Abstract
Neurodegenerative diseases are a common cause of morbidity and cognitive impairment in older adults. Most clinicians who care for the elderly are not trained to diagnose these conditions, perhaps other than typical Alzheimer’s disease (AD). Each of these disorders has varied epidemiology, clinical symptomatology, laboratory and neuroimaging features, neuropathology, and management. Thus, it is important that clinicians be able to differentiate and diagnose these conditions accurately. This review summarizes and highlights clinical aspects of several of the most commonly encountered neurodegenerative diseases, including AD, frontotemporal dementia (FTD) and its variants, progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), Parkinson’s disease (PD), dementia with Lewy bodies (DLB), multiple system atrophy (MSA), and Huntington’s disease (HD). For each condition, we provide a brief overview of the epidemiology, defining clinical symptoms and diagnostic criteria, relevant imaging and laboratory features, genetics, pathology, treatments, and differential diagnosis.
Neurodegenerative disease (ND) is a common and growing cause of mortality and morbidity worldwide, particularly in the elderly. The individual neurodegenerative disorders are heterogeneous in their clinical presentations and underlying physiology, although they often have overlapping features. Diagnostic accuracy is critical, as it allows for more reliable prognostication and often guides specific treatment and management. In this review, we provide a brief overview of several of the most common neurodegenerative diseases—particularly those associated with cognitive impairment—and discuss their clinical features and diagnosis, epidemiology, imaging results, genetics, relevant laboratory tests, differential diagnosis, and treatments. This review is not meant to provide an exhaustive overview of each diagnosis but rather to provide a basic background and stimulate further exploration. Many of the neurodegenerative diseases discussed here share clinical features with conditions traditionally categorized as prion diseases and often are considered in the differential diagnosis of prion diseases. Traditional prion diseases, such as sporadic Creutzfeldt–Jakob disease (sCJD), acquired forms of CJD, and genetic prion diseases, are discussed elsewhere in this collection. As is also discussed elsewhere in this collection, there is now increasing evidence that several neurodegenerative diseases behave in a “prion-like” manner and share similar pathophysiological mechanisms (Prusiner 2013; Watts et al. 2013; Walker and Jucker 2015).
ALZHEIMER’S DEMENTIA AND ALZHEIMER’S DISEASE
Although Alzheimer’s disease (AD) is often the term used to describe both the clinical syndrome and the pathological entity, some in the field prefer to use Alzheimer’s dementia to describe the clinical syndrome that is associated with a specific neuropathological process defined by two hallmark features: namely, the accumulation of extracellular neuritic plaques composed primarily of 42-amino-acid amyloid-beta (Aβ1−42), a cleavage product of the amyloid precursor protein (APP), and intracellular collections of neurofibrillary tangles composed of hyperphosphorylated species of microtubule-associated protein tau (MAPT). Thus, AD often is the name given to the pathological entity, and Alzheimer’s dementia is a term typically used to describe the clinical phenotype. For this review, we will use the term AD for both the clinical and pathological entities. The clinical phenotypes of AD are strikingly heterogeneous and reflect the variable neuroanatomical distribution of pathology and its effect on neural network functioning.
Epidemiology
AD is the most common form of dementia worldwide and makes up 60%–80% of all dementia cases, affecting an estimated 24 million people globally (Reitz et al. 2011; Mayeux and Stern 2012; Sosa-Ortiz et al. 2012). Although it can occur in younger persons, it is primarily a disease of the elderly. The prevalence of AD increases markedly with advancing age, with a greater than 15-fold increase reported between the ages of 65 and 85 (Evans et al. 1989; Mayeux and Stern 2012). One community-based U.S. study suggested that the prevalence is as high as 50% in people older than age 85 (Evans et al. 1989), although a European study estimated a lower prevalence of 22% at age 90 (Lobo et al. 2000). Although these reported distinctions may result from methodological differences (Corrada et al. 1995), there does appear to be global variation in the burden of disease (Sosa-Ortiz et al. 2012). The incidence rate also increases with age (Jorm and Jolley 1998; Mayeux and Stern 2012), and yearly risk ranges from 0.5% in individuals between the ages of 65 and 69 to 6% in those older than 85; AD occurs rarely before the age of 65, and these cases are considered “early-onset” AD. The incidence rate of AD doubles every 5 years (Brookmeyer et al. 1998; Mayeux and Stern 2012). There is recent evidence, however, that the incidence rates of dementia may be flattening or declining (Rocca et al. 2011; Schrijvers et al. 2012). More women have AD (Alzheimer’s Association 2016), and the detrimental effect of the ApoE ε4 gene on the risk of developing AD appears to be higher in women (Farrer et al. 1997).
There are a number of additional risk factors associated with an increased risk of developing AD, including the presence of the ApoE ε4 allele, cerebrovascular disease (approximately twofold), hyperlipidemia, smoking, diabetes (approximately twofold), obesity (1.6-fold), and traumatic brain injury. Protective factors include a higher cognitive reserve, consumption of a Mediterranean diet, and regular exercise. This is reviewed elsewhere (Mayeux and Stern 2012).
The majority of AD cases present with the typical, primarily amnestic form, whereas up to 15% of cases are considered atypical, presenting with early or prominent visual, frontal, motor, or other symptoms (Galton et al. 2000).
Clinical Symptoms and Diagnosis
Typical AD (also referred to as amnestic or limbic form) is characterized by the insidious onset and gradual progression of memory loss in association with other cognitive domains (often visuospatial and executive function) that leads to a loss of functional independence. The amnesia seen in typical AD primarily affects declarative episodic memory—autobiographical memories that are associated with specific events, times, places, and emotions—and is usually most evident for recent memories early in the disease course. This pattern of memory loss reflects dysfunction of mesial temporal structures and manifests in numerous ways. Individuals may misplace objects, repeat conversations or questions, or have difficulty keeping track of dates and appointments. Clinicians can formally assess memory by asking patients to recall and recognize a list of words or objects or to retell a brief story that is told to them. Other types of memory (e.g., procedural memory) that are processed outside of the hippocampal/parahippocampal structures are usually spared in AD (Markowitsch and Staniloiu 2012).
The original diagnostic criteria from the National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) required the presence of amnestic symptoms for diagnosis (McKhann et al. 2011a). Because of the relatively low sensitivity and specificity of these original criteria (∼70% for each parameter) when compared with underlying pathology, and the increasing recognition of nonamnestic “atypical” presentations of AD, the criteria were revised in 2011 to include a broader range of clinical phenotypes. See Box 1 for diagnostic criteria (McKhann et al. 2011a).
Box 1.
Clinical diagnostic criteria for Alzheimer’s disease (AD) (McKhann et al. 2011b)
I. Probable AD dementia (core clinical criteria)
- Meets criteria for dementia and has the following characteristics:
-
A.Insidious onset over months to years
-
B.Clear-cut history of worsening cognition by report or observation
-
C.Initial and most prominent cognitive deficits on history and examination are one of the following:
- Amnestic presentation: Impairment in learning and recall, deficits in other cognitive domains should be present
- Nonamnestic presentation
- Language presentation: Word-finding deficits, deficits in other domains should be present
- Visuospatial presentation: Spatial cognition-object agnosia, facial recognition, simultagnosia and alexia, deficits in other domains should be present
- Executive dysfunction: Impaired reasoning, judgment and problem solving, deficits in other domains should be present
-
D.There is no evidence of (a) cerebrovascular disease temporarily related to the onset of cognitive symptoms or presence of extensive infarcts or severe white matter hyperintensity burden, (b) core features of DLB other than dementia itself, (c) prominent features of bvFTD, (d) prominent features of semantic or nonfluent/agrammatic PPA, or (e) other active neurological disease, medical comorbidity, or use of medications with effects on cognition.
-
A.
II. Probable AD dementia with documented decline
Meets core clinical criteria, and
Has evidence of decline on subsequent evaluation based on informants and cognitive testing (formal neuropsychological evaluation or standardized mental status examinations)
III. Probable AD dementia in a carrier of a causative AD genetic mutation
Meets core clinical criteria, and
Has a known pathogenic mutation (APP, PSEN1 or PSEN2), not ApoE ε4
IV. Probable AD dementia with evidence of the AD pathophysiological process
Meets the core criteria, and
- Has the following biomarker data:
- High probability:
- positive amyloid (PET or CSF), AND positive CSF tau, FDG-PET, or structural MRI
- Intermediate probability:
- unavailable, conflicting, or indeterminate amyloid (PET or CSF), AND positive CSF tau, FDG-PET, or structural MRI, OR
- positive amyloid (PET or CSF), AND unavailable, conflicting, or indeterminate CSF tau, FDG-PET, or structural MRI
- Uninformative:
- unavailable, conflicting, or indeterminate amyloid (PET or CSF), AND unavailable, conflicting, or indeterminate CSF tau, FDG-PET, or structural MRI
V. Possible AD dementia (core clinical criteria)
Atypical: Meets core clinical criteria for AD but either has a sudden onset or shows insufficient historical detail or objective cognitive documentation or progressive decline
Etiologically mixed presentation: Meets the core criteria for AD but has evidence of (a) cerebrovascular disease, (b) features of DLB other than dementia itself, (c) evidence of another neurological disease or medical condition with known effects on cognition
VI. Possible AD dementia with evidence of the AD pathophysiological process
Atypical clinical presentation, and
- The following biomarker data
- High probability (but does not rule our second etiology):
- positive amyloid (PET or CSF), AND positive CSF tau, FDG-PET, or structural MRI
- Uninformative:
- Unavailable, conflicting, or indeterminate amyloid (PET or CSF), AND unavailable, conflicting, or indeterminate CSF tau, FDG-PET, or structural MRI
Atypical clinical presentations of AD include variants that reflect dysfunction outside the mesial temporal areas—namely, in the posterior parieto-occipital, frontal, motor, and language areas (Lee et al. 2011; Dubois et al. 2014; Sha and Rabinovici 2016). The posterior-predominant syndromes (including posterior cortical atrophy or PCA) include an occipitotemporal variant with visuoperceptive deficits (e.g., face, object, word recognition) and a biparietal variant with visuospatial deficits (e.g., Gerstmann or Balint syndrome, apraxia) (McMonagle et al. 2006; Alladi et al. 2007). The frontal variant presents with behavioral changes (e.g., apathy, disinhibition) and/or a dysexecutive cognitive profile (Ossenkoppele et al. 2015b). The language variant, often called the logopenic variant of primary progressive aphasia (lvPPA), presents primarily with word-retrieval difficulties and impaired sentence repetition with sparing of semantic knowledge and motor speech programs (Gorno-Tempini et al. 2011). AD also can present as corticobasal syndrome (CBS); in fact, about a quarter of the CBS cohort at our research center (UCSF Memory and Aging Center) have pathology-proven AD at autopsy (Lee et al. 2011).
There are multiple formal diagnostic criteria for AD (McKhann et al. 2011a; Dubois et al. 2014), which vary in their emphasis on the use of biomarkers in the diagnosis of the disease. The National Institute on Aging and Alzheimer’s Association (NIA-AAS) criteria allow the diagnosis of AD on purely clinical grounds (including atypical phenotypes) with biomarkers used to support and increase diagnostic certainty as to the underlying pathophysiology (McKhann et al. 2011a), whereas an International Working Group (IWG) requires both biomarker evidence and a suggestive clinical phenotype to make the diagnosis (Dubois et al. 2014).
Over the past several years, there has been great progress in the development of biomarkers for detecting underlying AD. These include both markers of AD pathophysiology (e.g., increased Aβ1-42 plaque formation and phosphorylated tau deposition) and those that reveal neuronal injury occurring in an anatomical distribution that is typical of AD (e.g., structural magnetic resonance imaging [MRI], fluorodeoxyglucose [FDG]-positron emission tomography [PET]).
Imaging
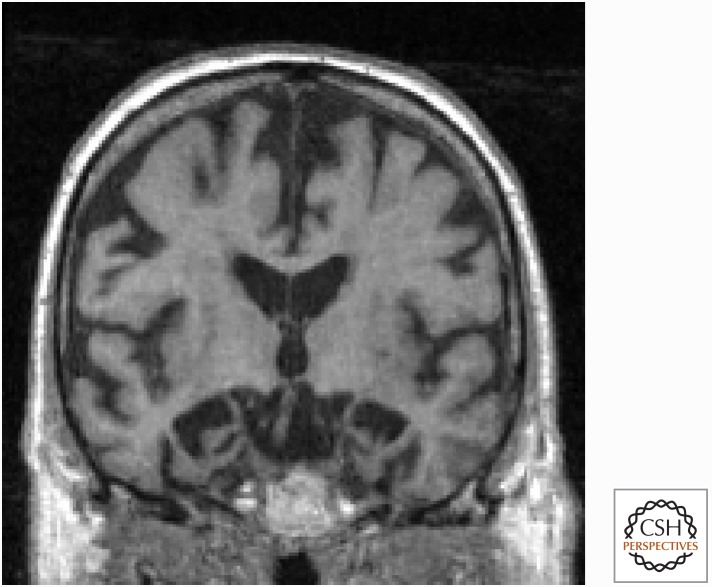
Structural MRI of patients with clinical AD shows disproportionate atrophy of the hippocampus and mesial temporal, lateral temporoparietal, and posterior cingulate/precuneus cortices bilaterally (Baron et al. 2001; Frisoni et al. 2002; Ishii et al. 2005), with the most characteristic finding being mesial temporal atrophy for typical AD (Fig. 1) (Wahlund et al. 2005; Kantarci et al. 2010; Whitwell et al. 2012). The degree of atrophy on MRI reflects the severity of pathological disease and the accumulation of neurofibrillary tangles (Silbert et al. 2003; Whitwell et al. 2008).
Figure 1.
Magnetic resonance imaging (MRI) of classic Alzheimer’s disease (AD). Coronal T1-weighted brain MRI of a 72-year-old right-handed man with memory problems for at least 4 years showing bilateral hippocampal, and less severe frontal and temporal cortical, atrophy. Orientation is radiologic (right side of figure is left side of brain). (From Sha and Rabinovici 2016, reprinted, with permission, from John Wiley and Sons.)
PET imaging can be used in different ways to evaluate patients with suspected AD. Consistent with atrophy on structural MRI, FDG-PET studies show hypometabolism within the mesial temporal and parietal areas (Hoffman et al. 2000; Silverman et al. 2001). PET studies that use tracers that specifically bind amyloid (C11-PiB [Klunk et al. 2004; Ikonomovic et al. 2008], F18-florbetapir [Wong et al. 2010; Clark et al. 2011, 2012], F18-flutemetamol [Vandenberghe et al. 2010; Wolk et al. 2011], and F18-florbetaben [Rowe et al. 2008]) can noninvasively assess if amyloid plaques are present in vivo. Although amyloid-PET imaging can reliably detect the presence or absence of amyloid with high sensitivity, amyloid commonly is found in elderly patients even without cognitive impairment (30%–40% at age 80) (Jansen et al. 2015; Ossenkoppele et al. 2015a). Thus, in this group, care must be taken not to attribute cognitive symptoms to AD merely because they have a positive scan, particularly when the clinical syndrome is not suggestive. Amyloid-PET scanning is widely available clinically, but often insurance carriers will not reimburse for the test. The large Imaging Dementia—Evidence for Amyloid Scanning (IDEAS) study in the United States, with >18,000 subjects and funded by Medicare, is currently assessing the clinical utility of amyloid PET to determine if Medicare should provide reimbursement in the future (Rabinovoci et al. 2015). PET tracers that bind to tau are under investigation and appear promising (Maruyama et al. 2013; Xia et al. 2013; Okamura et al. 2014; Johnson et al. 2016), but they are not yet clinically available.
Cerebrospinal Fluid and Other Laboratory Testing
Cerebrospinal fluid (CSF) analysis can also provide biomarker support for the diagnosis of AD. Elevated levels of tau and phosphorylated-tau (at residues 181 and 231) in combination with reduced levels of soluble Aβ1-42 amyloid distinguish AD patients from controls based on imaging tests (Shaw et al. 2009) and correlate with the presence of AD pathology at autopsy (Tapiola et al. 2009; reviewed in Blennow and Hampel 2003; Blennow et al. 2010). The presence of CSF AD biomarkers in patients with mild cognitive impairment increases their risk of developing AD (Hansson et al. 2006).
Genetics
The risk of developing AD increases with a positive family history of the disease. Having a first-degree relative with AD increases the risk by up to 3.5-fold, and this rises further if more relatives are affected (van Duijn et al. 1991). AD infrequently presents with an autosomal dominant inheritance pattern (<1% of cases), and when this occurs, it is usually caused by mutations in one of three genes: presenilin 1 (PSEN1), which is the most common; presenilin 2 (PSEN2); or amyloid precursor protein (APP). These genetic forms typically present decades earlier than sporadic AD, with a mean age of 46 years in a recent meta-analysis (Ryman et al. 2014). One study found that these inherited phenotypes account for 13% of patients with early-onset AD (Campion et al. 1999). The APP gene is on chromosome 21, which may help explain the relationship between trisomy 21 (Down’s syndrome) and the high rates of early-onset AD in individuals with this disease (Margallo-Lana et al. 2004).
The risk of developing sporadic AD is related to the presence of specific allelic variants (ε2, ε3, and ε4) of the polymorphic apolipoprotein E (APOE), with ε4 being associated with significantly higher risk (Jarvik et al. 1996). The frequency of the ε4 allele varies across ethnicities of individuals with the disease—from 9% in the Japanese population to 20% in African–Americans. The ε3 allele is the most common in the general population (72%–87%) and in those with AD (Myers et al. 1996). The presence of one ε4 allele increases the risk of sporadic AD two- to threefold, whereas two copies increase the risk 8- to 12-fold (Myers et al. 1996; Farrer et al. 1997; Slooter et al. 2004). ApoE ε4 is associated with decreased survival in men (Dal Forno et al. 2002), rapidity of cognitive decline (Martins et al. 2005), hippocampal volume loss (Mori et al. 2002), and the density of neuritic plaques shown at autopsy (Drzezga et al. 2009). The presence of the ε2 allele may be protective (Corder et al. 1994; Myers et al. 1996; Farrer et al. 1997).
Pathology
The hallmark pathological features of AD are mentioned above. The neuroanatomical distribution of neurofibrillary tangles and neuritic plaques differ, as observed by Braak and Braak (1991). Typically, neurofibrillary tangles are initially seen in the entorhinal cortex before spreading to the hippocampus (e.g., subiculum) and other paralimbic structures (e.g., basal forebrain nuclei, amygdala, anterodorsal thalamic nuclei). They then spread to the mesial temporal and parietal/retrosplenial isocortex and other subcortical structures and ultimately to the prefrontal areas. Primary motor, sensory, and visual areas tend to accumulate plaques only very late in the disease course (Braak and Braak 1991).
Amyloid plaque formation, however, tends to be more irregular and less reliable for use as a staging tool than is the deposition of neurofibrillary tangles. In general, plaques tend to form initially within the basal isocortex (frontal, temporal, occipital) followed by spread through the association cortices, and late involvement of the primary sensorimotor areas. The hippocampus is largely spared. Subcortical structures (including the striatum, thalamus, and hypothalamus) also accumulate amyloid (Braak and Braak 1991). Atypical pathological forms of AD, such as posterior cortical atrophy and frontal variants, tend to not conform to Braak’s staging and may spare the hippocampus (Murray et al. 2011).
Management/Treatment
There are currently no proven disease-modifying pharmacologic treatments for AD, although therapies targeting aspects of both amyloid and/or tau are under active investigation. Medical management of AD is therefore aimed at improving patient symptoms and optimizing both the patient’s and caregiver’s quality of life. Acetylcholine (ACh), a widely distributed neurotransmitter known to enhance cognition, is reduced in patients with AD. Raising the level of ACh via the use of acetylcholinesterase inhibitors (e.g., donepezil, rivastigmine, and galantamine) has been associated with improved cognition compared with placebo (Birks and Harvey 2003; Olin and Schneider 2001; Birks et al. 2015). Memantine, an N-methyl-d-aspartate (NMDA)-receptor antagonist believed to work by suppressing glutamate-mediated excitotoxicity, has been shown to reduce clinical deterioration on several scales in patients with moderate-to-severe AD compared with controls (Howard et al. 2012; Reisberg et al. 2003), but not in patients with mild disease (McShane et al. 2006). Combining acetylcholinesterase inhibition and memantine may have a marginal benefit compared with treatment with a single drug, although improved functional outcomes have not been shown (Farrimond et al. 2012). Moreover, the relatively modest benefits of these treatments should be considered alongside the potential side effects of each option. Controlling vascular risk factors (e.g., hypertension, hyperlipidemia, obstructive sleep apnea) is important to prevent and treat vascular cognitive impairment. AD patients may suddenly worsen as a result of a superimposed medical condition (e.g., infection, metabolic disturbance), and rapid deterioration in these patients warrants an evaluation for these etiologies.
Neuropsychiatric symptoms are common in AD, and nonpharmacologic management of these symptoms is preferred when possible. Psychiatric or behavioral manifestations of AD sometimes respond to standard symptomatic treatments for AD (acetylcholinesterase inhibitors or memantine), but often they require treatment with psychiatric medications. Selective serotonin reuptake inhibitors (SSRIs) with low anticholinergic properties (e.g., citalopram, escitalopram, fluoxetine) may treat depression, although supporting evidence is limited (Seitz et al. 2011). Neuroleptic medications should be avoided when possible given their limited efficacy (Sink et al. 2005) and increased risk of mortality; however, sometimes these medications are necessary for severe behavioral phenotypes when nonpharmacological or other treatments are unsuccessful.
Nonpharmacological interventions, such as cognitive rehabilitation (Woods et al. 2012), exercise (Forbes et al. 2015), and occupational therapy (Graff et al. 2008), help treat patients with dementia in some instances. Active social and mental engagement may also be helpful (Lyketsos et al. 2006).
Differential Diagnosis
The differential diagnosis of AD includes vascular dementia, other neurodegenerative diseases (e.g., frontotemporal lobar degeneration [FTLD], dementia with Lewy bodies [DLB]), limbic encephalopathies, vitamin deficiencies, and general medical conditions. Cerebrovascular disease and AD are frequently comorbid conditions, and distinguishing their relative contributions to a patient’s cognitive profile can be challenging.
DLB is a neurodegenerative disorder with cognitive features that overlap with AD (e.g., amnesia), although clinical features that can help distinguish DLB from AD are early hallucinations and illusions, parkinsonism, autonomic features, an antecedent rapid eye movement (REM) sleep behavioral disorder, and sensitivity to pharmacologic dopamine blockade. DLB is often pathologically comorbid with AD (Hamilton 2000). A recent study comparing patients with pathologically determined AD alone versus AD and DLB showed that patients with copathology tended to present earlier and are more likely to be men, have an ApoE ε4 allele, have more behavioral problems (delusions, hallucinations, sleep problems), and have more severe parkinsonian features (Chung et al. 2015).
