Abstract
Simian immunodeficiency virus (SIV) challenge of rhesus macaques provides an invaluable tool to evaluate the clinical prospects of HIV-1 vaccine concepts. However, as with any animal model of human disease, it is crucial to understand the advantages and limitations of this system to maximize the translational value of SIV vaccine studies. Here, we discuss the importance of assessing the efficacy of vaccine prototypes using stringent SIV challenge regimens that mimic HIV-1 transmission and pathogenesis. We also review some of the cautionary tales of HIV-1 vaccine research because they provide general lessons for the preclinical assessment of vaccine candidates.
Great Debates
What are the most interesting topics likely to come up over dinner or drinks with your colleagues? Or, more importantly, what are the topics that don't come up because they are a little too controversial? In Immune Memory and Vaccines: Great Debates, Editors Rafi Ahmed and Shane Crotty have put together a collection of articles on such questions, written by thought leaders in these fields, with the freedom to talk about the issues as they see fit. This short, innovative format aims to bring a fresh perspective by encouraging authors to be opinionated, focus on what is most interesting and current, and avoid restating introductory material covered in many other reviews.
The Editors posed 13 interesting questions critical for our understanding of vaccines and immune memory to a broad group of experts in the field. In each case, several different perspectives are provided. Note that while each author knew that there were additional scientists addressing the same question, they did not know who these authors were, which ensured the independence of the opinions and perspectives expressed in each article. Our hope is that readers enjoy these articles and that they trigger many more conversations on these important topics.
The development of a new vaccine is an expensive and complex enterprise that can take several years until an immunization protocol can be finally approved for use in people. It also entails great financial risks because the majority of candidates do not progress beyond early clinical trials. In view of these obstacles, new immunization regimens are thoroughly screened during preclinical stages to ensure that only the most promising ones advance into costly and lengthy human trials. Because the “go/no-go” criteria for prioritizing vaccine prototypes depend primarily on in vivo measures, animal models provide an invaluable resource for vaccine testing. In this regard, nonhuman primates (NHPs) have been used whenever possible to inform and guide the development of vaccines against human pathogens. Compared with standard laboratory mouse strains, NHPs are more genetically diverse and evolutionarily closer to humans—features that likely increase their translational value. Indeed, numerous NHPs were used to assess the pathogenicity and protective effects of the prototypes of the inactivated (Salk) and live-attenuated (Sabin) polio vaccines (Horstmann 1985). Moreover, NHP studies also enabled the generation of vaccines against hepatitis B (Wieland 2015), yellow fever (Norrby 2007), and anthrax (Friedlander et al. 2013). In the case of HIV, vaccine candidates are primarily evaluated using the simian immunodeficiency virus (SIV)/rhesus macaque model (Hatziioannou and Evans 2012). Although this animal system has yet to be validated, which can only be accomplished by the development of an effective HIV-1 vaccine that also works in the animal model, here we argue that properly designed SIV challenge studies can predict the outcomes of HIV-1 vaccine clinical trials. We also discuss some of the historical milestones in HIV-1 vaccine research that helped refine the SIV/rhesus macaque model because broadly applicable lessons can be learned from them.
Rhesus monkeys are not susceptible to HIV-1 infection, so vaccine efficacy is assessed by challenging animals with pathogenic viral surrogates of HIV-1, including SIVs and artificial simian/HIV (SHIV) chimeras. Many SIV and SHIV strains have been developed for in vivo challenge studies but, importantly, not all of these viruses effectively mimic the biology and pathogenesis of HIV-1. The SHIV89.6P strain, for example, is notorious in that vaccine efficacy against this virus supported the decision to evaluate recombinant adenovirus type-5 (rAd5) vectors encoding HIV-1 genes in clinical trials. SHIV89.6P was a popular model partly because of its exceptional virulence in naïve rhesus macaques, which resulted in consistent virologic outcomes after infection (Parker et al. 2001). Although this high pathogenicity was initially seen as a stringent test of vaccine performance, it soon became clear that SHIV89.6P differs from HIV-1 in crucial ways. For example, whereas most transmitted HIV-1 founder viruses are CCR5-tropic and replicate in memory CD4+ T cells (Shaw and Hunter 2012), SHIV89.6P uses CXCR4 as its coreceptor and targets naïve CD4+ T lymphocytes (Zhang et al. 2000). Additionally, the pathogenic profile of SHIV89.6P infection does not emulate the acute destruction of gut CD4+ T cells and erosion of the immune system that precede AIDS in most untreated HIV-1-infected individuals (Reimann et al. 1999; Moir et al. 2011). Last, and most important, vaccine containment of SHIV89.6P infection is surprisingly easy to achieve (Watkins et al. 2008), presumably because of the unusual sensitivity of the SHIV89.6P Env protein to autologous antibody neutralization (Montefiori et al. 1998; Letvin et al. 2004).
