Abstract
The effectiveness of bariatric surgery has been well-studied. However, complications after bariatric surgery have been understudied. This review assesses <30 day major complications associated with bariatric procedures, including anastomotic leak, myocardial infarction, pulmonary embolism. This review included 71 studies conducted in the United States between 2003 and 2014 and 107,874 patients undergoing either gastric bypass, adjustable gastric banding, or sleeve gastrectomy, with mean age 44 years and pre-surgery body mass index 46.5 kg/m2. <30 day anastomotic leak rate was 1.15%; myocardial infarction rate was 0.37%; pulmonary embolism rate was 1.17%. Among all patients, mortality rate following anastomotic leak, myocardial infarction, pulmonary embolism was 0.12%, 0.37%, and 0.18%, respectively. Among surgical procedures, <30 day after surgery, sleeve gastrectomy (1.21% [95% CI, 0.23%-2.19%]) had higher anastomotic leak rate than gastric bypass (1.14% [95% CI, 0.84%-1.43%]); gastric bypass had higher rates of myocardial infarction and pulmonary embolism than adjustable gastric banding or sleeve gastrectomy. During the review, we found that the quality of complication reporting is lower than the reporting of other outcomes. In summary, <30 day rates of the three major complications after either one of the procedures ranges from 0-1.55%. Mortality following these complications ranges from 0-0.64%. Future studies reporting complications after bariatric surgery should improve their reporting quality.
Keywords: Bariatric surgery, anastomotic leak, myocardial infarction, pulmonary embolism
1. INTRODUCTION
Obesity is prevalent in the United States (1-3) and is associated with serious chronic diseases and cancer (4, 5). As a result, the demand for surgical treatments of obesity has risen (6-8). Studies have shown that surgical treatment provides sustained weight loss and improvement in comorbid conditions as well as reductions in the relative risk of death (9-14). Nonetheless, bariatric surgery is also associated with adverse surgical sequelae, including serious complications and mortality.
Early major complications mainly include anastomotic leak (Leak), myocardial infarction (MI), and pulmonary embolism (PE), which could lead to death. Clinical trials have provided data on these adverse events for select surgical procedure(s) on targeted sets of patients. However, synthesized analyses regarding adverse events of surgical treatment of obesity remain understudied. Such questions include which bariatric procedure generates the lowest risk of these complications and death at different time points, and which complications are likely to be associated with a specific procedure.
Previous reviews shied away from meta-analyzing complications due to variably reported outcomes and durations of follow-up (15). Some have roughly summarized ad hoc complications of bariatric surgery as a by-product of the general review of bariatric surgery (16-18); others estimated an overall summary complication rate associated with each surgical procedure (19). To our knowledge, there has not been a systematic review or meta-analysis specifically estimating rates of complications after bariatric surgery that provides a detailed and comprehensive analysis of these complications.
The goal of the study is to quantify early major complications after various bariatric surgery procedures focusing on adult patients. Specifically, we focus on Leak, MI, and PE <30 days after surgery and report mortality associated with these complications. We conducted a systematic review and meta-analysis on relevant studies selected from recent (2003-2014) publications originating from the United States. We chose to focus on studies in the United States to control for the potential heterogeneity in complication outcomes due to heterogeneous patient populations, surgeons’ training, and clinical practices across countries and regions. The selected studies included both randomized clinical trials (RCTs) and observational studies (OBSs). For each study design (20), random-effects models (21) and appropriate meta-analytic techniques were used to analyze the data.
2. METHODS
This systematic review and meta-analysis was conducted and reported according to the established guidelines (22, 23). A review protocola was followed throughout.
2.1 Data Sources and Searches
A Master of Library and Information Science-qualified librarian helped develop the search strategy. We performed electronic searches on MEDLINE, EMBASE, SCOPUS, COCHRANE, CLINICALTRIALS, and PROQUEST using the timeframe of January 1st, 2003 to September 30th, 2014. Searches were performed using the Firefox browser (Mozilla), and results were imported to EndNote X5 (Thomson Reuters). Search terms can be found in the Appendix, Section 1.
2.2 Study Selection and Criteria
By screening abstracts, we excluded: publication of abstracts only, case reports, letters, comments, reviews, or meta-analyses; animal studies; languages other than English; duplicate studies; no surgical intervention; lack of outcomes of interest; not population of interest (adults age ≥18 years undergoing surgery to treat obesity); and studies conducted outside of the United States. Full articles from the remaining abstracts were obtained and screened thoroughly using the aforementioned exclusion criteria as well as the following: no clearly defined timeframe for each complication occurrence (e.g., ≤30 days after surgery), studies in which complications were not a primary study outcome.
2.3 Data Extraction
Data extraction was performed for studies if they reported that a surgical procedure was performed and at least one outcome of interest resulting from that surgery, if they presented data separately by surgical procedure when more than one procedure was performed, and if they recorded the initial study population size and sample size at all data collection points. We recorded study characteristics, including title, authors, publication year, country in which the study was conducted, study design, study arm, number of patients in each study arm, enrollment dates, patient inclusion and exclusion criteria. We also collected characteristics of the starting study sample, including age, race, sex, body height, pre-surgery weight and comorbidities when available. We extracted the information on surgical procedures performed, the target complications, including definition of complication/diagnostic criteria, occurrence, and time points, and complication-related mortality. Three trained reviewers independently reviewed the studies, abstracted data, and resolved disagreements by consensus.
