Abstract
Posthepatectomy liver failure (PHLF) represents the single most important cause of postoperative mortality after major liver resection, yet no effective treatment option is available. Extracorporeal liver support devices might be helpful, but systematic studies are lacking. Accordingly, we aimed to assess the safety and feasibility of the Molecular Adsorbent Recirculating System (MARS) in patients with PHLF. Between December 2012 and May 2015, a total of 206 patients underwent major or extended hepatectomy, and 10 consecutive patients with PHLF (according to the Balzan 50:50 criteria) were enrolled into the study. MARS treatment was initiated on postoperative day 5‐7, and five to seven consecutive treatment sessions were completed for each patient. In total, 59 MARS cycles were implemented, and MARS was initiated and completed without major complications in any patient. However, 1 patient developed an immense asymptomatic hyperbilirubinemia (without encephalopathy), 1 had repeated clotting problems in the MARS filter, and 2 patients experienced access problems with the central venous line. Otherwise, no adverse events were observed. In 9 patients, the bilirubin level and international normalized ratio decreased significantly (P < 0.05) during MARS treatment. The 60‐ and 90‐day mortality was 0% and 10%, respectively. Among the 9 survivors, 4 still had liver dysfunction at 90 days postoperatively. Five patients were alive 1 year postoperatively without any signs of liver dysfunction or disease recurrence. Conclusion: The use of MARS in PHLF is feasible and safe and improves liver function in patients with PHLF. In the present study, 60‐ and 90‐day mortality rates were unexpectedly low compared to a historical control group. The impact of MARS treatment on mortality in PHLF should be further evaluated in a randomized controlled clinical trial. (Hepatology Communications 2018;2:445‐454)
Abbreviations
- ACLF
acute on chronic liver failure
- ALF
acute liver failure
- BW
body weight
- CT
computed tomography
- ERCP
endoscopic retrograde cholangiopancreatography
- FLR
future liver remnant
- ICU
intensive care unit
- INR
international normalized ratio
- IQR
interquartile range
- MARS
Molecular Adsorbent Recirculating System
- MELD
Model for End‐Stage Liver Disease
- PHLF
posthepatectomy liver failure
- POD
postoperative day
Introduction
Partial hepatectomies for primary and secondary malignant liver tumors are performed with low mortality risk and good long‐term results at expert centers.1, 2 However, posthepatectomy liver failure (PHLF) still represents the leading single cause of postoperative morbidity and mortality.3, 4, 5 Although substantial advances have been made in all stages of operative care, treatment of PHLF, once it has occurred, remains limited to symptomatic treatment.6, 7 Therefore, there is a need to evaluate new treatment modalities in PHLF, such as extracorporeal liver support devices.
In recent decades, several techniques have been developed to support patients with both acute liver failure (ALF) and acute‐on‐chronic liver failure (ACLF).8 In this context, the Molecular Adsorbent Recirculating System (MARS) has emerged as a promising tool due to its capacity to remove water‐soluble and albumin‐bound toxins and to improve several clinically relevant hemodynamic and biochemical parameters in patients suffering from ALF or ACLF.9, 10, 11, 12, 13 Large, randomized, controlled trials did, however, fail to demonstrate a statistically significant survival benefit.14, 15 In the PHLF situation, only a few single‐center experiences have addressed the use of MARS as a rescue treatment option,16, 17, 18, 19, 20 and all of them suffer from heterogeneous patient groups and a lack of standardized treatment protocols. Recently, we reported our retrospective experience with MARS for PHLF at two tertiary referral centers where we found a trend toward improved 90‐day and long‐term survival in patients who received several MARS treatments early in the postoperative course.21 Based on these observations, we designed a prospective phase I study to evaluate the safety and feasibility together with the impact on patient outcome of early MARS treatment in patients with PHLF.
Patients and Methods
STUDY DESIGN
The study was designed as a prospective, single institution, phase I safety and feasibility study. The primary outcome was the safety and feasibility of early MARS treatment in patients suffering from PHLF according to the 50:50 criteria.22 Secondary outcomes were 60‐ and 90‐day mortality as well as impact of MARS treatment on liver regeneration (remnant volume) and function measured by liver‐specific blood samples (total and conjugated bilirubin, transaminases, international normalized ratio [INR], C‐reactive protein, creatinine, ammonia, and full blood cell count). Long‐term and disease‐free survival were assessed for 12 months following surgery.
PATIENT SELECTION AND STUDY INCLUSION
The study was conducted between December 1, 2012, and May 31, 2015, at Karolinska University Hospital, Huddinge, Stockholm, a tertiary referral center. Except for exclusions noted below, all patients with PHLF according to the Balzan (50:50) criteria after major/extended hepatectomy (removal of ≥4 Couinaud segments) were eligible for study enrollment. The applied Balzan criteria for PHLF predict a >50% 60‐day mortality in the case of a total bilirubin >50 μmol/L and a Quick value <50% (equivalent to INR >1.5) on postoperative day (POD) 5. Only patients with primary PHLF (caused by a too small liver remnant or by damage to the liver remnant prior to or during surgery due to, e.g., chemotherapy or bleeding) were eligible for inclusion.
