Abstract
Opiate misuse is a chronic relapsing disease that has become an epidemic in the United States. Methadone is the mainstay of treatment for opiate addiction and has been researched widely. Recently, new avenues of treatment have been researched and developed. The objective of this review is to study methadone in comparison to other pharmacological options available or being considered for opiate addiction treatment through a methodical search and review of evidence provided by recent clinical trials conducted in this regard. There is a paucity of high quality randomized controlled trials focusing on the comparison between buprenorphine and methadone for treatment of opiate use disorder. Buprenorphine should be researched more for patient retention and satisfaction, as well as for its prospect for better outcomes in neonatal abstinence syndrome to generate more decisive recommendations. Current data suggest monitoring of liver enzymes with the use of buprenorphine/naloxone for better liver outcomes. In light of the analyzed data, the authors conclude that methadone should still be considered the preferred treatment mode in comparison to slow-release oral morphine and heroin.
Keywords: Methadone, opiate, opiate addiction, heroin, buphernorphin, withdrawal, treatment
Opioid addiction is a widespread chronic disease in the United States. According to the National Institute on Drug Abuse, 1.9 million Americans have a substance-use disorder related to prescription pain relievers, and 586,000 Americans live with heroin addiction.1 Consequently, drug overdose was the leading cause of accidental deaths in 2014, with 18,893 deaths related to opioid analgesics and 10,574 deaths related to heroin.2
Pharmacotherapies for this disorder include methadone and levomethadyl acetate/levo-alpha-acetylmethadol (LAAM, opioid/mu full agonists); buprenorphine (partial mu agonist); naltrexone (opioid antagonist); a combination of buprenorphine and naltrexone; clonidine (and other alpha-2 adrenergic agonists)3; morphine4; and heroin.5 Recently, new avenues of treatment have been researched and developed. Current medications approved by the US Food and Drug Administration (FDA) for the management of opiate use disorder are methadone, buprenorphine, naltrexone, and naloxone.6,7
Since its introduction in 1965, methadone has been the most widely understood and well-researched treatment of all pharmacological and non-pharmacological treatment options.8–11 Systematic reviews have studied the efficacy and outcomes of methadone maintenance treatment (MMT) versus individual pharmacotherapies (e.g., morphine, buprenorphine, and/or naloxone). This review presents an updated overall look at how methadone fares in comparison to other treatment modalities in light of the latest evidence.
The objective of this review is to examine and evaluate the evidence provided by controlled trials examining various opiate substitution maintenance options for persons diagnosed with opiate use disorder published since 2010. Using this review, we answer the following questions:
I. What are the quality, weight, and applicability of evidence provided by recent randomized controlled trials (RCTs) regarding methadone’s superiority or non-inferiority compared to other modalities for maintenance treatment of opiate use disorder?
II. What recommendations do recent RCTs provide for addiction treatment specialists as well as policy makers regarding effective treatment options for chronic opioid addicts?
III. What should be the focus when conducting comparative RCTs on methadone treatment in opiate use disorder to find support of existing data or provide evidence where it is lacking?
IV. Which comparisons by researchers and clinical trials can yield more impactful outcomes in terms of applicability and translation to clinical recommendations?
METHODS
Eligibility criteria. Study design. Intervention-based RCTs.
Publications. Articles published from January 1, 2010, to June 23, 2016.
Participants. Adults diagnosed with opiate use disorder.
Interventions. Pharmacological, opiate agonist or partial-agonist, substitution-based maintenance interventions for opiate use disorder.
Comparator: Standard methadone maintenance treatment. Trials investigating methadone were selected if the comparator was an opiate substitution-based maintenance treatment for opiate use disorder.
Outcomes: Articles publishing primary outcomes of RCTs.
Location: Studies conducted in developed countries.
Search and study selection. Cochrane and US National Library of Medicine National Institutes of Health (NIH) databases were searched using the Wiley online library and PubMed, respectively, using the terms detailed in the Results section. References of related searches were also searched. The consequent lists were reviewed using predefined inclusion criteria at two different dates to confirm the consistency of application of inclusion criteria. Two researchers studied the results in detail to examine if all criteria were met. Conflicts were resolved through mutual discussion.
Assessment of quality and bias. The papers short-listed for study were assessed for study quality. The taxonomy of core biases from the Cochrane Handbook was used to identify biases. The Cochrane Collaboration Tool (Table 8.5.a in the Cochrane Handbook for Systematic Reviews of Interventions)12 was used to assess risk of bias and to rate studies as having high, low, or unclear risk in different areas.
Data collection process. A modification of the ERC data collection form13 was used to make charts of individual articles. Extracted data items are discussed in the text and charted in tables. A summary of the findings from individual studies is discussed in both narrative and tabulated form. Statistical details and data were used without any modifications.
Data synthesis. No meta-analysis or quantitative synthesis has been done because of the heterogeneity of the outcomes discussed.
RESULTS
Study selection: The search criteria and number of results returned are shown in Table 1.
TABLE 1.
Study selection
| PUBMED | |||
| TITLE/ABSTRACT SEARCH TERM | DATE OF PUBLICATION | DATE OF COMPLETION | RESULTS |
| Methadone treatment | 2010; 2016 | n/a | 366 |
| Methadone treatment AND trial AND buprenorphine | n/a | 01/01/2010 | 65 |
| Methadone treatment AND trial AND heroin | n/a | 01/01/2010; 3000 | 25 |
| Methadone treatment AND trial AND morphine | n/a | 01/01/2010; 3000 | 9 |
| COCHRANE DATABASE | |||
| TITLE, ABSTRACT, KEYWORD SEARCH TERM | RESULTS | ||
| Methadone treatment, opiate addiction, heroin | 11 | ||
| Methadone treatment, opiate addiction, morphine | 17 | ||
| Methadone treatment, buprenorphine in Cochrane Reviews | 221 | ||
| Methadone opiate addiction treatment | 183 | ||
The search process identified 897 articles that were then filtered manually. Sixty-nine articles were short-listed initially. The references of these articles were searched to identify more articles. Five articles were included in the review.14–18 Of these studies, two compared MMT to heroin-assisted treatment, one compared MMT to slow-release oral morphine (SROM), and two compared MMT to buprenorphine.
