Abstract
Zoonotic Visceral leishmaniasis (ZVL) is a neglected tropical disease that in the Americas is caused by the infection of Leishmania infantum and the domestic dog (Canis familiaris) is the main parasite reservoir in urban areas. The parasite is mainly transmitted by populations of the sibling species Lutzomyia longipalpis that has been spreading in countries including Brazil, Argentina, Paraguay and more recently Uruguay. Although bionomic parameters such as population survival and the duration of the gonotrophic cycle are critical in evaluating vector capacity, field studies have rarely been applied to sand fly populations. The present study sought to evaluate basic bionomic parameters related to the vectorial capacity of the (S)-9-methylgermacrene-B population of the Lu. longipalpis complex in a visceral leishmaniasis area of Sao Paulo state. The daily survival rate, the duration of the gonotrophic cycle and the dispersal pattern were evaluated through the mark- release-recapture method. A total of 1,547 males and 401 females were marked and released in five experiments carried out between February 2013 and February 2014. The higher recapture rates occurred within 100 meters of the release point and the estimated daily survival rates varied between 0.69 and 0.89 for females and between 0.69 and 0.79 for males. The minimum duration of the gonotrophic cycle observed was five days. The absolute population size, calculated ranged from 900 to 4,857 females and from 2,882 to 9,543 males. Our results demonstrate a high survival rate of this vector population and low dispersal that could be associated with the presence of all necessary conditions for its establishment and maintenance in the peridomiciles of this area. Our findings contribute to the basic data necessary for the understanding of ZVL dynamics and the evaluation of the implementation of prevention and control measures.
Author summary
American Visceral leishmaniasis (VL) is an anthropozoonosis resulting from infection by Leishmania infantum mainly transmitted by the bite of females of the sand fly Lutzomyia longipalpis which feed mainly on domestic dogs. It is a serious tropical disease that in some countries has spread in association with several factors such as human migration, precarious urbanization and vector adaptation to urban areas. In Brazil, although control measures such as the elimination of infected dogs, the application of residual insecticide and environmental management have been applied by the health authorities, however, they have not resulted in any significant reduction in VL incidence. The knowledge of bionomic parameters of vector populations is critical for the understanding of the transmission dynamic of VL and to evaluate prevention and control measures. Therefore, in the present study we applied the mark-release-recapture (MRR) method to evaluate under field conditions some basic bionomic parameters of a population of the Lutzomyia longipalpis complex. Our findings demonstrate a high survival rate of this vector and a low population dispersal. These results suggest that measures applied to dog populations to prevent sand fly bites could contribute to the reduction of the surviving vector population and indirectly to the reduction of the host biting rate. Additionally, the low dispersal suggests that environmental management could contribute to the reduction of vector density. Our data serve as the first estimates of vector survival rates of populations of the sibling species Lu. longipalpis under natural conditions and could be applied in the evaluation of prevention and control measures.
Introduction
Visceral leishmaniasis is a neglected tropical disease (NTD) that presents a complex dynamic influenced by socioeconomic factors such as poverty and limited access to health services [1,2] and also specific biological factors related to vector and host ecology parameters as well as natural interactions between etiological agents, hosts and vectors [3,4]. Although in recent years some initiatives have been undertaken with a view to the control of VL [2], some gaps in our knowledge of VL transmission dynamics could hinder these efforts [5]. In Latin America the need to clarify some key aspects of leishmaniasis transmission [6] and the failure of control programs together with the emergence of VL in new geographical areas in South America [7,8] stress the importance of this observation.
VL in the Americas is caused by Leishmania infantum and populations of the Lu. longipalpis complex are undoubtedly its main vector, although in the last years some sand fly species has been pointed as permissive vectors of this agent [9, 10, 11, 12]. The domestic dogs constitutes the main reservoir in urban areas [13,14,15]. In Brazil between 1999 and 2014, 53,067 human cases were notified [16] and currently represent around 95% of the cases notified in Latin America. Though the Brazilian northeast region is the most affected, recently VL has emerged in several areas of the Southeast region, an event associated mainly with the dispersion of populations of the Lu. longipalpis sibling species [17,18]. Ecological differences in these populations could be associated with different epidemiological patterns of VL transmission as suggested by Casanova et al [17] in relation to Sao Paulo State.
Beside the prevalence of host infection and the presence of the susceptible population, the maintenance of transmission cycles is dependent on ecological parameters that act directly on vector capacity [19,20] and therefore their estimation permits the evaluation of the role of sand fly species in VL transmission [4, 21, 22]. Among vector parameters, population size and the duration of the gonotrophic cycle are indirect indicators of the frequency and intensity of the host biting rate [23, 24, 25], while the vector life expectancy indicates the infective life and the proportion of the population capable of transmitting an agent [23, 25, 26]. Thus, these estimates have important applications in epidemiological surveillance and contributes to the understanding of VL dynamics [27, 28, 29].
Although these bionomic parameters can be evaluated under field conditions by means of the mark-release-recapture method (MRR), commonly used in malaria and arbovirus vector studies [19, 23], this method has rarely been applied to evaluate ecological parameters of neotropical sand fly populations [30,31,32,33] Interestingly, the knowledge of sand fly dispersal permits the analysis of the applicability of control programs such as those of environmental management and the application of residual insecticides. With the recognition that Lu. longipalpis constitutes a complex of cryptic species [34,35,], many gaps in vector ecology have come to light which hinder the understanding of zoonotic visceral leishmaniasis (ZVL) dynamics in Latin America.
In the present study, we have set out observations on the dispersal, population size, duration of gonotrophic cycle and estimated survival of a population of the Lu. longipalpis complex (S)-9-methylgermacrene-B) and analyzed the results in the light of their significance in relation to visceral leishmaniasis dynamics in a highly endemic area of VL in Brazil.
