Abstract
Based on their unique advantages, increasing interest has been shown in the use of aptamers as target ligands for specific cancer cell recognition and targeted cancer therapy. Recently, the development of aptamer-conjugated nanomaterials has offered new therapeutic opportunities for cancer treatment with better efficacy and lower toxicity. We highlight some of the promising classes of aptamer-conjugated nanomaterials for the specific recognition of cancer cells and targeted cancer therapy. Recent developments in the use of novel strategies that enable sensitive and selective cancer cell recognition are introduced. In addition to targeted drug delivery for chemotherapy, we also review how aptamer-conjugated nanomaterials are being incorporated into emerging technologies with significant improvement in efficiency and selectivity in cancer treatment.
Keywords: aptamer, cancer therapy, cell recognition, nanomaterials
INTRODUCTION
Despite advances in our understanding of molecular biology, chemotherapy, radiotherapy and conventional surgical procedures, cancer remains one of the leading causes of death in the world.1 Current cancer therapy, including chemotherapy and radiotherapy, often lacks tumor cell specificity, resulting in severe toxic effects for cancer patients undergoing these treatments. The ultimate goal in cancer therapy remains focused on the development of treatment modalities that effectively kill tumor cells without harming normal cells.2 Thus, novel strategies for targeted cancer therapy are in great demand for effective cancer treatment.
With the rapid development of nanotechnology, various nanostructured materials have been successfully synthesized for biomedical applications.3 Their diverse characteristics with multifunctional theranostic capability show promising potential in cancer therapy.4 These nanomaterials can nonspecifically accumulate in cancer tissue through the enhanced permeability and retention (EPR) effect, that is, by passive targeting, albeit with limited dosage and selectivity.5 Recently, however, the active, cell-specific targeting of nanomaterials has begun to represent a potentially powerful technology in cancer treatment. Active targeting is achieved by conjugating nanomaterials with targeting ligands that bind to overexpressed antigens or receptors on the target cells. This specific binding to targeted cells leads to an increased accumulation of nanomaterials on target cells while minimizing harmful toxicity to non-target cells.
Over the past several years, aptamers have become a new class of targeting ligands for diagnostic and therapeutic application in cancer therapy.6–8 Aptamers are short, synthetic, single-stranded oligonucleotides that specifically bind to various molecular targets, including small molecules, proteins, nucleic acids, and even cells and tissues with high affinity and specificity.9,10 Aptamers are derived from an iterative process called systematic evolution of ligands by exponential enrichment and represent a unique class of molecules that are larger than small-molecule drugs but smaller than antibodies.11,12 Compared with traditional ligands, including antibodies, peptides and small molecules, aptamers exhibit advantages such as low cost, low immunogenicity and toxicity, a small size to enable solid tumor penetration and high affinity to bind with the target, all of which make aptamers ideal candidates for targeted cancer therapy.13,14
By combining the inherent features of nanomaterials with the specific recognition ability of aptamers, aptamer-conjugated nanomaterials may provide a more efficient and less harmful approach to meet the growing demands for novel strategies in the fight against cancer.15–18 Herein, we focus on aptamer-conjugated nanomaterials for specific cancer cell recognition and the development of novel aptamer-nanomaterial-based strategies for targeted cancer therapy. This review first considers recent progress in the use of aptamer-tethered DNA/lipid nanostructured materials and aptamer-conjugated nanoparticles for specific cancer cell recognition. Novel strategies, such as photodynamic therapy (PDT) and photothermal therapy (PTT) using aptamer-conjugated nanomaterials, are also reviewed. This aptamer-targeted strategy demonstrates high efficacy and low side effects for cancer treatment, making aptamer-conjugated nanomaterials promising candidates for use in future cancer therapy.
APTAMER-CONJUGATED NANOMATERIALS FOR SPECIFIC CELL RECOGNITION
Distinguishing cancer cells from normal cells is important for effective cancer therapy. Methods that enable sensitive and selective cancer cell detection through precise molecular recognition are highly desired for the development of targeted cancer therapy and the potential efficacy of new therapeutic modalities. Inspired by aptamer technology and nanotechnology, several strategies developed for specific cell recognition are discussed.
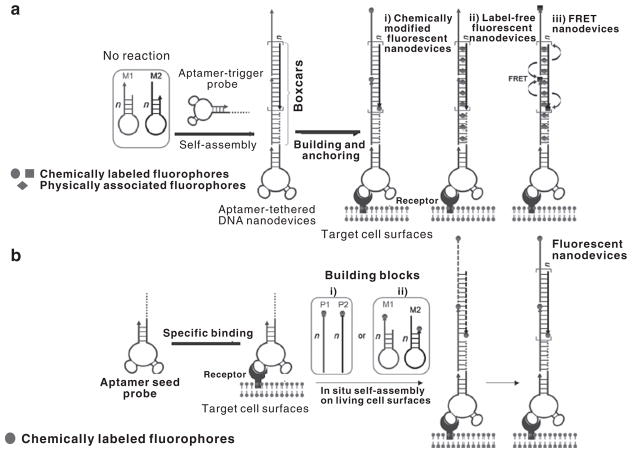
Based on aptamer-tethered DNA nanodevices (aptNDs), specific recognition and in situ self-assembly of aptNDs on target living cell surfaces have been achieved. To construct aptNDs, aptamer sgc8, which can bind to target human protein tyrosine kinase 7, was selected as a model. Protein tyrosine kinase 7 is overexpressed on the cell membrane of CCRF-CEM cells (human T-cell acute lymphocytic leukemia) but not on non-target Ramos cells. As illustrated in Figure 1, two partially complementary hairpin monomers, M1 and M2, and an aptamer probe were used to construct aptNDs through either a hybridization chain reaction-based self-assembly upon initiation by an aptamer-tethered trigger probe (Figure 1a) or by cascading alternative hybridization of two partially complementary monomers initiated by aptamer seed probes (Figure 1b(i)). The aptNDs could efficiently anchor or in situ self-assemble on the target cell surfaces. Either covalent chemical labeling of multiple copies of fluorophores or noncovalent physical association with multiple fluorogenic double-stranded DNA-intercalating fluorophores on each nanodevice provided enhanced fluorescence signals for effective cancer detection.19
Figure 1.
Construction of fluorescent DNA nanodevices on target living cell surfaces based on an aptamer-tethered DNA nanodevice platform, where (a) three types of fluorescent DNA nanodevices, preformed via hybridization chain reaction (HCR)-based self-assembly upon initiation by aptamer-tethered trigger probes, are anchored on target cell surfaces, or (b) aptamer seed probes initiate in situ self-assembly of fluorescent DNA nanodevices on target cell surfaces by either (i) cascading alternative hybridization of two partially complementary monomers or (ii) HCR (adapted from Zhu et al.19).
