Abstract
Enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR), widely used for the detection of plant viruses, are not easily performed, resulting in a demand for an innovative and more efficient diagnostic method. This paper summarizes the characteristics and research trends of biosensors focusing on the physicochemical properties of both interface elements and bioconjugates. In particular, the topological and photophysical properties of quantum dots (QDs) are discussed, along with QD-based biosensors and their practical applications. The QD-based Fluorescence Resonance Energy Transfer (FRET) genosensor, most widely used in the biomolecule detection fields, and QD-based nanosensor for Rev-RRE interaction assay are presented as examples. In recent years, QD-based biosensors have emerged as a new class of sensor and are expected to open opportunities in plant virus detection, but as yet there have been very few practical applications (Table 3). In this article, the details of those cases and their significance for the future of plant virus detection will be discussed.
Keywords: detection of plant virus, interface particle, quantum dot-based biosensors
The importance of diagnosis in the prevention of plant virus disease cannot be overemphasized. As the number of new plant viruses emerging increases due to climate change and expansion of trade in agricultural products, prevention of plant viruses by diagnosis is thought to be of critical importance. Since enzyme-linked immunosorbent assay (ELISA) or polymerase chain reaction (PCR), which are widely used for the detection of plant viruses, utilize specific antibodies or genes derived from the target virus, only the specific virus can be identified by these tests. This results in an inability to detect viruses that are beyond this range or which are newly emerging. In addition, the current ELISA and PCR diagnosis are expensive, time-consuming and require complex instruments and reagents. For these reasons, it is difficult to apply them on-site other than in specialized diagnostic institutions, so the development of a new diagnostic method with fewer limitations has been explored in various ways (Lopez et al., 2009). The recently developed loop mediated isothermal amplification (LAMP) is a technique that compensates for the disadvantages of the existing PCR-based method and is known to be able to rapidly amplify target genes with high specificity and sensitivity (Dhama et al., 2014; Notomi et al., 2000; Sakurai and Shibasaki, 2012).
On the other hand, fluorescent proteins, due to the advantages of easy observation and non-toxicity, are used in various fields such as membrane trafficking, detection of plant viruses, and quantitative analysis of gene expression (Berg and Beachy, 2008; Chalfie et al., 1994; Gerdes and Kaether, 1996; Misteli and Spector, 1997; Zimmer, 2002). However, since the fluorescent protein is an organic fluorophore with a narrow excitation wavelength range, there is a limitation in the light source used and there is a disadvantage that many fluorescent probes cannot be used at the same time because of their wide emission spectra. These also exhibit the following significant weaknesses; they are affected by various chemical metabolites in vivo, cause interference with their own fluorescent substances in plant cells and photobleaching reduces signal intensity rapidly in a short time (Berg and Beachy, 2008; Chudakov et al., 2010).
Nanotechnology, heralded as one of the core technologies of the future in the 2000s, exerts omnidirectional influence across all industries, and the biosensor field is no exception. The development of various biosensors using nanoparticles can be said to be typical examples of this effect. In particular, the semiconductor nanoparticles such as quantum dots (QDs) have excellent optical properties. QDs are size-tunable and relatively resistant to photobleaching seen in conventional organic dyes as well as having the potent intensity of emitted light, bringing about an innovation in optical imaging fields including biosensors (Algar et al., 2010; Martín-Palma et al., 2009; Wegner and Hildebrandt, 2015).
Although quantum dot-based biosensors (QD-based biosensors) have been applied in the life sciences for about five years for the detection of human pathogenic viruses, fungi, and harmful foodborne bacteria (Chan et al., 2002; Shamsipur et al., 2017; Sharma et al., 2015), they have not been applied to the field of plant virus detection until recently, with only a few cases in the published literature (Khater et al., 2017). It is therefore the aim of this paper to examine the current state of QD-based biosensors for the detection of plant viruses and to make predictions concerning their use in the future.
What are Biosensors?
The term biosensor refers to a device designed to measure the presence and amount of a specific biological substance by combining a mechanism that converts the physicochemical changes appearing in response to the analytes present in the sample with a receptor that can manifest this as a recognizable signal (Scheller et al., 2001). Due to their ability to selectively and rapidly detect only the substances to be analyzed, biosensors are actively used in many industrial fields such as medical diagnosis, development of new drugs, plant pathology, food safety testing, and environmental monitoring (van Dorst et al., 2010; Mello and Kubota, 2002; Rodriguez-Mozaz et al., 2006). Furthermore the development of key supporting elements such as various interfaces and nanotechnology have extended the application range of biosensors to point-of-care tests (POCT) and the Internet-of-things (IoT) (Ohashia and Osaka, 2017; Soper et al., 2006).
Biosensors can be classified according to the type of interface element, the labeling substance and the signal converter (Monošík et al., 2012). Typical examples include surface plasmon resonance (SPR) generated on metal surfaces, quartz crystal microbalance (QCM) due to the mass change of biomolecules, the refractive index change due to the bending phenomenon of the cantilever, and the fluorescence change due to fluorescence resonance energy transfer (FRET) (Baller et al., 2000; Batchelor-McAuley et al., 2009; Kairdolf et al., 2013; Yuqing et al., 2003). The advantages, disadvantages, and research trends of these representative biosensors are summarized in Table 1.
Table 1.
