Abstract
Cirrhosis and portal hypertension can lead to the formation of a spontaneous splenorenal shunt (SSRS) that may divert portal blood flow to the systemic circulation and reduce hepatic perfusion. Our aims were to evaluate SSRSs as an independent prognostic marker for mortality in patients with decompensated cirrhosis and the influence of SSRSs on liver transplantation (LT) outcomes. We retrospectively analyzed adult patients with decompensated cirrhosis undergoing LT evaluation from January 2001 to February 2016 at a large U.S. center. All patients underwent liver cross‐sectional imaging within 6 months of evaluation, and images were reviewed by two radiologists. Clinical variables were obtained by electronic health record review. The cohort was followed until death or receipt of LT, and the subset receiving LT was followed for death after LT or graft failure. Survival data were analyzed using multivariable competing risk and Cox proportional‐hazards regression models. An SSRS was identified in 173 (23%) of 741 included patients. Patients with an SSRS more often had portal vein thrombosis and less often had ascites (P < 0.01). An SSRS was independently associated with a nonsignificant trend for reduced mortality (adjusted subhazard ratio, 0.81; Gray's test P = 0.08) but had no association with receipt of LT (adjusted subhazard ratio, 1.02; Gray's test P = 0.99). Post‐LT outcomes did not differ according to SSRS for either death (hazard ratio, 0.85; log‐rank P = 0.71) or graft failure (hazard ratio, 0.71; log‐rank P = 0.43). Conclusion: Presence of an SSRS does not predict mortality in patients with decompensated cirrhosis or in LT recipients. (Hepatology Communications 2018;2:437‐444)
Abbreviations
- CI
confidence interval
- HR
hazard ratio
- LT
liver transplantation
- MELD
Model for End‐Stage Liver Disease
- SHR
subhazard ratio
- SSDMF
Social Security Death Master File
- SSRS
spontaneous splenorenal shunt
- TIPS
transjugular intrahepatic portosystemic shunt
- UNOS
United Network for Organ Sharing
Introduction
Chronic liver injury induces hepatic stellate cell activation, with progressive fibrosis culminating in liver cirrhosis and elevated hepatic vascular resistance leading to portal hypertension.1, 2 Portosystemic shunts are collateral blood vessels that form as a compensatory response to portal hypertension and divert blood flow to the systemic circulation. Spontaneous splenorenal shunts (SSRSs) are a common type of portosystemic shunt (occurring in an estimated 14% to 60% of patients with cirrhosis) that decompress the portal circulation through the left renal vein and inferior vena cava (Fig. 1).3, 4, 5 In clinical practice, an SSRS is relevant as a point of access to the portal circulation in the angiographic obliteration of bleeding varices or can be ligated to treat hepatic encephalopathy refractory to maximal medical therapy.6, 7
Figure 1.
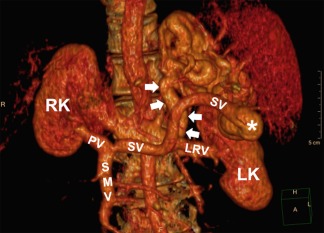
Three‐dimensional computerized tomographic reconstruction showing the anatomy of an SSRS, designated by the white arrows. SSRSs divert retrograde blood flow from the splenic vein in the portal circulation (confluence with the SSRS designated by the asterisk) to the left renal vein in the systemic circulation; blood then flows into the inferior vena cava. Abbreviations: LK, left kidney; LRV, left renal vein; PV, portal vein; RK, right kidney; SMV, superior mesenteric vein; SV, splenic vein.
