Abstract
Atherosclerosis is considered to be a chronic inflammatory disease that can lead to severe clinically important cardiovascular events. miR-150 is a small noncoding RNA that significantly enhances inflammatory responses by upregulating endothelial cell proliferation and migration, as well as intravascular environmental homeostasis. However, the exact role of miR-150 in atherosclerosis remains unknown. Here, we investigated the effect of miR-150 deficiency on atherosclerosis development. Using double-knockout (miR-150−/− and ApoE−/−) mice, we measured atherosclerotic lesion size and stability. Meanwhile, we conducted in vivo bone marrow transplantation to identify cellular-level components of the inflammatory response. Compared with mice deficient only in ApoE, the double-knockout mice had significantly smaller atherosclerotic lesions and displayed an attenuated inflammatory response. Moreover, miR-150 ablation promoted plaque stabilization via increases in smooth muscle cell and collagen content and decreased macrophage infiltration and lipid accumulation. The in vitro experiments indicated that an inflammatory response with miR-150 deficiency in atherosclerosis results directly from upregulated expression of the cytoskeletal protein, PDZ and LIM domain 1 (PDLIM1), in macrophages. More importantly, the decreases in phosphorylated p65 expression and inflammatory cytokine secretion induced by miR-150 ablation were reversed by PDLIM1 knockdown. These findings suggest that miR-150 is a promising target for the management of atherosclerosis.
Keywords: microRNA-150, inflammation, PDZ and LIM domain 1
Cardiovascular disease is the leading cause of death among a variety of chronic noncommunicable diseases affecting patients worldwide (1). Atherosclerosis, the critical pathophysiological process underlying CVD, is well-recognized as a complex multifactorial disease and is generally considered a chronic inflammatory response (2) that can lead to several severe clinical cardiovascular events, such as myocardial infarction, sudden cardiac death, and stroke. During the initiation of atherosclerosis, the injured endothelial cells attract circulating monocytes by expressing chemokines and adhesion molecules, which also mediate the adhesion and rolling of monocytes on endothelium. The monocytes subsequently migrate through the endothelium and differentiate into macrophages, a process that gives rise to foam cells following the excess engulfment of oxidized LDL (Ox-LDL). The continuous intensification of the inflammatory response from endothelium and macrophages promotes the expansion of vascular smooth muscle cells (VSMCs), accumulation of more macrophages, development of atherosclerotic plaques, and the formation of necrotic cores and vulnerable plaques (3). Therefore, exploring and targeting the key regulators of atherosclerosis-related inflammation may provide clinicians with an effective strategy for preventing atherosclerosis. The effectiveness of anti-inflammation has been verified recently by a clinical study (4), further strengthening the urgency of elucidating the mechanism underlying the development of inflammation during atherogenesis.
microRNAs are a class of small noncoding RNAs that are widely present in eukaryotic organisms and have important regulatory effects on cell metabolism, cell development, tumorigenesis, and immune inflammation (5–7). microRNAs fulfill their biological functions by negatively regulating the mRNA of their target molecules and have thus emerged as novel biomarkers for the diagnosis of various diseases and regulators of the progression of these diseases (8). miR-150 is a 22-nucleotide microRNA and is highly expressed in human lymph nodes, spleen, mature lymphocytes, regulatory T cells, and B cells under physiological conditions (9). miR-150 has also been shown to be upregulated in peripheral blood in patients with coronary heart disease (CHD) (10, 11). Previous studies have demonstrated that miR-150 plays important roles in regulating endothelial cell proliferation and migration and intravascular environmental homeostasis (12–14). In addition, miR-150-knockout mice displayed exacerbated obesity, tissue inflammation, and insulin resistance (15). Ox-LDL-mediated lipoprotein accumulation in macrophages is attenuated by miR-150 in vitro (16). Nevertheless, the specific role of miR-150 in atherosclerosis development has not been elucidated.
