Abstract
Background
This study establishes a novel and broadly-applicable defect classification system and flap selection algorithm for segmental mandibulectomy defects that emphasize the importance of the soft tissue deficit, in addition to that of the bony defect.
Methods
Between 1992 and 2011, 202 patients with mandibulectomy defects underwent immediate reconstruction by a single surgeon. Details of the bony and soft tissue defects, recommendations for the most appropriate reconstruction for each clinical scenario, and surgical outcomes are presented.
Results
A total of 211 flaps were performed in 202 patients. Forty-one (19%) were non-osseous only, and 170 (81%) were osseous-containing. The majority of osseous flaps were fibula osseous or osteocutaneous flaps (91%), and the majority of non-osseous flaps were vertical rectus abdominis myocutaneous (VRAM) flaps (68%). Flap selection was influenced by the number of soft tissue zones resected; defects of ≤1 soft tissue zone were predominantly reconstructed with an osseous flap, whereas defects that involved ≥4 zones underwent reconstruction with only a soft tissuey flap in 55% of cases.
Conclusion
The algorithm for reconstruction of the mandibulectomy defect must include both non-osseus and osseus flaps based on defect size, location, and number of soft tissue zones involved. As the extent of the soft tissue defect increases, non-osseus flaps are preferred due to greater reliability of the skin island. The surgical outcomes associated with this algorithm are similar to or better than what is published in the literature. This series represents the largest reported single surgeon experience with mandibulectomy defect reconstruction.
Introduction
Reconstruction following segmental mandibulectomy is nuanced and complicated, and subject to equally important functional and aesthetic considerations. After several decades managing patients with these defects, the senior author (P. G. C.) has developed a reconstructive algorithm. In doing so, existing mandibulectomy defect classification systems1-6 were found to be lacking in either precision or simplicity. Therefore, a classification system that included defect size, quality, and functional and aesthetic significance was also developed.
Classification system for segmental mandibulectomy defects
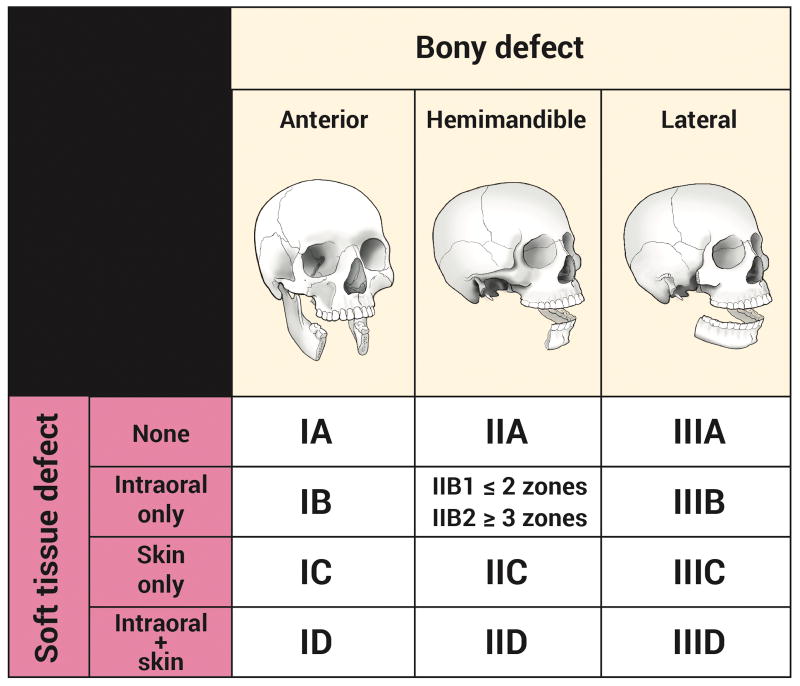
The classification system consists of a Roman numeral, a subdividing letter, and in some cases a subcategory number. The Roman numeral describes the bony defect, with “Type I” (anterior: any defect that includes the mandibular symphysis), “Type II” (hemimandible: includes the body, angle, and ascending ramus, with or without the condyle), and “Type III” (lateral: includes one or two of the body, angle, and ascending ramus, but not all three).
Unlike other classification systems, this system places an equivalent importance on the quality and location of the soft tissue defect (comprising any combination of skin, subcutaneous tissue, muscle, intraoral structures, and mucosal lining). This is accomplished via a letter following the Roman numeral, either “A” (no soft tissue defect), “B” (intraoral structure and/or mucosal lining defect only), “C” (skin defect only), or “D” (both intraoral structures/lining and skin defect).
The extent of the intraoral defect has been found to be an important determinant in the reconstruction of Type II (hemimandible) defects, so a sub-classification of “B1” and “B2” was created. Based on 5 zones of intraoral structures (buccal mucosa, floor of mouth, palate, tongue, and pharynx), excision of 2 or fewer zones is denoted “B1” and excision of 3 or more zones is denoted “B2.”
When the bony and soft tissue deficit designations are combined, a streamlined classification system of 13 defect types emerges: IA, IB, IC, ID, IIA, IIB1, IIB2, IIC, IID, IIIA, IIIB, IIIC, and IIID (Figure 1).
Figure 1. Mandibulectomy defect classification system.
The objective of this article is to introduce this mandibulectomy defect classification system and management algorithm, and to analyze flap selection over a 20-year period.
Materials and methods
Between 1992 and 2011, 202 patients with mandibulectomy defects underwent immediate reconstruction. Descriptors of the bony and soft tissue defects were recorded from a prospectively maintained database. This study was approved by the institutional review board of Memorial Sloan Kettering Cancer Center. Outcomes were analyzed using Fisher's exact test (Graphpad Software; La Jolla, California), and statistical significance was set at p<0.05.
The algorithm used to reconstruct mandibulectomy defects is conceptually based first on the location of the resected bone. This is followed by consideration of the required soft tissue, first assessing the volume, and then the surface area (the sum of the intraoral structures and/or skin). Of note, different areas of soft tissue have different reconstructive needs, and sometimes these supersede the bony needs. Related structures such as lips and condyle do not factor into the flap choice algorithm, and should be considered separate reconstructive concerns.
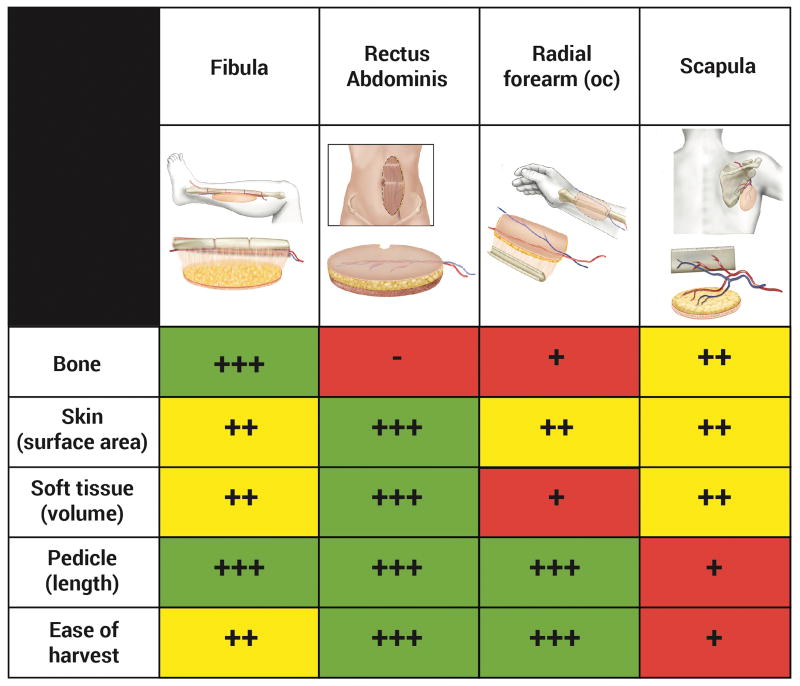
The four most common flaps used by the senior author for post-mandibulectomy reconstruction are the fibula, vertical rectus abdominis myocutaneous (VRAM), radial forearm, and scapula flaps. The relative strengths of these flaps are described in Figure 2.
