Abstract
Autoimmune hepatitis (AIH) is an immune‐mediated liver disease currently treated by immunosuppressive medications with significant side effects. Thus, novel mechanistic treatments are greatly needed. We performed prospective deep immunophenotyping of blood immune cells in patients with acute AIH before and after corticosteroid therapy. Blood samples from 26 patients with acute AIH (United Kingdom‐AIH Consortium) were phenotyped by flow cytometry at baseline and 4 months after starting corticosteroids. Pretreatment liver tissues were stained for forkhead box P3‐positive (FOXP3POS) regulatory T cells (Tregs), clusters of differentiation (CD)56POS natural killer (NK) cells, and chemokine (C‐X‐C motif) ligand 10. Chemokine secretion by cultured primary hepatocyte and biliary epithelial cells was measured by enzyme‐linked immunosorbent assay. Functional coculture assays with stimulated NK cells and Tregs were performed. CD161 ligand, lectin‐like transcript‐1 expression by intrahepatic immune cells was demonstrated with flow cytometry. Frequencies of NKbright cells declined with therapy (P < 0.001) and correlated with levels of alanine aminotransferase (P = 0.023). The Treg:NKbright ratio was lower pretreatment, and Tregs had an activated memory phenotype with high levels of CD39, cytotoxic T lymphocyte antigen 4, and FOXP3 but also high programmed death ligand 1, indicating exhaustion. Coculture experiments suggested the Tregs could not efficiently suppress interferon‐γ secretion by NK cells. Both Tregs and NK cells had high expression of liver infiltration and T helper 17 plasticity‐associated marker CD161 (P = 0.04). Pretreatment and CD161pos NK cells expressed high levels of perforin and granzyme B, consistent with an activated effector phenotype (P < 0.05). Lectin‐like transcript 1, a ligand for CD161, is expressed on intrahepatic B cells, monocytes, and neutrophils. Conclusion: Activated effector NK cells, which correlate with biochemical measurements of hepatitis, and exhausted memory Tregs are increased in the blood of patients with treatment‐naive AIH and decline with corticosteroid therapy. Inadequate regulation of NK cells by exhausted FOXP3pos Tregs may play a role in AIH pathogenesis and contribute to liver injury. (Hepatology Communications 2018;2:421‐436)
Abbreviations
- AIH
autoimmune hepatitis
- ALT
alanine aminotransferase
- CCR7
chemokine (C‐C motif) receptor 7
- CD
clusters of differentiation
- CTLA‐4
cytotoxic T lymphocyte antigen 4
- CXCL‐10
chemokine (C‐X‐C motif) ligand 10
- CXCR3
cysteine‐X‐cysteine receptor 3
- EM
effector memory
- FOXP3
forkhead box P3
- IFNγ
interferon‐γ
- IgG
immunoglobulin G
- IL
interleukin
- LLT1
lectin‐like transcript 1
- NK
natural killer
- NKT
natural killer T cells
- PD1
programmed death ligand 1
- Th
T helper
- TNFα
tumor necrosis factor α
- Treg
regulatory T cell
- UK‐AIH
United Kingdom Autoimmune Hepatitis
- ULN
upper limit of normal
Introduction
Autoimmune hepatitis (AIH) is an immune‐mediated liver disease characterized by interface and lobular hepatitis1 comprising infiltrates of both effector and regulatory T lymphocytes (Tregs).1, 2 There have been no new therapies for AIH for more than 3 decades, and it is becoming increasingly clear that there are limitations to the long‐term safety and efficacy of the nonspecific and empirical treatment in current use.3 Thus, there is a need for more effective mechanistically grounded approaches to treatment, and a better understanding of the immune make‐up of patients before they receive treatment is crucial for developing such novel immune cell/pathway‐targeted treatments for AIH.
One of the challenges in studying the immune status in patients who are treatment naive is the rapid initial response to corticosteroid treatment. This means that most patients are started on therapy before they can be investigated. In the vast majority of patients, this treatment is with corticosteroid or immunosuppressive therapy, which by nature alters immune activation status. Although studies have been performed to dissect the immune cell composition of patients with AIH on treatment, the immune balance between regulatory and effector cells in the treatment‐naive state and during longitudinal follow‐up of patients with acute AIH on maintenance immunosuppression is not known.
An imbalance between clusters of differentiation (CD)4positive[pos]CD25posCD127low Tregs4 and effector T cells has been proposed to contribute to the immune pathogenesis of AIH.2, 5, 6, 7 The differentiation and function of Tregs is controlled by transcription factor forkhead box P3 (FOXP3),8 and mutation in FOXP3 leads to a severe multiorgan autoimmune disorder (immunodysregulation polyendocrinopathy enteropathy X‐linked syndrome) in humans.9 CD56posCD3negative[neg] natural killer (NK) cells are a key component of the innate immune system and are involved in human autoimmune diseases, such as systemic lupus erythematosus10 and rheumatoid arthritis.11 NK cells are abundant in the liver.12 The activation and expansion of NK cells occurs in the early stages of AIH as NK cells function as a first response to liver injury13 and carry out diverse functions, including cytotoxicity, which may be directed at target cells, and cytokines interferon‐γ (IFNγ) and tumor necrosis factor α (TNFα) release, which can promote the maturation of antigen‐presenting cells to drive an adaptive immune response.14 Although NK cells have been shown to be particularly important in liver injury in viral hepatitis,15, 16 their frequency, function, and interaction with Tregs in the initial presentation of AIH, before corticosteroid therapy, and during longitudinal follow‐up is not known. In addition, recruitment and positioning of immune cells from peripheral circulation to the inflamed liver is also crucial for their function.2, 17
We hypothesized that dysregulation of effector innate NK cells and Tregs may play a role in acute AIH and that understanding this balance may help us to develop specific therapies and predict response to treatment. Based on this premise, we carried out a detailed, prospective, longitudinal immunophenotyping of immune cell subsets in patients with acute AIH before and 4 months after corticosteroid therapy and studied the influence of regulatory and effector lymphocytes on NK cells in functional assays. In addition, we screened for the presence of recruitment and positioning signals of liver‐infiltrated immune cells in treatment‐naive AIH livers.
Materials and Methods
ETHICS STATEMENT
Written informed consent was obtained from all subjects under United Kingdom‐AIH (UK‐AIH) ethics. Peripheral blood and liver tissues were collected with local research ethics committee approval (Newcastle, 14/LO/0303; Birmingham, CA/5192).
BLOOD AND LIVER TISSUE
We used 10 mL ethylene diamine tetraacetic acid‐chelated peripheral blood samples from patients diagnosed with AIH. AIH was diagnosed according to international AIH scoring criteria,18 which included scores from serologic, virology, and histologic information. Samples were transported overnight to the National Institute for Health Research Biomedical Research Unit, University of Birmingham, for next day analysis of immune phenotype by flow cytometry.