The present study showed that miR-150 expression was significantly upregulated in plaques from human patients with CHD and high-fat diet (HFD)-treated ApoE−/− mice. Specifically, miR-150 expression was upregulated in bone marrow-derived macrophages (BMDMs) upon Ox-LDL stimulation. miR-150 deficiency not only attenuated atherosclerotic plaque formation by decreasing inflammatory cytokine production but also protected against vulnerable plaque formation. By performing bone marrow transplantation and in vitro experiments, we showed that the effects of miR-150 on atherogenesis were largely dependent on its effects in macrophages, phenomena accompanied by inflammation alleviation. We noticed that miR-150 ablation reduced phosphorylated p65 expression, inflammatory cytokine secretion, and macrophage infiltration by directly upregulating PDZ and LIM domain 1 (PDLIM1) expression.
MATERIALS AND METHODS
Animals and diets
The animal study procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the Renmin Hospital. miR-150-knockout mice were purchased from the Jackson Laboratory (007750). To purify the background, the female miR-150−/− mice on a B6.Cg background were first cross-bred with male C57BL/6, and then the male F1 generation mice (miR-150 heterozygous) were mated with female C57BL/6 mice for the F2 generation mice. The F2 generation mice were then repeatedly crossed with C57BL/6J mice until the F9 generation (miR-150 heterozygous). Finally, these F9 mice were then crossed to yield miR-150−/− (pure C57BL/6J background) mice. ApoE−/− mice and miR-150−/− mice were cross-bred to obtain miR-150−/−ApoE−/− mice and ApoE−/− littermates. Eight-week-old mice were organized into two groups, which were fed a HFD (15.8% fat and 1.25% cholesterol) or normal chow (NC), respectively, for up to 28 weeks. These mice were euthanized via the intraperitoneal injection of pentobarbital sodium (50 mg/kg) at the time of tissue collection.
Mouse aorta dissection and en face aortic lesion area analysis
After receiving a HFD for 16 or 28 weeks, the mice were anesthetized through intraperitoneal injections of pentobarbital sodium. Their arteries were perfused with PBS and/or 4% paraformaldehyde through the left ventricle and then carefully separated from the base of the ascending aorta. For en face analysis, the entire aorta was stained with Oil Red O and atherosclerotic lesion sizes were quantified using Image-Pro Plus 6.0 (Image Metrology, Copenhagen, Denmark) software, as described previously (17). The heart and ascending aortic arch were subsequently dehydrated and embedded in paraffin for histological analysis. Consecutive 5 μm sections of the ascending aortic arch and the atrioventricular valve region of each heart were collected, while the latter tissues were stained with H&E for morphological analysis, picrosirius red for collagen deposition evaluation, and Oil Red O for lipid accumulation detection. The plaque stability score = [smooth muscle cell (SMC) area + collagen area]/(macrophage area + lipid area) (18).
Immunofluorescence
Aortic sinus cross-sections were used for immunofluorescence analyses, whose staining protocols were described previously (19). Tissue slides were blocked in 10% goat serum diluted with PBS for 1 h and then incubated with the following primary antibodies overnight at 4°C: anti-CD68 and anti-smooth muscle actin. The tissues were then incubated with the following secondary antibodies: Alexa Flour® 568 donkey anti-rat IgG (1:200 dilution; Invitrogen, A11011) and Alexa Flour® 488 donkey anti-rabbit IgG (1:200 dilution; Invitrogen, A11008). Images were acquired with a fluorescence microscope (Olympus, Tokyo, Japan) using DP2-BSW software (version 2.2) and were analyzed with Image-Pro Plus 6.0.
Determination of serum lipid and inflammatory cytokine levels
Blood samples were collected from the retro-orbital vein, while the mice were under isoflurane anesthesia. The supernatants were obtained by centrifugation at 10,000 g for 20 min at 25°C and were used to measure lipid metabolism indexes and inflammatory cytokine secretion.
Bone marrow transplantation study
In the bone marrow transplantation experiment, male ApoE−/− recipient mice aged 8 weeks were lethally irradiated with a total of 11 Gy of radiation (two doses of 5.5 Gy of radiation spaced 4 h apart). Bone marrow cells from the femurs and tibias of donor male mice (miR-150−/−ApoE−/− or ApoE−/−) were harvested under sterile conditions, and then each irradiated mouse was injected with 5 × 107 bone marrow donor cells through the orbital venous plexus. After 4 weeks, genomic DNA was collected from peripheral blood leukocytes and was genotyped using PCR. The mice were subsequently fed a HFD for an additional 16 weeks for this study.