Methods
Study area
The study was undertaken in the urban area of Panorama municipality, located in the western region of São Paulo state, Brazil. This locality belongs to the Atlantic forest region and presents a tropical climate Aw according to Koppen's classification [36]. Temperatures vary between 12 and 35°C with a rainy season occurring between October and March. The predominant soil is sand with flat topography. In 2012, this municipality was considered an area with intense transmission of ZVL according to the visceral leishmaniasis program classification of the health secretariate of Sao Paulo state. From 2013 to 2015, due to the decrease in the number of reported cases, this municipality was classified as an area of sporadic transmission. Previous study in this locality had identified six sand fly species belonging to the Nyssomyia, Evandromyia, Brumptomyia and Lutzomyia genera, with the predominance of the (S)-9-methylgermacrene-B population of the Lu. longipalpis complex [18].
Mark-release-recapture method
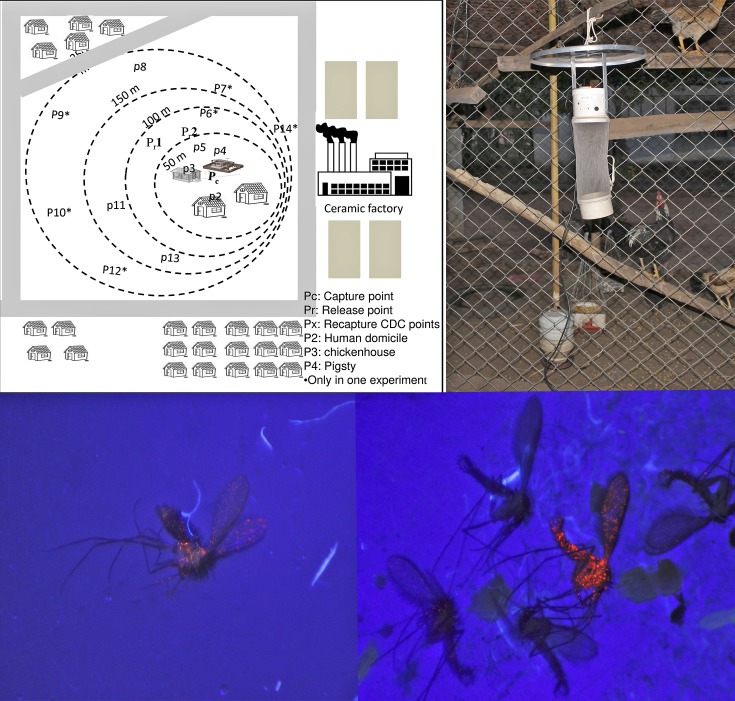
Five experiments were undertaken between 2013 (February, April, September, October) and 2014 (February). Two experiments were undertaken in a domicile located in the "Bela Vista" neighborhood (21°22'08''S, 51°51'12''W) and three other experiments in a domicile located in the "Areia Branca" neighborhood (21°21'47''S, 51°50'36W) in Panorama municipality. The distance between those domiciles is around 1.3 km.
The domiciles were selected due to the high frequencies of Lu. longipalpis and presence of favorable conditions (vegetation near the domiciles and animal husbandry) for the captures of this species. The residents of these domiciles provided formal consent to undertake the sand flies captures. The captures were performed with a Castro aspirator [37] in the animal shelters (chicken houses and pigsty) between 18:00 and 22:00 hours. This method was used to increase the chances of capturing naturally engorged females to estimate the duration of the gonotrophic cycle. All females were captured after a natural and full blood feeding on chicken or pigs. In each experiment, the specimens captured were sorted out by sex and subsequently marked with fluorescent powder (BioQuip) in color pink using a device described by Casanova et al. [31] and released after 24 hours. In the February (2013) experiment the sand flies marked were released at a distance of 60 m from the capture point to evaluate their dispersal and loyalty behavior. In the other four experiments the specimens were released at the capture point (0 meters).
Recapture method
The recapture attempts began within 24–72 hours after the release and were performed on 6–14 consecutive days. Detailed information (capture effort, temperature, distance of the release point from the CDC traps, temperature) for each experiment is presented in Table 1.
Table 1. General information related to the mark-release-recapture experiments and estimates of Lutzomyia longipalpis density, Panorama, São Paulo state, Brazil.
| Feb/13 | Apr/13 | Sep/13 | Oct/13 | Feb/14 | |
|---|---|---|---|---|---|
| Release data | 18/02 | 25/04 | 24/09 | 26/10 | 27/02 |
| Beginning of recaptures | 21/02 | 27/ 04 | 27/09 | 29/10 | 01/03 |
| Mean maximum temperature (°C) | 34.0 | 31.3 | 27.0 | 27.1 | 32.4 |
| Precipitation (mm) | 1.9 | 0 | 66.9 | 41 | 120 |
| Days of recapture attempts | 3–17 | 2–16 | 1–10 | 1–10 | 1–10 |
| Distance between the release and capture points (m) | 60 | 0 | 0 | 0 | 0 |
The recaptures were performed with a Castro aspirator from 19:00 to 22:00, on five consecutive days, on the walls of the domestic animal shelters and with 6–13 CDC light traps installed between 18:00 and 07:00 hours within 250 meters of the release point (Fig 1). All the specimens collected in each recapture attempts were euthanized (in freezer) and observed in the laboratory under stereoscopy illuminated with UV light to identify the insects marked with fluorescent power. Thereafter all the specimens were clarified and morphologically identified [38].
Fig 1. Schematic diagram of the distribution of the recapture points in the recapture attempts and marked and unmarked specimens.
