Abstract
Acute respiratory distress syndrome (ARDS) is a highly heterogeneous syndrome that can exhibit significant differences in the underlying causes, leading to different responses to treatment. It is required to identify subtypes of ARDS to guideline clinical treatment and trial design. The study aimed to identify subtypes of ARDS using latent class analysis (LCA). The study was a secondary analysis of the EDEN study, which was a randomized, controlled, multicenter trial conducted from January 2, 2008 to April 12, 2011. The primary study endpoint was death through 90-day follow up. LCA was performed incorporating variables on day 0 before randomization. The number of classes was chosen by a bootstrapped likelihood ratio test, Bayesian information criterion and the number of patients in each class. A total of 943 patients were enrolled in the study, including 219 (23.2%) non-survivors and 724 (76.8%) survivors. The LCA identified three classes of ARDS. Class 1 (hemodynamically unstable type) had significantly higher mortality rate (p = 0.003) than class 2 (intermediate type) and 3 (stable type) through 90 days follow up. There was significant interaction between cumulative fluid balance and the class (p = 0.02). While more fluid balance was beneficial for class 1, it was harmful for class 2 and 3. In conclusion, the study identified three classes of ARDS, which showed different clinical presentations, responses to fluid therapy and prognosis. The classification system used simple clinical variables and could help to design ARDS trials in the future.
Keywords: Latent class analysis, Acute respiratory distress syndrome, Fluid balance, Mortality
Background
Acute respiratory distress syndrome (ARDS) is a clinical syndrome manifested by acute onset of respiratory failure requiring medical intervention. Clinical outcomes may be greatly improved if ARDS is promptly identified at an early stage and treated properly. Despite strenuous efforts being made to improve the clinical outcomes of ARDS (Steinberg et al., 2006; Mansur et al., 2015; Zhang, Chen & Ni, 2015; Kacmarek et al., 2016; Artigas et al., 2017), the mortality rate remains unacceptably high (Frenzel et al., 2011; Dalhoff et al., 2012; Bellani et al., 2016; Wang et al., 2016). The recent LUNG SAFE study reports a hospital mortality of 40%, with an increase across the severity in the Berlin definition (Bellani et al., 2016). There are only a few interventions being proven to be effective in improving survival outcome which includes protective mechanical ventilation, conservative fluid management, neuromuscular blockade and prone positioning (Acute Respiratory Distress Syndrome Network et al., 2000; Wang et al., 2016; Murray et al., 2016; Matthay, McAuley & Ware, 2017). The challenge in the treatment of ARDS is probably attributable to the heterogeneity of the population. ARDS is classified as a syndrome simply because these patients share some common features in clinical presentations such as the acute onset, diffuse bilateral infiltrates in chest X-ray and low oxygenation as reflected by the PaO2/FiO2 (P/F) ratio (ARDS Definition Task Force et al., 2012). As a matter of fact, ARDS is a heterogeneous clinical syndrome exhibiting quite different clinical courses and responses to treatment, depending on the underlying causes, disease stage and comorbidities (Meyer & Christie, 2013; Shaver & Bastarache, 2014; Luo et al., 2017). Thus, it is important to find subclass of ARDS patients who are more likely to benefit from treatments, presumably because the subclass has a different underlying pathophysiology (Russell et al., 2017).
Latent class analysis (LCA) can help to find classes or subtypes of cases in multivariate data. It has been widely used in economics, business and psychology (Krauss et al., 2017; Nouwens et al., 2017). However, LCA is not widely understood in medicine, and there are often inadequate feature variables to characterize clinically meaningful latent classes. A variety of clinical and laboratory variables were collected in clinical trials involving ARDS patients. These data provided a good opportunity to establish a latent class model to identify subtypes of ARDS that can have different treatment responses. Previous studies have successfully classified ARDS into subphenotypes (Calfee et al., 2014; Famous et al., 2017). However, these studies employed inflammatory biomarkers such as interleukins and tumor necrosis factor which were not routinely available in routine clinical practice and the classification system might not be widely applicable. In this study, the author aimed to identify subtypes of ARDS by using simple clinical and laboratory variables. Furthermore, the mortality outcome was compared between subtypes. The response to fluid balance was also compared between ARDS subtypes.