2.4 Interventions and outcomes of interest
Surgical procedures were grouped into the following categories: (i) gastric bypass (GB), including any types of Roux-en-Y gastric bypass (RYGB, laparoscopic or open, any limb lengths), and biliopancreatic diversion with and without duodenal switch (BPD/DS); (ii) adjustable gastric banding (AGB), including any brand of bands or techniques; (iii) sleeve gastrectomy (SG); (iv) all surgical procedures (All), including all surgical procedures in GB, AGB, or SG, vertical banded gastroplasty categories, and any combinations of them (10).
Our outcomes of interest included the following surgical complications reported in the studies, which identified the incidence occurring within 30 days of the surgery: (1) Leak; (2) MI; (3) PE; and (4) mortality following these complications. We recorded the incidence of these outcomes in each study or study arm.
2.5 Quality Assessment
All studies were evaluated for quality on reporting Leak, MI, PE using a four-category system (24): (1) prospective or retrospective study; (2) clearly defined main outcomes; (3) the number of occurrences and the number of non-occurrences of a complication within the target timeframe were reported; and (4) clearly defined criteria and or/method of diagnosis for the complication. For (2), studies were categorized into three groups as follows: (a) the primary outcome of the study is specifically about Leak, MI, and/or PE; (b) the primary outcome of the study is a cluster of complications, not focusing on Leak, MI, or PE; and (c) the primary outcome of the study is complications in general.
2.6 Statistical Analysis
Analyses were performed using only the data from studies in the data extraction subset. Study and individual-level data were summarized using descriptive statistics.
All outcomes were synthesized by random-effects meta-analysis, when appropriate. A simple averaging method proposed by Bhaumik et al. (25) was used to avoid statistical problems caused by zero or rare events in each study.(25-27) In addition, a Bayesian random-effects meta-analysis (28, 29) was conducted as an alternative to the Bhaumik method. Both methods used in meta-analysis of bariatric surgery are detailed in Chang et al. (30)
Meta-analyses were conducted separately for RCTs and OBSs due to heterogeneity in study design. No conventional methods were performed to evaluate study heterogeneity (e.g., Cochran’s Q and I2 statistics) or publication bias (e.g., funnel plot and Egger’s test), because they are not appropriate in cases of rare events (31). Instead, we used a general Bayesian hierarchical model to account for both quantitative and qualitative heterogeneity in evidence (32) (see Appendix Section 3 and results presented in Table S3). <30-day complication/mortality rates were reported as percentages with the number of incidences as the numerator and the number of patients undergoing the target surgical procedure as denominator.
MATLAB (7.11, R2015a, MathWorks Inc, Natick, MA) was used to obtain the Bhaumik estimates and the numerical solutions of the standard errors. R (x64 3.2.3, R Development Core Team, Vienna, Austria) was used to perform the Bayesian random-effects meta-analyses with the JAGS, “runjags” package (2.0.2-8). We report Bhaumik estimates with 95% confidence intervals (CIs) reported in brackets, while estimates using other meta-analytic models are presented in the Appendix. When the outcome only included one data point, we report the data directly.
3. RESULTS
3.1 Data Retrieval
Figure 1 shows the study attrition diagram outlining the systematic review process. The electronic searches resulted in 8,381 records. After screening titles and abstracts for exclusion criteria, 7,923 abstracts were removed. Full articles were retrieved among the remaining 458 studies, and after further screening for exclusion and inclusion criteria, 71 articles were included in meta-analyses: 59 studies (3 RCTs and 55 OBSs) reported Leak data and 46 studies (3 RCTs and 43 OBSs) reported data on mortality following Leak; 7 studies (1 RCT and 6 OBSs) reported MI data and 4 studies (1 RCT and 3 OBSs) reported data on mortality following MI; 22 OBSs (no RCTs) reported PE data and 15 OBSs (no RCTs) reported data on mortality following PE. Studies can contribute to more than one analysis (see Appendix, Table S1).
Figure 1. Study attrition diagram.
