Abstract
Islet-1 expression identifies populations of progenitor cells in embryonic, fetal, and newborn murine hearts that are able to give rise to all cardiac cell lineages ex vivo and in vivo. Using systematic immunohistochemistry, we investigated whether islet-1-positive cells are present in adult mouse heart from the perspective of their potential therapeutic utility. The presence, localization, and nature of islet-1-positive cells were assessed in mice of different strains, ages, and conditions. Islet-1-positive cells were present in mouse heart from postnatal day 1 to young adulthood. Depending on the strain, these cells were organized in either 1 or 2 types of clusters localized to restricted areas, at a distance of 6%–35% of the heart length from the base. The first type of cluster was present in all strains and consisted of neural crest-derived cells that formed cardiac ganglia. The number of cells remained stable (a few hundred) from neonatal up to adult ages, and variations were noted between strains regarding their long-term persistency. The second type of cluster was essentially present in 129SvJ or Balb/C strains and absent from the other strains tested (C57BL/6J, C3H, SJL). It consisted of cells expressing highly ordered sarcomeric actin, consistent with their having cardiomyocyte identity. These cells disappeared in animals older than 4 months. Neither the number nor the type of islet-1-positive cells varied with time in a mouse model of dilated cardiomyopathy. Our studies demonstrate that islet-1-positive cells are relatively few in number in adult murine heart, being localized in restricted and rather inaccessible areas, and can represent both neural crest and cardiomyocyte lineages.
Introduction
Cardiovascular diseases are the primary cause of death in developed countries, and heart failure represents a major life-threatening condition. Pharmacological treatments, although ameliorative, are unable to prevent heart failure, and cardiac transplantation remains the ultimate therapeutic option. Unfortunately, heart transplantation faces 2 major drawbacks, shortage of potential donors and immunological problems. Autologous cell transplantation has become a promising new therapeutic strategy. A number of clinical trials have been performed using a variety of cell types, but true cardiac regeneration has never been observed. This failure probably reflects the intrinsic lack of cardiac differentiation of the presently elected candidates, that is, mainly bone marrow or skeletal muscle-derived cells [1]. This observation mandates the identification, isolation, and use of bona fide cardiac precursor cells.
Islet-1 is a transcription factor of the subfamily of Lin-11, Isl-1 and Mec-3 (LIM) homeodomain transcriptional regulators. First identified as an enhancer in the insulin gene [2], islet-1 is expressed in a variety of cell lineages of pancreatic endocrine origin during embryogenesis, as well as in normal adult islet cells [2,3]. Islet-1 also controls organogenesis of the pituitary and pancreas [4]. Islet-1 function is critical in the development and differentiation of the nervous system and the control of motoneuron identity [5–9], but is not restricted to neuroendocrine lineages [10]. Yuan and Schoenwolf [11] demonstrated that islet-1 is expressed asymmetrically in the early heart rudiments and during rotation of the foregut, thereby providing a new candidate in the left/right heart asymmetry signaling pathway. Islet-1 is required for formation of the heart, as islet-1 null mutant mice are missing outflow tract (OT), right ventricle, and much of the atrial tissue [12,13]. Lineage tracing showed that islet-1-expressing cells colonize the OT, the right ventricle, part of the atria, and a minor portion of the inner curvature of the left ventricle, thereby contributing substantially to embryonic heart formation. It was further demonstrated that murine or human islet-1-expressing cells not only contribute to cardiac contractile tissue, but also to the conduction system and endothelial/smooth muscle cells [14–18]. Further, Islet-1 is directly required for differentiation of cardiac progenitors into cardiomyocytes and smooth muscle cells [19]. Recently, a longitudinal study in fetal, neonatal, and young adult rats documented the contribution of these cells to the second heart field. Of note, some islet-1-positive cells persisted in young adulthood in OT areas and coexpressed the cardiac marker TnT [20]. Finally, murine and human islet-1-expressing cells can be purified, expanded, and differentiated into mature cardiomyocytes [15,21,22]. All these results identified cardiac islet-1-expressing cells as candidates for future cardiac stem cell therapy.
A candidate cell type for preclinical and clinical trials should fulfill specific requirements. To avoid viral safety issues and overcome immunological rejection, autologous approaches are preferred. This implies an ease of cell procurement, that is, small-volume biopsies may be obtained from anatomic sites dispensable for vital function. Further, the cells should be present in adulthood. Finally, cell selection should be based on a robust marker that will unambiguously identify the cell type of interest. Therefore, we asked whether islet-1-positive cells persist in the hearts of young and adult animals of different strains and so set up a thorough spatial and temporal investigation of islet-1-positive cells in mice from postnatal day 1 to early and late adulthood. We show that islet-1-positive cells were present in small numbers after birth, were organized as 1 or 2 types of clusters localized in very restricted areas, and disappeared between the ages of 4–12 months. Lastly, given the role of islet-1-expressing cells in cardiac formation and growth, a defect could be postulated to result in cardiomyopathy in adult life [23,24]. We observed that neither the number of islet-1-positive cells nor their localization varied during the development of a dilated cardiomyopathy in lamin A/C mice carrying a homozygous Lmna H222P missense mutation [25].
Methods
Animals
129SvJ, C57BL/6J, Balb/C, C3H, and SJL mice were purchased from Charles River (L'arbresle, France). The mouse model of cardiomyopathy, KI-LmnaH222P/H222P mice and their wild-type littermates KI-LmnaWT/WT were produced on a 129SvJ × C57BL/6J background and bred in our facility [25]. The islet-1-lacZ mouse model was created by the group of S. Evans by a knock-in of the sequence of nuclear lacZ (nlacZ) into the islet-1 locus in a C57BL/6J mouse background to generate heterozygous islet-1 nlacZ knock-in mice [16].