In response to the growing number of SHIV89.6P challenge studies performed in the early 2000s, Feinberg and Moore (2002) voiced their concerns about the adequacy of this strain for screening HIV-1 vaccines. At that time, developing a T-cell-based vaccine against HIV-1 was a priority in the field given the difficulty in engendering HIV-1-specific broadly neutralizing antibodies by vaccination. Rather than affording sterilizing immunity, vaccine-induced HIV-1-specific T-cell responses were intended to lower viremia in infected individuals as a means to delay progression to AIDS. Priority was given to rAd5 vectors because of their safety profile and impressive immunogenicity in NHPs (Shiver and Emini 2004). Importantly, although rAd5-vectored T-cell-based vaccines resulted in significant control of viremia in macaques challenged intravenously with SHIV89.6P (Shiver et al. 2002), similar regimens failed to durably control viral replication after rectal challenges with SIVmac239 (Casimiro et al. 2005). Like most transmitted HIV-1 founder viruses, SIVmac239 is CCR5-tropic and refractory to immunological control (Hatziioannou and Evans 2012), making it a more realistic challenge virus than SHIV89.6P. Notwithstanding these discordant results, the first efficacy trial of an rAd5-vectored T-cell-based HIV-1 vaccine (known as the “Step study”) began enrolling participants in 2004 but was then halted early in 2007 (Buchbinder et al. 2008). Unfortunately, neither Step nor any of the trials of rAd5/HIV-1 vectors that followed reported any protection against HIV-1 (Buchbinder et al. 2008; Gray et al. 2011; Hammer et al. 2013). In retrospect, the contradictory performances of rAd5/SIV vaccines against SHIV89.6P and SIVmac239 did not provide clear criteria for judging the potential of rAd5/HIV-1 vectors in humans. Nevertheless, these inconsistent outcomes should have at least prompted additional experiments to further validate the rAd5 platform in monkeys before advancing it into human efficacy trials. Indeed, we and others subsequently showed that an rAd5 vaccine regimen designed to mimic the immunization protocol used in the Step study did not substantially reduce viral replication after physiologically relevant SIV challenges (Qureshi et al. 2012; Reynolds et al. 2012). Of note, the use of intrarectal (IR) and penile SIV challenges in these monkey studies more accurately reflected the outcome of the Step trial. Thus, properly designed SIV vaccine experiments can inform and even predict the performance of similar regimens in clinical trials. In broader terms, these results also illustrate how the use of stringent challenge regimens that mimic the transmission route and disease course of human pathogens might increase the predictive value of animal models for vaccine research.
Importantly, the cautionary tale of SHIV89.6P and the Step study must not be taken as an indication that all SHIVs are inadequate for screening HIV-1 vaccine regimens. For instance, the pathogenic CCR5-tropic SHIVAD8 strain has been generated following serial passaging in rhesus macaques and appears to be a suitable surrogate of HIV-1 (Nishimura et al. 2010). Indeed, SHIVAD8-infected monkeys experience sustained viremia, gradual CD4+ T-lymphocyte loss, and AIDS-like symptoms. Furthermore, new chimeric strains bearing minimally adapted CCR5-tropic HIV-1 envs have been shown to replicate and cause AIDS-defining illnesses in rhesus macaques (Del Prete et al. 2014). Thus, these new SHIVs provide new tools for evaluating antibody-based vaccine regimens against HIV-1 in the preclinical stage.
Despite great efforts to approximate clinically relevant HIV-1 exposures, even the best SIV challenge models will be inherently flawed because new HIV-1 infections do not originate from atraumatic exposures to tissue-culture-produced viruses. Indeed, human intercourse often results in microabrasions of the genital mucosae caused by friction, which can facilitate virus entry into the tissue (Hladik and McElrath 2008). Furthermore, factors such as concurrent sexually transmitted diseases and needle sharing also increase the risk of HIV-1 transmission (Galvin and Cohen 2004; Patel et al. 2014). Because these variables are difficult (if not impossible) to recapitulate in NHPs, we should be cautious when considering the clinical prospects of successful SIV vaccines. This concern is particularly relevant for partially effective SIV vaccines because their clinical versions might turn out to be futile when faced against the reality of the aforementioned risk factors. In this regard, the recent announcement by the National Institute of Allergy and Infectious Diseases (NIAID) to move forward with the HVTN 702 phase 2b/3 HIV-1 vaccine trial merits discussion because the data on which this decision was based (NIAID 2016) is unpersuasive. HVTN 702 is set to take place in South Africa, where the incidence of HIV-1 infection among the adult population is as high as 18% (UNAIDS 2014). HVTN 702 aims to build on the results of the RV144 trial (Rerks-Ngarm et al. 2009), the only report of vaccine protection against HIV-1 to date. RV144 was conducted in low-risk communities in Thailand where HIV-1 prevalence among Thai adults is 1.4% (UNAIDS 2014). The immunization protocol in RV144 consisted of four inoculations of the canarypox vector ALVAC-HIV expressing Gag-Pro and a truncated form of gp120. The two last immunizations also included boosts with a bivalent gp120 subunit protein adjuvanted in alum. Although this ALVAC-HIV/gp120 (alum) regimen appeared to reduce HIV-1 infection rates among vaccinees, the effects were modest and did not reach statistical significance in an intention-to-treat analysis, including all randomized participants (Rerks-Ngarm et al. 2009). Similarly, no significant protective effects were detected in the per-protocol group, which comprised only the individuals who received all immunizations and remained uninfected through the vaccine phase (Rerks-Ngarm et al. 2009). However, when seven volunteers who tested positive for HIV-1 at baseline