Abstract
Objectives
The effect of smoking and human papilloma virus (HPV) on overall survival (OS) of oropharyngeal squamous cell carcinoma (OPSCC) patients undergoing concurrent chemoradiation (CCRT) remains unclear.
Study Design
Retrospective Review
Methods
Clinical characteristics of OPSCC patients treated between 2008– 2015 with CCRT were abstracted from medical records. OS curves and multivariate cox proportional hazard ratios (HR) were examined.
Results
Out of 120 evaluable patients, 71% had HPV+ tumors. Median follow-up duration for the entire cohort was 41.5 months (6–88 months). HPV+ current smokers experienced significantly worse 5 year OS (73% alive vs. 36% alive, p=0.01) with a similar trend in HPV− current smokers (66% alive vs. 31% alive, p=0.28) compared to former/never smokers undergoing CCRT. In a multivariate cox proportional hazard model adjusted for age, gender, and overall tumor stage, HPV+ current smokers experienced nearly a four-fold increase in overall mortality in comparison to HPV+ never/former smokers (HR= 3.68, 95% CI=1.35–10.0). Similarly, current smokers with HPV− tumors (HR= 6.8, 95% CI=1.11–41.67) had increased mortality compared to never/former smokers.
Conclusions
Current smoking is associated with poor prognosis, independent of HPV status, in OPSCC CCRT treated patients. Current smoking produced an approximately 4 to 7 fold increase in risk of mortality for HPV+ and HPV− patients respectively. Regardless of pack-years, efforts should be made to achieve smoking cessation before CCRT regardless of HPV status.
Keywords: Tobacco, Nicotine, HPV, SCCHN, base of tongue
Introduction
Squamous cell cancer of the head and neck (SCCHN) occurs annually in over 550,000 people worldwide 1. In the US in 2015 59,340 incident SCCHN cases are expected and 12,290 people are expected to die of SCCHN 2. Tobacco use has been the major risk factor for developing SCC of the head and neck 3. In recent decades, a higher rate of human papilloma virus (HPV) associated SCCHN has been reported, especially in tumors arising in the oropharynx 4,5.
The 2014 Surgeon General’s Report (SGR) concludes that smoking by cancer patients and survivors causes adverse outcomes in cancer patients, including increases in overall and cancer specific mortality (In: The Health Consequences of Smoking 2014) 6. Several large studies report current smoking increases overall mortality in head and neck cancer patients 7,8,9,10,11,12 and mortality is further linked to pack years 11,13. Importantly, current smoking by SCCHN patients is associated with increased overall and cancer specific mortality as compared with patients who quit smoking within the past year 14. However, there are limited data on the relationship between smoking, cessation, HPV, and mortality.
In 2010, Roswell Park Cancer Institute initiated an automated tobacco assessment and referral program where all patients were screened for tobacco use and automatically referred to a dedicated phone based cessation program 15. We have previously shown that lung cancer patients who quit smoking after diagnosis using this program had significant improvements in overall survival as compared with patients who continued to smoke 16. The purpose of this study is to evaluate the relationship between smoking, smoking cessation, HPV status, and survival in a cohort of OPSCC patients treated with definitive concurrent chemoradiotherapy (CCRT).
Materials and Methods
The Roswell Park Cancer Institute (RPCI) Institutional Review Board approved this retrospective study of OPSCC patients diagnosed and treated with CCRT between 2008 and 2015. To be included in this study the primary tumor had to be: (1) an invasive squamous cell carcinoma limited to the oropharynx or an oropharyngeal primary overlapping other adjacent anatomical sites (ICD-O-3: C09-C10), (2) evaluated for high risk HPV types 16 or 18 using in-situ hybridization (ISH) technique, (3) treated with curative CCRT and (4) have no prior history of SCCHN. No patients with metastatic disease were included. Overall, 198 consecutive OPSCC patients were diagnosed or treated at RPCI between 2008 and 2015. Of these, 120 (61%) patients qualified based on the above selection criteria. All of these 120 patients had HPV testing conducted on clinical request by in situ hybridization (HPV 16/18 biotinylated DNA probe -Y1412- Dako, Carpinteria, CA). In the first few years of this study period HPV testing was not routinely done and was performed only if specifically ordered. In the later years of the study, HPV testing was routine and omitted only if there was insufficient tumor sample due to diagnosis by fine needle aspiration. P16 staining as a surrogate marker for HPV positive has been routinely done for many years but was not available for the early patients in this cohort and was therefore not analyzed.
