Abstract
In this study, semiquantitative reverse transcription–PCR analysis showed that estrogen receptor α (ERα) and β (ERβ) mRNAs are developmentally regulated in the rat heart. We found that ERα mRNA was low in all heart chambers of 4-day-old rats, but was elevated in the atria (6- to 18-fold) and ventricles (3- to 4-fold) of adult rats. Western blotting analysis confirmed that these differences were efficiently translated into 67-kDa ERα protein. ERβ mRNA was expressed at its highest level in the left atrium and was 3- to 4-fold lower in other heart chambers of 4-day-old animals. In adult rats ERβ was decreased dramatically in the left atrium (20-fold) and, to a lesser extent in the other heart chambers (2- to 4-fold). Significant ER changes occurred already in the first week after birth. Accordingly, estrogen regulation in cells from neonatal hearts, as reported in several studies, may not correspond to that occurring in fully differentiated adult hearts, because of an altered degree of ER expression. In adult rats, ovariectomy decreases atrial ERα, the atria/body weight ratio, and atrial natriuretic peptide (ANP) transcription. Treatment of ovariectomized rats with 17-β-estradiol (25 μg, 10 days, s.c.) reversed these changes. In addition, there was no effect of ovariectomy and 17-β-estradiol supplementation on systolic blood pressure, but in ovariectomized rats a decreased heart rate followed 17-β-estradiol administration. Similar to the effects on ERα in the atria, ovariectomy lowered plasma ANP levels, and 17-β-estradiol administration restored ANP in the plasma of ovariectomized rats. Changes in plasma ANP correlated with changes in ANP content in the right atrium, as demonstrated by RIA. Increased ANP expression and secretion in response to ERα activation may be a protective mechanism in the heart.
Atrial natriuretic peptide (ANP) is a cardiac hormone produced predominantly in heart atria (1). It acts primarily on the ANP A receptor, that is, guanylyl cyclase, which converts GTP to cGMP. cGMP mediates the actions of ANP via protein kinase G. ANP plays an integral role in the regulation of hydromineral homeostasis under normal and pathological conditions, through potent biological effects, including vasorelaxation, diuresis, natriuresis, and reduction of venous return by a shift of plasma into the interstitium. The natriuretic and diuretic actions of ANP result from enhanced glomerular filtration and/or decreased tubular reabsorption of sodium and water, suppression of renin, aldosterone, and vasopressin secretion, as well as antagonism of most of the peripheral and central effects of angiotensin II. The heart not only secretes ANP but also expresses ANP receptors, which control heart functions through cGMP production in cardiomyocytes and fibroblasts (2).
The ANP gene is strongly activated in response to hypertrophic stimuli in the heart and prevents hypertrophy by inhibition of protein synthesis in cardiac myocytes via a cGMP-dependent process (3). Mice lacking ANP or its guanylyl cyclase A receptor exhibit marked cardiac hypertrophy that is disproportionately large for the very modest blood pressure increases found in these animals because of the loss of the vasodilating action of cGMP (4, 5). In addition, chronic normalization of blood pressure in these animals by administration of antihypertensive agents does not decrease the degree of cardiac hypertrophy (6). Interestingly, the hypertrophy is more evident in the atria than in the ventricles of these knockout mice, and hearts from knockout male mice are considerably more enlarged than those of female mice (4, 5), suggesting a parallel role of estrogens and ANP in the prevention of cardiac hypertrophy. The colocalization of estrogen receptors (ERs) and ANP in cardiac myocytes (7) and stimulation of ANP secretion from the isolated, perfused rat right atrium by 17-β-estradiol (8) provide arguments for the hypothesis that ANP acts as a mediator of estrogen action. In addition, ANP induces vasorelaxation of coronary arteries (9), inhibition of L-type Ca+2 channels in the myocardium (10), suppression of the renin-angiotensin system (11), and decreased sympathetic nervous system activity (12). These effects are recognized as being protective to the cardiovascular system and are also induced by estrogen (13).
Estrogen belongs to a class of steroid hormones that regulate target cells upon binding to intracellular and membrane receptors. Estrogen binding causes conformational changes to ERs, with homodimerization and attachment to cellular proteins serving as transcriptional coactivators (14). The dimeric receptors exhibit high affinity for specific DNA sequences sensitive to ER regulation. The result is alteration of cellular mRNA as well as changes in the type and level of cellular proteins (15). Although earlier studies showed binding of radiolabeled 17-β-estradiol to the heart (16), the presence of ER, required for the genomic effects on DNA and protein synthesis, was demonstrated only recently in cardiac myocytes and fibroblasts (17). Because of this ER localization, estrogen has been proposed to play an important role in cardiac hypertrophy and remodeling after myocardial infarction (18). On the other hand, studies of reperfusion injury in isolated, perfused hearts revealed that estrogen treatment of ovariectomized rats prevented myocardial damage and interstitial edema formation (19, 20). Furthermore, treatment of ovariectomized rats with 17-β-estradiol induces nitric oxide synthase in cardiac myocytes (17) and reduces heart contractility by inhibition of L-type Ca2+ channel activity (21, 22), which mediates negative inotropic actions on these cells (15).
Estradiol mediates gene transcription through the activation of two receptors: ERα and ERβ (23). ERβ is 96% identical to classical ERα in its DNA-binding domain (23) but lacks homology (18%) in the N-terminal transactivation domain. This lack of homology led to speculation that the two ERs may differentially regulate various genes (23). Recent observations suggest that estrogen effects in vascular cells, and possibly in the myocardium, depend on the relative expression of ERα and ERβ (24). However, the roles of ERα and/or ERβ in cardiac functions are not known.
