; Cotman et al. 2007; Udo et al. 2008). VEGF is involved in blood vessel formation (Prior et al. 2003; Gavin et al. 2004; Kraus et al. 2004) and can attenuate the aged-related decline in neurogenesis (Licht et al. 2016). The importance of VEGF for neurogenesis was first shown in songbirds. A seasonal increase in neurogenesis in the higher vocal center of male canaries involves a testosterone-linked VEGF to BDNF signaling pathway (Louissaint et al. 2002). In rodents, overexpression of VEGF enhanced blood vessel proliferation in the hippocampus in association with an increase in hippocampal neurogenesis (Cao et al. 2004; Licht et al. 2011). VEGF may be required for exercise-induced hippocampal neurogenesis. Using a systemic pharmacological inhibitor of VEGF, Fabel et al. (2003) showed that VEGF is necessary for voluntary running-enhanced hippocampal neurogenesis, supporting the link between physical activity, angiogenesis, and neurogenesis (Trejo et al. 2001; Voss et al. 2013).
PERIPHERAL ORGANS AND BRAIN FUNCTION
Parabiosis studies between young and aged animals have provided evidence that factors in blood can regulate vascular remodeling, CBF, and adult neurogenesis (Villeda et al. 2011; Katsimpardi et al. 2014). Peripheral organs such as skeletal muscle, adipose tissue, and liver secrete various molecules and vesicles into circulation to promote systemic homeostasis during exercise (Hansen et al. 2011; Pedersen and Febbraio 2012). Indeed, glucose and lipid metabolic adaptations to exercise are widely studied in these peripheral tissues. However, the effects of exercise-induced system metabolic changes on behavior and cognition as well as the underlying cellular mechanisms have largely remained unexplored. Below, we describe recent research that has begun to reveal metabolic energy metabolism pathways that may elicit and coordinate the adaptive responses to exercise in the brain.
Muscle
Skeletal muscle releases myokines (Pedersen and Febbraio 2008; Hawley et al. 2014) that may be linked to neural plasticity. This putative link is supported by clinical and basic research. For instance, children with Duchenne muscular dystrophy have cognitive deficits (Scheinfeld 1950; Black 1973; Florek and Karolak 1977; Bushby 1992; Lenk et al. 1993; Hinton et al. 2000). In addition, mdx (X-linked muscular dystrophy) mice have impaired memory function (Vaillend et al. 1995; Anderson et al. 2002). An important “master regulator” of muscle physiology is AMP-activated protein kinase (AMPK) (Hardie 2011). Activation of AMPK with an agonist, AICAR, decreased fat mass, increased oxygen consumption, and increased running endurance in sedentary mice by reprogramming muscle fibers to a type I phenotype in a PPARδ-dependent manner (Narkar et al. 2008; Guerrieri et al. 2017). To determine whether the effects on endurance extended to brain function, we treated mice with AICAR and spatial memory was tested (Kobilo et al. 2011b, 2014). The observed enhancement of memory function by AICAR was precluded by muscle-specific AMPK α2-subunit deficiency (Kobilo et al. 2014), supporting a link between muscle and cognition. Based on these findings, we set out to find the underlying mechanisms by treating L6 muscle cells with AICAR in culture and analyzing the conditioned media. Proteomic analysis led to identification of a novel myokine, cathepsin B (Ctsb), as a mediator of exercise-induced hippocampal plasticity (Moon et al. 2016).
Ctsb is a lysosomal thiol proteinase that has been implicated in a variety of physiological and pathological processes, including proteolytic maturation of proinsulin (Docherty et al. 1984; Steiner et al. 1984) and proalbumin (Judah and Quinn 1978; Quinn and Judah 1978; Matsuda et al. 1986), as well as cancer progression (Koblinski et al. 2002). The role of Ctsb in the brain has been mainly studied under disease conditions, with contradictory results. In a nonhuman primate model of transient ischemia, treatment with a Ctsb inhibitor prevented neuronal cell death (Yoshida et al. 2002). In Alzheimer’s disease (AD) mouse model studies, some researchers report that Ctsb increased pathological processes (Hook et al. 2008) or was neuroprotective with antiamyloidogenic functions (Mueller-Steiner et al. 2006; Wang et al. 2012; Embury et al. 2016). In our recent study, Ctsb was increased in plasma by exercise across mammalian species, including mice, monkeys, and humans (Moon et al. 2016). In adult hippocampal progenitor cell cultures, Ctsb application enhanced the expression of neurotrophin BDNF and DCX, a marker for neuronal differentiation. In Ctsb knockout mice, impaired spatial memory function and dentate granule cell physiology was observed. In addition, the neurogenic and cognitive response to exercise was blunted in the Ctsb-deficient mice as compared with their littermates. It is noteworthy that Ctsb deficiency did not affect general locomotor activity or mood-related behaviors such as sucrose preference and elevated T-maze. A role for Ctsb in cognition was also found in humans. The exercise-induced change in fitness was positively associated with performance on a complex-figure recall task that is considered to be hippocampus dependent (Vargha-Khadem et al. 1997; Maass et al. 2015). It will be of interest to determine in future studies whether there is a positive association between Ctsb levels and pattern separation performance.
Support for the idea that muscle energy metabolism affects brain function also comes from recent studies in mice that overexpress peroxisome proliferator-activated receptor γ coactivator (PGC-1α) in muscle (Wrann et al. 2013; Agudelo et al. 2014). Exercise activates this transcriptional coactivator (Pilegaard et al. 2003; Finck and Kelly 2006), and in muscle-specific PGC-1α-KO mouse, activity and maximal exercise capacity are reduced (Geng et al. 2010). Overexpression of PGC-1α in muscle increased production of fibronectin type III domain containing 5 (FNDC5), which is cleaved and is secreted as irisin, a myokine that is involved in oxygen consumption, transition of white fat into brown fat, and thermogenesis (Boström et al. 2012). FNDC5 is primarily expressed in the brain (Teufel et al. 2002), including the hippocampus. FNDC5 deficiency impairs neuronal development (Hashemi et al. 2013). Thirty days of voluntary exercise increased FNDC5 mRNA in the quadriceps and hippocampus, but not the whole brain. Increased FNDC5 expression in cortical neuron cultures enhanced BDNF levels. In addition, peripheral up-regulation of FNDC5 increased hippocampal BDNF gene expression (Wrann et al. 2013). Exercise also elevates irisin in human plasma (Jedrychowski et al. 2015). A link to cognitive function remains to be determined. In another study, overexpression of PGC-1α in mouse muscle regulated the kynurenine pathway and thereby protected these mice from stress-induced reduction of synaptic plasticity proteins in the brain, as well as from exhibiting depression-like behaviors (Agudelo et al. 2014).
Interleukin (IL)-6 is a cytokine with various physiological roles (Erta et al. 2012). IL-6 plasma level is significantly increased during exercise, partially from contracting skeletal muscles (Pedersen et al. 2001; Rasmussen et al. 2011). Exercise-induced IL-6 from skeletal muscle can increase glycogenolysis in the liver to maintain blood glucose levels during exercise (Keller et al. 2001; Pedersen et al. 2001). IL-6 also plays a major role in energy homeostasis in the central nervous system (CNS). Studies with IL-6-deficient mice indicate that IL-6 can regulate appetite, energy expenditure, and is related to the development of obesity (Wallenius et al. 2002). IL-6 peripheral administration can be absorbed into the cerebrospinal fluid (CSF) (Banks et al. 1994). In addition, IL-6 is increased in the adult mouse brain after exercise (Nybo et al. 2002; Rasmussen et al. 2011). IL-6 levels are reportedly elevated in mood-related disorders, such as depression (Bob et al. 2010), which are generally improved by exercise (Cooney et al. 2013). The role of IL-6 in neurogenesis is still controversial. Chronic astrocytic production of IL-6 reduces hippocampal neurogenesis in the subgranular zone (SGZ) of the DG in adult mice (Vallières et al. 2002). On the other hand, IL-6 increased differentiation in human fetal NPCs in the SGZ (Sarder et al. 1996). In addition, proliferation of neuronal cells is reduced and hippocampus--dependent learning is impaired in IL-6-deficient mice (Baier et al. 2009; Bowen et al. 2011). Thus, the role of IL-6 in mediating exercise-induced neurogenesis and improvement of brain function is still elusive. Further studies are needed to determine whether IL-6 is a critical factor in the improvement of cognitive function during exercise. Altogether, these studies suggest that myokines such as Ctsb, irisin, and IL-6 may modulate brain function during exercise. Because these myokines have various roles in different physiological and pathological conditions, care must be taken when extrapolating their role to brain function.
Adipose Tissue
Adipose tissue–derived cytokines, called adipokines, are also affected by exercise (Golbidi and Laher 2014). Adiponectin is involved in regulation of energy metabolism, immune systems, and brain functions, similar to exercise (Pedersen and Hoffman-Goetz 2000; Vu et al. 2007). Adiponectin increases fatty acid oxidation and glucose uptake in skeletal muscle (Yamauchi et al. 2014). In addition, it increases anti-inflammatory cytokines such as IL-10 in the macrophage (Moschen et al. 2012) and has beneficial effects on cardiovascular function (Han et al. 2007; Wang et al. 2009). In addition, adiponectin can enhance adult hippocampal neurogenesis in mice and in cell culture models (Zhang et al. 2011, 2016). Accordingly, Yau et al. (2014) suggest that adiponectin can cross the blood–brain barrier and that increased adiponectin levels may modulate hippocampal neurogenesis and ameliorate mood. Running-induced neurogenesis and antidepression-like behavior was not detected in adiponectin knockout mice (Yau et al. 2014). Overall, the discovery of adipokines, such as adiponectin, has led to important research showing that there is direct cross talk between adipose and brain tissues. Prospective studies should consider the potential secondary effects of adipokines on brain function, such as regulation of inflammation and reactive oxygen species (ROS) production during exercise.
Liver
Like adipose tissue and skeletal muscle, the liver releases proteins, termed hepatokines, for glucose and lipid homeostasis (Stefan and Häring 2013). Different kinds of hepatokines are known to be involved in organ cross talk during exercise (Hansen et al. 2011; von Holstein-Rathlou et al. 2016). Insulin-like growth factor 1 (IGF-1) consists of 70 amino acids and is mainly in the liver as an endocrine hormone, and has a molecular structure similar to insulin. Circulating IGF-1 binds to IGF-1 receptors and insulin/IGF-1 heteroreceptors, which activate the AKT signaling pathway enhancing insulin action (Moses et al. 1996). IGF-1 is a critical hormone in carbohydrate metabolic reactions, and administration of IGF-1 lowers glucose levels in humans (Guler et al. 1987). IGF-1 crosses the blood–brain barrier (Pardridge 1993; Pan and Kastin 2000) and mediates neuroplasticity and neuroprotection. Indeed, IGF-1 gene mutations cause microcephaly, sensorineural deafness, as well as mental retardation (Woods et al. 1996). Activation of the IGF-1 signaling cascade can regulate amyloid precursor protein (APP) metabolism through increasing levels of insulin-degrading enzyme, a thiol metalloendopeptidase able to degrade amyloid β (Wang et al. 2015).
During physical exercise, IGF-1 levels in skeletal muscle are rapidly up-regulated (Berg and Bang 2004). Increased levels of circulating IGF-1 also result in elevated IGF-1 levels in the brain (Carro et al. 2000). Intracerebroventricular injection of IGF-1 enhances cognitive function in old rats (Markowska et al. 1998). Peripheral administration of IGF-1 can increase the number of newborn neurons in the DG of rat hippocampi (Aberg et al. 2000). Blockade of circulating IGF-1 using antiserum inhibits the exercise-induced increase in adult hippocampal neurogenesis, indicating that circulating IGF-1 may be a critical factor for exercise-induced changes in the adult rat brain (Carro et al. 2000; Trejo et al. 2001). Despite these encouraging findings, it may be premature to state that IGF-1 is the systemic link between physical activity and brain function. In a meta-analysis of 115 research studies, >50% of studies found no difference in total circulating IGF-1 as a result of exercise (Orenstein and Friedenreich 2004). In addition, depletion of IGF-1 apparently has a protective role (Cohen et al. 2009; Gontier et al. 2015) in mouse models of Alzheimer’s disease.
The formation of ketones has recently been shown to play an important role in the effects of exercise on the brain. Acetyl-CoA induced by β-oxidation enters the citric acid cycle with oxaloacetate in the mitochondria (Newman and Verdin 2014). However, oxaloacetate is mainly involved in the gluconeogenic system in the liver during chronic exercise by hydrogenating into malate (Shimazu et al. 2013; Newman and Verdin 2014). The remaining acetyl-CoA is redirected into formation of ketone bodies, such as acetoacetate and β-hydroxybutyrate (BHA) (Laffel 1999). These ketone bodies are released from liver and used in other tissues as an energy source during cellular starvation (Feldman and Nelson 2004). BHA protects against oxidative stress by inhibiting class I HDACs, which regulate gene expression and chromatin structure involved in glucose metabolism and diabetes (Gregoretti et al. 2004). BHA has neuroprotective effects in various neurodegenerative diseases affecting the dopaminergic system (Tieu et al. 2003; Lim et al. 2011) and increases BDNF levels in cortical cells (Marosi et al. 2016). Recently, Sleiman and colleagues found that exercise-induced BHA can increase BDNF expression through HDAC2/HDAC3 inhibition and histone H3 acetylation in the hippocampus. They further showed that BHA can increase neurotransmitter release via TrkB receptors. Although their study did not include behavioral analyses after BHA administration, their findings suggest that peripheral BHA can link exercise and brain function through BDNF (Sleiman et al. 2016). Thus, in various physiological and pathological conditions, hepatokine levels are altered. These hepatokines have multiple roles and are particularly important for energy homeostasis. Further research will be needed to elucidate the interactions between hepatokines and exercise, and to understand their role in brain function.