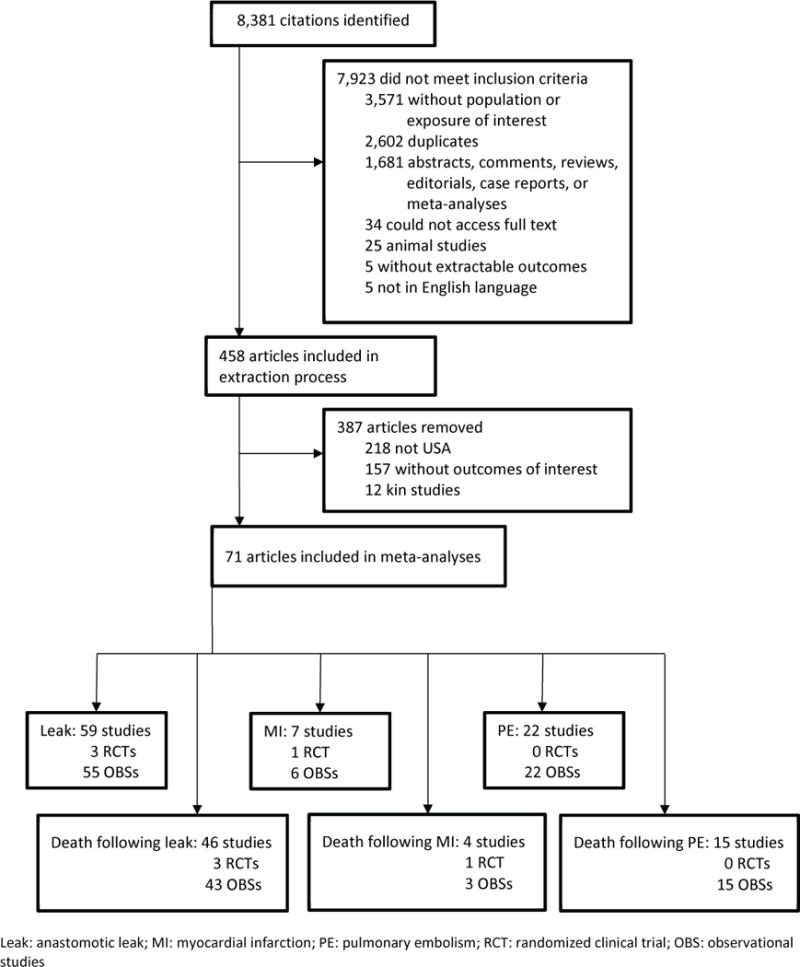
Leak: anastomotic leak; MI: myocardial infarction; PE: pulmonary embolism; RCT: randomized clinical trial; OBS: observational studies
3.2 Study and Patient Characteristics
Forty-one of the 71 included articles were published between 2003 and 2008, and 30 were published between 2009 and 2014 (Table 1). Four studies were RCTs and 67 studies were OBSs. Sixty-five studies reported GB outcomes; 12 studies reported AGB outcomes; 10 studies reported SG outcomes; no studies reported surgeries other than GB, AGB, or SG. Sixty-one studies reported patients’ mean age and their pre-surgery body mass index (BMI).
Table 1.
Study and patient characteristics
| Study characteristics | No. of studies | No. of patients | Patient characteristics | No./Total (mean or %) |
|---|---|---|---|---|
| Publication year | Age (years) | 44.00 | ||
| 2003-2008 | 41 | 23,814 | BMI (kg/m2) | 52.13 |
| 2009-2014 | 30 | 87,707 | Weight (kg) | 130.36 |
| Study design | Sex | |||
| RCT | 4 | 1,052 | Male | 16,256/72,725 (22.4) |
| OBS | 67 | 110,469 | Female | 56,469/72,725 (77.6) |
| Surgical procedure | Surgical procedure | |||
| GB | 65 | 74,063 | GB | 74,063/107,874 (68.7) |
| AGB | 12 | 30,671 | AGB | 30,671/107,874 (28.4) |
| SG | 10 | 3,140 | SG | 3,140/107,874 (2.9) |
| Age | 57 | 88,196 | ||
| BMI | 58 | 60,277 | ||
| Weight | 12 | 7,685 | ||
| Sex | 58 | 72,725 |
RCT: randomized clinical trial; OBS: observational studies; GB: gastric bypass; AGB: adjustable gastric banding; SG: sleeve gastrectomy; BMI: body mass index; kg: kilogram; m: meter.
A total of 107,874 patients were included in our analyses. Among them, 68.7% underwent GB, 28.4% underwent AGB. Among the 58 studies reporting patient sex (72,725 patients), 77.6% were females. Among the 57 studies reporting mean age of the participants at the time of surgery (88,196 patients), mean age at surgery was 44 years. Among the 58 studies reporting pre-surgery BMI (72,725 patients) and the 12 studies reporting pre-surgery weight (7,685 patients), patient mean BMI was 52.1 kg/m2 and mean weight was 130.4 kg before surgery.
3.3 Meta-analysis Results
Table 2 shows the meta-analytic results of major early complications and the following mortality after bariatric surgery using the Bhaumik meta-analytic method. We present meta-analytic results using the Bayesian RE models in Appendix, Table S2. We also present in Appendix Fig. S1-3 the percentages of the target complications and the following mortality reported in each study as well as the Bhaumik estimates for the meta-analysis results.
Table 2.
