Abstract
Nonalcoholic fatty liver disease (NAFLD) is associated with an increased risk of atherosclerotic cardiovascular disease. In our meta‐analysis, we aimed to assess the correlation of NAFLD and four surrogate markers of subclinical atherosclerosis. PubMed, Embase, and the Cochrane Library were searched up until April 2017. Original studies investigating the association between NAFLD and subclinical atherosclerosis were included. The outcome data were extracted and pooled for the effect estimate by using a random‐effects model. We used the Newcastle‐Ottawa Quality Assessment Scale to assess the quality of the included studies. Of the 434 initially retrieved studies, 26 studies involving a total of 85,395 participants (including 29,493 patients with NAFLD) were included in this meta‐analysis. The Newcastle‐Ottawa Quality Assessment Scale scores suggested the included studies were of high quality. The pooled effects estimate showed that subjects with NAFLD exhibited a significant independent association with subclinical atherosclerosis compared to the non‐NAFLD group (odds ratio, 1.60; 95% confidence interval, 1.45‐1.78). Subgroup analysis suggested that the presence of NAFLD yielded a remarkable higher risk of increased carotid artery intima‐media thickness/plaques, arterial stiffness, coronary artery calcification, and endothelial dysfunction with odds ratios (95% confidence interval) of 1.74 (1.47‐2.06), 1.56 (1.24‐1.96), 1.40 (1.22‐1.60), and 3.73 (0.99‐14.09), respectively. Conclusion: Our meta‐analysis revealed a close link between NAFLD and subclinical atherosclerosis in light of four different indices. Patients with NAFLD might benefit from screening and surveillance of early atherosclerosis, which would facilitate the prediction of potential cardiovascular disease burden, risk stratification, and appropriate intervention in the long term. (Hepatology Communications 2018;2:376‐392)
Abbreviations
- AS
arterial stiffness
- CAC
coronary artery calcification
- CIMT
carotid artery intima‐media thickness
- CI
confidence interval
- CVD
cardiovascular disease
- FMD
flow‐mediated dilation
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- OR
odds ratio
- PRISMA
preferred reporting items for systematic reviews and meta‐analyses
Introduction
Nonalcoholic fatty liver disease (NAFLD) has become an emerging global public health concern, with prevalence estimates ranging from 10% to 30% in the general population and a higher prevalence of 40% to 70% in individuals with obesity or diabetes mellitus.1 NAFLD is a condition histologically divided into nonalcoholic fatty liver (isolated steatosis with or without nonspecific inflammation) and nonalcoholic steatohepatitis (NASH), with varying degrees of hepatic fibrosis and more progressive natural history.2
In recent years, NAFLD has also been recognized as the hepatic manifestation of metabolic syndrome, sharing a series of risk factors with cardiovascular disease (CVD), including insulin resistance, hypertension, obesity, and dyslipidemia.3 Epidemiologic evidence from the early to mid‐2000s indicated that CVD events had increased and was presenting as the most common cause of death in patients with NAFLD (approximately 25%).4 As such, early evaluation for CVD in a preclinical stage in the high‐risk population is necessary to decrease cardiovascular morbidity and mortality.
Additionally, a growing body of evidence demonstrated that NAFLD not only behaved as a marker of atherosclerotic CVD but also might take part in its pathogenesis, providing insight regarding the relationship between NAFLD and early stage atherosclerosis.5, 6 Notably, a spectrum of studies reported that NAFLD is associated with markers of preclinical atherosclerosis, independent of traditional risk factors.7 At present, carotid artery intima‐media thickness (CIMT), arterial stiffness (AS), coronary artery calcification (CAC), and brachial arterial flow‐mediated dilation (FMD) are noninvasive techniques that generally serve as surrogate markers for subclinical atherosclerosis. They are used during initial assessment of potential cardiovascular events and risk stratification to determine appropriate therapeutic strategies for patients with latent CVD.
In this context, it is plausible that there is a relationship between the presence of NAFLD and subclinical atherosclerosis. A systematic review highlighted the association of NAFLD with various indices of subclinical atherosclerosis independent of established CVD risk factors. Unfortunately, that study failed to offer the effect estimates of the correlation, and uncertainty still exists with respect to the potential link between NAFLD and the aforementioned markers of subclinical atherosclerosis. We therefore performed a systematic review and meta‐analysis to attain a comprehensive understanding of this issue.
Materials and Methods
SEARCH STRATEGY
The protocol for this systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement.8 PubMed, Embase, and the Cochrane Library databases were extensively searched up until April 30, 2017, to identify potentially relevant publications without language or date restrictions. Medical subject heading terms were “nonalcoholic fatty liver disease” or “NAFLD” or “fatty liver” and “cardiovascular disease” or “subclinical atherosclerosis” or “preclinical atherosclerosis” or “intima‐media thickness” or “coronary calcification” or “coronary artery calcification” or “endothelial dysfunction” or “arterial stiffness” or “flow‐mediated dilation” or “pulse wave velocity.” Reference lists from cited articles were also manually searched for additional eligible trials.
INCLUSION CRITERIA
Criteria for inclusion of an article in this meta‐analysis were as follows: (1) prospective design, cross‐sectional design, or retrospective design; (2) original studies designed to evaluate the relationship between NAFLD and subclinical atherosclerosis; (3) odds ratios (ORs) or hazard ratios with confidence intervals (CIs) were provided or could be estimated with sufficient information; (4) diagnosis of NAFLD was based on ultrasound, computed tomography, or biopsy. Duplicated or overlapping reports were deleted if referring to the same title, author list, or publication date.
STUDY SELECTION AND DATA EXTRACTION
After the first screening of titles/abstracts, full articles of potentially eligible studies were independently reviewed by two investigators (Y.Z., X.Z.) with regard to the inclusion and exclusion criteria. The following relevant information of included studies was extracted: 1) study: the first author, year of publication, location, design; 2) participants: number, age, and sex of the NAFLD and non‐NAFLD groups; 3) evaluation methods of NAFLD and subclinical atherosclerosis; 4) adjusted confounders. Conventionally, the most adjusted estimate was selected when a study offered more than one risk estimate. Any discrepancies regarding the extraction of data were resolved by an additional investigator (M.Z.).
QUALITY ASSESSMENT
The methodologic quality of the included studies was evaluated by a “star system” based on the Newcastle‐Ottawa Quality Assessment Scale, which ranged from one to nine stars and consisted of three items: (1) patient selection; (2) comparability of groups or cohorts; (3) assessment of either the exposure or outcome of interest for case‐control or cohort studies, respectively. Two reviewers (Y.Z., X.Z) independently assessed the quality of the original article, with a third author addressing any subsequent disagreements.