The high specificity of aptamers to target cells has also led to selectivity improvement in the electrochemical and electrochemiluminescence detection of cancer cells.20–22 Using fluorescence and electrochemical methods, a signal amplification supersandwich strategy was developed for highly selective and sensitive detection of cancer cells using aptamer-DNA concatamer quantum dot probes. The proposed supersandwich cytosensor exhibited high sensitivity, with a detection limit of 50 cells per ml.23 Moreover, a novel cycle-amplifying technique using a DNA device on magnetic beads was further employed to improve the sensitivity of the electrochemiluminescence assay of cancer cells.24 In particular, a strategy using an aptamer and RNA polymerase-based amplification was also developed for highly sensitive and selective cancer cell detection.25
Because most biological samples exhibit virtually no magnetic background, the use of magnetic nanoparticles (MNPs) can lead to ultrasensitive detection. Based on a magnetic relaxation switch technique and a self-amplifying proximity assay utilizing the change of spin–spin relaxation time (ΔT2) of the surrounding water protons, Bamrungsap et al. designed aptamer-conjugated magnetic nanoparticles (ACMNPs) for cancer cell detection. The ACMNPs capitalize on the ability of the sgc8 aptamer to specifically bind target cancer cells, as well as the large surface areas of MNPs, to accommodate multiple aptamer-binding events. The ACMNPs can detect as few as 10 cancer cells in 250 μl of sample. Their specificity and sensitivity were also demonstrated by detection in cell mixtures and complex biological media, including fetal bovine serum, human plasma and whole blood. Furthermore, using an array of ACMNPs, various cell types were differentiated through pattern recognition, thus creating a cellular molecular profile that will allow clinicians to accurately identify cancer cells at the molecular and single-cell level.26
In another study, a DNA aptamer-polyethyleneglycol (PEG)-lipid composite was used to modify cell surfaces for specific cell recognition. Aptamer TD05, which selectively binds to IgG receptors on the surface of Ramos cells, a B-cell lymphoma cell line and sgc8 aptamer were used for testing. Leukemia cell lines were used to demonstrate that aptamers anchored on the cell surface could act as targeting ligands that specifically recognize their target cells. Furthermore, the potential of this probe was explored in adoptive cell therapy. Immune-effector cells modified by the probe demonstrated improved affinity, while remaining cytotoxic to target cancer cells. Surface modification of living cells by the aptamer-PEG-lipid provides an effective approach for cell recognition and shows considerable potential in cell-based therapy.27
Double aptamer-conjugated gold manganese oxide (Au@MnO) hybrid nanoflowers were also used as a multifunctional platform to specifically target CCRF-CEM cells and to capture ATP molecules from cell lysate. Moreover, these sgc8 aptamer- and ATP aptamer-modified nanoflowers were utilized as an efficient ionization substrate for laser desorption/ionization, leading to highly selective detection and analysis of metabolites from cancer cells. Single-platform nanoflower conjugates containing MnO and Au components provide an ideal all-in-one system for selective binding to the target molecule and for laser desorption ionization-mass spectrometry as an ionization substrate.28 These merits, together with the simple preparation of aptamer-conjugated nanomaterials, make such strategies very promising for effective diagnosis and targeted cancer therapy.
APTAMER-CONJUGATED NANOMATERIALS FOR TARGETED CHEMOTHERAPY
As targeting ligands, aptamers can distinguish between diseased and healthy cells, thus enabling the selective delivery of therapeutic drugs to target cells for efficient chemotherapy. Aptamers can be easily conjugated with biocompatible organic or inorganic nanomaterials, thus offering a sufficient number of platforms for conjugating multiple ligands and drug molecules. Aptamer-guided drug delivery systems, such as liposomes and micelles, polymeric nanoparticles and inorganic nanoparticles, have been exploited for anticancer drug delivery. Several representative aptamer-conjugated nanomaterials are discussed below.
APTAMER-CONJUGATED ORGANIC NANOMATERIALS
Biocompatible and biodegradable nanomaterials are the most commonly explored materials for targeted drug delivery. These materials can be formulated to encapsulate various drugs and can be modified with aptamers to increase specificity, allowing for accumulation of a drug in cancer cells with a corresponding decrease in systemic toxicity.
Liposomes are the most clinically established nanosystems for drug delivery. The improved specificity and efficacy of aptamer-guided liposome delivery systems were confirmed by Kang et al.,29 who modified liposomes with sgc8 aptamer and delivered drug cargos to target cells. After 30 min of incubation time, flow cytometry results revealed that the sgc8 aptamer-liposome conjugate could specifically bind to target leukemia CCRF-CEM cells with no binding to other non-target leukemia cancer cells (NB4 cells). In another study, liposomes decorated with thioated oligonucleotide aptamer (thioaptamer) against E-selectin (EST-Apt) were constructed. The intravenous administration of EST-Apt-liposome complexes resulted in their accumulation at the tumor vasculature of breast tumor xenografts without shortening the circulation half-life.30
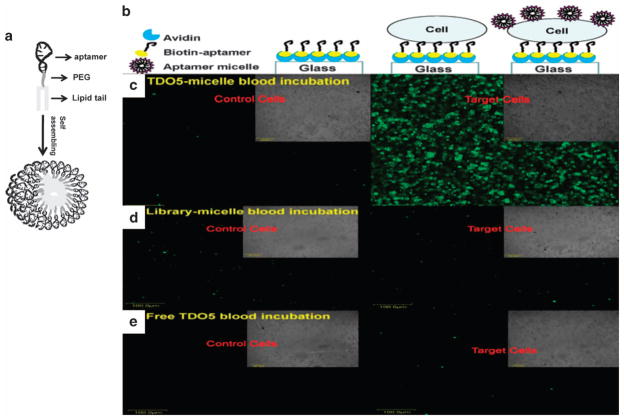
In addition to liposomes, aptamer-conjugated micelles have also been extensively studied for their potential application in drug delivery.31–33 To obtain DNA-micelle aggregates with good biocompatibility and high stability, Liu et al.34 synthesized a well-defined DNA-diacyllipid micelle with excellent thermal stability, further facilitating the development of aptamer-modified micelles as new delivery vehicles. As shown in Figure 2, the Tan group designed a self-assembling aptamer-micelle nanomaterial by attaching a lipid tail to the TD05 aptamer. Moreover, these authors mimicked a tumor site in the blood stream by immobilizing tumor cells onto the surface of a flow channel device. Flushing the aptamer-micelles through the channel demonstrated their selective recognition ability under flow circulation conditions in human whole-blood samples. By demonstrating good dynamic specificity in flow channel systems mimicking drug delivery in the blood system, aptamer-micelles have potential for cancer cell recognition and in vivo drug delivery applications.35
Figure 2.
Schematic illustration of aptamer-micelle formation (a). Stepwise immobilization scheme of the flow channel (b). Representative images of the bright field and fluorescent images of control cells (CCRF-CEM) and target cells (Ramos) captured on the flow channel surface incubated with FITC-TDO5-micelle (c), or FITC-library-micelle (d) or free FITC-TDO5 (e) spiked in a human whole-blood sample under continuous flow at 300 nl s−1 at 37 °C for 5 min. All the scale bars are 100 μm (adapted from Wu et al.35).
As polymer-based delivery systems, hydrogels have been employed in a selective target-responsive system. Using aptamers that cross-link with linear polyacrylamide chains, a general method for rapid and easy engineering of target-responsive hydrogels was demonstrated. Competitive binding of the target to the aptamer leads to decreased cross-linking density and dissolution of the hydrogel for potential drug release.36 Another novel polymer-based nanomaterial that can specifically bind to target cells with selective cytotoxicity was constructed using the T2-KK1B10 aptamer, sgc8c aptamer and TDO5 aptamer.37 Because of the selectivity of the aptamers, the toxic effect of the polymeric backbone was observed only upon internalization by the target cells, including drug-resistant cells. In other studies, aptamers that specifically bind to prostate-specific membrane antigen were intensively used as targeting ligands. Farokhzad et al.38–40 systematically studied the application of prostate-specific membrane antigen aptamer-conjugated polymeric nanoparticles loaded with various drugs for prostate cancer treatment. In addition to prostate cancer, targeted delivery of therapeutic polymeric nanoparticles is also a potentially powerful technology for treating infiltrative brain tumors using AS1411 and GMT8 aptamers, which, respectively, bind to nucleolin and U87 cells.41,42 Both in vitro and in vivo experiments demonstrated the improved antitumor cell growth effect.
