Abstract
Objectives
To characterize the functional and prognostic significance of oxygen uptake (VO2) kinetics following peak exercise in individuals with heart failure (HF).
Background
It is unknown to what extent patterns of VO2 recovery following exercise reflect circulatory response during exercise in HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF).
Methods
We investigated patients (30 HFpEF, 20 HFrEF, and 22 controls) who underwent cardiopulmonary exercise testing (CPET) with invasive hemodynamic monitoring and a second distinct HF cohort (n=106) who underwent non-invasive CPET with assessment of long-term outcomes. Fick cardiac output (CO) and cardiac filling pressures were measured at rest and throughout exercise in the initial cohort. A novel metric, VO2 recovery delay (VO2RD), defined as time until post-exercise VO2 falls permanently below peak VO2, was measured to characterize VO2 recovery kinetics.
Results
VO2RD in patients with HFpEF (median (IQR), 25 (9,39) seconds) and HFrEF (28 (2,52) seconds) was in excess of controls (5 (0,7) seconds, p<0.0001 and p=0.003 respectively). VO2RD was inversely related to CO augmentation during exercise in HFpEF (ρ=−0.70) and HFrEF (ρ=−0.73, both p<0.001). In the second cohort, VO2RD predicted transplant-free survival in univariate and multivariable Cox regression analysis (Cox hazard ratios were 1.49 and 1.37 per 10sec increase in VO2RD respectively, both p<0.005).
Conclusion
Post-exercise VO2RD is an easily recognizable, non-invasively derived pattern that signals impaired CO augmentation during exercise and predicts outcomes in HF. The presence and duration of VO2RD may complement established exercise measurements for assessment of cardiac reserve capacity. (Word count 249)
Keywords: Heart Failure, Cardiopulmonary Exercise Testing, Recovery Kinetics, Exercise Hemodynamics
INTRODUCTION
Impaired exercise capacity is a cardinal feature of heart failure (HF). Peak oxygen uptake (VO2) measured during cardiopulmonary exercise testing (CPET) reflects exercise capacity and is utilized to grade severity of HF (1). While the prognostic implications of reduced peak VO2 in HF patients are well known (2,3) other CPET gas exchange variables measured during exercise have emerged that offer insights into multi-organ physiologic reserve capacity and provide additive prognostic value when combined with peak VO2 (4–6).
Gas exchange patterns immediately following exercise provide information about the metabolic consequences of exercise exposure. Abnormally prolonged VO2 recovery to baseline resting values following exercise has been observed in patients with HF compared to healthy subjects (7,8). Prolonged VO2 and heart rate recovery following exercise both predict adverse outcomes in HF (9,10). However, attempts to fit various linear and exponential equations to VO2 recovery patterns have not translated into simple metrics that are routinely incorporated into clinical CPET interpretation in HF patients. Furthermore, mechanistic understanding of VO2 recovery patterns in HF remains limited. Finally, studies of VO2 recovery in HF have focused almost exclusively on the HFrEF population. We therefore conducted a comprehensive evaluation of VO2 recovery patterns and their relationships to metabolic and hemodynamic responses to exercise in carefully phenotyped HFpEF and HFrEF patients. We then investigated the prognostic significance of VO2 recovery patterns in a distinct patient cohort.
METHODS
Patient Population
We studied patients referred to Massachusetts General Hospital for CPET between June 2011 and July 2016. This study was approved by the Partners Human Research Committee. Patients with complete recovery gas exchange data during the three minutes after peak exercise were eligible for the study. The initial patient cohort was derived exclusively from consecutive patients who underwent CPET with invasive hemodynamic monitoring and met the following inclusion criteria; HFpEF: LVEF ≥ 0.50 with supine pulmonary artery wedge pressure (PAWP) ≥ 15 mmHg and NYHA Functional Class II–IV; HFrEF: LVEF < 0.45 and NYHA Functional Class II–IV; Controls: LVEF > 0.50, supine mean pulmonary artery pressure (mPAP) < 25 mmHg, supine PAWP < 15 mmHg, and a normal exercise capacity reflected by peak VO2 ≥ 85% predicted on the basis of age, gender, and height (11). Patients were excluded if they had any of the following conditions: 1) severe valvular heart disease; 2) intra-cardiac shunting; and 3) symptomatic, flow limiting coronary artery disease. Those who achieved only submaximal effort during exercise as reflected by a peak respiratory exchange ratio (RER) of < 1.00 and a peak heart rate (HR) < 85% of predicted were also excluded (6).
A second distinct patient cohort was studied to determine the prognostic value of VO2 recovery patterns. This cohort consisted of consecutive patients who were referred to the MGH for NYHA Class II–IV symptoms, had HFrEF with LVEF < 0.45, and underwent non-invasive cardiopulmonary exercise testing from June 2011 to October 2014. We focused on HFrEF patients in the non-invasive CPET cohort due to the well circumscribed phenotyping provided by documented low LVEF, as opposed to our limited capacity to definitively distinguish HFpEF from other conditions that limit exercise capacity in patients who undergo non-invasive CPET.
Cardiopulmonary Exercise Testing
Patients in the first cohort underwent placement of a pulmonary arterial catheter via the internal jugular vein and a systemic arterial catheter via the radial artery. First-pass radionuclide ventriculography of both ventricles was performed at rest (OnePass GVI Medical Devices, Twinsburg, OH).
Patients then underwent maximal incremental upright cycle ergometry (5–25 Watts/min continuous ramp following a 3-minute rest period and a 3-minute period of unloaded exercise, MedGraphics, St. Paul, MN). Breath-by-breath data were binned mid 5-of-7 by the metabolic cart for analysis of gas exchange patterns. Simultaneous hemodynamic measurements were obtained with exercise (Witt Biomedical Inc, Melbourne, FL), as previously described (12,13). Right atrial pressure, mPAP, PAWP, and systemic arterial pressures were measured in the upright position, at end-expiration at rest, and at one-minute intervals during exercise. Fick cardiac output (CO) was calculated at one minute intervals throughout exercise by measuring VO2 and simultaneous radial arterial and mixed venous O2 saturation to determine the oxygen extraction (C(a−v)O2) at each minute of exercise. VO2/work was defined as the slope of the relationship between VO2 and work from one minute after the initiation of loaded exercise to the end of exercise. Ventilatory efficiency or VE/VCO2 slope was defined as the relationship between expired carbon dioxide per minute and total ventilation per minute from the start of unloaded exercise to maximal exercise. Oxygen uptake efficiency slope (OUES) was defined as the relationship between VO2 and the natural log of total ventilation per minute throughout exercise (5). Following maximal exercise, the patients recovered over a 3-minute period, pedaling against no resistance for the first minute of recovery and sitting passively for the final two minutes of recovery. Prior to testing, patients were instructed to keep the mouthpiece in throughout recovery to ensure data completeness.
