Abstract
Ocean acidification significantly affects marine organisms in several ways, with complex interactions. Seaweeds might benefit from rising CO2 through increased photosynthesis and carbon acquisition, with subsequent higher growth rates. However, changes in seaweed chemistry due to increased CO2 may change the nutritional quality of tissue for grazers. In addition, organisms live in close association with a diverse microbiota, which can also be influenced by environmental changes, with feedback effects. As gut microbiomes are often linked to diet, changes in seaweed characteristics and associated microbiome can affect the gut microbiome of the grazer, with possible fitness consequences. In this study, we experimentally investigated the effects of acidification on the microbiome of the invasive brown seaweed Sargassum muticum and a native isopod consumer Synisoma nadejda. Both were exposed to ambient CO2 conditions (380 ppm, pH 8.16) and an acidification treatment (1,000 ppm, pH 7.86) for three weeks. Microbiome diversity and composition were determined using high-throughput sequencing of the variable regions V5-7 of 16S rRNA. We anticipated that as a result of acidification, the seaweed-associated bacterial community would change, leading to further changes in the gut microbiome of grazers. However, no significant effects of elevated CO2 on the overall bacterial community structure and composition were revealed in the seaweed. In contrast, significant changes were observed in the bacterial community of the grazer gut. Although the bacterial community of S. muticum as whole did not change, Oceanospirillales and Vibrionales (mainly Pseudoalteromonas) significantly increased their abundance in acidified conditions. The former, which uses organic matter compounds as its main source, may have opportunistically taken advantage of the possible increase of the C/N ratio in the seaweed under acidified conditions. Pseudoalteromonas, commonly associated to diseased seaweeds, suggesting that acidification may facilitate opportunistic/pathogenic bacteria. In the gut of S. nadejda, the bacterial genus Planctomycetia increased abundance under elevated CO2. This shift might be associated to changes in food (S. muticum) quality under acidification. Planctomycetia are slow-acting decomposers of algal polymers that could be providing the isopod with an elevated algal digestion and availability of inorganic compounds to compensate the shifted C/N ratio under acidification in their food.
In conclusion, our results indicate that even after only three weeks of acidified conditions, bacterial communities associated to ungrazed seaweed and to an isopod grazer show specific, differential shifts in associated bacterial community. These have potential consequences for seaweed health (as shown in corals) and isopod food digestion. The observed changes in the gut microbiome of the grazer seem to reflect changes in the seaweed chemistry rather than its microbial composition.
Keywords: Invasive seaweeds, Ocean acidification, Grazer microbiomes, Algae microbiomes, Metabarcoding, Sargassum muticum, Synisoma nadejda
Background
Ocean acidification significantly affects marine organisms in diverse ways (Fabry et al., 2008; Kroeker et al., 2013). In the case of species interactions (e.g., predator–prey), the outcome of such effects can be difficult to predict as antagonistic or synergistic effects may be observed (Asnaghi et al., 2013; Branch et al., 2013; Poore et al., 2013). This is particularly true for non-calcifying seaweeds, which in contrast to most other organisms can benefit from rising CO2 through increased photosynthesis and carbon acquisition, and subsequently acquire higher growth rates (Porzio, Buia & Hall-Spencer, 2011; Harley et al., 2012; Koch et al., 2013; Olischläger et al., 2013). However, changes in plant leaf chemistry in response to elevated carbon supply are expected to result in higher C:N and C:P ratios and, as such, reduce the nutritional quality of tissue for grazers (Urabe, Togari & Elser, 2003; Van De Waal et al., 2010) and the same is expected for seaweeds. Variations in the palatability of seaweeds may lead to changes in consumption rates by herbivores, which will have to absorb nutrients more efficiently or consume more to compensate for low concentrations of essential nutrients (Gutow et al., 2014). Thus, ocean acidification (OA) could have positive effects on seaweed growth rate, but may also induce behavioral changes on the herbivores and increased grazing rates. Therefore, it is important to understand the effects of ocean acidification on prey (bottom-up effects) and, as a top-down effect, on predation.
Interactions among organisms and their associated bacterial communities affect the holobiont physiology and health (Hollants et al., 2013; Egan et al., 2013), and play an important role in the functioning of hosts as, in the case of this study, seaweeds (Singh et al., 2011; Singh & Reddy, 2014). Seaweeds and marine organisms feeding on them live in a close association with diverse and abundant microbial communities (King et al., 2012; Hollants et al., 2013; Egan et al., 2013; Dudek et al., 2014). Seaweeds comprise dynamic species-specific bacterial communities (Aires et al., 2015; Aires, Serrão & Engelen, 2016; Vieira et al., 2016). The communities are recognized to have growth-promoting and nutritional effects (Head & Carpenter, 1975; Dimitrieva, Crawford & Yüksel, 2006), and to be involved in the production of biologically active (Chojnacka et al., 2012) and defensive (Burgess et al., 1999) compounds. At a higher trophic level, symbiotic bacteria inhabiting the guts of marine herbivores are also known to support important physiological functions (Hacquard et al., 2015), including the mediation of the digestion of food components by producing critical digestive enzymes for breaking down complex molecular structures (Mackie et al., 2004). In addition to digestive functions, grazers depend on seaweed-associated microbiota for nutrients found in the algal biofilm (i.e., proteins, polysaccharides, lipids, etc.; Tietjen, 2014). As such, diet represents an important factor in shaping microbial diversity in the intestinal systems of grazers. So, any changes in bacterial composition of the seaweed may result in diet-induced changes in the gut microbiota of grazers that may eventually affect their metabolism, as well as its fitness and biology (Mattila et al., 2014; Tietjen, 2014). Because carbon acquisition is expected to be facilitated for seaweeds at elevated CO2 levels, higher nutrient uptake is anticipated to help obtain other nutrients in the right balance with carbon. Part of these nutrients might be obtained through the microbiome and, therefore, the specific bacteria responsible for such acquisitions (e.g., phosphorous, nitrogen and iron) (Thomas et al., 2008; Burke et al., 2011b) might be positively selected and increase their abundance.
Because OA is expected to affect the interactions between marine herbivores and seaweeds through increased consumption of carbon enriched algal tissue (Gutow et al., 2014), the microbiomes of grazers might help with nutrient acquisition. While better understanding of the diversity and functions of associated symbiotic bacteria is needed, few studies have addressed the diversity and composition of gut microbiota of marine grazers (but see Hong et al., 2011; Devine, Pelletreau & Rumpho, 2012; Davis et al., 2013; Dudek et al., 2014).
To predict the responses of aquatic organisms to OA, it is necessary to understand responses of the host-associated microbiota to increasing CO2 and reduced pH. Little is known about the responses of associated microbiota to changes in pCO2 (partial pressure of carbon dioxide) including microbial metabolic capabilities or the ability to rapidly shift the host range (Morrow et al., 2015). Also, there is no consensus regarding whether a decrease in pH causes increase (Kerfahi et al., 2014), decrease (Taylor et al., 2014) or no changes (Hassenrück et al., 2016) in microbial richness and prevalence of dominant microbial taxa under acidification conditions. Furthermore, the current knowledge of acidification effects on the host-associated microbial communities is mostly based on the results of experiments conducted on corals. These experiments demonstrated that reduced pH initiates shifts in the coral microbiota towards microorganisms associated with stress and disease (Thurber et al., 2009; Meron et al., 2011; Webster et al., 2013). Therefore, there is a need for relevant studies on seaweeds, with a particular interest in species interactions as species may not respond similarly to OA and as effects can act synergistically. This response is particularly relevant to be examined in marine introduced seaweeds, because they are expected to benefit from future OA conditions, and thus increase their invasiveness.
In this study, we experimentally investigated the effects of acidification on the microbiomes of an emblematic invasive seaweed, the brown alga Sargassum muticum, and the gut microbiome of a native isopod consumer, Synisoma nadejda. This was done by following a three-week mesocosms exposure to elevated pCO2 followed by 16S amplicon sequencing in order to compare the bacterial community (hereafter microbiome) responses in these two hosts. Based on bacterial community characterization, our main hypotheses are that in acidified conditions (1) the seaweed-associated microbiome will have a different composition, (2) the grazers’ gut microbiome will mirror the changes in food source (assuming that seaweed nutritional content will change with acidification), when compared with ambient conditions. Considering the existing evidence for corals responses to OA (Thurber et al., 2009; Meron et al., 2011; Webster et al., 2013) and seaweeds responses to other environmental stresses (e.g., temperature, Case et al., 2011; Mensch et al., 2016) we expect seaweed microbiome to shift towards a community composed by stress related bacteria and putative pathogens. Also, raw plant consumers’ (e.g., fish, humans, etc.) gut microbiome and health is directly affected by environmental conditions (Sullam et al., 2012) or, following the “you are what you eat” premise, indirectly through changes in their food source/quality (Meziti et al., 2010; Berg et al., 2014), we predict that host’s “normal” gut bacterial composition could be affected by OA. An increased abundance of bacteria potentially assisting digestion could provide the isopod with an elevated algal digestion and availability of inorganic compounds to compensate the shifted C/N ratio under acidification in their food. Furthermore, if S. muticum showed a shift under OA conditions, we will investigate the presence of possible bacterial taxa that could assist the seaweed obtaining nutrients (e.g., nitrogen fixing bacteria) under elevated pCO2 conditions. These predictions, for both seaweed and gut microbiome, are limited to 16S taxonomic assignments and literature description and inherent reservations to this method will be considered.
Methodology
Experimental set-up
The experiment was performed at Centro de Ciencias do Mar (CCMAR) field station (Ramalhete) during the spring of 2014. Ambient (380 ppm CO2, and pH 8.16—global levels of today’s CO2 conditions) and elevated pCO2 (1,000) ppm CO2, and pH 7.86—the year 2100 predictions by IPCC, A1FI scenario (Houghton et al., 2001) conditions were controlled by two separate CO2 sensors systems. For acidified conditions, CO2 was injected in seawater deposits that provided seawater for the experimental units. In both systems salinity was 36, alkalinity 2,550 µmol kg−1 and seawater temperature 15 °C. Experimental units consisted of 3 L flowthrough mesocosms receiving each 30 L of seawater per hour. Experimental units were placed in one square meter tanks with 15 cm of the overflown seawater of the experimental units to stabilize temperature conditions in the units. Sargassum muticum and Syniosoma nadejda were sampled independently and kept isolated. Thus, the grazers were ‘naive’ and not previously on a diet containing S. muticum. After sampling, seaweeds and isopods were acclimated to ambient conditions in separate tanks for 1 week. A wet weight biomass of 1 g seaweed per experimental unit was used as a preliminary test and showed it did not affect the pH conditions in experimental units at the used volume and flow.
The two factors considered for the experiment were (1) CO2 conditions (two levels: 380 and 1,000 ppm), and (2) host conditions (three levels: seaweed with grazers, seaweed without grazers, the grazers that fed on the seaweed). These were combined in a factorial design (all possible combinations of all levels of the two factors). The number of replicates within treatment combination was four. Therefore, in total, 24 samples (2 CO2 × 3 hosts × 4 replicates) were collected (Fig. 1). The experiment ran for three weeks which was estimated sufficient for microbiomes to reflect the applied conditions considering the high turnover rate of food in the grazer gut and the multiplication rate of bacteria. During this period the units were cleaned, twice a week, to avoid epiphyte overgrowth on the experimental unit walls. In each experimental unit, seawater pH and the calibration of the automated CO2 injection system was manually checked daily to make sure the pH was stable. At the end of the three weeks, the isopods and seaweeds were flash frozen, transported to the laboratory in liquid nitrogen and stored there at −80 °C until further processing.
