Abstract
Repeat expansions in the promoter region of C9orf72 are the most common genetic cause of amyotrophic lateral sclerosis (ALS) and related disorders of the ALS/frontotemporal lobar degeneration (FTLD) spectrum. Remarkable clinical heterogeneity among patients with a repeat expansion has been observed, and genetic anticipation over different generations has been suggested. Genetic factors modifying the clinical phenotype have been proposed, including genetic variation in other known disease genes, the genomic context of the C9orf72 repeat, and expanded repeat size, which has been estimated between 45 and several thousand units. The role of variability in normal and expanded repeat sizes for disease risk and clinical phenotype is under debate. Different pathogenic mechanisms have been proposed, including loss of function, RNA toxicity, and dipeptide repeat (DPR) protein toxicity resulting from abnormal translation of the expanded repeat, but the major mechanism is yet unclear.
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease for which effective therapies aimed at delaying, halting, or preventing the disease are lacking. ALS patients show reduced control of voluntary muscle movement expressed in increased muscle weakness and disturbances in speech, swallowing, or breathing as a result of progressive upper and lower motor-neuron degeneration in the motor cortex, brain stem, and spinal cord. Up to 50% of ALS patients show mild disturbances in executive functions, whereas a minority also develops overt frontotemporal lobar degeneration (FTLD), presenting with either changes in personality and social conduct or language problems (Neary et al. 1998; Lomen-Hoerth et al. 2003; Ringholz et al. 2005). ALS is the most common neurodegenerative motor-neuron disorder (Rowland and Shneider 2001), and in the age group of <65 years, FTLD is the second most common neurodegenerative dementia after Alzheimer's disease (Rosso et al. 2003). There are families and individual patients in which both diseases occur (ALS-FTD) (Lomen-Hoerth et al. 2002), and TDP-43 inclusions (Arai et al. 2006; Neumann et al. 2006) in ALS and FTLD patients are indistinguishable (Tsuji et al. 2012), despite the pathological distribution being different for ALS and FTLD patients. There is ample evidence that common disease pathways are involved in ALS and FTLD because their clinical and pathological hallmarks overlap; hence, the pure forms of these diseases are considered the two extremes of one disease continuum (Lillo and Hodges 2009). In 10% of ALS patients and nearly 50% of FTLD patients, familial aggregation has been observed, suggesting a strong genetic component. Genetic studies identified mutations in the same genes in FTLD and ALS—for example, TBK1, TARDBP, FUS, VCP (Neumann et al. 2006; Kovacs et al. 2009; Johnson et al. 2010; Van Langenhove et al. 2010; Cirulli et al. 2015; Freischmidt et al. 2015; Pottier et al. 2015). The most convincing genetic evidence for a common disease pathomechanism was provided by the identification of the repeat expansion mutations in C9orf72 in patients with ALS, FTLD, and ALS-FTD (Gijselinck et al. 2010; DeJesus-Hernandez et al. 2011; Renton et al. 2011).
IDENTIFICATION OF C9orf72 REPEAT EXPANSIONS
In 2006, genetic linkage was reported at chromosome 9p21.3-9p21.1 in families with concomitant ALS and FTLD (Momeni et al. 2006; Morita et al. 2006; Vance et al. 2006). Since then, several families have been reported with conclusive or suggestive linkage to overlapping genomic regions (for review, see Gijselinck et al. 2012a). Despite the relatively small 3.6-Mb genomic segment shared by all conclusively linked families and major gene sequencing efforts by multiple research groups, the genetic defect remained long undetected. When genome-wide association studies in both ALS (van Es et al. 2009; Laaksovirta et al. 2010; Shatunov et al. 2010; Gijselinck et al. 2012b) and FTLD-TDP (Van Deerlin et al. 2010) identified a risk haplotype spanning three genes within the linked region, intensive mutation-detection strategies resulted in the identification of a pathological expansion of a noncoding G4C2 repeat in the 5′ region of C9orf72 (DeJesus-Hernandez et al. 2011; Renton et al. 2011; Gijselinck et al. 2012b), which explained both linkage and association (DeJesus-Hernandez et al. 2011; Renton et al. 2011; Gijselinck et al. 2012b).
STRUCTURE OF THE C9orf72 GENE
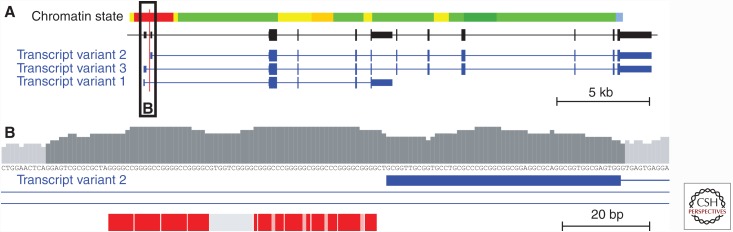
C9orf72 is transcribed in three major transcripts encoding two protein isoforms (C9orf72 a and b) (Fig. 1). A long isoform (481 amino acids) of the C9orf72 protein, isoform a, is encoded by transcript variants 1 and 3, whereas the shorter isoform b (222 amino acids) is encoded by transcript variant 2. Owing to the use of alternative first noncoding exons, the G4C2 repeat is either located in the upstream regulatory region of transcript 2 or in the first intron, following a noncoding exon 1, of transcripts 1 and 3 (Fig. 1). Based on ENCODE data, the repeat sequence is part of the functional core promoter of all three C9orf72 transcripts (Fig. 1) (Gijselinck et al. 2012b). The function of the gene is currently unclear, but it may play a role in autophagy and endosomal trafficking (Farg et al. 2014), and may be involved in regulating endoplasmatic reticulum stress (Zhang et al. 2014).
Figure 1.
Genomic organization of C9orf72 and the G4C2 hexanucleotide repeat at chromosome 9p21. (A) The C9orf72 gene and its major transcript variants numbered according to the National Center for Biotechnology Information (NCBI) RefSeq transcript collection. The red vertical line indicates the location of the noncoding G4C2 hexanucleotide repeat. The chromatin state illustrates transcriptional activity with the red bar indicating the active promoter based on combined analysis of histone marks in lymphoblastoid cell line GM12878 (Ernst et al. 2011). Black and blue boxes represent transcribed segments: wide boxes are coding, and narrow boxes are untranslated exonic regions. Horizontal connecting lines represent intronic regions. The black box labeled “B” is shown in detail in B. (B) Detailed view of the genomic context of hexanucleotide repeat sequence. The bar chart indicates relative 11 bp windowed GC content showing the contiguous GC-rich (>55%) low-complexity sequence in dark gray. Red boxes represent individual hexanucleotide repeat units in human reference genome sequence build GRCh37/hg19, with light red areas indicating nucleotide mismatches in the G4C2 consensus sequence. The gray box shows the common deletion in the 3′ flanking low-complexity sequence (LCS) containing the GTGGT motif, as frequently observed in expansion carriers. The blue box indicates the first noncoding exon of C9orf72 transcript variant 2, and blue lines indicate intronic regions.
FREQUENCY OF C9orf72 REPEAT EXPANSIONS
In both ALS (ALS Online Genetics Database [Abel et al. 2012]) and FTLD (AD and FTLD Mutation Database [Cruts et al. 2012]), C9orf72 repeat expansions are the most common mutation compared with other known genes. The highest mutation frequencies were recorded in familial patients with combined ALS and FTLD symptoms and a positive family history of these disorders. In the group of individuals with ALS-FTD, C9orf72 G4C2 repeat expansions are, together with TBK1 mutations, the only known common genetic causes. Numerous C9orf72 repeat expansion studies worldwide have confirmed that the G4C2 repeat expansion is the most common cause of disease in the ALS/FTLD continuum. When comparing mutation rates of known ALS genes, C9orf72 repeat expansions occurred one to two times more frequently than SOD1 mutations, together explaining 18%–50% of familial ALS patients (Smith et al. 2013; Stewart et al. 2012). Nevertheless, especially in ALS, in which family history is observed in only 10% of patients, sporadic ALS patients who carry an expanded repeat outnumber familial ALS patients. It is important to note that the frequency of C9orf72 G4C2 hexanucleotide repeat expansions greatly depends on ethnicity and geographical region. Globally, frequencies are the highest in the Caucasian populations of Europe and North America, ranging from 7% to 28% in ALS, 3.5% to 18% in FTLD, and 15% to more than 50% in ALS-FTD (Cruts et al. 2013). In European populations, frequencies were markedly elevated in ALS patients from Scandinavian countries (Majounie et al. 2012; Lindquist et al. 2013; Smith et al. 2013; van der Zee et al. 2013). In strong contrast, pathological repeat expansions were rarely observed in Asian populations, with frequencies of 0.4%–4.8% in ALS cases (Majounie et al. 2012; Tsai et al. 2012; Jang et al. 2013; Konno et al. 2013; Zou et al. 2013).
CLINICOPATHOLOGICAL HETEROGENEITY
Patients with a C9orf72 repeat expansion clinically present with widely variable symptoms on the ALS/FTLD spectrum, including ALS, FTLD, and ALS-FTD. The clinical characteristics of motor neuron disease (MND) associated with expanded C9orf72 repeats are in almost all instances compatible with a diagnosis of ALS. Spinal symptoms affecting muscles of the limbs were observed in ∼60% of reported repeat-expansion carriers diagnosed with ALS. Bulbar symptoms affecting muscles required for swallowing or speech were predominant in ∼40% and were significantly more frequent than the observed 25% in general ALS (Kiernan et al. 2011; Stewart et al. 2012). A generalized spinobulbar onset was seen in ∼1% (Brettschneider et al. 2012b; Millecamps et al. 2012; Smith et al. 2013). Dementia was enriched in ALS patients with a C9orf72 repeat expansion (Smith et al. 2013), with the dementia type usually being FTLD (Stewart et al. 2012). The clinical FTLD subtype was most often of the behavioral type, although progressive nonfluent aphasia (PNFA) ALS was also observed (Van Langenhove et al. 2013). Minor behavioral or cognitive abnormalities not meeting diagnostic criteria of FTLD were even more common (Byrne et al. 2012). In an extended European study, the fraction of ALS patients with symptoms of FTLD tripled from 10% in general ALS to 27% in repeat expansion carriers (Smith et al. 2013).
