Abstract
Prenatal inflammation is associated with poor neurobehavioral outcomes in exposed offspring. A common route of exposure for the fetus is intrauterine infection, which is often associated with preterm birth. Hippocampal development may be particularly vulnerable to an inflammatory insult during pregnancy as this region remains highly neurogenic both prenatally and postnatally. These studies sought to determine if intrauterine inflammation specifically altered hippocampal neurogenesis and migration of newly produced granule neurons during the early postnatal period. Microglial and astroglial cell populations known to play a role in the regulation of postnatal neurogenesis were also examined. We show that intrauterine inflammation significantly reduced hippocampal neurogenesis between postnatal days 7 (P7) and P14 as well as decreased granule cell density at P28. Ectopic migration of granule cells was observed in LPS-exposed mice at P14, but not at P28. Intrauterine inflammation had no effect on hippocampal astrocyte or microglia density or on apoptosis rate at the postnatal time points examined. Thus, exposure to intrauterine inflammation disrupts early postnatal neurogenesis and leads to aberrant migration of newly born granule cells.
Keywords: Intrauterine inflammation, Fetal brain injury, Hippocampal neurogenesis, Ectopic granule cells, Microglia, Astroglia
1. Introduction
Exposure to prenatal inflammation is associated with adverse long-term neurobehavioral outcomes for exposed infants, including learning and sensory-motor deficits, epilepsy, schizophrenia and autism spectrum disorder Allen, 2008; Brown et al., 2009; Ellman et al., 2009; Fatemi et al., 2002; Hultman et al., 1999; Knuesel et al., 2014; Mednick et al., 1988; Meyer, 2013. One of the most common clinical scenarios by which a fetus is exposed to inflammation is in the setting of preterm birth which is associated with local infection or inflammation in the uterus. It has been suggested that intrauterine inflammation disrupts a critical window of brain development, leading to an array of postnatal deficits Dammann and Leviton, 1997. Animal studies support a role for altered glial development (“white matter damage”) as well as neuronal injury as a proposed mechanism by which exposure to intrauterine inflammation leads to adverse neurological outcomes Boksa, 2010; Burd et al., 2012; Hagberg et al., 2015. However, the effect of prenatal inflammation on distinct brain areas with well characterized behavioral functions still remains largely unknown.
The role of the hippocampus in learning and memory has been well characterized Eichenbaum et al., 2012; Gaesser et al., 2013; Schacter et al., 2007 and is thought to rely on continuing neuronal production which takes place in the hippocampal subgranular zone (SGZ) of the postnatal brain Aimone et al., 2014; Lee et al., 2013; Ming and Song, 2011; Mu and Gage, 2011. The SGZ supports neural stem and progenitor cells (NPCs) which undergo repeated mitotic divisions Kempermann et al., 2015 producing differentiating cellular progeny which, upon completion of differentiation and maturation stages, become fully functional dentate granule neurons that are indiscernible morphologically and physiologically from granule cells born in utero Ambrogini et al., 2004; Ramirez-Amaya et al., 2006; Toni et al., 2008; van Praag et al., 2002. In the process of maturation, the majority of newborn cells navigate radially within the granule cell layer (GCL); however, some of these cells fail to retain their normal position and migrate further into the hilar area Hester and Danzer, 2014. These so called hilar ectopic granule cells (hEGCs) develop abnormal synaptic connectivity and show altered morphological and electrophysiological properties compared to the granule cells that reside within the GCL Scharfman and Pierce, 2012; Scharfman et al., 2007; Zhan et al., 2010. An unusually high presence of hEGCs has been documented in animal models of epilepsy and is thought to contribute to the development of this disorder Cayre et al., 2009; Hester and Danzer, 2014.
Hippocampal neurogenesis is a highly dynamic process known to be regulated by various environmental, physiological and pathological stimuli Aimone et al., 2014. In particular, it has been shown that systemic maternal inflammation during pregnancy can negatively affect postnatal neurogenesis in the hippocampal SGZ Green and Nolan, 2014. However, the role of intrauterine inflammation, as might occur in spontaneous preterm birth, on hippocampal neurogenesis has not been investigated in much detail Jiang et al., 2012. We hypothesize that exposure to intrauterine inflammation during a critical period of brain development will specifically alter hippocampal neurogenesis in the postnatal brain. In addition, microglial and astroglial cell populations have been shown to play a role in modulating neuronal production in the adult brain. In particular, microglia, which represent resident brain macrophages Kreutzberg, 1996; Town et al., 2005, are responsible for reducing the rate of postnatal neurogenesis following both systemic Monje et al., 2003 and local (within the brain) Ekdahl et al., 2003 inflammatory stimuli. Similarly, astrocyte function is also involved in the regulation of hippocampal neurogenesis under both normal Ashton et al., 2012; Környei et al., 2007 and inflammatory conditions Vallieres et al., 2002. Using our previously established model of inflammation-induced fetal brain injury Burd et al., 2010a,b; Elovitz et al., 2006, this study sought to address the effect of in utero inflammation on early postnatal neurogenesis and granule cell migration in the hippocampal SGZ as well as to examine possible alterations in microglial and astroglial cell populations.
2. Results
2.1. Exposure to intrauterine inflammation reduces early postnatal neurogenesis
Alterations in hippocampal neurogenesis were determined by labeling mitotically active NPCs with BrdU on postnatal days 7 and 8 and quantifying their neuronal progeny a week later, on P14 (Fig. 1A and B). Dual labeling with BrdU and the neuron-specific marker NeuN identified newly generated granule cells within the upper and lower blades of the dentate cell body layer (Fig. 1B). A significant reduction in the density of BrdU-labeled cells was detected in pups exposed to intrauterine inflammation as compared to control saline-injected animals (Fig. 1C, p = 0.023).
Fig. 1.
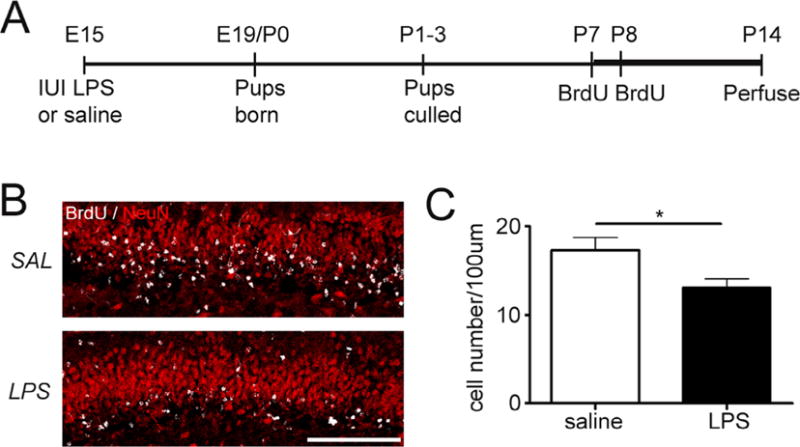
Exposure to intrauterine inflammation reduces early postnatal neurogenesis. (A) Schematic of experimental design. Pregnant dams were given intrauterine injections of lipopolysaccharide (LPS) [50 μg] or saline on embryonic day 15. Pups delivered at term were culled [5 pups/dam] and given injections of bromodeoxyuridine [BrdU, 75 mg/kg; s.c.] at postnatal days 7 and 8 (P7–P8) to label actively proliferating cells. Mice were transcardially perfused with paraformaldehyde at postnatal day 14 (B). Confocal maximum projection images of BrdU (white) and Neuronal Nuclei (NeuN, red) co-labeling in the dentate gyrus of mice exposed to intrauterine saline or LPS and sacrificed at P14. Scale bar = 100 μm. (C) Quantification of BrdU/NeuN density in the granule cell layer at P14. LPS: n = 10; saline: n = 8. *p<0.05. Data represent the mean ± SEM.