Because of the abundance of ER in atria but not in the ventricles of adult rats (16) and the presence of ER in myocytes secreting ANP (7), we hypothesize that estrogen induces ANP synthesis and release from the heart through an ER-dependent mechanism. Because ANP transcription changes during maturation, we investigated ERα and/or ERβ in newborn and adult rats. Subsequently, in ovariectomized rats, we evaluated the effect of 17-β-estradiol deficit and supplementation on ERα and/or ERβ activation in the atria and ventricles, and on cardiac hypertrophy, blood pressure, heart rate, ANP synthesis, and release.
Materials and Methods
Animals.
Experiments were conducted in accordance with guidelines of the Canadian Council on Animal Care with the approval of the institutional animal care committee of Centre Hospitalier de l'Université de Montréal. Sprague–Dawley rats were obtained from Charles River Breeding Laboratories. The ER developmental study was performed in dissected heart compartments obtained from 4- and 5-day-old male and female rats.
Estrogen effects also were investigated 14 days after surgery in ovariectomized rats and sham-operated controls (200–225 g). The serum estrogen concentrations increased by 2- to 3-fold between implantation (days 6–7 after copulation) and day 18 of rat pregnancy (25). This increase in serum estrogen is associated with plasma ANP elevation (26). To mimic this increase in both hormones, ovariectomized rats were treated with 17-β-estradiol. Two weeks after ovariectomy 17-β-estradiol injections were given (s.c.) daily for 10 days to rats randomly allocated to one of three treatment groups: (i) sham-operated controls receiving 100 μl of sunflower oil (vehicle); (ii) ovariectomized controls receiving 100 μl of vehicle; and (iii) ovariectomized rats receiving 25 μg of 17-β-estradiol acetate in 100 μl of vehicle. All three groups were housed in pairs in a light-controlled room (12 h light, 12 h dark) with ad libitum access to tap water and food. Body weight was recorded every 2 days. At the end of the experiment, the rats were decapitated, and their hearts were rapidly removed and blotted dry. The heart weight was recorded, after dissection of the left and right atria, right ventricle, and left ventricle with septum. Total heart, atria, and ventricle weight-to-body weight ratio expressed the degree of myocardial hypertrophy.
Plasma estrogen levels and the absence of the ovaries and uterine atrophy at necropsy verified the adequacy of ovariectomy. Bilateral ovariectomy produced a plasma 17-β-estradiol level of 5.7 ± 1.0 pg/ml, approximating values in similar studies (20), which indicate an extraovarian source of estradiol in plasma. This level increased to values occurring during early rat pregnancy (122.1 ± 13.3 pg/ml) after 17-β-estradiol administration, whereas the plasma of sham-operated controls contained 19.5 ± 3.9 pg/ml of 17-β-estradiol.
Physiologic Measurements.
Systolic blood pressures and heart rates were recorded by the tail-cuff method with the Visitech BP-2000 system (Apex, NC) after 3 days of training before injection and on the last days of the experiment from 9 a.m. to 11 a.m. daily.
ANP RIA.
To quantify ANP in plasma, 2 ml of blood was collected in chilled tubes containing 1 mg/ml EDTA, 10 mM PMSF (P7626; Sigma), and 5 mM pepstatin A (P4265; Sigma). After centrifugation for 20 min in 4°C at 4,000 rpm, the separated plasma was extracted immediately. Plasma ANP was measured by RIA after extraction in C18 Sep-Pak cartridges (Waters), as described (27).
For the determination of tissue ANP concentration, the heart chambers were homogenized in 0.1 M acetic acid containing protease inhibitors to a final concentration of 1 mg/ml of EDTA, 5 μM pepstatin A, and 10 μM PMSF, at 4°C in a Polytron homogenizer (setting: 10 × 3 for 20 s). After 20 min of centrifugation at 30,000 × g, the pellet was washed and rehomogenized in the same buffer. ANP concentration was determined by direct RIA in serial dilutions of the homogenates (27). The values were normalized to protein concentration and reported as μg/mg protein (28).
Semiquantitative Reverse Transcription–PCR (RT-PCR).
Total RNA was isolated from the rat hearts with the use of Trizol reagent (Life Technologies, Rockville, MD) according to the manufacturer's specifications. Total RNA was then treated with RNase-free DNase I under a standard protocol. The integrity and quality of the purified RNA were controlled by formaldehyde denaturing agarose gel electrophoresis and measurement of the A260/A280 ratio. First-strand cDNA was synthesized in a final volume of 40 μl containing first-strand buffer, 2 μg rat cardiac, or uterine RNA used as a control; 2 μg hexanucleotide primer (Amersham Pharmacia); and avian myeloblastosis virus reverse transcriptase (12 units/μg RNA; Life Technologies). Ten microliters of first-strand cDNA was added to a PCR mixture and amplified for 25–33 cycles by incubation at 95°C for 1 min, 57oC for 1 min, and 72°C for 1.5 min, with a final incubation at 72°C for 3 min, all in a Robocycler gradient 40 thermocycler (Stratagene).