Demographic and clinical characteristics of study subjects, including age, gender, social habits and previous cancer history, were collected by a detailed medical chart review. Clinical information regarding the current cancer such as stage, grade, anatomical sub-site, treatment modality, cancer recurrence, survival status, survival duration, and cause of death were also collected. The follow-up patient schedule as well as the cisplatin based chemotherapy (weekly or every three weeks) and intensity modulated radiation therapy regimens (70 Gray to the primary tumor and 56 Gray to the elective lymph nodes in 35 fractions) applied in this cohort have been previously described in detail 17,18.
Patients were categorized as current, former or never smokers based on the history noted on their clinical record at diagnosis. Patients identified as currently smoking at consult visits were referred to an automated smoking cessation service 15. Prior to 2010, the single treating radiation oncologist (AS) for this cohort provided formal smoking cessation support to all current smokers. Patients who reported having used tobacco within the 30 days prior to treatment were considered current smokers. Pack-years of smoking was calculated by multiplying packs of cigarettes smoked per day by the number of years smoked. All patients, if committed to smoking cessation, were given 30 days to try to quit smoking prior to the start of radiation therapy. If successful, these patients were categorized as former smokers.
Descriptive statistics of the overall cohort were summarized and clinical characteristics were compared between HPV+ and HPV− patients using Pearson Chi Square test for categorical variables and Wilcox Rank Sum test for continuous variables.
The effect of HPV status and the effect of smoking status on overall survival (OS) and disease-specific survival (DSS) were examined using multivariate cox proportional hazard regression models. Models were adjusted for age, gender, and overall tumor stage. Survival trends were estimated using Kaplan-Meier (K-M) survival curves. A p-value of < 0.05 was considered statistically significant. All analyses were conducted using SPSS software (SPSS for Windows, Version 16.0. Chicago, SPSS Inc.).
Results
Table 1 contains descriptive characteristics for the entire OPSCC cohort. The median age of this predominantly male cohort was 58.5 years old (36–85 years old). Among the 120 OPSCC patients included, 81% had an overall tumor stage of 4 and 75% had a nodal stage of N2 or N3. Current smoking was noted in 23% of patients (n=28) and 22% of patients (n=26) reported never smoking. Median follow-up duration for the entire cohort was 41.5 months (6–88 months).
Table 1.
Characteristics of the Overall Cohort (n=120)
| Characteristic | Value |
|---|---|
| Median (range) | |
| Median age at diagnosis | 58.5 (36-85) |
|
| |
| Median follow up time (months) | 41.5 (6-88) |
|
| |
| Characteristic | Value |
| Frequency(%) | |
|
| |
| Gender | |
| Male | 96 (80.0) |
| Female | 24 (20.0) |
|
| |
| Tobacco Status | |
| Never | 26 (21.7) |
| Former | 66 (55.0) |
| Current | 28 (23.3) |
|
| |
| Anatomical Subsite | |
| Base of the Tongue | 42 (35.0) |
| Tonsil | 49 (40.8) |
| Soft Palate | 2 (1.7) |
| Overlapping Subsites | 26 (21.7) |
| Oropharyngeal Wall | 1 (0.8) |
|
| |
| Overall Tumor Stage | |
| Stage II | 6 (5.0) |
| Stage III | 17 (14.2) |
| Stage IV | 97 (80.8) |
|
| |
| T-Stage | |
| T1 | 20 (16.8) |
| T2 | 49 (40.8) |
| T3 | 37 (30.8) |
| T4 | 6 (5.0) |
| T4a | 7 (5.8) |
| T4b | 1 (0.8) |
|
| |
| N-Stage | |
| N0 | 12 (10.0) |
| N1 | 18 (15.0) |
| N2 | 5 (4.2) |
| N2a | 13 (10.8) |
| N2b | 38 (31.7) |
| N2c | 26 (21.7) |
| N3 | 8 (6.7) |
|
| |
| Grade (n=114) | |
| Well Differentiated | 2 (1.8) |
| Moderately Differentiated | 51 (44.7) |
| Poorly Differentiated | 61 (53.5) |
Seventy-two percent of the patients were HPV+ and 28% were HPV− (Table 2). There was no statistically significant difference in age, gender, tumor stage, or nodal stage between HPV+ and HPV− patients. HPV− patients trended toward higher median pack years than HPV+ patients (30 vs. 18, p=0.075). HPV+ patients had a greater proportion of poorly differentiated tumors compared to HPV− patients (p<0.01).
