Abstract
Li–Fraumeni syndrome (LFS) is an autosomal dominant cancer predisposition syndrome caused by germline alterations in the tumor suppressor gene TP53. LFS is associated with numerous malignancies including astrocytoma. Sanger sequencing and chromosomal microarray studies of blood and tumor tissue from a 4-yr-old boy with glioblastoma demonstrated a germline TP53 mutation with loss of heterozygosity for the short arm of Chromosome 17 as the second inactivating event in the tumor. There was no family history of LFS, but the child's mother had recently died from metastatic choriocarcinoma after antecedent normal term delivery of a then 6-mo-old daughter. The choriocarcinoma contained the same TP53 mutation detected in the proband and the 6-mo-old daughter was confirmed to be a carrier. Unexpectedly, the germline TP53 mutation was found to be inherited from the unaffected father. We report here the second genetically confirmed case of TP53-mutated choriocarcinoma in the partner of an LFS patient. Based on this case and recent literature, female partners of LFS patients may have increased risk of choriocarcinoma due to transmission of germline TP53 mutation from male carriers. Although the Toronto protocol has established an effective approach to detect tumors and improve survival in children and adults with LFS, there is a need to expand the current criteria to include surveillance of female partners of LFS patients for choriocarcinoma and other gestational trophoblastic disease. Recognition of this unique mode of transmission of TP53 mutations should be considered in genetic counseling for cancer risk assessment and family planning.
Keywords: astrocytoma, brainstem glioma, choriocarcinoma, glioblastoma, uterine neoplasm
CASE HISTORY
A 4-yr-old boy with a previously unremarkable medical history presented with 6 wk of vomiting and horizontal gaze palsy. His family history was remarkable for the recent death of his mother reportedly from metastatic carcinoma at age 27, and he had a healthy 6-mo-old sister. His father was healthy at age 30, with no personal history of cancer, and his paternal grandfather had died of cancer of unknown type, possibly of renal origin.
Magnetic resonance imaging (MRI) revealed multiple lesions compatible with tumor involving the patient's brainstem and left temporal lobe. No resection was attempted, and the patient was treated with a presumptive diagnosis of infiltrative glioma with carboplatin and vincristine. After 1 mo, he reported worsening nausea and dizziness, and MRI showed tumor progression. He underwent biopsy and ventriculoperitoneal shunt placement.
Biopsy of the brainstem lesion showed a highly cellular infiltrative astrocytoma with marked proliferative activity (Fig. 1A–C) consistent with glioblastoma. The patient received treatment with involved-field radiation therapy and temozolomide. One-month follow-up MRI postcompletion of chemoradiotherapy showed diffuse leptomeningeal enhancement, and cerebrospinal fluid (CSF) cytology confirmed dissemination of tumor. His tumor tissue was sent to the Children's Hospital Los Angeles (CHLA) Center for Personalized Medicine for further analysis.
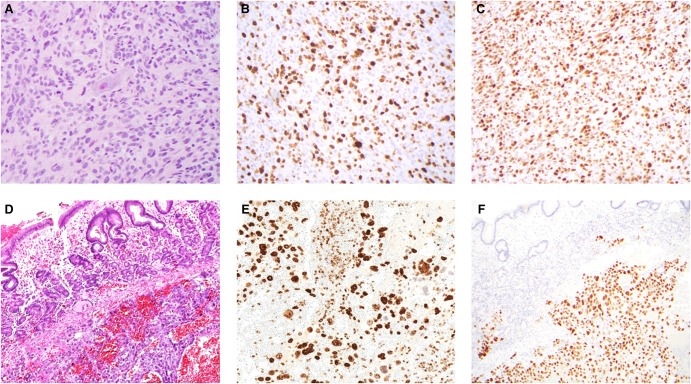
Figure 1.