CONCLUSION
Regular exercise has profound benefits for body and brain health. Emerging data suggests that the underlying mechanisms involve various physiological adaptations, including neural, immunological, vascular, and metabolic systems. However, caution should be used when extrapolating factors modulated by exercise to potential therapeutic interventions. For instance, IGF-1, when delivered systemically, has a potent proliferative action that promotes cancer (Arnaldez and Helman 2012) and decreases life span in animal models (Junnila et al. 2013). Thus, exercise may be most beneficial as a precision delivery tool that can distribute and up- or down-regulate important substrates with impeccable temporal and spatial resolution. Evidently, exercise stimulates neuronal activation in brain regions important for learning and memory, while engaging cross talk between peripheral organs (skeletal muscle, adipose tissue, liver) to supply critical substrates for brain health. The mechanisms by which different modes of exercise may affect brain function remain to be elucidated. To achieve this, specified intensity and time lines of exercise training paradigms as well as systematic analyses of brain function and adaptations will be needed.
ACKNOWLEDGMENTS
This work is supported by the Intramural Research Program of the National Institutes of Health’s (NIH) National Institute on Aging (NIA).
Footnotes
Editors: Juleen R. Zierath, Michael J. Joyner, and John A. Hawley
Additional Perspectives on The Biology of Exercise available at www.perspectivesinmedicine.org
REFERENCES
- Abel JL, Rissman EF. 2013. Running-induced epigenetic and gene expression changes in the adolescent brain. Int J Dev Neurosci 31: 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberg MA, Aberg ND, Hedbäcker H, Oscarsson J, Eriksson PS. 2000. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci 20: 2896–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham WC, Mason-Parker SE, Bear MF, Webb S, Tate WP. 2001. Heterosynaptic metaplasticity in the hippocampus in vivo: A BCM-like modifiable threshold for LTP. Proc Natl Acad Sci 98: 10924–10929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acil A, Dogan, Dogan. 2008. The effects of physical exercises to mental state and quality of life in patients with schizophrenia. J Psychiatr Ment Health Nurs 15: 808–815. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Perreau VM, Pop V, Cotman CW. 2005. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci 25: 4217–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agudelo LZ, Femenia T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, Correia JC, Izadi M, Bhat M, Schuppe-Koistinen I, et al. 2014. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 159: 33–45. [DOI] [PubMed] [Google Scholar]
- Ahn JH, Choi JH, Park JH, Kim IH, Cho JH, Lee JC, Koo HM, Hwangbo G, Yoo KY, Lee CH, et al. 2016. Long-term exercise improves memory deficits via restoration of myelin and microvessel damage, and enhancement of neurogenesis in the aged gerbil hippocampus after ischemic stroke. Neurorehabil Neural Repair 30: 894–905. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Deng W, Gage FH. 2011. Resolving new memories: A critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron 70: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainslie PN, Cotter JD, George KP, Lucas S, Murrell C, Shave R, Thomas KN, Williams MJA, Atkinson G. 2008. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol 586: 4005–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers K, Martinez-Canabal A, Restivo L. 2014. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 344: 598–602. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. 1965. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124: 319–335. [DOI] [PubMed] [Google Scholar]
- Alvarez-Saavedra M, De Repentigny Y, Yang D, O’Meara RW, Yan K, Hashem LE, Racacho L, Ioshikhes I, Bulman DE, Parks RJ, et al. 2016. Voluntary running triggers VGF-mediated oligodendrogenesis to prolong the lifespan of Snf2h-null ataxic mice. Cell Rep. 17: 862–875. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Rapp DN, Baek DH, McCloskey DP, Coburn-Litvak PS, Robinson JK. 2000. Exercise influences spatial learning in the radial arm maze. Physiol Behav 70: 425–429. [DOI] [PubMed] [Google Scholar]
- Anderson JL, Head SI, Rae C, Morley JW. 2002. Brain function in Duchenne muscular dystrophy. Brain 125: 4–13. [DOI] [PubMed] [Google Scholar]
- Arida RM, Scorza FA, dos Santos NF, Peres CA, Cavalheiro EA. 1999. Effect of physical exercise on seizure occurrence in a model of temporal lobe epilepsy in rats. Epilepsy Res 37: 45–52. [DOI] [PubMed] [Google Scholar]
- Arida RM, Sanabria ERG, Da Silva AC, Faria LC, Scorza FA, Cavalheiro EA. 2004. Physical training reverts hippocampal electrophysiological changes in rats submitted to the pilocarpine model of epilepsy. Physiol Behav 83: 165–171. [DOI] [PubMed] [Google Scholar]
- Arida RM, Scorza CA, Scorza FA, Gomes da Silva S, da Graça Naffah-Mazzacoratti M, Cavalheiro EA. 2007. Effects of different types of physical exercise on the staining of parvalbumin-positive neurons in the hippocampal formation of rats with epilepsy. Prog Neuropsychopharmacol Biol Psychiatry 31: 814–822. [DOI] [PubMed] [Google Scholar]
- Arida RM, Cavalheiro EA, Da Silva AC, Scorza FA. 2008. Physical activity and epilepsy: Proven and predicted benefits. Sport Med 38: 607–615. [DOI] [PubMed] [Google Scholar]
- Arida RM, Scorza FA, Terra VC, Cysneiros RM, Cavalheiro EA. 2009. Physical exercise in rats with epilepsy is protective against seizures: Evidence of animal studies. Arq Neuropsiquiatr 67: 1013–1016. [DOI] [PubMed] [Google Scholar]
- Arida RM, Scorza FA, Cavalheiro EA. 2010. Favorable effects of physical activity for recovery in temporal lobe epilepsy. Epilepsia 51: 76–9. [DOI] [PubMed] [Google Scholar]
- Arnaldez FI, Helman LJ. 2012. Targeting the insulin growth factor receptor 1. Hematol Oncol Clin North Am 26: 527–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami F, Movahedi A, Marandi SM, Abedi A. 2012. Kata techniques training consistently decreases stereotypy in children with autism spectrum disorder. Res Dev Disabil 33: 1183–1193. [DOI] [PubMed] [Google Scholar]
- Baier PC, May U, Scheller J, Rose-John S, Schiffelholz T. 2009. Impaired hippocampus-dependent and -independent learning in IL-6 deficient mice. Behav Brain Res 200: 192–196. [DOI] [PubMed] [Google Scholar]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Fishel MA, Watson GS, Cholerton BA, et al. 2010. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch Neurol 67: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M. 2012. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 74: 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow B, Broocks A, Pekrun G, George A, Meyer T, Pralle L, Bartmann U, Hillmer-Vogel U, Rüther E. 2000. The use of the panic and agoraphobia scale (P and A) in a controlled clinical trial. Pharmacopsychiatry 33: 174–181. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Gutierrez EG. 1994. Penetration of interleukin-6 across the murine blood–brain barrier. Neurosci Lett 179: 53–56. [DOI] [PubMed] [Google Scholar]
- Barnes JN. 2015. Exercise, cognitive function, and aging. Adv Physiol Educ 39: 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JN, Taylor JL, Kluck BN, Johnson CP, Joyner MJ. 2013. Cerebrovascular reactivity is associated with maximal aerobic capacity in healthy older adults. J Appl Physiol 114: 1383–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass MM, Duchowny CA, Llabre MM. 2009. The effect of therapeutic horseback riding on social functioning in children with autism. J Autism Dev Disord 39: 1261–1267. [DOI] [PubMed] [Google Scholar]
- Basso JC, Suzuki WA. 2017. The effects of acute exercise on mood, cognition, neurophysiology and neurochemical pathways: A review. Brain Plast 10.3233/BPL-160040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Jing DQ, Dincheva I, Neeb CC, Pattwell SS, Chao MV, Lee FS, Ninan I. 2012. BDNF Val66Met impairs fluoxetine-induced enhancement of adult hippocampus plasticity. Neuropsychopharmacology 37: 1297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia G, Alesi M, Inguglia M, Roccella M, Caramazza G, Bellafiore M, Palma A. 2013. Soccer practice as an add-on treatment in the management of individuals with a diagnosis of schizophrenia. Neuropsychiatr Dis Treat 9: 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe LH, Tian L, Morris N, Goodwin A, Allen SS, Kuldau J. 2005. Effects of exercise on mental and physical health parameters of persons with schizophrenia. Issues Ment Health Nurs 26: 661–676. [DOI] [PubMed] [Google Scholar]
- Benson C, Paylor JW, Tenorio G, Winship I, Baker G, Kerr BJ. 2015. Voluntary wheel running delays disease onset and reduces pain hypersensitivity in early experimental autoimmune encephalomyelitis (EAE). Exp Neurol 271: 279–290. [DOI] [PubMed] [Google Scholar]
- Belarbi K, Burnouf S, Fernandez-Gomez FJ, Laurent C, Lestavel S, Figeac M, Sultan A, Troquier L, Leboucher A, Caillierez R, et al. 2011. Beneficial effects of exercise in a transgenic mouse model of Alzheimer’s disease-like Tau pathology. Neurobiol Dis 43: 486–494. [DOI] [PubMed] [Google Scholar]
- Berg U, Bang P. 2004. Exercise and circulating insulin-like growth factor I. Hormone Res 62: 50–58. [DOI] [PubMed] [Google Scholar]
- Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, Mackay-Lyons M, Macko RF, Mead GE, Roth EJ, et al. 2014. Physical activity and exercise recommendations for stroke survivors: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45: 2532–2553. [DOI] [PubMed] [Google Scholar]
- Black F. 1973. Intellectual ability as related to age and stage of disease in muscular dystrophy: A brief note. J Psychol 84: 333–334. [DOI] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. 1990. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci 87: 5568–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland BH. 1986. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol 26: 1–54. [DOI] [PubMed] [Google Scholar]
- Bland BH, Oddie SD. 2001. Theta band oscillation and synchrony in the hippocampal formation and associated structures: The case for its role in sensorimotor integration. Behav Brain Res 127: 119–136. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. 1993. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 361: 31–39. [DOI] [PubMed] [Google Scholar]
- Bob P, Raboch J, Maes M, Susta M, Pavlat J, Jasova D, Vevera J, Uhrova J, Benakova H, Zima T. 2010. Depression, traumatic stress and interleukin-6. J Affect Disord 120: 231–234. [DOI] [PubMed] [Google Scholar]
- Bolz L, Heigele S, Bischofberger J. 2015. Running improves pattern separation during novel object recognition. Brain Plast 1: 129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. 2011. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 145: 1142–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach Ka, Boström EA, Choi JH, Long JZ, et al. 2012. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481: 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen KK, Dempsey RJ, Vemuganti R. 2011. Adult interleukin-6 knockout mice show compromised neurogenesis. NeuroReport 22: 126–130. [DOI] [PubMed] [Google Scholar]
- Brockett AT, LaMarca EA, Gould E. 2015. Physical exercise enhances cognitive flexibility as well as astrocytic and synaptic markers in the medial prefrontal cortex. PLoS ONE 10: e0124859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman-Fulks JJ, Storey KM. 2008. Evaluation of a brief aerobic exercise intervention for high anxiety sensitivity. Anxiety Stress Coping 21: 117–128. [DOI] [PubMed] [Google Scholar]
- Broocks A, Bandelow B, Pekrun G, George A, Meyer T, Bartmann U, Hillmer-Vogel U, Rüther E. 1998. Comparison of aerobic exercise, clomipramine, and placebo in the treatment of panic disorder. Am J Psychiatry 155: 603–609. [DOI] [PubMed] [Google Scholar]
- Brown RA, Abrantes AM, Read JP, Marcus BH, Jakicic J, Strong DR, Oakley JR, Ramsey SE, Kahler CW, Stuart G, et al. 2009. Aerobic exercise for alcohol recovery: Rationale, program description, and preliminary findings. Behav Modif 33: 220–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BM, Bourgeat P, Peiffer JJ, Burnham S, Laws SM, Rainey-Smith SR, Bartrés-Faz D, Villemagne VL, Taddei K, Rembach A, et al. 2014a. Influence of BDNF Val66Met on the relationship between physical activity and brain volume. Neurology 83: 1345–1352. [DOI] [PubMed] [Google Scholar]
- Brown RA, Abrantes AM, Minami H, Read JP, Marcus BH, Jakicic JM, Strong DR, Dubreuil ME, Gordon AA, Ramsey SE, et al. 2014b. A preliminary, randomized trial of aerobic exercise for alcohol dependence. J Subst Abuse Treat 47: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Veyrac A, Dufour F, Horwood J, Laroche S, Davis S. 2009. Inhibition of PI3K-Akt signaling blocks exercise-mediated enhancement of adult neurogenesis and synaptic plasticity in the dentate gyrus. PLoS ONE 4: e7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushby K. 1992. Recent advances in understanding muscular dystrophy. Arch Dis Child 67: 1310–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse M, Quinn L, Debono K, Jones K, Collett J, Playle R, Kelly M, Simpson S, Backx K, Wasley D, et al. 2013. A randomized feasibility study of a 12-week community-based exercise program for people with Huntington’s disease. J Neurol Phys Ther 37: 149–158. [DOI] [PubMed] [Google Scholar]
- Canning CG, Sherrington C, Lord SR, Close JCT, Heritier S, Heller GZ, Howard K, Allen NE, Latt MD, Murray SM, et al. 2015. Exercise for falls prevention in Parkinson disease: A randomized controlled trial. Neurology 84: 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. 2004. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet 36: 827–835. [DOI] [PubMed] [Google Scholar]
- Carmeli E, Barak S, Morad M, Kodesh E. 2009. Physical exercises can reduce anxiety and improve quality of life among adults with intellectual disability. Int Sport J 10: 77–85. [Google Scholar]