Gonotrophic cycle estimation
During three experiments (Feb-2013, Apr-2013 and Feb-2014) engorged females were captured in chicken houses, counted, marked and released 24 hours later. The recaptures were performed as described above. All the recaptured females were immobilized by cooling during 3 minutes at 4°C and observed under UV light, and those marked were individualized in vials containing a moist plaster layer at the bottom where the females laid their eggs or were dissected to observe their parity status. The duration of the gonotrophic cycle was considered to be the time between the blood meal (day of capture) and the median time of oviposition, in view of the fact that this Lu. longipalpis s.l population presents gonotrophic concordance as observed in previous studies [39].
Statistical analysis
Comparisons of the frequencies of males and females by capture method (manual captures vs CDC light traps) and distance were performed using the Chi-squared test and the probability and odds of capture by the distance from the release point were calculated.
The mortality was considered constant during the period of recapture. Thus, the daily survival was estimated horizontally on the basis of the numbers of specimens recaptured, which were transformed by the ln (y+1). A linear regression analysis was performed as a function of the delay (days) in recapture after the release [25, 26]. The daily survival estimate was obtained by the equation ln mt—ln (M0r)–t lns, where M0 is the number of specimens marked and released, r is the proportion of specimens recaptured, t is the period (days) of recapture attempts. Thus, the daily survival s is obtained from the antilog of the slope obtained in the regression analyses. The daily survival rate was estimated by the expression s = eb, where e is the natural logarithm base and b is the regression coefficient [26, 32, 40].
The Lincoln index, as corrected by Bailey [41], was used to estimate the population size (N). Using this deterministic method, the population size is estimated using a unique mark event. This estimation was undertaken using the equation N = a(n+1)/(r+1); where a is the number of mark-release specimens, n is the number of specimens recaptured, and r the number of marked specimens recaptured. The variance of N was estimated according to the equation a2n (n-r)/[(r+1)2 +(r+2)]. The confidence interval was estimated using 1.96 standard deviations (±1.96). The male and female density/km2 was estimated of the population size divided by the area covered by the CDC traps, 0.25 km2 [42].
Results
Description of the recapture data
In the five experiments a total of 1,547 males and 401 females were marked and released. During the recapture attempts 4,780 males and 1,096 females were captured and a total of 242 males and 36 females were recaptured, giving recapture rates of 15.64% and 8.98%, respectively. The number of males and females capture by method and distance from the point of origin is presented in Table 2.
Table 2. Number of females and males of Lutzomyia longipalpis captured and recaptured by method and distance from the release point during the mark release recapture experiments.
| Capture method | Distance from release point | Capture effort (hours) | Females captured | % | Females recaptured | % | Males captured | % | Males recaptured | % |
|---|---|---|---|---|---|---|---|---|---|---|
| CDC traps | 0–50 | 2760 | 277 | 25.3 | 3 | 8.3 | 994 | 20.8 | 25 | 10.3 |
| 51–100 | 1764 | 255 | 23.3 | 9 | 25.0 | 888 | 18.6 | 25 | 10.3 | |
| 101–150 | 1140 | 30 | 2.7 | 0 | 0.0 | 125 | 2.6 | 0 | 0.0 | |
| 151–250 | 636 | 4 | 0.4 | 0 | 0.0 | 16 | 0.3 | 2 | 0.8 | |
| Subtotal | 6300 | 566 | 51.6 | 12 | 33.3 | 2013 | 42.3 | 52 | 21.5 | |
| Manual captures | 0–100 | 72 | 530 | 48.4 | 24 | 66.7 | 2757 | 57.7 | 190 | 78.5 |
| Total | 6372 | 1096 | 100 | 36 | 100 | 4780 | 100 | 242 | 100 |
Considering all the specimens captured in the recapture attempts a statistical association between the methods of capture and the sex of species was observed and showed more females captured by the manual method (p<0.001).
Population dispersal
During the recapture attempts 97% of males and females (marked and unmarked) were captured within a distance of 100 m from the release point. No female was recaptured further than 100 meters and only two males were recaptured between 150 and 250 meters from the release point (Table 2). The proportion of captures was different according to the distance from the release point (p<0.001) with a greater chance of capture within 50 meters (odds ratio of 13.6) and decreasing with distance from the release point. In the first experiment in which the specimens were released at a distance of 60 m from the original capture point, 98% of the specimens recaptured were collected in the animal shelters where they had initially been captured.
As regards the captures with CDC light traps, a predominance of males (3:1) was observed and this proportion was no different in terms of the distance from the point of release in the two areas where the experiments were performed (domiciles 1 p = 0.717; domicile 2 p = 0.524).
Population survival and size
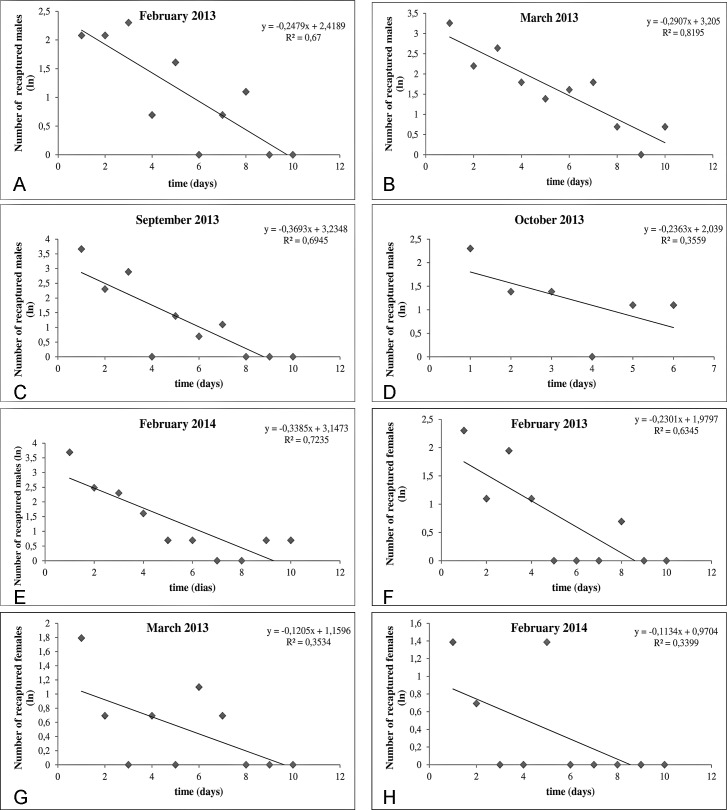
The daily survival rate for females was estimated during three experiments (month of high frequencies) and the values ranged from 0.79 to 0.89. In the regression analysis the highest coefficient of determination (0.63) was obtained in the February/2013 experiments. For the males, estimates of daily survival were obtained from the five experiments and the values ranged from 0.69 to 0.74. We considered a male daily survival rate of 0.74, a value estimated in the April/2013 experiment since it presented the highest coefficient of determination as representative of male survival (Table 3).