Recently, DNA-based nanomaterials for targeted drug transport have also been utilized in cancer therapy.43 A long aptamer-tethered DNA nanotrain assembled from short DNA sequences was designed as a carrier with the capability for high drug payload (for example, doxorubicin). Potent antitumor efficacy and reduced side effects of drug delivered by biocompatible aptamer-tethered DNA nanotrains were demonstrated in a mouse xenograft tumor model. Moreover, fluorophores on nanotrains and drug fluorescence dequenching upon release allowed intracellular signaling of nanotrains and drugs, making aptamer-tethered DNA nanotrains attractive for the development of novel targeted drug transport platforms for cancer theranostics.
APTAMER-CONJUGATED INORGANIC NANOMATERIALS
Large surface areas coupled with a unique size and shape, as well as composition-dependent physical and chemical properties, make inorganic nanomaterials very attractive in biomedical applications.44 Combined with aptamers, inorganic nanomaterials can provide multiple modalities, such as targeted recognition, detection, drug delivery and controlled-release, in one entity.45 Based on their favorable features, aptamer-conjugated iron oxide nanoparticles, gold nanomaterials and silica nanoparticles have been extensively studied.
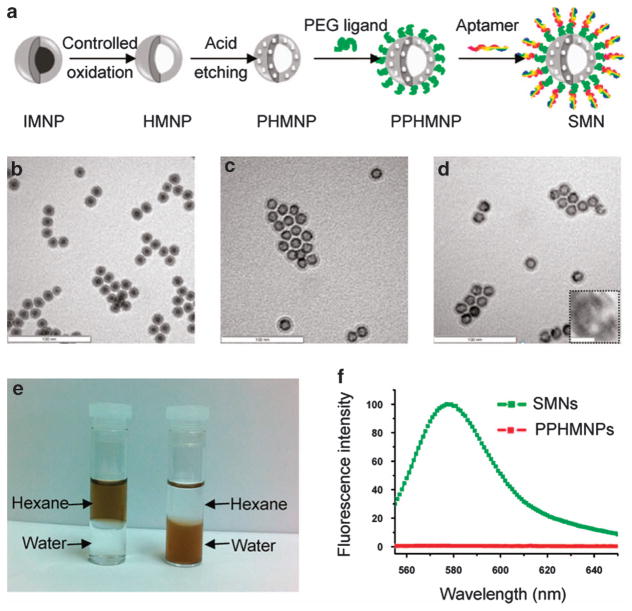
Among these biocompatible inorganic materials, MNPs have been proposed as drug carriers with a push toward clinical trials.46,47 ACMNPs have been formulated to meet the specific requirements of drug delivery and magnetic resonance imaging.48 As demonstrated in Figure 3, targeted chemotherapy and magnetic resonance imaging of cancer cells have been achieved using a smart multifunctional nanostructure (SMN) constructed from a porous hollow magnetite nanoparticle loaded with the anticancer drug doxorubicin, a heterobifunctional PEG ligand and sgc8 aptamer.49 Aptamers modified on the outer layer of SMN resulted in a multivalent effect, leading to enhanced specific binding and internalization of SMNs to target cancer cells. For the acid-labile pores, the lysosome localization of SMNs facilitates the release of doxorubicin from SMNs, enabling efficient killing of target cancer cells. In addition, T2 relaxation measurements and T2*-weighted magnetic resonance images revealed that this nanostructure can be used as a T2 contrast agent.
Figure 3.
Synthesis and characterization of smart multifunctional nanostructures (SMNs). (a) Schematic illustration of the synthesis of SMNs. TEM images of (b) iron-magnetite core-shell nanoparticles (IMNPs), (c) hollow magnetite nanoparticles (HMNPs) and (d) porous hollow magnetite nanoparticles (PHMNPs). The inset of d shows an enlarged image of a representative PHMNP. The scale bars are 100 nm (10 nm for the inset). (e) Dispersibility of PHMNPs (left) and PEGylated PHMNPs (PPHMNPs; right) in hexane and water. (f) Fluorescence intensity of PPHMNPs and SMNs (excitation: 545 nm). (adapted from Chen et al.49).
In another study, by employing different DNA fragments, a self-assembled multifunctional DNA polymer-coated superparamagnetic iron oxide nanostructure was constructed. This nanostructure combined imaging fluorescent tags, target recognition aptamers (AS1411 and sgc8) and targeted delivery drugs into one conjugated acceptor with high loading capacity and specificity.50
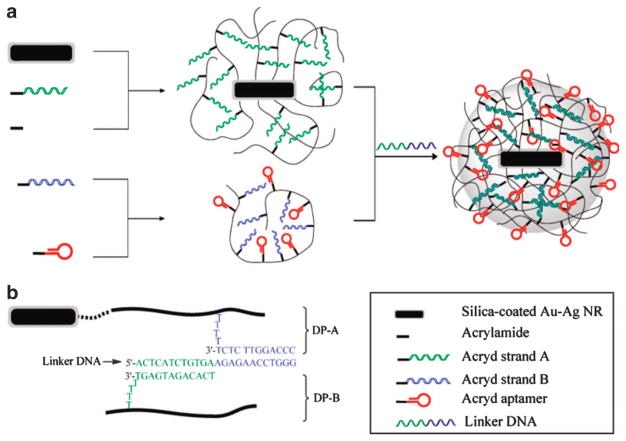
Gold nanomaterials have gained considerable attention as drug delivery platforms because of their inert and biocompatible properties, convenient synthesis and easy manipulation with a wide variety of targeting molecules.51,52 More importantly, their geometrically tunable optical characteristics and their strong photothermal response facilitate light-triggered gene/drug release in a nondestructive and controlled manner.53,54 As illustrated in Figure 4, Kang et al. constructed a near-infrared (NIR) light-responsive drug delivery platform based on Au-Ag nanorods (Au-Ag NRs) coated with DNA cross-linked polymeric shells. Exposure to a laser beam matching, the absorption peak of the Au-Ag NRs resulted in an increase in temperature leading to the rapid release of the encapsulated drug with high controllability. An in vitro study confirmed that aptamer-functionalized nanomaterials can be used as drug carriers for targeted drug delivery with remote control capability using NIR light with high spatial/temporal resolution.55 As the NIR region lies within the ‘biological window’ (700–1300 nm), where absorption and autofluorescence by tissues, blood and water are minimized,45 the design is appealing for in vivo applications.
Figure 4.
Schematic diagram illustrating the formation of an aptamer-functionalized core-shell nanogel (a). DNA sequences and linkages in the nanogel (b) (adapted from Kang et al.55). Au-Ag NR, Au-Ag nanorod.
Using typical DNA-silica surface conjugate chemistry,56 aptamer immobilization on silica nanoparticles has been developed for targeted recognition, drug delivery and stimuli-responsive release. Zhu et al. designed sgc8 aptamer-modified mesoporous silica nanoparticles for targeted drug delivery. These mesoporous silica nanoparticles were coated with polyelectrolyte multilayers to prevent premature leakage of drugs during the delivery process but controllable drug release under reducing conditions. The modification of aptamers permitted high cell recognition for this delivery vehicle, which could be used as a promising drug delivery system for specific intracellular delivery.57 In another work, an efficient cancer cell-specific fluorescent imaging and controlled release drug delivery system consisting of polyvalent mesoporous silica nanocarrier-aptamer bioconjugates was fabricated. A nanoporous core with a high surface area allowed high loading capacity with pH-dependent controlled release kinetics, and the surface-conjugated AS1411 aptamer facilitated the nanoparticle targeting of nucleolin overexpressed on MCF-7 cells.58 These studies illustrate the use of aptamers for cancer cell targeting, opening the door for the exploration of various aptamer-nanomaterial complexes that can be constructed to target multiple cancer types for the highly efficient delivery of therapeutic agents.
APTAMER-CONJUGATED NANOMATERIAL-BASED NOVEL STRATEGY FOR CANCER TREATMENT
Apart from their application as carriers for targeted drug delivery, nanomaterials have been utilized to develop novel strategies for cancer treatment.59,60 Their unique optical, electrochemical and magnetic properties combined with the specific recognition of aptamers allow for a range of novel cancer therapies to improve cancer treatment efficacy. Two of these emerging modalities are PDT and PTT.
