Abstract
Objective: Phantom limb pain is a condition frequently experienced after amputation. One treatment for phantom limb pain is traditional mirror therapy, yet some patients do not respond to this intervention, and immersive virtual reality mirror therapy offers some potential advantages. We report the case of a patient with severe phantom limb pain following an upper limb amputation and successful treatment with therapy in a custom virtual reality environment. Methods: An interactive 3-D kitchen environment was developed based on the principles of mirror therapy to allow for control of virtual hands while wearing a motion-tracked, head-mounted virtual reality display. The patient used myoelectric control of a virtual hand as well as motion-tracking control in this setting for five therapy sessions. Pain scale measurements and subjective feedback was elicited at each session. Results: Analysis of the measured pain scales showed statistically significant decreases per session [Visual Analog Scale, Short Form McGill Pain Questionnaire, and Wong-Baker FACES pain scores decreased by 55 percent (p=0.0143), 60 percent (p=0.023), and 90 percent (p=0.0024), respectively]. Significant subjective pain relief persisting between sessions was also reported, as well as marked immersion within the virtual environments. On followup at six weeks, the patient noted continued decrease in phantom limb pain symptoms. Conclusions: Currently available immersive virtual reality technology with myolectric and motion tracking control may represent a possible therapy option for treatment-resistant phantom limb pain.
Keywords: virtual reality, phantom limb pain, rehabilitation, amputation, therapy, pain
Phantom limb pain (PLP), the sensation of pain in an absent limb, is a frequent complication after amputation, with reported prevalence rates ranging from 40 to 85 percent.1,2 While the exact mechanism of PLP remains to be elucidated, peripheral and central neural factors are thought to contribute to this phenomenon.2 One current theory is that maladaptive neuroplastic changes in the sensory and motor cortices are associated with development of PLP.3 While no longer thought to be the sole cause of PLP, emotional factors also play a role in this condition. Significant psychological comorbidities have been noted among those with PLP, including anxiety (66%) and depression (41%).4 PLP is also a risk factor for developing depression in individuals with limb loss, with prevalence of significant depressive symptoms in this population close to 30 percent in a large cross-sectional study.5 Other risk factors for developing PLP include pre-existent pain, residual limb pain, and female gender.8 The prognosis for PLP is highly variable, with some patients having complete resolution of symptoms acutely, and others reporting chronic pain lasting for years.2
A variety of treatments for PLP, including pharmacologic agents (anticonvulsants, nonsteroidal anti-inflammatory drugs, opioids, antidepressants, etc.), local modalities (such as Transcutaneous Electrical Nerve Stimulation), psychological interventions (including biofeedback, mental imagery), and even surgical approaches have been reported, with varying degrees of success.6 Mental imagery is a technique in which patients imagine movements of the phantom limb while concentrating on sensations from various regions of the body. A study of PLP in upper limb amputees showed with mental imagery alone, pain was relieved significantly in 69 percent of patients and correlated with a reduction in cortical reorganization on fMRI.7 This therapeutic efficacy has led researchers to explore other therapies to incorporate mental imagery with a visual input, such as mirror and virtual reality therapies.
Traditional mirror therapy involves the patient viewing their native non-painful limb in a mirror, thus appearing to be their amputated limb.8 Activation of mirror neurons leading to modulation of somatosensory input is thought to contribute to analgesia noted during such therapy. Although mirror therapy has been widely used for many years, the evidence supporting it has been limited, with a recent systematic review of 20 studies noting the technique’s efficacy but lack of robust evidence.9 Traditional mirror therapy is limited by a variety of design constraints, and several recent studies have investigated the use of virtual reality (VR) mirror therapy instead. Such studies have demonstrated potential benefits in treating PLP, especially in patients unresponsive to traditional mirror therapy.10 Immersive VR offers the potential of customizable and engaging rehabilitation exercises, and typically utilizes a head-mounted display (HMD) with real-time motion tracking while viewing a computer-generated environment.11,12 While previously limited by availability and price, recent developments in the entertainment industry have increased general consumer access to VR and offer the potential for widespread clinical use.13,14,15
No published studies have investigated the use of the latest advancements in VR technology combined with peripheral myoelectric control to provide immersive treatment of PLP. Such technology has been demonstrated in training amputees to control a virtual prosthesis, neurorehabilitation in Parkinson’s disease, and analgesia for hospitalized patients.16,17,18 Given the rapid pace of development in this field and the potential for markedly engaging experiences, such VR might offer a unique modality for treatment of PLP within the rehabilitation setting.
