Abstract
In the context of oncology, liquid biopsies consist of harvesting cancer biomarkers, such as circulating tumor cells (CTCs), tumor-derived cell-free DNA (ctDNA), and extracellular vesicles (EVs), from bodily fluids. These biomarkers provide a source of clinically-actionable molecular information that can enable precision medicine. Herein, we review technologies for the molecular profiling of liquid biopsy markers with special emphasis on the analysis of low abundant markers from mixed-populations.
Keywords: circulating tumor cells, tumor cell-free DNA, extracellular vesicles, exosomes, polymerase chain reaction, next generation sequencing, mutation detection, gene expression profiling, precision medicine
Liquid Biopsy Biomarkers – CTCs, cfDNA, and EVs
Liquid biopsies rely on the isolation and analysis of cancer-related biomarkers in bodily fluids, such as blood, cerebrospinal fluid, saliva, or urine. The common biomarkers that comprise the liquid biopsy in the context of oncology are circulating tumor cells (CTC), tumor cell-free DNA (ctDNA), and extracellular vesicles (EVs). CTCs are shed from primary and/or metastatic tumor sites, carried through the blood circulation (detected at levels between 1–1,000/mL), and believed to form metastasis in distant organs, which is the major cause of cancer-associated mortality.1 Further, CTCs found in circulation can represent tumor heterogeneity (Fig. 1).2,3 There are numerous technologies that can efficiently isolate and purify CTCs from patients’ blood, but the detected CTC burden and CTC phenotypes will depend on the CTC isolation technology used.4 For example, CTCs expressing invasive phenotypes down-regulate and lose epithelial character in a process known as the epithelial-to-mesenchymal (EMT) transition. As such, CTCs likely consist of subpopulations with a diverse range phenotypes, and platforms that affinity-select only an epithelial subpopulation using anti-EpCAM antibodies may underrepresent the actual CTC burden by omitting CTCs with a mesenchymal character.4 Nevertheless, various technologies have established the prognostic value of EpCAM+ CTCs by showing correlation between high CTC burden and poor overall survival.4 However, studies have also demonstrated decreasing CTC burden during effective chemotherapy;5–7 EpCAM+ CTCs may not necessarily be correlated with tumor progression in some cases.6
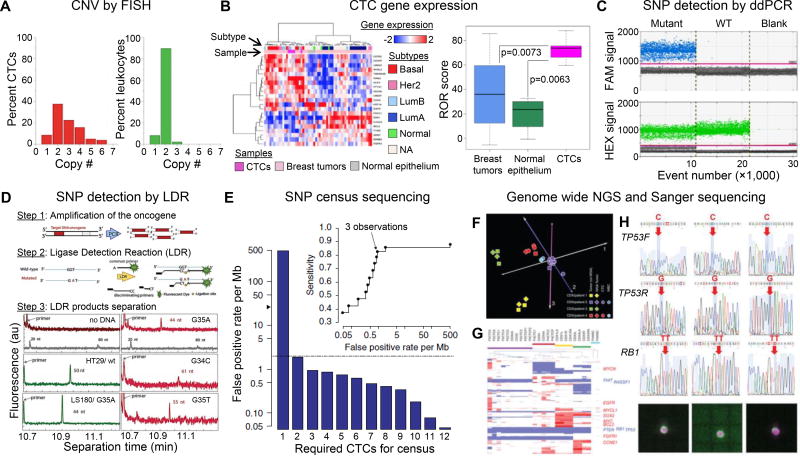
Figure 1. CTC molecular analyses.
(A) Histogram of the copy number of chromosome 8 detected in CTCs (red bars) and leukocytes surrounding CTCs (green bars) using FISH. Reproduced from41 with permission. (B) Exploratory analysis based on the subset of 21 genes from the PAM50 classifier. CTCs were clustered with the more aggressive breast cancer subtypes (HER2 positive and Luminal B based on a 90% confidence interval (p < 0.01) for assigning PAM50 subtype based on the available probes in the cDNA microarray. CTCs derived from Stage IV patients had a higher risk of recurrence score in comparison to I-SPY1 primary tumors. Reproduced from43 with permission. (C) 1D-Dot plot from ddPCR. The blue histogram indicates the number of droplets considered as positive for mutant KRAS according to the fluorescence threshold. The green histogram corresponds to the number of wild-type droplets. Mutant control, DNA containing a 1:1 mix of mutant and wildtype KRAS. WT control, DNA containing only KRAS wild-type. Blank, water. Reproduced from44 with permission. (D) Schematic of the polymerase chain reaction/ligase detection reaction (PCR/LDR) assay, and electropherograms of LDR products for: no gDNA; HT29 wt35 (50 nt); LS180 G35A (44 nt); and CTC isolated form metastatic pancreatic cancer patient: mesenchymal CTCs G35A (44 nt), epithelial CTCs G34C (61 nt); and another mesenchymal CTCs G35T (55 nt). The gray trace shows the DNA markers. Reproduced from6 with permission. (E) Estimation of false-positive rate per Mb among 19 independent CTC libraries after requiring the variant to be observed in at least nine of the CTC libraries. The gray dashed line indicates the reported mutation rate in bulk tumor sequencing of treated prostate cancer (~2 per Mb); the black arrowhead indicates the false-positive rate per Mb observed for a single CTC library. The inset shows sensitivity versus false-positive rate per Mb as a function of the required number of independent observations of the variant. Single CTCs, pools of ten CTCs and pools of ten WBCs isolated from patients were whole genome amplified along with 1 ng of DNA from CDXs. CNV analysis was carried out on the amplified material and unamplified tumor DNA. Reproduced from26 with permission. (F) Principal component analysis of genome-wide CNV data. (G) Hierarchical clustering of CNVs of 6,341 selected cancer-related genes. The positions of 13 genes showing frequent gains or losses in SCLC are indicated to the right of the heatmap. (H) Sanger sequencing of the RB1 and TP53 mutations (detected in CDX2) in six single CTCs, two pools of ten CTCs and a pool of ten WBCs isolated from patient 2, with images of a cytokeratin-stained single CTC from DEPArray. Red arrows indicate somatic mutations, and blue arrows indicate the corresponding unmutated regions. Panels F–H reproduced from37 with permission.
In addition to CTCs, tumors shed cfDNA and EVs into bodily fluids via physiological processes shared by normal cells. Both normal cells and cancer cells release cell-free DNA (cfDNA) through death or secretion,8,9 and each release mechanism provides cfDNA with unique properties (e.g., fragment size). The importance of cfDNA as a cancer biomarker stems from the shared genetic and epigenetic characteristics of tumor cells and their shed DNA (circulating tumor cell-free DNA – ctDNA). Interest in cfDNA/ctDNA analyses stems from the discovery that high cfDNA concentrations can be found in the blood of cancer patients, which can decrease following treatment.10 Since then, researchers have correlated cfDNA levels with cancer incidence/progression;11 the average cfDNA in the serum of healthy patients is ~13 ng/mL and ~180 ng/mL in cancer patients.12 However, it is now believed that cfDNA levels are not specific enough to be used as a diagnostic tool, because physiological conditions unrelated to cancer can generate high levels of cfDNA (autoimmune disease, stroke, sepsis, or trauma).13 Thus, recent research has focused on the mutational content in cfDNA/ctDNA.
