Abstract
Background:
The available evidence on the role of arsenic metabolism in individual susceptibility to the development of cancer, cardiovascular disease, and diabetes has not been formally and comprehensively reviewed.
Objectives:
Our goal was to systematically investigate the association of arsenic metabolism with cancer, cardiovascular disease, and diabetes-related outcomes in epidemiologic studies. As a secondary objective, we characterized the variation of arsenic metabolism in different populations worldwide.
Methods:
We searched Medline/PubMed and EMBASE from inception to January 2016 and applied predetermined exclusion criteria. Compositional data analysis was used to describe the distribution of arsenic metabolism biomarkers and evaluate the association between arsenic exposure and metabolism.
Results:
Twenty-eight studies met the inclusion criteria, 12 on cancer, nine on cardiovascular disease, and seven on diabetes-related outcomes. The median (interquartile range) for mean iAs%, MMA%, and DMA% was 11.2 (7.8–14.9)%, 13.0 (10.4–13.6)%, and 74.9 (69.8–80.0)%, respectively. Findings across studies suggested that higher arsenic exposure levels were associated with higher iAs% and lower DMA% and not associated with MMA%. For cancer, most studies found a pattern of higher MMA% and lower DMA% associated with higher risk of all-site, urothelial, lung, and skin cancers. For cardiovascular disease, higher MMA% was generally associated with higher risk of carotid atherosclerosis and clinical cardiovascular disease but not with hypertension. For diabetes-related outcomes, the pattern of lower MMA% and higher DMA% was associated with higher risk of metabolic syndrome and diabetes.
Conclusions:
Population level of iAs% and DMA%, but not MMA%, were associated with arsenic exposure levels. Overall, study findings suggest that higher MMA% was associated with an increased risk of cancer and cardiovascular disease, while lower MMA% was associated with an increased risk of diabetes and metabolic syndrome. Additional population-based studies and experimental studies are needed to further evaluate and understand the role of arsenic exposure in arsenic metabolism and the role of arsenic metabolism in disease development. https://doi.org/10.1289/EHP577
Introduction
Inorganic arsenic in water and food is a top priority toxicant for risk assessment and exposure reduction/mitigation (ATSDR 2011; IPCS 2010) as chronic arsenic exposure affects multiple organ systems, resulting in various cancers and cardiovascular disease, and possibly also in respiratory disease, diabetes, neurocognitive outcomes, and kidney disease (Kuo et al. 2013; Moon et al. 2012; NRC 2001; Naujokas et al. 2013; Tyler and Allan 2014; Wu et al. 2014b). Understanding the health effects of arsenic requires assessing the role of interindividual variation in inorganic arsenic metabolism. After absorption, inorganic arsenic (iAs including arsenate and arsenite) is mainly methylated into monomethylated and dimethylated compounds (MMA, DMA) in the liver, which are then excreted through the kidney together with unmethylated inorganic arsenic (Vahter 2002). Whether the methylation process is to detoxify or potentiate arsenic toxicity, however, remains an ongoing debate and may depend on the study outcome (Rossman 2003; Styblo et al. 2000).
Arsenic metabolism in urine is generally reported as the percentage of each arsenic species divided by their sum (iAs%, MMA%, and DMA%) and/or as the primary and secondary methylation indices (PMI, the ratio of MMA over iAs; and SMI, the ratio of DMA over MMA) (Del Razo et al. 1997). The relative proportion of arsenic metabolites in urine has been reported to be around 10–30% inorganic arsenic, 10–20% MMA, and 60–80% DMA, with substantial interpopulation and intrapopulation variations (Hernandez and Marcos 2008; Huang et al. 2009; Steinmaus et al. 2005; Vahter 2000). Higher levels of MMA% and lower levels of DMA% have been related to cancer and cardiovascular outcomes in populations from Taiwan, Bangladesh, and Argentina (Chen et al. 2013b; Hsueh et al. 1997; Steinmaus et al. 2006; Tseng et al. 2005). Lower levels of MMA% and higher levels of DMA%, on the other hand, have been related to higher body mass index and diabetes-related outcomes in populations from Mexico, Taiwan, and the United States (Chen et al. 2012; Gomez-Rubio et al. 2011; Gribble et al. 2013).
The available evidence on the role of arsenic metabolism in individual susceptibility to the development of cancer, cardiovascular disease, and diabetes has not been formally and comprehensively reviewed. Our objective was to conduct a systematic review to examine the role of arsenic metabolism, as measured in urine, in the development of cancer, cardiovascular disease, and diabetes-related outcomes. We evaluated whether the methylation patterns associated with higher risk of disease are consistent across diseases and across populations with varying arsenic exposure levels. In a secondary analysis, we characterized the urine arsenic metabolism profile in populations around the world.
Methods
Search Strategy and Study Selection
The systematic search and review processes were conducted in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines (Stroup et al. 2000). We searched PubMed/Medline (https://www.ncbi.nlm.nih.gov/pubmed) and EMBASE (https://www.embase.com/) for original epidemiologic studies investigating the role of arsenic metabolism in the risk of cancer, cardiovascular disease, and diabetes-related outcomes. For arsenic metabolism, we used the following MeSH terms and free text: “arsenic,” “methylation,” “metabolism,” “arsenic metabolism,” “arsenic methylation,” combined with other specific text-word terms related to the key research concepts. The PubMed and EMBASE database search strategies in the supplementary material [“PubMed and EMBASE database search strategies for arsenic metabolism and outcomes of interest Medline/PubMed (Jan 25, 2016)” and “EMBASE (Jan 25, 2016)”] show the full search strategy, and Figure S1 shows the flow diagram of study identification, selection, and exclusion. The search period was January 1966 through January 2016. There were no language restrictions. We also manually reviewed the reference lists from relevant original research and the investigators’ files.
Our primary exclusion criteria to screen records, after removing studies in nonhuman populations, were as follows: a) publications contained no original research (i.e., reviews, editorials, nonresearch letters); b) case reports and case series; c) studies did not measure cancer, cardiovascular outcomes (coronary heart disease, stroke, peripheral arterial disease, and hypertension), or diabetes and diabetes-related outcomes (including prediabetes and metabolic syndrome); and d) studies did not report information on arsenic metabolism, as measured by urine (percentage or ratios of urine arsenic species). After full text review, records were excluded if they met any of the following conditions: a) lacked report of the association between arsenic metabolism and the study outcome; b) focused only on arsenic-related skin lesions (premalignancy); c) were conducted among pregnant women (pregnancy may influence arsenic metabolism) (Hopenhayn et al. 2003), or d) were conducted among those less than 18 y of age(Su et al. 2012). Several studies that measured arsenic metabolism were excluded because they only reported the association for arsenic exposure with study outcomes stratified by arsenic metabolism levels, but not for arsenic metabolism per se (Engström et al. 2015; Leonardi et al. 2012; Tseng et al. 2005). For studies conducted in the same study population and with same outcome of interest [11 studies from the same population in Taipei (Chiang et al. 2014; Chung et al. 2008, 2010, 2011a, 2011b, 2013a, 2013b; Huang et al. 2016; Pu et al. 2007; Wu et al. 2013a; Wu et al. 2013b), 2 studies from the same population in Tainan (Chen et al. 2003a, 2005), and three studies in the endemic Blackfoot disease area (Hsu et al. 2015; Huang et al. 2008a, 2008b), all of above from Taiwan; four studies in Bangladesh (Chen et al. 2013a, 2013b; Wu et al. 2014a, 2015); two studies in Inner Mongolia (Li et al. 2013a, 2015)], we selected the publication with the largest sample size and with more complete information for the final statistical analysis (Chen et al. 2013b, 2003a; Huang et al. 2008a; Li et al. 2013a; Pu et al. 2007).
Data Abstraction
Two authors, C.-C.K. and K.A.M., independently abstracted data from articles that met the selection criteria. We used a standardized data extraction form to record the study characteristics (authors, journal, year of publication, country, study design, and study objectives); the participant characteristics [study population (general vs. hospital-based), number of participants, age, sex, and arsenic exposure levels as measured in urine or water]; measures of arsenic metabolism (iAs%, MMA%, DMA%, PMI, and SMI); outcome definitions; and the results of the association analysis, including the variables used for adjustment. To assess study quality, we adapted the criteria used by Longnecker et al. for observational studies (see Tables S1–S3) including the domains related to exposure assessment, outcome definition, and modeling approaches (Longecker et al. 1988). Disagreement between the two reviewers was resolved by consensus and discussion with a third reviewer if needed.
Statistical Methods
For mean age and sex distribution, mean arsenic concentrations in water or urine, and for each arsenic metabolism biomarker (iAs%, MMA%, DMA%, PMI, and SMI), we derived the numbers when not directly reported. For four studies included in the evaluation of metabolism distribution across populations that did not report arsenic concentrations in water or urine, the exposure level was imputed based on reported arsenic levels in water [ in Argentina and in California/Nevada in the United States (Steinmaus et al. 2006), in China (Li et al. 2013a), and in Northern Chile (Melak et al. 2014)] assuming that arsenic levels in urine and water are positively associated (Murcott 2012). Throughout the study, the estimated arsenic exposure level (eAs, ) was defined by derived arsenic concentrations in water (), urine ( or creatinine), or previous biomonitoring data indicating arsenic concentrations in water () relevant at the time of study recruitment. Most studies reported means of arsenic metabolism biomarkers in the population. For studies that did not, we calculated the means of each arsenic metabolism biomarker by dividing the mean concentrations of each arsenic species by the mean of total arsenic or the sum of inorganic and methylated arsenic. For three studies that provided medians of arsenic metabolism biomarkers among cases and noncases, we used the data from noncases to approximate the means of arsenic metabolism biomarkers (Gilbert-Diamond et al. 2013; Huang et al. 2008a; Lopez-Carrillo et al. 2014).
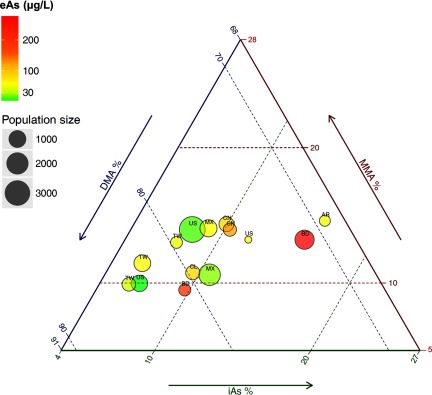
To describe arsenic metabolism across populations, we reported the median (interquartile range) of means in each study for each arsenic metabolism biomarker (iAs%, MMA%, and DMA%). The interpretation of these biomarkers is complicated because they are interdependent and their sum equals 100%. Higher MMA%, for example, could indicate either lower iAs% or lower DMA%. We used a three-axis diagram (triplot, or ternary plot) to represent the means of iAs%, MMA%, and DMA% across study populations. This inherent non-normality of the data makes the conventional linear regression modeling not feasible. We described the relationship between mean arsenic concentrations in urine (among studies without reported urine arsenic levels, concurrent measurements of arsenic levels in drinking water, or published arsenic biomonitoring data were used) and arsenic metabolism biomarkers (iAs%, MMA%, and DMA%) using the isometric log-ratio approach to compositional data analysis (van den Boogaart and Tolosana-Delgado 2013a). Essentially, we used log-ratio transformations of the arsenic metabolism components in order to apply standard multivariate regression techniques, and we estimated predicted values of each arsenic metabolism component by estimated arsenic exposure. A key principal of this method is scale invariance, meaning that only the relative, not absolute, magnitude of the components is important. Applying the log-ratio methodology with zero or missing values requires additional assumptions (van den Boogaart et al. 2015). For further information on compositional data analysis, including model diagnostics and variable selection, we refer the reader to the available textbooks and reviews (Aitchison 1986; Lovell et al. 2015; Pawlowsky-Glahn et al. 2007; Pawlowsky-Glahn and Buccianti 2011; van den Boogaart and Tolosana-Delgado 2013a). All statistical analyses and graphical displays for compositional data analysis were performed using packages ggtern (extension of ggplot2 for the creation of tertiary diagrams; Nicholas Hamilton) and compositions: Compositional Data Analysis (version 1.30-1; K. G. van den Boogaart, Raimon Tolosana, Matevz Bren) in R (version 3.0.0; R Development Core Team).