STATISTICAL ANALYSIS
The results of studies were pooled, and an overall estimate of ORs or hazard ratios with 95% CI were obtained. In the inverse variance approach, the weight given to each study is the inverse of the variance of the effect estimate (i.e., 1 over the square of its SEM). Clinical heterogeneity was assessed by the χ2 test and quantified by the I 2 statistic, which was minimal if <25%, moderate if 25%‐49%, and substantial if >50%. In light of the significant heterogeneity among studies, a random‐effects model by the Der Simonian and Laird method was used, resulting in a more conservative estimate compared to the fixed‐effects model. Two sources of variability in effects are assumed for weight in the inverse variance method with the random‐effects model; one is from sampling error and the other from study‐level differences, which represent the effects from variability across the population. Subgroup analysis was further conducted according to the following diagnostic indices of subclinical atherosclerosis: CIMT, CAC or plaques, AS, and FMD. Additionally, a sensitivity analysis was carried out by removing each individual study at a time and determining its effect on the ultimate effect estimate. P < 0.05 was considered statistically significant for all analyses. Funnel plots and Egger's regression test were performed to check for publication bias. Statistical analyses were performed with Review Manager version 5.3.9
Results
SEARCH RESULTS
The PRISMA flowchart of the literature search process is shown in Fig. 1.
Figure 1.
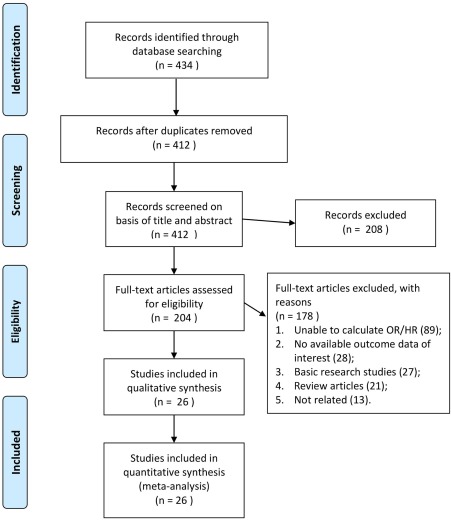
Flow diagram of the study selection process. Abbreviation: HR, hazard ratio.
Electronic and manual searches retrieved 434 potentially relevant publications, which after the initial screening resulted in removing 208 papers according to title and abstract. By reviewing the full articles, 178 papers were further excluded because (1) data were unavailable for a risk estimate (e.g., quantitative variables); (2) there were no outcome data of interest; or (3) the study was considered a basic research study/review article or not related. Eventually, 26 unique clinical studies were eligible for inclusion in this meta‐analysis.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35
BASELINE CHARACTERISTICS OF THE STUDIES
The main characteristics of the qualified studies in this meta‐analysis are summarized in Table 1. Overall, our analysis included 26 observational studies enrolling a total of 85,395 participants (including 29,493 patients with NAFLD). In both the NAFLD and non‐NAFLD groups, most subjects were middle‐aged male individuals, although two studies focused on the pediatric population. These studies were carried out in Asia (China, India, South Korea, Turkey, Iran, Israel, and Japan), Europe (Spain, Italy, Finland, Germany, and Sweden), and America (United States and Brazil). Among them, CIMT/carotid plaques, CAC, AS, and FMD were widely employed as surrogate markers of subclinical atherosclerosis. Twenty studies were cross‐sectional studies, four studies were case‐control studies, and two were prospective cohort studies (either population‐based or hospital‐based or outpatient cohorts). The majority of studies used ultrasonography or computed tomography for diagnosis of NAFLD, and four studies were based on liver biopsy. The Newcastle‐Ottawa Quality Assessment Scale scores suggested the included studies were of high quality (Table 2).
Table 1.
Characteristics of the Included Studies in the Meta‐analysis
| Location (Reference) | Study Design | Study Population NAFLD vs. Non‐NAFLD | Exclusion Criteria |
Age (Year): NAFLD vs. Non‐NAFLD |
Male (%) | Definition of Subclinical Atherosclerosis | Diagnosis of NAFLD | Variables of Multivariate Model |
|---|---|---|---|---|---|---|---|---|
| A. Carotid Intimal‐Medial Thickness or Plaques | ||||||||
| India10 | Cross‐sectional | Consecutive hospital‐based patients with type 2 diabetes (71 vs. 53) | Known hepatic disease, seropositivity for hepatitis B or C, ingestion of hepatotoxic drug(s), and alcoholic fatty liver | 57 vs. 61 | 60 | Mean CIMT >0.8 mm | Presence of an ultrasonographic pattern consistent with “bright liver,” with evident ultrasonographic contrast between hepatic and renal parenchyma, vessel blurring, and narrowing of the lumen of the hepatic veins in the absence of findings suggestive of chronic liver disease | Unadjusted |
| Turkey11 | Case control | Hospital‐based individuals (40 vs. 40) | Malignant disease, pancreas disease, adrenal/pituitary disease, chronic drug/alcohol use, and gastrointestinal surgery | 40 vs. 40 | 51 | Plaque was defined as a focal thickening of >1.2 mm in any carotid segment (near and far walls of right and left common carotid artery, bifurcation bulb, and internal carotid artery) | Liver biopsy | Unadjusted |
| Spain12 | Case control | Hospital‐based individuals (40 vs. 40) | Alcohol consumption, seropositivity for hepatitis B or C, or with serum transferrin saturation >45% | 53 vs. 52 | 50 | Plaque was defined as a focal thickening of ≥1.2 mm in any of 12 carotid segments (near and far walls of right and left common carotid artery, bifurcation, and internal carotid artery) | A “bright liver” (abnormally intense, high‐level echoes arising from the hepatic parenchyma, with an amplitude similar to that of echoes arising from the diaphragm) in the absence of chronic liver disease or cancer | Sex and age |
| Italy13 | Case control | Consecutive hospital‐based individuals matched for sex, age, and BMI (125 vs. 250) | History of DM, hypertension, CVD, viral /autoimmune hepatitis, alcohol consumption, hemochromatosis, drug‐induced liver disease, and Wilson's disease | 51 vs. 52 | 87 | Mean CIMT >0.64 mm | Diagnosis of NAFLD was based on ultrasonography and confirmed by biopsy in 54 patients | Sex, smoking, fasting glucose, lipid parameters, MetS, DM, and BMI |
| Korea14 | Cross‐sectional | Consecutive hospital ‐based patients without diabetes (320 vs. 313) | Alcohol consumption, viral hepatitis, autoimmune hepatitis, and use of hepatotoxic drugs | 54 vs. 52 | 53.6 | Increased IMT was considered as ≥1.0 mm in carotid arteries, and the presence of plaque was defined as localized lesions with protrusion into the arterial lumen or IMT ≥1.5 mm | Presence of diffuse hyperechoic echotexture, bright liver) increased liver echotexture compared with the kidneys, vascular blurring, and deep attenuation of the ultrasonic beam | Age, hypertension, hsCRP, BMI, WC, lipid profile, and liver enzymes |
