Abstract
Immunoglobulin G4 (IgG4)‐related disease is a fibroinflammatory systemic disorder with multiorgan involvement. Proximal bile duct involvement results in IgG4‐related sclerosing cholangitis, which is characterized by a lymphoplasmacytic infiltrate with abundant IgG4‐positive plasma cells and fibrosis. Differentiating between cholangiocarcinoma and IgG4‐sclerosing cholangitis can present a diagnostic dilemma. We describe an unusual presentation of a hepatic mass meeting multiple criteria for IgG4‐sclerosing cholangitis but was ultimately found to be cholangiocarcinoma. Several published case reports describe patients with suspected cholangiocarcinoma who are later found to have IgG4‐sclerosing cholangitis, but few reports have demonstrated the reverse. Distinguishing between cholangiocarcinoma and IgG4‐sclerosing cholangitis is challenging, and a high clinical suspicion for cholangiocarcinoma must always be maintained. (Hepatology Communications 2018;2:349‐353)
Abbreviations
- CA 19‐9
cancer antigen 19‐9
- CCA
cholangiocarcinoma
- CK
cytokeratin
- CT
computed tomography
- ERCP
endoscopic retrograde cholangiopancreatography
- IgG4
immunoglobulin G4
- RD
related disease
- RSC
sclerosing cholangitis
Introduction
Immunoglobulin G4 (IgG4)‐related disease (RD) is a multiorgan fibroinflammatory disorder.1, 2, 3, 4 Proximal bile duct involvement results in IgG4‐related sclerosing cholangitis (RSC), which is characterized by a lymphoplasmacytic infiltrate with abundant IgG4‐positive plasma cells and fibrosis classically in a storiform pattern.2, 4, 5 Differentiating between cholangiocarcinoma (CCA) and IgG4‐RSC can sometimes present a diagnostic dilemma.
Case Report
A 67‐year‐old Caucasian male patient presented to his local provider with generalized pruritus and painless jaundice. His past medical history was significant for Lynch syndrome and adenocarcinoma of the colon. This was complicated by three separate occurrences for which he underwent a complete proctocolectomy with end ileostomy 7 years prior to his current presentation at which time he showed signs of complete remission. He was found to have hyperbilirubinemia to 6 mg/dL (0.2‐1.3 mg/dL) and renal failure. A noncontrast computed tomography (CT) demonstrated an ill‐defined 3‐cm density in the left hepatic lobe (Fig. 1A). A plastic stent was placed into the left lobe during endoscopic retrograde cholangiopancreatography (ERCP) (Fig. 1B). CT and ultrasound‐guided biopsies were negative for malignancy. A primarily inflammatory chronic hepatitis was seen with focal bile duct proliferation with associated multinucleated giant cells. Cancer antigen 19‐9 (CA 19‐9), alpha‐fetoprotein, and carcinoembryonic antigen were normal.
Figure 1.
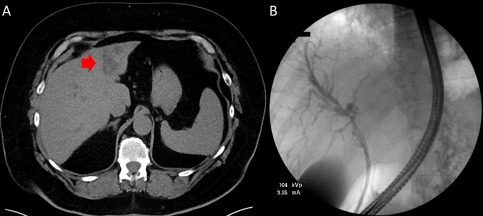
Initial images of the liver. (A) Initial CT abdomen without contrast. Ill‐defined 3.2 × 2.8‐cm mass‐like density in the left hepatic lobe (red arrow). (B) Initial ERCP with stent placed into the left intrahepatic biliary tree.