Distinguishing AD and frontotemporal dementia (FTD) and its related disorders requires attention to the clinical phenotypes under consideration (see section on FTD below). Behavioral variant FTD (bvFTD) is characterized by prominent behavioral features (e.g., apathy, loss of empathy, compulsions, and altered eating habits) and a dysexecutive neuropsychological profile, whereas these are rare presenting features of typical AD. Atypical cases of AD (see description above) can closely resemble FTD spectrum disorders (primary progressive aphasia [PPA], bvFTD), and in these cases MRI and AD biomarker studies (e.g., amyloid-PET, CSF Aβ1-42 amyloid, t-tau, and p-tau) can help distinguish the two diagnostic entities. Patients with AD, for example, often show more atrophy within the lateral parietal and occipital cortices on MRI than individuals with pathologically proven FTD. However, both groups show similar patterns of atrophy within the dorsolateral prefrontal cortex and medial temporal lobes (including the hippocampus and amygdala) (Rabinovici et al. 2007).
Other medical conditions can mimic aspects of AD, including metabolic abnormalities (e.g., hypothyroidism, electrolyte disturbances), nutritional deficiencies (e.g., Wernicke’s encephalopathy, pellagra, B12 deficiency), infection (e.g., syphilis, human immunodeficiency virus [HIV]), side effects of some medications (e.g., benzodiazepines, anticholinergics), normal pressure hydrocephalus, and psychiatric disease, among others. Other causes of structural brain disease, such as slow-growing tumors or chronic subdural hematoma, rarely mimic AD.
FRONTOTEMPORAL DEMENTIA
FTD is the umbrella term for a group of heterogeneous clinical syndromes resulting from neurodegeneration predominantly within the frontal and anterior temporal lobes, insular cortex, and subcortical structures. Early changes in emotion and behavior, language, and motor skills are the hallmark features of FTD and reflect dysfunction in the aforementioned structures. The clinically defined core syndromes within the FTD spectrum include bvFTD and PPA, the latter of which includes three distinct variants: semantic (svPPA), nonfluent/agrammatic (nfvPPA), and logopenic (lvPPA). There is considerable clinical overlap with other related neurodegenerative conditions, including progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and motor neuron disease co-occurring with other FTD phenotypes (FTD motor neuron disease [FTD-MND]), although these syndromes include symptoms that localize outside the frontal–temporal–insular networks and usually have prominent motor system involvement. A brief overview of FTD epidemiology, pathology, and genetics is provided below before focusing on the individual clinical entities.
Epidemiology
FTD is a common cause of early onset dementia in patients younger than 65. It is typically diagnosed in middle age and has an average age of onset of 56, although it has been reported in patients as early as their second decade (Stone et al. 2003), with ∼13% of cases occurring before age 50 (Onyike and Diehl-Schmid 2013). The overall incidence of FTD ranges from 1 to 17 cases per 100,000 people (Onyike and Diehl-Schmid 2013). In individuals of more than 70 years of age, the range narrows from 1 to 4 cases per 100,000 (Mercy et al. 2008; Knopman and Roberts 2011; Onyike and Diehl-Schmid 2013). Systematic analysis of eight population-based studies from Europe, Canada, and Japan yielded estimates of FTD prevalence that varied between 2 and 31 cases per 100,000 people (Onyike and Diehl-Schmid 2013). A more recent review of 26 population-based studies on FTD showed even more variation (100-fold) in the estimates of incidence and prevalence. In this analysis, the prevalence ranged from 1 to 461 people per 100,000 and the overall incidence from 0 to 33 cases per 100,000 person-years (Hogan et al. 2016). The overall rates of FTD among men and women appear to be equal (Hogan et al. 2016), although individual studies show variability (Onyike and Diehl-Schmid 2013; Coyle-Gilchrist et al. 2016). The distribution of subtypes is not equal; bvFTD, for example, is 1.5 to 2.5 times more common than nfvPPA and 1.8 to 3 times more common than svPPA (Johnson et al. 2005; Coyle-Gilchrist et al. 2016).
Pathology
The clinical entities that comprise FTD are distinguished from the multiple pathological processes that underlie them, and these pathological processes are referred to generally as frontotemporal lobar degeneration (FTLD). The clinical-pathologic relationships between FTD and FTLD are complex, and distinct clinical entities often show considerable heterogeneity of their underlying pathology. For example, bvFTD can be associated with several different pathologies, including tauopathies, TDP-43, and FUS (Ljubenkov and Miller 2016). Conversely, a single pathological process can produce diverse clinical phenotypes; PSP pathology can cause not only Steele–Richardson–Olszewski (i.e., Richardson’s) syndrome but also nfvPPA and CBS (Ljubenkov and Miller 2016) as discussed below.
Gross pathologic changes associated with FTLD include focal atrophy within the cortical and subcortical networks that support language and behavioral regulation, which manifests microscopically as neuron cell death, microvacuolization, swollen neurons, white matter myelin loss, and gliosis within the affected areas (Cairns et al. 2007). FTLD is associated with the accumulation of protein aggregates/inclusions within neurons and glia, and the particular molecular composition of these aggregates is used to define pathological subtypes of the disease. These aggregates include tau (FTLD-tau), transactive response DNA-binding protein 43 kDa (FTLD-TDP), fused in sarcoma protein (FTLD-FUS), and others (Sieben et al. 2012), with FTLD-tau and FTLD-TDP making up the vast majority of cases (∼90%) and being roughly equal in their frequency (Snowden et al. 2007; Rohrer et al. 2011). TDP is subdivided into four pathological subtypes, A–D (Mackenzie et al. 2011).
Genetics
Approximately 40% of patients with FTD have a first degree relative with dementia (Goldman et al. 2005), and 15% of cases have a family history that suggests autosomal dominant inheritance (Goldman et al. 2005; Coyle-Gilchrist et al. 2016). The majority of these genetic cases are explained by mutations in three genes: MAPT, chromosome 9 open reading frame 72 (C9ORF72), and granulin (GRN) (Galimberti and Scarpini 2012; Sieben et al. 2012). Familiality varies based on the FTD subtype, with svPPA showing the least amount of familial cases (17%) and FTD-MND showing the most (59%) (Goldman et al. 2005).
Clinical Symptoms, Diagnosis, Imaging, and Differential Diagnosis
Behavioral Variant FTD
bvFTD is the most common of the core FTD spectrum clinical syndromes (Hogan et al. 2016) and is characterized clinically by early changes in behavior, personality, emotion, and executive control. The defining features of the syndrome include early behavioral disinhibition (including socially inappropriate behavior, loss of decorum, and impulsiveness), apathy or inertia, loss of empathy or sympathy, perseverative, stereotyped, or compulsive/ritualistic behaviors, dietary changes (including changing food preferences, binge eating, and oral exploratory behaviors), and a neuropsychological profile that is primarily dysexecutive with sparing of memory and visuospatial skills (Rascovsky et al. 2011). See Box 2 for diagnostic criteria. These symptoms are thought to reflect dysfunction in the nondominant prefrontal cortex, anterior temporal lobe, paralimbic structures (anterior cingulate, frontal insular and lateral orbitofrontal cortices), hippocampus, and subcortical structures (ventral striatum and dorsomedial thalamus) (Rosen et al. 2005; Rankin et al. 2006; Seeley et al. 2008; Seeley 2010). The neuroanatomical substrates underlying the specific symptomatology in bvFTD are reviewed elsewhere (Lanata and Miller 2016).
Box 2.
Diagnostic criteria for behavioral variant FTD, svPPA, nfvPPA, and lvPPA (Gorno-Tempini et al. 2011; Rascovsky et al. 2011)
Diagnostic criteria for behavioral variant FTD
I. Neurodegenerative disease
The following symptom must be present to meet criteria for bvFTD
Shows progressive deterioration of behavior and/or cognition by observation or history (as provided by a knowledgeable informant).
II. Possible bvFTD
Three of the following behavioral/cognitive symptoms (A–F) must be present to meet criteria. Ascertainment requires that symptoms be persistent or recurrent, rather than single or rare events.
- Early* behavioral disinhibition (one of the following symptoms [A.1–A.3] must be present):
-
A.1.Socially inappropriate behavior
-
A.2.Loss of manners or decorum
-
A.3.Impulsive, rash, or careless actions
-
A.1.
- Early apathy or inertia (one of the following symptoms [B.1–B.2] must be present):
-
B.1.Apathy
-
B.2.Inertia
-
B.1.
- Early loss of sympathy or empathy (one of the following symptoms [C.1–C.2] must be present):
-
C.1.Diminished response to other people’s needs and feelings
-
C.2.Diminished social interest, interrelatedness, or personal warmth
-
C.1.
- Early perseverative, stereotyped, or compulsive/ritualistic behavior (one of the following symptoms [D.1–D.3] must be present):
-
D.1.Simple repetitive movements
-
D.2.Complex, compulsive, or ritualistic behaviors
-
D.3.Stereotypy of speech
-
D.1.
- Hyperorality and dietary changes (one of the following symptoms [E.1–E.3] must be present):
-
E.1.Altered food preferences
-
E.2.Binge eating, increased consumption of alcohol or cigarettes
-
E.3.Oral exploration or consumption of inedible objects
-
E.1.
- Neuropsychological profile: executive/generation deficits with relative sparing of memory and visuospatial functions (all of the following symptoms [F.1–F.3] must be present):
-
F.1.Deficits in executive tasks
-
F.2.Relative sparing of episodic memory
-
F.3.Relative sparing of visuospatial skills
-
F.1.
III. Probable bvFTD
All of the following symptoms (A–C) must be present to meet criteria.
Meets criteria for possible bvFTD
Exhibits significant functional decline (by caregiver report or as evidenced by Clinical Dementia Rating Scale or Functional Activities Questionnaire scores)
- Imaging results consistent with bvFTD (one of the following [C.1–C.2] must be present):
-
C.1.Frontal and/or anterior temporal atrophy on MRI or CT
-
C.2.Frontal and/or anterior temporal hypoperfusion or hypometabolism on PET or SPECT
-
C.1.
IV. Behavioral variant FTD with definite FTLD pathology
Criterion A and either criterion B or C must be present to meet criteria.
Meets criteria for possible or probable bvFTD
Histopathological evidence of FTLD on biopsy or at postmortem
Presence of a known pathogenic mutation
V. Exclusionary criteria for bvFTD
Criteria A and B must be answered negatively for any bvFTD diagnosis. Criterion C can be positive for possible bvFTD but must be negative for probable bvFTD.
Pattern of deficits is better accounted for by other nondegenerative nervous system or medical disorders
Behavioral disturbance is better accounted for by a psychiatric diagnosis
Biomarkers strongly indicative of Alzheimer’s disease or other neurodegenerative process
*As a general guideline “early” refers to symptom presentation within the first 3 years.
Diagnostic criteria for semantic variant PPA
I. Clinical diagnosis of semantic variant PPA
Both of the following core features must be present:
Impaired confrontation naming
Impaired single-word comprehension
At least three of the following other diagnostic features must be present:
Impaired object knowledge, particularly for low-frequency or low-familiarity items
Surface dyslexia or dysgraphia
Spared repetition
Spared speech production (grammar and motor speech)
II. Imaging-supported semantic variant PPA diagnosis
Both of the following criteria must be present:
Clinical diagnosis of semantic variant PPA
- Imaging must show one or more of the following results:
- Predominant anterior temporal lobe atrophy
- Predominant anterior temporal hypoperfusion or hypometabolism on SPECT or PET
III. Semantic variant PPA with definite pathology
Clinical diagnosis (criterion A below) and either criterion B or C must be present:
Clinical diagnosis of semantic variant PPA
Histopathologic evidence of a specific neurodegenerative pathology (e.g., FTLD-tau, FTLD-TDP, AD, other)
Presence of a known pathogenic mutation
Diagnostic criteria for nonfluent/agrammatic variant PPA
I. Clinical diagnosis of nonfluent/agrammatic variant PPA:
At least one of the following core features must be present:
Agrammatism in language production
Effortful, halting speech with inconsistent speech sound errors and distortions (apraxia of speech)
At least two of three of the following other features must be present:
Impaired comprehension of syntactically complex sentences
Spared single-word comprehension
Spared object knowledge
II. Imaging-supported nonfluent/agrammatic variant diagnosis
Both of the following criteria must be present:
Clinical diagnosis of nonfluent/agrammatic variant PPA
- Imaging must show one or more of the following results:
- Predominant left posterior frontoinsular atrophy on MRI or
- Predominant left posterior frontoinsular hypoperfusion or hypometabolism on SPECT or PET
III. Nonfluent/agrammatic variant PPA with definite pathology
Clinical diagnosis (criterion 1 below) and either criterion 2 or 3 must be present:
Clinical diagnosis of nonfluent/agrammatic variant PPA
Histopathologic evidence of a specific neurodegenerative pathology (e.g., FTLD-tau, FTLD-TDP, AD, other)
Presence of a known pathogenic mutation
Diagnostic criteria for logopenic variant PPA
I. Clinical diagnosis of logopenic variant PPA
Both of the following core features must be present:
Impaired single-word retrieval in spontaneous speech and naming
Impaired repetition of sentences and phrases
At least three of the following other features must be present:
Speech (phonologic) errors in spontaneous speech and naming
Spared single-word comprehension and object knowledge
Spared motor speech
Absence of frank agrammatism
II. Imaging-supported logopenic variant diagnosis
Both criteria must be present:
Clinical diagnosis of logopenic variant PPA
- Imaging must show one or more of the following results:
- Predominant left posterior perisylvian or parietal atrophy on MRI
- Predominant left posterior perisylvian or parietal hypoperfusion or hypometabolism on SPECT or PET
III. Logopenic variant PPA with definite pathology
Clinical diagnosis (criterion 1 below) and either criterion 2 or 3 must be present:
Clinical diagnosis of logopenic variant PPA
Histopathologic evidence of a specific neurodegenerative pathology (e.g., AD, FTLD-tau, FTLD-TDP, other)
Presence of a known pathogenic mutation
Formal diagnostic criteria allow a conclusion of “possible bvFTD” based on symptomatology alone, whereas “probable bvFTD” requires imaging findings and documentation of functional decline. Definitive diagnosis of “bvFTD with FTLD pathology” requires a histopathological analysis (via brain biopsy or autopsy) or the presence of a known pathological mutation (Rascovsky et al. 2011).
Neuroimaging can be helpful to assess patients who meet the clinical criteria for bvFTD. Although the brain may appear normal on structural imaging early in the disease course (Perry et al. 2006), more typical findings include volume loss within the right-side frontal, anterior temporal, and anterior insular cortices (Fig. 2A) (Rosen et al. 2002a; Perry et al. 2006; Seeley et al. 2008). SPECT and FDG-PET imaging are useful to distinguish FTD from AD and other neurodegenerative diseases based on patterns of regional hypometabolism (Foster et al. 2007; Mendez et al. 2007), although these techniques might not differentiate bvFTD from frontal variants of AD. Amyloid-PET can be helpful to assess for underlying AD pathology as a contributing etiology (Engler et al. 2008; Rabinovici et al. 2011).
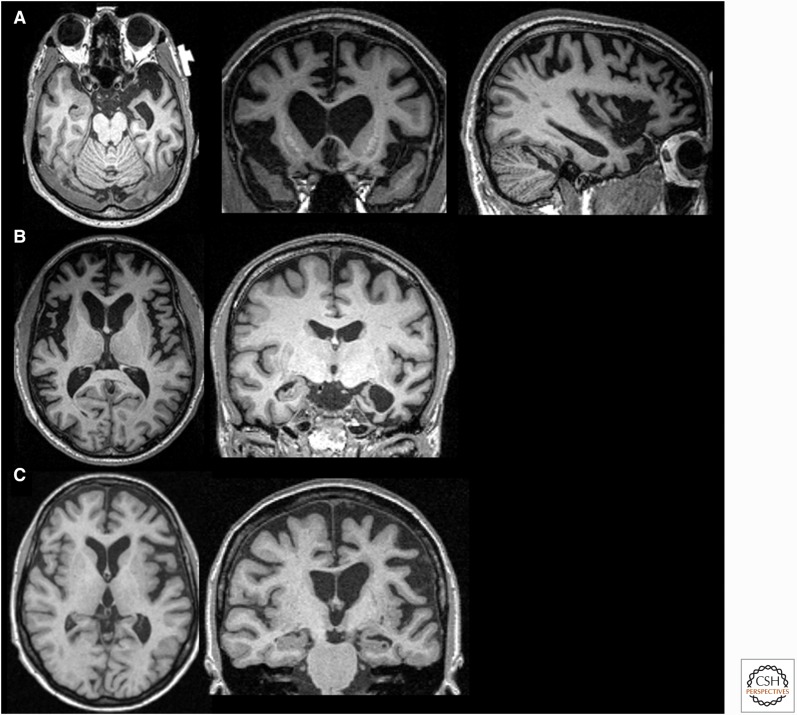
Figure 2.
Magnetic resonance imaging (MRI) in three variants of frontotemporal dementia (FTD). T1-weighted brain MRIs in behavioral variant FTD (bvFTD) (A), semantic variant primary progressive aphasia (svPPA) (B), and nonfluent variant (nfvPPA) (C). (A) A 55-year-old woman with a 4-year history of bvFTD with a score of 27/30 on the mini-mental status examination (MMSE) showing an axial, coronal, and sagittal (right side) MRI with significant bilateral (right more than left) frontal atrophy. (B) A 61-year-old man with svPPA showing symptoms for 1.5 years that included forgetting the names of friends and the names and knowledge of common objects. He also showed difficulty with planning, multitasking, and marked rigidity of daily routines. MRI shows severe left temporal pole atrophy. (C) A 74-year-old man with 2 years of progressive word-finding difficulty, slowed and effortful speech, phonemic paraphasias, and speech apraxia. MRI shows left insular and perisylvian atrophy consistent with nfvPPA. Orientation of coronal and axial MRIs are radiologic. (Images courtesy of Dr. David Perry.)
Differential Diagnosis
The differential diagnosis of bvFTD is broad, particularly early in the disease course, and includes psychiatric and other neurodegenerative disorders. Given its predominantly psychopathological manifestations (e.g., compulsions, disinhibitions), bvFTD is often misdiagnosed in patients as primary psychiatric disease (up to 50% of cases) (Woolley et al. 2011; Lanata and Miller 2016), including schizophrenia, schizoaffective disorder, bipolar disorder, depression (Velakoulis et al. 2009), obsessive compulsive disorder (Tonkonogy et al. 1994), and other psychiatric disorders (Lanata and Miller 2016). Patients with a static, nonprogressive, imaging-negative bvFTD are given the term “bvFTD phenocopy” (Rascovsky and Grossman 2013), and some of these patients have genetic alterations in the C9ORF72 gene (Khan et al. 2012). AD (Ossenkoppele et al. 2015b) and DLB can have overlapping features with bvFTD. FTD often can be distinguished from frontal AD variants by structural MRI, as patients with AD more often show mesial temporal and posterior atrophy compared with those with bvFTD (Ossenkoppele et al. 2015b), PET imaging (both with FDG and especially amyloid-binding tracers) (Rabinovici et al. 2011), and CSF biomarkers (total tau, phosphorylated tau, and Aβ1-42) (Ewers et al. 2015).
Primary Progressive Aphasia
PPA is a core clinical phenotype within the FTD spectrum and clinically is defined as the progressive loss of language function caused by neurodegeneration that interferes with daily life. Language deficits must be the earliest and primary cause of disability in the early stages of the illness (Mesulam 2003; Gorno-Tempini et al. 2011). PPA has three well-described variants—semantic (svPPA), nonfluent/agrammatic (nfvPPA), and logopenic (lvPPA)—and each reflect dysfunction within different aspects of the language system (Gorno-Tempini et al. 2011).
Semantic Variant
In terms of the epidemiology of svPPA, the mean age at diagnosis is 64–67 years, and median survival from symptom onset is 10.6–12.8 years, which is longer than other forms of FTD (Hodges et al. 2010; Coyle-Gilchrist et al. 2016). svPPA is the least likely of the FTD subtypes to be familial and occurs in an estimated 2%–7% of cases (Goldman et al. 2005; Hodges et al. 2010). Most patients with svPPA have underlying pathological features consistent with TDP-C, although Pick’s disease and AD are rarely also reported (Hodges et al. 2010; Harris et al. 2013).
svPPA is symptomatically characterized by the progressive degradation of semantic knowledge. Patients with svPPA have impairment in confrontational naming (i.e., the ability to produce the word for an object after seeing it or its picture), single-word comprehension, and object knowledge (particularly for uncommon objects), with spared repetition and speech sound production (Gorno-Tempini et al. 2011). Anomia usually begins with uncommon words (Kramer et al. 2003) and is accompanied by vague, empty-sounding speech. There is often surface dyslexia and dysgraphia (i.e., inability to spell, read, or recognize words with atypical spellings such as “yacht” or “colonel”). Fluency, repetition, and grammar are characteristically preserved. See Box 2 for diagnostic criteria. This syndrome is thought to result from dysfunction within the left anterior temporal lobe and its connections (Seeley et al. 2005). When the temporal lobar atrophy is right-sided or bilateral, clinical svPPA can be associated with early behavioral changes reminiscent of bvFTD and have semantic loss related to facial and emotional recognition (Chan et al. 2009; Henry et al. 2014). The behavioral phenotype of svPPA (right temporal form) can include hyper-religiosity, lack of empathy, obsessional behaviors, and lack of insight (Chan et al. 2009).
Imaging can be helpful in diagnosing svPPA. Structural MRI typically shows anterior temporal lobar atrophy, particularly along the inferior temporal gyrus (Fig. 2B) (Rosen et al. 2002a,b). Similar anatomical distributions can be seen with the imaging modalities single photon emission computed tomography (SPECT) and FDG-PET, which show hypoperfusion and hypometabolism, respectively (Gorno-Tempini et al. 2011).
Nonfluent Variant
nfvPPA accounts for ∼15% of all FTD-spectrum diagnoses (including CBD and PSP). Most patients diagnosed with nfvPPA present between the ages of 55 and 70 years (Hodges et al. 2010), with an average age of onset of 67 (Coyle-Gilchrist et al. 2016). Median survival after the onset of symptoms is 8–12 years (Hodges et al. 2010; Coyle-Gilchrist et al. 2016).
nfvPPA is characterized by progressive errors in motor speech production and grammatic structure (Gorno-Tempini et al. 2011), similar to Broca’s aphasia. The extent of these deficits varies between cases, although pure agrammatism is rare. nfvPPA often presents with slow, effortful speech with errors in the articulatory plan (i.e., apraxia of speech). Motor speech errors can be inconsistent and include distortions, deletions, substitutions, transpositions, and insertions; aprosodia is often an accompanying feature. Agrammatism manifests as difficulty in understanding sentences (particularly those with complex forms) with relatively preserved comprehension of single words. These deficits are thought to reflect dysfunction within the regions known to underlie motor speech planning, including a circuit involving the left inferior frontal gyrus, insula, premotor, and supplementary motor areas (Gorno-Tempini et al. 2004).