Methods
Study population
The study was a secondary analysis of the early versus delayed enteral feeding to treat people with acute lung injury or acute respiratory distress syndrome (EDEN) study, which was a randomized, controlled, multicenter trial conducted from January 2, 2008 to April 12, 2011 (National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network et al., 2012). The study randomized 1,000 patients within 48 h of developing ARDS requiring mechanical ventilation in approximately equal numbers to receive either trophic or full enteral feeding for the first six days. The effectiveness of both interventions was comparable with regard to clinical outcomes such as 60-day mortality, ventilator-free days and infectious complications. The secondary analysis utilized de-identified patient information and was approved by the institutional review board of Sir Run Run Shaw Hospital (20170313-2).
Definition of some variables
The study recorded three types of patient status at 90 days which were home with unassisted breathing, dead prior to home with unassisted breathing and last known status (neither dead nor home with unassisted breathing). For the purpose of de-identification, the date of these status was transformed to the number of days by subtracting the date of randomization. Death was considered as the event of interest.
Variables recorded on day 0 (before randomization) including age, heart rate, temperature, systolic blood pressure, diastolic blood pressure, sodium (plasma), bicarbonate (plasma), potassium (plasma), albumin (serum) and P/F ratio. If there were multiple records, the one closest to 8 o’clock was used. Fluid intakes and outputs were obtained for the first 24 h after randomization. Fluid balance for a single day was calculated as the difference between fluid intake and output.
Statistical analysis
Continuous variables were examined for their normality. Normal data were expressed as mean and standard deviation, and skewed data were expressed as median and interquartile range (Zhang, 2016a). Mann–Whitney U tests or t-tests were used for two group comparisons as appropriate. Variables normally distributed within latent classes were compared using analysis of variance, and non-normal data were compared using the Kruskal–Wallis test. Categorical variables were expressed as the number and proportions. Proportions were compared using Chi-square test or Fisher’s exact test (e.g., the former was used as long as 80% or more of cells had expected cell counts greater than five and all observed cell counts were at least one). Comparisons between latent classes were performed using the CBCgrps package in R (Zhang et al., 2017).
Latent class analysis is a subset of structural equation modeling where observed variables are used to identify unobserved or latent classes (Zhang, 2017). The number of classes was determined by using the Bayesian information criterion (BIC), a parametric bootstrapped likelihood ratio test, relative entropy and the number of individuals within each class (Nylund, Asparouhov & Muthén, 2007). The expectation-maximization algorithm was used to obtain the maximum likelihood estimates for the parameters. The distributions of observed variables were displayed across identified classes of ARDS.
A multivariable logistic regression model was built to examine the adjusted differences in 90-day mortality between ARDS classes (Zhang, 2016b). The interaction between class membership and randomization group were explored in the logistic regression model. To investigate the influence of ARDS classes on the effect of fluid balance on mortality risk, another logistic regression model was established by including the interaction between class and fluid balance on day 1.
All statistical analyses were performed using R (version 3.3.2) (R Core Team, 2016). Two-tailed p values less than 0.05 were considered statistically significant.
Results
A total of 943 patients were included in the study, including 219 (23.2%) non-survivors at 90 days and 724 (76.8%) survivors. Non-survivors were significantly older (60 (47, 72) vs. 51 (41, 60) years, p = 0.001), had higher baseline heart rate (98 (84, 110) vs. 94 (80, 108) per minute; p = 0.001), and potassium levels (4.0 (3.6, 4.5) vs. 3.9(3.6, 4.3) mmol/l; p = 0.07) than non-survivors. Non-survivors had significantly lower values of mean blood pressure (74 (67, 80) vs. 76 (69, 84) mmHg; p < 0.001) and P/F ratio (130 (92, 178) vs. 148 (108, 203); p = 0.002) than survivors (Table 1).