Table 2.
Cohort characteristics by HPV status (n=120)
| HPV− (n=34) | HPV + (n=86) | ||
|---|---|---|---|
| Characteristic | Median (range) | Median (range) | ap-value |
|
| |||
| Age at Diagnosis in years | 58.5 (36-85) | 58.5 (37-82) | 0.775 |
|
| |||
| Median Follow Up Time (months) | 45 (10-82) | 39 (6-88) | 0.309 |
|
| |||
| Tobacco pack-years (range) | 30 (0-68) | 18 (0-160) | 0.075 |
|
| |||
| Characteristic | Frequency (%) | Frequency (%) | ap-value |
|
| |||
| Gender | |||
| Male | 25 (73.5) | 71 (82.6) | |
| Female | 9 (26.5) | 15 (17.4) | 0.265 |
|
| |||
| Tobacco Status | |||
| Never | 4 (11.8) | 22 (25.6) | |
| Former | 22 (64.7) | 44 (51.2) | |
| Current | 8 (23.5) | 20 (23.3) | 0.228 |
|
| |||
| Overall tumor stage | |||
| Stage II | 2 (5.9) | 4 (4.7) | |
| Stage III | 6 (17.6) | 11 (12.8) | |
| Stage IV | 26 (76.5) | 71 (82.6) | 0.744 |
|
| |||
| T-Stage | |||
| T1/T2 | 17 (50.0) | 50 (58.1) | |
| T3/T4 | 17 (50.0) | 36 (41.9) | 0.418 |
|
| |||
| N-Stage | |||
| N0/N1 | 11 (32.4) | 19 (22.1) | |
| N2/N3 | 23 (67.6) | 57 (77.9) | 0.242 |
|
| |||
| Grade (n=114) | n=33 | n=81 | |
| Well/Moderately differentiated | 23 (69.7) | 30 (37.0) | |
| Poorly differentiated | 10 (30.3) | 51 (63.0) | 0.002 |
HN head and neck, T clinical tumor stage, N clinical nodal stage
Wilcoxon Rank Sum test (or Kruskal-Wallis test) was used to examine differences for age and tobacco pack-years and Pearson Chi-square test was used to examine differences for categorical variables
The Kaplan Meier curve for OS favored patients with HPV+ tumors vs. those with HPV− tumors (p=0.08) (Figure 1). When HPV status was stratified by smoking status, the Kaplan Meier curves for OS favored never/former smokers vs. current smokers but the difference only reached statistical significance for patients with HPV+ tumors (p=0.01) though there was a similar trend in HPV− patients (Figure 2). The cumulative survival rates at the end of 5 years for each group are as follows: HPV+ never/former smokers=73%, HPV+ current smokers, 36%, HPV− never/former smokers=66% and HPV− current smokers=31%.
Figure 1.
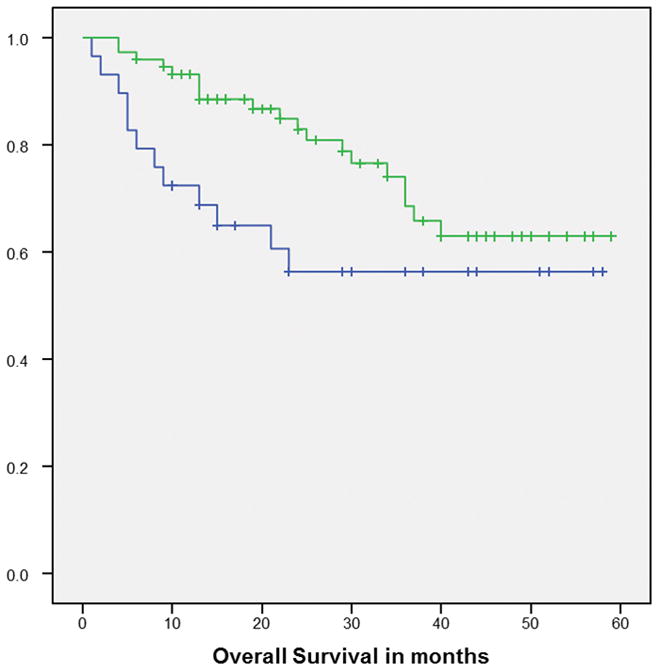
Kaplan-Meier overall survival curve for HPV+ (Green line) and HPV− (Blue line) p=0.08
Figure 2.