Histologic features of proband astrocytoma and maternal choriocarcinoma. The proband's brainstem astrocytoma showed a diffusely infiltrative pattern of growth (A) and a markedly elevated proliferative index (Ki-67) (B). Immunoreactivity for p53-D07 was strong and diffuse (C). The maternal submucosal gastric mass was hemorrhagic and necrotic with marked pleomorphism (D) and a very high proliferative index (Ki-67) (E). Immunoreactivity for p53-D07 was strong and diffuse in the choriocarcinoma cells, but absent in the adjacent gastric mucosa (F).
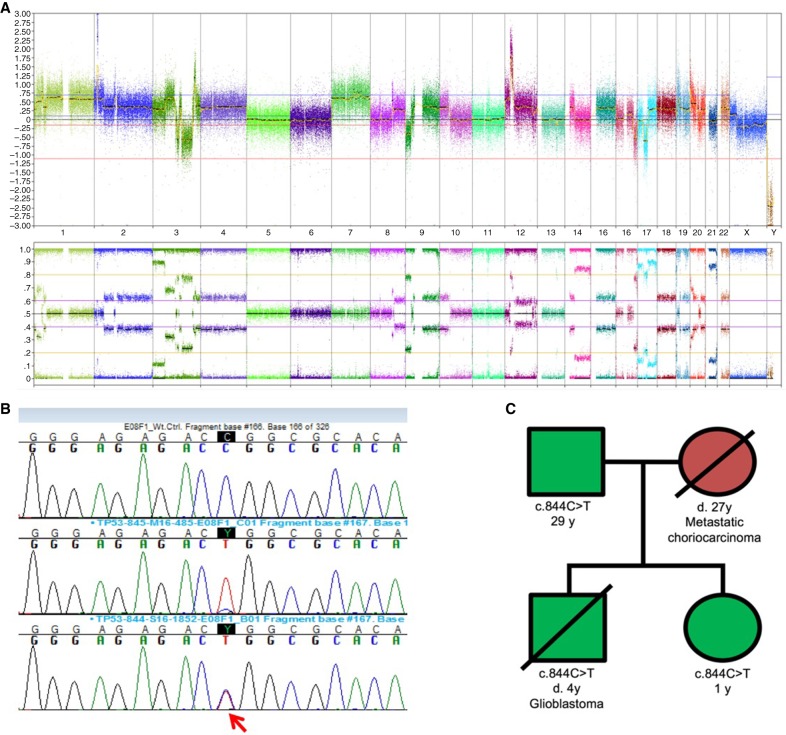
Chromosomal microarray testing performed on the astrocytoma tissue showed an extremely complex pattern of chromosomal gains and losses and three regions of chromosomal amplification that included MYCN and KRAS. Four copies of Chromosome 7, deletion of CDKN2A, and loss of heterozygosity (LOH) for the short arm of Chromosome 17 were also noted (Fig. 2A). The complex nature of the abnormalities was consistent with the histologic diagnosis of glioblastoma. Sanger sequencing of TP53 demonstrated the presence of a c.844C>T mutation, predicted to result in a p.Arg282Trp missense mutation at the protein level (Fig. 2B). The wild-type allele was absent, consistent with the LOH observed by chromosomal microarray analysis of the tumor. Given the pathogenic mutation in the tumor, germline TP53 mutation testing was recommended.
Figure 2.
Findings from molecular genetic analysis of tumor tissue. Chromosomal microarray performed on the proband's astrocytoma tissue. A complex pattern of gains and losses is observed. Key features include deletion of CDKN2A and loss of heterozygosity for 17p (A). Sanger sequencing. Top trace shows wild-type control, central trace shows the maternal gastric tumor with heterozygous pattern attributed to mixture of normal and tumor cells. The proband's astrocytoma with normal TP53 allele loss is shown in the lower trace, marked by an arrow (B). Pedigree from the proband's immediate family (C). Solid symbols (squares, males; circles, females) indicate clinically affected individuals; slashed symbols indicate deceased individuals. Green, Li–Fraumeni syndrome; red, metastatic choriocarcinoma.