The flow chart in Figure 1 describes the search method and stepwise filtration of the articles.
FIGURE 1.
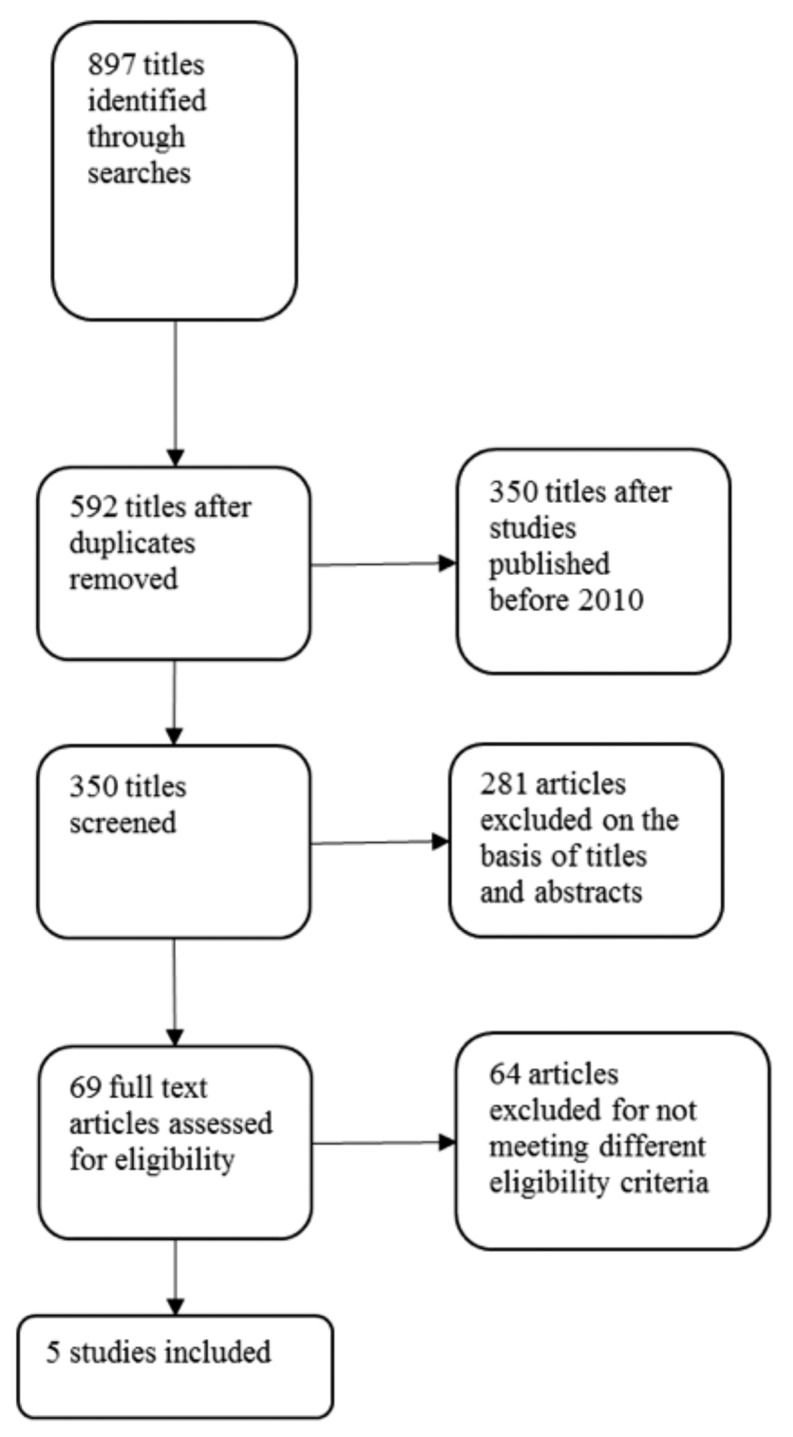
Flow chart of the search
Study characteristics. All five studies reviewed here are prospective, interventional RCTs. Detailed design characteristics of the trials are described in Table 2.
TABLE 2.
Trial designs and methods
| FIRST AUTHOR | LOCATION | DESIGN TERMS* | BLINDING* | RANDOMIZATION | TIMING** | ||
|---|---|---|---|---|---|---|---|
| METHOD* | RATIO | INTERVENTION# | MEASUREMENTS | ||||
| Jones 2010 | USA Canada Austria |
Double-blind double-dummy flexible-dosing | Yes, double | CESAR, stratified | 1:1 | Duration of pregnancy | For 10 days after birth, minimum |
| Strang 2010 | England | Prospective multisite open-label | No | Minimization, stratified | 1:1:1 | 26 weeks | 14–26 weeks |
| Beck 2013 | Switzerland Germany |
Multiple-dose open-label randomized cross-over | No | Random permuted blocks | 1:1 | 22 weeks | Every week for 22 weeks |
| Saxon 2013 | USA | RCT systematic prospective | No | Unclear method | 1:1, changed to 2:1 | 24 weeks | 1–24 weeks |
| Demaret 2015 | Belgium | Open-label RCT | No | Random permuted | 1:1 | 12 months | Every 3 months for 12 months |
Terms reported here exactly as described by researchers
Duration of interventions and points of measurement of outcomes reported. #Interventions studied in the randomized, controlled trial (RCT)
Jones et al14 randomized 175 pregnant participants to two intervention groups. One hundred and thirty-one participants completed treatment. These new mothers and neonates were followed for at least ten days after birth. Outcomes and data from participants that completed treatment have been analyzed and discussed in the article.
Strang et al15 randomized 127 participants to three study arms, with two experimental and one control medicine, for 26 weeks. They measured the outcomes during weeks 14 to 26 of the study. Data from all randomized participants have been analyzed by intention-to-treat (ITT) analysis. Data from 89 participants of the protocol sample (PP) have been analyzed and reported as well as compared with the ITT results.
