Abstract
Background
Cardiac autonomic neuropathy in type 2 dibetes mellitus (T2DM) patients is frequent and associated with high cardiovascular mortality. Heart rate variability (HRV) is the gold standard to measure cardiac autonomic neuropathy. We aimed to conduct a systematic review and meta–analysis to evaluate the impact of T2DM on HRV parameters.
Methods
The PubMed, Cochrane Library, Embase and Science Direct databases were searched on 1st October 2017 using the keywords “diabetes” AND (“heart rate variability” OR “HRV”). Included articles had to report HRV parameters in T2DM patients and healthy controls measured during 24 hours with a Holter–electrocardiogram. Measurements of HRV retieved were: RR–intervals (or Normal to Normal intervals—NN), standard deviation of RR intervals (SDNN), percetange of adjacent NN intervals differing by more than 50 milliseconds (pNN50), square root of the mean squared difference of successive RR intervals (RMSSD), total power, Low Frequency (LF), High Frequency (HF) and LF/HF ratio, as per Task Force recommendations.
Results
We included twenty-five case-control studies with 2,932 patients: 1,356 with T2DM and 1,576 healthy controls. T2DM patients had significantly (P<0.01) lower RR–intervals (effect size = –0.61; 95%CI –1.21 to –0.01), lower SDNN (–0.65; –0.83 to –0.47), lower RMSSD (–0.92; –1.37 to –0.47), lower pNN50 (–0.46; –0.84 to –0.09), lower total power (–1.52; –2.13 to –0.91), lower LF (–1.08; –1.46 to –0.69]), and lower HF (–0.79; –1.09 to –0.50). LF/HF did not differ between groups. Levels of blood glucose and HbA1c were associated with several HRV parameters, as well as Time from diagnosis of T2DM
Conclusions
T2DM was associated with an overall decrease in the HRV of T2DM patients. Both sympathetic and parasympathetic activity were decreased, which can be explained by the deleterious effects of altered glucose metabolism on HRV, leading to cardiac autonomic neuropathy.
Introduction
Type 2 diabetes mellitus (T2DM) is a public health concern [1]. T2DM is increasingly frequent in the world in association with the increase of sedentary behaviours, unhealthy diet, obesity and metabolic syndrome [2–6]. The number of people with T2DM is predicted to double within the next three decades [1]. Besides macrovascular [7–10] and microvascular complications [11–13], the leading cause of death in T2DM is cardiovascular mortality [9]. Cardiovascular mortality has been related to the cardiac autonomic neuropathy frequently associated with T2DM [1,5,14].
Screening for cardiac autonomic neuropathy has been recommended at the diagnosis of T2DM, particularly in patients with a history of poor glycaemic control, macro/micro vascular complications, and increased cardiovascular risk [15]. Despite standard cardiovascular reflex tests still belong to the gold standard for the assessment of cardiovascular autonomic neuropathy [16], one of the easiest and most reliable ways to assess cardiac autonomic neuropathy is through the measurement of heart rate variability (HRV). HRV is the variation between two consecutive beats: the higher the variation, the higher the parasympathetic activity. A high HRV reflects the fact that an individual can constantly adapt to micro–environmental changes [17]. Therefore, low HRV is a marker of cardiovascular risk [18]. Conveniently, the measurement of HRV is non–intrusive and pain–free [19]. Although the evaluation of HRV in T2DM has been assessed in several studies, conflicting results have been reported [20–22]. Moreover, there is no consensus on the decreased levels of HRV parameters in T2DM. Furthermore, despite HRV being linked with the severity of T2DM [23], no studies have comprehensively assessed the role of the most common variables, such as age [24], gender [25], blood glucose control [26], or medications treating for T2DM, on HRV parameters [27,28].
Therefore, we aimed to conduct a systematic review and meta–analysis on the impact of T2DM on HRV parameters. A secondary aim was to identify the most frequently reported explanatory variables.
Methods
Literature search
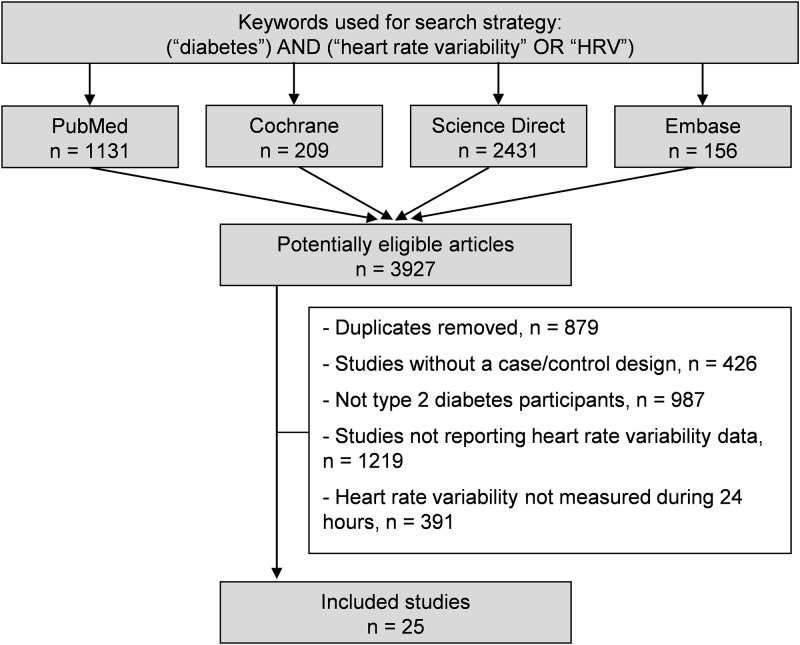
We reviewed all studies measuring HRV in T2DM patients and healthy controls. Animal studies were excluded. Between October 30th 2015 and October 1st 2017, the main articles databases (PubMed, Cochrane Library, Science Direct and Embase) were searched with the following keywords: “diabetes” AND (“heart rate variability” OR “HRV”). All articles compatible with our inclusions criteria were included, independently of article language and years of publication. To be included, case-control studies had to describe our main primary outcome, which was the measurement of HRV parameters in T2DM patients and healthy controls. We limited included studies to those reporting 24–hour measurements of HRV with Holter–electrocardiogram, following Task Force recommendations [29]. We imposed no limitation on the regional origin or the nature of the control group. Studies needed to be primary research. In addition, reference lists of all publications meeting the inclusion criteria were manually searched to identify any further studies that were not found with the electronic search. Ancestry searches were also completed on previous reviews to locate other potentially eligible primary studies. The search strategy is presented in Fig 1 and in S1 Appendix. One author (Thomas Benichou) conducted the literature searches, collated the articles, and extracted the data. Two authors (Thomas Benichou and Frédéric Dutheil) reviewed the abstracts independently and checked if article could be included in our metanalaysis according to inclusion critera. When consensus on suitability was not reached, a third author (Bruno Pereira) reviewed the debated articles. Then, all authors reviewed the eligible articles.
Fig 1. Search strategy.
Quality of the assessment
Despite not designed for quantifying the integrity of studies [30], the “STrengthening the Reporting of OBservational studies in Epidemiology” (STROBE) was used for checking the reporting quality of cohorts studies [31]. The STROBE Statement is a checklist of 22 items related to the title, abstract, introduction, methods, results and discussion sections of articles. Cohort, case control, and cross-sectional studies shared 18 items. Four items are specific to each of the three study designs. Six of the 22 items are splitted into several sub-items. We attributed one point per item or sub-item fulfilling the criteria. We calculated a percentage on a maximal score achievable of 32. The Consolidated Standards of Reporting Trials (CONSORT) for checking the reporting quality of randomized trials [32]. Similarly, the 25 items (with 11 of them splitted into several sub-items) from the CONSORT criteria could achieve a maximum score of 37, then converted into percentage.
HRV parameters
We included HRV parameters derived from a 24–hour Holter electrocardiogram following task force recommendations [29]. In the time domain, we analysed RR intervals, standard deviations of RR intervals (SDNN), the square root of the mean squared difference of successive RR intervals (RMSSD), and the percentage of adjacent NN intervals differing by more than 50 milliseconds (pNN50). The RMSSD and pNN50 are associated with high–frequency power (HF) and hence parasympathetic activity, whereas SDNN is correlated with low–frequency power (LF) [33]. In the spectral domain, we analysed LF (0.04–0.15 Hz), an index of both sympathetic and parasympathetic activity, and HF (0.15–0.4 Hz), representing the most efferent vagal (parasympathetic) activity to the sinus node. Very low frequency (VLF; 0.003–0.04 Hz) partially reflects thermoregulatory mechanisms, fluctuation in activity of the renin–angiotensin system, and the function of peripheral chemoreceptors. The LF/HF ratio, i.e. the sympathovagal balance, was also calculated.