APTAMER-CONJUGATED NANOMATERIALS FOR TARGETED PDT
PDT is a minimally invasive method that destroys cells in the presence of oxygen when light irradiates a photosensitizer, generating reactive oxygen species (mainly singlet oxygen). This process causes the destruction of cellular targets through direct cellular damage, vascular shutdown and activation of an immune response against targeted cells.61 Aptamer-conjugated nanomaterials have been applied for targeted PDT with the aim of improving the accumulation of photosensitizers in cancer tissue and selective photoinduced cancer damage.
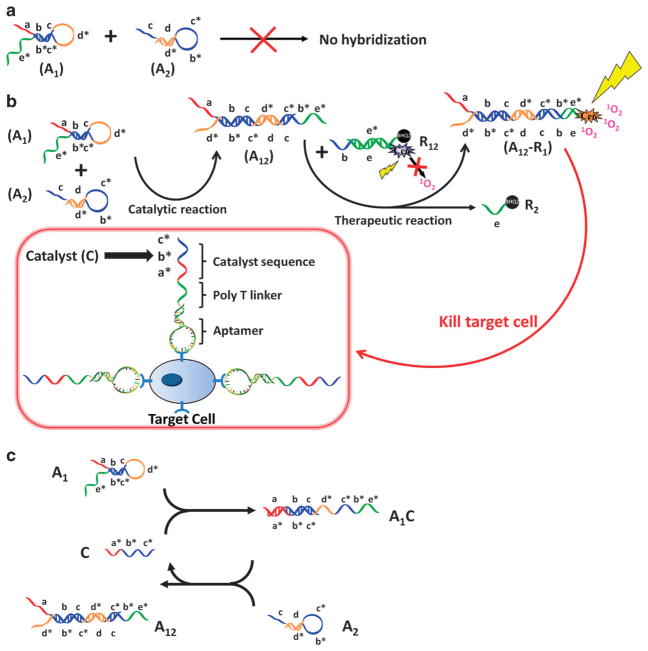
Direct conjugation of aptamers with photosensitizers or physical intercalation with photosensitizers are common methods of increasing the specific accumulation of photosensitizers at the target site.62–65 By introducing DNA self-assembly, a DNA-based nanocarrier was used for targeted PDT. As illustrated in Figure 5, the aptamers can selectively recognize target cancer cells and bind to the specific proteins on cell membranes. Then, the overhanging catalyst sequence on the aptamer can trigger a toehold-mediated catalytic strand displacement to activate the photosensitizer and achieve an amplified therapeutic effect. The specific binding-induced activation allows the DNA circuit to distinguish between diseased and healthy cells, thus reducing damage to nearby healthy cells. Moreover, the catalytic amplification reaction occurs close to the target cancer cells, resulting in a high local concentration of singlet oxygen for selective destruction.66
Figure 5.
Working scheme of DNA aptamer circuit on cell membrane. (a) Scheme of the circuit without catalyst. (b) Scheme of the circuit on the cell membrane. (c) Scheme of detailed reaction of DNA hairpins A1 and A2 catalyzed by C sequence. Different domains are labeled with different colors. All x domains are complementary to x* (adapted from Han et al.66).
The manipulation of singlet oxygen (1O2) production for targeted PDT was also performed using carbon nanotubes.67 In this study, aptamer conjugated with Ce6 was noncovalently bound with carbon nanotubes through π-stacking interactions. The attached single-stranded DNA aptamer brought the photosensitizer close to the carbon nanotube, which quenched singlet oxygen generation under light irradiation. Restoration of singlet oxygen generation occurred when aptamers were bound to target proteins. This study provides a novel strategy for PDT treatment with highly selective and controllable singlet oxygen generation.
In addition to traditional organic photosensitizers, nanomaterials used as photosensitizers offer an alternative approach to effective PDT. Liu et al. constructed an aptamer-fullerene photosensitizer and investigated the photodynamic effect. Conjugation of the R13 aptamer could effectively enhance the PDT efficiency of fullerene against A549 lung cancer cells in the presence of serum. Enhanced photodynamic efficiency and good biocompatibility in the dark make aptamer-fullerene conjugates highly promising photosensitizers in tumor-specific PDT applications.68
APTAMER-CONJUGATED NANOMATERIALS FOR TARGETED PTT
Similar to PDT, PTT is a relatively noninvasive and benign alternative for cancer treatment. This treatment modality exposes biological tissues to higher than normal temperatures to promote the destruction of abnormal cells.69 Thus far, the efficacy of this strategy has been demonstrated by successful tumor remission in mice.70,71
Gold nanomaterials are especially attractive candidates for exploration in PTT because of their tunable absorption in the NIR region. Initially, Huang et al.72 demonstrated the use of sgc8c aptamer-conjugated Au-Ag NRs for targeted PTT. By covalent linkage of aptamers on the nanorod surface, the specific cell targeting and selective photothermal destruction of human acute lymphoblastic leukemia cells was realized. Gold nanorods modified with two different aptamers were also used to destroy different cancer cells simultaneously. Aptamers selected against DU145 prostate cancer cells (aptamer CSC1) and their subpopulation of cancer stem cells (aptamer CSC13) were linked to the surface of gold nanorods, and the resulting conjugates were successfully used to target and kill both cancer cells and cancer stem cells using NIR laser irradiation.73
To further improve the photothermal efficacy of nanorods, a novel Ag-Au nanostructure was synthesized and modified with the S2.2 aptamer that specifically binds to MUC1 mucin. Superior to Au-Ag NRs, the Ag-Au nanostructures exhibit a high capability of absorbing NIR radiation and are able to perform PTT of MCF-7 cells at a very low irradiation power density (0.25 W cm−2) without destroying healthy cells and surrounding normal tissue. Because these synthesized nanostructures exhibit high surface enhanced Raman scattering activity, the synthesized nanostructures offer a protocol to specifically recognize and sensitively detect the cancer cells, making these nanostructures very promising for PTT of cancers.74
COMBINED STRATEGY FOR CANCER THERAPY USING APTAMER-CONJUGATED NANOMATERIALS
The development of combined PDT/PTT using aptamer-conjugated nanomaterials is currently being actively pursued to provide a highly specific and enhanced therapeutic outcome. Multimodal therapy using both AuNR/photosensitizer composites and hybrid nanomaterials under the guidance of aptamers could significantly enhance efficiency in cancer therapy.
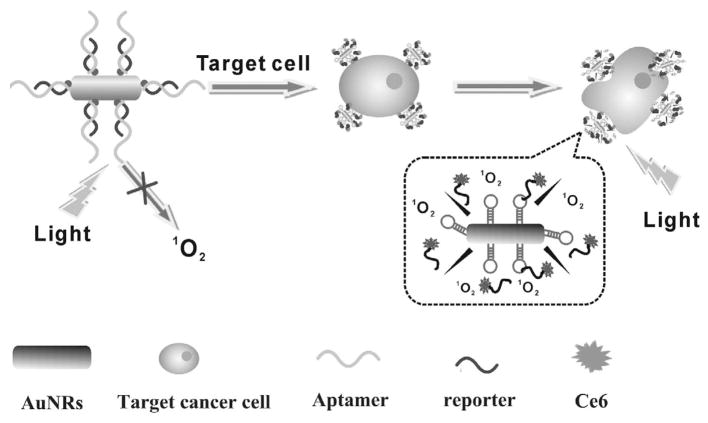
Recently, gold nanorods were used as a carrier to transport aptamers linked with chlorin 6 (Ce6) into target cancer cells.75 As illustrated in Figure 6, aptamer sgc8 was conjugated to an AuNR by a thiol-Au covalent bond and then hybridized with a Ce6-labeled photosensitizer/reporter to form a DNA double helix. When target cancer cells were absent, Ce6 was quenched and exhibited no PDTeffect. However, when target cancer cells were present, the aptamer changed structure to release Ce6 to produce singlet oxygen for PDT upon light irradiation. Importantly, by combining a photosensitizer with the photothermal effect of AuNR, dual PTT/PDT therapy was realized.