The methodology of semiquantitative RT-PCR and primer selection for ERα and ERβ transcript amplifications was adapted from Kuiper et al. (29). A 262-bp fragment of ERβ mRNA was amplified with the oligonucleotides erbkg1: 5′-TTCCCGGCAGCACCAGTAACC (+38 relative to ATG) and erbkg2: 5′-TCCCTCTTTGCGTTTGGACTA (+279 relative to ATG). A 344-bp fragment of rat ERα mRNA was amplified with the oligonucleotides kgb5: 5′-AATTCTGACAATCGACGCCAG (+472 relative to ATG) and kgb6: 5′-GTGCTTCAACATTCTCCCTCCTC (+794 relative to ATG). The oligonucleotides used for amplification of 18S RNA followed the manufacturer's protocol (Ambion, Austin, TX). Each cycle involved incubation at 94°C for 1 min, 59°C for 1 min, and 72°C for 2 min followed by a 5-min final extension at 72°C. For PCR quantitation, 10 μCi of [32P]d-CTP was added to PCR buffer. Ten microliters of the PCR products was electrophoresed on 1.5 agarose gel and vacuum-transferred onto Hybond N+ nylon membranes (Amersham Pharmacia) in 1.5 M NaCl and 0.5 M NaOH. For control of specificity of PCR amplification, after agarose gel electrophoresis and blotting to nitrocellulose filters, the unlabeled PCR products were hybridized to the internal oligonucleotides, ERUR 4: 5′-GGGACTCTTTTGAGGTTCTGC (+163–182 relative to ATG) for ERβ, and KG50: 5′-GCAGCGAGAAGGGAAACATGA (+518–538 relative to ATG) for ERα. RT-PCR for ANP transcript was performed according to the method of Chen et al. (30). Control RT-PCRs were conducted by omitting reverse transcriptase or RNA from the reaction mixture. Radioactive bands were counted and analyzed with the Storm 840 Imaging System and imagequant software (Version 4.2; Molecular Dynamics). To validate the use of this RT-PCR assay as a tool for the semiquantitative measurement of ER mRNA, dose–response curves were established for different amounts of total RNA extracted from heart tissue, and the samples were quantitated in the curvilinear phase of PCR amplification. These data were normalized to the corresponding values of 18S RNA PCR products in the same samples.
Western Blot Analysis.
Tissue samples were homogenized in sucrose buffer (20 mM Hepes/Tris with 250 mM sucrose, pH 7.4) and then centrifuged at 3,000 × g for 10 min at 4°C to remove cellular debris. The supernatant was centrifuged at 100,000 × g for 30 min at 4°C, and the pellet was suspended in sucrose buffer. The protein concentration was determined by a modified Bradford assay (27). An aliquot (25 μg) was subjected to SDS/PAGE under reducing conditions. The stacking gels contained 5% (wt/vol) acrylamide, and the separating gels were composed of 8% (wt/vol) acrylamide. The proteins were electrophoretically transferred from the gels onto nitrocellulose paper (Hybond-C; Amersham Pharmacia). The nitrocellulose blots were blocked with 5% (wt/vol) nonfat milk in Tris-buffered saline, containing 20 mmol/liter Tris⋅Cl (pH 8.0), 140 mmol/liter NaCl, and 0.05% (wt/vol) Tween-20. The membranes were then probed with a mouse mAb (1:2,000) directed against ERα (catalog no. sc-8002, Santa Cruz Biotechnology) or goat polyclonal antibody (1:1,000) directed against ERβ (catalog no. sc-6822, Santa Cruz Biotechnology). All antibody incubations and washes were performed in Tris-buffered saline with 0.05% Tween-20. The Amersham Pharmacia enhanced chemiluminescence system was used for detection. Membranes were visualized by exposure to Kodak X-Omat film.
Results
Differential Expression of ERs in Newborn and Adult Rat Hearts.
With the use of RT-PCR analysis, ERα and ERβ mRNA were detected in all heart chambers of adult and newborn rats (Fig. 1A). The resulting 344-bp and 262-bp PCR products were amplified from cardiac cDNA but not from corresponding RNA samples and confirmed as being ERα and ERβ by Southern blotting, with the use of a nested oligonucleotide probe. Additional controls included DNase treatment of RNA extracts before RT-PCR. The possibility that artificial products were amplified was further controlled by incomplete RT-PCRs, including omission of DNA polymerase or one of the primers. Total RNA samples from the hearts of newborn (1-day-old) rats were included in each experiment and used as standards (100%) for the calibration of ERα mRNA and ERβ mRNA levels.
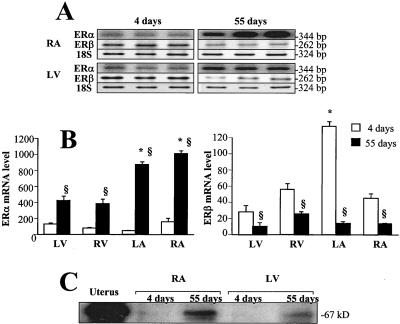
Figure 1.
RT-PCR analysis of ERα and β transcripts in the heart chambers of newborn (4-day-old) and adult (55-day-old) female rats. Total RNA (1 μg) was reverse-transcribed, and cDNA was exponentially amplified in the presence of gene-specific primers. (A) A PhosphorImager scan after separation of the radioactive PCR product by electrophoresis in 1.5% agarose. Representative bands obtained from three RNA samples of the right atrium (RA) and left ventricle (LV) are shown. (B) Bar graphs depict ERα mRNA (Left) and ERβ mRNA (Right) levels verified by 18S RNA product used as internal standard. The results were calculated as a ratio of the signal given by radioactive bands of ERα mRNA or ERβ mRNA to the corresponding 18S band. RNA samples from the whole heart of newborn (1-day-old) rats were included in each experiment and used as a standard (100%) for calibration of ERα mRNA or ERβ mRNA levels. Values are means ± SEM for the right atrium and left ventricle, as well as for the left atrium (LA) and right ventricle (RV). n = 6; §, P < 0.05 vs. 4-day-old; *, P < 0.05 vs. LV. (C) Expression of ERα protein in the right atrium and left ventricle of 4- and 55-day-old female rats. Twenty-five-microgram protein homogenates of the right atrium and left ventricle were subjected to SDS/PAGE, immunoblotted with an anti-ERα monoclonal antibody, and visualized by the chemiluminescence technique. The uterus homogenate was included as a positive control.
As shown in Fig. 1B Left, the highest levels of ERα mRNA were detected in the right atrium and left ventricle of 4-day-old rats with the lowest levels (2- to 3-fold, P < 0.05) in the left atrium. The ERα mRNA levels increased by 6- to 18-fold in the atria and by only 2- to 5-fold in the ventricles of rats on the 55th day of age, as compared with the values in 4-day-old animals. Relatively high ERα mRNA expression was found in adult rat atria in comparison with that in the ventricles. The expression of ERα mRNA was found to be significantly higher in the right than in the left atrium (P < 0.05).