Sanger sequencing demonstrated the presence of the c.844C>T mutation in the patient's blood. A recommendation for TP53 testing was extended to his immediate family, including his father and younger sister. The patient's father was shown to be a carrier of the c.844C>T mutation (Fig. 2C), and the child's sibling was found to have the same TP53 mutation. The sibling shows no evidence of disease (including normal serum β-hCG level) and is undergoing continued surveillance. Further testing of the paternal family has been recommended but is not yet complete.
The proband's brain tumor continued to progress, leading to his demise 8 months after initial diagnosis. Autopsy revealed extensive leptomeningeal dissemination of glioblastoma, which was strongly and diffusely positive for p53 immunoexpression.
REVIEW OF MATERNAL TUMOR PATHOLOGY
Because the patient's mother was originally presumed to be the carrier parent, tissue samples from her tumor were requested from the originating pathology department for further evaluation. Additional clinical history was also received. She initially presented with acute gastrointestinal bleeding 4 months after the birth of her second child and was found to have diffusely metastatic disease involving the stomach, lungs, liver, and brain. Upon pathologic review, the mother's tumor was determined to be metastatic choriocarcinoma to the stomach and lung (Fig. 1D–F). Key histologic features included marked nuclear pleomorphism, degenerative changes, syncytiotrophoblastic giant cells with immunoreactivity for inhibin and β-hCG, and markedly elevated proliferative activity (Ki-67 labeling index > 70%). Strong and diffuse p53 immunoreactivity was present in the gastric tumor sample but not in the adjacent gastric mucosa.
TP53 testing was performed on the mother's gastric tumor tissue at the CHLA Center for Personalized Medicine. The same c.844C>T TP53 mutation was detected in the maternal tumor tissue as was identified in the proband, father, and sibling. Sanger sequencing of a macro-dissected region of the histologically normal gastric mucosa was negative for the TP53 mutation, confirming that the mutation was not present in the mother's germline (Table 1). Chromosomal microarray analysis of the tumor revealed a complex pattern of copy-number gains and losses that was distinct from the child's brain tumor, and a mixed genotype pattern consistent with derivation from two individuals (data not shown).
Table 1.
Sequence variants identified from tumor samples
| Subject | Tumor | Gene | Chr | HGVS DNA | HGVS Protein | Variant type | Predicted effect | dbSNP ID | Genotype | Parent of origin |
|---|---|---|---|---|---|---|---|---|---|---|
| Proband | Astrocytoma | TP53 | 17 | NM_000546.5: c.844C>T | p.Arg282Trp | SNV | Missense | rs28934574 | Tumor homozygous (LOH Chr 17); germline heterozygous | Father |
| Mother | Gestational choriocarcinoma | TP53 | 17 | NM_000546.5: c.844C>T | p.Arg282Trp | SNV | Missense | rs28934574 | Tumor heterozygous; germline negative | N/A |
HGVS, Human Genome Variation Society; dbSNP, the Single Nucleotide Polymorphism database; SNV, single-nucleotide variant; LOH, loss of heterozygosity; N/A, not applicable.