Characteristics and research trends of biosensors
| Technology | Advantages | Disadvantages | Development trends | Remarks |
|---|---|---|---|---|
| SPR Biosensor | Real-time analysis possible | Better sensitivity required | Currently prevailing | Gold nanoparticles and AFM* applied research in progress |
| QCM Biosensor | Real-time analysis possible | Better sensitivity required | Replaced by SPR Biosensor | - |
| Electrochemical biosensor | Real-time analysis possible | Trouble of reproducibility and measurement errors | Just beginning | In progress at the research level |
| Fluorescent biosensor | Good sensitivity and simplicity | Fluorescent labeling required and dynamic analysis impossible | Already commercialized | Ongoing as a method for detecting major biomolecules |
Atomic force microscope.
Fluorescent biosensors have excellent sensitivity and simplicity and do not require expensive equipment, but interference due to biomaterials and short fluorescence persistence have been pointed out as disadvantages (Turner, 2013). However, recently they are facing a new turning point as they are combined with nanomaterials such as quantum dots or graphene quantum dots (GQDs) (Algar et al., 2010; Kairdolf et al., 2013; Shen et al., 2012).
Quantum Dots and QD-Based Biosensors
Quantum dots (QDs) are semiconductor nanocrystals with unique photophysical properties and are comprised of elements from the periodic groups II–VI, III–V or IV–VI. As nanostructured materials, QDs are also known as zero-dimensional materials. Cd-chalcogenide nanocrystals, one of the most typical QDs, consist of a core composed of a centrosome of about 2–10 nm and a shell composed of ZnS. When coated with a polymer on the outer surface of the shell, they usually have a size of 10–15 nm. CdSe, CdTe, and CdS are mainly used as the centrosome of quantum dots (Algar et al., 2010).
QDs have unique photophysical properties that other materials do not have. Compared to traditional fluorescent probes such as organic dyes and fluorescent proteins, QDs have special optical and electronic properties such as narrow and symmetric emission spectra, broad absorption spectra that enable the simultaneous excitation of multiple fluorescent colors and size-tunable light emissions. QDs are also remarkably brighter and more resistant to photobleaching than other materials (Jamieson et al., 2007; Kairdolf et al., 2013; Kuang et al., 2011; Wegner and Hildebrandt, 2015).
A biosensor using QDs as an interface element is called a quantum dot-based biosensor. QD-based biosensors have been named according to the type of molecular beacon conjugated on QDs and transduction signals; QD-based FRET genosensor, QD-based FRET immunosensor, and QD-based Bioluminescence Resonance Energy Transfer (BRET) immunosensor, etc. (Ishikawa-Ankerhold et al., 2012; Resch-Genger et al., 2008; Vinayaka and Thakur, 2011). The schematic principle of QD-based FRET genosensor, widely used in biological applications, is shown in Fig. 1.
Fig. 1.
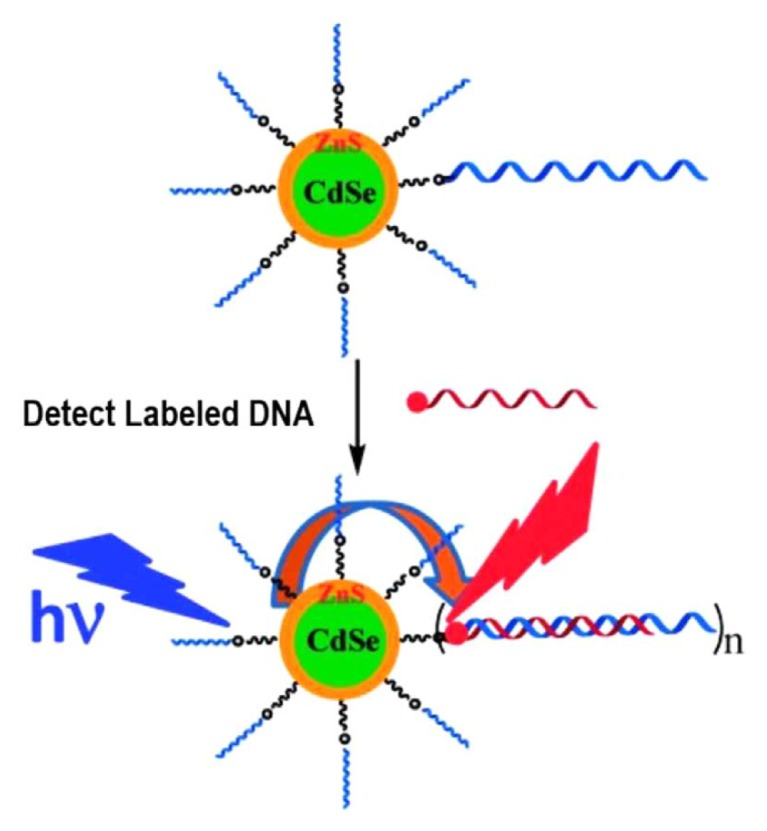
Schematic QD-based FRET genosensor. Hybridization of a complementary dye-labelled DNA probe with the QD-DNA conjugate leads to QD sensitized dye FRET signals as a readout for labelled DNA detection (Reproduced by courtesy of Zhang et al., 2013a).