Meta-analyses of major complications (anastomotic leak, myocardial infarction, pulmonary embolism, and mortality following them) after bariatric surgery
| GB | AGB | SG | Overall | ||
|---|---|---|---|---|---|
| Anastomotic leak | |||||
| RCT | Estimates† (%) | 0.09 [–, –] | – [–, –] | – [–, –] | 0.09 [–, –] |
| Study/arm/patient # | 3/3/855 | 0/0/0 | 0/0/0 | 3/3/855 | |
| OBS | Estimates† (%) | 1.14 [0.84, 1.43] | – [–, –] | 1.21 [0.23, 2.19] | 1.15 [0.86, 1.44] |
| Study/arm/patient # | 51/51/53,535 | 0/0/0 | 9/9/2,611 | 55/60/56,146 | |
| Mortality following anastomotic leak | |||||
| RCT | Estimates† (%) | 0.00 [–, –] | – [–, –] | – [–, –] | 0.00 [–, –] |
| Study/arm/patient # | 3/3/855 | 0/0/0 | 0/0/0 | 3/3/855 | |
| OBS | Estimates† (%) | 0.04 [0.01, 0.07] | – [–, –] | 0.64 [–, –] | 0.12 [0.00, 0.29] |
| Study/arm/patient # | 39/39/24,211 | 0/0/0 | 6/6/604 | 43/45/24,815 | |
| Myocardial infarction | |||||
| RCT | Estimates† (%) | 0.00 [–, –] | – [–, –] | – [–, –] | 0.00 [–, –] |
| Study/arm/patient # | 1/1/197 | 0/0/0 | 0/0/0 | 1/1/197 | |
| OBS | Estimates† (%) | 0.47 [0.00, 1.02] | 0.42 [0.00, 1.27] | 0.00 [–, –] | 0.37 [0.00, 0.76] |
| Study/arm/patient # | 5/5/36,086 | 4/4/21,936 | 2/2/950 | 6/11/58,972 | |
| Mortality following myocardial infarction | |||||
| RCT | Estimates† (%) | 0.00 [–, –] | – [–, –] | – [–, –] | 0.00 [–, –] |
| Study/arm/patient # | 1/1/197 | 0/0/0 | 0/0/0 | 1/1/197 | |
| OBS | Estimates† (%) | 0.02 [–, –] | 0.00 [–, –] | 0.00 [–, –] | 0.01 [–, –] |
| Study/arm/patient # | 2/2/2,456 | 2/2/94 | 1/1/6 | 3/5/2,556 | |
| Pulmonary embolism | |||||
| RCT | Estimates† (%) | – [–, –] | – [–, –] | – [–, –] | – [–, –] |
| Study/arm/patient # | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | |
| OBS | Estimates† (%) | 1.55 [0.41, 2.70] | 0.02 [–, –] | 0.25 [–, –] | 1.17 [0.31, 2.03] |
| Study/arm/patient # | 20/20/41,644 | 5/5/24,845 | 2/2/1,473 | 22/27/67,962 | |
| Mortality following pulmonary embolism | |||||
| RCT | Estimates† (%) | – [–, –] | – [–, –] | – [–, –] | – [–, –] |
| Study/arm/patient # | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | |
| OBS | Estimates† (%) | 0.22 [0.00, 0.49] | 0.01 [–, –] | 0.19 [–, –] | 0.18 [0.00, 0.39] |
| Study/arm/patient # | 13/13/4,792 | 3/3/3,003 | 1/1/529 | 15/17/8,324 | |
Means and 95% confidence intervals (CIs) are in brackets. GB: gastric bypass; AGB: adjustable gastric banding; SG: sleeve gastrectomy; Overall: all surgery; RCT: randomized controlled trials; OBS: observational studies. –: Not available; 95% CIs are not available when the number of study arms ≤6 or the number of patients <1,000.
3.3.1 Anastomotic leak and mortality following Leak
Sixty-three study arms (3 in RCTs and 60 in OBSs) had data on Leak (Table 2). All 3 RCTs reported Leak data on GB. Out of 855 patients in 3 RCTs, 1 RCT (760 patients) reported that 2 patients had Leak <30-day after surgery (Appendix, Fig. S1a), resulting in a Leak rate of 0.09% for GB (Table 2). For OBSs (56,146 patients), the overall <30-day Leak rate was 1.15% [0.86%-1.44%]. Among the 51 OBSs reporting Leak data on GB (53,535 patients), the rate was 1.14% [0.84%-1.43%]; and among the 9 OBSs reporting data on SG (2,611 patients), the rate was 1.21% [0.23%-2.19%].
All 3 RCTs and 43 OBSs reported mortality data following Leak. In RCTs, no patients (out of 855 GB patients) had mortality following Leak. In OBSs (24,815 patients), the overall mortality rate following Leak was 0.12% [0.00%-0.29%]. For GB, the mortality rate following Leak after GB was 0.04% [0.01%-0.07%] (39 OBSs, 24,211 patients). For SG, the mortality rate following Leak was 0.64% (1 death among 6 OBSs, 604 patients, Appendix, Fig. S1f).
3.3.2 Myocardial infarction and mortality following myocardial infarction
Twelve study arms (1 RCT and 6 OBSs) were included in the MI analyses. In the RCT (197 patients) reporting GB outcomes, no patients experienced MI <30 days. In OBSs (58,972 patients), the overall <30-day MI rate was 0.37% [0.00%-0.76%]. For GB, the <30-day MI rate was 0.47% [0.00%-1.02%] (5 OBSs, 36,086 patients). For AGB, the rate was 0.42% [0.00%-1.27%] (4 OBSs, 21,936 patients). For SG, 2 OBSs (2 study arms) reported that no patients had MI <30-day after surgery.