DISCUSSION
Remarkably, the transmission of a paternal TP53 mutation to a maternal partner has been previously reported: a 37-yr-old French male index case with cholangiocarcinoma had a child diagnosed at 5 mo of age with metastatic choriocarcinoma. Subsequently, choriocarcinoma was diagnosed in the child's mother 13 mo after delivery. Genotyping of metastases in both child and mother showed the tumors to be genetically similar and confirmed the paternal origin of the TP53 mutation. This remains, to our knowledge, the only other genetically confirmed case of transmission of TP53 mutation resulting in a neoplasm in a female partner. The authors of that report highlight data from the French LFS registry suggesting that this outcome may occur in up to 1% of the deliveries in partners of TP53 mutation carriers. Two of 213 pregnancies gave rise to gestational choriocarcinoma; however, these cases could not be genetically confirmed to harbor TP53 mutations. Of the two other presumed (not genetically confirmed) cases described, one was identified retrospectively: a young woman with breast cancer at age 36 was found to have a TP53 mutation of paternal origin and had a family history of choriocarcinoma in her mother (who died at age 22). Another case of gestational choriocarcinoma was reported in the female partner of a known TP53 mutation carrier. Tumor DNA was not available for molecular analysis of either of these cases. Overall the incidence of choriocarcinoma in partners of LFS patients could be up to 500-fold that of the general population (Patrier-Sallebert et al. 2015). Our review of publicly available data from the National Cancer Institute's LFS cohort did not reveal cases of gestational choriocarcinoma among LFS patients, but this registry may not routinely collect or report data from partners of LFS patients. Of note, a recent report described the first known case of choriocarcinoma in a patient who herself harbored a germline TP53 mutation (Brehin et al. 2018). Choriocarcinoma has not previously been considered a common LFS-associated malignancy.
Gestational choriocarcinoma is a subtype of the gestational trophoblastic neoplasms, a set of rare tumors that also includes placental site trophoblastic tumor and epithelioid trophoblastic tumor. These tumors are often associated with an antecedent molar pregnancy but can arise after a spontaneous abortion, ectopic pregnancy, or normal pregnancy, as is suspected in this case. Genotyping of choriocarcinomas from term pregnancies frequently reveals biparental genetic contribution, suggesting these tumors are genetically related to the antecedent pregnancy (Savage et al. 2017). In this case, the complex copy-number profile of the choriocarcinoma tissue precluded our ability to definitively interpret the parental contribution to the tumor. A mixture of wild-type TP53 with mutant TP53 could represent either a biparental genotype or, alternatively, a commingling of normal tissue (such as endothelial cells or inflammatory cells) with the tumor.
Length of time to presentation after the index pregnancy can vary significantly, and higher risk profiles are associated with an interval of >2 mo (Ngan et al. 2015). In this case, the patient also presented with disseminated disease including brain metastasis, a feature of the highest risk category (FIGO stage 4).
Here we report the second sequencing-confirmed case of paternal transmission of a germline TP53 mutation resulting in a choriocarcinoma arising in a female partner. The relationship of the TP53 mutation to the pathogenesis of choriocarcinoma is not fully understood. In separate studies, TP53 mutations or elevated levels of wild-type p53 protein have been demonstrated in choriocarcinoma cell lines, (Landers et al. 1994; Yaginuma et al. 1995), but there are conflicting reports regarding the prevalence of TP53 mutations in human choriocarcinoma tissue samples (Shi et al. 1996; Fulop et al. 1998). Alterations in ASPP proteins, which regulate p53, may be a more common tumorigenic pathway in choriocarcinoma, and they may account for the increased levels of p53 protein observed in human tissue samples (Mak et al. 2011, 2013).
Based on this case and recent literature, female partners of LFS patients may have an increased risk of choriocarcinoma after parental–fetal transmission of germline TP53 mutation from male carriers. Although the Toronto protocol has established an effective approach to detect tumors and improve overall survival in children and adults with inherited cancer susceptibility, there is a need to expand the current criteria to include surveillance of female partners of LFS patients for gestational trophoblastic disease, even after normal delivery. Early determination of carrier status of the child after birth should prompt even more thorough investigation of the mother. After molar pregnancy, current guidelines advise weekly serum β-hCG testing to monitor for gestational trophoblastic neoplasia (Ngan et al. 2015). In female partners of LFS patients after normal delivery, a similar approach could be used to follow serum β-hCG levels to zero. A plateaued or rising β-hCG should prompt further clinical investigation. The optimal length of time for monitoring is unclear, but given the reported choriocarcinoma in a female partner of an LFS patient at 13 mo after delivery, a year of monitoring may be appropriate (Patrier-Sallebert, Bougeard et al. 2015). Furthermore, detailed pathologic examination of the placenta should be performed to evaluate for intraplacental choriocarcinoma. Recognition of this unique mode of transmission of germline TP53 mutations may also further inform discussions about family planning with female partners of LFS patients; not only to prevent unwanted pregnancy, but also to reduce their own risk of developing cancer.
