Abstract
Many traits that are sexually dimorphic, appearing either differently or uniquely in one sex, are also sensitive to an organism’s condition. This phenomenon seems to have evolved to limit genetic conflict between traits that are under different selective pressures in each sex. Recent work has shed light on the molecular and developmental mechanisms that govern this condition sensitive growth, and this work has now expanded to encompass both sexual dimorphism as well as conditionally plastic growth, as it seems the two phenomena are linked on a molecular level. In all cases studied the gene doublesex, a conserved regulator of sex differentiation, controls both sexual dimorphism as well as the condition-dependent plastic responses common to these traits. However, the advent of next-generation -omics technologies has allowed researchers to decipher the common and diverged mechanisms of sexually dimorphic plasticity and expand investigations beyond the foundation laid by studies utilizing beetle weapons.
The evolution of sexually dimorphic plasticity
Across the animal kingdom, males and females adopt differences in morphology and behavior known as sexual dimorphism. Anisogamy, or the evolution of differently sized gametes, has provided a strong framework for investigations of the evolution of almost all sexual behaviors [1]. Differential costs in energy production of the gametes have been used to explain the evolution of sexual dimorphism as well as the evolution of selection for these differences. As organisms move away from having similarly sized gametes (isogamy), they open up the ability to engage in differential reproductive avenues and thus provide the foundation for the evolution of sex-based differences in somatic tissue [2], but see [3].
Sexual dimorphism, however, presents a potential problem for organismal fitness, as in most cases male and female organisms possess the same or similar genotype, and selective forces acting on genes that enhance fitness in one sex may reduce the fitness of the same genes in the opposite sex, and thus in many cases males and females will have different optimal fitness for sexually dimorphic traits, leading to genetic conflict. This situation has been documented in flies [4] and crickets [5], as well as in vertebrates [6,7] but is potentially universal [8]. In many cases, organisms can resolve this conflict by linking the expression of sexually dimorphic traits to other cues, such as organism condition [8]. By using organism condition to regulate the expression of the more exaggerated sexually dimorphic structures, organisms can not only reduce the genetic cost of the trait, but also the physiological cost [9], as many of these structures are energetically expensive to produce and maintain [10–13], but see [14–16]. Thus, by regulating the expression of sexually dimorphic traits through both sex-specific loci and the use of condition-dependent plasticity, organisms can achieve the best of both worlds- that is, they ensure that the strongest, healthiest organisms display the most prominently dimorphic structures [8,9] and they reduce the cost of these traits in the opposite sex.
There are many examples of insects whose sexually dimorphic traits exhibit condition-dependent, plastic responses. The most well-studied from a molecular point of view are scarab beetles, and particular attention has been paid to dung beetles in the family Scarabaeidae [17], stag beetles in the family Lucanidae [18,19], and rhinoceros beetles in the subfamily Dynastinae [20], although Gnatocerus flour beetles (family Tenebrionidae) [21] and the dipteran stalk-eyed flies (family Diopsidae) [22,23] have also been the subject of much research. Each of these groups contain members that exhibit striking sexual dimorphism, exhibiting weaponry that is conspicuously absent or greatly reduced in size in females while being exaggerated in males (but see O. sagittarius, [24]). In addition, in all of these organisms the final size of these structures is incredibly plastic, generally being determined by the level of access to food available to developing larvae [13,18,20,22,23,25–27], although in some cases the plastic response is mediated by infection status [28], with infected males having smaller weaponry than uninfected males. This review will focus predominantly on the insights gained through the study of beetle weaponry, but we will summarize what is known from other species where appropriate, and we also suggest new avenues of investigation that build on the foundation provided through the study of beetle weaponry that can serve as an important contrast to the data reviewed below.
Dsx links organism condition to sexually dimorphic trait expression
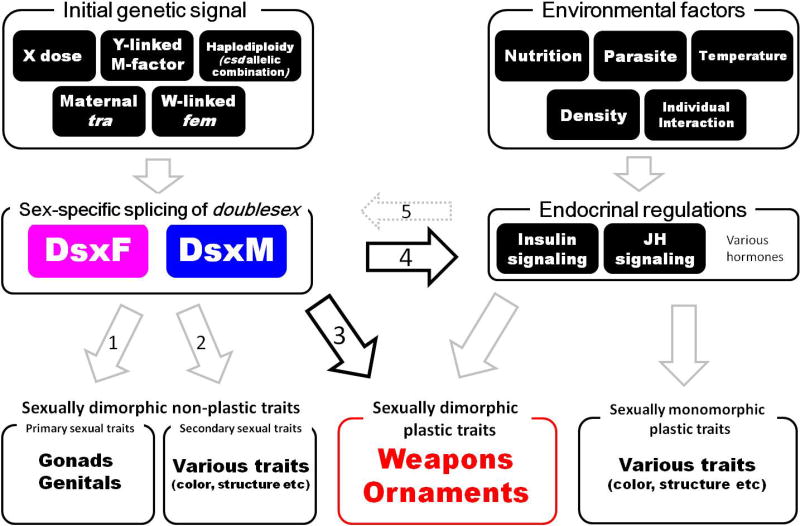
In insect, as in other organisms, sex-determination can be achieved by incredible diversity of genetic and environmental signals, but downstream effectors are relatively well conserved [29–31]. The most critical gene appears to be the transcription factor doublesex (dsx), an evolutionarily conserved key regulator of sex differentiation. Most insect species investigated possess only one dsx gene in their genome, yet express multiple dsx splice variants via sexspecific alternative splicing. Sex-specific Dsx isoforms promote sexual differentiation through the regulation of diverse gene sets [31,32] (Figure. 1).