Derived VO2 Recovery Kinetics
Based on the lack of any descent in VO2 during the early part of recovery in a subset of individuals, we termed a novel metric, VO2 recovery delay (VO2RD), as simply the time from the end of loaded exercise until the VO2 permanently falls below peak VO2, as illustrated in Figure 1. Peak VO2 was defined as the highest median breath-by-breath O2 consumption over a 30 second interval in the last minute of exercise. Because VO2RD measures the time until a permanent fall in VO2 below peak levels, this metric is also well-suited to HF patients with periodic breathing during and after exercise (or oscillatory ventilation) (14,15). Recovery VO2 kinetics were also described by T1/2, the time for VO2 to decrease to 50% of peak VO2 adjusted for resting VO2 (7,16–18) and HR recovery at 2 minutes, as previously described (10).
Figure 1. Defining VO2 recovery delay.
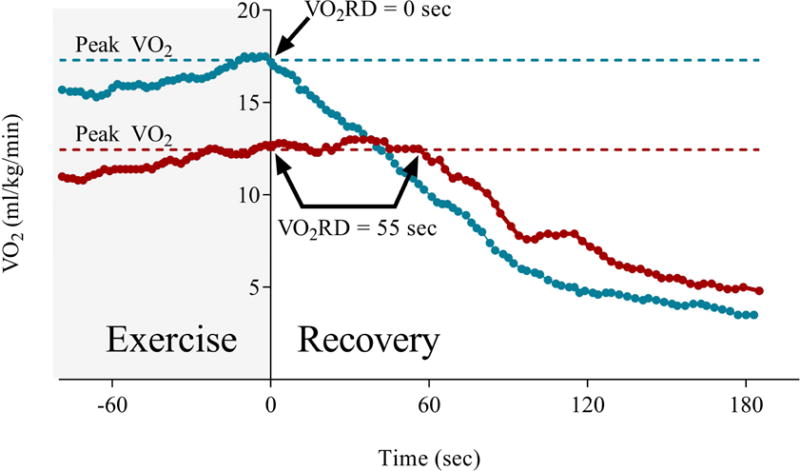
This illustration contains data from two patients with heart failure who demonstrate distinct patterns of VO2RD. The gray area illustrates the final portion of incremental ramp exercise. VO2RD was defined as the duration of time from end exercise until the time when oxygen consumption (VO2) fell permanently below peak VO2 (dashed lines). The blue line represents a patient who has an immediate decrement in VO2 following completion of the exercise period (shaded in gray) with a resultant VO2RD value of 0 seconds. In contrast, the second patient’s VO2 (red line) remained at values at or above those achieved at peak exercise for 55 seconds after exercise before beginning to decline.
Statistical Analyses
STATA 13.0 (StataCorp LLC) was used for all analyses. The Wilk-Shapiro test was used to determine the normality of each continuous variable. Continuous measurements are presented as mean ± SD for normally distributed variables and median (interquartile range, IQR) for non-normal variables. Categorical data are presented as percentages. Comparisons with continuous variables involving two groups were performed using either the Student t test or the Mann Whitney test, as appropriate. Comparisons with continuous variables involving three groups were made using either a 1-way ANOVA or Kruskal-Wallis test with post-hoc testing adjusted for multiple comparisons, as appropriate. Fisher’s exact test was used for comparisons of categorical data. Pearson or Spearman correlation analysis was performed, as appropriate. Mortality data were obtained from the Social Security Death Index. Kaplan-Meier survival with Log Rank testing and multivariable Cox regression analysis was used to determine if VO2 recovery patterns and other variables predict transplant-free survival. A p-value of < 0.05 was considered significant.
RESULTS
Population Characteristics
Baseline characteristics for control (n=22), HFpEF (n=30), and HFrEF (n=20) patients are summarized in Table 1. All three groups were similar in age. The HFrEF population was predominantly male. As expected, HFpEF patients had a greater body mass index compared to controls, as well as more frequent comorbidities of hypertension, diabetes mellitus, and hyperlipidemia. HFpEF and HFrEF patients exhibited very similar resting hemodynamic values with average resting supine mPAP of 26±6 and 26±7 mmHg and PAWP of 20±5 and 20±6 mmHg, respectively. Measurements performed during exercise testing are provided in Table 2. All three groups demonstrated peak exercise RERs consistent with maximal effort, as indicated by an average RER in excess of 1.10. HFpEF (13.3±2.8 ml/kg/min) and HFrEF (13.2±2.8 ml/kg/min) patients exhibited similarly reduced peak VO2 levels compared to controls (25.6±5.7 ml/kg/min).
Table 1.
Baseline Characteristics
| Characteristic | Controls | HFpEF | HFrEF | Cohort 2 |
|---|---|---|---|---|
| n | 22 | 30 | 20 | 106 |
| Age, years | 58±13 | 64±10 | 62±11 | 56±13 |
| Male sex, % | 59 | 50 | 90†‡ | 82 |
| Body mass index, kg/m2 | 26.6±3.8 | 31.5±6.5* | 28.2±6.5 | 28.0±4.6 |
| Hemoglobin, g/dl | 13.8±1.5 | 13.2±1.9 | 12.7±1.6 | 13.4±2.0 |
| Comorbidities, % | ||||
| Hypertension | 45 | 73* | 55 | 39 |
| Diabetes mellitus | 5 | 33* | 25 | 26 |
| Hyperlipidemia | 27 | 73* | 60 | 51 |
| Pharmacotherapies, % | ||||
| Diuretics | 9 | 63* | 65† | 84 |
| ACE inhibitor or ARB | 23 | 27 | 80†‡ | 75 |
| β-Adrenergic blocker | 9 | 67* | 80† | 92 |
| Aldosterone blockade | 0 | 13 | 45†‡ | 54 |
| Rest Hemodynamics | ||||
| LVEF, % | 65±6 | 66±7 | 30±11†‡ | 25±9 |
| Supine PAWP, mmHg | 9±3 | 20±5* | 20±6† | NA |
| Supine mPAP, mmHg | 15±4 | 26±6* | 26±7† | NA |
| Cardiac index, l/min/m2 | 3.1±0.5 | 2.4±0.6* | 2.2±0.5† | NA |
HFpEF indicates heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; LVEF, left ventricular ejection fraction; PAWP, pulmonary arterial wedge pressure; and mPAP, mean pulmonary arterial pressure.
p<0.05 for comparison of HFpEF and controls,
p<0.05 for comparison of HFrEF and controls,
p<0.05 for comparison of HFpEF and HFrEF
Table 2.