Formal research diagnostic criteria for nfvPPA include symptoms of either agrammatism or effortful, halting speech, with two out of the three following features: impaired comprehension of syntactically complex sentences, spared single-word comprehension, and spared object knowledge. The diagnosis of “imaging-supported” nfvPPA requires meeting the clinical criteria above as well as showing left posterior frontoinsular atrophy on MRI or corresponding metabolic/perfusion abnormalities on PET/SPECT. Definitive pathological diagnosis requires histological analysis or the presence of a known mutation (Gorno-Tempini et al. 2011). See Box 2 for diagnostic criteria.
Structural MRI often reveals atrophy within the aforementioned regions (Fig. 2C) (Gorno-Tempini et al. 2004; Josephs et al. 2006). FDG-PET (Grossman et al. 1996) and SPECT imaging (Mesulam 2003) show hypometabolism in the same regions. The underlying pathology is most often associated with FTLD-tau, although FTLD-TDP and AD pathology also occur (Harris and Jones 2014).
Logopenic Variant
A third well-described PPA clinical subtype is the logopenic variant (lvPPA), which presents with errors in word retrieval and sentence repetition (particularly for longer sentences and phrases). Speech is often slow and interrupted by word-finding pauses, and unlike nfvPPA, grammatical structures, prosody, and articulatory speech sounds (diction) remain largely intact. Phonologic paraphasic errors (using similar sounding words) are common. lvPPA deficits are hypothesized to emerge from errors in phonologic short-term memory (Gorno-Tempini et al. 2008, 2011). Clinical diagnostic criteria for lvPPA require impairment in both single-word retrieval (in spontaneous speech and naming) and repetition of sentences and phrases, as well as at least three of the following symptoms: phonologic errors (in spontaneous speech and naming), spared single-word comprehension and object knowledge, spared motor speech, and absence of frank agrammatism (Gorno-Tempini et al. 2011). See Box 2 for diagnostic criteria. Neuroimaging studies commonly show abnormalities within the left temporoparietal junction, including atrophy on structural MRI or hypometabolism on FDG-PET (Gorno-Tempini et al. 2004; Madhavan et al. 2013).
The vast majority of lvPPA cases have underlying AD pathology, although FTLD pathology is rarely reported (Rabinovici et al. 2008; Grossman 2010; Mesulam et al. 2014). In lvPPA, neurofibrillary tangles are generally distributed asymmetrically within the hemispheres, with the left more involved than the right (Mesulam et al. 2008; Gefen et al. 2012). Clinical lvPPA, therefore, is most often categorized as an atypical variant of AD. CSF analysis or amyloid imaging to determine the presence of AD biomarkers can be useful when the underlying pathology is unclear based on other clinical features. The presence of APOE ε4 does not predict pathology in lvPPA patients (Mesulam et al. 2008). Pharmacologic treatment of lvPPA is similar to that for patients with more typical presentations of AD (see section above on AD).
Treatment
Treatment of FTD-spectrum disorders is aimed at controlling symptoms, as there are no therapies proven to alter their underlying pathological processes, although clinical trials are in progress. In bvFTD, management strategies include the use of SSRIs (Swartz et al. 1997; Moretti et al. 2003; Anneser et al. 2007; Herrmann et al. 2012), trazodone (Lebert et al. 2004), dopamine blockade (Sink et al. 2005), and others. Nonpharmacologic interventions such as caregiver support and education, a Mediterranean diet, regular aerobic exercise, physical therapy for motor and gait impairment, swallow evaluation, optimization of home safety (including removal of firearms), stewardship over finances, and cessation of driving privileges are warranted depending on the clinical context (Ljubenkov and Miller 2016). Early referral to speech therapy is recommended for all PPAs. For lvPPA caused by AD, standard AD treatments, including acetylcholinesterase inhibitors, should be considered.
Frontotemporal Dementia Spectrum Syndromes with Prominent Motor Features
Frontotemporal Dementia-Motor Neuron Disease (FTD-MND)
There is substantial clinical overlap between patients with amyotrophic lateral sclerosis (ALS) and bvFTD, as 15% of bvFTD cases develop symptoms of ALS (Rascovsky et al. 2011) and 30% of ALS patients experience symptoms of bvFTD (Lomen-Hoerth 2011). The syndrome in which both illnesses coexist is referred to as FTD-MND.
FTD-MND is associated with a shorter survival (2.4 years from symptom onset) compared with bvFTD alone (6.6 years) (Lillo et al. 2010), classic ALS without cognitive changes (Olney et al. 2005), and other FTD syndromes (e.g., nfvPPA) (Hodges et al. 2003).
MND is characterized by findings that suggest both upper and lower motor neuron dysfunction. Upper motor neuron signs include hyperreflexia (e.g., clonus, spreading across multiple joints, positive Babinski and Hoffman signs), spasticity, and slow speech, whereas lower motor neuron findings include fasciculations, atrophy, and weakness. Electromyography can aid in diagnosis. Bulbar weakness appears to be overrepresented in cases of FTD-MND versus MND alone (Portet et al. 2001). Behavioral symptoms in cases of FTD-MND are typically of the bvFTD phenotype, and the presence of early delusional thinking in patients with bvFTD predicts subsequent development of FTD-MND (Lillo et al. 2010). Pseudobulbar affect is also common in cases of FTD-MND.
On structural MRI, patients with either ALS or FTD-MND show widespread atrophy of the frontotemporal cortices (including the premotor cortices), although the frontal regions are more atrophied in cases of FTD-MND (Chang et al. 2005).
The pathological changes seen in FTD-MND are typically associated with TDP-B (Mackenzie 2007; Mackenzie et al. 2011), although TDP-A (Rohrer et al. 2011) and FUS (Mackenzie et al. 2010) have also been reported. C9ORF72 expansions account for more than half of the inherited cases of FTD-MND (Cooper-Knock et al. 2015).
Progressive Supranuclear Palsy Syndrome
The mean age of onset of progressive supranuclear palsy syndrome (PSP-S) is 63 years (Golbe et al. 1988), and PSP-S rarely, if ever, occurs before the age of 40. Prevalence estimates range from 1.4 (Golbe et al. 1988) to 6.4 individuals per 100,000 (Schrag et al. 1999). Median survival after symptom onset is ∼6.9 years (Coyle-Gilchrist et al. 2016). Steele–Richardson–Olszewski syndrome (i.e., Richardson’s syndrome), the classic syndrome of PSP-S, is more rapidly progressive than other PSP variants (e.g., PSP-Parkinson’s) (O’Sullivan et al. 2008).
Steele–Richardson–Olszewski syndrome is clinically characterized by early postural instability, falls, and eye movement abnormalities, typically a vertical supranuclear gaze palsy or slowed vertical saccades. Accompanying features include early dysphagia and dysarthria, symmetric akinesia or rigidity (proximal more than distal), abnormal neck posturing (typically retrocollis), and a poor response to dopamine replacement (Litvan et al. 1996a). Typical parkinsonian features are common, including reduced eye blink with hypomimia, sitting “en bloc,” and bradykinesia. Prominent cognitive and behavioral changes often accompany the motor syndrome described above, and usually reflect frontal dysfunction, and include apathy, impulsivity, inattention, personality changes, and slowed processing speed, with memory, language, and visuospatial skills relatively spared (Litvan et al. 1996b; Donker Kaat et al. 2007; Bak et al. 2010). Depression is common (Schrag et al. 2010). Sleep disturbances are more commonly reported in PSP than in FTD (Bak et al. 2010). Well-described findings on the neurologic examination include the procerus sign (an involuntary furrowing of the brow that produces an expression of worry or exasperation), the “applause sign” in which the patient is unable to stop clapping despite being told to stop after three claps (a nonspecific sign of frontal-lobe dysfunction) (Dubois et al. 2005), a “wide-eyed” stare, and utilization behaviors. See Table 1 for diagnostic criteria.
Table 1.
Clinical diagnostic criteria for progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD)
| PSP | Mandatory inclusion criteria | Mandatory exclusion criteria | Supportive criteria |
|---|---|---|---|
| Possible | Gradually progressive disorder | Recent history of encephalitis | Symmetric akinesia or rigidity, proximal more than distal |
| Onset at age 40 or later | Alien limb syndrome, cortical sensory deficits, focal frontal or temporoparietal atrophy | Abnormal neck posture, especially retrocollis | |
| Either vertical (upward or downward gaze) supranuclear palsy or both slowing of vertical saccades and prominent postural instability with falls in the first year of disease conset | Hallucinations or delusions unrelated to dopaminergic therapy | Poor or absent response of parkinsonism to levodopa therapy | |
| No evidence of other diseases that could explain the foregoing features, as indicated by mandatory exclusion criteria | Cortical dementia of Alzheimer’s type (severe amnesia and aphasia or agnosia, according to NINCDS-ADRA criteria) | Early dysphagia and dysarthria | |
| Probable | Gradually progressive disorder | Severe, asymmetric parkinsonian signs (i.e., bradykinesia) | Early onset of cognitive impairment including at least two of the following: apathy, impairment in abstract thought, decreased verbal fluency, utilization behaviors, or frontal release signs |
| Onset at age 40 or later | Neuroradiologic evidence of relevant structural abnormality (i.e., basal ganglia or brainstem infarcts, lobar atrophy) | ||
| No evidence of other diseases that could explain the foregoing features, as indicated by mandatory exclusion criteria | Whipple’s disease, confirmed by polymerase chain reaction, if indicated | ||
| Definite | Clinically probable or possible PSP and histopathologic evidence of typical PSP | ||
| Diagnostic criteria of clinical phenotypes associated with corticobasal degeneration | |||
| Clinical phenotypes associated with CBD | Features | ||
| Probable corticobasal syndrome (CBS) | Asymmetric presentation of two of (i) limb rigidity or akinesia, (ii) limb dystonia, (iii) limb myoclonus plus two of (iv) orobuccal or limb apraxia, (v) cortical sensory deficit, (vi) alien limb phenomena (more than simple levitation) | ||
| Possible corticobsal syndrome (CBS) | May be symmetric: one of (i) limb rigidity or akinesia, (ii) limb dystonia, (iii) limb myoclonus plus 1 of (iv) orobuccal or limb apraxia, (v) cortical sensory deficit, (vi) alien limb phenomena (more than simple levitation) | ||
| Frontal behavioral-spatial syndrome | Two of (i) executive dysfunction, (ii) behavioral or personality changes, (iii) visuospatial deficits. | ||
| Nonfluent/agrammatic variant of primary progressive aphasia | Effortful, agramamtic speech plus at least one of (i) impaired grammar/sentence comprehension with relatively preserved single word comprehension, or (ii) groping, distorted speech production (apraxia of speech) | ||
| Progressive supranuclear palsy syndrome | Three of (i) axial or symmetric limb rigidity or akinesia, (ii) postural instability or falls, (iii) urinary incontinence, (iv) behavioral changes, (v) supranuclear gaze palsy or decreased velocity of vertical saccades | ||
Source: Litvan et al. 1996a; Armstrong et al. 2013.
NINCDS-ADRA, National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer’s Disease and Related Disorders Association.
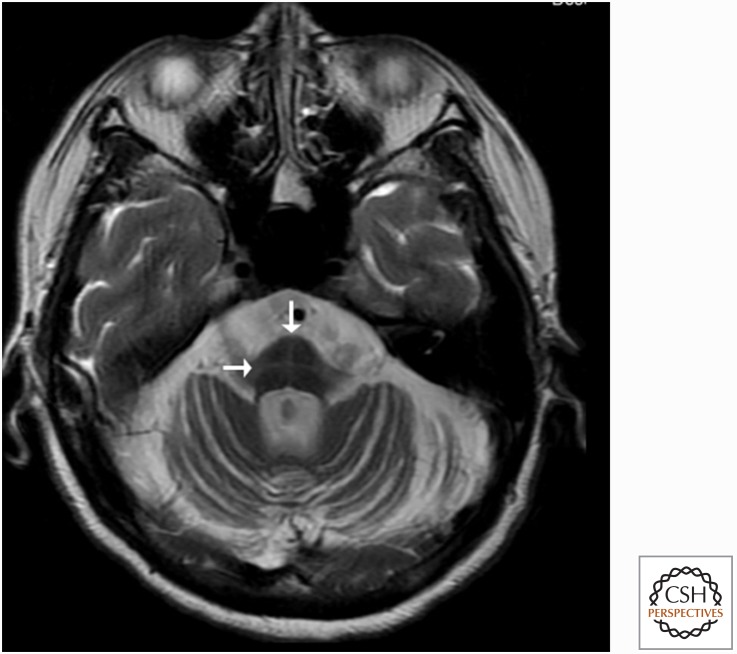
In PSP-S, structural MRI typically shows atrophy within the dorsal midbrain, pons, cerebellum, caudate, thalamus, and the frontal cortex with its associated subcortical white matter (Boxer et al. 2006; Josephs et al. 2008). Midbrain atrophy is significantly greater than in CBD (Boxer et al. 2006). When the midbrain atrophy is severe, it can appear as the “hummingbird sign” on MRI, in which on midsagittal view, the shape of the midbrain is reminiscent of a hummingbird with its beak extended (Graber and Staudinger 2009). Atrophy of the superior cerebellar peduncles is also seen in PSP (Tsuboi et al. 2003).
Pathologically, PSP is associated with atrophy within the basal ganglia, subthalamus, and brainstem, and is characterized microscopically by dense fibrillary four-repeat tau (4R tauopathy) filaments, globose-appearing neurofibrillary tangles, and glial fibrillary tangles in astrocytes and oligodendrocytes (Lee et al. 2001). These pathologic changes are distributed throughout the basal ganglia, midbrain (including the oculomotor nucleus), pons, and cerebellum (Hauw et al. 1994). Cortical involvement is variable and often correlates with the severity of cognitive impairment (Bigio et al. 1999).
Patients with histologic changes consistent with PSP pathology also are associated with a number of additional clinical phenotypes other than Steele–Richardson–Olszewski syndrome, including other PSP variants (PSP-parkinsonism [Williams et al. 2005], PSP-pure akinesia [Facheris et al. 2008], and PSP-primary progressive freezing gait [Compta et al. 2007], CBS, and FTD syndromes such as nfvPPA and bvFTD [Dickson et al. 2011]). Despite similar histopathology, these diverse phenotypes are often associated with distinct patterns of brain atrophy.
In terms of genetics, PSP-S is generally considered a sporadic disorder, although familial forms have been reported and are associated with mutations in MAPT (Donker Kaat et al. 2009). PSP is almost always associated with a particular tau haplotype (H1/H1) (Baker et al. 1999), although this genotype does not appear to affect age of onset, severity, or survival (Litvan et al. 2001).
The differential diagnosis primarily includes other neurodegenerative diseases with parkinsonism (e.g., Parkinson’s disease [PD], CBD, multiple system atrophy [MSA]), as well as vascular disease and other medical (e.g., Whipple’s causing oculomotor abnormalities) or structural (e.g., midbrain tumors) causes.
There are currently no available treatments for the underlying pathological processes of PSP, although such interventions are under investigation and treatment trials have begun. Early referral to physical, speech (for dysphagia and dysarthria), and occupational therapies are essential. Pharmacologic treatments are aimed at controlling symptoms and include medications for sleep, depression, or other behavioral changes. As PSP is usually not very responsive to carbidopa-levodopa, a trial may help diagnostically to differentiate PSP from PD; low-dose carbidopa-levodopa, however, can sometimes mildly improve some symptoms (Kompoliti et al. 1998).
Corticobasal Syndrome and Corticobasal Degeneration
The mean age of onset of CBS is 63 years (Wenning et al. 1998), with the youngest reported case occurring at the age of 45 years. The prevalence of CBS is unknown, although it is considered rare. The duration of survival after the onset of symptoms in CBS was recently reported to be 7.2 years (Coyle-Gilchrist et al. 2016). CBS is generally considered a sporadic disorder, although cases have been reported with mutations in the TREM2 gene.
CBS is the clinical entity characterized by the core motor features of limb rigidity and bradykinesia, dystonia, and myoclonus, as well as cortical dysfunction including apraxia (orobuccal or limb), cortical sensory loss (astereognosis, agraphesthesia, neglect), and alien limb phenomena (Armstrong et al. 2013). Clinical findings are typically asymmetric, although this is not always the case (Hassan et al. 2010). There may be cognitive and behavioral changes early in the course of CBS, and patients with CBS may later meet clinical criteria for bvFTD or PPA (Kertesz et al. 2005), or other clinical phenotypes (Armstrong et al. 2013). See Box 1 for diagnostic criteria. CBS is distinct from the neuropathologically defined CBD.
CBD is associated with gross asymmetric frontoparietal or paracentral lobar atrophy; numerous swollen and vacuolated “ballooned” neurons; and wispy, fine, filamentous 4R tau inclusions within cell bodies of the cerebral gray and white matter (Dickson 1999). The relationship between CBS and CBD is complex. CBS is associated with numerous underlying pathologies, including CBD, AD, PSP-tau, Pick’s-tau, TDP-43, Lewy bodies (LBs), and CJD (Boeve et al. 1999; Wadia and Lang 2007; Lee et al. 2011). CBD is associated with other clinical syndromes in addition to CBS, including progressive nonfluent aphasia, bvFTD, executive-motor syndrome, and posterior cortical atrophy (Wadia and Lang 2007; Lee et al. 2011). A large majority of CBD patients present with cognitive symptoms, whereas less than half initially show motor involvement (Lee et al. 2011).
Regardless of underlying pathology, patients with CBS typically show atrophy of the posteromedial frontal, perirolandic, and dorsal insular cortices on MRI (Lee et al. 2011). More prominent posterior involvement (e.g., parietal) may suggest underlying AD pathology, whereas frontal extension is associated with CBD pathology. Brainstem atrophy suggests PSP (Lee et al. 2011). FDG-PET studies show asymmetric hypometabolism within the posterior frontal, inferior parietal, and superior temporal regions, in addition to the subcortical structures (Coulier et al. 2003). In patients presenting with CBS, CSF analysis may also help to determine the presence of inflammation or AD biomarkers. Differential diagnosis includes other motor predominant neurodegenerative diseases, such as PD, PSP, MSA, DLB, CJD, and even AD.
SYNUCLEINOPATHIES (PARKINSONIAN NEURODEGENERATIVE DISEASES)
Idiopathic Parkinson’s Disease
Epidemiology
Idiopathic Parkinson’s disease (PD) is the second most common neurodegenerative disorder after AD. The prevalence of PD is estimated to be 0.3% in the general population, ∼1% in people older than age 60, and ∼3% in people age 80 years or older. The incidence rate of PD is 8–18 individuals per 100,000 person-years (Tanner and Goldman 1996; Nussbaum and Ellis 2003; de Lau and Breteler 2006). The median age of onset is 60 years, and the mean duration of the disease from diagnosis to death is 15 years (Lees et al. 2009). Men have 1.5–2 times higher prevalence and incidence than women (Moisan et al. 2016), and the age at onset is 2.1 years later in women than in men, or 53.4 years versus 51.3 years (Haaxma et al. 2007). Women are reported to present with milder symptoms, a higher rate of tremor (67% vs. 48% in men), and slower progression of motor disturbances.
Clinical Symptoms and Diagnosis
The cardinal motor symptoms of PD include bradykinesia, resting tremor, rigidity, and postural instability; other motor features include hypomimia, hypophonia, dysphagia, vision changes, micrographia, stooped posture, and gait freezing, among others. PD subtyping based on symptomatic features, however, suggests important differences between those with a tremor-predominant phenotype versus postural-instability and gait difficulties (PIGD), with the tremor-predominant group presenting at an earlier age but with a slower progression and a better response to dopamine replacement (Jankovic and Kapadia 2001; Thenganatt and Jankovic 2014). Patients with the PIGD type show more rapid cognitive decline and a higher incidence of dementia, whereas those who start with tremor tend to have dementia only after PIGD symptoms develop (Alves et al. 2006). Younger patients (onset before 40 years of age) with PD are more likely to have tremor, rigidity, dystonia, and levodopa-related motor complications as presenting symptoms and tend to progress more slowly, whereas patients with late-onset PD more likely present with the PIGD subtype and cognitive impairment and progress more rapidly (particularly for symptoms of mentation and freezing) (Jankovic et al. 1990; Jankovic and Kapadia 2001; Thenganatt and Jankovic 2014). The prevalence of cognitive decline in PD is variable early in the disease, with 19%–38% of patients reporting symptoms of mild cognitive impairment in the early stages of PD (Litvan et al. 2011). As the disease progresses, dementia becomes more common, with a prevalence of >75% in PD patients with >10 years disease duration (Hely et al. 2008).
In addition to motor symptoms, PD is associated with non-motor features, including dysautonomia (constipation, orthostasis, sphincter dysfunction), sleep disturbances (insomnia, REM behavioral parasomnias), mood disorders, anosmia, cognitive disturbances, and pain and sensory disturbances, all of which can negatively impact patient quality of life.
The diagnosis of PD is made solely based on clinical symptoms (bradykinesia, resting tremor, rigidity, and postural instability). MRI, other imaging studies, and laboratory tests are used to exclude other conditions.
Imaging
MRI is typically normal in PD and is primarily used to evaluate structural (e.g., vascular diseases, tumor, etc.) and other neurodegenerative causes of parkinsonism (e.g., multiple system atrophy, AD). PD can be comorbid with other conditions, and clinicians should be cautious not to interpret positive findings on structural neuroimaging as evidence against the diagnosis of PD when the clinical syndrome is suggestive. SPECT imaging using radioactively labeled tracers that bind the presynaptic striatal dopamine transporter (DaT) can be helpful to assess the integrity of the dopaminergic nigrostriatal pathways, which are characteristically dysfunctional in parkinsonian degenerative disorders. Reduced SPECT signal within the striatum suggests dysfunction in this pathway, as DaT is reduced in presynaptic terminals as a result of neuronal degeneration. DaT scanning is useful to distinguish PD from other causes of parkinsonism that do not affect dopaminergic nigrostriatal neurons (e.g., essential tremor, drug-induced and vascular parkinsonism) but not from parkinsonism from other degenerative disorders (e.g., MSA, PSP, CBD) (Kagi et al. 2010). Longitudinal studies show that younger patients with PD have reduced presynaptic monoamine transporter binding at symptom onset, but a slower rate of reduction thereafter (de la Fuente-Fernandez et al. 2011). Additionally, subregions within the striatum appear to lose their dopaminergic inputs during preclinical phases of the disease, whereas loss of dopaminergic inputs across the entire putamen correlates with disease progression (Lee et al. 2004).