Figure 1.
Sexually dimorphic plastic trait(s) develop downstream of both sexual regulation (mediated by Dsx) and environmental regulation (mediated by hormonal pathway(s)).
A variety of initial genetic signals determine the sex through upstream initiation of the sex determination cascade in insects. Examples include, X chromosome dose in Drosophila melanogaster [72], maternal input of transformer (tra) mRNA in Nasonia vitripennis [73], W-linked fem piRNA in Bombyx mori [74], the Y-linked M-factor, Mdmd in Musca domestica [75], and haplodiploidy and the csd allelic combination in Apis mellifera [76]. However, in all studied insects, these different determination signals converge on the conserved transcription factor Dsx, which functions as a downstream master switch gene for both sex determination and differentiation (see text). Various environmental factors can affect an animal’s physiological state, typically via hormonal regulation, and these endocrine signals the expression of various plastic traits. Accordingly, many traits that are both sex-specific and plastic are also under the control of this endocrine regulation, and thus these endocrine signals interact with Dsx in a variety of ways to ultimately generate trait expression that is sensitive not only to an organism’s sex, but also their condition.
Arrows 1–4 indicate various outcomes of Dsx regulation across insects. Dsx can regulate sexually dimorphic traits that do not exhibit plastic responses, such as yolk protein for ovary development and the gene lozenge during Drophila female genital disk development ([77], arrow 1), or through regulation of the gene bric-a-brac during development of abdominal pigmentation in Drosophila ([78], arrow 2). Critically to this review, Dsx can regulate the expression of sexually dimorphic plastic traits in two ways, either directly through changes in expression levels of dsx as in dung beetle horns ([33], arrow 3), or through sex-specific splice variants regulating responsiveness to endocrine signals ([19], arrow 4). It is possible, as suggested by the morph-specific expression patterns of Dsx target genes in dung beetle horns [33] that dsx may itself be regulated by endocrine signals (arrow 5), although there is no direct evidence of this relationship. It is important to note that, while the specific genes targeted by Dsx during the regulation of sexually dimorphic plasticity are unknown, evidence from next-generation sequencing experiments have suggested that developmental toolkit genes such as hedgehog may be directly regulated by Dsx expression level (arrow 3), and it is likely that JH signaling genes may also be regulated by Dsx (arrow 4). However, in both cases these predictions need to be confirmed through both functional experiments and through the use of techniques such as gSELEX, which allow for more targeted investigation of the binding sites regulated by Dsx, as well as allowing the investigation of genes that are regulated by Dsx that are not involved in the development of sexually dimorphic plastic traits (arrows 1 and 2).
Mandible growth in male stag beetles is promoted by increased levels of juvenile hormone (JH) in high condition males, while female mandibles do not respond to JH [18]. Through a combination of dsx RNAi knockdown and ectopic JH analog (JHA) treatment, Gotoh et al demonstrated that this sexually dimorphic response is regulated by Dsx. In dsx knockdown females, mandibles respond to JHA treatment and show exaggerated growth compared to GFP knockdown control females [19]. In males, both dsx knockdown and GFP knockdown individuals respond to ectopic JH treatment. These results suggest that the developmental “default” state can respond to JH, and thus exhibit plasticity, but that female-specific Dsx isoforms inhibit responsiveness. Thus, the sexually dimorphic plastic response of mandibles to JH is ultimately regulated through sex-specific Dsx isoforms [19]
In dung beetles, dsx also mediates sexually dimorphic plasticity. Kijimoto and colleagues [33], showed that knockdown of the dsx gene in Onthophagus taurus reduced the size of male horns in a condition-dependent manner. That is, larger males had a larger reduction in horn size after dsx RNAi than smaller males. In addition, females injected with dsx double-stranded RNA were induced to grow horns, and the size of these new horns was regulated by their body size. Unlike stag beetles, however, large and small males differed significantly in their level of expression of dsx in horn tissue. This suggests that male-specific horn plasticity in dung beetles is regulated by dsx expression levels, unlike in stag beetles where Dsx isoforms instead modulate the response to endocrine signals of condition. Thus, we now have two possible mechanisms through which a conserved signaling gene, dsx, can link the expression of sexually dimorphic traits to organism condition- either through limiting the response to a signal of condition, or alternatively through differences in expression level of sex-specific transcripts in response to condition (Figure 1).
The recent advances in understanding the developmental regulation of weaponed beetles described above have revealed that dsx may functions as a master switch gene for the development of sexually dimorphic plasticity in insects. However, many important mechanistic questions remain. For example, the precise mechanism through how Dsx inhibits responsiveness to JH is unknown. It is possible that expression of sex-specific forms of Dsx ultimately exert their action on sexually dimorphic plasticity by affecting the expression of the JH receptor, methoprene-tolerant. In addition, as Dsx is a transcription factor, it regulates the expression of various genes in both a positive and negative manner [34–36]. Thus, to fully understand the developmental mechanisms underlying sexually dimorphic plasticity, the identification of downstream developmental pathways regulated by Dsx is critical.
Screening of downstream targets of Dsx via Next-Generation Sequencing
The revolution of next-generation sequencing has allowed researchers to search for potential Dsx regulated genes through large-scale screening of gene expression data. The first investigation of the role of Dsx regulation during the development of sexually dimorphic plasticity was conducted by Ledón-Rettig and co-authors using RNA-seq analysis of dsx knockdowns in O. taurus [37].
