Abstract
Poultry carry zoonotic bacteria that can cause gastroenteritis in humans. Environmental transmission of pathogens from poultry operations may increase gastrointestinal infection risk in surrounding communities. To evaluate associations between residential proximity to high-density poultry operations and individual-level diarrheal illnesses, we conducted a nested case-control study among 514,488 patients in Pennsylvania (2006–2015). Using electronic health records, we identified cases of five gastrointestinal outcomes: three pathogen-specific infections, including Escherichia coli (n = 1425), Campylobacter (n = 567), and Salmonella (n = 781); infectious diarrhea (n = 781); and non-specific diarrhea (2012–2015; n = 28,201). We estimated an inverse-distance squared activity metric for poultry operations based on farm and patient addresses. Patients in the second and fourth (versus first) quartiles of the poultry operation activity metric had increased odds of Campylobacter (aOR [CI], Q2: 1.36 [1.01, 1.82]; Q3: 1.38 [0.98, 1.96]; Q4: 1.75 [1.31, 2.33]). Patients in the second, third, and fourth quartiles had increased odds of infectious diarrhea (Q2: 1.76 [1.29, 2.39]; Q3: 1.76 [1.09, 2.85]; Q4: 1.60 (1.12, 2.30]). Stratification revealed stronger relations of fourth quartile and both Campylobacter and infectious diarrhea in townships, the most rural community type in the study geography. Increasing extreme rainfall in the week prior to diagnosis strengthened fourth quartile Campylobacter associations. The poultry operation activity metric was largely unassociated with E. coli, Salmonella, and non-specific diarrhea. Findings suggest high-density poultry operations may be associated with campylobacteriosis and infectious diarrhea in nearby communities, highlighting additional public health concerns of industrial agriculture.
INTRODUCTION
Poultry are reservoirs of several zoonotic bacteria that cause acute gastroenteritis in humans, including Campylobacter, Salmonella, Escherichia coli, and Listeria (Berghaus et al. 2012; Blaak et al. 2015; Dahshan et al. 2016; Lee et al. 2016; Sahin et al. 2015). Among the most common causes of foodborne illness in the U.S., these pathogens cause significant morbidity and mortality, with serious sequelae such as Guillain-Barré syndrome (Campylobacter), reactive arthritis and irritable bowel syndrome (Campylobacter and Salmonella), end-stage renal disease (E. coli), and pre-term labor and fetal infection (Listeria) (Humphrey et al. 2007; Scallan et al. 2015). The risk of illness due to foodborne transmission of pathogenic bacteria from poultry meat is well documented (Batz et al. 2012). Environmental transmission of these pathogens from poultry operations to humans presents an additional, though far less-studied risk.
Research has previously linked industrial food animal production (IFAP)—which is characterized by large, homogeneous, and densely packed livestock operations—to increased risk of zoonotic diseases in nearby communities (Casey et al. 2015). In terms of zoonotic bacteria associated with gastroenteritis, a case-control study conducted in counties with high cattle density found that living or working on a dairy farm was positively associated with campylobacteriosis, with strong overlap between human and bovine bacterial isolates (Davis et al. 2013). A study conducted across multiple states that linked Campylobacter cases with socioeconomic and environmental data by zip code found that in top poultry and dairy producing regions, campylobacteriosis incidence rates were significantly higher in zip codes with broiler operations or dairy operations compared to zip codes without operations (Rosenberg Goldstein et al. 2016). Other ecological studies using disease surveillance data have reported associations of farm animal density and Campylobacter, and the percent of the population living on a farm with risk of E. coli infection (Chang et al. 2009; Green et al. 2006). Studies have also found that rural residents living in areas with swine or dairy IFAP experience greater occurrence of diarrhea as measured by interviews with area residents (Arnold 1999; Wing and Wolf 2000), although these studies did not assess specific pathogens and are subject to recall bias.
While studies have shown living or working on a poultry farm and contact with live poultry to be a risk factor for Campylobacter and antibiotic-resistant E. coli infection (Davis et al. 2013; Price et al. 2007; Studahl and Andersson 2000; Thorsteinsdottir et al. 2010; Wilson 2004), individual-level associations of poultry operations with risk of human infections in surrounding communities are largely unstudied. Environmental contamination from poultry operations has the potential to spread pathogens to nearby communities (Jonsson et al. 2010). Bacterial pathogens colonize animals at an early age and spread quickly through a flock (Blaak et al. 2015; Friese et al. 2013; Hermans et al. 2012; Sahin et al. 2015). From poultry houses, bacteria enter the community environment via aerosolized particles or in dust emitted through ventilation fans, through pests such as flies, and through land-disposal of poultry waste (Blaak et al. 2014; Blaak et al. 2015; Bull et al. 2006; Friese et al. 2013; Graham et al. 2009a; Graham et al. 2009b; Skora et al. 2016). Heavy rainfall can facilitate further transport of pathogens into surface and groundwater and is independently associated with gastrointestinal illness (Gleason and Fagliano 2017; Levy et al. 2016).
Given the limited research related to poultry IFAP and risk of relevant human infections in surrounding communities, the aim of this study was to evaluate associations between residential proximity to poultry operations and individual-level diarrheal illnesses. We conducted a case-control study of the association between residential proximity to poultry operations and five gastrointestinal outcomes. While past research utilized gravity models to analyze zoonotic disease risk in swine and bovine operations (e.g. Casey et al. 2013), to our knowledge this is the first study to use this geospatial method to assess infectious disease risks related to poultry IFAP and to evaluate associations of IFAP with gastrointestinal outcomes. We evaluated three pathogen-specific intestinal infection diagnoses that have been linked to poultry operations: E. coli, Campylobacter, and Salmonella. In addition, since the majority of patients seeking medical care for diarrhea are not tested for specific pathogens (Scallan et al. 2005), we sought to ascertain the relation of poultry operation proximity to less severe or persistent diarrheal illnesses by evaluating two other diagnoses, specifically infectious and non-specific diarrhea.
METHODS
Study Population
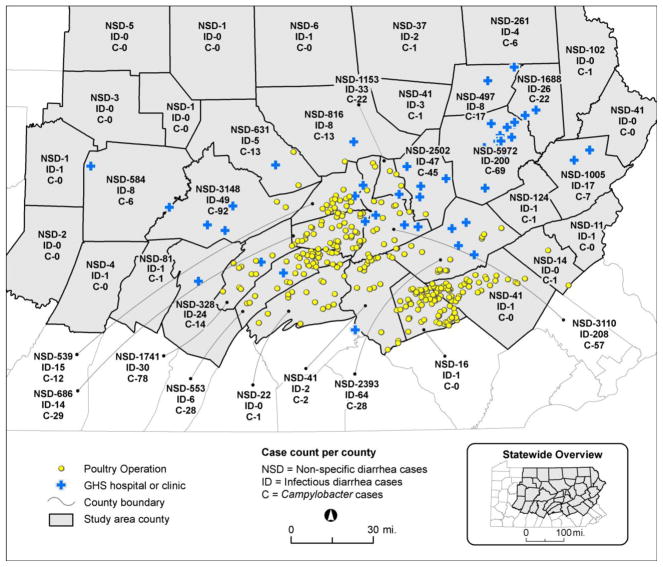
Using electronic health record (EHR) data, we identified child and adult patients with one of five gastrointestinal outcomes from the Geisinger Clinic, an integrated health system in Pennsylvania, USA. Geisinger primary care patients represent the age and sex distribution of the general population in central and northeastern Pennsylvania (J. A. Casey et al. 2016). The study area comprised 38 counties in Pennsylvania, including the health system’s primary care market and bordering counties (Figure 1). The latitude and longitude of patients’ addresses were geocoded using ArcGIS version 10.1 (Esri, Redlands, CA). For four of our study outcomes, we utilized EHR data from 514,488 patients who had contact with Geisinger from January 2006 to July 2015; for non-specific diarrhea, we limited data to 455,364 patients with contact from January 2012 to July 2015 due to inconsistencies in coding prior to 2012. The Geisinger Institutional Review Board approved the study and waived informed consent.
Figure 1.
Map of study area. Numbers within borders of each county indicate the number of cases of non-specific diarrhea, infectious diarrhea, and Campylobacter. Yellow circles show locations of poultry operations based on 2015 NMP data. Map created with ArcGIS (10.1, Esri, Redlands, CA).
Case Ascertainment and Control Selection
We identified cases from the five diagnostic groups using Geisinger system diagnostic codes and text descriptions cross-linked to International Classification of Diseases (ICD, 9th and 10th Revision) codes from outpatient, emergency department, and medication records. Indications from medication records were used to identify cases with potential call-in orders for diarrhea not associated with a patient encounter and to enhance incomplete coding from encounters. Diagnoses were identified as follows (ICD-9 codes, ICD-10 codes not documented as they were internally converted to ICD-9 during data extraction): intestinal infection related to Salmonella (003.0), E.coli (008.0, 008.00, 008.09, 041.4, 041.41, 041.49), or Campylobacter (008.43), infectious diarrhea/bacterial enteritis (008.5, 009.2, 009.3, excluding codes with text descriptions indicating travelers’ diarrhea), and non-specific diarrhea (787.91, 564.5). We used positive fecal sample laboratory test results to identify Salmonella and Campylobacter cases in addition to those identified through codes; negative test results received one week before or after a diagnostic code were used to exclude patients as cases. For Salmonella and Campylobacter cases, 28% and 36% had both a code and positive laboratory test, 25% and 19% had a code only, and 47% and 45% had a laboratory test only, respectively. There were an insufficient number of Listeria cases to evaluate. Patients with an inpatient diagnosis for infectious diarrhea and non-specific diarrhea were excluded as potential cases for one year following diagnosis to avoid counting hospital-acquired infections as cases. Given the ambiguity of the non-specific diarrhea ICD-9 code, we used additional exclusion criteria for these cases. Specifically, we did not include cases with text descriptions for “frequent defecation” or “frequent stool,” and we excluded cases for one-year after EHR notations for causes of non-infectious diarrhea (e.g., “chemotherapy-induced diarrhea,” “antibiotic-associated diarrhea”) or if there was a diagnosis for an acute condition associated with diarrhea (i.e., cholera, typhoid, Norwalk virus, receiving chemotherapy). All outcome analyses only included first incident cases. For all outcomes except non-specific diarrhea, 4% or less of cases had repeat diagnoses in subsequent years; 10% of non-specific diarrhea cases had repeat diagnoses, most of which had just one repeat diagnosis.