Similar ERβ mRNA analysis revealed age-related changes opposite those of ERα mRNA (Fig. 1B, Right). In 4-day-old rats, ERβ mRNA was about 2- to 3-fold lower than in newborn hearts, with the exception of the left atrium. ERβ mRNA was expressed at its highest level in the left atrium and was 3- to 4-fold lower in the other heart chambers of 4-day-old rats. In adult rats ERβ dramatically decreased in the left atrium (20-fold) and, to a lesser extent, in the other heart chambers (2- to 4-fold). Finally, in the adult heart, ERβ mRNA levels were similarly expressed in the left ventricle and both atria and were ≈2-fold higher in the right ventricle (P < 0.05). Comparison of changes in ER mRNA in the heart chambers revealed the most dramatic changes in both ERα and ERβ mRNA in the left atrium.
Because the presence of mRNA transcripts alone does not prove similar regulation of a translated product, we measured ER proteins. By Western blot analysis, we confirmed that increases in cardiac ERα transcripts are efficiently translated into 67-kDa ERα protein, which corresponds to the full sequence of the ERα molecule (Fig. 1C). Because of its low expression, ERβ was undetectable by Western blotting in rat heart extracts at all ages.
Effects of Ovariectomy and 17-β-Estradiol Supplementation.
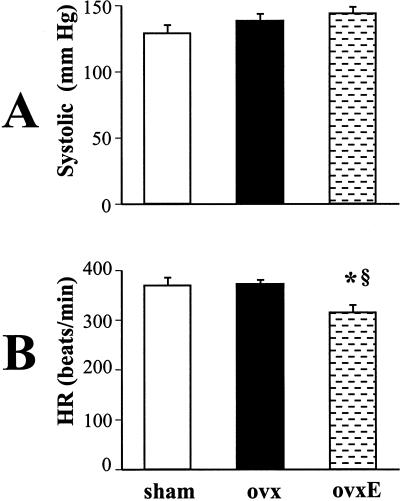
Another set of experiments elucidated the role of estrogen in the control of blood pressure, heart rate, heart, and body weight. Basal systolic blood pressure and heart rate were not different in sham-operated and ovariectomized rats. Pressure and heart rate measurements obtained before death (Fig. 2) show that administration of 17-β-estradiol to ovariectomized rats did not change blood pressure (138 ± 6 vs. 143 ± 5 mmHg). On the other hand, blood pressure was lowest in sham-operated animals (128 ± 9 mmHg). However, this result was not significantly different from that in ovariectomized rats treated with 17-β-estradiol or ovariectomized controls treated with vehicle. 17-β-Estradiol administration significantly decreased heart rate in ovariectomized rats to 314 ± 16 beats per min in comparison with vehicle-treated, ovariectomized (371 ± 9 beats per min, P < 0.05) or sham-operated controls (369 ± 9 beats per min, P < 0.05).
Figure 2.
Systolic blood pressure (A) and heart rate (B) in sham-operated (sham), ovariectomized (ovx), and ovariectomized rats supplemented with 17-β-estradiol (ovxE). n = 10; *, P < 0.05 vs. sham; §, P < 0.05 vs. ovx.
Table 1 shows that ovariectomized rats gained an average of 30 g more in body weight compared with their age- and weight-matched sham-operated controls. 17-β-Estradiol administration to ovariectomized animals decreased body weight by 9% compared with vehicle-treated, ovariectomized rats (P < 0.05), and their body weight was similar to that of sham-operated controls. However, total heart weight was comparable in control and 17-β-estradiol-treated, ovariectomized groups (872 ± 18 mg vs. 857 ± 23 mg, respectively) and was significantly higher than that of sham-operated controls (810 ± 21, P < 0.05). Thus, 17-β-estradiol treatment resulted in a higher heart-to-body weight ratio in ovariectomized rats (3.29 ± 0.08 mg/g) than in vehicle-treated controls (3.05 ± 0.05 mg/g, P < 0.05) that was not different from that of sham-operated animals (3.17 ± 0.04 mg/g). Further analysis demonstrated that the atria-to-body weight ratio was 35–40% lower in the ovariectomized, vehicle-treated group (0.19 ± 0.01 mg/g) than in sham-operated controls (0.32 ± 0.02 mg/g, P < 0.05) and ovariectomized rats receiving 17-β-estradiol (0.29 ± 0.02 mg/g, P < 0.05). In contrast, the ventricle-to-body weight ratio was comparable in the ovariectomized, vehicle-treated (2.84 ± 0.04 mg/g) and sham-operated groups (2.84 ± 0.04 mg/g) without a significant difference from ovariectomized rats treated with 17-β-estradiol (3.00 ± 0.08 mg/g). These results suggest that circulating 17-β-estradiol in plasma specifically targets the atria, which correlates with the predominant expression of ERα in these cardiac chambers.
Table 1.