This study identified over 400 potential Dsx targets uniquely expressed during growth of horns, with very few Dsx targets expressed in genitals and brain tissue, indicating that Dsx functions to coordinate growth of condition dependent sexually dimorphic tissues, but perhaps plays a much smaller role during development of sexually dimorphic structures that do not display condition dependence (i.e. genitals). However, as genital primordium is formed earlier than structures such as horns, the precise role of Dsx during genital development cannot be completely inferred from this experiment. Interestingly, the candidate genes identified through this analysis included members of the ecdysteroid and Hedgehog pathways, both of which have been previously implicated in condition dependent dimorphic growth [38,39]. Furthermore, using the list of putative Dsx targets identified in Onthophagus and comparing them to a list of genes differentially expressed between male and female horn tissue in the rhinoceros beetle Trypoxylus dichotomus [38, in revision] reveals 77 putative Dsx targets also differentially expressed in rhinoceros beetle horns, including the gene cubitus interruptus, another member of the Hedgehog pathway, further implicating both Dsx targets as well as the Hedgehog pathway as regulators of condition-dependent sexually dimorphic growth of weapons. The finding that members of the Hedgehog pathway both contain putative Dsx binding sites and are differentially expressed in two different beetle species suggest a role for this pathway not only for condition dependent plastic responses, but also sexually dimorphic plasticity, and further supporting that Hedgehog signaling is a downstream target of Dsx during the development of sexually dimorphic plasticity.
Another approach to identify the downstream target genes is through the identification of Dsx binding sites in an organism’s genome. In Drosophila, for example, the Dsx binding sequence was experimentally identified via genome wide screening [34,36]. These screenings led to the detection of specific genes involved with sexually dimorphic trait development and evolution [35,36]. Based on a genome-wide enrichment analysis of putative binding sequences in insect species with increasing phylogenetic distances to fruit flies, Luo et al suggested that the proposed 13-nucleotide sequence present in Drosophila is unlikely to be conserved outside of the Diptera [34]. However, although the binding sequence might not be completely identical, the similarity of the binding sequence between Drosophila and Coleoptera was predicted, allowing the application of computational prediction of putative Dsx binding sites as seen in [37,41]. Moreover, the whole genome of at least one of the organisms described above is in process (Onthophagus taurus; [42,43]), therefore, genome-wide screening of Dsx binding sites can be achieved by using techniques already applied in studies of Drosophila Dsx, such as ChIP-seq [36]. However, considering the poor availability of antibodies in non-model organisms, antibody-independent methods such as genomic SELEX which utilizes tagged recombinant DNA-binding protein and fragmented genomic DNA to identify target sites of a given focal binding protein (such as Dsx), could be used instead [44,45].
These direct screening approaches should be combined with the current wealth of transcription level based RNA-seq screening on focal traits in beetles, this data is available in O. taurus [37,46,47]; and is becoming available for at least one other beetle species [40], which can narrow down candidate genes to those regulated by Dsx. These large screening based approaches can shed light on the development of sexually dimorphic traits and the underlying mechanisms that generate plastic responses in these traits in the future. Importantly, combining large-scale screening efforts with functional investigations across a variety of beetles can help to understand the evolutionary history behind doublesex’s link to nutritional condition. Did this interaction evolve through gains of a Dsx binding site? If so, what kind of genes have gained Dsx binding sites? As described above, it seems as though genes in the Hedgehog signaling pathway may represent a universal acquisition of Dsx regulation to generate sexually dimorphic plasticity, but in order to fully answer this question we must leverage the quickly falling costs of next-generation sequencing [48] to investigate patterns of Dsx regulation across a larger variety of sexually dimorphic plastic weapons.
Perspectives
Much progress has been made in understanding the molecular basis of sexually dimorphic plasticity, at least in the Coleoptera, including understanding the physiology of upstream signals of condition [18,21,24,38,49], disentangling the plastic response of master regulator genes such as dsx to signals of condition [19,33,37,50], and identifying the downstream targets (such as the Hedgehog pathway) of master regulator genes [37,39]. However, there are many important questions remaining, and the current body of literature remains incredibly focused on beetle weaponry [51], possibly owing to the ease of RNAi knockdown in these insects, and the variability of RNAi effectiveness across other insect taxa [52]. We propose two new research foci for understanding the mechanisms of sexually dimorphic plasticity, namely an investigation into the interaction between infection status and Dsx function during development of beetle weaponry as well as breaking ground on a tractable insect model to find out if insights from beetle weapons are also shared by sexually dimorphic ornaments.
On the one hand, while it seems clear that Dsx is a critical link between nutritional condition and sexual dimorphism in beetle weaponry, nutrition is not the sole indicator of organismal condition. For example, while Dsx does control the development of condition dependent weaponry in Gnatocerus cornutus [53], the weaponry in this beetle demonstrates plastic responses to both nutrition condition (better-fed males developing larger mandible weapons, [12]) as well as infection status (males with a higher parasite load had smaller mandible weapons, [28]). Thus, to understand if Dsx is a universal link between sexually dimorphic traits and organismal condition, it is critical to understand how infection status interacts with Dsx, and we think that Gnatocerus represents an attractive model to understand this interaction.