We randomly selected outpatient controls with no history of diarrhea diagnoses and frequency-matched them to cases by age category (< 1, 1, 2–4, 5–9, 10–19, 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, 80–99, ≥ 100), sex, and year of encounter. If a control had multiple encounters in a year, one encounter was randomly selected for the activity metric assignment date.
Poultry Operation Data
Livestock operations are required to develop a Nutrient Management Plan (NMP) for manure handling if they exceed two animal equivalent units (AEUs, 1000 pounds of live weight on an annualized basis) per acre and have greater than eight AEUs (per Pennsylvania Act 38), or if they exceed 1000 total AEUs (per U.S. Clean Water Act). Operations can also voluntarily develop an NMP. NMPs provide information on livestock operation location and animal type and quantity. We obtained NMPs for poultry operations in the 38-county study area from County Conservation Districts. We located the latitude and longitude of poultry operations using Google Earth with visual confirmation of a poultry house on-site.
The study area contained five types of poultry operation: broilers (chickens raised for meat), layers (egg-laying hens), pullets (young hens produced for breeding operations), turkeys, and ducks. Poultry are reared in large flocks (e.g., approximately 20,000 broilers per flock) within grow-out houses and are produced in cycles throughout the year. Given their predominance in the poultry industry, research on zoonotic pathogens present in poultry operations generally focuses on chickens; however, there is evidence that bacteria such as E. coli, Campylobacter, and Salmonella are also prevalent in turkeys and ducks (Adzitey et al. 2012a; Adzitey et al. 2012b; Sahin et al. 2015; Siemer et al. 2004).
Poultry Operation Activity Metric Assignment
The poultry operation activity metric was calculated based on previously reported methods (Casey et al. 2013; Rasmussen et al. 2016) and took into account the total number of poultry operations in the study area, the distance between each patient’s residence and poultry operations, and the number (in AEUs) of poultry at each operation. We estimated the metric using inverse distance-squared gravity measures (Talen and Anselin 1998) in R version 3.3.1 (R Foundation for Statistical Computing):
where n was the number of operations in the 38-county region, ai was AEUs of poultry at operation i, and dij2 was the squared-distance (m2) between operation i and patient j. The activity metric was not normally distributed and was highly skewed, so was modeled as quartiles, with quartile one representing the lowest activity (and the reference category) and quartile four representing the highest activity.
Covariates
Demographic covariates were obtained from the EHR, including sex, age at diagnosis/encounter, race/ethnicity (non-Hispanic white versus all others), history of Medical Assistance (a proxy for low family socioeconomic status (Schwartz et al. 2014)), and smoking status (former, current, never, missing). Clinical covariates included an indicator (yes vs. no) for an antibiotic order in the EHR (outpatient records for the full study period, inpatient records starting in 2008) in the 30 and 60 days prior to diagnosis/encounter and another indicator for diagnosis with at least one of three gastrointestinal conditions, specifically Crohn’s disease (555.x), ulcerative colitis (556.x), and irritable bowel syndrome (564.1). Diagnosis with these gastrointestinal conditions was evaluated because they cause diarrhea and prior research has suggested that conditions resulting in inflammation of the gut may increase vulnerability to gastrointestinal infections (Mann and Saeed 2012). Covariates created using patients’ geocoded addresses included continuous distance (meters) between patient residence and the closest Geisinger facility, community type, and community socioeconomic deprivation. We hypothesized that distance to a Geisinger facility might influence care seeking for diarrhea differentially by location of residence. Community type was defined using a mixed definition of place that incorporated minor civil divisions (township, borough, city) and census tracts within cities; townships are the least population dense and range from rural to suburban, boroughs represent walkable small towns, and cities are the most population dense (Schwartz et al. 2011). Community socioeconomic deprivation, a factor previously associated with hospital admissions for gastrointestinal illness (Olowokure et al. 1999; Pockett et al. 2011), was assigned to communities and was based on a factor analysis of six indicators derived from the 2000 U.S. Census (proportion of population in poverty, unemployed, receiving public assistance, with less than a high school education, not in the labor force, and proportion of households without a car) (Nau et al. 2015). Weather-related covariates included season of diagnosis (defined as winter [December-February], spring [(March-May], summer [June-August], and fall [September-November]), average maximum temperature, total precipitation (mm), and number of extreme precipitation events (precipitation > 90th percentile for the 10-year study period [i.e., > 10.9 mm]) in the week prior to diagnosis/encounter. The week prior was chosen to approximate the incubation period of the assessed pathogens. Temperature and precipitation data came from the weather station closest to each patient’s residence and was obtained from the National Climatic Data Center (NOAA 2016).
Statistical Analysis
The goal of the analysis was to evaluate associations between the poultry operation activity metric and the five diagnostic groups representing specific gastrointestinal infections, infectious diarrhea, and non-specific diarrhea. We used logistic regression with robust standard errors clustered by community. Models were adjusted for sex, age, race/ethnicity, Medical Assistance, and smoking status (a risk factor for gastrointestinal disorders (Li et al. 2014)). Several additional covariates were assessed as potential confounders, including season of diagnosis/encounter, distance to closest Geisinger facility, community socioeconomic deprivation, prior antibiotic use, and diagnosis with a gastrointestinal condition (i.e., inflammatory and irritable bowel diagnoses). We repeated regression models for the five gastrointestinal outcomes stratified by community type to assess potential confounding by community type, as prior research in the study area has demonstrated that patients differ on individual-level and place-level variables by community type (J. A. Casey et al. 2016; Nau et al. 2015).
For models with significant associations for the poultry metric with gastrointestinal outcomes, we evaluated effect modification by several variables. We evaluated effect modification variables one at a time by including a cross-product term of the interacting variable with the poultry operation activity metric. Odds ratios and confidence intervals were calculated for poultry operation activity metric/outcome associations in models with statistically significant cross-product terms. Variables evaluated for effect modification included age, given the higher prevalence of diarrhea in young children and older adults (Scallan et al. 2005); Medical Assistance status, because socioeconomic status has been previously associated with gastrointestinal illness (Rose et al. 2016); prior antibiotic use, which can increase the vulnerability of the gut to bacterial infection and diarrhea due to alterations in the gut microbiota (Becattini et al. 2016); season of diagnosis/encounter; average maximum temperature; total precipitation; and extreme precipitation. Season and weather-related variables were evaluated because the incidence of some gastrointestinal illnesses fluctuate by seasonal factors such as temperature (Carlton et al. 2016) and extreme weather events, including heavy rainfall, have been reported to increase the risk of water-borne diseases like infectious diarrhea (Curriero et al. 2001; Gleason and Fagliano 2017).
Results are reported as adjusted odds ratios (aOR) with 95% confidence intervals (CI) and P values. Results were considered significant at P < 0.05 (two-tailed). We used Stata version 14.0 (StataCorp, LP, College Station, TX) for data analyses.
RESULTS
Description of Cases, Controls, and Poultry Operations
We identified 28,201 incident cases of non-specific diarrhea between 2012 and 2015, and 781 cases of infectious diarrhea, 1425 cases of E. coli-related intestinal infection, 567 cases of Campylobacter-related intestinal infection, and 293 cases of Salmonella gastroenteritis between 2006 and 2015 (Tables 1a and 1b). Cases overlapped across gastrointestinal outcomes, particularly between non-specific diarrhea and other case types; between 19.8% and 42.0% of the more specific diagnoses were also identified among patients with non-specific diarrhea diagnoses. Reflecting the Geisinger patient population, the majority of patients for all case types were non-Hispanic white and lived in townships. Across case types, a higher proportion of cases than controls had a history of Medical Assistance, and had an antibiotic order in the 30 days prior to diagnosis/encounter. Campylobacter and Salmonella diagnoses were substantially higher in the summer compared to other seasons.
Table 1a.