| Korea15 | Cross‐sectional | Hospital‐based individuals (507 vs. 514) | Know coronary heart disease or stroke, seropositive for hepatitis B, or excessive alcohol consumption | 51 vs. 52 | 54.5 | Increased IMT in this analysis was defined using the sex‐specific highest quintile: >0.86 mm for men and >0.83 mm for women | Degree of steatosis was assessed semiquantitatively (absent, mild, moderate, and severe) on the basis of abnormally intense high‐level echoes arising from the hepatic parenchyma, liver‐kidney differences in echo amplitude, echo penetration into the deep portion of the liver, and clarity of the blood vessel structure in the liver | Age, sex, WC, smoking, alcohol, SBP, fasting glucose, and total/HDL‐cholesterol ratio |
| Iran16 | Case control | Population‐based individuals (290 vs. 290) | Positive or suspicious results for HBsAg, anti‐HCV and HIV, any history of liver disease, major organ failure, alcohol consumption, pregnancy, weight loss or weight gain, and non‐Iranian | age matched | NA | Mean CIMT ≥0.8 mm | Presence of a ‘bright’ liver, with stronger echoes in the hepatic parenchyma than in the renal parenchyma, often associated with unusually fine liver echotexture and vessel blurring | Age, DM, hypertension, and WC |
| Italy18 | Cross‐sectional | Hospital‐based obese children (179 vs. 369) | Infectious and metabolic disorders | NA | 51.5 | Mean CIMT ≥0.5 mm | Ultrasonographic evidence of liver steatosis and the presence of persistently (>6 months) elevated ALT (>258 U/L for boys and >221 U/L for girls) | Age, sex, pubertal status, and BMI‐SDS |
| Italy17 | Cross‐sectional | Hospital‐based obese children (100 vs. 300) | Hepatic virus infections, autoimmune hepatitis, metabolic liver disease, antitrypsin deficiency, cystic fibrosis, Wilson's disease, history of hepatotoxic drug/alcohol use, blood transfusion, surgery, celiac disease, and hemochromatosis | 11 vs. 11 | 51.8 | Increased CIMT was defined as ≥90th percentile of values observed in healthy lean subjects | Ultrasound‐diagnosed fatty liver and persistently (>6 months) elevated ALT levels | Age, sex, Tanner stage, and MetS |
| Italy19 | Cross‐sectional | Consecutive hospital‐based obese subjects (189 vs. 172) | History of cardiovascu‐ lar disease, systemic disease, infection in the previous month, serious chronic illness, alcohol consumption, or use of drugs that interfere with insulin action | 46 vs. 43 | 29 | Mean CIMT >0.8 mm and/or plaques were present | Ultrasonographic evidence of liver steatosis was according to conventional criteria; histological features of steatosis, lobular inflammation, hepatocellular ballooning, and fibrosis were scored with the scoring system for NAFLD | Age |
| Italy20 | Cross‐sectional | Consecutive hospital‐based male (90 vs. 64) | Excessive alcohol consumption | 59 vs. 60 | 48.7 | Mean CIMT >0.9 mm | Presence of diffuse hyperechoic echotexture, bright liver, increased liver echotexture compared with the kidneys, vascular blurring, and deep attenuation of the ultrasonic beam | Age, sex, BMI, DM, smoking, hypertension, and dyslipidemia |
| Korea21 | Cross‐sectional | Hospital‐based individuals (4,303 vs. 3,717) | History of CVD/cancer, cirrhosis, seropositivity for hepatitis B or C, use of antithrombotic drugs, alcohol consumption, subclinical carotid atherosclerosis at baseline | NA | 100 | Mean CIMT >1.2 mm and/or plaques were present | Diagnosis of fatty liver was based on standard criteria, including parenchymal brightness, liver‐to‐kidney contrast, deep beam attenuation, and bright vessel walls | Age, BMI, alcohol, smoking, and MetS |
| India33 | Cross‐sectional | Hospital‐based individuals (52 vs. 28) | History of diabetes, CAD, seropositivity for hepatitis B or C and HIV, alcohol consumption, intake of drugs may cause fatty liver, severe illness or end organ dysfunction, current smokers, pregnant and lactating females | 42 vs. 37 | 67.5 | Mean CIMT was calculated by measuring the far wall at three sites: common carotid artery bifurcation, 10 mm proximal in common carotid, and 10 mm distal to bifurcation in internal carotid artery | Diffuse homogeneous increased echogenecity of the liver was diagnosed as fatty liver | Obesity, MetS, insulin resistance, and lipid parameters |
| B. Coronary artery calcification or plaques | ||||||||
| Japan22 | Cross‐sectional | Hospital‐based patients with NAFLD (n = 60) and controls (n = 238) | History of known liver disease, including viral, genetic, autoimmune, and drug‐induced liver disease, and alcohol consumption | 66 vs. 68 | 56.4 | Positive remodeling as having a remodeling index of >1.1 and calcified plaque was considered severe if >180 HU and mild if <180 HU | Average attenuation value of liver /average attenuation value of spleen <1.1 | Age, sex, alcohol, smoking, BMI |
| Israel23 | Cohort | Hospital‐based individuals (29 vs. 32) | Any other liver or biliary disorders and patients with high risk for CAD | 53 vs. 51 | 52 | Plaques were classified as calcified or noncalcified on a segmental basis, according to plaque features that included volume, attenuation, and calcification pattern. Calcified lesion was defined as a minimum of 2 pixels (area, 0.52 mm2) with a minimum attenuation of 130 HU | Hepatic steatosis was defined as an attenuation of ≥–10 HU or more (calculated as liver attenuation minus spleen attenuation) by using CT | Age, sex, smoking, lipid parameters, glucose levels, MetS, diabetes, BMI, and ALT |
| Taiwan24 | Cross‐sectional | Hospital‐based individuals (121 vs. 174) | Patients with unavailable hepatobiliary evaluation or incomplete laboratory data or positive for HBsAg or anti‐HCV Ab | NA | 66 | CAC scoring >100 | Ultrasonographic evidence: diffusely increased liver echogenicity with evident contrast between the liver and kidney, diffusely increased liver echogenicity with blurring of the intrahepatic vessels or diaphragm, or bright liver echogenicity with poor penetration of the posterior hepatic segment and intrahepatic vessels or invisibility of the diaphragm. CT evidence: liver attenuation less than the spleen, pronounced contrast attenuation between the liver and spleen with blurred intrahepatic vessels, or markedly reduced attenuation of the liver with evident contrast between the liver and intrahepatic vessels. | Age, sex, BMI, DM, smoking, hypertension, fasting glucose, lipid parameters, ALT/AST, serum uric acid, and gallbladder stones |