One RCT on GB and 3 OBSs (5 study arms) reported mortality data following MI. The RCT reported no patients (out of 197 GB patients) had mortality following MI. In OBSs (2,556 patients), the overall mortality rate following MI was 0.01% (1 death). For GB, 1 death following MI was reported among 2 OBSs (2,456 patients). For AGB (2 OBSs, 94 patients) and SG (1 OBS, 6 patients), there was no mortality following MI.
3.3.3 Pulmonary embolism and mortality following pulmonary embolism
Twenty-seven study arms (no RCTs, 22 OBSs) were included in the PE analyses. The overall <30-day PE rate was 1.17 % [0.31%-2.03%]. For GB, the <30-day PE rate was 1.55% [0.41%-2.70%] (20 OBSs, 41,644 patients). For AGB, the rate was 0.02% (5 OBSs, 24,845 patients). For SG, the rate was 0.25% (2 OBSs, 1,473 patients).
Seventeen study arms (no RCT, 15 OBSs, 8,324 patients) reported mortality following PE. Overall, the mortality rate following PE was 0.18% [0.00%-0.39%]. For GB, the mortality rate following PE was 0.22% [0.00%-0.49%]. For AGB, among 3 OBSs (3,003 patients), 1 patient in 1 OBS (2,909 patients) died following PE. For SG, the 1 included OBS reported 1 death following PE out of 529 patients.
3.4 Quality assessment on reporting complications of interest
The quality assessment on reporting complications of interest in the included studies is presented in the Appendix, Table S1. Eleven out of the 71 included articles are prospective studies (quality reporting category (1)).
Regarding whether the included studies clearly defined the target complications (category (2)), all studies reported the complications of interest in both the methods and the results sections (category (2d)). For the 59 studies reporting Leak, 27 reported Leak as the primary outcome; 5 studies reported a cluster of complications as the primary outcome, including Leak; and 26 studies whose primary outcome was overall complications reported data on Leak. For the 22 studies reporting PE, 6 reported PE as their primary outcome; 1 study reported a cluster of complications as the primary outcome, including PE; and 14 studies whose primary outcome was overall complications also reported PE. For the 7 studies reporting MI, no study reported MI as their primary outcome; 1 study reported a cluster of complications as the primary outcome; and 7 studies whose primary outcome was overall complications also reported MI.
Regarding category (3), 2 studies reporting Leak and no studies reporting PE or MI had determinable occurrence and non-occurrence of a complication within the target timeframe. For category (4), 28 studies reporting Leak, 4 studies reporting PE, and no studies reporting MI clearly defined criteria and or/method of diagnosis for the complication.
4. DISCUSSION
We conducted an up-to-date systematic review and meta-analysis of major complications, including Leak, MI, and PE, <30 days following bariatric surgery, based on literature published in the United States between 2003 and 2014. We found that the rates of these major early complications were low (<1.6%). The mortality rate following these complications were also low (<0.7%). We also identified a gap in complication reporting that needs to be addressed in future studies.
We estimated a much lower Leak rate after GB (0.09% in RCTs and 1.14% in OBSs) than that reported in a previously published meta-analysis of bariatric surgery, Maggard et al. (4.6%) (16). Furthermore, a higher rate of adverse events (including cardiac, stroke, or severe hypertension) was found in Maggard et al. (4.8% for GB and 0.7% for AGB), compared to <30-day MI rate of 0.47% for GB and 0.42% for AGB and <30-day PE rate of 1.55% for GB and 0.02% for AGB. The lower rates in our study can be explained by the difference in the inclusion criteria, e.g., time frames of the target complications and categories of complications. More importantly, our meta-analyses synthesized data from studies published between 2003 and 2014, while Maggard et al. synthesized data from studies published before 2003. The complication rates are reduced likely to be due to advances in technology (e.g., the increasing trend of minimally invasive surgery (33)) and accumulation of surgeons’ experience (10). In this regard, we also note that our estimates of these complication rates are applicable to the surgery performed in the United States in this timeframe (2003-2014). Our study was not able to include studies published after 2014 due to the lengthy process to prepare and conduct these meta-analyses. If we further include data from the most recently published studies, we anticipate that the rates of these complications are likely to be slightly lower than our estimates; in particular, studies have found that the Leak rate in SG decreased with surgeon’s experience and changes in techniques (34, 35) and the number of SG procedures increased significantly in recent years (36).
Our findings are consistent with previous literature that generally AGB has lower complication and mortality rates than GB (37, 38), even in terms of these major early complications, i.e., MI and PE. However, different from the overall complication rates reported in previous studies (10, 39), SG patients appeared to have higher rate of Leak and mortality following Leak than GB based on the OBSs included in our meta-analyses. Nonetheless, this conclusion cannot be drawn without noting that the smaller number of studies on SG and fewer patients were included in each analysis.
