Standfirst
In 2016, advances in atherosclerosis research were focused on the discovery and validation of new targets with genetic and mechanistic links to atherothrombotic heart disease. Novel targets include proteins involved in glycoprotein recognition and clearance, regulators of triglyceride-rich particle metabolism, inflammatory pathways that impair efferocytosis, and the gut microbiome.
Extending beyond genomic explorations that have successfully identified proatherosclerotic pathways such as LPA and PCSK9, several landmark papers this year reported on the identification of novel and rare genetic variants that highlight the involvement of additional processes in lipid metabolism which influence atherosclerotic risk (FIG. 1). Nioi and colleagues described genetic determinants that contribute to variations in the level of atherogenic lipoproteins1. Apolipoprotein B-containing particles can be captured globally by measuring non-HDL cholesterol (non-HDLc; calculated by deducting HDL cholesterol from total cholesterol). Non-HDLc is superior to LDL cholesterol in predicting coronary artery disease (CAD) risk, and the year 2016 marked the important discovery of a genetic association between variants affecting nonHDLc and CAD risk, and the protein asialoglycoprotein receptor 1 (ASGR1)1. ASGR1 is a lectin subunit of the transmembrane protein asialoglycoprotein receptor that has a critical role in serum glycoprotein homeostasis. In genetic discovery studies that initially involved approximately 400,000 Icelandic individuals, which were then validated in case–control studies from five European ancestry cohorts of approximately 300,000 individuals, carriers (approximately 1 in 120 Icelandic individuals) of a noncoding 12-basepair deletion in intron 4 of ASGR1 were found to have reduced non-HDLc levels, and an accompanying marked reduction in risk of CAD1. Carriers of this loss-of-function genetic variant had a mean 15mg/dl reduction in non-HDLc, a ~34% reduction in myocardial infarction risk, and a lifespan prolonged by 1.5 years compared with noncarriers1. The mechanisms underlying the pronounced atheroprotective effects of this variant, however, are unclear. This study thus reported that loss-of-function mutations in ASGR1 result in non-HDLc reductions and marked protection from CAD, and identify ASGR1 as a potential therapeutic target for the treatment and/or prevention of CAD1.
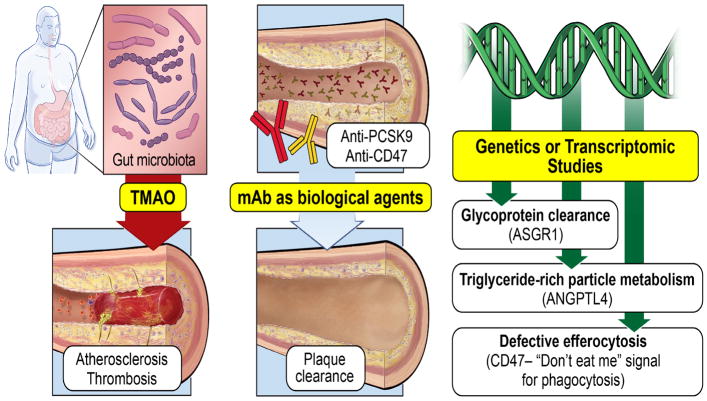
Figure 1. Newly discovered mechanistic pathways leading to development and progression of atherosclerosis.
Large-scale genomic studies revealed rare variants that altered the function of novel proteins in glycoprotein clearance (asialoglycoprotein receptor 1 [ASGR1]), triglyceride-rich particle metabolism (angiopoietin-related protein 4 [ANGPLT4]) and defective efferocytosis (CD47) are linked to coronary artery disease. Gut microbe-associated metabolism of dietary nutrients that are abundant in Western diets lead to the generation of the metabolite trimethylamine N-oxide (TMAO) generation, which is associated with enhanced coronary atherosclerotic plaque burden, heightened platelet responsiveness, and enhanced thrombotic complications. Therapeutic interventions with antibodies against PCSK9 in humans reduces LDL cholesterol levels, inhibits coronary atherosclerosis progression, and promotes plaque regression, whereas anti-CD47 therapy in animal models provides proof-of-concept validation for inhibition of atherosclerosis. mAB, monoclonal antibody.