| USA25 | Cohort | Population‐based individuals (512 vs. 2,502) | Pregnancy, weight >160 kg, uninterpretable CT scan results, and incomplete covariate information | NA | 49.5 | CAC scoring ≥90th percentile | Liver‐phantom ratio of 0.33 or lower by CT image | Age, sex, alcohol use, menopausal status, and hormone therapy |
| Korea26 | Cross‐sectional | Hospital‐based patients with NAFLD (n = 1,617) and controls (n = 2,406) | History of heart attack, coronary artery disease, and other cause of chronic liver disease | 58 vs. 57 | 60.7 | CAC scoring ≥100 | Ultrasonographic features consistent with “bright liver” and evident contrast between hepatic and renal parenchyma, vessel blurring, focal sparing, and narrowing of the lumen of the hepatic veins | Age, sex, BMI, WC, alcohol, smoking, DM, physical activity, hsCRP hypertension, lipid parameters |
| Korea27 | Cross‐sectional | Hospital‐based patients with NAFLD (n = 10,063) and controls (n = 11,272) | History of heart attack /CAD, seropositivity for viral hepatitis or other liver disease, alcohol consumption, and any missing data |
No‐obesity: 39 vs. 40; Obesity: 42 vs. 45 |
100 | Presence of CAC | Ultrasonographic evidence of a diffuse hyperechoic echotexture, hepatorenal echo contrast in reference to the cortex of the right kidney, and vascular blurring and deep‐echo attenuation | Age, DM, hypertension, smoking, and physical inactivity |
| Brazil28 | Cross‐sectional | Hospital‐based patients with steatosis (n = 204) and controls (n = 301) | NA | 48 vs. 46 | 100 | Presence of CAC | Ultrasonographic evidence of a bright liver, with evident contrast between hepatic and renal parenchyma | Age, BP, BMI, smoking, alcohol, dyslipidemia, fasting glucose, BP/lipid drugs, liver enzyme |
| C. Arterial stiffness | ||||||||
| Korea29 | Cross‐sectional | Hospital‐based patients with NAFLD (n = 1,249) and controls (n = 1,705) | History of peripheral artery disease, severe valvular heart disease, alcohol consumption, seropositivity for viral hepatitis and other types of hepatitis | 56 vs. 56 | 65 | Age‐ (10‐year interval) and sex‐specific highest quartile of the cardioankle vascular index | Ultrasonographic evidence of marked increase in bright echoes at a shallow depth, with deep attenuation and impaired visualization of the diaphragm and marked vascular blurring | Age, sex, and BMI WC, smoking, DM, and hypertension |
| Korea35 | Cross‐sectional | Hospital‐based patients with NAFLD (n = 1,667) and controls (n = 2,800) | Patients with unavailable hepatobiliary evaluation, incomplete information, positive for HBsAg or anti‐HCV Ab, abnormal level of liver enzymes, blood glucose, or BP | 51 vs. 52 | 77 | Increased pulse wave velocity was defined as ≥1,366 cm/second | Hepatic steatosis was defined using the standard criteria of fatty liver, including hepatorenal echo contrast, liver brightness, and vascular blurring | Age, sex, BMI, SBP, hsCRP, HR, lifestyle, fasting glucose, sCr, triglyceride, and HDL‐C |
| Korea30 | Cross‐sectional | Hospital‐based patients with NAFLD (n = 482) and controls (n = 960) | History of chronic liver disease, seropositivity for hepatitis B or C, alcohol consumption, subjects with comorbidities that affect WBC count, and missing covariate information | NA | 66.2 | Brachial‐ankle pulse wave velocity ≥1,496 cm/second for men and 1,482 cm/second for women | Ultrasonographic evidence of marked increase in bright echoes at a shallow depth, with deep attenuation and impaired visualization of the diaphragm and marked vascular blurring | Age, smoking, regular exercise, BMI, blood pressure, fasting plasma glucose, triglyceride, HDL, DM, and hypertension |
| China31 | Cross‐sectional | Hospital‐based patients with NAFLD (n = 7,469) and controls (n = 26,837) | History of viral hepatitis or other liver disease, alcohol consumption and subjects with overweight or underweight, missing ultrasonography or CAVI data | 49 vs. 41 | 41.5 | Cardioankle vascular index ≥8 m/second | Presence of at least two of three abnormal findings: diffusely increased echogenicity of the liver, ultrasound beam attenuation, and poor visualization of intrahepatic vessels and diaphragm | Age, sex, blood pressure, fasting plasma glucose, lipid parameters, uric acid, ALT, AST, and GGT |
| D. Flow‐mediated dilation | ||||||||
| Turkey32 | Cross‐sectional | Hospital‐based patients with NAFLD (n = 176) and controls (n = 90) | History of CVD, cerebrovascular disease, peripheral vascular disease, chronic liver disease, seropositivity of hepatitis B virus or C and alcohol consumption | 50 vs. 52 | 41 | Decreased flow‐mediated dilatation was determined as <10% | Ultrasonographic evidence of hepatorenal echogenic contrast, liver brightness, deep attenuation, and vascular blurring | Age, sex, BMI, and insulin resistance |
| India33 | Cross‐sectional | Hospital‐based individuals (52 vs. 28) | History of DM, CAD, seropositivity for hepatitis B or C and HIV, alcohol consumption, drug‐induced fatty liver, severe illness or organ dysfunction, current smokers, pregnant and lactating females | 42 vs. 37 | 67.5 | Mean CIMT was calculated by measuring the far wall at three sites: common carotid artery bifurcation, 10 mm proximal in common carotid, and 10 mm distal to bifurcation in internal carotid artery | Diffuse homogeneous increased echogenecity of the liver was diagnosed as fatty liver | Obesity, MetS, insulin resistance, and lipid parameters |
| Italy34 | Cross‐sectional | Hospital‐based patients with NAFLD (n = 52) and controls (n = 28) | History of seropositivity for hepatitis B or C, alcohol consumption, autoimmune hepatitis, primary biliary cirrhosis, celiac disease, genetic disease | 46 vs. 43 | 77.5 | Flow‐mediated vasodilation in the lower tertile (<5% vasodilation) | NAFLD cases were identified on the basis of chronically raised alanine aminotransferase levels (>1.5× upper normal values for 6 months or more) and a bright liver at ultrasound scan | Age, sex, BMI, and insulin resistance |
Abbreviations: ab, antibody; ALT, alanine aminotransferase; AST, aspartate transaminase; BP, blood pressure; BMI, body mass index; CAD, coronary artery disease; CT, computed tomography; CVD, cardiovascular disease; DM, diabetes mellitus; GGT, gamma‐glutamyl transferase; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HDL, high‐density lipoprotein; HDL‐C, high‐density lipoprotein cholesterol; HIV, human immunodeficiency virus; HR, heart rate; hsCRP, high‐sensitivity C‐reactive protein; HU, Hounsfield unit; MetS, metabolic syndrome; NA, not available; NAFLD, nonalcoholic fatty liver disease; sCr, serum creatinine; SBP, systolic blood pressure; SDS, standard deviation score; WC, waist circumference. SDS
Table 2.