Gastroesophageal varices are a well‐established predictor of adverse outcomes in cirrhosis due to their risk of hemorrhage, but the relationship of an SSRS with liver outcomes is uncertain. Hypothetically, the formation of an SSRS might exert a protective effect by lowering portal blood pressure and thereby reducing the likelihood of portal hypertensive complications. Indeed, the formation of gastroesophageal varices has been found to occur less frequently in patients with an SSRS.4 Alternatively, high blood flow through an SSRS could result in a “portal steal” phenomenon that deprives the already injured liver of blood, oxygen, and nutrients, accelerating disease progression and increasing the risk of adverse liver outcomes. The latter effect has led to postulation that SSRS ligation should be considered in patients undergoing liver transplantation (LT) to prevent ischemic injury to the allograft, particularly if portal inflow is suboptimal based on Doppler ultrasound.8
No prior studies have assessed the association of SSRSs with long‐term clinical outcomes in patients with decompensated cirrhosis. The aims of this study were to evaluate SSRSs as an independent predictor of clinical outcomes in patients undergoing evaluation for LT and to evaluate the natural history and transplant outcomes of LT recipients with an SSRS. We hypothesized that patients with decompensated cirrhosis and an SSRS would have lower survival relative to patients without an SSRS and that SSRSs would rapidly resolve after LT and not portend adverse post‐LT outcomes.
Participants and Methods
We retrospectively identified all patients ≥18 years of age who underwent evaluation for deceased donor liver transplantation at Oregon Health & Science University Hospital from September 2001 to February 2016. We excluded patients with hepatocellular carcinoma (as such patients represent a unique subpopulation less likely to have portal hypertension), acute liver failure, surgical shunts or transjugular intrahepatic portosystemic shunts (TIPS), prior LT recipients, and those without cross‐sectional liver imaging within 6 months of LT evaluation. All patients were confirmed to have cirrhosis based on radiologist review of cross‐sectional imaging. We chose to study patients with decompensated cirrhosis undergoing LT evaluation because such liver imaging is a routine component of the evaluation, thereby preventing the introduction of a selection bias related to the imaging indication.
DATA COLLECTION
Two body radiologists (A.F. and K.K.J.) reviewed all cross‐sectional liver imaging studies, which consisted of either dynamic multiphase computed tomography or magnetic resonance imaging, for the presence and size of SSRSs, ascites, gastroesophageal varices, and portal vein thrombosis. An SSRS was defined as any continuous vascular connection between the splenic vein near the splenic hilum and the left renal vein and was measured at the largest cross‐sectional diameter. In addition, electronic health records were reviewed to obtain demographic and clinical variables (age, sex, liver disease etiology, Model for End‐Stage Liver Disease [MELD] score, diuretic use, and histories of ascites, spontaneous bacterial peritonitis, and/or variceal bleeding), and endoscopy reports were reviewed to assess the endoscopic presence of gastroesophageal varices.
Whether accepted and listed on the LT wait list or declined for LT, patients were followed from LT evaluation to outcomes of death without LT or receipt of LT, or were censored at the time of last reported follow‐up without having received LT. Ascertainment of these outcomes was performed by reviewing the electronic health record and was corroborated using the United Network for Organ Sharing (UNOS) patient registry and linkage to the Social Security Death Master File (SSDMF).9, 10
We also evaluated post‐LT outcomes among the subset of patients who underwent LT. For that analysis, clinicodemographic variables were obtained at the time of LT from the electronic health record and the UNOS registry. Operative reports of the LT were reviewed to evaluate for SSRS ligation. No systematic change in the surgical technique for LT occurred over the study period at the study center. Patients were followed from the time of LT to outcomes of death or graft failure, or were censored at the last reported follow‐up visit without either outcome having occurred. These outcomes were obtained from the electronic health record, the UNOS registry, and the SSDMF. Among patients undergoing LT with a known SSRS, the two body radiologists also reviewed the most recent post‐LT liver imaging studies obtained at least 90 days after LT to evaluate for persistence or regression of the SSRS. No donor organs were obtained from executed prisoners or other institutionalized persons.