Body and organ weight, and organ weight/body weight ratio in sham-operated, ovariectomized, and ovariectomized rats supplemented with 17-β-estradiol
| Sham-operated n =
20
|
Ovariectomized n =
10
|
Ovariectomized + E2n = 10
|
||||
|---|---|---|---|---|---|---|
| Weight | Organ/BW, mg/g | Weight | Organ/BW, mg/g | Weight | Organ/BW, mg/g | |
| Body weight, g | 255 ± 2 | — | 285 ± 3* | — | 260.6 ± 3† | — |
| Uterus, mg | 448 ± 77 | 1.75 ± 0.30 | 119 ± 2* | 0.42 ± 0.01* | 292 ± 10† | 1.12 ± 0.03*† |
| Heart, mg | 810 ± 21 | 3.17 ± 0.04 | 872 ± 18* | 3.05 ± 0.05 | 857 ± 23* | 3.29 ± 0.08† |
| Left atrium, mg | 40 ± 2 | 0.16 ± 0.01 | 24 ± 2* | 0.08 ± 0.01* | 31 ± 3* | 0.12 ± 0.01*† |
| Right atrium, mg | 42 ± 3 | 0.17 ± 0.01 | 35 ± 5 | 0.12 ± 0.02* | 45 ± 4 | 0.17 ± 0.02† |
| Atria, mg | 83 ± 4 | 0.32 ± 0.02 | 59 ± 5* | 0.19 ± 0.01* | 76 ± 4† | 0.29 ± 0.02† |
| Left ventricle, mg | 599 ± 10 | 2.35 ± 0.03 | 668 ± 10* | 2.30 ± 0.03 | 633 ± 20 | 2.40 ± 0.07 |
| Right ventricle, mg | 128 ± 5 | 0.51 ± 0.02 | 145 ± 7 | 0.51 ± 0.02 | 148 ± 8* | 0.57 ± 0.03* |
| Ventricles, mg | 728 ± 10 | 2.84 ± 0.04 | 813 ± 17* | 2.84 ± 0.04 | 781 ± 23* | 3.00 ± 0.08 |
E2, 17-β-estradiol.
, P < 0.05 vs. sham-operated.
, P < 0.05 vs. ovariectomized control.
ERs and ANP in Response to Ovariectomy and 17-β-Estradiol Treatment.
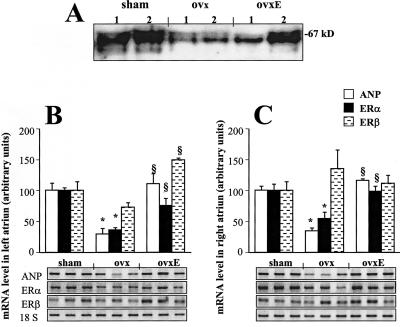
ER mRNA expression in the heart does not indisputably confirm the ability to respond directly to estrogen exposure. Therefore, experiments were performed to verify the hypothesis that estradiol effects on the atria/body weight ratio are reflected by ER changes. Indeed, as demonstrated in Fig. 3A, Western blot analysis revealed that 67-kDa protein encoding ERα decreased in the left and right atria after ovariectomy, and 17-β-estradiol treatment of ovariectomized rats increased ERα to normal levels in the right atrium and partially in the left atrium. The changes in ERα protein paralleled the changes in ERα mRNA (Fig. 3 B and C). Neither ovariectomy nor estradiol supplementation changed ERβ mRNA level in the right atrium (Fig. 3C), but in the left atrium (Fig. 3B) 17-β-estradiol administration to ovariectomized rats resulted in increased ERβ mRNA. ANP mRNAs also paralleled the results of ERα mRNA (Fig. 3 B and C), indicating that ANP synthesis is associated with the plasma 17-β-estradiol level as well as with ERα expression in the atria.
Figure 3.
(A) ERα protein expression in atria of sham-operated (sham), ovariectomized (ovx), and ovariectomized rats supplemented with 17-β-estradiol (ovxE). Twenty-five micrograms of protein homogenates of the left atrium (1) and right atrium (2) were subjected to SDS/PAGE, immunoblotted with an anti-ERα mAb, and visualized by the chemiluminescence technique. (B and C) RT-PCR analysis of ER transcripts and ANP in the left atrium (B) and right atrium (C) of sham-operated (sham), ovariectomized (ovx), and ovariectomized rats supplemented with 17-β-estradiol (ovxE). Total RNA (1 μg) was reverse-transcribed and cDNA was exponentially amplified in the presence of gene-specific primers. A PhosphorImager scan is presented after separation of the radioactive PCR product by electrophoresis in 1.5% agarose. Representative bands obtained in three RNA representative samples from sham, ovx, and ovxE rats are presented. The results were calculated as the ratio of the signal given by radioactive bands of ERα mRNA or ERβ mRNA to the corresponding 18S band. Values are means ± SEM adjusted to the values (in percentage) of sham-operated rats (100%). n = 6; *, P < 0.05 vs. sham; §, P < 0.05 vs. ovx.
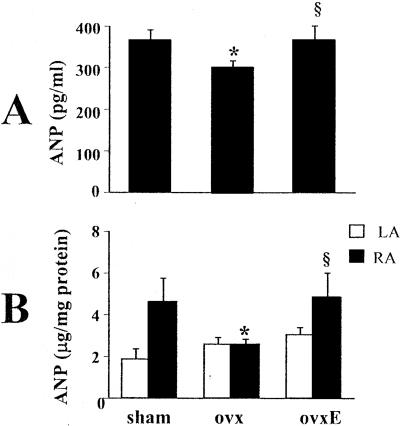
As shown in Fig. 4A, these ANP mRNA changes were paralleled by small but significant variations in plasma ANP levels. In ovariectomized, vehicle-treated rats, plasma ANP concentrations (300 ± 16 pg/ml) were lower than in sham-operated controls (365 ± 26 pg/ml, P < 0.05). Treatment with 17-β-estradiol significantly increased ANP in the plasma of ovariectomized rats (375 ± 37 pg/ml, P < 0.05 vs. vehicle-treated controls). RIA revealed that ovariectomy and estrogen treatment decreased and increased, respectively, ANP concentrations in the right but not in the left atrium (Fig. 4B). ANP in the right atrium decreased from 4.6 ± 1.1 μg/mg protein in sham-operated controls to 2.5 ± 0.31 μg/mg protein in ovariectomized animals (P < 0.05) and increased significantly in 17-β-estradiol-treated, ovariectomized rats to 4.8 ± 1.8 μg/mg protein (P < 0.05), similar to that in the sham-operated group.