Pretreatment, paraffin‐fixed, liver biopsy sections were obtained from 6 Birmingham patients for analysis by immunohistochemistry. Explanted diseased liver was obtained from patients undergoing liver transplantation for liver diseases, including primary sclerosing cholangitis, primary biliary cholangitis, alcoholic liver disease, and non alcoholic steatohepatitis; lymphocytes, hepatocytes, and biliary epithelial cells were isolated as described.19, 20, 21, 22, 23
STUDY DESIGN
The UK‐AIH Consortium immunophenotyping study reported here was set up as a prospective assessment comparing the changes in a set of predefined immune cell subset features for consecutive treatment‐naive patients recruited between May 2015 and November 2016 and evaluating these features in relation to changes in a set of predefined biochemical factors that indicate liver function (alanine aminotransferase [ALT], bilirubin, and immunoglobulin G [IgG]). Immune features compared included the frequencies of nine predefined, innate, and adaptive immune cell subsets (CD4, CD8, CD4CD8, and CD4‐CD8‐double‐negative CD3+ T cells; CD127+CD25+ CD4 Tregs; CD56+ NK T cells (NKT); CD19+ B cells; CD56 bright NK [NKbright] and CD56 dim NK [NKdim] cells) and their expression of four surface markers (cysteine‐X‐cysteine receptor 3 [CXCR3], interleukin [IL]‐6R, CD161, programmed death ligand 1 [PD1]). The initial body of data indicated important changes in NK and Treg subsets; thus, additional analyses were initiated for subsequent patients to also compare the effector phenotypes of NK subsets and the frequencies and phenotypes of effector and memory populations of CD4, CD8 T cells, and Treg populations before and after steroid therapy.
ISOLATION OF PERIPHERAL BLOOD MONONUCLEAR CELLS AND IMMUNE PHENOTYPING BY MULTICOLOR FLOW CYTOMETRY
Please see the http://onlinelibrary.wiley.com/doi/10.1002/hep4.1163/full.
FLOW CYTOMETRY ANALYSIS OF LECTIN‐LIKE TRANSCRIPT‐1 EXPRESSION BY LIVER‐INFILTRATING IMMUNE CELLS
Please see the http://onlinelibrary.wiley.com/doi/10.1002/hep4.1163/full.
IMMUNOHISTOCHEMISTRY STAINING OF CHEMOKINE (C‐X‐C MOTIF) LIGAND‐10, FOXP3, AND CD56 IN THE HUMAN LIVER
Pretreatment AIH liver biopsies were stained with anti‐chemokine (C‐X‐C motif) ligand 10 (CXCL‐10) (6D4; R&D Systems) and either anti‐FOXP3 (236A/E7; Abcam) or anti‐CD56 (CD564; Leica) on the Bond Rx system by using Bond Polymer Refine 3,3′‐diaminobenzidine tetrahydrochloride hydrate and Bond Polymer Refine red detection substrates (Novocastra).
ENZYME‐LINKED IMMUNOSORBENT ASSAY OF CXCL‐10 SECRETION BY STIMULATED PRIMARY HUMAN HEPATOCYTES AND BILIARY EPITHELIAL CELLS
CXCL‐10 concentrations in culture supernatants were measured using a bead‐based Bio‐Plex Pro assay (Bio‐Rad) according to the manufacturer's instructions.
NK AND T‐CELL COCULTURE ASSAYS
Please see the http://onlinelibrary.wiley.com/doi/10.1002/hep4.1163/full.
CLINICAL DATA COLLECTION
ALT, bilirubin, and IgG data were collected for each patient before and 4 months after immunosuppressive therapy. All patients on the study received steroid therapy. There were differences in the specific formulation of steroid given depending on the local practice and whether the patient had cirrhosis (prednisolone) or not (budesonide). Steroid dosage followed national and international guidelines.
STATISTICAL ANALYSIS
Comparisons between baseline and 4 months were performed using Wilcoxon tests. Changes between baseline and 4 months were compared to the changes in ALT, bilirubin, and IgG using Spearman's correlation coefficients. Comparisons across cell subsets or conditions of experiment were performed using Mann‐Whitney, Wilcoxon, or Friedman tests, with Dunn's post‐hoc analysis. Analyses were performed using SPSS 22 (IBM Corp., Armonk, NY) or GraphPad Prism software version 6 (San Diego, CA). Data were summarized graphically with error bars representing mean ± SEM. P < 0.05 was considered significant.
Results
We studied the baseline and month 4 peripheral immunophenotype in 26 patients (3 male, 23 female; median age 56 years, range 44‐66 years) with serologically and histologically confirmed diagnosis of acute AIH from different National Health Service hospitals across the United Kingdom. These patients had clinical markers of disease activity with presenting ALT (369 IU/L, range 82‐1,352), bilirubin (26 μmol/L, range 10‐121), and IgG (23 mg/dL, range 14‐38). The detailed clinical characterizations of the patients reported in the study and the medications they received are given in http://onlinelibrary.wiley.com/doi/10.1002/hep4.1163/full.
NK CELL FREQUENCIES ARE HIGHER IN PATIENTS WITH CORTICOSTEROID‐NAIVE AIH AND DECLINE WITH THERAPY
To investigate changes in circulating immune cells in the 26 patients with acute AIH, we compared their peripheral blood immune cell profiles presented at diagnosis (treatment naive/baseline) and after 4 months on corticosteroid therapy. Subsets were defined by the gating strategy in Fig. 1A. Strikingly, frequencies of NKbright cells were significantly higher in 25/26 patients at baseline and declined with therapy (median, 2.7% versus 0.9%; P < 0.001). Within the total lymphocyte pool, proportions of CD8 T cells (median, 14.4% versus 9.7%; P = 0.004; 20/26 patients), NKT (median, 0.63% versus 0.45%; P = 0.024; 19/26 patients), and Tregs (median, 1.6% versus 1.0%; P = 0.055; 19/26 patients) were also reduced with therapy (Fig. 1B). B‐cell frequencies were significantly lower at baseline (median, 23% versus 33%; P = 0.03), with increases after therapy observed in 80% of cases (20/25). Other cell subsets did not change significantly (Fig. 1A). Overall, the relative Treg/NKbright balance increased significantly with therapy (Fig. 1C), and compared to control patients with hemochromatosis, the Treg/NKbright ratio was significantly reduced in treatment‐naive patients (P < 0.0001). This was not only due to lower NKbright frequencies in hemochromatosis but also due to higher Treg frequencies in controls compared to patients with AIH (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1163/full). Furthermore, there was a significant correlation between the changes in ALT and frequencies of NKbright cells from baseline to 4‐month follow‐up (P = 0.02) (Fig. 1D; http://onlinelibrary.wiley.com/doi/10.1002/hep4.1163/full). None of the other effector populations (CD8 and NKT) that declined with therapy altered significantly in their balance with Tregs (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1163/full) or correlated with biochemical markers of disease activity (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1163/full). Corticosteroid therapy was thus associated with a change in the immune balance toward a higher proportion of Tregs over NKbright cells.
Figure 1.