For any mutational-focused ctDNA assay, sensitivity is affected by the amount of input of ctDNA, the number of probed mutations, and of course, the efficiency of the method selected for mutation detection.11 Thus, high efficiency for cfDNA isolation from a clinical sample is critical for the detection of rare ctDNA mutations. Isolation methods are challenged by interfering plasma proteins, potential gDNA contamination, and the small size of the ctDNA fragments; normal cfDNA fragments are ~166 bp long, approximately the length of one nucleosome, and overlap in size with generally shorter ctDNA fragments exists (Fig. 2A).14,15 Conventional phenol-chloroform DNA extraction is inefficient for the relatively short cfDNA/ctDNA fragments, and several commercially-available solid-phase extraction alternatives are widely used, although highly discrepant results have been obtained from a single sample analyzed by different methods.16 Further, pre-analytical variables, such as methods for blood processing and storage, can lead to blood cell apoptosis/necrosis and gDNA release17 and can lead to inter-laboratory variability for ctDNA-based analyses.18
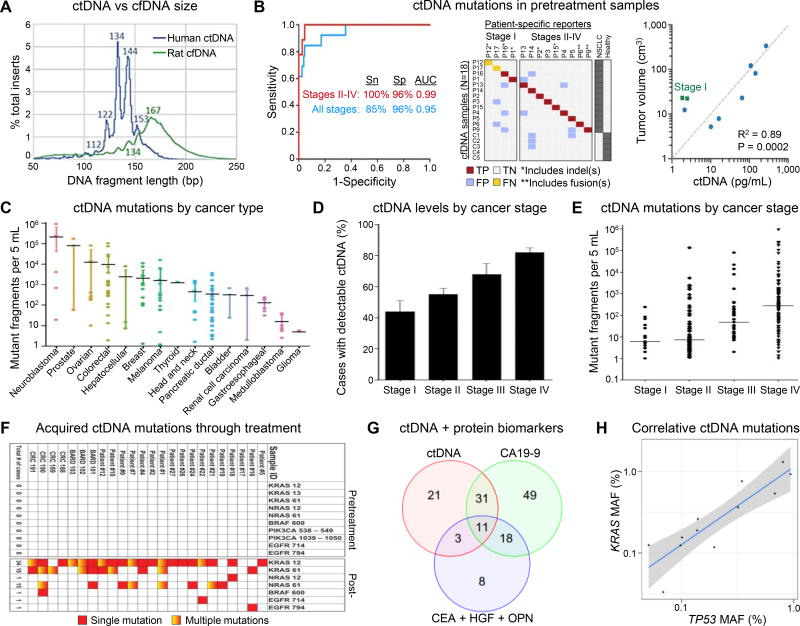
Figure 2. cfDNA and ctDNA analysis.
(A) Fragment sizes of ctDNA (human) and cfDNA (rat) in a GBM xenograft. Nearly identical cfDNA distributions were observed in healthy human plasma. Reproduced from15 with permission. (B) (left) Receiver Operating Characteristic (ROC) analysis of plasma DNA samples from samples from all stages (n = 13 patients) and stages II–IV (n = 9 patients). Area Under the Curve (AUC) values are significant at P < 0.0001. Sn, sensitivity; Sp, specificity. (middle) Raw data related to left panel. TP, true positive; FP, false positive; TN, true negative; FN, false negative. (right) Concordance between tumor volume, measured by CT or PET/CT, and pg/mL of ctDNA from pretreatment samples (n = 9), measured by CAPP-Seq. Reproduced from35 with permission. (C) Quantification of mutant fragments in the plasma of patients with different cancers. Error bars represent the 95% bootstrapped confidence interval of the mean. (D) Fraction of patients with detectable ctDNA and (E) quantification of mutant fragments in cancer cases categorized by stage. Error bars represent the standard error in the measurement (SEM). (F) Heat map of acquired resistance mutations to EGFR blockade in ctDNA from patients with metastatic CRC. Panels C-F reproduced from46 with permission. (G) Combining ctDNA and protein markers increases sensitivity because a large proportion of patients are detected by only one marker. The Venn diagram shows the number of patients detected by ctDNA KRAS mutations (red circle), CA19-9 (green circle), the three other protein biomarkers (blue circle), and by combinations thereof (overlapping regions). Eighty patients (36% of the total) were not detected by any of the three makers. (H) MAF of KRAS and TP53 mutations are strongly correlated in the plasma of the 12 patients whose plasma contained detectable amounts of both mutations providing validation of the reliability of the ctDNA assay and its quantitative nature. The shaded region represents the 95% confidence interval. Panels G,H reproduced from49 with permission.
EVs are nanovesicles secreted via multivesicular endosomes (MVEs; exosomes – 30–150 nm), membrane budding (microvesicles or ectosomes – 50–1,000 nm), or blebbing (apoptotic bodies – 50–2,000 nm). EVs are exceptionally stable19 and shuttle through bodily fluids, where they can be harvested as biomarkers. The “gold standard” for EV isolation is differential ultracentrifugation; cellular debris and larger EVs (>150 nm) are eliminated before pelleting remaining EVs at ≥100,000 g. Ultracentrifugation is laborious, difficult to automate, and requires additional density gradient centrifugation to eliminate co-precipitated proteins. New methods for EV purification are emerging – polymer precipitation, ion exchange columns, ultrafiltration, size-exclusion chromatography, affinity beads, and microfluidic devices (see Ref.20).
EV concentrations in, for example, normal plasma are confounded by the differing methodologies used for their enumeration (e.g., nanoparticle tracking analysis – NTA, fluorescence and colorimetric ELISA assays,20 and TEM20,21). Cryo-TEM and immuno-gold labeling demonstrated 5×107 EVs/mL for healthy plasma, of which >50% EVs were from erythrocytes and platelets.21 In additional to the EVs from normal blood cells, several studies have shown increased EV levels in cancer patients,20 but the clinical utility of increased EV levels in cancer treatment is arguably limited by potential false-positives from, for example, diabetes and/or cardiovascular diseases.22 Rather, EVs package several molecular biomarkers – proteins, a broad range of RNA types, including micro RNA (miRNA), long noncoding RNA (lncRNA), messenger RNA (mRNA),23–25 and DNA – that may constitute diverse EV-based liquid biopsies.23
Molecular Profiling Technologies for Liquid Biopsy Biomarkers
As discussed above, research has shown that enumeration of the liquid biopsy markers alone may not be adequate to enable precision therapy for cancer patients. Molecular profiling may provide more clinically useful information to the attending physician. However, molecular profiling of liquid biopsy markers is challenged by the low mass of the target isolated (for example, the amount of gDNA from one CTC is ~6 pg) and the low frequency of the target in normal material. Therefore, high analytical sensitivity and specificity, respectively, must be associated with the subsequent molecular assay to provide clinically useful information. While for CTCs, single cell analyses are possible, enough cells must be secured to obtain results that are meaningful from a clinical perspective.26 While some molecular profiling techniques applicable to liquid biopsy analyses are well established, new concepts for molecular profiling are evolving, some of which are applicable to clinical translation.
Polymerase chain reaction (PCR) based technologies – quantitative PCR (qPCR), reverse transcription qPCR (RT-qPCR), and digital droplet PCR and RT-PCR (ddPCR, ddRT-PCR) – are workhorse methods for liquid biopsy biomarkers, are very sensitive and specific to low frequency events, and are well suited for analyzing “known” targets (Table 1). ddPCR and ddRT-PCR provide absolute quantification of mutated clones and gene expression without the need of reference “housekeeping” genes, whereas RT-qPCR requires rigorous validation with reference genes, which can vary during cell cycle and between single cells. For example, standard RT-qPCR has been used in most EV-RNA studies (Fig. 3), although some target-specific modifications are employed, e.g., locked nucleic acids (LNAs) for multiplexed miRNA analysis. Alternatively, ddPCR offers high sensitivity and reproducibility27 and eliminates variabilities in endogenous controls that are apparent within EVs.19
Table 1.
| Method | Type of alteration | Analytical merits* | |
|---|---|---|---|
| PCR | Nested real-time | Known point mutations | Sensitivity 0.1–1% |
| ARMS/Scorpion | Sensitivity 0.1% | ||
| Mutant-allele specific68 | Sensitivity 0.01% | ||
| Bi-PAP/Bi-PAP-A | Sensitivity 0.01–0.1% | ||
| PNA-LNA PCR69 | Sensitivity 0.1% | ||
| Digital PCR | BEAMing | Known point mutations, genomic rearrangements | Sensitivity 0.001%–0.01% |
| ddPCR |
All techniques detect limited genomic loci and must consider ctDNA fragmentation for primer design.