Figure 2.
Relative strengths of the fibula, rectus abdominis, radial forearm, and scapula flaps.
The fibula flap has become the workhorse for mandibular reconstruction because of its extensive amount of bone with minimal donor site deficit; the ability to create multiple osteotomies without compromising vascularity (due to its dual blood supply); a sufficient bone caliber for dental implant osseointegration; a moderately sized skin island that can be separated into distinct chimeric skin islands; and additional soft tissue (most notably flexor hallucis longus) that can be harvested to optimize vascularity or fill volume deficits. However, the fibula flap's skin island is frequently stiff, sometimes of insufficient volume and/or surface area to satisfy particular defects, and of unpredictable vascularity7.
The radial forearm flap provides an excellent pedicle, the skin is highly reliable, soft and pliable, and though only a small amount of bone can be safely harvested, a portion of the radius can be included to create an osteocutaneous flap. Limitations of the bone include the minimal amount that can be harvested, the risk of donor site fracture, and that it can be easily devascularized by osteotomies. Additionally, the flap has a relatively small volume (though this is advantageous in certain settings).
The VRAM flap provides a very large surface area that can be separated into well-vascularized skin islands and a reliable pedicle that can be lengthened to 15-20 cm8 (the latter characteristic is particularly useful if the recipient vessels are in the contralateral neck9). These flaps can, however, be very bulky in obese patients. Furthermore, bone cannot be reliably.
The scapula osteocutaneous flap is rarely used due to its relatively poor-quality bone that cannot be reliably osteotomized or osseointegrated, as well as the need for intraoperative repositioning. The skin island is highly reliable, provides moderate volume and surface area, and can be oriented independent from the bone.