were excluded (out of 16,495 subjects enrolled in the RV144 trial) as part of a modified intention-to-treat analysis, the ALVAC-HIV/gp120 (alum) regimen showed a 31.2% reduction in the rate of HIV-1 acquisition (P = 0.04) (Rerks-Ngarm et al. 2009). Finally, the observed difference between HIV-1 acquisition in the vaccinees and placebo recipients took place soon after initiation of the trial. Inexplicably, the placebo group showed a sudden increase in numbers infected during the first year and this difference largely accounted for the observed effect (Rerks-Ngarm et al. 2009). The outcome of RV144 was also surprising, given the poor immunogenicity of ALVAC-HIV in previous trials and the failure of the bivalent gp120 component by itself to confer any protection against HIV-1 (Flynn et al. 2005; Pitisuttithum et al. 2006; Pantaleo et al. 2010). Furthermore, a post hoc statistical analysis of the data concluded that there was at most a 78% chance that the vaccine efficacy reported in RV144 was real (Gilbert et al. 2011).
HVTN 702 will also use an ALVAC-HIV/gp120 regimen but the vaccine content has been changed to match the most prevalent HIV-1 clade in South Africa and the vaccine protocol has been modified to enhance Env-specific humoral responses (NIAID 2016). One of these modifications is the replacement of alum with the oil-in-water emulsion MF59 as the adjuvant for the gp120 boosters. Interestingly, a recent monkey study compared the efficacy of ALVAC-SIV/gp120 adjuvanted with either alum or MF59 against repeated low dose IR challenges with SIVmac251, a biological isolate that is closely related to SIVmac239 (Hatziioannou and Evans 2012). Whereas the ALVAC-SIV/gp120 (alum) regimen partially reduced the risk of SIV infection per exposure, the ALVAC-SIV/gp120 (MF59) protocol conferred no protection (Vaccari et al. 2016). Of note, the first SIV exposure in this study occurred only 4 weeks after the last immunization and the challenge virus was largely homologous to the vaccine-encoded antigens. If the ALVAC-SIV/gp120 (MF59) protocol failed under such controlled conditions, its clinical version may not be effective against HIV-1 among South African participants. In light of these data and the uncertainty regarding the efficacy reported in RV144, we are skeptical that an ALVAC-HIV/gp120 (MF59) vaccine regimen will substantially reduce the incidence of HIV-1 infection in South Africa.
Because the primary goal of an HIV-1 vaccine is to prevent infection altogether, what then must be the criteria for deciding whether or not an SIV vaccine regimen warrants clinical evaluation? Unfortunately, in the absence of clear immune correlates of protection against HIV-1, this question cannot be answered accurately. However, instructive inferences can be made from what is known about HIV-1 transmission and epidemiology. For instance, HIV infection is most commonly acquired through unprotected sex (Shaw and Hunter 2012), so vaccine prototypes must show substantial efficacy against mucosal challenges with SIV before being considered for clinical trials. These challenges should consist of IR and intravaginal virus exposures because these routes are the most relevant portals of virus entry (Shaw and Hunter 2012). The size of the challenge inoculum is also important because exposing monkeys to high doses of SIV might overwhelm immune defenses and thereby mask potential protective effects afforded by vaccination (Vaccari et al. 2013). Furthermore, not every infectious exposure results in productive HIV-1 infection, considering the stochastic and selective forces that govern the population bottleneck of mucosal HIV-1 transmission (Joseph et al. 2015). In this regard, SIV stocks should be titrated first in naïve monkeys to determine a dose that reliably infects only a fraction of animals per challenge. To emulate the diversity of circulating HIV-1 isolates, there should also be some degree of sequence mismatch between vaccine-encoded antigens and the challenge virus (Watkins et al. 2008). However, in designing such heterologous SIV challenges, special attention must be given to the neutralization profile of the selected SIV strain because vaccine protection against easy-to-neutralize viruses has little translational value. For example, the biological isolate SIVsmE660, which has been used as a heterologous SIV challenge strain in previous experiments (Letvin et al. 2011; Patel et al. 2013; Roederer et al. 2013), is sensitive to antibody neutralization (Lopker et al. 2013). By comparison, the genetically close SIVmac239 and SIVmac251 strains display highly resistant neutralization profiles (Lopker et al. 2013). The relevance of these differences is illustrated by the discordant performances of an rDNA/rAd5 vaccine encoding SIVmac239 env and gag-pol against IR challenges with either SIVsmE660 or SIVmac251 (Letvin et al. 2011). Although this regimen significantly delayed acquisition of the former strain, it had no effect against the latter. Notably, this failure to prevent SIVmac251 infection was later recapitulated in the HVTN 505 trial in which a similar rDNA/rAd5 protocol did not protect men who have sex with men against infection with HIV-1 (Hammer et al. 2013). To circumvent the issue of neutralization sensitivity of the challenge strain and still keep it heterologous to the vaccine, recent experiments have immunized monkeys with SIVsmE543-3 sequences and subsequently challenged the animals with SIVmac251 (Barouch et al. 2012, 2015). The Env proteins of these two strains differ by ∼18%, which is comparable to the expected diversity of HIV-1 isolates within the same clade (Gaschen et al. 2002). Encouragingly, these studies have reported that Env-specific antibody responses induced by recombinant adenovirus/poxvirus or adenovirus/Env protein vaccine regimens can reduce the per-exposure risk of acquiring infection by 80%–90% after repeated IR challenges with SIVmac251. Phase 1/2a clinical trials aimed at investigating the safety and immunogenicity of these immunization strategies are already under way.