Figure 1. Schematic representation of the mesocosms experiment.
(A) Ambient (380 ppm) and (B) acidified (1,000 ppm) conditions each with four 3 L experimental units only containing 1 g wet weight (WW) S. muticum and four 3 L experimental units containing 1 g WW S. muticum along with the grazer S. nadejda were placed randomly in each CO2 treatment. Each unit represented a replicate from which sample(s) (seaweed or seaweed and grazer) were taken.
Hight-throughput sequencing of the microbiome
For both seaweeds and isopodes’s guts (also refered to as grazers, hereafter), DNA was extracted from all the 24 replicates using the Quick–gDNA kit (Zymo Research™, Irvine, CA, USA) according to the manufacturer protocol for “Solid Tissue Samples” (page 4 of the manual). Before extraction, isopods where dissected by removal of both ends and pulling out the intestinal tract. The total 16S rRNA was amplified using the universal primers 27F and 1492r with the following changes to the original protocol (Lane, 1991): an initial denaturation at 95 °C for 2 min, 35 cycles of denaturation at 95 °C for 20 s, annealing at 55 °C for 20 s, and extension at 72 °C for 90 s, with a final extension was at 72 °C for 3 min. The 25 µl reaction mixture contained 250 µM dNTPs, 0.6 µM of each primer, 1 × 2 PCR buffer mix, 2 µl of template DNA (with a final concentration of about 10 ng µl–1) and 0.3 µl of Taq polymerase (Advantage R2; Clontech, Mountain View, CA, USA). PCR products were cleaned using ExoFastAP enzyme following the Thermo Scientific™ protocol. Amplified DNA was sent to Molecular Research (MR DNA), Shallowater, Texas, where a nested–PCR was performed prior to sequencing. The modified 8 bp key–tagged primer 799F along with the reverse primer 1193R, covering the regions V5–V7 from 16S rRNA and amplifying a fragment of ∼400 bp, were used to avoid chloroplast cross amplification (Bodenhausen, Horton & Bergelson, 2013). PCR conditions were as follow: 95 °C for 3 min, 10 cycles of 95 °C for 20 s, 50 °C for 30 s, 72 °C for 30 s, and a final elongation of 72 °C for 3 min. All amplifications and sample preparation procedures were the same for both seaweeds and isopodes. Samples were pooled together in equal proportions based on their molecular weight (calculated based on the size of the amplicon) and DNA concentrations (using Qubit™; Invitrogen®, Carlsbad, CA, USA) and purified using calibrated Agencourt® AMPure® XP beads. DNA libraries were prepared by following Illumina TruSeq DNA library preparation protocol and paired–end (2 × 250 bp) sequencing performed at MR DNA (http://www.mrdnalab.com; Shallowater, TX, USA) on a MiSeq following the manufacturer’s guidelines.
Detailled protocols for sampling procedures, DNA extraction and PCR amplification mentioning all the important measures to avoid contamination, can be found in (Aires et al., 2018).
Sequence analysis and bioinformatics
A total of 3,204,094 partial 16S rRNA gene sequences were obtained from the 24 samples (i.e., two CO2 conditions X four replicates for seaweed in the presence and the absence of grazer and four grazer gut samples with two CO2 conditions). The bacterial community analyses were performed using Quantitative Insights into Microbial Ecology (QIIME version 1.8.0) software (Caporaso et al., 2010). Sequences were screened and filtered for a minimum read length of 350 bp (after reads were paired) and less than two undetermined nucleotides. Selected high-quality sequences were clustered into Operational Taxonomic Units (OTUs) within reads using denovo OTU picking method. Representative sequences for each OTU were selected using the “most-abundant” method and OTU sequence alignment was carried out using PyNAST (Caporaso et al., 2010) and Greengenes v.13.8 (McDonald et al., 2012). Taxonomic assignments were done using the UCLUST (Edgar, 2010) method with a 97% confidence threshold. To assign each OTU to the closest matching described taxon, searches were performed against the Greengenes taxonomy database v.13.8 for16S rRNA (McDonald et al., 2012), and sequences were putatively assigned to a described taxon with a minimum threshold of 0.001 (default value). Eukaryotes (i.e., chloroplasts and mitochondria) matching sequences were excluded from the OTU table in downstream analyses as well as rare OTUs (singletons and doubletons) and unassigned sequences (those sequences that did not match any of those from the Greengenes database, with a minimum threshold of 0.001).
Quality filtering resulted in 2,877,493 high-quality sequences, with an average of 119,896 ± 46,294 reads per sample, which were clustered into 42,730 unique operational taxonomic units (OTUs). The OTU table was rarefied to the minimum number of sequences (66,831). As a result, a total of 41,139 unique OTUs remained. Public access to the data can be done through: https://doi.org/10.6084/m9.figshare.5346316.v3.
All the statistical and diversity (alpha and beta) analysis were done using the filtered rarefied (to the minimum number of sequences—66,831) OTU table and considered significant at P < 0.05.
Alpha diversity indexes, including Chao I richness (Chao, 1984), observed number of species (OTUs) and Shannon diversity, were calculated using QIIME software. Bacterial community structure (beta diversity) was assessed by permutational multivariate analyses of variance (PERMANOVA) using Bray–Curtis dissimilarity matrices from square-root transformed data. PERMANOVA tested for differences among samples with different levels of a priori factors: Type of sample: Seaweed vs Grazer gut; for both CO2 treatments: Ambient vs Acidified; for Seaweed: Grazed vs Non-grazed, and the interactions among these factors. The homogeneity of multivariate dispersions (based on mean distance to group centroid for all groups within each factor) was tested using a resemblance based permutation test (PERMDISP). To visualize differences and to assess dissimilarity between samples, Canonical Analysis of Principal coordinates (CAP) plots were constructed to test the assignment/clustering of treatments interaction (S.muticum XGrazingxAcidification and Grazer gutxAcidification) as a priori factor. Similarities and dissimilarities in bacterial communities between acidification treatments were explored using, similarity percentage analyses (SIMPER). For those bacterial taxonomic groups that displayed a high contribution (concerning their differential abundances in the treatments being compared) for the differences between the grazer gut and the seaweed and the two CO2 levels, two-way analyses of variance (ANOVA) were performed (with the preliminary tests for normality and homogeneity of variances being implemented). Species (S. muticum and isopod) and acidification (CO2 ambient and elevated) were tested as factors affecting the structure/composition bacterial communities. For bacterial OTUs for which significant interaction were detected, a post-hoc t-test was implemented using a Bonferroni correction and a conservative alpha (considering the comparisons made: CO2 effect in S. muticum, CO2 effect on isopod gut), effect of type of tissue (seaweed/gut) in ambient CO2 and effect of type of tissue in elevated CO2 (P (T ≤ t) two tail < 0.0125).
All bacterial community structure statistical analyses were performed using the software program PRIMER-E + PERMANOVA v.6 (Clarke & Warwick, 1994; Clarke & Gorley, 2006).
Results
Bacterial communities associated with S. muticum and S. nadejda’s gut
Overall, 563 bacterial OTUs from 74 classes (apart from bacteria classified as ‘Other and non-ID’) distributed across 28 phyla (plus ‘Other and non-ID’) were identified. Among them, 551 bacterial OTUs distributed across 27 phyla (plus Other and non-ID) were associated with S. muticum (over all samples and treatments), compared to 450 OTUs distributed across 22 phyla (plus Other and non-ID) for gut biome. Note that those sequences labelled as “others” were ambiguous assignments by the classifier and “no-ID” sequences result from a good match with a reference sequence but that reference sequence is poorly defined (not named at a certain taxonomic level and below). Public access to the data can be done through: https://doi.org/10.6084/m9.figshare.5346316.v3.
Alpha diversity of associated bacterial communities (Table S1) was similar or slightly higher in S. muticum than in the grazer gut for Shannon index (2-way ANOVA, F = 2.507, P = 0.139), OTU richness (2-way ANOVA, F = 3.361, P = 0.092) and Chao 1 (2-way ANOVA, F = 6.226, P = 0.028), respectively. Acidification did not affect bacterial diversity at OTU level, for both seaweeds and isopodes gut, as estimated by diversity indexes: Shannon index (2-way ANOVA, F = 0.048, P = 0.831), unique OTU richness (2-way ANOVA, F = 0.178, P = 0.681) and Chao 1 (2-way ANOVA, F = 0.003, P = 0.960).
Despite the overall PERMANOVA results showing that bacterial community composition was significantly different for the different type of samples (grazed and non-grazed S. muticum and grazer gut, P = 0.001, Fig. 2, Table S2), predation (grazing) did not significantly change bacterial community structure of S. muticum (P = 0.372, Table S3A). The main differences among S. muticum (grazed and non-grazed) and the isopode gut (Table S2) were due to a higher abundance of Bacteroidetes (73.7%) associated to S. muticum (contributing 15.11% to the dissimilarity; SIMPER analysis) and to more Proteobacteria (47.2%) and Planctomycetes (32.4%) in the gut of S. nadejda, contributing 11.76 and 14.98% to the dissimilarity, respectively (SIMPER analysis).
Figure 2. Community structure.
Plot of canonical analysis of principal coordinates (CAP) based on Bray–Curtis distances calculated on square-root transformed bacterial abundances, showing the axes that best discriminate the bacterial assemblages across CO2 levels (blue-ambient versus red-acidified), grazing by S. nadejda on S. muticum (open squares-grazed seaweed vs filled squares-non-grazed seaweed) and the gut of the isopod on a diet of S. muticum (circles).
Bacterial phyla-specific to host (refered in PERMANOVA analysis as “Type of sample”) and specific conditions are presented in Table S4.
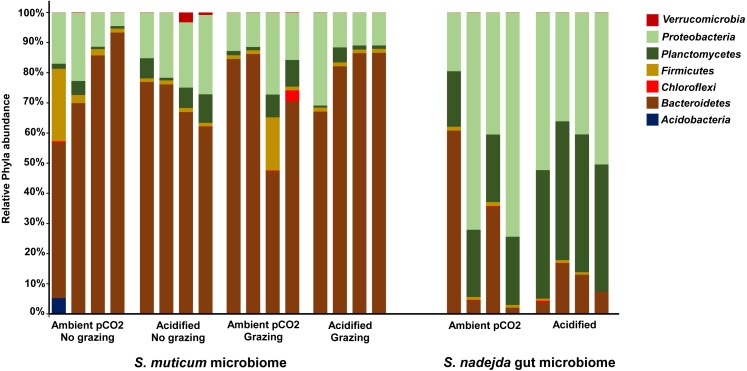
As mentioned before, grazing did not show significant effects on S. muticum bacterial community structure so, most of further analyses focused on the comparison of grazed S. muticum vs grazer gut. Grazed S. muticum associated bacterial communities were dominated by Bacteroidetes (75.4%, Fig. 3; Flavobacteriia 75.2%) and Proteobacteria (16.2%, Fig. 3; Alphaproteobacteria 12.2%), while the isopod gut communities were dominated by Proteobacteria (47.2%, Fig. 3; Alphaproteobacteria 43.5%), Planctomycetes (32.4%, Fig. 3; Planctomycetia 32.1%), and Bacteroidetes (17.7%, Fig. 3; Flavobacteriia 16.8%). Flavobacteriales (73.3%) was the most common bacterial order associated with S. muticum, while Rickettsiales (38.4%), Pirellulales (30.9%) and Flavobacteriales (16.8%) were the most abundant grazer gut-associated orders. The distribution of the bacterial genera belonging to the main phyla found for the two different systems and described above (S. muticum—Bacteroidetes and S. nadejda gut—Proteobacteria and Planctomycetes) showed a clear higher relative abundance of genera assigned as Non-ID and “other” Flavobacteraceae (Fig. 4A) for the seaweed microbiome and a isopode gut dominated by Neorickettsialles (Fig. 4B) and non-identified Pirellulaceae (Fig. 4C).