Quantitative real-time PCR and Western blotting
Total mRNA was extracted by trichloromethane, dissolved in DEPC-water, and then reverse transcribed into cDNA with a Transcriptor First Strand cDNA synthesis kit (Roche, Basel, Switzerland; 04897030001) as previously described (20). The expression levels of the target genes were quantified by real-time PCR using LightCycler 480 SYBR Green 1 Master Mix and a LightCycler 480 QPCR system (Roche Diagnostics, Indianapolis, IN). The relative transcription levels of the target genes were normalized against those of reference genes. Protein concentrations were determined by BCA assay. The proteins were separated by SDS-PAGE and then transferred to PVDF membranes, which were blocked in TBS for 1 h at room temperature before being incubated with the appropriate primary antibodies overnight at 4°C. The membranes were then incubated with the appropriate secondary antibodies and treated with enhanced chemiluminescence reagent (Thermo Scientific) before being visualized with Molecular Imager ChemiDoc™ XRS+ using Image Lab™ Software 5.1 (Bio-Rad, Bio-Legend Scientific Co. Ltd). The expression levels of specific proteins were normalized against those of internal references.
Cell culture and siRNA transfection
BMDMs were isolated and harvested from the femurs and tibias of miR-150−/−ApoE−/− and ApoE−/− mice under sterile conditions. Approximately 5 × 107 nucleated bone marrow cells were collected from each mouse and then cultured in 10 ml of RPMI with 10% fetal bovine serum and MCSF (50 ng/ml). The siRNA specific for PDLIM1 (s79437) and the control siRNA were purchased from Ambion (Silencer Select). The BMDMs were transfected with the above siRNA molecules according to the manufacturer’s protocol. The cells were then stimulated with 15 ng/ml Ox-LDL for 24 h after 24 h of serum starvation.
Human specimens
All human studies were conducted in accordance with the principles outlined in the Declaration of Helsinki and were approved by the ethics committee of Renmin Hospital of Wuhan University, China. The right coronary arteries of patients with CHD who had undergone heart transplantation were the sources of the atheromatous plaques assessed herein. The normal arteries used for these experiments were from donors whose hearts were unsuitable for transplantation. Written informed consent was obtained from the relevant families.
Statistical analysis
All statistical data were analyzed using SPSS software version 17.0 and are presented as the mean ± SD. Differences between two groups were analyzed by t-tests, while differences among multiple groups were analyzed by one-way ANOVA. P < 0.05 was considered statistically significant.