Abbreviations: PCR, polymerase chain reaction; ARMS, amplification refractory mutation system; Bi-PAP, bidirectional pyrophosphorolysis-activated polymerization; Bi-PAP-A, allele-specific amplification bidirectional; PNA-LNA, peptide nucleic acid–locked nucleic acid; BEAMing, beads, emulsion, amplification, magnetics; ddPCR, droplet digital PCR.
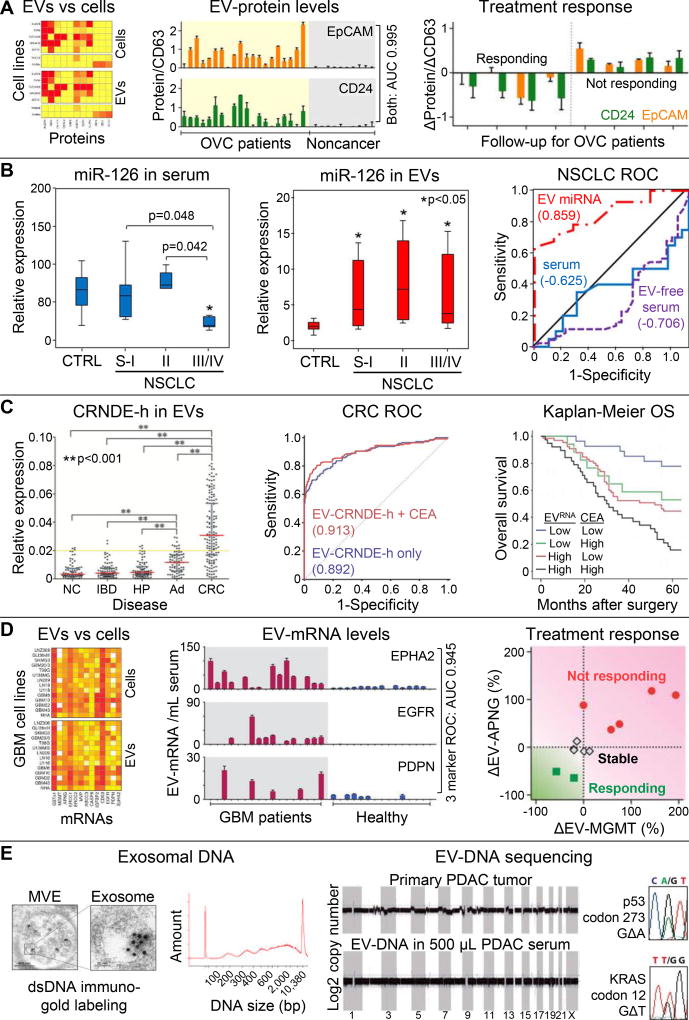
Figure 3. EV molecular analyses.
(A) Comparison of putative EOC markers and immune markers shows concordant protein expression on EVs (surface plasmon resonance-based analysis) and EOC and benign cell lines (flow cytometry). Elevated EV-EpCAM and EV-CD24 levels (normalized to EV-CD63) were associated with EOC ascites (N=20) whereas negligible signals were observed for non-cancer patients (N=10). Receiver operating characteristic (ROC) analysis showed an area under the curve (AUC) of 0.995 when the EV-EpCAM and EV-CD24 markers were combined. Longitudinal monitoring of EOC patients treated with chemotherapy (N=8) showed decreasing EV-EpCAM and EV-CD4 levels correlating with treatment response. Reproduced from50 with permission. (B) Levels of miR-126 in NSCLC patients were tested in bulk serum and within EVs specifically. EV-miR-126 fraction provide superior clinical sensitivity/specificity for NSCLC patients throughout localized (N=26) and metastatic (N=19) disease compared to healthy controls (N=31), which is illustrated by ROC analysis (AUC = 0.859). Reproduced from54 with permission. (C) Expression of the lncRNA marker CRNDE-h was elevated in EVs derived from CRC patients (N=148) as compared to patients with a normal colonoscopy (NC; N=80), hyperplastic polyp (HP; N=80), inflammatory bowel disease (IBD; N=80), and adenoma (AD; N=80). ROC analysis and Kaplan-Meier analysis of 5-year overall survival show that combination with the CEA biomarker shows improved sensitivity/specificity (AUC 0.913) and prognostic value of the EV-CRNDE-h marker. Reproduced from19 with permission. (D) EVs and parental GBM cell lines show concordant mRNA expression for 14 mRNA markers. When combined, three EV-mRNA markers could differentiate GBM patients (N=17) from healthy controls (N=15) with high clinical sensitivity/specificity (AUC 0.945), and downregulation of two other EV-mRNA markers correlated with chemotherapy response in 11 serial measurements of GBM patients (N=7). Reproduced from57 with permission. (E) TEM imaging of MVEs in pre-senescent fibroblasts with immuno-gold labeling of dsDNA, depicted as black dots, which shows the presences of dsDNA in exosomes that spans a broad size range (Bioanalyzer analysis). Reproduced from58 with permission. Shotgun whole genome sequencing of EV-DNA from a PDAC patient’s serum shows genome-wide coverage, but CNVs in the patient’s primary tumor are not reflected in EV-DNA. Sanger sequencing of EV-DNA from one PDAC patient revealed a KRAS and TP53 mutation. Reproduced from59 with permission.
Several advancements in molecular profiling have been applied to liquid biopsies. For identifying mutated genes in highly abundant wild-type DNA/cfDNA fragments, high melting temperature LNA clamp probes have been used to block wild-type amplification.28 Assays have also utilized ligase enzymes: PCR along with the ligase detection reaction (PCR/LDR) and ligation with BEAMing (beads, emulsions, amplification and magnetics).29–31 Multiplexed mass spectrometry can surveil wide panels of potentially mutated DNA,28 and exome-targeted next generation sequencing (NGS) assays, many of which are commercially-available, are widely used to evaluate multiple exon regions for potential mutations and aim at “actionable” targets (i.e., AKT1, EGFR, KRAS, KIT, TP53, PIK3CA, and others).32
In gene/mutation discovery-based research, whole genome NGS has been used. Technologies involving deep sequencing with Safe-Sequencing,33 Duplex Sequencing, CypherSeq, tagged amplicon deep sequencing,34 CAncer Personalized Profiling by deep Sequencing (CAPP-Seq),35 Ion AmpliSeq,36 and MiSeq37 have been demonstrated for cancer tissue and also rare liquid biopsy markers, such as ctDNA or CTCs (Table 2 and Fig. 4). These methods aim to improve detecting rare variants for liquid biopsy applications. Typical error rates of 0.1%–1% in NGS can be overcome by obtaining consensus sequences for high frequency events; however, it is difficult to distinguish errors from real mutations when marker (i.e., CTC or ctDNA) frequency is <5% of the total population. ctDNA occurrence in plasma is reported as low as ~0.1%.12,38,39
Table 2.