Figure 3. Host and treatment effects on associated bacteria phyla.
Relative abundance and distribution of the bacteria phyla associated to the brown seaweed Sargassum muticum, without (No grazing) and with (grazing) Synisoma nadejda isopods, and the gut of the isopod after three weeks on a Sargassum muticum diet, under ambient (380 ppm) and elevated/acidified (1,000 ppm) CO2 conditions.
Figure 4. Relative abundance of the genera belonging to the main bacterial phyla.
(A) Bacteroidetes, (B) Proteobacteria, and (C) Planctomycetes, associated with the brown seaweed Sargassum muticum grazed by Synisoma nadejda isopods (left side), and the gut microbiome of the isopod on a Sargassum muticum diet (right side), after three weeks under ambient (380 ppm; −CO2) and elevated/acidified (1,000 ppm; +CO2) CO2 conditions.
Several bacterial orders were significantly more abundant within grazed S. muticum than in the isopod gut: Flavobacteriales from the phylum Bacteroidetes (2-way ANOVA, F = 49.124, P < 0.001); Bdellovibrionales from the class Deltaproteobacteria (2-way ANOVA, F = 13.221, P = 0.003); as well as Acidithiobacillales (2-way ANOVA, F = 22.589, P = 0.0005), Alteromonadales (2-way ANOVA, F = 15.285, P = 0.002), and Oceanospirillales (2-way ANOVA, F = 6.407, P = 0.026) from the class Gammaproteobacteria (Fig. 5). In contrast, the isopod gut microbiome had higher abundances of Rickettsiales from the class Alphaproteobacteria (2-way ANOVA, F = 18.574, P = 0.001); the low abundance bacteria from the phylum TM6—class SJA-4 (2-way ANOVA, F = 12.491, P = 0.004); and the low abundance order AKAU3564-Phycisphaerae from the phylum Planctomycetes (2-way ANOVA, F = 7.325, P = 0.019) than S. muticum in the presence of grazer (Fig. 5).
Figure 5. Mean relative abundances of bacterial classes, and respective orders (A, Flavobacteriales and Ricketsiales; B, Bdellovibrionales and Oceanospirillales; C, Acidithiobacillales; D, Alteromonadales; E, Non-ID SJA-4 and AKAU3564 Phycisphaerae), significantly more abundant in either grazed Sargassum muticum or the gut of Synisoma nadejda.
After three weeks under ambient (380 ppm) and elevated/acidified (1,000 ppm) CO2 conditions. Alpha = 0.05, error bars show standard error per treatment (n = 4).
Bacterial diversity and composition under acidification conditions
Acidification did not affect the overall bacterial composition (PERMANOVA, P = 0.093, Table S2) and in particular that associated with S. muticum (P = 0.056, P = 0.584, Table S3B), but significantly affected the bacterial composition associated with the gut of S. nadejda (PERMANOVA, p = 0.022, Table S3B) (Fig. 2). However, under acidified conditions (and in the presence of grazers), the number of phyla in S. muticum bacterial community dropped from 26 to 17 (including one unidentified), compared to the ambient conditions (One-way ANOVA, F = 7.228, P = 0.009). In contrast, the number of phyla in the grazer gut-associated bacterial community under acidification treatment increased from 18 to 23 (including one unidentified), compared to the ambient conditions (One-way ANOVA, F = 1.923, P = 0.171).
The phyla specific to the microbiomes of S. muticum and gut of S. nadejda, under ambient vs acidified conditions, are presented in Table S4.
Some bacterial groups were unique to by their hosts under specific conditions (Fig. 6). The seaweed, in the presence of grazer and under the ambient conditions, had the highest number of unique bacterial OTUs (n = 28, 5%), while the grazer gut in the ambient conditions had the lowest number of unique bacterial OTUs (n = 2) (Fig. 6). S. muticum with the grazer present under the ambient conditions contained many OTUs belonging to Planctomycetes (3 OTUs, 24.8%), Proteobacteria (9 OTUs, 19.8%) and Bacteroidetes (9 OTUs, 19.5%) (Fig. 6). S. muticum with the grazer, but under acidification treatment, contained 13 unique OTUs, most of which belonged to Proteobacteria (7 OTUs, 74.9%) (Fig. 6). Sphingobacterium (Bacteroidetes) and Caldicoprobacter (Firmicutes) were unique to the gut of S. nadejda in the ambient conditions, compared to 23 OTUs unique to the grazer gut in the acidified conditions, a significant part of which belonged to Planctomycetes (2 OTUs, 50.7%) and Proteobacteria (8 OTUs, 15.8%) (Fig. 6).
Figure 6. Core communities.
Venn diagram representing the number of bacterial genera (present in at least 75% of samples) shared between the different CO2 treatments (ambient CO2− ↓ CO2; elevated/acidified CO2− ↑ CO2) and associated to grazed Sargassum muticum (Sm + G), and the gut microbiome of the isopod Synisoma nadejda on a Sargassum muticum diet. The bar plots show the distribution of Phyla of selected intersections.
A core bacterial community (present in at least 75% of samples) was composed of 181 bacterial OTUs (32.1%; including unidentified) (Fig. 6). Within this core bacterial community, the highest number of OTUs belonged to Bacteroidetes (n = 29; 55.3%), Proteobacteria (n = 90; 26.7%) and Planctomycetes (n = 7; 13.2%) (Fig. 5). Shared bacterial communities within S. muticum in the presence of grazer between the ambient and acidified conditions (n = 29; 5.2%) had the highest number of bacterial OTUs belonging to Proteobacteria (n = 14; 47.2%), Firmicutes (n = 8, 19.9%), and Bacteroidetes (n = 5; 18.5%) (Fig. 6). Shared bacterial communities in the gut of S. nadejda between the ambient and acidified conditions (n = 9; 1.6%), belonged mostly to Planctomycetes (n = 3; 54.8%) and Proteobacteria (n = 4, 32.3%) (Fig. 6).
Under elevated CO2 treatment, relative abundances of Gammaproteobacteria from the orders Oceanospirillales and Vibrionales (particularly Pseudoalteromonas), increased on S. muticum but only significantly in the absence of grazers (One-way ANOVA, P = 0.037 and P = 0.047, respectively; Figs. 7A and 7B). Under acidification, the abundance of Planctomycetia associated with the grazer gut was significantly higher than at the ambient CO2 levels (Two-tailed t-test, P < 0.001), as was their abundance (at elevated CO2) in the grazer gut than on S. muticum (Two-tailed t-test, P < 0.001) (Fig. 7C). Deeper analyses of bacterial abundance within Planctomycetia revealed that all bacterial orders detected within this class responded to acidification treatment (Figs. 7C–7E). A significant effect was confirmed within the grazer gut under different CO2 conditions and between the seaweed and the grazer gut under increased CO2. At elevated CO2 levels, the abundance of Pirellulales (Two-tailed t-test, P < 0.001, Fig. 7C), Planctomycetales (Two-tailed t-test, P = 0.008, Fig. 7D) and Gemmatales (Two-tailed t-test, P = 0.009, Fig. 7D), were increased in the isopode gut. Alongside, under the acidification treatment, the abundance of the bacterial orders Pirellulales (Two-tailed t-test, P < 0.001, Fig. 7C), Planctomycetales (Two-tailed t-test, P < 0.001, Fig. 7D) and Gemmatales (Two-tailed t-test, P = 0.008, Fig. 7D), was significantly higher in the grazer gut than associated with the seaweed (in the presence of grazers). The class C6-Planctomycetes also exhibited a significant increase in abundance under elevated CO2 conditions, but only within the grazer gut (Two-tailed t-test, P = 0.039) (Fig. 6). This was mostly due to the significant increase of bacteria from the order d113 (two-tailed t test, P = 0.039) (Fig. 6). The abundance of d113 under acidified conditions was significantly higher in the grazer gut than within S. muticum (two-tailed t test, P = 0.018) (Fig. 6). Overall, under acidification treatment, Planctomycetes increased in abundance (particularly within Planctomycetia) in the grazer gut from 20.9% to 43.8% (1-way ANOVA, F = 306.663, P < 0.001).
Figure 7. Mean relative abundances of associated bacterial orders.
(A and B) Sargassum muticum under grazing/non grazing influence after three weeks under ambient (380 ppm) and elevated/acidified (1,000 ppm) CO2 conditions and (C–E) grazed Sargassum muticum and the gut of Synisoma nadejda after three weeks under ambient (380 ppm) and elevated/acidified (1,000 ppm) CO2 conditions, that responded to acidification, but for which a significant interaction between acidification and type of sample (seaweed or grazer gut) was observed. Alpha = 0.05, error bars show standard error per treatment (n = 4).
Discussion
The results presented in this experimental study demonstrated that acidification affected specific bacterial groups, but hardly influenced the overall microbiome of the invasive brown seaweed S. muticum. In contrast, acidification caused significant changes in the gut microbiome of a native isopod consumer, S. nadejda. Interestingly, acidification increased abundances of Planctomycetia in the gut of S. nadejda and of Oceanospirillales and Vibrionales associated to S. muticum, raising hypotheses about their functional role under these conditions.
The guts of isopods are populated by symbiotic bacteria (e.g., Zimmer & Bartholmé, 2003; Wang, Brune & Zimmer, 2007; Fraune & Zimmer, 2008; Eberl, 2010) that assist in food utilization by the host (Zimmer, 2002; Zimmer et al., 2002; Zimmer & Bartholmé, 2003; Fraune & Zimmer, 2008). Because the diversity of gut bacterial communities is shaped by host diet (Tietjen, 2014), changes in seaweed characteristics and its microbiome, as a result of ocean acidification, were expected to affect the gut microbiome of the grazer with possible fitness consequences. Here, we documented that the most striking change resulting from acidification was a significantly increased abundance of Planctomycetes in the gut of S. nadejda, more specifically due to an increase of Non-ID Pirellulaceae. In this case, as seaweed microbiome was not overall affected by OA, these general changes in the grazer’s gut cannot be directly attributed to changes in its food microbiome. Although the functions of Pirellulaceae are hardly known, its presence was documented in the gastrointestinal tract of fish (Parris et al., 2016) and as part of the resident microbiomes of marine copepods but in very low abundance in starved specimens (Moisander, Sexton & Daley, 2015). Planctomycetes constituted the second most prevalent phylum (after Proteobacteria) in the gut of S. nadejda. These bacteria are known to widely colonize aquatic and terrestrial ecosystems (Lage & Bondoso, 2014) and were, until recently, considered environmental organisms. However, Planctomycetes have been reported associated to the gut of, not only marine taxa (Singh & Reddy, 2014), but also terrestrial herbivores (Fuerst & Sagulenko, 2011), mammals (Frey et al., 2006) and even humans (Cayrou et al., 2013).