Statistical considerations
We conducted meta–analyses on the HRV parameters in T2DM patients and healthy controls. P values less than 0.05 were considered statistically significant. For the statistical analysis, we used both Comprehensive Meta–analysis software (version 2, Biostat Corporation) [34–37] and Stata software (version 13, StataCorp, College Station, US) [34–36,38,39]. Main characteristics were synthetized for each study population and reported as the mean ± SD (standard–deviation) for continuous variables and the number (%) for categorical variables.
We evaluated heterogeneity in the study results by examining forest plots, confidence intervals (CI) and I2 statistic. Formal tests for homogeneity based on the I2 statistic are the most common metric for measuring the magnitude of between–study heterogeneity and are easily interpretable. I2 values range from 0 to 100%, and are considered low for <25%, modest for 25–50%, and high for >50%. We assume heterogeneity for a p–value of the I2 test <0.05. For example, a significant heterogeneity could be linked to the characteristics of the studies, such as those of the participants (age, sex, etc.), the time from T2DM diagnosis, the glycaemia or the HbA1c levels. We conducted random effects meta–analyses (DerSimonian and Laird approach) when data could be pooled [40]. To describe our results, we calculated the effect size (ES, standardised mean differences—SMD) [41] of each HRV parameter for each dependent variable. An ES is a unitless measure of the levels of the HRV data. The ES is centered at zero if the HRV data in T2DM patients are not different from those in healthy controls. A positive ES denoted higher levels of the tested HRV parameter in T2DM patients compared with heathy controls. An ES of 0.8 reflects a large effect, 0.5 a moderate effect, and 0.2 a small effect. We searched for potential publication bias using funnel plots of these meta–analyses. We verified the strength of our results by conducting further meta–analyses after exclusion of studies that were not evenly distributed around the base of the funnel.
When possible (sufficient sample size), meta–regressions were proposed to study the relationship between each HRV parameter (RR intervals, RMSSD, pNN50, SDNN, total power, LF, HF, LF/HF) and clinically relevant parameters such as gender, age, fasting blood glucose, and glycated haemoglobin (HbA1c). Results were expressed as regression coefficients and 95% CI.
Results
An initial search produced a possible 3927 articles (Fig 1). Removal of duplicates and use of the selection criteria reduced the number of articles reporting the evaluation of HRV on 24-hour recording in T2DM to 25 articles [42–66]. All included articles were written in English except one study which was written in Hungarian [55].
Quality of articles
The assessment of the quality of the twenty–five studies that were included was performed using the STROBE and CONSORT criteria. Results varied from 50% [59] to 71% [56] for the observational studies (STROBE), with a mean score of 59%. Results varied from 52% [59] to 67% [57] for the randomized trials (CONSORT), with a mean score of 59.5%. Overall, the studies performed best in the methods section and worst in the discussion section.
Objectives of included articles
All included articles aimed to compare HRV between T2DM patients and controls without T2DM [42–66]. Both the T2DM patients and the controls had cardiovascular diseases in five studies [42,48,51,56,60] and renal disease in three studies [43,46,52]. Five studies assessed the influence of high blood pressure on HRV in T2DM compared with healthy controls [49,50,54,55,58]. Other studies compared HRV between T2DM patients and controls based on blood catecholamine levels [44], circadian autonomic rhythm in insulinoresistant subjects [45], in cases of bowel preparation [63], metabolic syndrome [47], circadian rhythm in relation to blood adiponectin [62], or dimethylarginine levels [61], hypoglycaemic episodes [59], in acromegalic patients [53], and inhalation of ultrafine particles [57].
Inclusions and exclusions criteria
For T2DM patients: Inclusion criteria were: aged over 18 [43,48], and under 65 [51], 73 [53], or 75 years old [46,52], treated with oral antidiabetic agents [47], with normal [42,44,45] or high blood pressure [49,61]. The main exclusion criteria were: pregnancy [43,62], neurological disease [43]. Body Mass Index (BMI) over 25 kg/m2 [42,44,45], or 35 kg/m2 [58,62], chronic heart [42,44,45,50–52,57,58,60–63], liver [56,60,63], or renal [42,42,45,56,58,60–63] failure, insulin treatment [47,51], uncontrolled T2DM [47], thyroid disorder [50,52], or treatment that can influence HRV parameters [44–46,52,54,57–60].
For controls: All studies included controls without T2DM [42–66]. In each individual study, exclusion criteria were the same as for T2DM, i.e. pregnancy [43,62], neurological disease [43], BMI over 25 kg/m2 [42,44,45] or 35 kg/m2 [58,62], chronic heart [42,44,45,50–52,57,58,60–63], liver [56,60,63], or renal [42,42,45,56,58,60–63] failure, thyroid disorder [50,52], or treatment that can influence HRV parameters [44–46,52,54,57–60]. Controls had high blood pressure in five studies [49,56,58,60,62], and were on dialysis in three studies [43,46,52].
Healthy controls were paired with T2DM patients based on age [41,43,44,46,47,49,50,52,53,58,59,62], gender [42,44,45,47,51,59,60,63], body weight [50,59], BMI [44], and blood pressure [60].
Population
Sample size
Population sizes ranged from 12 [59], to 457 [56]. We included 2,932 patients in total: 1,356 with T2DM and 1,576 healthy controls.
Gender
The proportion of men varied from 35% [45] to 100% [50] in T2DM patients, with a mean of 52.2%, and from 28% [54] to 100% [60] in the control group, with a mean of 54.5%. One study did not specify the proportion of men with T2DM [53], while 3 studies did not specify it for the controls [53,57,59].
Age
The mean age of T2DM patients was 58.1±6.5 years, ranging from 45.9 [57] to 67.9 [52], and 55.9±7.6 years in the controls, ranging from 28.5 [57] to 65.7 [52]. Age was not reported for T2DM patients in two studies [50,53], and for the controls in three studies [50,53,59].
T2DM duration: the mean time from T2DM diagnosis was 7.8±4.4 years ranging from 3.0 [47,54] to 11.2 [51] years. T2DM duration was not reported in five studies [45,48,50,52,53].
Metabolic control
The mean HbA1c in T2DM patients was 7.6±0.8%, ranging from 6.5 [59] to 9.3% [58,62], and 5.2±0.5% in the controls, ranging from 4.1 [42] to 5.8% [58,62]. Eight studies did not report HbA1c in the T2DM patients [43,45,46,48,50,53,61,64] and nine studies did not specify for the controls [43,45–48,50,53,61,64]. Blood glucose levels were reported in 14 studies [42,44,46,47,51,52,55,58,60–63,65,66] with a mean blood glucose level of 147±16 mg/dL in T2DM patients, ranging from 117 [47] to 168 [58,62], and 90±6 in controls, ranging from 75 [46] to 95 [55]. Blood insulin levels were reported in one study [44]. No studies reported HOMA–IR. No studies reported neuropathy scores.
Body weight
Weight was reported in three studies [43,46,49], and waist circumference in two studies [47,52]. The mean BMI in T2DM patients was 27.2±3.5 kg/m2, ranging from 22.3 [44] to 33.7 [58,62], and 24.8±2.1 in controls, ranging from 21.8 [61] to 29.7 [54]. BMI was not reported in 11 studies in T2DM patients [42,43,46,48,50–53,55,57,64], and in 12 studies in controls [42,43,46,48,50–53,55,57,59,64].
Blood pressure
Mean systolic blood pressure in T2DM patients was 138.6±11.0 mmHg, ranging from 121.2 [44] to 156.0 [49], and 137.7±11.6 mmHg in controls, ranging from 120.2 [45] to 153.0 [49]. Mean diastolic blood pressure in T2DM patients was 80.8±4.3 mmHg, ranging from 69.9 [50] to 90.1 [49] and 82.2±5.4 mmHg in controls, ranging from 71.9 [50] to 92.9 [58,62]. Six studies did not report blood pressure in either T2DM patients or controls [42,48,52,53,57,64].
Blood lipid levels
Total cholesterol was reported in nine studies [42,47,51,54,55,61,62,65,66]. HDL and LDL cholesterol in six studies [47,55,60–62,65] and triglycerides in 12 studies [42,47,51,55,56,58–62,65,66].
Other characteristics
Smoking was reported in six studies [44,54–57,60], and alcohol in one study [44]. Marital status was never reported. Insufficient data precluded further analyses of those parameters.