CASE REPORT
A 49-year-old previously healthy male with a history of a right wrist disarticulation after a traumatic workplace injury was referred for evaluation. Prior to amputation, the patient did not have any pre-morbid pain, medical, or psychiatric history. His amputation had occurred five months prior to evaluation and resulted in severe PLP. He had already undergone a course of hand therapy that included traditional mirror therapy yet he reported little relief. Furthermore, he was also taking an oral analgesic (hydrocodone/acetaminophen), anticonvulsant (gabapentin), antidepressant (amitriptyline), and had a local nerve block. However, pain continued to be constant and unrelenting. He did not have any previous experience with a VR system.
We designed an immersive VR environment that included a virtual kitchen with interactive features. Various common household objects, such as apples, pots, and pans, were present in this 3-D space, and controllers that appeared as virtual hands (complete with supination, pronation, and grasp) allowed the user to manipulate these objects in VR. Additionally, two publically available VR games, Audioshield and Eleven: Table Tennis, were utilized. These games were selected based on their incorporation of simulated hand motions through motion controller tracking. The VR hardware setup consisted of a HMD, two handheld controllers, and two positional tracking sensors, all components of the Vive VR system (HTC) (Figure 1). An armband myoelectric controller, the Myo Band (Thalmic Labs) (Figure 2), was also used for kitchen environment. The VR system allowed users to freely walk in a measured space with their movements translated into the virtual environment through the HMD and controller positions. Finally, activation of selected forearm movements with the Myo Band resulted in the grasp and release of a virtual hand that corresponded with the position of the patient’s phantom limb. A minimum cleared space of 1.5m × 2.0m (5.0ft × 6.5ft) in a clinic room was required to set up the VR system to allow the patient to explore the virtual environment. A PC (Intel i5, Windows 10, Nvidia GTX 970) was utilized to run the VR setup.
FIGURE 1.
Virtual reality system layout
FIGURE 2.
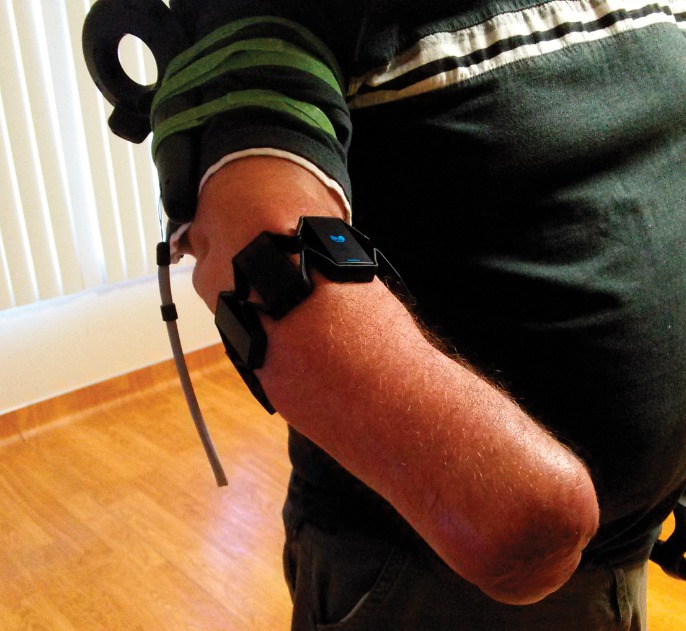
Myoelectric bracelet on amputee
With informed consent and Institutional Review Board approval, the patient was introduced to the immersive VR environment(s). He experienced five VR sessions that were approximately 45 minutes in length. These sessions were conducted over several weeks, no more than once a week. Visual Analog Scale (VAS), Short-form McGill Pain Questionnaire, and Wong-Baker FACES measurements were recorded before and after VR sessions. Subjective feedback of function and pain relief were also recorded before and after VR sessions.
During each session, the patient was instructed to visualize the virtual hands as if they were his native hands. He performed various visualization exercises using the virtual hands, interacted with the kitchen and game environments, all while guided by study personnel through audio cues. During the interactive kitchen scenario, the controller was strapped to the upper arm (Figure 2) with the Myo band placed distally on the forearm. For the remaining scenarios, the controller was strapped to the residual forearm (Figure 4).
FIGURE 4.