STATISTICAL ANALYSIS
We performed a sample size calculation assuming an estimated SSRS prevalence of 25% (based on prior literature), a control group survival of 50%, a hazard ratio (HR) for mortality of 1.4, and a 10% loss to follow‐up, which yielded a sample size of 758 (1:4 ratio of 152 patients with an SSRS to 606 patients without an SSRS) to achieve 80% power with a two‐sided P value of 0.05 in a log‐rank test. We expressed baseline variables as medians ± interquartile range if continuous and as proportions if categorical, and performed comparisons using the rank‐sum and χ2 tests, respectively. We evaluated binary outcome survival data using the Kaplan–Meier method and the log‐rank test to compare estimated survival functions and competing risk survival data using the Fine–Gray method and Gray's test to compare cumulative incidence functions.11, 12 We then developed multivariable competing risk regression models to evaluate outcomes after LT and Cox proportional‐hazards regression models to evaluate post‐LT outcomes. All regression models were evaluated for heterogeneity between SSRSs and all other variables, using interaction testing. The change in mean SSRS diameter after LT was compared using the paired t test. All analyses were performed using STATA/MP version 13.1 and R Studio version 0.99.486 for Macintosh OS X. The study was approved by the institutional review board.
Results
Over the study period, a total of 2,289 patients underwent LT evaluation at our institution. Of 741 patients enrolled based on the study's inclusion and exclusion criteria, 173 patients (23%) had an SSRS and 568 patients (77%) did not. At the time of LT evaluation, patients with and without an SSRS were similar with respect to age, MELD score, sex, and histories of variceal bleeding, spontaneous bacterial peritonitis, and diuretic use (P > 0.05 for all variables; Table 1). Patients with an SSRS were more likely to have a portal vein thrombosis (13% versus 4%; P < 0.01) and gastroesophageal varices on imaging (94% versus 85%; P < 0.01) and less likely to have ascites on imaging (43% versus 59%; P < 0.01). Use of diuretics did not differ among patients with an SSRS and those with (P = 0.63) or without ascites (P = 0.42). Liver disease etiology was similar between the two groups, although there was a trend toward a higher prevalence of cryptogenic/nonalcoholic steatohepatitis cirrhosis in patients with an SSRS (P = 0.08). Comparing patients who were listed for LT (47% of the cohort) to those who were not, listed patients had higher MELD scores (median 16 versus 14; P < 0.01), less often had hepatitis C (43% versus 51%; P = 0.03) or alcoholic liver disease (26% versus 43%; P = <0.01), more often had primary biliary cirrhosis or primary sclerosing cholangitis (16% versus 9%; P < 0.01), and were less often using diuretics (70% versus 77%; P = 0.03). However, the proportions of listed and not listed patients with an SSRS were similar (24% versus 23%; P = 0.73).
Table 1.
Baseline Characteristics by SSRS Status (N = 741)
| Median (IQR) or % |
SSRS n = 173 |
No SSRS n = 568 |
P |
|---|---|---|---|
| Age | 55 (49‐60) | 54 (49‐59) | 0.60 |
| Male | 54% | 57% | 0.40 |
| MELD score | 15 (12‐19) | 15 (12‐20) | 0.80 |
| CT imaging (vs. MRI) | 58% | 56% | 0.59 |
| Portal vein thrombosis | 13% | 4% | <0.01 |
| Gastroesophageal varices on endoscopy | 68% | 75% | 0.08 |
| Gastroesophageal varices on imaging | 94% | 85% | <0.01 |
| Ascites on imaging | 43% | 59% | <0.01 |
| Liver disease etiology | |||
| Hepatitis C | 47% | 47% | 0.90 |
| Hepatitis B | 4% | 2% | 0.16 |
| Alcohol | 31% | 36% | 0.17 |
| Primary biliary or sclerosing cholangitis | 13% | 12% | 0.84 |
| Cryptogenic/nonalcoholic steatohepatitis | 17% | 12% | 0.08 |
| Autoimmune hepatitis | 6% | 4% | 0.19 |
| Other | 5% | 6% | 0.70 |
| History of spontaneous bacterial peritonitis | 12% | 12% | 0.94 |
| History of variceal bleed | 25% | 28% | 0.42 |
| Diuretic use | 75% | 73% | 0.62 |
Abbreviations: CT, computerized tomography; IQR, interquartile range; MRI, magnetic resonance imaging.