Patient demographic and clinical characteristics for non-specific and infectious diarrhea diagnoses
| Characteristic | No. (%) unless specified | |||
|---|---|---|---|---|
|
| ||||
| Non-specific Diarrhea | Infectious Diarrhea | |||
| Cases | Controls | Cases | Controls | |
| n = 28201 | n = 84603 | n = 781 | n = 3901 | |
|
| ||||
| Male sex | 11330 (40.2) | 33987 (40.2) | 342 (43.8) | 1707 (43.8) |
|
| ||||
| Age at diagnosis, median (IQR) | 37 (14, 60) | 37 (14, 60) | 43 (23, 59) | 43 (23, 59) |
|
| ||||
| 0–4 | 4433 (15.7) | 13299 (15.7) | 79 (10.1) | 393 (10.1) |
|
| ||||
| 5–12 | 2242 (8.0) | 7041 (8.3) | 40 (5.1) | 216 (5.5) |
|
| ||||
| 13–18 | 1721 (6.1) | 5000 (5.9) | 36 (4.6) | 182 (4.7) |
|
| ||||
| 19–44 | 7815 (27.7) | 23285 (27.5) | 253 (32.4) | 261 (32.3) |
|
| ||||
| 45–61 | 5585 (19.8) | 16924 (20) | 208 (26.6) | 1052 (27.0) |
|
| ||||
| 62–74 | 3658 (13.0) | 11039 (13.1) | 105 (13.4) | 491 (12.6) |
|
| ||||
| ≥ 75 | 2747 (9.7) | 8015 (9.5) | 60 (7.7) | 306 (7.8) |
|
| ||||
| Diagnosis/encounter year | ||||
|
| ||||
| 2006 | --- | --- | 86 (11.0) | 426 (10.9) |
|
| ||||
| 2007 | --- | --- | 87 (11.1) | 432 (11.1) |
|
| ||||
| 2008 | --- | --- | 97 (12.4) | 481 (12.3) |
|
| ||||
| 2009 | --- | --- | 99 (12.7) | 503 (12.9) |
|
| ||||
| 2010 | --- | --- | 90 (11.5) | 454 (11.6) |
|
| ||||
| 2011 | --- | --- | 92 (11.8) | 460 (11.8) |
|
| ||||
| 2012 | 8698 (30.8) | 26094 (30.8) | 64 (8.2) | 316 (8.1) |
|
| ||||
| 2013 | 8307 (29.5) | 24921 (29.5) | 81 (10.4) | 405 (10.4) |
|
| ||||
| 2014 | 7428 (26.3) | 22284 (26.3) | 65 (8.3) | 324 (8.3) |
|
| ||||
| 2015 | 3768 (13.4) | 11304 (13.4) | 20 (2.6) | 100 (2.6) |
|
| ||||
| Race/ethnicity | ||||
|
| ||||
| Non-Hispanic White | 26024 (92.3) | 77793 (92.0) | 748 (95.8) | 3663 (93.9) |
|
| ||||
| Black | 764 (2.7) | 2469 (2.9) | 9 (1.2) | 87 (2.2) |
|
| ||||
| Hispanic | 1206 (4.3) | 3322 (3.9) | 17 (2.2) | 105 (2.7) |
|
| ||||
| Other | 190 (0.7) | 901 (1.1) | 6 (0.8) | 33 (0.9) |
|
| ||||
| Missing | 17 (0.1) | 118 (0.1) | 1 (0.1) | 13 (0.3) |
|
| ||||
| Smoking status | ||||
|
| ||||
| Current smoker | 4396 (15.6) | 11064 (13.1) | 131 (16.8) | 572 (14.7) |
|
| ||||
| Former smoker | 6077 (21.6) | 15281 (18.1) | 179 (22.9) | 773 (19.8) |
|
| ||||
| Never smoked | 13241 (47.0) | 39975 (47.3) | 359 (46.0) | 1645 (42.2) |
|
| ||||
| Missing | 4487 (15.9) | 18283 (21.6) | 112 (14.3) | 911 (23.4) |
|
| ||||
| History of Medical Assistance | 7469 (26.5) | 15188 (18.0) | 142 (18.2) | 587 (15.1) |
|
| ||||
| Antibiotic prescription in 30 days prior to diagnosis/encounter | 4133 (14.7) | 5830 (6.9) | 128 (16.4) | 262 (6.7) |
|
| ||||
| Diagnosis/encounter season | ||||
|
| ||||
| Winter | 7638 (27.1) | 22471 (26.6) | 175 (22.4) | 1009 (25.9) |
|
| ||||
| Spring | 9062 (32.1) | 23697 (28.0) | 213 (27.3) | 948 (24.3) |
|
| ||||
| Summer | 6079 (21.6) | 18441 (21.8) | 223 (28.6) | 901 (23.1) |
|
| ||||
| Fall | 5422 (19.2) | 19994 (23.6) | 170 (21.8) | 1043 (26.7) |
|
| ||||
| No. unique counties | 38 | 38 | 29 | 35 |
|
| ||||
| No. unique communities | 851 | 1046 | 294 | 613 |
|
| ||||
| Community type | ||||
|
| ||||
| City | 3709 (13.2) | 9871 (11.7) | 109 (14.0) | 372 (9.5) |
|
| ||||
| Borough | 8882 (31.5) | 25081 (29.7) | 251 (32.1) | 1115 (28.6) |
|
| ||||
| Township | 15459 (54.8) | 49293 (58.3) | 419 (53.7) | 2396 (61.4) |
|
| ||||
| Missing | 151 (0.5) | 358 (0.4) | 2 (0.3) | 18 (0.5) |
|
| ||||
| Community socioeconomic deprivationa, SD units, median (IQR) | −0.4 (−2.56, 2.33) | −0.62 (−2.72, 2.07) | 0.17 (−2.42, 3.54) | −0.83 (−2.96, 1.38) |
Abbreviations: IQR, interquartile range
Based on six U.S. Census indicators as described in the Methods.
Table 1b.
Patient demographic and clinical characteristics for pathogen-specific diagnoses
| Characteristic | No. (%) unless specified | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| E. coli | Campylobacter | Salmonella | ||||
| Cases | Controls | Cases | Controls | Cases | Controls | |
| n = 1425 | n = 7038 | n = 567 | n = 2818 | n = 293 | n = 1460 | |
|
| ||||||
| Male sex | 338 (23.7) | 1677 (23.8) | 300 (52.9) | 1489 (52.8) | 132 (45.1) | 658 (45.1) |
|
| ||||||
| Age at diagnosis, median (IQR) | 60 (30, 77) | 59 (31, 75) | 38 (16, 55) | 37 (14, 55) | 27 (5, 50) | 27 (5, 50) |
|
| ||||||
| 0–4 | 112 (7.9) | 559 (7.9) | 80 (14.1) | 398 (14.1) | 69 (23.6) | 343 (23.5) |
|
| ||||||
| 5–12 | 49 (3.4) | 284 (4.0) | 46 (8.1) | 260 (9.2) | 40 (13.7) | 166 (11.4) |
|
| ||||||
| 13–18 | 53 (3.7) | 258 (3.7) | 35 (6.2) | 164 (5.8) | 11 (3.8) | 86 (5.9) |
|
| ||||||
| 19–44 | 275 (19.3) | 1377 (19.6) | 177 (31.2) | 847 (30.1) | 77 (26.3) | 397 (27.2) |
|
| ||||||
| 45–61 | 257 (18.0) | 1250 (17.8) | 137 (24.2) | 685 (24.3) | 54 (18.4) | 257 (17.6) |
|
| ||||||
| 62–74 | 268 (18.8) | 1484 (21.1) | 76 (13.4) | 371 (13.2) | 28 (9.6) | 129 (8.8) |
|
| ||||||
| ≥ 75 | 411 (28.8) | 1826 (25.9) | 16 (2.8) | 93 (3.3) | 14 (4.8) | 82 (5.6) |
|
| ||||||
| Diagnosis/encounter year | ||||||
|
| ||||||
| 2006 | 111 (7.8) | 534 (7.6) | 28 (4.9) | 139 (4.9) | 34 (11.6) | 170 (11.6) |
|
| ||||||
| 2007 | 145 (10.2) | 717 (10.2) | 23 (4.1) | 115 (4.1) | 31 (10.6) | 155 (10.6) |
|
| ||||||
| 2008 | 131 (9.2) | 643 (9.1) | 47 (8.3) | 235 (8.3) | 40 (13.7) | 198 (13.6) |
|
| ||||||
| 2009 | 135 (9.5) | 667 (9.5) | 56 (9.9) | 277 (9.8) | 30 (10.2) | 150 (10.3) |
|
| ||||||
| 2010 | 138 (9.7) | 687 (9.8) | 54 (9.5) | 267 (9.5) | 22 (7.5) | 108 (7.4) |
|
| ||||||
| 2011 | 121 (8.5) | 587 (8.3) | 90 (15.9) | 445 (15.8) | 28 (9.6) | 140 (9.6) |
|
| ||||||
| 2012 | 124 (8.7) | 614 (8.7) | 70 (12.4) | 350 (12.4) | 30 (10.2) | 149 (10.2) |
|
| ||||||
| 2013 | 240 (16.8) | 1193 (17.0) | 93 (16.4) | 462 (16.4) | 30 (10.2) | 150 (10.3) |
|
| ||||||
| 2014 | 190 (13.3) | 946 (13.4) | 85 (15.0) | 423 (15.0) | 36 (12.3) | 180 (12.3) |
|
| ||||||
| 2015 | 90 (6.3) | 450 (6.4) | 21 (3.7) | 105 (3.7) | 12 (4.1) | 60 (4.1) |
|
| ||||||
| Race/ethnicity | ||||||
|
| ||||||
| Non-Hispanic White | 1326 (93.1) | 6664 (94.7) | 545 (96.1) | 2619 (92.9) | 278 (94.9) | 1329 (91.0) |
|
| ||||||
| Black | 32 (2.3) | 139 (2.0) | 5 (0.9) | 70 (2.5) | 7 (3.2) | 47 (3.2) |
|
| ||||||
| Hispanic | 54 (3.8) | 172 (2.4) | 14 (2.5) | 91 (3.2) | 6 (2.1) | 56 (3.8) |
|
| ||||||
| Other | 11 (0.8) | 53 (0.8) | 3 (0.5) | 31 (1.1) | 2 (0.7) | 22 (1.5) |
|
| ||||||
| Missing | 2 (0.1) | 10 (0.1) | 0 (0.0) | 7 (0.3) | 0 (0.0) | 6 (0.4) |
|
| ||||||
| Smoking status | ||||||
|
| ||||||
| Current smoker | 169 (11.9) | 781 (11.1) | 67 (11.8) | 367 (13.0) | 36 (12.3) | 183 (12.5) |
|
| ||||||
| Former smoker | 417 (29.3) | 1630 (23.2) | 116 (20.5) | 495 (17.6) | 49 (16.7) | 189 (13.0) |
|
| ||||||
| Never smoked | 632 (44.4) | 3413 (48.5) | 266 (46.9) | 1210 (42.9) | 128 (43.7) | 545 (37.3) |
|
| ||||||
| Missing | 207 (14.5) | 1214 (17.3) | 118 (20.8) | 746 (26.5) | 80 (27.3) | 543 (37.2) |
|
| ||||||
| History of Medical Assistance | 304 (21.4) | 789 (11.2) | 122 (21.5) | 483 (17.1) | 73 (24.9) | 293 (20.1) |
|
| ||||||
| Antibiotic prescription in 30 days prior to diagnosis/encounter | 393 (27.6) | 508 (7.2) | 153 (27.0) | 178 (6.3) | 83 (28.3) | 84 (5.8) |
|
| ||||||
| Diagnosis/encounter season | ||||||
|
| ||||||
| Winter | 363 (25.5) | 1825 (25.9) | 86 (15.2) | 767 (27.2) | 54 (18.4) | 351 (24.0) |
|
| ||||||
| Spring | 367 (25.8) | 1821 (25.9) | 102 (18.0) | 680 (24.1) | 70 (23.9) | 352 (24.1) |
|
| ||||||
| Summer | 347 (24.4) | 1594 (22.7) | 231 (40.7) | 602 (21.4) | 127 (43.3) | 352 (24.1) |
|
| ||||||
| Fall | 348 (24.4) | 1798 (25.6) | 148 (26.1) | 769 (27.3) | 42 (14.3) | 405 (27.7) |
|
| ||||||
| No. unique counties | 29 | 35 | 26 | 31 | 23 | 32 |
|
| ||||||
| No. unique communities | 378 | 675 | 280 | 556 | 185 | 464 |
|
| ||||||
| Community type | ||||||
|
| ||||||
| City | 196 (13.8) | 679 (9.7) | 38 (6.7) | 303 (10.8) | 26 (8.9) | 177 (12.1) |
|
| ||||||
| Borough | 460 (32.3) | 2104 (29.9) | 147 (25.9) | 846 (30.0) | 89 (30.4) | 429 (29.4) |
|
| ||||||
| Township | 763 (53.5) | 4215 (59.9) | 381 (67.2) | 1657 (58.8) | 176 (60.1) | 846 (58.0) |
|
| ||||||
| Missing | 6 (0.4) | 40 (0.6) | 1 (0.2) | 12 (0.4) | 2 (0.7) | 8 (0.6) |
|
| ||||||
| Community socioeconomic deprivation, SD units, median (IQR) | −0.05 (−2.37, 2.33) | −0.72 (−2.78, 1.92) | −1.05 (−2.93, 1.1) | −0.79 (−2.97, 1.69) | −0.59 (−2.97, 1.65) | −0.74 (−2.62, 2.07) |
Abbreviations: IQR, interquartile range
Based on six U.S. Census indicators as described in the Methods.