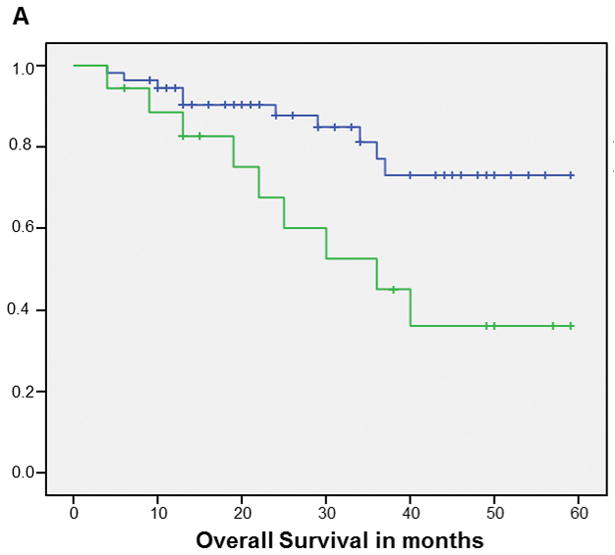
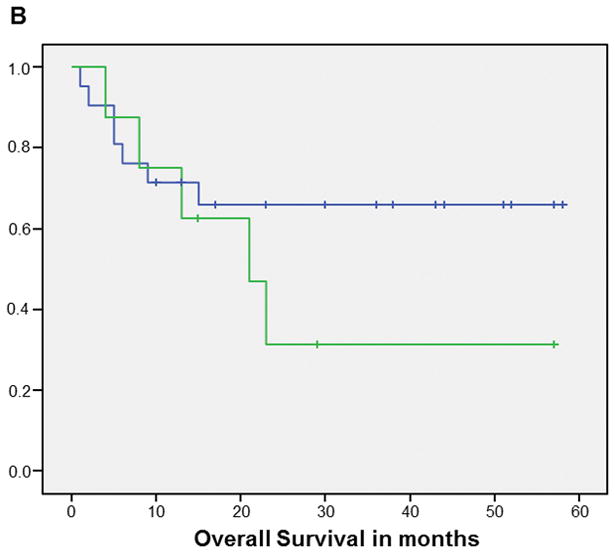
Kaplan-Meier curves of overall survival for HPV+ (A) (p=0.01) and HPV− (B) (p=0.28) patients: never/former smokers (Blue line) vs current smokers (Green line)
Results from the univariate and multivariate analysis for the effect of smoking status on overall survival stratified by HPV status are shown in Table 3. In a multivariate cox proportional hazard model adjusted for age, gender, and overall tumor stage, HPV+ current smokers experienced nearly a four-fold increase in overall mortality in comparison to HPV+ never/former smokers (HR= 3.68, 95% CI=1.35–10.0). Similarly, current smokers with HPV− tumors (HR= 6.8, 95% CI=1.11–41.67) had increased mortality compared to never/former smokers.
Table 3.
Cox Proportional Hazards Regression Modeling- Overall survival by HPV status and tobacco use
| Variable | n | Univariate | Multivariate | ||
|---|---|---|---|---|---|
|
| |||||
| Estimate (95% CI) | P-value | Estimate (95% CI) | P-value | ||
|
| |||||
| HPV Status | |||||
| HPV+ | 86 | 1.00 | 1.00 | ||
| HPV − | 34 | 1.71 (0.84, 3.50) | 0.142 | 1.61 (0.77, 3.37) | 0.209 |
|
| |||||
| HPV+ | |||||
| Never/former smokers (ref) | 66 | 1.00 | 1.00 | ||
| Current smokers | 20 | 2.80 (1.16, 6.80) | 0.022 | 3.68 (1.35, 10.0) | 0.011 |
|
| |||||
| HPV− | |||||
| Never/former smokers (ref) | 26 | 1.00 | 1.00 | ||
| Current Smokers | 8 | 2.46 (0.78, 7.75) | 0.125 | 6.8 (1.11, 41.67) | 0.038 |
The multivariate model is controlled for age, sex, HNC history, and overall stage.
The referent groups are never/former smokers.
As shown in Table 2, the cohort ultimately included 26, 66, and 28 never, former, and current smokers respectively. Starting in 2010, automated referrals to a smoking cessation program were completed for 32 patients identified prior to treatment. Of these 32 patients, 22 patients participated in at least one call and 14 participated in follow-up calls and support through the program. Eleven (78.6%) of the 14 patients that participated were alive at their last follow up. Of the 10 patients who quit smoking 30 days prior to CCRT, 9 (90%) were alive at last follow up. Of the 4 patients who reported continued smoking, 2 (50%) were alive at last follow-up.