NGS technologies used for liquid biopsy analyses.
| NGS Technology [% mutant detection] |
Description |
|---|---|
| Ion AmpliSeq™ MiSeq/HiSeq/MiniSeq Illumina [1%–10%] | “Sequencing by synthesis” – a complementary strand of DNA is built upon a template strand. Sequencing is based on the detection of hydrogen ion released (Ion) or use of fluorescent readout (Mi/Hi/MiniSeq) during DNA polymerization.36 |
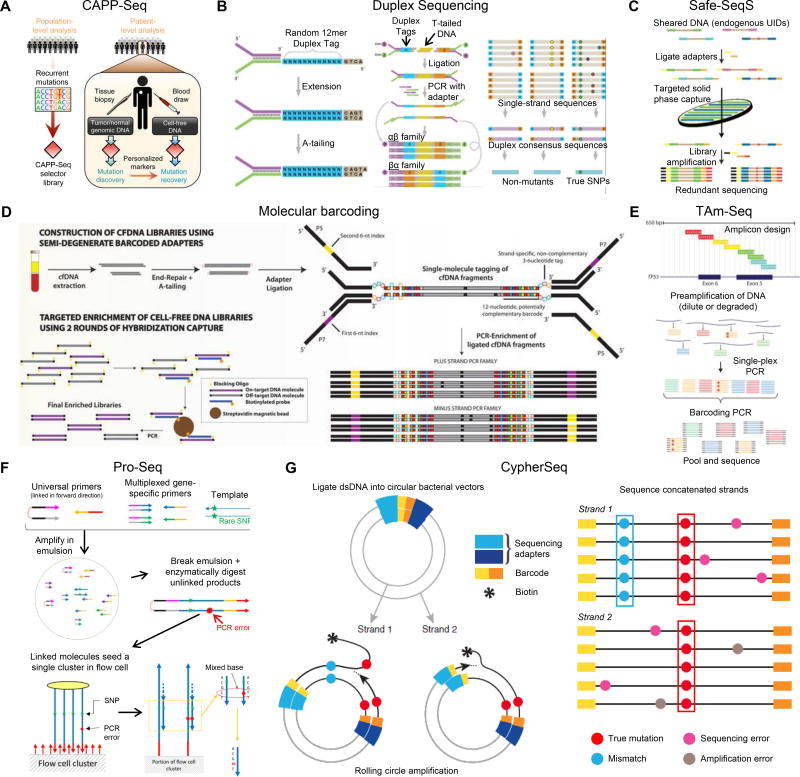
| CAncer Personalized Profiling by deep Sequencing (CAPP-Seq) [~0.02%] | Combines library preparation methods for low DNA input masses with a multiphase bioinformatics approach to design a 'selector', consisting of biotinylated DNA oligonucleotides that target recurrently mutated regions. To monitor ctDNA, the selector is applied to primary tumor DNA to identify a patient's cancer-specific genetic aberrations before being applied to ctDNA.35 (Fig. 4A) |
| Duplex Sequencing (Duplex-Seq) [0.01%] | Uses degenerate molecular tags to label each DNA molecule with its own unique DNA sequence. By tagging duplex DNA with adapters containing random but complementary double-stranded nucleotide sequences, it traces every sequence read back to one of the two strands of the original double-stranded DNA molecule. After adapter ligation, the individually labeled strands are PCR-amplified to create sequence families that share the same tag sequences derived from each of the two single parental strands. Following sequencing, members of each tag family are grouped, and a consensus sequence is established for each of the two strands to form “single-strand consensus sequences”. The two complementary consensus sequences derived from the two strands of an individual DNA duplex are then compared with each other, and the base identity at each position is retained only if the two strands match perfectly at that position, yielding a “duplex consensus sequence”. Polymerase errors appear in only one of the two DNA strands and thus are not counted as real mutations.70,71 (Fig. 4B) |
| Safe-sequencing system (Safe-Seq) [~0.02%] | A unique molecular identifier (UMI) approach to detect rare variants via amplification of each tagged template to create UMI families. The abundance of each UMI is used to distinguish between rare mutations and technical errors and can also be used to correct for PCR amplification bias.33 (Fig. 4C) |
| Molecular barcoding method [0.1%] | Relies on semi-degenerate barcoded adapters and personalized panels of biotinylated baits that target somatic mutations previously identified via the sequencing of tumor/liquid biopsies.72 (Fig. 4D) |
| Tagged amplicon deep sequencing method (TAm-Seq™) [0.25%–0.5%] | Combines library preparation and statistically-based analysis algorithms to identify and quantify cancer mutations.34 (Fig. 4E) |
| Proximity Sequencing (Pro-Seq) [0.003%] | Involves linking multiple copies of a single DNA template at the 5’ end early in the workflow so that the sequences of all molecules in a linked complex are nominally the same (except for any errors made in their derivation from the parent strand). The linking is arranged that both senses of the starting template can be represented in a single linked complex, providing duplex information. The linked complex is sequenced directly so that the multiple linked copies seed a single sequencing cluster/colony. A single cluster represents the aggregation of multiple redundant members of a family instead of a single molecule. Linked dsDNA fragments populate one cluster on an Illumina flow cell. dsDNA sequencing errors are compensated for by comparing the two strands of the DNA molecule.73 (Fig. 4F) |
| CypherSeq [0.001%–0.01%] | Two biotinylated, target-specific primers are used for each DNA strand. DNA is ligated into circular bacterial vectors that contain the targeted region of interest, and each strand is barcoded with a short sequence of nucleotides and then amplified by rolling-circle amplification (RCA). RCA product is a biotinylated strand concatenated with many copies of the template. Errors introduced during amplification are along the concatenated strand and are eliminated computationally. Concatenate is biotinylated; therefore, the strand can be pulled and template kept.74 (Fig. 4G) |
Figure 4. NGS sequencing methods.
Schematic diagrams depicting design of (A) CAPP-Seq selectors and their application for assessing ctDNA, Reproduced from35 with permission. (B) Duplex Sequencing: left panel: adapter synthesis, middle panel: Duplex Sequencing workflow, right panel: error correction. Reproduced from70 with permission. (C) Safe-Seq with endogenous UIDs plus capture. Reproduced from33 with permission. (D) Experimental workflow using semi-degenerate barcoded adapters and personalized panels of biotinylated baits. Reproduced from72 with permission. (E) tagged amplicon sequencing (TAm-Seq). Reproduced from34 with permission. (F) Pro-Seq workflow (left panel) and Pro-Seq sequencing (right panel). Reproduced from73 with permission. (G) CypherSeq construct and sequencing workflow. Reproduced from76 with permission.
What liquid biopsy information enables personalized medicine in the clinical setting?
While important, researchers and oncologists look beyond prognostic information. How can we help the patient by utilizing genetic information from the tumor? Is there information about the tumor that prevents us from administrating a particular treatment? To fulfill the “personalized medicine” concept, liquid biopsy markers such as CTCs, ctDNA, and EVs (Table 3) can provide molecular profiles and biological characterization of a patient’s cancer in a minimally invasive approach. This is particularly attractive for managing diseases where solid tissue biopsies are difficult to obtain, examples being lung and pancreatic cancer and cancers were sampling of the bone marrow is required (e.g., multiple myeloma and leukemias). Below, we present a few examples of how molecular liquid biopsy assays can secure valuable findings in the field of translational research or even clinical implementation.
Table 3.
How can liquid biopsy markers be implemented for precision medicine in clinical settings?
| Analysis Type | Information Provided |
|---|---|
| Enumeration |
|
| Gene Expression |
|
| Mutation assay |
|
| Sequencing |
|
Examples of Molecular CTC Liquid Biopsies
CTC analysis by miRNA in Situ Hybridization (MishCTC) was demonstrated by Ortega, et al.40 This method combined immunomagnetic CTC isolation, immunophenotyping based on cytokeratin expression, and LNA probes targeting miRNA-21 – a known onco-miRNA in cancer cells and absent in hematopoietic cells – with an enzyme-labeled fluorescence signal amplification approach for detection.40 In cells undergoing EMT, miRNA-21 expression was independent of the phenotype, suggesting that miRNA-21 analyses may detect both epithelial and mesenchymal CTCs.