We collected NMPs for 304 high-density poultry operations, which were located in 16 of the 38 study counties. A comparison with data from the U.S. Agricultural Census (USDA 2017) on the number of broilers, layers, and pullets produced in the study area indicated that the NMP data we collected accounted for roughly 95% of the animals produced. Missing data likely represented smaller poultry operations that were not required to submit an NMP. Broiler operations were most common (Table 2). Operations had a median of 154 poultry AEUs. As AEUs take into account animal weight and number of production days/year, this could translate, for example, to approximately 74,000 broilers raised over the course of six six-week cycles (252 production days) or 49,000 layers in production over a full year.
Table 2.
Summary of operations by poultry type
| Poultry operation type | No. operations (%) | No. counties in which operations are present | Mean AEUs | Median AEUs | AEU IQR |
|---|---|---|---|---|---|
| Total operations | 304 (100) | 16 | 248 | 154 | 101, 250 |
| Broiler | 160 (53) | 13 | 164 | 149 | 106, 213 |
| Layer | 73 (24) | 13 | 416 | 172 | 93, 337 |
| Turkey | 36 (12) | 11 | 396 | 227 | 149, 279 |
| Pullet | 26 (9) | 9 | 113 | 74 | 38, 151 |
| Duck | 12 (4) | 4 | 136 | 110 | 51, 158 |
Abbreviations: AEU, animal equivalent unit; IQR, interquartile range
Association of the Poultry Operation Activity Metric with Case Status
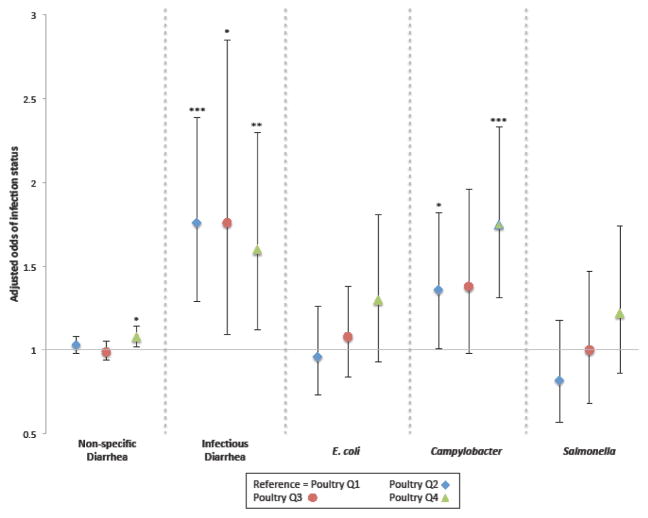
In an unadjusted model, patients with a poultry operation activity metric in the second, third, and fourth quartiles had significantly increased odds of diagnosis with infectious diarrhea compared with patients in the first quartile. Patients in the second and fourth quartiles had significantly increased odds of diagnosis with Campylobacter compared with patients in the first quartile (unadjusted results not shown). Adjustment for base covariates (sex, age, race/ethnicity, Medical Assistance, and smoking status) did not substantively change inferences (Figure 2). There were no associations for non-specific diarrhea in an unadjusted model; in an adjusted model, patients with a poultry operation activity metric in the fourth (versus first) quartile had slightly increased odds of diagnosis with non-specific diarrhea, crossing an inferential boundary (P < 0.05). We observed no associations for E. coli, or Salmonella in unadjusted or adjusted models. Although each of the following variables were associated with the outcomes, we found no evidence of confounding by season of diagnosis/encounter, community socioeconomic deprivation, prior antibiotic use, or inflammatory and irritable bowel diagnoses for any of the five outcomes, therefore these variables were not included in adjusted models. Adjusting for distance to the nearest Geisinger facility slightly increased the strength of the association between the poultry operation activity metric and Campylobacter. Since inferences did not change and given the limitations of the variable (i.e., distance between a patient’s residence and the closest Geisinger facility, without confirmation that they visited that facility), we opted to exclude this distance measure from the model.
Figure 2.
Adjusteda associations of the poultry operation activity metric with diarrheal illness/infection status
aMultivariable logistic regression models controlled for age, sex, race/ethnicity, Medical Assistance, and smoking status, as described in Methods. Non-specific diarrhea model also adjusted for centered age squared. * P < 0.05 ** P < 0.01 *** P < 0.001 comparing higher quartiles to first quartile reference group.
Stratification by Community Type
With the exception of E. coli, models stratified by community type revealed significant associations of the poultry operation activity metric and gastrointestinal outcomes by community type (Table 3). In townships, the odds of non-specific diarrhea were significantly higher in the second and fourth (versus first) quartiles of the poultry operation activity metric, the odds of infectious diarrhea were higher in the fourth quartile, and the odds of Campylobacter were higher in the third and fourth quartiles. Although statistically non-significant, results for E. coli demonstrated an exposure-effect trend. In boroughs, the odds of non-specific diarrhea were significantly higher in the fourth quartile, the odds of infectious diarrhea were significantly higher in the second and third quartiles, and the odds of Campylobacter were higher in the second quartile. In cities, the odds of non-specific diarrhea were significantly lower in the second and third quartiles, the odds of Salmonella gastroenteritis were lower in the second quartile, and the odds of infectious diarrhea were higher in the second quartile.
Table 3.
Adjusteda associations of the poultry operation activity metric with diarrhea outcomes, stratified by community type
| Poultry operation activity quantileb (range, AEU per kilometer2 × 10) | Township | Borough | City | |||
|---|---|---|---|---|---|---|
|
| ||||||
| AOR (CI) | P | AOR (CI) | P | AOR (CI) | P | |
|
| ||||||
| Non-specific Diarrhea | ||||||
| n = 64,752 | n = 33,963 | n = 13,580 | ||||
|
| ||||||
| 1 (0–0.08) | ref | ref | ref | |||
|
| ||||||
| 2 (0.08–0.15) | 1.08 (1.01, 1.16) | 0.02 | 1.05 (0.96, 1.14) | 0.33 | 0.85 (0.77, 0.93) | 0.001 |
|
| ||||||
| 3 (0.15–0.58) | 1.04 (0.98, 1.11) | 0.20 | 1.06 (0.96, 1.17) | 0.23 | 0.52 (0.41, 0.65) | <0.001 |
|
| ||||||
| 4 (0.58–702.62) | 1.10 (1.03, 1.19) | 0.01 | 1.12 (1.01, 1.24) | 0.03 | 0.94 (0.82, 1.07) | 0.36 |
|
| ||||||
| Infectious Diarrhea | ||||||
| n = 2815 | n = 1366 | n= 481 | ||||
|
| ||||||
| 1 (0–0.08) | ref | ref | ref | |||
|
| ||||||
| 2 (0.08–0.15) | 1.51 (0.99, 2.31) | 0.06 | 2.12 (1.14, 3.97) | 0.02 | 1.90 (1.08, 3.34) | 0.03 |
|
| ||||||
| 3 (0.15–0.58) | 1.57 (0.93, 2.64) | 0.09 | 2.55 (1.11, 5.87) | 0.03 | 0.53 (0.18, 1.62) | 0.27 |
|
| ||||||
| 4 (0.58–96.61) | 1.71 (1.02, 2.85) | 0.04 | 1.50 (0.89, 2.51) | 0.13 | 1.48 (0.57, 3.83) | 0.43 |
|
| ||||||
| E. coli | ||||||
| n = 4978 | n =2564 | n = 875 | ||||
|
| ||||||
| 1 (0–0.08) | ref | ref | ref | |||
|
| ||||||
| 2 (0.08–0.15) | 1.11 (0.71, 1.74) | 0.65 | 0.62 (0.38, 1.01) | 0.06 | 1.14 (0.76, 1.70) | 0.53 |
|
| ||||||
| 3 (0.15–0.58) | 1.28 (0.85, 1.95) | 0.24 | 0.97 (0.66, 1.41) | 0.86 | 0.69 (0.25, 1.92) | 0.48 |
|
| ||||||
| 4 (0.58–103.11) | 1.61 (0.95, 2.72) | 0.08 | 1.02 (0.62, 1.66) | 0.95 | 1.50 (0.86, 2.60) | 0.15 |
|
| ||||||
| Campylobacter | ||||||
| n = 2038 | n = 993 | n = 341 | ||||
|
| ||||||
| 1 (0–0.08) | ref | ref | ref | |||
|
| ||||||
| 2 (0.08–0.15) | 1.29 (0.87, 1.92) | 0.21 | 1.71 (1.08, 2.73) | 0.02 | 1.19 (0.54, 2.61) | 0.67 |
|
| ||||||
| 3 (0.15–0.58) | 1.69 (1.15, 2.50) | 0.01 | 0.93 (0.44, 1.99) | 0.85 | 0.60 (0.10, 3.57) | 0.57 |
|
| ||||||
| 4 (0.58–70.97) | 2.03 (1.40, 2.95) | <0.001 | 1.30 (0.80, 2.11) | 0.29 | 0.96 (0.32, 2.86) | 0.9 |
|
| ||||||
| Salmonellac | ||||||
| n = 1022 | n = 518 | n = 203 | ||||
|
| ||||||
| 1 (0–0.09) | ref | ref | ref | |||
|
| ||||||
| 2 (0.09–0.42) | 0.83 (0.54, 1.27) | 0.39 | 0.96 (0.52, 1.77) | 0.89 | 0.33 (0.13, 0.88) | 0.03 |
|
| ||||||
| 3 (0.42–55.17) | 1.17 (0.74, 1.83) | 0.51 | 1.39 (0.78, 2.48) | 0.27 | 1.00 (0.32, 3.13) | 0.99 |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; P, p-value; ref, reference group
Multivariable logistic regression models controlled for age, sex, race/ethnicity, Medical Assistance, and smoking status, as described in Methods. Non-specific diarrhea model also adjusted for centered age squared.