Discussion
This study has several important findings. First, HPV+ and HPV− OPSCC patients treated with CCRT and who were current smokers have a 4 and 7-fold increased risk of mortality respectively. HPV+ current smokers experienced significantly worse 5 year OS (73% alive vs. 36% alive, p=0.01) with a similar trend in HPV− current smokers (66% alive vs. 31% alive, p=0.28) compared to former/never smokers undergoing CCRT. Though preliminary and too few for definitive statistical comparisons, among the 14 smoking cessation program participants there was a greater proportion of deaths in the patients that did not quit smoking compared to the patients that did quit smoking (50% vs 10%). All deaths were due to progression of disease. These relative proportions suggest that quitting smoking 30 days prior to CCRT has a significant impact on mortality risk.
That current smoking portends a significantly poorer prognosis in head and neck cancer is consistent with the extensive literature summarized in in the SGR 6. It has been argued that smoking status should be an important part of staging and treatment for head/neck cancer patients 19. Previous studies have demonstrated that survival is improved in HPV+ OPSCC patients when compared to HPV− OPSCC13,20–25. In addition to HPV, Ang et al 13 showed that survival could be further stratified with a +/− 10 pack year variable. Such an analysis of this cohort (data not shown) produced similar curves to Ang et al. However, our data further highlights the need to consider current smoking as a prognostic factor independent of HPV. Indeed, current smoking was associated with worse survival as compared with former and never smokers in both HPV+ and HPV− groups (Fig 2) suggesting that pack-year status may be a less robust prognostic factor than current smoking in OPSCC patients undergoing CCRT. Moreover, previous pack-years smoked is immutable, whereas current smokers can chose to become former smokers thus opening an avenue for intervention to possibly improve outcome.
Smoking and constituents of tobacco increases tumor aggressiveness in cancer cells by stimulating proliferation, angiogenesis, migration, invasion, and decreased response to cytotoxic cancer agents such as chemotherapy and radiotherapy 26,27. The fact that smoking causes adverse survival and therapeutic outcomes in virtually all cancer disease sites and for all cancer treatments, as suggested by the 2014 SGR 6, suggests that many of the biologic effects of cigarette smoke may extend across a spectrum of histologic and genetic variants. The time and dose dependent biologic effects of cigarette smoking and/or smoking cessation and the interaction with HPV remain unknown.
Overall, this is consistent with data showing that 2 to 4 weeks of smoking cessation may be necessary to improve outcomes in cancer patients treated by surgery 28,29,30,31. Data from our study suggest that cessation for 4 weeks prior to start of therapy is associated with improved survival in the HPV+ group, and though there was a similar trend, too few patients were available to more definitively confirm this association in the HPV− group.
The exact mechanism by which smoking cessation improves outcome in patients treated with chemoradiation is unknown. In a study of 393 laryngeal cancer patients treated with radiation therapy (RT), those who quit smoking after diagnosis had reduced toxicity 32. Notably, in a cohort of head and neck cancer patients treated with radiation, 43% of smokers treated in the morning experienced Grade 3+ mucositis compared with 72% of smokers treated in the afternoon (p=0.04), suggesting that some effects of smoking on toxicity may be reduced within hours 33.
Based on such data, some may postulate that a benefit of smoking cessation may be related to the additive toxicity of smoking and CRT which may lead to reduced compliance with therapy or follow-up. We could not draw any conclusions, however, about toxicity as all but one patient in this series completed CCRT in 47 days or less17,34. Additionally, there was excellent compliance with follow-up in this cohort regardless of smoking status.
This study is limited by several factors. First, although the median follow-up was 41.5 months, the minimum follow-up was 6 months. Second, though structured tobacco assessments were used to evaluate tobacco use, there were no biochemical confirmation assessments. We and others have shown that approximately 30% of current smokers may misrepresent tobacco use 35,36,37. As a result, some proportion of self-reported former smokers may actually be current smokers. Moreover, even those patients who successfully quit during therapy may start smoking again following therapy. Though smoking status following completion of therapy was not captured in our database, the estimate of the single treating radiation oncologist (AS) who continues to follow this cohort is that approximately half of all patients who quit during therapy will return to smoking following therapy. Common re-inciting factors include: anxiety, death in the family, or job loss. Nonetheless, our data suggest that smoking cessation 4 weeks prior to initiation of CCRT in OPSCC patients may improve survival, which is analogous to our prior results showing improved survival with cessation in lung cancer patients 16.