CSF and Other Laboratory Testing
There are no specific CSF or laboratory tests for PD, but changes in some blood or CSF markers have been shown to correlate with clinical symptoms of PD (Chen-Plotkin et al. 2011; Kang et al. 2013).
Pathology
The core pathologic feature of PD is loss of dopaminergic neurons in the substantia nigra pars compacta. The microscopic pathological hallmark of PD is Lewy bodies (LBs), which are lamellated, eosinophilic, intracytoplasmic neuronal inclusions of insoluble, fibrillated aggregates that include α-synuclein and ubiquitin. Although motor symptoms are thought to reflect neuronal loss within the substania nigra, this is not the initial site involved. The anatomical distribution and spread of LBs throughout the central nervous system (CNS) is described by Braak et al. (2003) and begins in the dorsal motor nuclei of the vagus before ascending within the brainstem and ultimately to the cortex. α-Synuclein is also found in neuronal processes (Lewy neurites) as well as in astrocytes and oligodendroglial cells in PD (Spillantini et al. 1997; Kalia and Lang 2016).
Genetics
Although most cases of PD are thought to be sporadic, genetics likely plays an important role. Patients with PD, for example, are more than twice as likely to have a first-degree relative with the disease compared with controls (Marder et al. 1996). Rare familial forms of PD with both autosomal dominant and recessive inheritance have been described. Several genes have been associated with monogenic forms of the illness, including leucine-rich repeat kinase 2 (LRRK2), α-synuclein (SNCA) (Polymeropoulos et al. 1997), Parkin, phosphatase and tensin homolog–induced putative kinase-1 (PINK-1), DJ-1, ATPase type 13A2 (ATP13A2), PLA2G6, FBX07, VPS35, and DCTN1 (Singleton et al. 2013). LRRK2 mutations are the most common and are found in 5%–15% of familial parkinsonism cases; they are also associated with 1%–2% of sporadic PD cases (Gasser et al. 2011). Moreover, LRRK2 mutations usually manifest as a benign tremor-predominant phenotype (asymmetric parkinsonism) and have a decreased risk for cognitive and olfactory dysfunction (Healy et al. 2008). Mutations in Parkin, PINK-1, DJ-1, and ATP13A2 cause autosomal-recessive early-onset parkinsonism. Parkin mutations are associated with an early onset of disease and account for nearly half of the recessive familial forms with an onset before the age of 45 years; the clinical phenotype is largely benign, although with atypical features of psychiatric disease, cerebellar signs, and neuropathy (Lohmann et al. 2003; Singleton et al. 2013). Glucocerebrosidase mutations are known to increase the risk of developing PD more than fivefold (Lees et al. 2009). Other risk factor genes are discussed elsewhere in this collection (Nussbaum 2017).
Management/Treatment
Pharmacologic therapies that target the motor features of PD act by enhancing dopamine signaling, and mechanistically involve direct replacement (e.g., levodopa), dopamine receptor agonism (e.g., pramipexole, ropinirole, apomorphine), and reduced dopamine metabolism via monoamine oxidase-B (MAO-B) inhibition (e.g., selegiline) and catechol-O-methyltransferase (COMT) inhibition (e.g., entacapone). Anticholinergics (e.g., trihexyphenidyl, benzotropine) are effective for patients with a tremor-predominant phenotype. These medications are often most effective in the early stages of PD, and adverse effects such as motor fluctuations (“on-off” phenomena) and dyskinesias often develop at later stages after treatment for several years. Deep brain stimulation (DBS) can alleviate motor fluctuations and dyskinesias in patients with advanced, medication-refractory PD. DBS provides additional benefit for tremor, rigidity, and bradykinesia, but gait and balance are unlikely to improve, and cognition may be worsened (particularly verbal fluency) (Fasano et al. 2012). Electrodes placed in the globus pallidus internus or subthalamic nucleus regulate abnormal neural impulses, thereby relieving motor symptoms (Benabid et al. 1987; Siegfried and Lippitz 1994; Follett et al. 2010; Odekerken et al. 2016). DBS can reduce the dose or adverse effects of PD medications, but complications such as hemorrhage, infection, and lead migration should be considered when deciding on DBS treatment (Lyons et al. 2004; Guridi et al. 2012; Pouratian et al. 2012). Nonpharmacologic treatments such as speech, physical, and occupational therapies should also be considered depending on patient symptoms.
Differential Diagnosis
PD should be differentiated from other parkinsonian disorders, including vascular (e.g., striatal infarct), drug-induced (e.g., neuroleptics, antinausea), metabolic (e.g., Wilson’s, neuroacanthocytosis, liver disease), infectious (e.g., HIV, syphilis, CJD), toxic (e.g., carbon monoxide), normal pressure hydrocephalus, essential tremor, and other forms of neurodegenerative disease (e.g., MSA, PSP, CBS, DLB, and AD).
Dementia with Lewy Bodies and Parkinson’s Disease with Dementia
The clinical entities of DLB and PDD have overlapping features because both are characterized by progressive cognitive impairment, psychiatric and behavioral disturbances, and parkinsonian motor symptoms. The distinguishing feature between DLB and PDD is the timing of dementia onset: In DLB, cognitive impairment precedes or co-occurs with parkinsonian motor syndrome, whereas in PDD the motor syndrome precedes cognitive decline.
Epidemiology
The prevalence of dementia in patients living with PD in community-based studies is reported to be 30%, although the range varies from 10%–80% with the higher prevalence occurring in older groups of patients and those with longer disease duration; for example, the prevalence of dementia was estimated to be 83% in patients at 20 years of PD (Hely et al. 2008). The incidence of Parkinson disease dementia (PDD) steadily increases with age (Savica et al. 2013). DLB is the second most common dementia subtype after AD, affecting up to 30% of all dementia patients (Zaccai et al. 2005), although a more recent meta-analysis suggests a lower rate of 4.2% (Vann Jones and O’Brien 2014). The overall prevalence in the elderly population (age > 65) is 0.36% with an incidence of 0.87 cases per 1000 person-years. The mean age of DLB onset ranges from 59 to 78 years, as determined across several cohorts (Vann Jones and O’Brien 2014), and the incidence peaks in the sixth decade (Savica et al. 2013). In comparison to controls, DLB is associated with a history of depression, anxiety, stroke, a positive family history of PD, and the presence of ApoE ε4 alleles. In comparison to AD, patients with DLB are more likely to be male, have higher levels of educational attainment, and have a family history of PD (Boot et al. 2013).
Clinical Symptoms and Diagnosis
The hallmark clinical features of DLB are dementia associated with visual hallucinations, parkinsonism, and fluctuating mental status. The dementia of DLB tends to affect attention, executive functions, visuospatial skills, and memory recall. When compared with cognitively normal patients with PD, the parkinsonism of DLB and PDD tends to be more axial, with masked facies, postural instability, and gait difficulties, whereas rest tremor is less prominent (Burn et al. 2003). REM sleep behavior disorders, dysautonomia (syncope, urinary incontinence), psychiatric manifestations (depression, delusions), and hypersensitivity to neuroleptic medications are seen in DLB, PDD, and other synucleinopathies (McKeith et al. 2005). Specific delusional types are overrepresented in DLB and PDD, including “extracampine” hallucinations (the sensation of a “presence” just outside their peripheral visual field) and the Capgras delusion, in which patients believe that a person in their life has been replaced by an imposter (Josephs 2007; Chiba et al. 2015). One study comparing the clinical characteristics of DLB and PDD showed that a higher percentage of DLB patients experience hallucinations, cognitive fluctuations, and myoclonus (Savica et al. 2013).
Diagnostic criteria for DLB were first devised in 1996 and revised in 2005 by the DLB Consortium (McKeith et al. 2005). The distinctions “probable” and “possible” are made in the criteria. Diagnosis is primarily made based on clinical signs and symptoms, although dopamine imaging is also included. Clinical features are categorized as either central, core, suggestive, or supportive of DLB. Dementia is the central feature and essential for diagnosis. Core features include fluctuating cognition (particularly attention and alertness), visual hallucinations (usually well formed), and parkinsonism. Suggestive features are REM sleep behavior disorder, severe neuroleptic sensitivity, and a positive DaT scan. The diagnosis of “probable” DLB requires either two core features or one core plus one suggestive feature, whereas “possible” DLB requires one core feature (and no suggestive features), or one or more suggestive features. The presence of significant vascular disease or other confounding medical conditions make the diagnosis less likely. See Box 3 for diagnostic criteria.
Box 3.
Diagnostic criteria for DLB and MSA (McKeith et al. 2005; Gilman et al. 2008)
Diagnostic criteria for dementia with Lewy bodies (DLB)
- “Central” feature (essential for a diagnosis of possible or probable DLB)
- Dementia defined as progressive cognitive decline of sufficient magnitude to intefere with normal social or occupational function.
- Prominent or persistent memory impairment may not necessarily occur in the early stages but is usually evident with progression.
- Deficits on tests of attention, executive fucntion, and visuospatial ability may be especially prominent.
- “Core” features (two core features are sufficient for a diagnosis of probable DLB, one for possible DLB)
- Fluctuating cognition with pronounced variations in attention and alertness
- Recurrent visual hallucinations that are typically well-formed and detailed
- Spontaneous features of parkinsonism
- “Suggestive” features (If one or more of these is present in the presence of one or more core features, a diagnosis of probable DLB can be made. In the absence of any core features, one or more suggestive features is sufficient for possible DLB. Probable DLB should not be diagnosed on the basis of suggestive features alone.)
- REM sleep behavior disorder
- Severe neuroleptic sensitivity
- Low dopamine transporter uptake in the basal ganglia shown by SPECT or PET imaging
- “Supportive” features (commonly present but not proven to have diagnostic specificity)
- Repeated falls and syncope
- Transient, unexplained loss of consciousness
- Severe autonomic dysfunction, for example, orthostatic hypotension, urinary incontinence
- Hallucinations in other modalities
- Systematized delusions
- Depression
- Relative preservation of medial temporal lobe structures on CT/MRI scan
- Generalized low uptake on SPECT/PET perfusion scan with reduced occipital activity
- Abnormal (low uptake) meta-iodobenzylguanidine (MIBG) myocardial scintigraphy
- Prominent slow wave activity on electroencephalogram (EEG) with temporal lobe transient sharp waves
- A diagnosis of DLB is “less likely”
- In the presence of cerebrovascular disease evident as focal neurologic signs or on brain imaging
- In the presence of any other physical illness or brain disorder sufficient to account in part or in total for the clinical picture
- If parkinsonism only appears for the first time at a stage of severe dementia
- “Temporal sequence” of symptoms
- DLB should be diagnosed when dementia occurs before or concurrently with parkinsonism (if it is present). The term Parkinson disease dementia (PDD) should be used to describe dementia that occurs in the context of well-established Parkinson disease. In a practice setting, the term that is most appropriate to the clinical situation should be used, and generic terms such as LB disease are often helpful. In research studies in which distinction needs to be made between DLB and PDD, the existing one-year rule between the onset of dementia and parkinsonism DLB continues to be recommended. Adoption of other time periods will simply confound data pooling or comparison between studies. In other research settings that may include clinicopathologic studies and clinical trials, both clinical phenotypes may be considered collectively under categories such as LB disease or α-synucleinopathy.
Diagnostic criteria for MSA
I. Probable MSA
A sporadic, progressive, adult (>30 yr)-onset disease characterized by
Autonomic failure involving urinary incontinence (inability to control the release of urine from the bladder, with erectile dysfunction in males) or an orthostatic decrease of blood pressure within 3 min of standing by at least 30 mm Hg systolic or 15 mm Hg diastolic and
Poorly levodopa-response parkinsonism (bradykinesia with rigidity, tremor, or postural instability) or
A cerebellar syndrome (gait ataxia with cerebellar dysarthria, limb ataxia, or cerebellar oculomotor dysfunction)
II. Possible MSA
A sporadic, progressive, adult (>30 y)-onset disease characterized by
Parkinsonism (bradykinesia with rigidity, tremor, or postural instability) or
A cerebellar syndrome (gait ataxia with cerebellar dysarthria, limb ataxia, or cerebellar oculomotor dysfunction) and
At least one feature suggesting autonomic dysfunction (otherwise unexplained urinary urgency, frequency, or incomplete bladder emptying, erectile dysfunction in males, or significant orthostatic blood pressure decline that does not meet the level required in probable MSA) and
- At least one additional feature:
- Possible MSA-P or MSA-C:
- Babinski sign with hyperreflexia
- Stridor
- Possible MSA-P
- Rapidly progressive parkinsonism
- Poor response to levodopa
- Postural instability within 3 years of motor onset
- Gait ataxia, cerebellar dysarthria, limb ataxia, or cerebellar oculomotor dysfunction
- Dysphagia within 5 years of motor onset
- Atrophy on MRI of putamen, middle cerebellar peduncle, pons, or cerebellum
- Hypometabolism on FDG-PET in putamen, brainstem, or cerebellum
- Possible MSA-C
- Parkinsonism
- Atrophy on MRI of putamen, middle cerebellar peduncle, or pons
- Hypometabolism on FDG-PET in putamen
- Presynaptic nigrostriatal dopaminergic deneveration on SPECT or PET
Diagnostic criteria for PDD were suggested by the Movement Disorder Society Task Force in 2007 (Emre et al. 2007) and were put into use the same year (Dubois et al. 2007). PDD diagnosis requires patients to have antecedent PD (per Queen’s Square Brain Bank criteria [Hughes et al. 1992]) and a dementia syndrome affecting multiple cognitive domains (attention, executive functions, visuospatial skills, free recall memory) that is not otherwise explained by vascular disease or other medical conditions; the presence of behavioral symptoms (apathy, mood disorder, hallucinations, delusions, or excessive sleepiness) increase confidence in the diagnosis.
Imaging
Structural MRI is useful in evaluating patients for DLB and PDD, although its primary purpose is to assess for alternative etiologies that cause structural changes, including vascular disease, masses, or other forms of neurodegenerative disease. Despite the patients' advanced dementia, MRI scans of patients with DLB and PDD are often notable for their lack of atrophy (especially the medial temporal lobes), particularly when contrasted with AD (Barber et al. 2000; Whitwell et al. 2007; Watson et al. 2012). Despite the lack of global atrophy, DLB has been associated with volume loss within the mid and posterior cingulate, superior temporo-occipital, and lateral orbitofrontal cortices (Lebedev et al. 2013), putamen (Cousins et al. 2003), and dorsal midbrain (Whitwell et al. 2007), when compared with AD, although the clinical utility of these findings is undetermined.
FDG-PET findings show greater occipital hypometabolism in DLB and PDD patients compared with healthy controls (Perneczky et al. 2008; Klein et al. 2010) and individuals with AD (Okamura et al. 2001). PET and SPECT imaging can be used to assess the integrity of nigrostriatal dopaminergic pathways via the use of tracers that specifically bind dopamine (and other monoamine) transporters. Reduced tracer uptake within the striatum suggests dysfunction within these nigrostriatal projections, which is seen in DLB and PDD. Functional DaT imaging can help distinguish DLB and PDD from AD, as the nigrostriatal system is relatively preserved in the former (McKeith et al. 2007; Walker et al. 2007). Cortical cholinergic deficits, which are more prominent in DLB and PDD than in other types of dementia (Perry et al. 1994), have been shown using PET with ligands that bind acetylcholinesterase (Klein et al. 2010); these deficits are most pronounced in the occipital cortices. FDG-PET studies with Pittsburgh compound B (PiB) show greater β-amyloid deposition in DLB than in PDD, which is consistent with high rates of concurrent AD pathology in DLB (Edison et al. 2008). The amyloid burden is associated with cognitive impairment (Gomperts et al. 2012).
CSF and Other Laboratory Testing
There are no clinically available laboratory biomarkers (CSF, serum, or urine) that aid in diagnosing DLB or PDD, although CSF α-synuclein may be a potential biomarker (Mollenhauer et al. 2011). CSF β-amyloid and tau (total and phosphorylated) levels can assist the diagnosis of AD, but given the frequency of copathology, may not be useful in ruling out DLB.
Pathology
The pathologies of DLB and PDD are largely indistinguishable and are characterized by abnormalities of the α-synuclein and ubiquitin proteins, which aggregate in neurons to form LBs and Lewy neurites (LNs) (Lippa et al. 2007). These inclusions are located within the neocortex, limbic system, and brainstem. There is also a high rate of copathology with AD (McKeith et al. 2005). The presence of LBs in the neocortex correlates with cognitive impairment (Lippa et al. 2007). The distribution of LBs tends to correlate with symptomatology (Farlow 2016). Additionally, there is evidence that α-synuclein may spread through the CNS in a prion-like manner (Frost and Diamond 2010).
Genetics
DLB is largely considered a sporadic disorder, although genetic factors likely play a role. The disorder is associated with a positive family history of dementia in two-thirds of cases (Woodruff et al. 2006), and the risk of DLB is 2.3-fold if a patient has an affected sibling (Nervi et al. 2011). Although rare, familial cases have been described (Galvin et al. 2002), and extra copies of SNCA have been associated with inherited forms of DLB and PDD (Obi et al. 2008). The genetics of synucleinopathies have been recently reviewed (Nussbaum 2017).
Management/Treatment
Given the pronounced cholinergic deficits associated with LB disease, acetylcholinesterase inhibitors should be used as the first-line treatment for cognitive decline (attention and cognitive fluctuations), psychiatric symptoms (visual hallucinations, apathy, and anxiety), and sleep difficulties in both DLB and PDD (Samuel et al. 2000; Emre et al. 2004; Wesnes et al. 2005). By logical extension, anticholinergic medications should be avoided. Orthostatic hypotension, cardiac conduction arrhythmias, and vivid dreaming might occur or worsen with the use of cholinesterase inhibitors. Memantine is of unclear benefit (Wang et al. 2015), although one study showed improvement in the clinical global impression of change score (Aarsland et al. 2009), whereas others showed no improvement (Leroi et al. 2009).
Dopaminergic therapy is used to treat extrapyramidal symptoms in DLB and PDD, although symptomatic improvement with levodopa therapy is less than that observed in PD. Depression and anxiety in DLB and PDD can be treated with SSRIs or serotonin and norepinephrine reuptake inhibitors (SNRIs). Atypical antipsychotics can be beneficial for psychiatric symptoms but must be used very cautiously because of their adverse effects on movement and cognition. Traditional neuroleptics should be avoided because of neuroleptic hypersensitivity in DLB patients. Disease-modifying agents are not available yet clinically.
Differential Diagnosis
The differential diagnosis of DLB and PDD is similar to that of PD (see above) and includes cerebrovascular disease, drug or toxin effects (e.g., dopamine blockade, 1‐methyl‐4‐phenyl‐1,2,5,6‐tetrahydropyridine [MPTP], carbon monoxide), metabolic disease, infectious or postinfectious (e.g., HIV, syphilis, Whipple’s, CJD), normal pressure hydrocephalus, and other forms of neurodegenerative disease (especially AD) (Lippa and Possin 2016). When cognitive symptoms predominate, differentiating DLB from AD or some FTD-spectrum disorders can be challenging.
Multiple System Atrophy
Multiple system atrophy (MSA) is an α-synucleinopathy with progressive symptoms that span multiple neurologic systems, including cognitive, autonomic, cerebellar, and both pyramidal and extrapyramidal motor (Quinn 1989; Wenning et al. 1997; Geser et al. 2006; Fanciulli and Wenning 2015). MSA is classified into three types based on the predominant pattern of motor involvement: MSA-C (olivopontocerebellar atrophy), MSA-P (striatonigral degeneration), and MSA-mixed. MSA-C is characterized by prominent cerebellar features, whereas MSA-P manifests with parkinsonian symptoms. MSA-mixed has a combination of both symptoms (Gilman et al. 2008).
Epidemiology
The mean incidence of MSA over the age of 50 is 3 cases per 100,000 person-years (Bower et al. 1997), with a prevalence of 1.9–4.4 cases per 100,000 person-years (Schrag et al. 1999; Tison et al. 2000). The estimated mean age of onset is 54–61 years, with a wide range (ages 31–78) (Ben-Shlomo et al. 1997; Coon et al. 2015). MSA-C may have an earlier age of onset compared with MSA-P (58 vs. 62 years) (Coon et al. 2015). Geographically, MSA-P is more common than MSA-C in North America and Europe (Gilman et al. 2005; Kollensperger et al. 2010), although MSA-C is more common in Japan (Watanabe et al. 2002). The median survival is 6.2–7.5 years and is shorter with older age of onset, a parkinsonian phenotype (Ben-Shlomo et al. 1997; Wenning et al. 2013), and early dysautonomia (O’Sullivan et al. 2008; Coon et al. 2015). MSA progresses faster than PD.
Clinical Symptoms and Diagnosis
Although the diagnosis of MSA often is made at the time of motor involvement, nonmotor symptoms (autonomic failure, respiratory, and urogenital disorders) can precede motor symptoms by years and are considered the “premotor phase” (Jecmenica-Lukic et al. 2012). Motor features often include parkinsonism (bradykinesia, rigidity, and postural instability with postural tremor that is poorly responsive to dopamine replacement, cerebellar ataxia, pyramidal dysfunction [extensor plantar response, hyper-reflexia], dysarthria, camptocormia, anterocollis, and dystonia [Kollensperger et al. 2010; Fanciulli and Wenning 2015]). Early, prominent dysautonomia is characteristic of MSA and can include sphincter dysfunction (urinary incontinence, constipation), erectile dysfunction, orthostatic hypotension, respiratory stridor, and sweat gland dysfunction. Cognitive impairment is common and primarily affects the frontal/executive, visuospatial, memory (Stankovic et al. 2014), and emotional regulatory systems (Kollensperger et al. 2008). The MSA subtypes are defined by their prominent motor features, parkinsonism in the case of MSA-P and cerebellar ataxia in the case of MSA-C.
The diagnostic criteria (Gilman et al. 2008) for MSA are tiered (definite, probable, and possible) based on the likelihood that the clinical presentation aligns with the pathologic diagnosis. A definite diagnosis requires postmortem pathological analysis, whereas probable and possible diagnoses are based on clinical features. Both probable and possible MSA require the disorder to be sporadic, progressive, and adult-onset; probable MSA is defined by dysautonomia (urinary incontinence with erectile dysfunction or orthostatic hypotension) and either poorly dopamine-responsive parkinsonism (MSA-P) or a cerebellar syndrome (MSA-C). Possible MSA requires either parkinsonism (may be levodopa-responsive) or cerebellar dysfunction, evidence of dysautonomia (lower urinary tract symptoms, erectile dysfunction, mild orthostasis), and one additional clinical or neuroimaging feature of the disease. See Box 3 for diagnostic criteria.