All the procedures were conducted according to the Guide for the Care and Use of Laboratory animals (DHAW publication no. 85-23, Office of Science and Health Reports, DRR/NIH, Bethesda, MD). Mice were anesthetized (80 mg/kg ketamine, 16 mg/kg xylazine) and sacrificed at an age of 1, 5, 10, 15, 20, 25 days or 3, 4, 6, 7, 8, 10, 12 months (all wild-type strains and KI-Lmna animals), and 1.5–4 months (nlacZ knock-in mice). Hearts' weight and length were measured. Heart, thymus, and pancreas were collected and frozen in nitrogen-cooled isopentane. Pregnant wild-type female OFA rats were purchased from Charles River, anesthetized, and the spinal cords of E18 fetuses were collected and frozen in nitrogen-cooled isopentane.
Tissue immunohistology
Mouse hearts were systematically sectioned on cryostat from the base to the apex and all sections were collected (5-μm sections). Mouse pancreas sections (10 μm) were always used as positive controls for islet-1 labeling. Fetal rat neural tube sections (5 μm) were used for second validation of islet-1 labeling, and thymus (5 μm) sections for validation of Ki67 labeling. Islet-1 immunostaining was performed at room temperature on cryostat sections fixed in 4% paraformaldehyde (pH 7.4, 15 min), permeabilized with 0.1% Triton X-100 (20 min), blocked with 10% goat serum or 3% phosphate-buffered saline bovine serum albumin (1 h), and then incubated in rabbit polyclonal anti-islet-1 antibody (Ab) (Abcam, Cambridge, UK; 1/2000; 2 h) or mouse monoclonal 39.4D5 IgG2b and 40.2D6 IgG1 anti-Islet-1 Ab (DSHB, Iowa City, Iowa; 1/200; 2 h), respectively. A goat anti-rabbit or anti-mouse FITC-conjugated (Sigma, L'Isle d'Abeau, France; 1/2000; 1 h) secondary Ab was incubated to detect islet-1-positive cells. Slides were mounted with Vectashield® containing DAPI (Abcys, Paris, France) to visualize cell nuclei. Serial sections were stained with hematoxylin and eosin. The same staining procedure was used for colabeling of islet-1 (polyclonal Ab) and Ki-67 (rat monoclonal Ab; Dako AS, Glostrup, Denmark; 1/50; 1 h), or 165-kDa neurofilaments (DSHB, clone 2H3, 1/500, 1 h). Cardiomyocytes were identified by fluorescence using phalloidin-Fluoprobe 547H (Interchim SA, Montluçon, France) for staining actin according to manufacturer's instructions. α-Actinin staining was performed by employing the ZytoChem Plus AP Polymer Kit according to manufacturer's instruction (Zytomed Systems, Berlin, Germany) using an anti-α-actinin Ab (Sigma; clone EA53; 1/800; 1 h). Histological imaging was obtained using a Leica DMR Microscope and a Tri-CCD color video camera Sony DXC-950P driven by the TRIBVN ICS 1.3 (2002) software. Cell imaging was done using an Olympus IX10 microscope. Fluorescence imaging on tissue was done using a Zeiss Axioplan 2 Microscope and a Photometric CoolSnap fx Camera (Roper Scientific, Evry, France) controlled by the Metavue 6.2r6 (2004) software. Confocal microscopy was performed on a Leica TCS SP2 AOBS confocal laser-scanning microscope. When indicated, for a given heart representative of the group under study, the total number of islet-1-positive cells was obtained by adding up all the positive cells counted on every section of the heart.
LacZ staining of cardiac sections
Twelve-week-old heterozygous islet-1 nlacZ knock-in mice were sacrificed, hearts were frozen in a 30% sucrose/tissuetek mixture (1/1) immersed in liquid nitrogen. Ten-micrometer serial cryostat sections were cut. For staining, sections were air-dried for 30 min, fixed for 10 min with 4% paraformaldehyde, permeabilized in 0.02% NP40, 0.01% sodium deoxycholate, and 20 mM Tris (pH 7.4) for 1 h at 37°C, and then incubated in X-gal solution (5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 2 mM MgCl2, 2.5 mM X-gal; Sigma-Aldrich, Munich, Germany) in phosphate-buffered saline. For hematoxylin–eosin staining, sections were immersed in light hematoxylin and eosin after paraformaldehyde fixation.
Cholinesterase activity
Briefly, the sections were fixed in 2.5% glutaraldehyde for 1 h on ice and then incubated for 15 min in a solution containing acetic acid (100 mM), sodium acetate (100 mM), glycine (25 mM), and cupric sulfate (5 mM) in a Soerensen buffer. Then the sections were incubated in the same solution also containing acetylthiocholine iodide (4 mM) for 45 min. Following one rinse in water, the sections were incubated in ammonium sulfide (1%) for few minutes and rinsed in water. Cholinesterase activity is characterized by a brown staining.
Results
Distribution of the islet-1-positive cells in C57BL/6J and 129SvJ mouse heart
We set up a standardized protocol for the detection of islet-1-positive cells in sections from rat embryo neural tube and murine pancreas, serving as positive controls. Using mouse monoclonal 39.4D5 or 40.2D6 anti-islet-1 Ab, we consistently observed a high background because of the secondary anti-mouse Ab. This technical problem was circumvented using a rabbit polyclonal anti-islet-1 Ab, followed by a secondary anti-rabbit Ab. The specificity of the staining has been ascertained by a colabeling study of the same cells using both Abs and by western blot validation of the control recombinant protein (data not shown). All further experiments were then performed using the polyclonal Ab.
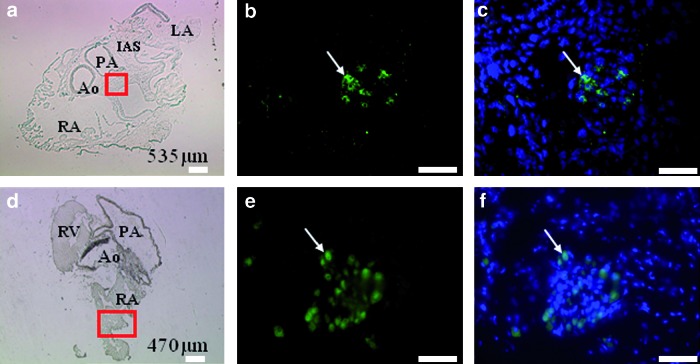
The distribution of islet-1-positive cells was first analyzed in the hearts of C57BL/6J mice, at different time points after birth. In 1–7-day-old mouse hearts, islet-1-positive cells were gathered in small clusters of 10–25 cells, characterized by large round nuclei and strong and homogeneous nuclear staining (Fig. 1a–c). Similar results were obtained at all time points up to 7 months of age (Fig. 1d–f and Table 1; n = 27/28 animals analyzed). By counting all the positive cells on all the serial sections covering entire hearts in representative animals, we could determine the total numbers of islet-1-positive cells in the given hearts. These numbers remained stable between 150 and 300 cells and then declined in oldest animals (Table 1). Beyond 7 months of age, no islet-1-positive cell could be detected in this strain (n = 0/3 animal analyzed). These numbers may be slightly overestimated, as the thickness of the sections (5 μm) was less than the average nucleus diameter.