2.2. Intrauterine inflammation leads to decreased neuronal density in the dentate gyrus at P28
Since we observed inflammation-induced decline in hippocampal neurogenesis, granule neuron density and GCL thickness were assessed at P14 and P28 using a neuronal marker Prox1. While granule cell density was not different between control and experimental groups at P14, it was reduced at P28 after exposure to inflammation (Fig. 2A, p = 0.002). GCL thickness was not affected at either time point (Fig. 2B).
Fig. 2.
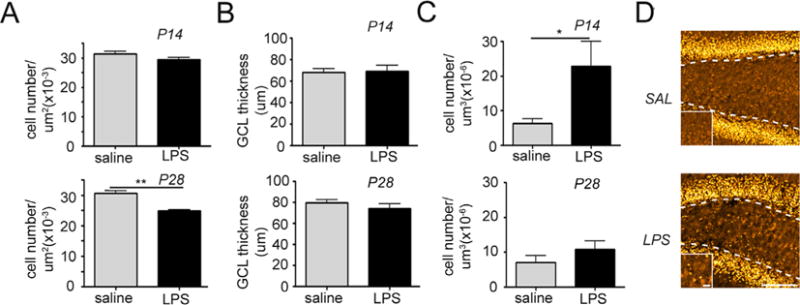
Intrauterine inflammation leads to decreased total neuronal density and transient migration abnormalities in the dentate gyrus. Quantification of granule cell density in the granule cell layer (GCL) (A), granule cell layer thickness (B), and ectopic granule cell density in the hilus (C) at postnatal days 14 and 28. *p < 0.05. Data represent the mean ± SEM. (D) Confocal maximum projection images of the granule cell-specific marker Prospero Homeobox 1 (Prox1) in the dentate gyrus of mice exposed to intrauterine saline or lypopolysaccharide (LPS) and sacrificed at P14 (LPS: n = 10; saline: n = 8). Dotted lines depict the granule cell layer (GCL)/hilar border. High magnification images depicted on the bottom left were taken from the center of the hilus. Scale bar = 100 μm, magnified panel = 10 μm.
2.3. Exposure to intrauterine inflammation generates ectopic granule cells
Prox1 labeled granule cells in the hilus of inflammation-exposed pups and saline-exposed pups were compared to ascertain if any granule cells were migrating improperly (Fig. 2C). Following exposure to intrauterine inflammation, the number of hEGCs was significantly increased at P14 (p = 0.023) (Fig. 2C and D). This effect was not apparent at P28 (Fig. 2C).
2.4. Intrauterine inflammation has no effect on total number and division rate of neural stem and progenitor cells at P14 and P28
The total number of hippocampal stem and progenitor cells was measured by counting nestin-positive cells in the SGZ of inflammation-exposed and control animals (Fig. 3A). The density of NPCs was not altered between exposed and unexposed pups at either P14 or P28 (Fig. 3B). Additionally, exposure to prenatal inflammation did not affect the number of mitotically active cells within the SGZ (Fig. 3C and D). Hence, there were no differences in Ki67-positive cell densities present at either time point.
Fig. 3.
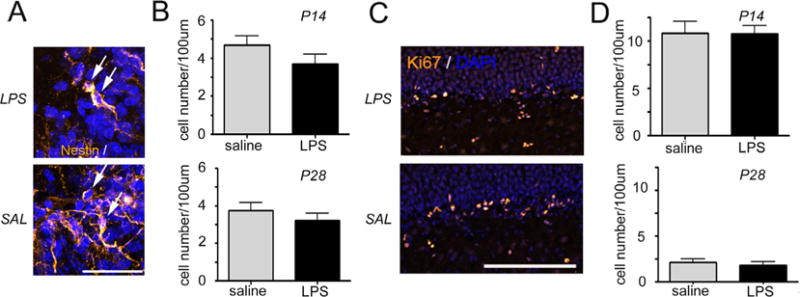
Intrauterine inflammation has no effect on the number and division rate of neuronal progenitor cells. Confocal maximum projection images of Nestin (A, yellow) and Ki67 (C, yellow) labeling of the neural progenitor cells in the dentate gyrus of mice exposed to intrauterine saline or lipopolysaccharide (LPS) and sacrificed at postnatal day 14 (P14). Arrows depict Nestin-positive neuronal progenitor cells in the subgranular zone (A). Cell nuclei are counterstained with 4′,6-diamidino-2-phenylindole (DAPI, blue). Scale bars are 20 μm (A) and 100 μm (C). Quantification of neuronal progenitor cell density (B) and the density of the Ki67 labeled cells (D) in the subgranular zone at P14 and P28. Data represent the mean ± SEM. P14: LPS, n = 10; saline, n = 8; P28: LPS n = 6; saline n = 6.
2.5. Intrauterine inflammation has no effect on postnatal apoptosis in the dentate gyrus
Apoptotic cells measured by labeling with an antibody against Cleaved Caspase 3 (Fig. 4A) were sparse in both treatment groups and their presence did not change in response to intrauterine inflammation at either time point (Fig. 4B).
Fig. 4.
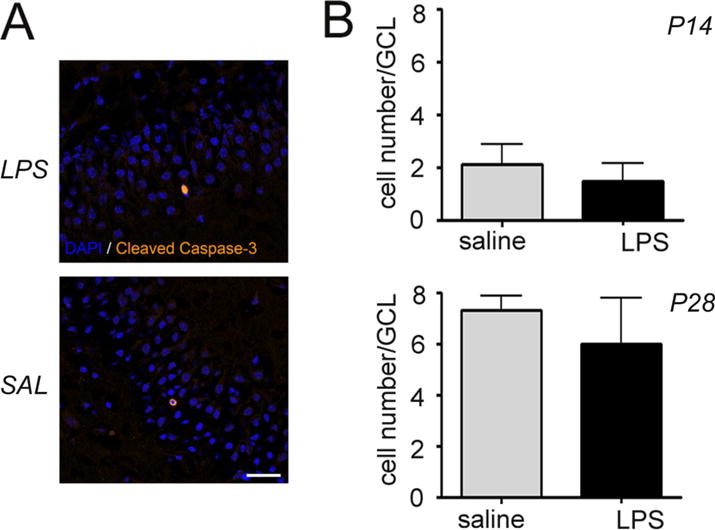
Intrauterine inflammation has no effect on postnatal apoptosis in the dentate gyrus. (A) Confocal maximum projection images through the dentate gyrus of mice exposed to intrauterine saline or lipopolysaccharide (LPS) and sacrificed at postnatal day 14 (P14). Apoptotic cells are labeled with antibodies against Cleaved Caspase-3 (yellow). 4′,6-diamidino-2-phenylindole (DAPI, blue) labels cell nuclei. Scale bar = 20 μm. (B) Quantification of Cleaved Caspase-3-expressing cells in the granule cell layer of the dentate gyrus at P14 and P28. Data represent the mean ± SEM. P14: LPS, n = 10; saline, n = 8; P28: LPS n = 6; saline n = 6.
