Abstract
Cancer metastasis is the second leading cause of death in the United States. Despite its morbidity, metastasis is an inefficient process that few cells can survive. However, cancer cells can often overcome these metastatic barriers via cellular responses to microenvironmental cues, such as through mechanotransduction. This review focuses on the mechanosensitive ion channels TRPV4 and P2X7, and their roles in metastasis, as both channels have been shown to have significant effects on tumor cell dissemination. Upon activation, these channels help form tumor neovasculature, promote transendothelial migration, and increase cell motility. Conversely, they have also been linked to forms of cancer cell death dependent upon levels of activation, implying the complex functionality of mechanosensitive ion channels. Understanding the roles of TRPV4, P2X7 and other mechanosensitive ion channels in these processes may reveal new possible drug targets that modify channel function to reduce a tumor’s metastatic potential.
Keywords: P2X7, TRPV4, Cancer, Metastasis, Mechanotransduction, Ion channels, Mechanosensitive
1. Introduction
Cancer metastasis is associated with ~90% of cancer-related death.1,2 Metastasis can be divided into multiple steps. First, the cancer cells must become motile and invade local tissue. Subsequently, the cells intravasate into lymphatic or blood circulation from tumor-induced neovasculature. The cells are transported by flow to distant organs and must bind to the vessel for extravasation to occur. Finally, the cells colonize a secondary tumor through proliferation in the distant tumor site.3–5 Due to the complexity and many physiological checkpoints involved in this process, less than 0.1% of circulating tumor cells (CTCs) form distal metastases,6 meaning successful colonization of a distant site is contingent upon several factors. Recently, mechanical stimuli such as shear stress have been receiving more attention in their effects on cancer progression (Figure 1).7 For instance, studies have shown that shear stress has been associated with enhanced metastasis8 and cancer cell death.9 In our lab, we demonstrated the synergistic effect of shear stress on TRAIL-induced apoptosis of CTCs (Figure 1C).10 These mechanical cues can be translated into biochemical responses in cells through the process of mechanotransduction.11 One form of cellular mechanotransuction occurs via the opening of mechanosensitive ion channels in response to mechanical stimuli. These ion channels transduce mechanical forces by facilitating the transport of second-messenger ions12 such as calcium, an ion that has been heavily implicated in cancer metastasis and apoptosis.13,14 Because of the many roles of calcium and other cations in cells, mechanosensitive ion channels represent an important area of study that could lead to novel anti-metastatic cancer therapy approaches. For example, the mechanosensitive ion channels TRPC1, TRPC3, TRPC6, TRPM4, TRPM7, TRPV4 and P2X7 have all been implicated in cancer metastasis.15–21 This review focuses on the mechanosensitive ion channels TRPV4 and P2X7, however, as these two ion channels have been more extensively studied with respect to cancer metastasis than the other channels mentioned, whereas the functions of other ion channels are only just beginning to emerge. Furthermore, both channels have been specifically linked to the progression of multiple steps in cancer metastasis and also in cancer cell death, illustrating the varied roles these ion channels can play in cancer progression (Table 1).20–23
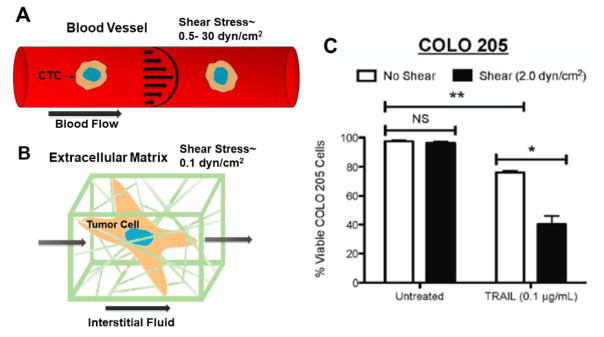
Figure 1. Changes is cell functionality in response to shear stress.
(A) Circulating tumor cell (CTC) exposed to shear stress in blood flow (B) Tumor cell within its extracellular matrix, exposed to interstitial flows (C) When highly metastatic colon cancer cells COLO 205 were exposed to shear stress, the cells were sensitized to TRAIL-induced apoptosis with a significant decrease in cell viability of approximately 30%. *P < 0.05. **P < 0.01. NS: non-significant. Reprinted from Mitchell and King, New J. Phys, 2013.10
Table 1.
Role of TRPV4 and P2X7 in Cancer Metastasis and Cell Death
| Metastatic Step | TRPV4 | P2X7 | ||
|---|---|---|---|---|
|
| ||||
| Promotion and Inhibition | Cell Lines | Promotion and Inhibition | Cell Lines | |
| Angiogenesis | Promoted by increasing tumor endothelial cell migration. | Breast and renal derived tumor endothelial cells | Promoted by increasing VEGF secretion. | B16, CT26, ACN |
| Inhibited angiogenesis by reducing Rho activity. | Lewis lung carcinoma cells | |||
| Intravasation | Promoted by Increased AKT activity, Wnt/β-catenin signaling, FAK activation, cell membrane protrusions and membrane deformability. Also by decreased E-cadherin, ERM proteins, and F-actin. | 4T07,4T1, MDA-MB-468, MDA-MB-435, MKN45, SGC-7901 | Promoted by increased secretion of AKT activity, MMPs, cysteine cathepsin B, and cell membrane protrusions. Also decreases E-cadherin expression and TIMPs activity. | MDA-MB-435, T47D, PC-9 |
| Extravasation | Promoted by increased cell deformability, and decreased E-cadherin integrin expression. | 4T07, MDA-MB-468, 4T1 | Promoted by increased MMP secretion. Also promotes colonization by increasing a cell’s survivability in nonoptimal conditions. | 4T07, MDA-MB-435, MDA-MB-231, WM239 |
|
| ||||
| Cell Death | TRPV4 | P2X7 | ||
|
| ||||
| Promotion and Inhibition | Cell Lines | Promotion and Inhibition | Cell Lines | |
| Apoptosis | Promoted by mitochondrial destabilization, releasing cytochrome c, and increasing caspase 3 and 7 activity. | MDA-MB-468 | Promoted by mitochondrial destabilization, releasing cytochrome c, and increasing caspase 3 and 7 activity. | ASPC-1, BXPC-3, CAPAN-1, MIAPACA-2, PANC-1 |
| Oncosis | Promoted by rapid calcium and sodium influx causing ATP depletion by sustained activation Na+/K+ ATPase and Ca2+ ATPase. | MDA-MB-468 | Promoted by rapid calcium and sodium influx causing ATP depletion by sustained activation Na+/K+ ATPase and Ca2+ ATPase. | Yet to be shown in cancer cells. Shown in human leukocytes. |
2. Mechanisms of TRPV4 and P2X7 Activation from Mechanical Stimuli
2.1. TRPV4 Activation
TRPV4 is a homotetrameric ion channel protein.24 TRPV4 contains six transmembrane regions, and one pore-forming subunit between transmembrane regions five and six that transports cations with a preference for calcium.25 This ion channel protein also contains multiple protein kinase C (PKC) and protein kinase A (PKA) phosphorylation sites,26 and a calmodulin binding site required for calcium-dependent activation (Figure 2).27
Figure 2.
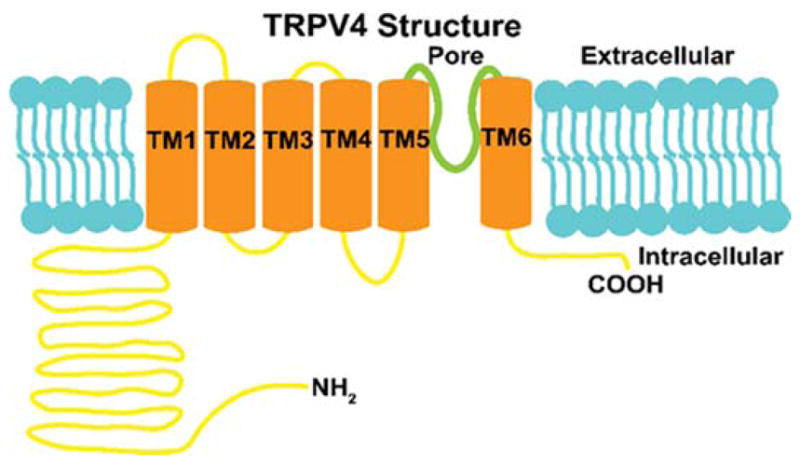
TRPV4 contains 6 transmembrane regions with a pore between transmembrane regions 5 and 6. The TRPV4 protein also has multiple PKA, PKC, and calmodulin binding sites that effect channel functionality.