To evaluate the association between arsenic metabolism biomarkers and health effects (cancer, cardiovascular disease and diabetes-related outcomes), we retrieved the relative risk estimates from the model adjusted for the most covariates. There were no large differences between the fully adjusted and crude models. To identify patterns across studies, we assessed the magnitude and direction of the association across studies without taking into account statistical significance but describing whether the individual studies were statistically significant or not. We did not perform a meta-analysis due to the small number of studies for most health endpoints evaluated and inconsistencies in the reporting format across studies.
Results
Arsenic Metabolism across Study Populations
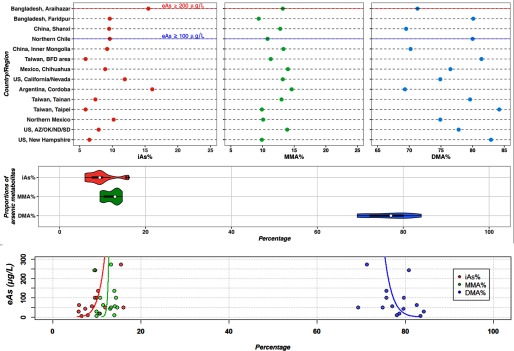
Twenty-six studies met the inclusion criteria, including 12 on cancer, 10 on cardiovascular diseases, and 5 on diabetes-related outcomes. Twelve studies were conducted in Taiwan (Chen et al. 2012, 2003a, 2003b; Chung et al. 2009; Hsueh et al. 1997; Huang et al. 2007, 2008a; YL Huang et al. 2009; Pu et al. 2007; Wang et al. 2011; Wu et al. 2006; Yu et al. 2000), four in the United States (Gilbert-Diamond et al. 2013; Kuo et al. 2015; Moon et al. 2013; Steinmaus et al. 2006), three in Bangladesh (Chen et al. 2013a, 2013b; Nizam et al. 2013), three in Mexico (Del Razo et al. 2011; Lopez-Carrillo et al. 2014; Mendez et al. 2016), 2 in China (Li et al. 2013a, 2013b), two in Argentina (Steinmaus et al. 2006, 2010), and one in Chile (Melak et al. 2014). The medians (interquartile range) for mean iAs%, MMA%, and DMA% of the 14 unique populations included were 9.4 (7.4–10.2)%, 12.9 (10.1–13.3)%, and 77.3 (71.3–80.1)%, respectively. The compositional means for iAs%, MMA%, and DMA% were 9.4%, 12.3%, and 78.4%, respectively. The distribution and variability of arsenic metabolism across study populations are summarized in Figure 1 and Figure 2, upper panel. Compared with iAs% and DMA%, MMA% showed lower interpopulation variance, with population means ranging from 9.4% in Faridpur, Bangladesh (Nizam et al. 2013) to 14.6% in Cordoba, Argentina (Steinmaus et al. 2006) (Figure 2, upper and middle panel). In an analysis of the relationship between arsenic exposure levels and arsenic metabolism biomarkers, a 2-fold increase in the estimated urine arsenic concentrations was associated with a 0.8% [95% confidence interval (CI): , 1.9] increase in iAs%, no association with MMA% (0.1% increase, 95% CI: , 0.8), and a 1.3% (95% CI: , 0.5) decrease in DMA%. In compositional data analysis, we confirmed a similar relationship between arsenic exposure levels and arsenic metabolism indicators at the population level (Figure 2, bottom panel). We conducted sensitivity analyses removing studies with urine arsenic concentrations estimated from imputed water arsenic levels (Li et al. 2013a; Melak et al. 2014; Steinmaus et al. 2006), with similar results: a 2-fold increase in the estimated urine arsenic concentrations was associated with a 0.9% (95% CI: , 1.9) increase in iAs%, no association with MMA% (0.1% increase, 95% CI: , 0.8), and a 1.2% (95% CI: , 0.6) decrease in DMA% (also see Figure S2).
Figure 1.
The distribution of urine arsenic metabolism profile across enrolled populations worldwide on ternery plot diagram. Three-axis graph (triplot) representing the compositional mean of arsenic metabolism biomarkers (iAs%, MMA%, and DMA%) in different study populations. The circles represent each study population and the country of the study is indicated with an acronym (AR, Argentina; BD, Bangladesh; CL, Chile; CN, China; MX, Mexico; TW, Taiwan; US, USA). The size of the circle corresponds to the study population size. The color of the circles reflects the estimated urine arsenic concentrations (eAs, ) as indicated in the top left legend (the highest two countries: BD and CN; while the lowest two: US and MX. For exact ranking of eAs, please refer to the top panel of Figure 2). The bottom side of the triplot represents iAs%, the right side represents MMA%, and the left side represents DMA%. For each population, the mean iAs%, MMA%, and DMA% can be estimated along parallel lines to the dashed green lines for iAs%, dashed red lines for MMA% and dashed blue lines for DMA%. For instance for the study from Chile (CL), the arsenic metabolism profile was 9.6% for iAs%, 10.8% for MMA% and 79.6% for DMA%. In this graph, we can observe that population means ranged between 5.9% and 16.1% for iAs%, between 9.4 and 14.6% for MMA%, and between 69.3% and 84.2% for DMA%.
Figure 2.
Variability of arsenic metabolism biomarkers in the study populations. Top panel: The distribution of each arsenic metabolism biomarker (iAs%, MMA%, DMA%) is plotted for each study listed in increasing order (from bottom to top) of the estimated urine arsenic levels in (eAs, ) in the study area. Middle panel, violin plot showing the median (open circle) with interquartile range (horizontal bar) and the kernel probability density for each arsenic metabolism biomarker (iAs%, MMA%, DMA%) across all studies. Bottom panel, the prediction curve (line) (right for iAs%, central for MMA%, and left for DMA%) derived from the compositional regression of each arsenic metabolism biomarker based on estimated urine arsenic levels (eAs, ). The right increasing curve supports that iAs% increases as eAs increases and the left decreasing curve supports that DMA% decreases as eAs increases. The central curve supports that MMA% does not change with changes in eAs concentrations as the line is vertical.
Study Design and Quality Assessment
By study design, seven studies were cross-sectional, 12 were case–control, and seven were prospective cohorts. The studies showed marked heterogeneity in reporting and modeling biomarkers of arsenic metabolism (see Tables S2–S4). Among case–control studies, most of them did not describe the timing of interview, response rate among noncases, and case–control selection, criteria commonly used to address potential surveillance, selection, and recall bias. Prospective studies, on the other hand, generally met important quality criteria, although associations between arsenic metabolism and health outcomes were not adjusted for total arsenic exposure in three studies (Chung et al. 2009; Hsueh et al. 1997; Wang et al. 2011).
Arsenic Metabolism and Cancer
Of the 12 studies on arsenic metabolism and cancer, 2 were prospective cohort studies (Chung et al. 2009; Huang et al. 2008a) and 10 were case–control studies (Table 1). Seven studies were conducted in Taiwan, six in the Blackfoot disease endemic area (arsenic in drinking water ) (Chen et al. 2003a, 2003b; Chung et al. 2009; Hsueh et al. 1997; Huang et al. 2008a; Yu et al. 2000), and one in Taipei City/County (arsenic in drinking water ) (Pu et al. 2007). Outside of Taiwan, the studies were conducted in populations from Argentina (Steinmaus et al. 2006, 2010), Chile (Melak et al. 2014), the United States (Gilbert-Diamond et al. 2013; Steinmaus et al. 2006), and Eastern Europe (Leonardi et al. 2012). Study populations from Argentina and Chile were exposed to arsenic levels in drinking water (Melak et al. 2014; Steinmaus et al. 2006). The U.S. study populations from California and Nevada were exposed to arsenic levels (Steinmaus et al. 2006), and the study population from New Hampshire was exposed to arsenic levels in drinking water (Gilbert-Diamond et al. 2013). Five studies evaluated urothelial cancers (Chen et al. 2003b; Huang et al. 2008a; Melak et al. 2014; Pu et al. 2007; Steinmaus et al. 2006), four evaluated skin cancer (Chen et al. 2003a; Gilbert-Diamond et al. 2013; Hsueh et al. 1997; Yu et al. 2000), two evaluated lung cancer (Melak et al. 2014; Steinmaus et al. 2010), one evaluated breast cancer (Lopez-Carrillo et al. 2014), and one evaluated all-cancer incidence (Chung et al. 2009) (Table 1). All studies confirmed incident cancer outcomes using pathological and medical information but did not provide analyses by histological subtypes (Table 1). Arsenic metabolism was defined based on the percentages of arsenic metabolites (iAs%, MMA%, and DMA%) in four studies (Hsueh et al. 1997; Melak et al. 2014; Steinmaus et al. 2006; Steinmaus et al. 2010), based on the methylation indices (PMI and SMI) in one study (Chen et al. 2003b), and based on both the arsenic species proportions and the methylation indices in seven studies (Chen et al. 2003a; Chung et al. 2009; Gilbert-Diamond et al. 2013; Huang et al. 2008a; Lopez-Carrillo et al. 2014; Pu et al. 2007; Yu et al. 2000) (Table 1).
Table 1.
Studies of arsenic metabolism and cancer.