Quality Assessment of Included Studies
|
Year (Reference) |
Selection | Comparability | Outcome/Exposure | Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
| A. Carotid intimal‐medial thickness or plaques | ||||||||||
| 201110 | * | * | * | ** | * | * | * | ******** | ||
| 200811 | * | * | * | ** | * | * | * | ******** | ||
| 200512 | * | * | * | ** | * | * | * | ******** | ||
| 200813 | * | * | * | ** | * | * | * | ******** | ||
| 201214 | * | * | * | ** | * | * | * | ******** | ||
| 200915 | * | * | * | ** | * | * | * | ******** | ||
| 201316 | * | * | * | ** | * | * | * | ******** | ||
| 201418 | * | * | * | ** | * | * | * | ******** | ||
| 201017 | * | * | * | ** | * | * | * | ******** | ||
| 201519 | * | * | * | ** | * | * | * | ******** | ||
| 200920 | * | * | * | ** | * | * | * | ******** | ||
| 201621 | * | * | * | ** | * | * | * | ******** | ||
| 201233 | * | * | * | ** | * | * | * | ******** | ||
| B. Coronary artery calcification or plaques | ||||||||||
| 200822 | * | * | * | ** | * | * | * | ******** | ||
| 201023 | * | * | * | ** | * | * | * | ******** | ||
| 201024 | * | * | * | ** | * | * | * | ******** | ||
| 201525 | * | * | * | ** | * | * | * | ******** | ||
| 201226 | * | * | * | ** | * | * | * | ******** | ||
| 201527 | * | * | * | ** | * | * | * | ******** | ||
| 200728 | * | * | * | ** | * | * | * | ******** | ||
| C. Arterial stiffness | ||||||||||
| 201529 | * | * | * | ** | * | * | * | ******** | ||
| 201235 | * | * | * | ** | * | * | * | ******** | ||
| 201230 | * | * | * | ** | * | * | * | ******** | ||
| 201531 | * | * | * | ** | * | * | * | ******** | ||
| D. Flow‐mediated dilation | ||||||||||
| 201632 | * | * | * | ** | * | * | * | ******** | ||
| 201233 | * | * | * | ** | * | * | * | ******** | ||
| 200534 | * | * | * | ** | * | * | * | ******** | ||
RELATIONSHIP BETWEEN NAFLD AND SUBCLINICAL ATHEROSCLEROSIS
Overall, the mosaic plot revealed that, compared to subjects without NAFLD, patients with NAFLD exhibited a significant higher risk of subclinical atherosclerosis in light of four different indices with OR (CI) values of 1.60 (1.45‐1.78) (Fig. 2). We found evidence of heterogeneity across the included studies (I 2 = 82%; P < 0.05), and an exclusion sensitivity analysis did not alter the above results. We then conducted a subgroup analysis for four types of indices accounting for subclinical atherosclerosis as mentioned below.
Figure 2.
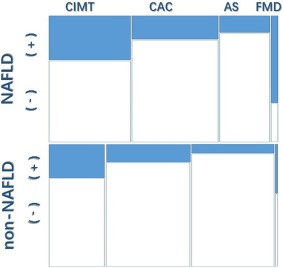
Mosaic plot showing that patients with NAFLD exhibited a significant higher risk of subclinical atherosclerosis compared to subjects without NAFLD.
Thirteen studies with 12,269 individuals were included that addressed CIMT/carotid plaques. Pooled data suggested that NAFLD was associated with a remarkably higher likelihood of pathologic CIMT/carotid plaques (OR, 1.74; 95% CI, 1.47‐2.06; P < 0.00001; I 2 = 86%) (Fig. 3).
Figure 3.
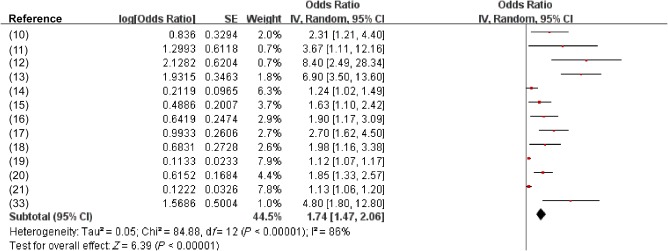
Effect estimate for the association between NAFLD and increased CIMT or plaques using a random‐effects model. Forest plot comparison of individuals without NAFLD versus patients with NAFLD. Red squares represent the OR, horizontal lines the CIs, black diamond represent the pooled OR.
Additionally, data on CAC were available for analysis from seven studies with 29,531 participants. The pooled analysis showed that NAFLD was significantly associated with a higher likelihood of increased CAC or plaques (OR, 1.40; 95% CI, 1.22‐1.60; P =0.02; I 2 = 59%) (Fig. 4).
Figure 4.
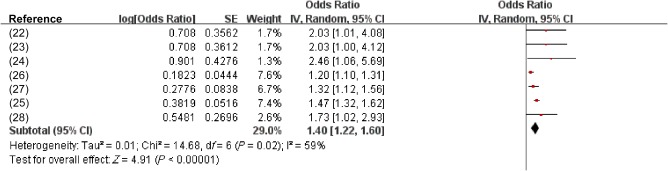
Effect estimate for the association between NAFLD and increased CAC or plaques using a random‐effects model. Forest plot comparison of individuals without NAFLD versus patients with NAFLD. Red squares represent the OR, horizontal lines the CIs, black diamond represent the pooled OR.
Four observational studies with 43,169 subjects were involved for AS. Compared with the non‐NAFLD group, the presence of NAFLD was significantly associated with a higher likelihood of AS (OR,1.56; CI, 1.24‐1.96). Potential heterogeneity across studies was observed (I 2 = 65%; P = 0.03) (Fig. 5).
Figure 5.

Effect estimate for the association between NAFLD and increased AS using a random‐effects model. Forest plot comparison of individuals without NAFLD versus patients with NAFLD. Red squares represent the OR, horizontal lines the CIs, black diamond represent the pooled OR.
We found three studies referring to FMD with available data from 426 participants. The likelihood of impaired FMD was higher in the NAFLD group compared to the non‐NAFLD group (OR, 3.73; 95% CI, 0.99‐14.09) with substantial heterogeneity (I 2 = 67%) (Fig. 6).