On the other hand, many traits that exhibit sexually dimorphic plasticity in insects are ornaments or signals, not beetle weapons, and are not expected to play by the same rules [54]. Examples of condition-dependent ornaments include wing pigmentation in damselflies [55,56], wing melanization in dragonflies [57], forelimbs in grasshoppers [58], calling songs in crickets [59], and pheromone production in beetles [60]. Unfortunately, the function of dsx itself, much less the effect of this gene on sexually dimorphic plasticity has been little studied outside of the context of condition-dependent weaponry and the evolution of insect sexual differentiation. One interesting model presents itself in the form of the sexually dimorphic, condition-dependent structures known as coremata present in many species of tiger moths [61]. The final size of these structures is dependent on the amount of pyrrolizidine alkaloids present in the larval diet [62,63], and they are used by male moths to release pheromones to attract females. Knockdown of sex-specific isoforms of Dsx in the model moth Bombyx mori led to disruption of sexually dimorphic traits [64], and it is likely that coremata development is similarly governed by sex-specific dsx splicing. As these structures are ornaments and not weapons, investigation of whether Dsx regulates sexually dimorphic plasticity in this organism would provide valuable insights into the universality of Dsx as a master regulator of plastic responses in insects, or determine whether the function of Dsx is only to link condition to the growth of weapons. Critically, the developmental morphology and histology of these structures has been well described for at least one moth species [65], RNA interference appears tractable in this moth family [66], and it also seems as though similar mechanisms to those seen in beetle weaponry (i.e. ecdysone signaling) may be critical for proliferation of these structures [67], thus making them an attractive new avenue of research.
It is also important to ask whether or not the mechanisms described above ultimately resolve genetic conflict as theory predicts [8]. One way to answer this question is to investigate whether there is evidence of large-scale sex bias in gene expression in traits demonstrating sexually dimorphic plasticity compared to other traits [68–70]. Next-generation sequencing data from dung beetles and stalk-eyed flies suggests that this is the case [47,68]. However, there is evidence from Gnatocerus beetles that the evolution of sexually dimorphic plastic responses does not, ultimately, resolve genetic conflict [71].
In summary, the evolutionary developmental model of beetle weaponry has provided a rich framework for the investigations into the molecular mechanisms underlying the development of sexually dimorphic plastic traits in insects. However, these studies have generally focused on the influence of a single signal of condition, nutrition, and it remains to be seen whether the results obtained in beetle weaponry can be generalized to other conditional signals, or to sexually dimorphic plastic traits that are not weapons. There is also much left unknown about the downstream targets of Dsx regulation and the precise mechanisms through which Dsx and endocrine signals of condition interact, although recent studies have also laid a strong foundation for further investigation of this question.
Box 1.
“Plasticity” is defined here as a nature of trait(s) whose expression pattern varies in response to an organism’s condition.
An organism’s condition is the sum of an organism’s genotype, physiological state, and epigenomic state [9,79].
When the response pattern of a trait is different between sexes, we defined such phenomena as “sexually dimorphic plasticity”.
Highlights.
Sexually dimorphic plasticity has evolved to limit the impact of sexual conflict.
Many insects have traits that are sexually dimorphic and plastically responsive to condition.
Signals of condition and the downstream responses to condition differ between species.
The gene doublesex seems to be critical to sexually dimorphic plasticity in all organisms studied.
Next-generation technology allows the investigation of regulatory responses to doublesex.
Acknowledgments
The authors would like to thank Yui Suzuki and David Angelini for the invitation to submit this review, and for the anonymous reviewers whose critical commentary helped shape the final manuscript.
This work was supported by a MEXT KAKENHI (16H011452) to TN, the Young Researcher Unit of Nagoya University to HG and TK, and by an NIH PERT fellowship #2K12GM000708-16 to RZ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Andersson MB. Sexual selection. Princeton University Press; 1994. [Google Scholar]
- 2.Parker GA, Baker RR, Smith VGF. The origin and evolution of gamete dimorphism and the male-female phenomenon. J. Theor. Biol. 1972;36:529–553. doi: 10.1016/0022-5193(72)90007-0. [DOI] [PubMed] [Google Scholar]
- 3.Randerson JP, Hurst LD. The uncertain evolution of the sexes. Trends Ecol. Evol. 2001;16:571–579. [Google Scholar]
- 4.Chippindale AK. Negative genetic correlation for adult fitness between sexes reveals ontogenetic conflict in Drosophila. Proc. Natl. Acad. Sci. 2001;98:1671–1675. doi: 10.1073/pnas.041378098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fedorka KM, Mousseau TA. Female mating bias results in conflicting sex-specific offspring fitness. Nature. 2004;429:65–67. doi: 10.1038/nature02492. [DOI] [PubMed] [Google Scholar]