| Reference | Study design (case/noncase) | Men (%) Age range Source | Outcome (Ascertainment method) | Arsenic exposure | Adjustment factors | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample (level) | iAs% | MMA% | DMA% | PMI | SMI | |||||
| Chung et al. 2009 | CO 17/191 | Putai, Taiwan | All cancer (registry) | Urine (mean total arsenic ) | NR | Age, sex, education | ||||
| 36% | vs. | vs. | vs | vs. | ||||||
| Mean 46 y | 0.6 (0.2, 1.7) | 2.4 (0.8, 9.1) | 0.9 (0.3, 2.7) | 0.4 (0.1, 1.4) | ||||||
| General | ||||||||||
| Chen et al. 2003b | CC 49/224 | Tainan, Taiwan | Urothelial (pathology) | Water (mean CAE ) | NR | NR | NR | 4.8 | 10.9 | Age, sex, education, smoking, BMI, hair dye, CAE |
| 63% | vs. | vs. | ||||||||
| 0.5 (0.2, 1.4) | 0.6 (0.3, 1.5) | |||||||||
| Hospital | ||||||||||
| Steinmaus et al. 2006 | CC 114/114 | Córdoba, Argentina | Urothelial (pathology) | Water (low-moderate) | NR | NR | Age, sex, smoking, bombilla use | |||
| 82% | NR | vs. | NR | |||||||
| 20-80 yrs | 1.3 (0.7, 2.4) | |||||||||
| General | ||||||||||
| 23/49 | California and Nevada, USA | Urothelial carcinoma (registry) | Water (low-moderate) | Age, sex, smoking | ||||||
| 80% | NR | vs. | NR | |||||||
| 40-85 y | 1.2 (0.4-3.7) | |||||||||
| General | ||||||||||
| Pu et al. 2007 | CC 177/313 | Taipei, Taiwan | Urothelial (pathology) | Urine (mean sum iAs, MMA, and DMA creatinine) | Age, sex, education, alcohol, paternal and maternal ethnicity, pesticide usage | |||||
| 66% | vs. | vs. | vs. | vs. | vs. | |||||
| 24-93 y | 1.2 (0.7, 2.0) | 2.8 (1.6, 4.8) | 0.4 (0.2, 0.7) | 3.1 (1.7, 5.6) | 0.3 (0.2, 0.6) | |||||
| Hospital | ||||||||||
| Huang et al. 2008a | CO 37/928 | Putai, Taiwan | Urothelial | Urine (median sum iAs, MMA, and DMA ) | Age, sex, education, smoking, CAE | |||||
| 43% | (registry and pathology) | vs. | vs. | vs. | vs. | vs. | ||||
| 1.4 (0.5, 3.6) | 1.7 (0.7, 4.0) | 0.3 (0.1, 0.9) | 0.8 (0.4, 2.0) | 0.5 (0.2, 1.3) | ||||||
| General | ||||||||||
| Melak et al. 2014 | CC | Northern Chile | Urothelial (medical records) | Water (lifetime average arsenic ) | NR | NR | Age, sex, smoking | |||
| Lung: 94/347; uro-thelial: 117/347 | 69% | Lung (medical records) | vs. | vs. | vs. | |||||
| Urothelial | Urothelial | Urothelial | ||||||||
| Hospital | 0.3 (0.2, 0.5) | 1.4 (0.9, 2.2) | 1.7 (1.1, 2.6) | |||||||
| Lung | Lung | Lung | ||||||||
| 1.1 (0.7, 1.8) | 2.3 (1.4, 3.8) | 0.6 (0.4, 1.1) | ||||||||
| Steinmaus et al. 2010 | CC | Córdoba, Argentina | Lung (medical records) | Water (low-moderate) | NR | NR | Age, sex, smoking, drinking-water arsenic exposure | |||
| 45/75 | 88% | NR | vs. | NR | ||||||
| 20-80 y | 3.1 (1.1, 8.1) | |||||||||
| Hospital | ||||||||||
| Hsueh et al. 1997 | CC 16/61 | Putai, Taiwan | Non-melanoma skin (pathology) | Water (artesian well ) | Age, sex, | |||||
| 42% | NR | Among CAE , vs. :3.0 (0.3-27.8) | NR | NR | NR | |||||
| General | ||||||||||
| Yu et al. 2000 | CC 26/26 | Putai, Taiwan | Non-melanoma skina (dermatologist diagnosis) | Urine (mean sum iAs, MMA, and DMA ) | 12.3 | 15.5 | 72.2 | NR | 4.6 | Age, sex |
| 54% | vs | vs | vs b | vs. | ||||||
| Mean 63 y | 3.5 (0.7, 16.9) | 5.5 (1.224.8) | 3.25 (1.1, 10.0) | 3.3 (0.9, 12.1)c | ||||||
| Hospital | ||||||||||
| Chen et al. 2003a | CC 76/224 | Tainan, Taiwan | Non-melanoma skin (pathology) | Urine (mean sum iAs, MMA, and DMA ) | Age, sex, education, smoking, alcohol, body mass index, sun exposure, CAE | |||||
| 60% | NR | Highest vs. lowest tertile | Highest vs. lowest tertile | vs. | vs. | |||||
| 1.4 (0.6, 3.4) | 0.8 (0.3, 1.9) | 1.3 (0.6, 2.9) | 0.9 (0.4, 2.1) | |||||||
| Hospital | ||||||||||
| Gilbert-Diamond et al. 2013 | CC 323/319 | New Hampshire, USA | Squamous cell (pathology and medical records) | Urine (median sum iAs, MMA, and DMA ) | ± median | ± median | ± median | ± median | ± median | Age, sex, education, smoking, BMI, urine creatinine, skin reaction to sun exposure, water arsenic |
| 59% | per 1% increase | per 1% increase | per 1% increase- | per 1% increase | per 1% increase | |||||
| 25–74 y | 1.00 (0.97, 1.04) | 1.01 (0.97, 1.05) | 0.99 (0.97, 1.02) | 1.08 (0.95, 1.23) | 1.00 (0.97, 1.03) | |||||
| General | ||||||||||
| López-Carrillo et al. 2014 | CC 1,016/1,028 | Northern Mexico | Breast cancer (pathology) | Urine (geometric mean iAs conc. creatinine) | Total inorganic arsenic, age, BMI, total breastfeeding, alcohol, smoking, age at first pregnancy, creatinine | |||||
| 0% | vs. | vs. | vs. | vs. | vs. | |||||
| Mean 54 y | 0.90 (0.65, 1.24) | 2.63 (1.89, 3.66) | 0.63 (0.45, 0.87) | 1.90 (1.39, 2.59) | 0.42 (0.31, 0.59) | |||||
Note:Values shown as mean±SD, highest vs. lowest category, and eRR (95% CI), unless otherwise specified. BMI, body mass index, Ca, cancer; CAE, cumulative arsenic exposure; CC, case–control study; CI, confidence interval; CO, prospective cohort study; DMA, dimethylarsinate; eRR, estimated relative risk; iAs, inorganic arsenic; MMA, monomethylarsonate; NR, not reported; NS, not significant; PMI, primary methylation index (MMA/iAs); SD, standard deviation; SMI, secondary methylation index (DMA/MMA).
Skin cancer refers to non-melanoma skin cancer.
Lowest vs. highest categories.
Association for the DMA/MMA, rather than MMA/DMA.
Urothelial cancer.
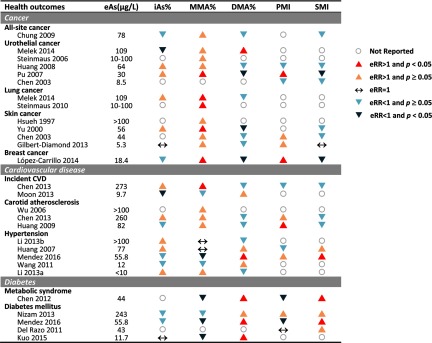
Of five studies on urothelial cancer (Chen et al. 2003b; Huang et al. 2008a; Melak et al. 2014; Pu et al. 2007; Steinmaus et al. 2006), four reported a positive association with MMA% (Huang et al. 2008a; Melak et al. 2014; Pu et al. 2007; Steinmaus et al. 2006), three with iAs% and DMA% (Huang et al. 2008a; Melak et al. 2014; Pu et al. 2007), and three with PMI and SMI (Chen et al. 2003b; Huang et al. 2008a; Pu et al. 2007). For MMA%, the direction of the estimated relative risks for urothelial cancer comparing the highest to lowest category of MMA% was consistent for all studies (relative risks (RR) range from 1.2 to 2.8), although only one study association was statistically significant (Table 1 and Figure 3) (Pu et al. 2007). For SMI, the corresponding estimated relative risks were also consistent across studies (RR from 0.3 to 0.6), although only one study association was statistically significant (Pu et al. 2007). The corresponding estimated relative risks for iAs%, DMA% and PMI were inconsistent across studies and ranged from 0.3 to 1.4 for iAs%, from 0.3 to 1.7 for DMA%, and from 0.5 to 3.1 for PMI (Table 1).
Figure 3.
Summary of the associations of arsenic methylation patterns with cancer, cardiovascular disease, and diabetes-related outcomes based on the estimated relative risk (eRR) and 95% confidence interval (CI) shown in Tables 1–3. Studies are sorted by estimated arsenic exposure (eAs) within each health outcome. The triangles indicate the direction of the association. An upward-pointing triangle stands for positive association while a downward-pointing triangle stands for a negative association. An upward-pointing triangle indicates a positive and statistically significant association (eRR above 1 and 95% CI not overlapping 1) and a lighter upward-pointing triangle indicates a positive but not statistically significant increase (eRR above 1 but 95% CI overlapping 1). A downward-pointing triangle indicates a negative and statistically significant association (eRR below 1 and 95% CI not overlapping 1) and a lighter downward-pointing triangle indicates a negative but not statistically significant association (eRR below 1 but 95% CI not overlapping 1). A horizontal arrow (↔) indicates a null association (eRR equal to 1). Gray open circles with no arrows indicate the data were not reported in the study. All the associations represented in this figure are also shown in Tables 1–3 as eRR and 95% CI.
Lung cancer.
Of the two studies on lung cancer (Melak et al. 2014; Steinmaus et al. 2010), both reported an association with MMA% (Melak et al. 2014; Steinmaus et al. 2010), and one also reported associations with iAs% and DMA% (Melak et al. 2014). No studies of lung cancer reported associations with PMI and SMI. For MMA%, the estimated relative risks comparing the highest to lowest category of MMA% were 2.3 and 3.1, both of them statistically significant (Table 1).
Skin cancer.
Of the four studies on skin cancer [three nonmelanoma skin cancer (Chen et al. 2003a; Hsueh et al. 1997; Yu et al. 2000), one squamous cell carcinoma (Gilbert-Diamond et al. 2013)], four reported on the association with MMA% (Chen et al. 2003a; Gilbert-Diamond et al. 2013; Hsueh et al. 1997; Yu et al. 2000), two with iAs% (Gilbert-Diamond et al. 2013; Yu et al. 2000), three with DMA% (Chen et al. 2003a; Gilbert-Diamond et al. 2013; Yu et al. 2000), two with PMI (Chen et al. 2003a; Gilbert-Diamond et al. 2013), and three with SMI (Chen et al. 2003a; Gilbert-Diamond et al. 2013; Yu et al. 2000). For MMA%, the estimated relative risks for skin cancer comparing the highest to lowest category of MMA% were consistent for all studies (RR range from 1.4 to 5.5), although only one study association was statistically significant (Yu et al. 2000). For iAs%, one study reported a null association and another showed a positive nonsignificant association (Table 1). For DMA%, one study found a positive significant association (RR of 3.25 (Yu et al. 2000)) and the others found inverse but nonstatistically significant associations (Chen et al. 2003a; Gilbert-Diamond et al. 2013). For PMI, both studies showed positive but nonsignificant associations (Chen et al. 2003a; Gilbert-Diamond et al. 2013). For SMI, one study was nonsignificantly positive (Yu et al. 2000), one study was inverse but nonsignificant (Chen et al. 2003a), and the other was null (Gilbert-Diamond et al. 2013).
Arsenic Metabolism and Cardiovascular Disease
From 10 studies reporting cardiovascular outcomes, five were conducted in Taiwan (Huang et al. 2007; YL Huang et al. 2009; Wang et al. 2011; Wu et al. 2006), 2 in Bangladesh (Chen et al. 2013a, 2013b), two in China (Li et al. 2013a, 2013b), one in the United States (Moon et al. 2013), and 1 in Mexico (Mendez et al. 2016). All study populations were characterized by high arsenic exposure ( in drinking water), except the study from the United States (Moon et al. 2013). Three were prospective cohort studies (Chen et al. 2013b; Moon et al. 2013; Wang et al. 2011), one was a nested case–control study (Wu et al. 2006), and six were cross-sectional studies (Chen et al. 2013a; Huang et al. 2007; YL Huang et al. 2009; Li et al. 2013a, 2013b; Mendez et al. 2016). Study outcomes included hypertension () (Huang et al. 2007; Li et al. 2013a, 2013b; Mendez et al. 2016; Wang et al. 2011), carotid atherosclerosis () (Chen et al. 2013a; YL Huang et al. 2009; Wu et al. 2006), and overall cardiovascular disease () (Chen et al. 2013b; Moon et al. 2013). Five studies reported results for percentages of each metabolite (iAs%, MMA%, and DMA%) (Li et al. 2013a, 2013b; Moon et al. 2013; Wang et al. 2011; Wu et al. 2006), whereas the other five studies reported both percentages of each metabolite and methylation indices (PMI and SMI) (Table 2) (Chen et al. 2013a, 2013b; Huang et al. 2007; YL Huang et al. 2009; Mendez et al. 2016).
Table 2.