RESULTS
miR-150 expression is upregulated in atheromatous plaques and macrophages
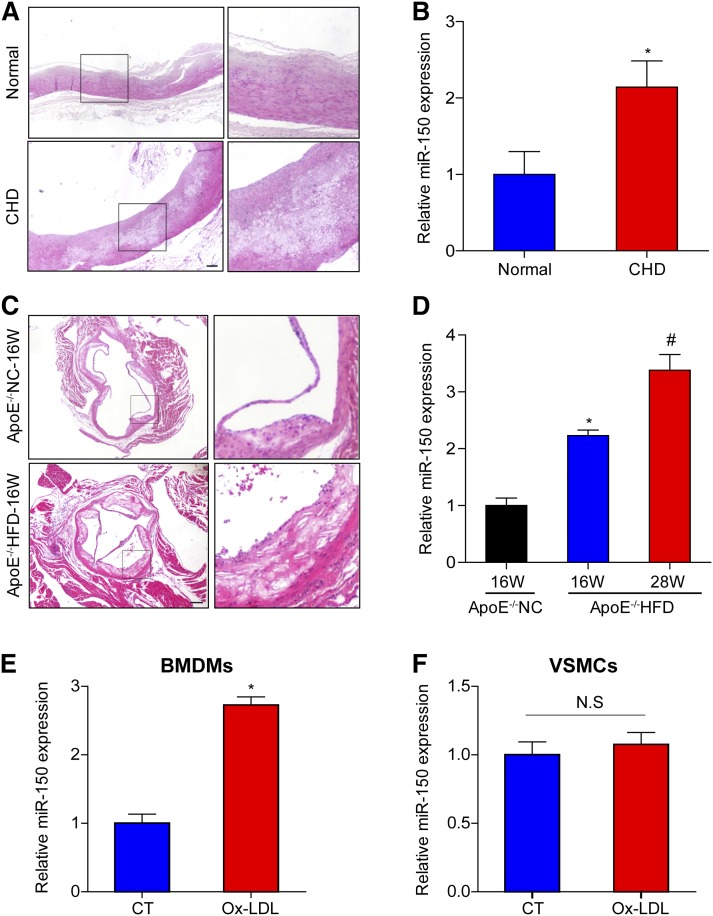
To determine the role of miR-150 in atherogenesis, we examined whether miR-150 expression was altered in atherosclerotic plaques. The atherosclerosis-prone regions of right coronary arteries from donors and patients with CHD were analyzed to determine the differences in miR-150 expression between the two groups. H&E staining demonstrated the presence of typical pathological changes in the arteries from the patients with CHD. The plaques in these arteries exhibited a thin fibrous cap and lipid cores (Fig. 1A). Quantitative (q)PCR analysis revealed that miR-150 expression was significantly upregulated in the arteries of patients with CHD (Fig. 1B). To determine whether the trends in miR-150 expression in the atherosclerotic plaques from the mouse model were consistent with those in the atherosclerotic plaques from the above patients, ApoE-deficient mice were fed with either NC for 16 weeks or a HFD for 16 or 28 weeks. HFD-treated ApoE−/− mice displayed larger plaques than chow-fed mice, as shown in the representative image (Fig. 1C). miR-150 expression levels were remarkably upregulated in HFD-treated ApoE−/− mice relative to those in NC-fed mice, which paralleled with duration of HFD administration (Fig. 1D). We also assessed the changes in miR-150 expression in BMDMs and VSMCs. We noted that miR-150 expression increased significantly in BMDMs (Fig. 1E), but did not change in VSMCs (Fig. 1F) upon Ox-LDL stimulation. These findings suggest that miR-150 is involved in the development of atherosclerosis by mainly functioning in macrophages.
Fig. 1.
miR-150 expression is increased in atherosclerotic plaques and macrophages A: H&E-stained right coronary arteries from a donor and a patient with CHD. Scale bar = 200 μm. B: qPCR analysis of miR-150 mRNA expression in human artery tissue samples (n = 4). *P < 0.05 versus normal group. C: H&E-stained aortic roots from ApoE−/− mice treated with NC or a HFD for 16 weeks (16W). Scale bar = 200 μm. D: qPCR analysis of miR-150 mRNA expression in the entire aorta in ApoE−/− mice treated with NC for 16 weeks or a HFD for 16 or 28 weeks (28W) (n = 3). *P < 0.05 versus ApoE−/− with 16W-NC treatment group; #P < 0.05 versus ApoE−/− with 16W-HFD treatment group. E, F: miR-150 mRNA expression in BMDMs or VSMCs stimulated with Ox-LDL. *P < 0.05 versus control (CT) group; N.S indicates not significant.