Poultry operation activity took into account the number of poultry operations in study area, distance between each patient’s residence and poultry operations, and number (in AEUs) of poultry at each operation. Activity increased over each quantile, with the lowest activity in quantile 1 (the reference category), as described in Methods.
For Salmonella, poultry operation activity quantiles were re-categorized as tertiles due to low cell counts in stratified models
Effect Modification Analyses
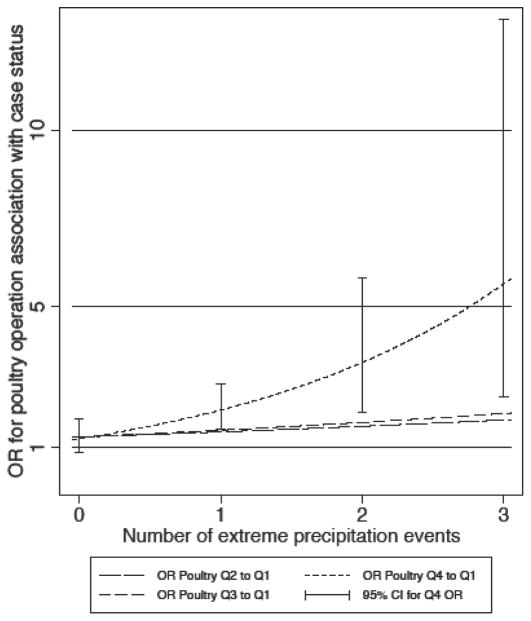
The number of extreme precipitation events in the week prior to diagnosis/encounter modified the association of poultry operations with Campylobacter diarrhea, strengthening the association between the fourth quartile of the poultry operation activity metric and Campylobacter as the number of extreme events increased from none to five (Figure 3; global P value = 0.004). Medical Assistance status also modified the relation between the poultry operation activity metric and Campylobacter diagnosis. Significant associations (aOR [CI], P value) were observed for the second, third, and fourth (versus first) quartiles of the poultry operation activity metric among Medical Assistance patients, with an exposure-effect relation (Q2: 2.29 [1.17, 4.48], 0.02; Q3: 2.84 [1.34, 6.05], 0.007; Q4: 3.29 [1.62, 6.65], < 0.001). Among non-Medical Assistance patients, only the fourth (versus first) quartile of the poultry operation activity metric was significantly associated with Campylobacter (Q4: 1.52 [1.11, 2.09], 0.01). We found evidence of effect modification by prior antibiotic use for non-specific diarrhea, observing a stronger association for the fourth (versus first) quartile of the poultry operation activity metric among those with an antibiotic order in the 30 days prior to diagnosis/encounter (Q4: 1.22 [1.08, 1.37], 0.002). We found no evidence of effect modification by age, season of diagnosis/encounter, average maximum temperature, or total precipitation for any of the five gastrointestinal outcome models.
Figure 3.
Plot of odds ratio of Campylobacter by poultry operation activity metric quartile (reference = Q1), modified by the numbera of extreme precipitation events in the week prior to diagnosis.
Abbreviations: OR, odds ratio; CI, confidence interval
aCategorized as 0, 1, 2, and 3 or more events since there were few instances of 4 or 5 events
DISCUSSION
In this study evaluating patients’ residential proximity to high-density poultry operations and diarrheal illnesses we found that patients who lived closer to a greater number or larger operations had a higher odds of diagnosis with campylobacteriosis and infectious diarrhea. The odds of Campylobacter increased further with the occurrence of more extreme precipitation events in the week prior to diagnosis. Additionally, proximity to poultry operations was more strongly associated with Campylobacter among patients with a history of Medical Assistance, a proxy for low socioeconomic status. We did not find substantive evidence that proximity to poultry operations was related to diagnosis with non-specific diarrhea or intestinal infections related to E. coli or Salmonella.
Compared to patients in the lowest quartile of the poultry operation activity metric, we observed that patients in the highest quartile had an elevated risk of diagnosis with Campylobacter, suggesting that high-density poultry operations may be a risk factor for campylobacteriosis in nearby communities. As one of the most common causes of foodborne illness, Campylobacter in poultry has been shown to cause the greatest disease burden among pathogen-food combinations, accounting for cost of illness, lost quality-adjusted life years, and number of illnesses, hospitalizations, and deaths (Batz et al. 2012). Well-adapted to avian hosts, Campylobacter is ubiquitous on poultry farms (Sahin et al. 2015). Ecological analyses have observed that in addition to broad population exposure to Campylobacter—presumably through a centralized food system endemically infected with the bacteria—rates of campylobacteriosis are higher in rural areas, with significant associations with farm animal density, particularly high chicken density (Green et al. 2006). Jonsson et al (2010) identified clusters of Campylobacter simultaneously occurring in humans and broiler flocks, providing additional evidence for potential environmental transmission of Campylobacter from poultry operations into nearby communities. By using geocoded patient addresses to evaluate individuals’ residential proximities to poultry operations, our findings provide more direct support for such transmission. The observed association could also indicate a common factor related to Campylobacter in poultry and humans (Jonsson et al. 2010), as Campylobacter is ubiquitous in the environment. Colonization of poultry chicks is believed to occur through horizontal transmission from external reservoirs such as other livestock and free-ranging animals, flies and other pests, contaminated water, and farm equipment (Hermans et al. 2012). Yet poultry flocks also act as a reservoir of Campylobacter, likely contributing to the persistence and abundance of bacteria in the environment.
Our primary analysis included all Campylobacter cases that occurred in the patient population, and therefore included all routes and sources of transmission, likely weakening observed associations with poultry operations. This was confirmed in analyses stratified by community type, which revealed a stronger, exposure-effect relation of the poultry operation activity metric and Campylobacter diagnoses in townships, the most rural community type in the study geography. Stratified models also revealed greater odds of Campylobacter for the second quartile of the poultry operation activity metric in boroughs, accounting for the elevated odds ratio observed in the second quartile of the non-stratified model. An individual living in a borough and in the second quartile of the poultry operation activity metric likely has limited residential exposure to poultry operations, suggesting that there is additional confounding not accounted for in our analysis. Such confounders may be unrelated to poultry operations, such as consuming unpasteurized milk or contaminated water (Carrique-Mas et al. 2005; Green et al. 2006; Jonsson et al. 2010). Although unevaluated in the current study, poultry slaughterhouses comprise another potential source of zoonotic bacteria, and are generally located in urban or peri-urban areas. Slaughterhouses discharge large amounts of wastewater into the environment that can be contaminated with bacteria such as Salmonella and Campylobacter, risking contamination of surface-water and groundwater in neighboring communities (Gerber et al. 2007).
A novel contribution of this study was the inclusion of temperature and precipitation data from local weather stations, allowing for analysis of the influence of weather-related factors on associations between the poultry operation activity metric and gastrointestinal outcomes. In temperate climates, campylobacteriosis incidence is generally highest in summer, as seen in our study, potentially reflecting the impact of temperature and precipitation on the survival of Campylobacter, along with changes in human activities that increase risk of infection (Weisent et al. 2014). We found that as the number of extreme precipitation events in the week prior to Campylobacter diagnosis increased from zero to five, the association between the highest quartile of the poultry operation activity metric and campylobacteriosis strengthened. Considering that most people exhibit clinical symptoms within four days of infection (Humphrey et al. 2007), these findings provide further support for the hypothesis that Campylobacter diagnoses were related to an environmental exposure and bolster our hypothesis that residential proximity to poultry operations increased risk of campylobacteriosis. Once bacteria enter the external environment, extreme precipitation events can transport pathogens into ground and surface water (Levy et al. 2016), potentially contaminating drinking water and recreational water bodies. Our findings relate to a Maryland study that found a positive association between extreme precipitation events and risk of campylobacteriosis; because this association was only observed in coastal counties that had poultry IFAP, the researchers speculated that runoff from fields applied with poultry waste during extreme precipitation events led to the contamination of water bodies and wells (Soneja et al. 2016). Studies demonstrate the runoff of bacteria from land applied with poultry litter following rainfall events (Soupir et al. 2006), higher prevalence of Campylobacter downstream of poultry farms (Vereen et al. 2013), and that bacteria can survive from days to months in manure and water, including through typical poultry litter storage practices (Graham et al. 2009a). It is possible that our poultry operation activity metric inadvertently captured nearby crop field application of poultry litter, since 38% of operations applied litter on crop fields on the home farm and an additional 39% of operations exported at least a portion of the waste to nearby farms. Discharge from wastewater treatment plants comprise another potential source of Campylobacter in the environment that increases with heavy rainfall (Rechenburg and Kistemann 2009); thus it is also possible that our findings reflect an underlying association between patients’ proximity to waterways downstream of potential discharges, or to septic overflows.