Studies of arsenic metabolism and cardiovascular diseases.
| Reference | Study design (case/noncase) | Men (%) Age range Source | Outcome (Ascertainment method) | Arsenic exposure | Adjustment factors | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample (level) | iAs% | MMA% | DMA% | PMI | SMI | |||||
| Moon et al. 2013 | CO Total | Arizona, Oklahoma, North/South Dakota, USA | CVD incidence and mortality (CHD, stroke and CHF; adjudicated by outcome assessment committee) | Urine (median sum iAs, MMA, and DMA creatinine) | NR | NR | Age, sex, education, smoking, BMI, LDL cholesterol, study site, total inorganic arsenic | |||
| 45.0% | 0.91 (0.84, 0.99) for incident CVD | 0.98 (0.90, 1.07) for incident CVD | 1.04 (0.96, 1.12) for incident CVD | |||||||
| 45–74 y | 0.83 (0.73, 0.94) for fatal CVD | 1.01 (0.87, 1.16) for fatal CVD | 1.03 (0.91, 1.17) for fatal CVD | |||||||
| General | ||||||||||
| Chen et al. 2013b | CCO 369/1,109 (subcohort) | Araihazar, Bangladesh | Fatal and nonfatal CVD (CHD and stroke; medical records, death certificate, outcome assessment committee) | Urine (mean sum iAs, MMA, and DMA creatinine) | Age, sex, education, smoking, hypertension, diabetes | |||||
| 51% | (17.4, 69.3) vs. (0.3, 12.4) | (14.4, 33.8) vs. (0.2, 10.3) | (75.6, 99.2) vs. (27.9, 68.6) | (1.06, 19.57) vs (0.01, 0.66) | (7.2, 32.3) vs. (1.4, 4.8) | |||||
| 1.1 (0.7, 1.6) | 1.6 (1.1, 2.2) | 0.8 (0.5, 1.1) | 0.9 (0.6, 1.3) | 0.5 (0.3, 0.9) | ||||||
| General | ||||||||||
| Wu et al. 2006 | NCC 163/163 | Ilan, Taiwan | Carotid atherosclerosis ( or the presence of ECCA plaque) | Water () | NR | NR | NR | NR | NR | Age, sex, smoking, total cholesterol, hypertension, CAE |
| 47% | vs. | |||||||||
| 0.5 (0.1, 2.0) | ||||||||||
| General | ||||||||||
| Huang et al. 2009 | CS 121/183 | Putai, Taiwan | Carotid atherosclerosis ( or the presence of ECCA plaque) | Urine (mean sum iAs, MMA, and DMA ) | 7.2 ±7.2 | Age, sex, smoking, hypertension, diabetes, total cholesterol, total urine arsenic | ||||
| 52% | Case (6.9) vs. control (7.4) | Case (15.1) vs. control (13.1) | Case (78.1) vs. control (79.5) | Case (3.9) vs. control (2.9) | Case (9.1) vs. control (16.0) | |||||
| General | ||||||||||
| Chen et al. 2013a | CS Total | Araihazar, Bangladesh | Carotid IMT | Urine (mean sum iAs, MMA, and DMA creatinine) | 15.5 | 13.0 | 71.6 | 0.98 | 6.7 | Age, sex, education, smoking, body mass index, systolic blood pressure, diabetes |
| 40% | Per 10% change: | Per 10% change | Per 10% change | Per 1-unit change | Per 1-unit change | |||||
| 4.1 (, 12.3) | 12.1 (0.4-23.8) | (, 0.2) | 1.5 ( , 8.1) | (, 0.4) | ||||||
| General | ||||||||||
| Huang et al. 2007 | CS 372/499 | Putai, Taiwan | HTN | Urine (mean sum iAs, MMA, and DMA ) | Age, sex, smoking, alcohol, body mass index, triglyceride, CAE | |||||
| 44% | (History and/or a or a ) | vs. | vs. | vs. | vs. | vs. | ||||
| (0.8-1.9) | 1.0 (0.7, 1.6) | 1.1 (0.7, 1.6) | 0.9 (0.6, 1.3) | 1.1 (0.7, 1.7) | ||||||
| General | ||||||||||
| Wang et al. 2011 | CO 110/242 | Putai, Taiwan | HTN | Water (mean CAE ) | NR | NR | NR | NR | NR | Age, sex, body mass index, glucose |
| 46% | (, a , or anti-HTN medication) | vs. | vs. | vs. | ||||||
| 0.7 (0.3, 1.6) | 0.6 (0.3, 1.3) | 1.4 (0.6, 3.2) | ||||||||
| General | ||||||||||
| Li et al. 2013a | CS 182/487 | Inner Mongolia, China | HTN ( or or anti-HTN medication) | Water (geometric mean CAE ) | 9.2 | 13.3 | 70.2 | NR | NR | Age, sex, smoking, alcohol, body mass index, diabetes |
| 43% | Per % change within water arsenic () categories : 0.8 (0.3, 2.3) 10–50: 1.5 (0.6, 3.9) : 2.0 (1.0, 4.1) | Per % change within water arsenic () categories or anti-HTN medication): 0.5 (0.05, 4.5)10–50: 0.8 (0.1, 7.1) : 1.8 (0.4, 7.9) | Per % change within water arsenic ( ) categories : 0.8 (0.1, 6.9) 10–50: 0.1 (0.002, 4.4) : 0.04 (0.002, 0.8) | |||||||
| Mean 50 y | ||||||||||
| General | ||||||||||
| Li et al. 2013b | CS 168/436 | Shanxi, China | HTN ( or or anti-HTN medication) | Urine (geometric mean sum iAs, MMA, and DMA creatinine) | 9.5 | 12.9 | 69.5 | NR | NR | Age, sex, smoking, alcohol, body mass index |
| 42% | ||||||||||
| -Mean 49 y | vs. | vs. | vs. | |||||||
| General | 1.5 (0.9, 2.5) | 1.00 (0.6, 1.7) | 0.7 (0.4, 1.2) | |||||||
| Mendez et al. 2016 | CS Total | Chihuahua, Mexico | HTN ( or or anti-HTN medication) | Urine (median total inorganic As 55.8 ()) | Total inorganic arsenic, age, sex, education, ethnicity, weight, waist circumference, smoking, alcohol, seafood intake, water source | |||||
| 31.4% | vs. | vs. | vs. | vs. | vs. | |||||
| Mean 46 y | ||||||||||
| General | () | () | () | () | () | |||||
Note: Values shown as mean±SD, highest vs. lowest category, and eRR (95% CI), unless otherwise specified. CAE, cumulative arsenic exposure; CCO, case-cohort study; CHF, congestive heart failure; CI, confidence interval; CO, prospective cohort study; CS, cross-sectional study; CVD, cardiovascular disease; DBP, diastolic blood pressure; DMA, dimethylarsinate; ECCA, extra-cranial carotid artery; eRR, estimated r4elative risk; HTN, hypertension; iAs, inorganic arsenic; IMT, intimal-medial thickness; MMA, monomethylarsonate; NCC, nested case–control study; NR, not reported; PMI, primary methylation index (MMA/iAs); SBP, systolic blood pressure; SD, standard deviation; SMI, secondary methylation index (DMA/MMA).
Table 3.
Studies of arsenic metabolism and metabolic syndrome and diabetes.
| Reference | Study design (case/noncase) | Men (%) Age range Source | Outcome (Ascertainment method) | Arsenic exposure | Adjustment factors | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample (level) | iAs% | MMA% | DMA% | PMI | SMI | |||||
| Chen et al. 2012 | CS 111/136 | Putai, Taiwan | Metabolic Syndrome ( Adult Treatment Panel III Criteria) | Urine (mean sum iAs, MMA, and DMA creatinine) | Age, betel nut chewing | |||||
| 43% | NR | vs. | vs. | vs. | vs. | |||||
| 0.4 (0.2, 0.7) | 2.0 (1.1, 3.9) | 0.4 (0.2, 0.8) | 2.6 (1.4, 5.1) | |||||||
| General | ||||||||||
| Del Razo et al. 2011 | CS 25/233 | Zimapán and Lagunera, Mexico | Diabetes (, , self-reported diagnosis, or medication) | Urine (mean sum iAs, MMA, and DMA ) | NR | NR | NR | 1.3 | 5.6 | Age, sex, obesity, hypertension |
| 33% | per IQR change | per IQR change | ||||||||
| Mean 34 y | 1.0 (0.9, 1.1) | 1.4 (0.9, 2.1) | ||||||||
| General | ||||||||||
| Nizam et al. 2013 | CC 140/180 | Faridpur, Bangladesh | Diabetes (self-reported diagnosis and , confirmed by medical records) | Urine (mean sum iAs, MMA, and DMA ) | 9.6 | 9.4 | 80.1 | 1.3 | 10.0 | Age, sex, location, smoking, body mass index, family history of diabetes, income, duration of drinking water, water arsenic |
| 43% | DM (8.6) vs. non-DM (10.4) | DM (8.8) vs. non-DM (9.7) | DM (82.6) vs. non-DM (79.9) | DM (1.4) vs. non-DM (1.2) | DM (11.6) vs. non-DM (10.0) | |||||
| General | ||||||||||
| Kuo et al. 2015 | CO 396/1,298 | Arizona, Oklahoma, North/South Dakota, USA | Diabetes (, , self-reported diagnosis, or medication) | Urine (mean sum iAs, MMA, and DMA creatinine) | 9.2 | 15.6 | 75.2 | NR | NR | Age, sex, education, smoking, alcohol, BMI, waist-to-hip ratio, study sites, total inorganic arsenic |
| 45.0% | per 5% change | per 5% change | per 5% change | |||||||
| 45–74 y | 1.0 (0.89, 1.12) | 0.84 (0.76, 0.94) | 1.07 (1.00, 1.15) | |||||||
| General | ||||||||||
| Mendez 2016 | CS Total | Chihuahua, Mexico | Diabetes (, , self-reported diagnosis, or medication) | Urine (median total inorganic As 55.8 ()) | Total inorganic arsenic, age, sex, education, ethnicity, weight, waist circumference, smoking, alcohol, seafood intake, water source | |||||
| 31.4% | vs. | vs. | vs. | vs. | vs. | |||||
| Mean 46 y | ||||||||||
| General | () | () | () | () | () | |||||
Note: Values shown as mean±SD, highest vs. lowest category, and eRR (95% CI), unless otherwise specified. ATPIII, National Cholesterol Education Program's Adult Treatment Panel III report; BMI, Body mass index (); CC, case–control study; CI, confidence interval; CS, cross-sectional study; DM, diabetes mellitus; DMA, dimethylarsinate; eRR, estimated relative risk; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; 2HPG, 2-h postprandial glucose; iAs, inorganic arsenic; IMT, intimal-medial thickness; MMA, monomethylarsonate; NR, not reported; OGTT, oral glucose tolerance test; PMI, primary methylation index (MMA/iAs); SD, standard deviation; SMI, secondary methylation index (DMA/MMA).
Cardiovascular disease incidence and mortality.
Clinical cardiovascular disease was defined as the occurrence of fatal and nonfatal stroke, coronary heart disease, heart failure, or other heart disease during the follow-up (Chen et al. 2013b; Moon et al. 2013). Two prospective cohort studies, one from Bangladesh and one from the United States, investigated the association between arsenic metabolism and incident cardiovascular disease based on MMA% (Table 2) (Chen et al. 2013b; Moon et al. 2013). The U.S. cohort (Moon et al. 2013) also investigated the association of arsenic metabolism with cardiovascular disease mortality. The Bangladesh cohort also reported iAs%, DMA%, PMI, and SMI. Higher MMA% and lower SMI were associated with higher risk of incident cardiovascular disease in Bangladesh, whereas the association for iAs%, DMA%, and PMI were around the null and nonsignificant (Chen et al. 2013b). In the U.S. cohort, lower iAs% was associated with increased risk of clinical cardiovascular incidence and mortality (Moon et al. 2013).
Carotid atherosclerosis.
The evaluation of carotid atherosclerosis was based on extracranial carotid Doppler ultrasound (Chen et al. 2013a; YL Huang et al. 2009; Wu et al. 2006). Of the three studies on carotid atherosclerosis, one was prospective (Wu et al. 2006) and two were cross-sectional. All reported the association with MMA% (Chen et al. 2013a; YL Huang et al. 2009; Wu et al. 2006), two with iAs% and DMA% (Chen et al. 2013a; YL Huang et al. 2009), and two with PMI and SMI (Chen et al. 2013a; YL Huang et al. 2009) (Table 2). Two studies found higher levels of carotid intima-media with higher MMA%, although only one was statistically significant (Chen et al. 2013a; YL Huang et al. 2009). For iAs%, the two studies showed inconsistent results (Chen et al. 2013a; YL Huang et al. 2009). For DMA%, the results were consistently but nonsignificantly inverse for higher DMA% and reduced risk of carotid atherosclerosis (Chen et al. 2013a; YL Huang et al. 2009). For PMI and SMI, the findings were consistently null for both studies.