Fluorescence in Situ Hybridization (FISH) has also been used to probe the centromeric regions of different chromosomes in prostate cancer CTCs (Fig. 1A). On average, CTCs contained abnormal chromosome copy number variations (CNVs), between 2.3 and 3.1, with heterogeneous aberrations observed between CTCs from different patients and even among CTCs from the same metastatic prostate cancer patient.41
Using RNA extracted from both CTCs and leukocytes, gene expression profiling by RT-qPCR identified CTC-specific genes (i.e., AGR2, S100A14, S100A16, FABP1 etc.) that could allow advanced cancer patients to be identified from healthy donors. Using a different gene panel, CTC expression profiles could differentiate 3 metastatic cancers (breast, prostate and colorectal) and classify the tissue of origin with ~80% accuracy,42 a critical step if CTC-based screening is to be realized. In another study, CTCs from metastatic breast cancer patients were surveyed with cDNA microarrays to profile the PAM50 genes; via unsupervised hierarchical clustering, CTC gene expression profiles could be clustered based on the primary breast tumor’s phenotype (HER2-enriched, Luminal B, or Basal-like). CTCs isolated from metastatic cancers with subtype HER2 and Luminal B had a higher risk of disease recurrence score (Fig. 1B).43
Considerable interest exists in analyzing point mutations in CTCs as a source of clinically actionable information, such as detecting KRAS mutations that preclude anti-EGFR therapies (e.g., cetuximab). In one study, CTCs were isolated from colorectal cancer (CRC) patients and interrogated for KRAS mutations after whole genome amplification (WGA). ddPCR could detect mutants at a frequency of 0.05% (less than one KRAS mutant cell/mL blood). ddPCR was a substantial improvement over TaqMelt PCR or high-resolution melting (0.5%; 50–75 cells/mL) and enabled CTC-based KRAS genotyping for 86% (30/35) of patient samples with a 77% concordance (sensitivity of 83%) with paired tumor tissues (Fig. 1C). As an alternative to ddPCR, PCR/LDR has the ability to detect single mutated copies in an excess of 500 wild-type molecules (0.2% frequency)30,31 and was able to identify KRAS mutations in epithelial and mesenchymal subpopulations of CTCs isolated from localized and metastatic pancreatic cancers (Fig. 1D).6 These represent powerful techniques for CTC-based SNP detection.44
To assess the potential of single cell CTC analysis, Lohr, et al.26 evaluated whole exome sequencing of single CTCs from metastatic prostate cancer patients. Replication errors during WGA could not be distinguished from true SNPs using only 1 CTC (25 false positive SNPs/Mb) (Fig. 1E). For “single cell” analysis, high quality NGS libraries were combined to call SNPs shared by ≥3 single CTCs (census sequencing) to reduce random amplification errors (0.9 false positive/Mb). Census sequencing affords statistical power that is not available to bulk sequencing; but factoring the variable success of WGA, even 10 CTCs sequenced in bulk can produce greater false positive rates (~10/Mb) than the expected mutation rate in prostate cancer (~2 per Mb). These results illustrate the importance of microfluidic-based technologies that can secure relatively high numbers of CTCs, even from a few mL of blood, and with unprecedented purity compared to the CellSearch™ assay.4 With higher CTC yields, CTCs can be analyzed in bulk with sequencing strategies such as exome-targeted NGS,6 for which a sequencing pipeline was recently reported for model cell lines and clinical CTCs using the Illumina MiSeq.32
Alternatively, single-cell RNA-sequencing can aid in identification of SNPs in rare cells because many RNA copies per cell enhance the statistical confidence for mutation detection. For example, Smart-Seq – an assay validated for sensitivity and variability as a function of input cells or TRNA/mRNA – is powerful in providing information on alternative splicing and gene expression by means of sampling full length cDNA. Smart-Seq also identified >4,000 high-confidence genomic SNPs via RNA sequencing, whereas genotype calls showed a significant number of false positive artifacts.45
CTCs isolated from small cell lung cancer patients were implanted into mice (CTC-derived explants, CDXs), and both the CTCs and CDXs were interrogated for CNVs by WGA and whole genome sequencing (Illumina MiSeq, paired-end 150 bp runs).37 Through principal component analysis and hierarchical clustering of more than 6,000 cancer-related genes, genomic comparisons revealed correlation between CTCs and the CDX tumors generated after implantation, which were notably distinct from unrelated CDXs and leukocytes (Fig. 1F,G).
Sequencing ctDNA in a cfDNA background
Novel NGS methods have been developed to detect low frequency biomarkers (i.e., mutated ctDNA) from the normal cfDNA background with high confidence. For example, CAPP-Seq was used to screen for ctDNA in non–small-cell lung cancer (NSCLC) patients’ plasma with a reported specificity of 96% for mutant frequencies of ~0.02%.35 Sequencing covered multiple somatic alterations that were identified in >95% of tumors, and ctDNA was detected in 100% of patients with advanced disease and in 50% of patients with stage I NSCLC (Fig. 2B).
The abundance of mutated ctDNA in different malignancies was evaluated using Safe-Seq, whole exome sequencing, PARE sequencing, and ligation-BEAMing methods (Fig. 2C).46 ctDNA mutations were detected with 87.2% sensitivity and 99.2% specificity, and the analysis of TP53 CNVs confirmed that variabilities in ctDNA mutational frequencies, with tumor type, tumor stage, and between patients (Fig. 2D,E), were intrinsic to the sample and not sequencing artifacts. ctDNA mutations were detected in 82% of metastatic patients and 52% of patients with localized disease. Importantly, 95% concordance in mutational status was found between ctDNA and matched tumor tissue, demonstrating the ability to survey a patient’s cancer via ctDNA analysis.
Further, the same authors designed a multiplex sequencing-based assay to query known mutational hotspots for several genes in the EGFR pathway. By comparing ctDNA mutational status before and after treatment, new mutations were observed to emerge following panitumumab or cetuximab treatment in 23/24 patients. These results included KRAS-negative and EGFR-negative CRC patients that first responded and then progressed while being treated with anti-EGFR therapy (Fig. 2F).46 Similarly, Azad et al.47 used comparative genomic hybridization of ctDNA and identified androgen receptor gene aberrations potentially responsible for apparent inefficiency of therapy in metastatic, castration-resistant prostate cancer patients that were ceasing abiraterone and enzalutamide treatment due to disease progression.
In addition to SNP detection, whole genome sequencing (HiSeq by Illumina) of hepatocellular carcinoma ctDNA elucidated genomic regions with CNV aberrations specific to a particular cancer site/type. Thus, the relative contributions of these particular tumors to the ctDNA pool was interrogated.48 Such methods provide a unique set of information but require high analytical power exclusively provided by deep sequencing, which is costly compared to targeted approaches.13
To improve the clinical sensitivity in early stage pancreatic cancer patients, Cohen et al.49 combined blood tests for KRAS gene mutations in ctDNA (HiSeq and MiSeq) with protein biomarkers (CA19-9, CEA, HGF, or OPN) (Fig. 2G,H). KRAS mutations were detected in the plasma in 30% patients, but when combined with protein markers, a sensitivity of 64% was observed (95% specificity). A 100% concordance was observed between plasma ctDNA mutations and tumor gDNA.
Molecular Profiling EVs and Their Cargo
New EV extraction technologies vary greatly in their complexity of operation and analytical figures of merit, such as recovery, purity, throughput, etc.20 But, consideration should be given to the final goal for EV analysis when selecting the extraction/isolation protocol. For example, several polymer precipitation kits are commercially available; while these methods have a relatively simple workflow, the isolated EVs are wrought with contaminants. Pretreating EVs with RNases/DNases before lysis is sufficient to enable downstream analysis of at least some cancer-specific biomarkers as demonstrated below. For other analyses, such as EV-mRNA expression profiling or EV-DNA SNP detection, it may be critical to eliminate normal EVs first, and affinity-purification would then be arguably better. Given the diversity of biomarkers found within EVs, this sets the stage for diverse technologies, each defined by achieving sufficient purity/recovery for the target with minimal experimental/financial cost.
EVs are known to shuttle proteins that cause cellular activation and degrade extracellular matrix in the tumor microenvironment.25 All EVs contain membrane (e.g., CD63) and cytosolic (e.g., TSG101) proteins that are commonly interrogated via Western blotting, but in the context of detecting cancer-specific signatures, EVs have been shown to contain protein profiles representative of the host cell (Fig. 3A),50 which enables affinity-selection via targets used in CTC isolation as an example.20 Im, et al.50 used a surface plasmon resonance system with sets of nanohole arrays functionalized with different antibodies for capturing and sensing EV subpopulations to test for EVs in epithelial ovarian cancer (EOC) ascites. Unlike EV-CD63 alone, monitoring EV-EpCAM and EV-CD24 levels normalized to EV-CD63 discerned EOC patients and treatment response (Fig. 3A). Thus, targeting cancer-associated proteins can illuminate signatures that would be obscured by normal EVs and can provide high sensitivity/specificity for cancer monitoring.