Hypertriglyceridaemia is another well-established cardiovascular risk factor. The important role of angiopoietin-related protein 4 (ANGPTL4) in modulating triglyceride levels by inhibiting lipoprotein lipase (LPL), and in reducing the risk of CAD were validated in two separate large-scale genomic analyses in 2016 (REFS 2, 3). Carriers of loss-of-function variants of SVEP1 (p.D2702G) and ANGPTL4 (p.E40K) had lower triglyceride levels than noncarriers; these mutations were also associated with protection from CAD2. Furthermore, a loss-of-function variant mutation in LPL (p.D36N) was associated with increased CAD risk, whereas a gain-of-function variant (p.S447*) was associated with reduced risk of CAD2. In an independent investigation reported this year, carriers of the E40K variant and 13 other monoallelic inactivating mutation variants of ANGPTL4 all had lower triglyceride levels and higher HDLc levels than noncarriers, and were less likely to have CAD3. In addition, monoclonal antibody inhibition of the ANGPTL4 protein in both mouse and nonhuman primate models resulted in a reduction in triglyceride levels3.
Despite the strong inverse association between HDLc and CAD risk, results from therapeutic intervention trials targeting HDLc levels have yielded frustratingly negative findings. Perhaps some of the confusion surrounding HDLc was clarified through genomic analyses published this year of rare variants in individuals with high HDLc. Mice with a Scarb1 knockdown have markedly elevated HDLc levels and paradoxically increased risk of atherosclerosis4. Targeted sequencing of coding regions of lipid-modifying genes in 328 individuals with high plasma HDLc levels led to the identification of a homozygote mutation for a loss-of-function variant (P376L) in SCARB1 that impairs post-translational processing of the scavenger receptor class B member 1 protein and abrogates selective HDLc uptake in transfected cells4. Carriers of this SCARB1 variant showed paradoxically elevated HDLc and an increased risk of CAD.
Progress has also been made this year in interventional studies for the treatment of atherosclerosis. Findings from the GLAGOV study5 suggest that PCSK9 inhibition might reverse coronary atherosclerosis via incremental LDL-cholesterol lowering, even in the presence of aggressive statin therapy. Statintreated patients (n=938) with angiographic CAD were prospectively randomly assigned to subcutaneous evolocumab (a PCSK9 inhibitor) or placebo for 76 weeks and assessed by serial intravascular coronary ultrasonography5. Compared with placebo, the evolocumab group had lower LDL-cholesterol levels (as low as 20mg/dl), had a greater decrease in atheroma volume, and showed plaque regression in a greater proportion of patients, without serious adverse effects. Although the study was not powered to assess clinical outcomes, major adverse cardiac events were numerically reduced with evolocumab therapy. These findings suggest that PCSK9 inhibition in the setting of intensive statin therapy can influence plaque progression and foster reversal of atherosclerosis.
Further mechanistic links between inflammation and atherosclerosis have also been explored6. Advanced lesions at risk of rupture are characterized by accumulation of diseased vascular cells and apoptotic cellular debris; why these cells are not cleared within atheroma remains unknown. Upregulation of CD47, an antiphagocytic molecule (a ‘don’t eat me’ signal) known to render malignant cells resistant to programmed cell removal (efferocytosis), occurs during atherogenesis (FIG. 1). Impaired efferocytosis might have a pathogenic role in cardiovascular disease. In a 2016 study, CD47 was upregulated in both human coronary and carotid atherosclerotic vessels, presumably via a tumour necrosis factor (TNF)-α and NFκB1-dependent process that results in inhibition of efferocytosis6. Moreover, administration of CD47-blocking antibodies reversed the defect in efferocytosis, normalized the clearance of diseased vascular tissue, and ameliorated atherosclerosis in multiple mouse models through the TNFα-driven programmed cell removal process6.