Reconstructive algorithm
Type I defects
The importance of the anterior mandible for maintaining projection and support of the lower face and the potential for dental implant osseointegration makes bony reconstruction the dominant consideration in Type I (anterior) defects.
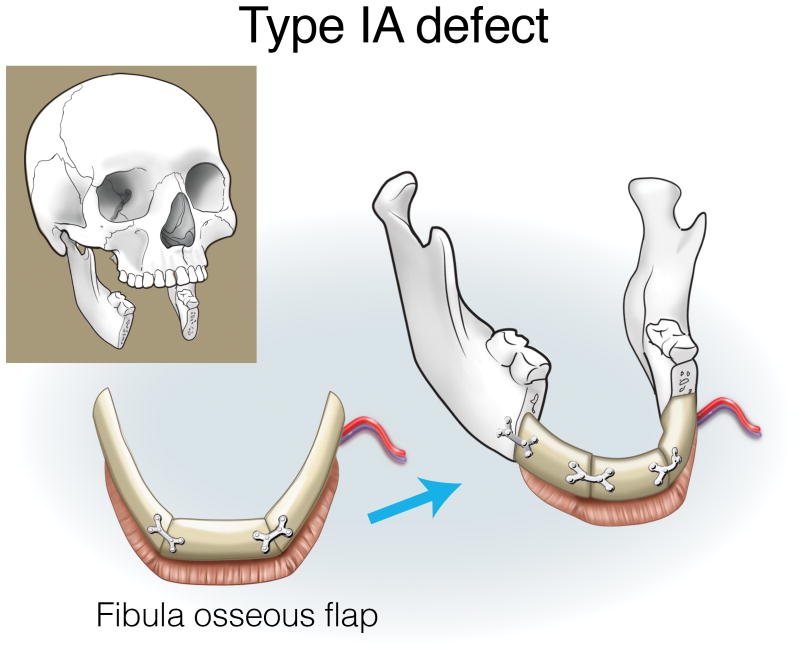
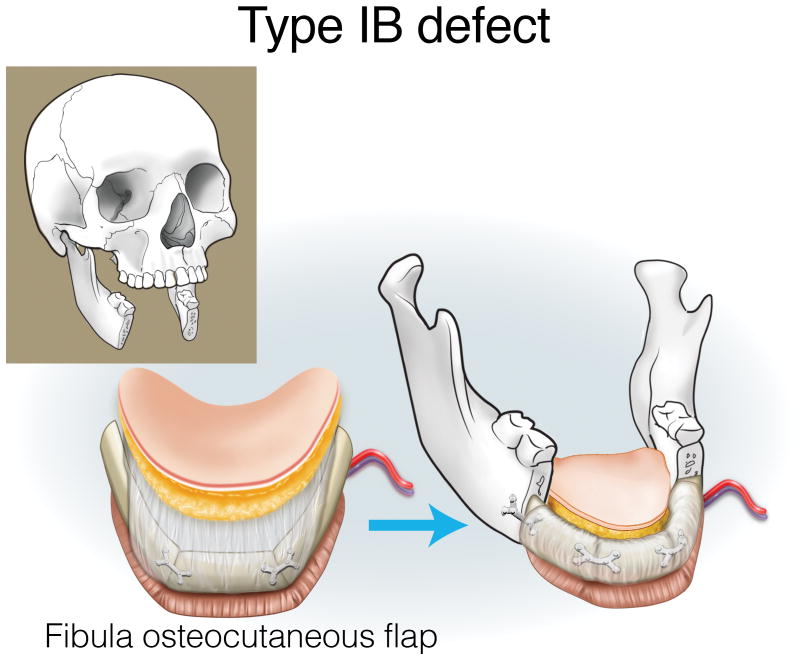
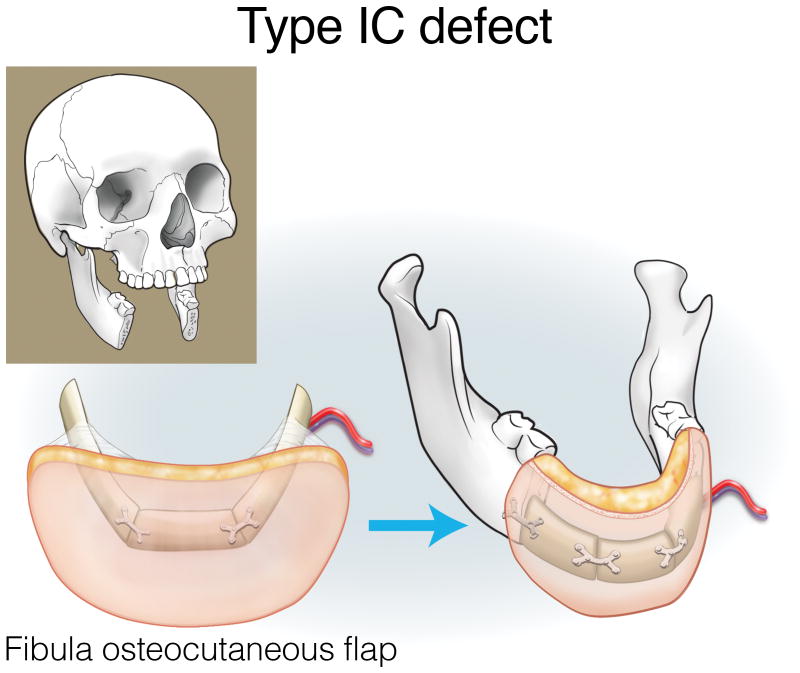
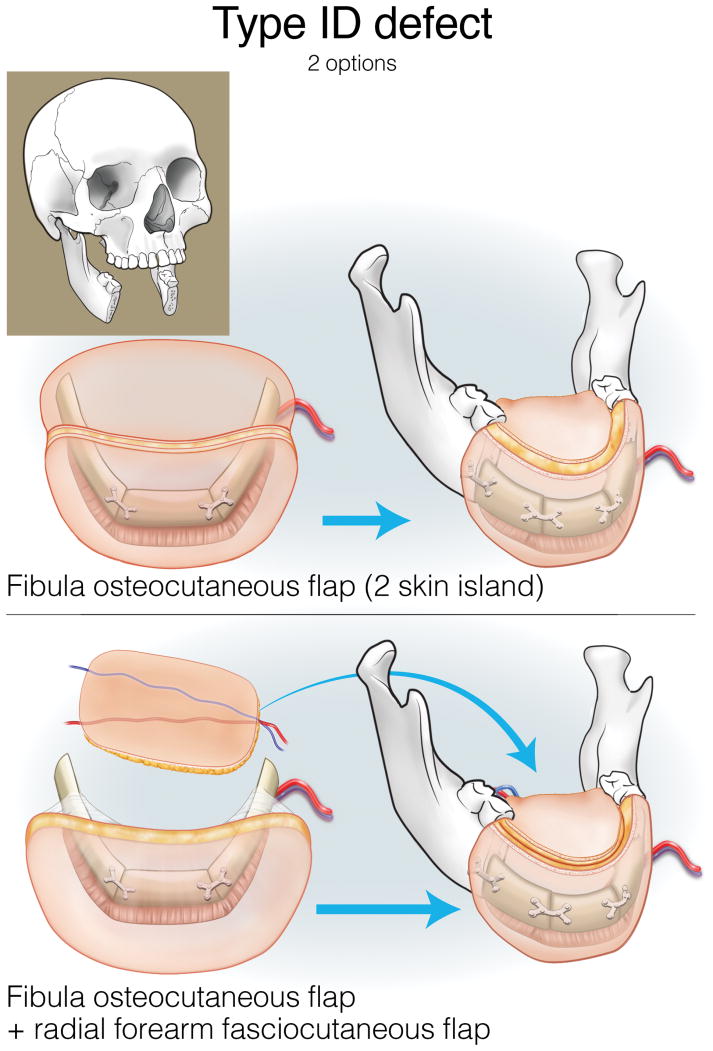
Type IA defects are appropriately reconstructed with an osseous (bone only) fibula flap (Figure 3). Type IB and IC defects can be reconstructed with an osteocutaneous fibula flap, with the skin island utilized for intraoral lining or skin replacement, respectively (Figures 4 and 5). Type ID defects require restoration of intraoral structures and skin. In some cases, a single fibula skin island can be folded with a strip of skin deepithelialized to effectively create two surfaces, or if more than one perforator is identified, two distinct skin islands can be inset separately. Alternatively, a separate radial forearm fasciocutaneous flap can be utilized (which is used for intraoral lining while the fibula is used for skin) (Figure 6).
Figure 3.
Reconstruction of Type IA mandibulectomy defects. ©2017, Memorial Sloan Kettering Cancer Center.
Figure 4.
Reconstruction of Type IB mandibulectomy defects. ©2017, Memorial Sloan Kettering Cancer Center.
Figure 5.
Reconstruction of Type IC mandibulectomy defects. ©2017, Memorial Sloan Kettering Cancer Center.
Figure 6.
Reconstruction of Type ID mandibulectomy defects. ©2017, Memorial Sloan Kettering Cancer Center.
Type II defects
Type II (hemimandible) defects encompass the entire hemimandible from the parasymphysis to the ipsilateral ascending ramus, and sometimes include the condyle. The degree of bony deficit is significant, therefore the fibula is indicated in most instances due to the bone's high quality and quantity. As an increasing magnitude of posterior soft tissue