Study designs
All studies described a prospective cohort design, except one study which was a randomly controlled prospective study [57].
HRV measurements and analysis
All included studies measured HRV over 24 consecutive hours using a Holter–electrocardiogram. HRV measurement recording was ambulatory with normal daily activity in most of the studies [42–45,47–51,53,54,56–58,60,62,64–66], ambulatory in non–dialysed patients and during hospitalization for dialysed patients in two studies [46,52], and exclusively during hospitalization in one study [59]. Three studies did not report the conditions of the measurements [55,61,63].
Nearly all studies had a distinct Holter monitoring system: Del Mar Reynolds Medical [61], CardioSmart Institutional CS 550 software [58], Holter cardio Light digital [62], Zymed Medical Instruments [48], Mortara Instruments [53,57], TM 2421 & 2425 systems [56], Holter AD35 TOP [46], Marquette Electronics [43], Meditech Cardiotens [54,55], Fukuda System [44], Spiderview Holter [52], Cardioscan DMS300–4 Model [63], Holter Digital Recorder AsPEKT [42,51], ArguSys Holter Monitor [47], CardioDay GETEMED [59]. Schiller Microvit MT–101 [65,66], and A&D System [49]. Four studies did not report the monitoring system [45,50,60,64], Fifteen studies explicitly mentioned that they followed task force recommandations [42,44,45,47,48,51–56,58,59,61,62]. Premature atrial and ventricular beats were automatically discarded and visually checked.
Meta–analyses of HRV values in T2DM
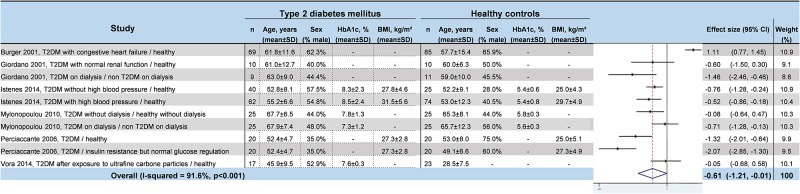
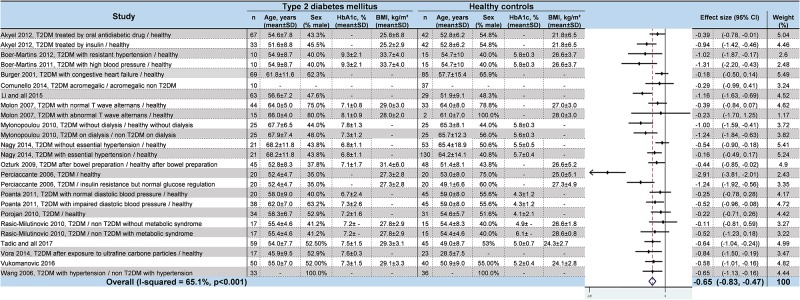
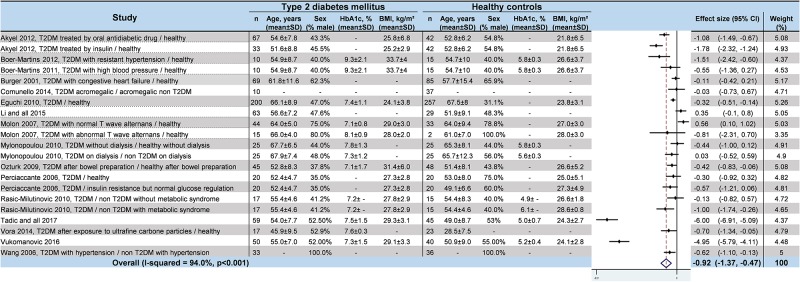
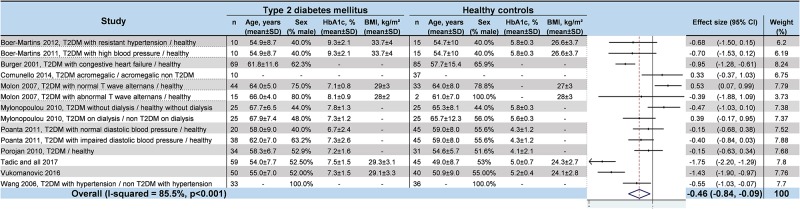
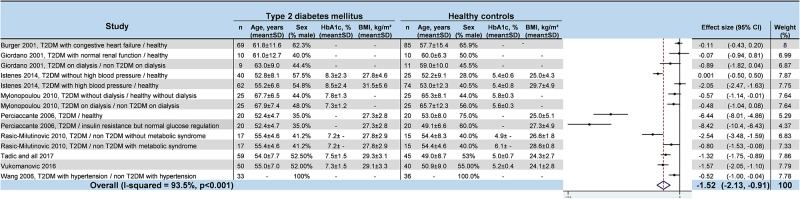
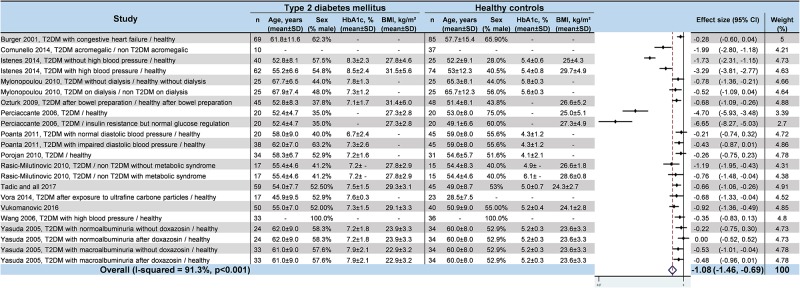
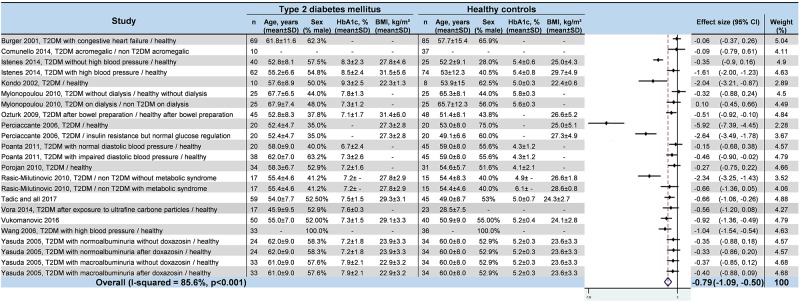
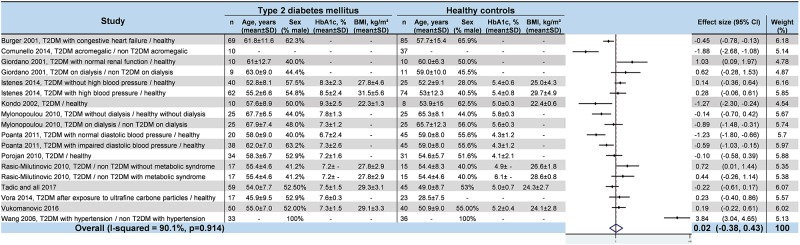
We noted strong evidence that T2DM patients had significantly lower RR intervals (effect size = –0.61; 95%CI –1.21 to –0.01, P = 0.01; I2 = 91.6%) (Fig 2) [45,46,48,52,54,57], lower SDNN (effect size = –0.65; 95%CI –0.83 to –0.47; P < 0.001; I2 = 65.1%) (Fig 3) [42,45,47,48,50–53,55,57,58,60–66], lower RMSSD (effect size = –0.92; 95%CI –1.37 to –0.47); P < 0.001; I2 = 94.0%) (Fig 4) [45,47,48,50,52,53,56–58,60–66], lower pNN50 (effect size = –0.46; 95%CI –0.84 to –0.09; P = 0.001; I2 = 85.5%) (Fig 5) [42,48,50–53,58,60,62,65,66], lower total power (effect size = –1.52; 95%CI –2.13 to –0.91; P < 0.001; I2 = 93.5%) (Fig 6) [45–48,50,52,54,65,66], lower LF (effect size = –1.08; 95%CI –1.46 to –0.69; p < 0.001; I2 = 91.3%) (Fig 7) [42,45,47–54,57,63,65,66], and lower HF (effect size = –0.79; 95%CI –1.09 to –0.50; P < 0.001; I2 = 85.6%) (Fig 8) [42,44,45,47–54,57,63,65,66]. LF/HF did not differ between groups (effect size = 0.02; 95%CI –0.38 to 0.43; P = 0.914; I2 = 90.1%) (Fig 9) [42,44,46–48,50–54,57,65,66]. Heterogeneity was significant (P < 0.001) for all meta–analyses. Funnel plots of meta–analyses analysing for potential publication bias are presented in the supplementary file. Meta–analyses were reperformed after the exclusion of studies that were not evenly distributed around the base of the funnel and showed similar results.