Peak Exercise and Hemodynamic Measurements
| Characteristic | Controls | HFpEF | HFrEF |
|---|---|---|---|
| n | 22 | 30 | 20 |
| Respiratory exchange ratio | 1.17±0.09 | 1.14±0.10 | 1.19±0.12 |
| Maximum workload, watts | 167±57 | 88±29* | 91±29† |
| VO2, % predicted | 102±11 | 72±20* | 55±13† |
| VO2, ml/kg/min | 25.6±5.7 | 13.3±2.8* | 13.2±2.8† |
| C(a−v)O2, ml/dl | 13.4±2.0 | 12.2±2.3 | 13.5±1.7 |
| Cardiac output, l/min | 16.1(12.4,16.9) | 9.3(7.3,12.8)* | 7.4(6.2,11.6)† |
| Heart rate, BPM | 150±18 | 117±26* | 113±25† |
| Stroke volume, ml | 100±22 | 88±30 | 77±19† |
| VO2/work slope, ml/min/watt | 10.4±0.8 | 8.3±2.0* | 8.7±1.9† |
| VE/VCO2 slope | 28.9±3.8 | 37.0±9.2* | 43.2±13.0† |
| O2 Saturation, % | 97(97,98) | 97(94,98) | 99(98,100) |
| PAWP, mmHg | 20±6 | 29±6* | 29±9† |
| mPAP, mmHg | 34±8 | 47±9* | 45±8† |
| HR recovery @ 2min, BPM | 41±11 | 23±17* | 23±15† |
HFpEF indicates heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; and HR, heart rate.
p<0.05 for comparison of HFpEF and controls,
p<0.05 for comparison of HFrEF and controls
Post-Exercise VO2 Recovery Kinetics
In controls, VO2 consistently declined almost immediately following peak exercise. However, in HF patients we commonly observed a prolonged VO2RD duration prior to a decrement in VO2 (Figure 1). Post-exercise VO2RD and T1/2 durations are displayed for the three groups in Figure 2. Controls exhibited minimal VO2RD durations with a median (IQR) value of 5 (0,7) seconds compared to 25 (7,43) seconds for HF patients (p<0.0001). HFpEF and HFrEF patients exhibited similarly prolonged VO2RD (25 (9,39) seconds vs. 28 (2,52) seconds, p=0.99). T1/2 was also significantly increased in HF patients compared to controls (107±28 seconds vs. 62±14 seconds, p<0.0001) and the T1/2 of HFpEF and HFrEF patients were similar (102±22 seconds vs. 114±36 seconds, p=0.21, Figure 2). Additionally, HR recovery 2 minutes post exercise was attenuated in HF patients relative to controls (Table 2).
Figure 2. VO2 recovery kinetics.
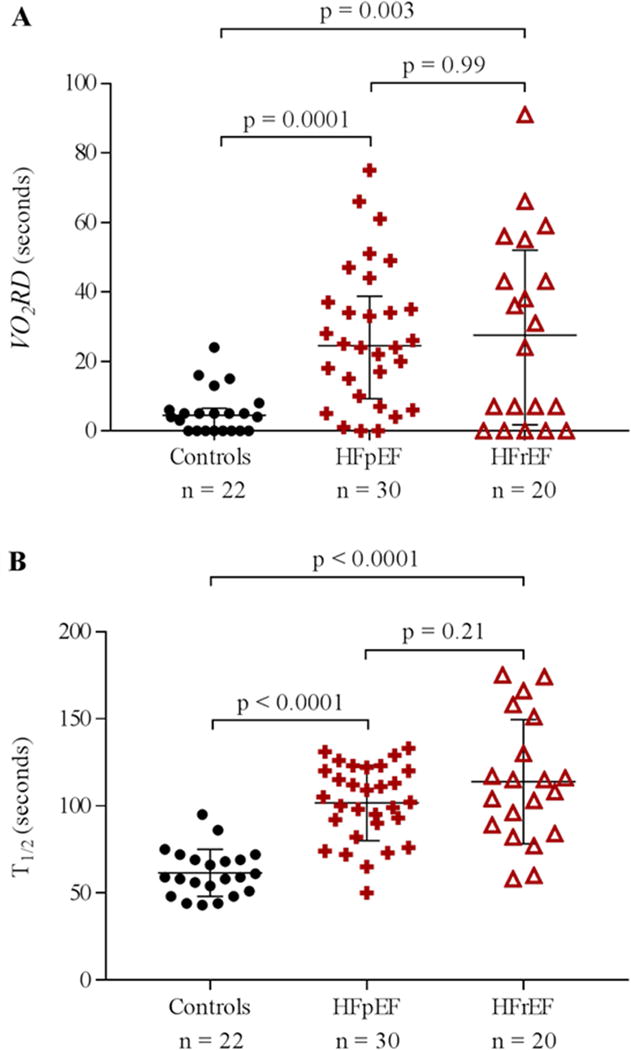
A) VO2RD with median (IQR) for controls and patients with HFpEF and HFrEF. B) T1/2 with mean ± SD for controls and patients with HFpEF and HFrEF.
Heart Failure Patients Stratified by the Median Recovery Delay
Since HFpEF and HFrEF patients exhibited similar post-exercise VO2RD durations, the two HF phenotypes were combined into one group and stratified by the median HF VO2RD duration of 25 seconds. The baseline characteristics of the stratified HF patients are summarized in Table 3. Baseline characteristics, comorbidities, and medication exposures were similar between the two strata. There was no difference in resting PAWP or cardiac index in those with prolonged VO2RD (≥ 25 seconds) compared with shorter VO2RD (< 25 seconds). In both the HFpEF and HFrEF cohorts, there was no difference in volitional effort between patients with VO2RD less than and greater than 25 seconds.
Table 3.