ALS patients with a C9orf72 repeat expansion have neuronal cytoplasmatic inclusions (NCI) immunoreactive to TDP-43 present in the spinal cord (Al-Sarraj et al. 2011). Compared with noncarriers, repeat expansion carriers showed a tendency toward more extensive microglial pathology in the medulla and motor cortex, which might correlate with the clinical enrichment of bulbar disease onset (Brettschneider et al. 2012a). In ALS patients without clinical signs of FTLD, extramotor neuronal TDP-43 pathology was limited (Al-Sarraj et al. 2011; Murray et al. 2011; Stewart et al. 2012). In addition to TDP-43 inclusions, p62 immunoreactive star-like inclusions have been associated with TDP-43 proteinopathies, including ALS-FTD linked to chromosome 9 (Boxer et al. 2011). p62 inclusions have a wider distribution than TDP-43 inclusions and far exceed them in number as well (Al-Sarraj et al. 2011; King et al. 2011; Troakes et al. 2011; Stewart et al. 2012; Simon-Sanchez et al. 2012). p62 inclusions are composed of dipeptide repeat (DPR) proteins resulting from unconventional repeat-associated non-ATG-initiated (RAN) translation of the expanded C9orf72 repeat in different reading frames (Fig. 2) (Ash et al. 2013; Mori et al. 2013c). In contrast to TDP-43 pathology, which might fit into different subclasses or even be lacking (Gijselinck et al. 2012b; Snowden et al. 2012a), DPR pathology is highly specific for C9orf72 positive cases.
Figure 2.
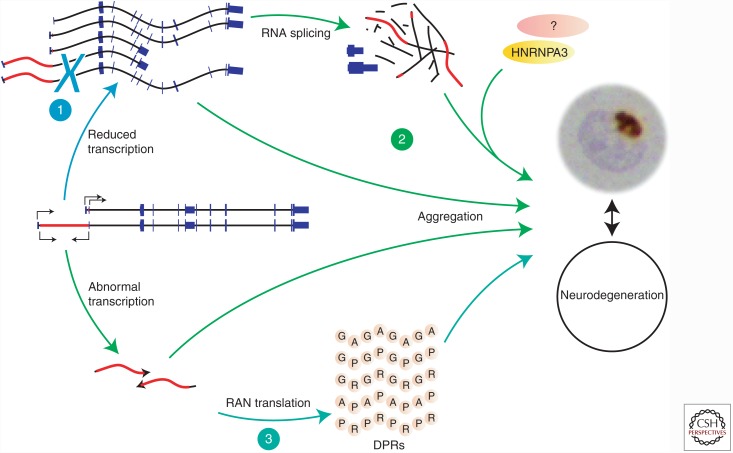
Putative, mutually nonexclusive pathological mechanisms associated with the hexanucleotide repeat expansion in C9orf72. (1) Haploinsufficiency resulting from reduced expression of the gene copy located on the repeat-containing haplotype (e.g., due to altered binding of transcription factors, DNA methylation, and/or position effects). (2) RNA gain-of-function toxicity due to the formation of aggregates of repeat-containing RNA species and sequestered RNA-binding proteins. (3) Abnormal bidirectional transcription of expanded repeat sequences followed by repeat-associated non-ATG-initiated (RAN) translation into aggregation-prone dipeptide repeat proteins (DPRs).
Irrespective of the presenting symptoms, onset age is highly variable, ranging from 27 (Majounie et al. 2012; Simon-Sanchez et al. 2012) to 83 (Sabatelli et al. 2012; Simon-Sanchez et al. 2012) years in ALS patients and from 30 to 76 years in FTLD (Majounie et al. 2012) patients. Also, carriers living up to 76 years without signs of disease were reported (Gijselinck et al. 2012b; Van Langenhove et al. 2013). Survival time was strongly associated with clinical diagnosis. For ALS, C9orf72 repeat expansions were associated with a disease duration between 3 and 96 months (Cooper-Knock et al. 2012; Millecamps et al. 2012; Stewart et al. 2012), whereas in FTLD patients, disease duration ranged from 1 to 22 years (DeJesus-Hernandez et al. 2011; Mahoney et al. 2012; Snowden et al. 2012b; Simon-Sanchez et al. 2012; van der Zee et al. 2013; Van Langenhove et al. 2013). As expected, when ALS symptoms became apparent, survival was dramatically compromised to an average of 1.8 years (Van Langenhove et al. 2013).
In recent studies of families segregating a G4C2 expansion, evidence showed a decreasing onset age of 7–11 years in each younger generation, which suggests possible genetic anticipation of the disease (Arighi et al. 2012; Boeve et al. 2012; Chió et al. 2012a; Ferrari et al. 2012; Gijselinck et al. 2012b; Hsiung et al. 2012; Stewart et al. 2012; Van Langenhove et al. 2013).
C9orf72 MODIFYING FACTORS: REPEAT SIZE VARIABILITY
The variability in clinical phenotype as described in the previous section is reminiscent of the presence of modifying factors of C9orf72 expansions and for genetic anticipation within families. Genetic modifiers have been proposed, including TMEM106B, which has been associated with a later age at onset and death in patients with FTLD (Gallagher et al. 2014) and with protecting carriers from developing FTLD (van Blitterswijk et al. 2014b), and ATXN2, which might predispose patients to the development of ALS rather than FTLD (van Blitterswijk et al. 2014a) and which could lead to the development of clinical signs featuring both FTLD and ALS in the presence of a C9orf72 repeat expansion (Lattante et al. 2014).
In most repeat expansion disorders, however, the size of a pathological repeat influences the expression of the clinical phenotype. For example, in myotonic dystrophy type I (Ashizawa et al. 1992; Harley et al. 1993; Gennarelli et al. 1996), the increasing number in successive generations of CTG units, located in the noncoding region of DMPK, correlates with more severe symptoms and an earlier age at onset. Also, in Friedreich's ataxia (FRDA), the onset age is influenced by the size of a GAA expansion within the first intron of FXN (Filla et al. 1996).
The size of the C9orf72 hexanucleotide repeats ranges from two repeat units to more than 4000 (DeJesus-Hernandez et al. 2011; Ishiura et al. 2012; Beck et al. 2013; Buchman et al. 2013; Dobson-Stone et al. 2013; Dols-Icardo et al. 2013; van Blitterswijk et al. 2013; Hubers et al. 2014; Waite et al. 2014; Gijselinck et al., 2015). Normal repeat sizes range between 2 and 24 units (Majounie et al. 2012; van der Zee et al. 2013), with those of 7–24 (van der Zee et al. 2013), 20–22 (Gomez-Tortosa et al. 2013), 12–21 (Benussi et al. 2014), 20–29 (Xi et al. 2012), 7–30 (Pamphlett et al. 2012), or 20–30 units (Nuytemans et al. 2013) considered as intermediate alleles. The pathogenic nature of the repeat depends on its size, but setting a sharp size cutoff between normal and pathogenic alleles is complicated. Some studies consider repeats of >30 units as pathogenic (Renton et al. 2011), whereas others use a cutoff of 60 units (Gijselinck et al. 2012b) depending on the upper limit of the repeat-primed polymerase chain reaction (PCR) detection method, which allows the detection of an expanded repeat allele as an allele being larger than about 30–60 repeat units in size, without further indication of the exact repeat length.
Exact sizing of the expanded G4C2 repeat has been limited because of its 100% GC content (Fig. 1), its large size, and the repetitive nature of its flanking sequences. Southern blot hybridization studies visualized the expanded alleles and estimated that the size of most repeat expansions ranged between several hundred and several thousand repeat units (DeJesus-Hernandez et al. 2011; Ishiura et al. 2012; Beck et al. 2013; Buchman et al. 2013; Dobson-Stone et al. 2013; Dols-Icardo et al. 2013; van Blitterswijk et al. 2013; Hubers et al. 2014; Waite et al. 2014; Gijselinck et al. 2016). The shortest pathological G4C2 expansion outside this pathogenic size range remains elusive. G4C2 hexanucleotide repeat sizes of 25–60 repeat units were rarely observed in ALS, FTLD, and related disorders (Dobson-Stone et al. 2012, 2013; Ratti et al. 2012; Simon-Sanchez et al. 2012; Xi et al. 2012; Beck et al. 2013; Garcia-Redondo et al. 2013; van Blitterswijk et al. 2013; Waite et al. 2014; Nordin et al. 2015); however, in most of the studies, cosegregation with disease or other arguments for pathogenicity were not observed in families, and older, healthy individuals heterozygous for alleles in the same size range have been reported (Xi et al. 2012). One study using Southern blot and a PCR assay to detect the size of short expansions up to 80 units identified short expansion sizes between 45 and 78 units in blood of 5.1% of expansion carriers cosegregating with disease and showing the same FTLD-TDP and DPR pathology as those with a long expansion of at least a few hundred units (Gijselinck et al. 2016). This indicates that short expansions might have the same pathogenic effect as long expansions. However, in different brain regions of the short expansion carrier, a pool of short and long expansion sizes was apparent, pointing to somatic mosaicism (Gijselinck et al. 2016). Several studies reported instability of the repeat from 16 units on due to somatic mosaicism across different tissues (Beck et al. 2013; van Blitterswijk et al. 2013; Nordin et al. 2015), which complicates determination of the repeat pathogenicity based on repeat sizing in blood-derived DNA. However, intra-individual variation of repeat number between tissues was higher than the variation within each tissue group (Nordin et al. 2015).