Fig 2. Meta–analysis of RR intervals of type 2 diabetes mellitus patients compared with controls.
-: non reported data (missing SD were also non reported). 95% CI: 95% confident intervals; BMI: Body Mass Index; RR: RR intervals; T2DM: Type 2 Diabetes Mellitus; SD: Standard Deviation.
Fig 3. Meta–analysis of SDNN of type 2 diabetes mellitus patients compared with controls.
-: non reported data (missing SD were also non reported). 95% CI: 95% confident intervals; BMI: Body Mass Index; SDNN: Standard Deviation of RR intervals; T2DM: Type 2 Diabetes Mellitus; SD: Standard Deviation.
Fig 4. Meta–analysis of RMSSD of type 2 diabetes mellitus patients compared with controls.
-: non reported data (missing SD were also non reported). 95% CI: 95% confident intervals; BMI: Body Mass Index; RMSSD: square root of mean squared differences of successive RR intervals; T2DM: Type 2 Diabetes Mellitus; SD: Standard Deviation.
Fig 5. Meta–analysis of pNN50 of type 2 diabetes mellitus patients compared with controls.
-: non reported data (missing SD were also non reported). 95% CI: 95% confident intervals; BMI: Body Mass Index; pNN50: percentage of RR intervals with more than 50 ms variation; T2DM: Type 2 Diabetes Mellitus; SD: Standard Deviation.
Fig 6. Meta–analysis of total power of type 2 diabetes mellitus patients compared with controls.
-: non reported data (missing SD were also non reported). 95% CI: 95% confident intervals; BMI: Body Mass Index; TP: Total Power; T2DM: Type 2 Diabetes Mellitus; SD: Standard Deviation.
Fig 7. Meta–analysis of the LF of type 2 diabetes mellitus patients compared with controls.
-: non reported data (missing SD were also non reported). 95% CI: 95% confident intervals; BMI: Body Mass Index; LF: Low Frequency; T2DM: Type 2 Diabetes Mellitus; SD: Standard Deviation.
Fig 8. Meta–analysis of the HF of type 2 diabetes mellitus patients compared with controls.
-: non reported data (missing SD were also non reported). 95% CI: 95% confident intervals; BMI: Body Mass Index; HF: High Frequency; T2DM: Type 2 Diabetes Mellitus; SD: Standard Deviation.
Fig 9. Meta–analysis of LF/HF of type 2 diabetes mellitus patients compared with controls.
-: non reported data (missing SD were also non reported). 95% CI: 95% confident intervals; BMI: Body Mass Index; LF/HF: Low Frequency/High Frequency ratio; T2DM: Type 2 Diabetes Mellitus; SD: Standard Deviation.
Meta–regressions
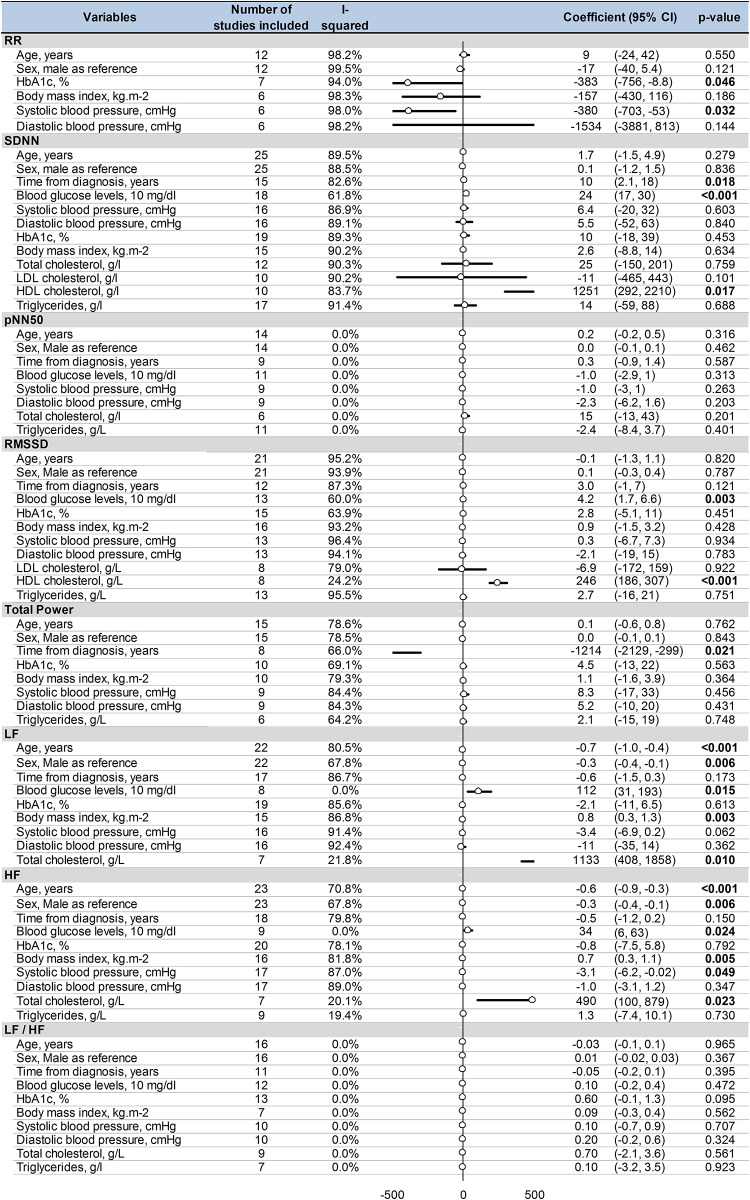
Age and male gender were associated with both a decrease in LF (coefficient = –0.7; 95%CI –1 to –0.4, P < 0.001; and coefficient = –0.3; 95%CI –0.4 to –0.1, P = 0.006, respectively) and a decrease in HF (coefficient = –0.6; 95%CI –0.9 to –0.3, P < 0.001; and coefficient = –0.3; 95%CI –0.4 to –0.1, P = 0.006). Similarly, both blood glucose levels and total cholesterol were associated with both an increase in LF (coefficient = 112; 95%CI 31–193, P = 0.015; coefficient = 1133; 95%CI 408–1858, P = 0.01; respectively) and in HF (coefficient = 34; 95%CI 6–63, P = 0.024; coefficient = 490; 95%CI 100–879, P = 0.023). Blood glucose levels were also significantly associated with an increase in RMSSD (coefficient = 4.2; 95%CI 1.7–6.6, P = 0.003) and with an increase in SDNN (coefficient = 24; 95%CI 17–30, P < 0.001). Higher levels of HbA1c were associated with shorter RR intervals (coefficient = –383; 95%CI –756 to –8.8, P = 0.046). HDL cholesterol levels were associated with both an increase in SDNN (coefficient = 1251; 95%CI 292–2210, P = 0.017) and RMSSD (coefficient = 246; 95%CI 186–307, P < 0.001). Body mass index was associated with both an increase in LF (coefficient = 0.8; 95%CI 0.3–1.3, P = 0.003) and in HF (coefficient = 0.7; 95%CI 0.3–1.1, P = 0.005). An increase in systolic blood pressure was linked with shorter RR intervals (coefficient = –380; 95%CI –703 to –53, P = 0.032) and a decrease in HF (coefficient = –3.1; 95%CI –6.2 to –0.02, P = 0.049). Time from diagnosis of T2DM was linked with a higher level of SDNN (coefficient = 10; 95%CI 2.1–18, P = 0.018) and a lower level of total power (coefficient = –1214; 95%CI –2129 to –299, P = 0.021) (Fig 10).
Fig 10. Meta–regression of factors influencing heart rate variability in type 2 diabetes mellitus.
95% CI: 95% confident intervals; RR: RR intervals; SDNN: Standard Deviation of RR intervals; pNN50: percentage of RR intervals with more than 50 ms variation; RMSSD: square root of mean squared differences of successive RR intervals; TP: Total Power; LF: Low Frequency; HF: High Frequency; LF/HF: Low Frequency/High Frequency ratio.
Discussion
The main findings were that T2DM patients exhibited a strong decrease in HRV. Both sympathetic and parasympathetic activity were decreased compared with non–T2DM patients, which can be explained by the deleterious metabolic effects of blood glucose levels on HRV. Dyslipdaemia and high blood pressure are linked with a decreased HRV in T2DM. Finally, age and the male gender were associated with both a decrease in sympathetic and parasympathetic activity.