Peripheral blood immune cell frequencies in patients with AIH before and after 4 months immunosuppression therapy. (A) Gating strategy for definition of immune cell subsets, including CD4, CD8, CD4‐CD8‐ (double‐negative) T cells; CD4posCD25posCD127neg regulatory T cells; CD56pos T cells; NKT; CD19+ B cells; CD56bright NK cells (NKbright), and CD56dim NK cells (NKdim). (B) Cell subset frequencies as a proportion of total lymphocytes at baseline and after 4 months. Wilcoxon test, *P < 0.05, **P < 0.01, ***P < 0.0001. Red dots indicate patients with high starting B cell frequency that declined dramatically with therapy. (C) Ratio of Tregs to NKbright cells in steroid‐naive, 4‐month follow‐up, and hemochromatosis (control) blood. Wilcoxon test, **P < 0.01; Mann‐Whitney U test, *P < 0.05, ****P < 0.0001. Error bars are means ± SEM. (D) Scatterplot and Spearman's correlation coefficient for the association between changes in ALT levels and NKbright cell frequencies between baseline and 4 months. Trend line calculated by linear regression is shown.
BOTH REGULATORY MEMORY AND EFFECTOR MEMORY T‐CELL FREQUENCIES ARE SIGNIFICANTLY HIGHER IN THE CIRCULATION IN TREATMENT‐NAIVE PATIENTS WITH AIH AND DECLINE AFTER THERAPY
We additionally explored whether T cells had been exposed to antigen by assessing the memory/naive status of the cells in a subgroup of the cohort (n = 12 treatment naive; n = 9 follow‐up). We defined memory and naive CD4, CD8, and Treg populations by their expression of CD45RA and chemokine (C‐C motif) receptor 7 (CCR7)24, 25 as central memory (CD45RAnegCCR7pos), effector memory (EM; CD45RAnegCCR7neg), naive (CD45RAposCCR7pos), and tissue‐resident terminally differentiated effector memory RApos (TEMRA; CD45RAposCCR7neg) (Fig. 2A). Within the CD4 and CD8 populations in the treatment‐naive state, naive CD4 T‐cell and TEMRA CD8 T‐cell subsets predominated (Fig. 2B). In contrast, the EM Treg subset was the significantly predominant Treg population (Fig. 2B). In all T‐cell classes, we observed significantly higher frequencies of EM cells at baseline compared to follow‐up (Fig. 2C).
Figure 2.
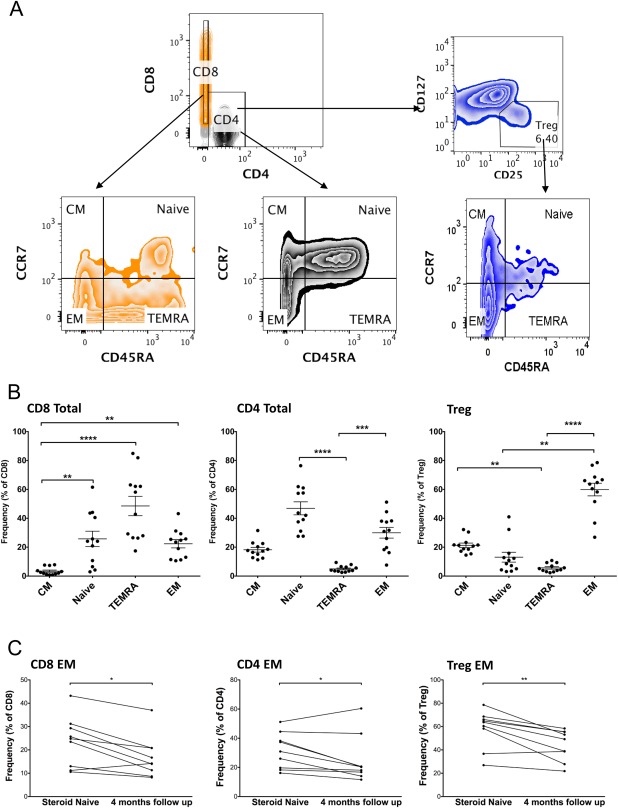
Frequencies of memory/naive CD8, CD4, and Treg subsets in treatment‐naive AIH before and after 4 months immunosuppression. (A) T‐cell memory and naive subsets were identified based on expression of CD45RA and CCR7. Representative fluorescence‐activated cell sorting plots illustrating the gating strategy are shown. (B) Frequencies before treatment. Friedman test with Dunn's post‐hoc analysis, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (C) Frequencies of EM CD4, CD8, and Tregs before and after 4 months immunosuppression. Wilcoxon test, *P < 0.05. Abbreviations: CM, central memory; TEMRA, terminally differentiated tissue resident effector memory RA‐positive.
MEMORY Tregs EXPRESS SUPPRESSIVE FUNCTIONAL MARKERS BUT ALSO PRESENT AN EXHAUSTED PHENOTYPE IN PATIENTS WITH CORTICOSTEROID‐NAIVE AIH
FOXP3 transcription factor controls Treg function.8 Cytotoxic T lymphocyte antigen 4 (CTLA‐4) leads to transendocytosis of CD80/86 on dendritic cells to down‐regulate antigen presentation,26 and CD39 generates immunosuppressive adenosine.27 Memory Tregs had significantly higher expression of these markers compared to other Treg groups in the treatment‐naive patients (Fig. 3A,D; http://onlinelibrary.wiley.com/doi/10.1002/hep4.1163/full).
Figure 3.
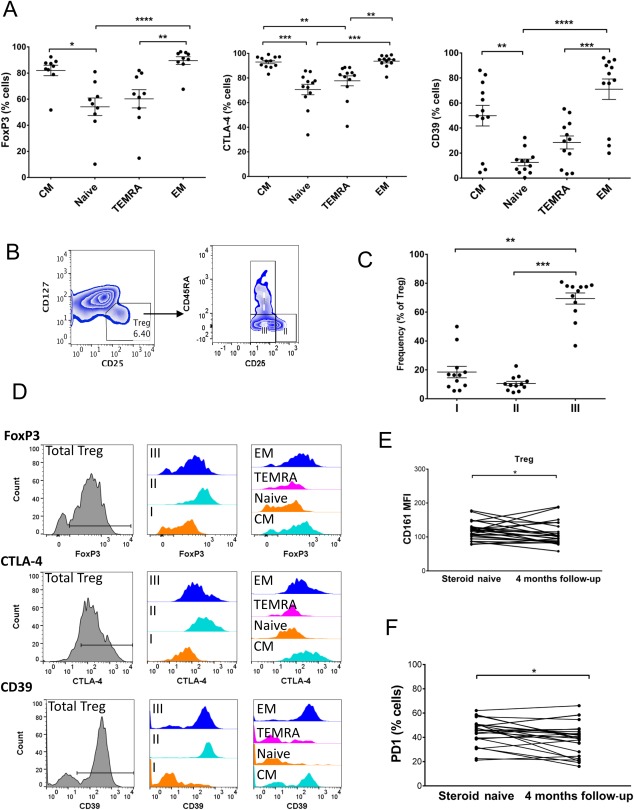
Treg phenotype in acute AIH. (A) Expression of Treg functional proteins FOXP3, CTLA‐4, and CD39 by memory/naive Treg subsets defined as in Fig. 2 by CD45RA and CCR7 expression. (B) Gating strategy for Treg fractions based on CD45RA and CD25 expression. (C) Frequencies of fractions in AIH pretreatment. In A and C, tests were Friedman with Dunn's post‐hoc analysis, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (D) Representative flow cytometry overlays showing expression of Treg functional proteins FOXP3, CTLA‐4, and CD39 by the total Treg population; fractions I, II, and III Tregs; and the memory and naive Treg subsets. (E) CD161 (MFI) and (F) PD1 (frequency) expression by total Tregs at baseline and after 4 months therapy. Wilcoxon test, *P < 0.05. Abbreviations: CM, central memory; MFI, median fluorescence intensity; TEMRA, terminally differentiated tissue resident effector memory RA‐positive.