TRPV4 has two primary non-independent mechanical activation pathways. One pathway of mechanical activation is by Phospholipase A2 (PLA2).28 PLA2 can access and cleave sn2-ester bonds of cellular phospholipids when cell membranes are stretched by mechanical strains, such as osmotic swelling or shear stress.29,30 This cleavage results in arachidonic acid (AA), which is metabolized by cytochrome p450 to epoxyeicosatrienoic acid (EET).31 EET is an agonist for TRPV4, causing the channel to open.28
Phospholipase C (PLC) is also important in the activation of TRPV4.32, PLC can be activated by the GPCR angiotensin II type 1 receptor (A2TR1) in response to mechanical stimulation.33,34 When A2TR1 is exposed to shear stress, an activating conformational shift of the receptor occurs, causing downstream activation of PLC.35,36 PLC subsequently cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol trisphosphate (IP3).37 DAG activates PKC,38,39 which phosphorylates TRPV4, sensitizing the ion channel to EET.40 IP3 also assists in the of activation TRPV4 through TRPV4’s calcium dependent calmodulin binding site.27 IP3 binds to IP3 receptors on the endoplasmic reticulum causing calcium to be released into the cytoplasm.41 The calcium then causes the calmodulin to bind to TRPV4, allowing for enhanced potentiation of the ion channel.27 Some PLA2 isoforms require calcium binding to their C2 domain for activation,42 suggesting IP3 induced calcium release may be important for sustained activation of TRPV4 as well.
2.2. P2X7 Activation
P2X7 is a trimeric purinergic receptor consisting of two transmembrane domains, an extracellular ATP ligand-binding domain, and an integral ion channel.43 In the trimeric structure of P2X7 there are 3 total ATP binding domains (Figure 3).44 Within milliseconds of ATP binding, its integral membrane pore opens, allowing for a small conductance of cations, such as calcium. When the P2X7 receptor has sustained activation of tens of seconds by ATP, a larger pore is formed that increases cation conductance, causing the pore to be permeable to 900 Da proteins.43,45
Figure 3.
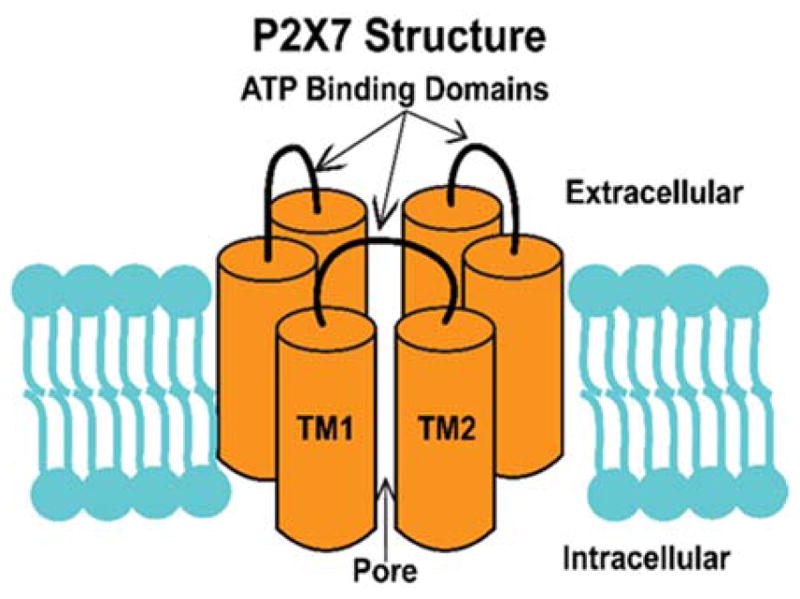
P2X7 is hometrimeric structure with 3 extracellular ATP binding domains. As P2X7 binds ATP, its inner pore region opens allowing for the flux of ions.
The time-dependent cation conductance of P2X7 can be explained by two contrasting mechanisms. (1) ATP binds to P2X7 causing a conformational change that dilates the initially small P2X7 cation pore. (2) Sustained P2X7 activation causes the opening of distinct protein pores via direct signaling or second-messengers.46 A reconciling mechanism is that when a single ATP molecule is bound to P2X7, the ligand-binding domain undergoes a conformational shift, making it more difficult for other ATPs to bind. After a second ATP is bound, the small cation pore opens, while simultaneously causing another conformational change, increasing steric hinderance for the binding of the third and final ATP. Once the third ATP binds, the P2X7 pore dilates allowing for large molecules and increased amounts of cations to enter the cell. The influx of cations can then activate other pores that are able to transport large proteins in addition to the dilated pore of P2X7.47 This also explains the time-dependence of P2X7’s pore dilation because more time is required to overcome the increasing difficulty of binding 3 ATPs.
P2X7’s method of mechanotransduction is indirect. Rather than a mechanical stimulus directly activating P2X7, another channel is altered and signals P2X7’s activation downstream. Panx-1 is a mechanosensitive channel that releases ATP into the extracellular fluid via shear stress and osmotic pressure.48–50 The Panx-1 released ATP then binds to P2X7’s binding domain causing the opening of the P2X7 and other associated pores.49
3. Role of TRPV4 and P2X7 in Cancer Metastasis
3.1. Angiogenesis
Angiogenesis is the growth of tumor neovasculature that develops in response to an increased nutrient and oxygen demand for the growing malignancy.51 In addition to providing nutrients, the tumor neovasculature promotes dissemination by creating a route of escape for cancer cells to enter the hematogenous circulation.52,53 The loose endothelial gap junctions within tumor vasculature allow for easier cancer cell intravasation compared to normal vessels.54
3.1.1. TRPV4
Arachidonic acid-based calcium entry has been linked to angiogenesis in breast derived tumor endothelial cells,55 leading to the suspicion that TRPV4 may play a significant role in angiogenesis. In another study, TRPV4 was upregulated in breast and renal tumor derived endothelial cells relative to healthy endothelial cells.56 TRPV4 stimulation by AA and subsequent calcium conductance was correlated with increased endothelial cell migration for breast tumor derived endothelial cells. Migration of healthy endothelial cells, however, was not observed. Endothelial cell migration is important in angiogenesis, as it allows endothelial cells to organize and form new blood vessels.57 In addition, calcium and AA inhibition eliminated tumor derived endothelial cell migration, further supporting TRPV4’s role in angiogenesis.56
Conversely, another study by Adapala et al. showed that TRPV4 expression was found to downregulate angiogenesis. In this study, TRPV4 expression was found to be downregulated in tumor endothelial cells with reduced calcium influx compared to healthy endothelial cells. When TRPV4 was activated by the small molecule agonist GSK1016790A, tumor vasculature was normalized.58 In a mouse angiogenesis study where mice were subcutaneously injected with Lewis lung carcinoma cells, TRPV4 knock out mice showed increased amounts of neovasculature with malformed blood vessels and large vessel diameters, in comparison to wild type mice. In this mouse study it was suggested that TRPV4 reduces angiogenesis by inhibiting Rho activity because a Rho kinase inhibitor in mice lacking TRPV4 was used to normalize tumor vasculature.59 TRPV4’s regulation of Rho activity is significant as Rho activity is known to enhance angiogenesis by altering the cellular cytoskeleton, allowing for increased motility of tumor endothelial cells.60
The disparate results may be explained by the different methods of studies used to study TRPV4. TRPV4 activation was correlated with enhanced migration for breast tumor derived endothelial cells with the use of a TRPV4 agonist.56 The TRPV4 mouse model study showed that TRPV4 deletion caused an increase in Rho activity, leading to increased angiogenesis.59 This suggests that basal levels of TRPV4 activation, as is the case with the later study, may inhibit angiogenesis, while over-activation of TRPV4 by an agonist or mechanical stimulation may increase angiogenesis.