In this study, acidification strongly increased the abundance of Planctomycetes in the bacterial gut communities of S. nadejda compared to ambient conditions. An increase of this Phylum in response to simulated OA has also been shown in sandy sediments (Currie et al., 2017). Although it is clear that in our study this was a consequence of acidification, the lack of seawater samples does not allow us to determine whether the observed Planctomycetes increase was due to acidification of the seawater or a direct response of the grazer’s gut to acidification. This phylum was also abundant (the third most abundant) in the bacterial community associated to S. muticum. These widespread bacteria have been often found associated to macroalgae (Lage & Bondoso, 2014), among which Caulerpa taxifolia (Meusnier et al., 2001) and the kelp Laminaria hyperborea (Bengtsson, Sjøtun & Øvreås, 2010). Bacteria from the phylum Planctomycetes are suggested to have potential benefits for their hosts, through their ability to mineralize organic molecules into inorganic compounds that match the nutritional requirements of macroalgae (Lage & Bondoso, 2014). Planctomycetes are also proposed to function as “slow-acting decomposers of organic matter” (i.e., algal polymer degradation; Bodelier & Dedysh, 2013) and important contributors to the global nitrogen cycle (i.e., anammox Planctomycetes, Fuerst & Sagulenko, 2011). So, the presence of these bacteria in the isopod gut might also be related to the ingestion of S. muticum. Its increased abundance (particularly those from the order Pirellulales) could also be a response to the change of the seaweed nutritional value under acidification, which likely increased its photosynthetic rate driven by high CO2 and consequent C/N ratio shifts (Cornwall, Revill & Hurd, 2015; Briggs, 2017). An increase in Planctomycetes abundance could provide the isopod with an elevated algal digestion capacity to compensate the highly carbonated food. Again, with the lack of control samples as starving isopods (empty guts) we can only raise new hypotheses about the direct (through its microbiome) or indirect (through its nutritional value/quality) influence of the food in the grazer’s gut microbiome. Yet, there is no doubt that acidification resulted in a significant overall change in the isopod gut microbiome that is not directly related to bacterial community shifts of S. muticum, which did not occur for the seaweed at elevated CO2.
Another important change resulting from acidification was a significant increase in Oceanospirillales (Non-ID Oceanospirillaceae) and Vibrionales (Pseudoalteromonas) associated with S. muticum in the absence of grazers. They all belong to Gammaproteobacteria which have been previously found in association with various seaweed species (e.g., Patel et al., 2003; Huggett et al., 2006). Members of the class Gammaproteobacteria are known to produce biologically active metabolites that mediate antifungal (Barbieri et al., 2001), antifouling (i.e., Alteromonas, Pseudomonas; Maki et al., 1988; Holmstrom, Rittschof & Kjelleberg, 1992; Avelin Mary et al., 1993; Holmström & Kjelleberg, 1999) and antibacterial activities (Hentschel et al., 2001). There are no studies documenting the effect of acidification on these bacteria in seaweeds, but environmental samples showed a high increase of Gammaproteobacteria under acidification, in particular from the order Oceanospirillales (Currie et al., 2017). Bacteria from the order Oceanospirillales are heterotrophic and capable of degrading complex organic compounds (Garrity et al., 2005; Goffredi, Johnson & Vrijenhoek, 2007). These bacteria use organic matter as food source and their increase in S. muticum under acidification might be opportunistic and related to the carbon content increase in the seaweed, and consequent seaweed enrichment, due to elevated CO2 levels.
Increased abundance of Vibrionales has often been associated with stressed and diseased marine invertebrates and they are also known as coral pathogens (Bourne & Munn, 2005; Bourne et al., 2008; Sunagawa et al., 2009; Meron et al., 2011). Vibrionales were also responsible for a number of infections in humans and animals (Vezzulli et al., 2016), and identified as potential pathogens of sablefish larvae (Schulze et al., 2006) and bivalve mollusks (Asplund et al., 2014). Interestingly, it has been shown that under low pH, the coral-associated pathogen Vibrio sp. increased in abundance (Meron et al., 2011), while the blue mussel pathogen Vibrio tubiashii became more infectious (Asplund et al., 2014). In this study, among the Vibrionales that experienced a significant increase were predominantly Pseudoalteromonas. While certain members of the genus Pseudoalteromonas were reported to have antibacterial activity in corals, providing it with defense against potential pathogens (Shnit-Orland, Sivan & Kushmaro, 2012), multiple studies identify Pseudoalteromonas as opportunistic pathogens of marine organisms (Liu et al., 2010; Song et al., 2012; Wang et al., 2012). Bacteria affiliated to the genus Pseudoalteromonas were found associated to the kelp Laminaria japonica affected by two different diseases (holle-rotten disease and red spot disease) (Sawabe et al., 1998; Gachon et al., 2010) where its isolation and posterior reinfection resulted in observable symptoms (Wang et al., 2008). Nevertheless, particular bacteria that may be otherwise commensal, under stress of the seaweed host, can become saprophytic (Egan et al., 2013) and this could hypothetically be the case of the Pseudoalteromonas found in S. muticum under acidification. Ocean acidification could potentially result in shifts from healthy associated bacterial communities within seaweeds towards a higher prevalence of pathogenic bacteria and/or increased vulnerability to disease. Unfortunately, our taxonomic assignments and our amplicon sequencing approach does not provide more detailed insights into possible bacterial pathogenicity.
Flavobacteriales from the Bacteroidetes phylum were among the bacterial groups that were significantly more abundant in association with S. muticum than in the isopod gut. Bacteroidetes colonize marine and freshwater environments widely (Thomas et al., 2011), populating a wide variety of surfaces, including macroalgae (Beleneva & Zhukova, 2006; Staufenberger et al., 2008) and marine sediments (Devine, Pelletreau & Rumpho, 2012). Bacteroidetes were isolated from Caulerpa taxifolia (Meusnier et al., 2001), Ulva australis and the red alga Delisea pulchra (Longford et al., 2007), suggesting that these typical marine bacteria are common seaweed associates (Tujula et al., 2010). This phylum represents some of the most abundant marine bacteria (Glöckner et al., 1999; Simon, Glöckner & Amann, 1999; Cottrell & Kirchman, 2000) and plays an important role as degraders of complex organic matter (Church, 2008). Flavobacteriia, the most prevalent class detected within the seaweed microbiome, are known to produce enzymes for polymer degradation (Fenchel, 2012). Most Flavobacteriia are able to degrade cellulose, chitin, proteins, and nucleic acids (Kirchman, 2002; Fenchel, 2012). However, Bacteroidetes are also related to stress conditions and often found in diseased corals (Barneah et al., 2007), as in the case of Porites compressa which when exposed to low pH showed an increase of disease-associated Flavobacteriia (Thurber et al., 2009). Flavobacteriales, the most widely represented Flavobacteriia order within S. muticum, are mostly associated with degradation of complex particle biomacromolecules, as well as algal debris (Kirchman, 2002), more specifically proteins, agars, xylan, fucoidan, cellulose, and chitin (Devine, Pelletreau & Rumpho, 2012). Bacteria from the genera Aquimarina and Tenacibaculum were among the most frequent OTUs and occur free-living in marine environments (Nedashkovskaya et al., 2006) and fixed to the surfaces of marine organisms (Suzuki et al., 2001). Bacteria from the genus Tenacibaculum are thought to induce morphogenesis in algae and possibly enhanced seaweed growth (Matsuo et al., 2003; Matsuo et al., 2005).
In this study, acidification resulted in a small (non-significant) decrease of seaweed-associated Flavobacteriales and just in the absence of grazer. This contrasts with a study conducted on biofilms from the Great Barrier Reef, which reported that with decreasing pH there was an increase in the relative abundance of Flavobacteriales (Flavobacteriaceae) (Witt et al., 2011). However, Witt et al. (2011) focused on natural biofilms on glass slides whereas biofilms from living organisms are the result of environment and host communication and may be differently influenced by any fluctuations that might have occurred in the seawater bacterial communities. Nevertheless, the lack of seawater bacterial community analysis in our study may limit the interpretation of some of these results. The decrease of these common seaweed associates (Hollants et al., 2013; Egan et al., 2013), although non significant, may result from the instablity of environmental pH changes. Natural seaweed bacterial assemblages can be disrupted by environmental pressures (Marzinelli et al., 2015) and that could contribute to the initial response of S. muticum to acidification.
Seaweed consuming iguanas, which have adapted to use macroalgae as their primary resource, were found to host a large propotion of Bacteroidetes in their gut when compared to terrestrial related species (Hong et al., 2011). Seaweed polysaccharides, many with sulfated sugars that are absent in terrestrial plants, are easily hydrolized by Bacteroides spp. present in the gastrointestinal tract (Shah & Gharbia, 1993). In particular, these bacteria have been described as contributing to the degradation of brown algal polysaccharides in the gastrointestinal tract of limpets (particularly Flavobacteriia; Dudek et al., 2014) and other gastropods that are seaweed consumers (Cardoso et al., 2012). As discussed above, Bacteroidetes were among the most abundant bacteria associated with S. muticum and, as shown on copepods (Moisander, Sexton & Daley, 2015), these were the third most abundant bacterial phylum in the intestinal tract of S. nadejda. Bacteroidetes are within the most abundant phyla in three different copepod species, for both starving and full gut specimens (Moisander, Sexton & Daley, 2015). These authors also found these bacteria to be the most abundant in seawater samples which lead to the assumption that they were not “gut permanent residents” but instead colonizers from the seawater. In our case, the lack of seawater samples and starved isopodes (empty gut) limit the interpretation of our results, so its relatively high abundance in S. nadejda gut might be both related with seawater and/or food.
In contrast to Bacteroidetes and Gammaproteobacteria prevailing within S. muticum, Alphaproteobacteria (Rickettsiales), as well as bacteria from the phylum TM6 (the class SJA-4) were more abundant in the grazer gut microbiome for both ambient and acidified conditions. Alphaproteobacteria were also reported as one of the most prevalent Classes in marine copepods (Moisander, Sexton & Daley, 2015). Bacteria from the order Rickettsiales, which dominated the gut of S. nadejda, are known as pathogenic to humans and animals (Perlman, Hunter & Zchori-Fein, 2006), and were found in the intestinal tract of infected isopod Armadillidium vulgare (Dittmer et al., 2016) and several other isopode species (Wang, Brune & Zimmer, 2007). Neorickettsia is known as a parasite to which the invertebrates usually serve as vectors (Greiman et al., 2014). This aspect is worth further investigation once the possibility of these herbivores to function as pathogen vectors may have consequences on human health if they are able to pass them along to some edible algae where they feed on.