2.6. Intrauterine inflammation has no effect on hippocampal microglia and astroglia densities
Microglial density was quantified in various regions of the hippocampus, including hilus, dentate gyrus (DG) and Cornu ammonis 1–3 (CA1-3), using antibodies against microglial marker Iba1 (Fig. 5A). Our data show that the number of microglial cells did not vary by exposure at P14 (Fig. 5B) or P28 (not shown).
Fig. 5.
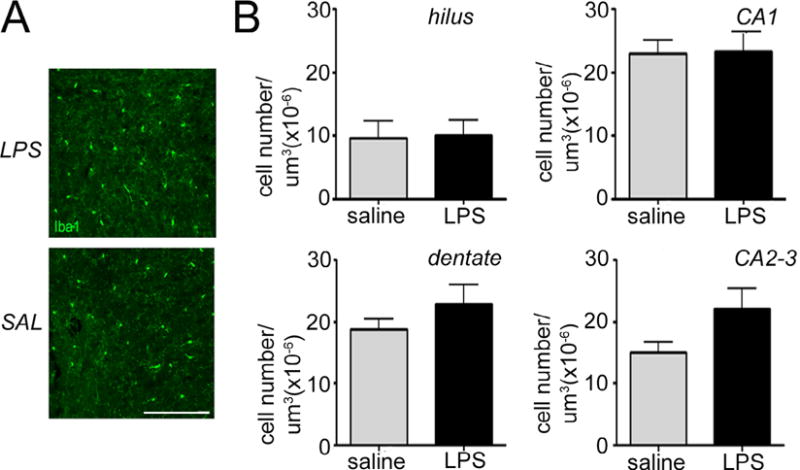
Intrauterine inflammation has no effect on hippocampal microglia density. (A) Confocal maximum projection images of the microglia-specific marker Ionized calcium-binding adapter molecule 1 (Iba1, green) in the Cornu Ammonis 1 (CA1) hippocampal region of mice exposed to intrauterine saline or lipopolysaccharide (LPS) and sacrificed at postnatal day 28 (P28). 4′,6-diamidino-2-phenylindole (DAPI) counterstains cell nuclei (blue). Scale bar = 100 μm. (B) Quantification of microglia density in the dentate gyrus, hilus, CA1, and CA2-3 regions at P14. Data represent the mean ± SEM. P14: LPS, n = 10; saline, n = 8; P28: LPS n = 6; saline n = 6.
The astrocyte specific marker Glial Fibrillary Acidic Protein (GFAP) was used for measuring astroglial density in the hippocampus (Fig. 6A). Similar to findings with microglia, exposure to intrauterine inflammation did not alter the number of astrocytes in the various regions of the hippocampus in any of the regions analyzed at P14 (Fig. 6B) or P28 (not shown).
Fig. 6.
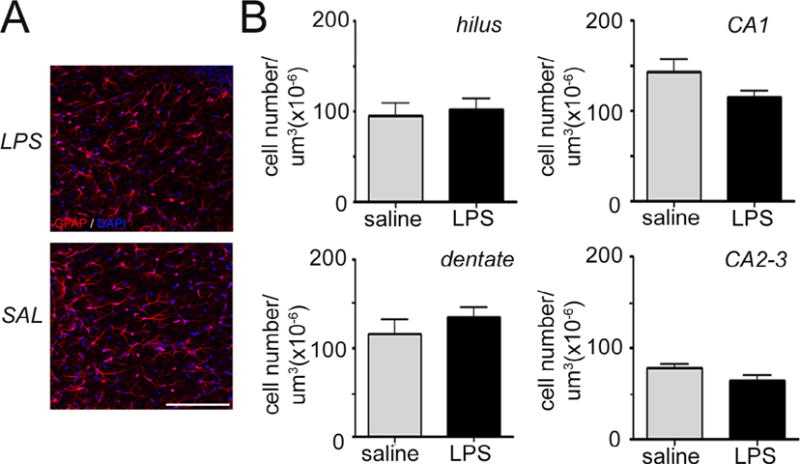
Intrauterine inflammation has no effect on hippocampal astrocyte density. (A) Confocal maximum projection images of the astrocyte-specific marker Glial Fibrillary Acidic Protein (GFAP, red) in the Cornu Ammonis 1 (CA1) hippocampal region of mice exposed to intrauterine saline or lipopolysaccharide (LPS) and sacrificed at postnatal day (P14). Cell nuclei are labeled with 4′,6-diamidino-2-phenylindole (DAPI) counterstain (blue). Scale bar = 100 μm. (B) Quantification of astrocyte density in the dentate gyrus, hilus, CA1, and CA2-3 regions at P14. Data represent the mean ± SEM. P14: LPS, n = 10; saline, n = 8.
3. Discussion
Exposure to prenatal inflammation has been strongly associated with a spectrum of adverse neurobehavioral outcomes in exposed offspring, including motor-sensory deficits, delayed learning and neurological disease, such as schizophrenia, autism spectrum disorder and epilepsy Anderson and Doyle, 2003; Cordeiro et al., 2015; Fazzi et al., 2009; Hack et al., 2005; Indredavik, 2010; Johnson et al., 2010a, b; Lee et al., 2011; Schieve et al., 2010. However, there is still a paucity of data on how an inflammatory environment in utero leads to fetal brain injury, causing neurobehavioral abnormalities in childhood and adulthood. In this study, we demonstrate that in addition to previously reported white matter damage and neuronal injury Burd et al., 2010a,b; Elovitz et al., 2006; Nitsos et al., 2006, early exposure to intrauterine inflammation results in a reduced rate of neurogenesis in the hippocampal SGZ and the accumulation of ectopic dentate granule cells known to be implicated in synaptic malfunction and increased neuronal excitability. As altered hippocampal function is associated with many neurological deficits, these findings provide a new mechanism by which exposure to intrauterine inflammation may lead to long term adverse outcomes.
The finding of decreased neuronal production in the hippocampal SGZ in response to intrauterine inflammation is consistent with a number of previous studies made in rodent model systems which reveal a reduction in postnatal neurogenesis following systemic inflammatory insults, including those evoked by E. coli LPS Cui et al., 2009; Girard et al., 2012; Graciarena et al., 2010, 2013; Lin and Wang, 2014; Mouihate, 2016. In contrast, a local E. coli administration in pregnant rats, which was shown to induce an inflammatory response in the uterus, increased NPC divisions between P3 and P28 but did not change neuronal and astroglial production measured at P28 Jiang et al., 2012. The inconsistency between the results of this study and our data can be attributed to multiple differences in experimental design including the application of different rodent models (rat vs mouse), infectious agents (E. coli vs LPS), routes of E. coli/LPS administration (endocervical vs intrauterine), BrdU labeling regimens (50 μg/kg BrdU for three or seven consecutive days at various time points vs two 75 μg/kg BrdU injections at P7 and P8) and time points of tissue collection and analysis. Thus, our data contribute to the accumulating evidence showing a negative effect of prenatal inflammation on hippocampal neurogenesis during early postnatal period. Importantly, this finding was obtained by applying a more clinically relevant route of evoking prenatal inflammation, intrauterine inflammation using LPS, as opposed to a systemic infectious or inflammatory exposure.
The observed reduction in novel neuronal production and total granule cell density can be caused by decreased abundance of cycling NPCs and/or increased apoptosis among differentiating granule cells Gage et al., 2008; Sierra et al., 2010. As our measurements showed no change in the numbers of total and dividing NPCs or the rate of apoptosis at both examined time points (P14 and P28), it is plausible that prenatal exposure to intrauterine inflammation exerts a negative effect on hippocampal neurogenesis by influencing the rate of NPC amplification earlier during postnatal development, between P7 and P14.
