Abstract
We report the case of a 27-yr-old male with visual field loss who had a 4.9-cm complex cystic mass in the right occipital lobe. Histologic examination showed pilocytic astrocytoma (PA) with anaplasia, and molecular characterization revealed FGFR1 duplication with additional variants of unknown significance in several genes (ARID1A, ARID1B, CHEK2, EPHA5, and MLL2). This is one of only a very few reported cases of anaplastic PA with characterization of molecular alterations.
Keywords: astrocytoma
CASE
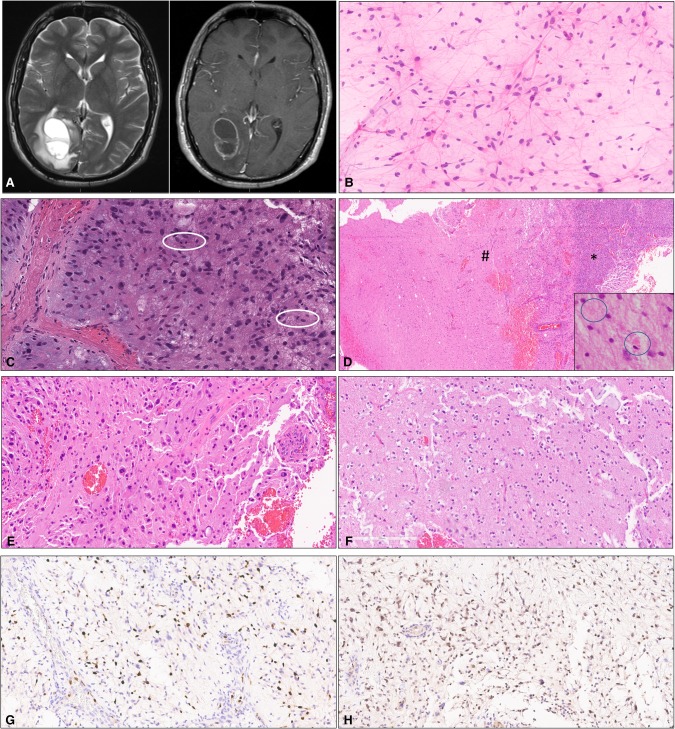
A 27-yr-old male presented to the emergency department with a complaint of left visual field loss after 2 mo of intermittent blurry vision. On examination, he had left homonymous hemianopsia. Magnetic resonance (MR) imaging demonstrated a 4.9 × 2.7 × 2.5-cm complex multiloculated ring-enhancing lesion in the right occipital lobe with hemorrhagic foci (Fig. 1A). Right parieto-occipital lobe craniotomy with gross total resection of the tumor was performed.
Figure 1.
(A) Axial T2-weighted MRI sequence (left) and T1-weighted MRI sequence with contrast (right) showing the presence of a complex lesion in the right occipital lobe with ring enhancement. (B) Cytologic smear preparation showing neoplastic astrocytes with long, delicate bipolar cytoplasmic processes (pilocytic morphology). (C) Mitotic activity and myxoid background. (D) Arcade of vascular proliferation (*) and relatively sharp demarcation with adjacent brain parenchyma (#). Rare eosinophilic granular bodies (EGBs) were present (inset). (E) Hypercellular brain parenchyma with pleomorphic tumor cells. (F) Tumor areas with myxoid appearance. (G) Ki67 antigen (MIB1) immunostain showing elevated labeling in the region with anaplasia. (H) Expression of p53 protein by tumor cells in the region with anaplasia.
Microscopic examination of the tissue showed a glial neoplasm with atypical cells displaying thin bipolar cytoplasmic processes (Fig. 1B), focally elevated mitotic activity (Fig. 1C), and prominent arcades of vascular proliferation that correlated with prominent ring enhancement seen on preoperative MR imaging (Fig. 1D). Tumor cell morphology varied from spindled to pleomorphic (Fig. 1E), with features of pilocytic astrocytoma (PA) and pleomorphic xanthoastrocytoma (PXA). Much of the tumor exhibited a prominent myxoid background (Fig. 1C,F); focal hyalinization of blood vessels was also observed. A relatively circumscribed interface with brain parenchyma was identified in several tissue fragments (Fig. 1D). Rosenthal fibers were not identified but EGBs were present (inset). Elevated mitotic activity was present (up to 7 mitoses per 10 high-power fields), and quantitative analysis yielded a correspondingly elevated Ki67 antigen (MIB1) labeling index (Fig. 1G).
Immunophenotyping showed focal areas of strong nuclear staining for p53 protein (Fig. 1H). Molecular profiling of the tumor was performed using a next-generation sequencing assay that interrogates 315 genes for mutation, as well as the introns of 28 genes involved in gene rearrangements (FoundationOne assay, Foundation Medicine, INC). A genomic alteration involving duplication of exons 10–18 of the FGFR1 gene was identified. In addition, five variants of unknown clinical significance were also identified (Table 1). Integration of the clinical, histologic, and molecular information available led to a final diagnosis of PA with anaplasia. Treatment with concurrent radiation and temozolomide was initiated.