Hypertension.
All studies on hypertension followed the World Health Organization standard protocol to measure blood pressure and define hypertension (Huang et al. 2007; Li et al. 2013a, 2013b; Mendez et al. 2016; Wang et al. 2011). Four studies were cross-sectional and one was a prospective cohort study (Wang et al. 2011). All reported the association with iAs%, MMA%, and DMA%, and two also reported on the association with PMI and SMI (Huang et al. 2007; Mendez et al. 2016). The association between MMA% and hypertension was inconsistent across studies, with studies finding positive (Li et al. 2013a), inverse (Mendez et al. 2016; Wang et al. 2011) and null associations (Huang et al. 2007; Li et al. 2013b), none of them statistically significant. The associations with iAs% and DMA% were also inconsistent (Table 2 and Figure 3).
Arsenic Metabolism and Diabetes-Related Outcomes
Five studies evaluated the associations of arsenic metabolism with diabetes-related outcomes (Chen et al. 2012; Del Razo et al. 2011; Kuo et al. 2015; Mendez et al. 2016; Nizam et al. 2013). All studies were published after 2011. Three studies were cross-sectional (Chen et al. 2012; Del Razo et al. 2011; Mendez et al. 2016), one was case–control (Nizam et al. 2013), and one was a prospective cohort (Kuo et al. 2015). The studies were conducted in populations from Taiwan (Chen et al. 2012), Bangladesh (Nizam et al. 2013), Mexico (Del Razo et al. 2011; Mendez et al. 2016), and the United States (Kuo et al. 2015). Most populations were exposed to high arsenic levels ( in drinking water) except in a study from the United States (Kuo et al. 2015). The study outcomes included diabetes () (Del Razo et al. 2011; Kuo et al. 2015; Mendez et al. 2016; Nizam et al. 2013) and metabolic syndrome () (Chen et al. 2012). The definitions of diabetes were consistent across studies using information on glycemia together with self-reported physician diagnosis or medication history (Del Razo et al. 2011; Kuo et al. 2015; Mendez et al. 2016; Nizam et al. 2013). Of the five studies, four reported the association with MMA%, iAs%, DMA% (Chen et al. 2012; Kuo et al. 2015; Mendez et al. 2016; Nizam et al. 2013), and four on the PMI and SMI (Chen et al. 2012; Del Razo et al. 2011; Mendez et al. 2016; Nizam et al. 2013). Four studies found that lower levels of MMA% and higher levels of DMA% were associated with higher risk of diabetes or metabolic syndrome (Chen et al. 2012; Kuo et al. 2015; Mendez et al. 2016; Nizam et al. 2013). The SMI was positively associated with higher risk of diabetes (Chen et al. 2012; Del Razo et al. 2011; Mendez et al. 2016; Nizam et al. 2013), although the association was significant only in two studies (Chen et al. 2012; Mendez et al. 2016). For PMI, the association with diabetes or metabolic syndrome was nonsignificantly positive in one study (Nizam et al. 2013), significantly inverse in two studies (Chen et al. 2012; Mendez et al. 2016), and null in one study (Del Razo et al. 2011).
Discussion
Main Findings
This systematic review of arsenic metabolism and disease found that the association with arsenic metabolism patterns was different across different chronic disease outcomes (Figure 3). For cancer, most studies found an association between cancer outcomes with higher MMA% and lower DMA%. For cardiovascular disease, most studies found that higher MMA% was associated with incident cardiovascular diseases and prevalent carotid atherosclerosis, although most of them were not statistically significant. For hypertension, no studies found any association with arsenic metabolism. For diabetes, lower MMA% and higher DMA% was associated with higher risk of diabetes or the metabolic syndrome in all studies. A major limitation in this area of research is the heterogeneity in the methods used for modeling arsenic metabolism and the selective reporting, which makes the comparison and interpretation of the results challenging. The research community should reach consensus on the appropriate methods for evaluating arsenic metabolism in epidemiologic studies.
In the evaluation of arsenic metabolism across populations, we found substantial interpopulation variability in the distribution of the arsenic metabolism measures. Interpopulation variability of arsenic metabolism has been recognized in previous studies (Loffredo et al. 2003; Vahter 2000). In the current review, we found that arsenic exposure (measured in drinking water or urine) was related to iAs% and DMA% (high arsenic in water being associated with higher iAs% and lower DMA%); however, there was a less pronounced association with MMA%. By comparing all the data on MMA% from multiple countries side by side, we also observed that the variability for MMA% across diverse populations was lower than variability of iAs% and DMA%. To our knowledge, few studies have directly investigated the association between arsenic exposure levels and arsenic metabolism. A similar pattern was also observed in the region of Lagunera in Mexico, although the sample size was small (Del Razo et al. 1997). Lower variability in MMA% in human populations is also consistent with an animal study in mice showing that an increase in sodium arsenate from 0.5 to increased iAs% by 14.4% and decreased DMA% by 16.1% but only increased MMA% by 1.3% (Hughes et al. 1994). In Southwestern Taiwan, a reduction in mean sum of inorganic arsenic exposure (baseline to ) over 15 y of follow-up was associated with a 4.9% decrease in iAs%, a 6.8% decrease in MMA%, and an 11.7% increase in DMA%, although this observation did not account for survival bias (YK Huang et al. 2009). Future research is needed to understand how arsenic metabolism capacity responds to environmental arsenic exposure.
Beyond arsenic exposure levels, genetic polymorphisms, particularly in the arsenic (III) methyltransferase (AS3MT) gene, have been considered major determinants in interindividual variability of the arsenic methylation patterns (Agusa et al. 2011; Eichstaedt et al. 2015). Age, sex, and body mass index may be other major determinants of arsenic metabolism (Jansen et al. 2015). Epidemiological studies of arsenic metabolism and disease need to carefully consider for which factors to adjust. At a minimum, all studies of arsenic metabolism should adjust for arsenic exposure levels, in order to assess the health effects beyond what could be related to differences in arsenic exposure. Adjustment for age and sex should also be required. In addition to age and sex, body mass index, nutritional status, and genetic variants are other major determinants of arsenic metabolism. For instance, the large variation in arsenic metabolism profile observed in the two study populations with comparable age and arsenic exposure level in Bangladesh could potentially be explained by differences in nutritional status as reflected in urine creatinine level ( in Faridpur vs. in Araihazar). If possible, epidemiologic studies should also evaluate potential effect modification between arsenic exposure levels and arsenic metabolism. In the current systematic review, due to the limited number of studies for each endpoint, unfortunately we could not evaluate the joint effect between arsenic exposure and metabolism in disease development. As the number of studies evaluating arsenic exposure and metabolism in disease development increases, meta-regression techniques could contribute to that understanding.
Implications for Public Health
The importance of evaluating the role of arsenic metabolism in explaining interindividual differences in arsenic-related disease was recognized in the late 1990s, with the first study on the higher risk of nonmelanoma skin cancer with higher MMA% in Southwestern Taiwan being published in 1997 (Hsueh et al. 1997). With advances in analytical technology, including sensitive methods to detect the relevant arsenic species in the urine, and the recognition of the importance of understanding the role of arsenic metabolism for the evaluation of susceptibility in risk assessment, the number of epidemiologic studies evaluating the association between arsenic metabolism and health effects has grown. Initially, the studies pointed at a higher risk of disease being associated with higher MMA%. This finding was consistent with the detoxification interpretation of methylation and the need to fully methylate inorganic arsenic to DMA to minimize arsenic health effects. A slower second methylation could result in higher concentrations of trivalent MMA (MMAIII) in human tissues. In cytotoxicity studies, MMAIII has shown to be as toxic or more than arsenite (Petrick et al. 2000). Most epidemiologic studies cannot measure MMAIII in urine, as it is a very unstable compound that quickly revert to pentavalent MMA. In a small study in central Mexico, participants with skin lesions had higher levels of MMAIII, despite similar total urine arsenic (Valenzuela et al. 2005). Recent studies on diabetes-related outcomes, however, have shown that the association of arsenic metabolism patterns with health outcomes can be more complex than originally thought and suggest that faster methylation or more complete methylation could also increase the risk of diabetes. A similar association has also been found between higher DMA% and obesity and increased body mass index (Gomez-Rubio et al. 2011; Gribble et al. 2013; Su et al. 2012). From a mechanistic perspective, experimental evidence from animal studies has shown that methylation could be a bioactive process with DMA (III) being a highly toxic dimethylated arsenic species targeting murine pancreatic islet cells (Douillet et al. 2013; Lu et al. 2011; Mass et al. 2001). Other indirect lines of evidence suggest that the synthesis and regulation of the main methyl donor for arsenic methylation, S-adenosylmethionine (SAM) generated through the one-carbon metabolism, may be critical for glucose and lipid homeostasis (Jackson et al. 2012; Locasale 2013; Ngo et al. 2014; Vahter 2007; Walker et al. 2011). These complex findings imply that manipulating individuals’ arsenic metabolism profile toward complete methylation via nutritional modification, for instance, through supplementation with folate (George et al. 2012; Hoque et al. 2000), may be more challenging than anticipated if increasing DMA% is related to a higher risk of diabetes-related metabolic complications. Additional long-term prospective epidemiological research and experimental research is needed to confirm and understand the role of arsenic metabolism and its connections with arsenic exposure, relevant exposure windows, genetics, nutrition, co-infections, and the microbiome (Chen 2014; Lu et al. 2014).
Limitations of Current Research
A major limitation in comparing studies, however, is the heterogeneity in the description and analysis of arsenic metabolism. For instance, when percentages of the species relative to their sum are presented, many studies only show the results for one of the species (Hsueh et al. 1997; Steinmaus et al. 2010; Wu et al. 2006). Similarly, only one of the two methylation indices are often presented. In addition to the selective reporting, the modeling methods used have generally failed to recognize that the three species (inorganic arsenic, MMA, and DMA) are interrelated and sum to 100%. Using conventional statistical methods designed for inherently compositional data may lead to inappropriate inferences (Aitchison 1986; Pawlowsky-Glahn and Egozcue 2006). In the 1980s, John Aitchison introduced compositional data analysis to address this common issue in geoscience (Aitchison 1981, 1986). However, this technique is not widely recognized even in the area of geochemical research (Buccianti and Grunsky 2014). Only recently, methods such as the leave-one-out approach or the use of triplots to represent the simultaneous distribution of arsenic metabolism have been used by environmental scientists (Gribble et al. 2013; Kuo et al. 2015).
The present findings should be interpreted cautiously. First, the possibility of publication bias cannot be ignored as null associations may be less likely to be published. Publication bias may overestimate the consistency of the relationship between arsenic metabolism and the selected health outcomes. Second, as most studies were cross-sectional or case–control designs, the possibility of reverse causation cannot be excluded. Also, adjustment for confounding was limited in some studies and overall we cannot exclude the possibility of residual confounding. However, the consistency of the findings adds support to conducting additional research to further assess the causality of these associations and a more comprehensive risk assessment targeting arsenic metabolism. Third, for the association between arsenic metabolism biomarkers and arsenic exposure we combined data across studies at different time periods, in different laboratories, and with different genetic background and nutritional status. Fourth, to obtain a fully comprehensive picture of the interplay between arsenic exposure and arsenic metabolism biomarkers, future reviews should expand to include all studies evaluating both arsenic exposure and metabolism. Fifth, in this review, we focused on the role of current arsenic exposure in arsenic metabolism rather than past exposure. Future research efforts should also be aimed to identify an appropriate population with both past and current arsenic exposure data to evaluate the effect of past arsenic exposure on the evolution of arsenic metabolism. Lastly, in this review, we focused on the role of current arsenic exposure in arsenic metabolism rather than past exposure. Future research efforts should identify a population with both past and current arsenic exposure data and longitudinal follow-up to evaluate the effect of past arsenic exposure on changes in arsenic metabolism.