- Carmeliet P. 2003. Blood vessels and nerves: Common signals, pathways and diseases. Nat Rev Genet 4: 710–720. [DOI] [PubMed] [Google Scholar]
- Carro E, Nuñez A, Busiguina S, Torres-Aleman I. 2000. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci 20: 2926–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassilhas RC, Lee KS, Fernandes J, Oliveira MGM, Tufik S, Meeusen R, De Mello MT. 2012. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience 202: 309–317. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Cummings DM, Hickey MA, Kleiman-Weiner M, Chen J, Watson JB, Levine MS. 2010. Rescuing the corticostriatal synaptic disconnection in the R6/2 mouse model of Huntington’s disease: Exercise, adenosine receptors and ampakines. PLoS Curr 10.1371/currents.RRN1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouloff F. 1989. Physical exercise and brain monoamines: A review. Acta Physiol Scand 137: 1–13. [DOI] [PubMed] [Google Scholar]
- Charrette AL, Lorenz LS, Fong J, O’Neil-Pirozzi TM, Lamson K, Demore-Taber M, Lilley R. 2016. Pilot study of intensive exercise on endurance, advanced mobility and gait speed in adults with chronic severe acquired brain injury. Brain Inj 30: 1213–1219. [DOI] [PubMed] [Google Scholar]
- Chen MD, Rimmer JH. 2011. Effects of exercise on quality of life in stroke survivors: A meta-analysis. Stroke 42: 832–837. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. 2004. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci 24: 4401–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DH, Lee KH, Lee J. 2016. Effect of exercise-induced neurogenesis on cognitive function deficit in a rat model of vascular dementia. Mol Med Rep 13: 2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie BR, Abraham WC. 1992. Priming of associative long-term depression in the dentate gyrus by theta frequency synaptic activity. Neuron 9: 79–84. [DOI] [PubMed] [Google Scholar]
- Christofoletti G, Oliani MM, Gobbi S, Stella F, Bucken Gobbi LT, Renato Canineu P. 2008. A controlled clinical trial on the effects of motor intervention on balance and cognition in institutionalized elderly patients with dementia. Clin Rehabil 22: 618–626. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. 2008. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience 155: 1048–1058. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Puchalski EK, Krone DA, Rhodes JS. 2009. Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus 19: 937–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Kohman RA, Miller DS, Bhattacharya TK, Brzezinska WJ, Rhodes JS. 2011. Genetic influences on exercise-induced adult hippocampal neurogenesis across 12 divergent mouse strains. Genes Brain Behav 10: 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, et al. 2009. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325: 210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Paulsson JF, Blinder P, Burstyn-Cohen T, Du D, Estepa G, Adame A, Pham HM, Holzenberger M, Kelly JW, et al. 2009. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell 139: 1157–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coiro V, Casti A, Saccani Jotti G, Rubino P, Manfredi G, Maffei ML, Melani A, Volta E, Chiodera P. 2007. Adrenocorticotropic hormone/cortisol response to physical exercise in abstinent alcoholic patients. Alcohol Clin Exp Res 31: 901–906. [DOI] [PubMed] [Google Scholar]
- Conradsson M, Littbrand H, Lindelof N, Gustafson Y, Rosendahl E. 2010. Effects of a high-intensity functional exercise programme on depressive symptoms and psychological well-being among older people living in residential care facilities: A cluster-randomized controlled trial. Aging Ment Health 14: 565–576. [DOI] [PubMed] [Google Scholar]
- Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, McMurdo M, Mead GE. 2013. Exercise for depression. Cochrane Database Syst Rev 9: CD004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. 2007. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci 30: 464–472. [DOI] [PubMed] [Google Scholar]
- Craft LL, Landers DM. 1998. The effect of exercise on clinical depression and depression resulting from mental illness: A meta-analysis. J Sport Exerc Psychol 20: 339–357. [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. 2010. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci 107: 2367–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K, Wilkie ME. 2004. Exercise reverses ethanol inhibition of neural stem cell proliferation. Alcohol 33: 63–71. [DOI] [PubMed] [Google Scholar]
- Cruise KE, Bucks RS, Loftus AM, Newton RU, Pegoraro R, Thomas MG. 2011. Exercise and Parkinson’s: Benefits for cognition and quality of life. Acta Neurol Scand 123: 13–19. [DOI] [PubMed] [Google Scholar]
- Cumming TB, Tyedin K, Churilov L, Morris ME, Bernhardt J. 2012. The effect of physical activity on cognitive function after stroke: A systematic review. Int Psychogeriatrics 24: 557–567. [DOI] [PubMed] [Google Scholar]
- Czurko A. 1999. Sustained activation of hippocampal pyramidal cells by “space clamping” in a running wheel. Eur J Neurosci 11: 344–352. [DOI] [PubMed] [Google Scholar]
- Daley A. 2008. Exercise and depression: A review of reviews. J Clin Psychol Med Settings 15: 140–147. [DOI] [PubMed] [Google Scholar]
- Danielsson L, Noras AM, Waern M, Carlsson J. 2013. Exercise in the treatment of major depression: A systematic review grading the quality of evidence. Physiother Theory Pract 29: 573–85. [DOI] [PubMed] [Google Scholar]
- Dashtipour K, Johnson E, Kani C, Kani K, Hadi E, Ghamsary M, Pezeshkian S, Chen JJ. 2015. Effect of exercise on motor and nonmotor symptoms of Parkinson’s disease. Parkinsons Dis 10.1155/2015/586378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David FJ, Robichaud JA, Leurgans SE, Poon C, Kohrt WM, Goldman JG, Comella CL, Vaillancourt DE, Corcos DM. 2015. Exercise improves cognition in Parkinson’s disease: The PRET-PD randomized, clinical trial. Mov Disord 30: 1657–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean CM, Rissel C, Sherrington C, Sharkey M, Cumming RG, Lord SR, Barker RN, Kirkham C, O’Rourke S. 2012. Exercise to enhance mobility and prevent falls after stroke: The Community Stroke Club Randomized Trial. Neurorehabil Neural Repair 26: 1046–1057. [DOI] [PubMed] [Google Scholar]
- Delp MD, Armstrong RB, Godfrey DA, Laughlin MH, Ross CD, Wilkerson MK. 2001. Exercise increases blood flow to locomotor, vestibular, cardiorespiratory and visual regions of the brain in miniature swine. J Physiol 533: 849–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zeeuw ELEJ, Tak ECPM, Dusseldorp E, Hendriksen IJM. 2010. Workplace exercise intervention to prevent depression: A pilot randomized controlled trial. Ment Health Phys Act 3: 72–77. [Google Scholar]
- Dietrich A, McDaniel WF. 2004. Endocannabinoids and exercise. Br J Sports Med 38: 536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MO, Andrews ZB, Horvath TL. 2008. Exercise-induced synaptogenesis in the hippocampus is dependent on UCP2-regulated mitochondrial adaptation. J Neurosci 28: 10766–10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillingham CM, Frizzati A, Nelson AJD, Vann SD. 2015. How do mammillary body inputs contribute to anterior thalamic function? Neurosci Biobehav Rev 54: 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Li J, Luan X, Ding YH, Lai Q, Rafols JA, Phillis JW, Clark JC, Diaz FG. 2004. Exercise pre-conditioning reduces brain damage in ischemic rats that may be associated with regional angiogenesis and cellular overexpression of neurotrophin. Neuroscience 124: 583–591. [DOI] [PubMed] [Google Scholar]
- Ding YH, Li J, Zhou Y, Rafols JA, Clark JC, Ding Y. 2006. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr Neurovasc Res 3: 15–23. [DOI] [PubMed] [Google Scholar]
- Dishman RK. 1997. Brain monoamines, exercise, and behavioral stress: Animal models. Med Sci Sports Exerc 29: 63–74. [DOI] [PubMed] [Google Scholar]
- Docherty K, Hutton JC, Steiner DF. 1984. Cathepsin B–related proteases in the insulin secretory granule. J Biol Chem 259: 6041–6044. [PubMed] [Google Scholar]
- Dumith SC, Hallal PC, Reis RS, Kohl HW. 2011. Worldwide prevalence of physical inactivity and its association with human development index in 76 countries. Prev Med 53: 24–28. [DOI] [PubMed] [Google Scholar]
- Duzel E, Van Praag H, Sendtner M. 2016. Can physical exercise in old age improve memory and hippocampal function? Brain 139: 662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eadie BD, Redila VA, Christie BR. 2005. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol 486: 39–47. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, et al. 2003. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112: 257–269. [DOI] [PubMed] [Google Scholar]
- Eggermont LHP, Knol DL, Hol EM, Swaab DF, Scherder EJA. 2009a. Hand motor activity, cognition, mood, and the rest-activity rhythm in dementia. A clustered RCT. Behav Brain Res 196: 271–278. [DOI] [PubMed] [Google Scholar]
- Eggermont LHP, Swaab DF, Hol EM, Scherder EJ. 2009b. Walking the line: A randomised trial on the effects of a short term walking programme on cognition in dementia. J Neurol Neurosurg Psychiatry 80: 802–804. [DOI] [PubMed] [Google Scholar]
- Ehringer MA, Hoft NR, Zunhammer M. 2009. Reduced alcohol consumption in mice with access to a running wheel. Alcohol 43: 443–452. [DOI] [PubMed] [Google Scholar]
- Embury CM, Dyavarshetty B, Lu Y, Wiederin JL, Ciborowski P, Gendelman HE, Kiyota T. 2016. Cathepsin B improves β-amyloidosis and learning and memory in models of Alzheimer's disease. J Neuroimmune Pharmacol 10.1007/s11481-016-9721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. 2011. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8: 566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom S, Lee MK, Park JH, Lee D, Kang HC, Lee JS, Jeon JY, Kim HD. 2016. The impact of a 35-week long-term exercise therapy on psychosocial health of children with benign epilepsy. J Child Neurol 31: 985–990. [DOI] [PubMed] [Google Scholar]
- Epp JR, Silva Mera R, Köhler S, Josselyn SA, Frankland PW. 2016. Neurogenesis-mediated forgetting minimizes proactive interference. Nat Commun 7: 10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wójcicki TR, McAuley E, Kramer AF. 2009. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 19: 1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, Pence BD, Martin SA, Vieira VJ, Woods JA, et al. 2010. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci 30: 5368–5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, et al. 2011. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci 108: 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Leckie RL, Weinstein AM. 2014. Physical activity, fitness, and gray matter volume. Neurobiol Aging 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen HR, Ellertsen B, Gronningsaeter H, Nakken KO, Loyning Y, Ursin H. 1994. Physical exercise in women with intractable epilepsy. Epilepsia 35: 1256–1264. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. 1998. Neurogenesis in the adult human hippocampus. Nat Med 4: 1313–1317. [DOI] [PubMed] [Google Scholar]
- Erta M, Quintana A, Hidalgo J. 2012. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci 8: 1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. 2003. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci 18: 2803–2812. [DOI] [PubMed] [Google Scholar]
- Fahimi A, Baktir M, Moghadam S, Mojabi F, Sumanth K, McNerney M, Ponnusamy R, Salehi A. 2016. Physical exercise induces structural alterations in the hippocampal astrocytes: Exploring the role of BDNF-TrkB signaling. Brain Struct Funct 10.1007/s00429-016-1308-8. [DOI] [PubMed] [Google Scholar]
- Falkai P, Malchow B, Wobrock T, Gruber O, Schmitt A, Honer WG, Pajonk FG, Sun F, Cannon TD. 2013. The effect of aerobic exercise on cortical architecture in patients with chronic schizophrenia: A randomized controlled MRI study. Eur Arch Psychiatry Clin Neurosci 263: 469–473. [DOI] [PubMed] [Google Scholar]
- Farioli-Vecchioli S, Mattera A, Micheli L, Ceccarelli M, Leonardi L, Saraulli D, Costanzi M, Cestari V, Rouault JP, Tirone F. 2014. Running rescues defective adult neurogenesis by shortening the length of the cell cycle of neural stem and progenitor cells. Stem Cells 32: 1968–1982. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, Van Praag H, Wodtke K, Gage FH, Christie BR. 2004. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague–Dawley rats in vivo. Neuroscience 124: 71–79. [DOI] [PubMed] [Google Scholar]
- Feldman E, Nelson R. 2004. Diabetic ketoacidosis. In Canine and feline endocrinology and reproduction, pp. 394–439. Elsevier, St. Louis. [Google Scholar]
- Finck BN, Kelly DP. 2006. PGC-1 coactivators: Inducible regulators of energy metabolism in health and disease. J Clin Invest 116: 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer TJ, Walker TL, Overall RW, Brandt MD, Kempermann G. 2014. Acute effects of wheel running on adult hippocampal precursor cells in mice are not caused by changes in cell cycle length or S phase length. Front Neurosci 8: 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florek M, Karolak S. 1977. Intelligence level of patients with the Duchenne type of progressive muscular dystrophy (pmd-d). Eur J Pediatr 126: 275–282. [DOI] [PubMed] [Google Scholar]
- Foley LS, Prapavessis H, Osuch EA, De Pace JA, Murphy BA, Podolinsky NJ. 2008. An examination of potential mechanisms for exercise as a treatment for depression: A pilot study. Ment Health Phys Act 1: 69–73. [Google Scholar]
- Forbes D, Forbes SC, Blake CM, Thiessen EJ, Forbes S. 2015. Exercise programs for people with dementia. Cochrane Database Syst Rev 4: CD006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsman AK, Nordmyr J, Wahlbeck K. 2011. Psychosocial interventions for the promotion of mental health and the prevention of depression among older adults. Health Promot Int 26: 85–107. [DOI] [PubMed] [Google Scholar]
- Fulk LJ, Stock HS, Lynn A, Marshall J, Wilson MA, Hand GA. 2004. Chronic physical exercise reduces anxiety-like behavior in rats. Int J Sports Med 25: 78–82. [DOI] [PubMed] [Google Scholar]