Figure 6.
Schematic diagram of aptamer-conjugated AuNR-Ce6 complex for targeted cancer therapy (adapted from Wang et al.75).
Because each gold nanorod is modified with many aptamer-Ce6 molecules, the gold nanorod-aptamer-Ce6 conjugate yields enhanced binding and therapeutic effects through the added ability to carry many photosensitizers. In addition, NIR laser irradiation of the gold nanorods enables further cell destruction via the photothermal effect. Consequently, significant cell death occurs upon light irradiation by simultaneous photodynamic and photothermal effects. This gold nanorod-aptamer-Ce6 conjugate offers a remarkably improved and synergistic therapeutic effect compared with PTT or PDT alone, providing high specificity and therapeutic efficiency, which can be generalized to other types of cancer therapies. Similarly, a switchable aptamer-based photosensitizer-AuNR platform has also been designed for multimodal cancer therapy.76
The use of hybrid nanomaterials composed of both gold nanomaterials and carbon nanotubes, which also exhibit strong optical absorption in the NIR region, will enable generation of much higher temperatures, in turn making the photothermal process much more effective and rapid. To achieve this goal, gold nanopopcorn were attached to single-walled carbon nanotube hybrid nanomaterials with S6 aptamer as the targeting molecule. The specific recognition of SK-BR-3 breast cancer cells was realized through interaction with the aptamer. Subsequent NIR irradiation induced the hyperthermia effect of the hybrid nanomaterial, leading to effective killing of cancer cells with high selectively.77 In another report, Khan et al. designed an A9 aptamer-conjugated gold nanocage decorated with single-walled carbon nanotubes for targeted imaging and photothermal destruction of prostate cancer cells. The bioconjugated hybrid nanomaterial-based imaging and therapy were highly selective and could distinguish between target and non-target cancer cell lines.78 As the photothermal response for the hybrid nanomaterial is far better than that for a single nanomaterial, it is promising to utilize the hybrid nanomaterials for highly effective in vivo photothermal cancer therapy.
CONCLUSIONS
The increasing inadequacies of conventional cancer therapy provide an impetus to the development of new therapeutic methods. By summarizing recent progress in integrating aptamers with various types of nanomaterials, we have demonstrated that these novel aptamer-conjugated nanomaterials benefit cancer therapy through increased specificity and efficacy as well as reduced toxicity. Because each nanomaterial has its own optical, electrochemical, magnetic and mechanical properties, aptamer-conjugated nanomaterials with diverse characteristics exhibit multifunctional theranostic capability for cancer therapy. The development of such multifunctional nanosystems combined with aptamers will eventually become a popular strategy for the design of novel nanoplatforms for successful cancer therapy. It should be noted that testing for some of the aptamer-conjugated nanoparticles has only been performed in vitro. Although aptamer-conjugated nanoparticles appear to hold potential for cancer therapy, considerable challenges and issues remain to be resolved, such as the poorly understood pharmacokinetics, toxicity and off-target effects. To realize the full potential of such multifunctional nanosystems, it is necessary to perform more stringent in vivo testing to demonstrate the effectiveness of these systems. The continued development of animal models for the evaluation of safety and efficacy of these promising therapeutic strategies will lay the foundation for use in humans.
FUTURE PERSPECTIVES
As excellent targeting ligands, aptamers have already succeeded in the sensitive and selective recognition of particular cancer cell populations or tissues. Bioconjugates integrating nanomaterials with aptamers will further prompt the development of efficient strategies for cancer therapy. Currently, liposomes, micelles and polymeric nanoparticles are the most promising materials for nanoparticle-based targeted drug delivery because of their biocompatibility and biodegradability. Compared with biodegradable organic macromolecules, inorganic nanomaterials may not have obvious advantages if simply used as drug carriers, as they hardly degrade in biological systems. However, combined with aptamers, biocompatible inorganic nanomaterials with unique optical, magnetic and electronic properties could provide relatively noninvasive and benign alternatives for targeted cancer therapy, leading to new approaches for cancer treatment. In addition to their utilization as platforms for targeted cancer therapy, aptamer-conjugated nanomaterials will find additional applications in the biomedical field, for example, in three-dimensional cell culture and tissue engineering. Future efforts will focus on developing aptamer-conjugated nanomaterials with multimodalities that combine both diagnostic and therapeutic components to address challenges such as multiple-drug resistance and ultimately to improve therapeutic outcomes and reduce costs.
Although aptamer-conjugated nanomaterials are emerging as a promising platform for cancer therapy, much work remains to be done before these materials can be used in clinical practice. This work includes minimizing the toxicity of the conjugate, improving target efficacy and studying the behavior of nanoparticles in biological microenvironments. To minimize systemic toxicity, key factors, such as surface charge, coating, particle size, as well as the biocompatibility and biodegradability of conjugates, should be carefully considered. To improve the target efficacy, it is necessary to optimize the surface modification of conjugates. In addition to modulating the density of aptamers, encapsulation of the nanoparticles with PEG coatings can prolong the circulation time of the conjugates, thereby effectively improving the targeted efficacy. Still, much effort is needed to achieve a successful cancer treatment. However, based on the promising multimodal theranostic nanoplatforms and the increasing demand for efficient cancer therapy, we will witness a continued and rapid development of aptamer-conjugated nanomaterials for cancer therapy in the near future.
Acknowledgments
This work is supported by the National Key Scientific Program of China (2011CB911000), NSFC grants (NSFC 21221003 and NSFC 21327009) and China National Instrumentation Program 2011YQ03012412, and by the National Institutes of Health (GM079359 and CA133086).
Biographies

Qiaoling Liu received her PhD (2010) in Chemistry from the Institute of Chemistry, Chinese Academy of Sciences, PR China, and carried out postdoctoral studies at the same institute from 2010 to 2013. Currently, she is an assistant professor in the State Key Laboratory for Chemo/Biosensing and Chemometrics at Hunan University. Her research interests focus on the development of smart novel hybrid nanomaterials for cancer applications and diagnostics.

Xiaohong Fang has been a professor at the Institute of Chemistry, Chinese Academy of Sciences, since 2001. She received her PhD (1996) in Chemistry from Peking University, PR China. After her postdoctoral research at the Department of Chemistry, University of Waterloo, Canada (1997–1998), she became a Research Associate at the Department of Chemistry, University of Florida, USA (1998–2001). She was the recipient of the National Distinguished Scholars in the New Century (2006), Distinguished Women Award, Chinese Academy of Sciences (2005), ‘Hundred Distinguished Young Scholars’ Achievement Award, Chinese Academy of Sciences (2005) and Distinguished Young Scientists Award, Chinese Chemical Society (2003). Her research interests lie in biophysical chemistry, nanobiotechnology, aptamer-based biosensors and cancer diagnostics.

Dr Zhuo Chen graduated with a Bachelor’s degree (2001) from Chemistry Department, Zhejiang University of China, and obtained PhD (2006) from the College of Chemistry and Molecular Engineering, Peking University, China. From 2006 to 2010 he worked as a postdoctoral scholar at the Chemistry Department of Stanford University. He has been a professor at Hunan University since 2010. His current research interests focus on bioanalysis and nanomaterials engineering, and specifically on the synthesis, functionalization and biosensing application of carbon nanomaterials.