3.1.2. P2X7
Tumor microenvironments are known to have high concentrations of ATP, whereas healthy tissues have negligible amounts of ATP in the extracellular fluid.61 This high extracellular concentration of ATP is due to increased secretion by cancer cells in response to mechanical stimuli, such as shear stress, cell swelling and stretching, resulting in P2X7 stimulation.62,63
P2X7 activation in B16 mouse melanoma cells, CT26 mouse colon cancer cells and ACN human neuroblastoma cells resulted in the secretion of vascular endothelial growth factor (VEGF).64–67 VEGF is important for the dissemination of cancer cells as it causes endothelial cells to proliferate and migrate to create neovasculature.68–70 This effect of P2X7 activation has made P2X7 a target for anti-cancer therapies. Mice injected with B16 cells were treated with benzoyl ATP (BzATP), an agonist of P2X7 or a selective inhibitor of P2X7, AZ10606120. Mice treated with AZ10606120 had significantly reduced VEGF secretion and vessel formation compared to mice treated with BzATP.64 Oxidized ATP, an inhibitor of P2X7, was used to block the development of a CT26 mouse model engineered to express P2X7.64 This inhibition resulted in the reduction of tumor size and VEGF secretion. The study was also successful in using shRNA against P2X7 to block tumor growth and VEGF secretion.64 Another study injected neuroblastoma ACN cells into immunocompromised mice and saw significant reduction in VEGF secretion and tumor growth using a P2X7 inhibitor.67 These studies indicate that P2X7 activation plays a prominent role in tumor angiogenesis by controlling the secretion of VEGF.
3.2. Intravasation
Efficient dissemination is contingent upon tumor cells’ ability to enter the vasculature.71 A major barrier in tumor cell intravasation is the basement membrane (BM), which acts as a barrier between the epithelium and the stroma which surrounds subjacent blood vessels.71 The BM is normally tightly controlled in healthy tissue; however, cancer cells have been shown to release proteases to promote proteolysis and subsequent degradation of the BM, thus providing a route for cells to enter the stroma. This active motility is not present in normal epithelial cells, and is selective to mutant cells that have undergone the epithelial-to-mesenchymal transition (EMT).72 This process takes an ordered epithelial cell with an apical-basal polarity and employs a series of morphological changes to transition into a less polar, spindle-shaped mesenchymal cell.72–74 In addition, the EMT results in the loss of many cellular junction proteins, such as fibronectin and E-cadherin, which creates a more motile cell that is able to invade the ECM.75
3.2.1. TRPV4
TRPV4 activation has been shown to promote factors characteristic of an invasive cancer phenotype in breast cancer20,22 and gastric cancer.76 In both cancer types, protein kinase B (AKT) activity was significantly upregulated in comparison to healthy control cells.20,76 Activated AKT has been found to promote EMT by reducing the expression of E-cadherin,73–75 such as in the breast cancer study of TRPV4’s role in metastasis in 4T07 cells,20 or by causing E-cadherin to localize to the cytoplasm76 as in prior studies of the gastric cancer cell lines MKN45 and SGC-7901.72 This lack of cell-cell adhesion results in increased cell motility.78,79 The downregulation of E-cadherin also frees its binding partner β-catenin to localize to the nucleus where it is available for participation in Wnt/β-catenin signaling, which is known to further promote EMT.78,80 By silencing or inhibiting TRPV4, AKT expression and Wnt/β-catenin signaling was reduced, while E-cadherin expression was increased.20,76 The prior TRPV4 4T07 breast cancer studies also showed significant upregulation of focal adhesion kinase (FAK) activation.20 FAK has been correlated with highly malignant human cancers81 and has been shown to promote cell motility by destabilizing the cytoskeleton through its interactions with small GTPases,82 producing podosomes and invadopodia.83
In another breast cancer study, TRPV4 upregulation in 4T07 and 4T1 cells was found to increase cell deformability, enhancing cell migration.22 This was supported by the fact that membrane blebbing has been found to enhance a cell’s ability to transmigrate across the endothelium by allowing cells to more easily deform to pass through the ECM84 and cell junctions in the endothelium.85 In cancer, this gives cells an enhanced ability to intravasate and extravasate. It was shown that TRPV4 interacts directly with the cytoskeleton,86 suggesting TRPV4 may directly modify cytoskeletal structure. MDA-MB-468 cells engineered to over-express TRPV4- also showed that TRPV4 expression regulates the expression of multiple proteins that effect cytoskeletal stabilization.22 The TRPV4 over-expressing MDA-MB-468 cells showed decreased expression of ezrin, radixin and moesin (ERM) proteins by 10–20%,22 which is notable as ERM proteins can bind to integral membrane proteins and actin to reduce membrane blebbing.87,88 In the TRPV4 over-expressing cells, cofilin phosphorylation was reduced by 1.45 fold.22 Reduced cofilin phosphorylation is significant as dephosphorylated cofilin promotes actin depolymerization.89 Furthermore, TRPV4 expression was shown to increase G-actin content and reduce F-actin, thereby increasing cell deformability.22
3.2.2. P2X7
Increased expression and activation of P2X7 has been linked with a variety of invasive cancer lineages, from lung cancer90 to thyroid cancer,91 lymphoma,92 pancreatic cancer,21 prostate cancer,93 and breast cancer.90,94,95 P2X7 activation correlates with increased activity of the specific metalloproteases (MMP) 3, 9, and 13 in 4T07, MDA-MB-435, and leukocytes.94–96 MMPs are proteolytic enzymes that play a key role in cancer metastasis as they can degrade virtually all types of ECM protein, assisting cancer cells in exiting the tumor and intravasating into the vasculature.97 The increased activity of MMP-9 is particularly important for intravasation, as shown by Kim et al., in which only cancer cells that expressed MMP-9 (ie. HEp3, HT1080, MDA-MB-231, and PC3) were capable of intravasating.98 That is, the cancer cells lacking MMP-9 expression, such as the MCF-7 cell line, exhibited negligible intravasation. One mechanism by which P2X7 regulates MMP activity is through the release of cysteine cathepsin B.95,99 Cathepsin B is a proteolytic enzyme that cleaves tissue inhibitors of metalloproteases (TIMP), rendering the TIMPs nonfunctional.100 The inactivation of TIMPs reduces the inhibition of MMPs, allowing for greater ECM degradation.99 This implies that P2X7 may play a major role in cancer invasion by decreasing MMP inhibition to promote ECM degradation.
P2X7 also aids in intravasation by promoting EMT. Like TRPV4, P2X7 activation has been linked to E-cadherin loss.93,94 The decrease in E-cadherin by P2X7 activation could be caused by multiple mechanisms, particularly through MMP-3 secretion as shown in MDA-MB-435 cells,93 and enhanced AKT signaling in T47D breast cancer cells.94,101 MMP-3 secretion by P2X7 is presumed to be significant to EMT because of MMP-3’s ability to cleave E-cadherin.102
Extracellular ATP has been shown to promote actin remodeling,103,104 implying P2X7 may have a role in this process as well. Actin remodeling is another behavior associated with EMT and has been known to increase cell motility through the formation of cellular protrusions, such as filipodia and lamellipodia 99,100,104,105 The proposed role of P2X7 in actin remodeling varies by study, but is not necessarily mutually exclusive between roles. In a breast cancer model of MDA-MB-435 cells, P2X7 caused a calcium influx that activates the calcium-dependent potassium channel SK3.95 SK3 channels have been previously shown to form cellular protrusions by actin remodeling and promote cell motility when intracellular calcium concentration is increased.106–108 When either P2X7 or SK3 activity was inhibited, cell protrusion formation was blocked.100 Another study in PC-9 human lung cancer cells supports the possible role of P2X7 in directing cell motility. When P2X7 expression was inhibited, the cells created lamellipodia and filipodia around the periphery of the cell membrane, causing directionless migration.109 This suggests that P2X7 may play a role in regulating lamellipodia formation by localizing it to SK3 channels.