is resected, however, closure of the soft tissue defect and restoration of the intraoral surfaces takes priority. For aesthetic restoration, it is conceptually ideal to replace the entire bony deficit in order to maintain the shape and contours of the lateral aspect of the lower face. In terms of function, there is minimal deficit if the anterior arch and contralateral hemimandible remain intact.
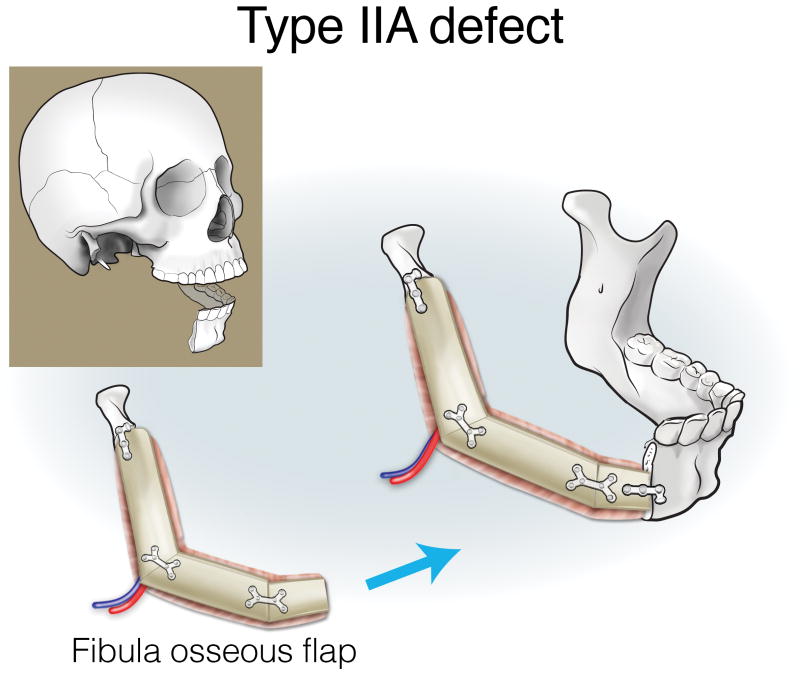
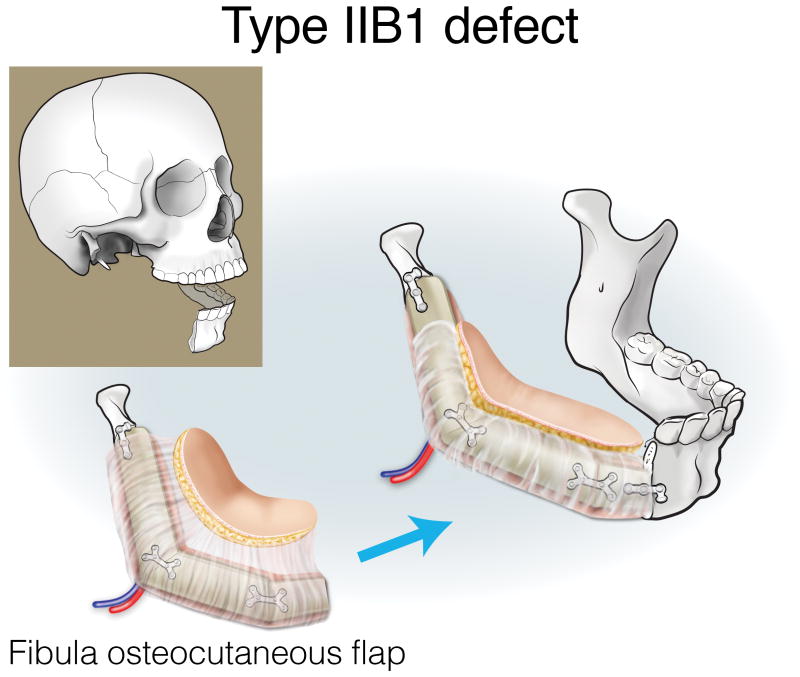
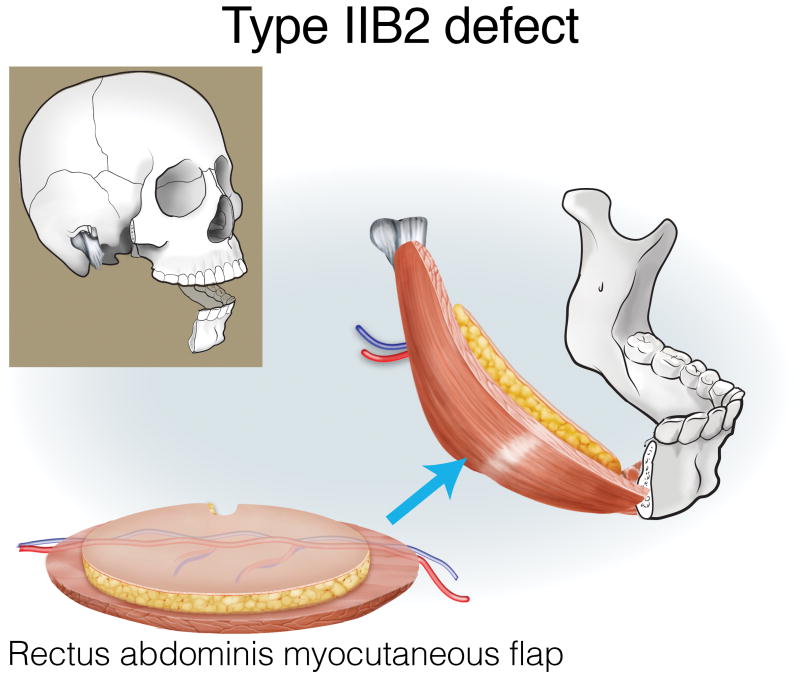
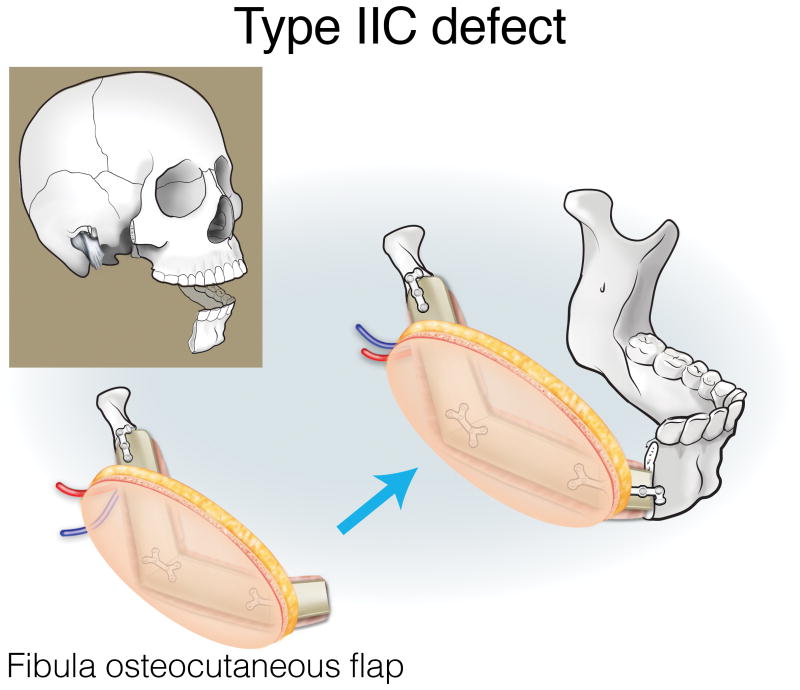
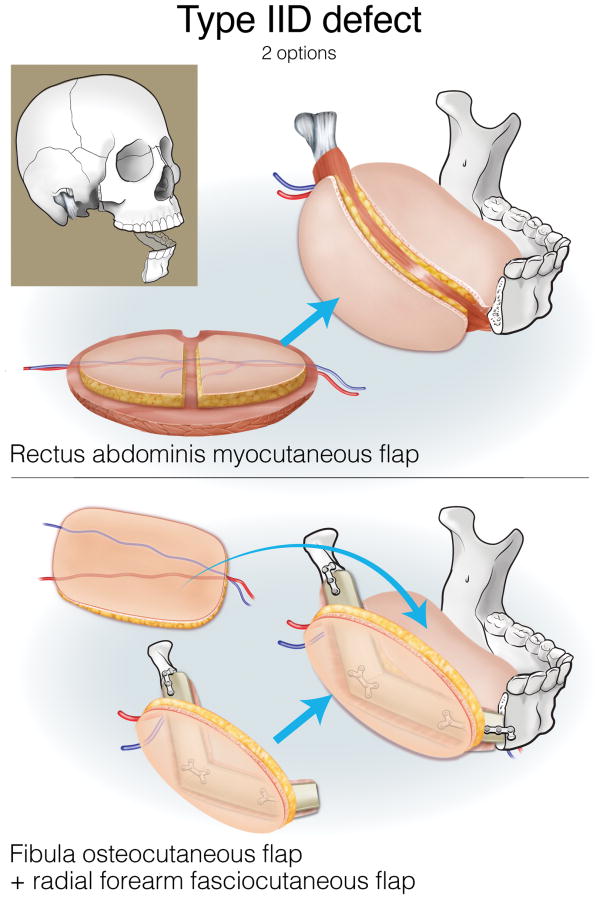
Type IIA defects are best reconstructed with an osseous fibula flap (Figure 7). Type IIB1 defects require restoration of a minimal-to-moderate amount of intraoral tissue, and can be reconstructed with an osteocutaneous fibula flap (Figure 8). Type IIB2 defects have an extensive intraoral tissue requirement, exceeding what the fibula can reliably provide. The VRAM flap provides abundant, reliable skin for intraoral resurfacing, in addition to substantial muscle and adipose tissue volume. This flap does not have any osseous component, but is well tolerated in terms of both function and aesthetics (Figure 9). Type IIC defects usually require a small-to-moderate volume of soft tissue and skin replacement in addition to the extensive bony requirements, and are thus best reconstructed with a fibula osteocutaneous flap (Figure 10). Type IID defects require extensive soft tissue volume replacement to resurface both the intraoral and external skin defects. Although a fibula osteocutaneous flap plus a radial forearm flap may fulfill the bony and soft tissue requirements, reconstruction of these defects in patients with advanced disease who require rapid, reliable healing and restoration of function is often best served by a folded, two-skin-island VRAM flap (Figure 11).
Figure 7.
Reconstruction of Type IIA mandibulectomy defects. ©2017, Memorial Sloan Kettering Cancer Center.