Exclusion criteria were age >80 years, uncontrolled bleeding or sepsis, any relevant and untreated surgical complication (such as mechanical bile duct obstruction, clotting of the hepatic artery, or portal vein thrombosis), and platelet count of <20 × 109/L. Patients with secondary PHLF (caused by any postoperative complication leading to PHLF later than POD 5) were not included in the study. Patients at risk were screened postoperatively and offered participation in the study when the inclusion criteria were fulfilled on POD 5 in the absence of exclusion criteria. Informed consent was obtained either from the patients or, in case of inability, from the closest relatives or legal representative. In order to validate the inclusion criteria, a historical control group was analyzed between January 1, 2010, and November 30, 2012, consisting of all patients who underwent major or extended hepatectomy at Karolinska University Hospital, Huddinge. The study was reviewed and approved by the regional ethical board, Stockholm (DNr 2010/1872‐31/2; DNr 2013/149‐31/2) and was registered at https://clinicaltrials.gov/ (NCT03011424) and performed according to the Helsinki Declaration.
MARS AND INTENSIVE CARE UNIT TREATMENT
MARS treatment was performed using a double lumen catheter inserted into the internal jugular or femoral vein. A standard continuous renal replacement therapy (MultiFiltrate; Fresenius Medical Care AG, Bad Homburg, Germany) system was used to run the MARS monitor (Baxter, Lund, Sweden). The blood flow on the MultiFiltrate machine was adjusted to 90‐150 mL/minute, and the albumin flow on the MARS monitor was set to 150 mL/minute. Dialysate and replacement fluid flow was set to receive a renal dialysis dose of 35 mL/kg/hour. Anticoagulation was achieved by local anticoagulation of the MARS circuit with citrate as described.23 Every session was planned to last between 6 and 12 hours. Intensive care unit (ICU) treatment was standardized prior to study onset and performed in accordance with the guidelines for treatment of acute liver failure, including renal hemodialysis (continuous veno‐venous hemofiltration) along with MARS treatment, mechanical ventilation, drainage of fluid collections, directed treatment with antibiotics and antifungals, and parenteral nutrition if needed, as reviewed elsewhere.24
EVALUATION OF THE SAFETY, FEASIBILITY, AND EFFICACY OF MARS TREATMENT
Prior to the index MARS session, blood samples were obtained and radiology was performed if appropriate to exclude significant surgery‐related complications. The Model for End‐Stage Liver Disease (MELD) score was calculated before the first and after the last MARS treatment. Encephalopathy was graded according to the West Haven criteria25 and repeated after each MARS treatment. Blood samples were obtained before and after every MARS session and during the clinical follow‐up as clinically indicated. Blood pressure, heart rate, and oxygen saturation were monitored continuously at the ICU.
Clinical evaluation, including documentation of adverse events, was performed before and after each MARS session. Adverse events were considered severe in the event that clinically significant complications occurred (the adverse event changed patient management, the patient required additional hospital care, the patient become permanently disabled, or the adverse event was considered to be life threatening). We assessed the following as primary safety outcomes of MARS treatment:
Bleeding complication and the need for blood transfusions.
Platelet count (termination of MARS or transfusion below 20 × 109/L).
Severe electrolyte or acid‐base derangements deemed secondary to local citrate anticoagulation of the MARS circuit resulting in early termination of MARS treatment.
Surgery‐related complications were classified according to the Clavien‐Dindo classification.26 Feasibility was mainly assessed by the number of screened patients eligible for study inclusion compared to the number finally included and treated according to the study protocol. We also assessed the number of patients not completing the planned treatment due to reasons other than safety reasons (logistical or technical reasons).
VOLUMETRIC ASSESSMENT
All patients underwent preoperative evaluation using four‐phase contrast‐enhanced computed tomography (CT) or liver‐specific magnetic resonance imaging. Future liver remnant (FLR) to body weight (BW) ratio and standardized FLR were calculated based on these investigations according to described formulas.27 CT was performed postoperatively based on clinical indications. All but 1 (patient number 1) patient underwent at least one CT scan between POD 5 and POD 49. Liver volumes before and after operation were calculated using the software Volume Viewer (Voxtool 11) for AW Volume Share 5 implemented on an AW Workstation (GE Healthcare, Fairfield, CT) as described elsewhere.28
DATA AND STATISTICAL ANALYSIS
Patient data were collected prospectively from the time point of inclusion (POD 5). Complementary data before POD 5 were collected retrospectively from the individual in‐hospital patient files. Categorical data were expressed as frequencies with percentages, and continuous variables were represented as medians with interquartile range (IQR). The Wilcoxon signed rank test was performed to assess paired nonparametric data (blood samples and MELD score), and the significance level was set at P < 0.05. One patient (patient number 8) was considered to be an outlier and was excluded from statistical analysis. Statistical analysis was performed using SPSS software version 24.0.0.0.