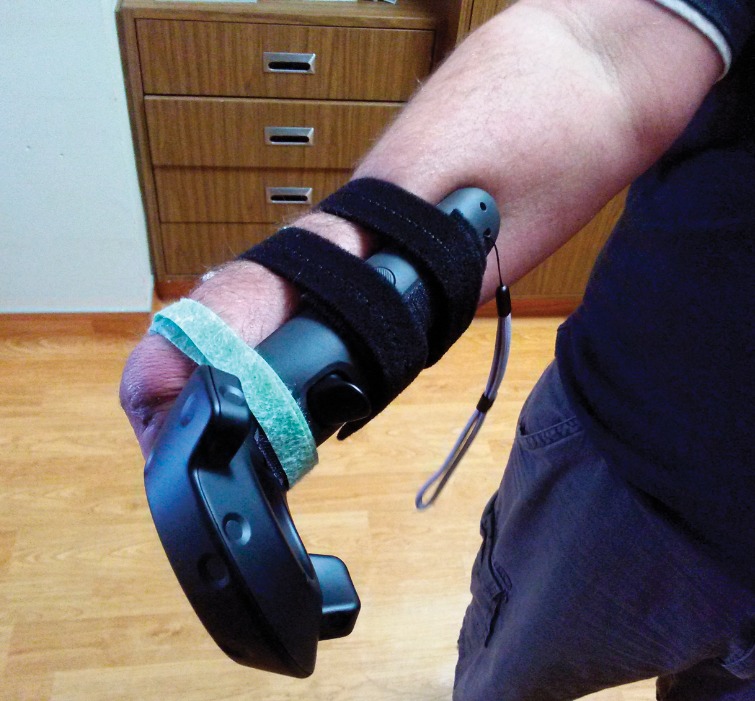
Motion controller setup on amputee
FIGURE 3.

Interactive virtual kitchen environment
RESULTS
The VR sessions were well-received, and the patient reported positive impressions for each immersive experience. He expressed interest in further participation and found the environments to be engaging without considerable difficulty. Training with the Myo band prior to each VR session was conducted to ensure adequate synchronization of muscle activity and device warm-up. When the Myo band synchronization was erroneous (occurred during one session), less engagement and pain relief was reported. The HMD was reportedly comfortable without distraction from the immersion of the VR experiences. The patient quickly became accustomed to the Myo band, unlike non-amputee subjects assisting with the study.
The patient was provided with the opportunity to provide subjective feedback during and between VR sessions. Comments included that the VR made him “forget the pain,” “took me away,” and “pain was not there” during sessions. A high degree of immersion and presence in VR was noted, as he felt the virtual hand representing his phantom hand was actually his “own hand,” that “I feel like my hand is back,” and that “it feels normal.” On comparison to previous traditional mirror therapy he had undergone, he stated that VR felt “like mirror therapy on steroids,” offering him significantly improved and longer lasting pain relief. The mirror therapy sessions with virtual hands and myoelectric control in the interactive kitchen were viewed as more beneficial by the patient than the VR games.
He noted significant subjective pain relief typically taking effect approximately 24 hours after each VR session. This decrease in pain would last for several days, progressively longer with each VR session. Given this improvement, the patient, without the direction of the research team, took himself off gabapentin for neuropathic pain. This resulted in worsened PLP, reflected in the worsened pre-VR pain scores seen in later sessions. Additionally, the patient missed a scheduled session during this time, leading to two weeks rather than one week between sessions. It was recommended by the research team that he continue his medications as prescribed and continue to follow up with his primary provider for further medication adjustments, which he did. He reported the clenched, flexed position of his phantom hand slowly changed with repeated VR sessions, particularly the kitchen environment, leading to the phantom fingers slowly moving into extension. The phantom pain also migrated from the entire hand to only the fingertips during one VR session.
Follow-up subjective feedback was recorded one week after the last VR session. The patient noted continued pain relief lasting over five days, and overall decrease in baseline pain levels. He reported his emotions had improved due to the pain relief, leading to his coworkers commenting on his improved mood. On six week followup, he reported the pain was still present, but generally decreased in severity and was much better tolerated overall.
No serious adverse reactions or events occurred during the sessions. The patient did experience a muscle cramp on his residual forearm after one session of using the Myo band. The cramp resolved with self-massage of the affected area and did not reoccur with guidance to decrease the frequency of movements. No motion sickness or headaches occurred during the VR sessions.
Statistical analysis. STATA12 software was utilized for statistical analysis of the data. A t-test was used to compare the pre and post VR pain mean pain scores. The statistical significant level was set at 0.05. All pain scales showed a statistically significant decrease in pain during each VR session. On average, VAS, Short-form McGill Pain Questionnaire (SF-MPQ), and Wong-Baker FACES pain scores (Figures 5–7) decreased by 55 percent (p=0.0143), 60 percent (p=0.023), and 90 percent (p=0.0024), respectively.