3.3. Extravasation
The steps of extravasation mirror the steps of intravasation.110 After arrest of CTCs at a distal site, a microcolony may form that causes the vasculature to rupture, exposing the CTCs to the surrounding tissue. Alternatively, cells can penetrate the endothelial wall by increasing hyperpermeability through the secretion of vascular permeability factors, such as VEGF.111 Once entering the tissue parenchyma, a mesenchymal to epithelial (MET) transition occurs, now promoting cell polarity and cadherin junctions to decrease motility.74 The notion that certain organs are predisposed to dissemination has been investigated for decades, starting with the “seed and soil” hypothesis proposed by Stephen Paget.112,113 This widely adopted theory recognizes that certain tumor cells preferentially extravasate and colonize distant organs with hospitable milieus. Just as a seed requires the appropriate soil to grow, metastatic cancer cell survival is contingent upon the organ microenvironment where extravasation occurs.
3.3.1. TRPV4
TRPV4 expression has been shown to correlate with a cell’s metastatic potential to extravasate. This has been demonstrated by showing that TRPV4 is upregulated in metastatic breast cancer cell lines 4T07 and 4T1 that have undergone extravasation, compared to basal levels expressed in primary tumors that have unsuccessfully extravasated to distant organs.22 In addition, expression of phosphorylated proteins in breast cancer cells expressing TRPV4 show similarities in protein phosphorylation to that of leukocytes during immune modulated extravasation.15 These phosphorylates include paxillin, β-catenin, and ezrin, all key proteins in promoting actin cytoskeleton deformation, implying that TRPV4 may be essential for the phosphorylation of these proteins that contribute to successful extravasation.112–114
It is evident that this channel upregulation plays a key role in priming migrating cancer cell morphology for extravasation by increasing cell deformability as seen in 4T07 and MDA-MB-468 cells.20,22 Similar to intravasation, this TRPV4 activation initiates actin depolymerization in the cell cortex, reducing cell shear modulus and stiffness. This morphological softening allows for a more motile cancer cell that is capable of exiting the vasculature by passing through loose endothelial junctions.117 This increased cell motility is also attributed to E-cadherin and integrin downregulation, as described previously.79,118 Holistically, the upregulation of TRPV4 consequently leads to a cancer cell with a more aggressive phenotype that is able to overcome physiological barriers in surviving hematogenous transit and initial arrest in distant tissues.20 This notion is supported by clinical samples of patient tumors, as TRPV4 upregulated tumors correlate with poor prognosis and an increase in metastatic lesions.20
3.3.2. P2X7
The role of P2X7 in extravasation is more complex than that of TRPV4. P2X7 can act as a growth-promoting or death-inducing receptor, contingent upon the levels of stimulation by extracellular ATP.119,120 Under normal conditions, P2X7 upregulation increases ATP production and induces cell proliferation in the absence of serum. This cellular response is likely to play a key role in promoting metastatic cell survival in foreign environments, enhancing survival and growth in the absence of microenvironmental nutrients present in the primary tumor niche.119
As previously mentioned, P2X7 activation has been linked to an increase in metalloprotease activity in the breast cancer lines 4T07 and MDA-MB-435.94–96 This MMP activation plays a major role in ECM degradation, making P2X7 an indirect protagonist in the promotion of extravasation. During the initial stages of transendothelial extravasation, MT-MMP’s localize to the leading edge of tumor cells, thereby anchoring the cells to endothelial junctions while promoting MMP activity to degrade the surrounding matrix.121 This MMP localization and production aids in proteolysis of the BM, promoting tumor growth and migration. Indeed, a study by Voura et al. found that for both MDA-MB-231 and WM239 cancer cells, extravasation can be reduced by over 35% j through MMP inhibition alone, motivating further research into inhibitory MMP drug candidates to reduce metastatic potential,121 for which P2X7 may be a promising target.
4. Cell Death
Although TRPV4 and P2X7 have been implicated extensively in the progression of metastasis, both ion channels have been shown to induce cancer cell death.23,122 TRPV4 and P2X7 induce apoptosis via the intrinsic pathway, permeabilizing the mitochondrial membrane to initiate the release of apoptotic enzymes.123,124 The apoptotic enzymes released consist of cytochrome c, apoptosis inducing factor (AIF), Smac/DIABLO, Omi/HtrA2 and endonuclease G.125 Cytochrome c is of particular importance because upon entering the cytosol, it forms the apoptosome with Apaf-1 and caspase 9.126,127 The formation of the apoptosome recruits the inactive forms of the effector caspases, caspase 3 and caspase 7.128 Caspases 3 and 7 are proteolytic enzymes that cleave proteins leading to cell death.129
TRAIL-induced apoptosis also results in caspase activity causing cell death.130 The implication is that mechanosensitive ion channels may sensitize colon cancer cells to TRAIL-induced apoptosis by further increasing caspase activity through a separate mechanism (Figure 1).10
TRPV4 has also been linked to the induction of cancer cell oncosis.23 P2X7 has yet to be linked to oncosis in cancer cells, but P2X7-induced oncosis has been shown in murine leukocytes.131 Oncosis is a form of cell death associated with cell swelling, organelle swelling, blebbing and increased membrane permeability caused by a critical depletion in ATP.23,132,133
4.1. TRPV4
Upon activation, TRPV4 induced apoptosis in the breast cancer cell line MDA-MB-468 by causing a major influx of calcium, resulting in a critical increase of cytosolic calcium.23,134 When TRPV4 activation raises the cytosolic calcium concentration sufficiently high, calcium is transported into the inner mitochondrial matrix where calcium can open the mitochondrial permeability transition pore (PTP).135 The opening of the PTP then causes the mitochondria to osmotically swell, rupturing the outer mitochondrial membrane.136,137 The rupturing of the outer membrane releases apoptotic enzymes, such as cytochrome c, which is stored in the space between the mitochondria’s outer membrane and inner membrane.138 TRPV4 activation also induces oncosis in MDA-MB-468 cells by promoting calcium and sodium influx. To remove the excess amounts of these ions from inside the cell, Na+/K+ ATPase and Ca2+ ATPase channels experience sustained activation, causing ATP depletion.23
4.2. P2X7
P2X7 also causes apoptosis in cancer cells by activating caspases 3 and 7.21,122,139 These apoptotic cancer cells were also associated with a significantly increased calcium influx,21 implying that P2X7 mediates apoptosis via the intrinsic pathway. Using pancreatic ductal adenocarcinoma cells including PANC1 and CAPAN1, it was found that the concentration of extracellular ATP determines if P2X7 will induce apoptosis or initiate the pro-metastatic changes described previously. When less ATP was present, P2X7 promoted cancer metastasis and growth. However, when more ATP was present, P2X7 caused cell death.21 This contradiction based on extracellular ATP concentration is most likely due to P2X7’s differing responses to sustained activation.140 When P2X7 does not have sufficient ATP available it only allows limited influx of calcium, causing pro-metastatic changes in cancer cells. However when more ATP is present, the calcium influx becomes toxic.
Oncosis is presumably caused by P2X7 in a similar manner to TRPV4. P2X7 creates a high cytosolic calcium concentration that must then be equilibrated using Ca2+ ATPases, depleting intracellular ATP content. P2X7 may further contribute to oncotic cell death through the opening and dilation of downstream pores that are capable of transporting ATP out of the cell,141 further lowering cellular ATP.
5. Clinical Trails
TRPV4 and P2X7 have yet to be explored as therapeutic targets for cancer treatment in clinical trials. However, antagonists for both ion channels are receiving attention with respect to other diseases. The P2X7 antagonists AZD9056 and CE-224,535 have both been tested in phase II clinical trials as rheumatoid arthritis treatments.142,143 Neither drug was deemed efficacious in treating rheumatoid arthritis, but both demonstrated acceptable safety, implying the potential of these drugs as safe anti-metastatic cancer therapies. The TRPV4 antagonist GSK2798745 was tested in a clinical trial for the treatment of congestive heart failure (https://clinicaltrials.gov indicator: NCT02497937). Unfortunately, the results and safety of this trial are yet to be reported at the time of publication of this article. To our knowledge, TRPV4 and P2X7 agonists have not yet been studied in clinical trials for any disease type. The lack of clinical trials using P2X7 and TRPV4 antagonists and agonists for cancer treatment leaves much room for the innovation of novel therapies based on these two ion channels as targets.