Baseline Characteristics and Exercise Measurements when Heart Failure Patients are Stratified by the Median VO2 Recovery Delay
| Characteristic | HF | HFpEF | HFrEF | |||
|---|---|---|---|---|---|---|
| VO2RD < 25s | VO2RD ≥ 25s | VO2RD < 25s | VO2RD ≥ 25s | VO2RD < 25s | VO2RD ≥ 25s | |
| Baseline | ||||||
| n | 25 | 25 | 15 | 15 | 10 | 10 |
| Age, years | 62±9 | 65±11 | 62±9 | 66±11 | 62±10 | 63±12 |
| Male sex, % | 68 | 64 | 53 | 47 | 90 | 90 |
| Body mass index, kg/m2 | 31.9±6.8 | 28.5±6.2 | 33.9±7.5 | 29.1±4.5† | 28.8±4.3 | 27.7±8.4 |
| Hemoglobin, g/dl | 12.9±1.7 | 13.1±1.8 | 12.9±1.6 | 13.5±2.1 | 12.8±1.9 | 12.6±1.2 |
| HFpEF, % | 60 | 60 | NA | NA | NA | NA |
| Comorbidities, % | ||||||
| Hypertension | 56 | 76 | 60 | 87 | 50 | 60 |
| Diabetes mellitus | 24 | 36 | 27 | 40 | 20 | 30 |
| Hyperlipidemia | 52 | 84* | 60 | 87 | 40 | 80 |
| Pharmacotherapies, % | ||||||
| Diuretics | 68 | 60 | 67 | 60 | 70 | 60 |
| ACE inhibitor or ARB | 48 | 48 | 20 | 33 | 90 | 70 |
| β-adrenergic blocker | 68 | 76 | 60 | 73 | 80 | 80 |
| Aldosterone blockade | 24 | 28 | 13 | 13 | 40 | 50 |
| Rest Hemodynamics | ||||||
| Supine PAWP, mmHg | 21±6 | 19±4 | 21±5 | 18±3 | 20±8 | 20±6 |
| Supine mPAP, mmHg | 27±7 | 25±6 | 28±7 | 25±5 | 26±8 | 26±6 |
| Cardiac index, l/min/m2 | 2.3±0.6 | 2.3±0.5 | 2.6±0.6 | 2.2±0.5 | 2.0±0.3 | 2.3±0.7 |
| Peak Exercise | ||||||
| Respiratory exchange ratio | 1.15±0.13 | 1.17±0.08 | 1.11±0.09 | 1.17±0.10 | 1.22±0.16 | 1.18±0.06 |
| Maximum workload, watts | 101±34 | 78±16* | 99±34 | 77±19† | 105±35 | 78±9‡ |
| VO2, % predicted | 72±21* | 57±15* | 81±23 | 62±12† | 60±5 | 49±17 |
| VO2, ml/kg/min | 14.5±2.7 | 12.0±2.2* | 14.5±2.7 | 12.2±2.5† | 14.5±3.0 | 11.9±1.8‡ |
| C(a−v)O2, ml/dl | 12.2±1.9 | 13.2±2.3 | 11.8±2.2 | 12.6±2.4 | 12.8±1.3 | 14.2±1.9 |
| Cardiac output, L/min | 11.4(9.2,14.0) | 7.1(5.9,8.7)* | 12.6(9.4,14.2) | 7.3(6.8,9.1)† | 10.7(8.1,12.9) | 6.7(5.9,7.0)‡ |
| Heart rate, BPM | 119±22 | 112±28 | 122±21 | 112±29 | 114±24 | 112±26 |
| Stroke volume, ml | 98±27 | 69±17* | 103±33 | 73±18† | 90±12 | 65±16‡ |
| VO2/work slope, ml/min/watt | 9.5±1.9 | 7.4±1.5* | 9.4±2.1 | 7.3±1.3† | 9.6±1.4 | 7.7±1.9‡ |
| VE/VCO2 slope | 36.4±9.3 | 42.6±12.1* | 35.6±9.4 | 38.5±9.1 | 37.6±9.5 | 48.8±13.9 |
| O2 Saturation, % | 97(96,99) | 98(97,99) | 97(95,97) | 97(95,99) | 99(98,100) | 99(98,99) |
| PAWP, mmHg | 28±6 | 30±8 | 28±5 | 30±7 | 28±9 | 30±10 |
| mPAP, mmHg | 46±10 | 46±8 | 47±11 | 47±9 | 45±9 | 44±7 |
| VO2RD, seconds | 7 (0,17) | 43 (34,56)* | 10 (5, 19) | 37 (33,50)† | 4 (0, 7) | 49 (39,58)‡ |
| T1/2, seconds | 94±25 | 119±26 | 94±21 | 110±19† | 95±31 | 133±30‡ |
| HR recovery @ 2min, BPM | 24±15 | 22±17 | 26±16 | 21±18 | 22±15 | 26±16 |
HF indicates heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; PAWP, pulmonary arterial wedge pressure; mPAP, mean pulmonary arterial pressure; VO2RD, VO2 recovery delay; and HR, heart rate
p<0.05 for comparison of HF ≥ 25s and HF < 25s,
p<0.05 for comparison of HFpEF ≥ 25s and HFpEF < 25s,
p<0.05 for comparison of HFrEF ≥ 25s and HFrEF < 25s
Exercise capacity, quantified by peak VO2 and maximal workload, was significantly reduced for patients with VO2RD ≥ 25 seconds compared to those with VO2RD < 25 seconds to a similar extent in HFrEF and HFpEF (Table 3). HF patients with VO2RD ≥ 25 seconds demonstrated relative inability to augment CO during exercise compared to those with VO2RD < 25 seconds (Figure 3A). Furthermore, strong negative correlations between VO2RD and augmentation of CO with exercise existed in both the HFpEF and HFrEF cohorts (Figure 3B and C). The evaluation of components of CO augmentation during exercise revealed that both HR and stroke volume augmentation during exercise were inversely related to VO2RD (ρ=−0.29, p=0.04 and ρ=−0.44, p=0.002, respectively) in HF patients. While we found a close relationship between VO2RD and the augmentation of CO in HF, there was no correlation between VO2RD and the augmentation in C(a−v)O2 during exercise (ρ=0.09, p=0.51). Augmentation in skeletal muscle oxygen extraction also did not differ between groups stratified by VO2RD (VO2RD≤25s vs. >25s, 5.94±1.61 vs. 6.45±1.74, p=0.29). Furthermore, there was no correlation between VO2RD and measurements of pulmonary function, including FEV1 and oxygen saturation during exercise (ρ=−0.15, p=0.32 and ρ=0.17, p=0.24, respectively). These findings suggest that VO2RD is specific for impairment in cardiac output reserve, rather than impairment in peripheral oxygen extraction in the HF patients investigated. VO2RD also did not correlate with 2 minute HR recovery (ρ=−0.11, p=0.48) or 30-second HR recovery (ρ=−0.27, p=0.10), indicating distinct physiologic information conferred by VO2RD in comparison to HR recovery.
Figure 3. Prolonged VO2 recovery delay is associated with impaired hemodynamic response to exercise.