Figure 6.

Effect estimate for the association between NAFLD and endothelial dysfunction (flow‐mediated vasodilation) using a random‐effects model. Forest plot comparison of individuals without NAFLD versus patients with NAFLD. Red squares represent the OR, horizontal lines the CIs, black diamond represent the pooled OR.
Discussion
We revealed a close link between NAFLD and subclinical atherosclerosis in this systematic review and meta‐analysis based on 26 observational studies involving a total of 85,395 participants and 29,493 NAFLD cases. Notably, subgroup analysis yielded a consistent result in the four different methods of CIMT/carotid plaques, CAC or plaques, AS, and FMD, albeit with substantial heterogeneity and potential publication bias. These findings are in agreement with a previous meta‐analysis assessing the correlation between NAFLD and pathologic CIMT (OR, 2.04; 95% CI, 1.65‐2.51) and carotid plaques (OR, 2.82; 95% CI, 1.87‐4.27).7 This is in contrast to patients with diabetes; however, no significant association was found between hepatic steatosis and CIMT/carotid calcium.36, 37, 38 Despite these controversial findings, our results are in line with a majority of epidemiologic studies demonstrating that NAFLD is closely associated with increased CIMT, AS, CAC score, and impaired endothelial function.39 This association is more prominent and significant in increased CIMT/plaque than other indicators of subclinical atherosclerosis. Although several meta‐analyses previously evaluated the association between NAFLD and individual indexes of subclinical atherosclerosis (CIMT, carotid plaque, and CAC), none provided a comprehensive determination with respect to carotid atherosclerosis, AS, CAC, or endothelial dysfunction.
CIMT, assessed by B‐mode ultrasound, may be used as a radiologic modality to demonstrate the presence and progression of subclinical atherosclerosis.40 Carotid atherosclerosis serves as a mirror of the generalized atherosclerotic burden, reflecting the probability of the presence of atherosclerotic lesions and structural abnormalities in other arteries.11 It correlates to the total number of vascular risk factors linearly and the prevalence and incidence of myocardial infarction and stroke and could facilitate early discrimination of patients likely to benefit from aggressive preventive measures.
AS is a reliable parameter of atherosclerotic vascular damage and early structural and functional arterial wall alteration, including high central pulse pressure, arterial remodeling, fat accumulation, inflammation, progression of stenosis, and plaques.41 Increasing systolic and pulse pressure is evaluated by measuring pulse wave velocity between two sites in the arterial tree, with a higher index indicating stiffer arteries; increasing epidemiologic evidence has suggested its significant association with CVD and NAFLD.29 As a low‐cost feasible method, the prognostic value of AS for cardiovascular events is well accepted in routine clinical practice, even for individuals who are asymptomatic.
The CAC score is validated as an excellent marker for the presence and instability of atherosclerotic plaque. As the predominant pathologic substrate of CVD, a vulnerable plaque is potentially related to myocardial infarction and cardiac death. Considering that the CAC score could indirectly reflect the total plaque burden, it is not surprising that a high CAC score is an independent predictor of CVD events after accounting for conventional risk factors.42 Previous studies suggested a linear relationship between the extent of the CAC score and all‐cause mortality, thus improving the sensitivity to predict severe coronary atherosclerosis if combined with the Framingham score.43
Endothelial dysfunction is a systemic pathologic state contributing to an imbalance between vasodilative and vasoconstrictive substances. FMD is assessed by high‐resolution ultrasonography, reflecting coronary endothelium‐dependent vasodilator function.44 An impaired FMD response indicates early functional and structural changes in the vascular endothelium. It plays an important part in the pathogenesis and clinical manifestations of atherosclerosis, yielding additive prognostic information of long‐term overt CVD events, even in a low‐risk population. Recent studies have shown that patients with NAFLD were significantly linked to endothelial dysfunction, which is potentially responsible for CVD in the long term.32, 34
The current understanding of the pathophysiology of NAFLD is based on the “multiple‐hits hypothesis.” The first hit initiates from simple steatosis as a consequence of insulin resistance and excessive lipid accumulation in hepatocytes. The second hit is the process involving oxidative stress and alteration of adipokines, contributing to the pathogenesis of NASH. The hepatocytes are then susceptible to multiple overwhelming insults, which lead to progressive liver disease, such as cirrhosis, liver failure, and ultimately hepatocellular carcinoma.45 Accumulating evidence suggests that NAFLD is not merely affected by insulin resistance but also could act as a stimulus for further insulin resistance and metabolic syndrome in turn, thus paving the path for the development and progression of atherosclerosis and overt CVD events.46
The biological mechanisms underlying the correlation between NAFLD and atherosclerosis remain to be elucidated. NASH and atherosclerosis were suggested as two aspects of a shared disease with a common etiology involving metabolic and inflammatory factors.47 However, overwhelming evidence suggests that NAFLD is unlikely to be an innocent bystander in the progression of atherosclerosis. Instead, the proatherogenic effect of NAFLD is implicated in the interplay between insulin resistance, abnormal lipoprotein metabolism, chronic low‐grade inflammation, excessive oxidative stress, and decreased adiponectin concentrations. Particularly, NAFLD renders a higher oxidative inflammatory response in the arterial wall, which may cause endothelial dysfunction and AS.30 In addition, plasminogen activator inhibitor‐1 and angiotensin II could exert a proatherogenic effect on blood vessels in patients with NAFLD. Further research is required to gain more insights into the crosstalk between NAFLD and atherosclerotic CVD.
Our meta‐analysis should be interpreted in view of certain limitations. First, the majority of the included studies in this analysis are observational rather than prospective studies, and this might affect the validity of the overall results. In particular, the cross‐sectional design fails to identify causal or temporal relationships between NAFLD and the development of subclinical atherosclerosis. Furthermore, data referring to the assessment of subclinical atherosclerosis from cross‐sectional studies and prospective cohort studies might convey different information. Specifically, cross‐sectional studies were more likely to be a single point on a subclinical atherosclerosis versus time curve, whereas the latter were more likely to correlate with the slope of that curve. Thus, processing progression data separately from cross‐sectional data would provide more useful information if more prospective cohort studies are available in the future. Given the hospital‐based nature of most included studies, it is also inevitably prone to selection bias and might lead to an overestimate of the effect. Second, the diagnostic criteria of subclinical atherosclerosis and the definition for NAFLD in each included study are not unified, and this might be responsible for the inevitable clinical heterogeneity in this meta‐analysis. Specifically, ultrasonography and computed tomography are the most common modalities for diagnosing NAFLD in the included studies, and this could lead to potential false‐negative results. Although ultrasonography is recommended as the first‐line noninvasive tool for diagnosis of NAFLD, it is less sensitive (60%‐90%) when hepatic fat infiltration is below approximately 30%.48 Due to invasiveness and ethical considerations, liver biopsy is still infrequently performed in clinical practice even though it is the gold standard for evaluating the severity of NAFLD. Third, the presence of heterogeneity might restrict the interpretation of the effect estimates and give misleading results that might have been used in the random‐effects model. The diversity in study design, surrogates of subclinical atherosclerosis, and ethnic and population characteristics are potentially responsible for a substantial heterogeneity across studies. Additionally, we were not able to exclude potential residual confounders, such as a history of medications, socioeconomic status, and lifestyle. Finally, possible publication bias exists according to the asymmetric funnel plot. Thus, the findings provided by this meta‐analysis should be interpreted with caution.