When we assessed the proportions of the Treg fractions defined by CD25 and CD45RA expression in treatment‐naive patients, we observed a predominance of fraction III (Fig. 3 B,C). However, fraction II had the highest level of Treg functional markers FOXP3, CTLA‐4, and CD39 (Fig. 3D; http://onlinelibrary.wiley.com/doi/10.1002/hep4.1163/full). Previous studies suggested that fraction III Tregs express CD161 and have a high propensity for differentiation toward a T helper (Th)17 phenotype.25, 28, 29 We found that CD161 expression on Tregs (median frequency, 12.9% versus 11.0%; P = 0.042; median fluorescence intensity, 118 versus 100; P = 0.037) was significantly higher before treatment (Fig. 3E). The frequency of CD161pos Tregs was also higher before therapy compared to the 4‐month follow‐up (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1163/full). Memory Tregs and fraction III Tregs had the highest expression of CD161 (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1163/full). In addition, PD1, a marker of lymphocyte exhaustion, was reduced on Tregs after 4 months of immunosuppressive therapy (median, 46 versus 40; P = 0.027) (Fig. 3F).
NK CELL CD161 EXPRESSION LEVELS ARE SIGNIFICANTLY HIGHER IN THE TREATMENT‐NAIVE STATE, AND CD161pos NK CELLS ARE CHARACTERIZED BY HIGH EXPRESSION OF GRANZYME B AND PERFORIN
With the exception of CD19pos B cells, all immune subsets contained CD161pos cells (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1163/full). The level of CD161 expression on NKdim cells (median fluorescence intensity, 274 versus 217; P < 0.001) was significantly higher before treatment compared with after 4 months corticosteroid (Fig. 4A; http://onlinelibrary.wiley.com/doi/10.1002/hep4.1163/full). We characterized the expression of functional cytolytic molecules by NK subsets subdivided according to CD161 expression and observed the highest frequencies of cytotoxic factors granzyme B and perforin in the CD161pos NK populations. CD161pos NKdim cells had significantly greater expression of both perforin and granzyme B on a cell by cell basis than NKbright cells (Fig. 4B,C; http://onlinelibrary.wiley.com/doi/10.1002/hep4.1163/full).
Figure 4.
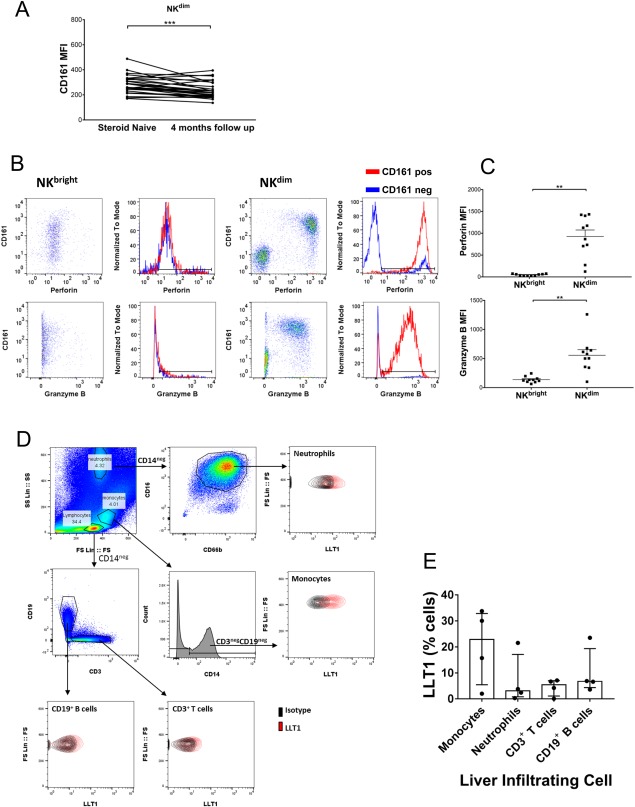
Characterization of CD161‐expressing NK cells and CD161 ligand LLT1 in AIH. (A) CD161 expression on NKdim cells at baseline and after 4 months therapy. Wilcoxon test, *P < 0.05. (B) Representative flow cytometry plots and overlays showing granzyme B and perforin expression by CD161pos and CD161neg NK cell populations pretreatment. (C) Summary data on granzyme B and perforin expression by CD161pos populations of NKbright and NKdim cells pretreatment; **P < 0.01. (D) Flow cytometry gating strategy for identification of immune cell subsets, including neutrophils, monocytes, T cells, and B cells, among isolated liver‐infiltrating immune cells and representative staining of LLT1 versus isotype control on these populations. (E) Summary data on LLT1 expression by liver‐infiltrating immune cells isolated from primary biliary cirrhosis, primary sclerosing cholangitis, alcoholic liver disease, and nonalcoholic steatohepatitis explanted livers. Abbreviation: MFI, median fluorescence intensity.
Because we detected high frequencies of CD161pos cells, we explored the expression of the CD161 ligand lectin‐like transcript 1 (LLT1), by liver‐infiltrating immune cells. We analyzed its expression by cells isolated from explanted autoimmune or inflammatory diseased liver tissue by flow cytometry (Fig. 4D) and detected LLT1 on T and B cells, monocytes, and neutrophils (Fig. 4E).
Tregs AND NK CELLS IN TREATMENT‐NAIVE AIH SHOW HIGH EXPRESSION OF CXCR3, AND CXCR3 LIGAND CXCL‐10 DERIVES FROM INFLAMED LIVER HEPATOCYTES AND BILIARY CELLS
CXCR3 was expressed by all subsets of immune cells, but expression levels varied (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1163/full). CXCR3pos Treg frequencies in the total Treg population were significantly higher in the treatment‐naive state than at follow‐up (P < 0.001) (Fig. 5A). The expression of CXCR3 was highest in the memory compared to naive Treg populations (P < 0.05) (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1163/full). We have previously reported a role for CXCR3 in lymphocyte homing to the liver,2, 17, 30 but to confirm its potential involvement in the pathogenesis of acute AIH, we stained pretreatment liver biopsies for the CXCR3‐binding chemokine CXCL‐10. CXCL‐10 was detected on bile ducts in the portal tracts and was closely associated with infiltrating FOXP3pos Treg cells (Fig. 5B) and CD56pos NK cells (Fig. 5C). Furthermore, we demonstrated that CXCL‐10 was secreted by human hepatocytes and biliary epithelial cells in response to inflammatory cytokines in vitro (Fig. 5D).