The patient was referred to our institution where he was admitted to the hospital for hypotension. Magnetic resonance imaging/ magnetic resonance cholangiopancreatography (Fig. 2A) demonstrated an ill‐defined hypoenhancing lesion in segment III measuring 4.0 × 2.9 cm, resulting in obstruction of the intrahepatic biliary ducts (left greater than right). Additionally, there were several hypoenhancing satellite lesions in segment II, II, and IVb. Several fluid collections with debris were noted along the anterior left lobe contiguous with the dilated intrahepatic bile ducts, consistent with bilomas. Repeat ERCP (Fig. 2B) was performed for cholangitis and showed stricture at the bifurcation. Brush cytology from the stricture yielded reactive changes, and a plastic stent was placed across the stricture. The outside biopsy specimen was reviewed at our institution, and IgG4 staining was performed (Fig. 3A,B). Multiple clusters of IgG4‐positive plasma cells were identified. No atypical epithelioid cells were seen. A paucicellular scar was seen, but the typical portal‐based, storiform, fibroinflammatory nodules were not visualized. Serum IgG4 was elevated at 208 mg/dL (11‐86 mg/dL), IgG1 was 623 mg/dL (330‐856 mg/dL), and total IgG was 1,216 mg/dL (695‐1,620 mg/dL). Total bilirubin improved from 2.7 to 1.5 mg/dL (0.2‐1.3 mg/dL) and aspartate aminotransferase from 151 to 103 mg/dL (0‐45 mg/dL), after ERCP with stent replacement. IgG4‐RSC was suspected, and prednisone 60 mg was started. Aspartate aminotransferase normalized after 2 weeks, and a repeat ERCP showed slight improvement. Prednisone was decreased to 40 mg after 6 weeks due to increased intraocular pressure.
Figure 2.
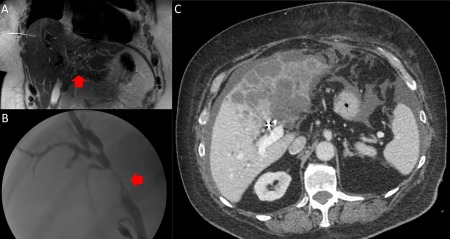
MRI and ERCP images. (A) Coronal T2 MRI demonstrating an ill‐defined 4.0 × 2.9‐cm hypo‐enhancing lesion in segment III (red arrow) causing obstruction of the intrahepatic biliary ducts (left greater than right). Additionally, there were several hypo‐enhancing satellite lesions in segment II, II, and IVb. (B) Second ERCP with biliary stricture at the bifurcation (red arrow). A plastic biliary was subsequently placed. (C) Axial and coronal images of CT abdomen 5 months after presentation. Bilobar low‐density hepatic masses, loculated ascites, and peritoneal nodular enhancement adjacent to the left hepatic lobe. Abbreviation: MRI, magnetic resonance imaging.
Figure 3.
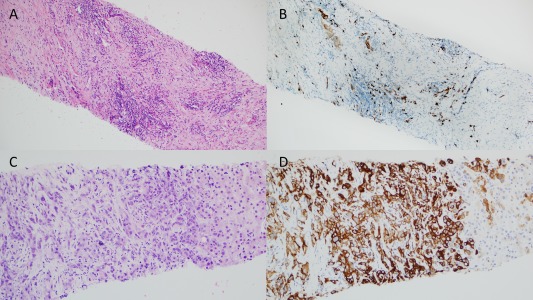
Biopsy staining. (A) Initial biopsy with H&E staining. No atypical epithelioid cells were seen. A paucicellular scar was seen, but the typical portal‐based, storiform, fibroinflammatory nodules were not. (B) Initial biopsy with IgG4 staining. Multiple clusters of IgG4‐positive plasma cells were identified. (C) Second biopsy with H&E staining; poorly differentiated carcinoma. (D) Second biopsy with CK7 staining consistent with cholangiocarcinoma. Abbreviation: H&E, hematoxylin and eosin.
Seven weeks after starting prednisone, the patient presented with pulmonary emboli and sepsis from enterococcal bacteremia. CT revealed extensive low‐density hepatic masses, loculated ascites, and peritoneal nodular enhancement (Fig. 2C). Ultrasound‐guided biopsy showed poorly differentiated carcinoma that was cytokeratin (CK)7 positive, CK20 negative, CK19 positive, arginase negative, Caudal Type Homeobox 2 negative, and special AT‐rich sequence‐binding protein 2 negative, favoring CCA (Fig. 3C,D). Serum CA 19‐9 was elevated at 281 U/mL (0‐37 U/mL). The patient elected for comfort care measures and died in home hospice care 6 weeks later.