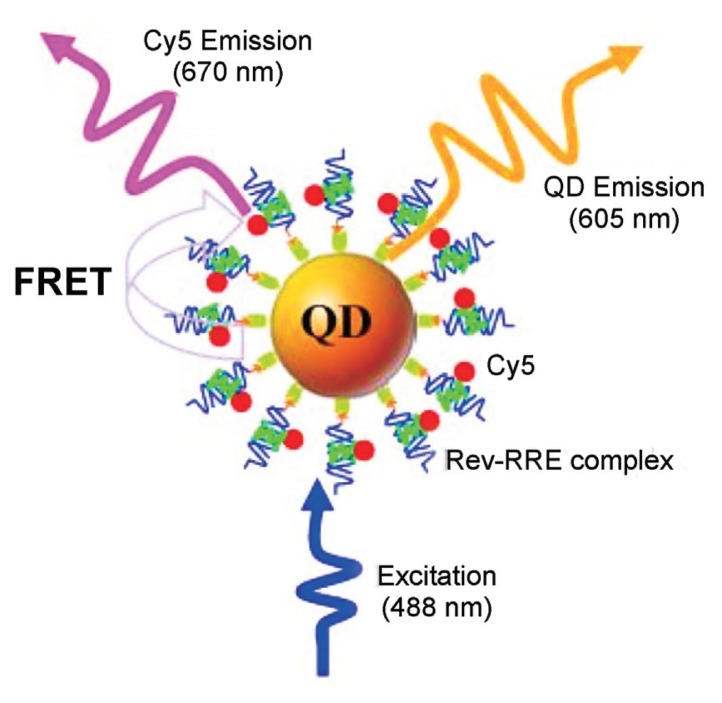
Zhang and Johnson (2006) reported a QD-based nanosensor for Rev-RRE interaction assay based on FRET between QD605 and Cy5 (Fig. 2). Rev is an important HIV-1 regulatory protein that binds the Rev responsive element (RRE) within the env gene of HIV-1 RNA genome; the binding of Rev to RRE is essential for HIV replication. The nucleotide sequence, 5′-GGUCUGGGCGCAGCGCAAGCUGACGGUACAGGCC, where the sequence identified as important for Rev binding shown in italics and Cy5-labelled peptide (TRQARRNRRRRWRERQR) were used for biotinylation and the probe, respectively. The binding of a Cy5-labeled Rev to a biotinylated RRE IIB RNA formed a Rev-RRE complex, which was caught on the surface of a QD605 to form a QD605/RRE-Rev/Cy5 assembly through specific streptavidin-biotin binding. FRET occurred between the QD605 and Cy5 upon illumination of the QD605/RRE-Rev/Cy5 assemblies with an excitation wavelength of 488 nm.
Fig. 2.
QD-based FRET genosensor for Rev-RRE interaction assay based on FRET (Reproduced by courtesy of Zhang and Johnson, 2006).
Recently, QD-based sensors have been emerging as a new class of sensor, because they can be both labeled and label-free. They are being applied in various fields for monitoring of environmental pollution indicators as well as detecting of human pathogenic viruses and harmful foodborne bacteria (Adegoke et al., 2016; Vinayaka and Thakur, 2010).
Detection of Plant Viruses Using Biosensors
Biosensors for the detection of plant viruses are classified into interface elements and bioconjugates. QCM, SPR, and FRET phenomena are used as physicochemical properties of the interface element (Khater et al., 2017). The specific nucleotides, antibody, or monoclonal antibody derived from viruses, and their aptamer or motif are used for bioconjugation (Candresse et al., 2007; Lautner et al., 2010). Since Eun et al. (2002) reported on a QCM-based genosensor for the detection of cymbidium mosaic potexvirus (CymMV) and odontoglossum ringspot tobamovirus (ORSV), a number of biosensors for the detection of plant viruses combining various bioconjugates with different interface elements have been developed (Florschütz et al., 2013; Sun et al., 2008).
The SPR biosensors have been extensively studied as biosensors for the detection of plant viruses; the potato virus Y (PVY) (Gutierrez-Aguirre et al., 2014), apple stem pitting virus (ASPV) (Lautner et al., 2010), maize chlorotic mottle virus (MCMV) (Zeng et al., 2013), barley stripe mosaic virus (BSMV) (Florschütz et al., 2013), and cowpea mosaic virus (CPMV) (Dubs et al., 1992). The details of SPR-based biosensors for detection of plant viruses are summarized in Table 2.
Table 2.
Detection of plant viruses by SPR-based biosensors
| Virus | Conjugate | LOD* | Reference |
|---|---|---|---|
| PVY | Monoclonal antibody | 0.31 mg/ml† | Gutierrez-Aguirre et al., 2014 |
| MCMV | Anti-MCMV antibody | 1 ng/ml | Zeng et al., 2013 |
| BSMV | Specific oligonucleotide from RNA-α | 14.7 ng/ml | Florschütz et al., 2013 |
| ASPV | DNA aptamer derived from coat protein | 500 mg/ml of total protein | Lautner et al., 2010 |
| CPMV | Monoclonal antibody | 16 mg/ml | Dubs et al., 1992 |
Limit of detection,
1 order of magnitude less sensitive than ELISA.