Figure 5.

CXCL‐10 distribution in treatment‐naive AIH livers colocalizes with CD56+ NK cells and FOXP3+ Tregs. (A) CXCR3 expression by total Tregs at baseline and after 4 months therapy. Wilcoxon test, *P < 0.05. (B,C) Dual staining of CXCL‐10 and either (B) CD56 or (C) FOXP3 on liver biopsy sections taken at diagnosis of AIH (CXCL‐10, brown staining; FOXP3+Tregs, CD56+NK cells, red staining). (D) CXCL‐10 concentrations in culture supernatants prepared from untreated and IFNγ + TNFα‐treated hepatocytes and biliary epithelial cells. Data are mean ± SEM. Abbreviation: BEC, biliary epithelial cell.
REGULATORY T‐CELL CONTROL OF NK CELLS
To investigate whether the significant increase in the ratio of NK cells to Tregs could have functional implications in AIH pathogenesis, we tested whether activated NK cell effector responses could be controlled by Tregs in vitro. CD3negCD56pos NK cells activated overnight with cytokines IL‐12 and IL‐15 secreted high levels of IFNγ but expressed little TNFα or CD107a (Fig. 6A). In agreement with the idea that there is inadequate Treg control over NK in the AIH disease process, we observed a very clear trend of suppression of NK activity by Tregs (P = 0.0625); marked suppression by Tregs occurred in five out of the six experiments performed. This was in contrast to the effects of coculture of NK cells with activated non‐Treg effectors, which led instead to significantly elevated IFNγ expression by NK (P = 0.0312) (Fig. 6B).
Figure 6.
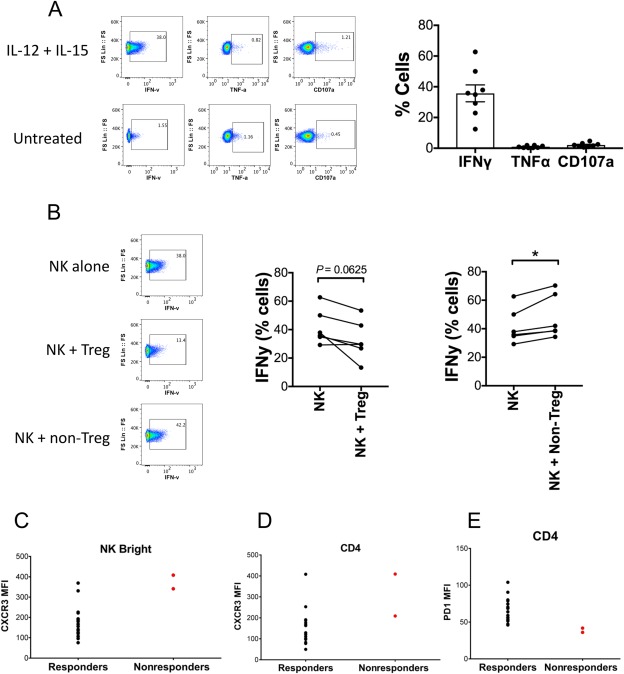
Regulation of NK cell function by activated regulatory and nonregulatory T cells. (A,B) NK cells, Tregs, and non‐Tregs were isolated from peripheral blood. NK cell degranulation (CD107a), IFNγ, and TNFα production, with overnight IL‐12 (20 ng/mL) + IL‐15 (20 ng/mL) stimulation monitored by flow cytometry in the presence or absence of activated Tregs or non‐Tregs. (A) Representative and summary data on NK degranulation and cytokine production with stimulation. (B) IFNγ production by NK cells cultured overnight alone or 1:1 with either Tregs or non‐Tregs under IL‐12 (20 ng/mL) + IL‐15 (20 ng/mL) stimulation. (C‐E) Expression of immune cell surface markers in responders and nonresponders after 4 months corticosteroid treatment. CXCR3 MFI on (C) NKbright cells and (D) CD4 T cells. (E) PD1 MFI on CD4 T cells. Abbreviation: MFI, median fluorescence intensity.
IMMUNOLOGICAL MARKERS AND PERIPHERAL BLOOD LIVER BIOCHEMISTRY AND IgG LEVEL
Finally, we explored whether any phenotypes examined in this study could define patients with treatment‐naive AIH who are nonresponders to corticosteroid therapy. We defined subjects as nonresponders based on their having 1.5 × upper limit of normal (ULN) values for ALT, 1.5 × ULN values for bilirubin, and 1.5 × ULN values for IgG at 4 months. Only 2 patients fulfilled these criteria, so any interpretation is limited; however, these patients were characterized by high levels of CXCR3 on their NKbright cells and CD4 T cells (Fig. 6C,D) and lower expression of the exhaustion marker PD1 on CD4 T cells (Fig. 6E).
Discussion
Type‐1 AIH is an immune‐mediated liver disease that usually responds to immunosuppression. Because patients are promptly treated with corticosteroids, it is difficult to study patients in the treatment‐naive state before immunosuppression is started. Our study is one of the first to characterize in detail the changes in immune cell composition before and after treatment in patients with AIH. The study was not designed to report the alterations in total cell numbers with therapy but rather to discuss the influence of therapy on the ratios of the different innate and adaptive immune subsets and to describe the changes in their individual phenotypes; as such, this study offers important insight into the subsets whose relative precedence may be key to AIH pathogenesis and thus crucial to target in future targeted therapies.
The two main findings of this study are a significant elevation of activated effector CD56bright NK cells in the blood prior to therapy and a concomitant increase in regulatory T cells. In the healthy state, NK cells constitute 5%‐15% of peripheral blood cells and are abundant in the liver.31 NK cells have been implicated in liver injury32 and in the pathogenesis of autoimmune diseases, such as systemic lupus erythematosus and diabetes.33, 34 They provide an early host defense to infection or injury and have the ability to kill other cells rapidly by cytolysis through their release of cytolytic mediators without prior stimulation.35 We observed a significant increase in CD56bright NK cells in untreated patients with AIH (25/26 cases) that fell after 4 months of immunosuppressive treatment. CD56bright cells are found in the liver but under normal circumstances are present at low frequencies in the blood. They are critical for local innate immune responses and are increased at sites of inflammation in autoimmune diseases.36 We also observed a higher frequency of NKdim cells (up to 40%) in treatment‐naive patients. It is possible that in treatment‐naive AIH, NK cells enter the peripheral circulation from the liver, but perhaps more likely, they are released into the blood from lymphoid tissues where they are found at high frequencies. In parallel to an increase in NK cell frequency, we also observed an increased Treg frequency in the circulation of patients with treatment‐naive AIH. Tregs are crucial to maintain peripheral immune tolerance and control effector immune cells.37, 38 The increase in the Treg population may thus be an attempt to suppress immune activation stimulated by the causative autoantigen in acute AIH. Although both NKbright and Treg cell frequencies were increased in treatment‐naive patients, the Treg versus NKbright cell ratio was reduced, suggesting a skewing of the immune balance toward the effector arm in the treatment‐naive state. Importantly, Tregs were weak suppressors of NK cell production of inflammatory cytokines; thus, the data suggest that loss of NK cell regulation could underlie hepatic damage in AIH. In support of this conclusion, the NKbright cell frequency correlated with levels of ALT in treatment‐naive patients.