Regulatory miRNAs have received significant attention for their roles in cancer progression.51 miRNAs guide complexed Argonaute-2 (Ago2), which inhibits translation or degrades mRNA. Freely circulating miRNAs, protected from the RNase-rich body fluids by Ago2-complexation, are likely byproducts of apoptosis/necrosis as no known biological mechanism controls miRNA shedding or uptake. Instead, EVs can carry functionally-effective miRNA51 and stabilize miRNA through circulation, sample handling, and storage.52
The ratio of vesicle-free miRNA to rare EV-miRNA remains unresolved.27,53,54 For 3 miRNAs in prostate cancer plasma, only 1 miRNA copy was found per 121 ±50 EVs, and 97% miRNAs were actually freely circulating.27 This has important implications for miRNA cancer biomarkers. As common phenol- or column-based methods for miRNA extraction from plasma lyse EVs,55 they acquire miRNA that freely circulates and EV-packaged miRNA. Careful distinction between EV-miRNA and freely circulating miRNA is of essence, as was demonstrated in NSCLC miRNA studies. Bulk serum miRNA levels (including both freely circulating and EV-miRNA) of tumor-suppressor miR-126 were normalized to endogenous and exogenous controls, and the results indicated that miR-126 levels decreased once metastasis occurred (Fig. 3B). However, via ultracentrifugation of EVs, Grimolizzi, et al. found EV-miR-126 levels had actually increased for localized and metastatic NSCLC patients (Fig. 3B). In vitro experiments suggested NSCLC may be actively modulating the microenvironment by exporting EV-miR-126, and EV purification was critical to detect these trends and improve clinical sensitivity/specificity (Fig. 3B).54 Whether this trend holds true for other miRNAs remains open.
Long noncoding RNA (lncRNAs) (>200 nt) play key roles in gene regulation, such as epigenetic modification and miRNA sponging, and lncRNA dysregulation affects several oncogenic pathways, e.g., TP53, c-MYC, and BRCA2.56 Several lncRNAs have been discovered within EVs24 including colorectal neoplasia which differentially expressed-h (CRNDE-h) lncRNA, a tissue-specific marker that has little or no expression in normal adult tissue. Liu, et al.19 used polymer precipitation and RT-qPCR to observe increased expression of EV-CRNDE-h in CRC patients (normalized against EV-GAPDH and EV-UBC mRNA) (Fig. 3C). EV-CRNDE-h abundance also had prognostic relevance, especially when combined with CEA levels (Fig. 3C). Given the diverse range of lncRNAs dysregulated in cancers,24 EV-lncRNA may prove an especially useful biomarker.
Along with other RNAs, EVs shuttle mRNA that can be translated into functional proteins by recipient cells, thereby promoting proliferation of endothelial and cancer cells for example.24,25 EV-mRNA profiling can recapitulate gene expression in the host cell (Fig. 3D)57 and may provide insight into a patient’s cancer subtype or drug resistance. Shao, et al.57 used anti-EGFR microbeads to purify EVs from 100 µL of glioblastoma multiforme (GBM) patient serum. An integrated microfluidic system then magnetically captured the beads, isolated RNA, and performed RT-qPCR – normalizing results to EV-GAPDH. Three EV-mRNAs (EV-EGFR, EV-EPHA2, EV-PDPN) were used for GBM identification, and an additional two EV-mRNAs for DNA repair enzymes (EV-MGMT and EV-APNG) correlated with chemotherapy response (Fig. 3D).
EVs contain different types of DNA including chromosomal dsDNA (Fig. 3E),58–60 at least some histone-bound.58 Rather than being derived solely from apoptotic bodies, Takahashi, et al. demonstrated that healthy cells transfer damaged chromosomal DNA into exosomes for extracellular export and thereby avoid an oxidative DNA damage response. It remains unclear if/how this mechanism is dysregulated in cancer,58 but the parallels between EV-DNA and cfDNA are clear.60 Shotgun NGS of DNA in EVs purified by ultracentrifugation showed genome-wide coverage58,59 but did not reflect CNVs in the primary tumor (Fig. 3E),59 perhaps due to dilution from normal DNA. Furthermore, PCR-Sanger sequencing showed high frequency KRAS and TP53 mutations in PDAC EV-DNA (Fig. 3E).59 Studies comparing EV-DNA to cfDNA are very recent.60 Careful control over EV-DNA and cfDNA cross-contamination and matched tumor samples will likely be required to determine if EV-DNA detects mutations that are retained in the cancer and provides additional information when compared to cfDNA.
Summary
The use of liquid biopsies with CTCs, ctDNA, and EVs and the cargo they carry, as compared to solid-tumor biopsies, provides a minimally invasive method for early and longitudinal assessment of predictive and prognostic information related to the disease.61 Each biomarker offers different clinical opportunities, therefore potentially providing complementary information to allow for effective management of many oncological diseases.62 For example, analysis of ctDNA exclusively may not provide information on the heterogeneity of cancer cells; as mutation status may vary between cells in a tumor tissue, one may miss information as to what tumor cell population the ctDNA originated. Analysis of different phenotypes of CTCs garnered from the heterogeneous tumor microenvironment may provide more specific information related to mutation status originating from specific subpopulations, thereby informing proper chemotherapies including combination therapies (i.e., precision medicine). The advantage of CTCs and EVs can be their valuable cargo (RNA, DNA, and proteins), which add another dimension to the molecular analysis that ctDNA cannot provide.
We are not in a position to provide an answer as to which biomarker will serve better because research continuously provides new insights, which is especially true for the accelerating EV field. Most in the research community believe that only one marker will not be able to deliver the diagnostic, prognostic information necessary to help navigate the treatment landscape, and as such, using the entire complement of information provided by all three markers would be beneficial.
All of these liquid biopsy markers are currently being evaluated in large studies to validate their use in a clinical setting. Among the challenges in the liquid biopsy field is the lack of standard guidelines. Rigorous sample quality assessment and controls will be essential for the validation of current and future clinical trials. CTCs have the advantage of being the most mature of all 3 markers,63 with a large body of research and 572 clinical trials registered on the NIH database as of January 2018.64 cfDNA/ctDNA, although discovered in the 1940’s,65 has only recently attracted attention primarily due to the advent of NGS but has already entered into randomized clinical trials with 131 studies registered.64,66 Lastly, EV research is in a preliminary stage of development with 4 trials registered on the NIH database.64 All these trials hope to verify the long-term clinical benefits for incorporating liquid biopsies into the field of precision medicine.66
Acknowledgments
The authors would like to express gratitude for financial support from the NIH (NIBIB P41-EB020594; IMAT R21-CA173279).