Figure 8.
Reconstruction of Type IIB1 mandibulectomy defects. ©2017, Memorial Sloan Kettering Cancer Center.
Figure 9.
Reconstruction of Type IIB2 mandibulectomy defects. ©2017, Memorial Sloan Kettering Cancer Center.
Figure 10.
Reconstruction of Type IIC mandibulectomy defects. ©2017, Memorial Sloan Kettering Cancer Center.
Figure 11.
Reconstruction of Type IID mandibulectomy defects. ©2017, Memorial Sloan Kettering Cancer Center.
Type III defects
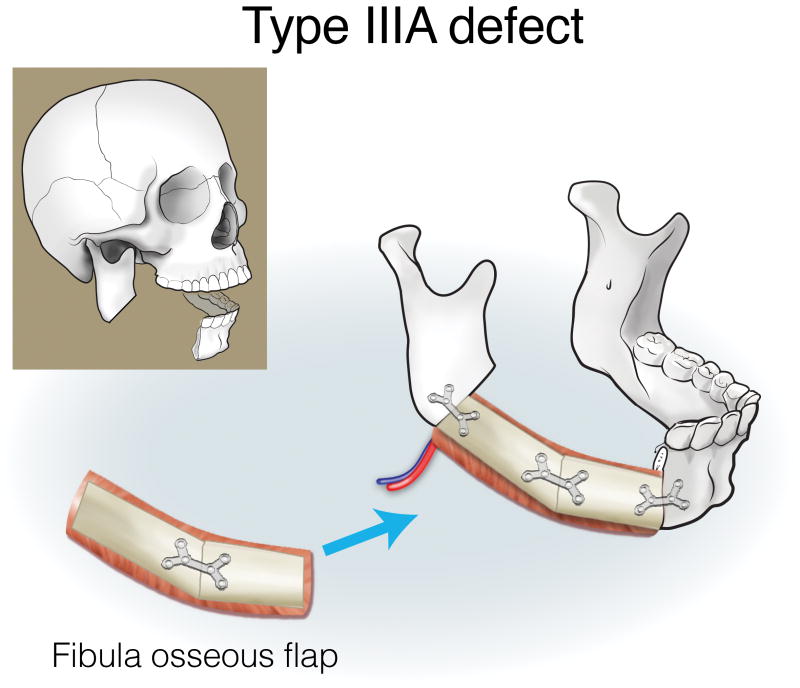
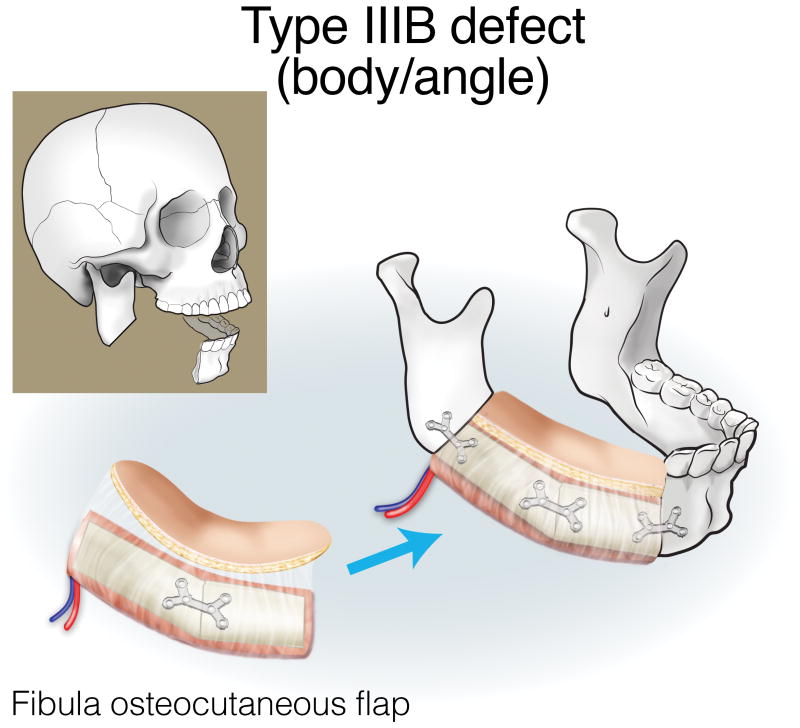
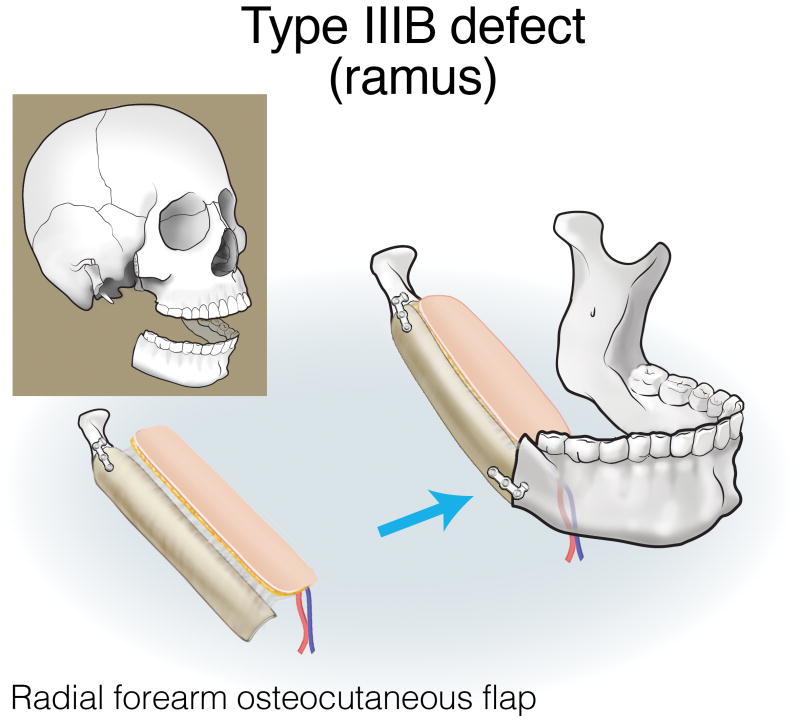
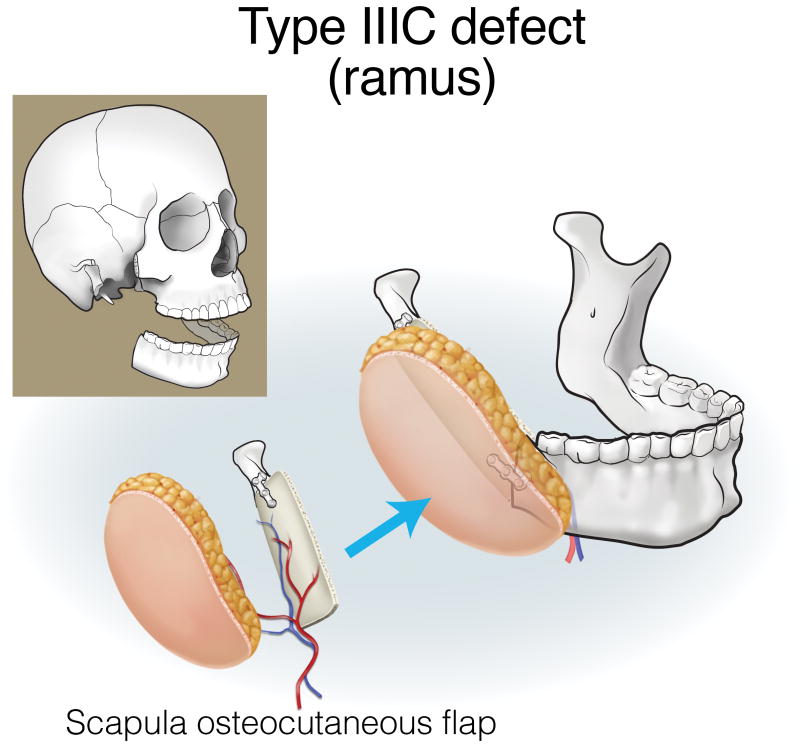
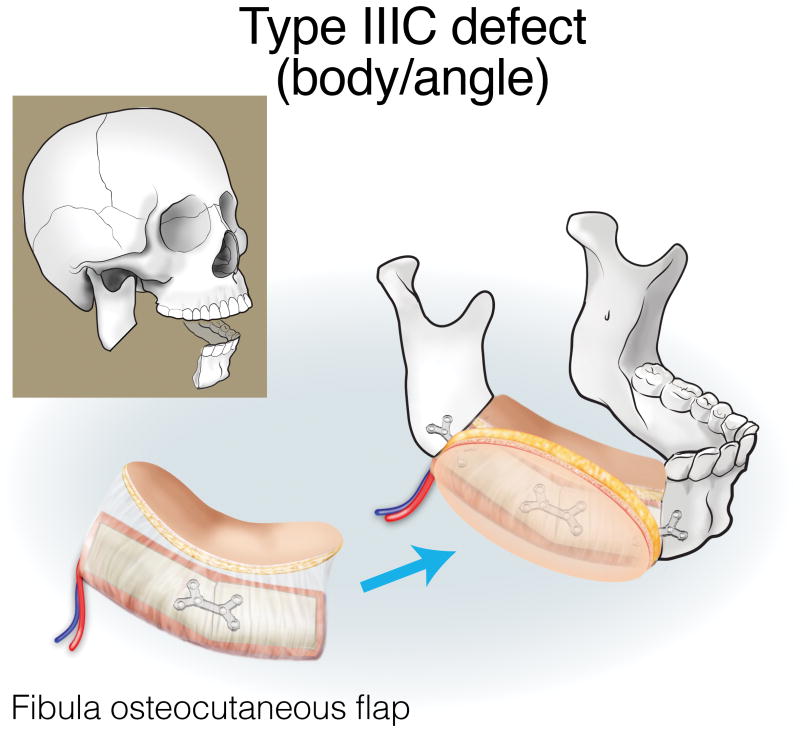
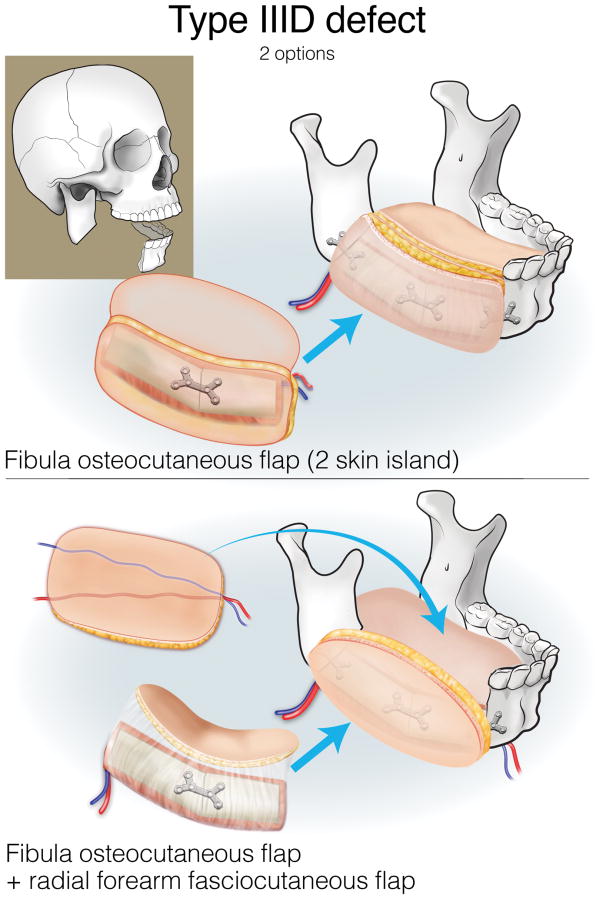
Finally, type III (lateral) defects have a relatively limited bony deficit (usually the body, angle, or ascending ramus). The fibula osseous flap is best for reconstructing a Type IIIA defect (Figure 12). Type IIIB defects of the body and angle are also best reconstructed with the fibula osteocutaneous flap (Figure 13). In contrast, when tumors involve the ascending ramus, often a large surface area of lateral pharyngeal mucosa needs to be replaced with a small segment of non-dentition-bearing bone. For this defect, a radial forearm osteocutaneous flap is ideal because it provides thin, pliable skin that resurfaces the lateral pharynx without obstructing the oropharyngeal cavity (as would occur with a fibula flap), while also providing adequate bone (Figure 14). Type IIIC defects can result from cutaneous or parotid gland tumors that involve skin, soft tissue, and bone, with a limited amount of ascending ramus. The bony elements of these defects are of minimal clinical significance, while there is a moderate-to-extensive skin and soft tissue requirement; for this reason, the scapula osteocutaneous flap is recommended, as it allows for well-matched skin resurfacing while still providing the limited amount of bone needed to reconstruct the ascending ramus (Figure 15). Alternatively, if the tumor involves the body (dentition-bearing) and external skin/soft tissue, then the fibula osteocutaneous flap is recommended (Figure 16). Type IIID defects are usually created after resection of tumors of gingival origin that extend inward to the floor of mouth and tongue, and outward into the mandible and skin. In these extensive soft tissue defects, a fibula osteocutaneous flap with a large, folded skin island or multiple skin islands may suffice. For even more extensive defects, a double flap (fibula osteocutaneous flap externally, radial forearm fasciocutaneous intraorally) may become necessary (Figure 17).
Figure 12.
Reconstruction of Type IIIA mandibulectomy defects. ©2017, Memorial Sloan Kettering Cancer Center.
Figure 13.
Reconstruction of Type IIIB (body/angle) mandibulectomy defects. ©2017, Memorial Sloan Kettering Cancer Center.
Figure 14.
Reconstruction of Type IIIB (ramus) mandibulectomy defects. ©2017, Memorial Sloan Kettering Cancer Center.
Figure 15.
Reconstruction of Type IIIC (ramus) mandibulectomy defects. ©2017, Memorial Sloan Kettering Cancer Center.
Figure 16.
Reconstruction of Type IIIC (body/angle) mandibulectomy defects. ©2017, Memorial Sloan Kettering Cancer Center.
Figure 17.
Reconstruction of Type ID mandibulectomy defects. ©2017, Memorial Sloan Kettering Cancer Center.
Results
Demographic and perioperative details
A total of 211 flaps were performed in 202 patients (58% male). Nine patients underwent reconstruction with two flaps (either both at the initial operation, or a second flap was utilized at a subsequent operation to address tissue loss after fibula osteocutaneous flap reconstruction). The average patient age was 52.3 years (range: 3-84 years), and the mean follow-up was 60.7 months (range: 1-203 months). Twenty-three patients (11%) underwent neoadjuvant therapy and 25 (12%) underwent adjuvant chemotherapy. Sixty-two (29%) underwent neoadjuvant therapy and 102 (48%) underwent adjuvant radiation. Mean operative time (including resection) was 11.7 hours, and the mean length of stay was 17.2 days.