An alteration of both sympathetic and parasympathetic activity
Adaptation to stress is characterized by an increase in sympathetic activity and a decrease in parasympathetic activity, inducing a state of alertness [17]. Interestingly, common diseases such as rheumatoid arthritis [67], depression [68], schizophrenia [69], multiple sclerosis [70], active ulcerative colitis [71], obesity and metabolic syndrome [72,73], myocardial infarctions [74], high blood pressure [75], smoking [76], and cancer [77], are associated with a decrease in parasympathetic activity and an activation of sympathetic activity. However, we demonstrated that T2DM patients had a decrease in both parasympathetic and sympathetic activity. An explanation could be that T2DM is a metabolic disease responsible for a cardiac autonomic neuropathy that affects both sympathetic and parasympathetic fibers. With the exception of the LF/HF ratio, which did not differ in our meta–analysis due to comparable changes in the LF and HF components, T2DM has a negative influence on almost all HRV parameters, reflecting the fact that diabetes leads to a cardiac autonomic dysfunction.
Deleterious effects of altered glucose metabolism
The significant relationship between altered glucose metabolism and HRV may explain the deleterious general metabolic effects on both parasympathetic and sympathetic activity. Interestingly, blood glucose levels were associated with both an increase in LF (sympathetic) and HF (parasympathetic), as well as SDNN (sympathetic) and RMSSD (parasympathetic), which may appear contradictory. In healthy individuals, parasympathetic activity is triggered by an increase in blood glucose levels through insulin responses [78]. Unfortunately, insulin levels were not reported in most studies included in our meta–analysis, which precluded further analysis. Another explanation of this relation that could appear contradictory is that blood glucose levels are not a good marker to evaluate diabetes control compared with HbA1c: blood glucose levels reflect an instantaneous level whereas HbA1c reflect diabetes control over the previous months. In almost all included studies, patients had blood sampling during hospitalisation, after a steady state and in a controlled environment for diet with a close monitoring of capillary blood glucose, which may explain our contradictory results. Another convincing point is that the relationships between HbA1c and HRV is logic. We demonstrated that higher levels of HbA1c were associated with shorter RR intervals, which were associated with an increased risk of ventricular arrhythmias [79]. There was also a tendency for higher LF/HF ratio (i.e. decreased HRV) with higher levels of HbA1c. Furthermore, time from diagnosis of T2DM was linked with a higher level of SDNN. Despite we demonstrated a decreased SDNN in T2DM patients, the metaregression is not contradictory but may simply highlights the fact that cardiac parasympathetic activity in T2DM is affected before sympathetic activity [80]. Time from diagnosis of T2DM was also linked with a lower level of total power, but not with LF and HF. Thus we can hypothesize that very low frequencies (VLF) could be decreased in T2DM. No studies included in our meta–analysis reported VLF. Although less commonly used, VLF are recognised as the most powerful independent predictors of mortality in patients with heart failure or in patients with chronic haemodialysis [81]. Despite few studies assessing VLF in diabetes, interesting relationships were reported between VLF and sleep apnea in diabetics [82]. The potential significance of VLF in diabetes should be studied further.
Other variables linked with HRV in T2DM
Similarly, total cholesterol was associated with both an increase in LF and HF, and HDL was associated with an increase in SDNN and RMSSD. To our knowledge, there is no data on hypercholesterolemia and HRV in the literature. Interestingly, some studies showed that a decrease in LDL by statin therapy could improve HRV parameters [83,84]. We demonstrated that an increase in systolic blood pressure was linked with shorter RR intervals and a decrease in HF. Despite no study previously assessing this relationship in diabetes, conflicting results were reported in the general population, with either high blood pressure associated with an increase in all spectral parameters [85], or a decrease in HRV [86]. It has also been suggested that the decrease in autonomic nervous function precedes the development of clinical hypertension [87]. Moreover, we found a significant relationship between BMI and HRV. Such relationships have been either found [88,89] or not [90,91] in the literature. However, the severity of obesity–related diseases is not directly linked to the accumulation of total body fat but rather to its distribution, and particularly to visceral localization [92]. HRV parameters have been previously correlated with sagittal abdominal diameter, anterior forearm skinfold thickness [93] and waist–hip ratio [90]. HRV parameters can also be improved after weight loss [94]. Finally, in line with the literature, we demonstrated a decrease in both LF and HF with age [95] and the male gender [96]. However, age and gender have a minor role on HRV parameters compared with the variables linked to T2DM.
Limitations
All meta–analyses have limitations [97]. Meta–analyses inherit the limitations of the individual studies of which they are composed and are subjected to a bias of selection of included studies. However, the use of broader keywords in the search strategy limits the number of missing studies. Despite our rigorous criteria for including studies in our meta–analysis, their quality varied. Indeed, most of the studies included were cross–sectional, with different measurement conditions for HRV parameters associated with a high inter and intra–individual variability. We demonstrated that all parameters measuring HRV were significantly decreased in T2DM compared with controls. Though there were similarities between the participants’ inclusion criteria, they were not identical. Moreover, the health status of controls was not detailed in all studies, which could have influenced HRV parameters. This may have also minimized the differences in HRV between T2DM patients and controls. In addition, some studies were monocentric, limiting the generalizability of our results. However, included studies were homogeneous according to funnel plots, and the populations investigated in the meta–analysis appeared to be equally distributed around the world. Similarly, the final number of patients included in the metaanalysis was not very high and may precluded generalizability, however the mean age could be considered as quite representative. Despite missing data, our meta–regressions demonstrated significant and interesting relationships, particularly between HRV parameters and variables linked with T2DM. Variables retrieved from declarative data in each study included are also a putative bias.
Conclusion
We reported strong evidence for an overall decrease in HRV in T2DM patients. Both sympathetic and parasympathetic activity were decreased, which can be explained by the deleterious effects of altered glucose metabolism on HRV. The benefits of an HRV evaluation in assessing and monitoring the severity of T2DM should be further studied, given its potential as a non–invasive, reliable and pain–free measurement.
Supporting information
(TIF)
(PDF)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by the University Hospital of Clermont-Ferrand, France.