Nevertheless, none of the published Southern blot hybridization techniques are suitable to detect all repeat expansion sizes accurately (DeJesus-Hernandez et al. 2011; Ishiura et al. 2012; Beck et al. 2013; Buchman et al. 2013; Dobson-Stone et al. 2013; van Blitterswijk et al. 2013; Nordin et al. 2015; Gijselinck et al. 2016). Often, because of somatic mosaicism, repeat expansions present as smears or as several distinct bands instead of single discrete bands. In addition, estimating repeat expansion sizes of >15 kb could not be determined accurately on normal agarose gel because of the lack of resolution in this size range. Furthermore, not all protocols could detect short expansion sizes (Beck et al. 2013). Therefore, no consensus could be established on the exact correlation between repeat size and onset age (Beck et al. 2013; Dols-Icardo et al. 2013; van Blitterswijk et al. 2013; Hubers et al. 2014; Waite et al. 2014; Nordin et al. 2015; Gijselinck et al. 2016). Three of these studies suggested a positive correlation of onset age with repeat size in the frontal cortex of FTLD patients (van Blitterswijk et al. 2013), in the cerebellum and parietal lobe of ALS patients (Nordin et al. 2015), or in the blood of expansion carriers with diverse neurodegenerative diseases (Beck et al. 2013). In contrast, one study compared two groups of short (45–80 units) and long (>80 units) expansion carriers and found a significant association between longer repeat expansion size and earlier onset age (Gijselinck et al. 2016). The use of a higher resolution gel electrophoresis technique (e.g., pulsed field gel electrophoresis) or more advanced genome-mapping technologies (e.g., the Irys technology of BioNano Genomics) would be useful to perform more detailed sizing of the very large expanded repeats and to detect expansions with higher throughput and sensitivity. Furthermore, implementation of single-molecule DNA sequencing technologies producing long sequence reads (e.g., Pacific Biosciences, Oxford Nanopore Technologies) will hopefully unravel the sequence of repeat expansions because interruptions in the repeat sequence might influence its stability, pathogenicity, and clinical manifestation in the patient.
Studies of repeat sizes between generations in C9orf72 families are almost lacking. One study identified a family with suggestive evidence for genetic anticipation over three generations (Gijselinck et al. 2016). Also, in several informative C9orf72 parent–child transmissions, Gijselinck et al. identified earlier onset ages, increasing expansion sizes, and/or increasing the methylation state of the CpG island 5′ flanking of the G4C2 repeat across two generations in 90% of parent–offspring pairs. These data were reminiscent of genetic anticipation in C9orf72 families because, next to the association between expansion size and onset age, they also found a positive association between methylation state of the 5′ flanking CpG island and expansion size (see the following section).
C9orf72 PROMOTER METHYLATION
Hypermethylation of CpG-rich promoters has been associated with transcriptional silencing in noncoding repeat expansion disorders like, for example, fragile X syndrome (Oberle et al. 1991; Sutcliffe et al. 1992; Knight et al. 1993; Willemsen et al. 2011), and the CpG methylation state has been directly correlated with repeat expansion size in FRDA (Castaldo et al., 2008). The C9orf72 G4C2 repeat is located in a region with high GC content (Fig. 1). Upstream of the G4C2 repeat, a CpG island is located showing hypermethylation in expansion carriers compared with normal repeat carriers in the blood, brain, and spinal cord (Xi et al. 2013, 2014; Belzil et al. 2014; Liu et al. 2014; Russ et al. 2014; Gijselinck et al. 2016). One study found a marginally significant association between hypermethylation and shorter repeat expansion size in the blood only (Russ et al. 2014), whereas another study showed a significant association between increased methylation and longer repeat expansion sizes in the blood and the brain, the latter finding correlating with an earlier disease onset (Gijselinck et al. 2016). They also suggested an association between hypermethylation of the G4C2 repeat itself and an increasing repeat length, which was confirmed by others using a qualitative assay (Xi et al. 2015). In addition, trimethylation of lysine residues on H3 and H4 histones was found in expansion carriers (Belzil et al. 2014).
Further investigation and replication studies of correlations of age at onset and methylation with repeat size in large cohorts are needed.
INCREASING RISK ASSOCIATED WITH INTERMEDIATE REPEAT ALLELES AND SMALL INDELS IN FLANKING REGION
The risk of carrying a normal intermediate repeat allele is yet unclear. Several studies in ALS and FTLD patients found no association between repeat number on nonexpanded alleles and disease risk and/or clinical presentation (Rutherford et al. 2012; Garcia-Redondo et al. 2013). In contrast, a 10-repeat allele was associated with four different neurodegenerative diseases including ALS and FTLD (Xi et al. 2012). Also, in Parkinson’s disease, intermediate alleles between 20 and 30 units were associated with disease risk in a Caucasian study (Nuytemans et al. 2013); however, this finding could not be replicated in a global study (Theuns et al. 2014). In addition, homozygous carriers of intermediate repeat alleles (7–24 units) showed a significantly increased risk for ALS and ALS-FTD (Gijselinck et al. 2016). These observations might be explained by the significant gradual decrease of C9orf72 transcriptional activity in vitro with an increasing number of G4C2 intermediate repeat units compared with the normal reference allele of 2 repeat units in human kidney and neuroblastoma cell lines (van der Zee et al. 2013; Gijselinck et al. 2016). In accordance, intermediate repeat carriers showed a significantly higher methylation degree of the flanking CpG island and the repeat itself compared with normal short repeats.
Small insertion and deletion polymorphisms (indels) were identified in the GC-rich low-complexity sequence (LCS) downstream from the G4C2 repeat (van der Zee et al. 2013; Rollinson et al. 2015), showing an increased frequency in G4C2 expansion carriers compared with normal repeat carriers (Fig. 1) (van der Zee et al. 2013). Moreover, in a Belgian ALS, ALS-FTD, and FTLD patient cohort without C9orf72 expansion, the frequency of a flanking indel was significantly higher than in the control cohort, suggesting the risk increasing effect on disease (Gijselinck et al. 2016). Indeed, a GTGGT deletion and a CGGGGCGGGCCCG GGGGCGGGCC deletion showed a highly significant decrease in C9orf72 promoter activity in vitro, thereby possibly affecting essential core promoter elements (Gijselinck et al. 2016).
ORIGIN OF C9orf72 EXPANSIONS
It is yet unclear by which mechanism a normal G4C2 repeat expands to a pathological size. Because of the tight genetic association of repeat expansions with a certain SNP-haplotype, it was hypothesized that all C9orf72 expansions are derived from a single common founder expansion on this risk haplotype (Majounie et al. 2012; Mok et al. 2012; Pliner et al. 2014). However, several lines of evidence suggest that the genomic context of the haplotype itself might render the repeat unstable, resulting in many expansion events on a predisposing haplotype. First, the appearance of pathological G4C2 expansions in apparently sporadic patients supports this hypothesis. Also, the absence of shared microsatellite haplotypes in small geographical regions implicated no recent shared ancestry of a large proportion of C9orf72 carriers (Beck et al. 2013). Furthermore, G4C2 intermediate repeats are significantly overrepresented on the same risk haplotype (DeJesus-Hernandez et al. 2011; van der Zee et al. 2013), suggesting that intermediate repeats are more prone to replication slippage triggering pathological expansions. Also, the overrepresentation of small indels in the 3′ flanking LCS of expansion carriers containing a deletion of a GTGGT motif in most cases possibly makes the repeat more prone to pathological expansion because of the formation of a longer imperfect G4C2 repeat (Fig. 1) (van der Zee et al. 2013).
DISEASE MECHANISMS
Different pathogenic mechanisms were proposed including loss of function, RNA toxicity, and DPR toxicity resulting from abnormal translation of the expanded repeat, but their relative contribution is yet unclear (Fig. 2).
In patients carrying a G4C2 expansion, allele-specific reduction of C9orf72 expression in brain tissue (DeJesus-Hernandez et al. 2011; Gijselinck et al. 2012b; Belzil et al. 2013; Ciura et al. 2013; Fratta et al. 2013; Waite et al. 2014), hypermethylation of the CpG island flanking the G4C2 repeat, and the G4C2 repeat itself in intermediate and expanded repeat carriers (Xi et al. 2013, 2014, 2015; Belzil et al. 2014; Liu et al. 2014; Russ et al. 2014; Gijselinck et al. 2016), reduced promoter activity of intermediate alleles (van der Zee et al. 2013; Gijselinck et al. 2016), and histone trimethylation (Belzil et al. 2013) were observed, suggesting a loss-of-function mechanism through transcriptional silencing of the promoter, as seen in other repeat expansion disorders like fragile X syndrome (Fig. 2) (Oberle et al. 1991; Sutcliffe et al. 1992; Knight et al. 1993; Gecz et al. 1996; Gu et al. 1996). An association between hypermethylation of a CpG island flanking an expanded repeat and repeat expansion size has also been found in the first intron of the FRDA gene FXN, which could possibly be correlated with reduced mRNA levels (Castaldo et al. 2008; Evans-Galea et al. 2012). In favor of this loss-of-function hypothesis are two C9orf72 loss-of-function models: a zebra fish knock-down model showing axonal degeneration of motor neurons (Ciura et al. 2013) and a Caenorhabditis elegans knockout model displaying age-dependent paralysis and neurodegeneration of GABAergic motor neurons (Therrien et al. 2013).