Alterations in neuronal production in the adult brain have been associated with significant behavioral changes. For example, in a mouse model, genetic or pharmacological ablation of hippocampal neurogenesis impairs animal performance in certain hippocampus-dependent learning and memory tasks Deng et al., 2010; Green and Nolan, 2014; Mu and Gage, 2011. In addition, a decrease in neuronal production in response to systemic inflammation is known to be associated with increased anxiety Bergami et al., 2009; Revest et al., 2009 and depression-like behaviors Ho and Wang, 2010; Lin and Wang, 2014; Snyder et al., 2011; Vollmayer et al., 2007. Reduced NPC proliferation has also been detected in the brains of schizophrenic patients Reif et al., 2006. On the contrary, reducing the neurogenesis rate in an animal model of status epilepticus appears to play a protective role as this manipulation decreases the frequency of acute seizures and prevents associated cognitive deficits Cho et al., 2015. It remains to be demonstrated how reduction in postnatal neuronal production in this model of intrauterine inflammation may lead to disruptions in behavioral function.
We also show for the first time that exposure to in utero inflammation is sufficient to cause an aberrant distribution of newly formed granule cells as manifested by the presence of hEGCs. Interestingly, while extra hEGCs are present in the hilus area at P14, they are no longer detectable at the later time point, P28, which suggests that the hippocampus may, at least temporarily, recover from the prenatal insult. Although the accumulation of hEGCs takes place during a limited developmental window, their presence may still adversely affect hippocampal function and behavioral performance which have been shown to be altered in response to intrauterine inflammation Kelley et al., 2017; Makinson et al., 2017. In particular, as shown in animal models of epilepsy, misplaced granule cells differ from other granule neurons in that they develop excessive excitatory innervation while being almost devoid of inhibitory inputs Pierce et al., 2005; Scharfman et al., 2007; Scharfman and Pierce, 2012; Zhan et al., 2010. As a result, these cells demonstrate increased spontaneous activity Dashtipour et al., 2001; Pierce et al., 2005 and frequent firing Scharfman et al., 2007 as compared to normally situated granule neurons, which is thought to contribute to epileptogenesis Bielefeld et al., 2014; Danzer, 2012; Hester and Danzer, 2014. Thus, the aberrant positioning of newly formed granule cells following exposure to prenatal inflammation may lead to the development of epilepsy-like symptoms and other behavioral abnormalities, such as certain memory deficits Myers et al., 2013, later in life. It has also been shown that the accumulation of hEGCs can be caused by increased levels of certain growth factors, such as Insulin-like Growth Factor I (IGF-I), Vascular Endothelial Growth Factor (VEGF) and Brain-derived Neurotrophic Factor (BDNF), in the brain Scharfman et al., 2007 and by inactivating the function of several genes, specifically, Reelin Stanfield and Cowan, 1979, p35 Patel et al., 2004, protein L-isoaspartate (D-aspartate) O-methyltransferase (Pmct-1) Farrar et al., 2005 and Bcl-2-associated X protein (Bax) Myers et al., 2013. Interestingly, intrauterine inflammation is known to lead to an elevated production of a number of neurotrophic factors in the fetal brain, including BDNF Elovitz et al., 2006, while maternal immune activation induced systemically reduces hippocampal expression of the Reelin protein in the offspring Depino, 2015; Fatemi et al., 2002; Forster et al., 2002; Ghiani et al., 2011; Harvey and Boksa, 2012; Meyer et al., 2006, 2008; Ratnayake et al., 2012. These data suggest that the displacement of granule cells in the dentate gyrus is a result of changes in neurotropic factors and/or modification of proteins involved in neurogenesis that can be directly induced by intrauterine inflammation.
Despite earlier reports showing microglial activation Burd et al., 2012; Dada et al., 2014; van den Heuij et al., 2014; Kannan et al., 2007; Lei et al., 2017; Makinson et al., 2017; Saadani-Makki et al., 2009; van den Williams et al., 2017; Zhang et al., 2016, astrogliosis Elovitz et al., 2006 and decreased neuronal survival Balakrishnan et al., 2013; Elovitz et al., 2006; Leitner et al., 2014; Yoon et al., 1997 after exposure to intrauterine inflammation, our study did not reveal similar changes at the examined time points (P14 and P28). This difference between our results and previously obtained data may derive from several variables in experimental conditions that are shown to affect the cellular and behavioral outcomes of prenatal inflammation, including the type of animal model, dose of infectious agent and the developmental time point at which the inflammation is induced Aguilar-Valles and Luheshi, 2011; Meyer et al., 2006. While microglial activation has been previously detected using the same mouse model of low dose LPS-induced inflammation (50 μg) as that utilized in our study, these results were achieved by administering LPS at a different time during fetal development Dada et al., 2014 and collecting brain samples at alternative time points Makinson et al., 2017. In addition, in both of these studies, FACS technique was implemented allowing for a more precise measurement of the sizes of microglial population. Consistent with our findings of unaltered astroglial densities in saline and LPS treated hippocampi, another study found that the expression of a commonly used astrocytic marker GFAP was unaffected in P7 hippocampus after exposure to intrauterine inflammation Elovitz et al., 2011. This finding, however, does not exclude the possibility that microglial and astroglial cells undergo corresponding functional changes in response to in utero inflammation at the developmental time points other than those analyzed in our study.
In conclusion, we have shown that, in addition to our previous reports of neuronal injury during the time of immediate exposure to intrauterine inflammation Burd et al., 2010a,b; Elovitz et al., 2003, 2006; Nitsos et al., 2006, in utero inflammatory challenge results in decreased hippocampal neurogenesis and aberrant positioning of newly generated granule cells in the hippocampus during the early postnatal period (P7–P28). These findings suggest that postnatal changes in hippocampal neurogenesis may be mechanistically involved in neurobehavioral abnormalities observed in affected offspring Brown et al., 2009; Gilmore and Jarskog, 1997; Mednick et al., 1988; Meyer, 2013. Understanding the mechanisms by which exposure to intrauterine inflammation, which is a common event in human pregnancy, leads to adverse neurobehavioral outcomes for exposed offspring can open new avenues for future therapeutic strategies. Further work is needed to demonstrate what specific behavioral disruptions are associated with these observed changes in neuronal development and function to further advance the field and decrease morbidity from this common exposure.
4. Experimental procedure
4.1. Mouse model of intrauterine inflammation
A previously described mouse model of intrauterine inflammation, which results in fetal and postnatal brain injury Burd et al., 2010a,b); Elovitz et al., 2006; Elovitz et al., 2011; Jansen et al., 2013 was utilized for these studies. For all experiments, CD-1, timed pregnant mice were purchased from Charles River Laboratories (Wilmington, MA). Animals were shipped 8–12 days after mating and allowed to acclimate in our facility for 3–7 days. A mini-laparotomy was performed under isoflurane anesthesia at gestational day 15 (E15) with normal gestation being 19–20 days. Briefly, the right uterus was exposed allowing visualization of the lowest two gestational sacs. Mice then received intrauterine injections of LPS from Escherichia coli (055:B5, Sigma, St Louis, MO, L2880, 50 μg/100 μl phosphate buffered saline/animal; LPS-treated group) or PBS (100 μl/animal; control, saline-treated group). Surgical incisions were closed using staples and dams were allowed to recover. Mice were provided food and water ad libitum and maintained on a 12:12 h light/dark cycle. Term delivering litters were culled to 5 pups/cage at P1-3 (P14: LPS, n = 10; saline, n = 8; P28: LPS n = 6; saline n = 6). All experiments were performed in accordance with the National Institute of Health Guidelines on Laboratory Animals with approval from the University of Pennsylvania’s Animal Care and Use Committee (protocol number 804658).