Dr Xiao-Bing Zhang received his MS in 1998, and PhD in 2001, both in chemistry from Hunan University. He worked at the Ecole Normale Superieure de Lyon (ENS de Lyon, France) and the Royal Institute of Technology (KTH, Sweden) as a post-doctoral fellow from 2003 to 2005, as an invited professor at ENS de Lyon in 2008, and as a visiting professor at the University of Illinois at Urbana-Champaign in 2009. He has been a professor at Hunan University since 2006. His research interests involve fluorescent probes and biosensors, and he has authored more than 60 publications.

Dr Weihong Tan, Distinguished Professor and VT and Louis Jackson Professor of Chemistry and Professor of Biomedical Engineering, earned his PhD (1993) in Chemistry at the University of Michigan. His research interests lie in molecular engineering, bionanotechnology and chemical biology. His group has engineered nucleic acid probes for biosensing platforms and DNA nanomotors. The Tan group has also developed numerous bioconjugated nanostructures for molecular imaging, efficient cell separation and sensitive cell detection. With the pioneer development of cell-based SELEX, the Tan group has generated various molecular probes, such as aptamers for cancer treatment and cancer biomarker discovery, in efforts to achieve molecular elucidation of diseases.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Mukerjee A, Ranjan AP, Vishwanatha JK. Combinatorial nanoparticles for cancer diagnosis and therapy. Curr Med Chem. 2012;19:3714–3721. doi: 10.2174/092986712801661176. [DOI] [PubMed] [Google Scholar]
- 2.Barbas AS, Mi J, Clary BM, White RR. Aptamer applications for targeted cancer therapy. Future Oncol. 2010;6:1117–1126. doi: 10.2217/fon.10.67. [DOI] [PubMed] [Google Scholar]
- 3.Bamrungsap S, Zhao Z, Chen T, Wang L, Li C, Fu T, Tan W. Nanotechnology in therapeutics: a focus on nanoparticles as a drug delivery system. Nanomedicine. 2012;7:1253–1271. doi: 10.2217/nnm.12.87. [DOI] [PubMed] [Google Scholar]
- 4.Tan W, Wang H, Chen Y, Zhang X, Zhu H, Yang C, Yang R, Liu C. Molecular aptamers for drug delivery. Trends Biotechnol. 2011;29:634–640. doi: 10.1016/j.tibtech.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu FX, Karnik R, Wang AZ, Alexis F, Levy-Nissenbaum E, Hong S, Langer RS, Farokhzad OC. Targeted nanoparticles for cancer therapy. Nanotoday. 2007;2:14–21. [Google Scholar]
- 6.Hu M, Zhang K. The application of aptamers in cancer research: an up-to-date review. Future Oncol. 2013;9:369–376. doi: 10.2217/fon.12.201. [DOI] [PubMed] [Google Scholar]
- 7.Shangguan D, Li Y, Tang Z, Cao ZC, Chen HW, Mallikaratchy P, Sefah K, Yang CJ, Tan W. Aptamers evolved from live cells as effective molecular probes for cancer study. Proc Natl Acad Sci USA. 2006;103:11838–11843. doi: 10.1073/pnas.0602615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sefah K, Bae KM, Phillips JA, Siemann DW, Su Z, McClellan S, Vieweg J, Tan W. Cell-based selection provides novel molecular probes for cancer stem cells. Int J Cancer. 2013;132:2578–2588. doi: 10.1002/ijc.27936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sefah K, Tang ZW, Shangguan DH, Chen H, Lopez-Colon D, Li Y, Parekh P, Martin J, Meng L, Phillips JA, Kim YM, Tan W. Molecular recognition of acute myeloid leukemia using aptamers. Leukemia. 2009;23:235–244. doi: 10.1038/leu.2008.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang X, Tan W. Aptamers generated from cell-SELEX for molecular medicine: a chemical biology approach. Acc Chem Res. 2010;43:48–57. doi: 10.1021/ar900101s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni X, Castanares M, Mukherjee A, Lupold SE. Nucleic acid aptamers: clinical applications and promising new horizons. Curr Med Chem. 2011;18:4206–4214. doi: 10.2174/092986711797189600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang YM, Donovan MJ, Tan W. Using aptamers for cancer biomarker discovery. J Nucleic Acids. 2013;2013:817350. doi: 10.1155/2013/817350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Hong H, Cai W. Tumor-targeted drug delivery with aptamers. Curr Med Chem. 2011;18:4185–4194. doi: 10.2174/092986711797189547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Zhao Q, Qiu L. Smart ligand: aptamer-mediated targeted delivery of chemotherapeutic drugs and siRNA for cancer therapy. J Control Release. 2013;171:152–162. doi: 10.1016/j.jconrel.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Yang R, Yang HL, Tan WH. Nucleic acid conjugated nanomaterials for enhanced molecular recognition. ACS Nano. 2009;3:2451–2460. doi: 10.1021/nn9006303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stadler N, Chi C, Lelie DVD, Gang O. DNA-incorporating nanomaterials in biotechnological applications. Nanomedicine. 2010;5:319–334. doi: 10.2217/nnm.10.2. [DOI] [PubMed] [Google Scholar]
- 17.Lee JH, Yigit MV, Mazumdar D, Lu Y. Molecular diagnostic and drug delivery agents based on aptamer-nanomaterial conjugates. Adv Drug Deliv Rev. 2010;62:592–605. doi: 10.1016/j.addr.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen T, Shukoor MI, Chen Y, Yuan Q, Zhu Z, Zhao Z, Gulbakan B, Tan W. Aptamer-conjugated nanomaterials for bioanalysis and biotechnology applications. Nanoscale. 2011;3:546–556. doi: 10.1039/c0nr00646g. [DOI] [PubMed] [Google Scholar]
- 19.Zhu G, Zhang S, Song E, Zheng J, Hu R, Fang X, Tan W. Building fluorescent DNA nanodevices on target living cell surfaces. Angew Chem Int Ed Engl. 2013;52:5490–5496. doi: 10.1002/anie.201301439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding C, Ge Y, Zhang S. Electrochemical and electrochemiluminescence determination of cancer cells based on aptamers and magnetic beads. Chem Eur J. 2010;16:10707–10714. doi: 10.1002/chem.201001173. [DOI] [PubMed] [Google Scholar]
- 21.Wu M, Yuan D, Xu J, Chen H. Sensitive electrochemiluminescence biosensor based on Au-ITO hybrid bipolar electrode amplification system for cell surface protein detection. Anal Chem. 2013;85:11960–11965. doi: 10.1021/ac402889z. [DOI] [PubMed] [Google Scholar]
- 22.Yan M, Sun G, Liu F, Lu J, Yu J, Song X. An aptasensor for sensitive detection of human breast cancer cells by using porous GO/Au composites and porous PtFe alloy as effective sensing platform and signal amplification labels. Anal Chim Acta. 2013;798:33–39. doi: 10.1016/j.aca.2013.08.046. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Xu S, He Z, Deng A, Zhu J. Supersandwich cytosensor for selective and ultrasensitive detection of cancer cells using aptamer-DNA concatamer-quantum dots probes. Anal Chem. 2013;85:3385–3392. doi: 10.1021/ac303789x. [DOI] [PubMed] [Google Scholar]
- 24.Jie G, Wang L, Yuan J, Zhang S. Versatile electrochemiluminescence assays for cancer cells based on dendrimer/CdSe-ZnS-quantum dot nanoclusters. Anal Chem. 2011;83:3873–3880. doi: 10.1021/ac200383z. [DOI] [PubMed] [Google Scholar]