The biosensors’ reaction times for detecting plant viruses are mostly within a few minutes, which is significantly faster than either ELISA or PCR. However, detection limits vary significantly depending on the interface particle, bioconjugate and target virus, ranging from several tens of pg/ml to several tens of mg/ml. In fact, Eun et al. (2002) reported the detection limits of QCM-DNA sensor of two plant viruses of CymMV and ORSV as 1 ng/ml and 10 ng/ml for RNA and vegetable sap, respectively. Meanwhile Dickert et al. (2004) reported the detection limit of QCM biosensor labeled with a TMV antibody as 100 ng/ml to 1 mg/ml. Malecka et al. (2014) reported that the detection limit of PPV was 12.8 pg/ml, using an ion-channel genosensor labeled with Plum Pox Virus (PPV)-derived 22-mer and 42-mer complementary ssDNA. Lin et al. (2014) reported that the detection time of a fiber optic particle plasmon resonance (FOPPR) immunosensor was within 10 minutes, and the detection limits of CymMV and ORSV were 48 pg/ml and 42 pg/ml, respectively, which is tens of times higher than the 1.2 ng/ml of ELISA. In addition, McClellan et al. (2012) reported that the detection limit and the detection time of bean pod mottle virus (BPMV) using silicon photonic microring resonator (SPMR) were 10 ng/ml and 45 min, respectively, which is slightly higher and slightly shorter than that of ELISA. Florschütz et al. (2013) reported that the detection limit of the SPR sensor labeled with the specific oligonucleotide of BSMV RNA-α was 14.7 ng/ml in infected plant sap. On the other hand, Gutiérrez-Aguirre et al. (2014) reported that the detection limit of the SPR sensor labeled with a monoclonal antibody derived from PVY coat protein was 0.31 mg/ml, which is tens of times less sensitive than the 0.019 mg/ml of ELISA. Based on these results, it can be suggested that the interface element selection is very important.
The detection limits were also reported to vary depending on the target virus. In fact, Dubs et al. (1992) reported that the detection limit of the SPR sensor labeled with a monoclonal antibody derived from CPMV coat protein was 16 μg/ml, which is tens of times less sensitive than the PVY detection limit (0.31 mg/ml) of the SPR sensor reported by Gutiérrez-Aguirre et al (2014).
Nucleic acids tend to be preferred as the most useful tool to mediate various physicochemical phenomena of interface particles because of their specificity among bioconjugates irrespective of biosensor interface element. As shown in Table 2, this seems to result from the fact that the detection limit of the biosensor labeled with nucleic acids as a probe is up to tens of thousands times more sensitive than that of the biosensor labeled with proteins such as antibodies or monoclonal antibodies.
Meanwhile, many investigations have revealed that the detection limit of the biosensors for plant viruses is more sensitive as the size of bioconjugate is smaller. This can be seen from the fact that the detection limit of the sensor labeled with a specific sequence of the gene or its aptamer is generally more sensitive than that of the sensor labeled with the antibody derived from the viral coat protein (Florschütz et al., 2013; Zeng et al., 2013). However, these are comparisons between different viral systems, so more research using the specific gene and antibody derived from the same virus would be needed for the precise measurement of the sensitivity between them.
Recently, quantum dot-based biosensors have been extensively studied in the field of human viruses (Adegoke et al., 2016; Shamsipur et al., 2017; Shen and Gao, 2015) because not only do quantum dots not require the expensive equipments that SPR or electrochemical biosensors do, but they can also reduce the non-specificity due to their own fluorescent material in the host cell. However, their application to the field of plant virus detection has only begun recently and only a very few cases have been reported (Khater et al., 2017). Medintz et al. (2005) labeled the CdSe quantum dots with histidine and lysine, the main constituent amino acids of CPMV coat protein. Sun et al. (2008) discussed the sensor labeled with the 23-mer oligonucleotide derived from the CaMV 35S promoter on a PbS nanoparticle. Investigations into the sensor labeled with the antibody derived from CTV coat protein on CdTe QDs (Safarnejad et al., 2017; Shojaei et al., 2016a) and the sensor labeled with the GVA antibody derived from GVA coat protein on the ZnO films (Tereshchenko et al., 2017) have followed. The details of QD-based biosensors for the detection of plant viruses are summarized in Table 3.
Table 3.