Table 1. Baseline characteristics between survivors and non-survivors.
| Variables | Total (n = 943) | Survivors (n = 724) | Non-survivors (n = 219) | p |
|---|---|---|---|---|
| P/F ratio | 144 (105, 200) | 148 (108, 203) | 130 (92, 178) | 0.002 |
| Heart rate (per minute, IQR) | 94 (81, 108) | 94 (80, 108) | 98 (84, 110) | 0.081 |
| Mean blood pressure (mmHg) | 76 (69, 84) | 76 (69, 84) | 74 (67, 80) | 0.001 |
| Sodium (mmol/l) | 138 (135, 142) | 138 (135, 142) | 139 (135, 142) | 0.859 |
| Albumin (mg/dl) | 2.2 (1.8, 2.7) | 2.3 (1.9, 2.7) | 2.1 (1.8, 2.7) | 0.010 |
| Potassium (mmol/l) | 3.9 (3.6, 4.4) | 3.9 (3.6, 4.3) | 4.0 (3.6, 4.5) | 0.070 |
| Bicarbonate (mmol/l) | 22 (19, 26) | 22.5 (20, 26) | 22 (18, 25) | 0.032 |
| Age (years, IQR) | 52 (42, 63) | 51 (41, 60) | 60 (46.5, 72) | 0.001 |
Note:
p Values were computed using Mann–Whitney U tests.
Latent class analysis
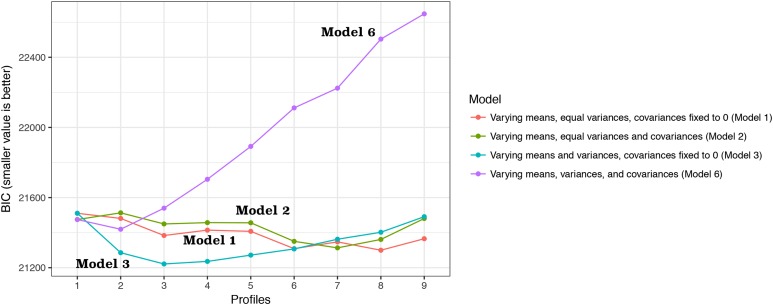
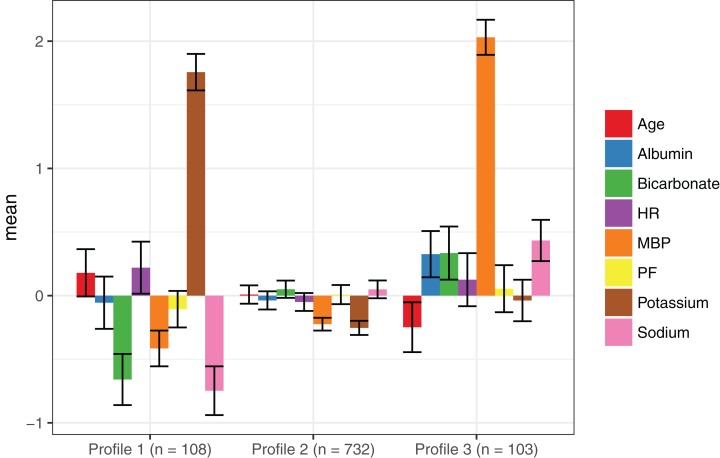
The BIC results are shown in Fig. 1. The three-class model showed the lowest BIC values in three models (1, 2 and 3). Model 6 with varying means, variances and covariances showed the lowest BIC value for two-class model. The three-class model had the highest entropy value (0.88). The number of patients in each class was sizable for the three-class model (108, 732 and 103 for class 1, 2 and 3, respectively). The bootstrapped likelihood ratio test also showed that the three-class model was significantly better than two-class model but not worse than four-class model (Table 2). As a result, the three-class model was chosen as the best fit model. Class 1 was characterized by older age, lower mean blood pressure, higher heart rate and lower bicarbonate (Table 3). Class 3 was characterized by younger age, lower heart rate and higher blood pressure. Class 2 was an intermediate type between class 1 and 3. The numbers of individuals were 108, 732 and 103 in class 1, 2 and 3, respectively (Fig. 2). Thus, class 1 could be named the hemodynamically unstable type; class 2 was named the intermediate type and class 3 was the stable type.
Figure 1. Bayesian information criterion (BIC) for choosing the number of classes.
Four models with different assumptions were employed for estimating BIC values. The three-class model showed the lowest BIC values in three models (1, 2 and 3). Model 6 with varying means, variances and covariances showed the lowest BIC value for two-class model.