ASSOCIATION OF SPONTANEOUS SPLENORENAL SHUNTS WITH DECOMPENSATED CIRRHOSIS OUTCOMES
Of patients with an SSRS evaluated for LT, 35% died and 25% underwent LT compared to 42% dying and 24% undergoing LT in patients without an SSRS (P = 0.19; Table 2). Median follow‐up time was longer in the SSRS group (507 versus 372 days; P = 0.06). In multiple regression, an SSRS was independently associated with a nonsignificant trend toward a lower risk of death (adjusted subhazard ratio [SHR], 0.81; 95% confidence interval [CI], 0.60‐1.13; Gray's test P = 0.08) (Table 3; Fig. 2A). An SSRS did not predict the risk of receiving LT (adjusted SHR, 1.02; 95% CI, 0.68‐1.54; Gray's test P = 0.99) (Table 3; Fig. 2B). The adjusted risk of death did not meaningfully change in a sensitivity analysis in which patients were required to have an SSRS ≥1 cm to be in the SSRS group (adjusted SHR, 0.90; 95% CI, 0.65‐1.24). No significant interactions were identified between SSRSs and any other variable in the multiple regression models.
Table 2.
Patient Outcomes After Liver Transplant Evaluation
| Outcomes |
SSRS n = 173 |
No SSRS n = 568 |
P |
|---|---|---|---|
| Days of follow‐up, median (IQR) | 507 (187‐1106) | 372 (121‐1082) | 0.06 |
| Alive without transplant, n (%) | 71 (41%) | 196 (35%) | 0.19 |
| Transplanted, n (%) | 42 (25%) | 134 (24%) | |
| Died, n (%) | 60 (35%) | 238 (42%) |
Abbreviation: IQR, interquartile range.
Table 3.
Patient Outcomes After Liver Transplant Evaluation in Competing Risks Analysis
| Incidence per 100 Person‐Years (95% CI) | Cumulative Incidence |
SHR (95% CI) |
Adjusted SHR (95% CI)a |
|||||
|---|---|---|---|---|---|---|---|---|
| Person‐Years | 1‐Year | 5‐Year | 10‐Year |
Gray's Test P |
||||
| Death | ||||||||
| No splenorenal shunt | 1,225 | 19.4 (17.1‐22.1) | 21.1% | 36.4% | 41.4% | 0.08 | Ref | Ref |
| Splenorenal shunt | 388 | 15.5 (12.0‐19.9) | 15.6% | 29.5% | 33.5% | 0.79 (0.60‐1.04) | 0.81 (0.60‐1.13) | |
| Liver transplantation | ||||||||
| No splenorenal shunt | 1,225 | 10.9 (9.2‐13.0) | 16.0% | 22.4% | 23.4% | 0.99 | Ref | Ref |
| Splenorenal shunt | 388 | 10.8 (8.0‐14.7) | 12.7% | 22.5% | 24.3% | 1.02 (0.73‐1.43) | 1.02 (0.68‐1.54) | |
Adjusted for age, sex, imaging modality, portal vein thrombosis, endoscopic varices, ascites, liver disease etiology, history of spontaneous bacterial peritonitis, history of variceal bleed, and use of diuretics.
Figure 2.
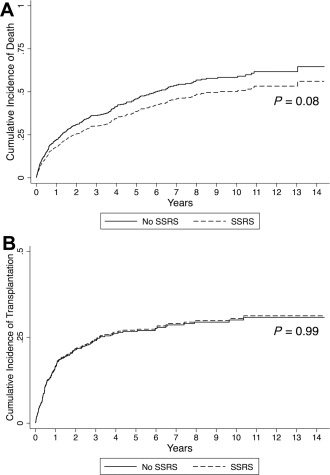
(A) Cumulative incidence of death or (B) receipt of liver transplantation in patients with decompensated cirrhosis who underwent liver transplant evaluation, according to the presence or absence of an SSRS.