Future Directions
This data extends prior findings from the SGR, and other studies, that current smoking by SCCHN patients decreases survival in both HPV+ and HPV− patients. Smoking cessation as a standard part of cancer care is now advocated by the American Association for Cancer Research (AACR) and the American Society of Clinical Oncology (ASCO) 38,39. Recent guidelines from NCCN now support smoking cessation as a standard part of cancer care for all patients who report using tobacco within the past 30 days and support for patients who have quit smoking to prevent relapse 40. However, smoking cessation is still not widely incorporated into clinical practice with most oncologists asking about tobacco use but few assisting patients with quitting tobacco use 41,42. Though smoking affects overall survival, cancer specific survival, and cancer treatment toxicity, many active cooperative group clinical trials do not assess tobacco use43. Much work is needed to implement structured smoking assessments and cessation support into cancer care 40. Unfortunately, even in our center where an automated smoking cessation program exists, more than half of all patients referred did not stop smoking. This suggests that further work is still needed to optimize smoking cessation programs.
Conclusions
Current smoking during CCRT in OPSCC patients in this cohort was strongly associated with a 4 to 7 fold increase in risk of mortality for HPV+ and HPV− patients respectively. Given this finding, and the number of acute and often reversible changes that occur with current smoking, regardless of pack-years already smoked, every effort should be made to motivate current smokers with OPSCC to stop smoking.
Table 4.
Summary statistic for smoking cessation patient referrals
| Status | Patients Referred to Smoking Cessation Program | Former/Never Smokers n (%) |
||
|---|---|---|---|---|
| Not Successfully Contacted n (%) |
Non-Participant n (%) |
Participant n (%) |
||
| Alive | 6 (33.3) | 4 (50.0) | 11 (78.6) | 77 (81.9) |
| Dead | 12 (66.7) | 4 (50.0) | 3 (21.4) | 17 (18.1) |
| Program Participants (n=14) | ||||
| Survival |
Quit Smoking n (%) |
Did Not Quit Smoking n (%) |
||
| Alive Dead |
9 (90.0) 1 (10.0) |
2 (50.0) 2 (50.0) |
||
Footnotes
Conflict of Interest: The authors report no conflict of interest.
Level of Evidence:4
Financial Disclosure: The authors no funding or financial relationships to disclose.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011 Mar-Apr;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Society AC. Cancer Facts & Figures 2015. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 3.Sturgis EM, Wei Q, Spitz MR. Descriptive epidemiology and risk factors for head and neck cancer. Semin Oncol. 2004 Dec;31(6):726–733. doi: 10.1053/j.seminoncol.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res. 2009 Nov 15;15(22):6758–6762. doi: 10.1158/1078-0432.CCR-09-0784. [DOI] [PubMed] [Google Scholar]
- 5.Cleveland JL, Junger ML, Saraiya M, Markowitz LE, Dunne EF, Epstein JB. The connection between human papillomavirus and oropharyngeal squamous cell carcinomas in the United States: implications for dentistry. J Am Dent Assoc. 2011 Aug;142(8):915–924. doi: 10.14219/jada.archive.2011.0298. [DOI] [PubMed] [Google Scholar]
- 6.The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): 2014. [Google Scholar]
- 7.Farshadpour F, Kranenborg H, Calkoen EV, et al. Survival analysis of head and neck squamous cell carcinoma: influence of smoking and drinking. Head Neck. 2011 Jun;33(6):817–823. doi: 10.1002/hed.21549. [DOI] [PubMed] [Google Scholar]
- 8.Meyer F, Bairati I, Fortin A, et al. Interaction between antioxidant vitamin supplementation and cigarette smoking during radiation therapy in relation to long-term effects on recurrence and mortality: a randomized trial among head and neck cancer patients. Int J Cancer. 2008 Apr 1;122(7):1679–1683. doi: 10.1002/ijc.23200. [DOI] [PubMed] [Google Scholar]
- 9.Shen GP, Xu FH, He F, et al. Pretreatment lifestyle behaviors as survival predictors for patients with nasopharyngeal carcinoma. PloS one. 2012;7(5):e36515. doi: 10.1371/journal.pone.0036515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duffy SA, Ronis DL, McLean S, et al. Pretreatment health behaviors predict survival among patients with head and neck squamous cell carcinoma. J Clin Oncol. 2009 Apr 20;27(12):1969–1975. doi: 10.1200/JCO.2008.18.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012 Jun 10;30(17):2102–2111. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khuri FR, Lee JJ, Lippman SM, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst. 2006 Apr 5;98(7):441–450. doi: 10.1093/jnci/djj091. [DOI] [PubMed] [Google Scholar]