Beck et al16 used a crossover design to compare SROM with oral methadone in 276 participants randomized to one of two sequences of medication administration (SROM → MET; MET → SROM). Participants were administered experimental or control medication for 11 weeks in Period 1 and crossed over to the other medication for the next 11 weeks. Outcomes were measured every week for 22 weeks. Data from all randomized participants (276) have been analyzed in the ITT analysis, and data from 157 participants meeting protocol criteria have been analyzed as per protocol sample.
Saxon et al17 conducted a phase 4, randomized, controlled study, examining the differences in liver outcomes in participants treated with buprenorphine/naloxone or methadone. In total, 1,269 participants were randomized to one of the two medications, at first in a 1:1 ratio and later switching to a 2:1 (BUP/naloxone:MET) ratio of allocation because of greater participant loss from the buprenorphine/naloxone group. Participants were treated for 24 weeks, and outcomes were measured eight times during this period. Results and data from participants meeting “evaluable” criteria have been analyzed and discussed. According to Saxon et al, “The criteria for ‘evaluable’ were completion of 24 weeks on assigned medication and provision of at least half of the eight liver tests scheduled between Weeks 1–24, at Weeks 1, 2, 4, 8, 12, 16, 20, and 24.”17
Demaret et al18 used an open-label RCT to study heroin-assisted treatment versus traditional methadone treatment in Belgium. Seventy-four participants were randomized to the experimental and control groups for a period of 12 months. Outcomes were measured every three months throughout this period. An ITT analysis was performed on all participants, and a per-protocol analysis was performed on the population meeting the protocol criteria.
Participant population. This review has a total of 1,921 randomized participants (Table 3), of which 1,339 were included in the final analyses of respective studies. Jones et al14 studied pregnant women, and as a result included zero men. However, the remaining participants were 72.68 percent men. Women in the study by Jones et al14 were less than 30 years old, while the rest of the participants were older in age.
TABLE 3.
Participant population
| AUTHOR | YEAR | TOTAL NO. RANDOMIZED | TOTAL NO. ANALYZED# | GROUP TYPES | ANALYZED?# YES/NO | GROUPS | NO. OF PATIENTS IN EACH GROUP | AGE YEARS | MEN | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MEAN | SD | NO. | % | ||||||||
| Jones | 2010 | 175 | 131 | Completed t/m | Yes | MET BUP |
73 58 |
27.7 25.3 |
0.7 0.7 |
0 | 0 |
| Did not complete | No | MET BUP |
16 28 |
29.7 29.1 |
1.6 1.7 |
0 0 |
0 0 |
||||
| Strang | 2010 | 127 | 127 | ITT | Yes | Inj. MET Inj. heroin Oral MET |
42 43 42 |
37.0 37.5 37.2 |
7.0 6.6 5.9 |
28 37 28 |
67 86 67 |
| Beck | 2013 | 276 | 276 | ITT PP |
Yes Yes |
ITT PP |
276 157 |
38.1 38.9 |
7.6 7.4 |
225 132* |
81.5 84.1* |
| Saxon | 2013 | 1269 | 731 | Evaluable (731) | Yes | BUP MET |
340 391 |
39.3 38.4 |
11.3 11.3 |
242 253 |
71.2 64.7 |
| Demaret | 2015 | 74 | 74 | ITT | Yes | Experimental (SROM) | 36 | 43 | 6 | 30 | 83 |
| Control (MET) | 38 | 42 | 7 | 35 | 92 | ||||||
| Combined | 2010 to 2015 | 1921 | 1339 | n/a | n/a | n/a | n/a | n/a | n/a | 878 | 65.56 |
| Excluding Jones 2010** | 2010 to 2015 | 1746 | 1208 | n/a | n/a | n/a | n/a | n/a | n/a | 878 | 72.68 |
Analyzed: Results for the group analyzed and discussed in the article.
These numbers not included in total sums, as they were included in the ITT group numbers listed.
This category used to show percentage of men in articles studying both sexes. SD: standard deviation; MET: methadone; BUP: buprenorphine; ITT: intention to treat; PP: per protocol; SROM: slow-release oral morphine
Strang et al15 and Demaret et al18 compared heroin and methadone maintenance treatments. A comparison of the baseline characteristics in their populations (Table 4) shows that Strang et al15 recruited an older population with a greater baseline use of alcohol and cocaine and a higher percentage of incarceration, although Demaret et al18 preferentially included participants with criminal backgrounds (Table 5). This fact should be kept in mind while interpreting the results of the two studies.
TABLE 4.
Participants in heroin versus MAT trials
| STUDY | GROUP# | NO. IN GROUP NO. | AGE MEAN±SD | IN PRISON NO. (%) | NO. OF EMPLOYED IN PAST MONTH (%) | BZD | ALCOHO | CRACK/COCAINE | NO. OF PREVIOUS ADDICTION T/M |
|---|---|---|---|---|---|---|---|---|---|
| IN PAST MONTH (30 DAYS) | |||||||||
| NO. (%) | NO. (%) | NO. (%) | |||||||
| Strang 2010 | Inj. MAT Inj. heroin Oral MAT |
42 43 42 |
37±7 37.5±6.6 37.2±5.9 |
26 (62) 33 (77) 34 (81) |
1 (2) 1 (2) 1 (2) |
13 (31) 13 (30) 18 (43) |
24 (57) 19 (44) 21 (50) |
29 (69) 34 (79) 31 (74) |
4.1±3.8 4.7±4.7 4.5±4.1 |
| Total* | 127 | 30 to 44.1 | 93 (73.2) | 3 (2) | 44 (34.6) | 64 (50.4) | 94 (74.0) | 0 to 9.4 | |
| Demaret 2015 | Heroin MAT | 36 38 |
43±6 42±7 |
26 (72) 21 (55) |
1 (3) 1 (3) |
18 (50) 13 (34) |
9 (25) 12 (32) |
14 (39) 20 (53) |
11±17 8±8 |
| Total | 74 | 36 to 50 | 47 (64) | 2 (3) | 31 (42) | 21 (28) | 34 (46) | 9±13 | |
Data used as reported in studies.