The experimentally controlled mesocosm data allowed us to isolate variables to infer their effects, even though they are not able to fully reconstruct the dynamic conditions of nature, as documented for corals that had different bacterial communities in laboratory and field (Kooperman et al., 2007; Meron et al., 2011). So, the cause of the observed bacterial community shifts can be easily identified but may not mimick exactly the processes in the field. Another limitation to realistic predictions is that global acidification may be happening quicker than IPCC models predicted. So, pH and CO2 conditions used in this and several other studies may not be realistic and underestimate the effects of OA that can be more catastrophic than what is expected to be in the next 100 years (the maximum IPCC prediction) (Thurber et al., 2009; Meron et al., 2011). However, those changes would happen over a large period of time and not over the course of three weeks, as in our experiment.
The results of this study demonstrate that bacterial communities associated within an isopod—seaweed predator–prey system are dynamic and responsive to changes in acidification. The observed changes in the associated bacterial communities of the seaweed and the grazer gut might be a type of acclimation that facilitates tolerance and survival, as suggested by Meron et al. (2011). The capacity of organisms to accommodate and modify a highly diverse microbiota may influence the fitness and success of the host and enhance its ability to survive changing environmental conditions (Morrow et al., 2015). Further research is required to better understand the processes and conditions under which different associated bacteria can increase tolerance of the host organisms to various disturbances.
Conclusion
The responses of bacteria associated with S. muticum and the gut of S. nadejda revealed in this study suggest that worst case acidification scenarios may not greatly affect overall bacterial community composition and diversity, but might affect specific bacterial groups.
Contrarily to what was expected, acidification to expected levels seems to have less significant impact on seaweed bacterial ecology than other environmental stressors, such as increased temperature (Mensch et al., 2016). However, specific groups had significant abundance shifts in seaweeds under acidification as was the case for Oceanospirillales and Vibrionales (mainly Pseudomonadales). The former might be related to the possible increase of the C/N ratio in the seaweed under acidified conditions and the latter, commonly found associated with diseased seaweeds, could be an indicator that acidification enables an increase of opportunistic/pathogenic bacteria. Unexpectedly, no particular bacteria increased abundance that could assist the seaweed in obtaining nutrients (like nitrogen fixing bacteria) under a higher carbon availability regime.
In contrast with the seaweed host, high CO2 levels globally changed the bacterial community associated to the isopod gut, particularly the abundance of members of the Class Planctomycetia. However, as no significant changes occurred in the global seaweed microbiome, the overall shift in the grazer gut bacterial community cannot be directly attributed to bacterial changes in the food. Instead, we can hypothesize that the food “quality” changed at elevated CO2 triggering a shift in the isopod gut Planctomycetia allowing it to better digest the seaweed and compensate the shifted C/N ratio as seaweed becomes less nutritional under acidification, as previously hypothesized. The generality of these findings must be addressed in further studies with other species.
Bacteroidetes (mainly Flavobacteriia), previously isolated and commonly found associated with other seaweed species (Huggett et al., 2006; Burke et al., 2011a; Lachnit et al., 2011), were the most dominant phyla, suggesting that they account for core roles in the metabolism of S. muticum. Both in ambient and acidified conditions, the isopod gut bacterial community was dominated by Neorickettsia (Alphaproteobacteria), for which invertebrates are usually the vectors. The pathogenicity of these bacteria has already been shown in other invertebrate species (Greiman et al., 2014) and the potential of isopod species as possible vectors for these bacteria has already been suggested (Yuksel, Thompson & Adams, 2006) and should be further investigated.
Concluding, our results show that after only three weeks of simulated acidification, bacterial communities associated to a seaweed host, when ungrazed, and to an isopod grazer gut, show shifts in composition. These bacterial community changes were particular and specific in the seaweed and only occurred when ungrazed, but were large and global community shifts in the isopod grazer gut. We hypothesized that these may have potential consequences for seaweed health and isopod food digestion. The observed changes in the gut microbiome of the grazer seem to be a reflection of changes in the seaweed chemistry rather than its microbial composition.
Supplemental Information
Acknowledgments
We would like to thank Dario Nobre and João Reis for conducting the acidification experiment.
Funding Statement
This study was made possible by the Erasmus Mundus Doctoral Programme MARES on Marine Ecosystem Health & Conservation (MARES_13_08: Acclimation and adaptation of invasive seaweeds) for Alexandra Serebryakova, and funds from FCT (Foundation for Science and Technology, Portugal) fellowships SFRH/BPD/63703/2009 and SFRH/BPD/107878/2015 to Aschwin H. Engelen and SFRH/BPD/116774/2016 to Tania Aires, and the EU SEAS-ERA project INVASIVES (SEAS-ERA/0001/2012) and CCMAR/Multi/04326/2013. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Tania Aires performed the experiments, analyzed the data, authored or reviewed drafts of the paper.
Alexandra Serebryakova performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper.
Frédérique Viard contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, funding.
Ester A. Serrão conceived and designed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper.
Aschwin H. Engelen conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, funding.
Data Availability
The following information was supplied regarding data availability:
Engelen, Aschwin (2018): Acidification + Grazing_Effects_Microbiome. figshare. https://doi.org/10.6084/m9.figshare.5346316.v3.
References
- Aires et al. (2015).Aires T, Moalic Y, Serrao EA, Arnaud-Haond S. Hologenome theory supported by co-occurrence networks of species-specific bacterial communities in siphonous algae (Caulerpa) FEMS Microbiology Ecology. 2015;91 doi: 10.1093/femsec/fiv067. fiv067. [DOI] [PubMed] [Google Scholar]
- Aires et al. (2018).Aires T, Muyzer G, Serrão EA, Engelen AH. Unraveling seaweeds bacteriomes—from field site to computer screen. In: Charrier B, Wichard T, Reddy CRK, editors. Protocols for macroalgae research. Taylor & Francis Group; Oxfordshire: 2018. pp. 97–112. [Google Scholar]
- Aires, Serrão & Engelen (2016).Aires T, Serrão EA, Engelen AH. Host and environmental specificity in bacterial communities associated to two highly invasive marine species (Genus Asparagopsis) Frontiers in Microbiology. 2016;7:559. doi: 10.3389/fmicb.2016.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnaghi et al. (2013).Asnaghi V, Chiantore M, Mangialajo L, Gazeau F, Francour P, Alliouane S, Gattuso JP. Cascading effects of ocean acidification in a rocky subtidal community. PLOS ONE. 2013;8:e61978. doi: 10.1371/journal.pone.0061978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asplund et al. (2014).Asplund ME, Baden SP, Russ S, Ellis RP, Gong N, Hernroth BE. Ocean acidification and host-pathogen interactions: blue mussels, Mytilus edulis, encountering Vibrio tubiashii. Environmental Microbiology. 2014;16:1029–1039. doi: 10.1111/1462-2920.12307. [DOI] [PubMed] [Google Scholar]
- Avelin Mary et al. (1993).Avelin Mary S, Vitalina Mary S, Rittschof D, Nagabhushanam R. Bacterial-barnacle interaction: potential of using juncellins and antibiotics to alter structure of bacterial communities. Journal of Chemical Ecology. 1993;19:2155–2167. doi: 10.1007/BF00979654. [DOI] [PubMed] [Google Scholar]
- Barbieri et al. (2001).Barbieri E, Paster BJ, Hughes D, Zurek L, Moser DP, Teske A, Sogin ML. Phylogenetic characterization of epibiotic bacteria in the accessory nidamental gland and egg capsules of the squid Loligo pealei (Cephalopoda: Loliginidae) Environmental Microbiology. 2001;3:151–167. doi: 10.1046/j.1462-2920.2001.00172.x. [DOI] [PubMed] [Google Scholar]
- Barneah et al. (2007).Barneah O, Ben-Dov E, Kramarsky-Winter E, Kushmaro A. Characterization of black band disease in Red Sea stony corals. Environmental Microbiology. 2007;9:1995–2006. doi: 10.1111/j.1462-2920.2007.01315.x. [DOI] [PubMed] [Google Scholar]
- Beleneva & Zhukova (2006).Beleneva IA, Zhukova NV. Bacterial communities of some brown and red algae from Peter the Great Bay, the Sea of Japan. Microbiology. 2006;75:348–357. doi: 10.1134/S0026261706030180. [DOI] [PubMed] [Google Scholar]
- Bengtsson, Sjøtun & Øvreås (2010).Bengtsson MM, Sjøtun K, Øvreås L. Seasonal dynamics of bacterial biofilms on the kelp Laminaria hyperborea. Aquatic Microbial Ecology. 2010;60:71–83. doi: 10.3354/ame01409. [DOI] [Google Scholar]
- Berg et al. (2014).Berg G, Grube M, Schloter M, Smalla K. The plant microbiome and its importance for plant and human health. Frontiers in Microbiology. 2014;5:491. doi: 10.1007/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodelier & Dedysh (2013).Bodelier PLE, Dedysh SN. Microbiology of wetlands. Frontiers in Microbiology. 2013;4 doi: 10.3389/fmicb.2013.00079. Article 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenhausen, Horton & Bergelson (2013).Bodenhausen N, Horton MW, Bergelson J. Bacterial communities associated with the leaves and the roots of Arabidopsis thaliana. PLOS ONE. 2013;8:e56329. doi: 10.1371/journal.pone.0056329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne et al. (2008).Bourne D, Iida Y, Uthicke S, Smith-Keune C. Changes in coral-associated microbial communities during a bleaching event. ISME Journal. 2008;2:350–363. doi: 10.1038/ismej.2007.112. [DOI] [PubMed] [Google Scholar]
- Bourne & Munn (2005).Bourne DG, Munn CB. Diversity of bacteria associated with the coral Pocillopora damicornis from the great barrier reef. Environmental Microbiology. 2005;7:1162–1174. doi: 10.1111/j.1462-2920.2005.00793.x. [DOI] [PubMed] [Google Scholar]