Table 2. Criteria to choose the best number of classes.
| Number of classes | BIC | Entropy | Number of individuals per class | Bootstrapped likelihood ratio test | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | p Value | |||
| 2 | 21481.114 | 0.78 | 374 | 569 | 0.001 | ||||
| 3 | 21383.724 | 0.88 | 108 | 732 | 103 | 0.001 | |||
| 4 | 21414.326 | 0.72 | 228 | 499 | 107 | 109 | 0.078 | ||
| 5 | 21407.395 | 0.75 | 175 | 562 | 86 | 19 | 101 | 0.067 | |
| 6 | 21308.773 | 0.76 | 179 | 552 | 84 | 19 | 103 | 6 | 0.079 |
Notes:
p Values were comparing with model with one fewer class.
BIC, Bayesian information criterion.
Table 3. Differences of feature variables among the three latent classes.
| Variables | Class 1 | Class 2 | Class 3 | p Value |
|---|---|---|---|---|
| P/F ratio | 140 (107, 193) | 145 (105, 200) | 155 (109, 203) | 0.621 |
| Heart rate (per minute, IQR) | 98 (83, 116) | 94 (80, 106) | 97 (80, 112) | 0.040 |
| Mean blood pressure (mmHg) | 71 (65, 78) | 74 (68, 81) | 102 (98, 107) | 0.001 |
| Sodium (mmol/l) | 135 (131, 139) | 139 (136, 142) | 141 (138, 143) | 0.001 |
| Albumin (mg/dl) | 2.2 (1.7, 2.7) | 2.2 (1.8, 2.7) | 2.4 (2.1, 3.0) | 0.001 |
| Potassium (mmol/l) | 5.0 (4.8, 5.4) | 3.8 (3.5, 4.2) | 4 (3.6, 4.4) | 0.001 |
| Bicarbonate (mmol/l) | 19 (15, 22) | 23 (20, 26) | 24 (20, 27) | 0.001 |
| Age (years, IQR) | 55 (45, 68) | 52 (42, 63) | 51 (37, 58) | 0.018 |
| Mortality | 36 (0.33) | 169 (0.23) | 14 (0.14) | 0.003 |
Notes:
Continuous variables were expressed as median and interquartile range, and categorical data were expressed as the number and proportions. p Values were computed using the Kruskal–Wallis test.
Figure 2. Latent class profile for the three-class model.
Class 1 was characterized by older age, lower mean blood pressure, higher heart rate and lower bicarbonate. Class 3 was characterized by younger age, lower heart rate and higher blood pressure. Class 2 is the intermediate type between class 1 and 3. Abbreviations: PF, P/F ratio; HR, baseline heart rate; MBP, mean blood pressure.
Clinical characteristics of the three classes
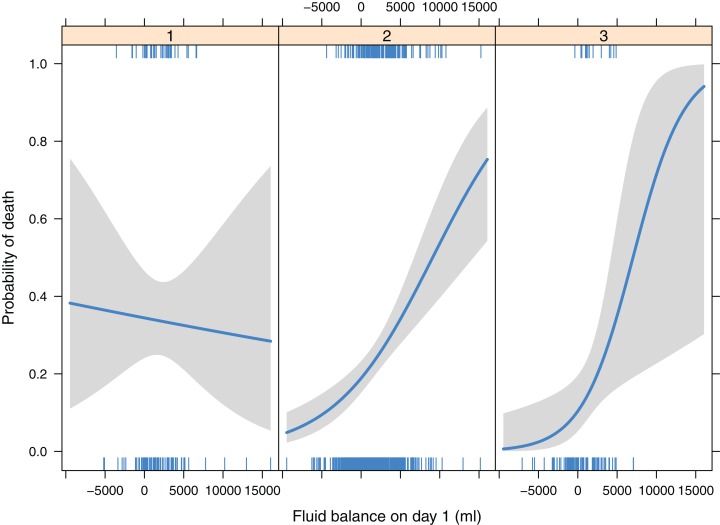
Logistic regression showed there was no significant interaction between randomization group and class membership (p = 0.553), and this interaction term was removed from the model. As compared with class 3, class 1 (OR: 2.76; 95% CI [1.38–5.82]) was associated with higher risk of death. Class 1 was associated with higher risk of death than class 2 (OR: 1.72; 95% CI [1.09–2.68]). Class 2 and 3 had similar mortality outcome (OR: 1.60; 95% CI [0.90–3.09]; p = 0.132). The randomization group had no independent effect on mortality outcome (p = 0.557). There was significant interaction between class membership and fluid balance on day 1 (p = 0.02). While more positive fluid intake was associated with lower risk of death in class 1, more negative fluid balance was associated with lower risk of death in class 2 and 3 (Table 4; Fig. 3).