Table 1.
Variants detected by next-generation sequencing analysis of tumor tissue
| Gene | Chromosome | Position | Variant type | Predicted effect |
|---|---|---|---|---|
| FGFR1 | 8p11.23 | Exons 10–18 | Duplication | Pathogenic |
| ARID1A | 1p36.11 | p.P21_S22INSPP | Insertion/deletion | VUS |
| ARID1B | 6q25.3 | p.C251G | Missense mutation | VUS |
| CHEK2 | 22q12.1 | p.T367fs*15 | Frameshift mutation | VUS |
| EPHA5 | 4q13.2 | p.D348G | Missense mutation | VUS |
| MLL2 | 12q13.12 | p.E5292D | Missense mutation | VUS |
VUS, variant of unknown significance.
TECHNICAL ANALYSIS
Molecular profiling of the tumor was performed using a next-generation sequencing assay that interrogates 315 genes for mutations, as well as the introns of 28 genes involved in gene rearrangements (FoundationOne assay, Foundation Medicine, INC). The test performs at a median depth of coverage of 500×.
DISCUSSION
The diagnostic challenges associated with this case relate to the presence of histologic features that overlap between PXA and PA and the increased mitotic activity with vascular proliferation, focally elevated Ki67 index, and p53 staining in a young patient. PA occurs in adults, with lobar localization being a frequent finding (∼44% in some series) (Stüer et al. 2007). However, PA with anaplastic features at initial presentation is uncommon (Stüer et al. 2007; Azad et al. 2009). Anaplasia in PA has been associated with shortened survival (Rodriguez et al. 2010b). The current WHO 2016 Classification of Tumors of the Central Nervous System recognizes the existence of PA with anaplasia; however, specific grading criteria for this entity have yet to be defined (Louis et al. 2016).
Mitogen-activated protein kinase (MAPK) pathway alterations are common in PA; in fact, PA is considered to be predominantly a single pathway disease resulting from MAPK dysregulation (Jones et al. 2013; Pathak et al. 2017). PXA and PA were considered in the differential diagnosis of this case, although the classic genetic alterations present in PXA or PA (BRAF p.V600E mutation and BRAF-KIAA1549 fusion, respectively) were not present. However, an FGFR1 duplication, involving exons 10–18, which contains the protein tyrosine kinase domain (TKD), was identified. Duplication of the FGFR1 TKD has been reported in low-grade astrocytomas (including PA), usually in an extracerebellar location, and in dysembryoplastic neuroepithelial tumor (DNET) (Jones et al. 2013; Zhang et al. 2013; Rivera et al. 2016; Fina et al. 2017). In one study, 24% of diffuse WHO 2007 grade II cerebral gliomas showed a duplication of the FGFR1 TKD. FGFR1 TKD duplication leads to autophosphorylation and activation of the MAPK/ERK and the PI3K pathways and has been shown to drive tumorigenesis (Zhang et al. 2013). The overall histologic features of this case are in favor of PA, and under the current classification system, this tumor is best classified as an anaplastic PA. Nonetheless, we recognize the possibility that the group of tumors with FGFR1 TKD duplication could represent a molecularly distinct subtype of glioma. However, further studies are required to confirm this assertion.
In addition to the FGFR1 TKD duplication, this case of PA with anaplasia showed additional genetic alterations of unknown biological and clinical significance (Table 1). The COSMIC, ExAC, and ClinVar databases were used to evaluate the potential significance of the additional variants detected in this case of PA with anaplasia. None of the variants is reported in the ExAC database. There is only a single entry of the ARID1A p21_S222INSPP variant in ClinVar (reported as likely benign), but we consider the evidence insufficient to make a final determination. There is one COSMIC entry for the CHEK2 T367fs*15 variant, and ClinVar shows 14 submissions of frameshift mutations in CHEK2, starting at codon 367. The variant is considered likely pathogenic in ClinVar. However, the significance of this variant in the context of our case is uncertain.
Genetic defects in ARID1A, ARID1B, and CHEK2 have been reported in brain tumors (Sallinen et al. 2005; Jones et al. 2012; Forbes et al. 2015; Park et al. 2015). It remains to be elucidated whether or not the FGFR1 duplication, or the additional genetic defects identified in this case, plays a direct role in the development of anaplastic features in PA. The molecular defects associated with PA have been characterized and primarily involve the MAPK pathway (e.g., BRAF-KIAA1549 fusion, BRAF p.V600E). However, the molecular defects associated with anaplasia in PA are not well known, although an association with neurofibromatosis type 1, the PI3K pathway, and p16 loss have been reported (Rodriguez et al. 2010a; Hsieh et al. 2012; Yeo et al. 2013). This case is one of only very few reports addressing the molecular defects involved in PA with anaplasia (Rodriguez et al. 2010a) and highlights the potential involvement of FGFR1 TKD in anaplastic PA.