- 13.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010 Jul 1;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warren GW, Kasza KA, Reid ME, Cummings KM, Marshall JR. Smoking at diagnosis and survival in cancer patients. Int J Cancer. 2013 Jan 15;132(2):401–410. doi: 10.1002/ijc.27617. [DOI] [PubMed] [Google Scholar]
- 15.Warren GW, Marshall JR, Cummings KM, et al. Automated tobacco assessment and cessation support for cancer patients. Cancer. 2014 Feb 15;120(4):562–569. doi: 10.1002/cncr.28440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobson Amato KA, Hyland A, Reed R, et al. Tobacco Cessation May Improve Lung Cancer Patient Survival. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2015 Jul;10(7):1014–1019. doi: 10.1097/JTO.0000000000000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Platek ME, McCloskey SA, Cruz M, et al. Quantification of the effect of treatment duration on local-regional failure after definitive concurrent chemotherapy and intensity-modulated radiation therapy for squamous cell carcinoma of the head and neck. Head Neck. 2013 May;35(5):684–688. doi: 10.1002/hed.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fung-Kee-Fung SD, Hackett R, Hales L, Warren G, Singh AK. A prospective trial of volumetric intensity-modulated arc therapy vs conventional intensity modulated radiation therapy in advanced head and neck cancer. World journal of clinical oncology. 2012 Apr 10;3(4):57–62. doi: 10.5306/wjco.v3.i4.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dahlstrom KR, Calzada G, Hanby JD, et al. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center: a staging system in need of repair. Cancer. 2013 Jan 1;119(1):81–89. doi: 10.1002/cncr.27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer. 2001 Aug 15;92(4):805–813. doi: 10.1002/1097-0142(20010815)92:4<805::aid-cncr1386>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Ritchie JM, Smith EM, Summersgill KF, et al. Human papillomavirus infection as a prognostic factor in carcinomas of the oral cavity and oropharynx. Int J Cancer. 2003 Apr 10;104(3):336–344. doi: 10.1002/ijc.10960. [DOI] [PubMed] [Google Scholar]
- 22.Ihloff AS, Petersen C, Hoffmann M, Knecht R, Tribius S. Human papilloma virus in locally advanced stage III/IV squamous cell cancer of the oropharynx and impact on choice of therapy. Oral Oncol. 2010 Oct;46(10):705–711. doi: 10.1016/j.oraloncology.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Ang KK, Sturgis EM. Human papillomavirus as a marker of the natural history and response to therapy of head and neck squamous cell carcinoma. Semin Radiat Oncol. 2012 Apr;22(2):128–142. doi: 10.1016/j.semradonc.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Maxwell JH, Kumar B, Feng FY, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010 Feb 15;16(4):1226–1235. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Worsham MJ, Stephen JK, Chen KM, et al. Improved survival with HPV among African Americans with oropharyngeal cancer. Clin Cancer Res. 2013 May 1;19(9):2486–2492. doi: 10.1158/1078-0432.CCR-12-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sobus SL, Warren GW. The biologic effects of cigarette smoke on cancer cells. Cancer. 2014 Dec 1;120(23):3617–3626. doi: 10.1002/cncr.28904. [DOI] [PubMed] [Google Scholar]
- 27.Warren GW, Sobus S, Gritz ER. The biological and clinical effects of smoking by patients with cancer and strategies to implement evidence-based tobacco cessation support. Lancet Oncol. 2014 Nov;15(12):e568–580. doi: 10.1016/S1470-2045(14)70266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaporciyan AA, Merriman KW, Ece F, et al. Incidence of major pulmonary morbidity after pneumonectomy: association with timing of smoking cessation. Ann Thorac Surg. 2002 Feb;73(2):420–425. doi: 10.1016/s0003-4975(01)03443-9. discussion 425–426. [DOI] [PubMed] [Google Scholar]
- 29.Mason DP, Subramanian S, Nowicki ER, et al. Impact of smoking cessation before resection of lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database study. Ann Thorac Surg. 2009 Aug;88(2):362–370. doi: 10.1016/j.athoracsur.2009.04.035. discussion 370–361. [DOI] [PubMed] [Google Scholar]