- 6.Royle NJ, Hartley IR, Parker GA. Sexual conflict reduces offspring fitness in zebra finches. Nature. 2002;416:733–736. doi: 10.1038/416733a. [DOI] [PubMed] [Google Scholar]
- 7.Mainguy J, Côté SD, Festa-Bianchet M, Coltman DW. Father–offspring phenotypic correlations suggest intralocus sexual conflict for a fitness-linked trait in a wild sexually dimorphic mammal. Proc. R. Soc. London B Biol. Sci. 2009;276 doi: 10.1098/rspb.2009.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonduriansky R. The genetic architecture of sexual dimorphism: the potential roles of genomic imprinting and condition-dependence. Sex, Size Gend. Roles. 2007 doi: 10.1093/acprof:oso/9780199208784.003.0020. [DOI] [Google Scholar]
- 9.Hill GE. Condition-dependent traits as signals of the functionality of vital cellular processes. Ecol. Lett. 2011;14:625–634. doi: 10.1111/j.1461-0248.2011.01622.x. [DOI] [PubMed] [Google Scholar]
- 10.Emlen DJ. Costs and the Diversification of Exaggerated Animal Structures. Science (80-.) 2001;291:1534–1536. doi: 10.1126/science.1056607. [DOI] [PubMed] [Google Scholar]
- 11.Moczek AP, Nijhout HF. Trade offs during the Development of Primary and Secondary Sexual Traits in a Horned Beetle. Am. Nat. 2004;163:184–191. doi: 10.1086/381741. [DOI] [PubMed] [Google Scholar]
- 12.Okada K, Miyatake T. Genetic correlations between weapons, body shape and fighting behaviour in the horned beetle Gnatocerus cornutus. Anim. Behav. 2009;77:1057–1065. [Google Scholar]
- 13.Yamane T, Okada K, Nakayama S, Miyatake T. Dispersal and ejaculatory strategies associated with exaggeration of weapon in an armed beetle. Proc. R. Soc. B Biol. Sci. 2010;277:1705–1710. doi: 10.1098/rspb.2009.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Painting CJ, Holwell GI. Exaggerated Trait Allometry, Compensation and Trade-Offs in the New Zealand Giraffe Weevil (Lasiorhynchus barbicornis) PLoS One. 2013;8:e82467. doi: 10.1371/journal.pone.0082467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCullough EL, Emlen DJ. Evaluating the costs of a sexually selected weapon: big horns at a small price. Anim. Behav. 2013;86:977–985. [Google Scholar]
- 16.House CM, Jensen K, Rapkin J, Lane S, Okada K, Hosken DJ, Hunt J. Macronutrient balance mediates the growth of sexually selected weapons but not genitalia in male broad-horned beetles. Funct. Ecol. 2015;30:769–779. [Google Scholar]
- 17.Snell-Rood EC, Cash A, Han MV, Kijimoto T, Andrews J, Moczek AP. Developmental Decoupling Of Alternative Phenotypes: Insights From The Transcriptomes Of Horn-Polyphenic Beetles. Evolution (N. Y) 2011;65:231–245. doi: 10.1111/j.1558-5646.2010.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotoh H, Cornette R, Koshikawa S, Okada Y, Lavine LC, Emlen DJ, Miura T. Juvenile Hormone Regulates Extreme Mandible Growth in Male Stag Beetles. PLoS One. 2011;6:e21139. doi: 10.1371/journal.pone.0021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19*.Gotoh H, Miyakawa H, Ishikawa A, Ishikawa Y, Sugime Y, Emlen DJ, Lavine LC, Miura T. Developmental Link between Sex and Nutrition; doublesex Regulates Sex-Specific Mandible Growth via Juvenile Hormone Signaling in Stag Beetles. PLoS Genet. 2014;10:e1004098. doi: 10.1371/journal.pgen.1004098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emlen DJ, Warren IA, Johns A, Dworkin I, Lavine LC. A Mechanism of Extreme Growth and Reliable Signaling in Sexually Selected Ornaments and Weapons. Science (80-.) 2012;337:860–864. doi: 10.1126/science.1224286. [DOI] [PubMed] [Google Scholar]
- 21.Okada Y, Gotoh H, Miura T, Miyatake T, Okada K. Juvenile hormone mediates developmental integration between exaggerated traits and supportive traits in the horned flour beetle Gnatocerus cornutus. Evol. Dev. 2012;14:363–371. doi: 10.1111/j.1525-142X.2012.00554.x. [DOI] [PubMed] [Google Scholar]
- 22.David P, Bjorksten T, Fowler K, Pomiankowski A. Condition-dependent signalling of genetic variation in stalk-eyed flies. Nature. 2000;406:186–188. doi: 10.1038/35018079. [DOI] [PubMed] [Google Scholar]
- 23.Cotton S, Fowler K, Pomiankowski A. Condition dependence of sexual ornament size and variation in the stalk-eyed fly Cyrtodiopsis dalmanni (Diptera: Diopsidae) Evolution (N. Y) 2004;58:1038. doi: 10.1111/j.0014-3820.2004.tb00437.x. [DOI] [PubMed] [Google Scholar]
- 24.Shelby JA, Madewell R, Moczek AP. Juvenile hormone mediates sexual dimorphism in horned beetles. J. Exp. Zool. Part B Mol. Dev. Evol. 2007;308B:417–427. doi: 10.1002/jez.b.21165. [DOI] [PubMed] [Google Scholar]
- 25.Emlen DJ. Alternative reproductive tactics and male-dimorphism in the horned beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae) Behav. Ecol. Sociobiol. 1997;41:335–341. [Google Scholar]
- 26.Iguchi Y. Horn Dimorphism of Allomyrina dichotoma septentrionalis (Coleoptera: Scarabaeidae) Affected by Larval Nutrition. Ann. Entomol. Soc. Am. 1998;91:845–847. [Google Scholar]
- 27.Johns A, Gotoh H, McCullough EL, Emlen DJ, Lavine LC. Heightened Condition-Dependent Growth of Sexually Selected Weapons in the Rhinoceros Beetle Trypoxylus dichotomus (Coleoptera: Scarabaeidae) Integr. Comp. Biol. 2014;54:614–621. doi: 10.1093/icb/icu041. [DOI] [PubMed] [Google Scholar]