Table 3. Estimates of daily survival for females and males of Lutzomyia longipalpis estimated horizontally from the decrease in the numbers of insects recaptured, transformed by ln (y +1) and subject to regression analysis as a function of time (days) since release.
| Month/year | Females | Males | ||||||
|---|---|---|---|---|---|---|---|---|
| b | R2 | P | s | b | R2 | P | s | |
| February/13 | -0.23 | 0.63 | 0.006 | 0.79 | -0.29 | 0.67 | 0.000 | 0.74 |
| April/13 | -0.12 | 0.35 | 0.012 | 0.88 | -0.29 | 0.81 | 0.000 | 0.74 |
| September/13 | - | - | - | - | -0,369 | 0.69 | 0.003 | 0.69 |
| October/13 | - | - | - | - | -0.236 | 0.36 | 0.030 | 0.79 |
| February/14 | -0.11 | 0.33 | 0.077 | 0.89 | -0.34 | 0.72 | 0.001 | 0.71 |
b:regression coefficient;R2: determination coefficient; P: p-value, s: survival rate
The scatter plots of the regression analysis of the number of males and females recaptured during the recapture attempts are presented in Fig 2.
Fig 2.
Scatter plot illustrating the relationship between the number of recaptured specimens and the time of recapture attempts in each experiment for males (A-E) and females (F-H).
The female population size calculated by the Lincoln Index ranged from 900 (September/2013) to 4,857 females (February/2014). For the males, the population size ranged from 2,882 (September/2013) to 9,543 males (February/2014). The density per km2 of females ranged between 3,600 and 19,426 and for males between11,528 and 38,172. A larger population of males than females was observed in all the experiments (Table 4).
Table 4. Estimates of population parameters for females and males of Lutzomyia longipalpis in Panorama municipality, São Paulo state, Brazil.
| Females | Males | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Month/year | a | r | N | PS | (SD) | CI | Density/Km2 | a | r | n | PS | (SD) | CI | Density/Km2 |
| February/13 | 174 | 19 | 286 | 2648 | 529 | 1610–3685 | 10,591 | 390 | 30 | 733 | 9543 | 1597 | 6412–12674 | 38,172 |
| April/13 | 70 | 9 | 404 | 3151 | 844 | 1496–4805 | 12,604 | 310 | 62 | 1678 | 8396 | 1013 | 6410–10382 | 33,584 |
| September/13 | 29 | 3 | 91 | 900 | 295 | 322–1478 | 3,600 | 332 | 59 | 511 | 2882 | 340 | 2214–3550 | 11,528 |
| October/13 | 45 | 1 | 100 | 4546 | 1299 | 2000–7091 | 18,184 | 200 | 20 | 548 | 5491 | 1093 | 3348–7634 | 21,964 |
| February/14 | 83 | 4 | 233 | 4857 | 1568 | 1782–7931 | 19,426 | 315 | 60 | 1494 | 7850 | 960 | 5968–9732 | 31,399 |
a number of specimens marked and released
r number of specimens recaptured
n total number of specimens captured during the recapture attempts
PS population size
SD standard deviation
CI confidence interval
Duration of gonotrophic cycle (GC)
As regards the duration of the gonotrophic cycle, in the February/2013 experiment: of the 174 engorged females marked and released, two were recaptured without either eggs or blood in their abdomen on the fifth day after their release. The temperature during the experiment ranged between 21 and 33°C and a minimum of 5 days was estimated for the duration of the GC. In the September/2013 experiment the temperature ranged between 17 and 32°C and one female was recaptured four days after its release. This female laid eggs on the sixth day, suggesting a minimum GC of 6 days.
Discussion
Knowledge of vector population parameters is fundamental to the understanding of the transmission dynamics of vector-borne diseases such as VL [42]. Although sand fly population survival rates influence other characteristics of vector capacity, such as population size and infective life time [23, 25], few studies have been undertaken under natural conditions to estimate this parameter [26, 43]. For neotropical sandflies some information has been obtained for vectors of cutaneous leishmaniasis by MRR [26,32], therefore this method has been used to estimate population dispersal in VL vectors [43]. In the present study we estimate using MRR the ecological parameters of one of the sibling species of Lu. longipalpis that occur in Sao Paulo state (S-9 methyl germacrene). We observed that recapture rates of females ranged from 2.2% to 12.8%, and for males from 7.7% to 20.7%. These results are similar to those described for other populations of Lu. longipalpis s.l in VL endemic areas in Colombia [44] and Brazil [45]. Lower recapture rates have been reported [33] in Campo Grande-MS (Brazil), however this result may have been affected by the long interval of the recapture attempts.