Figure 4.
Effect of rat ovariectomy and 17-β-estradiol supplementation on the ANP level in plasma (A) and on ANP concentration (B) in the left atrium (LA) and the right atrium (RA). The results are presented in samples from sham-operated (sham), ovariectomized (ovx), and ovariectomized rats supplemented with 17-β-estradiol (ovxE). n = 6; *, P < 0.05 vs. sham; §, P < 0.05 vs. ovx.
Discussion
The existence of two ER subtypes raises the possibility that variation in the presence and relative extent of ERs or ER-specific mechanisms mediates tissue-specific and physiological state-specific actions of estrogen. In this study, with the use of semiquantitative RT-PCR analysis and Western blotting, we discovered that ERs are developmentally regulated in the rat heart. Furthermore, we demonstrated that ERα expression rises from low levels in newborns in a chamber-specific manner, whereas ERβ mRNA decreases in the adult rat heart, suggesting a specific role of both receptors in heart maturation. High ERβ expression was observed in the newborn heart and at 4 days of postnatal life, when extensive hyperplasia of the rat heart occurs and the heart grows more rapidly than the body (31). Then, in adult rats, ERβ expression is low, in contrast to relatively high ERα mRNA levels. Age-dependent increases in ERα suggest that this receptor plays a role in heart maturation. The high ERα expression thereafter suggests that it functions as a predominant estrogenic mediator in the rat heart. In the adult rat heart, ERs α are significantly more expressed in the atria than in the ventricles. Atrial but not ventricular ERα levels decreased after ovariectomy and were partially reconstituted in response to estrogen treatment in ovariectomized rats. Ovariectomy also reduced the heart-to-body weight ratio and mainly the atrial weight-to-body weight ratio. The decrease in ERαs in the atria after ovariectomy was accompanied by decreased plasma ANP levels. 17-β-Estradiol, administrated at the dose that elevated plasma estradiol to supraphysiological levels, reconstituted these values in the plasma of ovariectomized rats. Interestingly, this increase was accompanied by a decreased heart rate. Variations in plasma ANP were correlated with changes in ANP concentration in the right atrium. The failure of ERα to be affected in the ventricle by altered estrogen levels suggests that these receptors are less responsive to estrogen.
Consistent with the concept that ERαs are the predominant estrogenic mediator in the heart in vivo, reduced 17-β-estradiol and ERα levels in adult ovariectomized rats resulted in a decreased heart-to-body weight ratio, which was restored by estrogen supplementation. Thus, the reduced 17-β-estradiol level in adult ovariectomized rats is associated with decreased ERα transcripts and protein. Similarly, ER concentrations in vascular tissue respond to changes in plasma estradiol level (32). Estradiol also increased ERα as well as ERβ transcripts in cultured cardiomyocytes (17). Thus, ERα-induced increased expression of certain genes may explain the greater heart-to-body weight ratio in 17-β-estradiol-treated ovariectomized rats than in vehicle-treated, ovariectomized controls, and recently Wallen et al. (33) found that female rats have a greater cardiac mass than males, which they attributed to estrogen.
In our experiments, the decrease in cardiac mass after ovariectomy and its reconstitution by 17-β-estradiol administration were well defined in the atria and paralleled the expression level of ERα and ANP in these heart chambers. In addition, we found that 17-β-estradiol treatment of ovariectomized rats resulted in a pronounced increase in ERα mRNA and ERα protein in the right atrium and, to a lesser extent, in the left atrium. These increases were associated with heightened plasma ANP, augmented ANP mRNA in both atria, and greater ANP concentration in the right atrium. Our findings of ERα down-regulation by ovariectomy and its reconstitution by 17-β-estradiol may explain the increased Ca2+ accumulation, myocardial damage, and myocardial edema observed after ischemia-reperfusion in ovariectomized rats (34), indicating that the absence of cardiac ERα results in deleterious effects on the heart and underlining the cardioprotective actions of ERα.
Further demonstrations that ERs are involved in myocardial regulation is the observation that ERα knockout in male mice leads to severe myocardial damage after ischemia-reperfusion (35), and the report that a man with a disruptive mutation in the ER gene had impaired flow-mediated, endothelium-dependent vasodilation (36) and premature coronary artery disease (37) in the presence of circulating estrogen. Recent studies have shown that ovariectomy increases and estrogen decreases L-type calcium channel density in rabbit ventricular myocytes (22). The effects may be mediated by the nitric oxide/cGMP pathway. Fraser et al. (20) reported that chronic administration of 17-β-estradiol to ovariectomized rats increases nitric oxide synthase activity, restores impaired cGMP production, and improves postischemic left ventricular work in hearts isolated from the same animals. Although up-regulation of ERs in cardiomyocytes was associated with an increase in nitric oxide synthase activity (38), a similar ERα-dependent mechanism mediated by ANP could also elevate cGMP and result in cardioprotection.
Plasma ANP augmented by 17-β-estradiol administration to ovariectomized rats was reported by Zhang et al. (26), who demonstrated that this effect was attenuated by combination of 17-β-estradiol with progesterone. In the uterus of ovariectomized mice, 17-β-estradiol stimulated (3-fold) C-type natriuretic peptide, whereas progesterone significantly attenuated this estrogen effect (39). Estrogen-stimulated increases in uterine C-type natriuretic peptide were ER-dependent, as demonstrated by inhibition with ICI-164.384, a nonselective ER antagonist. Progesterone also can act directly on ER in cardiac myocytes and, therefore, decreases ANP synthesis, because both progesterone receptors and ER are colocalized in these cells (40). Alternatively, progesterone by posttranslational mechanisms may decrease the number of oxytocin receptors, as demonstrated by Grazzini et al. (41), and this inhibition might lead to suppression of ANP secretion, as we observed (42).