We found that the reporting quality for the three target major complications is, in general, lower than the other outcomes, e.g., weight loss. During the review process, we noted that many studies did not clearly define a time frame when reporting complications. Moreover, many studies did not report non-occurrence of the target complications within a time frame. Both of these resulted in exclusion of these studies due to no outcomes of interest. The strength of this exclusion is to ensure our meta-analysis included only studies that met our criterion of reporting, and our estimates of complication rates would not be biased by imposing strong assumptions on unreported outcomes, e.g., assuming non-occurrence when studies did not report occurrence <30 day after surgery, which may underestimate the complication rates. The drawback of this exclusion is that we lost data points if those assumptions were true. As a result of more stringent exclusion criteria, the estimated rates in our meta-analyses are likely to be driven by the rates reported by the few included studies. For example, mortality following Leak for SG was estimated to be 0.64% based on the 6 included OBSs (604 patients), which is higher than the previously reported overall mortality rates of 0.29% (≤30 day) based on 10 OBSs (3,647 patients) and 0.34% (>30 day) based on 8 OBSs (3,099 patients) by Chang et al (10).
For the included articles, except for two studies (40, 41), we found, in the quality assessment, that almost all articles did not report both the number of occurrences and the number of non-occurrences of a complication within the target timeframe. This criterion sounds redundant when the timeframe of a complication is specified, but this is the only way to assure the non-occurrence of a target complication within the timeframe.
Our study has several strengths that should be highlighted. First, our meta-analysis included studies conducted in the last 12 years, which provides updated information on complication rates under current care and management after bariatric surgery. Second, we included outcomes following SG, a relatively new surgical procedure (42). Third, we used meta-analytic techniques designed to analyze rare event rates, which provides more reliable estimates than studies who used traditional meta-analytic techniques relying on the normality assumption. Fourth, we only included studies in our meta-analyses that did not require imposing strong assumptions, e.g., non-occurrence for not explicitly reported complication. Last, we carefully designed our study to minimize heterogeneity across included studies. For example, we excluded studies conducted outside of the United States due to heterogeneity in bariatric surgeons’ training, population characteristics, and health insurance system. Moreover, we separately analyzed RCTs and OBSs due to the difference in their study design. We also performed a Bayesian hierarchical model to account for the heterogeneity in study design.
Our study also has several limitations that should be noted. First, we categorized RYGB and BPD/DS into the GB category due to their malabsorptive and restrictive features (43). Some studies have found that complication rates for BPD/DS were higher than RYGB (44, 45), while others found comparable complication rates among them (46). As BPD/DS procedures were performed less common in the United States (8, 43), the impact of including both RYGB and BPD/DS in the same GB category should be minimal. Upon checking, among the 71 studies included in our meta-analyses, only three studies included BPD/DS, and after excluding them from the analyses, the complication rates did not vary, except for rates for Leak (from 1.14% to 1.12%) and mortality from PE (from 0.22% to 0.24%). Second, as mentioned above, many studies reporting information on complications were excluded due to the relatively low quality of reporting. Therefore, the rates are likely to be driven by the few included studies. Last, the numbers of study arms for AGB and SG in each analysis was not comparable to GB, making direct comparisons between procedures more difficult.
In conclusion, our study demonstrates that <30 day rate of major complications after GB, AGB, or SG operated in the United States between 2003 and 2014 ranges from 0-1.55%. Mortality following these complications is generally low, ranging from 0-0.64%. Among surgical procedures, <30 day after surgery, SG has higher Leak rate than GB; GB has higher MI and PE rates than AGB or SG. Our study also shows that the quality of complication reporting is relatively low, compared to the reporting of other outcomes. Future studies reporting complications after bariatric surgery should report both occurrence and non-occurrence within a definitive time frame to help evaluate risks of bariatric surgery and allow for valid comparison between surgical procedures.
Supplementary Material
Acknowledgments
We thank Ms. Angela Hardi, an MLIS qualified librarian at the Bernard Becker Medical Library at the Washington University in St. Louis, who helped develop search strategies and performed computerized searches.
Funding/Support: The Foundation for Barnes-Jewish Hospital and the National Institutes of Health (NIH) Grant U54 CA155496 supported this research. S-H. Chang is supported by the Agency for Healthcare Research and Quality (AHRQ) Grant K01 HS022330 and the NIH Grant R21 DK110530. G.A. Colditz is supported by the American Cancer Society Clinical Research Professorship.
Role of the Sponsor: The sponsor had no role in the design and conduct of the study, collection, management, analysis, or interpretation of the data; or the preparation, review, approval of the manuscript.