A previous in vivo study39 in diabetes suggested that Tregs suppress NK function by depleting IL‐2, which is critical for NK cell survival and optimal function, from the local environment. Although in the present study the suppression of NK cell production of inflammatory cytokines did not reach significance, there was a clear indication that Tregs offer a level of regulation over activated NK cells in the normal state. It is possible that in the treatment‐naive state of AIH, Tregs may be too exhausted, a conclusion supported by their elevated PD1 expression pretreatment, or too infrequent to effectively control NK cell activity. Overall, the data suggest that loss of NK cell regulation involving inadequate Treg‐mediated control could underlie hepatic damage in AIH. In support of this conclusion, the NKbright cell frequency correlated with levels of ALT in treatment‐naive patients.
The changes in peripheral blood described in this study are informative, although it is likely that the most important responses pathologically are those that occur within the liver. Our data suggest that the expanded peripheral populations we have seen have the potential to be recruited to the inflamed liver because they express high levels of the chemokine receptor CXCR3, which facilitates the recruitment of effector and regulatory T cells to the inflamed human liver2, 30 through interactions with its ligand CXCL‐10.2, 17, 30 We detected both CXCR3pos Tregs and NK cells residing close to CXCL‐10‐expressing bile ducts in liver biopsies from patients with acute AIH.
We examined the Treg subset in treatment‐naive patients in more detail and found that these cells were predominantly CD45RAneg memory, which suggests that they have been exposed to antigen. Specifically, Tregs and also CD4 and CD8 cells were predominantly of an EM phenotype before exposure to immunosuppression, and this proportion declined significantly in all subsets after therapy. Thus, it is likely that the EM component of Tregs is reflective of extensive efforts to suppress antigen‐primed effector T cells in the liver.40 Future studies into the T‐cell receptor repertoire of both CD4 and Tregs in treatment‐naive AIH would be of interest to determine whether these cells are clonally expanded subsets that respond to the same antigen. In addition, Treg functional markers FOXP3, CD39, and CTLA‐4 were highest on the memory population, suggesting that these are suppressive memory Tregs. However, these cells also expressed high levels of PD1, which has been associated with T‐cell exhaustion, suggesting that these cells may not be fully functional. This could explain in part why immune‐mediated liver injury progresses in spite of increased Treg frequencies.
Treg cells in peripheral blood can be divided into three fractions,25 with fraction III Tregs characterized by the expression of CD161,28, 29 which has been described as a potentially plastic Treg fraction that can change its lineage toward Th17.28, 29 CD161 is a lectin‐like receptor expressed on human NK cells and T lymphocytes.41 We have previously demonstrated that CD161pos T cells in the human liver include both Th17 and Tc17 cells42, 43 and that these cells are implicated not only in liver injury but also in the pathogenesis of autoimmune Crohn's disease.44 LLT1, a ligand for CD161,45 is present on antigen‐presenting cells, germinal center B cells, and Kupffer cells.46, 47 Interactions between CD161 on NK cells and LLT1 on target cells inhibit NK cell‐mediated cytotoxicity and cytokine production.46, 47, 48, 49 We observed significantly higher CD161 expression on both NK cells and Tregs in treatment‐naive AIH that declined after therapy and demonstrated that LLT1 is expressed on liver‐infiltrating monocytes, B cells, and neutrophils. Thus, CD161 could be activated by ligand‐bearing cells in the inflamed liver to down‐regulate effector responses; but if insufficient ligand is present, this feedback could be overwhelmed.
We noted that patients who did not respond to immunosuppressive therapy had a reduced exhausted T‐cell phenotype with lower PD1 expression, which agrees with a lack of control over effector cells in these patients with therapy. The B cell frequency was elevated in 20% (5/25 cases) of treatment‐naive patients, and this group might be one that would respond to anti‐B cell therapy, such as rituximab.39, 50
Our study has its limitations. As only small volumes of blood (5‐10 mL) were supplied by each participating hospital, the full volume was taken to isolate the peripheral blood mononuclear cells for immune phenotyping; count data on each subset in the whole blood were not collected. CD45 selection and dead cell exclusion were not included in the phenotyping panel. This decision was taken in the study design in order to maximize the depth of immune subset phenotyping with the small volume of sample available and access to only nine color instruments; it was also based on knowledge that CD45 frequencies of >99% and dead cell frequencies of 1%‐3% are consistently observed with peripheral blood samples processed under the same conditions and are seen to have negligible impact on the frequencies of the populations of interest in the study (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1163/full; http://onlinelibrary.wiley.com/doi/10.1002/hep4.1163/full). There were differences in the steroid regimen used by different contributing centers, and these might have influenced the magnitude of phenotypic change observed. However, an increase in variability would be more reasonable to expect; as such, the conclusions of disease‐relevant phenotypes made in this study are believed to be sound and relevant. Limitation of sample volume to under 10 mL with the current UK‐AIH ethics meant that in vitro assays to test the possibility that a lack of NK cell regulation by Tregs contributes to AIH pathogenesis could not be evaluated on patient blood as such analysis required around 50 mL. Despite these limitations, the study has identified interesting and novel phenotypic changes with therapy in a unique, rare, and poorly understood cohort, which can now guide the direction of further studies to address in more detail the pathologic mechanisms underlying AIH pathogenesis and direct improved and stratified therapeutic strategies with reduced side effects.
In conclusion, our study is the first to report longitudinal changes in detailed leucocyte subsets in AIH (Fig. 7). The changes in the balance of NK cells and Tregs in treatment‐naive patients suggest a switch in the effector/regulatory balance that might be amenable to therapy. The relevance of the NK cell changes is supported by the correlation with liver injury early in disease. Further studies using deep immunophenotyping with mass cytometry in well‐characterized cohorts of patients are required before immunophenotyping can be used for treatment stratification.
Figure 7.