FIG. 1.
Cluster of islet-1-positive cells in C57BL/6J mouse heart. Immunostaining was performed at 1 day postnatal (a–c) and 7-month-old heart (d–f). The a and d panels show the entire heart section (at the depths of 535 and 470 μm, respectively) and the positive area is delimited (red square). The b and e panels show the clusters of islet-1-positive cells. The nuclear colocalization of islet-1 and DAPI signal is presented in c and f panels. Arrows indicate islet-1-positive cells. Scale bars: (a, d) 100 μm; (b, c, e, f) 50 μm. RA, right atria; LA, left atria; RV, right ventricle; IAS, interatrial septum; Ao, aorta; PA, pulmonary artery. Color images available online at www.liebertonline.com/scd
Table 1.
Number and Localization of Islet-1-Positive Cells in C57BL/6J Mouse Heart
| Mouse age | na | Number of cellsb | Localizationc | Leveld |
|---|---|---|---|---|
| D1 | 10/10 | 302 | 101 OT, 201 RA | 12%–18% |
| D5 | 5/6 | 222 | 222 LV | 15%–23% |
| D10 | 2/2 | 163 | 131 OT, 32 IVS | 13%–27% |
| D15 | 1/1 | 306 | 192 RA, 54 IAS, 60 LA | 8%–19% |
| D20 | 5/5 | 180 | 14 RV, 133 IAS, 33 IVS | 12%–27% |
| D25 | 2/2 | 311 | 303 RA, 8 LV | 13%–26% |
| 4–7 months | 2/2 | 177; 30 | 149 RA, 28 LA; 30 RA | 17%–22%; 6% |
| 8–12 months | 0/3 | 0 | NA | NA |
Number of animals positive for Islet-1 labeling out of the total number in the group.
Represents the total number of cells counted in a given heart.
Number of cells in a specific localization: OT, outflow tract; RA, right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle; IAS, interauricular septum; IVS, interventricular septum.
The levels are normalized relatively to the height of the heart, from base to apex.
NA, not applicable.
At all time points, clusters of islet-1-positive cells were neither detected on all the sections nor randomly distributed along the entire length of the heart. As indicated in Table 1, sections containing clusters of islet-1-positive cells were gathered in areas located between 6%–12% and 27% of the heart length from the base to the apex. Further, within a section, the clusters were not randomly distributed (Table 1). The low number of islet-1-positive cells, as well as their localization deep inside the myocardium in areas that would not be dispensable for the survival of animals, may hamper their use for autologous cell therapy.
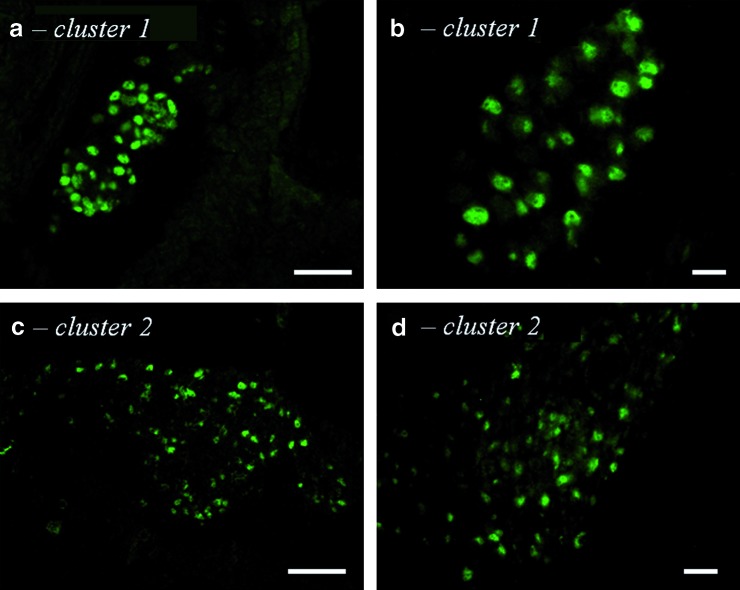
Surprisingly, we observed a different distribution in 129SvJ and 129SvJ × C57BL/6J mice (n = 35 animals analyzed). In agreement with our initial results, we detected islet-1-positive cells in approximately the same restricted area of the heart as in C57BL/6J mice. However, at variance with the previous observation, these cells were organized into 2 types of clusters, illustrated in Fig. 2. The first type corresponded to the type described in C57BL/6J hearts (Fig. 2a, b) and persisted beyond 10 months of age, that is, later than in C57BL/6J mice. The second type presented distinct features (Fig. 2c, d). The latter clusters were larger, containing up to 100 islet-1-positive cells with smaller more elongated nuclei, which had less homogeneous islet-1 staining. A typical distribution of the 2 types of clusters is presented in Table 2. This second type was observed in half of the animals before the age of 4 months (n = 17/31 animals analyzed) and disappeared later (n = 0/4 animals analyzed).
FIG. 2.
Clusters of islet-1-positive cells in 129SvJ and 129SvJ × C57BL/6J mouse hearts. Both day 1 postnatal 129SvJ (b, d) and 129SvJ × C57BL/6J (a, c) hearts contained clusters of the first type (a, b) and the second type (c, d). Scale bars: (a, c) 50 μm; (b, d) 5 μm. Color images available online at www.liebertonline.com/scd
Table 2.