Table 4. Interaction between fluid balance and latent class in multivariable model with 90-day survival outcome as the response variable.
| Variables | Odds ratio | Lower limit of 95% CI | Upper limit of 95% CI | p Value |
|---|---|---|---|---|
| Class 1 vs. 3 | 2.763 | 1.377 | 5.818 | 0.005 |
| Class 2 vs. 3 | 1.604 | 0.895 | 3.091 | 0.132 |
| Class 1 vs. 2 | 1.723 | 1.093 | 2.675 | 0.017 |
| Fluid balance on day 1 with each 2 l increase for class 1 | 0.966 | 0.726 | 1.260 | 0.801 |
| Fluid balance on day 1 with each 2 l increase for class 2 | 1.378 | 1.211 | 1.574 | 0.001 |
| Fluid balance on day 1 with each 2 l increase for class 3 | 1.849 | 1.146 | 3.132 | 0.015 |
| Randomization group (intervention vs. control) | 0.911 | 0.667 | 1.243 | 0.557 |
Notes:
p Values were computed using Wald statistic.
CI, confidence interval.
Figure 3. Interactions between ARDS class membership and fluid balance on day 1, by adjusting for randomization group.
Discussion
The study identified three classes of ARDS that had different 90-day mortality risk (e.g., class 1 had significantly higher mortality rate than class 2 and 3). Class 1 was a hemodynamically unstable type characterized by lower mean blood pressure and higher heart rate. Class 2 was an intermediate type that the SIRS response appears less severe than class 1. Class 3 was a group with stable vital signs. Multivariable regression model showed that while more fluid balance is potentially beneficial for class 1, more fluid balance was associated with increased risk of death for classes 2 and 3. The identification of these subtypes of ARDS may help to triage ARDS patients that respond differently to fluid therapy, and to design future clinical trials by using simple clinical variables.
It has long been noticed that ARDS is not a homogenous disease (Rezoagli, Fumagalli & Bellani, 2017), but it encompasses a highly heterogeneous patient population who have remarkable differences in genetic background, clinical characteristics, treatment responses and mortality outcomes (Meyer & Christie, 2013; Shaver & Bastarache, 2014; Matthay, McAuley & Ware, 2017). ARDS is clinically classified as a syndrome simply because it describes a collection of commonly encountered symptoms in critically ill patients, which typically involves acute onset, bilateral lung infiltrates, low oxygenation and requirement of respiratory support. Since ARDS is a heterogeneous clinical syndrome, many efforts have been made to categorize it into subtypes or classes. Brown and colleagues classified ARDS patients into four subtypes according to their six-month health status (Brown et al., 2017). However, this classification system required data over a six-month period and cannot be used at the beginning of disease onset. The strength of the present study was that clinical variables collected at the early stage of ARDS onset were employed, which can help clinicians to identify subtypes of ARDS that have different clinical outcomes and treatment responses. In another study, the authors employed inflammatory biomarkers to classify ARDS into two subphenotypes and the results showed that these subphenotypes had differences in clinical outcomes and responses to PEEP strategy (Calfee et al., 2014). Type 2 was characterized by hyperinflammatory responses, corresponding to the class 1 in our study. They further investigated the difference in the effect of liberal versus conservative fluid treatment strategy on clinical outcomes between the two subphenotypes. Fluid management strategy had significantly different effects on 90-day mortality in the two subphenotypes (p = 0.0039 for interaction). While conservative strategy resulted in higher mortality than liberal strategy in subphenotype 1 (26% vs. 18%), mortality in subphenotype 2 was lower with fluid-conservative strategy than the liberal strategy (40% vs. 50%). In this study, more positive fluid balance appeared to be associated with reduced risk of death in class 1, while the effect was opposite in class 2 and 3. The adverse effect of positive fluid balance was prominent in class 3 (corresponding to the hypo-inflammatory subphenotype). It is reasonable that ARDS patients without shock can benefit from conservative fluid strategy to reduce pulmonary edema. On the contrary, for patients with unstable hemodynamics, maintaining tissue perfusion by liberal fluid strategy is the priority. However, the biomarkers in Calfee’s study are not available in routine clinical practice, inhibiting its widespread use. In contrast, the present study employed simple variables that were readily available in all clinical institutions, making external validation of the classification system feasible. Famous and colleagues further showed that a parsimonious model involving only three variables interleukin-8, bicarbonate and tumor necrosis factor receptor-1 could accurately identify the two-class model. However, these biomarkers except for bicarbonate are still not routinely tested in real clinical practice, especially in some low-income countries.