- 25.Zhao J, Zhang L, Chen C, Jiang J, Yu R. A novel sensing platform using aptamer and RNA polymerase-based amplification for detection of cancer cells. Anal Chim Acta. 2012;745:106–111. doi: 10.1016/j.aca.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 26.Bamrungsap S, Chen T, Shukoor MI, Chen Z, Sefah K, Chen Y, Tan W. Pattern recognition of cancer cells using aptamer-conjugated magnetic nanoparticles. ACS Nano. 2012;6:3974–3981. doi: 10.1021/nn3002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong X, Liu H, Zhao Z, Altman MB, Lopez-Colon D, Yang CJ, Chang LJ, Liu C, Tan W. DNA aptamer-mediated cell targeting. Angew Chem Int Ed Engl. 2013;52:1472–1476. doi: 10.1002/anie.201207063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ocsoy I, Gulbakan B, Shukoor MI, Xiong X, Chen T, Powell DH, Tan W. Aptamer-conjugated multifunctional nanoflowers as a platform for targeting, capture, and detection in laser desorption ionization mass spectrometry. ACS Nano. 2013;7:417–427. doi: 10.1021/nn304458m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang H, O’Donoghue MB, Liu H, Tan W. A liposome-based nanostructure for aptamer directed delivery. Chem Commun. 2010;46:249–251. doi: 10.1039/b916911c. [DOI] [PubMed] [Google Scholar]
- 30.Mann AP, Bhavane RC, Somasunderam A, Liz Montalvo-Ortiz B, Ghaghada KB, Volk D, Nieves-Alicea R, Suh KS, Ferrari M, Annapragada A, Gorenstein DG, Tanaka T. Thioaptamer conjugated liposomes for tumor vasculature targeting. Oncotarget. 2011;2:298–304. doi: 10.18632/oncotarget.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Y, Duan S, Zeng X, Liu C, Davies NM, Li B, Forrest ML. Prodrug strategy for PSMA-targeted delivery of TGX-221 to prostate cancer cells. Mol Pharm. 2012;9:1705–1716. doi: 10.1021/mp3000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu W, Siddiqui IA, Nihal M, Pilla S, Rosenthal K, Mukhtar H, Gong S. Aptamer-conjugated and doxorubicin-loaded unimolecular micelles for targeted therapy of prostate cancer. Biomaterials. 2013;34:5244–5253. doi: 10.1016/j.biomaterials.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alemdaroglu FE, Alemdaroglu NC, Langguth P, Herrmann A. DNA block copolymer micelles-a combinatorial tool for cancer nanotechnology. Adv Mater. 2008;20:899–902. [Google Scholar]
- 34.Liu H, Zhu Z, Kang H, Wu Y, Sefah K, Tan W. DNA-based micelles: synthesis, micellar properties and size-dependent cell permeability. Chem Eur J. 2010;16:3791–3797. doi: 10.1002/chem.200901546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Y, Sefah K, Liu H, Wang R, Tan W. DNA aptamer-micelle as an efficient detection/delivery vehicle toward cancer cells. Proc Natl Acad Sci USA. 2010;107:5–10. doi: 10.1073/pnas.0909611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang H, Liu H, Kang H, Tan W. Engineering target-responsive hydrogels based on aptamer-target interactions. J Am Chem Soc. 2008;130:6320–6321. doi: 10.1021/ja801339w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang L, Meng L, Zhang X, Chen Y, Zhu G, Liu H, Xiong X, Sefah K, Tan W. Engineering polymeric aptamers for selective cytotoxicity. J Am Chem Soc. 2011;133:13380–13386. doi: 10.1021/ja201285y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, Richie JP, Langer R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci USA. 2006;103:6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. Proc Natl Acad Sci USA. 2008;105:17356–17361. doi: 10.1073/pnas.0809154105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhar S, Kolishetti N, Lippard SJ, Farokhzad OC. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc Natl Acad Sci USA. 2011;108:1850–1855. doi: 10.1073/pnas.1011379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo J, Gao X, Su L, Xia H, Gu G, Pang Z, Jiang X, Yao L, Chen J, Chen H. Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials. 2011;32:8010–8020. doi: 10.1016/j.biomaterials.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Gao H, Qian J, Yang Z, Pang Z, Xi Z, Cao S, Wang Y, Pan S, Zhang S, Wang W, Jiang X, Zhang Q. Whole-cell SELEX aptamer-functionalised poly(ethyleneglycol)-poly(ε-caprolactone) nanoparticles for enhanced targeted glioblastoma therapy. Biomaterials. 2012;33:6264–6272. doi: 10.1016/j.biomaterials.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 43.Zhu G, Zheng J, Song E, Donovan M, Zhang K, Liu C, Tan W. Self-assembled, aptamer-tethered DNA nanotrains for targeted transport of molecular drugs in cancer theranostics. Proc Natl Acad Sci USA. 2013;110:7998–8003. doi: 10.1073/pnas.1220817110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Z, Robinson JT, Tabakman SM, Yang K, Dai H. Carbon materials for drug delivery & cancer therapy. Mater Today. 2011;14:316–323. [Google Scholar]
- 45.Yang L, Zhang X, Ye M, Jiang J, Yang R, Fu T, Chen Y, Wang K, Liu C, Tan T. Aptamer-conjugated nanomaterials and their applications. Adv Drug Deliv Rev. 2011;63:1361–1370. doi: 10.1016/j.addr.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dobson J. Magnetic mico- and nano-particle-based targeting for drug and gene delivery. Nanomedicine. 2006;1:31–37. doi: 10.2217/17435889.1.1.31. [DOI] [PubMed] [Google Scholar]
- 47.Wang AZ, Bagalkot V, Vasilliou CC, Gu F, Alexis F, Zhang L, Shaikh M, Yuet K, Cima MJ, Langer R, Kantoff PW, Bander NH, Jon S, Farokhzad OC. Superparamagnetic iron oxide nanoparticle-aptamer bioconjugates for combined prostate cancer imaging and therapy. Chem Med Chem. 2008;3:1311–1315. doi: 10.1002/cmdc.200800091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu MK, Kim D, Lee I, So J, Jeong YY, Jon S. Image-guided prostate cancer therapy using aptamer-functionalized thermally cross-linked superparamagnetic iron oxide nanoparticles. Small. 2011;7:2241–2249. doi: 10.1002/smll.201100472. [DOI] [PubMed] [Google Scholar]
- 49.Chen T, Shukoor MI, Wang R, Zhao Z, Yuan Q, Bamrungsap S, Xiong X, Tan W. Smart multifunctional nanostructure for targeted cancer chemotherapy and magnetic resonance imaging. ACS Nano. 2011;5:7866–7873. doi: 10.1021/nn202073m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng J, Zhu G, Li Y, Li C, You M, Chen T, Song E, Yang R, Tan W. A spherical nucleic Acid platform based on self-assembled DNA biopolymer for high-performance cancer therapy. ACS Nano. 2013;7:6545–6554. doi: 10.1021/nn402344v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wijaya A, Schaffer SB, Pallares IG, Hamad-Schifferli K. Selective release of multiple DNA oligonucleotides from gold nanorods. ACS Nano. 2008;3:80–86. doi: 10.1021/nn800702n. [DOI] [PubMed] [Google Scholar]
- 52.Yang XJ, Liu X, Liu Z, Pu F, Ren JS, Qu XG. Near-infrared light-triggered, targeted drug delivery to cancer cells by aptamer gated nanovehicles. Adv Mater. 2012;24:2890–2895. doi: 10.1002/adma.201104797. [DOI] [PubMed] [Google Scholar]