Results
During the study period, from December 1, 2012, to May 31, 2015, 206 patients underwent major/extended hepatectomy at Karolinska University Hospital. Patients at risk (already fulfilling the 50:50 criteria or still increasing bilirubin and INR) were screened on POD 3 and 4. Fourteen patients fulfilled the inclusion criteria on POD 5. Two patients could not be included due to a lack of resources at the local ICU; 1 was unable to give informed consent due to language problems and 1 patient underwent multivisceral resection and was excluded due to uncontrolled sepsis. The remaining 10 patients (6 male patients, 4 female patients) with a median age of 69 years (range 49‐77) were included and treated according to the protocol (Fig. 1). These 10 patients were able to start MARS treatment within 7 days from surgery and thereby met the primary feasibility outcome. MARS treatment had to be interrupted in 2 patients (for 2 and 3 days) due to logistical reasons at the ICU. According to the treatment protocol, the interruption was not a protocol violation and did not influence feasibility. All patients received a minimum of five completed MARS sessions within 8 days from treatment onset. In 1 patient, clotting of the MARS filter occurred once and treatment had to be restarted with a new filter.
Figure 1.
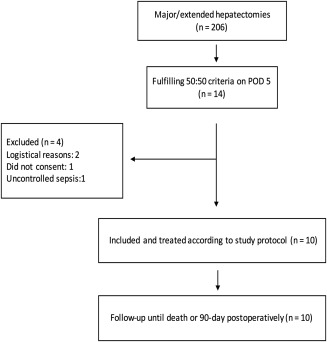
Study flow chart.
The indications for hepatectomy were colorectal cancer liver metastases (5 patients), perihilar cholangiocarcinoma (4 patients), and hepatocellular cancer (1 patient). The demographic data are detailed in Table 1. Preoperative volumetric analysis of the FLR revealed a mean FLR/BW ratio of 0.55 (IQR, 0.25) and a mean standardized FLR of 26.1% (IQR, 11.3%). Two patients underwent preoperative portal venous embolization and increased their FLR by 28% and 24%. Although the postoperative assessment of the FLR was obtained at various time points, we observed a substantial increase in liver volume over the first 25 postoperative days. Additional data regarding volumetric measurements are shown in Table 2.
Table 1.
Patient Characteristics of the Study Population
| Diabetes | CVD | Smoker | Cx | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | Age | Sex | BMI | 0 = no; 1 = yes | 0 = no; 1 = yes | 0 = no; 1 = yes | Indikation | 0 = no; 1 = yes | Surgery |
| 1 | 71 | m | 24 | 0 | 0 | 0 | CCC | 0 | Extended right hepatectomy |
| 2 | 75 | f | 31 | 0 | 0 | 1 | CRLM | 1 | Extended right hepatectomy |
| 3 | 65 | f | 21 | 1 | 0 | 0 | CCC | 0 | Extended right hepatectomy |
| 4 | 68 | m | 23 | 0 | 1 | 0 | CCC | 0 | Extended right hepatectomy |
| 5 | 66 | f | 23 | 1 | 1 | 0 | CCC | 0 | Extended right hepatectomy |
| 6 | 77 | f | 20 | 0 | 0 | 0 | CRLM | 1 | Right hepatectomy + seg 1 |
| 7 | 72 | m | 25 | 0 | 1 | 0 | HCC | 0 | Extended right hepatectomy |
| 8 | 57 | m | 31 | 0 | 0 | 0 | CRLM | 1 | Right hepatectomy + local seg 4 |
| 9 | 72 | m | 29 | 0 | 0 | 0 | CRLM | 0 | Right hepatectomy + local seg 4 |
| 10 | 49 | m | 26 | 0 | 0 | 0 | CRLM | 1 | Extended right hepatectomy |
| median (IQR) | 69,5 (10) | 24,5 (7) | |||||||
Abbreviations: BMI, body mass index; CCC, cholangiocarcinoma; CVD, cardio‐vascular disease; CRLM, colorectal liver metastasis; CVD, cardiovascular disease; Cx, neoadjuvant chemotherapy; f, female; HCC, hepatocellular carcinoma; m, male; PVE, portalvein embolisation; seg, liver segment.
Table 2.
Volumetric Measurements
| FLR | sFLR | Liver dysfunction** | TELV | Specimen | Postop | Increase postop | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient | FLR segments | preop mL | FLR/BWpreop | preop % | 0 = no; 1 = yes | pre op (calculated) | weight grams | Postop CT | volume mL | volume mL/day |
| 1 | 1‐3 | 140 | 0.22 | 10.2 | 0 | 1371 | 1117 | POD 94 | 730 | 6.3 |
| 2 | 1‐3 | 480 | 0.58 | 28.8 | 0 | 1665 | 1305 | POD 23 | 897 | 18.1 |
| 3 | 1‐3 | 190 | 0.33 | 14.9 | 0 | 1271 | 766 | POD 25 | 804 | 24.6 |
| 4 | 2‐3 | 250/320* | 0.45 | 20.4 | 1 | 1568 | 950 | POD 12 | 803 | 40.3 |
| 5 | 1‐3 | 290/360* | 0.62 | 28.8 | 1 | 1248 | 1295 | POD 49 | 945 | 119 |
| 6 | 2‐4 | 295 | 0.58 | 26.5 | 0 | 1114 | 726 | POD 5 | 488 | 38.6 |
| 7 | 2‐3 | 840 | 0.88 | 41.4 | 0 | 2028 | 1983 | POD 5 | 1388 | 109.6 |
| 8 | 1‐4 | 510 | 0.52 | 25.7 | 1 | 1983 | 1000 | POD 24 | 1525 | 42.3 |
| 9 | 1‐4 | 370 | 0.38 | 18.4 | 1 | 2011 | 1049 | POD 15 | 910 | 36 |
| 10 | 1‐3 | 535 | 0.61 | 28.3 | 1 | 1893 | 1320 | POD 23 | 1084 | 23.9 |
*pre/post portal venous embolization; ** persistent liver dysfunction on POD 90
Abbreviations: BW, body weight; CT, computed tomography; FLR, future liver remnant; preop, preoperatively; postop, postoperatively; sFLR, standardized future liver remnant; TELV, total estimated liver volume.