On the other hand, a toxic gain-of-function mechanism is supported by studies identifying sense and antisense RNA foci and DPR protein aggregates produced by the expanded C9orf72 G4C2 repeat in human neurons of different tissues involved in FTLD and ALS in vivo (DeJesus-Hernandez et al. 2011; Ash et al. 2013; Donnelly et al. 2013; Gendron et al. 2013; Lagier-Tourenne et al. 2013; Lee et al. 2013; Mizielinska et al. 2013; Mori et al. 2013a,c; Zu et al. 2013) and RNA foci in induced pluripotent stem-cell (iPSC)-derived human neurons (Fig. 2) (Almeida et al. 2013; Donnelly et al. 2013; Sareen et al. 2013). In addition, RNA-binding proteins were sequestered in the pathological deposits of repeat expansion carriers like hnRNPA3 (Donnelly et al. 2013; Lee et al. 2013; Mori et al. 2013b; Xu et al. 2013). Moreover, RNA foci burden in the frontal cortex showed a significant inverse correlation with onset age (Mizielinska et al. 2013) and repeat length (Mizielinska et al. 2014). Also, a later onset age was observed in short repeat expansion carriers than in long repeat expansion carriers (Gijselinck et al. 2016), which might be in favor of a toxicity mechanism as unstable, growing repeat lengths will result in a gradually more harmful effect, as seen in other repeat expansion diseases like myotonic dystrophy type I (Ashizawa et al. 1992; Harley et al. 1993; Gennarelli et al. 1996).
Some observations might be benign side effects, but, more likely, different mechanisms acting on specific transcripts together are involved in the disease process (reviewed in Gendron et al. 2014; see also Gendron and Petrucelli 2017). Alternatively, hypermethylation might be a rescue mechanism to prevent the formation of RNA foci (Liu et al. 2014) and might therefore be neuroprotective (Russ et al. 2014; McMillan et al. 2015).
Furthermore, increasing evidence suggests the role of DPR proteins in neurodegeneration. DPR toxicity, in particular of arginine-rich DPRs, was shown in a Drosophila model (Mizielinska et al. 2014), and DPR neurotoxicity was observed in primary neurons (May et al. 2014; Wen et al. 2014). Modifier screens of the DPR toxicity in Drosophila and yeast suggested involvement of nucleocytoplasmic transport defects (Jovičić et al. 2015; Boeynaems et al. 2016).
DOUBLE GENE HITS
A pathological C9orf72 repeat expansion was repeatedly detected in patients who also carry a mutation in another Mendelian disease gene. Especially in ALS, occurrences of double gene hits is not uncommon: mutations in TARDBP (Chió et al. 2012b; Cooper-Knock et al. 2012; van Blitterswijk et al. 2012), TBK1 (Van Mossevelde et al. 2016), FUS (Millecamps et al. 2012; van Blitterswijk et al. 2012), SOD1 (Millecamps et al. 2012; van Blitterswijk et al. 2012), OPTN (Cooper-Knock et al. 2012; Millecamps et al. 2012), ANG (Millecamps et al. 2012), UBQLN2 (Millecamps et al. 2012), DAO (Millecamps et al. 2012), GRN (Ferrari et al. 2012), SQSTM1 (Almeida et al. 2016), and PSEN2 (Ferrari et al. 2012b) have been reported, including mutations of proven clinical significance. One study has noted an exceptionally early onset age of 37 years in an ALS patient carrying a pathological C9orf72 repeat expansion and an established TARDBP Ala321Val mutation, suggesting a synergistic effect of both genetic changes on the disease pathogenesis (Cooper-Knock et al. 2012). Similarly, in a family segregating the TBK1 Glu643del mutation and a C9orf72 repeat expansion, two siblings carrying both mutations had a remarkably early onset age of 41 and 51 years, respectively, whereas a sibling carrying only the repeat expansion was clinically asymptomatic at age 62 (Van Mossevelde et al. 2016). Further research of the pathogenic nature of these double mutations not only will provide information on the contribution of C9orf72 to disease in these patients, but also will shed new light on the impact of specific mutations in the previously established ALS and FTLD genes.
BIOMARKERS AND THERAPEUTIC STRATEGIES
Sensitive and specific biomarkers are extremely helpful in earlier diagnosis, evaluation of disease progression, and assessment of drug efficacy during clinical trials aimed at treating the disease. To date, no biomarkers for C9orf72 expansions have been validated. One study specifically detected the GP DPR protein in the cerebrospinal fluid of C9orf72 expansion carriers, which could potentially serve as a biomarker for therapies targeting RNA foci or DPRs (Su et al. 2014). In addition, a functional magnetic resonance imaging study revealed promising data in detecting early stage altered network connectivity in FTLD patients with a C9orf72 expansion (Lee et al. 2014). Also, the use of antisense oligonucleotides targeting and decreasing RNA foci will be promising as a potential therapeutic approach (Donnelly et al. 2013; Lagier-Tourenne et al. 2013; Sareen et al. 2013). Furthermore, the correlation of repeat size with onset age and methylation state of the C9orf72 region indicates that methylation might serve as a potential biomarker. Aberrant DNA methylation is becoming a promising therapeutic target in FTLD and ALS since hypermethylation of the GRN promoter was shown to be involved in FTLD (Banzhaf-Strathmann et al. 2013), and several reports have suggested that abnormal DNA methylation might be involved in ALS disease mechanisms (Morahan et al. 2009; Martin 2010; Chestnut et al. 2011).
CONCLUDING REMARKS
In conclusion, repeat expansions in the promoter region of C9orf72 are the most common genetic cause of the ALS/FTLD spectrum of diseases. Understanding the effect of the variability in normal and expanded repeat size and repeat sequence content on disease risk and clinical phenotype is of major importance in assessing clinical presentation, disease risk, and severity, and in providing better diagnostic guidelines for molecular genetic testing and counseling. Elucidating the exact disease mechanism(s) will have a major impact on understanding the underlying biology of ALS, FTLD, and related disorders and will contribute to the development of new therapeutic targets leading to relevant therapies for halting or preventing the disease.
ACKNOWLEDGMENTS
The research in the author’s laboratory is, in part, funded by the Belgian Science Policy Office Interuniversity Attraction Poles Program; the Flemish government–supported European Initiative on Centers of Excellence in Neurodegeneration (CoEN); the Flemish government–initiated Methusalem Excellence Program; the Flemish government–initiated Flanders Impulse Program on Networks for Dementia Research (VIND); the Alzheimer Research Foundation (SAO-FRA); the Medical Foundation Queen Elisabeth (QEMF); the Research Foundation Flanders (FWO); the Agency for Innovation by Science and Technology Flanders (IWT) and the University of Antwerp Research Fund, Belgium; and the MetLife Foundation Award for Medical Research, United States. We thank the neurologists of the different neurology centers and memory clinics from within the Belgian Neurology Consortium for their contributions to the clinical evaluation of patients and the personnel of the Genetic Service Facility at the VIB Department of Molecular Genetics (www.vibgeneticservicefacility.be) and the Antwerp Biobank at the Institute Born-Bunge for their expert support. The FWO provided a postdoctoral fellowship to I.G.
Footnotes
Editor: Stanley B. Prusiner
Additional Perspectives on Prion Diseases available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Abel O, Powell JF, Andersen PM, Al-Chalabi A. 2012. ALSoD: A user-friendly online bioinformatics tool for amyotrophic lateral sclerosis genetics. Hum Mutat 33: 1345–1351. [DOI] [PubMed] [Google Scholar]
- Almeida S, Gascon E, Tran H, Chou HJ, Gendron TF, Degroot S, Tapper AR, Sellier C, Charlet-Berguerand N, Karydas A, et al. 2013. Modeling key pathological features of frontotemporal dementia with C9ORF72 repeat expansion in iPSC-derived human neurons. Acta Neuropathol 126: 385–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida MR, Letra L, Pires P, Santos A, Rebelo O, Guerreiro R, van der Zee J, Van Broeckhoven C, Santana I. 2016. Characterization of an FTLD-PDB family with the coexistence of SQSTM1 mutation and hexanucleotide (G4C2) repeat expansion in C9orf72 gene. Neurobiol Aging 40: 191.e1–191.e8. [DOI] [PubMed] [Google Scholar]
- Al-Sarraj S, King A, Troakes C, Smith B, Maekawa S, Bodi I, Rogelj B, Al-Chalabi A, Hortobagyi T, Shaw CE. 2011. p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol 122: 691–702. [DOI] [PubMed] [Google Scholar]
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, et al. 2006. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351: 602–611. [DOI] [PubMed] [Google Scholar]
- Arighi A, Fumagalli GG, Jacini F, Fenoglio C, Ghezzi L, Pietroboni AM, De RM, Serpente M, Ridolfi E, Bonsi R, et al. 2012. Early onset behavioral variant frontotemporal dementia due to the C9ORF72 hexanucleotide repeat expansion: Psychiatric clinical presentations. J Alzheimers Dis 31: 447–452. [DOI] [PubMed] [Google Scholar]