Several limitations need to be acknowledged in the study. The study was a secondary analysis of a clinical trial, which was subject to inherent limitations of the post hoc analysis. Sample size and statistical power were not designed for the LCA statistical model. Fluid strategy was not randomized in the original study, raising the problem of selection bias, e.g., more fluid was given to class 1 patients. In this case, class membership is a confounder for the relationship between fluid balance and mortality outcome. In the present study, any such confounding effect was adjusted for by the use of multivariable regression model. Another limitation of the study is that LCA results are not readily transferable to a score or some other algorithm to help triage ARDS patients. The next step may be to build a multinomial logistic regression model by regressing latent class on candidate predictors to obtain the weight (regression coefficient) for each of the predictors. In this way, a mathematical equation or nomogram can be built to predict the probability of belonging to a subclass for a given patient with known predictors (Zhang & Kattan, 2017).
Conclusion
In conclusion, this study identified three classes of ARDS, which showed different clinical outcomes and treatment responses to fluid therapy. The classification system used simple clinical variables and could help to design ARDS trials in the future.
Funding Statement
The study was supported by Zhejiang Provincial Natural Science Foundation of China (LGF18H150005). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The author declares that they have no competing interests.
Author Contributions
Zhongheng Zhang conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Clinical Trial Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The study was approved by the institutional review board of Sir Run Run Shaw Hospital (20170313-2).
Clinical Trial Registration
The following information was supplied regarding Clinical Trial registration:
Trial registration: NCT00609180, registered February 6, 2008.
Data Availability
The following information was supplied regarding data availability:
All data are available at: https://biolincc.nhlbi.nih.gov/studies/eden/?q=EDEN.
References
- Acute Respiratory Distress Syndrome Network et al. (2000).Acute Respiratory Distress Syndrome Network. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New England Journal of Medicine. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- ARDS Definition Task Force et al. (2012).ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- Artigas et al. (2017).Artigas A, Camprubí-Rimblas M, Tantinyà N, Bringué J, Guillamat-Prats R, Matthay MA. Inhalation therapies in acute respiratory distress syndrome. Annals of Translational Medicine. 2017;5(14):293. doi: 10.21037/atm.2017.07.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellani et al. (2016).Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A, LUNG SAFE Investigators. ESICM Trials Group Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- Brown et al. (2017).Brown SM, Wilson EL, Presson AP, Dinglas VD, Greene T, Hopkins RO, Needham DM, with the National Institutes of Health NHLBI ARDS Network Understanding patient outcomes after acute respiratory distress syndrome: identifying subtypes of physical, cognitive and mental health outcomes. Thorax. 2017;72(12):1094–1103. doi: 10.1136/thoraxjnl-2017-210337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfee et al. (2014).Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA, NHLBI ARDS Network Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respiratory Medicine. 2014;2(8):611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalhoff et al. (2012).Dalhoff K, Abele-Horn M, Andreas S, Bauer T, Baum von H, Deja M, Ewig S, Gastmeier P, Gatermann S, Gerlach H, Grabein B, Höffken G, Kern WV, Kramme E, Lange C, Lorenz J, Mayer K, Nachtigall I, Pletz M, Rohde G, Rosseau S, Schaaf B, Schaumann R, Schreiter D, Schütte H, Seifert H, Sitter H, Spies C, Welte T, German Society for Anaesthesiology and Intensive Care Medicine, German Society for Infectious Diseases, German Society for Hygiene and Microbiology, German Respiratory Society, Paul-Ehrlich-Society for Chemotherapy Epidemiology, diagnosis and treatment of adult patients with nosocomial pneumonia. S-3 Guideline of the German Society for Anaesthesiology and Intensive Care Medicine, the German Society for Infectious Diseases, the German Society for Hygiene and Microbiology, the German Respiratory Society and the Paul-Ehrlich-Society for Chemotherapy. Pneumologie. 2012;66(12):707–765. doi: 10.1055/s-0032-1325924. [DOI] [PubMed] [Google Scholar]