Opportunities and Challenges for Future Studies
Future studies with large sample size, appropriate baseline and prospective arsenic metabolism estimation, and sufficient long-term follow-up will help verify the independent association between arsenic metabolism and various health effects, and the interaction between arsenic exposure and metabolism on the development of chronic diseases. Developing a simple and interpretable modeling approach for arsenic metabolism is a research priority, although it might be difficult due to population specific complexities and the contribution of unmeasured covariates such as diet and genetic determinants. For example, interpretation of ratio measures of methylation (both PMI and SMI) is challenging, despite their previous popularity in epidemiological studies (Del Razo et al. 1997). It is difficult to predict whether changes in the numerator or the denominator will have a dominant effect, and thus associations with a methylation index obscures whether the association lies with the numerator or the denominator.
The biological meaning of arsenic metabolism may extend beyond increasing risks of arsenic toxicity. Arsenic metabolism may be an integrated marker reflecting genetic variations on arsenic sensitivity and long-term environmental arsenic exposure on genetic modification (Eichstaedt et al. 2015). Arsenic metabolism may also reflect differential microbiome distribution across humans and human populations, as it has been shown that gut microorganisms can methylate inorganic arsenic (Dietert and Silbergeld 2015). Arsenic metabolism is also related to one-carbon nutrients as demonstrated in clinical trials from Bangladesh (Gamble et al. 2007; Peters et al. 2015). The interplay between one-carbon metabolism, arsenic metabolism, and DNA methylation provides an opportunity to explore the genomic coding, metabolism regulation, and phenotype expression from a mechanistic perspective (Baccarelli et al. 2010). One-carbon metabolism is composed of three key cycles including the methionine cycle, the folate cycle, and the cysteine-cystathionine cycle (Locasale 2013; Stipanuk 2004). The major methyl donor in humans, S-adenosylmethionine, is generated through the methionine cycle and facilitates more than 50 methylation reactions, including DNA methylation and arsenic methylation (Agusa et al. 2011; Stead et al. 2006). The methionine cycle is completed by the re-methylation of homocysteine back to methionine, through the folate cycle (Stover 2004), or by irreversibly degrading homocysteine into cysteine (Wijekoon et al. 2006). Dysfunction of the methionine cycle has been linked to chronic diseases including cancer and cardiovascular disease (Baccarelli et al. 2010; Locasale 2013). The cysteine–cystathionine cycle involves glutathione-transsulfuration and generates antioxidant thio buffers, which are critical to maintaining intracellular reduction–oxidation status (Deplancke and Gaskins 2002). The imbalance of redox status has also been linked to cancer metabolism (Cairns et al. 2011; Moore et al. 2007), cardiovascular disease (Zimmet and Hare 2006), and diabetes (Dugan et al. 2013). Indeed, increasing epidemiological evidence supports the association between arsenic exposure and global DNA methylation status (Niedzwiecki et al. 2013; Ren et al. 2011). Moreover, mathematical modeling to approach the complexity of one-carbon metabolism and the interaction between the one-carbon and arsenic metabolism has been initiated (Lawley et al. 2011; Nijhout et al. 2008).
Conclusions
This is the first systematic review evaluating the association between arsenic metabolism and several chronic disease outcomes. Specific methylation patterns were associated with increased disease risk, in different directions for different diseases. Cancer and cardiovascular disease outcomes were related to higher MMA%, whereas diabetes and the metabolic syndrome were in general related to lower MMA% and higher DMA%. Our analysis scope and conclusions were constrained due to small sample size, limited prospective evidence, and inconsistent arsenic metabolism reporting and statistical approach across studies. These findings call for a consensus on reporting standards for the evaluation of the health effects of arsenic metabolism. Conducting large prospective cohort studies in populations exposed to a wide range of arsenic exposure levels is critical to better characterize the interplay of arsenic metabolism with factors that influence the individual metabolism patterns, including arsenic exposure levels and genetic determinants of arsenic metabolism. Understanding the biological and epidemiological meaning of arsenic metabolism could improve the risk assessment of arsenic toxicity and provide a potential tool for disease prediction, prevention, and control.
Supplemental Material
Acknowledgments
This work was supported by the National Institutes of Health (grants R01ES021367, 1R01ES025216, P42ES010349, and P30 ES009089), and by the Ministry of Science and Technology of Taiwan (grant 105-2314-B-039-040).
References
- Agusa T, Fujihara J, Takeshita H, Iwata H. 2011. Individual variations in inorganic arsenic metabolism associated with AS3MT genetic polymorphisms. Int J Mol Sci 12(4):2351–2382, PMID: 21731446, 10.3390/ijms12042351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitchison J. 1981. A new approach to null correlations of proportions. Math Geol 13:175–189, 10.1007/BF01031393. [DOI] [Google Scholar]
- Aitchison J. 1986. The Statistical Analysis of Compositional Data. London, UK:Chapman & Hall, Ltd. [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). 2011. “Priority List of Hazardous Substances.” Atlanta, GA:ATSDR. [Google Scholar]
- Baccarelli A, Rienstra M, Benjamin EJ. 2010. Cardiovascular epigenetics: basic concepts and results from animal and human studies. Circ Cardiovasc Genet 3(6):567–573, PMID: 21156932, 10.1161/CIRCGENETICS.110.958744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccianti A, Grunsky E. 2014. Compositional data analysis in geochemistry: are we sure to see what really occurs during natural processes?. J Geochem Explor 141:1–5, 10.1016/j.gexplo.2014.03.022. [DOI] [Google Scholar]
- Cairns RA, Harris IS, Mak TW. 2011. Regulation of cancer cell metabolism. Nat Rev Cancer 11(2):85–95, PMID: 21258394, 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- Chen CJ. 2014. Health hazards and mitigation of chronic poisoning from arsenic in drinking water: Taiwan experiences. Rev Environ Health 29(1–2):13–19, PMID: 24552958, 10.1515/reveh-2014-0007. [DOI] [PubMed] [Google Scholar]
- Chen JW, Wang SL, Wang YH, Sun CW, Huang YL, Chen CJ, et al. 2012. Arsenic methylation, GSTO1 polymorphisms, and metabolic syndrome in an arseniasis endemic area of southwestern Taiwan. Chemosphere 88(4):432–438, PMID: 22440634, 10.1016/j.chemosphere.2012.02.059. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wu F, Graziano JH, Parvez F, Liu M, Paul RR, et al. 2013a. Arsenic exposure from drinking water, arsenic methylation capacity, and carotid intima-media thickness in Bangladesh. Am J Epidemiol 178(3):372–381, PMID: 23788675, 10.1093/aje/kwt001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wu F, Liu M, Parvez F, Slavkovich V, Eunus M, et al. 2013b. A prospective study of arsenic exposure, arsenic methylation capacity, and risk of cardiovascular disease in Bangladesh. Environ Health Perspect 121(7):832–838, PMID: 23665672, 10.1289/ehp.1205797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Guo YL, Su HJ, Hsueh YM, Smith TJ, Ryan LM, et al. 2003a. Arsenic methylation and skin cancer risk in southwestern Taiwan. J Occup Environ Med 45(3):241–248, PMID: 12661181, 10.1097/01.jom.0000058336.05741.e8. [DOI] [PubMed] [Google Scholar]
- Chen YC, Su HJ, Guo YL, Houseman EA, Christiani DC. 2005. Interaction between environmental tobacco smoke and arsenic methylation ability on the risk of bladder cancer. Cancer Causes Control 16(2):75–81, PMID: 15868449, 10.1007/s10552-004-2235-1. [DOI] [PubMed] [Google Scholar]
- Chen YC, Su HJ, Guo YL, Hsueh YM, Smith TJ, Ryan LM, et al. 2003b. Arsenic methylation and bladder cancer risk in Taiwan. Cancer Causes Control 14(4):303–310, PMID: 12846360, 10.1023/A:1023905900171. [DOI] [PubMed] [Google Scholar]
- Chiang CI, Huang YL, Chen WJ, Shiue HS, Huang CY, Pu YS, et al. 2014. XRCC1 Arg194Trp and Arg399Gln polymorphisms and arsenic methylation capacity are associated with urothelial carcinoma. Toxicol Appl Pharmacol 279(3):373–379, PMID: 25018058, 10.1016/j.taap.2014.06.027. [DOI] [PubMed] [Google Scholar]
- Chung CJ, Hsueh YM, Bai CH, Huang YK, Huang YL, Yang MH, et al. 2009. Polymorphisms in arsenic metabolism genes, urinary arsenic methylation profile and cancer. Cancer causes Control 20(9):1653–1661, PMID: 19680750, 10.1007/s10552-009-9413-0. [DOI] [PubMed] [Google Scholar]
- Chung CJ, Huang CJ, Pu YS, Su CT, Huang YK, Chen YT, et al. 2008. Urinary 8-hydroxydeoxyguanosine and urothelial carcinoma risk in low arsenic exposure area. Toxicol Appl Pharmacol 226(1):14–21, PMID: 17950770, 10.1016/j.taap.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Chung CJ, Huang CY, Pu YS, Shiue HS, Su CT, Hsueh YM. 2013a. The effect of cigarette smoke and arsenic exposure on urothelial carcinoma risk is modified by glutathione S-transferase M1 gene null genotype. Toxicol Appl Pharmacol 266(2):254–259, PMID: 23159782, 10.1016/j.taap.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Chung CJ, Huang YL, Huang YK, Wu MM, Chen SY, Hsueh YM, et al. 2013b. Urinary arsenic profiles and the risks of cancer mortality: a population-based 20-year follow-up study in arseniasis-endemic areas in Taiwan. Environ Res 122:25–30, PMID: 23276485, 10.1016/j.envres.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Chung CJ, Pu YS, Chen YT, Su CT, Wu CC, Shiue HS, et al. 2011a. Protective effects of plasma alpha-tocopherols on the risk of inorganic arsenic-related urothelial carcinoma. Sci Total Environ 409(6):1039–1045, PMID: 21227482, 10.1016/j.scitotenv.2010.11.037. [DOI] [PubMed] [Google Scholar]
- Chung CJ, Pu YS, Su CT, Chen HW, Huang YK, Shiue HS, et al. 2010. Polymorphisms in one-carbon metabolism pathway genes, urinary arsenic profile, and urothelial carcinoma. Cancer causes Control 21(10):1605–1613, PMID: 20532609, 10.1007/s10552-010-9589-3. [DOI] [PubMed] [Google Scholar]
- Chung CJ, Pu YS, Su CT, Huang CY, Hsueh YM. 2011b. Gene polymorphisms of glutathione S-transferase omega 1 and 2, urinary arsenic methylation profile and urothelial carcinoma. Sci Total Environ 409(3):465–470, PMID: 21094982, 10.1016/j.scitotenv.2010.10.053. [DOI] [PubMed] [Google Scholar]
- Del Razo LM, García-Vargas GG, Valenzuela OL, Castellanos EH, Sánchez-Peña LC, Currier JM, et al. 2011. Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: a cross-sectional study in the Zimapán and Lagunera regions in Mexico. Environ Health 10:73, 10.1186/1476-069X-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Razo LM, García-Vargas GG, Vargas H, Albores A, Gonsebatt ME, Montero R, et al. 1997. Altered profile of urinary arsenic metabolites in adults with chronic arsenicism. A pilot study. Arch Toxicol 71(4):211–217, PMID: 9101036, 10.1007/s002040050378. [DOI] [PubMed] [Google Scholar]
- Deplancke B, Gaskins HR. 2002. Redox control of the transsulfuration and glutathione biosynthesis pathways. Curr Opin Clin Nutr Metab Care 5:85–92, PMID: 11790955. [DOI] [PubMed] [Google Scholar]