Two patients experienced severe postoperative complications (Clavien‐Dindo ≥3b); 1 patient required re‐operation for hemorrhage and 1 required endoscopic retrograde cholangiopancreatography (ERCP) for common bile duct stenting. Further details regarding histopathology and intra‐operative/postoperative complications are shown in Table 3. Following the operation, all patients were transferred to our Intermediate Care Unit, and none of the included patients were initially in need of treatment at the ICU. On POD 5, the median bilirubin was 98 μmol/L (IQR, 65) and the median INR was 1.8 (IQR, 0.43). In total, 59 consecutive MARS sessions were completed. Every treatment cycle comprised a minimum of three consecutive MARS sessions (12 sessions in total). Details regarding MARS and ICU treatments are presented in Table 4.
Table 3.
Histopathology and Complications
| Inflammation | Fibrosis | Steatosis | Specimen weight | |||
|---|---|---|---|---|---|---|
| Patient | Pathology | grade | grade | grade | grams | Complication intra‐/postoperatively |
| 1 | GBC, T3 N1 R1 | 1 | 2 | 2 | 1117 | Postop bleeding, re‐op POD 4 |
| 2 | CRLM, focal R1 | 0 | 2 | 3 | 1305 | HE |
| 3 | IG4 cholangitis | 0 | 2 | 0 | 766 | Bile leakage, conservative treatment, HE |
| 4 | CCC, T2b N2 R1 | 1 | 1 | 1 | 950 | Ascites, sepsis |
| 5 | GBC, T3 N0 R1 | 1 | 3 | 0 | 1295 | HE |
| 6 | CRLM, focal R1 | 0 | 1 | 2 | 726 | HE, systemic infection |
| 7 | HCC, T3b V1 R0 | 1 | 2 | 2 | 1983 | HE, bile leakage, conservative treatment |
| 8 | CRLM, focal R1 | 1 | 2 | 2 | 1000 | Intra‐op injury of the left bileduct, ERCP, Stent |
| 9 | CRLM, focal R1 | 0 | 1 | 2 | 1049 | None |
| 10 | CRLM, focal R1 | 0 | 1 | 1 | 1320 | Intraop bleeding, ascites |
Pathology grading according to the latest tumor related TNM classification (T=size of primary tumor, N=lymph nodes, R=resection margins, V=invasion into vein).
Abbreviations: CCC, cholangiocarcinoma; CRCm, colo‐rectal liver metastasis; CRLM, colorectal liver metastasis; ERCP, endoscopic retrograde cholangio‐pancreatography; GBC, gallbladder cancer; HCC, hepatocellular carcinoma; HE, hepatic encephalopathy (Westhaven grade 2 or higher); IG4, Immunoglobulin G4‐related cholangitis.
Table 4.
ICU and MARS Treatment
| MELD | MELD | MARS | CRRT/ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | SAPS Score | before MARS | after MARS | MARS start | MARS end | MARSsessions | pause days | renal failure | Mechanical ventilation |
| 1 | 80 | 18 | 22 | POD 7 | POD 14 | 7 (4+3) | 2 | 1 | 0 |
| 2 | 67 | 23 | 18 | POD 6 | POD 12 | 5 | 0 | 0 | 0 |
| 3 | 72 | 19 | 12 | POD 5 | POD 12 | 5 | 0 | 0 | 0 |
| 4 | 60 | 22 | 24 | POD 7 | POD 17 | 7 (3+4) | 3 | 0 | 0 |
| 5 | 67 | 21 | 20 | POD 6 | POD 13 | 7 | 0 | 1 | 0 |
| 6 | 72 | 20 | 14 | POD 6 | POD 11 | 5 | 0 | 0 | 0 |
| 7 | 91 | 20 | 13 | POD 5 | POD 10 | 5 | 0 | 0 | 3 days |
| 8 | 61 | 24 | 31 | POD 7 | POD 13 | 7 | 0 | 1 | 0 |
| 9 | 61 | 19 | 17 | POD 6 | POD 10 | 5 | 0 | 0 | 0 |
| 10 | 68 | 21 | 16 | POD 7 | POD 12 | 5 | 0 | 0 | 0 |
| median (IQR) | 67,5 (13) | 21 (5) | 19 (9) |
Abbreviations: CRRT, continuous renal replacement therapy; MELD, model for end‐stage liver disease; POD, post‐operative day; SAPS, simplified acute physiology score.