Detection of plant viruses by QD-based biosensors
| Virus | Interface | Conjugate | LOD* | Reference |
|---|---|---|---|---|
| CPMV2 | CdSe-ZnS core | Surface-immobilized CPMV | - | Medintz et al., 2005 |
| CaMV | PbS nanoparticle | 23-mer derived from CaMV 35S | 4.38 × 10−12 mol/l | Sun et al., 2008 |
| CTV | InP | Antibody to CTV coat protein | 2 nM for antibody | Moreau et al., 2012 |
| CTV | CdTe | CTV-CP antibody | 220 ng/ml | Shojaei et al., 2016b |
| CTV | AuNPs/QD | AuNPs-CTV-CP/QDs-CTV-CP antibody | 130 ng/ml3 | Shojaei et al., 2016a |
| CTV | CdTe | CTV-CP antibody | 198 ng/ml | Safarnejad et al., 2017 |
| GVA | ZnO films | Grapevine virus A-type proteins | 1 pg/ml-10 ng/ml | Tereshchenko et al., 2017 |
| ToRSV, BPMV & ArMV | Fe3O4/SiO2 MNPs & SiO2/UCNPs4 | Antibody | 100 ng/ml | Zhang et al., 2013b |
Limit of detection
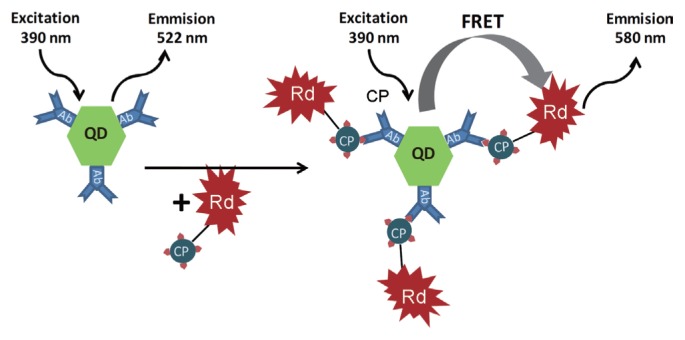
Citrus tristeza virus (CTV) was the most extensively researched. The sensor, labeled with a CTV coat protein-derived antibody on the CdTe quantum dot, is a representative example (Safarnejad et al., 2017; Shojaei et al., 2016b; Moreau et al., 2012). In fact, using antibody derived from CTV coat protein, alternative types of biosensors were reported to enhance the specificity and sensitivity of fluorescence resonance energy transfer (FRET)-based biosensor. Shojaei et al. (2016a) reported the complex sensor using the mixture of CdTe quantum dots and gold nanoparticles (AuNPs) labeled with an antibody derived from CTV coat protein. Safarnejad et al. (2017) reported on the donor-acceptor complex sensor; QDs conjugated with antibody derived from CTV coat protein and rhodamine 123 conjugated with the recombinant coat protein of the CTV were used as donor and acceptor, respectively (Fig. 3).
Fig. 3.
Schematic presentation of specific CTV nanobiosensor (Reproduced by courtesy of Safarnejad et al., 2017).
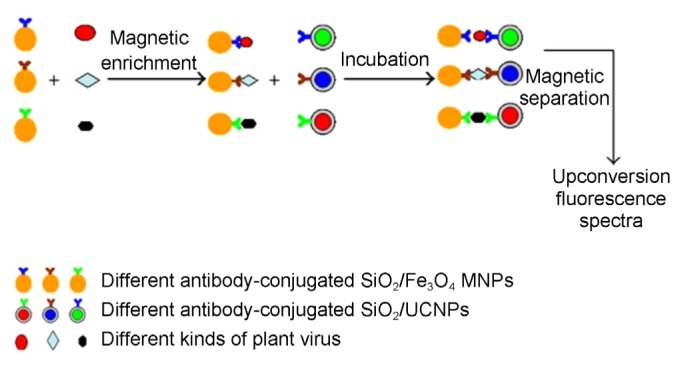
Conversely, Zhang et al. (2013b) reported that mixed infections can be detected at a limit of 100 ng/ml by reacting magnetic nanoparticles (MNPs) and upconversion nanoparticles (UCNPs) in sandwich form (Fig. 4). Fe3O4/SiO2 MNPs (~100 nm) were labeled with different antibodies derived from coat protein of ToRSV, BPMV and ArMV and employed to capture and enrich the target viruses. Then SiO2 UCNPs conjugated with specific antibodies as signal probes were added to form sandwich complexes.
Fig. 4.
Schematic presentation of immunomagnetic separation and fluorescence detection of target viruses in sandwich-type system (Reproduced by courtesy of Zhang et al., 2013b).
Concluding Remarks
This paper aims to both outline the characteristics of biosensors according to the physicochemical properties of interface elements and bioconjugates, and review the related research trends in the field. The topological and unique photophysical properties of quantum dots (QDs) and QD-based biosensors using quantum dots (QDs) as an interface element are also discussed. A QD-based nanosensor for Rev-RRE interaction assay (Zhang and Johnson, 2006) is presented as one of the practical applications.
In the field of plant virus detection, the utility of SPR biosensors for major plant viruses including TMV, PVY, MCMV and BSMV have been extensively researched. Even though there are relatively few studies of plant virus detection by QD-based biosensors compared to the active research in the human virus detection field, several cases have been reported (Table 3). It is believed that the evidence reviewed here strongly suggests that QDs have specific characteristics which make them particularly suited to the detection of plant viruses.
QDs, compared to conventional organic dyes and fluorescent proteins, have unique photophysical properties such as narrow and symmetric emission spectra, broad absorption spectra that enable the simultaneous excitation of multiple fluorescent colors, size-tunable light emission and resistance to photobleaching (Kairdolf et al., 2013; Wegner and Hildebrandt, 2015). Even though the application of QD-based biosensors to the detection of plant viruses has been limited, if consideration is given to the unique photophysical properties of the quantum dots as an interface element, it can be expected that they will contribute to a future paradigm shift in the field of plant virus detection.
Acknowledgments
This work was supported by Seokyeong University in 2016.