References
- 1.da Rocha Fernandes J, Ogurtsova K, Linnenkamp U, Guariguata L, Seuring T, Zhang P, et al. IDF Diabetes Atlas estimates of 2014 global health expenditures on diabetes. Diabetes Res Clin Pract. 2016;117: 48–54. doi: 10.1016/j.diabres.2016.04.016 [DOI] [PubMed] [Google Scholar]
- 2.Meigs JB. Epidemiology of type 2 diabetes and cardiovascular disease: translation from population to prevention: the Kelly West award lecture 2009. Diabetes Care. 2010;33: 1865–1871. doi: 10.2337/dc10-0641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaacks LM, Siegel KR, Gujral UP, Narayan KMV. Type 2 diabetes: A 21st century epidemic. Best Pract Res Clin Endocrinol Metab. 2016;30: 331–343. doi: 10.1016/j.beem.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 4.Ginter E, Simko V. Global prevalence and future of diabetes mellitus. Adv Exp Med Biol. 2012;771: 35–41. [DOI] [PubMed] [Google Scholar]
- 5.Ginter E, Simko V. Type 2 diabetes mellitus, pandemic in 21st century. Adv Exp Med Biol. 2012;771: 42–50. [DOI] [PubMed] [Google Scholar]
- 6.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and Type 2 Diabetes: The American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33: e147–e167. doi: 10.2337/dc10-9990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox CS. Cardiovascular Disease Risk Factors, Type 2 Diabetes Mellitus, and the Framingham Heart Study. Trends Cardiovasc Med. 2010;20: 90–95. doi: 10.1016/j.tcm.2010.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laakso M. Cardiovascular Disease in Type 2 Diabetes From Population to Man to Mechanisms: The Kelly West Award Lecture 2008. Diabetes Care. 2010;33: 442–449. doi: 10.2337/dc09-0749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tancredi M, Rosengren A, Svensson A-M, Kosiborod M, Pivodic A, Gudbjörnsdottir S, et al. Excess Mortality among Persons with Type 2 Diabetes. N Engl J Med. 2015;373: 1720–1732. doi: 10.1056/NEJMoa1504347 [DOI] [PubMed] [Google Scholar]
- 10.Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Lancet Diabetes Endocrinol. 2015;3: 105–113. doi: 10.1016/S2213-8587(14)70219-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponto KA, Koenig J, Peto T, Lamparter J, Raum P, Wild PS, et al. Prevalence of diabetic retinopathy in screening-detected diabetes mellitus: results from the Gutenberg Health Study (GHS). Diabetologia. 2016;59: 1913–1919. doi: 10.1007/s00125-016-4013-5 [DOI] [PubMed] [Google Scholar]
- 12.Ksvh Kumar, Modi K, Basile A, Kota S. Profile of microvascular disease in type 2 diabetes in a tertiary health care hospital in India. Ann Med Health Sci Res. 2012;2: 103 doi: 10.4103/2141-9248.105654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hazari A, Maiya AG, Shivashankara KN, Agouris I, Monteiro A, Jadhav R, et al. Kinetics and kinematics of diabetic foot in type 2 diabetes mellitus with and without peripheral neuropathy: a systematic review and meta-analysis. SpringerPlus. 2016;5: 1819 doi: 10.1186/s40064-016-3405-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh JP, Larson MG, O’Donnell CJ, Wilson PF, Tsuji H, Lloyd-Jones DM, et al. Association of hyperglycemia with reduced heart rate variability (The Framingham Heart Study). Am J Cardiol. 2000;86: 309–312. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler D, Zentai CP, Perz S, Rathmann W, Haastert B, Döring A, et al. Prediction of mortality using measures of cardiac autonomic dysfunction in the diabetic and nondiabetic population: the MONICA/KORA Augsburg Cohort Study. Diabetes Care. 2008;31: 556–561. doi: 10.2337/dc07-1615 [DOI] [PubMed] [Google Scholar]
- 16.Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care. 1985;8: 491–498. [DOI] [PubMed] [Google Scholar]
- 17.Hufnagel C, Chambres P, Bertrand PR, Dutheil F. The Need for Objective Measures of Stress in Autism. Front Psychol. 2017;8 doi: 10.3389/fpsyg.2017.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boudet G, Walther G, Courteix D, Obert P, Lesourd B, Pereira B, et al. Paradoxical dissociation between heart rate and heart rate variability following different modalities of exercise in individuals with metabolic syndrome: The RESOLVE study. Eur J Prev Cardiol. 2017;24: 281–296. doi: 10.1177/2047487316679523 [DOI] [PubMed] [Google Scholar]
- 19.Dutheil F, Chambres P, Hufnagel C, Auxiette C, Chausse P, Ghozi R, et al. “Do Well B.”: Design Of WELL Being monitoring systems. A study protocol for the application in autism. BMJ Open. 2015;5: e007716–e007716. doi: 10.1136/bmjopen-2015-007716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solanki JD, Basida SD, Mehta HB, Panjwani SJ, Gadhavi BP. Comparative study of cardiac autonomic status by heart rate variability between under-treatment normotensive and hypertensive known type 2 diabetics. Indian Heart J. doi: 10.1016/j.ihj.2016.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lotric MB, Stefanovska A, Stajer D, Urbancic-Rovan V. Spectral components of heart rate variability determined by wavelet analysis. Physiol Meas. 2000;21: 441–457. [DOI] [PubMed] [Google Scholar]
- 22.Roy B, Ghatak S. Nonlinear methods to assess changes in heart rate variability in type 2 diabetic patients. Arq Bras Cardiol. 2013;101: 317–327. doi: 10.5935/abc.20130181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerritsen J, Dekker JM, TenVoorde BJ, Kostense PJ, Heine RJ, Bouter LM, et al. Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension, or a history of cardiovascular disease the Hoorn study. Diabetes Care. 2001;24: 1793–1798. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J. Effect of Age and Sex on Heart Rate Variability in Healthy Subjects. J Manipulative Physiol Ther. 2007;30: 374–379. doi: 10.1016/j.jmpt.2007.04.001 [DOI] [PubMed] [Google Scholar]
- 25.Saleem S, Hussain MM, Majeed SMI, Khan MA. Gender differences of heart rate variability in healthy volunteers. JPMA J Pak Med Assoc. 2012;62: 422–425. [PubMed] [Google Scholar]
- 26.Rothberg LJ, Lees T, Clifton-Bligh R, Lal S. Association Between Heart Rate Variability Measures and Blood Glucose Levels: Implications for Noninvasive Glucose Monitoring for Diabetes. Diabetes Technol Ther. 2016;18: 366–376. doi: 10.1089/dia.2016.0010 [DOI] [PubMed] [Google Scholar]
- 27.Kilit C, Demir S, Pasali Kilit T, Melek M. The effects of metformin and rosiglitazone on heart rate variability in type 2 diabetes mellitus. Int Soc Electrocardiol Annu Meet—Istanb 200734th Int Congr Electrocardiol. 2007;40: S40–S41.
- 28.Kumarathurai P, Anholm C, Larsen BS, Olsen RH, Madsbad S, Kristiansen O, et al. Effects of Liraglutide on Heart Rate and Heart Rate Variability: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Diabetes Care. 2016; dc161580. doi: 10.2337/dc16-1580 [DOI] [PubMed] [Google Scholar]
- 29.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93: 1043–1065. [PubMed] [Google Scholar]
- 30.da Costa BR, Cevallos M, Altman DG, Rutjes AWS, Egger M. Uses and misuses of the STROBE statement: bibliographic study. BMJ Open. 2011;1: e000048 doi: 10.1136/bmjopen-2010-000048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elm E v., Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335: 806–808. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340: c869–c869. doi: 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otzenberger H, Gronfier C, Simon C, Charloux A, Ehrhart J, Piquard F, et al. Dynamic heart rate variability: a tool for exploring sympathovagal balance continuously during sleep in men. Am J Physiol. 1998;275: H946–950. [DOI] [PubMed] [Google Scholar]
- 34.Benoist d’Azy C, Pereira B, Naughton G, Chiambaretta F, Dutheil F. Antibioprophylaxis in Prevention of Endophthalmitis in Intravitreal Injection: A Systematic Review and Meta-Analysis. PloS One. 2016;11: e0156431 doi: 10.1371/journal.pone.0156431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benoist d’Azy C, Pereira B, Chiambaretta F, Dutheil F. Oxidative and Anti-Oxidative Stress Markers in Chronic Glaucoma: A Systematic Review and Meta-Analysis. PloS One. 2016;11: e0166915 doi: 10.1371/journal.pone.0166915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Courtin R, Pereira B, Naughton G, Chamoux A, Chiambaretta F, Lanhers C, et al. Prevalence of dry eye disease in visual display terminal workers: a systematic review and meta-analysis. BMJ Open. 2016;6: e009675 doi: 10.1136/bmjopen-2015-009675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ollier M, Chamoux A, Naughton G, Pereira B, Dutheil F. Chest CT scan screening for lung cancer in asbestos occupational exposure: a systematic review and meta-analysis. Chest. 2014;145: 1339–1346. doi: 10.1378/chest.13-2181 [DOI] [PubMed] [Google Scholar]