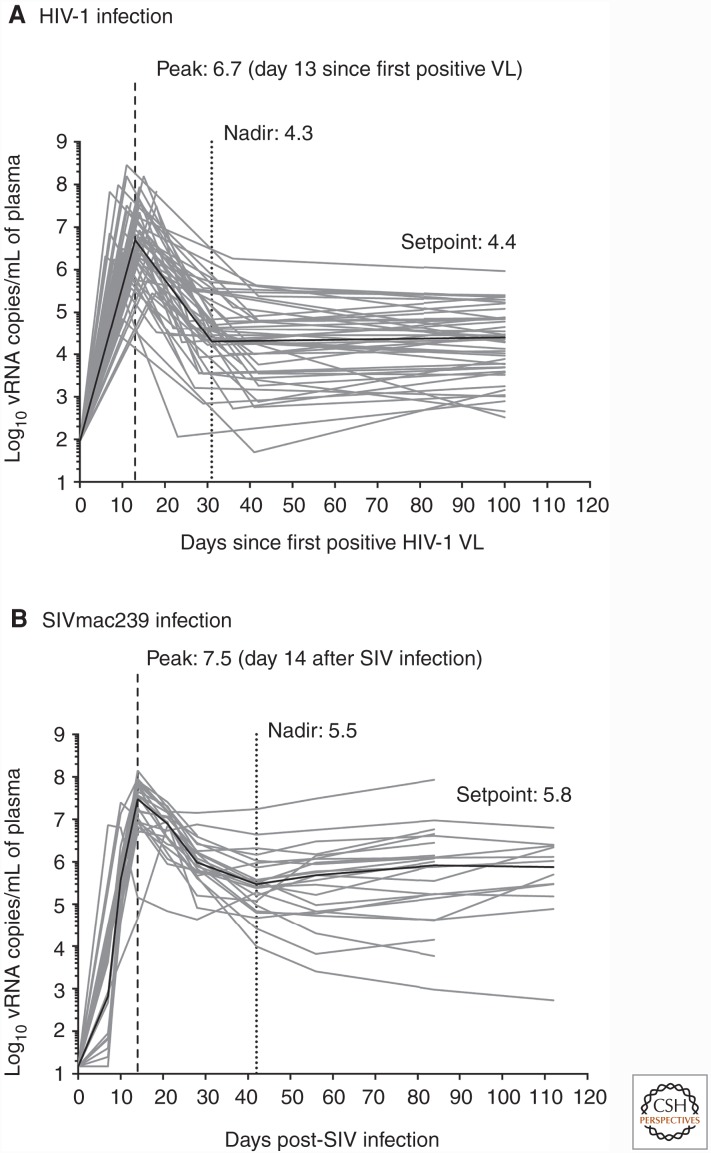
Most of the SIV vaccine experiments conducted in our laboratory have used repeated marginal dose IR challenges with SIVmac239 for three main reasons. First, the viral load dynamics in SIVmac239-infected rhesus macaques show clear parallels with primary HIV-1 infection in humans (Fig. 1) (Dang and Hirsch 2008; Robb et al. 2016), underscoring the translational use of this animal model. Second, receptive anal intercourse carries the highest risk of sexually transmitting HIV-1 (Patel et al. 2014). As a result, preclinical vaccine studies focused on preventing SIV acquisition via the IR route are relevant for the still-growing number of HIV-1 infections among gay and bisexual men in several parts of the world (UNAIDS 2014). Additionally, they may also be directly relevant for heterosexual transmission because it is difficult to estimate the role of anal intercourse in acquisition of HIV in heterosexual couples. Third, substantial vaccine-mediated containment of SIVmac239 infection is exceedingly hard to achieve, even in the setting of homologous challenges (Wilson et al. 2006; Bilello et al. 2011; Martins et al. 2014). In fact, live-attenuated SIV vaccines remain the best option for protection against challenge with SIVmac239 (Koff et al. 2006), although safety concerns preclude the deployment of this concept in humans. Thus, any immunization regimen that effectively contains SIVmac239 infection deserves further investigation. In this regard, a recent vaccine modality using live rhesus cytomegalovirus (RhCMV) vectors engineered to express SIV genes is noteworthy given its remarkable performance against SIVmac239 challenges. RhCMV is a member of the Herpesviridae family and, similar to live-attenuated SIV vaccines, establishes a persistent infection in monkeys, leading to intermittent low-level antigen exposure. This type of immune stimulation favors the generation of effector memory T-cell (TEM) responses that recirculate through extra lymphoid tissues and are endowed with immediate antiviral activity (Masopust and Picker 2012). Remarkably, approximately half of RhCMV-vaccinated macaques control viral replication shortly after SIVmac239 infection (Hansen et al. 2011). Except for occasional viral load “blips” in the ensuing weeks, these successful vaccinees remain aviremic in the chronic phase and ultimately clear SIVmac239 infection (Hansen et al. 2013a). The 68.1 RhCMV strain used in these experiments elicits CD8+ TEM responses of unconventional MHC restriction that are only now beginning to be characterized (Hansen et al. 2013b, 2016). Although vaccination with 68.1 RhCMV vectors did not block acquisition of SIV infection, the profound virologic control afforded by this vaccine modality and its potential to eradicate SIV infection are novel and warrant clinical testing. Indeed, a phase 1 trial of a human CMV-vectored vaccine against HIV-1 is slated to begin in 2017.
Figure 1.
Log-transformed viral loads over the courses of untreated HIV-1 and SIVmac239 infections. (A) Longitudinal viral loads (VLs) are plotted against the number of days since the first blood draw tested positive for HIV-1 RNA in 50 HIV-1-infected subjects (based on data in Robb et al. 2016). (B) Longitudinal VLs from 24 rhesus macaques that were rectally infected with SIVmac239 as part of previous and ongoing studies conducted by our laboratory (Martins et al. 2014; M Martins, unpubl.). Peak VL was defined as the highest plasma viral (v)RNA measurement within the initial 42 days since the first positive HIV-1 VL (A) or since the monkeys were infected with SIVmac239 (B). Nadir VL was defined as the lowest plasma vRNA measurement after peak viremia through day 42 in both A and B. The HIV-1 setpoint was calculated as the average VL of all samples collected after day 42 and until antiretroviral therapy was initiated up to 1 year after