References
- Adegoke O, Seo M, Kato T, Kawahito S, Park EY. An ultrasensitive SiO2-encapsulated alloyed CdZnSeS quantum dot-molecular beacon nanobiosensor for norovirus. Biosens Bioelectron. 2016;86:135–142. doi: 10.1016/j.bios.2016.06.027. [DOI] [PubMed] [Google Scholar]
- Algar WR, Tavares AJ, Krull UJ. A review of the application of quantum dots as integrated components of assays, bioprobes, and biosensors utilizing optical transduction. Anal Chim Acta. 2010;673:1–25. doi: 10.1016/j.aca.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Baller MK, Lang HP, Fritz J, Gerber C, Gimzewski JK, Drechsler U, Rothuizen H, Despont M, Vettiger P, Battiston FM, Fornaro P, Meyer E, Guntherodt HJ. A cantilever array-based artificial nose. Ultramicroscopy. 2000;82:1–9. doi: 10.1016/S0304-3991(99)00123-0. [DOI] [PubMed] [Google Scholar]
- Batchelor-McAuley C, Wildgoose GG, Compton RG. The physicochemical aspects of DNA sensing using electrochemical methods. Biosens Bioelectron. 2009;24:3183–3190. doi: 10.1016/j.bios.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Berg RH, Beachy RN. Fluorescent protein applications in plants. Methods Cell Biol. 2008;85:153–177. doi: 10.1016/S0091-679X(08)85008-X. [DOI] [PubMed] [Google Scholar]
- Candresse T, Lot H, German-Retana S, Krause-Sakate R, Thomas J, Souche S, Delaunay T, Lanneau M, le Gall O. Analysis of the serological variability of lettuce mosaic virus using monoclonal antibodies and surface plasmon resonance technology. J Gen Virol. 2007;88:2605–2610. doi: 10.1099/vir.0.82980-0. [DOI] [PubMed] [Google Scholar]
- Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Chan WCW, Maxwell DJ, Gao X, Bailey RE, Han M, Nie S. Luminescent quantum dots for multiplexed biological detection and imaging. Curr Opin Biotechnol. 2002;13:40–46. doi: 10.1016/S0958-1669(02)00282-3. [DOI] [PubMed] [Google Scholar]
- Chudakov DM, Matz MV, Lukyanov S, Lukyanov KA. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol Rev. 2010;90:1103–1163. doi: 10.1152/physrev.00038.2009. [DOI] [PubMed] [Google Scholar]
- Dhama K, Karthik K, Chakraborty S, Tiwari R, Kapoor S, Kumar A, Thomas P. Loop-mediated isothermal amplification of DNA (LAMP): a new diagnostic tool lights the world of diagnosis of animal and human pathogens: a review. Pak J Biol Sci. 2014;17:151–166. doi: 10.3923/pjbs.2014.151.166. [DOI] [PubMed] [Google Scholar]
- Dickert FL, Hayden O, Bindeus R, Mann KJ, Blaas D, Waigmann E. Bioimprinted QCM sensors for virus detection-screening of plant sap. Anal Bioanal Chem. 2004;378:1929–1934. doi: 10.1007/s00216-004-2521-5. [DOI] [PubMed] [Google Scholar]
- Dubs MC, Altschuh D, van Regenmortel MHV. Interaction between viruses and monoclonal antibodies studied by surface plasmon resonance. Immunol Lett. 1992;31:59–64. doi: 10.1016/0165-2478(92)90011-C. [DOI] [PubMed] [Google Scholar]
- Eun AJ, Huang L, Chew F, Li SF, Wong S. Detection of two orchid viruses using quartz crystal microbalance-based DNA biosensors. Phytopathology. 2002;92:654–658. doi: 10.1094/PHYTO.2002.92.6.654. [DOI] [PubMed] [Google Scholar]
- Florschütz K, Schröter A, Schmieder S, Chen W, Schweizer P, Sonntag F, Danz N, Baronian K, Kunze G. ‘Phytochip’: on-chip detection of phytopathogenic RNA viruses by a new surface plasmon resonance platform. J Virol Methods. 2013;189:80–86. doi: 10.1016/j.jviromet.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Gerdes H, Kaether C. Green fluorescent protein: applications in cell biology. FEBS Lett. 1996;389:44–47. doi: 10.1016/0014-5793(96)00586-8. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Aguirre I, Hodnik V, Glais L, Rupar M, Jacquot E, Anderluh G, Ravnikar M. Surface plasmon resonance for monitoring the interaction of Potato virus Y with monoclonal antibodies. Anal Biochem. 2014;447:74–81. doi: 10.1016/j.ab.2013.10.032. [DOI] [PubMed] [Google Scholar]
- Ishikawa-Ankerhold HC, Ankerhold R, Drummen GPC. Advanced fluorescence microscopy techniques-FRAP, FLIP, FLAP, FRET and FLIM. Molecules. 2012;17:4047–4132. doi: 10.3390/molecules17044047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson T, Bakhshi R, Petrova D, Pocock R, Imani M, Seifalian AM. Biological applications of quantum dots. Biomaterials. 2007;28:4717–4732. doi: 10.1016/j.biomaterials.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Kairdolf BA, Smith AM, Stokes TH, Wang MD, Young AN, Nie S. Semiconductor quantum dots for bioimaging and biodiagnostic applications. Annu Rev Anal Chem (Palo Alto Calif) 2013;6:143–162. doi: 10.1146/annurev-anchem-060908-155136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khater M, de la Escosura-Muñiz A, Merkoçi A. Biosensors for plant pathogen detection. Biosens Bioelectron. 2017;93:72–86. doi: 10.1016/j.bios.2016.09.091. [DOI] [PubMed] [Google Scholar]