- Ash PE, Bieniek KF, Gendron TF, Caulfield T, Lin WL, DeJesus-Hernandez M, van Blitterswijk MM, Jansen-West K, Paul JW III, Rademakers R, et al. 2013. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron 77: 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashizawa T, Dubel JR, Dunne PW, Dunne CJ, Fu YH, Pizzuti A, Caskey CT, Boerwinkle E, Perryman MB, Epstein HF, et al. 1992. Anticipation in myotonic dystrophy. II. Complex relationships between clinical findings and structure of the GCT repeat. Neurology 42: 1877–1883. [DOI] [PubMed] [Google Scholar]
- Banzhaf-Strathmann J, Claus R, Mücke O, Rentzsch K, van der Zee J, Engelborghs S, De Deyn PP, Cruts M, Van Broeckhoven C, Plass C, et al. 2013. Promoter DNA methylation regulates progranulin expression and is altered in FTLD. Acta Neuropathol 1: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J, Poulter M, Hensman D, Rohrer JD, Mahoney CJ, Adamson G, Campbell T, Uphill J, Borg A, Fratta P, et al. 2013. Large C9orf72 hexanucleotide repeat expansions are seen in multiple neurodegenerative syndromes and are more frequent than expected in the UK population. Am J Hum Genet 92: 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzil VV, Bauer PO, Prudencio M, Gendron TF, Stetler CT, Yan IK, Pregent L, Daughrity L, Baker MC, Rademakers R, et al. 2013. Reduced C9orf72 gene expression in c9FTD/ALS is caused by histone trimethylation, an epigenetic event detectable in blood. Acta Neuropathol 126: 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzil VV, Bauer PO, Gendron TF, Murray ME, Dickson D, Petrucelli L. 2014. Characterization of DNA hypermethylation in the cerebellum of c9FTD/ALS patients. Brain Res 1584: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benussi L, Rossi G, Glionna M, Tonoli E, Piccoli E, Fostinelli S, Paterlini A, Flocco R, Albani D, Pantieri R, et al. 2014. C9ORF72 hexanucleotide repeat number in frontotemporal lobar degeneration: A genotype-phenotype correlation study. J Alzheimers Dis 38: 799–808. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Boylan KB, Graff-Radford NR, DeJesus-Hernandez M, Knopman DS, Pedraza O, Vemuri P, Jones D, Lowe V, Murray ME, et al. 2012. Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain 135: 765–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeynaems S, Bogaert E, Michiels E, Gijselinck I, Sieben A, Jovičić A, De Baets G, Scheveneels W, Steyaert J, Cuijt I, et al. 2016. Drosophila screen connects nuclear transport genes to DPR pathology in c9ALS/FTD. Sci Rep 6: 20877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer AL, Mackenzie IR, Boeve BF, Baker M, Seeley WW, Crook R, Feldman H, Hsiung GY, Rutherford N, Laluz V, et al. 2011. Clinical, neuroimaging and neuropathological features of a new chromosome 9p-linked FTD-ALS family. J Neurol Neurosurg Psychiatry 82: 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettschneider J, Toledo JB, Van Deerlin VM, Elman L, McCluskey L, Lee VM, Trojanowski JQ. 2012a. Microglial activation correlates with disease progression and upper motor neuron clinical symptoms in amyotrophic lateral sclerosis. PLoS ONE 7: e39216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettschneider J, Van Deerlin VM, Robinson JL, Kwong L, Lee EB, Ali YO, Safren N, Monteiro MJ, Toledo JB, Elman L, et al. 2012b. Pattern of ubiquilin pathology in ALS and FTLD indicates presence of C9ORF72 hexanucleotide expansion. Acta Neuropathol 123: 825–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman VL, Cooper-Knock J, Connor-Robson N, Higginbottom A, Kirby J, Razinskaya OD, Ninkina N, Shaw PJ. 2013. Simultaneous and independent detection of C9ORF72 alleles with low and high number of GGGGCC repeats using an optimised protocol of Southern blot hybridisation. Mol Neurodegener 8: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne S, Elamin M, Bede P, Shatunov A, Walsh C, Corr B, Heverin M, Jordan N, Kenna K, Lynch C, et al. 2012. Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: A population-based cohort study. Lancet Neurol 11: 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaldo I, Pinelli M, Monticelli A, Acquaviva F, Giacchetti M, Filla A, Sacchetti S, Keller S, Avvedimento VE, Chiariotti L, et al. 2008. DNA methylation in intron 1 of the frataxin gene is related to GAA repeat length and age of onset in Friedreich ataxia patients. J Med Genet 45: 808–812. [DOI] [PubMed] [Google Scholar]
- Chestnut BA, Chang Q, Price A, Lesuisse C, Wong M, Martin LJ. 2011. Epigenetic regulation of motor neuron cell death through DNA methylation. J Neurosci 31: 16619–16636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chió A, Borghero G, Restagno G, Mora G, Drepper C, Traynor BJ, Sendtner M, Brunetti M, Ossola I, Calvo A, et al. 2012a. Clinical characteristics of patients with familial amyotrophic lateral sclerosis carrying the pathogenic GGGGCC hexanucleotide repeat expansion of C9ORF72. Brain 135: 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chió A, Restagno G, Brunetti M, Ossola I, Calvo A, Canosa A, Moglia C, Floris G, Tacconi P, Marrosu F, et al. 2012b. ALS/FTD phenotype in two Sardinian families carrying both C9ORF72 and TARDBP mutations. J Neurol Neurosurg Psychiatry 83: 730–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli ET, Lasseigne BN, Petrovski S, Sapp PC, Dion PA, Leblond CS, Couthouis J, Lu YF, Wang Q, Krueger BJ, et al. 2015. Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science 347: 1436–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciura S, Lattante S, Le BI, Latouche M, Tostivint H, Brice A, Kabashi E. 2013. Loss of function of C9orf72 causes motor deficits in a zebrafish model of amyotrophic lateral sclerosis. Ann Neurol 74: 180–187. [DOI] [PubMed] [Google Scholar]
- Cooper-Knock J, Hewitt C, Highley JR, Brockington A, Milano A, Man S, Martindale J, Hartley J, Walsh T, Gelsthorpe C, et al. 2012. Clinico-pathological features in amyotrophic lateral sclerosis with expansions in C9ORF72. Brain 135: 751–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruts M, Theuns J, Van Broeckhoven C. 2012. Locus-specific mutation databases for neurodegenerative brain diseases. Hum Mutat 33: 1340–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, Van Langenhove T, van der Zee J, Van Broeckhoven C. 2013. Current insights into the C9orf72 repeat expansion diseases of the FTLD/ALS spectrum. Trends Neurosci 36: 450–459. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, et al. 2011. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72: 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson-Stone C, Hallupp M, Bartley L, Shepherd CE, Halliday GM, Schofield PR, Hodges JR, Kwok JB. 2012. C9ORF72 repeat expansion in clinical and neuropathologic frontotemporal dementia cohorts. Neurology 79: 995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson-Stone C, Hallupp M, Loy CT, Thompson EM, Haan E, Sue CM, Panegyres PK, Razquin C, Seijo-Martinez M, Rene R, et al. 2013. C9ORF72 repeat expansion in Australian and Spanish frontotemporal dementia patients. PLoS ONE 8: e56899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dols-Icardo O, Garcia-Redondo A, Rojas-Garcia R, Sanchez-Valle R, Noguera A, Gomez-Tortosa E, Pastor P, Hernandez I, Esteban-Perez J, Suarez-Calvet M, et al. 2013. Characterization of the repeat expansion size in C9orf72 in amyotrophic lateral sclerosis and frontotemporal dementia. Hum Mol Genet 23: 749–754. [DOI] [PubMed] [Google Scholar]
- Donnelly CJ, Zhang PW, Pham JT, Heusler AR, Mistry NA, Vidensky S, Daley EL, Poth EM, Hoover B, Fines DM, et al. 2013. RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention. Neuron 80: 415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J, Kheradpour P, Mikkelsen TS, Shoresh N, Ward LD, Epstein CB, Zhang X, Wang L, Issner R, Coyne M, et al. 2011. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature 473: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans-Galea MV, Carrodus N, Rowley SM, Corben LA, Tai G, Saffery R, Galati JC, Wong NC, Craig JM, Lynch DR, et al. 2012. FXN methylation predicts expression and clinical outcome in Friedreich ataxia. Ann Neurol 71: 487–497. [DOI] [PubMed] [Google Scholar]
- Farg MA, Sundaramoorthy V, Sultana JM, Yang S, Atkinson RA, Levina V, Halloran MA, Gleeson PA, Blair IP, Soo KY, et al. 2014. C9ORF72, implicated in amytrophic lateral sclerosis and frontotemporal dementia, regulates endosomal trafficking. Hum Mol Genet 23: 3579–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari R, Mok K, Moreno JH, Cosentino S, Goldman J, Pietrini P, Mayeux R, Tierney MC, Kapogiannis D, Jicha GA, et al. 2012. Screening for C9ORF72 repeat expansion in FTLD. Neurobiol Aging 33: 1850.e1–1850.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filla A, De Michele G, Cavalcanti F, Pianese L, Monticelli A, Campanella G, Cocozza S. 1996. The relationship between trinucleotide (GAA) repeat length and clinical features in Friedreich ataxia. Am J Hum Genet 59: 554–560. [PMC free article] [PubMed] [Google Scholar]
- Fratta P, Poulter M, Lashley T, Rohrer JD, Polke JM, Beck J, Ryan N, Hensman D, Mizielinska S, Waite AJ, et al. 2013. Homozygosity for the C9orf72 GGGGCC repeat expansion in frontotemporal dementia. Acta Neuropathol 126: 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freischmidt A, Wieland T, Richter B, Ruf W, Schaeffer V, Müller K, Marroquin N, Nordin F, Hübers A, Weydt P, et al. 2015. Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat Neurosci 18: 631–636. [DOI] [PubMed] [Google Scholar]