- Fuss J, Gass P. 2010. Endocannabinoids and voluntary activity in mice: Runner’s high and long-term consequences in emotional behaviors. Exp Neurol 224: 103–105. [DOI] [PubMed] [Google Scholar]
- Gabriels RL, Agnew JA, Holt KD, Shoffner A, Pan ZX, Ruzzano S, Clayton GH, Mesibov G. 2012. Pilot study measuring the effects of therapeutic horseback riding on school-age children and adolescents with autism spectrum disorders. Res Autism Spectr Disord 6: 578–588. [Google Scholar]
- Gallego X, Cox RJ, Funk E, Foster RA, Ehringer MA. 2015. Voluntary exercise decreases ethanol preference and consumption in C57BL/6 adolescent mice: Sex differences and hippocampal BDNF expression. Physiol Behav 138: 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mesa Y, López-Ramos JC, Giménez-Llort L, Revilla S, Guerra R, Gruart A, Laferla FM, Cristòfol R, Delgado-García JM, Sanfeliu C. 2011. Physical exercise protects against Alzheimer’s disease in 3xTg-AD mice. J Alzheimers Dis 24: 421–454. [DOI] [PubMed] [Google Scholar]
- Gavin TP, Robinson CB, Yeager RC, England JA, Nifong LW, Hickner RC. 2004. Angiogenic growth factor response to acute systemic exercise in human skeletal muscle. J Appl Physiol (1985) 96: 19–24. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh ELK, Sailor KA, Kitabatake Y, Ming G, Song H. 2006. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439: 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. 2007. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron 54: 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebara E, Sultan S, Kocher-Braissant J, Toni N. 2013. Adult hippocampal neurogenesis inversely correlates with microglia in conditions of voluntary running and aging. Front Neurosci 7: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebara E, Bonaguidi MA, Beckervordersandforth R, Sultan S, Udry F, Gijs PJ, Lie DC, Ming GL, Song H, Toni N. 2016. Heterogeneity of radial glia-like cells in the adult hippocampus. Stem Cells 34: 997–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng T, Li P, Okutsu M, Yin X, Kwek J, Zhang M, Yan Z. 2010. PGC-1α plays a functional role in exercise-induced mitochondrial biogenesis and angiogenesis but not fiber-type transformation in mouse skeletal muscle. Am J Physiol Cell Physiol 298: C572–C579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golbidi S, Laher I. 2014. Exercise induced adipokine changes and the metabolic syndrome. J Diabetes Res 10.1155/2014/726861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin P, Ziv M, Jazaieri H, Gross JJ. 2012. Randomized controlled trial of mindfulness-based stress reduction versus aerobic exercise: Effects on the self-referential brain network in social anxiety disorder. Front Hum Neurosci 6: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin P, Ziv M, Jazaieri H, Hahn K, Gross JJ. 2013. MBSR vs aerobic exercise in social anxiety: fMRI of emotion regulation of negative self-beliefs. Soc Cogn Affect Neurosci 8: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes da Silva S, Unsain N, Mascó DH, Toscano-Silva M, de Amorim HA, Silva Araújo BH, Simões PS, Naffah-Mazzacoratti Mda G, Mortara RA, Scorza FA, et al. 2012. Early exercise promotes positive hippocampal plasticity and improves spatial memory in the adult life of rats. Hippocampus 22: 347–358. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Zhuang Y, Feng J, Ying Z, Fan G. 2011. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur J Neurosci 33: 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves JT, Bloyd CW, Shtrahman M, Johnston ST, Schafer ST, Parylak SL, Tran T, Chang T, Gage FH. 2016. In vivo imaging of dendritic pruning in dentate granule cells. Nat Neurosci 19: 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gontier G, George C, Chaker Z, Holzenberger M, Aïd S. 2015. Blocking IGF signaling in adult neurons alleviates Alzheimer’s disease pathology through amyloid-β clearance. J Neurosci 35: 11500–11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotze W, Kubicki S, Munter M, Teichmann J. 1967. Effect of physical exercise on seizure threshold (investigated by electroencephalographic telemetry). Dis Nerv Syst 28: 664–667. [PubMed] [Google Scholar]
- Graven C, Brock K, Hill K, Joubert L. 2011. Are rehabilitation and/or care co-ordination interventions delivered in the community effective in reducing depression, facilitating participation and improving quality of life after stroke? Disabil Rehabil 33: 1501–1520. [DOI] [PubMed] [Google Scholar]
- Greenstein YJ, Pavlides C, Winson J. 1988. Long-term potentiation in the dentate gyrus is preferentially induced at theta rhythm periodicity. Brain Res 438: 331–334. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Kennedy S, Smith TP, Campeau S, Day HEW, Fleshner M. 2003. Voluntary freewheel running selectively modulates catecholamine content in peripheral tissue and c-Fos expression in the central sympathetic circuit following exposure to uncontrollable stress in rats. Neuroscience 120: 269–281. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Brooks L, Fleshner M. 2008. Anxiety-like behaviors produced by acute fluoxetine administration in male Fischer 344 rats are prevented by prior exercise. Psychopharmacology (Berl) 199: 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Spence KG, Crevling DM, Clark PJ, Craig WC, Fleshner M. 2013. Exercise-induced stress resistance is independent of exercise controllability and the medial prefrontal cortex. Eur J Neurosci 37: 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire CA, Bonenfant D, Le Nguyen A, Aumont A, Fernandes KJL. 2014. Untangling the influences of voluntary running, environmental complexity, social housing and stress on adult hippocampal neurogenesis. PLoS ONE 9: e86237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoretti IV, Lee YM, Goodson HV. 2004. Molecular evolution of the histone deacetylase family: Functional implications of phylogenetic analysis. J Mol Biol 338: 17–31. [DOI] [PubMed] [Google Scholar]
- Griffin ÉW, Bechara RG, Birch AM, Kelly ÁM. 2009. Exercise enhances hippocampal-dependent learning in the rat: Evidence for a BDNF-related mechanism. Hippocampus 19: 973–980. [DOI] [PubMed] [Google Scholar]
- Gu W, Jiang W, Wester P. 2003. Real-time cortical cerebral blood flow follow-up in conscious, freely moving rats by laser Doppler flowmetry. Methods 30: 172–177. [DOI] [PubMed] [Google Scholar]
- Guerrieri D, Moon HY, van Praag H. 2017. Exercise in a pill: The latest on exercise-mimetics. Brain Plast 10.3233/BPL-160043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler HP, Zapf J, Froesch ER. 1987. Short-term metabolic effects of recombinant human insulin-like growth factor I in healthy adults. N Engl J Med 317: 137–140. [DOI] [PubMed] [Google Scholar]
- Guthold R, Ono T, Strong KL, Chatterji S, Morabia A. 2008. Worldwide variability in physical inactivity. A 51-country survey. Am J Prev Med 34: 486–494. [DOI] [PubMed] [Google Scholar]
- Hackert VH, den Heijer T, Oudkerk M, Koudstaal PJ, Hofman A, Breteler MM. 2002. Hippocampal head size associated with verbal memory performance in nondemented elderly. Neuroimage 17: 1365–1372. [DOI] [PubMed] [Google Scholar]
- Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U, Alkandari JR, Bauman AE, Blair SN, Brownson RC, et al. 2012. Global physical activity levels: Surveillance progress, pitfalls, and prospects. Lancet 380: 247–257. [DOI] [PubMed] [Google Scholar]
- Han SH, Quon MJ, Kim J, Koh KK. 2007. Adiponectin and cardiovascular disease: Response to therapeutic interventions. J Am Coll Cardiol 49: 531–538. [DOI] [PubMed] [Google Scholar]
- Hansen J, Brandt C, Nielsen AR, Hojman P, Whitham M, Febbraio MA, Pedersen BK, Plomgaard P. 2011. Exercise induces a marked increase in plasma follistatin: Evidence that follistatin is a contraction-induced hepatokine. Endocrinology 152: 164–171. [DOI] [PubMed] [Google Scholar]
- Hardie DG. 2011. AMP-activated protein kinase—An energy sensor that regulates all aspects of cell function. Genes Dev 25: 1895–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DJ, Busse M, Openshaw R, Rosser AE, Dunnett SB, Brooks SP. 2013. Exercise attenuates neuropathology and has greater benefit on cognitive than motor deficits in the R6/1 Huntington’s disease mouse model. Exp Neurol 248: 457–469. [DOI] [PubMed] [Google Scholar]
- Hashemi MS, Ghaedi K, Salamian A, Karbalaie K, Emadi-Baygi M, Tanhaei S, Nasr-Esfahani MH, Baharvand H. 2013. Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience 231: 296–304. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. 2014. Integrative biology of exercise. Cell 159: 738–749. [DOI] [PubMed] [Google Scholar]
- Heise J, Buckworth J, McAuley JW, Long L, Kirby TE. 2002. Exercise training results in positive outcomes in persons with epilepsy. Clin Exerc Physiol 4: 79. [Google Scholar]
- Henke PG. 1990. Hippocampal pathway to the amygdala and stress ulcer development. Brain Res Bull 25: 691–695. [DOI] [PubMed] [Google Scholar]
- Herring MP, Jacob ML, Suveg C, Dishman RK, O’Connor PJ. 2011. Feasibility of exercise training for the short-term treatment of generalized anxiety disorder: A randomized controlled trial. Psychother Psychosom 81: 21–28. [DOI] [PubMed] [Google Scholar]
- Herring MP, Kline CE, O’Connor PJ. 2015. Effects of exercise on sleep among young women with generalized anxiety disorder. Ment Health Phys Act 9: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn P, Abreu BC, Ottenbacher KJ. 2004. The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Arch Phys Med Rehabil 85: 1694–1704. [DOI] [PubMed] [Google Scholar]
- Hill LE, Droste SK, Nutt DJ, Linthorst AC, Reul JM. 2010. Voluntary exercise alters GABAA receptor subunit and glutamic acid decarboxylase-67 gene expression in the rat forebrain. J Psychopharmacol 24: 745–756. [DOI] [PubMed] [Google Scholar]
- Himi N, Takahashi H, Okabe N, Nakamura E, Shiromoto T, Narita K, Koga T, Miyamoto O. 2016. Exercise in the early stage after stroke enhances hippocampal brain-derived neurotrophic factor expression and memory function recovery. J Stroke Cerebrovasc Dis 25: 2987–2994. [DOI] [PubMed] [Google Scholar]
- Hinton VJ, De Vivo DC, Nereo NE, Goldstein E, Stern Y. 2000. Poor verbal working memory across intellectual level in boys with Duchenne dystrophy. Neurology 54: 2127–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JR, Ostfeld I, Kaplan Z, Zohar J, Cohen H. 2015. Exercise enhances the behavioral responses to acute stress in an animal model of PTSD. Med Sci Sports Exerc 47: 2043–2052. [DOI] [PubMed] [Google Scholar]
- Hook VY, Kindy M, Hook G. 2008. Inhibitors of cathepsin B improve memory and reduce Aβ in transgenic Alzheimer’s disease mice expressing the wild-type, but not the Swedish mutant, β-secretase APP site. J Biol Chem 283: 7745–7753. [DOI] [PubMed] [Google Scholar]
- Hovland A, Nordhus IH, Sjøbø T, Gjestad BA, Birknes B, Martinsen EW, Torsheim T, Pallesen S. 2013. Comparing physical exercise in groups to group cognitive behaviour therapy for the treatment of panic disorder in a randomized controlled trial. Behav Cogn Psychother 41: 408–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Zhao X. Genetics and epigenetics in adult neurogenesis. 2016. Cold Spring Harb Perspect Biol 8: a018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang H, Choi Y. 2010. The effects of the dance therapy program through rhythmic exercise on cognitive memory performance of the elderly with dementia. Proc 21st Pan-Asian Congr Sport Phys Educ 4: 12–17. [Google Scholar]
- Ieraci A, Mallei A, Musazzi L, Popoli M. 2015. Physical exercise and acute restraint stress differentially modulate hippocampal brain-derived neurotrophic factor transcripts and epigenetic mechanisms in mice. Hippocampus 25: 1380–1392. [DOI] [PubMed] [Google Scholar]
- Ieraci A, Madaio A, Mallei A, Lee F, Popoli M. 2016. Brain-derived neurotrophic factor Val66Met human polymorphism impairs the beneficial exercise-induced neurobiological changes in mice. Neuropsychopharmacology 41: 3070–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer KA, Cotman CW. 2013. Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiol Dis 57: 47–55. [DOI] [PubMed] [Google Scholar]
- Jazaieri H, Goldin PR, Werner K, Ziv M, Gross JJ. 2012. A randomized trial of MBSR versus aerobic exercise for social anxiety disorder. J Clin Psychol 68: 715–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM, Nair KS, Gygi SP, Spiegelman BM. 2015. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab 22: 734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson T, Lindwall M, Archer T, Josefsson T. 2014. Physical exercise intervention in depressive disorders: Meta-analysis and systematic review. Scand J Med Sci Sport 24: 259–272. [DOI] [PubMed] [Google Scholar]
- Judah JD, Quinn PS. 1978. Calcium ion-dependent vesicle fusion in the conversion of proalbumin to albumin. Nature 271: 384–385. [DOI] [PubMed] [Google Scholar]
- Junnila RK, List EO, Berryman DE, Murrey JW, Kopchick JJ. 2013. The GH/IGF-1 axis in ageing and longevity. Nat Rev Endocrinol 9: 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanarek RB, Marks-Kaufman R, D’Anci KE, Przypek J. 1995. Exercise attenuates oral intake of amphetamine in rats. Pharmacol Biochem Behav 51: 725–729. [DOI] [PubMed] [Google Scholar]
- Kannangara TS, Lucero MJ, Gil-Mohapel J, Drapala RJ, Simpson JM, Christie BR, Van Praag H. 2011. Running reduces stress and enhances cell genesis in aged mice. Neurobiol Aging 32: 2279–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. 2014. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344: 630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller C, Steensberg A, Pilegaard H, Osada T, Saltin B, Pedersen BK, Neufer PD. 2001. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: Influence of muscle glycogen content. FASEB J 15: 2748–2750. [DOI] [PubMed] [Google Scholar]
- Kemoun G, Thibaud M, Roumagne N, Carette P, Albinet C, Toussaint L, Paccalin M, Dugué B. 2010. Effects of a physical training programme on cognitive function and walking efficiency in elderly persons with dementia. Dement Geriatr Cogn Disord 29: 109–114. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. 2004. Milestones of neuronal development in the adult hippocampus. Trends Neurosci 27: 447–452. [DOI] [PubMed] [Google Scholar]