ITT group numbers and characteristics.
Totals calculated using reported numbers in Strang 2010. No.: number; SD: standard deviation; BZD: benzodiazepine; t/m: treatment. MAT: Methadone
TABLE 5.
Eligibility criteria
| STUDY | KEY ELIGIBILITY CRITERIA* |
|---|---|
| Jones 2010 | Women Singleton pregnancy (6–30 weeks gestation) |
| Strang 2010 | Chronic heroin addicts Receiving conventional oral maintenance treatment (>6 months) Continuing to inject street heroin regularly |
| Beck 2013 | Participating in MMT for >26 weeks Methadone dose of 50mg/day or more at time of inclusion |
| Saxon 2013 | ALT or AST not more than 5 times ULN ALP not more than 3 times ULN |
| Demaret 2015 | Heroin dependency for at least 5 years (Almost) daily use of street heroin At least one previous experience of MMT (with a minimum dose of 60mg) Heroin use through injection or inhalation Poor (physical or mental) health or criminal involvement |
Only features distinguished from other studies included here. All studies have eligibility criteria of adult participants with diagnosis of opiate use disorder and without other significant illnesses, so these are not included here. MMT: methadone maintenance treatment; ULN: upper limit of normal; ALT: alanine amino transferase; AST: aspartate amino transferase; ALP: alkaline phosphatase.
Jones et al14 and Demaret et al18 reported “no significant baseline differences” between groups. Strang et al15 reported stratification of data based on baseline differences between groups. Saxon et al17 reported significant differences in baseline drug use indicators and accounted for them in the analysis of results.
All articles described the eligibility criteria (Table 5) for participant selection in detail.
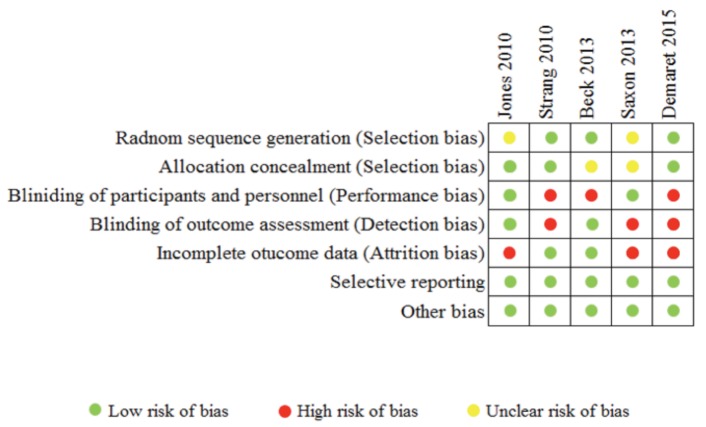
Risk of bias within studies. Figure 3 depicts an assessment of risk of bias within individual studies. Jones et al14 used blinding techniques; however, they did not discuss a randomization process.
Figure 3.
Risk of bias in individual studies
Saxon et al17 studied changes in enzyme levels, an outcome not influenced by the subjective feeling of the user. There was no outcome accessor blinding, and the randomization process was not clearly described. Also, there was an unequal loss of randomized patients, and data from “evaluable” participants were analyzed. This trial was a phase IV study, conducted and funded in collaboration with Reckitt Benckiser Pharmaceuticals, leading to a conflict of interest.
Strang et al15 and Demaret et al18 compared interventions administered via injections or inhalation with orally administered interventions, consequently rendering blind techniques a difficult mark.
Strang et al15 used objective measures of outcomes, i.e., laboratory tests, yet the outcomes themselves were highly susceptible to the influence of participants’ subjective perceptions and experiences with the drugs.
Demaret et al18 used 3 subjective outcome measures, only 2 of which were validated objectively. Also, they reported greater loss of participant population in the control group, and missing participants were assumed to be non-responders. The majority of participants missing from the control group never presented to the allocation centers after randomization. The authors detailed, “In the control group, among the 17 (45%) participants who were on MMT outside a partner centre at baseline, 12 (32%) were still on MMT outside the partner centres at 12 months. This explained the high rate of drop out after randomisation.”18 This raises concern regarding attrition bias.
Beck et al16 did not describe any allocation concealment procedures. The authors discussed a lack of blinding of participants and personnel as a limiting factor of the study, but used objective measures of outcomes.
Interventions studied. The included articles were studies that compared opiate substitution medications (buprenorphine alone, buprenorphine with naloxone, morphine, heroin, and methadone) with methadone. Buprenorphine (with/without naloxone) was studied in two articles.14,17 Strang et al15 and Demaret et al18 studied heroin (diamorphine/diacetyl morphine) in comparison with methadone. Strang et al15 conducted a comparison of injectable versus oral methadone as well. The article by Beck et al16 was the only finalized article comparing morphine versus methadone.
Details of the administration and dosage of interventions can be seen in Table 6.
TABLE 6.
Interventions studied
| STUDY | INTERVENTION | COMPARATOR | ||||
|---|---|---|---|---|---|---|
| DRUG | ROUTE | DOSE | DRUG | ROUTE | DOSE | |
| Jones 2010 | Buprenorphine | Sublingual | 2–32mg Flexible | Methadone | Oral | 20–140mg/day |
| Strang 2010 | Diamorphine or diacetyl morphine + optional oral methadone for nightly cravings | Injection, not specified | Week 7–26; Mean daily doses: 398.9mg (SD=163.6)+41.8mg (SD=12.7) supplementary oral methadone; twice daily, usually equally divided | Methadone | Oral | Week 7–26; mean daily dose: 107.3mg (SD=39.9); once daily |
| Methadone + optional oral methadone for nightly cravings | Injection, not specified | Mean daily doses: 128.3mg (SD=38.3)+31.4mg (SD=13.0) supplementary oral methadone | Methadone | Oral | Mean daily dose: 107.3mg (SD=39.9); once daily | |
| Beck 2013 | Slow-release oral morphine | Oral | 580–970mg, once daily | Methadone | Oral | 80–120mg/day Once daily |
| Saxon 2013 | Buprenorphine + naloxone | Not available | Mean maximum daily dose: 22.1mg (SD=8.2; median=24mg) | Methadone | Mean maximum daily dose: 93.2mg (SD=42.2; median=90mg) | |
| Demaret 2015 | Diacetyl morphine + optional oral methadone for nightly cravings | Injection or inhalation | Avg. daily dose: 573±230mg; patients came to methadone center 2.3 times a day | Methadone | Oral | Mean daily dose: 77±21mg |
Primary outcomes, measurement, and analysis. Neonatal effects of exposure during pregnancy were studied by Jones et al,14 while the rest of the articles examined outcomes in the adult participants themselves.