- 38.Lanhers C, Pereira B, Naughton G, Trousselard M, Lesage F-X, Dutheil F. Creatine Supplementation and Upper Limb Strength Performance: A Systematic Review and Meta-Analysis. Sports Med. 2017;47: 163–173. doi: 10.1007/s40279-016-0571-4 [DOI] [PubMed] [Google Scholar]
- 39.Lanhers C, Pereira B, Naughton G, Trousselard M, Lesage F-X, Dutheil F. Creatine Supplementation and Lower Limb Strength Performance: A Systematic Review and Meta-Analyses. Sports Med. 2015;45: 1285–1294. doi: 10.1007/s40279-015-0337-4 [DOI] [PubMed] [Google Scholar]
- 40.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7: 177–188. [DOI] [PubMed] [Google Scholar]
- 41.Citrome L, Magnusson K. Paging Dr Cohen, Paging Dr Cohen... An effect size interpretation is required STAT!: visualising effect size and an interview with Kristoffer Magnusson. Int J Clin Pract. 2014;68: 533–534. doi: 10.1111/ijcp.12435 [DOI] [PubMed] [Google Scholar]
- 42.Porojan M, Costin S, Poantă L, Cerghizan A, Pop D, Dumitraşcu DL. Autonomic neuropathy and plasma catecholamine in patients with diabetes mellitus. Romanian J Intern Med Rev Roum Médecine Interne. 2010;48: 341–345. [PubMed] [Google Scholar]
- 43.Hathaway DK, Cashion AK, Wicks MN, Milstead EJ, Gaber AO. Cardiovascular dysautonomia of patients with end-stage renal disease and type I or type II diabetes. Nurs Res. 1998;47: 171–179. [DOI] [PubMed] [Google Scholar]
- 44.Kondo K, Matsubara T, Nakamura J, Hotta N. Characteristic patterns of circadian variation in plasma catecholamine levels, blood pressure and heart rate variability in Type 2 diabetic patients. Diabet Med J Br Diabet Assoc. 2002;19: 359–365. [DOI] [PubMed] [Google Scholar]
- 45.Perciaccante A, Fiorentini A, Paris A, Serra P, Tubani L. Circadian rhythm of the autonomic nervous system in insulin resistant subjects with normoglycemia, impaired fasting glycemia, impaired glucose tolerance, type 2 diabetes mellitus. BMC Cardiovasc Disord. 2006;6: 19 doi: 10.1186/1471-2261-6-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giordano M, Manzella D, Paolisso G, Caliendo A, Varricchio M, Giordano C. Differences in heart rate variability parameters during the post-dialytic period in type II diabetic and non-diabetic ESRD patients. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc—Eur Ren Assoc. 2001;16: 566–573. [DOI] [PubMed] [Google Scholar]
- 47.Rasic-Milutinovic ZR, Milicevic DR, Milovanovic BD, Perunicic-Pekovic GB, Pencic BD. Do components of metabolic syndrome contribute to cardiac autonomic neuropathy in non-diabetic patients? Saudi Med J. 2010;31: 650–657. [PubMed] [Google Scholar]
- 48.Burger AJ, Aronson D. Effect of diabetes mellitus on heart rate variability in patients with congestive heart failure. Pacing Clin Electrophysiol PACE. 2001;24: 53–59. [DOI] [PubMed] [Google Scholar]
- 49.Yasuda G, Hasegawa K, Kuji T, Ogawa N, Shimura G, Umemura S, et al. Effects of doxazosin on ambulatory blood pressure and sympathetic nervous activity in hypertensive Type 2 diabetic patients with overt nephropathy. Diabet Med J Br Diabet Assoc. 2005;22: 1394–1400. doi: 10.1111/j.1464-5491.2005.01636.x [DOI] [PubMed] [Google Scholar]
- 50.Wang G. Effects of impaired glucose metabolism on heart rate variability and blood pessure variability in essential hpertensive patients. J Huazhong Univ Sci Technol. 2006;26: 654–656. [DOI] [PubMed] [Google Scholar]
- 51.Poanta L, Porojan M, Dumitrascu DL. Heart rate variability and diastolic dysfunction in patients with type 2 diabetes mellitus. Acta Diabetol. 2011;48: 191–196. doi: 10.1007/s00592-011-0256-2 [DOI] [PubMed] [Google Scholar]
- 52.Mylonopoulou M, Tentolouris N, Antonopoulos S, Mikros S, Katsaros K, Melidonis A, et al. Heart rate variability in advanced chronic kidney disease with or without diabetes: midterm effects of the initiation of chronic haemodialysis therapy. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc—Eur Ren Assoc. 2010;25: 3749–3754. doi: 10.1093/ndt/gfq226 [DOI] [PubMed] [Google Scholar]
- 53.Comunello A, Dassie F, Martini C, De Carlo E, Mioni R, Battocchio M, et al. Heart rate variability is reduced in acromegaly patients and improved by treatment with somatostatin analogues. Pituitary. 2015;18: 525–534. doi: 10.1007/s11102-014-0605-6 [DOI] [PubMed] [Google Scholar]
- 54.Istenes I, Körei AE, Putz Z, Németh N, Martos T, Keresztes K, et al. Heart rate variability is severely impaired among type 2 diabetic patients with hypertension. Diabetes Metab Res Rev. 2014;30: 305–312. [DOI] [PubMed] [Google Scholar]
- 55.Nagy K, Sipos E, El Hadj Othmane T. [Heart rate variability is significantly reduced in non-diabetic patients with hypertension]. Orv Hetil. 2014;155: 865–870. doi: 10.1556/OH.2014.29886 [DOI] [PubMed] [Google Scholar]
- 56.Eguchi K, Schwartz JE, Pickering TG, Hoshide S, Ishikawa J, Shimada K, et al. Increased heart rate variability during sleep is a predictor for future cardiovascular events in patients with type 2 diabetes. Hypertens Res Off J Jpn Soc Hypertens. 2010;33: 737–742. doi: 10.1038/hr.2010.61 [DOI] [PubMed] [Google Scholar]
- 57.Vora R, Zareba W, Utell MJ, Pietropaoli AP, Chalupa D, Little EL, et al. Inhalation of ultrafine carbon particles alters heart rate and heart rate variability in people with type 2 diabetes. Part Fibre Toxicol. 2014;11: 31 doi: 10.1186/s12989-014-0031-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boer-Martins L, Figueiredo VN, Demacq C, Martins LC, de Faria APC, Moraes Cde H, et al. Leptin and aldosterone in sympathetic activity in resistant hypertension with or without type 2 diabetes. Arq Bras Cardiol. 2012;99: 642–648. [DOI] [PubMed] [Google Scholar]
- 59.Soydan N, Bretzel RG, Fischer B, Wagenlehner F, Pilatz A, Linn T. Reduced capacity of heart rate regulation in response to mild hypoglycemia induced by glibenclamide and physical exercise in type 2 diabetes. Metabolism. 2013;62: 717–724. doi: 10.1016/j.metabol.2012.12.003 [DOI] [PubMed] [Google Scholar]
- 60.Molon G, Costa A, Bertolini L, Zenari L, Arcaro G, Barbieri E, et al. Relationship between abnormal microvolt T-wave alternans and poor glycemic control in type 2 diabetic patients. Pacing Clin Electrophysiol PACE. 2007;30: 1267–1272. doi: 10.1111/j.1540-8159.2007.00849.x [DOI] [PubMed] [Google Scholar]
- 61.Akyel A, Çengel A, Tavil Y, Şahinarslan A, Topal S, Yayla Ç, et al. Relationship between plasma asymmetric dimethylarginine level and autonomic dysfunction in diabetic patients. Türk Kardiyol Derneği Arş Türk Kardiyol Derneğinin Yayın Organıdır. 2012;40: 148–154. [DOI] [PubMed] [Google Scholar]
- 62.Boer-Martins L, Figueiredo VN, Demacq C, Martins LC, Consolin-Colombo F, Figueiredo MJ, et al. Relationship of autonomic imbalance and circadian disruption with obesity and type 2 diabetes in resistant hypertensive patients. Cardiovasc Diabetol. 2011;10: 24 doi: 10.1186/1475-2840-10-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ozturk NA, Gokturk HS, Demir M, Erdogan D, Unler GK, Gur G, et al. The effect of autonomous neuropathy on bowel preparation in type 2 diabetes mellitus. Int J Colorectal Dis. 2009;24: 1407–1412. doi: 10.1007/s00384-009-0757-4 [DOI] [PubMed] [Google Scholar]
- 64.Li X, Yu S, Chen H, Lu C, Zhang K, Li F. Cardiovascular autonomic function analysis using approximate entropy from 24-h heart rate variability and its frequency components in patients with type 2 diabetes. J Diabetes Investig. 2015;6: 227–235. doi: 10.1111/jdi.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vukomanovic V, Tadic M, Suzic-Lazic J, Kocijancic V, Celic V. The relationship between heart rate variability and left ventricular layer-specific deformation in uncomplicated diabetic patients. Int J Cardiovasc Imaging. 2017;33: 481–490. doi: 10.1007/s10554-016-1023-9 [DOI] [PubMed] [Google Scholar]
- 66.Tadic M, Vukomanovic V, Cuspidi C, Suzic-Lazic J, Stanisavljevic D, Celic V. Left atrial phasic function and heart rate variability in asymptomatic diabetic patients. Acta Diabetol. 2017;54: 301–308. doi: 10.1007/s00592-016-0962-x [DOI] [PubMed] [Google Scholar]