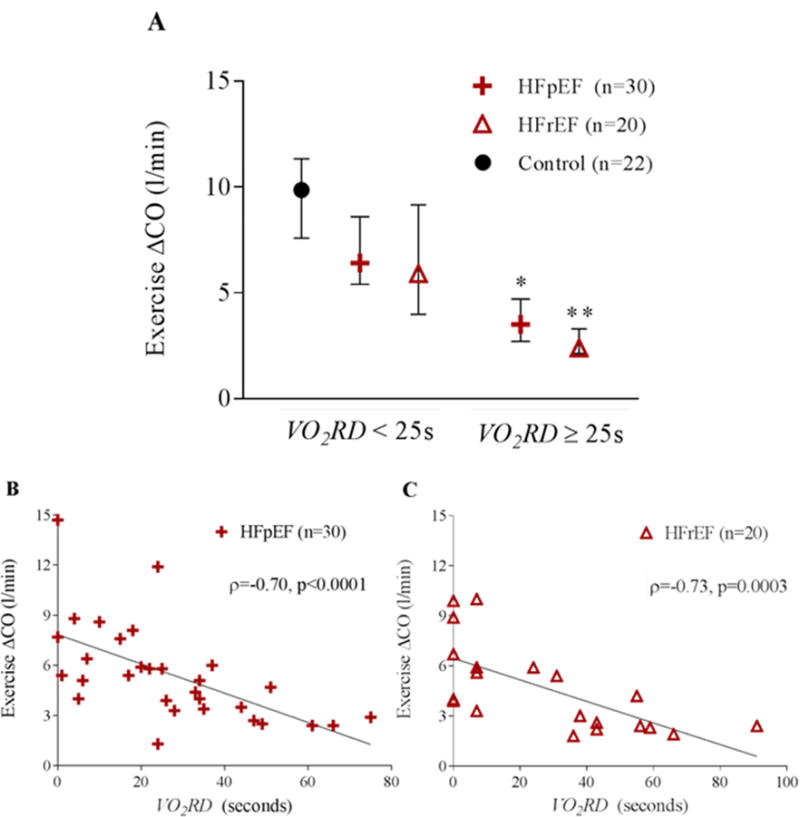
A) Cardiac output augmentation during exercise for the controls, HFpEF, and HFrEF groups is depicted as median with IQR. HFpEF and HFrEF groups are stratified by the median HF VO2RD (25s). (* indicates p=0.0015 between HFpEF < 25s and HFpEF ≥25s and ** indicates p=0.003 between HFrEF < 25s and HFrEF ≥25s. A scatter plot of cardiac output augmentation during exercise versus VO2RD for B) HFpEF and C) HFrEF. Spearman rank correlation is included.
An abnormally low VO2 versus work slope is indicative of poor oxygen utilization and a greater reliance on anaerobic metabolism for a given workload with an increase in O2 deficit (19). We tested the hypothesis that a low VO2 versus work slope during exercise, would be associated with prolonged VO2 recovery delay due to the need to “repay the O2 deficit” accumulated with exercise during recovery (Figure 4A). VO2/work slope was reduced in HFpEF (8.3±2.0 ml/min/watt) and HFrEF (8.7±1.9 ml/min/watt) compared to controls (10.4±0.8 ml/min/watt) in whom this relationship was within normal expected values of 10±1.5 ml/min/watt (p=0.0001 and p=0.003, respectively) (6,19). There was an inverse relationship between VO2/work slope and VO2RD duration in HF patients and the majority of HF patients with VO2RD ≥ 25 seconds demonstrated below normal oxygen utilization per watt of work performed (i.e. < 8.5 ml/min/watt; Figure 4B).
Figure 4. Prolonged VO2 recovery delay is associated with reduced VO2/work slope.
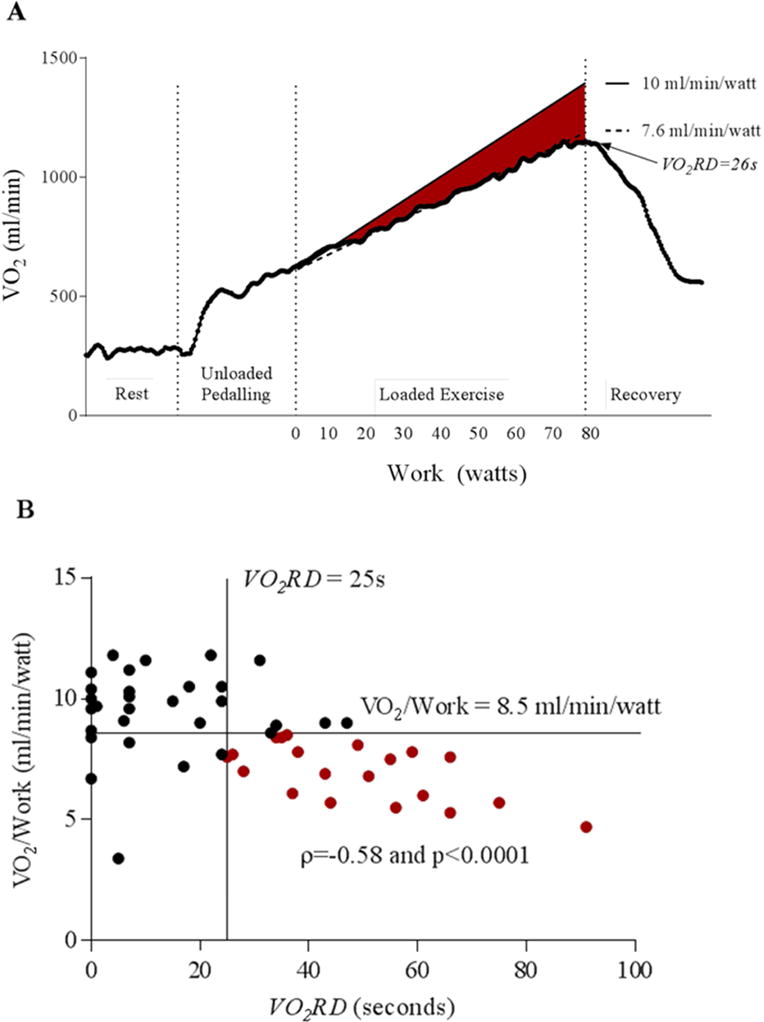
A) Oxygen uptake plotted against workload during progression of an exercise test in a representative patient with HF and a prolonged VO2RD of 26s. The red area highlights the difference between normal VO2/work (10 ml/min/watt) and the subject’s reduced VO2/work of 7.6 ml/min/watt, which represents an O2 deficit at the tissue. B) A scatter plot of VO2/work versus VO2RD for the combined HF group (n=50). Spearman rank correlation is included. The HF patients with prolonged VO2RD and abnormal VO2/work (< 8.5 mL/watt) are denoted in red (n=20 of 25 with prolonged VO2RD)
Prognostic Value of Recovery Delay in HFrEF
We utilized a larger patient cohort (n=106; Supplemental Table 1) undergoing noninvasive CPET to determine whether VO2RD predicts transplant-free survival in HFrEF. The median follow-up time was 2.5 years and 23 patients died or underwent cardiac transplantation (14 deaths, 9 heart transplants), while 17 additional patients underwent placement of a LVAD, which was censored for transplant-free survival analysis. As a continuous variable, VO2RD predicted transplant-free survival in both univariate (Cox hazard ratio 1.49 per 10 second increase in recovery delay, 95% CI 1.25–1.78, p<0.001) and multivariable Cox regression analysis adjusting for VE/VCO2 slope, OUES, HR recovery at 2 minutes, and Wasserman VO2 % predicted (Cox hazard ratio 1.37 per 10 second increase in recovery delay, 95% CI 1.10–1.71, p=0.005) (Table 4). As a dichotomous variable, VO2RD ≥ 25 seconds was associated with worse transplant-free survival with a Cox hazard ratio of 4.9 (95% CI 1.4–16.4, p=0.01). Kaplan-Meier curves stratified by VO2RD are shown in Figure 5A, with those HF patients having a prolonged VO2RD exhibiting poorer outcome (Log Rank p=0.0048) with 20 out of 23 events observed in the prolonged VO2RD group. Baseline and exercise characteristics of these HF patients stratified by VO2RD of 25 seconds are shown in Supplemental Table 1. Furthermore, VO2RD was a better predictor of cardiac transplant-free survival than T1/2 in multivariable analysis (Cox hazard ratio, 1.32 per 10 second increase in VO2RD, 95% CI 1.05–1.66, p=0.018 and Cox hazard ratio, 1.16 per 20 second increase in T1/2, 95% CI 0.93–1.40, p=0.12).