Multiple advances were reported this year on the role of the gut microbiome in atherothrombosis. A link between gut microorganisms and atherosclerotic plaque development was first established in 2011, in which untargeted metabolomic and functional studies led to the discovery of the atherogenic metabolite trimethylamine N-oxide (TMAO)7. In a 2016 study of patients with stable CAD undergoing coronary angiography and plaque characterization, elevated plasma level of TMAO was shown to predict coronary atherosclerotic burden more powerfully than traditional cardiac risk factors8. Microbial production of TMAO was also reported to serve as a novel mechanism linking diet and specific gut microbiota with induction of heightened platelet responsiveness and enhanced thrombosis potential in vivo in both humans and other animals9. Initial clinical studies (n = 4,007) reported that plasma levels of TMAO independently predicted the risk of thrombotic events, and subsequent mechanistic studies showed that TMAO enhanced submaximal stimulus-dependent platelet activation by multiple agonists through augmented Ca2+ release from intracellular stores9. Complementary animal-model studies employing dietary choline or TMAO, germ-free mice, and microbial transplantation collectively confirmed a role for gut microbiota and TMAO in modulating platelet hyperresponsiveness and thrombosis potential in vivo, and identified microbial taxa associated with plasma TMAO and thrombosis potential9. In another study, nonlethal inhibition of the rate-limiting microbial enzyme involved in generating TMAO from dietary choline was also shown to serve as a novel approach for the treatment of atherosclerosis10. These studies highlight the potential of targeting TMAO levels for the prevention and treatment of atherothrombosis.
In summary, numerous notable advances in atherosclerosis research were made in 2016. The power of genomics and other ‘omics’ technologies for discovery of new pathways linked to CAD development has begun to be realized, and has the potential to expand the number of therapeutic targets for atherosclerosis.
Key advances.
Asialoglycoprotein receptor 1 (ASGR1) can regulate non-HDL-cholesterol levels and influence the risk of coronary artery disease (CAD)1
Variants in the ANGPTL4 gene are linked to hypertriglyceridaemia and CAD risk2,3
PCSK9 inhibition in the setting of intensive statin therapy can decrease LDL-cholesterol levels, reduce coronary atherosclerotic plaque progression, and induce significant reversal of atherosclerosis5
An increase in the gut microorganism-dependent metabolite trimethylamine N-oxide is associated with coronary atherosclerotic burden, and linked to heightened platelet responsiveness and thrombosis potential8,9
Acknowledgments
W.H.W.T. and S.L.H. are supported by grants from the National Institutes of Health and the Office of Dietary Supplements (R01HL103866, P20HL113452, R01DK106000, R01HL126827).
Biographies
Dr. Hazen is chair of the Department of Cellular & Molecular Medicine, and section head of Preventive Cardiology at the Cleveland Clinic. A practicing physician and scientist, his research focuses on elucidation of biochemical mechanisms operative in atherosclerosis other inflammatory diseases.
Dr. Tang is the director of the Center for Clinical Genomics at the Cleveland Clinic. A cardiologist and clinician scientist, he specializes in heart failure/transplant, cardio-renal diseases, and genomic and metabolomic studies to enumerate contributors to cardiac diseases.
Footnotes
Competing interests statement
S.L.H. is named as inventor on pending patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. He is also a paid consultant for Esperion and P&G, and has received research funds from Astra Zeneca, P&G, Pfizer Inc., Roche Diagnostics, and Takeda. S.L.H. has also received royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland HeartLab, Esperion, Frantz Biomarkers, LLC, and Siemens. W.H.W.T. declares no competing interests.
References
- 1.Nioi P, et al. Variant ASGR1 Associated with a Reduced Risk of Coronary Artery Disease. N Engl J Med. 2016;374:2131–2141. doi: 10.1056/NEJMoa1508419. [DOI] [PubMed] [Google Scholar]
- 2.Stitziel NO, et al. Coding Variation in ANGPTL4, LPL, and SVEP1 and the Risk of Coronary Disease. N Engl J Med. 2016;374:1134–1144. doi: 10.1056/NEJMoa1507652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dewey FE, et al. Inactivating Variants in ANGPTL4 and Risk of Coronary Artery Disease. N Engl J Med. 2016;374:1123–1133. doi: 10.1056/NEJMoa1510926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zanoni P, et al. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016;351:1166–1171. doi: 10.1126/science.aad3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholls SJ, et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. Jama. 2016 doi: 10.1001/jama.2016.16951. [DOI] [PubMed] [Google Scholar]
- 6.Kojima Y, et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536:86–90. doi: 10.1038/nature18935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Senthong V, et al. Plasma Trimethylamine N-Oxide, a Gut Microbe-Generated Phosphatidylcholine Metabolite, Is Associated With Atherosclerotic Burden. J Am Coll Cardiol. 2016;67:2620–2628. doi: 10.1016/j.jacc.2016.03.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu W, et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]