miR-150 deficiency attenuates atherosclerotic plaque formation
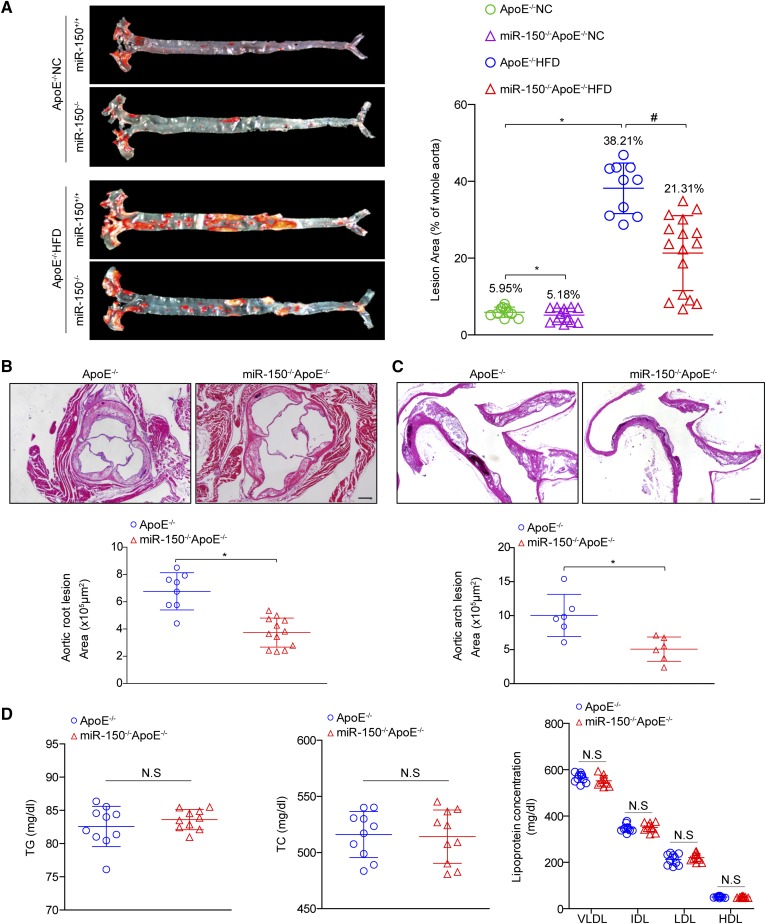
To explore the contribution of miR-150 upregulation to atheromatous plaque formation, we crossed miR-150-deficient mice with ApoE−/− mice and obtained miR150−/−ApoE−/− mice. We observed that the areas of the atherosclerotic lesions in the aortic tree were significantly decreased in miR-150−/−ApoE−/− mice (21.31%) compared with ApoE−/− mice (38.21%) after 28 weeks of HFD feeding (Fig. 2A), while slightly significant differences in lesion areas between the two groups were observed when feeding on NC (5.95% versus 5.18%). We further assessed plaque formation in the atherosclerosis-prone regions of the aortic root and aortic arch to assess the effect of miR-150 on atherogenesis in mice. Quantitative analysis demonstrated that arch and root lesion sizes were significantly decreased in HFD-fed miR-150−/−ApoE−/− mice compared with HFD-fed ApoE−/− mice (Fig. 2B, C). Dyslipidemia is a risk factor for atherosclerosis development; however, there were no differences between the two groups with respect to total cholesterol and triglyceride levels and lipoprotein profiles (VLDL, IDL, LDL, and HDL levels) after HFD treatment (Fig. 2D).
Fig. 2.
miR-150 ablation protects mice from atherosclerosis. A: Oil Red O staining of atherosclerotic lesions in the entire aorta in ApoE−/− and miR-150−/−ApoE−/− mice treated with NC or a HFD for 28 weeks. Quantitative lesion data are shown in the right panel (n = 10–16). *P < 0.05 versus ApoE−/− NC group; #P < 0.05 versus ApoE−/− HFD group. B, C: H&E staining for the morphology of the plaques in the aortic roots (B) and ascending aortic arches (C) of ApoE−/− and miR-150−/−ApoE−/− mice treated with a HFD for 28 weeks (n = 6–12). Scale bar = 200 μm. *P < 0.05 versus ApoE−/− group. D: The lipid metabolism parameters of ApoE−/− and miR-150−/−ApoE−/− mice (n = 10 each group). Not significant (N.S) versus ApoE−/− littermates. TC, total cholesterol; TG, triglyceride.