Imaging
Structural MRI is useful to evaluate patients with suspected MSA to identify additional etiologies that may present with overlapping symptoms (e.g., vascular disease, masses) and also to look for characteristic features of the disease. MSA is associated with atrophy of the pons, cerebellum, putamen, and middle cerebellar peduncles, T2 hyperintensities within the lateral putaminal rim and middle cerebellar peduncles, and a T2 hypointensity within the posterior putamen (Brooks et al. 2009). The often-described “hot-cross-bun sign”—a cruciform hyperintensity seen in the pons on axial T2/FLAIR sequences—reflects selective loss of myelinated transverse pontocerebellar fibers in the pontine raphe but preservation of the corticospinal tracts and tegmentum (Fig. 3). The hot-cross-bun sign, however, is not specific to MSA and can be seen in other diseases with overlapping cerebellar involvement, such as many of the spinocerebellar ataxias (Brooks et al. 2009). Although supportive, no single MRI feature is sensitive or specific for the diagnosis. On serial MRI, MSA-P is associated with increased atrophy and iron deposition within the putamen, compared with MSA-C (Lee et al. 2015).
Figure 3.
“Hot-cross-bun” sign of multiple system atrophy (MSA) of the cerebellar-predominant subtype (MSA-C). Axial T2-weighted magnetic resonance imaging (MRI) of the brain of a 52-year-old patient four years after the onset of MSA-C shows a cruciform T2 hyperintensity in the pons called the “hot-cross-bun sign” (indicated by arrows). Cerebellum shows atrophy. Orientation is radiological. Note that this sign is not specific for MSA, as it occurs in other cerebellar degenerative disorders, such as some of the spinocerebellar ataxias.
Patients with MSA show regional hypometabolism within the striatum, brainstem, and cerebellum on FDG-PET (Fulham et al. 1991; Gilman et al. 1994). Presynaptic dopamine PET or SPECT imaging cannot reliably distinguish patients with MSA from patients with other parkinsonian conditions (Brooks et al. 2009), although asymmetric tracer uptake within the striatum might suggest MSA rather than PD (Perju-Dumbrava et al. 2012).
CSF and Other Laboratory Testing
There are no laboratory tests that can confirm a diagnosis of MSA, although CSF biomarkers are under active investigation. One study found that CSF neurofilament light-chain levels are elevated in MSA patients compared with controls and PD cases (Herbert et al. 2015). In contrast to PD, MSA is associated with elevated levels of CSF DJ-1 and total tau, and the combination of these proteins shows a sensitivity of 82% and a specificity of 81% in differentiating MSA from PD (Herbert et al. 2014).
Pathology
Gross pathological features of MSA include atrophy within the olivopontocerebellar and striatonigral systems and the frontal lobe. Histological features include neuronal loss, gliosis, myelin loss, and axonal degeneration within the olivopontocerebellar and striatonigral regions, hypothalamus, and intermediolateral cell column of the spinal cord (Wenning et al. 1997). The defining neuropathological feature of MSA is the presence of fibrillized α-synuclein inclusions within oligodendrocytes, called glial cytoplasmic inclusions (Papp et al. 1989; Ahmed et al. 2012).
Genetics
MSA is considered a sporadic disorder. A recent estimate of heritability is low, at 2% to 6% (Federoff et al. 2016). Familial forms with autosomal dominant inheritance patterns have been reported (Wullner et al. 2009; Stemberger et al. 2011), and mutations in the COQ2 gene have been identified in cases of familial MSA (Multiple-System Atrophy Research Collaboration 2013).
Several risk factor genes have been identified for the disease. Single nucleotide polymorphisms (SNPs) in SNCA were reported to be associated with PD and MSA (Scholz et al. 2009; Simon-Sanchez et al. 2009), and mutations in COQ2 were found in familial MSA cases in Japan (Multiple-System Atrophy Research Collaboration 2013). A recent genome-wide association study (GWAS) showed no association of SNCA or COQ2 genes with MSA, although SNPs in the genes FBXO47, ELOVL7, EDN1, and MAPT were reported (Sailer et al. 2016). The MAPT H1 haplotype was also thought to be associated with MSA (Vilarino-Guell et al. 2011).
Management/Treatment
There are no disease-modifying therapies that target the underlying pathological mechanisms of MSA; available treatments are designed to alleviate bothersome symptoms. Despite prominent features of parkinsonism in MSA, a lasting symptomatic response to dopaminergic medications is minimal, although transient improvement with levodopa occurs in up to 40% of patients (Kollensperger et al. 2010). A small trial of amantadine showed a trend toward improvement in motor symptoms, although it was not significant (Wenning and Working Group on Atypical Parkinsonism of the Austrian Parkinson’s Society 2005). Standard pharmacologic and nonpharmacologic strategies should be considered to treat nonmotor symptoms and should be used based on the severity and nature of the symptom, including urinary symptoms (e.g., straight catheterization for retention), orthostatic hypotension (e.g., salt intake, midodrine, fludrocortisone), erectile dysfunction (e.g., sildenafil), and stridor (e.g., continuous positive airway pressure [CPAP]) (Fanciulli and Wenning 2015). Enrollment in physical, occupational, and speech therapies is recommended based on clinical symptoms.
Differential Diagnosis
The differential diagnosis for MSA-P includes conditions that lead to parkinsonism (see differential diagnosis for PD above). MSA-P can be mistaken for PD, particularly early in the disease course. Distinguishing features include early significant autonomic failure, poor response to dopaminergic therapy, history of REM sleep behavioral disorders, and inspiratory stridor (Kollensperger et al. 2008). MSA tends to have a more aggressive course than PD and patients become more rapidly disabled (frequent falls, autonomic problems, and difficulties with swallowing and speech). For MSA-C, differential diagnoses include the causes of chronic cerebellar ataxias (e.g., alcohol use, vitamin E deficiency, celiac disease, HIV, Whipple’s disease, anti-GAD65 antibodies, sarcoid, prion disease including Gerstmann–Sträussler–Scheinker [GSS], spinocerebellar ataxias (SCAs), Wilson’s, fragile X premutations, mitochondrial disease) or chronic autonomic failure (e.g., small fiber neuropathy, pure autonomic failure, antiganglionic nicotinic acetylcholine receptor antibodies, medications).
Huntington’s Disease
Epidemiology
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disorder with symptoms of involuntary movements, personality changes, and dementia that is caused by excessive expansion of CAG repeats in the huntingtin gene on chromosome 4. HD is rare, with a recent meta-analysis (Pringsheim et al. 2012) estimating the service-based worldwide prevalence of 2.7 cases per 100,000, with higher rates in Europe, North America, and Australia compared with Asia. The incidence was estimated to be 0.38 cases per 100,000 person-years. The median age of diagnosis of HD is ∼40 years (Newcombe 1981), although the timing of onset is partially determined by the number of CAG repeats (Brinkman et al. 1997). Both juvenile (i.e., Westphal variant) (Seneca et al. 2004) and late-onset (Myers et al. 1985) forms are described. There are an estimated 50,000 people with HD in the United States and Canada (Fisher and Hayden 2014).
Clinical Symptoms and Diagnosis
The hallmark symptomatology of HD is progressive dysfunction across multiple neurologic systems, including motor, cognitive (dementia with dysexecutive features), and psychiatric (anxiety, irritability, aggression, disinhibition, antisocial behaviors, apathy, psychosis). Although clinical diagnosis requires motor involvement, many of the nonmotor features are present years before the onset of motor symptoms. Chorea (involuntary jerking, dance-like movements involving the proximal and distal limbs) is often the most prominent motor symptom, although patients may be unaware of these movements at early stages of the illness. Other motor symptoms include dystonia, ataxia (gait, limb, and speech), motor impersistence, atypical parkinsonism (bradykinesia, rigidity), and eye movement abnormalities (slow volitional saccades with delayed initiation). Progressive motor disturbances are a major cause of life-threatening conditions such as dysphagia (weight loss, aspiration) and falls. Weight loss is common in HD, even before dysphagia, which is likely due to mitochondrial dysfunction.
Cognitive impairment often develops before the onset of motor symptoms and is usually present at the time of diagnosis. Cognitive deficits primarily involve executive functions (multitasking, planning, set-shifting, processing speed, word generation, memory recall), with cortically mediated processes such as memory, language, and praxis relatively spared. Patients with HD also often lack insight into their motor and cognitive impairment. This is reviewed elsewhere (Paulsen 2011).
Neuropsychological/behavioral symptoms usually precede the onset of motor symptoms and include depression, anxiety, irritability, aggression, disinhibition, antisocial behaviors, apathy, and psychosis (for review, see Eddy et al. 2016). The lifetime prevalence of major depression in HD is much higher than in the general population (ranges from 20% to 56%) (Shiwach 1994; Julien et al. 2007), and HD is associated with high rates of suicide and suicide attempts (Di Maio et al. 1993; Paulsen et al. 2005). Depressive symptoms occur less frequently with advancing stages of illness (Paulsen et al. 2005; Thompson et al. 2012). Anxiety disorders are common in HD and affect 13%–71% of cases, particularly generalized anxiety disorder and panic disorder (Dale and van Duijn 2015). HD is associated with impairment in social cognition and alexithymia (the reduced ability to interpret and describe one’s internal emotional state).
The diagnosis of HD is based on the presence of unequivocal motor signs of HD as defined by the Unified Huntington’s Disease Rating Scale (UHDRS) (Kremer et al. 1996) in a patient who carries a known CAG-repeat expanded allele (Hogarth et al. 2005; Reilmann et al. 2014). The gold standard for genetic confirmation is the demonstration of CAG expansion of at least 36 repeats on the huntingtin gene on chromosome 4. Usually CAG repeats of 36 to 39 are considered reduced penetrance and ≥40 repeats are fully penetrant (MacDonald et al. 1993). Cognitive and psychiatric manifestations are supportive but not essential for the diagnosis (Roos 2010). There are ongoing attempts to recategorize the disease based on broader aspects of the natural history (including cognitive symptoms, biomarkers, and functional decline), with a recent proposed diagnostic classification scheme of “presymptomatic,” “prodromal,” and “manifest” or “motor” HD (Reilmann et al. 2014).
Imaging
Structural MRI shows focal regions of tissue volume loss in HD, most notably in the striatum (but also in the white matter and neocortex) (Fig. 4). These changes are evident in gene carriers years before the onset of motor symptoms (Roos 2010) and often correlate with CAG-repeat length. The rate of striatal volume loss on longitudinal MRI is higher in gene carriers compared with controls (Aylward et al. 2011) and is predictive of the onset of motor symptoms in gene carriers (Paulsen et al. 2014). Neocortical atrophy can be global or focal, and regional variability correlates with specific symptoms (Rosas et al. 2008). Diffusion tensor imaging methods reveal reduced white matter integrity in the corpus callosum and reduced fractional anisotropy in the basal ganglion in patients with HD genetic mutations, and these abnormalities correlate with prognosis and severity of symptoms (Ross et al. 2014). FDG-PET shows hypometabolism within the striatum (Feigin et al. 2001). The neuroimaging of HD is reviewed elsewhere (Niccolini and Politis 2014; Ross et al. 2014).
Figure 4.
Magnetic resonance imaging (MRI) in healthy normal subject versus patient with Huntington’s disease (HD). Coronal T2-weighted fluid-attenuated inversion recovery (FLAIR) MRI in (A) a healthy control subject and (B) an HD patient. (A) A 68-year-old healthy individual shows normal caudate size (indicated by arrows). (B) A 67-year-old patient with moderate stage HD, 7 years after onset shows diffuse cortical atrophy with disproportionate caudate atrophy (arrows) and corresponding enlargement of the lateral ventricles. Orientation is radiological.
CSF and Other Laboratory Testing
Genetic testing is the gold standard for the molecular diagnosis of HD. There are currently no validated CSF or serum biomarkers of HD pathology, although this is an area of active investigation.
Pathology
Gross pathological changes in HD include atrophy in the striatum, cerebral cortex, and subcortical white matter. The hallmark microscopic pathological features include medium spiny neuronal loss within the striatum (caudate more than putamen) and regions of the cerebral cortex. In advanced cases, there is more widespread neuronal loss, including within the cerebellum, thalamus, hippocampus, and brainstem nuclei (Heinsen et al. 1994; Vonsattel and DiFiglia 1998; Rub et al. 2013, 2014).
Genetics
The chromosomal location of the huntingtin gene was discovered in 1983 (Gusella et al. 1983) and was characterized as a disorder of CAG-repeat expansion in 1993 (MacDonald et al. 1993). It is inherited with an autosomal dominant pattern. In non-HD controls, the average number of CAG repeat units within the huntingtin gene is 17 to 20. Phenotypic HD often occurs when the number of CAG repeat units expands to 36 and does so invariably when the number reaches 40 or greater. Although HD is rarely seen when the number of repeat units is 27 to 35, this intermediate number makes the allele genetically unstable and apt to expand further in successive generations (e.g., genetic anticipation), and makes future generations at risk of having fully penetrant HD. The rate of expansion with successive generations can be higher with paternal inheritance. Genetic anticipation is a hallmark of the disease. Increasing numbers of CAG repeat units correlate with disease severity (Rosenblatt et al. 2006) and earlier onset (Lee et al. 2012).
Management/Treatment
HD has no cure or disease-modifying agents, and, therefore, treatment only alleviates symptoms. The benefits of treatment need to be carefully balanced with any potential side effects. Both pharmacologic and nonpharmacologic strategies can be used to achieve this end. Several reviews of symptomatic treatments and pharmacotherapy for HD are available (Ross and Tabrizi 2011; Eddy et al. 2016).
Chorea requires treatment when a patient’s safety, quality of life, or functionality is affected. The American Academy of Neurology recently recommended the most effective treatments for HD-associated chorea (Armstrong et al. 2012). The first-line therapy is tetrabenazine, which is effective and Food and Drug Administration (FDA)-approved for the condition, and it acts mechanistically by decreasing the levels of dopamine (and serotonin/norepinephrine). Adverse effects include parkinsonism, depression, and suicide. Benzodiazepines and amantadine may be effective as well. There is insufficient data to support the use of neuroleptics, although anecdotal reports suggest they may have a potential benefit; any benefit, however, needs to be weighed against the risk of cardiac arrhythmia and somnolence. In clinical practice, however, atypical neuroleptics are commonly used to treat chorea, as well as concomitant psychiatric symptoms should they occur. Dopaminergic agents used to treat PD are usually effective in patients with the Westphal variant of HD, which typically presents with parkinsonism and not chorea.
Treating the psychiatric manifestations of HD can improve quality of life for patients and their loved ones. SSRIs have been used to treat anxiety, depression, irritability, perseverative thinking, and apathy. Neuroleptics (Squitieri et al. 2001) and benzodiazepines (Orth et al. 2011) have also been used to treat anxiety. Additionally, neuroleptics can help manage psychosis (Orth et al. 2011). In severe cases, electroconvulsive therapy has been effective for refractory depression (Cusin et al. 2013). Acetylcholinesterase inhibitors (e.g., donepezil, rivastigmine, memantine) have not been shown to improve cognition in HD patients, although these compounds may be effective in some patients. Nonpharmacologic strategies such as physical therapy, occupational therapy, speech therapy, use of walkers, home safety evaluations, dietary consultation, structured daily schedules, and social work services are all imperative, particularly as the disease progresses.
Differential Diagnosis
The differential diagnosis for HD includes psychiatric disease, other dementias, and causes of chorea. The differential diagnosis for chorea is broad and includes genetic disorders such as benign hereditary chorea, C9ORF72 mutations, spinocerebellar ataxias (including Machado-Joseph disease), neuroacanthocytosis, dentatorubropallidoluysian atrophy (DRPLA), and Wilson disease; rheumatic disorders such as Sydenham chorea and chorea gravidarum; less commonly, infectious disorders such as HIV and CJD; systemic disorders such as systemic lupus erythematosus and thyrotoxicosis; neoplastic/paraneoplastic conditions such as polycythemia vera or antibody-mediated disorders; and medication side effects such as from neuroleptics, oral contraceptives, phenytoin, levodopa, and cocaine. Obtaining an accurate family history, including knowledge of early death, gait disorders, and psychiatric illness, is important in the examination. In one study, ∼1% of the cases clinically diagnosed as HD did not have CAG-repeat expansion of the huntingtin gene and were caused by other conditions (e.g., HD phenocopy), including HDL1, HDL2, HDL3, SCA17, and SCA 1/2/3 (Roos 2010).
HD pathogenesis and therapies have been recently reviewed (Jimenez-Sanchez et al. 2016; Pearce and Kopito 2017).
CONCLUDING REMARKS
Neurodegenerative diseases are a common cause of cognitive impairment in older adults. Diagnosing dementia can be difficult, but identifying certain key features or findings that we have discussed above can facilitate a correct diagnosis. Although there are no cures or disease-modifying therapies currently available for any of these conditions, treatment trials are underway. With the understanding that many of these diseases share prion-like properties, this knowledge might be a large step forward in preventing or halting the disease process.
ACKNOWLEDGMENTS
M.D.G. has no relevant conflicts of interest but serves on the board of directors for San Francisco Bay Area Physicians for Social Responsibility, on the editorial board of Dementia & Neuropsychologia, and serves or has served as a consultant for Advanced Medical, Inc., Best Doctors, Inc., Biohaven Pharmaceuticals Inc., Gerson Lehrman Group, Inc., Grand Rounds, Inc., Guidepoint Global, LLC, Lewis Brisbois Bisgaard & Smith LLP, Lundbeck Inc., Kendall Brill & Kelly LLP, MEDACorp, NeuroPhage, Franciscan Physician Network, Teva Pharmaceuticals, LCN Consulting, Optio Biopharma Solutions, and Quest Diagnostics. M.D.G. receives research/grant support from the National Institute on Aging (NIA) (R01 AG031189), the Michael J. Homer Family Fund, Quest Diagnostics, CurePSP, and the Tau Consortium.
This research was also supported by the National Institutes of Health (NIH)/NIA R01 AG031189, P01 AG019724, P50 AG023501, the Michael J. Homer Family Fund, and the Tau Consortium. Dr. Erkkinen and Dr. Kim report no conflicts of interest. Dr. David Perry is affiliated with the University of California, San Francisco, Memory and Aging Center.