- Branch et al. (2013).Branch TA, DeJoseph BM, Ray LJ, Wagner CA. Impacts of ocean acidification on marine seafood. Trends in Ecology and Evolution. 2013;28:178–186. doi: 10.1016/j.tree.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Briggs (2017).Briggs LM. The effects of ocean warming and acidification on seawed growth and urchin grazing. California State Polytechnic University; Pomona: 2017. [Google Scholar]
- Burgess et al. (1999).Burgess JG, Jordan EM, Bregu M, Mearns-Spragg A, Boyd KG. Microbial antagonism: a neglected avenue of natural products research. Journal of Biotechnology. 1999;70:27–32. doi: 10.1016/S0168-1656(99)00054-1. [DOI] [PubMed] [Google Scholar]
- Burke et al. (2011a).Burke C, Steinberg P, Rusch D, Kjelleberg S, Thomas T. Bacterial community assembly based on functional genes rather than species. Proceedings of the National Academy of Sciences of the United States of America. 2011a;108:14288–14293. doi: 10.1073/pnas.1101591108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke et al. (2011b).Burke C, Thomas T, Lewis M, Steinberg P, Kjelleberg S. Composition, uniqueness and variability of the epiphytic bacterial community of the green alga Ulva australis. The ISME Journal. 2011b;5:590–600. doi: 10.1038/ismej.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso et al. (2010).Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, Mcdonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nature Publishing Group. 2010;7:335–336. doi: 10.1038/nmeth0510-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso et al. (2012).Cardoso AM, Cavalcante JJV, Vieira RP, Lima JL, Grieco MAB, Clementino MM, Vasconcelos ATR, Garcia ES, De Souza W, Albano RM, Martins OB. Gut bacterial communities in the giant land snail Achatina fulica and their modification by sugarcane-based diet. PLOS ONE. 2012;7:e33440. doi: 10.1371/journal.pone.0033440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case et al. (2011).Case RJ, Longford SR, Campbell AH, Low A, Tujula N, Steinberg PD, Kjelleberg S. Temperature induced bacterial virulence and bleaching disease in a chemically defended marine macroalga. Environmental Microbiology. 2011;13:529–537. doi: 10.1111/j.1462-2920.2010.02356.x. [DOI] [PubMed] [Google Scholar]
- Cayrou et al. (2013).Cayrou C, Sambe B, Armougom F, Raoult D, Drancourt M. Molecular diversity of the Planctomycetes in the human gut microbiota in France and Senegal. Apmis. 2013;121:1082–1090. doi: 10.1111/apm.12087. [DOI] [PubMed] [Google Scholar]
- Chao (1984).Chao A. Nonparametric estimation of the number of Classes in a population. Scandinavian Journal of Statistics. 1984;11:265–270. [Google Scholar]
- Chojnacka et al. (2012).Chojnacka K, Saeid A, Witkowska Z, Tuhy Ł. Biologically active compounds in seaweed extracts—the prospects for the application. The Open Conference Proceedings Journal. 2012;3:20–28. doi: 10.2174/1876326X01203020020. [DOI] [Google Scholar]
- Church (2008).Church MJ. Resource control of bacterial dynamics in the sea. In: Kirchman DL, editor. Microbial ecology of the oceans. John Wiley & Sons; Hoboken: 2008. pp. 335–382. [Google Scholar]
- Clarke & Gorley (2006).Clarke K, Gorley RN. Primer-E Ltd; Plymouth: 2006. 192p. [Google Scholar]
- Clarke & Warwick (1994).Clarke KR, Warwick RM. Change in marine communities. An approach to statistical analysis and interpretation. Natural Environment Research Council; Plymouth: 1994. pp. 1–172. [Google Scholar]
- Cornwall, Revill & Hurd (2015).Cornwall CE, Revill AT, Hurd CL. High prevalence of diffusive uptake of CO2 by macroalgae in a temperate subtidal ecosystem. Photosynthesis Research. 2015;124:181–190. doi: 10.1007/s11120-015-0114-0. [DOI] [PubMed] [Google Scholar]
- Cottrell & Kirchman (2000).Cottrell MT, Kirchman DL. Community composition of marine bacterioplankton determined by 16S rRNA gene clone libraries and fluorescence in situ hybridization. Applied and Environmental Microbiology. 2000;66:5116–5122. doi: 10.1128/aem.66.12.5116-5122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie et al. (2017).Currie AR, Tait K, Parry H, De Francisco-Mora B, Hicks N, Mark Osborn A, Widdicombe S, Stahl H. Marine microbial gene abundance and community composition in response to ocean acidification and elevated temperature in two contrasting coastal marine sediments. Frontiers in Microbiology. 2017;8 doi: 10.3389/fmicb.2017.01599. Article 1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis et al. (2013).Davis J, Fricke WF, Hamann MT, Esquenazi E, Dorrestein PC, Hill RT. Characterization of the bacterial community of the chemically defended Hawaiian sacoglossan Elysia rufescens. Applied and Environmental Microbiology. 2013;79:7073–7081. doi: 10.1128/AEM.01568-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine, Pelletreau & Rumpho (2012).Devine SP, Pelletreau KN, Rumpho ME. 16S rDNA-based metagenomic analysis of bacterial diversity associated with two populations of the kleptoplastic sea slug Elysia chlorotica and its algal prey Vaucheria litorea. The Biological Bulletin. 2012;223:138–154. doi: 10.1086/BBLv223n1p138. [DOI] [PubMed] [Google Scholar]
- Dimitrieva, Crawford & Yüksel (2006).Dimitrieva GY, Crawford RL, Yüksel GÜ. The nature of plant growth-promoting effects of a Pseudoalteromonad associated with the marine algae Laminaria japonica and linked to catalase excretion. Journal of Applied Microbiology. 2006;100:1159–1169. doi: 10.1111/j.1365-2672.2006.02831.x. [DOI] [PubMed] [Google Scholar]
- Dittmer et al. (2016).Dittmer J, Lesobre J, Moumen B, Bouchon D. Host origin and tissue microhabitat shaping the microbiota of the terrestrial isopod Armadillidium vulgare. FEMS Microbiology Ecology. 2016;92 doi: 10.1093/femsec/fiw063. Article fiw063. [DOI] [PubMed] [Google Scholar]
- Dudek et al. (2014).Dudek M, Adams J, Swain M, Hegarty M, Huws S, Gallagher J. Metaphylogenomic and potential functionality of the limpet Patella pellucida’s gastrointestinal tract microbiome. International Journal of Molecular Sciences. 2014;15:18819–18839. doi: 10.3390/ijms151018819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl (2010).Eberl R. Sea-land transitions in isopods: pattern of symbiont distribution in two species of intertidal isopods Ligia pallasii and Ligia occidentalis in the Eastern Pacific. Symbiosis. 2010;51:107–116. doi: 10.1007/s13199-010-0057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar (2010).Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Egan et al. (2013).Egan S, Harder T, Burke C, Steinberg P, Kjelleberg S, Thomas T. The seaweed holobiont: understanding seaweed-bacteria interactions. FEMS Microbiology Reviews. 2013;37:462–476. doi: 10.1111/1574-6976.12011. [DOI] [PubMed] [Google Scholar]
- Fabry et al. (2008).Fabry VJ, Seibel BA, Richard AF, Orr JC. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES Journal of Marine Science. 2008;65:414–432. doi: 10.1093/icesjms/fsn048. [DOI] [Google Scholar]
- Fenchel (2012).Fenchel T. Chapter 3—degradation of organic polymers and hydrocarbons. Elsevier; Amsterdam: 2012. [DOI] [Google Scholar]
- Fraune & Zimmer (2008).Fraune S, Zimmer M. Host-specificity of environmentally transmitted Mycoplasma-like isopod symbionts. Environmental Microbiology. 2008;10:2497–2504. doi: 10.1111/j.1462-2920.2008.01672.x. [DOI] [PubMed] [Google Scholar]
- Frey et al. (2006).Frey JC, Rothman JM, Pell AN, Nizeyi B, Cranfield MR, Angert ER, Nizeyi JB. Fecal bacterial diversity in a wild gorilla. Applied and Environmental Microbiology. 2006;72:3788–3792. doi: 10.1128/AEM.72.5.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst & Sagulenko (2011).Fuerst JA, Sagulenko E. Beyond the bacterium: Planctomycetes challenge our concepts of microbial structure and function. Nature Reviews Microbiology. 2011;9:403–413. doi: 10.1038/nrmicro2578. [DOI] [PubMed] [Google Scholar]
- Gachon et al. (2010).Gachon CMM, Sime-Ngando T, Strittmatter M, Chambouvet A, Kim GH. Algal diseases: spotlight on a black box. Trends in Plant Science. 2010;15:633–640. doi: 10.1016/j.tplants.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Garrity et al. (2005).Garrity GM, Bell JA, Lilburn T, Garrity GM, Bell JA, Lilburn T. Oceanospirillales ord. nov. In: Brenner D, Krieg N, Garrity G, Boone D, De Vos P, editors. Bergey’s manual of systematics of archaea and bacteria. John Wiley & Sons; Hoboken: 2005. pp. 270–323. [DOI] [Google Scholar]
- Glöckner et al. (1999).Glöckner FO, Fuchs BM, Fuchs BM, Glo FO, Amann R. Bacterioplankton compositions of lakes and oceans: a first comparison based on fluorescence in situ hybridization. Applied and Environmental Microbiology. 1999;65:3721–3726. doi: 10.1128/aem.65.8.3721-3726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffredi, Johnson & Vrijenhoek (2007).Goffredi SK, Johnson SB, Vrijenhoek RC. Genetic diversity and potential function of microbial symbionts associated with newly discovered species of Osedax polychaete worms. Applied and Environmental Microbiology. 2007;73:2314–2323. doi: 10.1128/AEM.01986-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiman et al. (2014).Greiman SE, Tkach VV, Pulis E, Fayton TJ, Curran SS. Large scale screening of digeneans for Neorickettsia endosymbionts using real-time PCR reveals new Neorickettsia genotypes, host associations and geographic records. PLOS ONE. 2014;9:e98453. doi: 10.1371/journal.pone.0098453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutow et al. (2014).Gutow L, Rahman MM, Bartl K, Saborowski R, Bartsch I, Wiencke C. Ocean acidification affects growth but not nutritional quality of the seaweed Fucus vesiculosus (Phaeophyceae, Fucales) Journal of Experimental Marine Biology and Ecology. 2014;453:84–90. doi: 10.1016/j.jembe.2014.01.005. [DOI] [Google Scholar]
- Hacquard et al. (2015).Hacquard S, Garrido-Oter R, González A, Spaepen S, Ackermann G, Lebeis S, McHardy AC, Dangl JL, Knight R, Ley R, Schulze-Lefert P. Microbiota and host nutrition across plant and animal kingdoms. Cell Host and Microbe. 2015;17:603–616. doi: 10.1016/j.chom.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Harley et al. (2012).Harley CDG, Anderson KM, Demes KW, Jorve JP, Kordas RL, Coyle TA, Graham MH. Effects of climate change on global seaweed communities. Journal of Phycology. 2012;48:1064–1078. doi: 10.1111/j.1529-8817.2012.01224.x. [DOI] [PubMed] [Google Scholar]