We also evaluated predictors of death among patients with SSRS after their LT evaluation (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1157/full). An alcohol‐related liver disease etiology was significantly associated with death (SHR 1.99; 95% CI, 1.21‐3.29). All other considered variables did not significantly predict death among patients with SSRS who were evaluated for LT, including variables representing portal hypertension (ascites, diuretic use, portal vein thrombosis, endoscopic varices, and prior spontaneous bacterial peritonitis or variceal bleeding).
PATIENT AND SPLENORENAL SHUNT OUTCOMES AFTER LT
Follow‐up data were available for 170 of 176 patients who underwent LT (96.5%). The remaining 6 patients underwent LT at other institutions, and none of these patients had an SSRS at the time of their LT evaluations. Forty‐two of the 170 analyzed LT recipients had an SSRS (24.7%). Based on review of LT operative reports, none of the patients with an SSRS received intraoperative SSRS ligation. Patient outcomes after LT are shown in Table 4. Although the post‐LT mortality rate was lower among patients with an SSRS (2.9 deaths per 100 person‐years versus 3.4 deaths per 100 person‐years without SSRS), presence of an SSRS was not a significant predictor of lower mortality (HR, 0.85; 95% CI, 0.36‐1.99; log‐rank P = 0.71) (Fig. 3A). Similarly, graft failure incidence was lower with an SSRS (2.9 events per 100 person‐years versus 3.9 per 100 person‐years without), but the presence of an SSRS was not a significant predictor of lower graft failure (HR, 0.71; 95% CI, 0.31‐1.66; log‐rank P = 0.43) (Fig. 3B).
Table 4.
Patient Outcomes After Liver Transplantation in Cox Proportional‐Hazards Regression
| Incidence per 100 Person‐Years (95% CI) | Overall or Graft Survival |
Hazard Ratio (95% CI) |
|||||
|---|---|---|---|---|---|---|---|
| Person‐Years | 1‐Year | 5‐Year | 10‐Year | Log‐Rank P | |||
| Death | |||||||
| No splenorenal shunt | 670 | 3.4 (2.3‐5.2) | 96.9% | 86.9% | 73.0% | 0.71 | Ref |
| Splenorenal shunt | 240 | 2.9 (1.4‐6.1) | 97.5% | 83.3% | 78.1% | 0.85 (0.36‐1.99) | |
| Graft failure | |||||||
| No splenorenal shunt | 659 | 3.9 (2.7‐5.8) | 93.7% | 85.5% | 67.7% | 0.43 | Ref |
| Splenorenal shunt | 240 | 2.9 (1.4‐6.1) | 97.5% | 83.2% | 78.0% | 0.71 (0.31‐1.66) | |
Figure 3.
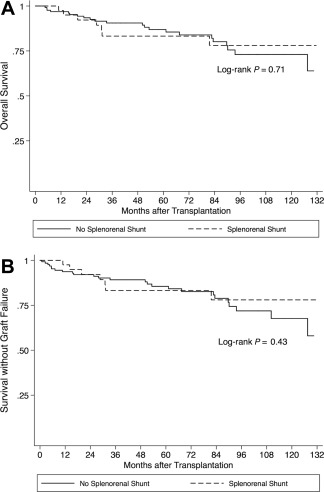
(A) Overall survival and (B) survival without liver allograft failure in patients who underwent transplantation for decompensated cirrhosis, according to the presence or absence of an SSRS.
Of the 42 patients who underwent LT with an SSRS identified on imaging during their LT evaluations, 25 had cross‐sectional computed tomography or magnetic resonance imaging days after LT (interquartile range, 298‐1,840 days). The mean SSRS diameter was 12 mm prior to LT and 10 mm after LT (P = 0.03), and the SSRS decreased in diameter in 12 patients (48%), stayed the same diameter in 11 patients (44%), and increased in diameter in 2 patients (8%) (Fig. 4).