With prior studies demonstrating positive and negative associations, the relationship between socioeconomic status and campylobacteriosis remains unclear (Green et al. 2006; Newman et al. 2015; Rosenberg Goldstein et al. 2016). We found that Campylobacter cases were more likely than controls to have a history of Medical Assistance, a proxy for low socioeconomic status. Additionally, Medical Assistance modified the poultry operation/Campylobacter relation: proximity to poultry operations was more strongly associated with Campylobacter diagnosis among patients with a history of Medical Assistance. Our results may be explained by differential healthcare-seeking behavior for gastrointestinal illness by socioeconomic status, as has previously been reported (Scallan et al. 2006; Tam et al. 2003). Alternatively, these findings may indicate the existence of risk factors among patients with low socioeconomic status—such as agricultural employment—that increase their vulnerability to the risk of campylobacteriosis related to poultry operations.
We observed a trend of higher odds of E. coli diagnoses among patients in townships with greater proximity to poultry operations, though these results were not statistically significant. We found no association between residential proximity to poultry operations and Salmonella. Without bacteria samples from the poultry operations, the surrounding environment, and patient residences, we can only speculate as to why we detected associations for Campylobacter, but not E. coli or Salmonella. It is possible that there was a low prevalence of E. coli and Salmonella in the poultry operations included in this study. Research assessing the microbiological composition of poultry litter—one indicator of the presence of bacteria in poultry operations—demonstrated that bacterial composition varies by region and other factors (Terzich et al. 2000), and the prevalence of bacteria on poultry farms has been shown to vary with management practices (Berghaus et al. 2012). Considering environmental persistence differs by type of bacteria, it is also possible that environmental conditions hampered transmission of E. coli and Salmonella. Campylobacter, E. coli, and Salmonella are able to survive in the open environment, but factors such as the availability of nutrients, soil pH, moisture, and temperature affect bacterial persistence (Blaak et al. 2015; Cook et al. 2014; van Elsas et al. 2011). The complexity and diversity of the natural environment impedes predictions regarding bacterial survival (van Elsas et al. 2011).
Although we observed increased odds of infectious diarrhea for quartiles two, three, and four of the poultry operation activity metric compared to the first quartile, odds of this outcome were highest among patients in the second and third, rather than fourth, quartiles. Models stratified by community type revealed that in townships, the odds of infectious diarrhea were significantly higher for the fourth quartile, providing some support that residential proximity to poultry operations may increase risk for infectious diarrhea. Stratified models also showed that the elevated odds ratios in the second and third quartiles were due to increased odds in boroughs and cities. A closer look at the data revealed that these associations were driven by a high number of cases in just three boroughs and two cities, which we believe indicates a spatial clustering of infectious diarrhea cases picked up by our models but unrelated to poultry operations. Again, this suggests additional confounding that was not accounted for in our analysis. Identifying potential confounders is problematic given the non-specificity of infectious diarrhea as an outcome, which has innumerable causes.
We examined infectious and non-specific diarrhea as outcomes in order to capture gastrointestinal infections whose relatively lower severity or persistence did not warrant testing for specific pathogens. The lack of specificity for the associated diagnostic codes presents a drawback to the inclusion of these outcomes, because many of these patients likely had diarrheal illnesses unrelated to environmental transmission. This was particularly problematic for non-specific diarrhea, which included diarrheal symptoms whose link to an infectious agent was not characterized. Any associations with poultry IFAP would likely be eclipsed by the abundance of other, more predominant causes of non-specific diarrhea (e.g., medication or chronic illness). Although we did observe significantly higher odds of non-specific diarrhea in the fourth (versus first) quartile of the poultry operation activity metric, and some significant associations in the stratified analysis, the effect sizes were small, as would be expected for this non-specific outcome, in which only a small number of patients within the larger study sample have possible poultry IFAP links.
Interestingly, we observed an interaction with prior antibiotic use for non-specific diarrhea: within the fourth quartile of the poultry operation activity metric, the odds of non-specific diarrhea were significantly higher among patients with an antibiotic order in the 30 days prior to diagnosis/encounter. In evaluating effect modification by prior antibiotic use, we hypothesized that antibiotics may increase the vulnerability of the gut to bacterial infection and diarrhea due to alterations in the gut microbiota (Becattini et al. 2016), including infections originating from poultry operations. Results for non-specific diarrhea provide some support for this hypothesis, but are tempered by the lack of evidence of effect modification by prior antibiotic use for the other outcomes. We lacked data on inpatient antibiotic orders prior to 2008, but this seems unlikely to have affected our findings.
A strength of this study was the use of EHR data to evaluate five diarrheal illnesses; however, although we incorporated fecal culture results in case definitions for Campylobacter and Salmonella, the use of diagnostic codes limited the specificity of other case definitions. Additionally, considering that the majority of patients with acute gastroenteritis do not seek medical care (Scallan et al. 2006), it is likely that our studied patients represent the more severe or long-lasting diarrheal illnesses in the general population. Such selection bias may have biased our results toward the null, since infections from poultry-related bacteria such as Campylobacter are generally self-limiting. Bacterium-specific acquired host immunity may have subjected our findings to misclassification bias (Havelaar et al. 2009). Once infected, acquired immunity against Campylobacter, Salmonella, and E. coli can limit illness duration and severity during subsequent infections with homologous bacterial strains—likely diminishing healthcare seeking—and protect individuals from subsequent illness (Gomes et al. 2016; Griffin and McSorley 2011; Havelaar et al. 2009). As evidenced by research on occupational exposure to Campylobacter, acquired immunity is more likely to occur with persistent exposure to bacteria—as would be the case with living near poultry operations—than with exposure to a high-dose or an unfamiliar bacterial type (Havelaar et al. 2009). Thus, without a comprehensive account of study patients’ medical history, including all diarrheal episodes, instances of adaptive immunity could have lead to misclassification of cases as controls, biasing estimates toward the null. The small proportion of repeat diagnoses in our study population could be a signal of acquired immunity to persistent bacterial exposure, or may indicate that the trend of not seeking care for diarrheal illness is amplified for subsequent diarrheal episodes.
Another strength of this study included primary collection of NMP data to estimate the poultry operation activity metric. Comparison with U.S. Agricultural Census data suggested that NMP data was quite comprehensive. Missing data likely represented smaller operations and likely would have biased results toward the null. Geospatial analysis allowed us to link IFAP activity to patient residence and demonstrate initial evidence that residential proximity to poultry operations may increase risk for campylobacteriosis and infectious diarrhea. Parallel results in townships in stratified models reinforced these findings, irrespective of the presence of residual spatial confounding, as previously discussed. However, our proximity model is limited in that we cannot determine if the observed associations with Campylobacter and infectious diarrhea are, in fact, related to environmental transmission of bacteria from poultry operations rather than another factor captured by the poultry operation activity metric, such as occupational exposure to poultry and other livestock, environmental transmission of bacteria from other types of livestock operations, or obtaining drinking water from a household well (a factor that likely correlates with rural residence) (Carrique-Mas et al. 2005; Green et al. 2006; Potter et al. 2003). Future research with explicit exposure assessment is needed to validate our findings.
We were also unable to evaluate patients’ exposure to poultry litter applied to crop fields as fertilizer. Historically, more than 90% of poultry litter was applied to agricultural land, usually within a few miles from where it was produced (Moore Jr. et al. 1995). Although NMPs provide information on crop field application when it is used on-site or directly exported to nearby farms, we found that 49% of NMPs in our study exported poultry litter through the use of a broker, and so lacked sufficient information to identify the crop fields to which litter was applied. According to some brokers, poultry litter is transported up to 50 miles from the source farm, and is used both on crop fields and mushroom farms (personal communication). Brokers are not required to document where they distribute purchased poultry litter or how it is used, further limiting access to information about IFAP and constraining environmental health research related to pathogen transmission via poultry litter application. Finally, while we evaluated high-density livestock operations, zoonotic disease risks are not unique to such operations; for example, chickens raised in free-range systems may be more likely to carry Campylobacter than housed animals, potentially due to greater exposure to pathogen reservoirs in the natural environment (Heuer et al. 2001). However, IFAP is the predominant model of poultry production in the U.S., and, increasingly, globally (Casey et al. 2015; Lam et al. 2016), reinforcing the importance of studying IFAP-related disease risks.
CONCLUSION
In one of the first studies to evaluate patients’ residential proximity to poultry operations and diarrheal illnesses, we found an association between residing closer to more or larger poultry operations and campylobacteriosis. This association was stronger as the number of extreme precipitation events increased in the week prior to diagnosis, evidence of biologically plausible effect modification that provides further support to a causal inference that Campylobacter diagnoses were related to this environmental exposure. We also found that proximity to poultry operations increased risk of infectious diarrhea among patients living in townships, the most rural community type in the study geography. These findings contribute to growing concern regarding the public health impacts of IFAP.