the first positive VL. The simian immunodeficiency virus (SIV) setpoint was calculated as the average VL of all measurements performed within days 42 and 112 postinfection. Log-transformed medians of peak (dashed vertical line), nadir (dotted vertical line), and setpoint VLs are shown in each graph. The time to peak VL is shown in parenthesis after the median peak VL in each panel and corresponds to the median number of days since HIV-1 vRNA was first detected in plasma (A) or since the establishment of productive SIV infection (B). The solid black line in each panel denotes the median of VLs measured at each time point.
CONCLUDING REMARKS
Ultimately, there is no substitute for clinical data for determining the efficacy of a vaccine regimen. Nonetheless, given the high costs of human trials and the consequences of failure, we must ensure that only the most promising concepts progress to phase 2b/3 trials. In the case of HIV-1, the SIV/rhesus macaque model provides an invaluable tool for screening and prioritizing vaccine candidates at the preclinical stage, although it is worth noting that we will not know the true predictive value of this system until new HIV-1 vaccine regimens start showing reliable levels of protection in people. Meanwhile, however, we should rely on repeated mucosal challenges with in vivo–titrated doses of pathogenic neutralization-resistant SIV strains as a stringent and realistic test of the clinical prospects of HIV-1 vaccine prototypes.
Lastly, it is important to highlight that the experience accrued from the SIV/rhesus macaque model can also inform vaccine development against other human infectious diseases. The recent outbreak of Zika virus (ZIKV) in the Americas, for example, has been declared an international health emergency by the World Health Organization, prompting the generation of ZIKV NHP models for testing vaccines and other prophylactic approaches. The expertise acquired from SIV challenge studies in rhesus macaques has been directly relevant for this enterprise as shown by the significant contributions made by HIV/SIV researchers in tackling ZIKV. Indeed, Dudley et al. (2016) have developed a rhesus macaque model of ZIKV infection in which they described the kinetics of viral replication in pregnant and nonpregnant monkeys, the in vivo impact of different ZIKV challenge doses, and the susceptibility of ZIKV-infected animals to reinfection. The ontogeny of immune responses after infection and the extent to which the virus replicated in anatomic locations other than blood were also evaluated (Dudley et al. 2016), analogous to the importance of these immunological and virologic parameters for the outcome of primate lentivirus infections (Dang and Hirsch 2008; Moir et al. 2011). Encouragingly, recent experiments have shown that ZIKV Env-specific antibodies elicited by clinically relevant vaccine platforms have completely protected rhesus macaques from ZIKV challenge (Abbink et al. 2016; Dowd et al. 2016; Larocca et al. 2016). These results are completely different from almost all SIV challenge studies conducted to date, suggesting that engendering protective immunity against ZIKV in humans might be relatively straightforward. Furthermore, our group has also initiated monkey experiments designed to test whether monoclonal antibody prophylaxis can prevent ZIKV infection. The challenge virus for this experiment will consist of a minimally passaged Brazilian ZIKV isolate in an attempt to avoid confounding factors related to the passage history of virus strains, as previously reported for HIV-1 and SIV (Sawyer et al. 1994; Moore and Ho 1995; Langlois et al. 1998). In conclusion, the extensive knowledge generated by honing the SIV/rhesus macaque model is applicable not only to the field of HIV-1/AIDS research, but also to the development of immune interventions against globally relevant human pathogens.