Regarding population dispersal, in the five experiments the majority of the specimens were recaptured at distances of less than 100 meters from the release point, contrasting with the observations made in Colombia that described high recapture ratios at distances of between 100 and 300 m and dispersion of almost 500 meters [44]. This difference could be explained by the distinct environmental characteristics of the areas studied, since in the Colombian rural areas researched, the blood sources were more widely dispersed and there was denser vegetation cover with more potential resting sites for sandflies. Corroborating our results, the limited dispersion of Lu. longipalpis populations has been described in other endemic VL areas in Brazil [31,43], as well as that for other sand fly species [30,31,32,46, 47].
The high male recapture rates and limited dispersion could be related to the characteristic aggregation (leks) of Lu. longipalpis s.l. [45] and the presence in the peridomicile of the basic resources (organic matter, humidity, protection from sunlight) favoring the development of immature forms, as well as of resting sites and blood feeding sources for adults, such as have been suggested for other sand fly species [30,31,32,46]. Thus, our results could suggest that environmental characteristic such as topography, vegetation cover and urbanization could also determine the natural population dispersal, since studies have demonstrated that this species can travel distances of up to 960 meters in two days [44]. Further to the dispersal observations, in the first experiment when the specimens marked were released at 60 meters from the original capture point, it was observed that they returned to the animal shelters where they had originally been captured. This observation suggests that the insects are able to memorize their area of activity and that there is an olfactory memory or loyalty mediated by the kairomones emitted by the host, corroborating previous observations for this species [49]. This behavior does not seem to be exclusive to this sand fly since similar observations have been made for Ny. neivai [47] and Ny. whitmani [48].
On the other hand, we estimate a daily survival rate ranging between 0.79 and 0.89 for females and 0.69 and 0.79 for males. These estimates could be understated due to uncontrolled factors such as emigration, deaths and recapture success [25,26]. However, these results constitute the first published data for populations of Lu. longipalpis under natural conditions. Our results suggest a high daily survival of the males and females of this species as compared to those described for Ny. neivai (0.64) and Ny. intermedia (0.57), vectors of tegumentary leishmaniasis in Brazil [26,32]. The survival estimates for the female population is difficult due to their low density in urban areas, as has been observed in previous studies [44]. The low recapture rate for females observed in the present study could be related to the fact that the females were engorged when released. This fact would influence their recapture rate since they could stay in their natural shelters until oviposition, making their capture more difficult.
In the first experiment a male was captured on the 13th day after release and a female on the 11th day, these periods being respectively the maximum recapture times observed. These values are in accordance with the recapture time described for Lu. longipalpis of 8 days [44]. However, dispersal studies have shown that males of this species can be recaptured even more than a month after release [33].
The estimated population size presents the lowest value in September/2013, with 2,883 males and 667 females. The maximum values were obtained in February/2013 with an estimated 9,234 males and 2,497 females. The greater male population seems to be related to their high level of activity of males in the animal shelters and also to the aggregation behavior (lek) observed for this species [45]. The estimated population size shows the high frequencies of Lu. longipalpis in this municipality in the rainy season as observed in a study with monthly captures in this focus [18] where females carrying eggs were also captured in the intradomicile. This information suggests that in this period the probability of contact between competent vector and infected and susceptible host increased. Therefore, the high survival of females together with the population size observed in the present study support the hypothesis that in the rainy season the risk of transmission of Leishmania infantum to the human and canine population in this locality increases.
The estimates of the duration of the gonotrophic cycle should be interpreted with care in view of the low frequencies of females captured and the low recapture rate. Although an alternative manner to evaluate this parameter under natural conditions would be to release engorged females reared in the laboratory as described by Morrison et al. [44], this practice is impossible in urban areas because of the ethical implications. An alternative method is the capture of naturally engorged females in animal shelters or the offer of a blood meal by the exposure of a host. Using this methodology, Dye et al. [45] released engorged females of Lu. longipalpis s.l and recaptured some of them without blood or eggs three days later. That suggests this period as being the minimum GC for this species in an Amazonian area. In the present study a minimum time ranging between five and six days for the GC was observed, a variation affected mainly by the temperature variation during the experiments (Table 1), however, other variables such as the physiological age must be considered. Under laboratory conditions a minimum GC duration of four days, with a median of five days, has been observed [39]. For Phlebotomus papatasi, under natural conditions, females take a blood meal every six days [50] and for the neotropical species Ny. neivai, Casanova et al [31] estimated a GC of four days.
Epidemiological importance of the findings
Our observations demonstrated the long daily survival of one population of the Lu. longipalpis complex under field conditions, for the first time. Considering a daily survival of 0.79 for Lu. longipalpis (Table 3) and a minimum extrinsic incubation period of 7 days for Le. infantum [51], our data suggest that after a first infective blood meal on a competent reservoir, at least 19% of the potentially infective females will be able to take a second blood meal. According to our estimates of the size of the female population, it is to be expected that in a population of 4,857 females (Table 3), 923 females will still be alive on the fifth day. Thus, in this case, the infective population of the vector will depend on some other ecological parameter such as the biting rate or the prevalence and movement of a competent reservoir [19,20].
In view of the high vector survival rate observed, the use of control measures aiming to impact the survival of the vector population (e.g. the use of residual insecticides and canine host protection measures such as insecticide impregnated collars or topical products) could impact Le. infantum transmission in endemic areas. This reduction in the infective life of the vector population and the decrease in the host biting rate have been demonstrated both in field studies and mathematical models to affect the transmission dynamic [27,52].