Jones et al14 particularized five primary outcomes, all neonatal and all (except head circumference) measured by personnel/physicians subjectively (i.e., subject to assessor’s evaluation or treatment staff’s standards). Actions taken to minimize differences and enhance objectivity included training of all assessors by an expert at the outset as well as retraining during the course of the trial and a uniform standard across individuals and locations. The primary outcomes are listed in Table 7. The included outcomes covered the key areas of neonatal abstinence syndrome that are most likely to be affected by choice of exposure during pregnancy. However, analysis was performed only on data from participants who completed treatment. The O’Brien-Fleming spending function was used to set the α level at 0.0091 for each primary outcome. Outcomes were reported on continuous scales as odds ratios.
TABLE 7.
Results of individual studies
| STUDY | COMPARISON | LIST OF PRIMARY OUTCOMES* | RESULTS REPORTED |
|---|---|---|---|
| Jones | BUP vs. MET |
|
No significant difference No significant difference 89% less morphine in BUP group 43% less hospital stay in BUP group No significant difference |
| Strang 2010 | Inj. heroin and Inj. MET vs. standard MET | Reduction of regular use of street heroin: 50% or more of negative specimens for street heroin on weekly urinalysis during Weeks 14–26 | Significant difference between inj. heroin and oral methadone in favor of inj. heroin in ITT as well as PP population |
| Beck 2013 | SROM vs. MET | The primary efficacy endpoint: the proportion of positive urine samples per patient and per treatment for coconsumption of heroin | Non-inferiority of SROM to MET reported in ITT and PP populations |
| Saxon 2013 | BUP/naloxone vs. MET |
|
No significant difference between medication groups. No significant difference between medication groups. No significant difference between medication groups. No significant difference between medication groups. No significant difference between medication groups. |
| Demaret 2015 | HAT vs. MET | Street heroin use during the previous month decreased significantly more in the experimental group than in the control group Main effect of group not statistically significant in Results section but stated as significantly lower in Discussion section Main effect of group not statistically significant |
Primary outcomes as described in the article.
Evaluable participants defined by the article: “The criteria for ‘evaluable’ were completion of 24 weeks on assigned medication and provision of at least half of the eight liver tests scheduled between weeks 1–24, at weeks 1, 2, 4, 8, 12, 16, 20, and 24.” BUP: buprenorphine; MET: methadone; NAS: neonatal syndrome; ITT: intention to treat; PP: per protocol; SROM: slow-release oral morphine; BUP/naloxone: buprenorphine and naloxone combination; ULN: upper limit of normal; ALT: alanine amino transferase; AST: aspartate amino transferase.
Drug/Alcohol section of the European Addiction Severity Index.
Maudsley Addiction Profile - Health Symptoms Scale (MAP-HSS).
Symptom Check-List (SCL-90-R). HAT: Heroine Assisted Treatment
Strang et al15 used reduction in regular use of heroin as the primary outcome and employed an objective test (not subject to the assessor’s evaluation), i.e., weekly urinalysis for quantifying the outcome, which was reported using logistic regression scales to calculate odds ratios for the ITT and PP samples.
Beck et al16 also used weekly urinalyses under blinded conditions for assessment of the primary outcome. An analysis of variance (ANOVA) was used for the primary analysis, and the Welch t-test was used to test for unequal carryover effect in the second period of the crossover study. A non-inferiority analysis was performed on the PP and ITT populations with a 2-sided, 95-percent confidence interval, set at a 10-percent margin for decision of non-inferiority. The p-value was calculated with a logistic regression model using generalized estimating equations.
Saxon et al17 measured changes in liver enzyme levels and used five categories of changes as the primary outcomes for performing a shift-table analysis on data from “evaluable” participants (defined earlier in the review). A hazard ratio was calculated for only one category.
Demaret et al18 used subjective (subject to participants’ reporting and judgment) but standardized scales. The results for two of the outcomes measured were validated via urine toxicological analyses and criminal records. Results were reported on a dichotomous multidomain index, rating participants as responders or non-responders. Efficacy evaluation was based on the percentage difference between groups. The statistical methods used included Fischer’s exact test for contingency tables and ANOVA for secondary analysis. Statistical analyses were performed using STATISTICA 10.
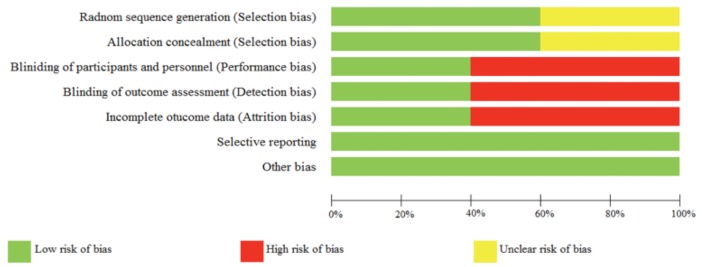
Risk of bias across studies. An assessment of risk of bias across the included studies is presented in Figure 2. No bias was noted in locating or appraising studies. However, only published articles were reviewed in this article; thus, publication bias should be noted.
FIGURE 2.