miR-150 ablation protects against vulnerable plaque formation
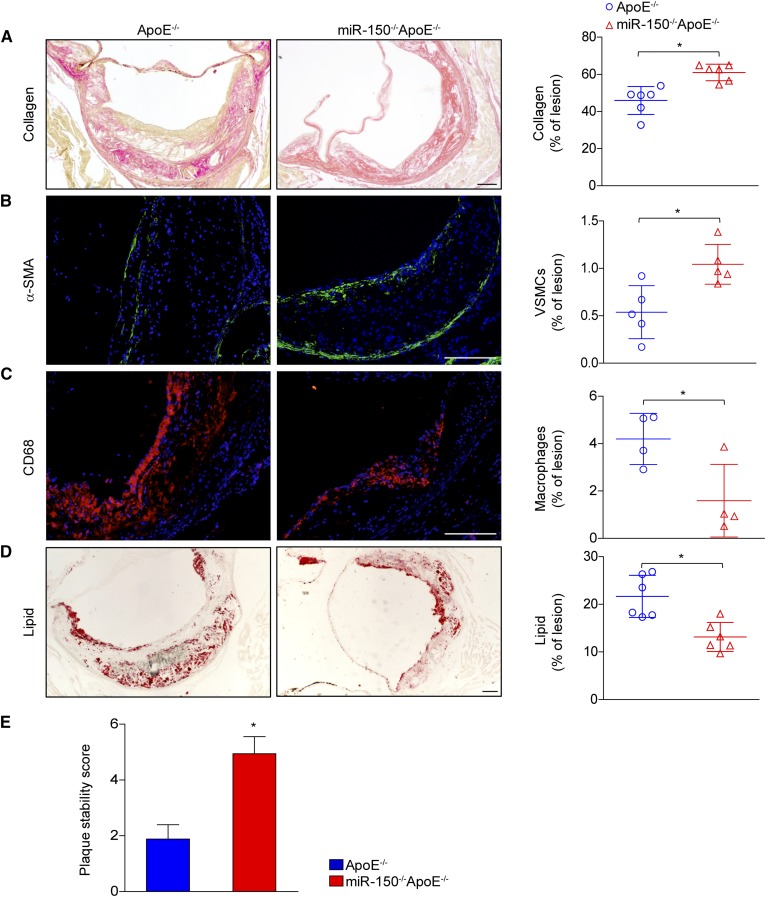
Although large plaques can occlude vessels, the contents of plaques, including lipids, collagen deposits, SMCs, and infiltrated macrophages, are more responsible than the size of plaques for the poor outcomes associated with clinical CVD complications. The composition of the fibrous cap, containing collagen similar to that found in smooth muscle, is the core determinant of plaque stability. We observed that SMC abundance and collagen levels were increased in miR-150−/−ApoE−/− mice compared with ApoE−/− mice (Fig. 3A, B). We also noticed that the numbers of infiltrated macrophages (Fig. 3C) and lipid accumulation (Fig. 3D) were dramatically decreased in the absence of miR-150. Finally, the improved stability characteristics of plaque were found in miR-150 ablation according to the plaque stability score (Fig. 3E). Taken together, these data suggested that miR-150 deficiency was effective in maintaining atherosclerotic plaque stability.
Fig. 3.
miR-150 deficiency increases plaque stability. A–D: Cross-sections of aortic roots from ApoE−/− and miR-150−/−ApoE−/− mice were stained with picrosirius red to assess collagen deposition (A) and α-smooth muscle actin (α-SMA) expression (green) to determine SMC compositions (B). The tissues were also stained with CD68 (red) to assess macrophage infiltration (C) and Oil Red O to assess lipid accumulation (D). The quantitative results for each image are shown in the right panels. E: The assessment of plaque stability score in ApoE−/− and miR-150−/−ApoE−/− mice (n = 4–6). Scale bar = 100 μm.*P < 0.05 versus ApoE−/− group.
The inflammatory response was ameliorated in miR-150-deficient mice
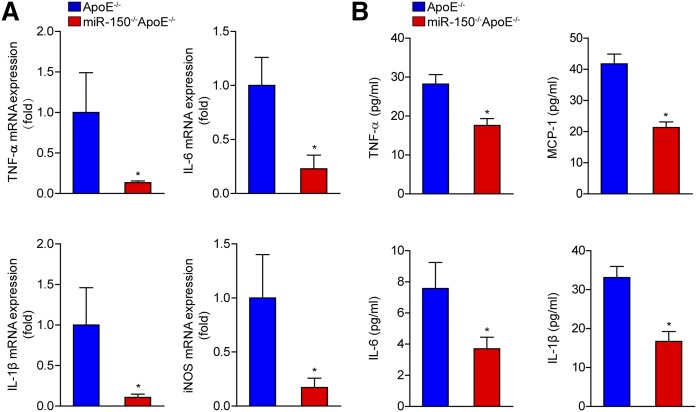
Given that inflammation is an important mediator of atherosclerosis development, we examined the expression of pro-inflammatory cytokine in the absence of miR-150. We measured the serum and mRNA levels of secreted cytokines. We found that mRNA levels of TNF-α, interleukin (IL)-6, IL-1β, and inducible nitric oxide synthase (iNOS) in the aortas were decreased in miR-150−/−ApoE−/− mice compared with ApoE−/− mice (Fig. 4A). Consistently, serum levels of TNF-α, monocyte chemotactic protein 1 (MCP-1), IL-6, and IL-1β were significantly lower in the miR-150−/−ApoE−/− mice compared with those in the ApoE−/− controls (Fig. 4B).