- Gallagher MD, Suh E, Grossman M, Elman L, McCluskey L, van Swieten JC, Al-Sarraj S, Neumann M, Gelpi E, Ghetti B, et al. 2014. TMEM106B is a genetic modifier of frontotemporal lobar degeneration with C9orf72 hexanucleotide repeat expansions. Acta Neuropathol 127: 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Redondo A, Dols-Icardo O, Rojas-Garcia R, Esteban-Perez J, Cordero-Vazquez P, Munoz-Blanco JL, Catalina I, Gonzalez-Munoz M, Varona L, Sarasola E, et al. 2013. Analysis of the C9orf72 gene in patients with amyotrophic lateral sclerosis in Spain and different populations worldwide. Hum Mutat 34: 79–82. [DOI] [PubMed] [Google Scholar]
- Gecz J, Gedeon AK, Sutherland GR, Mulley JC. 1996. Identification of the gene FMR2, associated with FRAXE mental retardation. Nat Genet 13: 105–108. [DOI] [PubMed] [Google Scholar]
- *.Gendron TF, Petrucelli L. 2017. Disease mechanisms of C9ORF72 repeat expansions. Cold Spring Harb Perspect Med 10.1101/cshperspect.a024224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron TF, Bieniek KF, Zhang YJ, Jansen-West K, Ash PE, Caulfield T, Daughrity L, Dunmore JH, Castanedes-Casey M, Chew J, et al. 2013. Antisense transcripts of the expanded C9ORF72 hexanucleotide repeat form nuclear RNA foci and undergo repeat-associated non-ATG translation in c9FTD/ALS. Acta Neuropathol 126: 829–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron TF, Belzil VV, Zhang YJ, Petrucelli L. 2014. Mechanisms of toxicity in C9FTLD/ALS. Acta Neuropathol 127: 359–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarelli M, Novelli G, Andreasi BF, Martorell L, Cornet M, Menegazzo E, Mostacciuolo ML, Martinez JM, Angelini C, Pizzuti A, et al. 1996. Prediction of myotonic dystrophy clinical severity based on the number of intragenic [CTG]n trinucleotide repeats. Am J Med Genet 65: 342–347. [DOI] [PubMed] [Google Scholar]
- Gijselinck I, Engelborghs S, Maes G, Cuijt I, Peeters K, Mattheijssens M, Joris G, Cras P, Martin JJ, De Deyn PP, et al. 2010. Identification of 2 loci at chromosomes 9 and 14 in a multiplex family with frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Arch Neurol 67: 606–616. [DOI] [PubMed] [Google Scholar]
- Gijselinck I, Sleegers K, Van Broeckhoven C, Cruts M. 2012a. A major genetic factor at chromosome 9p implicated in amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD). In Amyotrophic lateral sclerosis (ed. Maurer MH), pp. 537–554. InTech, Rijeka, Croatia. [Google Scholar]
- Gijselinck I, Van Langenhove T, van der Zee J, Sleegers K, Philtjens S, Kleinberger G, Janssens J, Bettens K, Van Cauwenberghe C, Pereson S, et al. 2012b. A C9orf72 promoter repeat expansion in a Flanders-Belgian cohort with disorders of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum: A gene identification study. Lancet Neurol 11: 54–65. [DOI] [PubMed] [Google Scholar]
- Gijselinck I, Van Mossevelde S, van der Zee J, Sieben A, Engelborghs S, De Bleecker J, Ivanoiu A, Deryck O, Edbauer D, Zhang M, et al. 2016. The C9orf72 repeat size correlates with onset age of disease, DNA methylation and transcriptional downregulation of the promoter. Mol Psychiatry 21: 1112–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Tortosa E, Gallego J, Guerrero-Lopez R, Marcos A, Gil-Neciga E, Sainz MJ, Diaz A, Franco-Macias E, Trujillo-Tiebas MJ, Ayuso C, et al. 2013. C9ORF72 hexanucleotide expansions of 20–22 repeats are associated with frontotemporal deterioration. Neurology 80: 366–370. [DOI] [PubMed] [Google Scholar]
- Gu Y, Shen Y, Gibbs RA, Nelson DL. 1996. Identification of FMR2, a novel gene associated with the FRAXE CCG repeat and CpG island. Nat Genet 13: 109–113. [DOI] [PubMed] [Google Scholar]
- Harley HG, Rundle SA, MacMillan JC, Myring J, Brook JD, Crow S, Reardon W, Fenton I, Shaw DJ, Harper PS. 1993. Size of the unstable CTG repeat sequence in relation to phenotype and parental transmission in myotonic dystrophy. Am J Hum Genet 52: 1164–1174. [PMC free article] [PubMed] [Google Scholar]
- Hsiung GY, DeJesus-Hernandez M, Feldman HH, Sengdy P, Bouchard-Kerr P, Dwosh E, Butler R, Leung B, Fok A, Rutherford NJ, et al. 2012. Clinical and pathological features of familial frontotemporal dementia caused by C9ORF72 mutation on chromosome 9p. Brain 135: 709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubers A, Marroquin N, Schmoll B, Vielhaber S, Just M, Mayer B, Hogel J, Dorst J, Mertens T, Just W, et al. 2014. Polymerase chain reaction and Southern blot-based analysis of the C9orf72 hexanucleotide repeat in different motor neuron diseases. Neurobiol Aging 35: 1214–1216. [DOI] [PubMed] [Google Scholar]
- Ishiura H, Takahashi Y, Mitsui J, Yoshida S, Kihira T, Kokubo Y, Kuzuhara S, Ranum LP, Tamaoki T, Ichikawa Y, et al. 2012. C9ORF72 repeat expansion in amyotrophic lateral sclerosis in the Kii peninsula of Japan. Arch Neurol 69: 1154–1158. [DOI] [PubMed] [Google Scholar]
- Jang JH, Kwon MJ, Choi WJ, Oh KW, Koh SH, Ki CS, Kim SH. 2013. Analysis of the C9orf72 hexanucleotide repeat expansion in Korean patients with familial and sporadic amyotrophic lateral sclerosis. Neurobiol Aging 34: 1311–1319. [DOI] [PubMed] [Google Scholar]
- Johnson JO, Mandrioli J, Benatar M, Abramzon Y, Van Deerlin VM, Trojanowski JQ, Gibbs JR, Brunetti M, Gronka S, Wuu J, et al. 2010. Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron 68: 857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovičić A, Mertens J, Boeynaems S, Bogaert E, Chai N, Yamada SB, Paul JW 3rd, Sun S, Herdy JR, Bieri G, et al. 2015. Modifiers of C9orf72 dipeptide repeat toxicity connect nucleocytoplasmic transport defects to FTD/ALS. Nat Neurosci 18: 1226–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC. 2011. Amyotrophic lateral sclerosis. Lancet 377: 942–955. [DOI] [PubMed] [Google Scholar]
- King A, Maekawa S, Bodi I, Troakes C, Al-Sarraj S. 2011. Ubiquitinated, p62 immunopositive cerebellar cortical neuronal inclusions are evident across the spectrum of TDP-43 proteinopathies but are only rarely additionally immunopositive for phosphorylation-dependent TDP-43. Neuropathology 31: 239–249. [DOI] [PubMed] [Google Scholar]
- Knight SJ, Flannery AV, Hirst MC, Campbell L, Christodoulou Z, Phelps SR, Pointon J, Middleton-Price HR, Barnicoat A, Pembrey ME. 1993. Trinucleotide repeat amplification and hypermethylation of a CpG island in FRAXE mental retardation. Cell 74: 127–134. [DOI] [PubMed] [Google Scholar]
- Konno T, Shiga A, Tsujino A, Sugai A, Kato T, Kanai K, Yokoseki A, Eguchi H, Kuwabara S, Nishizawa M, et al. 2013. Japanese amyotrophic lateral sclerosis patients with GGGGCC hexanucleotide repeat expansion in C9ORF72. J Neurol Neurosurg Psychiatry 84: 398–401. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Murrell JR, Horvath S, Haraszti L, Majtenyi K, Molnar MJ, Budka H, Ghetti B, Spina S. 2009. TARDBP variation associated with frontotemporal dementia, supranuclear gaze palsy, and chorea. Mov Disord 24: 1843–1847. [DOI] [PubMed] [Google Scholar]
- Laaksovirta H, Peuralinna T, Schymick JC, Scholz SW, Lai SL, Myllykangas L, Sulkava R, Jansson L, Hernandez DG, Gibbs JR, et al. 2010. Chromosome 9p21 in amyotrophic lateral sclerosis in Finland: A genome-wide association study. Lancet Neurol 9: 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier-Tourenne C, Baughn M, Rigo F, Sun S, Liu P, Li HR, Jiang J, Watt AT, Chun S, Katz M, et al. 2013. Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc Natl Acad Sci 110: E4530–E4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattante S, Millecamps S, Stevanin G, Rivaud-Pechoux S, Moigneu C, Camuzat A, Da Baroca S, Mundwiller E, Couarch P, Salachas F, et al. 2014. Contribution of ATXN2 intermediary polyQ expansions in a spectrum of neurodegenerative disorders. Neurology 83: 990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YB, Chen HJ, Peres JN, Gomez-Deza J, Attig J, Stalekar M, Troakes C, Nishimura AL, Scotter EL, Vance C, et al. 2013. Hexanucleotide repeats in ALS/FTD form length-dependent RNA foci, sequester RNA binding proteins, and are neurotoxic. Cell Rep 5: 1178–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Khazenzon AM, Trujillo AJ, Guo CC, Yokoyama JS, Sha SJ, Takada LT, Karydas AM, Block NR, Coppola G, et al. 2014. Altered network connectivity in frontotemporal dementia with C9orf72 hexanucleotide repeat expansion. Brain 137: 3047–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillo P, Hodges JR. 2009. Frontotemporal dementia and motor neurone disease: Overlapping clinic-pathological disorders. J Clin Neurosci 16: 1131–1135. [DOI] [PubMed] [Google Scholar]
- Lindquist SG, Duno M, Batbayli M, Puschmann A, Braendgaard H, Mardosiene S, Svenstrup K, Pinborg LH, Vestergaard K, Hjermind LE, et al. 2013. Corticobasal and ataxia syndromes widen the spectrum of C9ORF72 hexanucleotide expansion disease. Clin Genet 83: 279–283. [DOI] [PubMed] [Google Scholar]
- Liu EY, Russ J, Wu K, Neal D, Suh E, McNally AG, Irwin DJ, Van Deerlin VM, Lee EB. 2014. C9orf72 hypermethylation protects against repeat expansion-associated pathology in ALS/FTD. Acta Neuropathol 128: 525–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomen-Hoerth C, Anderson T, Miller B. 2002. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology 59: 1077–1079. [DOI] [PubMed] [Google Scholar]