- 30.Kuri M, Nakagawa M, Tanaka H, Hasuo S, Kishi Y. Determination of the duration of preoperative smoking cessation to improve wound healing after head and neck surgery. Anesthesiology. 2005 May;102(5):892–896. doi: 10.1097/00000542-200505000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Sardari Nia P, Weyler J, Colpaert C, Vermeulen P, Marck EV, Schil PV. Prognostic value of smoking status in operated non-small cell lung cancer. Lung Cancer. 2005 Mar-/47(3):351–359. doi: 10.1016/j.lungcan.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 32.van der Voet JC, Keus RB, Hart AA, Hilgers FJ, Bartelink H. The impact of treatment time and smoking on local control and complications in T1 glottic cancer. Int J Radiat Oncol Biol Phys. 1998 Sep 1;42(2):247–255. doi: 10.1016/s0360-3016(98)00226-0. [DOI] [PubMed] [Google Scholar]
- 33.Bjarnason GA, Mackenzie RG, Nabid A, et al. Comparison of toxicity associated with early morning versus late afternoon radiotherapy in patients with head-and-neck cancer: a prospective randomized trial of the National Cancer Institute of Canada Clinical Trials Group (HN3) Int J Radiat Oncol Biol Phys. 2009 Jan 1;73(1):166–172. doi: 10.1016/j.ijrobp.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 34.McCloskey SA, Jaggernauth W, Rigual NR, et al. Radiation treatment interruptions greater than one week and low hemoglobin levels (12 g/dL) are predictors of local regional failure after definitive concurrent chemotherapy and intensity-modulated radiation therapy for squamous cell carcinoma of the head and neck. Am J Clin Oncol. 2009 Dec;32(6):587–591. doi: 10.1097/COC.0b013e3181967dd0. [DOI] [PubMed] [Google Scholar]
- 35.Alberg AJ, Worley ML, Tooze JA, et al. The Validity of Self-reported Recent Smoking in Head and Neck Cancer Surgical Patients. Otolaryngol Head Neck Surg. 2015 Dec;153(6):990–995. doi: 10.1177/0194599815594385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warren GW, Arnold SM, Valentino JP, et al. Accuracy of self-reported tobacco assessments in a head and neck cancer treatment population. Radiother Oncol. 2012 Apr;103(1):45–48. doi: 10.1016/j.radonc.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khuri FR, Kim ES, Lee JJ, et al. The impact of smoking status, disease stage, and index tumor site on second primary tumor incidence and tumor recurrence in the head and neck retinoid chemoprevention trial. Cancer Epidemiol Biomarkers Prev. 2001 Aug;10(8):823–829. [PubMed] [Google Scholar]
- 38.Hanna N, Mulshine J, Wollins DS, Tyne C, Dresler C. Tobacco cessation and control a decade later: American society of clinical oncology policy statement update. J Clin Oncol. 2013 Sep 1;31(25):3147–3157. doi: 10.1200/JCO.2013.48.8932. [DOI] [PubMed] [Google Scholar]
- 39.Toll BA, Brandon TH, Gritz ER, et al. Assessing tobacco use by cancer patients and facilitating cessation: an American Association for Cancer Research policy statement. Clin Cancer Res. 2013 Apr 15;19(8):1941–1948. doi: 10.1158/1078-0432.CCR-13-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ostroff JS, Goffin JR, Khuri FR, Warren GW. Perspective on the National Comprehensive Cancer Network’s Clinical Practice Guidelines for Smoking Cessation. Journal of oncology practice / American Society of Clinical Oncology. 2015 Sep 15; doi: 10.1200/JOP.2015.006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warren GW, Marshall JR, Cummings KM, et al. Practice patterns and perceptions of thoracic oncology providers on tobacco use and cessation in cancer patients. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013 May;8(5):543–548. doi: 10.1097/JTO.0b013e318288dc96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warren GW, Marshall JR, Cummings KM, et al. Addressing tobacco use in patients with cancer: a survey of american society of clinical oncology members. Journal of oncology practice / American Society of Clinical Oncology. 2013 Sep 1;9(5):258–262. doi: 10.1200/JOP.2013.001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters EN, Torres E, Toll BA, et al. Tobacco assessment in actively accruing National Cancer Institute Cooperative Group Program Clinical Trials. J Clin Oncol. 2012 Aug 10;30(23):2869–2875. doi: 10.1200/JCO.2011.40.8815. [DOI] [PMC free article] [PubMed] [Google Scholar]