ADDITIONAL INFORMATION
Data Deposition and Access
The variants were submitted to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) and can be found under accession numbers SCV000692539, SCV000692540, SCV000692560, and SCV000692561.
Ethics Statement
The study was performed with approval of the Institutional Review Board (IRB) at the University of Texas MD Anderson Cancer Center (# PA17-0216) with waiver of informed consent.
Competing Interest Statement
The authors have declared no competing interest.
Referees
Mariarita Santi
Kar-Ming Fung
Anonymous
REFERENCES
- Azad A, Deb S, Cher L. 2009. Primary anaplastic pilocytic astrocytoma. J Clin Neurosci 16: 1704–1706. [DOI] [PubMed] [Google Scholar]
- Fina F, Barets D, Colin C, Bouvier C, Padovani L, Nanni-Metellus I, Ouafik L, Scavarda D, Korshunov A, Jones DTW, et al. 2017. Droplet digital PCR is a powerful technique to demonstrate frequent FGFR1 duplication in dysembryoplastic neuroepithelial tumors. Oncotarget 8: 2104–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes SA, Beare D, Gunasekaran P, Leung K, Bindal N, Boutselakis H, Ding M, Bamford S, Cole C, Ward S, et al. 2015. COSMIC: exploring the world's knowledge of somatic mutations in human cancer. Nucleic Acids Res 43: D805–D811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh M-S, Ho JT-M, Lin L-W, Tu P-H, Perry A, Huang AP-H. 2012. Cerebellar anaplastic pilocytic astrocytoma in a patient of neurofibromatosis type-1: case report and review of the literature. Clin Neurol Neurosurg 114: 1027–1029. [DOI] [PubMed] [Google Scholar]
- Jones S, Li M, Parsons DW, Zhang X, Wesseling J, Kristel P, Schmidt MK, Markowitz S, Yan H, Bigner D, et al. 2012. Somatic mutations in the chromatin remodeling gene ARID1A occur in several tumor types. Hum Mutat 33: 100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DTW, Hutter B, Jäger N, Korshunov A, Kool M, Warnatz H-J, Zichner T, Lambert SR, Ryzhova M, Quang DAK, et al. 2013. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet 45: 927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Ellison DW, Figarella-Branger D, Perry A, Reifenberger G, von Deimling A, eds. 2016. WHO classification of tumours of the central nervous system, 4th ed WHO, Geneva. [DOI] [PubMed] [Google Scholar]
- Park C-K, Park I, Lee S, Sun C-H, Koh Y, Park S-H, Kim JE, Yun H, Lee S-H. 2015. Genomic dynamics associated with malignant transformation in IDH1 mutated gliomas. Oncotarget 6: 43653–43666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak P, Kumar A, Jha P, Purkait S, Faruq M, Suri A, Suri V, Sharma MC, Sarkar C. 2017. Genetic alterations related to BRAF-FGFR genes and dysregulated MAPK/ERK/mTOR signaling in adult pilocytic astrocytoma. Brain Pathol 27: 580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera B, Gayden T, Carrot-Zhang J, Nadaf J, Boshari T, Faury D, Zeinieh M, Blanc R, Burk DL, Fahiminiya S, et al. 2016. Germline and somatic FGFR1 abnormalities in dysembryoplastic neuroepithelial tumors. Acta Neuropathol 131: 847–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez EF, Scheithauer BW, Giannini C, Rynearson A, Cen L, Hoesley B, Gilmer-Flynn H, Sarkaria JN, Jenkins S, Long J, et al. 2010a. PI3K/AKT pathway alterations are associated with clinically aggressive and histologically anaplastic subsets of pilocytic astrocytoma. Acta Neuropathol 121: 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez FJ, Scheithauer BW, Burger PC, Jenkins S, Giannini C. 2010b. Anaplasia in pilocytic astrocytoma predicts aggressive behavior. Am J Surg Pathol 34: 147–160. [DOI] [PubMed] [Google Scholar]
- Sallinen S-L, Ikonen T, Haapasalo H, Schleutker J. 2005. CHEK2 mutations in primary glioblastomas. J Neurooncol 74: 93–95. [DOI] [PubMed] [Google Scholar]
- Stüer C, Vilz B, Majores M, Becker A, Schramm J, Simon M. 2007. Frequent recurrence and progression in pilocytic astrocytoma in adults. Cancer 110: 2799–2808. [DOI] [PubMed] [Google Scholar]
- Yeo YH, Byrne NP, Counelis GJ, Perry A. 2013. Adult with cerebellar anaplastic pilocytic astrocytoma associated with BRAF V600E mutation and p16 loss. Clin Neuropathol 32: 159–164. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, Orisme W, Punchihewa C, Parker M, Qaddoumi I, et al. 2013. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet 45: 602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]