ACKNOWLEDGMENTS
We are grateful to Dr. Ron Desrosiers for providing insightful comments on this manuscript.
Footnotes
Editors: Shane Crotty and Rafi Ahmed
Additional Perspectives on Immune Memory and Vaccines: Great Debates available at www.cshperspectives.org
REFERENCES
- Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, Kirilova M, Li Z, Ng’ang’a D, Nanayakkara O, et al. 2016. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 353: 1129–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, et al. 2012. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482: 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, Alter G, Broge T, Linde C, Ackerman ME, Brown EP, Borducchi EN, Smith KM, Nkolola JP, Liu J, et al. 2015. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science 349: 320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilello JP, Manrique JM, Shin YC, Lauer W, Li W, Lifson JD, Mansfield KG, Johnson RP, Desrosiers RC. 2011. Vaccine protection against simian immunodeficiency virus in monkeys using recombinant γ-2 herpesvirus. J Virol 85: 12708–12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, et al. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372: 1881–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro DR, Wang F, Schleif WA, Liang X, Zhang ZQ, Tobery TW, Davies ME, McDermott AB, O’Connor DH, Fridman A, et al. 2005. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with DNA and recombinant adenoviral vaccine vectors expressing Gag. J Virol 79: 15547–15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Q, Hirsch VM. 2008. Rapid disease progression to AIDS due to Simian immunodeficiency virus infection of macaques: Host and viral factors. Adv Pharmacol 56: 369–398. [DOI] [PubMed] [Google Scholar]
- Del Prete GQ, Ailers B, Moldt B, Keele BF, Estes JD, Rodriguez A, Sampias M, Oswald K, Fast R, Trubey CM, et al. 2014. Selection of unadapted, pathogenic SHIVs encoding newly transmitted HIV-1 envelope proteins. Cell Host Microbe 16: 412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd KA, Ko SY, Morabito KM, Yang ES, Pelc RS, DeMaso CR, Castilho LR, Abbink P, Boyd M, Nityanandam R, et al. 2016. Rapid development of a DNA vaccine for Zika virus. Science 354: 237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley DM, Aliota MT, Mohr EL, Weiler AM, Lehrer-Brey G, Weisgrau KL, Mohns MS, Breitbach ME, Rasheed MN, Newman CM, et al. 2016. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun 7: 12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg MB, Moore JP. 2002. AIDS vaccine models: Challenging challenge viruses. Nat Med 8: 207–210. [DOI] [PubMed] [Google Scholar]
- Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. 2005. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis 191: 654–665. [DOI] [PubMed] [Google Scholar]
- Friedlander AM, Grabenstein JD, Brachman PS. 2013. Anthrax vaccines. In Vaccines, 6th ed. (ed. Plotkin SA, et al. ), pp. 127–140. Saunders, Philadelphia. [Google Scholar]
- Galvin SR, Cohen MS. 2004. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol 2: 33–42. [DOI] [PubMed] [Google Scholar]
- Gaschen B, Taylor J, Yusim K, Foley B, Gao F, Lang D, Novitsky V, Haynes B, Hahn BH, Bhattacharya T, et al. 2002. Diversity considerations in HIV-1 vaccine selection. Science 296: 2354–2360. [DOI] [PubMed] [Google Scholar]
- Gilbert PB, Berger JO, Stablein D, Becker S, Essex M, Hammer SM, Kim JH, Degruttola VG. 2011. Statistical interpretation of the RV144 HIV vaccine efficacy trial in Thailand: A case study for statistical issues in efficacy trials. J Infect Dis 203: 969–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray GE, Allen M, Moodie Z, Churchyard G, Bekker LG, Nchabeleng M, Mlisana K, Metch B, de Bruyn G, Latka MH, et al. 2011. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: A double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis 11: 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer SM, Sobieszczyk ME, Janes H, Karuna ST, Mulligan MJ, Grove D, Koblin BA, Buchbinder SP, Keefer MC, Tomaras GD, et al. 2013. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med 369: 2083–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. 2011. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473: 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Piatak MJ, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, et al. 2013a. Immune clearance of highly pathogenic SIV infection. Nature 502: 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, Gilbride RM, Lewis MS, Gilliam AN, Ventura AB, et al. 2013b. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science 340: 1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Wu HL, Burwitz BJ, Hughes CM, Hammond KB, Ventura AB, Reed JS, Gilbride RM, Ainslie E, Morrow DW, et al. 2016. Broadly targeted CD8+ T cell responses restricted by major histocompatibility complex E. Science 351: 714–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatziioannou T, Evans DT. 2012. Animal models for HIV/AIDS research. Nat Rev Microbiol 10: 852–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladik F, McElrath MJ. 2008. Setting the stage: Host invasion by HIV. Nat Rev Immunol 8: 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann DM. 1985. The poliomyelitis story: A scientific hegira. Yale J Biol Med 58: 79–90. [PMC free article] [PubMed] [Google Scholar]