We observed no major complications or mortality related to the MARS treatment with respect to safety. According to the main safety outcome measures, MARS was safe in all patients as there was no bleeding, no need to transfuse blood products, and no severe derangement of electrolytes or acid‐base balance during MARS treatment. Two patients had problems with low flow in the central venous line. The central line was replaced 3 times in these 2 patients without procedure‐related complications. One patient (patient number 8) had a marked increase in both total and conjugated bilirubin (peak total bilirubin about 1,000 μmol/L). INR increased as well (range between 2 and 3) without any other signs of severe liver failure. During hepatectomy, this patient suffered from a minimal injury to the left bile duct and was treated with ERCP and stent insertion intra‐operatively. By repeated postoperative investigations, both with radiology (CT, magnetic resonance, and magnetic resonance cholangiopancreatography) and ERCP, any mechanical obstruction of the bile ducts could be excluded. Despite comprehensive discussions about the patient with expert hepatologists, the reason behind this hyperbilirubinemia remained unclear.
Considering patient number 8 as a statistical outlier, bilirubin and INR decreased significantly under MARS treatment (bilirubin P = 0.042; INR P = 0.023) when comparing values before the first and after the last treatment. Changes in creatinine, C‐reactive protein, ammonia, and platelets were not significant (Fig. 2). The median MELD score was 21 (IQR, 5) before MARS and 19 (IQR, 7) after MARS, and the difference was not significant. In 4 patients, hepatic encephalopathy was observed at ICU admittance (Westhaven grade II or higher). Initially, 1 patient required mechanical ventilation due to encephalopathy and respiratory failure but recovered after 3 days of MARS treatment. In the other 3 patients, MARS treatment led to clinical improvement after the first two treatment cycles and parenteral nutrition and mechanical ventilation could be avoided in all 3. In the study population, 60‐day mortality was 0% and 90‐day mortality was 1/10 (10%). Five patients were alive 1 year after surgery without any signs of liver dysfunction or disease recurrence. The remaining 4 patients died between POD 130 and 348; all had advanced disease recurrence (perihilar cholangiocarcinoma in 1 patient and colorectal liver metastasis in 3 patients), and 3 patients had chronic liver dysfunction. Details of patient outcome are shown in Table 5.
Figure 2.
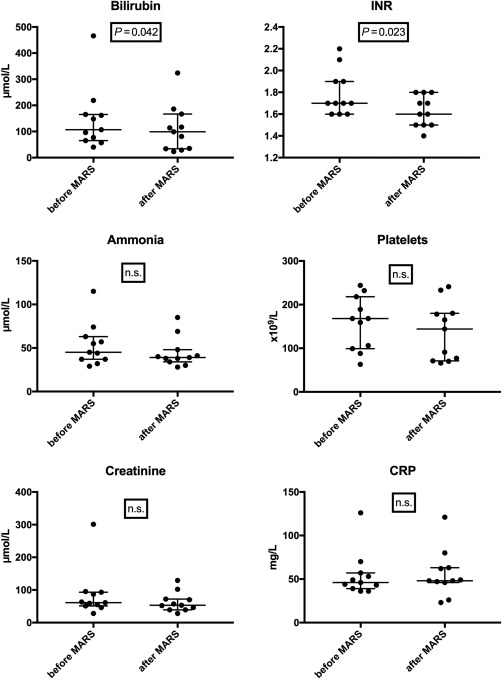
Blood samples before/after MARS treatment. Abbreviation: n.s., not significant.
Table 5.
Patient Outcome and Survival
| Hospital stay/days | Bilirubin | 60‐day mortality | 90‐day mortality | Liver dysfunction** | ||
|---|---|---|---|---|---|---|
| Patient | (until 1. demission) | POD 90mikromol/L | INR POD 90 | 0 = no; 1 = yes | 0 = no; 1 = yes | 0 = no; 1 = yes |
| 1 | 35 | 18 | 1.3 | 0 | 0 | 0 |
| 2 | 46 | 18 | 1 | 0 | 0 | 0 |
| 3 | 35 | 37 | 1.3 | 0 | 0 | 0 |
| 4 | 128 | 427 | 1.4 | 0 | 0 | 1 |
| 5 | 90 | 619 | 2.5 | 0 | 1 | 1 |
| 6 | 20 | n.a. | n.a. | 0 | 0 | 0 |
| 7 | 39 | 353 | 1.2 | 0 | 0 | 1 |
| 8 | 46 | 380 | 1.8 | 0 | 0 | 1 |
| 9 | 24 | 38* | 1.4* | 0 | 0 | 1 |
| 10 | 30 | 24 | 1.5 | 0 | 0 | 0 |
| median (IQR) | 37 (29) | 195 (396) | 1,4 (1,0) |
*on POD 144; †liver dysfunction on POD 90. * = on POD 144; ** = liver dysfunction on POD 90
Abbreviations: n.a., not available; POD, post operative day; until 1. demission, until 1. demission from hospital after index operation.