- Lomen-Hoerth C, Murphy J, Langmore S, Kramer JH, Olney RK, Miller B. 2003. Are amyotrophic lateral sclerosis patients cognitively normal? Neurology 60: 1094–1097. [DOI] [PubMed] [Google Scholar]
- Mahoney CJ, Beck J, Rohrer JD, Lashley T, Mok K, Shakespeare T, Yeatman T, Warrington EK, Schott JM, Fox NC, et al. 2012. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: Clinical, neuroanatomical and neuropathological features. Brain 135: 736–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majounie E, Renton AE, Mok K, Dopper EG, Waite A, Rollinson S, Chió A, Restagno G, Nicolaou N, Simon-Sanchez J, et al. 2012. Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol 11: 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ. 2010. Mitochondrial and cell death mechanisms in neurodegenerative diseases. Pharmaceuticals (Basel) 3: 839–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May S, Hornburg D, Schludi MH, Arzberger T, Rentzsch K, Schwenk BM, Grasser FA, Mori K, Kremmer E, Banzhaf-Strathmann J, et al. 2014. C9orf72 FTLD/ALS-associated Gly-Ala dipeptide repeat proteins cause neuronal toxicity and Unc119 sequestration. Acta Neuropathol 128: 485–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan CT, Russ J, Wood EM, Irwin DJ, Grossman M, McCluskey L, Elman L, Van Deerlin V, Lee EB. 2015. C9orf72 promoter hypermethylation is neuroprotective: Neuroimaging and neuropathologic evidence. Neurology 84: 1622–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecamps S, Boillee S, Le Ber I, Seilhean D, Teyssou E, Giraudeau M, Moigneu C, Vandenberghe N, Danel-Brunaud V, Corcia P, et al. 2012. Phenotype difference between ALS patients with expanded repeats in C9ORF72 and patients with mutations in other ALS-related genes. J Med Genet 49: 258–263. [DOI] [PubMed] [Google Scholar]
- Mizielinska S, Lashley T, Norona FE, Clayton EL, Ridler CE, Fratta P, Isaacs AM. 2013. C9orf72 frontotemporal lobar degeneration is characterised by frequent neuronal sense and antisense RNA foci. Acta Neuropathol 126: 845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizielinska S, Gronke S, Niccoli T, Ridler CE, Clayton EL, Devoy A, Moens T, Norona FE, Woollacott IO, Pietrzyk J, et al. 2014. C9orf72 repeat expansions cause neurodegeneration in Drosophila through arginine-rich proteins. Science 345: 1192–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok KY, Koutsis G, Schottlaender LV, Polke J, Panas M, Houlden H. 2012. High frequency of the expanded C9ORF72 hexanucleotide repeat in familial and sporadic Greek ALS patients. Neurobiol Aging 33: 1851–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momeni P, Schymick J, Jain S, Cookson MR, Cairns NJ, Greggio E, Greenway MJ, Berger S, Pickering-Brown S, Chió A, et al. 2006. Analysis of IFT74 as a candidate gene for chromosome 9p-linked ALS-FTD. BMC Neurol 6: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morahan JM, Yu B, Trent RJ, Pamphlett R. 2009. A genome-wide analysis of brain DNA methylation identifies new candidate genes for sporadic amyotrophic lateral sclerosis. Amyotroph Lateral Scler 10: 418–429. [DOI] [PubMed] [Google Scholar]
- Mori K, Arzberger T, Grasser FA, Gijselinck I, May S, Rentzsch K, Weng SM, Schludi MH, van der Zee J, Cruts M, et al. 2013a. Bidirectional transcripts of the expanded C9orf72 hexanucleotide repeat are translated into aggregating dipeptide repeat proteins. Acta Neuropathol 126: 881–893. [DOI] [PubMed] [Google Scholar]
- Mori K, Lammich S, Mackenzie IR, Forne I, Zilow S, Kretzschmar H, Edbauer D, Janssens J, Kleinberger G, Cruts M, et al. 2013b. hnRNP A3 binds to GGGGCC repeats and is a constituent of p62–positive/TDP43-negative inclusions in the hippocampus of patients with C9orf72 mutations. Acta Neuropathol 125: 413–423. [DOI] [PubMed] [Google Scholar]
- Mori K, Weng SM, Arzberger T, May S, Rentzsch K, Kremmer E, Schmid B, Kretzschmar HA, Cruts M, Van Broeckhoven C, Haass C, Edbauer D. 2013c. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science 339: 1335–1338. [DOI] [PubMed] [Google Scholar]
- Morita M, Al Chalabi A, Andersen PM, Hosler B, Sapp P, Englund E, Mitchell JE, Habgood JJ, de Belleroche J, Xi J, et al. 2006. A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology 66: 839–844. [DOI] [PubMed] [Google Scholar]
- Murray ME, DeJesus-Hernandez M, Rutherford NJ, Baker M, Duara R, Graff-Radford NR, Wszolek ZK, Ferman TJ, Josephs KA, Boylan KB, et al. 2011. Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol 122: 673–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, et al. 1998. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology 51: 1546–1554. [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, et al. 2006. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314: 130–133. [DOI] [PubMed] [Google Scholar]
- Nordin A, Akimoto C, Wuolikainen A, Alstermark H, Jonsson P, Birve A, Marklund SL, Graffmo KS, Forsberg K, Brannstrom T, et al. 2015. Extensive size variability of the GGGGCC expansion in C9orf72 in both neuronal and non-neuronal tissues in 18 patients with ALS or FTD. Hum Mol Genet 24: 3133–3142. [DOI] [PubMed] [Google Scholar]
- Nuytemans K, Bademci G, Kohli MM, Beecham GW, Wang L, Young JI, Nahab F, Martin ER, Gilbert JR, Benatar M, et al. 2013. C9ORF72 intermediate repeat copies are a significant risk factor for Parkinson disease. Ann Hum Genet 77: 351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberle I, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A, Boue J, Bertheas MF, Mandel JL. 1991. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science 252: 1097–1102. [DOI] [PubMed] [Google Scholar]
- Pamphlett R, Cheong PL, Trent RJ, Yu B. 2012. Transmission of C9orf72 hexanucleotide repeat expansions in sporadic amyotrophic lateral sclerosis: An Australian trio study. Neuroreport 23: 556–559. [DOI] [PubMed] [Google Scholar]
- Pliner HA, Mann DM, Traynor BJ. 2014. Searching for Grendel: Origin and global spread of the C9ORF72 repeat expansion. Acta Neuropathol 127: 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottier C, Bieniek KF, Finch N, van de Vorst M, Baker M, Perkersen R, Brown P, Ravenscroft T, van Blitterswijk M, Nicholson AM, et al. 2015. Whole-genome sequencing reveals important role for TBK1 and OPTN mutations in frontotemporal lobar degeneration without motor neuron disease. Acta Neuropathol 130: 77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratti A, Corrado L, Castellotti B, Del BR, Fogh I, Cereda C, Tiloca C, D’Ascenzo C, Bagarotti A, Pensato V, et al. 2012. C9ORF72 repeat expansion in a large Italian ALS cohort: evidence of a founder effect. Neurobiol Aging 33: 2858.e7–2858.e14. [DOI] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, et al. 2011. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72: 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringholz GM, Appel SH, Bradshaw M, Cooke NA, Mosnik DM, Schulz PE. 2005. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology 65: 586–590. [DOI] [PubMed] [Google Scholar]
- Rollinson S, Bennion CJ, Young K, Ryan SJ, Druyeh R, Rohrer JD, Snowden J, Richardson A, Jones M, Harris J, et al. 2015. A small deletion in C9orf72 hides a proportion of expansion carriers in FTLD. Neurobiol Aging 36: 1601–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso SM, Donker KL, Baks T, Joosse M, de KI, Pijnenburg Y, de Jong D, Dooijes D, Kamphorst W, Ravid R, et al. 2003. Frontotemporal dementia in The Netherlands: Patient characteristics and prevalence estimates from a population-based study. Brain 126: 2016–2022. [DOI] [PubMed] [Google Scholar]
- Rowland LP, Shneider NA. 2001. Amyotrophic lateral sclerosis. N Engl J Med 344: 1688–1700. [DOI] [PubMed] [Google Scholar]
- Russ J, Liu EY, Wu K, Neal D, Suh E, Irwin DJ, McMillan CT, Harms MB, Cairns NJ, Wood EM, et al. 2014. Hypermethylation of repeat expanded C9orf72 is a clinical and molecular disease modifier. Acta Neuropathol 129: 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford NJ, Heckman MG, DeJesus-Hernandez M, Baker MC, Soto-Ortolaza AI, Rayaprolu S, Stewart H, Finger E, Volkening K, Seeley WW, et al. 2012. Length of normal alleles of C9ORF72 GGGGCC repeat do not influence disease phenotype. Neurobiol Aging 33: 2950–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatelli M, Conforti FL, Zollino M, Mora G, Monsurro MR, Volanti P, Marinou K, Salvi F, Corbo M, Giannini F, et al. 2012. C9ORF72 hexanucleotide repeat expansions in the Italian sporadic ALS population. Neurobiol Aging 33: 1848.e15–1848.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareen D, O’Rourke JG, Meera P, Muhammad AK, Grant S, Simpkinson M, Bell S, Carmona S, Ornelas L, Sahabian A, et al. 2013. Targeting RNA foci in iPSC-derived motor neurons from ALS patients with a C9ORF72 repeat expansion. Sci Transl Med 5: 208ra149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatunov A, Mok K, Newhouse S, Weale ME, Smith B, Vance C, Johnson L, Veldink JH, van Es MA, van den Berg LH, et al. 2010. Chromosome 9p21 in sporadic amyotrophic lateral sclerosis in the UK and seven other countries: A genome-wide association study. Lancet Neurol 9: 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Sanchez J, Dopper EG, Cohn-Hokke PE, Hukema RK, Nicolaou N, Seelaar H, de Graaf JR, de KI, van Schoor NM, Deeg DJ, et al. 2012. The clinical and pathological phenotype of C9orf72 hexanucleotide repeat expansions. Brain 135: 723–735. [DOI] [PubMed] [Google Scholar]