Abbreviations
- Leak
anastomotic leak
- MI
myocardial infarction
- PE
pulmonary embolism
- RCT
randomized clinical trial
- OBS
observational study
- GB
gastric bypass
- AGB
adjustable gastric banding
- SG
sleeve gastrectomy
- CI
confidence interval
- BMI
body mass index
Footnotes
Authors’ contributions: Dr. Chang had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chang, Freeman, Eagon, Colditz
Analysis and interpretation of data: Chang, Freeman, Lee, Eagon, Colditz
Drafting of the manuscript: Chang, Freeman
Critical revision of the manuscript for important intellectual content: Chang, Freeman, Lee, Stoll, Calhoun, Eagon, Colditz
Statistical expertise: Chang, Freeman, Lee
Obtained funding: Chang, Colditz
Administrative, technical, or material support: Chang, Colditz
Study supervision: Chang, Colditz
Disclaimer: The conclusions and opinions presented herein are solely the responsibility of the authors and do not necessarily represent the official views of AHRQ, NIH, or the Foundation for Barnes-Jewish Hospital.
Supplemental material: Appendix
Conflict of interests None.
The review protocol is available on the website: http://publichealthsciences.wustl.edu/Research/Major-Early-Complications-Following-Bariatric-Surg.
References
- 1.Farzadfar F, Finucane MM, Danaei G, Pelizzari PM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in serum total cholesterol since 1980: systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3.0 million participants. Lancet. 2011;377(9765):578–86. doi: 10.1016/S0140-6736(10)62038-7. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 4.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 5.Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004;23(38):6365–78. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 6.Livingston EH. The incidence of bariatric surgery has plateaued in the U.S. Am J Surg. 2010;200(3):378–85. doi: 10.1016/j.amjsurg.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tessier DJ, Eagon JC. Surgical management of morbid obesity. Current problems in surgery. 2008;45(2):68–137. doi: 10.1067/j.cpsurg.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Buchwald H, Oien DM. Metabolic/bariatric surgery Worldwide 2008. Obes Surg. 2009;19(12):1605–11. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]
- 9.Bradley D, Conte C, Mittendorfer B, Eagon JC, Varela JE, Fabbrini E, et al. Gastric bypass and banding equally improve insulin sensitivity and beta cell function. The Journal of clinical investigation. 2012;122(12):4667–74. doi: 10.1172/JCI64895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang S-H, Stoll CR, Song J, Varela JE, Eagon JC, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA surgery. 2014;149(3):275–87. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang S-H, Stoll CRT, Colditz GA. Cost-effectiveness of bariatric surgery: should it be universally available? Maturitas. 2011;69(3):230–8. doi: 10.1016/j.maturitas.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Christou NV, Sampalis JS, Liberman M, Look D, Auger S, McLean AP, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240(3):416–23. doi: 10.1097/01.sla.0000137343.63376.19. discussion 23-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marsk R, Naslund E, Freedman J, Tynelius P, Rasmussen F. Bariatric surgery reduces mortality in Swedish men. Br J Surg. 2010;97(6):877–83. doi: 10.1002/bjs.6985. [DOI] [PubMed] [Google Scholar]
- 14.Matarasso A, Roslin MS, Kurian M. Bariatric surgery: an overview of obesity surgery. Plast Reconstr Surg. 2007;119(4):1357–62. doi: 10.1097/01.prs.0000254785.31020.e6. [DOI] [PubMed] [Google Scholar]
- 15.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 16.Maggard MA, Shugarman LR, Suttorp M, Maglione M, Sugerman HJ, Livingston EH, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142(7):547–59. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 17.Padwal R, Klarenbach S, Wiebe N, Birch D, Karmali S, Manns B, et al. Bariatric surgery: a systematic review and network meta-analysis of randomized trials. Obes Rev. 2011;12(8):602–21. doi: 10.1111/j.1467-789X.2011.00866.x. [DOI] [PubMed] [Google Scholar]
- 18.Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. Bmj. 2013;347:f5934. doi: 10.1136/bmj.f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang S-H, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA surgery. 2014;149(3):275–87. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golder S, Loke YK, Bland M. Meta-analyses of adverse effects data derived from randomised controlled trials as compared to observational studies: methodological overview. PLoS medicine. 2011;8(5):e1001026. doi: 10.1371/journal.pmed.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosteller F, Colditz GA. Understanding research synthesis (meta-analysis) Annual review of public health. 1996;17:1–23. doi: 10.1146/annurev.pu.17.050196.000245. [DOI] [PubMed] [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 24.Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(3 Suppl):21–35. doi: 10.1016/s0749-3797(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 25.Bhaumik DK, Amatya A, Normand SL, Greenhouse J, Kaizar E, Neelon B, et al. Meta-Analysis of Rare Binary Adverse Event Data. Journal of the American Statistical Association. 2012;107(498):555–67. doi: 10.1080/01621459.2012.664484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23(9):1351–75. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 27.Sutton AJ, Cooper NJ, Lambert PC, Jones DR, Abrams KR, Sweeting MJ. Meta-analysis of rare and adverse event data. Expert review of pharmacoeconomics & outcomes research. 2002;2(4):367–79. doi: 10.1586/14737167.2.4.367. [DOI] [PubMed] [Google Scholar]