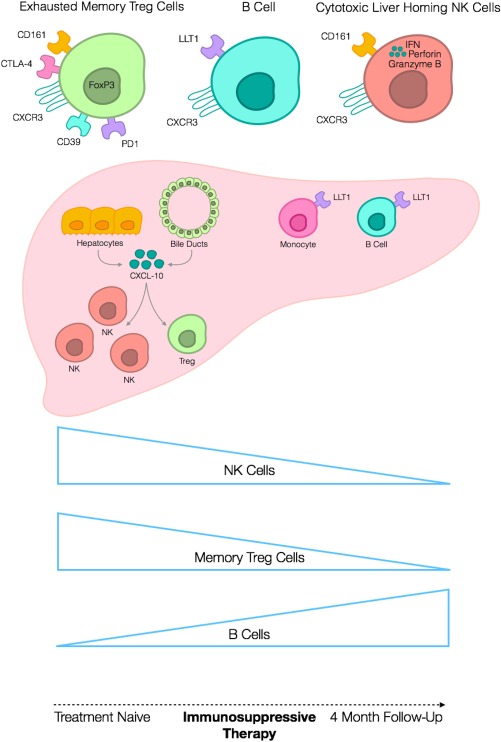
Diagrammatic illustration of the immune cell composition changes occurring in treatment‐naive AIH with 4 months corticosteroid therapy. Regulatory T cells in the peripheral blood of patients with treatment‐naive acute AIH are predominantly effector memory in phenotype (CD45RAnegCCR7neg) and express functional proteins CD39, CTLA‐4, and FOXP3. They also express the liver‐homing receptor CXCR3 and exhaustion marker PD1. B cells and monocytes in inflamed AIH livers express the CD161 ligand LLT1, and CD161 expression by both NK cells and Tregs is elevated before therapy in patients with acute AIH. CXCL‐10 secreted by inflamed hepatocytes and bile ducts attracts CXCR3‐expressing NK cells and Tregs to the site of hepatitis in AIH. Both NK cells and memory Tregs are expanded significantly in the peripheral immune cell composite of patients with acute AIH before the start of therapy compared to after 4 months on corticosteroid. In contrast, the B cell frequency is significantly lower at the treatment‐naive acute stage and recovers with corticosteroid.
Author names in bold designate shared co‐first authorship.
Supporting information
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1163/full.
Supporting Information Figure 1
Supporting Information Figure 2
Supporting Information Figure 3
Supporting Information Figure 4
Supporting Information Figure 5
Supporting Information Figure 6
Supporting Information Figure 7
Supporting Information Figure 8
Supporting Information Figure 9
Supporting Information Figure 10
Supporting Information Figures
Supporting Information Tables
Supporting Information
Acknowledgment
We are indebted to all the local principle investigators and members of the clinical and research teams who have recruited to UK‐AIH treatment‐naive patients for this study.
Potential conflict of interest: Nothing to report.
Supported by a Medical Research Council Clinician Scientist Award (G1002552 to Y.H.O.); Queen Elizabeth Hospital Birmingham Charity and National Institute for Health Research (NIHR) Birmingham Biomedical Research Unit (H.C.J.); United Kingdom Autoimmune Hepatitis (UK‐AIH) Translational Research Collaboration (M.K.B.); Wellcome Trust (109965MA) and NIHR (P.K.); NIHR Biomedical Research Centre Oxford; NIHR Newcastle Biomedical Research Centre and the NIHR Rare Diseases Translational Research Collaboration (J.K.D.); NIHR Rare Diseases Translational Research Collaboration, United Kingdom (UK‐AIH study).
This study was conducted on behalf of the UK‐AIH Consortium.
This paper presents independent research supported by the NIHR Biomedical Research Unit Birmingham and Biomedical Research Centres, Newcastle and Oxford, and the NIHR Rare Diseases Translational Research Collaboration, United Kingdom. The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health.
REFERENCES
- 1. Kerr JF, Cooksley WG, Searle J, Halliday JW, Halliday WJ, Holder L, et al. The nature of piecemeal necrosis in chronic active hepatitis. Lancet 1979;2:827‐828. [DOI] [PubMed] [Google Scholar]
- 2. Oo YH, Weston CJ, Lalor PF, Curbishley SM, Withers DR, Reynolds GM, et al. Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver. J Immunol 2010;184:2886‐2898. [DOI] [PubMed] [Google Scholar]
- 3. Wong LL, Kendrick, S , Dyson, JK , Jones D. Understanding the unmet need in autoimmune hepatitis, The United Kingdom Autoimmune Hepatitis Study (UK‐AIH). Hepatology 2016:64:815A. [Google Scholar]
- 4. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self‐tolerance maintained by activated T cells expressing IL‐2 receptor alpha‐chains (CD25). Breakdown of a single mechanism of self‐tolerance causes various autoimmune diseases. J.Immunol 1995;155:1151‐1164. [PubMed] [Google Scholar]
- 5. Peiseler M, Sebode M, Franke B, Wortmann F, Schwinge D, Quaas A, et al. FOXP3+ regulatory T cells in autoimmune hepatitis are fully functional and not reduced in frequency. J Hepatol 2012;57:125‐132. [DOI] [PubMed] [Google Scholar]
- 6. Taubert R, Hardtke‐Wolenski M, Noyan F, Wilms A, Baumann AK, Schlue J, et al. Intrahepatic regulatory T cells in autoimmune hepatitis are associated with treatment response and depleted with current therapies. J Hepatol 2014;61:1106‐1114. [DOI] [PubMed] [Google Scholar]
- 7. Jeffery HC, Jeffery LE, Lutz P, Corrigan M, Webb GJ, Hirschfield GM, et al. Low‐dose interleukin‐2 promotes STAT5 phosphorylation, Treg survival and CTLA‐4‐dependent function in autoimmune liver diseases. Clin Exp Immunol 2017;188:394‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003;299:1057‐1061. [PubMed] [Google Scholar]
- 9. Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X‐linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 2001;27:20‐21. [DOI] [PubMed] [Google Scholar]
- 10. Schepis D, Gunnarsson I, Eloranta ML, Lampa J, Jacobson SH, Karre K, et al. Increased proportion of CD56bright natural killer cells in active and inactive systemic lupus erythematosus. Immunology 2009;126:140‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pridgeon C, Lennon GP, Pazmany L, Thompson RN, Christmas SE, Moots RJ. Natural killer cells in the synovial fluid of rheumatoid arthritis patients exhibit a CD56bright,CD94bright,CD158negative phenotype. Rheumatology (Oxford) 2003;42:870‐878. [DOI] [PubMed] [Google Scholar]
- 12. Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology 2006;43(Suppl. 1):S54‐S62. [DOI] [PubMed] [Google Scholar]
- 13. Werner JM, Heller T, Gordon AM, Sheets A, Sherker AH, Kessler E, et al. Innate immune responses in hepatitis C virus‐exposed healthcare workers who do not develop acute infection. Hepatology 2013;58:1621‐1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gerosa F, Baldani‐Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med 2002;195:327‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schuch A, Hoh A, Thimme R. The role of natural killer cells and CD8(+) T cells in hepatitis B virus infection. Front Immunol 2014;5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maini MK, Peppa D. NK cells: a double‐edged sword in chronic hepatitis B virus infection. Front Immunol 2013;4:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oo YH, Banz V, Kavanagh D, Liaskou E, Withers DR, Humphreys E, et al. CXCR3‐dependent recruitment and CCR6‐mediated positioning of Th‐17 cells in the inflamed liver. J Hepatol 2012;57:1044‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hennes EM, Zeniya M, Czaja AJ, Pares A, Dalekos GN, Krawitt EL, et al.; International Autoimmune Hepatitis Group . Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 2008;48:169‐176. [DOI] [PubMed] [Google Scholar]
- 19. Bhogal RH, Hodson J, Bartlett DC, Weston CJ, Curbishley SM, Haughton E, et al. Isolation of primary human hepatocytes from normal and diseased liver tissue: a one hundred liver experience. PLoS One 2011;6:e18222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen YY, Jeffery HC, Hunter S, Bhogal R, Birtwistle J, Braitch MK, et al. Human intrahepatic regulatory T cells are functional, require IL‐2 from effector cells for survival and are susceptible to Fas ligand‐mediated apoptosis. Hepatology 2016;64:138‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holt AP, Haughton EL, Lalor PF, Filer A, Buckley CD, Adams DH. Liver myofibroblasts regulate infiltration and positioning of lymphocytes in human liver. Gastroenterology 2009;136:705‐714. [DOI] [PubMed] [Google Scholar]