- 53.Chen CC, Lin YP, Wang CW, Tzeng HC, Wu CH, Chen YC, Chen CP, Chen LC, Wu YC. DNA-gold nanorod conjugates for remote control of localized gene expression by near infrared irradiation. J Am Chem Soc. 2006;128:3709–3715. doi: 10.1021/ja0570180. [DOI] [PubMed] [Google Scholar]
- 54.You J, Zhang G, Li C. Exceptionally high payload of doxorubicin in hollow gold nanospheres for near-infrared light-triggered drug release. ACS Nano. 2010;4:1033–1041. doi: 10.1021/nn901181c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang H, Trondoli AC, Zhu G, Chen Y, Chang Y, Liu H, Huang Y, Zhang X, Tan W. Near-infrared light-responsive core-shell nanogels for targeted drug delivery. ACS Nano. 2011;5:5094–5099. doi: 10.1021/nn201171r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hilliard LR, Zhao XJ, Tan WH. Immobilization of oligonucleotides onto silica nanoparticles for DNA hybridization studies. Anal Chim Acta. 2002;470:51–56. [Google Scholar]
- 57.Zhu CL, Song XY, Zhou WH, Yang HH, Wen YH, Wang XR. An efficient cell-targeting and intracellular controlled-release drug delivery system based on MSN-PEM-aptamer conjugates. J Mater Chem. 2009;19:7765–7770. [Google Scholar]
- 58.Li LL, Yin Q, Cheng J, Lu Y. Polyvalent mesoporous silica nanoparticle-aptamer bioconjugates target breast cancer cells. Adv Healthcare Mater. 2012;1:567–572. doi: 10.1002/adhm.201200116. [DOI] [PubMed] [Google Scholar]
- 59.Park H, Yang J, Lee J, Haam S, Choi IH, Yoo KH. Multifunctional nanoparticles for combined doxorubicin and photothermal treatments. ACS Nano. 2009;3:2919–2926. doi: 10.1021/nn900215k. [DOI] [PubMed] [Google Scholar]
- 60.Park JH, von Maltzahn G, Xu MJ, Fogal V, Kotamraju VR, Ruoslahti E, Bhatia SN, Sailor M. J Cooperative nanomaterial system to sensitize, target, and treat tumors. Proc Natl Acad Sci USA. 2010;107:981–986. doi: 10.1073/pnas.0909565107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bugaj AM. Targeted photodynamic therapy-a promising strategy of tumor treatment. Photochem Photobiol Sci. 2011;10:1097–1109. doi: 10.1039/c0pp00147c. [DOI] [PubMed] [Google Scholar]
- 62.Ferreira CSM, Cheung MC, Missailidis S, Bisland S, GariÕpy J. Phototoxic aptamers selectively enter and kill epithelial cancer cells. Nucleic Acids Res. 2009;37:866–876. doi: 10.1093/nar/gkn967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mallikaratchy P, Tang Z, Tan W. Cell specific aptamer-photosensitizer conjugates as a molecular tool in photodynamic therapy. Chem Med Chem. 2008;3:425–428. doi: 10.1002/cmdc.200700260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang K, You M, Chen Y, Han D, Zhu Z, Huang J, Williams K, Yang CJ, Tan W. Self-assembly of a bifunctional DNA carrier for drug delivery. Angew Chem Int Ed Engl. 2011;50:6098–6101. doi: 10.1002/anie.201008053. [DOI] [PubMed] [Google Scholar]
- 65.Shieh Y, Yang S, Wei M, Shieh M. Aptamer-based tumor-targeted drug delivery for photodynamic therapy. ACS Nano. 2010;4:1433–1442. doi: 10.1021/nn901374b. [DOI] [PubMed] [Google Scholar]
- 66.Han D, Zhu G, Wu C, Zhu Z, Chen T, Zhang X, Tan W. Engineering a cell-surface aptamer circuit for targeted and amplified photodynamic cancer therapy. ACS Nano. 2013;7:2312–2319. doi: 10.1021/nn305484p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu Z, Tang Z, Phillips JA, Yang R, Wang H, Tan W. Regulation of singlet oxygen generation using single-walled carbon nanotubes. J Am Chem Soc. 2008;130:10856–10857. doi: 10.1021/ja802913f. [DOI] [PubMed] [Google Scholar]
- 68.Liu Q, Xu L, Zhang X, Li N, Zheng J, Guan M, Fang X, Wang C, Shu C. Enhanced photodynamic efficiency of an aptamer-guided fullerene photosensitizer toward tumor cells. Chem Asian J. 2013;8:2370–2376. doi: 10.1002/asia.201300039. [DOI] [PubMed] [Google Scholar]
- 69.Fisher JW, Sarkar S, Buchanan CF, Szot CS, Whitney J, Hatcher HC, Torti SV, Rylander CG, Rylander MN. Photothermal response of human and murine cancer cells to multiwalled carbon nanotubes after laser irradiation. Cancer Res. 2010;70:9855–9864. doi: 10.1158/0008-5472.CAN-10-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang HW, Lu YJ, Lin KJ, Hsu SC, Huang CY, She SH, Liu HL, Lin CW, Xiao MC, Wey SP, Chen PY, Yen TC, Wei KC, Ma CC. EGRF conjugated PEGylated nanographene oxide for targeted chemotherapy and photothermal therapy. Biomaterials. 2013;34:7204–7214. doi: 10.1016/j.biomaterials.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 71.Xiao Q, Zheng X, Bu W, Ge W, Zhang S, Chen F, Xing H, Ren Q, Fan W, Zhao K, Hua Y, Shi J. A core/satellite multifunctional nanotheranostic for in vivo imaging and tumor eradication by radiation/photothermal synergistic therapy. J Am Chem Soc. 2013;135:13041–13048. doi: 10.1021/ja404985w. [DOI] [PubMed] [Google Scholar]
- 72.Huang YF, Sefah K, Bamrungsap S, Chang HT, Tan W. Selective photothermal therapy for mixed cancer cells using aptamer-conjugated nanorods. Langmuir. 2008;24:11860–11865. doi: 10.1021/la801969c. [DOI] [PubMed] [Google Scholar]
- 73.Wang J, Sefah K, Altman MB, Chen T, You M, Zhao Z, Huang CZ, Tan W. Aptamer-conjugated nanorods for targeted photothermal therapy of prostate cancer stem cells. Chem Asian J. 2013;8:2417–2422. doi: 10.1002/asia.201300375. [DOI] [PubMed] [Google Scholar]
- 74.Wu P, Gao Y, Zhang H, Cai C. Aptamer-guided silver-gold bimetallic nanostructures with highly active surface-enhanced Raman scattering for specific detection and near-infrared photothermal therapy of human breast cancer cells. Anal Chem. 2012;84:7692–7699. doi: 10.1021/ac3015164. [DOI] [PubMed] [Google Scholar]
- 75.Wang J, You M, Zhu G, Shukoor MI, Chen Z, Zhao Z, Altman MB, Yuan Q, Zhu Z, Chen Y, Huang CZ, Tan W. Photosensitizer-gold nanorod composite for targeted multimodal therapy. Small. 2013;9:3678–3684. doi: 10.1002/smll.201202155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang J, Zhu G, You M, Song E, Shukoor MI, Zhang K, Altman MB, Chen Y, Zhu Z, Huang CZ, Tan W. Assembly of aptamer switch probes and photosensitizer on gold nanorods for targeted photothermal and photodynamic cancer therapy. ACS Nano. 2012;6:5070–5077. doi: 10.1021/nn300694v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beqa L, Fan Z, Singh AK, Senapati D, Ray PC. Gold nano-popcorn attached SWCNT hybrid nanomaterial for targeted diagnosis and photothermal therapy of human breast cancer cells. ACS Appl Mater Interfaces. 2011;3:3316–3324. doi: 10.1021/am2004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khan SA, Kanchanapally R, Fan Z, Beqa L, Singh AK, Senapati D, Ray PC. A gold nanocage-CNT hybrid for targeted imaging and photothermal destruction of cancer cells. Chem Commun. 2012;48:6711–6713. doi: 10.1039/c2cc32313c. [DOI] [PubMed] [Google Scholar]