Taken together, the present study demonstrates an independent correlation between NAFLD and subclinical atherosclerosis. The clinical implication of our results might consider patients with NAFLD to be recognized as at a high risk of atherosclerotic CVD. Awareness of the association is important for clinicians when various indices accounting for subclinical atherosclerosis are feasible in clinical practice. Future studies are still needed to further elucidate whether the association is derived from an overlapping etiology or if NAFLD contributes to a substantial risk of atherosclerosis progression. Hence, patients with NAFLD may benefit from early evaluation of atherosclerosis, thus facilitating prediction of CVD morbidity and mortality, risk stratification for appropriate intervention, and improvement of long‐term clinical outcomes.
Author names in bold designate shared co‐first authorship.
Potential conflict of interest: Nothing to report.
Supported by grants from the National Natural Science Foundation of China (81500665), Scientific Research Foundation of Wenzhou (Y20160223), High Level Creative Talents from the Department of Public Health in Zhejiang Province, and Project of New Century 551 Talent Nurturing in Wenzhou.
Contributor Information
Shen‐Wen Fu, Email: 476654976@qq.com.
Ming‐Hua Zheng, Email: zhengmh@wmu.edu.cn.
REFERENCES
- 1. Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology 2007;46:582‐589. [DOI] [PubMed] [Google Scholar]
- 2. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274‐285. [DOI] [PubMed] [Google Scholar]
- 3. Bhatia LS, Curzen NP, Calder PC, Byrne CD. Non‐alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur Heart J 2012;33:1190‐1200. [DOI] [PubMed] [Google Scholar]
- 4. Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population‐based cohort study. Gastroenterology 2005;129:113‐121. [DOI] [PubMed] [Google Scholar]
- 5. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non‐alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta‐analysis. J Hepatol 2016;65:589‐600. [DOI] [PubMed] [Google Scholar]
- 6. Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non‐alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia 2008;51:1947‐1953. [DOI] [PubMed] [Google Scholar]
- 7. Ampuero J, Gallego‐Durán R, Romero‐Gómez M. Association of NAFLD with subclinical atherosclerosis and coronary‐artery disease: meta‐analysis. Rev Esp Enferm Dig 2015;107:10‐16. [PubMed] [Google Scholar]
- 8. Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta‐analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg 2011;39:91‐92. [DOI] [PubMed] [Google Scholar]
- 9. Review Manger (RevMan) [computer program]. Version 5.3. Copenhagen, Denmark: The Nordic Cochrane Center, The Cochrane Collaboration; 2014. [Google Scholar]
- 10. Agarwal AK, Jain V, Singla S, Baruah BP, Arya V, Yadav R, et al. Prevalence of non‐alcoholic fatty liver disease and its correlation with coronary risk factors in patients with type 2 diabetes. J Assoc Physicians India 2011;59:351‐354. [PubMed] [Google Scholar]
- 11. Aygun C, Kocaman O, Sahin T, Uraz S, Eminler AT, Celebi A, et al. Evaluation of metabolic syndrome frequency and carotid artery intima‐media thickness as risk factors for atherosclerosis in patients with nonalcoholic fatty liver disease. Dig Dis Sci 2008;53:1352‐1357. [DOI] [PubMed] [Google Scholar]
- 12. Brea A, Mosquera D, Martín E, Arizti A, Cordero JL, Ros E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case‐control study. Arterioscler Thromb Vasc Biol 2005;25:1045‐1050. [DOI] [PubMed] [Google Scholar]
- 13. Fracanzani AL, Burdick L, Raselli S, Pedotti P, Grigore L, Santorelli G, et al. Carotid artery intima‐media thickness in nonalcoholic fatty liver disease. Am J Med 2008;121:72‐78. [DOI] [PubMed] [Google Scholar]
- 14. Kang JH, Cho KI, Kim SM, Lee JY, Kim JJ, Goo JJ, et al. Relationship between nonalcoholic fatty liver disease and carotid artery atherosclerosis beyond metabolic disorders in non‐diabetic patients. J Cardiovasc Ultrasound 2012;20:126‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim HC, Kim DJ, Huh KB. Association between nonalcoholic fatty liver disease and carotid intima‐media thickness according to the presence of metabolic syndrome. Atherosclerosis 2009;204:521‐525. [DOI] [PubMed] [Google Scholar]
- 16. Lankarani KB, Mahmoodi M, Lotfi M, Zamiri N, Heydari ST, Ghaffarpasand F, et al. Common carotid intima‐media thickness in patients with non‐alcoholic fatty liver disease: a population‐based case‐control study. Korean J Gastroenterol 2013;62:344‐351. [DOI] [PubMed] [Google Scholar]
- 17. Pacifico L, Anania C, Martino F, Cantisani V, Pascone R, Marcantonio A, et al. Functional and morphological vascular changes in pediatric nonalcoholic fatty liver disease. Hepatology 2010;52:1643‐1651. [DOI] [PubMed] [Google Scholar]
- 18. Pacifico L, Bonci E, Andreoli G, Romaggioli S, Di Miscio R, Lombardo CV, et al. Association of serum triglyceride‐to‐HDL cholesterol ratio with carotid artery intima‐media thickness, insulin resistance and nonalcoholic fatty liver disease in children and adolescents. Nutr Metab Cardiovasc Dis 2014;24:737‐743. [DOI] [PubMed] [Google Scholar]
- 19. Puig J, Blasco G, Daunis‐I‐Estadella J, Loshuertos E, Codina J, Cuba V, et al. Nonalcoholic fatty liver disease and age are strong indicators for atherosclerosis in morbid obesity. Clin Endocrinol (Oxf) 2015;83:180‐186. [DOI] [PubMed] [Google Scholar]
- 20. Ramilli S, Pretolani S, Muscari A, Pacelli B, Arienti V. Carotid lesions in outpatients with nonalcoholic fatty liver disease. World J Gastroenterol 2009;15:4770‐4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sinn DH, Cho SJ, Gu S, Seong D, Kang D, Kim H, et al. Persistent nonalcoholic fatty liver disease increases risk for carotid atherosclerosis. Gastroenterology 2016;151:481‐488.e1. [DOI] [PubMed] [Google Scholar]