Our data are consistent with other studies showing that the decrease in heart rate may be a direct effect of estrogen administration (43). Thus, changes in heart rate can be due to ER-mediated changes, inasmuch as experiments on the isolated rat heart (44) show that 17-β-estradiol decreased the heart rate, together with a dose-dependent negative chronotropic effect in the right atrium but not in the left atrium. Correspondingly, we have already demonstrated that the rat right atrium is the primary source of ANP release in response to volume expansion in association with heart rate reduction upon activation of oxytocin receptors (42, 45). Our experiments show that, in addition, 17-β-estradiol treatment increased ANP release and synthesis concomitantly with bradycardia. ANP acts via ANP receptors in the right atrium to activate particulate guanylyl cyclase, leading to increased cGMP accumulation. Then, cGMP decreases the rate of cardiac contraction by acting on the sinoatrial node. The observation that both estrogen (21, 22, 46) and ANP (10) inhibit Ca2+ channels in cardiomyocytes is consistent with linkage to cGMP elevation. In addition, the negative chronotropic effects of ANP may be mediated by stimulation of Ca2+ and voltage-activated potassium channel activity through activation of cGMP-dependent protein kinase (47). This stimulation of Ca2+ and voltage-activated potassium channel activity may reduce oxygen consumption and produce cardioprotection.
Acknowledgments
We are indebted to Suhayla Mukkadam-Daher for her helpful discussions. We acknowledge the editorial assistance of Ovid Da Silva, Centre de Recherche, Centre Hospitalier de l'Université de Montréal. These studies were supported by grants from the Medical Research Council of Canada (MT-15049 to M.J. and J.G., MT-11674 to J.G.), a grant from the Heart and Stroke Foundation of Canada (to J.G.), and a grant from the National Institute of Mental Health (MH-51853 to S.M.M.).
Abbreviations
- ANP
atrial natriuretic peptide
- ER
estrogen receptor
- RT-PCR
reverse transcription–PCR
References
- 1.De Bold A J, Borenstein H B, Veress A T, Sonnenberg H. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 2.Lin X, Hanze J, Heese F, Sodmann R, Lang R E. Circ Res. 1995;77:750–758. doi: 10.1161/01.res.77.4.750. [DOI] [PubMed] [Google Scholar]
- 3.Horio T, Nishikimi T, Yoshihara F, Matsuo H, Takishita S, Kangawa K. Hypertension. 2000;35:19–24. doi: 10.1161/01.hyp.35.1.19. [DOI] [PubMed] [Google Scholar]
- 4.John S W, Krege J H, Oliver P M, Hagaman J R, Hodgin J B, Pang S C, Flynn T G, Smithies O. Science. 1995;267:679–681. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- 5.Oliver P M, John S W, Purdy K E, Kim R, Maeda N, Goy M F, Smithies O. Proc Natl Acad Sci USA. 1998;95:2547–2551. doi: 10.1073/pnas.95.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knowles J W, Esposito G, Mao L, Hagaman J R, Fox J E, Smithies O, Rockman H A, Maeda N. J Clin Invest. 2001;107:975–984. doi: 10.1172/JCI11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Back H, Forssmann W G, Stumpf W E. Cell Tissue Res. 1989;255:673–674. doi: 10.1007/BF00218808. [DOI] [PubMed] [Google Scholar]
- 8.Deng Y, Kaufman S. Life Sci. 1993;53:689–696. doi: 10.1016/0024-3205(93)90245-x. [DOI] [PubMed] [Google Scholar]
- 9.Supaporn T, Wennberg P W, Wei C M, Kinoshita M, Matsuda Y, Burnett J C. Clin Sci. 1996;90:357–362. doi: 10.1042/cs0900357. [DOI] [PubMed] [Google Scholar]
- 10.Tohse N, Nakaya H, Takeda Y, Kanno M. Br J Pharmacol. 1995;114:1076–1082. doi: 10.1111/j.1476-5381.1995.tb13316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richards A M, Ikram H, Yandle T G, Nicholls M G, Webster M W, Espiner E A. Lancet. 1985;i:545–549. doi: 10.1016/s0140-6736(85)91207-3. [DOI] [PubMed] [Google Scholar]
- 12.Imaizumi T, Takeshita A. J Cardiovasc Electrophysiol. 1993;4:719–729. doi: 10.1111/j.1540-8167.1993.tb01257.x. [DOI] [PubMed] [Google Scholar]
- 13.Mendelsohn M E, Karas R H. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 14.Katzenellenbogen B S, Montano M M, Ediger T R, Sun J, Ekena K, Lazennec G, Martini P G, McInerney E M, Delage-Mourroux R, Weis K, et al. Recent Prog Horm Res. 2000;55:163–193. [PubMed] [Google Scholar]
- 15.Calovini T, Haase H, Morano I. J Cell Biochem. 1995;59:69–78. doi: 10.1002/jcb.240590109. [DOI] [PubMed] [Google Scholar]
- 16.Stumpf W E, Sar M, Aumuller G. Science. 1977;196:319–321. doi: 10.1126/science.847474. [DOI] [PubMed] [Google Scholar]
- 17.Grohe C, Kahlert S, Lobbert K, Vetter H. J Endocrinol. 1998;156:R1–R7. doi: 10.1677/joe.0.156r001. [DOI] [PubMed] [Google Scholar]