Flap selection
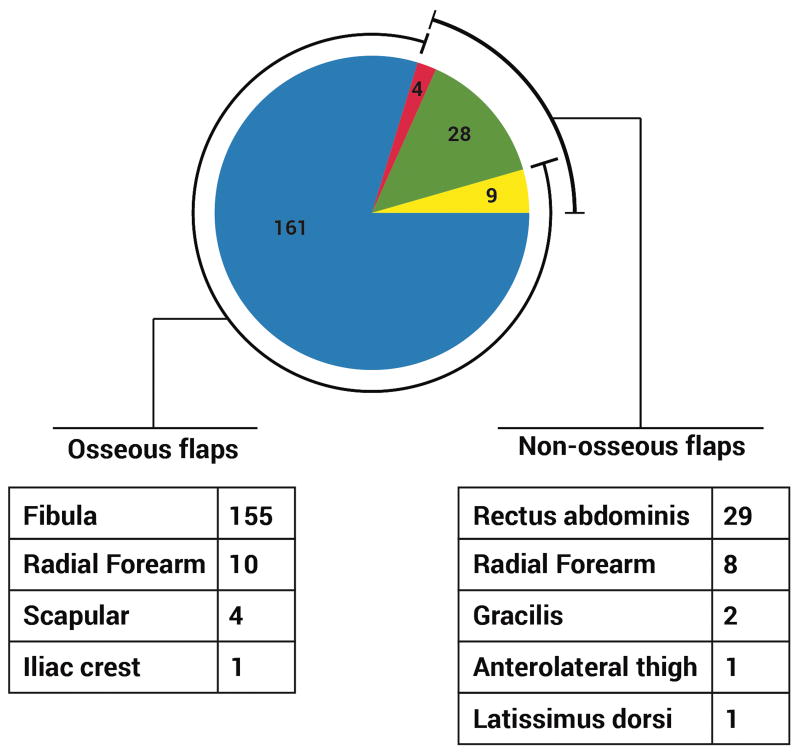
Of the 211 flaps, 41 (19%) were non-osseous only, and 170 (81%) were osseous-containing (9 of which were osseous flaps performed simultaneously with non-osseous flaps). Most osseous flaps were fibula osseous or osteocutaneous flaps (91%), and the majority of non-osseous flaps were VRAM flaps (68%) (Figure 18).
Figure 18. Flap selection, by type of flap.
Flap selection was influenced by the number of soft tissue zones resected; defects of up to 1 soft tissue zone were reconstructed with an osseous flap in 97% (99 of 102), whereas defects that involved 4-5 zones were reconstructed only with soft tissue flaps in 55%.
Surgical outcomes
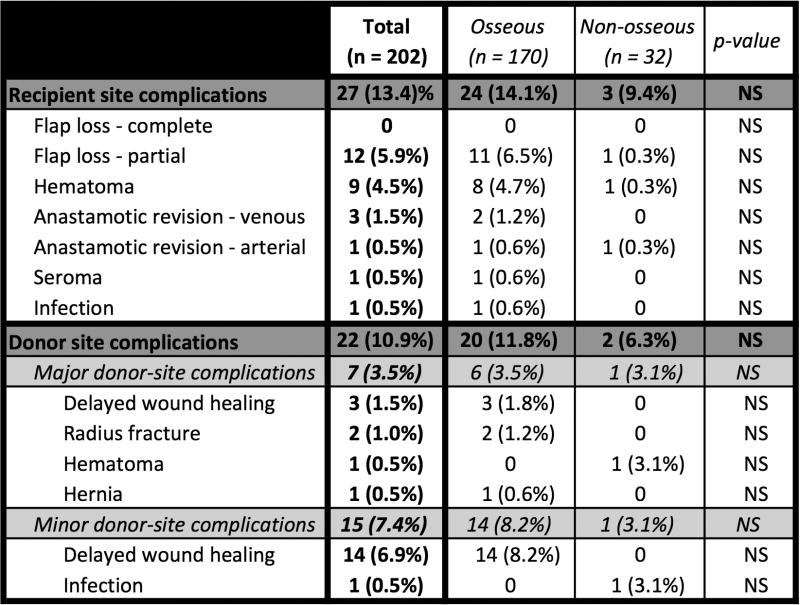
The overall recipient site complication rate was 13.4% (Figure 19); the rate was 14.1% in osseous-containing flaps, compared with 9.4% for non-osseous flaps (p=NS). The complete flap loss rate was 0%, and the partial flap loss rate was 5.9%; there was no statistically significant difference in flap loss between patients who received osseous versus non-osseous flaps. A second free flap was required in 1.0% of all patients due to partial flap loss. The venous thrombosis rate was 1.5%, and the arterial thrombosis rate was 0.5% (flaps were salvaged in all cases). The donor site complication rate was 10.9%; 3.5% were classified as major, and 7.4% were classified as minor.
Figure 19. Surgical complications.
Discussion
The classification system and reconstructive algorithm presented here are based on both bony and soft tissue requirements, and simplify the approach to post-mandibulectomy reconstruction, while maximizing clinical outcomes. As they are meant for the clinician who must decide on the reconstructive plan, we feel that the clinical relevance and applicability of both elements are extremely high, and that sets them apart from classification systems and algorithms that have been previously described. Most notably, the Jewer classification is more of an anatomic description, and does not lend itself well to reconstructive decision-making. We believe that the bony characterization is made more complicated than necessary by making different classifications (C, LC, HC, LCL, HCL, and HH) for what we believe always requires the same flap (fibula). And we believe that it underrepresents the importance of the soft tissue deficit. Even when “s” (skin) and/or “m” (mucosa) is included in the classification system, it fails to distinguish between a defect that will require a minimal amount of soft tissue and one that will require a significant amount; this is clinically relevant because the most appropriate flap is different depending on the magnitude of soft tissue resection.
From the perspective of the 3 types of bony defects, the fibula flap remains the workhorse because of its versatility and near-ideal bony characteristics. But, there are times when the soft tissue qualities of alternative (sometimes non-osseous) flaps outweigh its benefits.
For Type I (anterior) mandibulectomy defects, the most important aesthetic requirement is maintaining anterior projection. While this could theoretically be attained by reconstruction plates alone, experience has shown that it often leads to plate exposure and extrusion10. The combination of reconstruction plate with a non-osseous free flap for soft tissue coverage (such as a radial forearm fasciocutaneous flap) has been advocated in the literature11, though not in anterior defects where future osseointegration is an important element of the overall reconstructive approach. Therefore, an osseous flap is mandatory in this setting. The only other flap that can recreate the sharp angle of the mandibular symphysis is the scapular tip, though this flap cannot as safely undergo osteotomy or osseointegration, and should only be considered when a fibula flap is not possible. As the algorithm indicates, the only setting in which a fibula flap alone is not recommended is when there is an extensive amount of soft tissue needed, in which case a fibula combined with a radial forearm fasciocutaneous flap provides the best combination of bone and skin surface area.