- 22. Shetty S, Bruns T, Weston CJ, Stamataki Z, Oo YH, Long HM, et al. Recruitment mechanisms of primary and malignant B cells to the human liver. Hepatology 2012;56:1521‐1531. [DOI] [PubMed] [Google Scholar]
- 23. Lalor PF, Edwards S, McNab G, Salmi M, Jalkanen S, Adams DH. Vascular adhesion protein‐1 mediates adhesion and transmigration of lymphocytes on human hepatic endothelial cells. J Immunol 2002;169:983‐992. [DOI] [PubMed] [Google Scholar]
- 24. Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999;401:708‐712. [DOI] [PubMed] [Google Scholar]
- 25. Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009;30:899‐911. [DOI] [PubMed] [Google Scholar]
- 26. Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, et al. Trans‐endocytosis of CD80 and CD86: a molecular basis for the cell‐extrinsic function of CTLA‐4. Science 2011;332:600‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007;204:1257‐1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Afzali B, Mitchell PJ, Edozie FC, Povoleri GA, Dowson SE, Demandt L, et al. CD161 expression characterizes a subpopulation of human regulatory T cells that produces IL‐17 in a STAT3‐dependent manner. Eur J Immunol 2013;43:2043‐2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pesenacker AM, Bending D, Ursu S, Wu Q, Nistala K, Wedderburn LR. CD161 defines the subset of FoxP3+ T cells capable of producing proinflammatory cytokines. Blood 2013;121:2647‐2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Curbishley SM, Eksteen B, Gladue RP, Lalor P, Adams DH. CXCR3 activation promotes lymphocyte transendothelial migration across human hepatic endothelium under fluid flow. Am J Pathol 2005;167:887‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ajuebor MN, Wondimu Z, Hogaboam CM, Le T, Proudfoot AE, Swain MG. CCR5 deficiency drives enhanced natural killer cell trafficking to and activation within the liver in murine T cell‐mediated hepatitis. Am J Pathol 2007;170:1975‐1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vermijlen D, Luo D, Froelich CJ, Medema JP, Kummer JA, Willems E, et al. Hepatic natural killer cells exclusively kill splenic/blood natural killer‐resistant tumor cells by the perforin/granzyme pathway. J Leukoc Biol 2002;72:668‐676. [PubMed] [Google Scholar]
- 33. Hou Y, Zhang C, Xu D, Sun H. Association of killer cell immunoglobulin‐like receptor and human leucocyte antigen‐Cw gene combinations with systemic lupus erythematosus. Clin Exp Immunol 2015;180:250‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dotta F, Censini S, van Halteren AG, Marselli L, Masini M, Dionisi S, et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent‐onset type 1 diabetic patients. Proc Natl Acad Sci U S A 2007;104:5115‐5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meier UC, Owen RE, Taylor E, Worth A, Naoumov N, Willberg C, et al. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol 2005;79:12365‐12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gross CC, Schulte‐Mecklenbeck A, Runzi A, Kuhlmann T, Posevitz‐Fejfar A, Schwab N, et al. Impaired NK‐mediated regulation of T‐cell activity in multiple sclerosis is reconstituted by IL‐2 receptor modulation. Proc Natl Acad Sci U S A 2016;113:E2973‐2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008;133:775‐787. [DOI] [PubMed] [Google Scholar]
- 38. Jeffery HC, Braitch MK, Brown S, Oo YH. Clinical potential of regulatory T cell therapy in liver diseases: an overview and current perspectives. Front Immunol 2016;7:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sitrin J, Ring A, Garcia KC, Benoist C, Mathis D. Regulatory T cells control NK cells in an insulitic lesion by depriving them of IL‐2. J Exp Med 2013;210:1153‐1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gantner F, Leist M, Lohse AW, Germann PG, Tiegs G. Concanavalin A‐induced T‐cell‐mediated hepatic injury in mice: the role of tumor necrosis factor. Hepatology 1995;21:190‐198. [DOI] [PubMed] [Google Scholar]
- 41. Fergusson JR, Smith KE, Fleming VM, Rajoriya N, Newell EW, Simmons R, et al. CD161 defines a transcriptional and functional phenotype across distinct human T cell lineages. Cell reports 2014;9:1075‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kang YH, Seigel B, Bengsch B, Fleming VM, Billerbeck E, Simmons R, et al. CD161(+)CD4(+) T cells are enriched in the liver during chronic hepatitis and associated with co‐secretion of IL‐22 and IFN‐gamma. Front Immunol 2012;3:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Billerbeck E, Kang YH, Walker L, Lockstone H, Grafmueller S, Fleming V, et al. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue‐homing properties. Proc Natl Acad Sci U S A 2010;107:3006‐3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, et al. Circulating and gut‐resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med 2009;206:525‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Llibre A, Klenerman P, Willberg CB. Multi‐functional lectin‐like transcript‐1: a new player in human immune regulation. Immunol Lett 2016;177:62‐69. [DOI] [PubMed] [Google Scholar]
- 46. Llibre A, Lopez‐Macias C, Marafioti T, Mehta H, Partridge A, Kanzig C, et al. LLT1 and CD161 expression in human germinal centers promotes B cell activation and CXCR4 downregulation. J Immunol 2016;196:2085‐2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rosen DB, Cao W, Avery DT, Tangye SG, Liu YJ, Houchins JP, et al. Functional consequences of interactions between human NKR‐P1A and its ligand LLT1 expressed on activated dendritic cells and B cells. J Immunol 2008;180:6508‐6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chalan P, Bijzet J, Huitema MG, Kroesen BJ, Brouwer E, Boots AM. Expression of lectin‐like transcript 1, the ligand for CD161, in rheumatoid arthritis. PLoS One 2015;10:e0132436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Llibre A, Garner L, Partridge A, Freeman GJ, Klenerman P, Willberg CB. Expression of lectin‐like transcript‐1 in human tissues. F1000Res 2016;5:2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Burak KW, Swain MG, Santodomingo‐Garzon T, Lee SS, Urbanski SJ, Aspinall AI, et al. Rituximab for the treatment of patients with autoimmune hepatitis who are refractory or intolerant to standard therapy. Can J Gastroenterol 2013;27:273‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1163/full.
Supporting Information Figure 1
Supporting Information Figure 2
Supporting Information Figure 3
Supporting Information Figure 4
Supporting Information Figure 5
Supporting Information Figure 6
Supporting Information Figure 7
Supporting Information Figure 8
Supporting Information Figure 9
Supporting Information Figure 10
Supporting Information Figures
Supporting Information Tables
Supporting Information