6. Conclusions
TRPV4 and P2X7 exemplify the complex roles that mechanosensitive ion channels have in cancer cell dissemination, and motivate the need for further study of these types of channels. Due to the transport of calcium, TRPV4 and P2X7 are host to a variety of pro-metastatic pathways, making the channels appear to be attractive targets for anti-metastatic cancer therapies. However, these channels have also been implicated in causing cancer cell death. This presents a challenge for the targeting of mechanosensitive ion channels for cancer therapies, as a tight therapeutic window is needed to maintain the beneficial effects of the ion channels, while preventing adverse behaviors. Determining the proper levels of activation for TRPV4, P2X7 and other mechanosensitive ion channels to leverage the apoptotic effects of these channels, while preventing the stimulation of pro-metastatic pathways, could lead to novel cancer therapies.
References
- 1.Chaffer CL, Weinberg RA. A Perspective on Cancer Cell Metastasis. Science. 2011;331(6024):1559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 2.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6(6):449–58. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 3.Su Z, Yang Z, Xu Y, Chen Y, Yu Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer. 2015;14(1):48. doi: 10.1186/s12943-015-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eger A, Mikulits W. Models of epithelial–mesenchymal transition. Drug Discov Today Dis Models. 2005;2(1):57–63. [Google Scholar]
- 5.van Zijl F, Krupitza G, Mikulits W. Initial steps of metastasis: Cell invasion and endothelial transmigration. Mutat Res Mutat Res. 2011;728(1–2):23–34. doi: 10.1016/j.mrrev.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mack GS, Marshall A. Lost in migration. Nat Biotechnol. 2010;28(3):214–29. doi: 10.1038/nbt0310-214. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell MJ, King MR. Computational and experimental models of cancer cell response to fluid shear stress. Front Oncol. 2013;3:44. doi: 10.3389/fonc.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng. 2014;16:321–46. doi: 10.1146/annurev-bioeng-071813-105259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell MJ, Denais C, Chan MF, Wang Z, Lammerding J, King MR. Lamin A/C deficiency reduces circulating tumor cell resistance to fluid shear stress. Am J Physiol - Cell Physiol. 2015;309(11):C736–46. doi: 10.1152/ajpcell.00050.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell MJ, King MR. Fluid Shear Stress Sensitizes Cancer Cells to Receptor-Mediated Apoptosis via Trimeric Death Receptors. New J Phys. 2013;15:015008. doi: 10.1088/1367-2630/15/1/015008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paluch EK, Nelson CM, Biais N, et al. Mechanotransduction: use the force(s) BMC Biol. 2015;13:47. doi: 10.1186/s12915-015-0150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinac B. Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci. 2004;117(12):2449–60. doi: 10.1242/jcs.01232. [DOI] [PubMed] [Google Scholar]
- 13.Prevarskaya N, Skryma R, Shuba Y. Calcium in tumour metastasis: new roles for known actors. Nat Rev Cancer. 2011;11(8):609–18. doi: 10.1038/nrc3105. [DOI] [PubMed] [Google Scholar]
- 14.Schwarz EC, Qu B, Hoth M. Calcium, cancer and killing: The role of calcium in killing cancer cells by cytotoxic T lymphocytes and natural killer cells. Biochim Biophys Acta BBA - Mol Cell Res. 2013;1833(7):1603–11. doi: 10.1016/j.bbamcr.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 15.He B, He B, Liu F, et al. Silencing TRPC1 expression inhibits invasion of CNE2 nasopharyngeal tumor cells. Oncol Rep. 2012;27(5):1548–54. doi: 10.3892/or.2012.1695. [DOI] [PubMed] [Google Scholar]
- 16.Yang SL, Cao Q, Zhou KC, Feng YJ, Wang YZ. Transient receptor potential channel C3 contributes to the progression of human ovarian cancer. Oncogene. 2009;28(10):1320–8. doi: 10.1038/onc.2008.475. [DOI] [PubMed] [Google Scholar]
- 17.Yang L-L, Liu B-C, Lu X-Y, et al. Inhibition of TRPC6 reduces non-small cell lung cancer cell proliferation and invasion. Oncotarget. 2016;8(3):5123–34. doi: 10.18632/oncotarget.14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holzmann C, Kappel S, Kilch T, et al. Transient receptor potential melastatin 4 channel contributes to migration of androgen-insensitive prostate cancer cells. Oncotarget. 2015;6(39):41783–93. doi: 10.18632/oncotarget.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Middelbeek J, Kuipers AJ, Henneman L, et al. TRPM7 is required for breast tumor cell metastasis. Cancer Res. 2012;72(16):4250–61. doi: 10.1158/0008-5472.CAN-11-3863. [DOI] [PubMed] [Google Scholar]
- 20.Lee WH, Choong LY, Jin TH, et al. TRPV4 plays a role in breast cancer cell migration via Ca2+-dependent activation of AKT and downregulation of E-cadherin cell cortex protein. Oncogenesis. 2017;6(5):e338. doi: 10.1038/oncsis.2017.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannuzzo A, Pedersen SF, Novak I. The P2X7 receptor regulates cell survival, migration and invasion of pancreatic ductal adenocarcinoma cells. Mol Cancer. 2015;14:203. doi: 10.1186/s12943-015-0472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee WH, Choong LY, Mon NN, et al. TRPV4 Regulates Breast Cancer Cell Extravasation, Stiffness and Actin Cortex. Sci Rep. 2016;6:27903. doi: 10.1038/srep27903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters AA, Jamaludin SYN, Yapa KTDS, et al. Oncosis and apoptosis induction by activation of an overexpressed ion channel in breast cancer cells. Oncogene. 2017 doi: 10.1038/onc.2017.234. [DOI] [PubMed] [Google Scholar]
- 24.White JPM, Cibelli M, Urban L, Nilius B, McGeown JG, Nagy I. TRPV4: Molecular Conductor of a Diverse Orchestra. Physiol Rev. 2016;96(3):911–73. doi: 10.1152/physrev.00016.2015. [DOI] [PubMed] [Google Scholar]
- 25.Nilius B, Watanabe H, Vriens J. The TRPV4 channel: structure-function relationship and promiscuous gating behaviour. Pflüg Arch. 2003;446(3):298–303. doi: 10.1007/s00424-003-1028-9. [DOI] [PubMed] [Google Scholar]
- 26.Nilius B, Vriens J, Prenen J, Droogmans G, Voets T. TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol - Cell Physiol. 2004;286(2):C195–205. doi: 10.1152/ajpcell.00365.2003. [DOI] [PubMed] [Google Scholar]
- 27.Strotmann R, Schultz G, Plant TD. Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C-terminal calmodulin binding site. J Biol Chem. 2003;278(29):26541–9. doi: 10.1074/jbc.M302590200. [DOI] [PubMed] [Google Scholar]
- 28.Berna-Erro A, Izquierdo-Serra M, Sepúlveda RV, et al. Structural determinants of 5′,6′-epoxyeicosatrienoic acid binding to and activation of TRPV4 channel. Sci Rep. 2017;7(1):10522. doi: 10.1038/s41598-017-11274-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehtonen JY, Kinnunen PK. Phospholipase A2 as a mechanosensor. Biophys J. 1995;68(5):1888–94. doi: 10.1016/S0006-3495(95)80366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen S, Lambert IH, Thoroed SM, Hoffmann EK. Hypotonic cell swelling induces translocation of the alpha isoform of cytosolic phospholipase A2 but not the gamma isoform in Ehrlich ascites tumor cells. Eur J Biochem. 2000;267(17):5531–9. doi: 10.1046/j.1432-1327.2000.01615.x. [DOI] [PubMed] [Google Scholar]
- 31.Vriens J, Owsianik G, Fisslthaler B, et al. Modulation of the Ca2 Permeable Cation Channel TRPV4 by Cytochrome P450 Epoxygenases in Vascular Endothelium. Circ Res. 2005;97(9):908–15. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi N, Hamada-Nakahara S, Itoh Y, et al. TRPV4 channel activity is modulated by direct interaction of the ankyrin domain to PI(4,5)P2. Nat Commun. 2014;5:4994. doi: 10.1038/ncomms5994. [DOI] [PubMed] [Google Scholar]