Footnotes
Editor: Stanley B. Prusiner
Additional Perspectives on Prion Biology available at www.cshperspectives.org
REFERENCES
- Aarsland D, Ballard C, Walker Z, Bostrom F, Alves G, Kossakowski K, Leroi I, Pozo-Rodriguez F, Minthon L, Londos E. 2009. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: A double-blind, placebo-controlled, multicentre trial. Lancet Neurol 8: 613–618. [DOI] [PubMed] [Google Scholar]
- Ahmed Z, Asi YT, Sailer A, Lees AJ, Houlden H, Revesz T, Holton JL. 2012. The neuropathology, pathophysiology and genetics of multiple system atrophy. Neuropathol Appl Neurobiol 38: 4–24. [DOI] [PubMed] [Google Scholar]
- Alladi S, Xuereb J, Bak T, Nestor P, Knibb J, Patterson K, Hodges JR. 2007. Focal cortical presentations of Alzheimer’s disease. Brain 130: 2636–2645. [DOI] [PubMed] [Google Scholar]
- Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. 2006. Changes in motor subtype and risk for incident dementia in Parkinson’s disease. Mov Disord 21: 1123–1130. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association. 2016. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement 12: 459–509. [DOI] [PubMed] [Google Scholar]
- Anneser JM, Jox RJ, Borasio GD. 2007. Inappropriate sexual behaviour in a case of ALS and FTD: successful treatment with sertraline. Amyotroph Lateral Scler 8: 189–190. [DOI] [PubMed] [Google Scholar]
- Armstrong MJ, Miyasaki JM, American Academy of Neurology. 2012. Evidence-based guideline: Pharmacologic treatment of chorea in Huntington disease: Report of the guideline development subcommittee of the American Academy of Neurology. Neurology 79: 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M, et al. 2013. Criteria for the diagnosis of corticobasal degeneration. Neurology 80: 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward EH, Nopoulos PC, Ross CA, Langbehn DR, Pierson RK, Mills JA, Johnson HJ, Magnotta VA, Juhl AR, Paulsen JS, et al. 2011. Longitudinal change in regional brain volumes in prodromal Huntington disease. J Neurol Neurosurg Psychiatry 82: 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak TH, Crawford LM, Berrios G, Hodges JR. 2010. Behavioural symptoms in progressive supranuclear palsy and frontotemporal dementia. J Neurol Neurosurg Psychiatry 81: 1057–1059. [DOI] [PubMed] [Google Scholar]
- Baker M, Litvan I, Houlden H, Adamson J, Dickson D, Perez-Tur J, Hardy J, Lynch T, Bigio E, Hutton M. 1999. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet 8: 711–715. [DOI] [PubMed] [Google Scholar]
- Barber R, Ballard C, McKeith IG, Gholkar A, O’Brien JT. 2000. MRI volumetric study of dementia with Lewy bodies: A comparison with AD and vascular dementia. Neurology 54: 1304–1309. [DOI] [PubMed] [Google Scholar]
- Baron JC, Chetelat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, Eustache F. 2001. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer’s disease. Neuroimage 14: 298–309. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J. 1987. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol 50: 344–346. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y, Wenning GK, Tison F, Quinn NP. 1997. Survival of patients with pathologically proven multiple system atrophy: A meta-analysis. Neurology 48: 384–393. [DOI] [PubMed] [Google Scholar]
- Bigio EH, Brown DF, White CL III. 1999. Progressive supranuclear palsy with dementia: Cortical pathology. J Neuropathol Exp Neurol 58: 359–364. [DOI] [PubMed] [Google Scholar]
- Birks JS, Harvey R. 2003. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst Rev 10.1002/14651858.CD001190. [DOI] [PubMed] [Google Scholar]
- Birks JS, Chong LY, Grimley Evans J. 2015. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst Rev 9: CD001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Hampel H. 2003. CSF markers for incipient Alzheimer’s disease. Lancet Neurol 2: 605–613. [DOI] [PubMed] [Google Scholar]
- Blennow K, Hampel H, Weiner M, Zetterberg H. 2010. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol 6: 131–144. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Maraganore DM, Parisi JE, Ahlskog JE, Graff-Radford N, Caselli RJ, Dickson DW, Kokmen E, Petersen RC. 1999. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology 53: 795–800. [DOI] [PubMed] [Google Scholar]
- Boot BP, Orr CF, Ahlskog JE, Ferman TJ, Roberts R, Pankratz VS, Dickson DW, Parisi J, Aakre JA, Geda YE, et al. 2013. Risk factors for dementia with Lewy bodies: A case-control study. Neurology 81: 833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JH, Maraganore DM, McDonnell SK, Rocca WA. 1997. Incidence of progressive supranuclear palsy and multiple system atrophy in Olmsted County, Minnesota, 1976 to 1990. Neurology 49: 1284–1288. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Geschwind MD, Belfor N, Gorno-Tempini ML, Schauer GF, Miller BL, Weiner MW, Rosen HJ. 2006. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch Neurol 63: 81–86. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. 1991. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82: 239–259. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. 2003. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24: 197–211. [DOI] [PubMed] [Google Scholar]
- Brinkman RR, Mezei MM, Theilmann J, Almqvist E, Hayden MR. 1997. The likelihood of being affected with Huntington disease by a particular age, for a specific CAG size. Am J Hum Genet 60: 1202–1210. [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Gray S, Kawas C. 1998. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health 88: 1337–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DJ, Seppi K, Neuroimaging Working Group on MSA. 2009. Proposed neuroimaging criteria for the diagnosis of multiple system atrophy. Mov Disord 24: 949–964. [DOI] [PubMed] [Google Scholar]
- Burn DJ, Rowan EN, Minett T, Sanders J, Myint P, Richardson J, Thomas A, Newby J, Reid J, O’Brien JT, et al. 2003. Extrapyramidal features in Parkinson’s disease with and without dementia and dementia with Lewy bodies: A cross-sectional comparative study. Mov Disord 18: 884–889. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL III, Schneider JA, Grinberg LT, Halliday G, et al. 2007. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: Consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 114: 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campion D, Dumanchin C, Hannequin D, Dubois B, Belliard S, Puel M, Thomas-Anterion C, Michon A, Martin C, Charbonnier F, et al. 1999. Early-onset autosomal dominant Alzheimer disease: Prevalence, genetic heterogeneity, and mutation spectrum. Am J Hum Genet 65: 664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D, Anderson V, Pijnenburg Y, Whitwell J, Barnes J, Scahill R, Stevens JM, Barkhof F, Scheltens P, Rossor MN, et al. 2009. The clinical profile of right temporal lobe atrophy. Brain 132: 1287–1298. [DOI] [PubMed] [Google Scholar]
- Chang JL, Lomen-Hoerth C, Murphy J, Henry RG, Kramer JH, Miller BL, Gorno-Tempini ML. 2005. A voxel-based morphometry study of patterns of brain atrophy in ALS and ALS/FTLD. Neurology 65: 75–80. [DOI] [PubMed] [Google Scholar]
- Chen-Plotkin AS, Hu WT, Siderowf A, Weintraub D, Goldmann Gross R, Hurtig HI, Xie SX, Arnold SE, Grossman M, Clark CM, et al. 2011. Plasma epidermal growth factor levels predict cognitive decline in Parkinson disease. Ann Neurol 69: 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba Y, Fujishiro H, Ota K, Kasanuki K, Arai H, Hirayasu Y, Sato K, Iseki E. 2015. Clinical profiles of dementia with Lewy bodies with and without Alzheimer’s disease-like hypometabolism. Int J Geriatr Psychiatry 30: 316–323. [DOI] [PubMed] [Google Scholar]
- Chung EJ, Babulal GM, Monsell SE, Cairns NJ, Roe CM, Morris JC. 2015. Clinical features of Alzheimer disease with and without lewy bodies. JAMA Neurol 72: 789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, Pontecorvo MJ, Hefti F, Carpenter AP, Flitter ML, et al. 2011. Use of florbetapir-PET for imaging β-amyloid pathology. JAMA 305: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, Fleisher AS, Reiman EM, Sabbagh MN, Sadowsky CH, et al. 2012. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: A prospective cohort study. Lancet Neurol 11: 669–678. [DOI] [PubMed] [Google Scholar]
- Compta Y, Valldeoriola F, Tolosa E, Rey MJ, Marti MJ, Valls-Sole J. 2007. Long lasting pure freezing of gait preceding progressive supranuclear palsy: A clinicopathological study. Mov Disord 22: 1954–1958. [DOI] [PubMed] [Google Scholar]
- Coon EA, Sletten DM, Suarez MD, Mandrekar JN, Ahlskog JE, Bower JH, Matsumoto JY, Silber MH, Benarroch EE, Fealey RD, et al. 2015. Clinical features and autonomic testing predict survival in multiple system atrophy. Brain 138: 3623–3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper-Knock J, Kirby J, Highley R, Shaw PJ. 2015. The spectrum of C9orf72-mediated neurodegeneration and amyotrophic lateral sclerosis. Neurotherapeutics 12: 326–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC Jr, Rimmler JB, Locke PA, Conneally PM, Schmader KE. 1994. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet 7: 180–184. [DOI] [PubMed] [Google Scholar]
- Corrada M, Brookmeyer R, Kawas C. 1995. Sources of variability in prevalence rates of Alzheimer’s disease. Int J Epidemiol 24: 1000–1005. [DOI] [PubMed] [Google Scholar]
- Coulier IM, de Vries JJ, Leenders KL. 2003. Is FDG-PET a useful tool in clinical practice for diagnosing corticobasal ganglionic degeneration?. Mov Disord 18: 1175–1178. [DOI] [PubMed] [Google Scholar]
- Cousins DA, Burton EJ, Burn D, Gholkar A, McKeith IG, O’Brien JT. 2003. Atrophy of the putamen in dementia with Lewy bodies but not Alzheimer’s disease: An MRI study. Neurology 61: 1191–1195. [DOI] [PubMed] [Google Scholar]
- Coyle-Gilchrist IT, Dick KM, Patterson K, Vazquez Rodriquez P, Wehmann E, Wilcox A, Lansdall CJ, Dawson KE, Wiggins J, Mead S, et al. 2016. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology 86: 1736–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusin C, Franco FB, Fernandez-Robles C, DuBois CM, Welch CA. 2013. Rapid improvement of depression and psychotic symptoms in Huntington’s disease: A retrospective chart review of seven patients treated with electroconvulsive therapy. Gen Hosp Psychiatry 35: 678.e3–678.e5. [DOI] [PubMed] [Google Scholar]
- Dal Forno G, Carson KA, Brookmeyer R, Troncoso J, Kawas CH, Brandt J. 2002. APOE genotype and survival in men and women with Alzheimer’s disease. Neurology 58: 1045–1050. [DOI] [PubMed] [Google Scholar]
- Dale M, van Duijn E. 2015. Anxiety in Huntington’s disease. J Neuropsychiatry Clin Neurosci 27: 262–271. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Schulzer M, Kuramoto L, Cragg J, Ramachandiran N, Au WL, Mak E, McKenzie J, McCormick S, Sossi V, et al. 2011. Age-specific progression of nigrostriatal dysfunction in Parkinson’s disease. Ann Neurol 69: 803–810. [DOI] [PubMed] [Google Scholar]
- de Lau LM, Breteler MM. 2006. Epidemiology of Parkinson’s disease. Lancet Neurol 5: 525–535. [DOI] [PubMed] [Google Scholar]
- Di Maio L, Squitieri F, Napolitano G, Campanella G, Trofatter JA, Conneally PM. 1993. Suicide risk in Huntington’s disease. J Med Genet 30: 293–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW. 1999. Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol 246: II6–II15. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Kouri N, Murray ME, Josephs KA. 2011. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J Mol Neurosci 45: 384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donker Kaat L, Boon AJ, Kamphorst W, Ravid R, Duivenvoorden HJ, van Swieten JC. 2007. Frontal presentation in progressive supranuclear palsy. Neurology 69: 723–729. [DOI] [PubMed] [Google Scholar]
- Donker Kaat L, Boon AJ, Azmani A, Kamphorst W, Breteler MM, Anar B, Heutink P, van Swieten JC. 2009. Familial aggregation of parkinsonism in progressive supranuclear palsy. Neurology 73: 98–105. [DOI] [PubMed] [Google Scholar]
- Drzezga A, Grimmer T, Henriksen G, Muhlau M, Perneczky R, Miederer I, Praus C, Sorg C, Wohlschlager A, Riemenschneider M, et al. 2009. Effect of APOE genotype on amyloid plaque load and gray matter volume in Alzheimer disease. Neurology 72: 1487–1494. [DOI] [PubMed] [Google Scholar]
- Dubois B, Slachevsky A, Pillon B, Beato R, Villalponda JM, Litvan I. 2005. “Applause sign” helps to discriminate PSP from FTD and PD. Neurology 64: 2132–2133. [DOI] [PubMed] [Google Scholar]
- Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA, Dickson D, Duyckaerts C, Cummings J, Gauthier S, et al. 2007. Diagnostic procedures for Parkinson’s disease dementia: Recommendations from the movement disorder society task force. Mov Disord 22: 2314–2324. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, et al. 2014. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol 13: 614–629. [DOI] [PubMed] [Google Scholar]
- Eddy CM, Parkinson EG, Rickards HE. 2016. Changes in mental state and behaviour in Huntington’s disease. Lancet Psychiatry 3: 1079–1086. [DOI] [PubMed] [Google Scholar]
- Edison P, Rowe CC, Rinne JO, Ng S, Ahmed I, Kemppainen N, Villemagne VL, O’Keefe G, Nagren K, Chaudhury KR, et al. 2008. Amyloid load in Parkinson’s disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatry 79: 1331–1338. [DOI] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Albanese A, Byrne EJ, Deuschl G, De Deyn PP, Durif F, Kulisevsky J, van Laar T, Lees A, et al. 2004. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med 351: 2509–2518. [DOI] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, et al. 2007. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22: 1689–1707; quiz 1837. [DOI] [PubMed] [Google Scholar]
- Engler H, Santillo AF, Wang SX, Lindau M, Savitcheva I, Nordberg A, Lannfelt L, Langstrom B, Kilander L. 2008. In vivo amyloid imaging with PET in frontotemporal dementia. Eur J Nucl Med Mol Imaging 35: 100–106. [DOI] [PubMed] [Google Scholar]
- Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, Hebert LE, Hennekens CH, Taylor JO. 1989. Prevalence of Alzheimer’s disease in a community population of older persons. Higher than previously reported. JAMA 262: 2551–2556. [PubMed] [Google Scholar]
- Ewers M, Mattsson N, Minthon L, Molinuevo JL, Antonell A, Popp J, Jessen F, Herukka SK, Soininen H, Maetzler W, et al. 2015. CSF biomarkers for the differential diagnosis of Alzheimer’s disease: A large-scale international multicenter study. Alzheimers Dement 11: 1306–1315. [DOI] [PubMed] [Google Scholar]
- Facheris MF, Maniak S, Scaravilli F, Schule B, Klein C, Pramstaller PP. 2008. Pure akinesia as initial presentation of PSP: A clinicopathological study. Parkinsonism Relat Disord 14: 517–519. [DOI] [PubMed] [Google Scholar]
- Fanciulli A, Wenning GK. 2015. Multiple-system atrophy. N Engl J Med 372: 1375–1376. [DOI] [PubMed] [Google Scholar]
- Farlow M. 2016. Epidemiology, pathology, and pathogenesis of dementia with Lewy bodies. In UpToDate (ed. DeKosky ST). UpToDate, Waltham, MA. [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. 1997. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 278: 1349–1356. [PubMed] [Google Scholar]
- Farrimond LE, Roberts E, McShane R. 2012. Memantine and cholinesterase inhibitor combination therapy for Alzheimer’s disease: A systematic review. BMJ Open 2: e000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Daniele A, Albanese A. 2012. Treatment of motor and non-motor features of Parkinson’s disease with deep brain stimulation. Lancet Neurol 11: 429–442. [DOI] [PubMed] [Google Scholar]
- Federoff M, Price TR, Sailer A, Scholz S, Hernandez D, Nicolas A, Singleton AB, Nalls M, Houlden H. 2016. Genome-wide estimate of the heritability of multiple system atrophy. Parkinsonism Relat Disord 22: 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin A, Leenders KL, Moeller JR, Missimer J, Kuenig G, Spetsieris P, Antonini A, Eidelberg D. 2001. Metabolic network abnormalities in early Huntington’s disease: An [18F]FDG PET study. J Nucl Med 42: 1591–1595. [PubMed] [Google Scholar]
- Fisher ER, Hayden MR. 2014. Multisource ascertainment of Huntington disease in Canada: Prevalence and population at risk. Mov Disord 29: 105–114. [DOI] [PubMed] [Google Scholar]
- Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, Marks WJ Jr, Rothlind J, Sagher O, Moy C, et al. 2010. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med 362: 2077–2091. [DOI] [PubMed] [Google Scholar]
- Forbes D, Forbes SC, Blake CM, Thiessen EJ, Forbes S. 2015. Exercise programs for people with dementia. Cochrane Database Syst Rev 4: CD006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster NL, Heidebrink JL, Clark CM, Jagust WJ, Arnold SE, Barbas NR, DeCarli CS, Turner RS, Koeppe RA, Higdon R, et al. 2007. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain 130: 2616–2635. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Testa C, Zorzan A, Sabattoli F, Beltramello A, Soininen H, Laakso MP. 2002. Detection of grey matter loss in mild Alzheimer’s disease with voxel based morphometry. J Neurol Neurosurg Psychiatry 73: 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B, Diamond MI. 2010. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci 11: 155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulham MJ, Dubinsky RM, Polinsky RJ, Brooks RA, Brown RT, Curras MT, Baser S, Hallett M, Di Chiro G. 1991. Computed tomography, magnetic resonance imaging and positron emission tomography with [18F]fluorodeoxyglucose in multiple system atrophy and pure autonomic failure. Clin Auton Res 1: 27–36. [DOI] [PubMed] [Google Scholar]
- Galimberti D, Scarpini E. 2012. Genetics of frontotemporal lobar degeneration. Front Neurol 3: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Xuereb JH, Hodges JR. 2000. Atypical and typical presentations of Alzheimer’s disease: A clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain 123: 484–498. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Lee SL, Perry A, Havlioglu N, McKeel DW Jr, Morris JC. 2002. Familial dementia with Lewy bodies: Clinicopathologic analysis of two kindreds. Neurology 59: 1079–1082. [DOI] [PubMed] [Google Scholar]
- Gasser T, Hardy J, Mizuno Y. 2011. Milestones in PD genetics. Mov Disord 26: 1042–1048. [DOI] [PubMed] [Google Scholar]
- Gefen T, Gasho K, Rademaker A, Lalehzari M, Weintraub S, Rogalski E, Wieneke C, Bigio E, Geula C, Mesulam MM. 2012. Clinically concordant variations of Alzheimer pathology in aphasic versus amnestic dementia. Brain 135: 1554–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geser F, Wenning GK, Seppi K, Stampfer-Kountchev M, Scherfler C, Sawires M, Frick C, Ndayisaba JP, Ulmer H, Pellecchia MT, et al. 2006. Progression of multiple system atrophy (MSA): A prospective natural history study by the European MSA Study Group (EMSA SG). Mov Disord 21: 179–186. [DOI] [PubMed] [Google Scholar]
- Gilman S, Koeppe RA, Junck L, Kluin KJ, Lohman M, St Laurent RT. 1994. Patterns of cerebral glucose metabolism detected with positron emission tomography differ in multiple system atrophy and olivopontocerebellar atrophy. Ann Neurol 36: 166–175. [DOI] [PubMed] [Google Scholar]
- Gilman S, May SJ, Shults CW, Tanner CM, Kukull W, Lee VM, Masliah E, Low P, Sandroni P, Trojanowski JQ, et al. 2005. The North American Multiple System Atrophy Study Group. J Neural Transm (Vienna) 112: 1687–1694. [DOI] [PubMed] [Google Scholar]
- Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, et al. 2008. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71: 670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golbe LI, Davis PH, Schoenberg BS, Duvoisin RC. 1988. Prevalence and natural history of progressive supranuclear palsy. Neurology 38: 1031–1034. [DOI] [PubMed] [Google Scholar]
- Goldman JS, Farmer JM, Wood EM, Johnson JK, Boxer A, Neuhaus J, Lomen-Hoerth C, Wilhelmsen KC, Lee VM, Grossman M, et al. 2005. Comparison of family histories in FTLD subtypes and related tauopathies. Neurology 65: 1817–1819. [DOI] [PubMed] [Google Scholar]
- Gomperts SN, Locascio JJ, Marquie M, Santarlasci AL, Rentz DM, Maye J, Johnson KA, Growdon JH. 2012. Brain amyloid and cognition in Lewy body diseases. Mov Disord 27: 965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL. 2004. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 55: 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, Perani D, Garibotto V, Cappa SF, Miller BL. 2008. The logopenic/phonological variant of primary progressive aphasia. Neurology 71: 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, et al. 2011. Classification of primary progressive aphasia and its variants. Neurology 76: 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber JJ, Staudinger R. 2009. Teaching neuroimages: “Penguin” or “hummingbird” sign and midbrain atrophy in progressive supranuclear palsy. Neurology 72: e81. [DOI] [PubMed] [Google Scholar]
- Graff MJ, Adang EM, Vernooij-Dassen MJ, Dekker J, Jonsson L, Thijssen M, Hoefnagels WH, Rikkert MG. 2008. Community occupational therapy for older patients with dementia and their care givers: Cost effectiveness study. BMJ 336: 134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M. 2010. Primary progressive aphasia: Clinicopathological correlations. Nat Rev Neurol 6: 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Mickanin J, Onishi K, Hughes E, D’Esposito M, Ding XS, Alavi A, Reivich M. 1996. Progressive nonfluent aphasia: Language, cognitive, and PET measures contrasted with probable Alzheimer’s disease. J Cogn Neurosci 8: 135–154. [DOI] [PubMed] [Google Scholar]
- Guridi J, Rodriguez-Oroz MC, Alegre M, Obeso JA. 2012. Hardware complications in deep brain stimulation: Electrode impedance and loss of clinical benefit. Parkinsonism Relat Disord 18: 765–769. [DOI] [PubMed] [Google Scholar]
- Gusella JF, Wexler NS, Conneally PM, Naylor SL, Anderson MA, Tanzi RE, Watkins PC, Ottina K, Wallace MR, Sakaguchi AY. 1983. A polymorphic DNA marker genetically linked to Huntington’s disease. Nature 306: 234–238. [DOI] [PubMed] [Google Scholar]
- Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, Booij J, Dluzen DE, Horstink MW. 2007. Gender differences in Parkinson’s disease. J Neurol Neurosurg Psychiatry 78: 819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RL. 2000. Lewy bodies in Alzheimer’s disease: A neuropathological review of 145 cases using α-synuclein immunohistochemistry. Brain Pathol 10: 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. 2006. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol 5: 228–234. [DOI] [PubMed] [Google Scholar]
- Harris JM, Jones M. 2014. Pathology in primary progressive aphasia syndromes. Curr Neurol Neurosci Rep 14: 466-014-0466-4. [DOI] [PubMed] [Google Scholar]
- Harris JM, Gall C, Thompson JC, Richardson AM, Neary D, du Plessis D, Pal P, Mann DM, Snowden JS, Jones M. 2013. Classification and pathology of primary progressive aphasia. Neurology 81: 1832–1839. [DOI] [PubMed] [Google Scholar]
- Hassan A, Whitwell JL, Boeve BF, Jack CR Jr, Parisi JE, Dickson DW, Josephs KA. 2010. Symmetric corticobasal degeneration (S-CBD). Parkinsonism Relat Disord 16: 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I. 1994. Preliminary NINDS neuropathologic criteria for Steele–Richardson–Olszewski syndrome (progressive supranuclear palsy). Neurology 44: 2015–2019. [DOI] [PubMed] [Google Scholar]