- 28**.Demuth JP, Naidu A, Mydlarz LD. Sex, War, and Disease: The Role of Parasite Infection on Weapon Development and Mating Success in a Horned Beetle (Gnatocerus cornutus) PLoS One. 2012;7:e28690. doi: 10.1371/journal.pone.0028690. This paper demonstrates strong evidence for the role of infection status affecting the expression of a sexually dimorphic plastic trait. Importantly, the majority of studies on sexually dimorphic plasticity focus on the role of nutrition during the development of these traits, and this paper fills a vital gap. In addition, despite investigation into the function of dsx in this organism, this research above has not been followed up by further work, and represents a key avenue of investigation for future studies of the role of Dsx in mediating condition dependent sexual dimorphism in response to infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams TM, Carroll SB. Genetic and molecular insights into the development and evolution of sexual dimorphism. Nat. Rev. Genet. 2009;10:797–804. doi: 10.1038/nrg2687. [DOI] [PubMed] [Google Scholar]
- 30.Shukla JN, Nagaraju J. Two female-specific DSX proteins are encoded by the sex-specific transcripts of dsx, and are required for female sexual differentiation in two wild silkmoth species Antheraea assama and Antheraea mylitta (Lepidoptera, Saturniidae) Insect Biochem. Mol. Biol. 2010;40:672–682. doi: 10.1016/j.ibmb.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Kopp A. Dmrt genes in the development and evolution of sexual dimorphism. Trends Genet. 2012;28:175–184. doi: 10.1016/j.tig.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gempe T, Beye M. Function and evolution of sex determination mechanisms, genes and pathways in insects. BioEssays. 2010;33:52–60. doi: 10.1002/bies.201000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kijimoto T, Moczek AP, Andrews J. Diversification of doublesex function underlies morph-, sex-, and species-specific development of beetle horns. Proc. Natl. Acad. Sci. 2012;109:20526–20531. doi: 10.1073/pnas.1118589109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo SD, Shi GW, Baker BS. Direct targets of the D. melanogaster DSXF protein and the evolution of sexual development. Development. 2011;138:2761–2771. doi: 10.1242/dev.065227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35**.Luo SD, Baker BS. Constraints on the evolution of a doublesex target gene arising from doublesex’s pleiotropic deployment. Proc. Natl. Acad. Sci. 2015;112:E852–E861. doi: 10.1073/pnas.1501192112. Both [35] and [36] identified many genes involved in sexually dimorphic trait development and evolution. This paper investigated the regulation of one of these genes (Flavin-containing monooxygenase-2 (Fmo-2)) in a variety of tissues in Drosophila melanogaster finding that sexually dimorphic expression of this gene is due to the deployment of separate cis-regulatory modules acting on the conserved dsx binding site in this gene. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Clough E, Jimenez E, Kim Y-A, Whitworth C, Neville MC, Hempel LU, Pavlou HJ, Chen Z-X, Sturgill D, Dale RK, et al. Sex- and Tissue-Specific Functions of Drosophila Doublesex Transcription Factor Target Genes. Dev. Cell. 2014;31:761–773. doi: 10.1016/j.devcel.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Ledón-Rettig CC, Zattara EE, Moczek AP. Asymmetric interactions between doublesex and tissue- and sex-specific target genes mediate sexual dimorphism in beetles. Nat. Commun. 2017;8:14593. doi: 10.1038/ncomms14593. In addition to the discussion in the text, these authors provide a methodolgical framework for identifying candidate genes possessing dsx bindings sites in non-model organisms. Briefly, the authors used PoSSuM to compare the similarity of predicted sites to the binding preference of Drosophila doublesex obtained from the CIS-BP database located at< http://cisbp.ccbr.utoronto.ca>. They then identifed genes differentially expressed between control and dsx-knockdown organisms that also contained a minimum of five putative Dsx-binding sites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emlen DJ, Nijhout HF. Hormonal control of male horn length dimorphism in the dung beetle Onthophagus taurus (Coleoptera: Scarabaeidae) J. Insect Physiol. 1999;45:45–53. doi: 10.1016/s0022-1910(98)00096-1. [DOI] [PubMed] [Google Scholar]
- 39**.Kijimoto T, Moczek AP. Hedgehog signaling enables nutrition-responsive inhibition of an alternative morph in a polyphenic beetle. Proc. Natl. Acad. Sci. U. S. A. 2016;113:5982–7. doi: 10.1073/pnas.1601505113. In this paper, the authors use RNAi against two members of the Hedgehog signaling pathway hedgehog (hh) and smoothend (smo), to downregulate expression of these genes during horn development. Crucially, disruption of either of these genes allowed males of any size to develop head horns, in effect removing the condition-dependent plasticity of this trait. As discussed above, later authors have identified other members of this pathway as being regulated by dsx, thus providing a mechanism through which sex-specific signals can interact and regulate sexually dimorphic plasticity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40*.Zinna R, Emlen D, Lavine L, Johns A, Gotoh H, Niimi T, Dworkin I. Sexual dimorphism and heightened conditional expression in a sexually selected weapon in the Asian rhinoceros beetle. Mol. Ecol. 2017 doi: 10.1111/mec.14907. [in revision] [DOI] [PubMed] [Google Scholar]