- Kuang H, Zhao Y, Ma W, Xua L, Wang L, Xu C. Recent developments in analytical applications of quantum dots. Trends Analyt Chem. 2011;30:1620–1636. doi: 10.1016/j.trac.2011.04.022. [DOI] [Google Scholar]
- Lautner G, Balogh Z, Bardoczy V, Meszaros T, Gyurcsanyi RE. Aptamer-based biochips for label-free detection of plant virus coat proteins by SPR imaging. Analyst. 2010;135:918–926. doi: 10.1039/b922829b. [DOI] [PubMed] [Google Scholar]
- Lin HY, Huang CH, Lu SH, Kuo IT, Chau LK. Direct detection of orchid viruses using nanorod-based fiber optic particle plasmon resonance immunosensor. Biosens Bioelectron. 2014;51:371–378. doi: 10.1016/j.bios.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Lopez MM, Llop P, Olmos A, Marco-Noales E, Cambra M, Bertolini E. Are molecular tools solving the challenges posed by detection of plant pathogenic bacteria and viruses? Curr Issues Mol Biol. 2009;11:13–46. [PubMed] [Google Scholar]
- Malecka K, Michalczuk L, Radecka H, Radecki1 J. Ion-channel genosensor for the detection of specific DNA sequences derived from plum pox virus in plant extracts. Sensors. 2014;14:18611–18624. doi: 10.3390/s141018611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Palma RJ, Manso M, Torres-Costa V. Optical biosensors based on semiconductor nanostructures. Sensors. 2009;9:5149–5172. doi: 10.3390/s90705149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan MS, Domier LL, Bailey RC. Label-free virus detection using silicon photonic microring resonators. Biosens Bioelectron. 2012;31:388–392. doi: 10.1016/j.bios.2011.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medintz IL, Sapsford KE, Konnert JH, Chatterji A, Lin T, Johnson JE, Mattoussi H. Decoration of discretely immobilized cowpea mosaic virus with luminescent quantum dots. Langmuir. 2005;21:5501–5510. doi: 10.1021/la0468287. [DOI] [PubMed] [Google Scholar]
- Mello LD, Kubota LT. Review of the use of biosensors as analytical tools in the food and drink industries. Food Chem. 2002;77:237–256. doi: 10.1016/S0308-8146(02)00104-8. [DOI] [Google Scholar]
- Misteli T, Spector DL. Applications of the green fluorescent protein in cell biology and biotechnology. Nat Biotechnol. 1997;15:961–964. doi: 10.1038/nbt1097-961. [DOI] [PubMed] [Google Scholar]
- Monošík R, Streďanský M, Šturdík E. Biosensors-classification, characterization and new trends. Acta Chim Slov. 2012;5:109–120. [Google Scholar]
- Moreau ALD, Janissen R, Santos CA, Peroni LA, Stach-Machado DR, de Souza AA, de Souza AP, Cotta MA. Highly-sensitive and label-free indium phosphide biosensor for early phytopathogen diagnosis. Biosens Bioelectron. 2012;36:62–68. doi: 10.1016/j.bios.2012.03.038. [DOI] [PubMed] [Google Scholar]
- Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:e63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashia K, Osaka T. Industrialization trial of a biosensor technology. ECS Trans. 2017;75:1–9. doi: 10.1149/07539.0001ecst. [DOI] [Google Scholar]
- Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T. Quantum dots versus organic dyes as fluorescent labels. Nat Methods. 2008;5:763–775. doi: 10.1038/nmeth.1248. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mozaz S, de Alda MJL, Barceló D. Biosensors as useful tools for environmental analysis and monitoring. Anal Bioanal Chem. 2006;386:1025–1041. doi: 10.1007/s00216-006-0574-3. [DOI] [PubMed] [Google Scholar]
- Safarnejad MR, Samiee F, Tabatabie M, Mohsenifar A. Development of quantum dot-based nanobiosensors against citrus tristeza virus (CTV) Sensors & Transducers J. 2017;213:54–60. [Google Scholar]
- Sakurai A, Shibasaki F. Updated values for molecular diagnosis for highly pathogenic avian influenza virus. Viruses. 2012;4:1235–1257. doi: 10.3390/v4081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller FW, Wollenberger U, Warsinke A, Lisdat F. Research and development in biosensors. Curr Opin Biotechnol. 2001;12:35–40. doi: 10.1016/S0958-1669(00)00169-5. [DOI] [PubMed] [Google Scholar]
- Shamsipur M, Nasirian V, Mansouri K, Barati A, Veisi-Raygani A, Kashanian S. A highly sensitive quantum dots-DNA nanobiosensor based on fluorescence resonance energy transfer for rapid detection of nanomolar amounts of human papillomavirus 18. J Pharm Biomed Anal. 2017;136:140–147. doi: 10.1016/j.jpba.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Sharma A, Rao KV, Kamboj DV, Gaur R, Upadhyay S, Shaik M. Relative efficiency of zinc sulfide (ZnS) quantum dots (QDs) based electrochemical and fluorescence immunoassay for the detection of Staphylococcal enterotoxin B (SEB) Biotechnol Rep (Amst) 2015;6:129–136. doi: 10.1016/j.btre.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Zhu Y, Yang X, Li C. Graphene quantum dots: emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem Commun. 2012;48:3686–3699. doi: 10.1039/c2cc00110a. [DOI] [PubMed] [Google Scholar]