Number and Localization of Islet-1-Positive Cells in 129SvJ × C57BL/6J Mouse Heart
| Cluster 1 | Cluster 2 | |||||
|---|---|---|---|---|---|---|
| Age, n | na | Numberb | Localization, levelc | na | Numberb | Localization, levelc |
| D1–2 months | 24/27 | 487 | RA, LA | 15/27 | 1748 | RA, IAS |
| n = 27 | 22%–35% | 13%–23% | ||||
| 3–4 months | 3/4 | 719 | RA, LA, IAS, IVS | 2/4 | 841 | IAS |
| n = 4 | 21%–33% | 21%–26% | ||||
| 6–10 months | 4/4 | 426, 258, | RA, IAS, IVS | 0/4 | 0 | NA |
| n = 4 | 381 | 11%–29% | ||||
Number of animals positive for islet-1 labeling out of the total number in the group.
Represents the total number of cells counted in a given heart.
Number of cells in a specific localization: RA, right atrium; LA, left atrium; IAS, interauricular septum; IVS, interventricular septum. The levels are normalized relatively to the height of the heart, from base to apex.
To investigate whether this result was a peculiar, unique feature of the 129SvJ strain, we looked for the presence of islet-1-positive cells in hearts of 3 other mouse strains frequently used in biological studies: C3H, Balb/C, and SJL. Balb/C, but not C3H or SJL strains, also showed the presence of 2 types of clusters (data not shown). Therefore, the presence of 2 types of clusters of islet-1-positive cells is not an exclusive feature of the 129SvJ strain.
As one of our goals was to determine whether the number and localization of islet-1-positive cells would be altered in the course of heart failure, we repeated these experiments using mice that harbor a knock-in mutation in the Lamin A/C gene and develop progressive cardiomyopathy, the KI-LmnaH222P/H222P model. This mouse line was derived from embryonic stem cells produced by the 129SvJ strain and was backcrossed into the C57BL/6J strain [25]. The 2 types of clusters were detected, and neither the number of islet-1-positive cells nor their localization exhibited differences that could be attributed to the development of dilated cardiomyopathy (data not shown).
Characterization and distinction of the 2 types of clusters
Islet-1-positive cells are quiescent
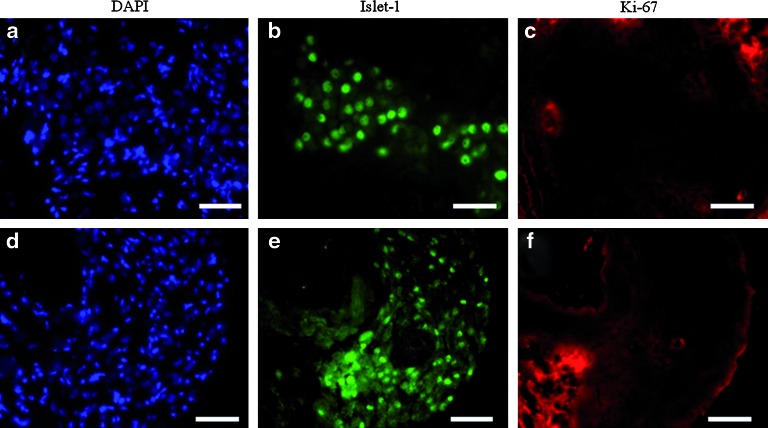
We tested whether islet-1-positive cells in adult hearts were proliferating, using an anti-Ki-67 Ab (n = 5 animals analyzed). As illustrated, neither the first type of cluster (Fig. 3a–c) nor the second (Fig. 3d–f) showed any significant costaining with anti-Ki-67 and anti-islet-1 Abs, indicating that islet-1-positive cells were in a mitotically quiescent state. This result was further confirmed using bromodeoxyuridine staining coupled with anti-islet-1 staining following a pulse administration of bromodeoxyuridine. No colocalization was observed (n = 2; data not shown).
FIG. 3.
Research of proliferating cells in clusters of islet-1-positive cells. Coimmunostaining for islet-1 and Ki-67 were performed on 21-day-old 129SvJ mouse heart sections. No Ki-67-positive cells (c, f) could be observed within islet-1-positive cells constituting the first type (a–c) or the second type (d–f) of clusters. Scale bars: 50 μm. Color images available online at www.liebertonline.com/scd
Islet-1-positive cells in the first type of cluster express neurofilament protein
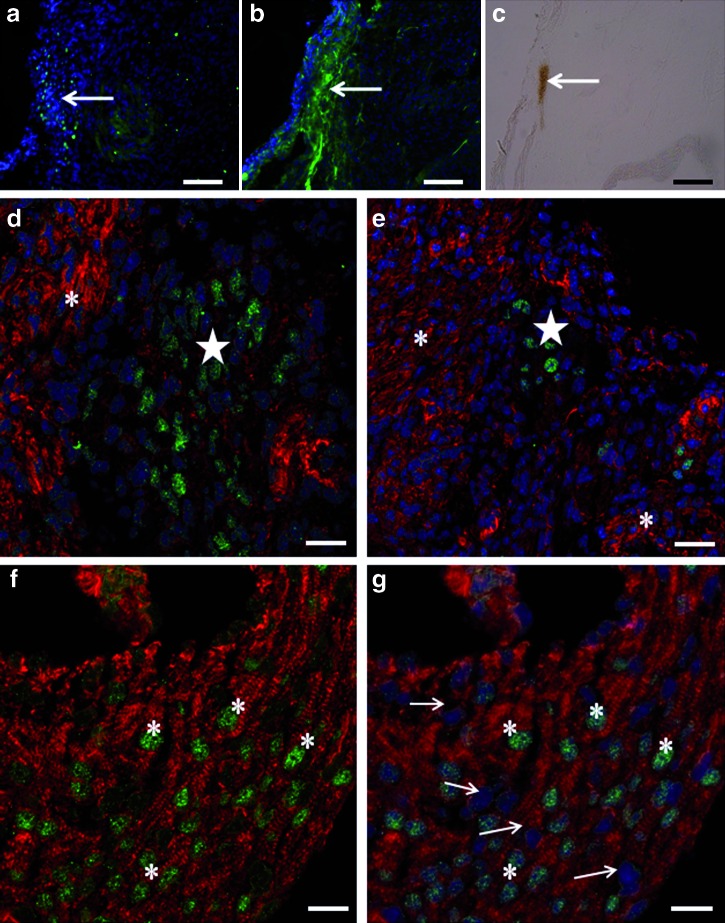
As it has been recently described that cardiac ganglia cells express islet-1 in hearts of E14.5 and 3-day-old mice [16], we tested which type of cluster corresponded to these cells. Using an Ab directed against the 165-kDa neurofilament protein, we found that only cells in the first type of cluster were labeled. Indeed, these cells were costained with Abs directed against islet-1 and neurofilament protein (Fig. 4a–c). Acetylcholinesterase-positive staining of the same area suggested the cholinergic nature of these cells (n = 6). Of note, more cells were stained for acetylcholinesterase than for islet-1. Taken together, these observations suggest a role for some islet-1-positive cells in the composition of the cardiac ganglia.