- Famous et al. (2017).Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT, Calfee CS, ARDS Network Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. American Journal of Respiratory and Critical Care Medicine. 2017;195(3):331–338. doi: 10.1164/rccm.201603-0645OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenzel et al. (2011).Frenzel J, Gessner C, Sandvoss T, Hammerschmidt S, Schellenberger W, Sack U, Eschrich K, Wirtz H. Outcome prediction in pneumonia induced ALI/ARDS by clinical features and peptide patterns of BALF determined by mass spectrometry. PLOS ONE. 2011;6(10):e25544. doi: 10.1371/journal.pone.0025544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacmarek et al. (2016).Kacmarek RM, Villar J, Sulemanji D, Montiel R, Ferrando C, Blanco J, Koh Y, Soler JA, Martínez D, Hernández M, Tucci M, Borges JB, Lubillo S, Santos A, Araujo JB, Amato MBP, Suárez-Sipmann F, Open Lung Approach Network Open lung approach for the acute respiratory distress syndrome: a pilot, randomized controlled trial. Critical Care Medicine. 2016;44(1):32–42. doi: 10.1097/CCM.0000000000001383. [DOI] [PubMed] [Google Scholar]
- Krauss et al. (2017).Krauss MJ, Rajbhandari B, Sowles SJ, Spitznagel EL, Cavazos-Rehg P. A latent class analysis of poly-marijuana use among young adults. Addictive Behaviors. 2017;75:159–165. doi: 10.1016/j.addbeh.2017.07.021. [DOI] [PubMed] [Google Scholar]
- Luo et al. (2017).Luo L, Shaver CM, Zhao Z, Koyama T, Calfee CS, Bastarache JA, Ware LB. Clinical predictors of hospital mortality differ between direct and indirect ARDS. Chest. 2017;151(4):755–763. doi: 10.1016/j.chest.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansur et al. (2015).Mansur A, Steinau M, Popov AF, Ghadimi M, Beissbarth T, Bauer M, Hinz J. Impact of statin therapy on mortality in patients with sepsis-associated acute respiratory distress syndrome (ARDS) depends on ARDS severity: a prospective observational cohort study. BMC Medicine. 2015;13(1):128. doi: 10.1186/s12916-015-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay, McAuley & Ware (2017).Matthay MA, McAuley DF, Ware LB. Clinical trials in acute respiratory distress syndrome: challenges and opportunities. Lancet Respiratory Medicine. 2017;5(6):524–534. doi: 10.1016/S2213-2600(17)30188-1. [DOI] [PubMed] [Google Scholar]
- Meyer & Christie (2013).Meyer NJ, Christie JD. Genetic heterogeneity and risk of acute respiratory distress syndrome. Seminars in Respiratory and Critical Care Medicine. 2013;34(4):459–474. doi: 10.1055/s-0033-1351121. [DOI] [PubMed] [Google Scholar]
- Murray et al. (2016).Murray MJ, DeBlock H, Erstad B, Gray A, Jacobi J, Jordan C, McGee W, McManus C, Meade M, Nix S, Patterson A, Sands MK, Pino R, Tescher A, Arbour R, Rochwerg B, Murray CF, Mehta S. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Critical Care Medicine. 2016;44(11):2079–2103. doi: 10.1097/CCM.0000000000002027. [DOI] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network et al. (2012).National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, Moss M, Morris A, Dong N, Rock P. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307(8):795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouwens et al. (2017).Nouwens PJG, Lucas R, Smulders NBM, Embregts PJCM, van Nieuwenhuizen C. Identifying classes of persons with mild intellectual disability or borderline intellectual functioning: a latent class analysis. BMC Psychiatry. 2017;17(1):257. doi: 10.1186/s12888-017-1426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylund, Asparouhov & Muthén (2007).Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Structural Equation Modeling: A Multidisciplinary Journal. 2007;14(4):535–569. doi: 10.1080/10705510701575396. [DOI] [Google Scholar]