This case underscores the importance of integrating molecular techniques into tumor diagnostics. Particularly in the pediatric population, germline alterations can be suspected on the basis of tumor sequencing or copy-number-based genetic studies. In this specific case, recognition of this unusual LFS family was facilitated by the genetic analysis of their child's brain tumor tissue. The complex pattern of copy-number variation observed in the tumor tissue was considered suspicious for a TP53-driven tumor, and subsequent Sanger sequencing was confirmatory. Codon 282 of TP53 is a known hotspot for single base substitution, and R282W (arginine to tryptophan) mutant is significantly associated with shortened survival time and earlier onset age in LFS (Xu et al. 2014). Identification of this mutation at an early age can allow for close surveillance of this patient's sibling for malignancy. Surveillance protocols developed for individuals with LFS have demonstrated improved long-term survival for these patients (Plon 2016; Villani et al. 2016).
Not only has molecular testing in tumor pathology established a key role in the diagnosis, prognostication, and management of individual patients, but it can also reveal information of profound clinical significance to their family members. This case is an excellent example of how the routine performance of molecular testing in neuropathology and other surgical pathology specialties is expanding our understanding of familial tumor syndromes. It also demonstrates the significance of interpreting cancer genetics in the context of constitutional testing.
METHODS
Genomic DNA was extracted from frozen tissue from the proband's brain tumor using a commercially available kit (QIAGEN QIAamp DNA Mini Tissue Kit). Genomic DNA from the maternal gastric tumor sample was extracted from formalin fixed paraffin embedded (FFPE) tissue, using a commercially available kit (QIAGEN QIAamp DNA FFPE Tissue Kit). Chromosomal microarray analysis of the brain tumor sample was performed according to the manufacturer's protocols (OncoScan FFPE Assay Kit, ThermoFisher Scientific).
Exon 8 of the TP53 gene (NM_000546.5) was amplified using primers developed by CHLA (forward: 5′-GCTCCAGAAAGGACAAGGG-3′; reverse: 5′-GTTATGCCTCAGATTCACTTTT-3′). Sanger sequencing was performed using the Big Dye Terminator v1.1 (Life Technologies, ThermoFisher Scientific) on an automated fluorescent sequencer (ABI 3730 Genetic Analyzer, Applied Biosystems, ThermoFisher Scientific).
Analysis of saliva samples from the father and children was performed at Ambry Genetics. The CHLA Center for Personalized Medicine provides fee-for-service TP53 sequencing and chromosomal microarray testing.
ADDITIONAL INFORMATION
Data Deposition and Access
The variants identified in this family were submitted to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) and can be found under accession number SCV000680092.
Ethics Statement
Written and verbal consent for all studies was obtained by the coauthors from the proband's father prior to testing. The CHLA IRB protocol to cover the analysis and publication is CCI-06-00198 (CHLA Tumor Registry).
Acknowledgments
We thank Dr. Milton Kiyabu for his initial pathologic review of the maternal tumor. Additional histology services were provided by the CHLA Pediatric Biorepository and Pathology Core Laboratory. The authors thank Cindy Fong, Jennifer Han, and Dolores Estrine for technical assistance.
Author Contributions
J.A.C. reviewed the proband's original biopsy and autopsy cases and wrote the manuscript. L.S. reviewed the original biopsy and autopsy cases. C.K. and L.B. provided genetic counseling to the family. A.M., G.D., and B.T. were and are involved with clinical management of the proband and his sibling. G.I.V. provided slides and tissue samples from the proband's mother's tumor. D.M.P. and A.R.J. provided additional pathologic review. J.A.B. performed analysis of sequencing and microarray data. All coauthors reviewed the manuscript.