FIG. 4.
Characterization of islet-1-positive cells. Costainings for islet-1 (a), 165-kDa neurofilaments (b), acetylcholinesterase activity (c), and phalloidin (d–g) were performed on 5-day-old 129SvJ × C57BL/6J serial mouse heart sections. A cluster containing islet-1-positive cells (a) stained positively for 165-kDa neurofilaments (b) and acetylcholinesterase (c, arrows). In this first type of cluster, islet-1-positive cells (d, e, stars) were negative for phalloidin staining, whereas the surrounding cardiac tissue was positive (d, e, asterisks). Sarcomeric features colocalized with islet-1 expression in nuclei in the second type of cluster (f). Islet-1-positive cells stained by phalloidin (asterisks) are intricated within myocardial tissue and in close contact with phalloidin-positive and islet-1-negative nuclei of cardiomyocytes (g, arrows). Scale bars: (a–c) 50 μm; (d–g) 5 μm. Color images available online at www.liebertonline.com/scd
Islet-1-positive cells in the second type of cluster express a cardiomyocyte-specific marker
Using phalloidin, we analyzed the expression of actin. As expected, most cells on the heart sections, being differentiated cardiomyocytes, exhibited a typical sarcomeric organization. However, cells in the second type of islet-1-positive cluster also exhibited a sarcomeric organization of actin (Fig. 4f, g; n = 5), whereas this was not the case for cells in the first type of cluster (Fig. 4d, e). This result was surprising, as islet-1 transcription is generally turned off as cells differentiate into a cardiac type [26].
LacZ-positive cells in the islet-1 nlacZ mouse model
Our data were confirmed using an islet-1 nlacZ mouse model. Clusters of lacZ-positive cells were detected in 1.5–4-month-old animals (n = 6/6), on serial sections located in a very similar region as observed in other mouse models, being situated between the atria and next to the OT (Fig. 5a–d), or close to the atria (Fig. 5e–g). Multiple labeling on adjacent sections showed colocalization of nlacZ, acetylcholinesterase-positive staining, and Islet-1 expression, although all the cells in clusters may not be at the same level of commitment, differentiation, and organization (Fig. 5e–g). In these clusters, cells were negative for α-actinin and sarcomeric actin. The clusters were arranged in a nest-like fashion (n = 3/3).
FIG. 5.
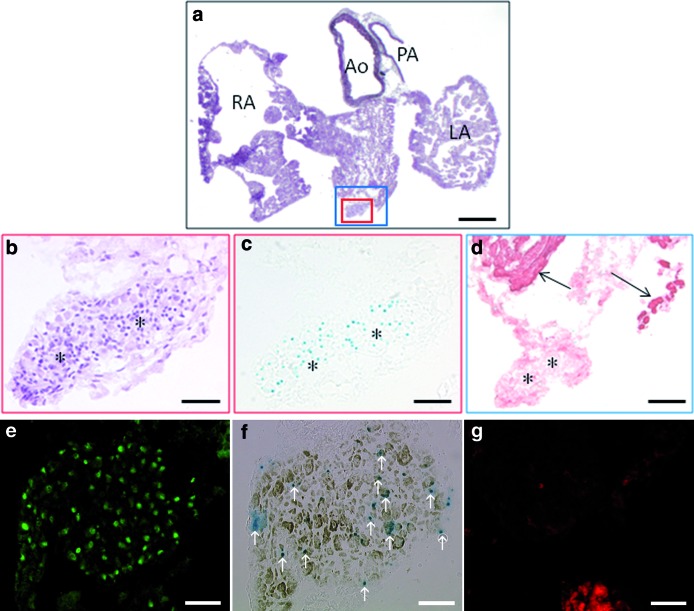
LacZ-positive cells in islet-1 nlacZ knock-in mouse model. (a–d) LacZ-positive cells are located around the outflow tract in a 12-week-old islet-1 nlacZ knock-in mouse. (b–d) Serial sections showing magnifications of the insets delineated by small (b, c) or large (d) squares in a. Cells are arranged in a cluster (b, hematoxylin–eosin staining); some of them express islet-1 in the nucleus (c, X-gal staining), and they are α-actinin negative (d). Asterisks indicate similar areas in b–d; arrows point out remote cardiac areas positive for α-actinin in d. (e–g) Adjacent sections showing a cluster located in RA, containing cells expressing islet-1 in the nucleus (e), nlacZ (f, pointed by arrows), and/or acetylcholinesterase (f), but no sarcomeric structures (g, phalloidin staining). Scale bars: (a) 800 μm; (b, c) 100 μm; (d) 200 μm; (e–g) 75 μm. Color images available online at www.liebertonline.com/scd
Discussion
Using a rabbit polyclonal Ab directed against the transcription factor islet-1, we have identified islet-1-positive cells in the mouse heart from birth to adult stages. These cells are organized in 1 or 2 types of clusters that are localized to a consistent position situated between 6% and 35% of the height of the heart from the basal plate, covering the OT, restricted areas of the right atrium and left atrium, of the interventricular septum and interauricular septum, and of the upper part of the left ventricle. The number of islet-1-positive cells is limited to a few hundred, and it remains stable for 2 (second cluster) or several (first cluster) months after birth.