FIGURE 5.

Visual Analog Scale for Pain (VAS) pain score
FIGURE 7.
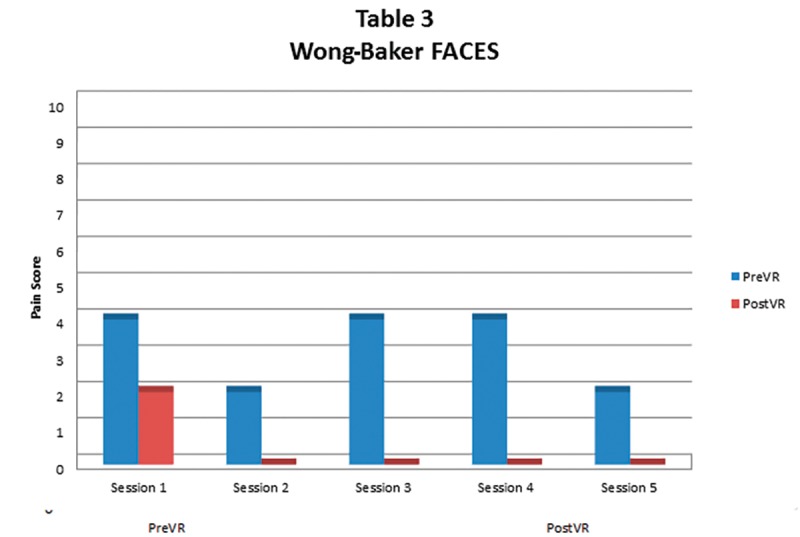
Wong-Baker FACES Pain Scale score
DISCUSSION
From our review of the literature, this is the first report of utilizing the latest current immersive VR technology with full motion tracking combined with myoelectric control of a virtual limb for treating PLP. We demonstrated the design and use of such a system in an outpatient clinical environment for PLP. The patient reported positive impressions of the VR sessions, substantial analgesia (both subjective and objective), interest in continuing the therapy, and without significant adverse reactions. Additionally, the patient noted significant immersion during the sessions, demonstrated by his report of ownership over the VR phantom hand. The only unexpected issue that occurred was the patient’s self-adjustment of his medications based on his own pain relief.
While the patient did report lasting pain relief between sessions, his pain scale measurements did not always reflect this. At times, he presented with increased pain scale values from prior visits (Figures 5 and 6). However, he consistently reported subjective improvements in his perceived pain between sessions, despite these measurements. A possible explanation for this paradox is that perhaps the pain scales more accurately tracked immediate pain relief and not longer term changes in neuropathic pain severity perception. A novelty effect was not seen, with the VR therapy continuing to offer relief at each session. Various hypotheses regarding how virtual reality might contribute to analgesia have been proposed, including the Gate Control Theory, activation of descending inhibitory pathways, production of endogenous opioids, mirror neuron activation, and beneficial neuroplasticity.19,20 The exact mechanism is likely multi-factorial and different in acute versus chronic pain. Based on the results, we are confident immersive VR technology might be considered as an adjuvant for phantom limb pain, not only in the upper extremity, but also for the lower and polyamputee population, with software modifications to project these limbs in the VR setting. The VR environment might be incorporated into outpatient therapy following amputation, and further research to investigate this system’s use after acute amputations with PLP might be considered for patients undergoing inpatient rehabilitation. Additionally, other forms of neuropathic pain that respond to imagery and mirror therapy techniques may also be treatable with VR, such as complex regional pain syndrome.
FIGURE 6.

Short-form McGill Pain Questionnaire (SF-MPQ) pain score
CONCLUSION
In this case, a patient with severe, treatment-resistant PLP following an upper limb amputation reported marked improvements with five sessions of VR therapy. While this report is limited in scope, immersive VR hand therapy with myoelectric control and motion tracking features is a possible option for the management of upper limb PLP. Additional investigation to establish a common protocol with a wide variety of patients might be considered for future research.
ACKNOWLEDGEMENTS
Special thanks to Brandon McCowan and Graham Kampmeier (Loma Linda University Health Interactives Studio), Michael Davidson and Joshua Swinehart (Orthotics and Prosthetics, Loma Linda University Health), and Justin Dunn and Elizabeth Yeo (Loma Linda University School of Medicine) for their contributions to this project. Illustration of VR setup (Figure 1) is an original image and is used with permission from artist Graham Kampmeier.