- Joseph SB, Swanstrom R, Kashuba AD, Cohen MS. 2015. Bottlenecks in HIV-1 transmission: Insights from the study of founder viruses. Nat Rev Microbiol 13: 414–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff WC, Johnson PR, Watkins DI, Burton DR, Lifson JD, Hasenkrug KJ, McDermott AB, Schultz A, Zamb TJ, Boyle R, et al. 2006. HIV vaccine design: Insights from live attenuated SIV vaccines. Nat Immunol 7: 19–23. [DOI] [PubMed] [Google Scholar]
- Langlois AJ, Desrosiers RC, Lewis MG, KewalRamani VN, Littman DR, Zhou JY, Manson K, Wyand MS, Bolognesi DP, Montefiori DC. 1998. Neutralizing antibodies in sera from macaques immunized with attenuated simian immunodeficiency virus. J Virol 72: 6950–6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocca RA, Abbink P, Peron JP, Zanotto PM, Iampietro MJ, Badamchi-Zadeh A, Boyd M, Ng’ang’a D, Kirilova M, Nityanandam R, et al. 2016. Vaccine protection against Zika virus from Brazil. Nature 536: 474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letvin NL, Huang Y, Chakrabarti BK, Xu L, Seaman MS, Beaudry K, Korioth-Schmitz B, Yu F, Rohne D, Martin KL, et al. 2004. Heterologous envelope immunogens contribute to AIDS vaccine protection in rhesus monkeys. J Virol 78: 7490–7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letvin NL, Rao SS, Montefiori DC, Seaman MS, Sun Y, Lim SY, Yeh WW, Asmal M, Gelman RS, Shen L, et al. 2011. Immune and genetic correlates of vaccine protection against mucosal infection by SIV in monkeys. Sci Transl Med 3: 81ra36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopker M, Easlick J, Sterrett S, Decker JM, Barbian H, Learn G, Keele BF, Robinson JE, Li H, Hahn BH, et al. 2013. Heterogeneity in neutralization sensitivities of viruses comprising the simian immunodeficiency virus SIVsmE660 isolate and vaccine challenge stock. J Virol 87: 477–5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins MA, Wilson NA, Piaskowski SM, Weisgrau KL, Furlott JR, Bonaldo MC, Veloso de Santana MG, Rudersdorf RA, Rakasz EG, Keating KD, et al. 2014. Vaccination with Gag, Vif, and Nef gene fragments affords partial control of viral replication after mucosal challenge with SIVmac239. J Virol 88: 7493–7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Picker LJ. 2012. Hidden memories: Frontline memory T cells and early pathogen interception. J Immunol 188: 5811–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir S, Chun TW, Fauci AS. 2011. Pathogenic mechanisms of HIV disease. Annu Rev Pathol 6: 223–248. [DOI] [PubMed] [Google Scholar]
- Montefiori DC, Reimann KA, Wyand MS, Manson K, Lewis MG, Collman RG, Sodroski JG, Bolognesi DP, Letvin NL. 1998. Neutralizing antibodies in sera from macaques infected with chimeric simian-human immunodeficiency virus containing the envelope glycoproteins of either a laboratory-adapted variant or a primary isolate of human immunodeficiency virus type 1. J Virol 72: 3427–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, Ho DD. 1995. HIV-1 neutralization: The consequences of viral adaptation to growth on transformed T cells. AIDS 9: S117–S136. [PubMed] [Google Scholar]
- NIAID. 2016. Large-scale HIV vaccine trial to launch in South Africa. In NIH-funded study will test safety, efficacy of vaccine regimen. NIAID, Bethesda, MD. [Google Scholar]
- Nishimura Y, Shingai M, Willey R, Sadjadpour R, Lee WR, Brown CR, Brenchley JM, Buckler-White A, Petros R, Eckhaus M, et al. 2010. Generation of the pathogenic R5-tropic simian/human immunodeficiency virus SHIVAD8 by serial passaging in rhesus macaques. J Virol 84: 4769–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby E. 2007. Yellow fever and Max Theiler: The only Nobel Prize for a virus vaccine. J Exp Med 204: 2779–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G, Esteban M, Jacobs B, Tartaglia J. 2010. Poxvirus vector-based HIV vaccines. Curr Opin HIV AIDS 5: 391–396. [DOI] [PubMed] [Google Scholar]