Two different types of clusters were observed. Both types consisted of quiescent cells, but each type contained cells with a homogeneous and distinct phenotype. One type of cluster was previously described up to the 3rd day postbirth and likely consists of cardiac ganglia cells [16,27]. The other type of cluster was present essentially in given strains of mice (129SvJ and Balb/C) and consisted of cells deeply embedded in the myocardium. Cells of this type presented a sarcomeric organization of actin and were presumably cardiomyocytes.
The presence and nature of islet-1-positive cells were dependent on murine strain. Indeed, the first, neural-like type of cluster was present in all strains, but persisted longer in life in adult 129SvJ animals, whereas the second, cardiogenic-like type of cluster was present only in young animals of 129SvJ and Balb/C strains. Differences in neural progenitor cells' proliferation, migration, and survival have been reported between C57Bl/6J and 129SvJ strains [28], and the genetic background affects cardiac cell biology and physiology [29]. Strikingly, C57Bl/6J mice have been described to develop earlier and more severe heart failure pathologies than 129SvJ mice in induced [30] and transgenic [31] models. Whether the differential presence of islet-1-positive cells relates to differences in late fetal developmental kinetics or to different cardiac performances between strains is not known and would deserve further studies.
Some differences were noted between the present results and the original descriptions. Laugwitz et al. illustrated the presence of clustered islet-1-positive cells in a postnatal day 1 rat heart [21]. The localization of these clusters and number of cells involved are similar to what we have observed. These clusters were not further characterized, so that we cannot conclude whether they would be equivalent to our first or second type. Also, taking advantage of an inducible islet-1-Cre and an islet-1-mER-Cre-mER × R26-LacZ double heterozygous mouse model, Laugwitz et al. [21] were able to identify islet-1-expressing cells from postnatal hearts by lineage tracing, which have the potential to give rise to functional cardiomyocytes when selected and expanded in vitro. Upon tamoxifen injection in their inducible model, isolated and dispersed LacZ-expressing cells were observed in postnatal animals. In our present study, we did not identify isolated and dispersed islet-1-positive cells. We can only hypothesize that isolated cardiogenic progenitors would transiently express islet-1 up to shortly after birth, but would still be amenable to characterization because of the persistence of lacZ activity for a short time in the inducible model [21].
Genead et al. detailed the presence and localization of islet-1-positive cells in rat embryos, neonates, and young adults (up to 13 weeks old) [20]. In adult animals, they observed persistent islet-1-positive cells around the OT area. Similar to our description, the cells were mainly gathered in the upper part of the heart. At variance with our results, their cells mainly expressed cardiac markers and therefore may correspond to the second type of cluster observed in some, but not all, mice strains. We observed that this type of cluster disappeared between fourth and sixth month of adulthood, which exceeds the duration of their study. Genead et al. also described the presence of a minority of so-called undifferentiated islet-1-positive cells, which may correspond to the ganglia cells observed in our first type of cluster. Indeed, ganglia cells represent a minority in 129SvJ and BalbC mice strains (both first and second types of cluster are present), but the totality in C57Bl/6J, C3H, and SJL mice strains (first type of cluster is only present). Taken together, these observations suggest species and strain variations regarding the persistence of islet-1-positive cells in adult animals.
Our data suggest that islet-1 is expressed, in both cases, by noncycling, quiescent cells, that is, peripheral nervous system ganglia in the first type of cluster and cardiomyocytes in second type. In chick embryos, cells from the neural tube and dorsal root ganglia express islet-1 after cell cycle exiting, whereas islet-1-positive cells from the sympathetic ganglia are still dividing [32]. In mouse, the expression of islet-1 is generally considered to be shut-off upon commitment into cardiac cells [12,16,21,26,33]. Indeed, lineage tracing studies indicate that most cells previously expressing islet-1 did not express it after E9 and formation of cardiac structures. However, islet-1 is still expressed in distinct heart subdomains at postnatal day 3, including the sino-atrial node, the base of aorta and pulmonary arteries, and cardiac ganglia [16]. In rat, some differentiated islet-1-positive cells expressed cardiac markers [20], and a few dividing islet-1-positive cells were observed at postnatal stage. Our data indicate that, in the first type of cluster, islet-1 expression coincided with the presence of neurofilaments and acetylcholinesterase activity, suggesting a parasympathic nature of these ganglia. In the second type of cluster, islet-1 expression coincided with the presence of actin cross-striation, a hallmark of cardiomyocyte differentiation. Both observations argue against the idea that islet-1 expression is always shutdown during the process of cardiac differentiation. At least, a significant number of cells still have been expressing islet-1 for weeks or months after birth, and noteworthy, the second type of cluster may be considered as the most transient, as no islet-1-positive cells of this type were found after the age of 4 months. Our finding that this type of cluster is present in some strains of mice only (129SvJ, Balb/C) may explain why these cells are not systematically identified.
Given the role postulated for islet-1-expressing cells in cardiac formation and growth, a mutation of the gene itself or a defect in pathways leading to its expression could result in alterations of cardiogenesis, leading to cardiomyopathy [23,24]. Indeed, we recently identified a mutation within the islet-1 gene in a patient presenting with dilated cardiomyopathy [34]. Here, we did not observe any variation in the number and organization of islet-1-positive cells in a mouse model of dilated cardiomyopathy due to a mutation in the lamin A/C gene. This observation suggests that islet-1-positive cells in adult heart are not depleted in the context of this model of cardiomyopathy and that lamin A/C mutation did not impact the developmental expression of islet-1.