Figure 4.
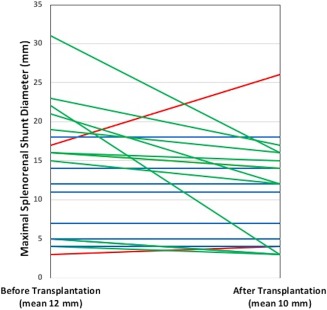
Change in the maximal diameter of SSRSs after liver transplantation. A reduction in SSRS diameter is denoted with green lines, no change in diameter with blue lines, and an increase in diameter with red lines.
Discussion
Portal hypertension is a key mediator of adverse outcomes in patients with cirrhosis.13 Although the development of gastroesophageal varices imparts risk to patients with cirrhosis in the form of portal hypertensive bleeding, it remains uncertain whether other common sources of portosystemic collateralization impart a similar risk because such bleeding rarely occurs and their decompressive effects might reduce portal hypertension and its negative consequences; alternatively, such collaterals could exert a harmful effect by diverting oxygenated blood away from the liver, inducing a relative ischemia of that organ or inciting the development of hepatic encephalopathy. We observed a net beneficial effect of SSRSs, with patients less often having ascites (despite no difference in diuretic use) and their presence conferring a reduced risk of death in advanced chronic liver disease (albeit not significant at the P < 0.05 threshold). SSRSs were not associated with increased risks for mortality or graft failure after LT, and the plurality of patients with subsequent post‐LT imaging experienced a reduction in SSRS diameter despite none undergoing SSRS ligation at the time of LT. Therefore, SSRSs may play an important role in liver disease outcomes, predominately mediated through decompression of the portal circulation, but this pathophysiology either resolves or becomes clinically inconsequential after the portal hypertension is corrected with LT.
The insertion of a TIPS imposes a similar physiology to the SSRS and was shown to predict a similar beneficial effect on mortality risk in patients with decompensated cirrhosis. Among patients listed for LT in the national UNOS patient registry, the presence of TIPS was associated with a modest but significantly reduced risk of death (adjusted SHR, 0.95), attributed to less frequent portal hypertensive complications.14 One key distinction in evaluating the effect of TIPS is that its presence inherently introduces a selection bias relative to patients without a TIPS because the TIPS patients had to have been considered healthy enough to receive one a priori, whereas the non‐TIPS patients had no such requirement for good health. Accordingly, TIPS patients were found to have lower MELD scores and were less likely to receive LT compared to their non‐TIPS counterparts (adjusted SHR, 0.92). However, we believe that our study represents a less biased evaluation of the clinical consequences of a similar pathophysiology because SSRSs form naturally rather than as a consequence of a particular clinical event and are not known to develop in accordance with a patient's health status (all patients undergo imaging at the time of the LT evaluation and the assessment for an SSRS was not biased by an imaging indication). In addition, the MELD score of our SSRS and non‐SSRS patients did not significantly differ. Thus, it is conceivable that the risk of receiving LT may not be lower in TIPS patients had it been possible to conduct an analysis unbiased by the indication for the shunt procedure and for differences in liver function at the time of LT evaluation. The few other studies evaluating the relationship of SSRSs with liver disease severity or outcomes have yielded variable results and were not adjusted for confounding variables or designed to evaluate time‐dependent risks.3, 4, 5
Based on concerns for inadequate hepatic allograft perfusion and hepatic encephalopathy due to persistent portosystemic shunting, intraoperative SSRS ligation during LT has been explored using a variety of surgical techniques.8, 15, 16, 17, 18, 19, 20 These case series observed that SSRSs frequently persisted after LT, particularly when larger than 10 mm, reported an increase in portal vein flow after shunt ligation, and documented the relative safety of the procedure. However, these series were not designed to evaluate the efficacy of the intervention relative to expectant management, and several were limited to living‐donor LT recipients, who inherently have a smaller portal vein diameter and may be more prone to develop inadequate portal vein inflow. We also found that although SSRSs often persisted after LT, the absence of shunt ligation in all patients was not associated with a higher risk of mortality or allograft loss, even when restricting the comparison to larger SSRSs (≥10 mm). Based on our retrospective design, we were unable to evaluate portal vein inflow or perform neuropsychologic testing for hepatic encephalopathy; therefore, it is possible that the SSRS patients experienced more adverse physiologic or clinical consequences that did not herald an increased risk of death or graft failure.