- Kern L, Koegel RL, Dyer K, Blew PA, Fenton LR. 1982. The effects of physical exercise on self-stimulation and appropriate responding in autistic children. J Autism Dev Disord 12: 399–419. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Rolls ET. 2015. A computational theory of hippocampal function, and tests of the theory: New developments. Neurosci Biobehav Rev 48: 92–147. [DOI] [PubMed] [Google Scholar]
- Khalil H, Quinn L, van Deursen R, Dawes H, Playle R, Rosser A, Busse M. 2013. What effect does a structured home-based exercise programme have on people with Huntington’s disease? A randomized, controlled pilot study. Clin Rehabil 27: 646–658. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Tannenholz L, Hen R. 2012. NR2B-dependent plasticity of adult-born granule cells is necessary for context discrimination. J Neurosci 32: 8696–8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Han PL. 2016. Physical exercise counteracts stress-induced upregulation of melanin-concentrating hormone in the brain and stress-induced persisting anxiety-like behaviors. Exp Neurobiol 25: 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BK, Seo JH. 2013. Treadmill exercise alleviates post-traumatic stress disorder-induced impairment of spatial learning memory in rats. J Exerc Rehabil 9: 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Shin MS, Seo TB, Ji ES, Baek SS, Lee SJ, Park JK, Kim CJ. 2013. Treadmill exercise ameliorates motor disturbance through inhibition of apoptosis in the cerebellum of valproic acid-induced autistic rat pups. Mol Med Rep 8: 327–334. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Song BK, So B, Lee O, Song W, Kim Y. 2014a. Increase of circulating BDNF levels and its relation to improvement of physical fitness following 12 weeks of combined exercise in chronic patients with schizophrenia: A pilot study. Psychiatry Res 220: 792–796. [DOI] [PubMed] [Google Scholar]
- Kim TW, Kang HS, Park JK, Lee SJ, Baek S Bin, Kim CJ. 2014b. Voluntary wheel running ameliorates symptoms of MK-801-induced schizophrenia in mice. Mol Med Rep 10: 2924–2930. [DOI] [PubMed] [Google Scholar]
- Kimhy D, Vakhrusheva J, Bartels MN, Armstrong HF, Ballon JS, Khan S, Chang RW, Hansen MC, Ayanruoh L, Lister A, et al. 2015. The impact of aerobic exercise on brain-derived neurotrophic factor and neurocognition in individuals with schizophrenia: A single-blind, randomized clinical trial. Schizophr Bull 41: 859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhy D, Lauriola V, Bartels M, Armstrong H, Vakhrusheva J, Ballon J, Sloan R. 2016. Aerobic exercise for cognitive deficits in schizophrenia—The impact of frequency, duration, and fidelity with target training intensity. Schizophr Bull 172: 213–215. [DOI] [PubMed] [Google Scholar]
- Kiuchi T, Lee H, Mikami T. 2012. Regular exercise cures depression-like behavior via VEGF-Flk-1 signaling in chronically stressed mice. Neuroscience 207: 208–217. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. 2002. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci 99: 10825–10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Rasińska J, Empl L, Sparenberg M, Poshtiban A, Hain EG, Iggena D, Rivalan M, Winter Y, Steiner B. 2016. Physical exercise counteracts MPTP-induced changes in neural precursor cell proliferation in the hippocampus and restores spatial learning but not memory performance in the water maze. Behav Brain Res 307: 227–238. [DOI] [PubMed] [Google Scholar]
- Klempin F, Beis D, Mosienko V, Kempermann G, Bader M, Alenina N. 2013. Serotonin is required for exercise-induced adult hippocampal neurogenesis. J Neurosci 33: 8270–8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintsova AY, Hamilton GF, Boschen KE. 2012. Long-term consequences of developmental alcohol exposure on brain structure and function: Therapeutic benefits of physical activity. Brain Sci 3: 1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloos AD, Fritz NE, Kostyk SK, Young GS, Kegelmeyer D. 2013. Video game play (Dance Dance Revolution) as a potential exercise therapy in Huntington’s disease: A controlled clinical trial. Clin Rehabil 27: 972–982. [DOI] [PubMed] [Google Scholar]
- Knierim JJ. 2015. From the GPS to HM: Place cells, grid cells, and memory. Hippocampus 25: 719–725. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Neunuebel JP, Deshmukh SS. 2014. Functional correlates of the lateral and medial entorhinal cortex: Objects, path integration and local–global reference frames. Philos Trans R Soc Lond B Biol Sci 369: 20130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. 2011a. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem 18: 605–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Yuan C, van Praag H. 2011b. Endurance factors improve hippocampal neurogenesis and spatial memory in mice. Learn Mem 18: 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Guerrieri D, Zhang Y, Collica SC, Becker KG, van Praag H. 2014. AMPK agonist AICAR improves cognition and motor coordination in young and aged mice. Learn Mem 21: 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblinski JE, Dosescu J, Sameni M, Moin K, Clark K, Sloane BF. 2002. Interaction of human breast fibroblasts with collagen I increases secretion of procathepsin B. J Biol Chem 277: 32220–32227. [DOI] [PubMed] [Google Scholar]
- Kodali M, Megahed T, Mishra V, Shuai B, Hattiangady B, Shetty A. 2016. Voluntary running exercise-mediated enhanced neurogenesis does not obliterate retrograde spatial memory. J Neurosci 36: 8112–8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl HW, Craig CL, Lambert EV, Inoue S, Alkandari JR, Leetongin G, Kahlmeier S. 2012. The pandemic of physical inactivity: Global action for public health. Lancet 380: 294–305. [DOI] [PubMed] [Google Scholar]
- Kondo M, Nakamura Y, Ishida Y, Shimada S. 2015. The 5-HT3 receptor is essentialfor exercise-induced hippocampal neurogenesis and antidepressant effects. Mol Psychiatry 20: 1428–1437. [DOI] [PubMed] [Google Scholar]
- Korhonen T, Kujala UM, Rose RJ, Kaprio J. 2009. Physical activity in adolescence as a predictor of alcohol and illicit drug use in early adulthood: A longitudinal population-based twin study. Twin Res Hum Genet 12: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus RM, Stallings HW III, Yeager RC, Gavin TP. 2004. Circulating plasma VEGF response to exercise in sedentary and endurance-trained men. J Appl Physiol (1985) 96: 1445–1450. [DOI] [PubMed] [Google Scholar]
- Krogh J, Nordentoft M, Sterne JA, Lawlor DA. 2011. The effect of exercise in clinically depressed adults: Systematic review and meta-analysis of randomized controlled trials. J Clin Psychiatry 72: 529–538. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G. 2003. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol 467: 455–463. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. 2006. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging 27: 1505–1513. [DOI] [PubMed] [Google Scholar]
- Kropff E, Carmichael JE, Moser M-B, Moser EI. 2015. Speed cells in the medial entorhinal cortex. Nature 523: 419–424. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. 1996. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci 16: 2027–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Eisch AJ, Spalding K, Peterson DA. 2016. Detection and phenotypic characterization of adult neurogenesis. Cold Spring Harb Perspect Biol 8: a025981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffel L. 1999. Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev 15: 412–426. [DOI] [PubMed] [Google Scholar]
- Lange-Asschenfeldt C, Kojda G. 2008. Alzheimer’s disease, cerebrovascular dysfunction and the benefits of exercise: From vessels to neurons. Exp Gerontol 43: 499–504. [DOI] [PubMed] [Google Scholar]
- Lautenschlager NT, Cox KL, Flicker L, Foster JK, Bockxmeer FM Van, Xiao J, Greenop K, Almeida O, Lautenschlager NT, Cox KL, et al. 2008. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease. JAMA 300: 1027–1037. [DOI] [PubMed] [Google Scholar]
- Lee CD, Folsom AR, Blair SN. 2003. Physical activity and stroke risk: A meta-analysis. Stroke 34: 2475–2481. [DOI] [PubMed] [Google Scholar]
- Lee H, Ohno M, Ohta S, Mikami T. 2013. Regular moderate or intense exercise prevents depression-like behavior without change of hippocampal tryptophan content in chronically tryptophan-deficient and stressed mice. PLoS ONE 8: e66996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenk U, Hanke R, Thiele H, Speer A. 1993. Point mutations at the carboxy terminus of the human dystrophin gene: Implications for an association with mental retardation in DMD patients. Hum Mol Genet 2: 1877–1881. [DOI] [PubMed] [Google Scholar]
- Le Page C, Ferry A, Rieu M. 1994. Effect of muscular exercise on chronic relapsing experimental autoimmune encephalomyelitis. J Appl Physiol 77: 2341–2347. [DOI] [PubMed] [Google Scholar]
- Le Page C, Bourdoulous S, Béraud E, Couraud PO, Rieu M, Ferry A. 1996. Effect of physical exercise on adoptive experimental auto-immune encephalomyelitis in rats. Eur J Appl Physiol Occup Physiol 73: 130–135. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser M, Moser EI, Moser I. 2007. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 315: 961–966. [DOI] [PubMed] [Google Scholar]
- Levinson LJ, Reid G. 1993. The effects of exercise intensity on the stereotypic behaviors of individuals with autism. Adapt Phys Act Q 10: 255–268. [Google Scholar]
- Li J, Ding YH, Rafols JA, Lai Q, McAllister JP, Ding Y. 2005. Increased astrocyte proliferation in rats after running exercise. Neurosci Lett 386: 160–164. [DOI] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. 2008. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron 59: 399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht T, Goshen I, Avital A, Kreisel T, Zubedat S, Eavri R, Segal M, Yirmiya R, Keshet E. 2011. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci 108: 5081–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht T, Rothe G, Kreisel T, Wolf B, Benny O, Rooney AG, Ffrench-Constant C, Enikolopov G, Keshet E. 2016. VEGF preconditioning leads to stem cell remodeling and attenuates age-related decay of adult hippocampal neurogenesis. Proc Natl Acad Sci 113: E7828–E7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Chesser AS, Grima JC, Rappold PM, Blum D, Przedborski S, Tieu K. 2011. D- β -hydroxybutyrate is protective in mouse models of Huntington’s disease. PLoS ONE 6: e24620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HL, Zhao G, Cai K, Zhao HH, Shi LD. 2011. Treadmill exercise prevents decline in spatial learning and memory in APP/PS1 transgenic mice through improvement of hippocampal long-term potentiation. Behav Brain Res 218: 308–314. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez C, LeRoith D, Torres-Aleman I. 2004. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci 101: 9833–9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louissaint A Jr, Rao S, Leventhal C, Goldman SA. 2002. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron 34: 945–960. [DOI] [PubMed] [Google Scholar]
- Lu J, Xu Y, Hu W, Gao Y, Ni X, Sheng H, Liu Y. 2014. Exercise ameliorates depression-like behavior and increases hippocampal BDNF level in ovariectomized rats. Neurosci Lett 573: 13–18. [DOI] [PubMed] [Google Scholar]
- Maass A, Duzel S, Goerke M, Becke A, Sobieray U, Neumann K, Lovden M, Lindenberger U, Backman L, Braun-Dullaeus R, et al. 2015. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol Psychiatry 20: 585–593. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Mooney M, Sonntag WE. 1998. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience 87: 559–569. [DOI] [PubMed] [Google Scholar]
- Marlatt MW, Potter MC, Lucassen PJ, van Praag H. 2012. Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Dev Neurobiol 72: 943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt MW, Potter MC, Bayer TA, van Praag H, Lucassen PJ. 2013. Prolonged running, not fluoxetine treatment, increases neurogenesis, but does not alter neuropathology, in the 3xTg mouse model of Alzheimer’s disease. Curr Top Behav Neurosci 15: 313–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marosi K, Kim SW, Moehl K, Scheibye-Knudsen M, Cheng A, Cutler R, Camandola S, Mattson MP. 2016. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J Neurochem 139: 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Ogushi F, Ogawa K, Katunuma N. 1986. Structure and properties of albumin Tokushima and its proteolytic processing by cathepsin B in vitro. J Biochem 100: 375–379. [DOI] [PubMed] [Google Scholar]
- McAuley JW, Long L, Heise J, Kirby T, Buckworth J, Pitt C, Lehman KJ, Moore JL, Reeves AL. 2001. A prospective evaluation of the effects of a 12-week outpatient exercise program on clinical and behavioral outcomes in patients with epilepsy. Epilepsy Behav 2: 592–600. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. 2007. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 317: 94–99. [DOI] [PubMed] [Google Scholar]
- Mika A, Bouchet CA, Bunker P, Hellwinkel JE, Spence KG, Day HEW, Campeau S, Fleshner M, Greenwood BN. 2015. Voluntary exercise during extinction of auditory fear conditioning reduces the relapse of fear associated with potentiated activity of striatal direct pathway neurons. Neurobiol Learn Mem 125: 224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklič Š, Jurič DM, Čaman-Kržan M. 2004. Differences in the regulation of BDNF and NGF synthesis in cultured neonatal rat astrocytes. Int J Dev Neurosci 22: 119–130. [DOI] [PubMed] [Google Scholar]
- Miladi-Gorji H, Rashidy-Pour A, Fathollahi Y, Semnanian S, Jadidi M. 2014. Effects of voluntary exercise on hippocampal long-term potentiation in morphine-dependent rats. Neuroscience 256: 83–90. [DOI] [PubMed] [Google Scholar]
- Mitchell KS, Dick AM, Dimartino DM, Smith BN, Niles B, Koenen KC, Street A. 2014. A pilot study of a randomized controlled trial of yoga as an intervention for PTSD symptoms in women. J Trauma Stress 27: 121–128. [DOI] [PubMed] [Google Scholar]