Type II (hemimandible) defects are often accompanied by the need for extensive soft tissue coverage (external and/or intraoral). The relatively limited surface area of the skin island of the fibula osteocutaneous flap makes it a good choice when the soft tissue defect is moderate-sized, but when the defect is larger, the soft tissue limitations outweigh the bony benefits, and alternative flaps are more appropriate. The principle of replacing “like with like” calls for osseous reconstruction in all cases, and as recently as 1999 we recommended this approach9. Our reconstructive approach has evolved, however, as we realized that the advantageous soft tissue elements of non-osseous flaps – particularly the VRAM flap – outweigh the advantages of any osseous flap when extensive soft tissue surface area and volume are needed (a belief echoed in the literature, as issues like mandibular alignment are well-preserved even without bony replacement 12, 13). Furthermore, the desire to minimize wound healing problems and rapidly initiate oral intake and adjuvant therapy may additionally steer decision-making towards non-osseous flaps. Further factoring into decision-making is the fact that bony reconstruction requires rigid fixation, which can be complicated by hardware infections, plate exposure, and bony non-union.
Recommendations for Type III (lateral) defects are influenced by the lesser importance of load-bearing bone (especially if limited to the ascending ramus) and greater importance of the nuances of soft tissue: as thin as possible on the intraoral surface and as closely matched as possible for the volume, texture, and color of the external skin. There are some who have advocated for non-vascularized bone in such cases14, but the frequent need for soft tissue coverage and the high incidence of radiation treatment in this population makes vascularized options preferable.
When the algorithm is analyzed from the perspective of the different flap options, it is clear that the fibula osteocutaneous flap remains the reconstructive workhorse because of its bony, soft tissue, and skin qualities – it is the flap of choice in most clinical scenarios. The radial forearm flap is utilized more rarely: as an osteocutaneous flap when a small amount of bone and a substantial amount of thin, pliable lining is required (Type IIIB), or as a fasciocutaneous flap in combination with a fibula osteocutaneous flap when large amounts of intraoral and skin coverage are needed (Types ID, IID, and IIID). The scapula osteocutaneous flap is recommended when a small amount of lateral bone is needed along with an extensive amount of skin and moderate volume of soft tissue for external covering (Type IIIC). The VRAM flap is selected when extensive soft tissue is needed, and often is separated into two skin islands (Type IIB2, IID). Appreciation of the greater importance of soft tissue and the lesser importance of bony reconstitution in hemimandible defects with a large posterior soft tissue requirement has grown over time.
Utilization of this algorithm that includes both osseous and non-osseous flaps facilitates reconstruction that is reliable and has a high success rate. The surgical complications in the current series are comparable to, or better than, most large series in the literature. Complete flap loss (0%), hematoma (4.5%) and anastomotic revision (2.0%) are all less frequent than has previously been published 2,3,5.
It is important to note that this combination of defect classification system and reconstructive algorithm is applicable to all types of mandibulectomy defects, even though it was developed as a result of treating patients with cancer. This population is often older with multiple comorbidities, and often needs to undergo adjuvant radiation and chemotherapy in an expedited fashion. Therefore, more than other groups, these patients require a reconstructive approach that is reliable and straightforward. However, mandibular defects created by other etiologies (benign, congenital, trauma) could also be classified using this system, and equally effectively reconstructed using this algorithm – a system that specifically addresses both bony and soft tissue defects is critical to maximizing aesthetic and functional outcomes, no matter the etiology.
In conclusion, this defect classification system and management algorithm for the reconstruction of mandibulectomy defects is simple, comprehensive, effective, and broadly applicable.
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Financial Disclosure Statement: The authors have nothing to disclose.
Presented at: none
- Made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data;
- Drafted the article or revising it critically for important intellectual conten
- Had final approval of the version to be published;
- Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.David DJ, Tan E, Katsaros J, Sheen R. Mandibular reconstruction with vascularized iliac crest: a 10-year experience. Plast Reconstr Surg. 1988;82(5):792–801. doi: 10.1097/00006534-198811000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Jewer DD, Boyd JB, Manktelow RT, Zuker RM, Rosen IB, Gullane PJ, Rotstein LE, Freeman JE. Orofacial and mandibular reconstruction with the iliac crest free flap: a review of 60 cases and a new method of classification. Plast Reconstr Surg. 1989;84(3):391–403. [PubMed] [Google Scholar]
- 3.Urken ML, Weinberg H, Vickery C, Buchbinder D, Lawson W, Biller HF. Oromandibular reconstruction using microvascular composite free flaps. Arch Otolaryngol Head Neck Surg. 1991;117:733–44. doi: 10.1001/archotol.1991.01870190045010. [DOI] [PubMed] [Google Scholar]
- 4.Boyd JB, Gullane PJ, Rotstein LE, Brown DH, Irish JC. Classification of mandibular defects. Plast Reconst Surg. 1993;92:1266–75. [PubMed] [Google Scholar]
- 5.Schultz BD, Sosin M, Nam A, Mohan R, Zhang P, Khalifian S, Vranis N, Manson PN, Bojovic B, Rodriguez ED. Classification of mandible defects and algorithm for microvascular reconstruction. Plast Reconstr Surg. 2015;135:743–54e. doi: 10.1097/PRS.0000000000001106. [DOI] [PubMed] [Google Scholar]
- 6.Brown JS, Barry C, Ho M, Shaw R. A new classification for mandibular defects after oncological resection. Lancet Oncol. 2016;17:e23–30. doi: 10.1016/S1470-2045(15)00310-1. [DOI] [PubMed] [Google Scholar]
- 7.Hidalgo DA. Fibula free flap: a new method of mandible reconstruction. Plast Reconstr Surg. 1989;84(1):71–9. [PubMed] [Google Scholar]
- 8.Cordeiro PG, Santamaria E. The extended, pedicled rectus abdominis free tissue transfer for head and neck reconstruction. Ann Plast Surg. 1997;39(1):53–9. doi: 10.1097/00000637-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Cordeiro PG, Disa JJ, Hidalgo DA, Hu QY. Reconstruction of the mandible with osseous free flaps: a 10-year experience with 150 consecutive patients. Plast Reconstr Surg. 1999;104:1314–20. doi: 10.1097/00006534-199910000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Schusterman MA, Reece GP, Kross SS, Weldon ME. Use of the AO plate for immediate mandibular reconstruction in cancer patients. Plast Reconstr Surg. 1991;88(4):588–93. doi: 10.1097/00006534-199110000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Zenn MR, Hidalgo DA, Cordeiro PG, Shah JP, Strong EW, Kraus DH. Current role of the radial forearm free flap in mandibular reconstruction. Plast Reconstr Surg. 1997;99:1012–7. doi: 10.1097/00006534-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Hanasono MM, Zevallos JP, Skoraci RJ, Yu P. A prospective analysis of bony versus soft-tissue reconstruction for posterior mandibular defects. Plast Reconst Surg. 2010;125:1413–21. doi: 10.1097/PRS.0b013e3181d62aef. [DOI] [PubMed] [Google Scholar]
- 13.Kroll SS, Robb GL, Miller MJ, Reese GP, Evan GP. Reconstruction of posterior mandibular defects with soft tissue using the rectus abdominis free flap. Br J Plast Surg. 1998;51:503–7. doi: 10.1054/bjps.1998.0001. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Sung HM, Jang JD, Park YW, Min SK, Kim EC. Successful reconstruction of 15-cm segmental defects by bone marrow stem cells and resected autogenous bone graft in central hemangioma. J Oral Maxillofac Surg. 2010;68(1):188–94. doi: 10.1016/j.joms.2009.08.031. [DOI] [PubMed] [Google Scholar]