- R Core Team (2016).R Core Team . R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2016. [Google Scholar]
- Rezoagli, Fumagalli & Bellani (2017).Rezoagli E, Fumagalli R, Bellani G. Definition and epidemiology of acute respiratory distress syndrome. Annals of Translational Medicine. 2017;5(14):282. doi: 10.21037/atm.2017.06.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell et al. (2017).Russell JA, Lee T, Singer J, Boyd JH, Walley KR, Gordon AC, Cook DJ, Presneill JJ, Storms MM, Boulton CLH, Jones S, Bernard GR, Slutsky AS, Wells GA, Gasparini A, Savage B, Ayers D, Woods R, Wu K, Maralit M, Howe B, Higgins A, LeBlanc ME, Sutherland AM, Sham A, McLeod A, Dorscheid DR, Hameed M, Lazosky L, Helderweirt S, Foley K, Honeyman C, Terins T, Chittock D, Ronco J, Smith L, Logie S, Wood G, Auld F, Boulton CH, Stedham V, Mantle M, Fox L, McCauley GD, Rolf JD, Erbacher C, Martinka G, Goulding S, Silverwood S, Leung L, Keenan S, Murray J, Van Osch M, Light B, Dominique M, Gray P, Stimpson R, Rosser S, Bell D, Janz W, Hebert PC, McArdle T, Watpool I, Granton JT, Steinberg M, Matte-Martyn A, McDonald E, Clarke F, Tkaczyk A, Zytaruk N, Mehta S, Stewart T, Suri A, Martinez-Motta C, MacDonald R, Sivanantham V, Hodder R, Foxall J, Lewis M, Ward M, Santos Dos C, Friedrich J, Scales D, Smith O, DeCampos I, Richards A, Michalopoulos H, Bakshi U, Sibbald W, Smith T, Code K, Bojilov B, Dale C, Keogh M, Meade M, Hand L. The Septic Shock 3.0 definition and trials: a vasopressin and Septic Shock trial experience. Critical Care Medicine. 2017;45(6):940–948. doi: 10.1097/CCM.0000000000002323. [DOI] [PubMed] [Google Scholar]
- Shaver & Bastarache (2014).Shaver CM, Bastarache JA. Clinical and biological heterogeneity in acute respiratory distress syndrome: direct versus indirect lung injury. Clinics in Chest Medicine. 2014;35(4):639–653. doi: 10.1016/j.ccm.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg et al. (2006).Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M, National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. New England Journal of Medicine. 2006;354(16):1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2016).Wang C, Wang X, Chi C, Guo L, Guo L, Zhao N, Wang W, Pi X, Sun B, Lian A, Shi J, Li E. Lung ventilation strategies for acute respiratory distress syndrome: a systematic review and network meta-analysis. Scientific Reports. 2016;6(1):22855. doi: 10.1038/srep22855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang (2016a).Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Annals of Translational Medicine. 2016a;4(5):91. doi: 10.21037/atm.2016.02.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang (2016b).Zhang Z. Model building strategy for logistic regression: purposeful selection. Annals of Translational Medicine. 2016b;4(6):111. doi: 10.21037/atm.2016.02.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang (2017).Zhang Z. Structural equation modeling in the context of clinical research. Annals of Translational Medicine. 2017;5(5):102. doi: 10.21037/atm.2016.09.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Chen & Ni (2015).Zhang Z, Chen L, Ni H. The effectiveness of corticosteroids on mortality in patients with acute respiratory distress syndrome or acute lung injury: a secondary analysis. Scientific Reports. 2015;5(1):17654. doi: 10.1038/srep17654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2017).Zhang Z, Gayle AA, Wang J, Zhang H, Cardinal-Fernández P. Comparing baseline characteristics between groups: an introduction to the CBCgrps package. Annals of Translational Medicine. 2017;5:484. doi: 10.21037/atm.2017.09.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang & Kattan (2017).Zhang Z, Kattan MWK. Drawing Nomograms with R: applications to categorical outcome and survival data. Annals of Translational Medicine. 2017;5(10):211. doi: 10.21037/atm.2017.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
All data are available at: https://biolincc.nhlbi.nih.gov/studies/eden/?q=EDEN.