There were several limitations to our study. First, we restricted our analysis to patients with decompensated cirrhosis undergoing LT evaluation, and this may reduce the generalizability of our results. While this approach offered epidemiologic advantages (elimination of bias arising from the indication for abdominal imaging, a low rate of missing data in this closely monitored patient group, and long follow‐up times), it also represents a cohort with more advanced liver disease, fewer medical comorbidities, and higher socioeconomic status than patients with cirrhosis in general. We attempted to better simulate real‐world conditions and community practice by including patients who were evaluated but declined for LT, tracking their mortality through linkage to the SSDMF. However, an SSRS itself could be a marker for liver dysfunction associated with an increased risk for LT referral; this would lead to an artefactually high estimate of SSRS prevalence relative to a general population of patients with cirrhosis. Second, as stated above, we were unable to methodically evaluate subgroups of SSRS patients at higher risk for an adverse clinical course and likely to benefit more from intraoperative SSRS ligation at LT, such as those with impaired portal vein inflow or risk factors for persistent hepatic encephalopathy in the post‐LT period. Thus, our results do not disprove a salutary effect of SSRS ligation for a variety of “softer” outcomes that we did not evaluate. Finally, because all included patients underwent evaluation for LT, a large proportion ultimately underwent LT who would have died without it. However, we analyzed survival data using competing risk regression to account for the interdependence of each outcome with the alternate competing outcome, allowing unbiased risk estimates to be obtained.21
In summary, the presence of an SSRS in a large cohort of patients with cirrhosis undergoing LT evaluation was associated with a statistically insignificant reduced risk of death and no effect on the risk of receiving LT. Patients undergoing LT with an SSRS had no increased risk of death or allograft failure despite none undergoing SSRS ligation. Future research on this topic should be directed at a prospective clinical trial to evaluate the efficacy and safety of SSRS ligation in LT recipients. This should focus on subgroups likely to be at the highest risk for adverse patient and graft outcomes, such as those with very large shunts or low portal vein flow.
Supporting information
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1157/full.
Supporting Information 1
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Zhou W‐C, Zhang Q‐B, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroentero 2014;20:7312‐7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garcia‐Tsao G. Current management of the complications of cirrhosis and portal hypertension: variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Gastroenterology 2001;120:726‐748. [DOI] [PubMed] [Google Scholar]
- 3. Zardi EM, Uwechie V, Caccavo D, Pellegrino NM, Cacciapaglia F, Di Matteo F, et al. Portosystemic shunts in a large cohort of patients with liver cirrhosis: detection rate and clinical relevance. J Gastroenterol 2009;44:76‐83. [DOI] [PubMed] [Google Scholar]
- 4. Tarantino G, Citro V, Conca P, Riccio A, Tarantino M, Capone D, et al. What are the implications of the spontaneous spleno‐renal shunts in liver cirrhosis? BMC Gastroenterol 2009;9:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Carlis L, Del Favero E, Rondinara G, Belli LS, Sansalone CV, Zani B, et al. The role of spontaneous portosystemic shunts in the course of orthotopic liver transplantation. Transpl Int 1992;5:9‐14. [DOI] [PubMed] [Google Scholar]
- 6. Choi YH, Yoon CJ, Park JH, Chung JW, Kwon JW, Choi GM. Balloon‐occluded retrograde transvenous obliteration for gastric variceal bleeding: its feasibility compared with transjugular intrahepatic portosystemic shunt. Korean J Radiol 2003;4:109‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vavasseur D, Duvoux C, Cherqui D, Derhy S, Rahmouni A, Dhumeaux D, et al. Chronic hepatic encephalopathy due to spontaneous splenorenal shunt: successful treatment by transhepatic shunt embolization. Cardiovasc Intervent Radiol 1994;17:298‐300. [DOI] [PubMed] [Google Scholar]
- 8. Horrow MM, Phares MA, Viswanadhan N, Zaki R, Araya V, Ortiz J. Vascular steal of the portal vein after orthotopic liver transplant: intraoperative sonographic diagnosis. J Ultrasound Med 2010;29:125‐128. [DOI] [PubMed] [Google Scholar]
- 9. U.S. Department of Health and Human Services, Organ Procurement and Transplantation Network . Data: citing data. http://optn.transplant.hrsa.gov/data/citing-data. Accessed July 2017.