- 28.Smith TC, Spiegelhalter DJ, Thomas A. Bayesian approaches to random-effects meta-analysis: a comparative study. Stat Med. 1995;14(24):2685–99. doi: 10.1002/sim.4780142408. [DOI] [PubMed] [Google Scholar]
- 29.Warn DE, Thompson SG, Spiegelhalter DJ. Bayesian random effects meta-analysis of trials with binary outcomes: methods for the absolute risk difference and relative risk scales. Stat Med. 2002;21(11):1601–23. doi: 10.1002/sim.1189. [DOI] [PubMed] [Google Scholar]
- 30.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The Effectiveness and Risks of Bariatric Surgery: An Updated Systematic Review and Meta-analysis, 2003-2012. JAMA Surg. 2013 doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shuster JJ, Walker MA. Low-event-rate meta-analyses of clinical trials: implementing good practices. Stat Med. 2016;35(14):2467–78. doi: 10.1002/sim.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prevost TC, Abrams KR, Jones DR. Hierarchical models in generalized synthesis of evidence: an example based on studies of breast cancer screening. Stat Med. 2000;19(24):3359–76. doi: 10.1002/1097-0258(20001230)19:24<3359::aid-sim710>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 33.Johnson EE, Simpson AN, Harvey JB, Lockett MA, Byrne KT, Simpson KN. Trends in bariatric surgery, 2002-2012: do changes parallel the obesity trend? Surg Obes Relat Dis. 2016;12(2):398–404. doi: 10.1016/j.soard.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Stroh C, Kockerling F, Volker L, Frank B, Stefanie W, Christian K, et al. Results of More Than 11,800 Sleeve Gastrectomies: Data Analysis of the German Bariatric Surgery Registry. Ann Surg. 2016;263(5):949–55. doi: 10.1097/SLA.0000000000001559. [DOI] [PubMed] [Google Scholar]
- 35.Noel P, Nedelcu M, Gagner M. Impact of the Surgical Experience on Leak Rate After Laparoscopic Sleeve Gastrectomy. Obes Surg. 2016;26(8):1782–7. doi: 10.1007/s11695-015-2003-1. [DOI] [PubMed] [Google Scholar]
- 36.Khorgami Z, Shoar S, Andalib A, Aminian A, Brethauer SA, Schauer PR. Trends in utilization of bariatric surgery, 2010-2014: sleeve gastrectomy dominates. Surg Obes Relat Dis. 2017;13(5):774–8. doi: 10.1016/j.soard.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 37.Angrisani L, Lorenzo M, Borrelli V. Laparoscopic adjustable gastric banding versus Roux-en-Y gastric bypass: 5-year results of a prospective randomized trial. Surg Obes Relat Dis. 2007;3(2):127–32. doi: 10.1016/j.soard.2006.12.005. discussion 32-3. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen NT, Slone JA, Nguyen XM, Hartman JS, Hoyt DB. A prospective randomized trial of laparoscopic gastric bypass versus laparoscopic adjustable gastric banding for the treatment of morbid obesity: outcomes, quality of life, and costs. Ann Surg. 2009;250(4):631–41. doi: 10.1097/SLA.0b013e3181b92480. [DOI] [PubMed] [Google Scholar]
- 39.Hutter MM, Schirmer BD, Jones DB, Ko CY, Cohen ME, Merkow RP, et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg. 2011;254(3):410–20. doi: 10.1097/SLA.0b013e31822c9dac. discussion 20-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerhard GS, Chokshi R, Still CD, Benotti P, Wood GC, Freedman-Weiss M, et al. The influence of iron status and genetic polymorphisms in the HFE gene on the risk for postoperative complications after bariatric surgery: A prospective cohort study in 1,064 patients. Patient Safety in Surgery. 2011;5(1) doi: 10.1186/1754-9493-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin MYC, Mehdi Tavakol M, Sarin A, Amirkiai SM, Rogers SJ, Carter JT, et al. Laparoscopic sleeve gastrectomy is safe and efficacious for pretransplant candidates. Surgery for Obesity and Related Diseases. 2013;9(5):653–8. doi: 10.1016/j.soard.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Rosenthal RJ. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of >12,000 cases. Surgery for Obesity and Related Diseases. 2012;8(1):8–19. doi: 10.1016/j.soard.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 43.Anderson B, Gill RS, de Gara CJ, Karmali S, Gagner M. Biliopancreatic diversion: the effectiveness of duodenal switch and its limitations. Gastroenterol Res Pract. 2013;2013:974762. doi: 10.1155/2013/974762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deveney CW, MacCabee D, Marlink K, Welker K, Davis J, McConnell DB. Roux-en-Y divided gastric bypass results in the same weight loss as duodenal switch for morbid obesity. Am J Surg. 2004;187(5):655–9. doi: 10.1016/j.amjsurg.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Neff KJ, Olbers T, le Roux CW. Bariatric surgery: the challenges with candidate selection, individualizing treatment and clinical outcomes. BMC medicine. 2013;11:8. doi: 10.1186/1741-7015-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biertho L, Lebel S, Marceau S, Hould FS, Lescelleur O, Moustarah F, et al. Perioperative complications in a consecutive series of 1000 duodenal switches. Surg Obes Relat Dis. 2013;9(1):63–8. doi: 10.1016/j.soard.2011.10.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