- Healy DG, Falchi M, O’Sullivan SS, Bonifati V, Durr A, Bressman S, Brice A, Aasly J, Zabetian CP, Goldwurm S, et al. 2008. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: A case-control study. Lancet Neurol 7: 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsen H, Strik M, Bauer M, Luther K, Ulmar G, Gangnus D, Jungkunz G, Eisenmenger W, Gotz M. 1994. Cortical and striatal neurone number in Huntington’s disease. Acta Neuropathol 88: 320–333. [DOI] [PubMed] [Google Scholar]
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. 2008. The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov Disord 23: 837–844. [DOI] [PubMed] [Google Scholar]
- Henry ML, Wilson SM, Ogar JM, Sidhu MS, Rankin KP, Cattaruzza T, Miller BL, Gorno-Tempini ML, Seeley WW. 2014. Neuropsychological, behavioral, and anatomical evolution in right temporal variant frontotemporal dementia: A longitudinal and post-mortem single case analysis. Neurocase 20: 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert MK, Eeftens JM, Aerts MB, Esselink RA, Bloem BR, Kuiperij HB, Verbeek MM. 2014. CSF levels of DJ-1 and tau distinguish MSA patients from PD patients and controls. Parkinsonism Relat Disord 20: 112–115. [DOI] [PubMed] [Google Scholar]
- Herbert MK, Aerts MB, Beenes M, Norgren N, Esselink RA, Bloem BR, Kuiperij HB, Verbeek MM. 2015. CSF neurofilament light chain but not FLT3 ligand discriminates parkinsonian disorders. Front Neurol 6: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann N, Black SE, Chow T, Cappell J, Tang-Wai DF, Lanctot KL. 2012. Serotonergic function and treatment of behavioral and psychological symptoms of frontotemporal dementia. Am J Geriatr Psychiatry 20: 789–797. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Davies R, Xuereb J, Kril J, Halliday G. 2003. Survival in frontotemporal dementia. Neurology 61: 349–354. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Mitchell J, Dawson K, Spillantini MG, Xuereb JH, McMonagle P, Nestor PJ, Patterson K. 2010. Semantic dementia: Demography, familial factors and survival in a consecutive series of 100 cases. Brain 133: 300–306. [DOI] [PubMed] [Google Scholar]
- Hoffman JM, Welsh-Bohmer KA, Hanson M, Crain B, Hulette C, Earl N, Coleman RE. 2000. FDG PET imaging in patients with pathologically verified dementia. J Nucl Med 41: 1920–1928. [PubMed] [Google Scholar]
- Hogan DB, Jette N, Fiest KM, Roberts JI, Pearson D, Smith EE, Roach P, Kirk A, Pringsheim T, Maxwell CJ. 2016. The prevalence and incidence of frontotemporal dementia: A systematic review. Can J Neurol Sci 43: S96–S109. [DOI] [PubMed] [Google Scholar]
- Hogarth P, Kayson E, Kieburtz K, Marder K, Oakes D, Rosas D, Shoulson I, Wexler NS, Young AB, Zhao H. 2005. Interrater agreement in the assessment of motor manifestations of Huntington’s disease. Mov Disord 20: 293–297. [DOI] [PubMed] [Google Scholar]
- Howard R, McShane R, Lindesay J, Ritchie C, Baldwin A, Barber R, Burns A, Dening T, Findlay D, Holmes C, et al. 2012. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med 366: 893–903. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. 1992. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, Lopresti BJ, Ziolko S, Bi W, Paljug WR, et al. 2008. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease. Brain 131: 1630–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Kawachi T, Sasaki H, Kono AK, Fukuda T, Kojima Y, Mori E. 2005. Voxel-based morphometric comparison between early- and late-onset mild Alzheimer’s disease and assessment of diagnostic performance of Z score images. AJNR Am J Neuroradiol 26: 333–340. [PMC free article] [PubMed] [Google Scholar]
- Jankovic J, Kapadia AS. 2001. Functional decline in Parkinson disease. Arch Neurol 58: 1611–1615. [DOI] [PubMed] [Google Scholar]
- Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, Huber S, Koller W, Olanow C, Shoulson I. 1990. Variable expression of Parkinson’s disease: A base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology 40: 1529–1534. [DOI] [PubMed] [Google Scholar]
- Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, Visser PJ, Amyloid Biomarker Study Group, Aalten P, Aarsland D, et al. 2015. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA 313: 1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvik G, Larson EB, Goddard K, Schellenberg GD, Wijsman EM. 1996. Influence of apolipoprotein E genotype on the transmission of Alzheimer disease in a community-based sample. Am J Hum Genet 58: 191–200. [PMC free article] [PubMed] [Google Scholar]
- Jecmenica-Lukic M, Poewe W, Tolosa E, Wenning GK. 2012. Premotor signs and symptoms of multiple system atrophy. Lancet Neurol 11: 361–368. [DOI] [PubMed] [Google Scholar]
- Jimenez-Sanchez M, Licitra F, Underwood BR, Rubinsztein DC. 2016. Huntington’s disease: Mechanisms of pathogenesis and therapeutic strategies. Cold Spring Harb Perspect Med 10.1101/cshperspect.a024240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JK, Diehl J, Mendez MF, Neuhaus J, Shapira JS, Forman M, Chute DJ, Roberson ED, Pace-Savitsky C, Neumann M, et al. 2005. Frontotemporal lobar degeneration: Demographic characteristics of 353 patients. Arch Neurol 62: 925–930. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, Mormino E, Chhatwal J, Amariglio R, Papp K, et al. 2016. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol 79: 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF, Jolley D. 1998. The incidence of dementia: A meta-analysis. Neurology 51: 728–733. [DOI] [PubMed] [Google Scholar]
- Josephs KA. 2007. Capgras syndrome and its relationship to neurodegenerative disease. Arch Neurol 64: 1762–1766. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, Hauser MF, Witte RJ, Boeve BF, Knopman DS, et al. 2006. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 129: 1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Dickson DW, Boeve BF, Knopman DS, Petersen RC, Parisi JE, Jack CR Jr. 2008. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging 29: 280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien CL, Thompson JC, Wild S, Yardumian P, Snowden JS, Turner G, Craufurd D. 2007. Psychiatric disorders in preclinical Huntington’s disease. J Neurol Neurosurg Psychiatry 78: 939–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagi G, Bhatia KP, Tolosa E. 2010. The role of DAT-SPECT in movement disorders. J Neurol Neurosurg Psychiatry 81: 5–12. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Lang AE. 2016. Parkinson disease in 2015: Evolving basic, pathological and clinical concepts in PD. Nat Rev Neurol 12: 65–66. [DOI] [PubMed] [Google Scholar]
- Kang JH, Irwin DJ, Chen-Plotkin AS, Siderowf A, Caspell C, Coffey CS, Waligorska T, Taylor P, Pan S, Frasier M, et al. 2013. Association of cerebrospinal fluid β-amyloid 1–42, T-tau, P-tau181, and α-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol 70: 1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Avula R, Senjem ML, Samikoglu AR, Zhang B, Weigand SD, Przybelski SA, Edmonson HA, Vemuri P, Knopman DS, et al. 2010. Dementia with Lewy bodies and Alzheimer disease: Neurodegenerative patterns characterized by DTI. Neurology 74: 1814–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. 2005. The evolution and pathology of frontotemporal dementia. Brain 128: 1996–2005. [DOI] [PubMed] [Google Scholar]
- Khan BK, Yokoyama JS, Takada LT, Sha SJ, Rutherford NJ, Fong JC, Karydas AM, Wu T, Ketelle RS, Baker MC, et al. 2012. Atypical, slowly progressive behavioural variant frontotemporal dementia associated with C9ORF72 hexanucleotide expansion. J Neurol Neurosurg Psychiatry 83: 358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JC, Eggers C, Kalbe E, Weisenbach S, Hohmann C, Vollmar S, Baudrexel S, Diederich NJ, Heiss WD, Hilker R. 2010. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology 74: 885–892. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, et al. 2004. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 55: 306–319. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Roberts RO. 2011. Estimating the number of persons with frontotemporal lobar degeneration in the US population. J Mol Neurosci 45: 330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollensperger M, Geser F, Seppi K, Stampfer-Kountchev M, Sawires M, Scherfler C, Boesch S, Mueller J, Koukouni V, Quinn N, et al. 2008. Red flags for multiple system atrophy. Mov Disord 23: 1093–1099. [DOI] [PubMed] [Google Scholar]
- Kollensperger M, Geser F, Ndayisaba JP, Boesch S, Seppi K, Ostergaard K, Dupont E, Cardozo A, Tolosa E, Abele M, et al. 2010. Presentation, diagnosis, and management of multiple system atrophy in Europe: Final analysis of the European multiple system atrophy registry. Mov Disord 25: 2604–2612. [DOI] [PubMed] [Google Scholar]
- Kompoliti K, Goetz CG, Litvan I, Jellinger K, Verny M. 1998. Pharmacological therapy in progressive supranuclear palsy. Arch Neurol 55: 1099–1102. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, Miller BL. 2003. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol 16: 211–218. [DOI] [PubMed] [Google Scholar]
- Kremer HPH, Huntington Study Group. 1996. Unified Huntington’s disease rating scale: Reliability and consistency. Mov Disord 11: 136–142. [DOI] [PubMed] [Google Scholar]
- Lanata SC, Miller BL. 2016. The behavioural variant frontotemporal dementia (bvFTD) syndrome in psychiatry. J Neurol Neurosurg Psychiatry 87: 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedev AV, Westman E, Beyer MK, Kramberger MG, Aguilar C, Pirtosek Z, Aarsland D. 2013. Multivariate classification of patients with Alzheimer’s and dementia with Lewy bodies using high-dimensional cortical thickness measurements: An MRI surface-based morphometric study. J Neurol 260: 1104–1115. [DOI] [PubMed] [Google Scholar]
- Lebert F, Stekke W, Hasenbroekx C, Pasquier F. 2004. Frontotemporal dementia: A randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord 17: 355–359. [DOI] [PubMed] [Google Scholar]
- Lee VM, Goedert M, Trojanowski JQ. 2001. Neurodegenerative tauopathies. Annu Rev Neurosci 24: 1121–1159. [DOI] [PubMed] [Google Scholar]
- Lee CS, Schulzer M, de la Fuente-Fernandez R, Mak E, Kuramoto L, Sossi V, Ruth TJ, Calne DB, Stoessl AJ. 2004. Lack of regional selectivity during the progression of Parkinson disease: Implications for pathogenesis. Arch Neurol 61: 1920–1925. [DOI] [PubMed] [Google Scholar]
- Lee SE, Rabinovici GD, Mayo MC, Wilson SM, Seeley WW, DeArmond SJ, Huang EJ, Trojanowski JQ, Growdon ME, Jang JY, et al. 2011. Clinicopathological correlations in corticobasal degeneration. Ann Neurol 70: 327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Ramos EM, Lee JH, Gillis T, Mysore JS, Hayden MR, Warby SC, Morrison P, Nance M, Ross CA, et al. 2012. CAG repeat expansion in Huntington disease determines age at onset in a fully dominant fashion. Neurology 78: 690–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kim TH, Mun CW, Kim TH, Han YH. 2015. Progression of subcortical atrophy and iron deposition in multiple system atrophy: A comparison between clinical subtypes. J Neurol 262: 1876–1882. [DOI] [PubMed] [Google Scholar]
- Lees AJ, Hardy J, Revesz T. 2009. Parkinson’s disease. Lancet 373: 2055–2066. [DOI] [PubMed] [Google Scholar]
- Leroi I, Overshott R, Byrne EJ, Daniel E, Burns A. 2009. Randomized controlled trial of memantine in dementia associated with Parkinson’s disease. Mov Disord 24: 1217–1221. [DOI] [PubMed] [Google Scholar]
- Lillo P, Garcin B, Hornberger M, Bak TH, Hodges JR. 2010. Neurobehavioral features in frontotemporal dementia with amyotrophic lateral sclerosis. Arch Neurol 67: 826–830. [DOI] [PubMed] [Google Scholar]
- Lippa CF, Possin KL. 2016. Lewy Body Dementia (DLB/PDD). In Non-Alzheimer’s and atypical dementia (ed. Geshwind M, Belkoura C), pp. 64 Wiley-Blackwell, West Sussex, United Kingdom. [Google Scholar]
- Lippa CF, Duda JE, Grossman M, Hurtig HI, Aarsland D, Boeve BF, Brooks DJ, Dickson DW, Dubois B, Emre M, et al. 2007. DLB and PDD boundary issues: Diagnosis, treatment, molecular pathology, and biomarkers. Neurology 68: 812–819. [DOI] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, et al. 1996a. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele–Richardson–Olszewski syndrome): Report of the NINDS-SPSP international workshop. Neurology 47: 1–9. [DOI] [PubMed] [Google Scholar]
- Litvan I, Mega MS, Cummings JL, Fairbanks L. 1996b. Neuropsychiatric aspects of progressive supranuclear palsy. Neurology 47: 1184–1189. [DOI] [PubMed] [Google Scholar]
- Litvan I, Baker M, Hutton M. 2001. Tau genotype: No effect on onset, symptom severity, or survival in progressive supranuclear palsy. Neurology 57: 138–140. [DOI] [PubMed] [Google Scholar]
- Litvan I, Aarsland D, Adler CH, Goldman JG, Kulisevsky J, Mollenhauer B, Rodriguez-Oroz MC, Troster AI, Weintraub D. 2011. MDS Task Force on mild cognitive impairment in Parkinson’s disease: Critical review of PD-MCI. Mov Disord 26: 1814–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubenkov PA, Miller BL. 2016. A clinical guide to frontotemporal dementias. Focus 14: 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo A, Launer LJ, Fratiglioni L, Andersen K, Di Carlo A, Breteler MM, Copeland JR, Dartigues JF, Jagger C, Martinez-Lage J, et al. 2000. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 54: S4–S9. [PubMed] [Google Scholar]
- Lohmann E, Periquet M, Bonifati V, Wood NW, De Michele G, Bonnet AM, Fraix V, Broussolle E, Horstink MW, Vidailhet M, et al. 2003. How much phenotypic variation can be attributed to parkin genotype? Ann Neurol 54: 176–185. [DOI] [PubMed] [Google Scholar]
- Lomen-Hoerth C. 2011. Clinical phenomenology and neuroimaging correlates in ALS-FTD. J Mol Neurosci 45: 656–662. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Colenda CC, Beck C, Blank K, Doraiswamy MP, Kalunian DA, Yaffe K, Task Force of American Association for Geriatric Psychiatry. 2006. Position statement of the American Association for Geriatric Psychiatry regarding principles of care for patients with dementia resulting from Alzheimer disease. Am J Geriatr Psychiatry 14: 561–572. [DOI] [PubMed] [Google Scholar]
- Lyons KE, Wilkinson SB, Overman J, Pahwa R. 2004. Surgical and hardware complications of subthalamic stimulation: A series of 160 procedures. Neurology 63: 612–616. [DOI] [PubMed] [Google Scholar]
- MacDonald ME, Ambrose CM, Duyao MP, Myers RH, Lin C, Srinidhi L, Barnes G, Taylor SA, James M, Groot N, et al. 1993. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72: 971–983. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR. 2007. The neuropathology of FTD associated with ALS. Alzheimer Dis Assoc Disord 21: S44–S49. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Rademakers R, Neumann M. 2010. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol 9: 995–1007. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DM, Lee VM. 2011. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 122: 111–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan A, Whitwell JL, Weigand SD, Duffy JR, Strand EA, Machulda MM, Tosakulwong N, Senjem ML, Gunter JL, Lowe VJ, et al. 2013. FDG PET and MRI in logopenic primary progressive aphasia versus dementia of the Alzheimer’s type. PLoS ONE 8: e62471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder K, Tang MX, Mejia H, Alfaro B, Cote L, Louis E, Groves J, Mayeux R. 1996. Risk of Parkinson’s disease among first-degree relatives: A community-based study. Neurology 47: 155–160. [DOI] [PubMed] [Google Scholar]
- Margallo-Lana M, Morris CM, Gibson AM, Tan AL, Kay DW, Tyrer SP, Moore BP, Ballard CG. 2004. Influence of the amyloid precursor protein locus on dementia in Down syndrome. Neurology 62: 1996–1998. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Staniloiu A. 2012. Amnesic disorders. Lancet 380: 1429–1440. [DOI] [PubMed] [Google Scholar]
- Martins CA, Oulhaj A, de Jager CA, Williams JH. 2005. APOE alleles predict the rate of cognitive decline in Alzheimer disease: A nonlinear model. Neurology 65: 1888–1893. [DOI] [PubMed] [Google Scholar]
- Maruyama M, Shimada H, Suhara T, Shinotoh H, Ji B, Maeda J, Zhang MR, Trojanowski JQ, Lee VM, Ono M, et al. 2013. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron 79: 1094–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeux R, Stern Y. 2012. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med 2: 10.1101/cshperspect.a006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, et al. 2005. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology 65: 1863–1872. [DOI] [PubMed] [Google Scholar]
- McKeith I, O’Brien J, Walker Z, Tatsch K, Booij J, Darcourt J, Padovani A, Giubbini R, Bonuccelli U, Volterrani D, et al. 2007. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: A phase III, multicentre study. Lancet Neurol 6: 305–313. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, et al. 2011a. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, et al. 2011b. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMonagle P, Deering F, Berliner Y, Kertesz A. 2006. The cognitive profile of posterior cortical atrophy. Neurology 66: 331–338. [DOI] [PubMed] [Google Scholar]
- McShane R, Areosa Sastre A, Minakaran N. 2006. Memantine for dementia. Cochrane Database Syst Rev 2: CD003154. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Shapira JS, McMurtray A, Licht E, Miller BL. 2007. Accuracy of the clinical evaluation for frontotemporal dementia. Arch Neurol 64: 830–835. [DOI] [PubMed] [Google Scholar]
- Mercy L, Hodges JR, Dawson K, Barker RA, Brayne C. 2008. Incidence of early-onset dementias in Cambridgeshire, United Kingdom. Neurology 71: 1496–1499. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. 2003. Primary progressive aphasia—A language-based dementia. N Engl J Med 349: 1535–1542. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, Weintraub S, Bigio EH. 2008. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol 63: 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH. 2014. Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia. Brain 137: 1176–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisan F, Kab S, Mohamed F, Canonico M, Le Guern M, Quintin C, Carcaillon L, Nicolau J, Duport N, Singh-Manoux A, et al. 2016. Parkinson disease male-to-female ratios increase with age: French nationwide study and meta-analysis. J Neurol Neurosurg Psychiatry 87: 952–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, Sixel-Doring F, Trenkwalder C, Schlossmacher MG. 2011. α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: A cohort study. Lancet Neurol 10: 230–240. [DOI] [PubMed] [Google Scholar]
- Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. 2003. Frontotemporal dementia: Paroxetine as a possible treatment of behavior symptoms. A randomized, controlled, open 14-month study. Eur Neurol 49: 13–19. [DOI] [PubMed] [Google Scholar]
- Mori E, Lee K, Yasuda M, Hashimoto M, Kazui H, Hirono N, Matsui M. 2002. Accelerated hippocampal atrophy in Alzheimer’s disease with apolipoprotein E epsilon4 allele. Ann Neurol 51: 209–214. [DOI] [PubMed] [Google Scholar]
- Multiple-System Atrophy Research Collaboration. 2013. Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med 369: 233–244. [DOI] [PubMed] [Google Scholar]
- Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. 2011. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: A retrospective study. Lancet Neurol 10: 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RH, Sax DS, Schoenfeld M, Bird ED, Wolf PA, Vonsattel JP, White RF, Martin JB. 1985. Late onset of Huntington’s disease. J Neurol Neurosurg Psychiatry 48: 530–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RH, Schaefer EJ, Wilson PW, D’Agostino R, Ordovas JM, Espino A, Au R, White RF, Knoefel JE, Cobb JL, et al. 1996. Apolipoprotein E ɛ4 association with dementia in a population-based study: The Framingham study. Neurology 46: 673–677. [DOI] [PubMed] [Google Scholar]
- Nervi A, Reitz C, Tang MX, Santana V, Piriz A, Reyes D, Lantigua R, Medrano M, Jimenez-Velazquez IZ, Lee JH, et al. 2011. Familial aggregation of dementia with Lewy bodies. Arch Neurol 68: 90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe RG. 1981. A life table for onset of Huntington’s chorea. Ann Hum Genet 45: 375–385. [DOI] [PubMed] [Google Scholar]
- Niccolini F, Politis M. 2014. Neuroimaging in Huntington’s disease. World J Radiol 6: 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum RL. 2017. Genetics of synucleinopathies. Cold Spring Harb Perspect Med 10.1101/cshperspect.a024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum RL, Ellis CE. 2003. Alzheimer’s disease and Parkinson’s disease. N Engl J Med 348: 1356–1364. [DOI] [PubMed] [Google Scholar]
- Obi T, Nishioka K, Ross OA, Terada T, Yamazaki K, Sugiura A, Takanashi M, Mizoguchi K, Mori H, Mizuno Y, et al. 2008. Clinicopathologic study of a SNCA gene duplication patient with Parkinson disease and dementia. Neurology 70: 238–241. [DOI] [PubMed] [Google Scholar]
- Odekerken VJ, Boel JA, Schmand BA, de Haan RJ, Figee M, van den Munckhof P, Schuurman PR, de Bie RM, NSTAPS Study Group. 2016. GPi vs STN deep brain stimulation for Parkinson disease: Three-year follow-up. Neurology 86: 755–761. [DOI] [PubMed] [Google Scholar]
- Okamura N, Arai H, Higuchi M, Tashiro M, Matsui T, Hu XS, Takeda A, Itoh M, Sasaki H. 2001. [18F]FDG-PET study in dementia with Lewy bodies and Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 25: 447–456. [DOI] [PubMed] [Google Scholar]
- Okamura N, Furumoto S, Fodero-Tavoletti MT, Mulligan RS, Harada R, Yates P, Pejoska S, Kudo Y, Masters CL, Yanai K, et al. 2014. Non-invasive assessment of Alzheimer’s disease neurofibrillary pathology using 18F-THK5105 PET. Brain 137: 1762–1771. [DOI] [PubMed] [Google Scholar]
- Olin J, Schneider L. 2001. Galantamine for Alzheimer’s disease. Cochrane Database Syst Rev 1: CD001747. [DOI] [PubMed] [Google Scholar]
- Olney RK, Murphy J, Forshew D, Garwood E, Miller BL, Langmore S, Kohn MA, Lomen-Hoerth C. 2005. The effects of executive and behavioral dysfunction on the course of ALS. Neurology 65: 1774–1777. [DOI] [PubMed] [Google Scholar]
- Onyike CU, Diehl-Schmid J. 2013. The epidemiology of frontotemporal dementia. Int Rev Psychiatry 25: 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth M, European Huntington’s Disease Network, Handley OJ, Schwenke C, Dunnett S, Wild EJ, Tabrizi SJ, Landwehrmeyer GB. 2011. Observing Huntington’s disease: The European Huntington’s Disease Network’s REGISTRY. J Neurol Neurosurg Psychiatry 82: 1409–1412. [DOI] [PubMed] [Google Scholar]
- Ossenkoppele R, Jansen WJ, Rabinovici GD, Knol DL, van der Flier WM, van Berckel BN, Scheltens P, Visser PJ, Amyloid PET Study Group, Verfaillie SC, et al. 2015a. Prevalence of amyloid PET positivity in dementia syndromes: A meta-analysis. JAMA 313: 1939–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Pijnenburg YA, Perry DC, Cohn-Sheehy BI, Scheltens NM, Vogel JW, Kramer JH, van der Vlies AE, La Joie R, Rosen HJ, et al. 2015b. The behavioural/dysexecutive variant of Alzheimer’s disease: Clinical, neuroimaging and pathological features. Brain 138: 2732–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan SS, Massey LA, Williams DR, Silveira-Moriyama L, Kempster PA, Holton JL, Revesz T, Lees AJ. 2008. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain 131: 1362–1372. [DOI] [PubMed] [Google Scholar]
- Papp MI, Kahn JE, Lantos PL. 1989. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci 94: 79–100. [DOI] [PubMed] [Google Scholar]