Reviewers’ assessment of risk of bias in all studies
DISCUSSION
An increasing awareness about the opiate abuse epidemic19 has led researchers on a dynamic quest to ascertain the best therapeutic option for management of this disorder. Methadone is considered the mainstay of maintenance treatment for opiate use disorder. Various quality studies, reviews, and meta-analyses conducted up until now have concluded superiority of methadone maintenance over no treatment or abstinence alone.8–11 Safety concerns regarding methadone use20,21 have led to a vigorous search for alternate and safer opiate substitutes. However, methadone still retains its status as the most-used opiate substitution therapy, and more evidence is needed to prove or disprove this status.22
While conducting a search for this review, it was seen, as expected, that most research was observational and longitudinal. This research was not included in the review.
Five RCTs meeting eligibility criteria for the search were analyzed in detail for design, methods, participants, outcomes, assessments, and results. Careful examination of quality was carried out to identify and define bias and evaluate significance and worth of evidence reported by each study.
Buprenorphine versus methadone. Jones et al14 conducted a multicenter study, providing data from a blinded controlled trial that indicated buprenorphine’s superiority in better neonatal outcomes, specifically, use of morphine and number of hospital stay days needed for treatment of neonatal abstinence syndrome in neonates born to mothers treated with buprenorphine or methadone for opiate substitution during pregnancy. The issue of higher morphine dose needed in methadone treatment does not translate to serious or non-serious adverse events, as reported in the study. In fact, all neonatal outcomes other than the two aforementioned ones were similar between the two groups. Weight of the outcome should be explored further.
Although not deliberated as primary outcomes, better patient retention and satisfaction seen in the methadone group cannot be ignored, especially under blinded conditions. A major issue in the management of opiate addiction is prevention of relapse, even more so in pregnant patients. Methadone’s better retention profile, which is both generalizable and applicable, helps make it the treatment of choice in the eyes of most addiction treatment specialists. It is postulated by the authors that individual variation in buprenorphine absorption and easier tapering-off from the drug might have been contributing factors to the attrition in the buprenorphine population.
Opiate substitution during pregnancy warrants special recommendations and attention, owing to the maternal and neonatal parameters affected by opioids. Both methadone and buprenorphine are classified as category C (lacking adequate, well-controlled studies in pregnant women) drugs by the FDA and carry the risk of neonatal morbidity and mortality. However, both can be used for maintenance treatment of opiate use disorder.23 Opiate substitution in a structured careful manner during pregnancy prevents fluctuations of opioid levels in serum and prevents relapse to street opiate use. Methadone has been considered an acceptable treatment for opiate use disorder during pregnancy since as early as the 1970s.24,25 Methadone is, in effect, considered the standard of care for opiate use disorder treatment during pregnancy,22,23,26 as it improves maternal and neonatal outcomes when instituted with comprehensive prenatal care.27
However, recent trials have presented buprenorphine as a possible alternative to methadone as a first-line treatment of select pregnant patients.28–30 One major criterion favoring selection of buprenorphine is accessibility of patients to a licensed care center and ability to attend a clinic on a more frequent basis. Buprenorphine can be prescribed in an office-based setting by healthcare practitioners holding specific licensure,31–33 while methadone can only be prescribed in licensed centers. In 2016, the Comprehensive Addiction and Recovery Act allowed nurse practitioners and physician assistants to provide office-based, buprenorphine-assisted therapies after appropriate training and certification.34 This makes buprenorphine more accessible and desirable for many patients. Also, the lesser drug interaction posed by buprenorphine might make it the preferable choice for physicians while managing patients with multiple medical issues. However, it is recommended to screen out patients based on their liver health status and past experience with the two drugs.
Methadone is still considered the standard treatment for management of opiate addiction in pregnancy for most of the pregnant population. Saxon et al17 studied the effects of buprenorphine/naloxone on liver health, using methadone as a comparator. The results showed no significant difference; however, the high risks of bias in multiple areas, specifically attrition bias, limit the quality of evidence. Also, better retention was seen with methadone, but it was not one of the outcomes studied. Although some researchers have indicated an increase in liver enzymes with buprenorphine treatment in patients with hepatitis and have recommended careful monitoring during buprenorphine treatment,35,36 other articles found no effect of buprenorphine on liver enzymes.37,38 Treatment retention is an aspect that needs to be researched and addressed as well, as both the included articles and previously published data indicate methadone’s superiority in this regard.
Careful monitoring of liver enzymes is still warranted while treating patients with buprenorphine. More research is needed before any conclusions can be made in this regard.
Heroin versus methadone. Strang et al15 and Demaret et al18 studied the use of heroin as an opiate substitute in comparison to methadone maintenance treatment. Both studies showed significant reduction in heroin use in participants being treated with heroin, as compared to methadone. Nonetheless, lack of blinding has a greater impact in this specific context because of subjective preference and motivational factors. As discussed earlier, the results should be seen in view of the participant population. The study by Strang et al,15 with higher baseline age, alcohol and cocaine use, and criminality, showed more significant results compared to the study by Demaret et al,18 thus necessitating further research on a larger size population before any recommendations or conclusions can be made.
Previously, research has been performed on the use of heroin in other countries39–43 with results ranging from promising to ambiguous. No studies with concluding evidence have been conducted in the United States so far.
SROM versus methadone. Beck et al16 performed an RCT that showed non-inferiority of SROM to methadone in treatment of opiate use disorder expressed as efficacy of treatment. This was a good quality study, but it only showed non-inferiority. Statistically significant differences between outcomes for SROM and MET are seen in favor of MET; however, they are within the 10 percent inferiority margin. Consequently, they still do not provide evidence for supporting recommendation of SROM as an alternate to MMT. This RCT does legitimize further studies and wider investigation regarding this intervention, especially keeping in mind the oral route and once-daily administration. Previous research does not provide any conclusive evidence, as stated by two systematic reviews conducted in this regard.44,45
Across the review of various articles, it was seen that different researchers employ a wide array of survey formats and units for measuring baseline drug use and addiction treatments. This factor impedes assimilation of data and identification of relationships and patterns among factors like duration of addiction, injection drug use, concurrent cocaine/alcohol/benzodiazepine use, duration and forms of previous treatments, and efficacy of intervention of interest. Development and use of standard questionnaires and charts for this purpose will help promote greater usability and better analysis of RCTs.