In order to validate the 50:50 criteria, we analyzed a historical cohort in our own institution. Between January 2010 and November 2012, 248 patients underwent major or extended hepatectomy. In total, 11 patients (4.4%) met the 50:50 criteria. Both the 60‐ and 90‐day mortality rates were 64% (7/11) and thereby in line with the original work of Balzan et al.22
Discussion
PHLF continues to be a major threat for patient survival after extended hepatectomy as there is no specific treatment available.29 The present study demonstrates that it is safe and feasible to use MARS for patients with PHLF. In the study population, 60‐ and 90‐day mortality were low compared to the expected mortality according to the 50:50 criteria (>50%); in addition, 50% of all included patients experienced a disease‐free 1‐year survival.
MARS appeared safe and feasible, and we observed no major complications. Problems with the central venous access were not related to PHLF. In addition, clotting of the MARS filter using local citrate anticoagulation of the MARS circuit occurred only once out of all patients as described,23 and we observed no systemic bleeding or clotting complications. However, 1 patient developed a rapid and massive increase in serum bilirubin without any other clinical signs of liver failure (e.g., encephalopathy). Each MARS session resulted in a transient decrease in bilirubin, but this did not prevent a progressive overall increase in bilirubin. A paradoxical increase in bilirubin during MARS treatment has not been described, and the mechanisms in this patient remain obscure.
We identified no major safety issues but did observe some problems regarding feasibility. MARS treatment requires the transfer of patients to the ICU in our hospital. As ICU resources are limited, our patients, who would otherwise not have been in need of ICU treatment, competed with other more diseased patients in need of ICU. MARS was therefore interrupted for 2 and 3 days in 2 patients. Nevertheless, all patients followed the treatment protocol, and just one out of 59 treatment sessions had to be restarted due to problems with the MARS filter. In conclusion, feasibility was good once MARS was initiated.
Extracorporeal liver support has attracted much interest over the past 2 decades as a treatment for acute liver diseases. Despite potentially positive effects on clinical and laboratory parameters, no study has been able to demonstrate a significant survival benefit in ALF15 or ACLF patients.14 A systematic evaluation of MARS has not been undertaken with respect to PHLF. Previous reports consist of small case series with heterogeneous patient cohorts, and all lack standardized treatment protocols.16, 17, 18, 19 To our knowledge, the present study is the first prospective study that systematically investigates the safety and feasibility of MARS for PHLF.
Today, liver regeneration after partial hepatectomy is well understood.30 Risk factors for PHLF are patient‐related factors, such as age and comorbidities; liver‐related factors, such as the quality of liver tissue and, most importantly, the extent of liver resection as a liver remnant that is too small unavoidably leads to PHLF.6 The pathophysiology is different in PHLF 31 compared to ALF 32 or ACLF.33 However, the argument for using MARS in PHLF is that early support of liver function should result in an enhanced regeneration of an otherwise insufficient liver remnant. Several MARS effects might contribute to achieve this goal. First, albumin serves as an important scavenger for reactive oxygen species released from inflammatory cells.34 Oxidative stress was found to severely impair liver regeneration after partial hepatectomy in mice due to a reduction in insulin/ insulin‐like growth factor 1 signaling.35 Thus, detoxification of the circulating albumin might contribute to a reduction in oxidative stress and thereby improve liver regeneration. Second, growth factors are crucial for liver regeneration after hepatectomy,30 and MARS might contribute to improved regeneration through an increase in plasma hepatocyte growth factor as found by Donati et al.10 in selected patients with ALF and ACLF. Third, portal hypertension has been described as a major risk factor for the development of PHLF,36 and MARS treatment has been shown to reduce portal pressure in ACLF patients.9 However, all these potentially beneficial effects need further investigation in PHLF.
The small‐for‐size syndrome represents a major risk factor for the development of PHLF.37 The majority of our patients had an FLR/BW ratio that allowed for safe hepatectomy according to recent recommendations.27, 38 However, 3 patients had a low FLR/BW ratio (<0.4) but initially showed a good response to MARS treatment with improvement of liver function. Two of these patients recovered entirely, and 1 had impaired liver function beyond POD 90. Radiology after MARS treatment was not performed primarily to assess liver volume but was performed based on clinical indications. Therefore, investigations were not carried out at standardized time points, making it difficult to draw concise conclusions about FLR volume increase. However, in all patients who underwent CT in the early post‐MARS phase, we were able to detect a substantial increase in liver volume, albeit with huge interindividual differences. Based on the volumetric findings, we were not able to distinguish between the patients who recovered and those with persistent liver dysfunction on POD 90. These findings illustrate the need for better understanding of the function/volume correlation in liver regeneration as well as the need for better tools to accurately predict PHLF.
Earlier reports of patients with other forms of liver failure suggest that at least three consecutive MARS sessions are needed to achieve optimal treatment outcomes.15, 39, 40 In the present study, we strived to avoid “under treatment” of patients and prescheduled five to seven MARS sessions for each patient. The number of treatment sessions needed to achieve a sufficient regeneration and function of the liver remnant needs to be established in future studies. Because liver regeneration starts immediately after completion of the hepatectomy,30 MARS treatment should possibly start even earlier than POD 5. To date, there are no other predictive tools with good reliability available to delineate those at high risk of developing PHLF earlier than POD 5. Thus, the 50:50 criteria currently represent the best available tool to indicate potentially lethal liver failure.