Discussion
While many published case reports describe patients with suspected CCA who are later found to have IgG4‐RSC, few reports have demonstrated the reverse.6, 7 Here, we examine a case that on imaging was very concerning for malignancy, but serum IgG4, histology, and partial response to prednisone suggested IgG4‐RSC. According to a recent meta‐analysis, >135 mg/dL serum IgG4 level confers a sensitivity of 87.2% and specificity of 82.6% for the diagnosis of IgG4‐RD.8 Some authors have advocated using a 2‐fold upper limit of normal, but this yielded a sensitivity of 63% and a specificity of 94.8% in the same meta‐analysis. Moreover, in a cohort study of 126 CCA patients, 4 patients still had a >2‐fold increase in IgG4 and only a >4‐fold cutoff was 100% specific for IgG4‐RD, particularly in patients with coexisting primary RSC.9 Another approach suggests using the ratio of IgG4:IgG >0.1 or IG4:IgG1 >0.24 as cutoffs.1 In our patient, the ratio was 0.17 and 0.33, which would be consistent with IgG4‐RSC. The initial biopsy was also suggestive, but IgG4‐positive plasma cells can be seen in the peritumoral infiltrate of CCA and pancreatic adenocarcinoma (≥20 IgG4‐positive plasma cells per high‐power field) and in up to 40% of extrahepatic CCA related to interleukin‐10 expression.10, 11 Using a cutoff of ≥50 IgG4‐positive plasma cells per high‐power field on biopsy specimens may be more specific for IgG4‐RD and avoid missing malignancy.12 Very elevated CA 19‐9 levels are more suggestive of CCA. A cohort of 275 patients with CCA had a mean CA 19‐9 of 2,788.2 ± 10,563.8 U/mL compared to a cohort of 30 patients with IgG4‐RSC having a mean CA19‐9 of 134.7 ± 257.2 U/mL (P < 0.001).13 Involvement of other organs, often seen in IgG4‐RD, was not seen in this patient, which was another clue that this patient had CCA. In a cohort of 53 patients with IgG4‐RSC, 92% had pancreatic involvement (most common) followed by kidney, retroperitoneum, salivary glands, lymph nodes, and lung.5
We should also consider the possibility of this patient having both IgG4‐related disease and CCA. Some studies have suggested a long‐term increased risk of malignancy with IgG4‐related disease, but a cohort of 113 patients with IgG4‐related disease was followed for a mean of 73 months and no significant increase in malignancy was noted.14, 15 Additionally, the time of onset of this patient's symptoms to his ultimate death due to progressive CCA would favor just a single process. This patient did have a history of Lynch syndrome (specific mutation unknown), and the literature suggests a possible slight increase in hepatobiliary malignancies with mismatch repair gene mutations.16 In the setting of increased cell turnover due to IgG4‐RSC, it is conceivable that progression to CCA could have happened, but it seems unlikely.
Nevertheless, distinguishing between CCA and IgG4‐RSC remains challenging, and a high clinical suspicion for CCA must always be maintained, particularly in the setting of isolated biliary disease. Interpretation of IgG4 serum levels and staining can be problematic as neither is completely sensitive or specific.
Acknowledgment
We thank Dr. Sarah E. Umetsu for providing images from pathology slides.
Potential conflict of interest: Dr. Hameed received grants from Gilead, Intercept, Conatus and Genfit.
Supported by the Advanced/Transplant Hepatology Fellowship Award from the American Association for the Study of Liver Diseases Foundation (to V.A.)