- Hassenrück et al. (2016).Hassenrück C, Fink A, Lichtschlag A, Tegetmeyer HE, De Beer D, Ramette A. Quantification of the effects of ocean acidification on sediment microbial communities in the environment: the importance of ecosystem approaches. FEMS Microbiology Ecology. 2016;92 doi: 10.1093/femsec/fiw027. Article fiw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head & Carpenter (1975).Head WD, Carpenter EJ. Nitrogen fixation associated with the marine macroalga Codium fragile. Limnology and Oceanography. 1975;20:815–823. doi: 10.1017/CBO9781107415324.004. [DOI] [Google Scholar]
- Hentschel et al. (2001).Hentschel U, Schmid M, Wagner M, Fieseler L, Gernert C, Hacker J. Isolation and phylogenetic analysis of bacteria with antimicrobial activities from the Mediterranean sponges Aplysina aerophoba and Aplysina cavernicola. FEMS Microbiology Ecology. 2001;35:305–312. doi: 10.1111/j.1574-6941.2001.tb00816.x. [DOI] [PubMed] [Google Scholar]
- Hollants et al. (2013).Hollants J, Leliaert F, De Clerck O, Willems A. What we can learn from sushi: a review on seaweed-bacterial associations. FEMS Microbiology Ecology. 2013;83:1–16. doi: 10.1111/j.1574-6941.2012.01446.x. [DOI] [PubMed] [Google Scholar]
- Holmström & Kjelleberg (1999).Holmström C, Kjelleberg S. Marine Pseudoalteromonas species are associated with higter organisms and produce biologically active extracellular agents. FEMS Microbiology Ecology. 1999;30:285–293. doi: 10.1111/j.1574-6941.1999.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Holmstrom, Rittschof & Kjelleberg (1992).Holmstrom C, Rittschof D, Kjelleberg S. Inhibition of settlement by larvae of Balanus amphitrite and Ciona intestinalis by a surface-colonizing marine bacterium. Applied and Environmental Microbiology. 1992;58:2111–2115. doi: 10.1128/aem.58.7.2111-2115.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong et al. (2011).Hong PY, Wheeler E, Cann IKO, Mackie RI. Phylogenetic analysis of the fecal microbial community in herbivorous land and marine iguanas of the Galápagos Islands using 16S rRNA-based pyrosequencing. ISME Journal. 2011;5:1461–1470. doi: 10.1038/ismej.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton et al. (2001).Houghton JT, Ding Y, Griggs DJ, Noguer M, Van der Linden PJ, Dai X, Maskell K, Johnson C, editors. Climate Change 2001: the Scientific Basis. Cambridge University Press; Cambridge: 2001. IPCC, 2001: climate change 2001: the scientific basis, contribution of working group I to the third assessment report of the intergovernmental panel on climate change; p. 881. [DOI] [Google Scholar]
- Huggett et al. (2006).Huggett MJ, Williamson JE, De Nys R, Kjelleberg S, Steinberg PD. Larval settlement of the common Australian sea urchin Heliocidaris erythrogramma in response to bacteria from the surface of coralline algae. Oecologia. 2006;149:604–619. doi: 10.1007/s00442-006-0470-8. [DOI] [PubMed] [Google Scholar]
- Kerfahi et al. (2014).Kerfahi D, Hall-Spencer JM, Tripathi BM, Milazzo M, Lee J, Adams JM. Shallow water marine sediment bacterial community shifts along a Natural CO2 gradient in the mediterranean sea off Vulcano, Italy. Microbial Ecology. 2014;67:819–828. doi: 10.1007/s00248-014-0368-7. [DOI] [PubMed] [Google Scholar]
- King et al. (2012).King GM, Judd C, Kuske CR, Smith C. Analysis of stomach and gut microbiomes of the Eastern Oyster (Crassostrea virginica) from Coastal Louisiana, USA. PLOS ONE. 2012;7:e51475. doi: 10.1371/journal.pone.0051475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman (2002).Kirchman DL. The ecology of Cytophaga-Flavobacteria in aquatic environments. FEMS Microbiology Ecology. 2002;39:91–100. doi: 10.1016/S0168-6496(01)00206-9. [DOI] [PubMed] [Google Scholar]
- Koch et al. (2013).Koch M, Bowes G, Ross C, Zhang XH. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Global Change Biology. 2013;19:103–132. doi: 10.1111/j.1365-2486.2012.02791.x. [DOI] [PubMed] [Google Scholar]
- Kooperman et al. (2007).Kooperman N, Ben-Dov E, Kramarsky-Winter E, Barak Z, Kushmaro A. Coral mucus-associated bacterial communities from natural and aquarium environments. FEMS Microbiology Letters. 2007;276:106–113. doi: 10.1111/j.1574-6968.2007.00921.x. [DOI] [PubMed] [Google Scholar]
- Kroeker et al. (2013).Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, Duarte CM, Gattuso JP. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Global Change Biology. 2013;19:1884–1896. doi: 10.1111/gcb.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachnit et al. (2011).Lachnit T, Meske D, Wahl M, Harder T, Schmitz R. Epibacterial community patterns on marine macroalgae are host-specific but temporally variable. Environmental Microbiology. 2011;13:655–665. doi: 10.1111/j.1462-2920.2010.02371.x. [DOI] [PubMed] [Google Scholar]
- Lage & Bondoso (2014).Lage OM, Bondoso J. Planctomycetes and macroalgae, a striking association. Frontiers in Microbiology. 2014;5:1–9. doi: 10.3389/fmicb.2014.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane (1991).Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. John Wiley and Sons; Chichester: 1991. pp. 115–175. [Google Scholar]
- Liu et al. (2010).Liu H, Zheng F, Sun X, Hong X, Dong S, Wang B, Tang X, Wang Y. Identification of the pathogens associated with skin ulceration and peristome tumescence in cultured sea cucumbers Apostichopus japonicus (Selenka) Journal of Invertebrate Pathology. 2010;105:236–242. doi: 10.1016/j.jip.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Longford et al. (2007).Longford S, Tujula N, Crocetti G, Holmes A, Holmström C, Kjelleberg S, Steinberg P, Taylor M. Comparisons of diversity of bacterial communities associated with three sessile marine eukaryotes. Aquatic Microbial Ecology. 2007;48:217–229. doi: 10.3354/ame048217. [DOI] [Google Scholar]
- Mackie et al. (2004).Mackie RI, Rycyk M, Ruemmler RL, Aminov RI, Wikelski M. Biochemical and microbiological evidence for fermentative digestion in free-living land iguanas (Conolophus pallidus) and marine iguanas (Amblyrhynchus cristatus) on the Galápagos archipelago. Physiological and Biochemical Zoology. 2004;77:127–138. doi: 10.1086/383498. [DOI] [PubMed] [Google Scholar]
- Maki et al. (1988).Maki JS, Rittschof D, Costlow JD, Mitchell R. Inhibition of attachment of larval barnacles, Balanus amphitrite, by bacterial surface films. Marine Biology. 1988;97:199–206. doi: 10.1007/BF00391303. [DOI] [Google Scholar]
- Marzinelli et al. (2015).Marzinelli EM, Campbell AH, Zozaya Valdes E, Vergés A, Nielsen S, Wernberg T, De Bettignies T, Bennett S, Caporaso JG, Thomas T, Steinberg PD. Continental-scale variation in seaweed host-associated bacterial communities is a function of host condition, not geography. Environmental Microbiology. 2015;17:4078–4088. doi: 10.1111/1462-2920.12972. [DOI] [PubMed] [Google Scholar]
- Matsuo et al. (2005).Matsuo Y, Imagawa H, Nishizawa M, Shizuri Y. Isolation of an algal morphogenesis inducer from a marine bacterium. Science. 2005;307:1598. doi: 10.1126/science.1105486. [DOI] [PubMed] [Google Scholar]
- Matsuo et al. (2003).Matsuo Y, Suzuki M, Kasai H, Shizuri Y, Harayama S. Isolation and phylogenetic characterization of bacteria capable of inducing differentiation in the green alga Monostroma oxyspermum. Environmental Microbiology. 2003;5:25–35. doi: 10.1046/j.1462-2920.2003.00382.x. [DOI] [PubMed] [Google Scholar]
- Mattila et al. (2014).Mattila JM, Zimmer M, Vesakoski O, Jormalainen V. Habitat-specific gut microbiota of the marine herbivore Idotea balthica (Isopoda) Journal of Experimental Marine Biology and Ecology. 2014;455:22–28. doi: 10.1016/j.jembe.2014.02.010. [DOI] [Google Scholar]
- McDonald et al. (2012).McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. The ISME Journal. 2012;6:610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensch et al. (2016).Mensch B, Neulinger SC, Graiff A, Pansch A, Künzel S, Fischer MA, Schmitz RA. Restructuring of epibacterial communities on Fucus vesiculosus forma mytili in response to elevated pCO2 and increased temperature levels. Frontiers in Microbiology. 2016;7 doi: 10.3389/fmicb.2016.00434. Article 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meron et al. (2011).Meron D, Atias E, Iasur Kruh L, Elifantz H, Minz D, Fine M, Banin E. The impact of reduced pH on the microbial community of the coral Acropora eurystoma. ISME Journal. 2011;5:51–60. doi: 10.1038/ismej.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meusnier et al. (2001).Meusnier I, Olsen JL, Stam WT, Destombe C, Valero M. Phylogenetic analyses of Caulerpa taxifolia (Chlorophyta) and of its associated bacterial microflora provide clues to the origin of the Mediterranean introduction. Molecular Ecology. 2001;10:931–946. doi: 10.1046/j.1365-294X.2001.01245.x. [DOI] [PubMed] [Google Scholar]
- Meziti et al. (2010).Meziti A, Ramette A, Mente E, Kormas KA. Temporal shifts of the Norway lobster (Nephrops norvegicus) gut bacterial communities. FEMS Microbiology Ecology. 2010;74:472–484. doi: 10.1111/j.1574-6941.2010.00964.x. [DOI] [PubMed] [Google Scholar]
- Moisander, Sexton & Daley (2015).Moisander PH, Sexton AD, Daley MC. Stable associations masked by temporal variability in the marine copepod microbiome. PLOS ONE. 2015;10:e0138967. doi: 10.1371/journal.pone.0138967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow et al. (2015).Morrow KM, Bourne DG, Humphrey C, Botté ES, Laffy P, Zaneveld J, Uthicke S, Fabricius KE, Webster NS. Natural volcanic CO2 seeps reveal future trajectories for host-microbial associations in corals and sponges. The ISME Journal. 2015;9:894–908. doi: 10.1038/ismej.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedashkovskaya et al. (2006).Nedashkovskaya OI, Vancanneyt M, Christiaens L, Kalinovskaya NI, Mikhailov VV, Swings J. Aquimarina intermedia sp. nov., reclassification of Stanierella latercula (Lewin 1969) as Aquimarina latercula comb. nov and Gaetbulimicrobium brevivitae Yoon, et al. 2006 as Aquimarina brevivitae comb. nov., and amended description of the genus Aquimarina. International Journal of Systematic and Evolutionary Microbiology. 2006;56:2037–2041. doi: 10.1099/ijs.0.64155-0. [DOI] [PubMed] [Google Scholar]
- Olischläger et al. (2013).Olischläger M, Bartsch I, Gutow L, Wiencke C. Effects of ocean acidification on growth and physiology of Ulva lactuca (Chlorophyta) in a rockpool-scenario. Phycological Research. 2013;61:180–190. doi: 10.1111/pre.12006. [DOI] [Google Scholar]