Fig. 4.
The inflammatory response is inhibited in the absence of miR-150. A: The mRNA expression levels of secreted cytokines, namely, TNF-α, IL-6, IL-1β, and iNOS, in the entire aortas of ApoE−/− and miR-150−/−ApoE−/− mice were measured by qPCR (n = 4). *P < 0.05 versus ApoE−/− group. B: The serum levels of secreted cytokines in ApoE−/− and miR-150−/−ApoE−/− mice (n = 6). *P < 0.05 versus ApoE−/− group.
miR-150 deficiency plays an athero-protective role in macrophages
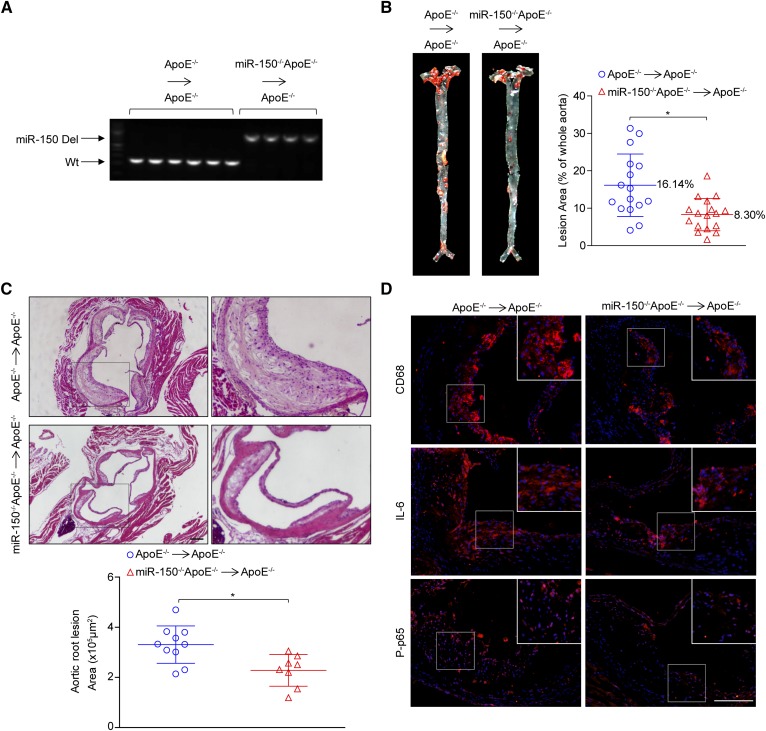
miR-150 expression was upregulated in BMDMs, as shown in Fig. 1E, we next investigated whether miR-150 deficiency in hematopoietic cells contributes to the development of the phenotype observed in the global-knockout mice. We transplanted bone marrow from miR-150−/−ApoE−/− or ApoE−/− donors into lethally irradiated ApoE−/− recipient mice and then treated the mice with a HFD for 16 additional weeks. The success of the chimera generation procedure was determined by analyzing the genomic DNA isolated from the white blood cells (Fig. 5A). We found that miR-150 deficiency in macrophages attenuated atherosclerotic plaque development throughout the entire aorta (Fig. 5B) and aortic root (Fig. 5C). Moreover, the assessment of infiltration of macrophages, IL-6 secretion, and phosphorylated p65 levels indicated that miR-150 deficiency in macrophages significantly ameliorated inflammatory responses (Fig. 5D). These results indicated that the downregulation of miR-150 expression in bone marrow-derived cells was vital for the athero-protective effects of miR-150 deficiency.