- Shen W, Gao Z. Quantum dots and duplex-specific nuclease enabled ultrasensitive detection and serotyping of Dengue viruses in one step in a single tube. Biosens Bioelectron. 2015;65:327–332. doi: 10.1016/j.bios.2014.10.060. [DOI] [PubMed] [Google Scholar]
- Shojaei TR, Salleh MAM, Sijam K, Rahim RA, Mohsenifar A, Safarnejad R, Tabatabaei M. Detection of Citrus tristeza virus by using fluorescence resonance energy transfer-based biosensor. Spectrochim Acta A Mol Biomol Spectrosc. 2016a;169:216–222. doi: 10.1016/j.saa.2016.06.052. [DOI] [PubMed] [Google Scholar]
- Shojaei TR, Salleh MAM, Sijam K, Rahim RA, Mohsenifar A, Safarnejad R, Tabatabaei M. Fluorometric immunoassay for detecting the plant virus Citrus tristeza using carbon nanoparticles acting as quenchers and antibodies labeled with CdTe quantum dots. Mikrochim Acta. 2016b;183:2277–2287. doi: 10.1007/s00604-016-1867-7. [DOI] [Google Scholar]
- Soper SA, Brown K, Ellington A, Frazier B, Garcia-Maneroe G, Gauf V, Gutman SI, Hayes DF, Kortei B, Landers JL, Larson D. Point-of-care biosensor systems for cancer diagnostics/prognostics. Biosens Bioelectron. 2006;21:1932–1942. doi: 10.1016/j.bios.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Sun W, Zhong J, Qin P, Jiao K. Electrochemical biosensor for the detection of cauliflower mosaic virus 35S gene sequences using lead sulfide nanoparticles as oligonucleotide labels. Anal Biochem. 2008;377:115–119. doi: 10.1016/j.ab.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Tereshchenko A, Fedorenko V, Smyntyna V, Konup I, Konup A, Eriksson M, Yakimova R, Ramanavicius A, Balme S, Bechelany M. ZnO films formed by atomic layer deposition as an optical biosensor platform for the detection of Grapevine virus A-type proteins. Biosens Bioelectron. 2017;92:763–769. doi: 10.1016/j.bios.2016.09.071. [DOI] [PubMed] [Google Scholar]
- Turner APF. Biosensors: sense and sensibility. Chem Soc Rev. 2013;42:3184–3196. doi: 10.1039/c3cs35528d. [DOI] [PubMed] [Google Scholar]
- van Dorst B, Mehta J, Bekaert K, Rouah-Martin E, de Coen W, Dubruel P, Blust R, Robbens J. Recent advances in recognition elements of food and environmental biosensors: a review. Biosens Bioelectron. 2010;26:1178–1194. doi: 10.1016/j.bios.2010.07.033. [DOI] [PubMed] [Google Scholar]
- Vinayaka AC, Thakur MS. Focus on quantum dots as potential fluorescent probes for monitoring food toxicants and foodborne pathogens. Anal Bioanal Chem. 2010;397:1445–1455. doi: 10.1007/s00216-010-3683-y. [DOI] [PubMed] [Google Scholar]
- Vinayaka AC, Thakur MS. Photoabsorption and resonance energy transfer phenomenon in CdTe-protein bioconjugates: an insight into QD-biomolecular interactions. Bioconjug Chem. 2011;22:968–975. doi: 10.1021/bc200034a. [DOI] [PubMed] [Google Scholar]
- Wegner KD, Hildebrandt N. Quantum dots: bright and versatile in vitro and in vivo fluorescence imaging biosensors. Chem Soc Rev. 2015;44:4792–4834. doi: 10.1039/C4CS00532E. [DOI] [PubMed] [Google Scholar]
- Yuqing M, Jianguo G, Jianrong C. Ion sensitive field effect transducer-based biosensors. Biotechnol Adv. 2003;21:527–534. doi: 10.1016/S0734-9750(03)00103-4. [DOI] [PubMed] [Google Scholar]
- Zeng C, Huang X, Xu J, Li G, Ma J, Ji HF, Zhu S, Chen H. Rapid and sensitive detection of maize chlorotic mottle virus using surface plasmon resonance-based biosensor. Anal Biochem. 2013;440:18–22. doi: 10.1016/j.ab.2013.04.026. [DOI] [PubMed] [Google Scholar]
- Zhang C, Johnson LW. Quantum-dot-based nanosensor for RRE IIB RNA-rev peptide interaction assay. J Am Chem Soc. 2006;128:5324–5325. doi: 10.1021/ja060537y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Feng G, Guo Y, Zhou D. Robust and specific ratiometric biosensing using a copper-free clicked quantum dot-DNA aptamer sensor. Nanoscale. 2013a;5:10307–10315. doi: 10.1039/c3nr02897f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Chen W, Chen X, Zhang Y, Lin X, Wu Z, Li M. Multiplex immunoassays of plant viruses based on functionalized upconversion nanoparticles coupled with immunomagnetic separation. J Nanomater. 2013b2013:317437. [Google Scholar]
- Zimmer M. Green Fluorescent Protein (GFP): applications, structure, and related photophysical behavior. Chem Rev. 2002;102:759–782. doi: 10.1021/cr010142r. [DOI] [PubMed] [Google Scholar]