- Dietert RR, Silbergeld EK. 2015. Biomarkers for the 21st century: listening to the microbiome. Toxicol Sci 144(2):208–216, PMID: 25795652, 10.1093/toxsci/kfv013. [DOI] [PubMed] [Google Scholar]
- Douillet C, Currier J, Saunders J, Bodnar WM, Matousek T, Styblo M. 2013. Methylated trivalent arsenicals are potent inhibitors of glucose stimulated insulin secretion by murine pancreatic islets. Toxicol Appl Pharmacol 267(1):11–15, PMID: 23261974, 10.1016/j.taap.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves AE, et al. 2013. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest 123(11):4888–4899, PMID: 24135141, 10.1172/JCI66218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichstaedt CA, Antao T, Cardona A, Pagani L, Kivisild T, Mormina M. 2015. Positive selection of AS3MT to arsenic water in Andean populations. Mutat Res 780:97–102, PMID: 26366667, 10.1016/j.mrfmmm.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström KS, Vahter M, Fletcher T, Leonardi G, Goessler W, Gurzau E, et al. 2015. Genetic variation in arsenic (+3 oxidation state) methyltransferase (AS3MT), arsenic metabolism and risk of basal cell carcinoma in a European population. Environ Mol Mutagen 56(1):60–69, PMID: 25156000, 10.1002/em.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble MV, Liu X, Slavkovich V, Pilsner JR, Ilievski V, Factor-Litvak P, et al. 2007. Folic acid supplementation lowers blood arsenic. Am J Clin Nutr 86(4):1202–1209, PMID: 17921403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George CM, van Geen A, Slavkovich V, Singha A, Levy D, Islam T, et al. 2012. A cluster-based randomized controlled trial promoting community participation in arsenic mitigation efforts in Bangladesh. Environ Health 11:41, PMID: 22713347, 10.1186/1476-069X-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert-Diamond D, Li Z, Perry AE, Spencer SK, Gandolfi AJ, Karagas MR. 2013. A population-based case–control study of urinary arsenic species and squamous cell carcinoma in New Hampshire, USA. Environ Health Perspect 121(10):1154–1160, PMID: 23872349, 10.1289/ehp.1206178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Rubio P, Roberge J, Arendell L, Harris RB, O’Rourke MK, Chen Z, et al. 2011. Association between body mass index and arsenic methylation efficiency in adult women from southwest U.S. and northwest Mexico. Toxicol Appl Pharmacol 252(2):176–182, PMID: 21320519, 10.1016/j.taap.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble MO, Crainiceanu CM, Howard BV, Umans JG, Francesconi KA, Goessler W, et al. 2013. Body composition and arsenic metabolism: a cross-sectional analysis in the Strong Heart Study. Environ Health 12:107, PMID: 24321145, 10.1186/1476-069X-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A, Marcos R. 2008. Genetic variations associated with interindividual sensitivity in the response to arsenic exposure. Pharmacogenomics 9(8):1113–1132, PMID: 18681785, 10.2217/14622416.9.8.1113. [DOI] [PubMed] [Google Scholar]
- Hopenhayn C, Huang B, Christian J, Peralta C, Ferreccio C, Atallah R, et al. 2003. Profile of urinary arsenic metabolites during pregnancy. Environ Health Perspect 111(16):1888–1891, PMID: 14644662, 10.1289/ehp.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque BA, Mahmood AA, Quadiruzzaman M, Khan F, Ahmed SA, Shafique SA, et al. 2000. Recommendations for water supply in arsenic mitigation: a case study from Bangladesh. Public health 114(6):488–494, PMID: 11114764, 10.1016/S0033-3506(00)00395-4. [DOI] [PubMed] [Google Scholar]
- Hsu LI, Wu MM, Wang YH, Lee CY, Yang TY, Hsiao BY, et al. 2015. Association of environmental arsenic exposure, genetic polymorphisms of susceptible genes, and skin cancers in Taiwan. BioMed Res Int 2015:892579, PMID: 26295053, 10.1155/2015/892579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh YM, Chiou HY, Huang YL, Wu WL, Huang CC, Yang MH, et al. 1997. Serum beta-carotene level, arsenic methylation capability, and incidence of skin cancer. Cancer Epidemiol Biomarkers Prev 6(8):589–596, PMID: 9264271. [PubMed] [Google Scholar]
- Huang CY, Pu YS, Shiue HS, Chen WJ, Lin YC, Hsueh YM. 2016. Polymorphisms of human 8-oxoguanine DNA glycosylase 1 and 8-hydroxydeoxyguanosine increase susceptibility to arsenic methylation capacity-related urothelial carcinoma. Arch Toxicol 90(8):1917–1927, PMID: 26359225, 10.1007/s00204-015-1590-x. [DOI] [PubMed] [Google Scholar]
- Huang YK, Huang YL, Hsueh YM, Yang MH, Wu MM, Chen SY, et al. 2008a. Arsenic exposure, urinary arsenic speciation, and the incidence of urothelial carcinoma: a twelve-year follow-up study. Cancer Causes Control 19(8):829–839, PMID: 18351295, 10.1007/s10552-008-9146-5. [DOI] [PubMed] [Google Scholar]
- Huang YK, Huang YL, Hsueh YM, Wang JT, Yang MH, Chen CJ. 2009. Changes in urinary arsenic methylation profiles in a 15-year interval after cessation of arsenic ingestion in southwest Taiwan. Environ Health Perspect 117(12):1860–1866, PMID: 20049204, 10.1289/ehp.0900560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YK, Pu YS, Chung CJ, Shiue HS, Yang MH, Chen CJ, et al. 2008b. Plasma folate level, urinary arsenic methylation profiles, and urothelial carcinoma susceptibility. Food Chem Toxicol 46(3):929–938, PMID: 18054417, 10.1016/j.fct.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Huang YK, Tseng CH, Huang YL, Yang MH, Chen CJ, Hsueh YM. 2007. Arsenic methylation capability and hypertension risk in subjects living in arseniasis-hyperendemic areas in southwestern Taiwan. Toxicol Appl Pharmacol 218(2):135–142, PMID: 17173945, 10.1016/j.taap.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Huang YL, Hsueh YM, Huang YK, Yip PK, Yang MH, Chen CJ. 2009. Urinary arsenic methylation capability and carotid atherosclerosis risk in subjects living in arsenicosis-hyperendemic areas in southwestern Taiwan. Sci Total Environ 407(8):2608–2614, PMID: 19187952, 10.1016/j.scitotenv.2008.12.061. [DOI] [PubMed] [Google Scholar]
- Hughes MF, Menache M, Thompson DJ. 1994. Dose-dependent disposition of sodium arsenate in mice following acute oral exposure. Fundam Appl Toxicol 22(1):80–89, PMID: 8125217, 10.1006/faat.1994.1011. [DOI] [PubMed] [Google Scholar]
- IPCS (International Programme on Chemical Safety). 2010. Ten chemicals of major public health concern, Geneva, Switzerland:World Health Organization, http://www.who.int/ipcs/assessment/public_health/chemicals_phc/en/ [accessed 21 March 2015]. [Google Scholar]
- Jackson MI, Cao J, Zeng H, Uthus E, Combs GF Jr. 2012. S-adenosylmethionine-dependent protein methylation is required for expression of selenoprotein p and gluconeogenic enzymes in HepG2 human hepatocytes. J Biol Chem 287(43):36455–36464, PMID: 22932905, 10.1074/jbc.M112.412932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RJ, Argos M, Tong L, Li J, Rakibuz-Zaman M, Islam MT, et al. 2015. Determinants and consequences of arsenic metabolism efficiency among 4,794 individuals: demographics, lifestyle, genetics, and toxicity. Cancer Epidemiol Biomarkers Prev 25(2):381–390, PMID: 26677206, 10.1158/1055-9965.EPI-15-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CC, Howard BV, Umans JG, Gribble MO, Best LG, Francesconi KA, et al. 2015. Arsenic exposure, arsenic metabolism, and incident diabetes in the Strong Heart Study. Diabetes Care 38(4):620–627, PMID: 25583752, 10.2337/dc14-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CC, Moon K, Thayer KA, Navas-Acien A. 2013. Environmental chemicals and type 2 diabetes: an updated systematic review of the epidemiologic evidence. Curr Diab Rep 13(6):831–849, PMID: 24114039, 10.1007/s11892-013-0432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley SD, Cinderella M, Hall MN, Gamble MV, Nijhout HF, Reed MC. 2011. Mathematical model insights into arsenic detoxification. Theor Biol Med Model 8:31, PMID: 21871107, 10.1186/1742-4682-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi G, Vahter M, Clemens F, Goessler W, Gurzau E, Hemminki K, et al. 2012. Inorganic arsenic and basal cell carcinoma in areas of Hungary, Romania, and Slovakia: a case–control study. Environ Health Perspect 120(5):721–726, PMID: 22436128, 10.1289/ehp.1103534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li B, Xi S, Zheng Q, Lv X, Sun G. 2013a. Prolonged environmental exposure of arsenic through drinking water on the risk of hypertension and type 2 diabetes. Environ Sci Pollut Res Int 20(11):8151–8161, PMID: 23649600, 10.1007/s11356-013-1768-9. [DOI] [PubMed] [Google Scholar]
- Li X, Li B, Xi S, Zheng Q, Wang D, Sun G. 2013b. Association of urinary monomethylated arsenic concentration and risk of hypertension: a cross-sectional study from arsenic contaminated areas in northwestern China. Environ Health 12:37, PMID: 23602086, 10.1186/1476-069X-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang D, Li X, Zheng Q, Sun G. 2015. A potential synergy between incomplete arsenic methylation capacity and demographic characteristics on the risk of hypertension: findings from a cross-sectional study in an arsenic-endemic area of Inner Mongolia, China. Int J Environ Res Public Health 12(4):3615–3632, PMID: 25837203, 10.3390/ijerph120403615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale JW. 2013. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer 13(8):572–583, PMID: 23822983, 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo CA, Aposhian HV, Cebrian ME, Yamauchi H, Silbergeld EK. 2003. Variability in human metabolism of arsenic. Environ Res 92(2):85–91, PMID: 12854687, 10.1016/S0013-9351(02)00081-6. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Berlin JA, Orza MJ, Chalmers TC. 1988. A meta-analysis of alcohol consumption in relation to risk of breast cancer. JAMA. 260(5):652–656, PMID: 3392790, 10.1001/jama.260.5.652. [DOI] [PubMed] [Google Scholar]
- Lopez-Carrillo L, Hernandez-Ramirez RU, Gandolfi AJ, Ornelas-Aguirre JM, Torres-Sanchez L, Cebrian ME. 2014. Arsenic methylation capacity is associated with breast cancer in northern Mexico. Toxicol Appl Pharmacol 280(1):53–59, PMID: 25062773, 10.1016/j.taap.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Lovell D, Pawlowsky-Glahn V, Egozcue JJ, Marguerat S, Bähler J. 2015. Proportionality: a valid alternative to correlation for relative data. PLoS Comput Biol 11(3):e1004075, PMID: 25775355, 10.1371/journal.pcbi.1004075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Abo RP, Schlieper KA, Graffam ME, Levine S, Wishnok JS, et al. 2014. Arsenic exposure perturbs the gut microbiome and its metabolic profile in mice: an integrated metagenomics and metabolomics analysis. Environ Health Perspect 122(3):284–291, PMID: 24413286, 10.1289/ehp.1307429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TH, Su CC, Chen YW, Yang CY, Wu CC, Hung DZ, et al. 2011. Arsenic induces pancreatic β-cell apoptosis via the oxidative stress-regulated mitochondria-dependent and endoplasmic reticulum stress-triggered signaling pathways. Toxicol Lett 201(1):15–26, PMID: 21145380, 10.1016/j.toxlet.2010.11.019. [DOI] [PubMed] [Google Scholar]
- Mass MJ, Tennant A, Roop BC, Cullen WR, Styblo M, Thomas DJ, et al. 2001. Methylated trivalent arsenic species are genotoxic. Chem Res Toxicol 14(4):355–361, PMID: 11304123, 10.1021/tx000251l. [DOI] [PubMed] [Google Scholar]