- 41*.Shukla JN, Palli SR. Doublesex target genes in the red flour beetle Tribolium castaneum. Sci. Rep. 2012;2 doi: 10.1038/srep00948. In this work, the authors identify 12 genes whose expression level is reduced upon injection of Tcdsx RNAi. Importantly, eight of these genes contained the 13bp consensus sequence for the Dsx binding site predicted from Drosophila, indicating direct regulation of these genes, while the other four gene lacked this sequence, and were concluded to be indirect targets. This paper thus provides direct evidence for conservation of Dsx binding sites between Drosophila and Tribolium, and provides a solid basis for the assumption of conservation utilized by [46]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Zattara E, Hughes DST, Richards S, Kijimoto T, Mozcek A. Onthophagus taurus Genome Annotations v0.5.3. 2016 doi: 10.15482/USDA.ADC/1255153. [DOI] [Google Scholar]
- 43*.Zattara E, Mozcek A, Murali SC, Bandaranaike D, Hernandez B, Chao H, Dinh H, Doddapaneni H, Dugan-Rocha S, Elkadiri S, et al. Onthophagus taurus Genome Assembly 1.0. 2016 doi: 10.15482/USDA.ADC/1255156. [DOI] [Google Scholar]
- 44.Zimmermann B, Bilusic I, Lorenz C, Schroeder R. Genomic SELEX: A discovery tool for genomic aptamers. Methods. 2010;52:125–132. doi: 10.1016/j.ymeth.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45**.Kojima T, Kunitake E, Ihara K, Kobayashi T, Nakano H. A Robust Analytical Pipeline for Genome-Wide Identification of the Genes Regulated by a Transcription Factor: Combinatorial Analysis Performed Using gSELEX-Seq and RNA-Seq. PLoS One. 2016;11:e0159011. doi: 10.1371/journal.pone.0159011. As the name suggests, this work provides a functional pipeline for identifying cohorts of genes regulated by a given transcription factor. Importantly, this work uses the combined power of gSELEX-Seq and RNA-seq to rule out false positives in the pool of potential transcription factor targets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46**.Ledón-Rettig CC, Moczek AP. The transcriptomic basis of tissue- and nutrition-dependent sexual dimorphism in the beetle Onthophagus taurus. Ecol. Evol. 2016;6:1601–1613. doi: 10.1002/ece3.1933. In this analysis, the principal finding of importance to the current review was the discovery that gene expression showed the greatest amount of sex and nutrition bias in expression in a sexually dimorphic and condition-dependent trait (i.e. head horns). In addition, this set of biased genes was relatively small, which lends support for condition-dependent sexually dimorphic plasticity being regulated by a few key regulators, such as dsx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47*.Kijimoto T, Snell-Rood EC, Pespeni MH, Rocha G, Kafadar K, Moczek AP. The nutritionally responsive transcriptome of the polyphenic beetle Onthophagus taurus and the importance of sexual dimorphism and body region. Proc. R. Soc. B Biol Sci. 2014;281:20142084. doi: 10.1098/rspb.2014.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hou Z, Jiang P, Swanson SA, Elwell AL, Nguyen BKS, Bolin JM, Stewart R, Thomson JA. A cost-effective RNA sequencing protocol for large-scale gene expression studies. Sci. Rep. 2015;5:9570. doi: 10.1038/srep09570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zinna R, Gotoh H, Brent CS, Dolezal A, Kraus A, Niimi T, Emlen D, Lavine LC. Endocrine Control of Exaggerated Trait Growth in Rhinoceros Beetles. Integr. Comp. Biol. 2016;56:247–259. doi: 10.1093/icb/icw042. [DOI] [PubMed] [Google Scholar]
- 50.Ito Y, Harigai A, Nakata M, Hosoya T, Araya K, Oba Y, Ito A, Ohde T, Yaginuma T, Niimi T. The role of doublesex in the evolution of exaggerated horns in the Japanese rhinoceros beetle. EMBO Rep. 2013;14:561–567. doi: 10.1038/embor.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51*.Toubiana W, Khila A. The benefits of expanding studies of trait exaggeration to hemimetabolous insects and beyond morphology. Curr. Opin. Genet. Dev. 2016;39:14–20. doi: 10.1016/j.gde.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 52.Bellés X. Beyond Drosophila: RNAi In Vivo and Functional Genomics in Insects. Annu. Rev. Entomol. 2010;55:111–128. doi: 10.1146/annurev-ento-112408-085301. [DOI] [PubMed] [Google Scholar]
- 53*.Gotoh H, Ishiguro M, Nishikawa H, Morita S, Okada K, Miyatake T, Yaginuma T, Niimi T. Molecular cloning and functional characterization of the sex-determination gene doublesex in the sexually dimorphic broad-horned beetle Gnatocerus cornutus (Coleoptera, Tenebrionidae) Sci. Rep. 2016;6 doi: 10.1038/srep29337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54*.McCullough EL, Miller CW, Emlen DJ. Why Sexually Selected Weapons Are Not Ornaments. Trends Ecol. Evol. 2016;31:742–751. doi: 10.1016/j.tree.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 55.Hooper RE, Tsubaki Y, Siva-Jothy MT. Expression of a costly, plastic secondary sexual trait is correlated with age and condition in a damselfly with two male morphs. Physiol. Entomol. 1999;24:364–369. [Google Scholar]
- 56.Contreras-Garduno J, Buzatto BA, Serrano-Meneses MA, Najera-Cordero K, Cordoba-Aguilar A. The size of the red wing spot of the American rubyspot as a heightened condition-dependent ornament. Behav. Ecol. 2008;19:724–732. [Google Scholar]
- 57.Moore MP, Martin RA. Intrasexual selection favours an immune-correlated colour ornament in a dragonfly. J. Evol. Biol. 2016;29:2256–2265. doi: 10.1111/jeb.12953. [DOI] [PubMed] [Google Scholar]
- 58.Valverde JP, Eggert H, Kurtz J, Schielzeth H. Condition-dependence and sexual ornamentation: Effects of immune challenges on a highly sexually dimorphic grasshopper. Insect Sci. 2017 doi: 10.1111/1744-7917.12448. [DOI] [PubMed] [Google Scholar]