REFERENCES
- 1. Zen Y, Kawakami H, Kim JH. IgG4‐related sclerosing cholangitis: all we need to know. J Gastroenterol 2016;51:295‐312. [DOI] [PubMed] [Google Scholar]
- 2. Ohara H, Okazaki K, Tsubouchi H, Inui K, Kawa S, Kamisawa T, et al.; Research Committee of IgG4‐related Diseases ; Research Committee of Intractable Diseases of Liver and Biliary Tract ; Ministry of Health, Labor and Welfare, Japan ; Japan Biliary Association . Clinical diagnostic criteria of IgG4‐related sclerosing cholangitis 2012. J Hepatobiliary Pancreat Sci 2012;19:536‐542. [DOI] [PubMed] [Google Scholar]
- 3. Okazaki K, Uchida K, Koyabu M, Miyoshi H, Ikeura T, Takaoka M. IgG4 cholangiopathy: current concept, diagnosis, and pathogenesis. J Hepatol 2014;61:690‐695. [DOI] [PubMed] [Google Scholar]
- 4. Deshpande V. The pathology of IgG4‐related disease: critical issues and challenges. Semin Diagn Pathol 2012;29:191‐196. [DOI] [PubMed] [Google Scholar]
- 5. Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, et al. Immunoglobulin G4–associated cholangitis: clinical profile and response to therapy. Gastroenterology 2008;134:706‐715. [DOI] [PubMed] [Google Scholar]
- 6. Zhang YA, Shen XZ, Zhu JM, Liu TT. Extensive metastatic cholangiocarcinoma associated with IgG4‐related sclerosing cholangitis misdiagnosed as isolated IgG4‐related sclerosing cholangitis: a case report and literature review. Medicine (Baltimore) 2015;94:e2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kerdsirichairat T, Manivel JC. An unusual lesion presenting as cholangiopathy. Gastroenterology 2016;150:e3‐5. [DOI] [PubMed] [Google Scholar]
- 8. Hao M, Liu M, Fan G, Yang X, Li J. Diagnostic value of serum IgG4 for IgG4‐related disease: a PRISMA‐compliant systematic review and meta‐analysis. Medicine (Baltimore) 2016;95:e3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oseini AM, Chaiteerakij R, Shire AM, Ghazale A, Kaiya J, Moser CD, et al. Utility of serum immunoglobulin G4 in distinguishing immunoglobulin G4‐associated cholangitis from cholangiocarcinoma. Hepatology 2011;54:940‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Resheq YJ, Quaas A, von Renteln D, Schramm C, Lohse AW, Luth S. Infiltration of peritumoural but tumour‐free parenchyma with IgG4‐positive plasma cells in hilar cholangiocarcinoma and pancreatic adenocarcinoma. Dig Liver Dis 2013;45:859‐865. [DOI] [PubMed] [Google Scholar]
- 11. Harada K, Shimoda S, Kimura Y, Sato Y, Ikeda H, Igarashi S, et al. Significance of immunoglobulin G4 (IgG4)‐positive cells in extrahepatic cholangiocarcinoma: molecular mechanism of IgG4 reaction in cancer tissue. Hepatology 2012;56:157‐164. [DOI] [PubMed] [Google Scholar]
- 12. Lin J, Cummings OW, Greenson JK, House MG, Liu X, Nalbantoglu I, et al. IgG4‐related sclerosing cholangitis in the absence of autoimmune pancreatitis mimicking extrahepatic cholangiocarcinoma. Scand J Gastroenterol 2015;50:447‐453. [DOI] [PubMed] [Google Scholar]
- 13. Du S, Liu G, Cheng X, Li Y, Wang Q, Li J, et al. Differential diagnosis of immunoglobulin G4‐associated cholangitis from cholangiocarcinoma. J Clin Gastroenterol 2016;50:501‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Douhara A, Mitoro A, Otani E, Furukawa M, Kaji K, Uejima M, et al. Cholangiocarcinoma developed in a patient with IgG4‐related disease. World J Gastrointest Oncol 2013;5:181‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hirano K, Tada M, Sasahira N, Isayama H, Mizuno S, Takagi K, et al. Incidence of malignancies in patients with IgG4‐related disease. Intern Med 2014;53:171‐176. [DOI] [PubMed] [Google Scholar]
- 16. Win AK, Lindor NM, Young JP, Macrae FA, Young GP, Williamson E, et al. Risks of primary extracolonic cancers following colorectal cancer in lynch syndrome. J Natl Cancer Inst 2012;104:1363‐1372. [DOI] [PMC free article] [PubMed] [Google Scholar]