Acknowledgments
Funding: This research was funded by a Fisher Center Discovery Program grant from the Sherrilyn and Ken Fisher Center for Environmental Infectious Diseases in the Johns Hopkins School of Medicine. The funder had no involvement in the study design; collection, analysis, and interpretation of data; writing of the report; or the decision to submit the article for publication.
We thank Mona Mohammed, Shai Gerstle, and Matthew Geiger at Bucknell University for their assistance with data collection, as well as Joe Dewalle of the Geisinger Health System for his assistance developing variables and maps.
Abbreviations
- IFAP
industrial food animal production
- NMP
Nutrient Management Plan
- AEU
animal equivalent unit
Footnotes
Conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adzitey F, Huda N, Ali GR. Prevalence and antibiotic resistance of campylobacter, salmonella, and l. Monocytogenes in ducks: A review. Foodborne Pathog Dis. 2012a;9:498–505. doi: 10.1089/fpd.2011.1109. [DOI] [PubMed] [Google Scholar]
- Adzitey F, Liew CY, Aronal AP, Huda N. Isolation of escherichia coli from ducks and duck related samples. Asian Journal of Animal and Veterinary Advances. 2012b;7:351–355. [Google Scholar]
- Arnold SD. Dairy herds and rural communities in southern new mexico. Journal of Environmental Health. 1999;62:9–17. [Google Scholar]
- Batz MB, Hoffmann S, Morris JG., Jr Ranking the disease burden of 14 pathogens in food sources in the united states using attribution data from outbreak investigations and expert elicitation. J Food Prot. 2012;75:1278–1291. doi: 10.4315/0362-028X.JFP-11-418. [DOI] [PubMed] [Google Scholar]
- Becattini S, Taur Y, Pamer EG. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol Med. 2016;22:458–478. doi: 10.1016/j.molmed.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghaus RD, Mathis DL, Bramwell RK, Macklin KS, Wilson JL, Wineland MJ, et al. Multilevel analysis of environmental salmonella prevalences and management practices on 49 broiler breeder farms in four south-eastern states, USA. Zoonoses Public Health. 2012;59:365–374. doi: 10.1111/j.1863-2378.2012.01464.x. [DOI] [PubMed] [Google Scholar]
- Blaak H, Hamidjaja RA, van Hoek AH, de Heer L, de Roda Husman AM, Schets FM. Detection of extended-spectrum beta-lactamase (esbl)-producing escherichia coli on flies at poultry farms. Appl Environ Microbiol. 2014;80:239–246. doi: 10.1128/AEM.02616-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaak H, van Hoek AH, Hamidjaja RA, van der Plaats RQ, Kerkhof-de Heer L, de Roda Husman AM, et al. Distribution, numbers, and diversity of esbl-producing e. Coli in the poultry farm environment. PLoS One. 2015;10:e0135402. doi: 10.1371/journal.pone.0135402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull SA, Allen VM, Domingue G, Jorgensen F, Frost JA, Ure R, et al. Sources of campylobacter spp. Colonizing housed broiler flocks during rearing. Appl Environ Microbiol. 2006;72:645–652. doi: 10.1128/AEM.72.1.645-652.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton EJ, Woster AP, DeWitt P, Goldstein RS, Levy K. A systematic review and meta-analysis of ambient temperature and diarrhoeal diseases. Int J Epidemiol. 2016;45:117–130. doi: 10.1093/ije/dyv296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrique-Mas J, Andersson Y, Hjertqvist M, Svensson A, Torner A, Giesecke J. Risk factors for domestic sporadic campylobacteriosis among young children in sweden. Scand J Infect Dis. 2005;37:101–110. doi: 10.1080/00365540510027165. [DOI] [PubMed] [Google Scholar]
- Casey JA, Curriero FC, Cosgrove SE, Nachman KE, Schwartz BS. High-density livestock operations, crop field application of manure, and risk of community-associated methicillin-resistant staphylococcus aureus infection in pennsylvania. JAMA Intern Med. 2013;173:1980–1990. doi: 10.1001/jamainternmed.2013.10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey JA, Kim BF, Larsen J, Price LB, Nachman KE. Industrial food animal production and community health. Curr Environ Health Rep. 2015;2:259–271. doi: 10.1007/s40572-015-0061-0. [DOI] [PubMed] [Google Scholar]
- Casey JA, James P, Rudolph KE, Wu CD, Schwartz BS. Greenness and birth outcomes in a range of pennsylvania communities. Int J Environ Res Public Health. 2016:13. doi: 10.3390/ijerph13030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey JA, Savitz DA, Rasmussen SG, Ogburn EL, Pollak J, Mercer DG, et al. Unconventional natural gas development and birth outcomes in pennyslvania, USA. Epidemiology. 2016;27:163–172. doi: 10.1097/EDE.0000000000000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M, Groseclose SL, Zaidi AA, Braden CR. An ecological analysis of sociodemographic factors associated with the incidence of salmonellosis, shigellosis, and e. Coli o157:H7 infections in us counties. Epidemiol Infect. 2009;137:810–820. doi: 10.1017/S0950268808001477. [DOI] [PubMed] [Google Scholar]
- Cook KL, Netthisinghe AM, Gilfillen RA. Detection of pathogens, indicators, and antibiotic resistance genes after land application of poultry litter. J Environ Qual. 2014;43:1546–1558. doi: 10.2134/jeq2013.10.0432. [DOI] [PubMed] [Google Scholar]
- Curriero FC, Patz JA, Rose JB, Lele S. The association between extreme precipitation and waterborne disease outbreaks in the united states, 1948–1994. Am J Public Health. 2001;91:1194–1199. doi: 10.2105/ajph.91.8.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahshan H, Merwad AM, Mohamed TS. Listeria species in broiler poultry farms: Potential public health hazards. J Microbiol Biotechnol. 2016;26:1551–1556. doi: 10.4014/jmb.1603.03075. [DOI] [PubMed] [Google Scholar]
- Davis MA, Moore DL, Baker KN, French NP, Patnode M, Hensley J, et al. Risk factors for campylobacteriosis in two washington state counties with high numbers of dairy farms. J Clin Microbiol. 2013;51:3921–3927. doi: 10.1128/JCM.01433-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese A, Schulz J, Zimmermann K, Tenhagen BA, Fetsch A, Hartung J, et al. Occurrence of livestock-associated methicillin-resistant staphylococcus aureus in turkey and broiler barns and contamination of air and soil surfaces in their vicinity. Appl Environ Microbiol. 2013;79:2759–2766. doi: 10.1128/AEM.03939-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P, Opio C, Steinfeld H. Poultry in the 21st century: Avian influenza and beyond. Bangkok, Thailand: Food and Agriculture Organization of the United Nations; 2007. Poultry production and the environment - a review. [Google Scholar]
- Gleason JA, Fagliano JA. Effect of drinking water source on associations between gastrointestinal illness and heavy rainfall in new jersey. PLoS One. 2017;12:e0173794. doi: 10.1371/journal.pone.0173794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes TA, Elias WP, Scaletsky IC, Guth BE, Rodrigues JF, Piazza RM, et al. Diarrheagenic escherichia coli. Braz J Microbiol. 2016;47(Suppl 1):3–30. doi: 10.1016/j.bjm.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JP, Evans SL, Price LB, Silbergeld EK. Fate of antimicrobial-resistant enterococci and staphylococci and resistance determinants in stored poultry litter. Environ Res. 2009a;109:682–689. doi: 10.1016/j.envres.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Graham JP, Price LB, Evans SL, Graczyk TK, Silbergeld EK. Antibiotic resistant enterococci and staphylococci isolated from flies collected near confined poultry feeding operations. Sci Total Environ. 2009b;407:2701–2710. doi: 10.1016/j.scitotenv.2008.11.056. [DOI] [PubMed] [Google Scholar]
- Green CG, Krause DO, Wylie JL. Spatial analysis of campylobacter infection in the canadian province of manitoba. Int J Health Geogr. 2006;5:2. doi: 10.1186/1476-072X-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin AJ, McSorley SJ. Development of protective immunity to salmonella, a mucosal pathogen with a systemic agenda. Mucosal Immunol. 2011;4:371–382. doi: 10.1038/mi.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelaar AH, van Pelt W, Ang CW, Wagenaar JA, van Putten JP, Gross U, et al. Immunity to campylobacter: Its role in risk assessment and epidemiology. Crit Rev Microbiol. 2009;35:1–22. doi: 10.1080/10408410802636017. [DOI] [PubMed] [Google Scholar]
- Hermans D, Pasmans F, Messens W, Martel A, Van Immerseel F, Rasschaert G, et al. Poultry as a host for the zoonotic pathogen campylobacter jejuni. Vector Borne Zoonotic Dis. 2012;12:89–98. doi: 10.1089/vbz.2011.0676. [DOI] [PubMed] [Google Scholar]
- Heuer OE, Pedersen K, Andersen JS, Madsen M. Prevalence and antimicrobial susceptibility of thermophilic campylobacter in organic and conventional broiler flocks. Lett Appl Microbiol. 2001;33:269–274. doi: 10.1046/j.1472-765x.2001.00994.x. [DOI] [PubMed] [Google Scholar]
- Humphrey T, O’Brien S, Madsen M. Campylobacters as zoonotic pathogens: A food production perspective. Int J Food Microbiol. 2007;117:237–257. doi: 10.1016/j.ijfoodmicro.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Jonsson ME, Heier BT, Norstrom M, Hofshagen M. Analysis of simultaneous space-time clusters of campylobacter spp. In humans and in broiler flocks using a multiple dataset approach. Int J Health Geogr. 2010;9:48. doi: 10.1186/1476-072X-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam Y, Fry JP, Hu E, Kim BF, Nachman KE. Industrial food animal production in low- and middle-income countries: A landscape assessment. Center for a Livable Future; 2016. [Google Scholar]