We also have presented evidence that supports the hypothesis of loyalty behavior by the recapture of specimens in the animal shelters of previous nocturnal activity and the low population dispersal associated with conditions favorable to its development. This information supports the idea that measures such as environmental management in the peridomiciliary environment could impact the vector density as has been demonstrated in some endemic areas [53,54]. Because it seems that in these peridomiciles the sand fly population find all the elements necessary for their development (breeding sites, natural shelters and blood feeding sources) [12]; therefore, with the modification of these conditions the pathogen’s transmission can be reduced.
With the current expansion of VL to new areas in Brazil, estimates of vector population parameters are critical for the assessment of the vector capacity of the populations of this cryptic species, information necessary for the development of models to evaluate prevention and control measures as well as to increase our knowledge of VL dynamics [5].
Conclusion
In conclusion, the S-9 methyl population of Lu. longipalpis has a long daily survival rate that can influence other parameters of its vectorial capacity such as infective life expectancy and the host biting rate. Its low dispersal seems to be associated with loyalty behavior and the presence of all the necessary conditions for the establishment and maintenance of the vector population. Our findings related to the population’s daily survival rate provide the first published information on Neotropical species involved in the transmission of the agent of VL in the Americas. Some prevention and control measures aiming at the decrease of vector density, such as environmental management and reduction of the vector survival rate by the use of insecticide impregnated collars or topical products on the canine population are strongly to be recommended. Our observation is of great value in the evaluation of control measures and in the development of mathematical models of VL dynamics.
Acknowledgments
We would like to thank the owners of the domiciles where the field experiments were undertaken and the team of the epidemiological surveillance of Panorama municipality by its assistance in the captures.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by the São Paulo Research Foundation - Brazil (FAPESP, to EABG 212/03751-4 and Fellowship to FG-O 2011/23541-1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Molyneux DH, Savioli L, Engels D. Neglected tropical diseases: progress towards addressing the chronic pandemic. Lancet. 2017;389(10066):312–25. doi: 10.1016/S0140-6736(16)30171-4 [DOI] [PubMed] [Google Scholar]
- 2.Kamhawi S. The yin and yang of leishmaniasis control. PLoS Negl Trop Dis. 2017; 20;11(4):e0005529 doi: 10.1371/journal.pntd.0005529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Killick-Kendrick R. The biology and control of phlebotomine sand flies. Clin Dermatol. 1999;17:279–89. doi: 10.1016/S0738-081X(99)00046-2 [DOI] [PubMed] [Google Scholar]
- 4.Ready PD. Biology of phlebotomine sand flies as vectors of disease agents. 2013. Annu Rev Entomol. 2013;58:227–50. doi: 10.1146/annurev-ento-120811-153557 [DOI] [PubMed] [Google Scholar]
- 5.Cameron MM, Acosta-Serrano A, Bern C, Boelaert M, Den Boer M, Burza S, et al. Understanding the transmission dynamics of Leishmania donovani to provide robust evidence for interventions to eliminate visceral leishmaniasis in Bihar, India. Parasit Vectors. BioMed Central Ltd; 2016; 27:9 doi: 10.1186/s13071-016-1309-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero GA, Boelaert M. Control of visceral leishmaniasis in Latin America- a systematic review. PLoS Negl Trop Dis. 2010;19;4(1):e584. doi: 10.1371/journal.pntd.0000584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrio A, Parodi CM, Locatelli F, Mora MC, Basombrío MA, Korenaga M, et al. Leishmania infantum and human visceral leishmaniasis, Argentina. Emerg Infect Dis. 2012;18(2):354–355. doi: 10.3291/eid1802.110924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satragno D, Faral-Tello P, Canneva B, Verger L, Lozano A, Vitale E, et al. Autochthonous outbreak and expansion of canine visceralleishmaniasis, Uruguay. Emerg Infect Dis. 2017;23(3):536–538. doi: 10.3201/eid2303.160377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Travi BL, Montoya J, Gallego J, Jaramillo C, Llano R, Velez ID. Bionomics of Lutzomyia evansi (Diptera: Psychodidae) vector of visceral leishmaniasis in northern Columbia. J Med Entomol. 1996;33(3):278–85. doi: 10.1093/jmedent/33.3.278 [DOI] [PubMed] [Google Scholar]
- 10.Salomón OD, Quintana MG, Bezzi G, Morán ML, Betbeder E, Valdéz DV. Lutzomyia migonei as putative vector of visceral leishmaniasis in La Banda, Argentina. Acta Trop. 2010;113(1):84–7. doi: 10.1016/j.actatropica.2009.08.024 [DOI] [PubMed] [Google Scholar]