This study was initiated with a goal of identifying and localizing a specific cell type that could potentially be used as a tool to reconstitute heart tissue following transplantation in a failing heart. The rationale for focusing on islet-1-positive cells was based on previous results showing that the transcription factor islet-1 was expressed by cells that not only contribute to the formation of cardiac contractile tissue but also to the conduction system and the emergence of endothelial/smooth muscle cells [12–18,21,33]. Our hypothesis was that islet-1-expressing cells could represent, in the adult, a remnant population of cardiac precursor cells, which might be mobilized in the setting of cardiomyopathy or might be useful for transplant. Our results underline the phenotypic and functional heterogeneity of islet-1-positive cells. Indeed, islet-1-positive cells are not numerous, their number decreases with age, and their localization precludes their extraction from living subjects. Moreover, in a clinical context, these cells were not observed in remote heart biopsies prepared from cohorts of aged patients amenable to cell therapy [35]. Finally, the most persistent clusters of islet-1-positive cells coexpress neural markers, suggesting that islet-1 alone, as a marker, would not be sufficient to select and isolate pure cardiac precursors.
In conclusion, our results show that islet-1-positive cells are present and organized in clusters in postnatal and adult mouse heart and may disappear later in adulthood. These cells do not represent a pure cardiac precursor population and cannot be used immediately as tools for cell transplantation in regenerative heart medicine. Yet, their transient expression and peculiar organization suggest a specific biological role in the postnatal heart that warrants further investigation.
Acknowledgments
This work was supported by the Leducq Foundation through the dedicated Cardiac Progenitor Transatlantic Alliance (CaPTAA) network, the French Association against Myopathies (AFM), the INSERM, and the sixth Framework Program of the European Union (Marie Curie EXT-014051). The authors thank the CaPTAA members and our collaborators for fruitful discussions, especially Drs. Ken Chien, Philippe Menasché, Cyril Catelain, Karine Vauchez, Karl Laugwitz, Alessandra Moretti, Peter Gruber, Leslie Caron, Catherine Clusel, and Alain Cimino. The authors also thank Dr. Yves Fromes for helpful histological interpretations, Drs. Valerie Decostre, Laure Renou, and Aurélie Papadopoulos for providing the KI-LmnaH222P/H222P murine model, and Dr. Anne Bertrand and Mrs. Andrée Rouche for their help in illustrations. This work is dedicated to the loving and respectful memory of Dr. Ketty Schwartz (1937–2007), who pioneered the field and initiated the CaPTAA network.
This work was supported in part by NIH grant RO1HL074066 to SME.
Author Disclosure Statement
Jean-Thomas Vilquin is involved in the Biotechnology Company Myosix SA as initial founder, but neither has consultancy practice nor receives fees in relation to this study.
References
- 1.Menasché P. Cell-based therapy for heart disease: a clinically oriented perspective. Mol Ther. 2009;17:758–766. doi: 10.1038/mt.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karlsson O. Thor S. Norberg T. Ohlsson H. Edlund T. Insulin gene enhancer binding protein Isl-1 is a member of a novel class of proteins containing both a homeo- and a Cys-His domain. Nature. 1990;344:879–882. doi: 10.1038/344879a0. [DOI] [PubMed] [Google Scholar]
- 3.Dong J. Asa SL. Drucker DJ. Islet cell and extrapancreatic expression of the LIM domain homeobox gene isl-1. Mol Endocrinol. 1991;5:1633–1641. doi: 10.1210/mend-5-11-1633. [DOI] [PubMed] [Google Scholar]
- 4.Ahlgren U. Pfaff SL. Jessell TM. Edlund T. Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385:257–260. doi: 10.1038/385257a0. [DOI] [PubMed] [Google Scholar]
- 5.Ericson J. Thor S. Edlund T. Jessell TM. Yamada T. Early stages of motor neuron differentiation revealed by expression of homeobox gene Islet-1. Science. 1992;256:1555–1560. doi: 10.1126/science.1350865. [DOI] [PubMed] [Google Scholar]
- 6.Thaler JP. Koo SJ. Kania A. Lettieri K. Andrews S. Cox C. Jessell TM. Pfaff SL. A postmitotic role for Isl-class LIM homeodomain proteins in the assignment of visceral spinal motor neuron identity. Neuron. 2004;41:337–350. doi: 10.1016/s0896-6273(04)00011-x. [DOI] [PubMed] [Google Scholar]
- 7.Thor S. Andersson SG. Tomlinson A. Thomas JB. A LIM-homeodomain combinatorial code for motor-neuron pathway selection. Nature. 1999;397:76–80. doi: 10.1038/16275. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchida T. Ensini M. Morton SB. Baldassare M. Edlund T. Jessell TM. Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 9.Radde-Gallwitz K. Pan L. Gan L. Lin X. Segil N. Chen P. Expression of Islet1 marks the sensory and neuronal lineages in the mammalian inner ear. J Comp Neurol. 2004;477:412–421. doi: 10.1002/cne.20257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M. Drucker DJ. The LIM domain homeobox gene isl-1: conservation of human, hamster, and rat complementary deoxyribonucleic acid sequences and expression in cell types of nonneuroendocrine lineage. Endocrinology. 1994;134:1416–1422. doi: 10.1210/endo.134.3.7907017. [DOI] [PubMed] [Google Scholar]