- 33.Guo DF, Sun YL, Hamet P, Inagami T. The angiotensin II type 1 receptor and receptor-associated proteins. Cell Res. 2001;11(3):165–80. doi: 10.1038/sj.cr.7290083. [DOI] [PubMed] [Google Scholar]
- 34.Mercado J, Baylie R, Navedo MF, et al. Local control of TRPV4 channels by AKAP150-targeted PKC in arterial smooth muscle. J Gen Physiol. 2014;143(5):559–75. doi: 10.1085/jgp.201311050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang W, Strachan RT, Lefkowitz RJ, Rockman HA. Allosteric Modulation of β-Arrestin-biased Angiotensin II Type 1 Receptor Signaling by Membrane Stretch. J Biol Chem. 2014;289(41):28271–83. doi: 10.1074/jbc.M114.585067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou Y, Akazawa H, Qin Y, et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol. 2004;6(6):499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- 37.Putney JW, Tomita T. Phospholipase C Signaling and Calcium Influx. Adv Biol Regul. 2012;52(1):152–64. doi: 10.1016/j.advenzreg.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47(6):859–66. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- 39.You J-S, Lincoln HC, Kim C-R, et al. The Role of Diacylglycerol Kinase ζ and Phosphatidic Acid in the Mechanical Activation of Mammalian Target of Rapamycin (mTOR) Signaling and Skeletal Muscle Hypertrophy. J Biol Chem. 2014;289(3):1551–63. doi: 10.1074/jbc.M113.531392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan H-C, Zhang X, McNaughton PA. Activation of the TRPV4 Ion Channel Is Enhanced by Phosphorylation. J Biol Chem. 2009;284(41):27884–91. doi: 10.1074/jbc.M109.028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta BBA - Mol Cell Res. 2009;1793(6):933–40. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50(Suppl):S237–242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei L, Caseley E, Li D, Jiang L-H. ATP-induced P2X Receptor-Dependent Large Pore Formation: How Much Do We Know? Front Pharmacol. 2016;7:5. doi: 10.3389/fphar.2016.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chataigneau T, Lemoine D, Grutter T. Exploring the ATP-binding site of P2X receptors. Front Cell Neurosci. 2013;7:273. doi: 10.3389/fncel.2013.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiley JS, Sluyter R, Gu BJ, Stokes L, Fuller SJ. The human P2X7 receptor and its role in innate immunity. Tissue Antigens. 2011;78(5):321–32. doi: 10.1111/j.1399-0039.2011.01780.x. [DOI] [PubMed] [Google Scholar]
- 46.Pelegrín P. Many ways to dilate the P2X7 receptor pore. Br J Pharmacol. 2011;163(5):908–11. doi: 10.1111/j.1476-5381.2011.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan Z, Khadra A, Li S, Tomic M, Sherman A, Stojilkovic SS. Experimental characterization and mathematical modeling of P2X7 receptor channel gating. J Neurosci Off J Soc Neurosci. 2010;30(42):14213–24. doi: 10.1523/JNEUROSCI.2390-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 2006;25(21):5071–82. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia J, Lim JC, Lu W, et al. Neurons respond directly to mechanical deformation with pannexin-mediated ATP release and autostimulation of P2X7 receptors. J Physiol. 2012;590(10):2285–304. doi: 10.1113/jphysiol.2012.227983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Križaj D, Ryskamp DA, Tian N, et al. From Mechanosensitivity to Inflammatory Responses: New Players in the Pathology of Glaucoma. Curr Eye Res. 2014;39(2):105–19. doi: 10.3109/02713683.2013.836541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baeriswyl V, Christofori G. The angiogenic switch in carcinogenesis. Semin Cancer Biol. 2009;19(5):329–37. doi: 10.1016/j.semcancer.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Bielenberg DR, Zetter BR. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J Sudbury Mass. 2015;21(4):267–73. doi: 10.1097/PPO.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 54.Dudley AC. Tumor Endothelial Cells. Cold Spring Harb Perspect Med. 2012;2(3):a006536. doi: 10.1101/cshperspect.a006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pla AF, Grange C, Antoniotti S, et al. Arachidonic Acid–Induced Ca2+ Entry Is Involved in Early Steps of Tumor Angiogenesis. Mol Cancer Res. 2008;6(4):535–45. doi: 10.1158/1541-7786.MCR-07-0271. [DOI] [PubMed] [Google Scholar]
- 56.Fiorio Pla A, Ong HL, Cheng KT, et al. TRPV4 mediates tumor-derived endothelial cell migration via arachidonic acid-activated actin remodeling. Oncogene. 2012;31(2):200–12. doi: 10.1038/onc.2011.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Michaelis UR. Mechanisms of endothelial cell migration. Cell Mol Life Sci. 2014;71(21):4131–48. doi: 10.1007/s00018-014-1678-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adapala RK, Thoppil RJ, Ghosh K, et al. Activation of mechanosensitive ion channel TRPV4 normalizes tumor vasculature and improves cancer therapy. Oncogene. 2016;35(3):314–22. doi: 10.1038/onc.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thoppil RJ, Cappelli HC, Adapala RK, Kanugula AK, Paruchuri S, Thodeti CK. TRPV4 channels regulate tumor angiogenesis via modulation of Rho/Rho kinase pathway. Oncotarget. 2016;7(18):25849–61. doi: 10.18632/oncotarget.8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoang MV, Whelan MC, Senger DR. Rho activity critically and selectively regulates endothelial cell organization during angiogenesis. Proc Natl Acad Sci. 2004;101(7):1874–9. doi: 10.1073/pnas.0308525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Virgilio FD. Increased Level of Extracellular ATP at Tumor Sites: In Vivo Imaging with Plasma Membrane Luciferase. PLOS ONE. 2008;3(7):e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta BBA - Mol Cell Res. 2008;1783(5):673–94. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 63.Pedersen S, Pedersen SF, Nilius B, Lambert IH, Hoffmann EK. Mechanical stress induces release of ATP from Ehrlich ascites tumor cells. Biochim Biophys Acta BBA - Biomembr. 1999;1416(1–2):271–84. doi: 10.1016/s0005-2736(98)00228-4. [DOI] [PubMed] [Google Scholar]
- 64.Adinolfi E, Raffaghello L, Giuliani AL, et al. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res. 2012;72(12):2957–69. doi: 10.1158/0008-5472.CAN-11-1947. [DOI] [PubMed] [Google Scholar]
- 65.Adinolfi E, Capece M, Amoroso F, De Marchi E, Franceschini A. Emerging roles of P2X receptors in cancer. Curr Med Chem. 2015;22(7):878–90. doi: 10.2174/0929867321666141012172913. [DOI] [PubMed] [Google Scholar]
- 66.Hattori F, Ohshima Y, Seki S, et al. Feasibility study of B16 melanoma therapy using oxidized ATP to target purinergic receptor P2X7. Eur J Pharmacol. 2012;695(1–3):20–6. doi: 10.1016/j.ejphar.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 67.Amoroso F, Capece M, Rotondo A, et al. The P2X7 receptor is a key modulator of the PI3K/GSK3β/VEGF signaling network: evidence in experimental neuroblastoma. Oncogene. 2015;34(41):5240–51. doi: 10.1038/onc.2014.444. [DOI] [PubMed] [Google Scholar]
- 68.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359(6398):845–8. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 69.Hoeben A, Landuyt B, Highley MS, Wildiers H, Oosterom ATV, Bruijn EAD. Vascular Endothelial Growth Factor and Angiogenesis. Pharmacol Rev. 2004;56(4):549–80. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 70.Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69(Suppl 3):4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- 71.Gupta GP, Massagué J. Cancer Metastasis: Building a Framework. Cell. 2006;127(4):679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 72.Yang J, Weinberg RA. Epithelial-Mesenchymal Transition: At the Crossroads of Development and Tumor Metastasis. Dev Cell. 2008;14(6):818–29. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 73.Chaffer CL, San Juan BP, Lim E, Weinberg RA. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016;35(4):645–54. doi: 10.1007/s10555-016-9648-7. [DOI] [PubMed] [Google Scholar]