- 11.Guimarães VC, Pruzinova K, Sadlova J, Volfova V, Myskova J, Filho SP, Volf P. Lutzomyia migonei is a permissive vector competent for Leishmania infantum. Parasit Vectors. 2016; 17;9:159 doi: 10.1186/s13071-016-1444-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galvis-Ovallos F, da Silva MD, Bispo GB, de Oliveira AG, Neto JR, Malafronte RD, Galati EA. Canine visceral leishmaniasis in the metropolitan area of São Paulo: Pintomyia fischeri as potential vector of Leishmania infantum. Parasite. 2017;24:2 doi: 10.1051/parasite/2017002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deane MP, Deane LM. Infecção natural do Phlebotomus longipalpis por leptomonas, provavelmente de Leishmania donovani, em foco de calazar, no Ceará. Hospital.1954; 45: 697–702 [PubMed] [Google Scholar]
- 14.Salomón OD, Feliciangeli MD, Quintana MG, Afonso MM, Rangel EF. Lutzomyia longipalpis urbanisation and control. Memórias do Instituto Oswaldo Cruz. 2015;110:831–46. doi: 10.1590/0074-02760150207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lainson R, Rangel EF. Lutzomyia longipalpis and the eco-epidemiology of American visceral leishmaniasis, with particular reference to Brazil: a review. Memórias do Instituto Oswaldo Cruz. 2005;100:811–27. [DOI] [PubMed] [Google Scholar]
- 16.Brasil M da S do. Casos confirmados de leishmaniose visceral, Brasil, Grandes Regiões e Unidades Federadas. 1990 a 2015. In: Secretaria de vigilancia em Saúde/ Ministério da Saúde. [Internet]. 2015. http://portalarquivos.saude.gov.br/images/pdf/2017/marco/03/LV-Casos.pdf
- 17.Casanova C, Colla-Jacques FE, Hamilton JG, Brazil RP, Shaw JJ. Distribution of Lutzomyia longipalpis chemotype populations in São Paulo state, Brazil. PLoS Negl Trop Dis. 2015;9(3):e0003620 doi: 10.1371/journal.pntd.0003620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galvis-Ovallos F, Casanova C, Sevá ADP, Galati EAB. Ecological parameters of the (S)-9-methylgermacrene-B population of the Lutzomyia longipalpis complex in a visceral leishmaniasis area in São Paulo state, Brazil. Parasit Vectors. 2017; 30;10(1):269 doi: 10.1186/s13071-017-2211-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reisen WK. Estimation of vectorial capacity: introduction. Bull Soc Vector Ecol. 1989;14:39–40. [Google Scholar]
- 20.Reisen WK. Landscape epidemiology of vector-borne diseases. Annu Rev Entomol. 2010;55:461–83. doi: 10.1146/annurev-ento-112408-085419 [DOI] [PubMed] [Google Scholar]
- 21.Killick-Kendrick R Phlebotomine vectors of the leishmaniases: a review. Med Vet Entomol. 1990;4(1):1–24. doi: 10.1111/j.1365-2915.1990.tb00255.x [DOI] [PubMed] [Google Scholar]
- 22.Killick-Kendrick R. The biology and control of phlebotomine sand flies. Clin Dermatol. 1999;17(3):279–89. doi: 10.1016/S0738-081X(99)00046-2 [DOI] [PubMed] [Google Scholar]
- 23.Garrett-Jones C, Shidrawi GR. Malaria vectorial capacity of a population of Anopheles gambiae An exercise in epidemiological entomology. Bull World Health Organ. 1969; 40:531–545. [PMC free article] [PubMed] [Google Scholar]
- 24.Freier JE. Estimation of vectorial capacity: Vector abundance in relation to man. Bull Soc Vect Ecol 1989; 14:41–46. [Google Scholar]
- 25.Milby MM, Reisen WK. Estimation of vectorial capacity: vector survivorship. Bull Soc Vector Ecol. 1989;14:47–54. [Google Scholar]
- 26.Casanova C, Natal D, Santos FAM. Survival, population size and gonotrophic cycle duration of Nyssomyia neivai (Diptera: Psychodidae) at an endemic area of American cutaneous leishmaniasis in southern Brazil. J Med Entomol. 2009;46: 42–50. doi: 10.1603/033.046.0106 [DOI] [PubMed] [Google Scholar]
- 27.Sevá AP, Ovallos FG, Amaku M, Carrillo E, Moreno J, Galati EA, et al. Canine-based strategies for prevention and control of visceral leishmaniasis in Brazil. PLoS One. 2016;8;11(9):e0162854 doi: 10.1371/journal.pone.0162854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao S, Kuang Y, Wu CH, Ben-Arieh D, Ramalho-Ortigao M, Bi K. Zoonotic visceral leishmaniasis transmission: modeling, backward bifurcation, and optimal control. J Math Biol;73(6–7):1525–1560. doi: 10.1007/s00285-016-0999-z [DOI] [PubMed] [Google Scholar]
- 29.Shimozako HJ, Wu J, Massad E. Mathematical modelling for zoonotic visceral leishmaniasis dynamics: A new analysis considering updated parameters and notified human Brazilian data. Infectious Disease Modelling. 2017, 2(2): 143–160 doi: 10.1016/j.idm.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexander JB, Young DG. Dispersal of phlebotomine sand flies (Diptera: Psychodidae) in a Colombian focus of Leishmania (Viannia) braziliensis. Memórias do Instituto Oswaldo Cruz. 1992;87: 397–403. [DOI] [PubMed] [Google Scholar]
- 31.Casanova C, Costa AI, Natal D. Dispersal pattern of the sand fly Lutzomyia neivai (Diptera: Psychodidae) in a cutaneous leishmaniasis endemic rural area in Southeastern Brazil. Memórias do Instituto Oswaldo Cruz. 2006;100:719–724. [DOI] [PubMed] [Google Scholar]
- 32.Galati EA, Fonseca MB, Marassá AM, Bueno EF. Dispersal and survival of Nyssomyia intermedia and Nyssomyia neivai (Diptera: Psychodidae: Phlebotominae) in a cutaneous leishmaniasis endemic area of the speleological province of the Ribeira Valley, state of São Paulo, Brazil. Memórias do Instituto Oswaldo Cruz. 2009;104:1148–1158. [DOI] [PubMed] [Google Scholar]