- Melak D, Ferreccio C, Kalman D, Parra R, Acevedo J, Pérez L, et al. 2014. Arsenic methylation and lung and bladder cancer in a case-control study in northern Chile. Toxicol Appl Pharmacol 274(2):225–231, PMID: 24296302, 10.1016/j.taap.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MA, González-Horta C, Sánchez-Ramírez B, Ballinas-Casarrubias L, Cerón RH, Morales DV, et al. 2016. Chronic exposure to arsenic and markers of cardiometabolic risk: a cross-sectional study in Chihuahua, Mexico. Environ Health Perspect 124(1):104–111, PMID: 26068977, 10.1289/ehp.1408742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon K, Guallar E, Navas-Acien A. 2012. Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep 14(6):542–555, PMID: 22968315, 10.1007/s11883-012-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, et al. 2013. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. a prospective cohort study. Ann Intern Med 159(10):649–659, PMID: 24061511, 10.7326/0003-4819-159-10-201311190-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LE, Malats N, Rothman N, Real FX, Kogevinas M, Karami S, et al. 2007. Polymorphisms in one-carbon metabolism and trans-sulfuration pathway genes and susceptibility to bladder cancer. Int J Cancer 120(11):2452–2458, PMID: 17311259, 10.1002/ijc.22565. [DOI] [PubMed] [Google Scholar]
- Murcott S. 2012. Arsenic Contamination in the World: An International Sourcebook 2012. London, UK:IWA Publishing. [Google Scholar]
- Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, et al. 2013. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 121(3):295–302, PMID: 23458756, 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo S, Li X, O’Neill R, Bhoothpur C, Gluckman P, Sheppard A. 2014. Elevated S-adenosylhomocysteine alters adipocyte functionality with corresponding changes in gene expression and associated epigenetic marks. Diabetes 63(7):2273–2283, PMID: 24574043, 10.2337/db13-1640. [DOI] [PubMed] [Google Scholar]
- Niedzwiecki MM, Hall MN, Liu X, Oka J, Harper KN, Slavkovich V, et al. 2013. A dose-response study of arsenic exposure and global methylation of peripheral blood mononuclear cell DNA in Bangladeshi adults. Environ Health Perspect 121(11–12):1306–1312, PMID: 24013868, 10.1289/ehp.1206421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhout HF, Reed MC, Ulrich CM. 2008. Mathematical models of folate-mediated one-carbon metabolism. Vitam Horm 79:45–82, PMID: 18804691, 10.1016/S0083-6729(08)00402-0. [DOI] [PubMed] [Google Scholar]
- Nizam S, Kato M, Yatsuya H, Khalequzzaman M, Ohnuma S, Naito H, et al. 2013. Differences in urinary arsenic metabolites between diabetic and non-diabetic subjects in Bangladesh. Int J Environ Res Public Health 10(3):1006–1019, PMID: 23481591, 10.3390/ijerph10031006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC (National Research Council). 2001. Arsenic in Drinking Water, 2001 Update. Washington, DC:National Academies Press. [Google Scholar]
- Pawlowsky-Glahn V, Buccianti A. 2011. Compositional Data Analysis: Theory and Applications. Hoboken, NJ:John Wiley & Sons, Ltd. [Google Scholar]
- Pawlowsky-Glahn V, Egozcue JJ. 2006. Compositional data and their analysis: an introduction. Geological Society, London, Special Publications 264(1):1–10, 10.1144/GSL.SP.2006.264.01.01. [DOI] [Google Scholar]
- Pawlowsky-Glahn V, Egozcue JJ, Tolosana-Delgado R. 2007. Lecture notes on compositional data analysis. http://dugi-doc.udg.edu/bitstream/handle/10256/297/CoDa-book.pdf?sequence=1 [accessed 5 July 2015].
- Peters BA, Hall MN, Liu X, Parvez F, Sanchez TR, van Geen A, et al. 2015. Folic acid and creatine as therapeutic approaches to lower blood arsenic: a randomized controlled trial. Environ Health Perspect 123(12):1294–1301, PMID: 25978852, 10.1289/ehp.1409396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE, Vasken Aposhian H. 2000. Monomethylarsonous acid (MMAIII) is more toxic than arsenite in Chang human hepatocytes. Toxicol Appl Pharmacol 163(2):203–207, 10.1006/taap.1999.8872. [DOI] [PubMed] [Google Scholar]
- Pu YS, Yang SM, Huang YK, Chung CJ, Huang SK, Chiu AW, et al. 2007. Urinary arsenic profile affects the risk of urothelial carcinoma even at low arsenic exposure. Toxicol Appl Pharmacol 218(2):99–106, PMID: 17196235, 10.1016/j.taap.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Ren X, McHale CM, Skibola CF, Smith AH, Smith MT, Zhang L. 2011. An emerging role for epigenetic dysregulation in arsenic toxicity and carcinogenesis. Environ Health Perspect 119(1):11–19, PMID: 20682481, 10.1289/ehp.1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman TG. 2003. Mechanism of arsenic carcinogenesis: an integrated approach. Mutat Res 533(1–2):37–65, PMID: 14643412, 10.1016/j.mrfmmm.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Stead LM, Brosnan JT, Brosnan ME, Vance DE, Jacobs RL. 2006. Is it time to reevaluate methyl balance in humans?. Am J Clin Nutr 83(1):5–10, PMID: 16400042. [DOI] [PubMed] [Google Scholar]
- Steinmaus C, Bates MN, Yuan Y, Kalman D, Atallah R, Rey OA, et al. 2006. Arsenic methylation and bladder cancer risk in case–control studies in Argentina and the United States. J Occup Environ Med 48(5):478–488, PMID: 16688004 10.1097/01.jom.0000200982.28276.70. [DOI] [PubMed] [Google Scholar]
- Steinmaus C, Yuan Y, Kalman D, Atallah R, Smith AH. 2005. Intraindividual variability in arsenic methylation in a U.S. population. Cancer Epidemiol Biomarkers Prev 14(4):919–924, PMID: 15824164, 10.1158/1055-9965.EPI-04-0277. [DOI] [PubMed] [Google Scholar]
- Steinmaus C, Yuan Y, Kalman D, Rey OA, Skibola CF, Dauphine D, et al. 2010. Individual differences in arsenic metabolism and lung cancer in a case-control study in Cordoba, Argentina. Toxicol Appl Pharmacol 247(2):138–145, PMID: 20600216, 10.1016/j.taap.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipanuk MH. 2004. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr 24:539–577, PMID: 15189131, 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- Stover PJ. 2004. Physiology of folate and vitamin B12 in health and disease. Nutr Rev 62:S3–S12, PMID: 15298442, 10.1111/j.1753-4887.2004.tb00070.x. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. 2000. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 283(15):2008–2012, 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, et al. 2000. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol 74(6):289–299, PMID: 11005674, 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- Su CT, Lin HC, Choy CS, Huang YK, Huang SR, Hsueh YM. 2012. The relationship between obesity, insulin and arsenic methylation capability in Taiwan adolescents. Sci Total Environ 414:152–158, PMID: 22104380, 10.1016/j.scitotenv.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Tseng CH, Huang YK, Huang YL, Chung CJ, Yang MH, Chen CJ, et al. 2005. Arsenic exposure, urinary arsenic speciation, and peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Toxicol Appl Pharmacol 206(3):299–308, PMID: 16039941, 10.1016/j.taap.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Tyler CR, Allan AM. 2014. The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Curr Environ Health Rep 1(2):132–147, PMID: 24860722, 10.1007/s40572-014-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M. 2000. Genetic polymorphism in the biotransformation of inorganic arsenic and its role in toxicity. Toxicol Lett 112-113:209–217, PMID: 10720733, 10.1016/S0378-4274(99)00271-4. [DOI] [PubMed] [Google Scholar]
- Vahter M. 2002. Mechanisms of arsenic biotransformation. Toxicology 181-182:211–217, PMID: 12505313, 10.1016/S0300-483X(02)00285-8. [DOI] [PubMed] [Google Scholar]
- Vahter ME. 2007. Interactions between arsenic-induced toxicity and nutrition in early life. J Nutr 137(12):2798–2804, PMID: 18029502. [DOI] [PubMed] [Google Scholar]
- Valenzuela OL, Borja-Aburto VH, Garcia-Vargas GG, Cruz-Gonzalez MB, Garcia-Montalvo EA, Calderon-Aranda ES, et al. 2005. Urinary trivalent methylated arsenic species in a population chronically exposed to inorganic arsenic. Environ Health Perspect 113(3):250–254, PMID: 15743710, 10.1289/ehp.7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boogaart K, Tolosana-Delgado R. 2013a. Analyzing Compositional Data with R. Berlin, Germany:Springer. [Google Scholar]
- van den Boogaart KG, Tolosana-Delgado R, Templ M. 2015. Regression with compositional response having unobserved components or below detection limit values. Stat Modelling 15(12):191–213, 10.1177/1471082X14535527. [DOI] [Google Scholar]
- Walker AK, Jacobs RL, Watts JL, Rottiers V, Jiang K, Finnegan DM, et al. 2011. A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell 147(4):840–852, PMID: 22035958, 10.1016/j.cell.2011.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SL, Li WF, Chen CJ, Huang YL, Chen JW, Chang KH, et al. 2011. Hypertension incidence after tap-water implementation: a 13-year follow-up study in the arseniasis-endemic area of southwestern Taiwan. Sci Total Environ 409(21):4528–4535, PMID: 21872294, 10.1016/j.scitotenv.2011.07.058. [DOI] [PubMed] [Google Scholar]
- Wijekoon EP, Brosnan ME, Brosnan JT. 2006. Homocysteine metabolism. In: Biochemistry of Atherosclerosis. Cheema SK, ed. New York, NY:Springer, 329–357. [Google Scholar]
- Wu CC, Chen MC, Huang YK, Huang CY, Lai LA, Chung CJ, et al. 2013a. Environmental tobacco smoke and arsenic methylation capacity are associated with urothelial carcinoma. J Formos Med Assoc 112(9):554–560, PMID: 23871550, 10.1016/j.jfma.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Wu CC, Huang YK, Chung CJ, Huang CY, Pu YS, Shiue HS, et al. 2013b. Polymorphism of inflammatory genes and arsenic methylation capacity are associated with urothelial carcinoma. Toxicol Appl Pharmacol 272(1):30–36, PMID: 23727622, 10.1016/j.taap.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Wu F, Jasmine F, Kibriya MG, Liu M, Cheng X, Parvez F, et al. 2014a. Interaction between arsenic exposure from drinking water and genetic susceptibility in carotid intima-media thickness in Bangladesh. Toxicol Appl Pharmacol 276(3):195–203, PMID: 24593923, 10.1016/j.taap.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Jasmine F, Kibriya MG, Liu M, Cheng X, Parvez F, et al. 2015. Interaction between arsenic exposure from drinking water and genetic polymorphisms on cardiovascular disease in Bangladesh: a prospective case-cohort study. Environ Health Perspect 123(5):451–457, PMID: 25575156, 10.1289/ehp.1307883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Molinaro P, Chen Y. 2014b. Arsenic exposure and subclinical endpoints of cardiovascular diseases. Curr Environ Health Rep 1(2):148–162, PMID: 25013752, 10.1007/s40572-014-0011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MM, Chiou HY, Hsueh YM, Hong CT, Su CL, Chang SF, et al. 2006. Effect of plasma homocysteine level and urinary monomethylarsonic acid on the risk of arsenic-associated carotid atherosclerosis. Toxicol Appl Pharmacol 216(1):168–175, PMID: 16806340, 10.1016/j.taap.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Yu RC, Hsu KH, Chen CJ, Froines JR. 2000. Arsenic methylation capacity and skin cancer. Cancer Epidemiol Biomarkers Prev 9(11):1259–1262, PMID: 11097236. [PubMed] [Google Scholar]
- Zimmet JM, Hare JM. 2006. Nitroso-redox interactions in the cardiovascular system. Circulation 114(14):1531–1544, PMID: 17015805, 10.1161/CIRCULATIONAHA.105.605519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.