Table 4.
VO2 Recovery Delay Predicts Cardiovascular Transplant-Free Survival in HFrEF Cohort 2 (n=106) Independently of Other Prognostic CPET Variables
| Parameter | Cox Hazard Ratio | 95% Confidence Interval | P-value |
|---|---|---|---|
| VO2RD (for every 10 sec increase) | 1.37 | 1.10 – 1.71 | 0.005 |
| VE/VCO2 Slope (for every 1 increase) | 1.04 | 0.97 – 1.11 | 0.271 |
| OUES (for every 0.1 increase) | 1.11 | 1.01 – 1.21 | 0.023 |
| HR recovery @ 2min (for every 5 BPM) | 0.77 | 0.62 – 0.95 | 0.018 |
| VO2 percent predicted (for every 1% increase) | 0.95 | 0.92 – 0.99 | 0.006 |
VO2RD, indicates VO2 recovery delay; OUES, oxygen uptake efficiency slope; HR, heart rate
Figure 5. VO2 recovery delay is a prognostic indicator in HFrEF.
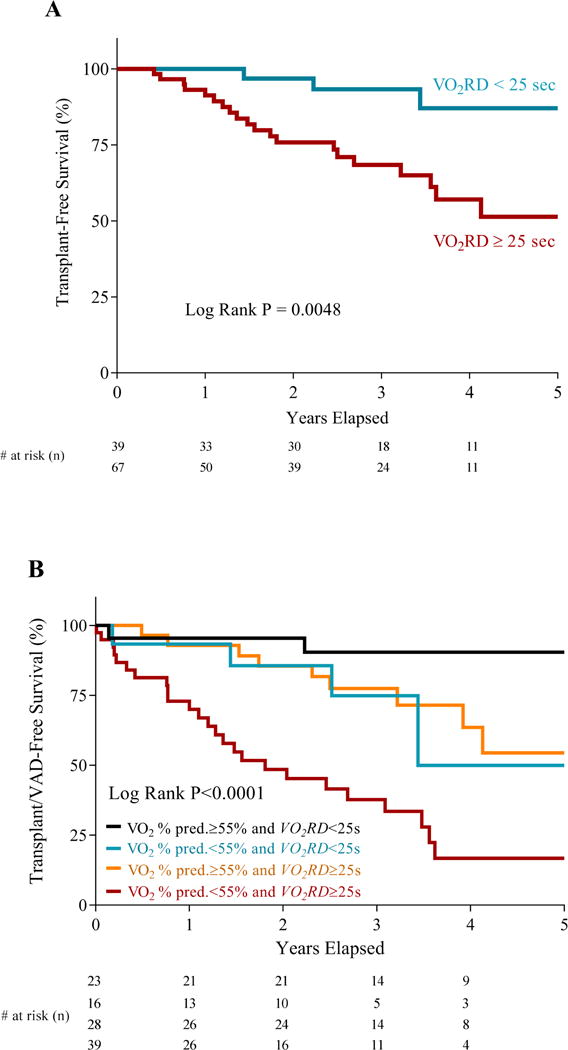
A) Kaplan-Meier transplant-free survival curves for HFrEF patients (n=106) dichotomized by a VO2RD of 25s. B) Kaplan-Meier VAD/transplant-free survival curves for HFrEF patients (n=106) stratified by VO2 % predicted and VO2RD
A multi-outcome sensitivity analysis demonstrated that VO2RD consistently predicted a range of clinical outcomes in both univariate and multivariable analyses (Supplemental Table 2). When assessing transplant/LVAD-free survival, a VO2RD ≥ 25 seconds had a Cox hazard ratio of 4.0 (95% CI 1.7–9.4, p=0.002) in univariate analysis and was a significant predictor independent of peak VO2 % predicted, VE/VCO2 slope, OUES, and HRR in multivariable analysis (Cox HR 3.1, p=0.02). In HFrEF patients, there is a close relationship between degree of impairment in % predicted peak VO2 (as determined by the Wasserman equation (6)) and prognosis (20). Amongst those HF patients with a relatively preserved peak VO2 % predicted > 55% (n=51), those with a prolonged VO2RD ≥ 25 seconds (n=28) exhibited a trend towards worse transplant/LVAD-free survival compared to those with a shorter VO2RD (Log Rank p=0.067; Figure 5B). Furthermore, amongst those with reduced peak VO2 % predicted < 55% (n=55), those with a prolonged VO2RD (n=39) exhibited worse transplant/LVAD-free survival compared to those with a shorter VO2RD (Log Rank p=0.028; Figure 5B). Finally, the presence of peak VO2 % predicted of < 55% and prolonged VO2RD conferred significantly increased risk compared to the absence of both findings (Log Rank p<0.0001; Figure 5B).
DISCUSSION
In this study, we defined a novel, easily discernible pattern of sustained VO2 elevation following exercise in patients with heart failure, which we term VO2 recovery delay. We found that the duration of VO2RD was directly related to the degree of impaired cardiac output augmentation in response to exercise in HFpEF and HFrEF. In addition, VO2RD was prolonged in HF patients with lower than normal oxygen utilization per watt of work performed, suggesting that a prolonged VO2RD reflects an increased need to repay oxygen deficit that accumulates during exercise when cardiac output augmentation lags behind the metabolic demands imposed by exercise. VO2RD also predicted transplant/LVAD-free survival, independently of peak VO2 % predicted. Taken together, our findings indicate that VO2RD is a simple non-invasive measure of the metabolic consequences of exercise exposure in HF patients that provides additional prognostic value beyond peak VO2.
The utility of performing precise quantification of exercise responses with CPET in patients with HF and other cardiorespiratory conditions is firmly supported by an expanding evidence basis. Multiple recent scientific statements have advocated for increased routine use of CPET in clinical practice in addition to CMS-mandated use of CPET in patient selection for advanced HF interventions (21). Recommended standardized CPET reports within these scientific statements contain numerous gas exchange CPET variables, but not a single recovery gas exchange measurement. This study addresses several limitations of studies done to date characterizing VO2 recovery patterns (10,16,17,22). First, divergent methods have been used to fit exponential equations to recovery patterns, but the multi-component nature of the recovery patterns often observed in HF (i.e. a recovery overshoot or plateau period followed by an exponential decline; Figure 1) indicates that a single equation will not suffice to describe VO2 recovery in HF patients. Second, most studies of recovery VO2 kinetics have not included comprehensive hemodynamic measurements during exercise to provide mechanistic insights into prolonged VO2 recovery. Finally, assessment of VO2 recovery patterns have been confined to patients with known HFrEF despite the fact that there is an unmet need to define metrics that accurately reflect impaired cardiac reserve in patients with HFpEF.