- 33.Oliveira EF, Silva EA, Casaril AE, Fernandes CE, Paranhos Filho AC, Gamarra RM et al. Behavioral aspects of Lutzomyia longipalpis (Diptera: Psychodidae) in urban area endemic for visceral leishmaniasis. J Med Entomol. 2013;50:277–84. [DOI] [PubMed] [Google Scholar]
- 34.Uribe S. The status of the Lutzomyia longipalpis species complex and possible implications for Leishmania transmission. Memórias do Instituto Oswaldo Cruz. 1999;94:729–734. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton JGC, Maingon RDC, Alexander B, Ward RD, Brazil RP. Analysis of the sex pheromone extract of individual male Lutzomyia longipalpis sandflies from six regions in Brazil. Med Vet Entomol. 2005;19:480–48 doi: 10.1111/j.1365-2915.2005.00594.x [DOI] [PubMed] [Google Scholar]
- 36.Peel MC, Finlayson BL, McMahon TA. Updated world map of the Köppen-Geiger climate classification. Hydrol Earth Syst Sci. 2007;11:1633–1644. doi: 10.5194/hess-11-1633-2007 [Google Scholar]
- 37.Alexander B. Sampling methods for phlebotomine sandflies. Med Vet Entomol 2000;14: 109–122. doi: 10.1046/j.1365-2915.2000.00237.x [DOI] [PubMed] [Google Scholar]
- 38.Galati EAB. Apostila de Bioecologia e Identificação de Phlebotominae (Diptera, Psychodidae) Departamento de Epidemiologia, Faculdade de Saude Publica da USP, Sao Paulo, Brasil. 2013. available from: http://www.fsp.usp.br/~egalati/
- 39.Galvis-Ovallos F. Leishmaniose visceral americana: avaliação dos parâmetros da capacidade vetorial de Lutzomyia longipalpis no município de Panorama, estado de São Paulo, Brasil. [Thesis]. Faculdade de Saúde Pública (FSP). Universidade de São Paulo;2016. Avaliable from: http://www.teses.usp.br/teses/disponiveis/6/6132/tde-17032016-133512/en.php
- 40.Nelson RL, Tempelis CH, Reeves WC, Fine PEM. Estimates of survival, population size, and emergence of Culex tarsalis at an isolated site. Ann Entomol Soc Am. 1978;71:801–808. doi: 10.1093/aesa/71.5.801 [Google Scholar]
- 41.Bailey NT. Improvements in the interpretation of recapture data. J Anim Ecol. 1952; 21: 120–127. doi: 10.2307/1913 [Google Scholar]
- 42.Reisen WK, Lothrop HD. Population ecology and dispersal of Culex tarsalis (Diptera: Culicidae) in the Coachella Valley of California. J Med Entomol. 1995;32:490–502 [DOI] [PubMed] [Google Scholar]
- 43.Rock KS, Quinnell RJ, Medley GF, Courtenay O. Progress in the mathematical modelling of visceral leishmaniasis. Adv Parasitol. 2016;94:49–131. doi: 10.1016/bs.apar.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 44.Morrison AC, Ferro C, Morales A, Tesh RB, Wilson ML. Dispersal of the sand fly Lutzomyia longipalpis (Diptera: Psychodidae) at an endemic focus of visceral leishmaniasis in Colombia. J Med Entomol. 1993;30:427–435. [DOI] [PubMed] [Google Scholar]
- 45.Dye C, Davies CR, Lainson R. Communication among phlebotomine sandflies: a field study of domesticated Lutzomyia longipalpis populations in Amazonian Brazil. Anim Behav. 1991;42:183–192. [Google Scholar]
- 46.Chaniotis BN, Correa MA, Tesh RD, Johnson KM. Horizontal and vertical movements of phlebotomine sand flies in a Panamanian rain forest. J Med Entomol. 1974;11:363–375 [DOI] [PubMed] [Google Scholar]
- 47.Freitas JS, Reinhold-Castro KR, Casanova C, Silva JP, Previdelli I, Teodoro U. Spatial and/or olfactory memory in sandflies in an endemic area for American cutaneous leishmaniasis, southern Brazil. Rev Soc Bras Med Trop.2009; 42:151–155. [DOI] [PubMed] [Google Scholar]
- 48.Campbell-Lendrum DH, Brandão-Filho SP, Ready PD, Davies CR. Host and/or site loyalty of Lutzomyia whitmani (Diptera: Psychodidae) in Brazil. Med and Vet Entomol. 1999;13:209–211. [DOI] [PubMed] [Google Scholar]
- 49.Kelly DW, Dye C. Pheromones, kairomones and aggregation dynamics of the sandfly Lutzomyia longipalpis. Anim Behav. 1997;53:721–731. [Google Scholar]
- 50.Killick-Kendric R, Rioux JA. Mark-release-recapture of sand flies fed on leishmanial dogs: the natural life-cycle of Leishmania infantum in Phlebotomus ariasi. Parassitologia. 2002;44: 67–71. [PubMed] [Google Scholar]
- 51.Lainson R, Ward RD, Shaw JJ. Experimental transmission of Leishmania chagasi, causative agent of neotropical visceral leishmaniasis, by the sandfly Lutzomyia longipalpis. Nature. 1977;266:628–630. [DOI] [PubMed] [Google Scholar]
- 52.Reithinger R, Coleman PG, Alexander B, Vieira EP, Assis G, Davies CR. Are insecticide-impregnated dog collars a feasible alternative to dog culling as a strategy for controlling canine visceral leishmaniasis in Brazil?. Int J Parasitol. 2004;34(1):55–62. doi: 10.1016/j.ijpara.2003.09.006 [DOI] [PubMed] [Google Scholar]
- 53.Lara-Silva FO, Michalsky ÉM, Fortes-Dias CL, Fiuza VOP, Dias ES. Evaluation of chemical spraying and environmental management efficacy in areas with minor previous application of integrated control actions for visceral leishmaniasis in Brazil. Acta Trop. 2017;176:109–113. doi: 10.1016/j.actatropica.2017.07.029 [DOI] [PubMed] [Google Scholar]
- 54.Teodoro U, Thomaz-Soccol V, Kühl JB, Santos DR, Santos ES, Santos AR, et al. Reorganization and cleanness of peridomiciliar area to control sand flies (Diptera, Psychodidae, Phlebotominae) in South Brazil. Braz Arch Biol Technol. 2004;47:205–212. doi: 10.1590/S1516-89132004000200007 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.