- Lee S, Lee J, Ha J, Choi Y, Kim S, Lee H, et al. Clinical relevance of infections with zoonotic and human oral species of campylobacter. J Microbiol. 2016;54:459–467. doi: 10.1007/s12275-016-6254-x. [DOI] [PubMed] [Google Scholar]
- Levy K, Woster AP, Goldstein RS, Carlton EJ. Untangling the impacts of climate change on waterborne diseases: A systematic review of relationships between diarrheal diseases and temperature, rainfall, flooding, and drought. Environ Sci Technol. 2016;50:4905–4922. doi: 10.1021/acs.est.5b06186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LF, Chan RL, Lu L, Shen J, Zhang L, Wu WK, et al. Cigarette smoking and gastrointestinal diseases: The causal relationship and underlying molecular mechanisms (review) Int J Mol Med. 2014;34:372–380. doi: 10.3892/ijmm.2014.1786. [DOI] [PubMed] [Google Scholar]
- Mann EA, Saeed SA. Gastrointestinal infection as a trigger for inflammatory bowel disease. Curr Opin Gastroenterol. 2012;28:24–29. doi: 10.1097/MOG.0b013e32834c453e. [DOI] [PubMed] [Google Scholar]
- Moore PA, Jr, Daniel TC, Sharpley AN, Wood CW. Poultry manure management: Environmentally sound options. Journal of Soil and Water Conservation. 1995;50:321–327. [Google Scholar]
- Nau C, Schwartz BS, Bandeen-Roche K, Liu A, Pollak J, Hirsch A, et al. Community socioeconomic deprivation and obesity trajectories in children using electronic health records. Obesity (Silver Spring) 2015;23:207–212. doi: 10.1002/oby.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman KL, Leon JS, Rebolledo PA, Scallan E. The impact of socioeconomic status on foodborne illness in high-income countries: A systematic review. Epidemiol Infect. 2015;143:2473–2485. doi: 10.1017/S0950268814003847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOAA. National oceanic and atmospheric climatic data center (ncdc) 2016 [Google Scholar]
- Olowokure B, Hawker J, Weinberg J, Gill N, Sufi F. Deprivation and hospital admission for infectious intestinal diseases. Lancet. 1999;353:807–808. doi: 10.1016/S0140-6736(99)00611-X. [DOI] [PubMed] [Google Scholar]
- Pockett RD, Adlard N, Carroll S, Rajoriya F. Paediatric hospital admissions for rotavirus gastroenteritis and infectious gastroenteritis of all causes in england: An analysis of correlation with deprivation. Curr Med Res Opin. 2011;27:777–784. doi: 10.1185/03007995.2011.555757. [DOI] [PubMed] [Google Scholar]
- Potter RC, Kaneene JB, Hall WN. Risk factors for sporadic campylobacter jejuni infections in rural michigan: A prospective case-control study. Am J Public Health. 2003;93:2118–2123. doi: 10.2105/ajph.93.12.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LB, Graham JP, Lackey LG, Roess A, Vailes R, Silbergeld E. Elevated risk of carrying gentamicin-resistant escherichia coli among u.S. Poultry workers. Environ Health Perspect. 2007;115:1738–1742. doi: 10.1289/ehp.10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SG, Ogburn EL, McCormack M, Casey JA, Bandeen-Roche K, Mercer DG, et al. Association between unconventional natural gas development in the marcellus shale and asthma exacerbations. JAMA Intern Med. 2016;176:1334–1343. doi: 10.1001/jamainternmed.2016.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechenburg A, Kistemann T. Sewage effluent as a source of campylobacter sp. In a surface water catchment. Int J Environ Health Res. 2009;19:239–249. doi: 10.1080/09603120802460376. [DOI] [PubMed] [Google Scholar]
- Rose TC, Adams N, Taylor-Robinson DC, Barr B, Hawker J, O’Brien S, et al. Relationship between socioeconomic status and gastrointestinal infections in developed countries: A systematic review protocol. Syst Rev. 2016;5:13. doi: 10.1186/s13643-016-0187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg Goldstein RE, Cruz-Cano R, Jiang C, Palmer A, Blythe D, Ryan P, et al. Association between community socioeconomic factors, animal feeding operations, and campylobacteriosis incidence rates: Foodborne diseases active surveillance network (foodnet), 2004–2010. BMC Infect Dis. 2016;16:354. doi: 10.1186/s12879-016-1686-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin O, Kassem II, Shen Z, Lin J, Rajashekara G, Zhang Q. Campylobacter in poultry: Ecology and potential interventions. Avian Dis. 2015;59:185–200. doi: 10.1637/11072-032315-Review. [DOI] [PubMed] [Google Scholar]
- Scallan E, Majowicz SE, Hall G, Banerjee A, Bowman CL, Daly L, et al. Prevalence of diarrhoea in the community in australia, canada, ireland, and the united states. Int J Epidemiol. 2005;34:454–460. doi: 10.1093/ije/dyh413. [DOI] [PubMed] [Google Scholar]
- Scallan E, Jones TF, Cronquist A, Thomas S, Frenzen P, Hoefer D, et al. Factors associated with seeking medical care and submitting a stool sample in estimating the burden of foodborne illness. Foodborne Pathog Dis. 2006;3:432–438. doi: 10.1089/fpd.2006.3.432. [DOI] [PubMed] [Google Scholar]
- Scallan E, Hoekstra RM, Mahon BE, Jones TF, Griffin PM. An assessment of the human health impact of seven leading foodborne pathogens in the united states using disability adjusted life years. Epidemiol Infect. 2015;143:2795–2804. doi: 10.1017/S0950268814003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz BS, Stewart WF, Godby S, Pollak J, Dewalle J, Larson S, et al. Body mass index and the built and social environments in children and adolescents using electronic health records. Am J Prev Med. 2011;41:e17–28. doi: 10.1016/j.amepre.2011.06.038. [DOI] [PubMed] [Google Scholar]
- Schwartz BS, Bailey-Davis L, Bandeen-Roche K, Pollak J, Hirsch AG, Nau C, et al. Attention deficit disorder, stimulant use, and childhood body mass index trajectory. Pediatrics. 2014;133:668–676. doi: 10.1542/peds.2013-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemer BL, Harrington CS, Nielsen EM, Borck B, Nielsen NL, Engberg J, et al. Genetic relatedness among campylobacter jejuni serotyped isolates of diverse origin as determined by numerical analysis of amplified fragment length polymorphism (aflp) profiles. J Appl Microbiol. 2004;96:795–802. doi: 10.1111/j.1365-2672.2004.02205.x. [DOI] [PubMed] [Google Scholar]
- Skora J, Matusiak K, Wojewodzki P, Nowak A, Sulyok M, Ligocka A, et al. Evaluation of microbiological and chemical contaminants in poultry farms. Int J Environ Res Public Health. 2016;13:192. doi: 10.3390/ijerph13020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneja S, Jiang C, Romeo Upperman C, Murtugudde R, CSM, Blythe D, et al. Extreme precipitation events and increased risk of campylobacteriosis in maryland, u.S.A. Environ Res. 2016;149:216–221. doi: 10.1016/j.envres.2016.05.021. [DOI] [PubMed] [Google Scholar]
- Soupir ML, Mostaghimi S, Yagow ER, Hagedorn C, Vaughan DH. Transport of fecal bacteria from poultry litter and cattle manures applied to pastureland. Water, Air, and Soil Pollution. 2006;169:125–136. [Google Scholar]
- Studahl A, Andersson Y. Risk factors for indigenous campylobacter infection: A swedish case-control study. Epidemiol Infect. 2000;125:269–275. doi: 10.1017/s0950268899004562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talen E, Anselin L. Assessing spatial equity: An evaluation of measures of accessbility to public playgrounds. Environment and Planning A. 1998;30:595–613. [Google Scholar]
- Tam CC, Rodrigues LC, O’Brien SJ. The study of infectious intestinal disease in england: What risk factors for presentation to general practice tell us about potential for selection bias in case-control studies of reported cases of diarrhoea. Int J Epidemiol. 2003;32:99–105. doi: 10.1093/ije/dyg007. [DOI] [PubMed] [Google Scholar]
- Terzich M, Pope MJ, Cherry TE, Hollinger J. Survey of pathogens in poultry litter in the united states. Journal of Applied Poultry Research. 2000;9:287–291. [Google Scholar]
- Thorsteinsdottir TR, Haraldsson G, Fridriksdottir V, Kristinsson KG, Gunnarsson E. Prevalence and genetic relatedness of antimicrobial-resistant escherichia coli isolated from animals, foods and humans in iceland. Zoonoses Public Health. 2010;57:189–196. doi: 10.1111/j.1863-2378.2009.01256.x. [DOI] [PubMed] [Google Scholar]
- USDA. Quick stats. National Agriculture Statistics Service; 2017. [Google Scholar]
- van Elsas JD, Semenov AV, Costa R, Trevors JT. Survival of escherichia coli in the environment: Fundamental and public health aspects. ISME J. 2011;5:173–183. doi: 10.1038/ismej.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereen E, Jr, Lowrance RR, Jenkins MB, Adams P, Rajeev S, Lipp EK. Landscape and seasonal factors influence salmonella and campylobacter prevalence in a rural mixed use watershed. Water Res. 2013;47:6075–6085. doi: 10.1016/j.watres.2013.07.028. [DOI] [PubMed] [Google Scholar]
- Weisent J, Seaver W, Odoi A, Rohrbach B. The importance of climatic factors and outliers in predicting regional monthly campylobacteriosis risk in georgia, USA. Int J Biometeorol. 2014;58:1865–1878. doi: 10.1007/s00484-014-0788-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IG. Airborne campylobacter infection in a poultry worker: Case report and review of the literature. Commun Dis Public Health. 2004;7:349–353. [PubMed] [Google Scholar]
- Wing S, Wolf S. Intensive livestock operations, health, and quality of life among eastern north carolina residents. Environ Health Perspect. 2000;108:233–238. doi: 10.1289/ehp.00108233. [DOI] [PMC free article] [PubMed] [Google Scholar]