4.2. BrdU administration, neonatal brain perfusion and tissue collection
The thymidine analogue bromodeoxyuridine (BrdU) (Sigma LifeSciences, B5002-500 mg; Sigma-Aldrich, St Louis, MO) was dissolved in phosphate-buffered saline (PBS) and injected subcutaneously to all pups at P7 and P8 (75 mg/kg). Pups were anesthetized with a Ketamine/Xylazine cocktail (200 mg/kg; 10 mg/kg, i.p. respectively) and transcardially perfused with 10 U/mL heparin, 2.5% paraformaldehyde, and 4% sucrose in PBS, pH 7.4 at P14 (n = 18). Brains were removed, fixed overnight in the same solution, cryoprotected in 30% sucrose (in PBS), and flash frozen in isopentane at −25 °C. Brains were sectioned coronally at 50 μm, mounted to gelatin-coated slides (2–3 sections/slide), and stored at −80 °C.
4.3. Immunohistochemistry
Slide-mounted brain sections from dorsal hippocampus were processed for histology. Hippocampal sections were identified based on general morphological features of the area Paxinos and Frankin, 2013 and immunostained with antibodies against the following molecular markers: a DNA intercalating agent, BrdU (sheep anti-BrdU, 1:200; GeneTex), a marker of microglial cells Iba1 (rabbit anti-Iba1, 1:1000; Wako), a marker specific for dentate granule neurons and late neuronal progenitor cells, Prox1 (rabbit anti-Prox1, 1:500; Millipore), an apoptotic cell marker, Cleaved Caspase 3 (rabbit anti-cleaved caspase 3, 1:200; Cell Signaling), a marker of mitotic cells, Ki67 (rabbit anti-Ki67, 1:100; Abcam), an astroglial cell marker, GFAP (goat anti-GFAP, 1:300; Abcam), a neuronal cell marker, NeuN (mouse anti-NeuN, 1:300; Millipore), and a marker of neural stem and progenitor cells, Nestin (mouse anti-Nestin, 1:200; Abcam). Secondary labeling was performed with AlexaFluor 488 donkey anti-rabbit (Abcam), AlexaFluor 647 donkey anti-sheep (Abcam), AlexaFluor 488 donkey anti-mouse (Life Technologies), AlexaFluor 568 donkey anti-goat (Life Technologies), and AlexaFluor 568 donkey anti-mouse (Life Technologies). Brain sections were dehydrated in alcohol, cleared in xylenes, and coverslips were affixed with mounting media (Krystalon; Harleco).
4.4. Confocal microscopy and histological analyses
Images were collected from a Zeiss LSM 710 confocal system set up on an AxioObserver inverted microscope and a Leica TCS SP8 confocal system set up on a DMI 6000 inverted microscope. Dorsal hippocampal sections (~2 mm posterior to bregma; Paxinos and Franklin, 2001) were imaged by investigators blind to treatment group. All images were imported into ImageJ software for quantification.
The rate of postnatal neurogenesis was determined by analyzing confocal image stacks made from coronal brain slices immunostained with antibodies against BrdU and Ki67 protein. Each three-dimensional z-stack contained 10 images collected at 1 μm increments. For P7-P14 neurogenesis, mature neurons labeled with BrdU were imaged from the midpoint of the upper and lower blades (field size 420 × 420 μm) of the dentate cell body layer along the length of the SGZ. For P14 and P28 neurogenesis, Ki67 expressing neural progenitor cells were imaged from the upper and lower blades (field size 420 × 420 μm) of the SGZ of the DG. Ki67-expressing cells were counted if their cell bodies were proximal (<30 μm) to the SGZ and exhibited a characteristic granule cell morphology (round shape, ~10 μm in cell body diameter). Neurons meeting the selection criterion were scored in three dimensional Z-stacks if their cell body came into focus when focusing from the surface of the tissue to the bottom Howell et al., 2002. The length of the SGZ was measured for each dentate blade using Fiji software and the sum of the length of both blades was used to normalize cell counts. For simplicity and to avoid confusion with area counts neuronal cell densities are presented as cell number per 100 μm of the SGZ instead of cell number per SGZ length multiplied by the depth of an image Z-stack.
Granule cell density and the thickness of the granule cell layer were determined by analyzing confocal images of Prox1 labeling. Counting frames (100 × 25 μm) were positioned at the midpoints of the upper and lower dentate blades in one hemisphere for each animal. Cell bodies that crossed the upper or right frame boundaries were included, and excluded if they crossed the lower or left frame boundaries. Granule cell density is presented as granule cells per square micrometer of tissue and granule cell thickness is shown in micrometers.
Hilar ectopic granule cell density was determined by analyzing confocal image stacks of Prox1 labeling. Images were collected at 1 μm increments through 10 μm of tissue to create three-dimensional confocal z-stacks. Granule cells were scored as ectopic if their cell bodies were at least 20 μm from the granule cell/hilar border and came into focus when focusing from the surface of the tissue to the bottom. The hilar areas within which ectopic granule cells were counted were outlined and measured using Fiji software. These areas were multiplied by the depth of a three-dimensional image Z-stack (10 × 1 μm = 10 μm) to calculate tissue volume. Obtained cell counts were divided by tissue volume to calculate cell density which is presented as cell number per cubic micrometer of tissue.
Neural stem and progenitor cell density was determined by analyzing confocal images of Nestin labeling. Nestin expressing NPCs were imaged from the upper and lower blades (field size 135 × 135 μm) of the SGZ of the DG. Images were collected at 1 μm increments through 10 μm of tissue to create three-dimensional confocal z-stacks. Nestin/DAPI expressing cells were counted if their cell bodies were proximal (<30 μm) to the SGZ and their cell body came into focus when focusing from the surface of the tissue to the bottom. The length of the SGZ was measured for each dentate blade using Fiji software, and data are presented as cell number per 100 μm of the SGZ.
To assess apoptosis in the dentate granule cell layer, dentate gyri were screened under epifluorescent illumination for the presence of Cleaved Caspase-3 labeling. Manual cell counts were conducted throughout the SGZ and GCL in one hemisphere for each animal. Data are presented as the total number of Cleaved Caspase-3 expressing cells per GCL.
Microglia and astrocyte densities were determined by analyzing confocal image stacks of Iba1/DAPI and GFAP/DAPI labeling, respectively. Four hippocampal regions were assessed (DG, Hilus, CA1, CA2-3; field size 290 μm × 290 μm). Images were collected at 1 μm increments through 10 μm of tissue to create three-dimensional confocal z-stacks. Microglia and astrocytes were scored if their cell body came into focus when focusing from the surface of the tissue to the bottom. Respective hippocampal areas were outlined and measured using Fiji software. Tissue volume was calculated by multiplying area measurements by the depth of an image Z-stack (10 μm) and used for normalizing cell counts. Data are presented as cell number per cubic micrometer of tissue.