References
- 1.Seyfried TN, Huysentruyt LC. On the Origin of Cancer Metastasis. Critical reviews in oncogenesis. 2013;18:43–73. doi: 10.1615/critrevoncog.v18.i1-2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allard WJ, Matera J, Miller MC, et al. Tumor Cells Circulate in the Peripheral Blood of All Major Carcinomas but not in Healthy Subjects or Patients With Nonmalignant Diseases. Clinical Cancer Research. 2004;10:6897–904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 3.Wang M, Zhao J, Zhang L, et al. Role of tumor microenvironment in tumorigenesis. Journal of Cancer. 2017;8:761–73. doi: 10.7150/jca.17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson JM, Witek MA, Kamande JW, Soper SA. Materials and microfluidics: enabling the efficient isolation and analysis of circulating tumour cells. Chemical Society Reviews. 2017;46:4245–80. doi: 10.1039/c7cs00016b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stott SL, Hsu C-H, Tsukrov DI, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18392–7. doi: 10.1073/pnas.1012539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witek MA, Aufforth RD, Wang H, et al. Discrete microfluidics for the isolation of circulating tumor cell subpopulations targeting fibroblast activation protein alpha and epithelial cell adhesion molecule. npj Precision Oncology. 2017;1:24. doi: 10.1038/s41698-017-0028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–9. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thierry AR, El Messaoudi S, Gahan PB, Anker P, Stroun M. Origins, structures, and functions of circulating DNA in oncology. Cancer and Metastasis Reviews. 2016;35:347–76. doi: 10.1007/s10555-016-9629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. 2017 doi: 10.1038/nrc.2017.7. [DOI] [PubMed] [Google Scholar]
- 10.Leon SA, Shapiro B, Sklaroff DM, Yaros MJ. Free DNA in the serum of cancer patients and the effect of therapy. Cancer research. 1977;37:646–50. [PubMed] [Google Scholar]
- 11.Butler TM, Spellman PT, Gray J. Circulating-tumor DNA as an early detection and diagnostic tool. Current Opinion in Genetics & Development. 2017;42:14–21. doi: 10.1016/j.gde.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Jahr S, Hentze H, Englisch S, et al. DNA Fragments in the Blood Plasma of Cancer Patients: Quantitations and Evidence for Their Origin from Apoptotic and Necrotic Cells. Cancer Research. 2001;61:1659–65. [PubMed] [Google Scholar]
- 13.Zeerleder S. The struggle to detect circulating DNA. Critical Care. 2006;10:142. doi: 10.1186/cc4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mouliere F, Rosenfeld N. Circulating tumor-derived DNA is shorter than somatic DNA in plasma. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:3178–9. doi: 10.1073/pnas.1501321112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Underhill HR, Kitzman JO, Hellwig S, et al. Fragment Length of Circulating Tumor DNA. PLOS Genetics. 2016;12:e1006162-e. doi: 10.1371/journal.pgen.1006162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mauger F, Dulary C, Daviaud C, Deleuze JF, Tost J. Comprehensive evaluation of methods to isolate, quantify, and characterize circulating cell-free DNA from small volumes of plasma. Analytical and Bioanalytical Chemistry. 2015:6873–8. doi: 10.1007/s00216-015-8846-4. [DOI] [PubMed] [Google Scholar]
- 17.El Messaoudi S, Rolet F, Mouliere F, Thierry AR. Circulating cell free DNA: Preanalytical considerations. Clinica Chimica Acta. 2013;424:222–30. doi: 10.1016/j.cca.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Malentacchi F, Pizzamiglio S, Verderio P, et al. Influence of storage conditions and extraction methods on the quantity and quality of circulating cell-free DNA (ccfDNA): the SPIDIA-DNAplas External Quality Assessment experience. Clin Chem Lab Med. 2015;53:1935–42. doi: 10.1515/cclm-2014-1161. [DOI] [PubMed] [Google Scholar]
- 19.Liu T, Zhang X, Gao S, et al. Exosomal long noncoding RNA CRNDE-h as a novel serum-based biomarker for diagnosis and prognosis of colorectal cancer. Oncotarget. 2016;7:85551–63. doi: 10.18632/oncotarget.13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Contreras-Naranjo JC, Wu H-JJ, Ugaz VM. Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine. Lab Chip. 2017;17:3558–77. doi: 10.1039/c7lc00592j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arraud N, Linares R, Tan S, et al. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost. 2014;12:614–27. doi: 10.1111/jth.12554. [DOI] [PubMed] [Google Scholar]
- 22.Jansen F, Nickenig G, Werner N. Extracellular Vesicles in Cardiovascular Disease. Circ Res. 2017;120:1649–57. doi: 10.1161/CIRCRESAHA.117.310752. [DOI] [PubMed] [Google Scholar]
- 23.Tkach M, Thery C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 164:1226–32. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 24.Kim K, Abdelmohsen K, Mustapic M, Kapogiannis D, Gorospe M. RNA in extracellular vesicles. Wiley Interdiscip Rev RNA. 2017;8:c1413. doi: 10.1002/wrna.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo W, Gao Y, Li N, et al. Exosomes: New players in cancer (Review) Oncol Rep. 2017;38:665–75. doi: 10.3892/or.2017.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohr JG, Adalsteinsson VA, Cibulskis K, et al. Whole-exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat Biotech. 2014;32:479–84. doi: 10.1038/nbt.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chevillet JR, Kang Q, Ruf IK, et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci U S A. 111:14888–93. doi: 10.1073/pnas.1408301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arcila M, Lau C, Nafa K, Ladanyi M. Detection of KRAS and BRAF mutations in colorectal carcinoma roles for high-sensitivity locked nucleic acid-PCR sequencing and broad-spectrum mass spectrometry genotyping. J Mol Diagn. 2011;13:64–73. doi: 10.1016/j.jmoldx.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diaz LA, Williams R, Wu J, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486:537–40. doi: 10.1038/nature11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khanna M, Cao W, Zirvi M, Paty P, Barany F. Ligase detection reaction for identification of low abundance mutations. Clinical Biochemistry. 1999;32:287–90. doi: 10.1016/s0009-9120(99)00020-x. [DOI] [PubMed] [Google Scholar]
- 31.Pingle M, Rundell M, Das S, Golightly LM, Barany F. PCR/LDR/Universal Array Platforms for the Diagnosis of Infectious Disease. Methods in molecular biology (Clifton, NJ) 2010;632:141–57. doi: 10.1007/978-1-60761-663-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu HE, Triboulet M, Zia A, et al. Workflow optimization of whole genome amplification and targeted panel sequencing for CTC mutation detection. npj Genomic Medicine. 2017;2:34. doi: 10.1038/s41525-017-0034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinde I, Wu J, Papadopoulos N, Kinzler KW, Vogelstein B. Detection and quantification of rare mutations with massively parallel sequencing. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9530–5. doi: 10.1073/pnas.1105422108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forshew T, Murtaza M, Parkinson C, et al. Noninvasive Identification and Monitoring of Cancer Mutations by Targeted Deep Sequencing of Plasma DNA. Science Translational Medicine. 2012;4:136ra68–ra68. doi: 10.1126/scitranslmed.3003726. [DOI] [PubMed] [Google Scholar]
- 35.Newman AM, Bratman SV, To J, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nature Medicine. 2014;20:548. doi: 10.1038/nm.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rothé F, Laes JF, Lambrechts D, et al. Plasma circulating tumor DNA as an alternative to metastatic biopsies for mutational analysis in breast cancer. Annals of Oncology. 2014;25:1959–65. doi: 10.1093/annonc/mdu288. [DOI] [PubMed] [Google Scholar]
- 37.Hodgkinson CL, Morrow CJ, Li Y, et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nature Medicine. 2014;20:897. doi: 10.1038/nm.3600. [DOI] [PubMed] [Google Scholar]
- 38.Volik S, Alcaide M, Morin RD, Collins C. Cell-free DNA (cfDNA): Clinical Significance and Utility in Cancer Shaped By Emerging Technologies. Molecular Cancer Research. 2016;14:898–908. doi: 10.1158/1541-7786.MCR-16-0044. [DOI] [PubMed] [Google Scholar]
- 39.Gormally E, Hainaut P, Caboux E, et al. Amount of DNA in plasma and cancer risk: A prospective study. International Journal of Cancer. 2004;111:746–9. doi: 10.1002/ijc.20327. [DOI] [PubMed] [Google Scholar]