- Parker RA, Regan MM, Reimann KA. 2001. Variability of viral load in plasma of rhesus monkeys inoculated with simian immunodeficiency virus or simian-human immunodeficiency virus: Implications for using nonhuman primate AIDS models to test vaccines and therapeutics. J Virol 75: 11234–11238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Jalah R, Kulkarni V, Valentin A, Rosati M, Alicea C, von Gegerfelt A, Huang W, Guan Y, Keele BF, et al. 2013. DNA and virus particle vaccination protects against acquisition and confers control of viremia upon heterologous simian immunodeficiency virus challenge. Proc Natl Acad Sci 110: 2975–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. 2014. Estimating per-act HIV transmission risk: A systematic review. AIDS 28: 1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, Hu D, Tappero JW, Choopanya K. 2006. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis 194: 1661–1671. [DOI] [PubMed] [Google Scholar]
- Qureshi H, Ma ZM, Huang Y, Hodge G, Thomas MA, DiPasquale J, DeSilva V, Fritts L, Bett AJ, Casimiro DR, et al. 2012. Low-dose penile SIVmac251 exposure of rhesus macaques infected with adenovirus type 5 (Ad5) and then immunized with a replication-defective Ad5-based SIV gag/pol/nef vaccine recapitulates the results of the phase IIb step trial of a similar HIV-1 vaccine. J Virol 86: 2239–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann KA, Watson A, Dailey PJ, Lin W, Lord CI, Steenbeke TD, Parker RA, Axthelm MK, Karlsson GB. 1999. Viral burden and disease progression in rhesus monkeys infected with chimeric simian-human immunodeficiency viruses. Virology 256: 15–21. [DOI] [PubMed] [Google Scholar]
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361: 2209–2220. [DOI] [PubMed] [Google Scholar]
- Reynolds MR, Weiler AM, Piaskowski SM, Piatak MJ, Robertson HT, Allison DB, Bett AJ, Casimiro DR, Shiver JW, Wilson NA, et al. 2012. A trivalent recombinant Ad5 gag/pol/nef vaccine fails to protect rhesus macaques from infection or control virus replication after a limiting-dose heterologous SIV challenge. Vaccine 30: 4465–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb ML, Eller LA, Kibuuka H, Rono K, Maganga L, Nitayaphan S, Kroon E, Sawe FK, Sinei S, Sriplienchan S, et al. 2016. Prospective study of acute HIV-1 infection in adults in East Africa and Thailand. N Engl J Med 374: 2120–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roederer M, Keele BF, Schmidt SD, Mason RD, Welles HC, Fischer W, Labranche C, Foulds KE, Louder MK, Yang ZY, et al. 2013. Immunological and virological mechanisms of vaccine-mediated protection against SIV and HIV. Nature 505: 502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer LS, Wrin MT, Crawford-Miksza L, Potts B, Wu Y, Weber PA, Alfonso RD, Hanson CV. 1994. Neutralization sensitivity of human immunodeficiency virus type 1 is determined in part by the cell in which the virus is propagated. J Virol 68: 1342–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GM, Hunter E. 2012. HIV transmission. Cold Spring Harb Perspect Med 2: a006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiver JW, Emini EA. 2004. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu Rev Med 55: 355–372. [DOI] [PubMed] [Google Scholar]
- Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, et al. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415: 331–335. [DOI] [PubMed] [Google Scholar]
- UNAIDS. 2014. The gap report: Joint United Nations programme on HIV/AIDS; Geneva. [Google Scholar]
- Vaccari M, Keele BF, Bosinger SE, Doster MN, Ma ZM, Pollara J, Hryniewicz A, Ferrari G, Guan Y, Forthal DN, et al. 2013. Protection afforded by an HIV vaccine candidate in macaques depends on the dose of SIVmac251 at challenge exposure. J Virol 87: 3538–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari M, Gordon SN, Fourati S, Schifanella L, Liyanage NP, Cameron M, Keele BF, Shen X, Tomaras GD, Billings E, et al. 2016. Adjuvant-dependent innate and adaptive immune signatures of risk of SIVmac251 acquisition. Nat Med 22: 762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DI, Burton DR, Kallas EG, Moore JP, Koff WC. 2008. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat Med 14: 617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland SF. 2015. The chimpanzee model for hepatitis B virus infection. Cold Spring Harb Perspect Med 5: a021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NA, Reed J, Napoe GS, Piaskowski S, Szymanski A, Furlott J, Gonzalez EJ, Yant LJ, Maness NJ, May GE, et al. 2006. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J Virol 80: 5875–5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lou B, Lal RB, Gettie A, Marx PA, Moore JP. 2000. Use of inhibitors to evaluate coreceptor usage by simian and simian/human immunodeficiency viruses and human immunodeficiency virus type 2 in primary cells. J Virol 74: 6893–6910. [DOI] [PMC free article] [PubMed] [Google Scholar]