- 67.Adlan AM, Paton JFR, Lip GYH, Kitas GD, Fisher JP. Increased sympathetic nerve activity and reduced cardiac baroreflex sensitivity in rheumatoid arthritis. J Physiol. 2017;595: 967–981. doi: 10.1113/JP272944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen X, Yang R, Kuang D, Zhang L, Lv R, Huang X, et al. Heart rate variability in patients with major depression disorder during a clinical autonomic test. Psychiatry Res. 2017;256: 207–211. doi: 10.1016/j.psychres.2017.06.041 [DOI] [PubMed] [Google Scholar]
- 69.Montaquila JM, Trachik BJ, Bedwell JS. Heart rate variability and vagal tone in schizophrenia: A review. J Psychiatr Res. 2015;69: 57–66. doi: 10.1016/j.jpsychires.2015.07.025 [DOI] [PubMed] [Google Scholar]
- 70.Studer V, Rocchi C, Motta C, Lauretti B, Perugini J, Brambilla L, et al. Heart rate variability is differentially altered in multiple sclerosis: implications for acute, worsening and progressive disability. Mult Scler J—Exp Transl Clin. 2017;3: 2055217317701317. doi: 10.1177/2055217317701317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Furlan R, Ardizzone S, Palazzolo L, Rimoldi A, Perego F, Barbic F, et al. Sympathetic overactivity in active ulcerative colitis: effects of clonidine. Am J Physiol Regul Integr Comp Physiol. 2006;290: R224–232. doi: 10.1152/ajpregu.00442.2005 [DOI] [PubMed] [Google Scholar]
- 72.Thorp AA, Schlaich MP. Relevance of Sympathetic Nervous System Activation in Obesity and Metabolic Syndrome. J Diabetes Res. 2015;2015: 341583 doi: 10.1155/2015/341583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liao D, Sloan RP, Cascio WE, Folsom AR, Liese AD, Evans GW, et al. Multiple metabolic syndrome is associated with lower heart rate variability. The Atherosclerosis Risk in Communities Study. Diabetes Care. 1998;21: 2116–2122. [DOI] [PubMed] [Google Scholar]
- 74.Malliani A, Montano N. Sympathetic overactivity in ischaemic heart disease. Clin Sci Lond Engl 1979. 2004;106: 567–568. doi: 10.1042/CS20040068 [DOI] [PubMed] [Google Scholar]
- 75.Julius S, Nesbitt S. Sympathetic overactivity in hypertension. A moving target. Am J Hypertens. 1996;9: 113S–120S. [DOI] [PubMed] [Google Scholar]
- 76.Niedermaier ON, Smith ML, Beightol LA, Zukowska-Grojec Z, Goldstein DS, Eckberg DL. Influence of cigarette smoking on human autonomic function. Circulation. 1993;88: 562–571. [DOI] [PubMed] [Google Scholar]
- 77.Fagundes CP, Murray DM, Hwang BS, Gouin J-P, Thayer JF, Sollers JJ, et al. Sympathetic and parasympathetic activity in cancer-related fatigue: More evidence for a physiological substrate in cancer survivors. Psychoneuroendocrinology. 2011;36: 1137–1147. doi: 10.1016/j.psyneuen.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stockhorst U, Huenig A, Ziegler D, Scherbaum WA. Unconditioned and conditioned effects of intravenous insulin and glucose on heart rate variability in healthy men. Physiol Behav. 2011;103: 31–38. doi: 10.1016/j.physbeh.2011.01.014 [DOI] [PubMed] [Google Scholar]
- 79.Chen-Scarabelli C, Scarabelli TM. Suboptimal glycemic control, independently of QT interval duration, is associated with increased risk of ventricular arrhythmias in a high-risk population. Pacing Clin Electrophysiol PACE. 2006;29: 9–14. doi: 10.1111/j.1540-8159.2006.00298.x [DOI] [PubMed] [Google Scholar]
- 80.Goit RK, Khadka R, Sharma SK, Limbu N, Paudel BH. Cardiovascular autonomic function and vibration perception threshold in type 2 diabetes mellitus. J Diabetes Complications. 2012;26: 339–342. doi: 10.1016/j.jdiacomp.2012.03.026 [DOI] [PubMed] [Google Scholar]
- 81.Huang J-C, Kuo I-C, Tsai Y-C, Lee J-J, Lim L-M, Chen S-C, et al. Heart Rate Variability Predicts Major Adverse Cardiovascular Events and Hospitalization in Maintenance Hemodialysis Patients. Kidney Blood Press Res. 2017;42: 76–88. doi: 10.1159/000469716 [DOI] [PubMed] [Google Scholar]
- 82.Amra B, Behjati M, Penzel T, Fietze I, Schoebel C, Sarrafzadegan N. Nocturnal heart rate variation in diabetic and non-diabetic patients with sleep apnea syndrome. Sleep Med. 2017;29: 57–60. doi: 10.1016/j.sleep.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 83.Pehlivanidis AN, Athyros VG, Demitriadis DS, Papageorgiou AA, Bouloukos VJ, Kontopoulos AG. Heart rate variability after long-term treatment with atorvastatin in hypercholesterolaemic patients with or without coronary artery disease. Atherosclerosis. 2001;157: 463–469. doi: 10.1016/S0021-9150(00)00746-2 [DOI] [PubMed] [Google Scholar]
- 84.Badea AR, Nedelcu L, Valeanu M, Zdrenghea D. The relationship between serum lipid fractions and heart rate variability in diabetic patients with statin therapy. Clujul Med. 2014;87: 152 doi: 10.15386/cjmed-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Askin L, Cetin M, Turkmen S. Ambulatory blood pressure results and heart rate variability in patients with premature ventricular contractions. Clin Exp Hypertens. 2017; 1–6. doi: 10.1080/10641963.2017.1356846 [DOI] [PubMed] [Google Scholar]
- 86.de Andrade PE, do Amaral JAT, Paiva L da S, Adami F, Raimudo JZ, Valenti VE, et al. Reduction of heart rate variability in hypertensive elderly. Blood Press. 2017; 1–9. doi: 10.1080/08037051.2017.1354285 [DOI] [PubMed] [Google Scholar]
- 87.Schroeder EB, Liao D, Chambless LE, Prineas RJ, Evans GW, Heiss G. Hypertension, blood pressure, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Hypertens Dallas Tex 1979. 2003;42: 1106–1111. doi: 10.1161/01.HYP.0000100444.71069.73 [DOI] [PubMed] [Google Scholar]
- 88.Al-Trad BA, Faris M a. IE, Al-Smadi M, Bashir A, Mansi M, Alaraj M, et al. Cardiac autonomic dysfunction in young obese males is not associated with disturbances in pituitary-thyroid axis hormones. Eur Rev Med Pharmacol Sci. 2015;19: 1689–1695. [PubMed] [Google Scholar]
- 89.Mustafa G, Kursat FM, Ahmet T, Alparslan GF, Omer G, Sertoglu E, et al. The relationship between erythrocyte membrane fatty acid levels and cardiac autonomic function in obese children. Rev Port Cardiol Orgao Of Soc Port Cardiol Port J Cardiol Off J Port Soc Cardiol. 2017;36: 499–508. doi: 10.1016/j.repc.2016.10.013 [DOI] [PubMed] [Google Scholar]
- 90.Yadav RL, Yadav PK, Yadav LK, Agrawal K, Sah SK, Islam MN. Association between obesity and heart rate variability indices: an intuition toward cardiac autonomic alteration—a risk of CVD. Diabetes Metab Syndr Obes Targets Ther. 2017;Volume 10: 57–64. doi: 10.2147/DMSO.S123935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoo HJ, Hwang SY, Choi KM, Baik SH, Lee EM, Kim EJ, et al. Clinical implication of body size phenotype on heart rate variability. Metabolism. 2016;65: 1589–1596. doi: 10.1016/j.metabol.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 92.Dutheil F, Gordon BA, Naughton G, Crendal E, Courteix D, Chaplais E, et al. Cardiovascular risk of adipokines: a review. J Int Med Res. 2017; 300060517706578. doi: 10.1177/0300060517706578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rastović M, Srdić-Galić B, Barak O, Stokić E. Association between anthropometric measures of regional fat mass and heart rate variability in obese women: Anthropometry and heart rate variability in obese women. Nutr Diet. 2017;74: 51–60. doi: 10.1111/1747-0080.12280 [DOI] [PubMed] [Google Scholar]
- 94.Karason K, Mølgaard H, Wikstrand J, Sjöström L. Heart rate variability in obesity and the effect of weight loss. Am J Cardiol. 1999;83: 1242–1247. doi: 10.1016/S0002-9149(99)00066-1 [DOI] [PubMed] [Google Scholar]
- 95.Reardon M, Malik M. Changes in heart rate variability with age. Pacing Clin Electrophysiol PACE. 1996;19: 1863–1866. [DOI] [PubMed] [Google Scholar]
- 96.Pavithran P, Madanmohan T, Nandeesha H. Sex differences in short-term heart rate variability in patients with newly diagnosed essential hypertension. J Clin Hypertens Greenwich Conn. 2008;10: 904–910. doi: 10.1111/j.1751-7176.2008.00052.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.LeLorier J, Grégoire G, Benhaddad A, Lapierre J, Derderian F. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med. 1997;337: 536–542. doi: 10.1056/NEJM199708213370806 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(PDF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.