CONCLUSION AND RECOMMENDATIONS
I. More intervention-based clinical trials are needed to provide a strong alternative candidate therapy for management of opiate use disorder.
II. Buprenorphine, although a promising candidate, should be researched more regarding treatment retention and patient satisfaction before any conclusions can be made regarding its standard use for opiate substitution maintenance treatment of pregnant patients with opiate use disorder.
III. Heroin-assisted treatment still cannot be considered in opiate addicts refractory to MMT; more data should be collected regarding efficacy in larger participant populations under blinded conditions. Safety, generalizability, and diversion should be studied in further detail.
IV. Based on the present data, SROM is not a more effective alternative to methadone. Further clinical research with sound methodology is warranted to explore its potential use in this respect.
REFERENCES
- 1.Rockville MD. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. 2015. Substance Abuse and Mental Health Services Administration. Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. http://www.samhsa.gov/data/sites/default/files/NSDUH-FRR1-2014/NSDUH-FRR1-2014.pdf Available at.
- 2.Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System, Mortality File 2015. Atlanta, GA: Center for Disease Control and Prevention. Number and Age-Adjusted Rates of Drug-poisoning Deaths Involving Opioid Analgesics and Heroin: United States, 2000-2014. http://www.cdc.gov/nchs/data/health_policy/AADR_drug_poisoning_involving_OA_Heroin_US_2000-2014.pdf Available at.
- 3.Stotts AL, Dodrill CL, Kosten TR. Opioid dependence treatment: options in pharmacotherapy. Expert Opin Pharmacother. 2009;10(11):1727–1740. doi: 10.1517/14656560903037168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giacomuzzi S, Kemmler G, Ertl M, Riemer Y. Opioid addicts at admission vs. slow-release oral morphine, methadone, and sublingual buprenorphine maintenance treatment participants. Subst Use Misuse. 2006;41(2):223–244. doi: 10.1080/10826080500391845. [DOI] [PubMed] [Google Scholar]
- 5.Fischer B, Oviedo-Joekes E, Blanken P, et al. Heroin-assisted treatment (HAT) a decade later: a brief update on science and politics. J Urban Health. 2007;84(4):552–562. doi: 10.1007/s11524-007-9198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The ASAM National Practice Guideline for the Use of Medications in the Treatment of Addictions Involving Opioid Use. http://www.asam.org/docs/default-source/practice-support/guidelines-and-consensus-docs/asam-national-practice-guideline-supplement.pdf?sfvrsn=24 Available at.
- 7.Bart G. Maintenance medication for opiate addiction: the foundation of recovery. J Addict Dis. 2012;31(3):207–225. doi: 10.1080/10550887.2012.694598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dole VP, Nyswander M. A medical treatment for diacetylmorphine (heroin) addiction. JAMA. 1965;193:80–84. doi: 10.1001/jama.1965.03090080008002. [DOI] [PubMed] [Google Scholar]
- 9.Hubbard RL, Rachal JV, Craddock SG. Cavanaugh ER. Treatment Outcome Prospective Study (TOPS): client characteristics and behaviors before, during, and after treatment. In: Tims FM, Ludford JP, editors. Drug Abuse Treatment Evaluation: Strategies, Progress, and Prospects. [PubMed] [Google Scholar]
- 10.Institute of Medicine (US) Committee on Federal Regulation of Methadone Treatment. Federal Regulation of Methadone Treatment. In: Rettig RA, Yarmolinsky A, editors. Washington, DC: National Academies Press (US); 1995. p. 2.http://www.ncbi.nlm.nih.gov/books/NBK232112/ Pharmacology and Medical Aspects of Methadone Treatment. Available from. [PubMed] [Google Scholar]
- 11.Sees KL, Delucchi KL, Masson C, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA. 2000;283(10):1303–1310. doi: 10.1001/jama.283.10.1303. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JPT, Green S, editors. The Cochrane Collaboration, 2011. Cochrane Handbook for Systematic Reviews of Interventions. www.cochrane-handbook.org Version 5.1.0 [updated March 2011] Available from.
- 13.Cochrane Collaboration Glossary, 2010. http://www.cochrane.org/training/cochrane-handbook Available from.
- 14.Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363(24):2320–2331. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strang J, Metrebian N, Lintzeris N, et al. Supervised injectable heroin or injectable methadone versus optimised oral methadone as treatment for chronic heroin addicts in England after persistent failure in orthodox treatment (RIOTT): a randomised trial. Lancet. 2010;375(9729):1885–1895. doi: 10.1016/S0140-6736(10)60349-2. [DOI] [PubMed] [Google Scholar]
- 16.Beck T, Haasen C, Verthein U, et al. Maintenance treatment for opioid dependence with slow-release oral morphine: a randomized cross-over, non-inferiority study versus methadone. Addiction. 2014;109(4):617–626. doi: 10.1111/add.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saxon AJ, Ling W, Hillhouse M, et al. Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug Alcohol Depend. 2013;128(1-2):71–76. doi: 10.1016/j.drugalcdep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demaret I, Quertemont E, Litran G, et al. Efficacy of heroin-assisted treatment in Belgium: a randomised controlled trial. Eur Addict Res. 2015;21:179–187. doi: 10.1159/000369337. [DOI] [PubMed] [Google Scholar]
- 19.The White House. Office of the Press Secretary. https://www.whitehouse.gov/the-press-office/2015/10/21/fact-sheet-obama-administration-announces-public-and-private-sector
- 20.Weimer MB, Chou R. Research gaps on methadone harms and comparative harms: findings from a review of the evidence for an American Pain Society and College on Problems of Drug Dependence clinical practice guideline. J Pain. 2014;15(4):366–376. doi: 10.1016/j.jpain.2014.01.496. [DOI] [PubMed] [Google Scholar]
- 21.Chou R, Weimer MB, Dana T. Methadone overdose and cardiac arrhythmia potential: findings from a review of the evidence for an American Pain Society and College on Problems of Drug Dependence clinical practice guideline. J Pain. 2014;15(4):338–365. doi: 10.1016/j.jpain.2014.01.495. [DOI] [PubMed] [Google Scholar]
- 22.Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. (Treatment Improvement Protocol Series 43) Rockville, MD: US Department of Health and Human Services; 2005.