- 74.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-Mesenchymal Transitions in Development and Disease. Cell. 2009;139(5):871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 75.Waclaw B, Bozic I, Pittman ME, Hruban RH, Vogelstein B, Nowak MA. A spatial model predicts that dispersal and cell turnover limit intratumour heterogeneity. Nature. 2015;525(7568):261–4. doi: 10.1038/nature14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xie R, Xu J, Xiao Y, et al. Calcium promotes human gastric cancer via a novel coupling of calcium-sensing receptor and TRPV4 channel. Cancer Res. 2017 doi: 10.1158/0008-5472.CAN-17-0360. canres.0360.2017. [DOI] [PubMed] [Google Scholar]
- 77.Fujita Y, Krause G, Scheffner M, et al. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol. 2002;4(3):222–31. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- 78.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-Cadherin Promotes Metastasis via Multiple Downstream Transcriptional Pathways. Cancer Res. 2008;68(10):3645–54. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 79.Frixen UH, Behrens J, Sachs M, et al. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991;113(1):173–85. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Su Y-J, Chang Y-W, Lin W-H, Liang C-L, Lee J-L. An aberrant nuclear localization of E-cadherin is a potent inhibitor of Wnt/β-catenin-elicited promotion of the cancer stem cell phenotype. Oncogenesis. 2015;4(6):e157. doi: 10.1038/oncsis.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cance WG, Harris JE, Iacocca MV, et al. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin Cancer Res Off J Am Assoc Cancer Res. 2000;6(6):2417–23. [PubMed] [Google Scholar]
- 82.Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in Mechanotransduction. J Biol Chem. 2004;279(13):12001–4. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 83.Hauck CR, Hsia DA, Ilic D, Schlaepfer DD. v-Src SH3-enhanced interaction with focal adhesion kinase at beta 1 integrin-containing invadopodia promotes cell invasion. J Biol Chem. 2002;277(15):12487–90. doi: 10.1074/jbc.C100760200. [DOI] [PubMed] [Google Scholar]
- 84.Krakhmal NV, Zavyalova MV, Denisov EV, Vtorushin SV, Perelmuter VM. Cancer Invasion: Patterns and Mechanisms. Acta Naturae. 2015;7(2):17–28. [PMC free article] [PubMed] [Google Scholar]
- 85.Fackler OT, Grosse R. Cell motility through plasma membrane blebbing. J Cell Biol. 2008;181(6):879–84. doi: 10.1083/jcb.200802081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goswami C, Kuhn J, Heppenstall PA, Hucho T. Importance of non-selective cation channel TRPV4 interaction with cytoskeleton and their reciprocal regulations in cultured cells. PloS One. 2010;5(7):e11654. doi: 10.1371/journal.pone.0011654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paluch EK, Raz E. The role and regulation of blebs in cell migration. Curr Opin Cell Biol. 2013;25(5):582–90. doi: 10.1016/j.ceb.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Niggli V, Rossy J. Ezrin/radixin/moesin: Versatile controllers of signaling molecules and of the cortical cytoskeleton. Int J Biochem Cell Biol. 2008;40(3):344–9. doi: 10.1016/j.biocel.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 89.Mizuno K. Signaling mechanisms and functional roles of cofilin phosphorylation and dephosphorylation. Cell Signal. 2013;25(2):457–69. doi: 10.1016/j.cellsig.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 90.Jelassi B, Anchelin M, Chamouton J, et al. Anthraquinone emodin inhibits human cancer cell invasiveness by antagonizing P2X7 receptors. Carcinogenesis. 2013;34(7):1487–96. doi: 10.1093/carcin/bgt099. [DOI] [PubMed] [Google Scholar]
- 91.Gu L-Q, Li F-Y, Zhao L, et al. Association of XIAP and P2X7 receptor expression with lymph node metastasis in papillary thyroid carcinoma. Endocrine. 2010;38(2):276–82. doi: 10.1007/s12020-010-9384-7. [DOI] [PubMed] [Google Scholar]
- 92.Ren S, Zhang Y, Wang Y, et al. Targeting P2X7 receptor inhibits the metastasis of murine P388D1 lymphoid neoplasm cells to lymph nodes. Cell Biol Int. 2010;34(12):1205–11. doi: 10.1042/CBI20090428. [DOI] [PubMed] [Google Scholar]
- 93.Qiu Y, Li W-H, Zhang H-Q, Liu Y, Tian X-X, Fang W-G. P2X7 mediates ATP-driven invasiveness in prostate cancer cells. PloS One. 2014;9(12):e114371. doi: 10.1371/journal.pone.0114371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xia J, Yu X, Tang L, Li G, He T. P2X7 receptor stimulates breast cancer cell invasion and migration via the AKT pathway. Oncol Rep. 2015;34(1):103–10. doi: 10.3892/or.2015.3979. [DOI] [PubMed] [Google Scholar]
- 95.Jelassi B, Chantôme A, Alcaraz-Pérez F, et al. P2X7 receptor activation enhances SK3 channels- and cystein cathepsin-dependent cancer cells invasiveness. Oncogene. 2011;30(18):2108–22. doi: 10.1038/onc.2010.593. [DOI] [PubMed] [Google Scholar]
- 96.Gu BJ, Wiley JS. Rapid ATP-induced release of matrix metalloproteinase 9 is mediated by the P2X7 receptor. Blood. 2006;107(12):4946–53. doi: 10.1182/blood-2005-07-2994. [DOI] [PubMed] [Google Scholar]
- 97.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):nrc745. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 98.Kim J, Yu W, Kovalski K, Ossowski L. Requirement for Specific Proteases in Cancer Cell Intravasation as Revealed by a Novel Semiquantitative PCR-Based Assay. Cell. 1998;94(3):353–62. doi: 10.1016/s0092-8674(00)81478-6. [DOI] [PubMed] [Google Scholar]
- 99.Murphy N, Lynch MA. Activation of the P2X7 receptor induces migration of glial cells by inducing cathepsin B degradation of tissue inhibitor of metalloproteinase 1. J Neurochem. 2012;123(5):761–70. doi: 10.1111/jnc.12031. [DOI] [PubMed] [Google Scholar]
- 100.Kostoulas G, Lang A, Nagase H, Baici A. Stimulation of angiogenesis through cathepsin B inactivation of the tissue inhibitors of matrix metalloproteinases. FEBS Lett. 1999;455(3):286–90. doi: 10.1016/s0014-5793(99)00897-2. [DOI] [PubMed] [Google Scholar]
- 101.Larue L, Bellacosa A. Epithelial–mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24(50):7443–54. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 102.Noe V, Fingleton B, Jacobs K, et al. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114(1):111–8. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- 103.Pubill D, Dayanithi G, Siatka C, et al. ATP induces intracellular calcium increases and actin cytoskeleton disaggregation via P2x receptors. Cell Calcium. 2001;29(5):299–309. doi: 10.1054/ceca.2000.0194. [DOI] [PubMed] [Google Scholar]
- 104.Shankar J, Nabi IR. Actin cytoskeleton regulation of epithelial mesenchymal transition in metastatic cancer cells. PloS One. 2015;10(3):e0119954. doi: 10.1371/journal.pone.0119954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Haynes J, Srivastava J, Madson N, Wittmann T, Barber DL. Dynamic actin remodeling during epithelial-mesenchymal transition depends on increased moesin expression. Mol Biol Cell. 2011;22(24):4750–64. doi: 10.1091/mbc.E11-02-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barfod ET, Moore AL, Roe MW, Lidofsky SD. Ca2+-activated IK1 channels associate with lipid rafts upon cell swelling and mediate volume recovery. J Biol Chem. 2007;282(12):8984–93. doi: 10.1074/jbc.M607730200. [DOI] [PubMed] [Google Scholar]
- 107.Chantome A, Girault A, Potier M, et al. KCa2. 3 channel-dependent hyperpolarization increases melanoma cell motility. Exp Cell Res. 2009;315(20):3620–30. doi: 10.1016/j.yexcr.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 108.Potier M, Tran TA, Chantome A, et al. Altered SK3/KCa2. 3-mediated migration in adenomatous polyposis coli (Apc) mutated mouse colon epithelial cells. Biochem Biophys Res Commun. 2010;397(1):42–7. doi: 10.1016/j.bbrc.2010.05.046. [DOI] [PubMed] [Google Scholar]