In our department, MARS treatment required patient care at the ICU, but most of the enrolled patients would otherwise not have met the criteria for treatment at the ICU. The higher level of care experienced by these patients at an ICU might have an overall beneficial effect on patient survival and thus represents a possible bias in the mortality assessment.
In conclusion, the use of MARS in PHLF is feasible and safe. In this study, the 60‐ and 90‐day mortality rates were much lower than expected. However, 5 patients did not regain normal liver function on POD 90. Thus, the final role of MARS in PHLF should be clarified in a randomized controlled clinical trial.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Gilg S, Sparrelid E, Isaksson B, Lundell L, Nowak G, Stromberg C. Mortality‐related risk factors and long‐term survival after 4460 liver resections in Sweden‐a population‐based study. Langenbecks Arch Surg 2017;402:105‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Farges O, Goutte N, Bendersky N, Falissard B. Incidence and risks of liver resection: an all‐inclusive French nationwide study. Ann Surg 2012;256:697‐704. [DOI] [PubMed] [Google Scholar]
- 3. Hammond JS, Guha IN, Beckingham IJ, Lobo DN. Prediction, prevention and management of postresection liver failure. Br J Surg 2011;98:1188‐1200. [DOI] [PubMed] [Google Scholar]
- 4. Helling TS. Liver failure following partial hepatectomy. HPB (Oxford) 2006;8:165‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Mierlo KM, Schaap FG, Dejong CH, Olde Damink SW. Liver resection for cancer: New developments in prediction, prevention and management of postresectional liver failure. J Hepatol 2016;65:1217‐1231. [DOI] [PubMed] [Google Scholar]
- 6. Lafaro K, Buettner S, Maqsood H, Wagner D, Bagante F, Spolverato G, et al. Defining post hepatectomy liver insufficiency: where do we stand? J Gastrointest Surg 2015;19:2079‐2092. [DOI] [PubMed] [Google Scholar]
- 7. Qadan M, Garden OJ, Corvera CU, Visser BC. Management of postoperative hepatic failure. J Am Coll Surg 2016;222:195‐208. [DOI] [PubMed] [Google Scholar]
- 8. Nevens F, Laleman W. Artificial liver support devices as treatment option for liver failure. Best Pract Res Clin Gastroenterol 2012;26:17‐26. [DOI] [PubMed] [Google Scholar]
- 9. Catalina MV, Barrio J, Anaya F, Salcedo M, Rincon D, Clemente G, et al. Hepatic and systemic haemodynamic changes after MARS in patients with acute on chronic liver failure. Liver Int 2003;23(Suppl. 3):39‐43. [DOI] [PubMed] [Google Scholar]
- 10. Donati G, La Manna G, Cianciolo G, Grandinetti V, Carretta E, Cappuccilli M, et al. Extracorporeal detoxification for hepatic failure using molecular adsorbent recirculating system: depurative efficiency and clinical results in a long‐term follow‐up. Artif Organs 2014;38:125‐134. [DOI] [PubMed] [Google Scholar]
- 11. Mitzner SR, Stange J, Klammt S, Peszynski P, Schmidt R, Noldge‐Schomburg G. Extracorporeal detoxification using the molecular adsorbent recirculating system for critically ill patients with liver failure. J Am Soc Nephrol 2001;12(Suppl. 17):S75‐S82. [PubMed] [Google Scholar]
- 12. Schmidt LE, Wang LP, Hansen BA, Larsen FS. Systemic hemodynamic effects of treatment with the molecular adsorbents recirculating system in patients with hyperacute liver failure: a prospective controlled trial. Liver Transpl 2003;9:290‐297. [DOI] [PubMed] [Google Scholar]
- 13. Heemann U, Treichel U, Loock J, Philipp T, Gerken G, Malago M, et al. Albumin dialysis in cirrhosis with superimposed acute liver injury: a prospective, controlled study. Hepatology 2002;36:949‐958. [DOI] [PubMed] [Google Scholar]
- 14. Banares R, Nevens F, Larsen FS, Jalan R, Albillos A, Dollinger M, et al.; RELIEF study group . Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute‐on‐chronic liver failure: the RELIEF trial. Hepatology 2013;57:1153‐1162. [DOI] [PubMed] [Google Scholar]
- 15. Saliba F, Camus C, Durand F, Mathurin P, Letierce A, Delafosse B, et al. Albumin dialysis with a noncell artificial liver support device in patients with acute liver failure: a randomized, controlled trial. Ann Intern Med 2013;159:522‐531. [DOI] [PubMed] [Google Scholar]
- 16. Kellersmann R, Gassel HJ, Buhler C, Thiede A, Timmermann W. Application of Molecular Adsorbent Recirculating System in patients with severe liver failure after hepatic resection or transplantation: initial single‐centre experiences. Liver 2002;22(Suppl. 2):56‐58. [DOI] [PubMed] [Google Scholar]
- 17. van de Kerkhove MP, de Jong KP, Rijken AM, de Pont AC, van Gulik TM. MARS treatment in posthepatectomy liver failure. Liver Int 2003;23(Suppl. 3):44‐51. [DOI] [PubMed] [Google Scholar]