- 59.Holzer B, Jacot A, Brinkhof MWG. Condition-dependent signaling affects male sexual attractiveness in field crickets Gryllus campestris. Behav. Ecol. 2003;14:353–359. [Google Scholar]
- 60.Rantala MJ, Kortet R, Kotiaho JS, Vainikka A, Suhonen J. Condition dependence of pheromones and immune function in the grain beetle Tenebrio molitor. Funct. Ecol. 2003;17:534–540. [Google Scholar]
- 61.Conner WE, Iyengar VK. Male Pheromones in Moths. In: Allison JD, Cardé RT, editors. Pheomone Communication in Moths: Evolution, Behavior, and Application. University of California Press; 2016. pp. 191–210. [Google Scholar]
- 62.Jordan AT, Conner WE. Dietary basis for the developmental plasticity of an adroconial structure in the salt marsh moth Estigmene acrea (Drury) (Lepidoptera: Arctiidae) J. Lepid. Soc. 2007;61:32–37. [Google Scholar]
- 63.Jordan AT, Jones TH, Conner WE. Morphogenetic effects of alkaloidal metabolites on the development of the coremata in the salt marsh moth Estigmene acrea (Dru.) (Lepidoptera: Arctiidae) Arch. Insect Biochem. Physiol. 2007;66:183–189. doi: 10.1002/arch.20211. [DOI] [PubMed] [Google Scholar]
- 64.Xu J, Zhan S, Chen S, Zeng B, Li Z, James AA, Tan A, Huang Y. Sexually dimorphic traits in the silkworm Bombyx mori, are regulated by doublesex. Insect Biochem. Mol. Biol. 2017;80:42–51. doi: 10.1016/j.ibmb.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 65.Egelhaaf A, Rick-Wagner S, Schneider D. Development of the male scent organ of Creatonotos transiens (Lepidoptera, Arctiidae) during metamorphosis. Zoomorphology. 1992;111:125–139. [Google Scholar]
- 66.Kim YIl, Kim HJ, Kwon YM, Kang YJ, Lee IH, Jin BR, Han YS, Kim I, Cheon HM, Ha NG, et al. RNA interference mediated knockdown of apolipophorin-III leads to knockdown of manganese superoxide dismutase in Hyphantria cunea. Comp. Biochem. Physiol. - A Mol. Integr. Physiol. 2011;159:303–312. doi: 10.1016/j.cbpa.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 67.Schmitz B, Buck M, Egelhaaf A, Schneider D. Ecdysone and a dietary alkaloid interact in the development of the pheromone gland of a male moth (Creatonotos, Lepidoptera: Arctiidae) Roux’s Arch. Dev. Biol. Off. organ EDBO. 1989;198:1–7. doi: 10.1007/BF00376363. [DOI] [PubMed] [Google Scholar]
- 68.Wilkinson GS, Johns PM, Metheny JD, Baker RH. Sex-Biased Gene Expression during Head Development in a Sexually Dimorphic Stalk-Eyed Fly. PLoS One. 2013;8:e59826. doi: 10.1371/journal.pone.0059826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stuglik MT, Babik W, Prokop Z, Radwan J. Alternative reproductive tactics and sex-biased gene expression: the study of the bulb mite transcriptome. Ecol. Evol. 2014;4:623–632. [Google Scholar]
- 70.Joag R, Stuglik M, Konczal M, Plesnar-Bielak A, Skrzynecka A, Babik W, Radwan J. Transcriptomics of Intralocus Sexual Conflict: Gene Expression Patterns in Females Change in Response to Selection on a Male Secondary Sexual Trait in the Bulb Mite. Genome Biol. Evol. 2016;8:2351–2357. doi: 10.1093/gbe/evw169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harano T, Okada K, Nakayama S, Miyatake T, Hosken DJ. Intralocus Sexual Conflict Unresolved by Sex-Limited Trait Expression. Curr. Biol. 2010;20:2036–2039. doi: 10.1016/j.cub.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 72.Erickson JW, Quintero JJ. Indirect effects of ploidy suggest X chromosome dose, not the X:A ratio, signals sex in Drosophila. PLoS Biol. 2007;5:2821–2830. doi: 10.1371/journal.pbio.0050332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verhulst EC, Beukeboom LW, van de Zande L. Maternal Control of Haplodiploid Sex Determination in the Wasp Nasonia. Science (80-.) 2010;328 doi: 10.1126/science.1185805. [DOI] [PubMed] [Google Scholar]
- 74.Kiuchi T, Koga H, Kawamoto M, Shoji K, Sakai H, Arai Y, Ishihara G, Kawaoka S, Sugano S, Shimada T, et al. A single female-specific piRNA is the primary determiner of sex in the silkworm. Nature. 2014;509:633–636. doi: 10.1038/nature13315. [DOI] [PubMed] [Google Scholar]
- 75.Sharma A, Heinze SD, Wu Y, Kohlbrenner T, Morilla I, Brunner C, Wimmer EA, van de Zande L, Robinson MD, Beukeboom LW, et al. Male sex in houseflies is determined by Mdmd, a paralog of the generic splice factor gene CWC22. Science (80-.) 2017;356:642–645. doi: 10.1126/science.aam5498. [DOI] [PubMed] [Google Scholar]
- 76.Beye M, Hasselmann M, Fondrk MK, Page RE, Omholt SW. The gene csd is the primary signal for sexual development in the honeybee and encodes an SR-type protein. Cell. 2003;114:419–429. doi: 10.1016/s0092-8674(03)00606-8. [DOI] [PubMed] [Google Scholar]
- 77*.Wagamitsu S, Takase D, Aoki F, Suzuki MG. Identification of the Doublesex protein binding sites that activate expression of lozenge in the female genital disc in Drosophila melanogaster. Mech. Dev. 2017;143:26–31. doi: 10.1016/j.mod.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 78.Williams TM, Selegue JE, Werner T, Gompel N, Kopp A, Carroll SB. The Regulation and Evolution of a Genetic Switch Controlling Sexually Dimorphic Traits in Drosophila. Cell. 2008;134:610–623. doi: 10.1016/j.cell.2008.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Warren IA, Gotoh H, Dworkin IM, Emlen DJ, Lavine LC. A general mechanism for conditional expression of exaggerated sexually-selected traits. BioEssays. 2013;35:889–899. doi: 10.1002/bies.201300031. [DOI] [PubMed] [Google Scholar]