- 11.Yuan S. Schoenwolf GC. Islet-1 marks the early heart rudiments and is asymmetrically expressed during early rotation of the foregut in the chick embryo. Anat Rec. 2000;260:204–207. doi: 10.1002/1097-0185(20001001)260:2<204::AID-AR90>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Cai CL. Liang X. Shi Y. Chu PH. Pfaff SL. Chen J. Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin L. Bu L. Cai CL. Zhang X. Evans S. Isl1 is upstream of sonic hedgehog in a pathway required for cardiac morphogenesis. Dev Biol. 2006;295:756–763. doi: 10.1016/j.ydbio.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 14.Garry DJ. Olson EN. A common progenitor at the heart of development. Cell. 2006;12:1101–1104. doi: 10.1016/j.cell.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 15.Moretti A. Caron L. Nakano A. Lam JT. Bernshausen A. Chen Y. Qyang Y. Bu L. Sasaki M. Martin-Puig S. Sun Y. Evans SM. Laugwitz KL. Chien KR. Multipotent embryonic isl1 + progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y. Liang X. Najafi N. Cass M. Lin L. Cai CL. Chen J. Evans SM. Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells. Dev Biol. 2007;304:286–296. doi: 10.1016/j.ydbio.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu SM. Fujiwara Y. Cibulsky SM. Clapham DE. Lien CL. Schultheiss TM. Orkin SH. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Bu L. Jiang X. Martin-Puig S. Caron L. Zhu S. Shao Y. Roberts DJ. Huang PL. Domian IJ. Chien KR. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460:113–117. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- 19.Kwon C. Qian L. Cheng P. Nigam V. Arnold J. Srivastava D. A regulatory pathway involving Notch1/beta-catenin/Isl1 determines cardiac progenitor cell fate. Nat Cell Biol. 2009;11:951–957. doi: 10.1038/ncb1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Genead R. Danielsson C. Andersson AB. Corbasci M. Franco-Cereceda A. Sylven C. Grinnemo KH. Islet-1 cells are cardiac progenitors present during the entire lifespan: from the embryonic stage to adulthood. Stem Cells Dev. 2010;19:1–15. doi: 10.1089/scd.2009.0483. [DOI] [PubMed] [Google Scholar]
- 21.Laugwitz KL. Moretti A. Lam J. Gruber P. Chen Y. Woodard S. Lin LZ. Cai CL. Lu MM. Reth M. Platoshyn O. Yuan JX. Evans S. Chien KR. Postnatal isl1 + cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qyang Y. Martin-Puig S. Chiravuri M. Chen S. Xu H. Bu L. Jiang X. Lin L. Granger A. Moretti A. Caron L. Wu X. Clarke J. Taketo MM. Laugwitz KL. Moon RT. Gruber P. Evans SM. Ding S. Chien KR. The renewal and differentiation of Isl1 + cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007;1:165–179. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 23.Olivotto I. Cecchi F. Poggesi C. Yacoub MH. Developmental origins of hypertrophic cardiomyopathy phenotypes: a unifying hypothesis. Nat Rev Cardiol. 2009;6:317–321. doi: 10.1038/nrcardio.2009.9. [DOI] [PubMed] [Google Scholar]
- 24.Stevens KN. Hakonarson H. Kim CE. Doevendans PA. Koeleman BP. Mital S. Raue J. Glessner JT. Cole JG. Moreno V. Granger A. Gruber SB. Gruber PJ. Common variation in ISL1 confers genetic susceptibility for human congenital heart disease. PLoS One. 2010;5:e10855. doi: 10.1371/journal.pone.0010855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arimura T. Helbling-Leclerc A. Massart C. Varnous S. Niel F. Lacène E. Fromes Y. Toussaint M. Mura AM. Keller DI. Amthor H. Isnard R. Malissen M. Schwartz K. Bonne G. Mouse model carrying H222P-Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Hum Mol Genet. 2005;14:155–169. doi: 10.1093/hmg/ddi017. [DOI] [PubMed] [Google Scholar]
- 26.Moretti A. Lam J. Evans SM. Laugwitz KL. Biology of Isl1 + cardiac progenitor cells in development and disease. Cell Mol Life Sci. 2007;64:674–682. doi: 10.1007/s00018-007-6520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batulevicius D. Skripka V. Pauziene N. Pauza DH. Topography of the porcine epicardiac nerve plexus as revealed by histochemistry for acetylcholinesterase. Auton Neurosci. 2008;138:64–75. doi: 10.1016/j.autneu.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Schauwecker PE. Genetic influence on neurogenesis in the dentate gyrus of two strains of adult mice. Brain Res. 2006;1120:83–92. doi: 10.1016/j.brainres.2006.08.086. [DOI] [PubMed] [Google Scholar]
- 29.Shah AP. Siedlecka U. Gandhi A. Navaratnarajah M. Abou Al-Saud S. Yacoub MH. Terracciano CM. Genetic background affects function and intracellular calcium regulation of mouse hearts. Cardiovasc Res. 2010;87:683–693. doi: 10.1093/cvr/cvq111. [DOI] [PubMed] [Google Scholar]
- 30.Barrick CJ. Rojas M. Schoonhoven R. Smyth SS. Threadgill DW. Cardiac response to pressure overload in 129S1/SvImJ and C57BL/6J mice: temporal- and background-dependent development of concentric left ventricular hypertrophy. Am J Physiol Heart Circ Physiol. 2007;292:H2119–H2130. doi: 10.1152/ajpheart.00816.2006. [DOI] [PubMed] [Google Scholar]
- 31.Barrick CJ. Roberts RB. Rojas M. Rajamannan NM. Suitt CB. O'Brien KD. Smyth SS. Threadgill DW. Reduced EGFR causes abnormal valvular differentiation leading to calcific aortic stenosis and left ventricular hypertrophy in C57BL/6J but not 129S1/SvImJ mice. Am J Physiol Heart Circ Physiol. 2009;297:H65–H75. doi: 10.1152/ajpheart.00866.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avivi C. Goldstein RS. Differential expression of Islet-1 in neural crest-derived ganglia: Islet-1 + dorsal root ganglion cells are post-mitotic and Islet-1 + sympathetic ganglion cells are still cycling. Brain Res Dev Brain Res. 1999;115:89–92. doi: 10.1016/s0165-3806(99)00054-1. [DOI] [PubMed] [Google Scholar]
- 33.Laugwitz KL. Moretti A. Caron L. Nakano A. Chien KR. Islet1 cardiovascular progenitors: a single source for heart lineages? Development. 2008;135:193–205. doi: 10.1242/dev.001883. [DOI] [PubMed] [Google Scholar]
- 34.Friedrich F. Dilanian G. Charron P. Isnard R. Chien KR. Eschenhagen T. Villard E. Carrier L. Identification of a Mef2c gain-of-function mutation in the Isl1 transcription factor in patients with familial cardiomyopathies (Abstract) Naunyn-Schmiedebergs Arch Pharmacol. 2009;379(S236):50. [Google Scholar]
- 35.Pouly J. Bruneval P. Mandet C. Proksch S. Peyrard S. Amrein C. Bousseaux V. Guillemain R. Deloche A. Fabiani JN. Menasché P. Cardiac stem cells in the real world. J Thorac Cardiovasc Surg. 2008;135:673–678. doi: 10.1016/j.jtcvs.2007.10.024. [DOI] [PubMed] [Google Scholar]