4.5. Statistics
Statistical analyses were performed using SigmaStat software (version 12.5). T-tests were used for data that met assumptions of normality and equal variance, whereas Mann-Whitney U-tests were used when data violated one or both assumptions. P values <0.05 were considered significant. Values are presented as mean s ± SEM.
Acknowledgments
We thank the laboratories of Dr. Ted Abel and Dr. Mariella De Biasi for making available their Cryostat instruments for our studies. We are also grateful to Laura Heiser for proof reading the manuscript.
Funding
This work was supported by the National Institutes of Health [R01 HD076032]. The funding source had no involvement in the study design, collection, analysis and interpretation of data or in the writing and submitting of the article for publication.
Footnotes
Author contributions statement
The manuscript was reviewed by all authors prior to submission. Michael Hester, Amy Brown and Guillermo Barila were responsible for animal husbandry and conducting surgeries and tissue dissection. Michael Hester and Natalia Tulina performed cryo-sectioning, immunostainings and confocal microscopy and analyzed the results. Natalia Tulina, Amy Brown and Michal Hester interpreted the data and wrote the manuscript. Michal Elovitz provided scientific guidance and edited the manuscript.
Additional information
The authors declare no conflict of interest.
References
- Aguilar-Valles A, Luheshi GN. Alterations in cognitive function and behavioral response to amphetamine induced by prenatal inflammation are dependent on the stage of pregnancy. Psychoneuroendocrinology. 2011;36:634–648. doi: 10.1016/j.psyneuen.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH. Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev. 2014;94:991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MC. Neurodevelopmental outcomes of preterm infants. Curr Opin Neurol. 2008;21:123–128. doi: 10.1097/WCO.0b013e3282f88bb4. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Lattanzi D, Ciuffoli S, Agostini D, Bertini L, Stocchi V, Santi S, Cuppini R. Morpho-functional characterization of neuronal cells at different stages of maturation in granule cell layer of adult rat dentate gyrus. Brain Res. 2004;1017:21–31. doi: 10.1016/j.brainres.2004.05.039. [DOI] [PubMed] [Google Scholar]
- Anderson P, Doyle LW. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA. 2003;289:3264–3272. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- Ashton RS, Conway A, Pangarkar C, Bergen J, Lim KI, Shah P, Bissell M, Schaffer DV. Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nat Neurosci. 2012;15:1399–1406. doi: 10.1038/nn.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan B, Dai H, Janisse J, Romero R, Kannan S. Maternal endotoxin exposure results in abnormal neuronal architecture in the newborn rabbit. Dev Neurosci. 2013;35:396–405. doi: 10.1159/000353156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergami M, Berninger B, Canossa M. Conditional deletion of TrkB alters adult hippocampal neurogenesis and anxiety-related behavior. Commun Integr Biol. 2009;2:14–16. doi: 10.4161/cib.2.1.7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeld P, van Vliet EA, Gorter JA, Lucassen PJ, Fitzsimons CP. Different subsets of newborn granule cells: a possible role in epileptogenesis? Eur J Neurosci. 2014;39:1–11. doi: 10.1111/ejn.12387. [DOI] [PubMed] [Google Scholar]
- Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. 2010;24:881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Brown AS, Vinogradov S, Kremen WS, Poole JH, Deicken RF, Penner JD, McKeague IW, Kochetkova A, Kern D, Schaefer CA. Prenatal exposure to maternal infection and executive dysfunction in adult schizophrenia. Am J Psychiatry. 2009;166:683–690. doi: 10.1176/appi.ajp.2008.08010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd I, Balakrishnan B, Kannan S. Models of fetal brain injury, intrauterine inflammation, and preterm birth. Am J Reprod Immunol. 2012;67:287–294. doi: 10.1111/j.1600-0897.2012.01110.x. [DOI] [PubMed] [Google Scholar]
- Burd I, Bentz AI, Chai J, Gonzalez J, Monnerie H, Le Roux PD, Cohen AS, Yudkoff M, Elovitz MA. Inflammation-induced preterm birth alters neuronal morphology in the mouse fetal brain. J Neurosci Res. 2010a;88:1872–1881. doi: 10.1002/jnr.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd I, Breen K, Friedman A, Chai J, Elovitz MA. Magnesium sulfate reduces inflammation-associated injury in fetal mice. Am J Obstet Gynecol. 2010b;202:292.e1–292.e9. doi: 10.1016/j.ajog.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayre M, Canoll P, Goldman JE. Cell migration in the normal and pathological postnatal mammalian brain. Prog Neurobiol. 2009;88:41–63. doi: 10.1016/j.pneurobio.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KO, Lybrand ZR, Ito N, Brulet R, Tafacory F, Zhang L, Good L, Ure K, Kernie SG, Birnbaum SG, Scharfman HE, Eisch AJ, Hsieh J. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat Commun. 2015;6:6606. doi: 10.1038/ncomms7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro CN, Tsimis M, Burd I. Infections and brain development. Obstet Gynecol Surv. 2015;70:644–655. doi: 10.1097/OGX.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui K, Ashdown H, Luheshi GN, Boksa P. Effects of prenatal immune activation on hippocampal neurogenesis in the rat. Schizophr Res. 2009;113:288–297. doi: 10.1016/j.schres.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Dada T, Rosenzweig JM, Al Shammary M, Firdaus W, Al Rebh S, Borbiev T, Tekes A, Zhang J, Alqahtani E, Mori S, Pletnikov MV, Johnston MV, Burd I. Mouse model of intrauterine inflammation: sex-specific differences in long-term neurologic and immune sequelae. Brain Behav Immun. 2014;38:142–150. doi: 10.1016/j.bbi.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damman O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42:1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Danzer SC. Depression, stress, epilepsy and adult neurogenesis. Exp Neurol. 2012;233:22–32. doi: 10.1016/j.expneurol.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashtipour K, Tran PH, Okazaki MM, Nadler JV, Ribak CE. Ultrastructural features and synaptic connections of hilar ectopic granule cells in the rat dentate gyrus are different from those of granule cells in the granule cell layer. Brain Res. 2001;890:261–271. doi: 10.1016/s0006-8993(00)03119-x. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosc. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depino AM. Early prenatal exposure to LPS results in anxiety- and depression-related behaviors in adulthood. Neuroscience. 2015;299:56–65. doi: 10.1016/j.neuroscience.2015.04.065. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci Biobehav Rev. 2012;36:1597–1608. doi: 10.1016/j.neubiorev.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman LM, Yolken RH, Buka SL, Torrey EF, Cannon TD. Cognitive functioning prior to the onset of psychosis: the role of fetal exposure to serologically determined influenza infection. Biol Psychiatry. 2009;65:1040–1047. doi: 10.1016/j.biopsych.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovitz MA, Brown AG, Breen K, Anton L, Maubert M, Burd I. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int J Dev Neurosci. 2011;29:663–671. doi: 10.1016/j.ijdevneu.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovitz MA, Mrinalini C, Sammel MD. Elucidating the early signal transduction pathways leading to fetal brain injury in preterm birth. Pediatr Res. 2006;59:50–55. doi: 10.1203/01.pdr.0000191141.21932.b6. [DOI] [PubMed] [Google Scholar]
- Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol. 2003;163:2103–2111. doi: 10.1016/S0002-9440(10)63567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar CE, Huang CS, Clarke SG, Houser CR. Increased cell proliferation and granule cell number in the dentate gyrus of protein repair-deficient mice. J Comp Neurol. 2005;493:524–537. doi: 10.1002/cne.20780. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle J, Kanodia R, Kist D, Emamian ES, Patterson PH, Shi L, Sidwell R. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol. 2002;22:25–33. doi: 10.1023/a:1015337611258. [DOI] [PubMed] [Google Scholar]
- Fazzi E, Bova S, Giovenzana A, Signorini S, Uggetti C, Bianchi P. Cognitive visual dysfunctions in preterm children with periventricular leukomalacia. Dev Med Child Neurol. 2009;51:974–981. doi: 10.1111/j.1469-8749.2009.03272.x. [DOI] [PubMed] [Google Scholar]
- Forster E, Tielsch A, Saum B, Weiss KH, Johanssen C, Graus-Porta D, Muller U, Frotscher M. Reelin, disabled 1, and beta 1integrins are required for the formation of the radial glial scaffold in the hippocampus. Proc Natl Acad Sci USA. 2002;99:13178–13183. doi: 10.1073/pnas.202035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaesser B, Spreng RN, McLelland VC, Addis DR, Schacter DL. Imagining the future: evidence for a hippocampal contribution to constructive processing. Hippocampus. 2013;23:1150–1161. doi: 10.1002/hipo.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH, Kempermann G, Song H. Adult Neurogenesis. Cold Spring Harbor Laboratory Press; 2008. [Google Scholar]
- Ghiani CA, Mattan NS, Nobuta H, Malvar JS, Boles J, Ross MG, Waschek JA, Carpenter EM, Fisher RS, de Vellis J. Early effects of lipopolysaccharide-induced inflammation on foetal brain development in rat. ASN Neuro. 2011;3:e00068. doi: 10.1042/AN20110027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Jarskog LF. Exposure to infection and brain development: cytokines in the pathogenesis of schizophrenia. Schizophr Res. 1997;24:365–367. doi: 10.1016/s0920-9964(96)00123-5. [DOI] [PubMed] [Google Scholar]
- Girard S, Sébire H, Brochu ME, Briota S, Sarret P, Sébire G. Postnatal administration of IL-1Ra exerts neuroprotective effects following perinatal inflammation and/or hypoxic-ischemic injuries. Brain Behav Immun. 2012;26:1331–1339. doi: 10.1016/j.bbi.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graciarena M, Depino AM, Pitossi FJ. Prenatal inflammation impairs adult neurogenesis and memory related behavior through persistent hippocampal TGFbeta1 downregulation. Brain Behav Immun. 2010;24:1301–1309. doi: 10.1016/j.bbi.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Graciarena M, Roca V, Mathieu P, Depino AM, Pitossi FJ. Differential vulnerability of adult neurogenesis by adult and prenatal inflammation: role of TGF-beta1. Brain Behav Immun. 2013;34:17–28. doi: 10.1016/j.bbi.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Green HF, Nolan YM. Inflammation and the developing brain: consequences for hippocampal neurogenesis and behavior. Neurosci Biobehav Rev. 2014;40:20–34. doi: 10.1016/j.neubiorev.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Hack M, Taylor HG, Drotar D, Schluchter M, Cartar L, Andreias L, Wilson-Costello D, Klein N. Chronic conditions, functional limitations, and special healthcare needs of school age kids born with extremely low birth weight in the 1990th. JAMA. 2005;294:318–325. doi: 10.1001/jama.294.3.318. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Mallard C, Ferriero DM, Vannucci SJ, Levison SW, Vexler ZS, Gressens P. The role of inflammation in perinatal brain injury. Nat Rev Neurol. 2015;11:192–208. doi: 10.1038/nrneurol.2015.13. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey L, Boksa P. A stereological comparison of GAD67 and reelin expression in the hippocampal stratum oriens of offspring from two mouse models of maternal inflammation during pregnancy. Neuropharmacology. 2012;62:1767–1776. doi: 10.1016/j.neuropharm.2011.11.022. [DOI] [PubMed] [Google Scholar]
- Hester MS, Danzer SC. Hippocampal granule cell pathology in epilepsy – a possible structural basis for epileptic co-morbidities? Epilepsy Behav. 2014;38:105–116. doi: 10.1016/j.yebeh.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho YC, Wang S. Adult neurogenesis is reduced in the dorsal hippocampus of rats displaying learned helpless behavior. Neuroscience. 2010;171:153–161. doi: 10.1016/j.neuroscience.2010.08.062. [DOI] [PubMed] [Google Scholar]
- Howell K, Hopkins N, McLoughlin P. Combined confocal microscopy and stereology: a highly efficient and unbiased approach to quantitative structural measurement in tissues. Exp Physiol. 2002;87:747–756. doi: 10.1113/eph8702477. [DOI] [PubMed] [Google Scholar]
- Hultman CM, Sparén P, Takei N, Murray RM, Cnattingius S. Prenatal and perinatal risk factors for schizophrenia, affective psychosis, and reactive psychosis of early onset: case-control study. BMJ. 1999;318:421–426. doi: 10.1136/bmj.318.7181.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indredavik MS. Extremely preterm children at increased risk of autism spectrum disorders. Evid Based Ment Health. 2010;13:92. doi: 10.1136/ebmh.13.3.92. [DOI] [PubMed] [Google Scholar]
- Jansen M, Wang W, Greco D, Bellenchi GC, di Porzio U, Brown AJ, Ikonen E. What dictates the accumulation of desmosterol in the developing brain? FASEB J. 2013;27:865–870. doi: 10.1096/fj.12-211235. [DOI] [PubMed] [Google Scholar]
- Jiang P, Sun Y, Zhu T, Zhan C, Gu W, Yuan T, Yu H. Endogenous neurogenesis in the hippocampus of developing rat after intrauterine infection. Brain Res. 2012;1459:1–14. doi: 10.1016/j.brainres.2012.03.058. [DOI] [PubMed] [Google Scholar]
- Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Psychiatric disorders in extremely preterm children: longitudinal finding at age 11 years in the EPICure study. L Am Acad Child Adolesc Psychiatry. 2010a;49(543–563):e1. [PubMed] [Google Scholar]
- Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Autism spectrum disorders in extremely preterm children. J Pediatr. 2010b;156(525–531):e2. doi: 10.1016/j.jpeds.2009.10.041. [DOI] [PubMed] [Google Scholar]
- Kannan S, Saadani-Makki F, Muzik O, Chakraborty P, Mangner TJ, Janisse J, Romero R, Chugani DC. Microglial activation in perinatal rabbit brain induced by intrauterine inflammation: detection with 11C-(R)-PK11195 and small-animal PET. J Nucl Med. 2007;48:946–954. doi: 10.2967/jnumed.106.038539. [DOI] [PubMed] [Google Scholar]
- Kelley MH, Wu WW, Lei J, McLane M, Xie H, Hart KD, Pereira L, Burd I, Maylie J. Functional changes in hippocampal synaptic signaling in offspring survivors of a mouse model of intrauterine inflammation. J Neuroinflamm. 2017;14:180–187. doi: 10.1186/s12974-017-0951-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Song H, Gage FH. Neurogenesis in the adult hippocampus. Cold Spring Harb Perspect Biol. 2015;7:018812. doi: 10.1101/cshperspect.a018812. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, Toovey S, Prinssen EP. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol. 2014;10:643–660. doi: 10.1038/nrneurol.2014.187. [DOI] [PubMed] [Google Scholar]