- 18. Rittler P, Ketscher C, Inthorn D, Jauch KW, Hartl WH. Use of the molecular adsorbent recycling system in the treatment of postoperative hepatic failure and septic multiple organ dysfunction‐‐preliminary results. Liver Int 2004;24:136‐141. [DOI] [PubMed] [Google Scholar]
- 19. Inderbitzin D, Muggli B, Ringger A, Beldi G, Gass M, Gloor B, et al. Molecular absorbent recirculating system for the treatment of acute liver failure in surgical patients. J Gastrointest Surg 2005;9:1155‐1161. [DOI] [PubMed] [Google Scholar]
- 20. Chiu A, Chan LMY, Fan ST. Molecular adsorbent recirculating system treatment for patients with liver failure: the Hong Kong experience. Liver Int 2006;26:695‐702. [DOI] [PubMed] [Google Scholar]
- 21. Gilg S, Escorsell A, Fernandez J, Garcia Valdecasas JC, Saraste L, Wahlin S, et al. Albumin dialysis with mars in post‐hepatectomy liver failure (PHLF): experiences from two HPB centers. Surgery Curr Res 2015;5:252. [Google Scholar]
- 22. Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The “50‐50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg 2005;242:824‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meijers B, Laleman W, Vermeersch P, Nevens F, Wilmer A, Evenepoel P. A prospective randomized open‐label crossover trial of regional citrate anticoagulation vs. anticoagulation free liver dialysis by the Molecular Adsorbents Recirculating System. Crit Care 2012;16:R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Den Broek MA, Olde Damink SW, Dejong CH, Lang H, Malagó M, Jalan R, et al. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int 2008;28:767‐780. [DOI] [PubMed] [Google Scholar]
- 25. Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy‐‐definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002;35:716‐721. [DOI] [PubMed] [Google Scholar]
- 26. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chun YS, Ribero D, Abdalla EK, Madoff DC, Mortenson MM, Wei SH, et al. Comparison of two methods of future liver remnant volume measurement. J Gastrointest Surg 2008;12:123‐128. [DOI] [PubMed] [Google Scholar]
- 28. Sparrelid E, Gilg S, Brismar TB, Lundell L, Isaksson B. Rescue ALPPS is efficient and safe after failed portal vein occlusion in patients with colorectal liver metastases. Langenbecks Arch Surg 2017;402:69‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Truant S, El Amrani M, Skrzypczyk C, Boleslawski E, Sergent G, Hebbar M, et al. Factors associated with fatal liver failure after extended hepatectomy. HPB (Oxford). 2017;19:682‐687. [DOI] [PubMed] [Google Scholar]
- 30. Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol 2010;176:2‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Siu J, McCall J, Connor S. Systematic review of pathophysiological changes following hepatic resection. HPB (Oxford). 2014;16:407‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bernal W, Wendon J. Acute liver failure. N Engl J Med 2013;369:2525‐2534. [DOI] [PubMed] [Google Scholar]
- 33. Sen S, Williams R, Jalan R. The pathophysiological basis of acute‐on‐chronic liver failure. Liver 2002;22(Suppl. 2):5‐13. [DOI] [PubMed] [Google Scholar]
- 34. Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology 2005;41:1211‐1219. [DOI] [PubMed] [Google Scholar]
- 35. Beyer TA, Xu W, Teupser D, auf dem Keller U, Bugnon P, Hildt E, et al. Impaired liver regeneration in Nrf2 knockout mice: role of ROS‐mediated insulin/IGF‐1 resistance. EMBO J 2008;27:212‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Allard MA, Adam R, Bucur PO, Termos S, Cunha AS, Bismuth H, et al. Posthepatectomy portal vein pressure predicts liver failure and mortality after major liver resection on noncirrhotic liver. Ann Surg 2013;258:822‐829. [DOI] [PubMed] [Google Scholar]
- 37. Tucker ON, Heaton N. The ‘small for size’ liver syndrome. Curr Opin Crit Care 2005;11:150‐155. [DOI] [PubMed] [Google Scholar]
- 38. Kim HJ, Kim CY, Park EK, Hur YH, Koh YS, Kim HJ, et al. Volumetric analysis and indocyanine green retention rate at 15 min as predictors of post‐hepatectomy liver failure. HPB (Oxford) 2015;17:159‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Camus C, Lavoue S, Gacouin A, Compagnon P, Boudjema K, Jacquelinet C, et al. Liver transplantation avoided in patients with fulminant hepatic failure who received albumin dialysis with the molecular adsorbent recirculating system while on the waiting list: impact of the duration of therapy. Ther Apher Dial 2009;13:549‐555. [DOI] [PubMed] [Google Scholar]
- 40. Hassanein T, Oliver D, Stange J, Steiner C. Albumin dialysis in cirrhosis with superimposed acute liver injury: possible impact of albumin dialysis on hospitalization costs. Liver Int 2003;23(Suppl. 3):61‐65. [DOI] [PubMed] [Google Scholar]