- Smith BN, Newhouse S, Shatunov A, Vance C, Topp S, Johnson L, Miller J, Lee Y, Troakes C, Scott KM, et al. 2013. The C9ORF72 expansion mutation is a common cause of ALS+/-FTD in Europe and has a single founder. Eur J Hum Genet 21: 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Rollinson S, Thompson JC, Harris JM, Stopford CL, Richardson AM, Jones M, Gerhard A, Davidson YS, Robinson A, et al. 2012a. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain 135: 693–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Rollinson S, Thompson JC, Harris JM, Stopford CL, Richardson AM, Jones M, Gerhard A, Davidson YS, Robinson A, et al. 2012b. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain 135: 693–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart H, Rutherford NJ, Briemberg H, Krieger C, Cashman N, Fabros M, Baker M, Fok A, DeJesus-Hernandez M, Eisen A, et al. 2012. Clinical and pathological features of amyotrophic lateral sclerosis caused by mutation in the C9ORF72 gene on chromosome 9p. Acta Neuropathol 123: 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Zhang Y, Gendron TF, Bauer PO, Chew J, Yang WY, Fostvedt E, Jansen-West K, Belzil VV, Desaro P, et al. 2014. Discovery of a biomarker and lead small molecules to target r(GGGGCC)-associated defects in c9FTD/ALS. Neuron 83: 1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JS, Nelson DL, Zhang F, Pieretti M, Caskey CT, Saxe D, Warren ST. 1992. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet 1: 397–400. [DOI] [PubMed] [Google Scholar]
- Therrien M, Rouleau GA, Dion PA, Parker JA. 2013. Deletion of C9ORF72 results in motor neuron degeneration and stress sensitivity in C. elegans. PLoS ONE 8: e83450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theuns J, Verstraeten A, Sleegers K, Wauters E, Gijselinck I, Smolders S, Crosiers D, Corsmit E, Elinck E, Sharma M, et al. 2014. Global investigation and meta-analysis of the C9orf72 (G4C2)n repeat in Parkinson disease. Neurology 83: 1906–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troakes C, Maekawa S, Wijesekera L, Rogelj B, Siklos L, Bell C, Smith B, Newhouse S, Vance C, Johnson L, et al. 2011. An MND/ALS phenotype associated with C9orf72 repeat expansion: Abundant p62–positive, TDP-43-negative inclusions in cerebral cortex, hippocampus and cerebellum but without associated cognitive decline. Neuropathology 32: 505–514. [DOI] [PubMed] [Google Scholar]
- Tsai CP, Soong BW, Tu PH, Lin KP, Fuh JL, Tsai PC, Lu YC, Lee IH, Lee YC. 2012. A hexanucleotide repeat expansion in C9ORF72 causes familial and sporadic ALS in Taiwan. Neurobiol Aging 33: 2232. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Arai T, Kametani F, Nonaka T, Yamashita M, Suzukake M, Hosokawa M, Yoshida M, Hatsuta H, Takao M, et al. 2012. Molecular analysis and biochemical classification of TDP-43 proteinopathy. Brain 135: 3380–3391. [DOI] [PubMed] [Google Scholar]
- van Blitterswijk M, DeJesus-Hernandez M, Niemantsverdriet E, Murray ME, Heckman MG, Diehl NN, Brown PH, Baker MC, Finch NA, Bauer PO, et al. 2013. Association between repeat sizes and clinical and pathological characteristics in carriers of C9ORF72 repeat expansions (Xpansize-72): A cross-sectional cohort study. Lancet Neurol 12: 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Blitterswijk M, van Es MA, Hennekam EA, Dooijes D, van Rheenen W, Medic J, Bourque PR, Schelhaas HJ, van der Kooi AJ, de Visser M, et al. 2012. Evidence for an oligogenic basis of amyotrophic lateral sclerosis. Hum Mol Genet 21: 3776–3784. [DOI] [PubMed] [Google Scholar]
- van Blitterswijk M, Mullen B, Heckman MG, Baker MC, DeJesus-Hernandez M, Brown PH, Murray ME, Hsiung GY, Stewart H, Karydas AM, et al. 2014a. Ataxin-2 as potential disease modifier in C9ORF72 expansion carriers. Neurobiol Aging 35: 2421–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Blitterswijk M, Mullen B, Nicholson AM, Bieniek KF, Heckman MG, Baker MC, DeJesus-Hernandez M, Finch NA, Brown PH, Murray ME, et al. 2014b. TMEM106B protects C9ORF72 expansion carriers against frontotemporal dementia. Acta Neuropathol 127: 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C, Al Chalabi A, Ruddy D, Smith BN, Hu X, Sreedharan J, Siddique T, Schelhaas HJ, Kusters B, Troost D, et al. 2006. Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.p2–21.3. Brain 129: 868–876. [DOI] [PubMed] [Google Scholar]
- Van Deerlin V, Sleiman PM, Martinez-Lage M, Chen-Plotkin A, Wang LS, Graff-Radford NR, Dickson DW, Rademakers R, Boeve BF, Grossman M, et al. 2010. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet 42: 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zee J, Gijselinck I, Dillen L, Van Langenhove T, Theuns J, Engelborghs S, Philtjens S, Vandenbulcke M, Sleegers K, Sieben A, et al. 2013. A pan-European study of the C9orf72 repeat associated with FTLD: Geographic prevalence, genomic instability, and intermediate repeats. Hum Mutat 34: 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es MA, Veldink JH, Saris CG, Blauw HM, Van Vught PW, Birve A, Lemmens R, Schelhaas HJ, Groen EJ, Huisman MH, et al. 2009. Genome-wide association study identifies 19p13.3 (UNC13A) and 9p21.2 as susceptibility loci for sporadic amyotrophic lateral sclerosis. Nat Genet 41: 1083–1087. [DOI] [PubMed] [Google Scholar]
- Van Langenhove T, van der Zee J, Sleegers K, Engelborghs S, Vandenberghe R, Gijselinck I, Van den Broeck M, Mattheijssens M, Peeters K, De Deyn PP, et al. 2010. Genetic contribution of FUS to frontotemporal lobar degeneration. Neurology 74: 366–371. [DOI] [PubMed] [Google Scholar]
- Van Langenhove T, van der Zee J, Gijselinck I, Engelborghs S, Vandenberghe R, Vandenbulcke M, De Bleecker J, Sieben A, Versijpt J, Ivanoiu A, et al. 2013. Distinct clinical characteristics of C9orf72 expansion carriers compared With GRN, MAPT, and nonmutation carriers in a Flanders–Belgian FTLD cohort. JAMA Neurol 70: 365–373. [DOI] [PubMed] [Google Scholar]
- Van Mossevelde S, van der Zee J, Gijselinck I, Engelborghs S, Sieben A, Van Langenhove T, De Bleecker J, Baets J, Vandenbulcke M, Van Laere K, et al. 2016. Clinical features of TBK1 carriers compared with C9orf72, GRN and non-mutation carriers in a Belgian cohort. Brain 139: 452–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite AJ, Baumer D, East S, Neal J, Morris HR, Ansorge O, Blake DJ. 2014. Reduced C9orf72 protein levels in frontal cortex of amyotrophic lateral sclerosis and frontotemporal degeneration brain with the C9ORF72 hexanucleotide repeat expansion. Neurobiol Aging 35: 1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Tan W, Westergard T, Krishnamurthy K, Markandaiah SS, Shi Y, Lin S, Shneider NA, Monaghan J, Pandey UB, et al. 2014. Antisense proline-arginine RAN dipeptides linked to C9ORF72-ALS/FTD form toxic nuclear aggregates that initiate in vitro and in vivo neuronal death. Neuron 84: 1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen R, Levenga J, Oostra BA. 2011. CGG repeat in the FMR1 gene: Size matters. Clin Genet 80: 214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z, Zinman L, Grinberg Y, Moreno D, Sato C, Bilbao JM, Ghani M, Hernandez I, Ruiz A, Boada M, et al. 2012. Investigation of c9orf72 in 4 neurodegenerative disorders. Arch Neurol 69: 1583–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z, Zinman L, Moreno D, Schymick J, Liang Y, Sato C, Zheng Y, Ghani M, Dib S, Keith J, Robertson J, Rogaeva E. 2013. Hypermethylation of the CpG island near the G4C2 repeat in ALS with a C9orf72 expansion. Am J Hum Genet 92: 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z, Rainero I, Rubino E, Pinessi L, Bruni AC, Maletta RG, Nacmias B, Sorbi S, Galimberti D, Surace EI, et al. 2014. Hypermethylation of the CpG-island near the C9orf72 G4C2 -repeat expansion in FTLD patients. Hum Mol Genet 23: 5630–5637. [DOI] [PubMed] [Google Scholar]
- Xi Z, Zhang M, Bruni AC, Maletta RG, Colao R, Fratta P, Polke JM, Sweeney MG, Mudanohwo E, Nacmias B, et al. 2015. The C9orf72 repeat expansion itself is methylated in ALS and FTLD patients. Acta Neuropathol 129: 715–727. [DOI] [PubMed] [Google Scholar]
- Xu Z, Poidevin M, Li X, Li Y, Shu L, Nelson DL, Li H, Hales CM, Gearing M, Wingo TS, Jin P. 2013. Expanded GGGGCC repeat RNA associated with amyotrophic lateral sclerosis and frontotemporal dementia causes neurodegeneration. Proc Natl Acad Sci 110: 7778–7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, Jansen-West K, Xu YF, Gendron TF, Bieniek KF, Lin WL, Sasaguri H, Caulfield T, Hubbard J, Daughrity L, et al. 2014. Aggregation-prone c9FTD/ALS poly(GA) RAN-translated proteins cause neurotoxicity by inducing ER stress. Acta Neuropathol 128: 505–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou ZY, Li XG, Liu MS, Cui LY. 2013. Screening for C9orf72 repeat expansions in Chinese amyotrophic lateral sclerosis patients. Neurobiol Aging 34: 1710–1716. [DOI] [PubMed] [Google Scholar]
- Zu T, Liu Y, Banez-Coronel M, Reid T, Pletnikova O, Lewis J, Miller TM, Harms MB, Falchook AE, Subramony SH, et al. 2013. RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc Natl Acad Sci 110: E4968–E4977. [DOI] [PMC free article] [PubMed] [Google Scholar]