Our study is the first to investigate the easily recognizable and measurable pattern of a delay in VO2 recovery following exercise. VO2RD is minimal (i.e. usually ≤5 seconds) in controls, even from a referral cohort of patients undergoing evaluation of dyspnea on exertion who proved to have normal physiologic responses during exercise. In contrast, VO2RD ≥ 25 seconds was observed in half of the HF patients in our initial cohort and more than half in our second cohort.
While VO2RD is a novel parameter that does not lend itself to comparison to previous studies, the mean T1/2 of HF patients in our study of 107±28 seconds was intermediate between that reported by Nanas et al. (90±24 seconds) and Scrutinio et al. (152±54 seconds) in HFrEF populations (16,22). Notably, the patients studied by Nanas et al. included individuals with NYHA class I and average peak VO2 was higher than in our study population (16.7 ml/kg/min vs. 13.3 ml/kg/min), hence it is to be expected that recovery kinetics were more rapid in the Nanas study compared to this study.
Our findings relating VO2RD to impaired exercise CO augmentation and poor prognosis are also consistent with those of other investigators who have linked measures of impaired VO2 recovery kinetics to functional capacity and prognosis in patients with dilated cardiomyopathy (17) and HFrEF (7,23–25). For example, Tanabe et al. described a strong correlation between T1/2 and cardiac index at peak exercise in HFrEF (18). Our findings extend those of Tanabe by introducing a measurement that correlates with exercise cardiac indices that are independent of resting hemodynamic state (i.e. exercise change in CO). Furthermore, we characterized VO2RD in HFpEF patients in whom surrogate markers for impaired CO response to exercise are desirable in light of the numerous contributing factors to exercise intolerance among patients with HFpEF. We found that VO2RD was closely related to CO augmentation in HFrEF and HFpEF and it was more closely linked to inability to augment SV than HR. Therefore, HR augmentation and recovery patterns alone do not sufficiently capture the information provided by VO2RD.
The close correlations observed between impaired augmentation in CO and prolonged VO2RD, along with the observed low VO2/work slope in patients with prolonged VO2RD, support the hypothesis that VO2RD reflects the need to repay oxygen deficit that accumulates during exercise when CO augmentation lags behind metabolic demands imposed by exercise. The VO2/work slope below 8.5 ml/min/watt observed in patients with prolonged VO2RD is indicative of a requisite shift toward anaerobic metabolism for a greater proportion of work performed during exercise, with progressive development of oxygen deficit.
While impairment in peak VO2 is an established potent predictor of outcomes in heart failure, our study shows that VO2 recovery delay is an additional, independent predictor of cardiac transplant-free survival that offers prognostic value beyond peak VO2 and other prognostic CPET variables. We compared the prognostic strength of recovery delay against that of T1/2 and found that recovery delay outperformed T1/2 as a predictor of transplant-free survival. Additionally, after adjustment for VE/VCO2 slope, OUES, HRR, and VO2 % predicted every ten second increase in recovery delay conferred a 37% greater hazard for cardiac transplantation or death.
Study Limitations
Limitations to our study must be considered. First, CPET measurements are among the many variables used to select individuals for advanced HF interventions, making it possible that abnormal CPET findings contributed to the development of the transplant or LVAD endpoints. However, VO2RD data was not available in any of the patients at the time of transplantation or LVAD and the transplanted patients included in this study were uniformly UNOS Status 1B or 1A, while patients undergoing LVAD implantation were uniformly INTERMACs Patient Profile 4 or less, indicating use of both interventions more as “rescue therapy” to avert mortality rather than purely elective interventions. Our patient cohort sizes were limited because the collection of three minutes of recovery gas exchange data was not routinely performed in our laboratory prior to May 2013, when a dedicated recovery protocol was created. The ease of measurement of VO2RD will lend itself to confirmatory studies of its prognostic significance in larger HF studies. Additional studies are also warranted in other disease states to further understand the specificity of VO2RD for a circulatory insufficiency in comparison to other sources of exercise intolerance in conditions other than heart failure.
The control population was limited in size (n=22) due to the infrequency with which subjects without significant cardiopulmonary disease are referred for CPET with invasive hemodynamic monitoring. Furthermore, the control population cannot be considered as completely normal given that its constituents underwent CPET for the evaluation of dyspnea. “Normal controls” were normal based on their exercise capacity, ejection fractions, and hemodynamic measurements at rest and during exercise, but did harbor some CVD such as hypertension. Use of these control patients, however, would tend to underestimate the differences between HF patients and completely healthy controls.
Supplementary Material
CLINICAL PERSPECTIVE.
Presence of a prolonged VO2 recovery delay after exercise reflects circulatory insufficiency in both HFrEF and HFpEF, correlating strongly with otherwise invasively determined measures of hemodynamic response to exercise. VO2RD further proves to be a strong prognostic marker for HFrEF that is independent of other commonly used CPET variables for HF prognostication, including peak VO2, ventilatory efficiency and OUES. These findings suggest VO2RD complements exercise gas exchange measurements for assessment of cardiac reserve capacity during non-invasive exercise testing. Furthermore, no recovery gas exchange measures are routinely included in CPET report templates endorsed by recent societal scientific statements (21). These findings add to the growing evidence base supporting clinical use of CPET in HF patients and emphasizes the importance of extending gas exchange measurements into the recovery period.
TRANSLATIONAL OUTLOOK.
There has been significant recent growth in the recognition of gas exchange patterns during and immediately after exercise that predict prognosis and offer insight into mechanisms of exercise intolerance in heart failure. VO2 recovery delay is a novel metric to easily quantify circulatory insufficiency and delayed O2 kinetics during exercise. Future studies will focus on whether VO2RD will perform similarly well in predicting outcomes in earlier stages of cardiovascular disease. In addition, it is not known whether conditions beyond HF that impair exercise capacity will be associated with similar VO2RD.
Acknowledgments
Funding: Support was obtained from the National Institutes of Health 5R01HL131029 (G.D.L) and K08HL111210 (R.M), the American Heart Association 15GPSGC24800006 (G.D.L), and the Hassenfeld Clinical Scholars Program (G.D.L., R.M., C.S.B, and L.T.W).
Abbreviations
- VO2
oxygen uptake
- CPET
cardiopulmonary exercise testing
- PAWP
pulmonary arterial wedge pressure
- mPAP
mean pulmonary arterial pressure
- RER
respiratory exchange ratio
- HF
heart failure
- HR
heart rate
- VO2RD
VO2 recovery delay
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No authors have any relevant disclosures.