- 10. Social Security Administration . Social Security Death Master File. Limited Access Death Master File (DMF) ‐ FAQS. https://www.ssdmf.com/RelId/2239598/ISvars/default/F_A_Q_.htm. Accessed January 2018.
- 11. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc 1999;94:496‐509. [Google Scholar]
- 12. Dignam JJ, Kocherginsky MN. Choice and interpretation of statistical tests used when competing risks are present. J Clin Oncol 2008;26:4027‐4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garcia‐Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology 2010;51:1445‐1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berry K, Lerrigo R, Liou IW, Ioannou GN. Association between transjugular intrahepatic portosystemic shunt and survival in patients with cirrhosis. Clin Gastroenterol Hepatol;14:118‐123. [DOI] [PubMed] [Google Scholar]
- 15. Sadamori H, Yagi T, Matsukawa H, Matsuda H, Shinoura S, Umeda Y, et al. The outcome of living donor liver transplantation with prior spontaneous large portasystemic shunts. Transpl Int 2008;21:156‐162. [DOI] [PubMed] [Google Scholar]
- 16. Kim H, Yoon KC, Lee KW, Yi NJ, Lee HW, Choi Y, et al. Tips and pitfalls in direct ligation of large spontaneous splenorenal shunt during liver transplantation. Liver Transpl 2017;23:899‐906. [DOI] [PubMed] [Google Scholar]
- 17. Golse N, Bucur PO, Faitot F, Bekheit M, Pittau G, Ciacio O, et al. Spontaneous splenorenal shunt in liver transplantation: results of left renal vein ligation versus renoportal anastomosis. Transplantation 2015;99:2576‐2585. [DOI] [PubMed] [Google Scholar]
- 18. Awad N, Horrow MM, Parsikia A, Brady P, Zaki R, Fishman MD, et al. Perioperative management of spontaneous splenorenal shunts in orthotopic liver transplant patients. Exp Clin Transplant 2012;10:475‐481. [DOI] [PubMed] [Google Scholar]
- 19. Braun MM, Bar‐Nathan N, Shaharabani E, Aizner S, Tur‐Kaspa R, Belenky A, et al. Postshunt hepatic encephalopathy in liver transplant recipients. Transplantation 2009;87:734‐739. [DOI] [PubMed] [Google Scholar]
- 20. Margarit C, Lazaro JL, Charco R, Hidalgo E, Revhaug A, Murio E. Liver transplantation in patients with splenorenal shunts: intraoperative flow measurements to indicate shunt occlusion. Liver Transpl Surg 1999;5:35‐39. [DOI] [PubMed] [Google Scholar]
- 21. Jepsen P, Vilstrup H, Andersen PK. The clinical course of cirrhosis: the importance of multistate models and competing risks analysis. Hepatology 2015;62:292‐302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1157/full.
Supporting Information 1