- 22. Akabame S, Hamaguchi M, Tomiyasu K, Tanaka M, Kobayashi‐Takenaka Y, Nakano K, et al. Alcoholic fatty liver disease (NAFLD) by 64‐detector multislice computed tomography (MSCT). Circ J 2008;72:618‐625. [DOI] [PubMed] [Google Scholar]
- 23. Assy N, Djibre A, Farah R, Grosovski M, Marmor A. Presence of coronary plaques in patients with nonalcoholic fatty liver disease. Radiology 2010;254:393‐400. [DOI] [PubMed] [Google Scholar]
- 24. Chen CH, Nien CK, Yang CC, Yeh YH. Association between nonalcoholic fatty liver disease and coronary artery calcification. Dig Dis Sci 2010;55:1752‐1760. [DOI] [PubMed] [Google Scholar]
- 25. Mellinger JL, Pencina KM, Massaro JM, Hoffmann U, Seshadri S, Fox CS, O'Donnell CJ, Speliotes EK: Hepatic steatosis and cardiovascular disease outcomes: An analysis of the Framingham Heart Study. J Hepatol 2015;63:470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim D, Choi SY, Park EH, Lee W, Kang JH, Kim W, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology 2012;56:605‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee MK, Park HJ, Jeon WS, Park SE, Park CY, Lee WY, et al. Higher association of coronary artery calcification with non‐alcoholic fatty liver disease than with abdominal obesity in middle‐aged Korean men: the Kangbuk Samsung Health Study. Cardiovasc Diabetol 2015;14:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Santos RD, Nasir K, Conceição RD, Sarwar A, Carvalho JA, Blumenthal RS. Hepatic steatosis is associated with a greater prevalence of coronary artery calcification in asymptomatic men. Atherosclerosis 2007;194:517‐519. [DOI] [PubMed] [Google Scholar]
- 29. Chung GE, Choi SY, Kim D, Kwak MS, Park HE, Kim MK, et al. Nonalcoholic fatty liver disease as a risk factor of arterial stiffness measured by the cardioankle vascular index. Medicine (Baltimore) 2015;94.e654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee YJ, Shim JY, Moon BS, Shin YH, Jung DH, Lee JH, et al. The relationship between arterial stiffness and nonalcoholic fatty liver disease. Dig Dis Sci 2012;57:196‐203. [DOI] [PubMed] [Google Scholar]
- 31. Luo ZX, Zeng Q, Luo R, Wang Y, Ge Q. Relative contributions of ectopic liver and abdominal fat accumulation to arterial stiffness. Endocr Pract 2015;21:574‐580. [DOI] [PubMed] [Google Scholar]
- 32. Sapmaz F, Uzman M, Basyigit S, Ozkan S, Yavuz B, Yeniova A, et al. Steatosis grade is the most important risk factor for development of endothelial dysfunction in NAFLD. Medicine (Baltimore) 2016;95:e3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thakur ML, Sharma S, Kumar A, Bhatt SP, Luthra K, Guleria R, et al. Nonalcoholic fatty liver disease is associated with subclinical atherosclerosis independent of obesity and metabolic syndrome in Asian Indians. Atherosclerosis 2012;223:507‐511. [DOI] [PubMed] [Google Scholar]
- 34. Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology 2005;42:473‐480. [DOI] [PubMed] [Google Scholar]
- 35. Kim BJ, Kim NH, Kim BS, Kang JH. The association between nonalcoholic fatty liver disease, metabolic syndrome and arterial stiffness in nondiabetic, nonhypertensive individuals. Cardiology 2012;123:54‐61. [DOI] [PubMed] [Google Scholar]
- 36. McKimmie RL, Daniel KR, Carr JJ, Bowden DW, Freedman BI, Register TC, et al. Hepatic steatosis and subclinical cardiovascular disease in a cohort enriched for type 2 diabetes: the Diabetes Heart Study. Am J Gastroenterol 2008;103:3029‐3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Petit JM, Guiu B, Terriat B, Loffroy R, Robin I, Petit V, et al. Nonalcoholic fatty liver is not associated with carotid intima‐media thickness in type 2 diabetic patients. J Clin Endocrinol Metab 2009;94:4103‐4106. [DOI] [PubMed] [Google Scholar]
- 38. Guo K, Zhang L, Lu J, Yu H, Wu M, Bao Y, et al. Non‐alcoholic fatty liver disease is associated with late but not early atherosclerotic lesions in Chinese inpatients with type 2 diabetes. J Diabetes Complications 2017;31:80‐85. [DOI] [PubMed] [Google Scholar]
- 39. Oni ET, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis 2013;230:258‐267. [DOI] [PubMed] [Google Scholar]
- 40. Baldassarre D, Amato M, Bondioli A, Sirtori CR, Tremoli E. Carotid artery intima‐media thickness measured by ultrasonography in normal clinical practice correlates well with atherosclerosis risk factors. Stroke 2000;31:2426‐2430. [DOI] [PubMed] [Google Scholar]
- 41. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al.; European Network for Non‐invasive Investigation of Large Arteries . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006;27:2588‐2605. [DOI] [PubMed] [Google Scholar]
- 42. Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O'Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three‐year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol 2005;46:807‐814. [DOI] [PubMed] [Google Scholar]
- 43. Burke AP, Taylor A, Farb A, Malcom GT, Virmani R. Coronary calcification: insights from sudden coronary death victims. Z Kardiol 2000;89(Suppl. 2):49‐53. [DOI] [PubMed] [Google Scholar]
- 44. Moens AL, Goovaerts I, Claeys MJ, Vrints CJ. Flow‐mediated vasodilation: a diagnostic instrument, or an experimental tool? Chest 2005;127:2254‐2263. [DOI] [PubMed] [Google Scholar]
- 45. Yilmaz Y. Review article: is non‐alcoholic fatty liver disease a spectrum, or are steatosis and non‐alcoholic steatohepatitis distinct conditions? Aliment Pharmacol Ther 2012;36:815‐823. [DOI] [PubMed] [Google Scholar]
- 46. Gaggini M, Morelli M, Buzzigoli E, DeFronzo R, Bugianesi E, Gastaldelli A. Non‐alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients 2013;5:1544‐1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bieghs V, Rensen PC, Hofker MH, Shiri‐Sverdlov R. NASH and atherosclerosis are two aspects of a shared disease: central role for macrophages. Atherosclerosis 2012;220:287‐293. [DOI] [PubMed] [Google Scholar]
- 48. Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta‐analysis. Hepatology 2011;54:1082‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]