References
- 1.Balady GJ, Arena R, Sietsema K, et al. Clinician’s Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 2.O’Neill JO, Young JB, Pothier CE, Lauer MS. Peak oxygen consumption as a predictor of death in patients with heart failure receiving beta-blockers. Circulation. 2005;111:2313–8. doi: 10.1161/01.CIR.0000164270.72123.18. [DOI] [PubMed] [Google Scholar]
- 3.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–86. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 4.Guazzi M, Dickstein K, Vicenzi M, Arena R. Six-minute walk test and cardiopulmonary exercise testing in patients with chronic heart failure: a comparative analysis on clinical and prognostic insights. Circulation Heart failure. 2009;2:549–55. doi: 10.1161/CIRCHEARTFAILURE.109.881326. [DOI] [PubMed] [Google Scholar]
- 5.Malhotra R, Bakken K, D’Elia E, Lewis GD. Cardiopulmonary Exercise Testing in Heart Failure. JACC Heart failure. 2016;4:607–16. doi: 10.1016/j.jchf.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. The American review of respiratory disease. 1984;129:S49–55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- 7.Cohen-Solal A, Laperche T, Morvan D, Geneves M, Caviezel B, Gourgon R. Prolonged kinetics of recovery of oxygen consumption after maximal graded exercise in patients with chronic heart failure. Analysis with gas exchange measurements and NMR spectroscopy. Circulation. 1995;91:2924–32. doi: 10.1161/01.cir.91.12.2924. [DOI] [PubMed] [Google Scholar]
- 8.Nanas S, Nanas J, Kassiotis C, et al. Early recovery of oxygen kinetics after submaximal exercise test predicts functional capacity in patients with chronic heart failure. European journal of heart failure. 2001;3:685–92. doi: 10.1016/s1388-9842(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 9.Arena R, Guazzi M, Myers J, Peberdy MA. Prognostic value of heart rate recovery in patients with heart failure. American heart journal. 2006;151:851.e7–13. doi: 10.1016/j.ahj.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Fortin M, Turgeon PY, Nadreau E, et al. Prognostic Value of Oxygen Kinetics During Recovery From Cardiopulmonary Exercise Testing in Patients With Chronic Heart Failure. The Canadian journal of cardiology. 2015;31:1259–65. doi: 10.1016/j.cjca.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Jones NL, Makrides L, Hitchcock C, Chypchar T, McCartney N. Normal standards for an incremental progressive cycle ergometer test. The American review of respiratory disease. 1985;131:700–8. doi: 10.1164/arrd.1985.131.5.700. [DOI] [PubMed] [Google Scholar]
- 12.Murphy RM, Shah RV, Malhotra R, et al. Exercise oscillatory ventilation in systolic heart failure: an indicator of impaired hemodynamic response to exercise. Circulation. 2011;124:1442–51. doi: 10.1161/CIRCULATIONAHA.111.024141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhakal BP, Malhotra R, Murphy RM, et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circulation Heart failure. 2015;8:286–94. doi: 10.1161/CIRCHEARTFAILURE.114.001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guazzi M, Myers J, Peberdy MA, Bensimhon D, Chase P, Arena R. Exercise oscillatory breathing in diastolic heart failure: prevalence and prognostic insights. European heart journal. 2008;29:2751–9. doi: 10.1093/eurheartj/ehn437. [DOI] [PubMed] [Google Scholar]
- 15.Arena R, Myers J, Abella J, et al. Prognostic value of timing and duration characteristics of exercise oscillatory ventilation in patients with heart failure. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2008;27:341–7. doi: 10.1016/j.healun.2007.11.574. [DOI] [PubMed] [Google Scholar]
- 16.Nanas S, Nanas J, Kassiotis C, et al. Respiratory muscles performance is related to oxygen kinetics during maximal exercise and early recovery in patients with congestive heart failure. Circulation. 1999;100:503–8. doi: 10.1161/01.cir.100.5.503. [DOI] [PubMed] [Google Scholar]
- 17.de Groote P, Millaire A, Decoulx E, Nugue O, Guimier P, Ducloux Kinetics of oxygen consumption during and after exercise in patients with dilated cardiomyopathy. New markers of exercise intolerance with clinical implications. Journal of the American College of Cardiology. 1996;28:168–75. doi: 10.1016/0735-1097(96)00126-x. [DOI] [PubMed] [Google Scholar]
- 18.Tanabe Y, Takahashi M, Hosaka Y, Ito M, Ito E, Suzuki K. Prolonged recovery of cardiac output after maximal exercise in patients with chronic heart failure. Journal of the American College of Cardiology. 2000;35:1228–36. doi: 10.1016/s0735-1097(00)00517-9. [DOI] [PubMed] [Google Scholar]
- 19.Tanabe Y, Nakagawa I, Ito E, Suzuki K. Hemodynamic basis of the reduced oxygen uptake relative to work rate during incremental exercise in patients with chronic heart failure. International journal of cardiology. 2002;83:57–62. doi: 10.1016/s0167-5273(02)00013-x. [DOI] [PubMed] [Google Scholar]
- 20.Arena R, Myers J, Abella J, et al. Determining the preferred percent-predicted equation for peak oxygen consumption in patients with heart failure. Circulation Heart failure. 2009;2:113–20. doi: 10.1161/CIRCHEARTFAILURE.108.834168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ. 2016 Focused Update: Clinical Recommendations for Cardiopulmonary Exercise Testing Data Assessment in Specific Patient Populations. Circulation. 2016;133:e694–711. doi: 10.1161/CIR.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 22.Scrutinio D, Passantino A, Lagioia R, Napoli F, Ricci A, Rizzon P. Percent achieved of predicted peak exercise oxygen uptake and kinetics of recovery of oxygen uptake after exercise for risk stratification in chronic heart failure. International journal of cardiology. 1998;64:117–24. doi: 10.1016/s0167-5273(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 23.Hayashida W, Kumada T, Kohno F, et al. Post-exercise oxygen uptake kinetics in patients with left ventricular dysfunction. International journal of cardiology. 1993;38:63–72. doi: 10.1016/0167-5273(93)90205-u. [DOI] [PubMed] [Google Scholar]
- 24.Sietsema KE, Ben-Dov I, Zhang YY, Sullivan C, Wasserman K. Dynamics of oxygen uptake for submaximal exercise and recovery in patients with chronic heart failure. Chest. 1994;105:1693–700. doi: 10.1378/chest.105.6.1693. [DOI] [PubMed] [Google Scholar]
- 25.Kriatselis CD, Nedios S, Kelle S, Helbig S, Gottwik M, von Bary C. Oxygen Kinetics and Heart Rate Response during Early Recovery from Exercise in Patients with Heart Failure. Cardiology research and practice. 2012;2012:512857. doi: 10.1155/2012/512857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