- Környei Z, Gócza E, Rühl R, Orsolits B, Vörös E, Szabó B, Vágovits B, Madarász E. Astroglia-derived retinoic acid is a key factor in glia-induced neurogenesis. FASEB J. 2007;21:2496–2509. doi: 10.1096/fj.06-7756com. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lee JD, Park HJ, Park ES, Oh MK, Park B, Rha DW, Cho SR, Kim EY, Park JY, Kim CH, Kim DG, Park CI. Motor pathway in injury in patients with periventrivular leukomalacia and spastic diplegia. Brain. 2011;134:1199–12110. doi: 10.1093/brain/awr021. [DOI] [PubMed] [Google Scholar]
- Lee MM, Reif A, Schmitt AG. Major depression: a role for hippocampal neurogenesis? Curr Top Behav Neurosci. 2013;14:153–179. doi: 10.1007/7854_2012_226. Review. [DOI] [PubMed] [Google Scholar]
- Lei J, Rosenzweig JM, Mishra MK, Alshehri W, Brancusi F, McLane M, Almalki A, Bahabry R, Arif H, Rozzah R, Alyousif G, Shabi Y, Alhehaily N, Zhong W, Facciabene A, Kannan S, Kannan RM, Burd I. Maternal dendrimer-based therapy for inflammation-induced preterm birth and perinatal brain injury. Sci Rep. 2017;7:6106. doi: 10.1038/s41598-017-06113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner K, Al Shammary M, McLane M, Johnston MV, Elovitz MA, Burd I. IL-1 receptor blockade prevents fetal cortical brain injury but not preterm birth in a mouse model of inflammation-induced preterm birth and perinatal brain injury. Am J Reprod Immunol. 2014;71:418–426. doi: 10.1111/aji.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YL, Wang S. Prenatal lipopolysaccharide exposure increases depression-like behaviors and reduces hippocampal neurogenesis in adult rats. Behav Brain Res. 2014;259:24–34. doi: 10.1016/j.bbr.2013.10.034. [DOI] [PubMed] [Google Scholar]
- Makinson R, Lloyd K, Rayasam A, McKee S, Brown A, Barila G, Grissom N, George R, Marini M, Fabry Z, Elovitz M, Reyes TM. Intrauterine inflammation induces sex-specific effects on neuroinflammation, white matter, and behavior. Brain Behav Immun. 2017 doi: 10.1016/j.bbi.2017.07.016. 2017/07/26 (in publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45:189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- Meyer U. Developmental neuroinflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:20–34. doi: 10.1016/j.pnpbp.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Mouihate A. Prenatal Activation of Toll-Like Receptor-4 Dampens Adult Hippocampal Neurogenesis in An IL-6 Dependent Manner. Front Cell Neurosci. 2016;10:173. doi: 10.3389/fncel.2016.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener. 2011;6:85. doi: 10.1186/1750-1326-6-85. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CE, Bermudez-Hernandez K, Scharfman HE. The influence of ectopic migration of granule cells into the hilus on dentate gyrus-CA3 function. PLoS One. 2013;8:e68208. doi: 10.1371/journal.pone.0068208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsos I, Rees SM, Duncan J, Kramer BW, Harding R, Newnham JP, Moss TJ. Chronic exposure to intra-amniotic lipopolysaccharide affects the ovine fetal brain. J Soc Gynecol Investig. 2006;13:239–247. doi: 10.1016/j.jsgi.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Patel LS, Wenzel HJ, Schwartzkroin PA. Physiological and morphological characterization of dentate granule cells in the p35 knock-out mouse hippocampus: evidence for an epileptic circuit. J Neurosci. 2004;24:9005–9014. doi: 10.1523/JNEUROSCI.2943-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Frankin K. The mouse brain in stereotaxic coordinates. Elsevier; 2013. [Google Scholar]
- Pierce JP, Melton J, Punsoni M, McCloskey DP, Scharfman HE. Mossy fibers are the primary source of afferent input to ectopic granule cells that are born after pilocarpine-induced seizures. Exp Neurol. 2005;196:316–331. doi: 10.1016/j.expneurol.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26:12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnayake U, Quinn TA, Castillo-Melendez M, Dickinson H, Walker DW. Behaviour and hippocampus-specific changes in spiny mouse neonates after treatment of the mother with the viral-mimetic Poly I: C at mid-pregnancy. Brain Behav Immun. 2012;26:1288–1299. doi: 10.1016/j.bbi.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009;14:959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- Saadani-Makki F, Kannan S, Makki M, Muzik O, Janisse J, Romero R, Chugani D. Intrauterine endotoxin administration leads to white matter diffusivity changes in newborn rabbits. J Child Neurol. 2009;24:1179–1189. doi: 10.1177/0883073809338213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, McCloskey D. Ectopic granule cells of the rat dentate gyrus. Dev Neurosci. 2007;29:14–27. doi: 10.1159/000096208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Pierce JP. New insights into the role of hilar ectopic granule cells in the dentate gyrus based on quantitative anatomic analysis and three-dimensional reconstruction. Epilepsia. 2012;53:109–115. doi: 10.1111/j.1528-1167.2012.03480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieve LA, Baio J, Rice CE, Durkin M, Kirby RS, Drews-Botsch C, Miller LA, Nicholas JS, Cunniff CM. Risk for cognitive deficit in a population-based sample of U.S. children with autism spectrum disorders: variation by perinatal health factors. Disabil Health J. 2010;3:202–212. doi: 10.1016/j.dhjo.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behavior. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield BB, Cowan WM. Morphology of the hippocampus and dentate gyrus in normal and reeler mice. J Comp Neurol. 1979;185:393–422. doi: 10.1002/cne.901850302. [DOI] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town T, Nikolic V, Tan J. The microglial “activation” continuum: from innate to adaptive responses. J Neuroinflammation. 2005;2:24. doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallières L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22(2):486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuij LG, Mathai S, Davidson JO, Lear CA, Booth LC, Fraser M, Gunn AJ, Bennet L. Synergistic white matter protection with acute-on-chronic endotoxin and subsequent asphyxia in preterm fetal sheep. J Neuroinflamm. 2014;11:89. doi: 10.1186/1742-2094-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmayr B, Mahlstedt MM, Henn FA. Neurogenesis and depression: what animal models tell us about the link. Eur Arch Psychiatry Clin Neurosci. 2007;257:300–303. doi: 10.1007/s00406-007-0734-2. [DOI] [PubMed] [Google Scholar]
- Williams M, Zhang Z, Nance E, Drewes JL, Lesniak WG, Singh S, Chugani DC, Rangaramanujam K, Graham DR, Kannan S. Maternal inflammation results in altered tryptophan metabolism in rabbit placenta and fetal brain. Dev Neurosci. 2017 doi: 10.1159/000471509. in publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BH, Kim CJ, Romero R, Jun JK, Park KH, Choi ST, Chi JG. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol. 1997;177:797–802. doi: 10.1016/s0002-9378(97)70271-0. [DOI] [PubMed] [Google Scholar]
- Zhan RZ, Timofeeva O, Nadler JV. High ratio of synaptic excitation to synaptic inhibition in hilar ectopic granule cells of pilocarpine-treated rats. J Neurophysiol. 2010;104:3293–3304. doi: 10.1152/jn.00663.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Bassam B, Thomas AG, Williams M, Liu J, Nance E, Rojas C, Slusher BS, Kannan S. Maternal inflammation leads to impaired glutamate homeostasis and up-regulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain. Neurobiol Dis. 2016;94:116–128. doi: 10.1016/j.nbd.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