- 23.Effective medical treatment of opiate addiction. National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction. JAMA. 1998;280(22):1936–1943. [PubMed] [Google Scholar]
- 24.Sharpe C, Kuschel C. Outcomes of infants born to mothers receiving methadone for pain management in pregnancy. Arch Dis Child Fetal Neonatal Ed. 2004;89(1):F33–6. doi: 10.1136/fn.89.1.F33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kandall SR, Doberczak TM, Jantunen M, Stein J. The methadone-maintained pregnancy. Clin Perinatol. 1999;26(1):173–183. [PubMed] [Google Scholar]
- 26.Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy. Effects and management. Obstet Gynecol Clin North Am. 1998;25(1):139–151. doi: 10.1016/s0889-8545(05)70362-4. [DOI] [PubMed] [Google Scholar]
- 27.Finnegan LP, editor. DHEW Publication No. (ADM) 79-678; Washington, DC: U.S. Govt. Print. Off: 1979. Drug Dependence in Pregnancy: Clinical Management of Mother and Child. National Institute on Drug Abuse. [Google Scholar]
- 28.Treatment Center for S A. (2005). Chapter 13. Medication-Assisted Treatment for Opioid Addiction during Pregnancy. Substance Abuse and Mental Health Services Administration (US) http://www.ncbi.nlm.nih.gov/books/NBK64148/ Retrieved from. [PubMed]
- 29.Fischer G, Johnson RE, Eder H, et al. Treatment of opioid-dependent pregnant women with buprenorphine. Addiction. 2000;95(2):239–244. doi: 10.1046/j.1360-0443.2000.95223910.x. [DOI] [PubMed] [Google Scholar]
- 30.Lacroix I, Berrebi A, Chaumerliac C, et al. Buprenorphine in pregnant opioid-dependent women: first results of a prospective study. Addiction. 2004;99(2):209–214. doi: 10.1046/j.1360-0443.2003.00600.x. [DOI] [PubMed] [Google Scholar]
- 31.Opioid Abuse, Dependence, and Addiction in Pregnancy. The American College of Obstetricians and Gynecologists Committee Opinion Paper Number 524 May 2012 (Reaffirmed in 2016)
- 32.Institute of Medicine. Federal regulation of methadone treatment. Washington DC: National Academy Press; 1995. [Google Scholar]
- 33.Drug Addiction Treatment Act 2000. https://www.deadiversion.usdoj.gov/pubs/docs/dwp_buprenorphine.htm
- 34. PUBLIC LAW 114 - 198 – Comprehensive Addiction and Recovery Act of 2016, 114 stat 1221.
- 35.Petry NM, Bickel WK, Piasecki D, Marsch LA, Badger GJ. Elevated liver enzyme levels in opioid-dependent patients with hepatitis treated with buprenorphine. Am J Addict. 2000;9(3):265–269. doi: 10.1080/10550490050148099. [DOI] [PubMed] [Google Scholar]
- 36.Petry NM, Bickel WK, Piasecki D, Marsch LA, Badger GJ. Elevated liver enzyme levels in opioid-dependent patients with hepatitis treated with buprenorphine. Am J Addict. 2000;9(3):265–269. doi: 10.1080/10550490050148099. [DOI] [PubMed] [Google Scholar]
- 37.Bruce RD, Altice FL. Case series on the safe use of buprenorphine/naloxone in individuals with acute hepatitis C infection and abnormal hepatic liver transaminases. Am J Drug Alcohol Abuse. 2007;33(6):869–874. doi: 10.1080/00952990701653875. [DOI] [PubMed] [Google Scholar]
- 38.Lucas GM, Young A, Donnell D, et al. HPTN 058 study group. Hepatotoxicity in a 52-week randomized trial of short-term versus long-term treatment with buprenorphine/naloxone in HIV-negative injection opioid users in China and Thailand. Drug Alcohol Depend. 2014;142:139–145. doi: 10.1016/j.drugalcdep.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blanken P, Hendriks VM, van Ree JM, van den Brink W. Outcome of long-term heroin-assisted treatment offered to chronic, treatment-resistant heroin addicts in the Netherlands. Addiction. 2010;105(2):300–308. doi: 10.1111/j.1360-0443.2009.02754.x. [DOI] [PubMed] [Google Scholar]
- 40.Haasen C, Verthein U, Degkwitz P, et al. Heroin-assisted treatment for opioid dependence: randomised controlled trial. Br J Psychiatry. 2007;191(1):55–62. doi: 10.1192/bjp.bp.106.026112. [DOI] [PubMed] [Google Scholar]
- 41.Rehm J, Gschwend P, Steffen T, et al. Feasibility, safety, and efficacy of injectable heroin prescription for refractory opioid addicts: a follow-up study. Lancet. 2001;358(9291):1417–1423. doi: 10.1016/S0140-6736(01)06529-1. [DOI] [PubMed] [Google Scholar]
- 42.Haro G, Martínez-Raga J, Castellano M, et al. Heroin prescribing: Is there scientific evidence of its efficacy for the treatment of its dependence. Actas Esp Psiquiatr. 2001;29(5):343–348. Review. Spanish. [PubMed] [Google Scholar]
- 43.Lintzeris N. Prescription of heroin for the management of heroin dependence: current status. CNS Drugs. 2009;23(6):463–476. doi: 10.2165/00023210-200923060-00002. [DOI] [PubMed] [Google Scholar]
- 44.Jegu J, Gallini A, Soler P, Montastruc J-L, Lapeyre-Mestre M. Slow-release oral morphine for opioid maintenance treatment: a systematic review. Br J Clin Pharmacol. 2011;71(6):832–843. doi: 10.1111/j.1365-2125.2011.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferri M, Minozzi S, Bo A, Amato L. Slow-release oral morphine as maintenance therapy for opioid dependence. Cochrane Database Syst Rev. 2013;6:CD009879. doi: 10.1002/14651858.CD009879.pub2. [DOI] [PubMed] [Google Scholar]