- Mokhtari-Zaer A, Ghodrati-Jaldbakhan S, Vafaei AA, Miladi-Gorji H, Akhavan MM, Bandegi AR, Rashidy-Pour A. 2014. Effects of voluntary and treadmill exercise on spontaneous withdrawal signs, cognitive deficits and alterations in apoptosis-associated proteins in morphine-dependent rats. Behav Brain Res 271: 160–170. [DOI] [PubMed] [Google Scholar]
- Moon HY, Kim SH, Yang YR, Song P, Yu HS, Park HG, Hwang O, Lee-Kwon W, Seo JK, Hwang D, et al. 2012. Macrophage migration inhibitory factor mediates the antidepressant actions of voluntary exercise. Proc Natl Acad Sci 109: 13094–13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon HY, Becke A, Berron D, Mattison JA, Duzel E, Van Praag H, Moon HY, Becke A, Berron D, Becker B, et al. 2016. Running-induced systemic cathepsin B secretion is associated with memory function. Cell Metab 24: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SA, Hallsworth K, Jakovljevic DG, Blamire AM, He J, Ford GA, Rochester L, Trenell MI. 2014. Effects of community exercise therapy on metabolic, brain, physical, and cognitive function following stroke: A randomized controlled pilot trial. Neurorehabil Neural Repair 29: 623–635. [DOI] [PubMed] [Google Scholar]
- Moschen AR, Wieser V, Tilg H. 2012. Adiponectin: Key player in the adipose tissue-liver crosstalk. Curr Med Chem 19: 5467–5473. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. 1995. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci 92: 9697–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses AC, Young SC, Morrow LA, O’Brien M, Clemmons DR. 1996. Recombinant human insulin-like growth factor I increases insulin sensitivity and improves glycemic control in type II diabetes. Diabetes 45: 91–100. [DOI] [PubMed] [Google Scholar]
- Motaghinejad M, Ghaleni MA, Motaghinejad O. 2014. Preventive effects of forced exercise against alcohol-induced physical dependency and reduction of pain perception threshold. Int J Prev Med 5: 1299–1307. [PMC free article] [PubMed] [Google Scholar]
- Motaghinejad M, Bangash MY asan, Motaghinejad O. 2015. Attenuation of alcohol withdrawal syndrome and blood cortisol level with forced exercise in comparison with diazepam. Acta Med Iran 53: 311–316. [PubMed] [Google Scholar]
- Motl RW, Pilutti LA. 2012. The benefits of exercise training in multiple sclerosis. Nat Rev Neurol 8: 487–497. [DOI] [PubMed] [Google Scholar]
- Motl RW, Snook EM. 2008. Physical activity, self-efficacy, and quality of life in multiple sclerosis. Ann Behav Med 35: 111–115. [DOI] [PubMed] [Google Scholar]
- Motl RW, Snook EM, Wynn DR, Vollmer T. 2008. Physical activity correlates with neurological impairment and disability in multiple sclerosis. J Nerv Ment Dis 196: 492–495. [DOI] [PubMed] [Google Scholar]
- Movahedi A, Bahrami F, Marandi SM, Abedi A. 2013. Improvement in social dysfunction of children with autism spectrum disorder following long term Kata techniques training. Res Autism Spectr Disord 7: 1054–1161. [Google Scholar]
- Mueller-Steiner S, Zhou Y, Arai H, Roberson ED, Sun B, Chen J, Wang X, Yu G, Esposito L, Mucke L, et al. 2006. Antiamyloidogenic and neuroprotective functions of Cathepsin B: Implications for Alzheimer’s disease. Neuron 51: 703–714. [DOI] [PubMed] [Google Scholar]
- Murrell CJ, Cotter JD, Thomas KN, Lucas SJE, Williams MJA, Ainslie PN. 2013. Cerebral blood flow and cerebrovascular reactivity at rest and during sub-maximal exercise: Effect of age and 12-week exercise training. Age (Omaha) 35: 905–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychasiuk R, Hehar H, Ma I, Candy S, Esser MJ. 2016. Reducing the time interval between concussion and voluntary exercise restores motor impairment, short-term memory, and alterations to gene expression. Eur J Neurosci 44: 2407–2417. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Uchida S, Suzuki A, Hotta H, Aikawa Y. 2003. The effect of walking on regional blood flow and acetylcholine in the hippocampus in conscious rats. Auton Neurosci Basic Clin 103: 83–92. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, et al. 2002. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 297: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. 2003. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron 38: 305–315. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. 2004. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci 5: 361–372. [DOI] [PubMed] [Google Scholar]
- Nakken KO, Bjørholt PG, Johannessen SI, Løyning T LE. 1990. Effect of physical training on aerobic capacity, seizure occurrence, and serum level of antiepileptic drugs in adults with epilepsy. Epilepsia 31: 88–94. [DOI] [PubMed] [Google Scholar]
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, et al. 2008. AMPK and PPARδ agonists are exercise mimetics. Cell 134: 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento CMC, Ayan C, Cancela JM, Gobbi LTB, Gobbi S, Stella F. 2014. Effect of a multimodal exercise program on sleep disturbances and instrumental activities of daily living performance on Parkinson’s and Alzheimer’s disease patients. Geriatr Gerontol Int 14: 259–266. [DOI] [PubMed] [Google Scholar]
- Neeper S, Gomez-Pinilla F, Choi J, Cotman C. 1995. Exercise and brain neurotrophins. Nature 373: 109. [DOI] [PubMed] [Google Scholar]
- Neeper SA, GomezPinilla F, Choi J, Cotman CW. 1996. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res 726: 49–56. [PubMed] [Google Scholar]
- Newman JC, Verdin E. 2014. β-hydroxybutyrate: Much more than a metabolite. Diabetes Res Clin Pract 106: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson H, Kehle TJ, Bray MA, Van Heest J. 2011. The effects of antecedent physical activity on the academic engagement of children with autism spectrum disorder. Psychol Sch 48: 198–213. [Google Scholar]
- Nishijima T, Soya H. 2006. Evidence of functional hyperemia in the rat hippocampus during mild treadmill running. Neurosci Res 54: 186–191. [DOI] [PubMed] [Google Scholar]
- Nishijima T, Okamoto M, Matsui T, Kita I, Soya H. 2012. Hippocampal functional hyperemia mediated by NMDA receptor/NO signaling in rats during mild exercise. J Appl Physiol 112: 197–203. [DOI] [PubMed] [Google Scholar]
- Nishijima T, Torres-Aleman I, Soya H. 2016. Exercise and cerebrovascular plasticity. Prog Brain Res 225: 243–268. [DOI] [PubMed] [Google Scholar]
- Nybo L, Nielsen B, Pedersen BK, Møller K, Secher NH. 2002. Interleukin-6 release from the human brain during prolonged exercise. J Physiol 542: 991–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan RM, Griffin EEW, Kelly AM. 2009. Long-term treadmill exposure protects against age-related neurodegenerative change in the rat hippocampus. Hippocampus 19: 1019–1029. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Ainslie PN. 2009. Cerebral blood flow during exercise: Mechanisms of regulation. J Appl Physiol 107: 1370–1380. [DOI] [PubMed] [Google Scholar]
- Oldridge N, Guyatt G, Jones N, Crowe J, Singer J, Feeny D, McKelvie R, Runions J, Streiner D, Torrance G. 1991. Effects on quality of life with comprehensive rehabilitation after acute myocardial infarction. Am J Cardiol 67: 1084–1089. [DOI] [PubMed] [Google Scholar]
- Oldridge N, Streiner D, Hoffmann R, Guyatt G. 1995. Profile of mood states and cardiac rehabilitation after acute myocardial infarction. Med Sci Sports Exerc 27: 900–905. [PubMed] [Google Scholar]
- Orenstein M, Friedenreich C. 2004. Review of physical activity and the IGF family. J Phys Act Heal 1: 291–320. [Google Scholar]
- Oriel KN, George CL, Peckus R, Semon A. 2011. The effects of aerobic exercise on academic engagement in young children with autism spectrum disorder. Pediatr Phys Ther 10: 187–193. [DOI] [PubMed] [Google Scholar]
- Orr G, Rao G, Houston FP, McNaughton BL, Barnes CA. 2001. Hippocampal synaptic plasticity is modulated by theta rhythm in the fascia dentata of adult and aged freely behaving rats. Hippocampus 11: 647–654. [DOI] [PubMed] [Google Scholar]
- Osborne PG. 1997. Hippocampal and striatal blood flow during behavior in rats: Chronic laser Doppler flowmetry study. Physiol Behav 61: 485–492. [DOI] [PubMed] [Google Scholar]
- Otsuka A, Shiuchi T, Chikahisa S, Shimizu N, Séi H. 2015. Voluntary exercise and increased food intake after mild chronic stress improve social avoidance behavior in mice. Physiol Behav 151: 264–271. [DOI] [PubMed] [Google Scholar]
- Pajonk F-G, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, Kierer A, Müller S, Oest M, Meyer T, et al. 2010. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry 67: 133–143. [DOI] [PubMed] [Google Scholar]
- Pan CY. 2010. Effects of water exercise swimming program on aquatic skills and social behaviors in children with autism spectrum disorders. Autism 14: 9–28. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. 2000. Interactions of IGF-1 with the blood–brain barrier in vivo and in situ. Neuroendocrinology 72: 171–178. [DOI] [PubMed] [Google Scholar]
- Pang TYC, Stam NC, Nithianantharajah J, Howard ML, Hannan AJ. 2006. Differential effects of voluntary physical exercise on behavioral and brain-derived neurotrophic factor expression deficits in Huntington’s disease transgenic mice. Neuroscience 141: 569–584. [DOI] [PubMed] [Google Scholar]
- Pan-Vazquez A, Rye N, Ameri M, McSparron B, Smallwood G, Bickerdyke J, Rathbone A, Dajas-Bailador F, Toledo-Rodriguez M. 2015. Impact of voluntary exercise and housing conditions on hippocampal glucocorticoid receptor, miR-124 and anxiety. Mol Brain 8: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. 1993. Transport of insulin-related peptides and glucose across the blood–brain barrier. Ann NY Acad Sci 692: 126–137. [DOI] [PubMed] [Google Scholar]
- Park YJ. 2014. Effects of communal exercise with visual and auditory feedback provided by a smart application on gait ability and fear of falling in Parkinson’s disease patients. J Exerc Rehabil 10: 286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JK, Lee SJ, Kim TW. 2014. Treadmill exercise enhances NMDA receptor expression in schizophrenia mice. J Exerc Rehabil 10: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DI, White LJ. 2013. Effect of 10-day forced treadmill training on neurotrophic factors in experimental autoimmune encephalomyelitis. Appl Physiol Nutr Metab 38: 194–199. [DOI] [PubMed] [Google Scholar]
- Patki G, Li L, Allam F, Solanki N, Dao AT, Alkadhi K, Salim S. 2014. Moderate treadmill exercise rescues anxiety and depression-like behavior as well as memory impairment in a rat model of posttraumatic stress disorder. Physiol Behav 130: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten AR, Yau SY, Fontaine CJ, Meconi A, Wortman RC, Christie BR. 2015. The benefits of exercise on structural and functional plasticity in the rodent hippocampus of different disease models. Brain Plast 1: 97–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Greenstein YJ, Grudman M, Winson J. 1988. Long-term potentiation in the dentate gyrus is induced preferentially on the positive phase of theta-rhythm. Brain Res 439: 383–387. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. 2008. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol Rev 88: 1379–1406. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. 2012. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat Rev Endocrinol 8: 457–465. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Hoffman-Goetz L. 2000. Exercise and the immune system: Regulation, integration, and adaptation. Physiol Rev 80: 1055–1081. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Steensberg A, Fischer C, Keller C, Ostrowski K, Schjerling P. 2001. Exercise and cytokines with particular focus on muscle-derived IL-6. Exerc Immunol Rev 7: 18–31. [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Small SA, Brown TR. 2007. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci 104: 5638–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatti VC, Davies-Sala MG, Espósito MS, Mongiat LA, Trinchero MF, Schinder AF. 2011. The timing for neuronal maturation in the adult hippocampus is modulated by local network activity. J Neurosci 31: 7715–7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilegaard H, Saltin B, Neufer PD. 2003. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol 546: 851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploughman M, Granter-Button S, Chernenko G, Tucker BA, Mearow KM, Corbett D. 2005. Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-I and insulin-like growth factor I after focal ischemia. Neuroscience 136: 991–1001. [DOI] [PubMed] [Google Scholar]
- Potter MC, Yuan C, Ottenritter C, Mughal M, van Praag H. 2010. Exercise is not beneficial and may accelerate symptom onset in a mouse model of Huntington’s disease. PLoS Curr 10.1371/currents.RRN1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MB, Medina JL, Burns S, Kauffman BY, Monfils M, Asmundson GJG, Diamond A, McIntyre C, Smits JAJ. 2015. Exercise augmentation of exposure therapy for PTSD: Rationale and pilot efficacy data. Cogn Behav Ther 44: 314–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior BM, Lloyd PG, Yang HT, Terjung RL. 2003. Exercise-induced vascular remodeling. Exerc Sport Sci Rev 31: 26–33. [DOI] [PubMed] [Google Scholar]
- Prupas A, Reid G. 2001. Effects of exercise frequency on stereotypic behaviors of children with developmental disabilities. Educ Train Ment Retard Dev Disabil 36: 196–206. [Google Scholar]
- Pryor WM, Freeman KG, Larson RD, Edwards GL, White LJ. 2015. Chronic exercise confers neuroprotection in experimental autoimmune encephalomyelitis. J Neurosci Res 93: 697–706. [DOI] [PubMed] [Google Scholar]
- Quaney BM, Boyd LA, McDowd JM, Zahner LH, He J, Mayo MS, Macko RF. 2009. Aerobic exercise improves cognition and motor function poststroke. Neurorehabil Neural Repair 23: 879–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querido JS, Sheel AW. 2007. Regulation of cerebral blood flow during exercise. Sport Med 37: 765–782. [DOI] [PubMed] [Google Scholar]