- 40.Ortega FG, Lorente JA, Garcia Puche JL, et al. miRNA in situ hybridization in circulating tumor cells - MishCTC. Scientific Reports. 2015;5:9207. doi: 10.1038/srep09207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swennenhuis JF, Tibbe AGJ, Levink R, Sipkema RCJ, Terstappen LWMM. Characterization of circulating tumor cells by fluorescence in situ hybridization. Cytometry Part A. 2009;75A:520–7. doi: 10.1002/cyto.a.20718. [DOI] [PubMed] [Google Scholar]
- 42.Smirnov DA, Zweitzig DR, Foulk BW, et al. Global Gene Expression Profiling of Circulating Tumor Cells. Cancer Research. 2005;65:4993–7. doi: 10.1158/0008-5472.CAN-04-4330. [DOI] [PubMed] [Google Scholar]
- 43.Lang JE, Scott JH, Wolf DM, et al. Expression profiling of circulating tumor cells in metastatic breast cancer. Breast cancer research and treatment. 2015;149:121–31. doi: 10.1007/s10549-014-3215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denis JA, Patroni A, Guillerm E, et al. Droplet digital PCR of circulating tumor cells from colorectal cancer patients can predict KRAS mutations before surgery. Molecular Oncology. 2016;10:1221–31. doi: 10.1016/j.molonc.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramsköld D, Luo S, Wang Y-C, et al. Full-Length mRNA-Seq from single cell levels of RNA and individual circulating tumor cells. Nature biotechnology. 2012;30:777–82. doi: 10.1038/nbt.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bettegowda C, Sausen M, Leary RJ, et al. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Science Translational Medicine. 2014;6:224ra24–ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azad AA, Volik SV, Wyatt AW, et al. Androgen receptor gene aberrations in circulating cell-free DNA: Biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clinical Cancer Research. 2015;21:2315–24. doi: 10.1158/1078-0432.CCR-14-2666. [DOI] [PubMed] [Google Scholar]
- 48.Chan KCA, Jiang P, Zheng YWL, et al. Cancer Genome Scanning in Plasma: Detection of Tumor-Associated Copy Number Aberrations, Single-Nucleotide Variants, and Tumoral Heterogeneity by Massively Parallel Sequencing. Clinical Chemistry. 2013;59:211–24. doi: 10.1373/clinchem.2012.196014. [DOI] [PubMed] [Google Scholar]
- 49.Cohen JD, Javed AA, Thoburn C, et al. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proceedings of the National Academy of Sciences. 2017;114:10202–7. doi: 10.1073/pnas.1704961114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Im H, Shao H, Park Y, et al. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat Biotechnol. 2014;32:490–5. doi: 10.1038/nbt.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larrea E, Sole C, Manterola L, et al. New Concepts in Cancer Biomarkers: Circulating miRNAs in Liquid Biopsies. Int J Mol Sci. 2016;17:627. doi: 10.3390/ijms17050627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Köberle V, Pleli T, Schmithals C, et al. Differential stability of cell-free circulating microRNAs: implications for their utilization as biomarkers. PloS one. 2013;8:e75184. doi: 10.1371/journal.pone.0075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gallo A, Tandon M, Alevizos I, Illei GG. The Majority of MicroRNAs Detectable in Serum and Saliva Is Concentrated in Exosomes. PLoS ONE. 2012;7:e30679. doi: 10.1371/journal.pone.0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grimolizzi F, Monaco F, Leoni F, et al. Exosomal miR-126 as a circulating biomarker in non-small-cell lung cancer regulating cancer progression. Scientific reports. 2017;7:15277. doi: 10.1038/s41598-017-15475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eldh M, Lötvall J, Malmhäll C, Ekström K. Importance of RNA isolation methods for analysis of exosomal RNA: evaluation of different methods. Mol Immunol. 2012;50:278–86. doi: 10.1016/j.molimm.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253–61. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
- 57.Shao H, Chung J, Lee K, et al. Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nature Commun. 2015;6:6999. doi: 10.1038/ncomms7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi A, Okada R, Nagao K, et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nature Commun. 2017;8:15287. doi: 10.1038/ncomms15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kahlert C, Melo SA, Protopopov A, et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem. 2014;289:3869–75. doi: 10.1074/jbc.C113.532267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klump J, Philipp U, Follo M, E-A, Medicine B. Extracellular Vesicles or free circulating DNA: where to search for BRAF and cKIT mutations? Nanomed NBM. 2017 doi: 10.1016/j.nano.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 61.Gold B, Cankovic M, Furtado LV, Meier F, Gocke CD. Do circulating tumor cells, exosomes, and circulating tumor nucleic acids have clinical utility?: A report of the association for molecular pathology. Journal of Molecular Diagnostics. 2015;17:209–24. doi: 10.1016/j.jmoldx.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Durendez-Saez E, Azkarate A, Meri M, et al. New insights in non-small-cell lung cancer: circulating tumor cells and cell-free DNA. J Thorac Dis. 2017;9:S1332–S45. doi: 10.21037/jtd.2017.06.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang W, Xia W, Lv Z, Ni C, Xin Y, Yang L. Liquid Biopsy for Cancer: Circulating Tumor Cells, Circulating Free DNA or Exosomes? Cell Physiol Biochem. 2017;41:755–68. doi: 10.1159/000458736. [DOI] [PubMed] [Google Scholar]
- 64.US National Library of Medicine - Clinical Trials. [Accessed January, 2018]; at https://clinicaltrials.gov/ct2/results?cond=&term=%22circulating+tumor+cells%22&cntry=&state=&city=&dist=
- 65.Mandel P, Metais P. Les acides mucléiques du plasma sanguin chez l'Homme. Comptes rendus des seances de la Societe de biologie et de ses filiales. 1948;142:241–3. [PubMed] [Google Scholar]
- 66.Perakis S, Auer M, Belic J, Heitzer E. In: Advances in Circulating Tumor DNA Analysis. Makowski GSBTAiCC, editor. Elsevier; 2017. pp. 73–153. [DOI] [PubMed] [Google Scholar]
- 67.Bidard FC, Weigelt B, Reis-Filho JS. Going with the Flow: From Circulating Tumor Cells to DNA. Science Translational Medicine. 2013;5 doi: 10.1126/scitranslmed.3006305. 207ps14-ps14. [DOI] [PubMed] [Google Scholar]
- 68.Zapparoli GV, Jorissen RN, Hewitt CA, McBean M, Westerman DA, Dobrovic A. Quantitative threefold allele-specific PCR (QuanTAS-PCR) for highly sensitive JAK2 V617F mutant allele detection. BMC Cancer. 2013;13:206. doi: 10.1186/1471-2407-13-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miyazawa H, Tanaka T, Nagai Y, et al. Peptide nucleic acid–locked nucleic acid polymerase chain reaction clamp-based detection test for gefitinib-refractory T790M epidermal growth factor receptor mutation. Cancer Science. 2008;99:595–600. doi: 10.1111/j.1349-7006.2007.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmitt MW, Kennedy SR, Salk JJ, Fox EJ, Hiatt JB, Loeb LA. Detection of ultra-rare mutations by next-generation sequencing. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:14508–13. doi: 10.1073/pnas.1208715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmitt MW, Fox EJ, Prindle MJ, et al. Sequencing small genomic targets with high efficiency and extreme accuracy. Nature methods. 2015;12:423–5. doi: 10.1038/nmeth.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alcaide M, Yu S, Davidson J, et al. Targeted error-suppressed quantification of circulating tumor DNA using semi-degenerate barcoded adapters and biotinylated baits. Scientific Reports. 2017;7:10574. doi: 10.1038/s41598-017-10269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pel J, Choi WWY, Leung AO, et al. Duplex Proximity Sequencing (Pro-Seq): A method to improve DNA sequencing accuracy without the cost of molecular barcoding redundancy. bioRxiv. 2017 doi: 10.1371/journal.pone.0204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gregory MT, Bertout JA, Ericson NG, et al. Targeted single molecule mutation detection with massively parallel sequencing. Nucleic Acids Research. 2016;44:e22-e. doi: 10.1093/nar/gkv915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beveridge R. Circulating tumor cells in the management of metastatic breast cancer patients. Community Oncology. 2007;4:79–82. [Google Scholar]
- 76.Marx V. Cancer: hunting rare somatic mutations. Nature Methods. 2016;13:295. doi: 10.1038/nmeth.3803. [DOI] [PubMed] [Google Scholar]