REFERENCES
- 1.Hommer D, McCallin J. Advances in the treatment of phantom limb pain. Curr Phys Med Rehabil Rep. 2014;2(4):250–254. [Google Scholar]
- 2.Luo Y, Anderson T. Phantom limb pain: a review. Int Anesthesiol Clin. 2016;54:121–139. doi: 10.1097/AIA.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 3.Flor H, Nikolajsen L, Jensen T. Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci. 2006;7:873–881. doi: 10.1038/nrn1991. [DOI] [PubMed] [Google Scholar]
- 4.Padovani M, Martins M, Venancio A, et al. Anxiety, depression and quality of life in individuals with phantom limb pain. Acta Ortop Bras. 2015;23:107–110. doi: 10.1590/1413-78522015230200990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darnell B, Ephraim P, Wegener S, et al. Depressive symptoms and mental health service utilization among persons with limb loss: results of a national survey. Arch Phys Med Rehabil. 2005;86:650–658. doi: 10.1016/j.apmr.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 6.Subedi B, Grossberg G. Phantom limb pain: mechanisms and treatment approaches. Pain Res Treat. 2011:864605. doi: 10.1155/2011/864605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maclver K, Lloyd D, Kelly S, et al. Phantom limb pain, cortical reorganization and the therapeutic effect of mental imagery. Brain. 2008;131:2181–2191. doi: 10.1093/brain/awn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramachandra V, Rogers-Ramachandra D. Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci. 1996;263:377–386. doi: 10.1098/rspb.1996.0058. [DOI] [PubMed] [Google Scholar]
- 9.Barbin J, Seetha V, Casillas J, et al. The effects of mirror therapy on pain and motor control of phantom limb in amputees: A systematic review. Ann Phys Rehabil Med. 2016;59:270–275. doi: 10.1016/j.rehab.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Dunn J, Yeo E, Moghaddampour P, et al. Virtual and augmented reality in the treatment of phantom limb pain: a literature review. Neurorehabilitation. 2017:1–7. doi: 10.3233/NRE-171447. Preprint. [DOI] [PubMed] [Google Scholar]
- 11.Teo W, Muthalib M, Yamin S, et al. Does a combination of virtual reality, neuromodulation and beuroimaging provide a comprehensive platform for neurorehabilitation? A narrative review of the literature. Front Hum Neurosci. 2016;10:284. doi: 10.3389/fnhum.2016.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravi D, Kumar N, Singhi P. Effectiveness of virtual reality rehabilitation for children and adolescents with cerebral palsy: an updated evidence-based systematic review. Physiotherapy. 2016 doi: 10.1016/j.physio.2016.08.004. Preprint. [DOI] [PubMed] [Google Scholar]
- 13.Dascal J, Reid M, Ishak W, et al. Virtual reality and medical inpatients: a systematic review of randomized, controlled trials. Innov Clin Neurosci. 2017;14(1-2):14–21. [PMC free article] [PubMed] [Google Scholar]
- 14.Firth N. First wave of virtual reality games will let you live the dream. New Sci. 2013;218:19–20. [Google Scholar]
- 15.Greenwald W. The best VR (virtual reality) headsets of 2017. 2017. [Accessed May 20, 2017]. http://www.pcmag.com/article/342537/the-best-virtual-reality-vr-headsets PCMAG website. May, 18.
- 16.Phelan I, Arden M, Garcia C. 2015. Exploring virtual reality and prosthetic training. Virtual Reality IEEE. [Google Scholar]
- 17.Kim A, Darakjian N, Finley J. Walking in fully immersive virtual environments: an evaluation of potential adverse effects in older adults and individuals with Parkinson’s disease. J Neuroeng Rehabil. 2017;14:1. doi: 10.1186/s12984-017-0225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosadeghi S, Reid M, Martinez B, et al. Feasibility of an Immersive Virtual Reality Intervention for Hospitalized Patients: An Observational Cohort Study. JMIR Ment Health. 2016;3(2) doi: 10.2196/mental.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gold J, Belmont K, Thomas D. The neurobiology of virtual reality pain attenuation. Cyberpsychol Behav. 2007;10:536–544. doi: 10.1089/cpb.2007.9993. [DOI] [PubMed] [Google Scholar]
- 20.Sato K, Fukumori S, Matsusaki T, et al. Nonimmersive virtual reality mirror visual feedback therapy and its application for the treatment of complex regional pain syndrome: an open-label pilot study. Pain Med. 2010;11(4):622–29. doi: 10.1111/j.1526-4637.2010.00819.x. [DOI] [PubMed] [Google Scholar]