- Quinn PS, Judah JD. 1978. Calcium-dependent Golgi-vesicle fusion and cathepsin B in the conversion of proalbumin into albumin in rat liver. Biochem J 172: 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji CA, Lopez OL, Kuller LH, Carmichael OT, Becker JT. 2009. Age, Alzheimer disease, and brain structure. Neurology 73: 1899–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambo LM, Ribeiro LR, Oliveira MS, Furian AF, Lima FD, Souza MA, Silva LFA, Retamoso LT, Corte CLD, Puntel GO, et al. 2009. Additive anticonvulsant effects of creatine supplementation and physical exercise against pentylenetetrazol-induced seizures. Neurochem Int 55: 333–340. [DOI] [PubMed] [Google Scholar]
- Rand D, Eng JJ, Liu-Ambrose T, Tawashy AE. 2010. Feasibility of a 6-month exercise and recreation program to improve executive functioning and memory in individuals with chronic stroke. Neurorehabil Neural Repair 24: 722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen P, Vedel JC, Olesen J, Adser H, Pedersen MV, Hart E, Secher NH, Pilegaard H. 2011. In humans IL-6 is released from the brain during and after exercise and paralleled by enhanced IL-6 mRNA expression in the hippocampus of mice. Acta Physiol (Oxf) 201: 475–482. [DOI] [PubMed] [Google Scholar]
- Reiss JI, Dishman RK, Boyd HE, Robinson JK, Holmes PV. 2009. Chronic activity wheel running reduces the severity of kainic acid-induced seizures in the rat: Possible role of galanin. Brain Res 1266: 54–63. [DOI] [PubMed] [Google Scholar]
- Renoir T, Pang TYC, Zajac MS, Chan G, Du X, Leang L, Chevarin C, Lanfumey L, Hannan AJ. 2012. Treatment of depressive-like behaviour in Huntington’s disease mice by chronic sertraline and exercise. Br J Pharmacol 165: 1375–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethorst CD, Wipfli BM, Landers DM. 2009. The antidepressive effects of exercise. Sport Med 39: 491–511. [DOI] [PubMed] [Google Scholar]
- Riedel G, Micheau J, Lam AG, Roloff EL, Martin SJ, Bridge H, de Hoz L, Poeschel B, McCulloch J, Morris RG. 1999. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat Neurosci 2: 898–905. [DOI] [PubMed] [Google Scholar]
- Rodríguez JJ, Noristani HN, Olabarria M, Fletcher J, Somerville TD, Yeh CY, Verkhratsky A. 2011. Voluntary running and environmental enrichment restores impaired hippocampal neurogenesis in a triple transgenic mouse model of Alzheimer’s disease. Curr Alzheimer Res 8: 707–717. [DOI] [PubMed] [Google Scholar]
- Rosenbaum S, Sherrington C, Tiedemann A. 2015a. Exercise augmentation compared with usual care for post-traumatic stress disorder: A randomized controlled trial. Acta Psychiatr Scand 131: 350–359. [DOI] [PubMed] [Google Scholar]
- Rosenbaum S, Vancampfort D, Steel Z, Newby J, Ward PB, Stubbs B. 2015b. Physical activity in the treatment of post-traumatic stress disorder: A systematic review and meta-analysis. Psychiatry Res 230: 130–136. [DOI] [PubMed] [Google Scholar]
- Rosenblatt LE, Gorantla S, Torres JA, Yarmush RS, Rao S, Park ER, Denninger JW, Benson H, Fricchione GL, Bernstein B, et al. 2011. Relaxation response–based yoga improves functioning in young children with autism: A pilot study. J Altern Complement Med 17: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal-Malek A, Mitchell S. 1997. The effects of exercise on the self-stimulatory behaviors and positive responding of adolescents with autism. J Autism Dev Disord 27: 193–202. [DOI] [PubMed] [Google Scholar]
- Rossi S, Furlan R, De Chiara V, Musella A, Lo Giudice T, Mataluni G, Cavasinni F, Cantarella C, Bernardi G, Muzio L, et al. 2009. Exercise attenuates the clinical, synaptic and dendritic abnormalities of experimental autoimmune encephalomyelitis. Neurobiol Dis 36: 51–59. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt A, Beard RC, Cotman CW. 1999. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology 21: 679–682. [DOI] [PubMed] [Google Scholar]
- Sadeghi K, Ahmadi SM, Ahmadi SM, Rezaei M, Miri J, Abdi A, Khamoushi F, Salehi M, Jamshidi K. 2016a. A comparative study of the efficacy of cognitive group therapy and aerobic exercise in the treatment of depression among the students. Glob J Health Sci 8: 84171. [DOI] [PubMed] [Google Scholar]
- Sadeghi M, Peeri M, Hosseini MJ. 2016b. Adolescent voluntary exercise attenuated hippocampal innate immunity responses and depressive-like behaviors following maternal separation stress in male rats. Physiol Behav 163: 177–183. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. 2011. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472: 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarder M, Abe K, Saito H, Nishiyama N. 1996. Comparative effect of IL-2 and IL-6 on morphology of cultured hippocampal neurons from fetal rat brain. Brain Res 715: 9–16. [DOI] [PubMed] [Google Scholar]
- Saur L, Baptista PPA, De Senna PN, Paim MF, Nascimento P Do, Ilha J, Bagatini PB, Achaval M, Xavier LL. 2014. Physical exercise increases GFAP expression and induces morphological changes in hippocampal astrocytes. Brain Struct Funct 219: 293–302. [DOI] [PubMed] [Google Scholar]
- Scheewe TW, Takken T, Kahn RS, Cahn W, Backx FJG. 2012. Effects of exercise therapy on cardiorespiratory fitness in patients with schizophrenia. Med Sci Sports Exerc 44: 1834–1842. [DOI] [PubMed] [Google Scholar]
- Scheewe TW, Backx FJG, Takken T, Jörg F, van Strater ACP, Kroes AG, Kahn RS, Cahn W. 2013a. Exercise therapy improves mental and physical health in schizophrenia: A randomised controlled trial. Acta Psychiatr Scand 127: 464–473. [DOI] [PubMed] [Google Scholar]
- Scheewe TW, van Haren NEM, Sarkisyan G, Schnack HG, Brouwer RM, de Glint M, Hulshoff Pol HE, Backx FJG, Kahn RS, Cahn W. 2013b. Exercise therapy, cardiorespiratory fitness and their effect on brain volumes: A randomised controlled trial in patients with schizophrenia and healthy controls. Eur Neuropsychopharmacol 23: 675–685. [DOI] [PubMed] [Google Scholar]
- Scheinfeld A. 1950. The new you and heredity. Lippincott, Philadelphia. [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. 2004. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429: 184–187. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TJ, Rada P, Pieruzzini PR, Hsueh B, Gould E. 2013. Physical exercise prevents stress-induced activation of granule neurons and enhances local inhibitory mechanisms in the dentate gyrus. J Neurosci 33: 7770–7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. 2000. Loss of recent memory after bilateral hippocampal lesions. J Neuropsychiatry Clin Neurosci 12: 103–113. [DOI] [PubMed] [Google Scholar]
- Secher NH, Seifert T, Van Lieshout JJ. 2008. Cerebral blood flow and metabolism during exercise: Implications for fatigue. J Appl Physiol 104: 306–314. [DOI] [PubMed] [Google Scholar]
- Sen S, Nesse RM, Stoltenberg SF, Li S, Gleiberman L, Chakravarti A, Weder AB, Burmeister M. 2003. A BDNF coding variant is associated with the NEO personality inventory domain neuroticism, a risk factor for depression. Neuropsychopharmacology 28: 397–401. [DOI] [PubMed] [Google Scholar]
- Seo TB, Cho HS, Shin MS, Kim CJ, Ji ES, Baek SS. 2013. Treadmill exercise improves behavioral outcomes and spatial learning memory through up-regulation of reelin signaling pathway in autistic rats. J Exerc Rehabil 9: 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setkowicz Z, Kosonowska E, Kaczyńska M, Gzieło-Jurek K, Janeczko K. 2016. Physical training decreases susceptibility to pilocarpine-induced seizures in the injured rat brain. Brain Res 1642: 20–32. [DOI] [PubMed] [Google Scholar]
- Sforzo GA. 1989. Opioids and exercise: An update. Sport Med 7: 109–124. [DOI] [PubMed] [Google Scholar]
- Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, et al. 2013. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339: 211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman LM, Katzel LI, Ivey FM, Sorkin JD, Favors K, Anderson KE, Smith BA, Reich SG, Weiner WJ, Macko RF. 2013. Randomized clinical trial of 3 types of physical exercise for patients with Parkinson disease. JAMA Neurol 70: 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira H, Moraes H, Oliveira N, Coutinho ESF, Laks J, Deslandes A. 2013. Physical exercise and clinically depressed patients: A systematic review and meta-analysis. Neuropsychobiology 67: 61–68. [DOI] [PubMed] [Google Scholar]
- Sim YJ, Kim SS, Kim JY, Shin MS, Kim CJ. 2004. Treadmill exercise improves short-term memory by suppressing ischemia-induced apoptosis of neuronal cells in gerbils. Neurosci Lett 372: 256–261. [DOI] [PubMed] [Google Scholar]
- Sim YJ, Kim H, Kim JY, Yoon SJ, Kim SS, Chang HK, Lee TH, Lee HH, Shin MC, Shin MS, et al. 2005. Long-term treadmill exercise overcomes ischemia-induced apoptotic neuronal cell death in gerbils. Physiol Behav 84: 733–738. [DOI] [PubMed] [Google Scholar]
- Sinyor D, Brown T, Rostant L, Seraganian P. 1982. The role of a physical fitness program in the treatment of alcoholism. J Stud Alcohol 43: 380–386. [DOI] [PubMed] [Google Scholar]
- Sleiman SF, Henry J, Al-Haddad R, El Hayek L, Abou Haidar E, Stringer T, Ulja D, Karuppagounder SS, Holson EB, Ratan RR, et al. 2016. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. eLife 5: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Schmidt KT, Iordanou JC, Mustroph ML. 2008. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend 98: 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JAJ, Berry AC, Rosenfield D, Powers MB, Behar E, Otto MW. 2008. Reducing anxiety sensitivity with exercise. Depress Anxiety 25: 689–699. [DOI] [PubMed] [Google Scholar]
- Sobieraj JC, Kim A, Fannon MKJ, Mandyam CD. 2016. Chronic wheel running-induced reduction of extinction and reinstatement of methamphetamine seeking in methamphetamine dependent rats is associated with reduced number of periaqueductal gray dopamine neurons. Brain Struct Funct 221: 261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, et al. 2010. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science 327: 863–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza P, Goncalves E, Pedroso G, Farias H, Junqueira S, Marcon R, Tuon T, Cola M, Silveira P, Santos A, et al. 2016. Physical exercise attenuates experimental autoimmune encephalomyelitis by inhibiting peripheral immune response and blood–brain barrier disruption. Mol Neurobiol 10.1007/s12035-016-0014-0. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, et al. 2013. Dynamics of hippocampal neurogenesis in adult humans. Cell 153: 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speisman RB, Kumar A, Rani A, Foster TC, Ormerod BK. 2013. Daily exercise improves memory, stimulates hippocampal neurogenesis and modulates immune and neuroimmune cytokines in aging rats. Brain Behav Immun 28: 25–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. 1992. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol Rev 99: 195–231. [DOI] [PubMed] [Google Scholar]
- Stathopoulou G, Powers MB, Berry AC, Smits JAJ, Otto MW. 2006. Exercise interventions for mental health: A quantitative and qualitative review. Clin Psychol Sci Pract 13: 179–193. [Google Scholar]
- Stefan N, Häring HU. 2013. The role of hepatokines in metabolism. Nat Rev Endocrinol 9: 144–152. [DOI] [PubMed] [Google Scholar]
- Steib K, Scha I, Jagasia R, Ebert B, Lie DC. 2014. Mitochondria modify exercise-induced development of stem cell-derived neurons in the adult brain. J Neurosci 34: 6624–6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner DF, Docherty K, Carroll R. 1984. Golgi/granule processing of peptide hormone and neuropeptide precursors: A minireview. J Cell Biochem 24: 121–130. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. 2007. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus 17: 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stummer W, Weber K, Tranmer B, Baethmann a, Kempski O. 1994. Reduced mortality and brain damage after locomotor activity in gerbil forebrain ischemia. Stroke 25: 1862–1869. [DOI] [PubMed] [Google Scholar]
- Suh H, Deng W, Gage FH. 2009. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol 25: 253–275. [DOI] [PubMed] [Google Scholar]
- Sultan S, Li L, Moss J, Petrelli F, Cassé F, Gebara E, Lopatar J, Pfrieger FW, Bezzi P, Bischofberger J, et al. 2015. Synaptic integration of adult-born hippocampal neurons is locally controlled by astrocytes. Neuron 88: 957–972. [DOI] [PubMed] [Google Scholar]
- Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Engberg K, Lauterbur PC, Greenough WT. 2003. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience 117: 1037–1046. [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. 1999. Genetic enhancement of learning and memory in mice. Nature 401: 63–69. [DOI] [PubMed] [Google Scholar]
- Tang YP, Wang H, Feng R, Kyin M, Tsien JZ. 2001. Differential effects of enrichment on learning and memory function in NR2B transgenic mice. Neuropharmacology 41: 779–790. [DOI] [PubMed] [Google Scholar]
- Tantimonaco M, Ceci R, Sabatini S, Catani MV, Rossi A, Gasperi V, Maccarrone M. 2014. Physical activity and the endocannabinoid system: An overview. Cell Mol Life Sci 71: 2681–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarumi T, Zhang R. 2014. Cerebral hemodynamics of the aging brain: Risk of Alzheimer disease and benefit of aerobic exercise. Front Physiol 5: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. 2006. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature 442: 929–933. [DOI] [PubMed] [Google Scholar]
- Telenius EW, Engedal K, Bergland A. 2015a. Effect of a high-intensity exercise program on physical function and mental health in nursing home residents with dementia: An assessor blinded randomized controlled trial. PLoS ONE 10: e0126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telenius EW, Engedal K, Bergland A. 2015b. Long-term effects of a 12 weeks high-intensity functional exercise program on physical function and mental health in nursing home residents with dementia: A single blinded randomized controlled trial. BMC Geriatr 15: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Brinke LF, Bolandzadeh N, Nagamatsu LS, Hsu CL, Davis JC, Miran-Khan K, Liu-Ambrose T. 2015. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: A 6-month randomised controlled trial. Br J Sports Med 49: 248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry-McElrath YM, O’Malley PM. 2011. Substance use and exercise participation among young adults: Parallel trajectories in a national cohort-sequential study. Addiction 106: 1855–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teufel A, Malik N, Mukhopadhyay M, Westphal H. 2002. Frcp1 and Frcp2, two novel fibronectin type III repeat containing genes. Gene 297: 79–83. [DOI] [PubMed] [Google Scholar]