- Paulsen JS. 2011. Cognitive impairment in Huntington disease: Diagnosis and treatment. Curr Neurol Neurosci Rep 11: 474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Nehl C, Hoth KF, Kanz JE, Benjamin M, Conybeare R, McDowell B, Turner B. 2005. Depression and stages of Huntington’s disease. J Neuropsychiatry Clin Neurosci 17: 496–502. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Long JD, Ross CA, Harrington DL, Erwin CJ, Williams JK, Westervelt HJ, Johnson HJ, Aylward EH, Zhang Y, et al. 2014. Prediction of manifest Huntington’s disease with clinical and imaging measures: A prospective observational study. Lancet Neurol 13: 1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce MM, Kopito RR. 2017. Prion-like characteristics of polyglutamine-containing proteins. Cold Spring Harb Perspect Med 10.1101/cshperspect.a024257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perju-Dumbrava LD, Kovacs GG, Pirker S, Jellinger K, Hoffmann M, Asenbaum S, Pirker W. 2012. Dopamine transporter imaging in autopsy-confirmed Parkinson’s disease and multiple system atrophy. Mov Disord 27: 65–71. [DOI] [PubMed] [Google Scholar]
- Perneczky R, Drzezga A, Boecker H, Forstl H, Kurz A, Haussermann P. 2008. Cerebral metabolic dysfunction in patients with dementia with Lewy bodies and visual hallucinations. Dement Geriatr Cogn Disord 25: 531–538. [DOI] [PubMed] [Google Scholar]
- Perry EK, Haroutunian V, Davis KL, Levy R, Lantos P, Eagger S, Honavar M, Dean A, Griffiths M, McKeith IG. 1994. Neocortical cholinergic activities differentiate Lewy body dementia from classical Alzheimer’s disease. Neuroreport 5: 747–749. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Graham A, Williams G, Rosen H, Erzinclioglu S, Weiner M, Miller B, Hodges J. 2006. Patterns of frontal lobe atrophy in frontotemporal dementia: A volumetric MRI study. Dement Geriatr Cogn Disord 22: 278–287. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. 1997. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science 276: 2045–2047. [DOI] [PubMed] [Google Scholar]
- Portet F, Cadilhac C, Touchon J, Camu W. 2001. Cognitive impairment in motor neuron disease with bulbar onset. Amyotroph Lateral Scler Other Motor Neuron Disord 2: 23–29. [DOI] [PubMed] [Google Scholar]
- Pouratian N, Thakkar S, Kim W, Bronstein JM. 2012. Deep brain stimulation for the treatment of Parkinson’s disease: Efficacy and safety. Degener Neurol Neuromuscul Dis 10.2147/DNND.S25750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringsheim T, Wiltshire K, Day L, Dykeman J, Steeves T, Jette N. 2012. The incidence and prevalence of Huntington’s disease: A systematic review and meta-analysis. Mov Disord 27: 1083–1091. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. 2013. Biology and genetics of prions causing neurodegeneration. Annu Rev Genet 47: 601–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn N. 1989. Multiple system atrophy—The nature of the beast. J Neurol Neurosurg Psychiatry 1989: 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici GD, Seeley WW, Kim EJ, Gorno-Tempini ML, Rascovsky K, Pagliaro TA, Allison SC, Halabi C, Kramer JH, Johnson JK, et al. 2007. Distinct MRI atrophy patterns in autopsy-proven Alzheimer’s disease and frontotemporal lobar degeneration. Am J Alzheimers Dis Other Demen 22: 474–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, O’Neil JP, Lal RA, Dronkers NF, Miller BL, et al. 2008. Aβ amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol 64: 388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici GD, Rosen HJ, Alkalay A, Kornak J, Furst AJ, Agarwal N, Mormino EC, O’Neil JP, Janabi M, Karydas A, et al. 2011. Amyloid vs FDG-PET in the differential diagnosis of AD and FTLD. Neurology 77: 2034–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovoci GD, Hillner B, Whitmer RA, Carrillo M, Gatsonis C, Siegel B. 2015. Imaging dementia-evidence for amyloid scanning (IDEAS)—A national study to evaluate the clinical utility of amyloid PET. Alzheimers Dement (Amst) 11: P263–P264. [Google Scholar]
- Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, Miller BL. 2006. Structural anatomy of empathy in neurodegenerative disease. Brain 129: 2945–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Grossman M. 2013. Clinical diagnostic criteria and classification controversies in frontotemporal lobar degeneration. Int Rev Psychiatry 25: 145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, et al. 2011. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134: 2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilmann R, Leavitt BR, Ross CA. 2014. Diagnostic criteria for Huntington’s disease based on natural history. Mov Disord 29: 1335–1341. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ, Memantine Study Group. 2003. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 348: 1333–1341. [DOI] [PubMed] [Google Scholar]
- Reitz C, Brayne C, Mayeux R. 2011. Epidemiology of Alzheimer disease. Nat Rev Neurol 7: 137–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Petersen RC, Knopman DS, Hebert LE, Evans DA, Hall KS, Gao S, Unverzagt FW, Langa KM, Larson EB, et al. 2011. Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement 7: 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Lashley T, Schott JM, Warren JE, Mead S, Isaacs AM, Beck J, Hardy J, de Silva R, Warrington E, et al. 2011. Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain 134: 2565–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos RA. 2010. Huntington’s disease: A clinical review. Orphanet J Rare Dis 5: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Salat DH, Lee SY, Zaleta AK, Pappu V, Fischl B, Greve D, Hevelone N, Hersch SM. 2008. Cerebral cortex and the clinical expression of Huntington’s disease: Complexity and heterogeneity. Brain 131: 1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, Feiwell R, Kramer JH, Miller BL. 2002a. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 58: 198–208. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Kramer JH, Gorno-Tempini ML, Schuff N, Weiner M, Miller BL. 2002b. Patterns of cerebral atrophy in primary progressive aphasia. Am J Geriatr Psychiatry 10: 89–97. [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. 2005. Neuroanatomical correlates of behavioural disorders in dementia. Brain 128: 2612–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt A, Liang KY, Zhou H, Abbott MH, Gourley LM, Margolis RL, Brandt J, Ross CA. 2006. The association of CAG repeat length with clinical progression in Huntington disease. Neurology 66: 1016–1020. [DOI] [PubMed] [Google Scholar]
- Ross CA, Tabrizi SJ. 2011. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet Neurol 10: 83–98. [DOI] [PubMed] [Google Scholar]
- Ross CA, Aylward EH, Wild EJ, Langbehn DR, Long JD, Warner JH, Scahill RI, Leavitt BR, Stout JC, Paulsen JS, et al. 2014. Huntington disease: Natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol 10: 204–216. [DOI] [PubMed] [Google Scholar]
- Rowe CC, Ackerman U, Browne W, Mulligan R, Pike KL, O’Keefe G, Tochon-Danguy H, Chan G, Berlangieri SU, Jones G, et al. 2008. Imaging of amyloid β in Alzheimer’s disease with 18F-BAY94–9172, a novel PET tracer: Proof of mechanism. Lancet Neurol 7: 129–135. [DOI] [PubMed] [Google Scholar]
- Rub U, Hoche F, Brunt ER, Heinsen H, Seidel K, Del Turco D, Paulson HL, Bohl J, von Gall C, Vonsattel JP, et al. 2013. Degeneration of the cerebellum in Huntington’s disease (HD): Possible relevance for the clinical picture and potential gateway to pathological mechanisms of the disease process. Brain Pathol 23: 165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rub U, Hentschel M, Stratmann K, Brunt E, Heinsen H, Seidel K, Bouzrou M, Auburger G, Paulson H, Vonsattel JP, et al. 2014. Huntington’s disease (HD): Degeneration of select nuclei, widespread occurrence of neuronal nuclear and axonal inclusions in the brainstem. Brain Pathol 24: 247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryman DC, Acosta-Baena N, Aisen PS, Bird T, Danek A, Fox NC, Goate A, Frommelt P, Ghetti B, Langbaum JB, et al. 2014. Symptom onset in autosomal dominant Alzheimer disease: A systematic review and meta-analysis. Neurology 83: 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer A, Scholz SW, Nalls MA, Schulte C, Federoff M, Price TR, Lees A, Ross OA, Dickson DW, Mok K, et al. 2016. A genome-wide association study in multiple system atrophy. Neurology 87: 1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel W, Caligiuri M, Galasko D, Lacro J, Marini M, McClure FS, Warren K, Jeste DV. 2000. Better cognitive and psychopathologic response to donepezil in patients prospectively diagnosed as dementia with Lewy bodies: A preliminary study. Int J Geriatr Psychiatry 15: 794–802. [DOI] [PubMed] [Google Scholar]
- Savica R, Grossardt BR, Bower JH, Boeve BF, Ahlskog JE, Rocca WA. 2013. Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA Neurol 70: 1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz SW, Houlden H, Schulte C, Sharma M, Li A, Berg D, Melchers A, Paudel R, Gibbs JR, Simon-Sanchez J, et al. 2009. SNCA variants are associated with increased risk for multiple system atrophy. Ann Neurol 65: 610–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag A, Ben-Shlomo Y, Quinn NP. 1999. Prevalence of progressive supranuclear palsy and multiple system atrophy: A cross-sectional study. Lancet 354: 1771–1775. [DOI] [PubMed] [Google Scholar]
- Schrag A, Sheikh S, Quinn NP, Lees AJ, Selai C, Mathias C, Litvan I, Lang AE, Bower JH, Burn DJ, et al. 2010. A comparison of depression, anxiety, and health status in patients with progressive supranuclear palsy and multiple system atrophy. Mov Disord 25: 1077–1081. [DOI] [PubMed] [Google Scholar]
- Schrijvers EM, Verhaaren BF, Koudstaal PJ, Hofman A, Ikram MA, Breteler MM. 2012. Is dementia incidence declining?: Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology 78: 1456–1463. [DOI] [PubMed] [Google Scholar]
- Seeley WW. 2010. Anterior insula degeneration in frontotemporal dementia. Brain Struct Funct 214: 465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Bauer AM, Miller BL, Gorno-Tempini ML, Kramer JH, Weiner M, Rosen HJ. 2005. The natural history of temporal variant frontotemporal dementia. Neurology 64: 1384–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, Gorno-Tempini ML. 2008. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol 65: 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz DP, Adunuri N, Gill SS, Gruneir A, Herrmann N, Rochon P. 2011. Antidepressants for agitation and psychosis in dementia. Cochrane Database Syst Rev 2: CD008191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneca S, Fagnart D, Keymolen K, Lissens W, Hasaerts D, Debulpaep S, Desprechins B, Liebaers I, De Meirleir L. 2004. Early onset Huntington disease: A neuronal degeneration syndrome. Eur J Pediatr 163: 717–721. [DOI] [PubMed] [Google Scholar]
- Sha S, Rabinovici G. 2016. Atypical Alzheimer’s disease. In Non-Alzheimer’s and atypical dementia (ed. Geshwind M, Belkoura C), pp. 17 Wiley-Blackwell, West Sussex, United Kingdom. [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, et al. 2009. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 65: 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiwach R. 1994. Psychopathology in Huntington’s disease patients. Acta Psychiatr Scand 90: 241–246. [DOI] [PubMed] [Google Scholar]
- Sieben A, Van Langenhove T, Engelborghs S, Martin JJ, Boon P, Cras P, De Deyn PP, Santens P, Van Broeckhoven C, Cruts M. 2012. The genetics and neuropathology of frontotemporal lobar degeneration. Acta Neuropathol 124: 353–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried J, Lippitz B. 1994. Bilateral chronic electrostimulation of ventroposterolateral pallidum: A new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery 35: 1126–1129; discussion 1129–1130. [DOI] [PubMed] [Google Scholar]
- Silbert LC, Quinn JF, Moore MM, Corbridge E, Ball MJ, Murdoch G, Sexton G, Kaye JA. 2003. Changes in premorbid brain volume predict Alzheimer’s disease pathology. Neurology 61: 487–492. [DOI] [PubMed] [Google Scholar]
- Silverman DH, Small GW, Chang CY, Lu CS, Kung De Aburto MA, Chen W, Czernin J, Rapoport SI, Pietrini P, Alexander GE, et al. 2001. Positron emission tomography in evaluation of dementia: Regional brain metabolism and long-term outcome. JAMA 286: 2120–2127. [DOI] [PubMed] [Google Scholar]
- Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, et al. 2009. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet 41: 1308–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer MJ, Bonifati V. 2013. The genetics of Parkinson’s disease: Progress and therapeutic implications. Mov Disord 28: 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KM, Holden KF, Yaffe K. 2005. Pharmacological treatment of neuropsychiatric symptoms of dementia: A review of the evidence. JAMA 293: 596–608. [DOI] [PubMed] [Google Scholar]
- Slooter AJ, Cruts M, Hofman A, Koudstaal PJ, van der Kuip D, de Ridder MA, Witteman JC, Breteler MM, Van Broeckhoven C, van Duijn CM. 2004. The impact of APOE on myocardial infarction, stroke, and dementia: The Rotterdam Study. Neurology 62: 1196–1198. [DOI] [PubMed] [Google Scholar]
- Snowden J, Neary D, Mann D. 2007. Frontotemporal lobar degeneration: Clinical and pathological relationships. Acta Neuropathol 114: 31–38. [DOI] [PubMed] [Google Scholar]
- Sosa-Ortiz AL, Acosta-Castillo I, Prince MJ. 2012. Epidemiology of dementias and Alzheimer’s disease. Arch Med Res 43: 600–608. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. 1997. α-synuclein in Lewy bodies. Nature 388: 839–840. [DOI] [PubMed] [Google Scholar]
- Squitieri F, Cannella M, Porcellini A, Brusa L, Simonelli M, Ruggieri S. 2001. Short-term effects of olanzapine in Huntington disease. Neuropsychiatry Neuropsychol Behav Neurol 14: 69–72. [PubMed] [Google Scholar]
- Stankovic I, Krismer F, Jesic A, Antonini A, Benke T, Brown RG, Burn DJ, Holton JL, Kaufmann H, Kostic VS, et al. 2014. Cognitive impairment in multiple system atrophy: A position statement by the Neuropsychology Task Force of the MDS Multiple System Atrophy (MODIMSA) study group. Mov Disord 29: 857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemberger S, Scholz SW, Singleton AB, Wenning GK. 2011. Genetic players in multiple system atrophy: Unfolding the nature of the beast. Neurobiol Aging 32: 1924.e5–1924.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Griffiths TD, Rastogi S, Perry RH, Cleland PG. 2003. Non-Picks frontotemporal dementia imitating schizophrenia in a 22-year-old man. J Neurol 250: 369–370. [DOI] [PubMed] [Google Scholar]
- Swartz JR, Miller BL, Lesser IM, Darby AL. 1997. Frontotemporal dementia: Treatment response to serotonin selective reuptake inhibitors. J Clin Psychiatry 58: 212–216. [PubMed] [Google Scholar]
- Tanner CM, Goldman SM. 1996. Epidemiology of Parkinson’s disease. Neurol Clin 14: 317–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H, Pirttila T. 2009. Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol 66: 382–389. [DOI] [PubMed] [Google Scholar]
- Thenganatt MA, Jankovic J. 2014. Parkinson disease subtypes. JAMA Neurol 71: 499–504. [DOI] [PubMed] [Google Scholar]
- Thompson JC, Harris J, Sollom AC, Stopford CL, Howard E, Snowden JS, Craufurd D. 2012. Longitudinal evaluation of neuropsychiatric symptoms in Huntington’s disease. J Neuropsychiatry Clin Neurosci 24: 53–60. [DOI] [PubMed] [Google Scholar]
- Tison F, Yekhlef F, Chrysostome V, Sourgen C. 2000. Prevalence of multiple system atrophy. Lancet 355: 495–496. [DOI] [PubMed] [Google Scholar]
- Tonkonogy JM, Smith TW, Barreira PJ. 1994. Obsessive-compulsive disorders in Pick’s disease. J Neuropsychiatry Clin Neurosci 6: 176–180. [DOI] [PubMed] [Google Scholar]
- Tsuboi Y, Slowinski J, Josephs KA, Honer WG, Wszolek ZK, Dickson DW. 2003. Atrophy of superior cerebellar peduncle in progressive supranuclear palsy. Neurology 60: 1766–1769. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Van Laere K, Ivanoiu A, Salmon E, Bastin C, Triau E, Hasselbalch S, Law I, Andersen A, Korner A, et al. 2010. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: A phase 2 trial. Ann Neurol 68: 319–329. [DOI] [PubMed] [Google Scholar]
- van Duijn CM, Clayton D, Chandra V, Fratiglioni L, Graves AB, Heyman A, Jorm AF, Kokmen E, Kondo K, Mortimer JA, et al. 1991. Familial aggregation of Alzheimer’s disease and related disorders: A collaborative re-analysis of case-control studies. Int J Epidemiol 20: S13–S20. [DOI] [PubMed] [Google Scholar]
- Vann Jones SA, O’Brien JT. 2014. The prevalence and incidence of dementia with Lewy bodies: A systematic review of population and clinical studies. Psychol Med 44: 673–683. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Walterfang M, Mocellin R, Pantelis C, McLean C. 2009. Frontotemporal dementia presenting as schizophrenia-like psychosis in young people: Clinicopathological series and review of cases. Br J Psychiatry 194: 298–305. [DOI] [PubMed] [Google Scholar]
- Vilarino-Guell C, Soto-Ortolaza AI, Rajput A, Mash DC, Papapetropoulos S, Pahwa R, Lyons KE, Uitti RJ, Wszolek ZK, Dickson DW, et al. 2011. MAPT H1 haplotype is a risk factor for essential tremor and multiple system atrophy. Neurology 76: 670–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonsattel JP, DiFiglia M. 1998. Huntington disease. J Neuropathol Exp Neurol 57: 369–384. [DOI] [PubMed] [Google Scholar]
- Wadia PM, Lang AE. 2007. The many faces of corticobasal degeneration. Parkinsonism Relat Disord 13: S336–S340. [DOI] [PubMed] [Google Scholar]
- Wahlund LO, Almkvist O, Blennow K, Engedahl K, Johansson A, Waldemar G, Wolf H. 2005. Evidence-based evaluation of magnetic resonance imaging as a diagnostic tool in dementia workup. Top Magn Reson Imaging 16: 427–437. [DOI] [PubMed] [Google Scholar]
- Walker LC, Jucker M. 2015. Neurodegenerative diseases: Expanding the prion concept. Annu Rev Neurosci 38: 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker Z, Jaros E, Walker RW, Lee L, Costa DC, Livingston G, Ince PG, Perry R, McKeith I, Katona CL. 2007. Dementia with Lewy bodies: A comparison of clinical diagnosis, FP-CIT single photon emission computed tomography imaging and autopsy. J Neurol Neurosurg Psychiatry 78: 1176–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HF, Yu JT, Tang SW, Jiang T, Tan CC, Meng XF, Wang C, Tan MS, Tan L. 2015. Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies: Systematic review with meta-analysis and trial sequential analysis. J Neurol Neurosurg Psychiatry 86: 135–143. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Saito Y, Terao S, Ando T, Kachi T, Mukai E, Aiba I, Abe Y, Tamakoshi A, Doyu M, et al. 2002. Progression and prognosis in multiple system atrophy: An analysis of 230 Japanese patients. Brain 125: 1070–1083. [DOI] [PubMed] [Google Scholar]
- Watson R, O’Brien JT, Barber R, Blamire AM. 2012. Patterns of gray matter atrophy in dementia with Lewy bodies: A voxel-based morphometry study. Int Psychogeriatr 24: 532–540. [DOI] [PubMed] [Google Scholar]
- Watts JC, Giles K, Oehler A, Middleton L, Dexter DT, Gentleman SM, DeArmond SJ, Prusiner SB. 2013. Transmission of multiple system atrophy prions to transgenic mice. Proc Natl Acad Sci 110: 19555–19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning GK, Tison F, Ben Shlomo Y, Daniel SE, Quinn NP. 1997. Multiple system atrophy: A review of 203 pathologically proven cases. Mov Disord 12: 133–147. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Litvan I, Jankovic J, Granata R, Mangone CA, McKee A, Poewe W, Jellinger K, Ray Chaudhuri K, D’Olhaberriague L, et al. 1998. Natural history and survival of 14 patients with corticobasal degeneration confirmed at postmortem examination. J Neurol Neurosurg Psychiatry 64: 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning GK, Working Group on Atypical Parkinsonism of the Austrian Parkinson’s Society. 2005. Placebo-controlled trial of amantadine in multiple-system atrophy. Clin Neuropharmacol 28: 225–227. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Geser F, Krismer F, Seppi K, Duerr S, Boesch S, Kollensperger M, Goebel G, Pfeiffer KP, Barone P, et al. 2013. The natural history of multiple system atrophy: A prospective European cohort study. Lancet Neurol 12: 264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesnes KA, McKeith I, Edgar C, Emre M, Lane R. 2005. Benefits of rivastigmine on attention in dementia associated with Parkinson disease. Neurology 65: 1654–1656. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Weigand SD, Shiung MM, Boeve BF, Ferman TJ, Smith GE, Knopman DS, Petersen RC, Benarroch EE, Josephs KA, et al. 2007. Focal atrophy in dementia with Lewy bodies on MRI: A distinct pattern from Alzheimer’s disease. Brain 130: 708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Josephs KA, Murray ME, Kantarci K, Przybelski SA, Weigand SD, Vemuri P, Senjem ML, Parisi JE, Knopman DS, et al. 2008. MRI correlates of neurofibrillary tangle pathology at autopsy: A voxel-based morphometry study. Neurology 71: 743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Dickson DW, Murray ME, Weigand SD, Tosakulwong N, Senjem ML, Knopman DS, Boeve BF, Parisi JE, Petersen RC, et al. 2012. Neuroimaging correlates of pathologically defined subtypes of Alzheimer’s disease: A case-control study. Lancet Neurol 11: 868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, de Silva R, Paviour DC, Pittman A, Watt HC, Kilford L, Holton JL, Revesz T, Lees AJ. 2005. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson’s syndrome and PSP-parkinsonism. Brain 128: 1247–1258. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Grachev ID, Buckley C, Kazi H, Grady MS, Trojanowski JQ, Hamilton RH, Sherwin P, McLain R, Arnold SE. 2011. Association between in vivo fluorine 18-labeled flutemetamol amyloid positron emission tomography imaging and in vivo cerebral cortical histopathology. Arch Neurol 68: 1398–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Rosenberg PB, Zhou Y, Kumar A, Raymont V, Ravert HT, Dannals RF, Nandi A, Brasic JR, Ye W, et al. 2010. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir [corrected] F 18). J Nucl Med 51: 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods B, Aguirre E, Spector AE, Orrell M. 2012. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev 2: CD005562. [DOI] [PubMed] [Google Scholar]
- Woodruff BK, Graff-Radford NR, Ferman TJ, Dickson DW, DeLucia MW, Crook JE, Arvanitakis Z, Brassler S, Waters C, Barker W, et al. 2006. Family history of dementia is a risk factor for Lewy body disease. Neurology 66: 1949–1950. [DOI] [PubMed] [Google Scholar]
- Woods B, Aguirre E, Spector AE, Orrell M. 2012. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev 2: CD005562. [DOI] [PubMed] [Google Scholar]
- Woolley JD, Khan BK, Murthy NK, Miller BL, Rankin KP. 2011. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: Rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J Clin Psychiatry 72: 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullner U, Schmitt I, Kammal M, Kretzschmar HA, Neumann M. 2009. Definite multiple system atrophy in a German family. J Neurol Neurosurg Psychiatry 80: 449–450. [DOI] [PubMed] [Google Scholar]
- Xia CF, Arteaga J, Chen G, Gangadharmath U, Gomez LF, Kasi D, Lam C, Liang Q, Liu C, Mocharla VP, et al. 2013. [18F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimers Dement 9: 666–676. [DOI] [PubMed] [Google Scholar]
- Zaccai J, McCracken C, Brayne C. 2005. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing 34: 561–566. [DOI] [PubMed] [Google Scholar]