- 109.Takai E, Tsukimoto M, Harada H, Kojima S. Autocrine signaling via release of ATP and activation of P2X7 receptor influences motile activity of human lung cancer cells. Purinergic Signal. 2014;10(3):487–97. doi: 10.1007/s11302-014-9411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Valastyan S, Weinberg RA. Tumor Metastasis: Molecular Insights and Evolving Paradigms. Cell. 2011;147(2):275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437(7058):497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- 112.Langley RR, Fidler IJ. The seed and soil hypothesis revisited - the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer J Int Cancer. 2011;128(11):2527–35. doi: 10.1002/ijc.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fidler IJ. The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 114.Tsubouchi A, Sakakura J, Yagi R, et al. Localized suppression of RhoA activity by Tyr31/118-phosphorylated paxillin in cell adhesion and migration. J Cell Biol. 2002;159(4):673–83. doi: 10.1083/jcb.200202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hugh TJ, Dillon SA, O’Dowd G, et al. beta-catenin expression in primary and metastatic colorectal carcinoma. Int J Cancer. 1999;82(4):504–11. doi: 10.1002/(sici)1097-0215(19990812)82:4<504::aid-ijc6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 116.Bulut G, Hong S-H, Chen K, et al. Small molecule inhibitors of ezrin inhibit the invasive phenotype of osteosarcoma cells. Oncogene. 2012;31(3):269–81. doi: 10.1038/onc.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen J, Zhou W, Jia Q, et al. Efficient extravasation of tumor-repopulating cells depends on cell deformability. Sci Rep. 2016;6:srep19304. doi: 10.1038/srep19304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2(2):91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 119.Adinolfi E, Callegari MG, Ferrari D, et al. Basal Activation of the P2X7 ATP Receptor Elevates Mitochondrial Calcium and Potential, Increases Cellular ATP Levels, and Promotes Serum-independent Growth. Mol Biol Cell. 2005;16(7):3260–72. doi: 10.1091/mbc.E04-11-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Constantinescu P, Wang B, Kovacevic K, et al. P2X7 receptor activation induces cell death and microparticle release in murine erythroleukemia cells. Biochim Biophys Acta. 2010;1798(9):1797–804. doi: 10.1016/j.bbamem.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 121.Voura EB, English JL, Yu H-YE, et al. Proteolysis during Tumor Cell Extravasation In Vitro: Metalloproteinase Involvement across Tumor Cell Types. PLOS ONE. 2013;8(10):e78413. doi: 10.1371/journal.pone.0078413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.White N, Butler PEM, Burnstock G. Human melanomas express functional P2X7 receptors. Cell Tissue Res. 2005;321(3):411–8. doi: 10.1007/s00441-005-1149-x. [DOI] [PubMed] [Google Scholar]
- 123.Elmore S. Apoptosis: A Review of Programmed Cell Death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fulda S, Debatin K-M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25(34):4798–811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 125.Uren RT, Dewson G, Bonzon C, Lithgow T, Newmeyer DD, Kluck RM. Mitochondrial Release of Pro-apoptotic Proteins ELECTROSTATIC INTERACTIONS CAN HOLD CYTOCHROME c BUT NOT Smac/DIABLO TO MITOCHONDRIAL MEMBRANES. J Biol Chem. 2005;280(3):2266–74. doi: 10.1074/jbc.M411106200. [DOI] [PubMed] [Google Scholar]
- 126.Goldstein JC, Muñoz-Pinedo C, Ricci J-E, et al. Cytochrome c is released in a single step during apoptosis. Cell Death Differ. 2005;12(5):453–62. doi: 10.1038/sj.cdd.4401596. [DOI] [PubMed] [Google Scholar]
- 127.Bratton SB, Salvesen GS. Regulation of the Apaf-1–caspase-9 apoptosome. J Cell Sci. 2010;123(19):3209–14. doi: 10.1242/jcs.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tait SWG, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11(9):621–32. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 129.Cullen SP, Martin SJ. Caspase activation pathways: some recent progress. Cell Death Differ. 2009;16(7):935–8. doi: 10.1038/cdd.2009.59. [DOI] [PubMed] [Google Scholar]
- 130.Choi K, Ryu S-W, Song S, Choi H, Kang SW, Choi C. Caspase-dependent generation of reactive oxygen species in human astrocytoma cells contributes to resistance to TRAIL-mediated apoptosis. Cell Death Differ. 2010;17(5):833–45. doi: 10.1038/cdd.2009.154. [DOI] [PubMed] [Google Scholar]
- 131.Labasi JM, Petrushova N, Donovan C, et al. Absence of the P2X7 Receptor Alters Leukocyte Function and Attenuates an Inflammatory Response. J Immunol. 2002;168(12):6436–45. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 132.Weerasinghe P, Buja LM. Oncosis: An important non-apoptotic mode of cell death. Exp Mol Pathol. 2012;93(3):302–8. doi: 10.1016/j.yexmp.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 133.Del Nagro C, Xiao Y, Rangell L, Reichelt M, O’Brien T. Depletion of the Central Metabolite NAD Leads to Oncosis-mediated Cell Death. J Biol Chem. 2014;289(51):35182–92. doi: 10.1074/jbc.M114.580159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mattson MP, Chan SL. Calcium orchestrates apoptosis. Nat Cell Biol. 2003;5(12):1041–3. doi: 10.1038/ncb1203-1041. [DOI] [PubMed] [Google Scholar]
- 135.Gogvadze V, Orrenius S, Zhivotovsky B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim Biophys Acta BBA - Bioenerg. 2006;1757(5):639–47. doi: 10.1016/j.bbabio.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 136.Contreras L, Drago I, Zampese E, Pozzan T. Mitochondria: The calcium connection. Biochim Biophys Acta BBA - Bioenerg. 2010;1797(6):607–18. doi: 10.1016/j.bbabio.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 137.Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27(50):6407–18. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Szalai G, Krishnamurthy R, Hajnóczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. EMBO J. 1999;18(22):6349–61. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Salvestrini V, Orecchioni S, Talarico G, et al. Extracellular ATP induces apoptosis through P2X7R activation in acute myeloid leukemia cells but not in normal hematopoietic stem cells. Oncotarget. 2016;8(4):5895–908. doi: 10.18632/oncotarget.13927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Khadra A, Tomić M, Yan Z, Zemkova H, Sherman A, Stojilkovic SS. Dual Gating Mechanism and Function of P2X7 Receptor Channels. Biophys J. 2013;104(12):2612–21. doi: 10.1016/j.bpj.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sauer H, Hescheler J, Wartenberg M. Mechanical strain-induced Ca2+waves are propagated via ATP release and purinergic receptor activation. Am J Physiol - Cell Physiol. 2000;279(2):C295–307. doi: 10.1152/ajpcell.2000.279.2.C295. [DOI] [PubMed] [Google Scholar]
- 142.Keystone EC, Wang MM, Layton M, Hollis S, McInnes IB D1520C00001 Study Team. Clinical evaluation of the efficacy of the P2X7 purinergic receptor antagonist AZD9056 on the signs and symptoms of rheumatoid arthritis in patients with active disease despite treatment with methotrexate or sulphasalazine. Ann Rheum Dis. 2012;71(10):1630–5. doi: 10.1136/annrheumdis-2011-143578. [DOI] [PubMed] [Google Scholar]
- 143.Stock TC, Bloom BJ, Wei N, et al. Efficacy and Safety of CE-224,535, an Antagonist of P2X7 Receptor, in Treatment of Patients with Rheumatoid Arthritis Inadequately Controlled by Methotrexate. J Rheumatol. 2012;39(4):720–7. doi: 10.3899/jrheum.110874. [DOI] [PubMed] [Google Scholar]