- Thinschmidt JS, Kinney GG, Kocsis B. 1995. The supramammillary nucleus: Is it necessary for the mediation of hippocampal theta rhythm? Neuroscience 67: 301–312. [DOI] [PubMed] [Google Scholar]
- Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan S Du, Naini A, Vila M, Jackson-Lewis V, Ramasamy R, et al. 2003. d-β-Hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest 112: 892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson CL, Patel S, Meek C, Herd CP, Clarke CE, Stowe R, Shah L, Sackley CM, Deane KHO, Wheatley K, et al. 2013. Physiotherapy versus placebo or no intervention in Parkinson’s disease. Cochrane Database Syst Rev 9: CD002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. 2001. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci 21: 1628–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutkun E, Ayyildiz M, Agar E. 2010. Short-duration swimming exercise decreases penicillin-induced epileptiform ECoG activity in rats. Acta Neurobiol Exp (Wars) 70: 382–389. [DOI] [PubMed] [Google Scholar]
- Uc EY, Doerschug KC, Magnotta V, Dawson JD, Thomsen TR, Kline JN, Rizzo M, Newman SR, Mehta S, Grabowski TJ, et al. 2014. Phase I/II randomized trial of aerobic exercise in Parkinson disease in a community setting. Neurology 83: 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo H, Yoshida Y, Kino T, Ohnuki K, Mizunoya W, Mukuda T, Sugiyama H. 2008. Enhanced adult neurogenesis and angiogenesis and altered affective behaviors in mice overexpressing vascular endothelial growth factor 120. J Neurosci 28: 14522–14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillend C, Rendon A, Misslin R, Ungerer A. 1995. Influence of dystrophin-gene mutation on mdx mouse behavior. I: Retention deficits at long delays in spontaneous alternation and bar-pressing tasks. Behav Genet 25: 569–579. [DOI] [PubMed] [Google Scholar]
- Vallières L, Campbell IL, Gage FH, Sawchenko PE. 2002. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci 22: 486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancampfort D, Probst M, De Hert M, Soundy A, Stubbs B, Stroobants M, De Herdt A. 2014. Neurobiological effects of physical exercise in schizophrenia: A systematic review. Disabil Rehabil 36: 1–6. [DOI] [PubMed] [Google Scholar]
- van Dellen A, Cordery PM, Spires TL, Blakemore C, Hannan AJ. 2008. Wheel running from a juvenile age delays onset of specific motor deficits but does not alter protein aggregate density in a mouse model of Huntington’s disease. BMC Neurosci 9: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Borght K, Havekes R, Bos T, Eggen BJL, Van der Zee EA. 2007. Exercise improves memory acquisition and retrieval in the Y-maze task: Relationship with hippocampal neurogenesis. Behav Neurosci 121: 324–334. [DOI] [PubMed] [Google Scholar]
- Van der Borght K, Kobor-Nyakas DE, Klauke K, Eggen BJL, Nyakas C, Van Der Zee EA, Meerlo P. 2009. Physical exercise leads to rapid adaptations in hippocampal vasculature: Temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus 19: 928–936. [DOI] [PubMed] [Google Scholar]
- Van Der Kolk BA, Stone L, West J, Rhodes A, Emerson D, Suvak M, Spinazzola J. 2014. Yoga as an adjunctive treatment for posttraumatic stress disorder: A randomized controlled trial. J Clin Psychiatry 75: e559–e565. [DOI] [PubMed] [Google Scholar]
- Van de Winckel A, Feys H, De Weerdt W, Dom R. 2004. Cognitive and behavioural effects of music-based exercises in patients with dementia. Clin Rehabil 18: 253–260. [DOI] [PubMed] [Google Scholar]
- Vann SD. 2010. Re-evaluating the role of the mammillary bodies in memory. Neuropsychologia 48: 2316–2327. [DOI] [PubMed] [Google Scholar]
- van Praag H. 2008. Neurogenesis and exercise: Past and future directions. Neuro Molecular Med 10: 128–140. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. 1999a. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci 96: 13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. 1999b. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2: 266–270. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. 2002. Functional neurogenesis in the adult hippocampus. Nature 415: 1030–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. 2005. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci 25: 8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. 1997. Differential effects of early hippocampal pathology on episodic and semantic memory. Science 277: 376–380. [DOI] [PubMed] [Google Scholar]
- Vasuta C, Caunt C, James R, Samadi S, Schibuk E, Kannangara T, Titterness AK, Christie BR. 2007. Effects of exercise on NMDA receptor subunit contributions to bidirectional synaptic plasticity in the mouse dentate gyrus. Hippocampus 17: 1201–1208. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. 2003. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience 122: 647–657. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. 2004. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci 20: 2580–2590. [DOI] [PubMed] [Google Scholar]
- Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F. 2006. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res 1070: 124–130. [DOI] [PubMed] [Google Scholar]
- Vedamurthachar A, Janakiramaiah N, Hegde JM, Shetty TK, Subbakrishna DK, Sureshbabu S V, Gangadhar BN. 2006. Antidepressant efficacy and hormonal effects of Sudarshana Kriya Yoga (SKY) in alcohol dependent individuals. J Affect Disord 94: 249–253. [DOI] [PubMed] [Google Scholar]
- Venturelli M, Scarsini R, Schena F. 2011. Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. Am J Alzheimers Dis Other Demen 26: 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen M, van der Meij A, van Deurzen PA, Janzing JGE, Arias-Vásquez A, Buitelaar JK, Franke B. 2010. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: Effects of gender and ethnicity. Mol Psychiatry 15: 260–271. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Viana Di Prisco G. 2004. Theta rhythm of the hippocampus: Subcortical control and functional significance. Behav Cogn Neurosci Rev 3: 173–200. [DOI] [PubMed] [Google Scholar]
- Viboolvorakul S, Patumraj S. 2014. Exercise training could improve age-related changes in cerebral blood flow and capillary vascularity through the upregulation of VEGF and eNOS. Biomed Res Int 2014: 230731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, et al. 2011. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477: 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar C, Potter MC, Choi J, Lee JY, Stringer TP, Callaway EM, Gage FH, Suh H, van Praag H. 2012. Monosynaptic inputs to new neurons in the dentate gyrus. Nat Commun 3: 1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar C, Potter MC, van Praag H. 2013. All about running: Synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr Top Behav Neurosci 15: 189–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar C, Peterson BD, van Praag H. 2016. Running rewires the neuronal network of adult-born dentate granule cells. Neuroimage 131: 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkers KM, Scherder EJA. 2011. The effect of regular walks on various health aspects in older people with dementia: Protocol of a randomized-controlled trial. BMC Geriatr 11: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Holstein-Rathlou S, Bondurant LD, Peltekian L, Naber MC, Yin TC, Claflin KE, Urizar AI, Madsen AN, Ratner C, Holst B, et al. 2016. FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab 23: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Vivar C, Kramer AF, van Praag H. 2013. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci 17: 525–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu V, Riddell MC, Sweeney G. 2007. Circulating adiponectin and adiponectin receptor expression in skeletal muscle: Effects of exercise. Diabetes Metab Res Rev 23: 600–611. [DOI] [PubMed] [Google Scholar]
- Vukovic J, Colditz MJ, Blackmore DG, Ruitenberg MJ, Bartlett PF. 2012. Microglia modulate hippocampal neural precursor activity in response to exercise and aging. J Neurosci 32: 6435–6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. 2002. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 8: 75–79. [DOI] [PubMed] [Google Scholar]
- Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, Scheffler B, Steindler DA. 2006. Microglia instruct subventricular zone neurogenesis. Glia 54: 815–825. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gao E, Tao L, Lau WB, Yuan Y, Goldstein BJ, Lopez BL, Christopher TA, Tian R, Koch W, et al. 2009. AMP-activated protein kinase deficiency enhances myocardial ischemia/reperfusion injury but has minimal effect on the antioxidant/antinitrative protection of adiponectin. Circulation 119: 835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Sun B, Zhou Y, Grubb A, Gan L. 2012. Cathepsin B degrades amyloid-β in mice expressing wild-type human amyloid precursor protein. J Biol Chem 287: 39834–39841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Wang Y, Wang Y, Li R, Zhou C. 2014. Impact of physical exercise on substance use disorders: A meta-analysis. PLoS ONE 9: e110728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, He F, Wang Y. 2015. Association between polymorphisms of the insulin-degrading enzyme gene and late-onset Alzheimer disease. J Geriatr Psychiatry Neurol 28: 94–98. [DOI] [PubMed] [Google Scholar]
- Ward SC, Whalon K, Rusnak K, Wendell K, Paschall N. 2013. The association between therapeutic horseback riding and the social communication and sensory reactions of children with autism. J Autism Dev Disord 43: 2190–2198. [DOI] [PubMed] [Google Scholar]
- Wedekind D, Broocks A, Weiss N, Engel K, Neubert K, Bandelow B. 2010. A randomized, controlled trial of aerobic exercise in combination with paroxetine in the treatment of panic disorder. World J Biol Psychiatry 11: 904–913. [DOI] [PubMed] [Google Scholar]
- Weinstein A, Chin L, Collins J, Goel D, Keyser R, Chan L. 2016. Effect of aerobic exercise training on mood in people with traumatic brain injury: A pilot study. J Head Trauma Rehabil 10.1097/HTR.0000000000000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman AS, Young DE, Budson AE, Stern CE, Schon K. 2016. Entorhinal volume, aerobic fitness, and recognition memory in healthy young adults: A voxel-based morphometry study. NeuroImage 126: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods KA, Fraser NC, Postel-Vinay MC, Savage MO, Clark AJL. 1996. A homozygous splice site mutation affecting the intracellular domain of the growth hormone (GH) receptor resulting in Laron syndrome with elevated GH-binding protein. J Clin Endocrinol Metab 81: 1686–1690. [DOI] [PubMed] [Google Scholar]
- Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM. 2013. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab 18: 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Iwabu M, Okada-Iwabu M, Kadowaki T. 2014. Adiponectin receptors: A review of their structure, function and how they work. Best Pract Res Clin Endocrinol Metab 28: 15–23. [DOI] [PubMed] [Google Scholar]
- Yau SY, Li A, Hoo RLC, Ching YP, Christie BR, Lee TMC, Xu A, So KF. 2014. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc Natl Acad Sci 111: 15810–15815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Yamashima T, Zhao L, Tsuchiya K, Kohda Y, Tonchev AB, Matsuda M, Kominami E. 2002. Primate neurons show different vulnerability to transient ischemia and response to cathepsin inhibition. Acta Neuropathol 104: 267–272. [DOI] [PubMed] [Google Scholar]
- Zafra F, Lindholm D, Castrén E, Hartikka J, Thoenen H. 1992. Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J Neurosci 12: 4793–4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zedlitz AMEE, Rietveld TCM, Geurts AC, Fasotti L. 2012. Cognitive and graded activity training can alleviate persistent fatigue after stroke: A randomized, controlled trial. Stroke 43: 1046–1051. [DOI] [PubMed] [Google Scholar]
- Zhang D, Guo M, Zhang W, Lu XY. 2011. Adiponectin stimulates proliferation of adult hippocampal neural stem/progenitor cells through activation of p38 mitogen-activated protein kinase (p38MAPK)/glycogen synthase kinase 3β (GSK-3β)/β-catenin signaling cascade. J Biol Chem 286: 44913–44920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Wang X, Lu X. 2016. Adiponectin exerts neuotrophic effects on dendritic arborization, spinogenesis, and neurogenesis of the dentate gyrus of male mice. Endocrinology 157: 2853–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Ming GL, Gage FH. 2006. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci 26: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. 2008. Mechanisms and functional implications of adult neurogenesis. Cell 132: 645–660. [DOI] [PubMed] [Google Scholar]
- Zhao C, Jou J, Wolff LJ, Sun H, Gage FH. 2014. Spine morphogenesis in newborn granule cells is differentially regulated in the outer and middle molecular layers. J Comp Neurol 522: 2756–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Liu HL, Zhang H, Tong XJ. 2015. Treadmill exercise enhances synaptic plasticity, but does not alter β-amyloid deposition in hippocampi of aged APP/PS1 transgenic mice. Neuroscience 298: 357–366. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. 2006. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci 9: 268–275. [DOI] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Gliddon LA, Carroll ME. 2010. Reduction of extinction and reinstatement of cocaine seeking by wheel running in female rats. Psychopharmacology (Berl) 209: 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Carroll ME. 2012. Exercise to reduce the escalation of cocaine self-administration in adolescent and adult rats. Psychopharmacology (Berl) 224: 387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]