- Parris et al. (2016).Parris DJ, Brooker RM, Morgan MA, Dixson DL, Stewart FJ. Whole gut microbiome composition of damselfish and cardinalfish before and after reef settlement. PeerJ. 2016;4:e2412. doi: 10.7717/peerj.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel et al. (2003).Patel P, Callow ME, Joint I, Callow JA. Specificity in the settlement—modifying response of bacterial biofilms towards zoospores of the marine alga Enteromorpha. Environmental Microbiology. 2003;5:338–349. doi: 10.1046/j.1462-2920.2003.00407.x. [DOI] [PubMed] [Google Scholar]
- Perlman, Hunter & Zchori-Fein (2006).Perlman SJ, Hunter MS, Zchori-Fein E. The emerging diversity of Rickettsia. Proceedings of the Royal Society B: Biological Sciences. 2006;273:2097–2106. doi: 10.1098/rspb.2006.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poore et al. (2013).Poore AGB, Graba-Landry A, Favret M, Sheppard Brennand H, Byrne M, Dworjanyn SA. Direct and indirect effects of ocean acidification and warming on a marine plant-herbivore interaction. Oecologia. 2013;173:1113–1124. doi: 10.1007/s00442-013-2683-y. [DOI] [PubMed] [Google Scholar]
- Porzio, Buia & Hall-Spencer (2011).Porzio L, Buia MC, Hall-Spencer JM. Effects of ocean acidification on macroalgal communities. Journal of Experimental Marine Biology and Ecology. 2011;400:278–287. doi: 10.1016/j.jembe.2011.02.011. [DOI] [Google Scholar]
- Sawabe et al. (1998).Sawabe T, Makino H, Tatsumi M, Nakano K, Tajima K, Iqbal MM, Yumoto I, Ezura Y, Christen R. Pseudoalteromonas bacteriolytica sp. nov., a marine bacterium that is the causative agent of red spot disease of Laminaria japonica. International Journal of Systematic Bacteriology. 1998;3:769–774. doi: 10.1099/00207713-48-3-769. [DOI] [PubMed] [Google Scholar]
- Schulze et al. (2006).Schulze AD, Alabi AO, Tattersall-Sheldrake AR, Miller KM. Bacterial diversity in a marine hatchery: balance between pathogenic and potentially probiotic bacterial strains. Aquaculture. 2006;256:50–73. doi: 10.1016/j.aquaculture.2006.02.008. [DOI] [Google Scholar]
- Shah & Gharbia (1993).Shah HN, Gharbia SE. Ecophysiology and taxonomy of bacteroides and related taxa. Clinical Infectious Diseases. 1993;16:S160–S167. doi: 10.1093/clinids/16.Supplement_4.S160. [DOI] [PubMed] [Google Scholar]
- Shnit-Orland, Sivan & Kushmaro (2012).Shnit-Orland M, Sivan A, Kushmaro A. Antibacterial activity of pseudoalteromonas in the coral holobiont. Microbial Ecology. 2012;64:851–859. doi: 10.1007/s00248-012-0086-y. [DOI] [PubMed] [Google Scholar]
- Simon, Glöckner & Amann (1999).Simon M, Glöckner FO, Amann R. Different community structure and temperature optima of heterotrophic picoplankton in various regions of the Southern Ocean. Aquatic Microbial Ecology. 1999;18:275–284. doi: 10.3354/ame018275. [DOI] [Google Scholar]
- Singh et al. (2011).Singh RP, Mantri VA, Reddy CRK, Jha B. Isolation of seaweed-associated bacteria and their morphogenesis-inducing capability in axenic cultures of the green alga Ulva fasciata. Aquatic Biology. 2011;12:13–21. doi: 10.3354/ab00312. [DOI] [Google Scholar]
- Singh & Reddy (2014).Singh RP, Reddy CRKRK. Seaweed-microbial interactions: key functions of seaweed-associated bacteria. FEMS Microbiology Ecology. 2014;88:213–230. doi: 10.1111/1574-6941.12297. [DOI] [PubMed] [Google Scholar]
- Song et al. (2012).Song JK, Kim D, Eun JB, Choi BD, Oh MJ, Jung SJ. Identification of cellulolytic bacteria associated with tunic softness syndrome in the sea squirt, Halocynthia roretzi. Food Science and Biotechnology. 2012;21:1405–1411. doi: 10.1007/s10068-012-0185-z. [DOI] [Google Scholar]
- Staufenberger et al. (2008).Staufenberger T, Thiel V, Wiese J, Imhoff JF. Phylogenetic analysis of bacteria associated with Laminaria saccharina. FEMS Microbiology Ecology. 2008;64:65–77. doi: 10.1111/j.1574-6941.2008.00445.x. [DOI] [PubMed] [Google Scholar]
- Sullam et al. (2012).Sullam KE, Essinger SD, Lozupone CA, O’Connor MP, Rosen GL, Knight R, Kilham SS, Russell JA. Environmental and ecological factors that shape the gut bacterial communities of fish: a meta-analysis. Molecular Ecology. 2012;21:3363–3378. doi: 10.1111/j.1365-294X.2012.05552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunagawa et al. (2009).Sunagawa S, Desantis TZ, Piceno YM, Brodie EL, Desalvo MK, Voolstra CR, Weil E, Andersen GL, Medina M. Bacterial diversity and white Plague disease-associated community changes in the caribbean coral Montastraea faveolata. ISME Journal. 2009;3:512–521. doi: 10.1038/ismej.2008.131. [DOI] [PubMed] [Google Scholar]
- Suzuki et al. (2001).Suzuki M, Nakagawa Y, Harayama S, Yamamoto S. Phylogenetic analysis and taxonomic study of marine Cytophaga-like bacteria: proposal for Tenacibaculum gen. nov. with Tenacibaculum maritimum comb. nov. and Tenacibaculum ovolyticum comb. nov. and description of Tenacibaculum mesophilum sp. nov. and Ten. International Journal of Systematic and Evolutionary Microbiology. 2001;51:1639–1652. doi: 10.1099/00207713-51-5-1639. [DOI] [PubMed] [Google Scholar]
- Taylor et al. (2014).Taylor JD, Ellis R, Milazzo M, Hall-Spencer JM, Cunliffe M. Intertidal epilithic bacteria diversity changes along a naturally occurring carbon dioxide and pH gradient. FEMS Microbiology Ecology. 2014;89:670–678. doi: 10.1111/1574-6941.12368. [DOI] [PubMed] [Google Scholar]
- Thomas et al. (2008).Thomas T, Evans FF, Schleheck D, Mai-Prochnow A, Burke C, Penesyan A, Dalisay DS, Stelzer-Braid S, Saunders N, Johnson J, Ferriera S, Kjelleberg S, Egan S. Analysis of the Pseudoalteromonas tunicata genome reveals properties of a surface-associated life style in the marine environment. PLOS ONE. 2008;3:e3252. doi: 10.1371/journal.pone.0003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas et al. (2011).Thomas F, Hehemann JH, Rebuffet E, Czjzek M, Michel G. Environmental and gut Bacteroidetes: the food connection. Frontiers in Microbiology. 2011;2 doi: 10.3389/fmicb.2011.00093. Article 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurber et al. (2009).Thurber RV, Willner-Hall D, Rodriguez-Mueller B, Desnues C, Edwards RA, Angly F, Dinsdale E, Kelly L, Rohwer F. Metagenomic analysis of stressed coral holobionts. Environmental Microbiology. 2009;11:2148–2163. doi: 10.1111/j.1462-2920.2009.01935.x. [DOI] [PubMed] [Google Scholar]
- Tietjen (2014).Tietjen M. “You are what you eat”: how diet can influence the gut microbiota of marine invertebrates. The Plymouth Student Scientist. 2014;7:203–211. [Google Scholar]
- Tujula et al. (2010).Tujula NA, Crocetti GR, Burke C, Thomas T, Holmström C, Kjelleberg S. Variability and abundance of the epiphytic bacterial community associated with a green marine Ulvacean alga. The ISME Journal. 2010;4:301–311. doi: 10.1038/ismej.2009.107. [DOI] [PubMed] [Google Scholar]
- Urabe, Togari & Elser (2003).Urabe J, Togari J, Elser JJ. Stoichiometric impacts of increased carbon dioxide on a planktonic herbivore. Global Change Biology. 2003;9:818–825. doi: 10.1046/j.1365-2486.2003.00634.x. [DOI] [Google Scholar]
- Van De Waal et al. (2010).Van De Waal DB, Verschoor AM, Verspagen JMH, Van Donk E, Huisman J. Climate-driven changes in the ecological stoichiometry of aquatic ecosystems. Frontiers in Ecology and the Environment. 2010;8:145–152. doi: 10.1890/080178. [DOI] [Google Scholar]
- Vezzulli et al. (2016).Vezzulli L, Grande C, Reid PC, Hélaouët P, Edwards M, Höfle MG, Brettar I, Colwell RR, Pruzzo C. Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E5062–E5071. doi: 10.1073/pnas.1609157113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira et al. (2016).Vieira C, Engelen AH, Guentas L, Aires T, Houlbreque F, Gaubert J, Serrão EA, Clerck O De, Payri CE. Species specificity of bacteria associated to the brown seaweeds Lobophora (Dictyotales, Phaeophyceae) and their potential for induction of rapid coral bleaching in Acropora muricata. Frontiers in Microbiology. 2016;7 doi: 10.3389/fmicb.2016.00316. Article 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Brune & Zimmer (2007).Wang Y, Brune A, Zimmer M. Bacterial symbionts in the hepatopancreas of isopods: diversity and environmental transmission. FEMS Microbiology Ecology. 2007;61:141–152. doi: 10.1111/j.1574-6941.2007.00329.x. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2012).Wang Y, Feng N, Li Q, Ding J, Zhan Y, Chang Y. Isolation and characterization of bacteria associated with a syndrome disease of sea urchin Strongylocentrotus intermedius in North China. Aquaculture Research. 2012;44:691–700. doi: 10.1111/j.1365-2109.2011.03073.x. [DOI] [Google Scholar]
- Wang et al. (2008).Wang G, Shuai L, Li Y, Lin W, Zhao X, Duan D. Phylogenetic analysis of epiphytic marine bacteria on Hole-Rotten diseased sporophytes of Laminaria japonica. Journal of Applied Phycology. 2008;20:403–409. doi: 10.1007/s10811-007-9274-4. [DOI] [Google Scholar]
- Webster et al. (2013).Webster NS, Negri AP, Flores F, Humphrey C, Soo R, Botté ES, Vogel N, Uthicke S. Near-future ocean acidification causes differences in microbial associations within diverse coral reef taxa. Environmental Microbiology Reports. 2013;5:243–251. doi: 10.1111/1758-2229.12006. [DOI] [PubMed] [Google Scholar]
- Witt et al. (2011).Witt V, Wild C, Anthony KRN, Diaz-Pulido G, Uthicke S. Effects of ocean acidification on microbial community composition of, and oxygen fluxes through, biofilms from the Great Barrier Reef. Environmental Microbiology. 2011;13:2976–2989. doi: 10.1111/j.1462-2920.2011.02571.x. [DOI] [PubMed] [Google Scholar]
- Yuksel, Thompson & Adams (2006).Yuksel SA, Thompson KD, Adams A. Rickettsial infections of fish. Turkish Journal of Fisheries and Aquatic Sciences. 2006;6:63–78. doi: 10.1016/S0959-8030(96)00001-4. [DOI] [Google Scholar]
- Zimmer (2002).Zimmer M. Nutrition in terrestrial isopods (Isopoda: Oniscidea): an evolutionary-ecological approach. Biological Reviews of the Cambridge Philosophical Society. 2002;77:455–493. doi: 10.1017/S1464793102005912. [DOI] [PubMed] [Google Scholar]
- Zimmer & Bartholmé (2003).Zimmer M, Bartholmé S. Bacterial endosymbionts in Asellus aquaticus (Isopoda) and Gammarus pulex (Amphipoda), and their contribution to digestion. Limnology and Oceanography. 2003;48:2208–2213. doi: 10.4319/lo.2003.48.6.2208. [DOI] [Google Scholar]
- Zimmer et al. (2002).Zimmer M, Danko JP, Pennings SC, Danford AR, Carefoot TH, Ziegler A, Uglow RF. Cellulose digestion and phenol oxidation in coastal isopods (Crustacea: Isopoda) Marine Biology. 2002;140:1207–1213. doi: 10.1007/s00227-002-0800-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
Engelen, Aschwin (2018): Acidification + Grazing_Effects_Microbiome. figshare. https://doi.org/10.6084/m9.figshare.5346316.v3.