- 18.Pelzer T, Shamim A, Wolfges S, Schumann M, Neyses L. Adv Exp Med Biol. 1997;432:83–89. doi: 10.1007/978-1-4615-5385-4_9. [DOI] [PubMed] [Google Scholar]
- 19.Delyani J A, Murohara T, Nossuli T O, Lefer A M. J Mol Cell Cardiol. 1996;28:1001–1008. doi: 10.1006/jmcc.1996.0093. [DOI] [PubMed] [Google Scholar]
- 20.Fraser H, Davidge S T, Clanachan A S. Cardiovasc Res. 2000;46:111–118. doi: 10.1016/s0008-6363(99)00424-1. [DOI] [PubMed] [Google Scholar]
- 21.Johnson B D, Zheng W, Korach K S, Scheuer T, Catterall W A, Rubanyi G M. J Gen Physiol. 1997;110:135–140. doi: 10.1085/jgp.110.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patterson E, Ma L, Szabo B, Robinson C P, Thadani U. J Pharmacol Exp Ther. 1998;284:586–591. [PubMed] [Google Scholar]
- 23.Gustafsson J A. J Endocrinol. 1999;163:379–383. doi: 10.1677/joe.0.1630379. [DOI] [PubMed] [Google Scholar]
- 24.Hodges Y K, Tung L, Yan X D, Graham J D, Horwitz K B, Horwitz L D. Circulation. 2000;101:1792–1798. doi: 10.1161/01.cir.101.15.1792. [DOI] [PubMed] [Google Scholar]
- 25.Bridges R S. Endocrinology. 1984;114:930–940. doi: 10.1210/endo-114-3-930. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Novak K, Kaufman S. J Physiol (London) 1995;488:509–514. doi: 10.1113/jphysiol.1995.sp020985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutkowska J. Nucl Med Biol. 1987;14:323–331. doi: 10.1016/0883-2897(87)90009-2. [DOI] [PubMed] [Google Scholar]
- 28.Bradford M M. Anal Biochem. 1976;72:248–252. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 29.Kuiper G G, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson J A. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y F, Elton T S, Oparil S. Hypertension. 1992;19:296–300. doi: 10.1161/01.hyp.19.3.296. [DOI] [PubMed] [Google Scholar]
- 31.Clubb F J, Bishop S P. Lab Invest. 1984;50:571–577. [PubMed] [Google Scholar]
- 32.Vargas R, Wroblewska B, Rego A, Hatch J, Ramwell P W. Br J Pharmacol. 1993;109:612–617. doi: 10.1111/j.1476-5381.1993.tb13616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallen W J, Cserti C, Belanger M P, Wittnich C. Hypertension. 2000;36:774–779. doi: 10.1161/01.hyp.36.5.774. [DOI] [PubMed] [Google Scholar]
- 34.Zhai P, Eurell T E, Cotthaus R, Jeffery E H, Bahr J M, Gross D R. Am J Physiol. 2000;279:H2766–H2775. doi: 10.1152/ajpheart.2000.279.6.H2766. [DOI] [PubMed] [Google Scholar]
- 35.Zhai P, Eurell T E, Cooke P S, Lubahn D B, Gross D R. Am J Physiol. 2000;278:H1640–H1647. doi: 10.1152/ajpheart.2000.278.5.H1640. [DOI] [PubMed] [Google Scholar]
- 36.Sudhir K, Chou T M, Messina L M, Hutchison S J, Korach K S, Chatterjee K, Rubanyi G M. Lancet. 1997;349:1146–1147. doi: 10.1016/S0140-6736(05)63022-X. [DOI] [PubMed] [Google Scholar]
- 37.Sudhir K, Chou T M, Chatterjee K, Smith E P, Williams T C, Kane J P, Malloy M J, Korach K S, Rubanyi G M. Circulation. 1997;96:3774–3777. doi: 10.1161/01.cir.96.10.3774. [DOI] [PubMed] [Google Scholar]
- 38.Nuedling S, Kahlert S, Loebbert K, Doevendans P A, Meyer R, Vetter H, Grohe C. Cardiovasc Res. 1999;43:666–674. doi: 10.1016/s0008-6363(99)00093-0. [DOI] [PubMed] [Google Scholar]
- 39.Acuff C G, Huang H, Steinhelper M E. Am J Physiol. 1997;273:H2672–H2677. doi: 10.1152/ajpheart.1997.273.6.H2672. [DOI] [PubMed] [Google Scholar]
- 40.Knowlton A A, Sun L. Am J Physiol. 2001;280:H455–H464. doi: 10.1152/ajpheart.2001.280.1.H455. [DOI] [PubMed] [Google Scholar]
- 41.Grazzini E, Guillon G, Mouillac B, Zingg H H. Nature (London) 1998;392:509–512. doi: 10.1038/33176. [DOI] [PubMed] [Google Scholar]
- 42.Gutkowska J, Jankowski M, Lambert C, Mukaddam-Daher S, Zingg H H, McCann S M. Proc Natl Acad Sci USA. 1997;94:11704–11709. doi: 10.1073/pnas.94.21.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He X R, Wang W, Crofton J T, Share L. Am J Physiol. 1998;275:R1202–R1208. doi: 10.1152/ajpregu.1998.275.4.R1202. [DOI] [PubMed] [Google Scholar]
- 44.Eckstein N, Nadler E, Barnea O, Shavit G, Ayalon D. Am J Obstet Gynecol. 1994;171:844–848. doi: 10.1016/0002-9378(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 45.Favaretto A L, Ballejo G O, Albuquerque-Araujo W I, Gutkowska J, Antunes-Rodrigues J, McCann S M. Peptides (Tarrytown, NY) 1997;18:1377–1381. doi: 10.1016/s0196-9781(97)00209-x. [DOI] [PubMed] [Google Scholar]
- 46.Jiang C, Poole-Wilson P A, Sarrel P M, Mochizuki S, Collins P, MacLeod K T. Br J Pharmacol. 1992;106:739–745. doi: 10.1111/j.1476-5381.1992.tb14403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White R E, Darkow D J, Lang J L. Circ Res. 1995;77:936–942. doi: 10.1161/01.res.77.5.936. [DOI] [PubMed] [Google Scholar]