Competing Interest Statement
The authors have declared no competing interest.
REFERENCES
- Brehin AC, Patrier-Sallebert S, Bougeard G, Side-Pfennig G, Llamas Gutierrez F, Lamy A, Colasse E, Kandel-Aznar C, Delnatte C, Vuillemin E, et al. 2018. Gestational choriocarcinoma associated with a germline TP53 mutation. Fam Cancer 17: 113–117. [DOI] [PubMed] [Google Scholar]
- Fulop V, Mok SC, Genest DR, Gati I, Doszpod J, Berkowitz RS. 1998. p53, p21, Rb and mdm2 oncoproteins. Expression in normal placenta, partial and complete mole, and choriocarcinoma. J Reprod Med 43: 119–127. [PubMed] [Google Scholar]
- Landers JE, Haines DS, Strauss JF III, George DL. 1994. Enhanced translation: a novel mechanism of mdm2 oncogene overexpression identified in human tumor cells. Oncogene 9: 2745–2750. [PubMed] [Google Scholar]
- Mak VC, Lee L, Siu MK, Wong OG, Lu X, Ngan HY, Wong ES, Cheung AN. 2011. Downregulation of ASPP1 in gestational trophoblastic disease: correlation with hypermethylation, apoptotic activity and clinical outcome. Mod Pathol 24: 522–532. [DOI] [PubMed] [Google Scholar]
- Mak VC, Lee L, Siu MK, Wong OG, Lu X, Ngan HY, Wong ES, Cheung AN. 2013. Downregulation of ASPP2 in choriocarcinoma contributes to increased migratory potential through Src signaling pathway activation. Carcinogenesis 34: 2170–2177. [DOI] [PubMed] [Google Scholar]
- Ngan HY, Seckl MJ, Berkowitz RS, Xiang Y, Golfier F, Sekharan PK, Lurain JR. 2015. Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynaecol Obstet 131(Suppl 2): S123–S126. [DOI] [PubMed] [Google Scholar]
- Patrier-Sallebert S, Bougeard G, Baert-Desurmont S, Lamy A, Flaman JM, Mansuy L, Bronner M, Lasset C, Brugières L, Golfier F, et al. 2015. Transmission of germline TP53 mutations from male carriers to female partners. J Med Genet 52: 145–146. [DOI] [PubMed] [Google Scholar]
- Plon SE. 2016. Improvement of outcomes for TP53 carriers. Lancet Oncol 17: 1184–1186. [DOI] [PubMed] [Google Scholar]
- Savage J, Adams E, Veras E, Murphy KM, Ronnett BM. 2017. Choriocarcinoma in women: analysis of a case series with genotyping. Am J Surg Pathol 41: 1593–1606. [DOI] [PubMed] [Google Scholar]
- Shi YF, Xie X, Zhao CL, Ye DF, Lu SM, Hor JJ, Pao CC. 1996. Lack of mutation in tumour-suppressor gene p53 in gestational trophoblastic tumours. Br J Cancer 73: 1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani A, Shore A, Wasserman JD, Stephens D, Kim RH, Druker H, Gallinger B, Naumer A, Kohlmann W, Novokmet A, et al. 2016. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li–Fraumeni syndrome: 11 year follow-up of a prospective observational study. Lancet Oncol 17: 1295–1305. [DOI] [PubMed] [Google Scholar]
- Xu J, Wang J, Hu Y, Qian J, Xu B, Chen H, Zou W, Fang JY. 2014. Unequal prognostic potentials of p53 gain-of-function mutations in human cancers associate with drug-metabolizing activity. Cell Death Dis 5: e1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaginuma Y, Yamashita T, Takuma N, Katayama H, Ishikawa M. 1995. Analysis of the p53 gene in human choriocarcinoma cell lines. Br J Cancer 71: 9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]