Fig. 5.
miR-150 deficiency in marrow-derived cells attenuates atherosclerosis. A: The efficacy of bone marrow transplantation was determined with genomic DNA isolated from white blood cells after the chimera generation procedure. B: Oil Red O staining of atherosclerotic lesions in the entire aortas of ApoE−/−→ApoE−/− and miR-150−/−ApoE−/−→ApoE−/− mice treated with an HFD for 16 weeks. Quantitative data for the lesions are shown in the right panel (n = 13–17). *P < 0.05 versus ApoE−/−→ApoE−/− group. C: H&E-stained plaques in the aortic roots of ApoE−/−→ApoE−/− and miR-150−/−ApoE−/−→ApoE−/− mice (n = 8–10). Scale bar = 200 μm. D: The immunofluorescence staining of CD68, IL-6, and phosphorylated p65 (P-p65) (red). Scale bar = 100 μm. *P < 0.05 versus ApoE−/−→ApoE−/− group.
miR-150 deficiency in macrophages decreases inflammatory cytokine secretion and macrophage attraction
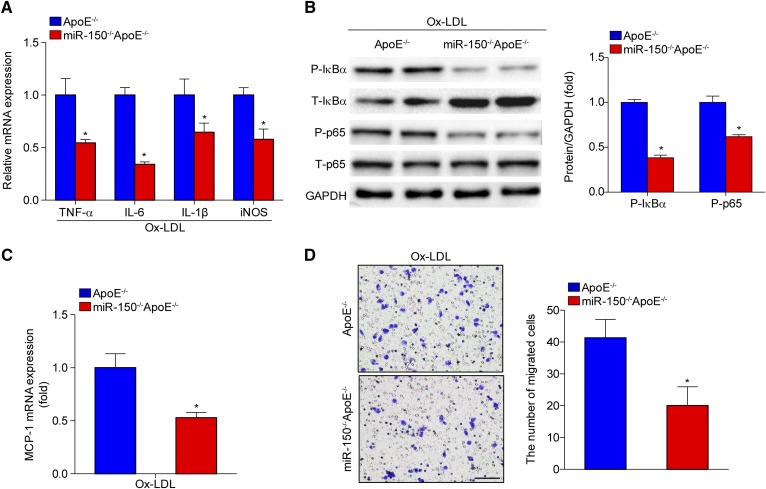
We next assessed the secretion of inflammatory cytokines from BMDMs from miR-150−/−ApoE−/− or ApoE−/− mice and observed that several pro-inflammatory mediators, such as TNF-α, IL-6, IL-1β, and iNOS (Fig. 6A), were downregulated. Nuclear transcription factor-κB (NF-κB) signaling pathway activation is widely recognized as an important player in the mediation of inflammation in the development of atherosclerosis. Western blot analysis showed that phosphorylated IκBα and p65 levels were decreased in BMDMs from miR-150−/−ApoE−/− mice compared with those from ApoE−/− mice (Fig. 6B). Interestingly, we also observed that MCP-1 mRNA expression was reduced in BMDMs from miR-150−/−ApoE−/− mice (Fig. 6C). MCP-1 is critical for the attraction of monocytes in peripheral blood and the subsequent formation of macrophages in plaques. Thus, we tested macrophage infiltration ability and found that miR-150 deficiency significantly inhibited macrophage migration (Fig. 6D).
Fig. 6.
The inflammatory response and macrophage migration are attenuated by miR-150 ablation. A: qPCR analysis of the expression of inflammatory cytokines secreted by BMDMs from miR-150−/−ApoE−/− or ApoE−/− mice stimulated with Ox-LDL. *P < 0.05 versus the ApoE−/− group. B: Western blot analysis of the expression levels of phosphorylated (P)-IκBα and P-p65 in BMDMs from miR-150−/−ApoE−/− or ApoE−/− mice. *P < 0.05 versus the ApoE−/− group. C: MCP-1 mRNA expression in BMDMs from miR-150−/−ApoE−/− or ApoE−/− mice. *P < 0.05 versus the ApoE−/− group. D: Macrophage infiltration ability was tested with transwell experiments and crystal violet staining. Scale bar = 100 μm. *P < 0.05 versus the ApoE−/− group.
Deletion of miR-150 promotes PDLIM1 expression
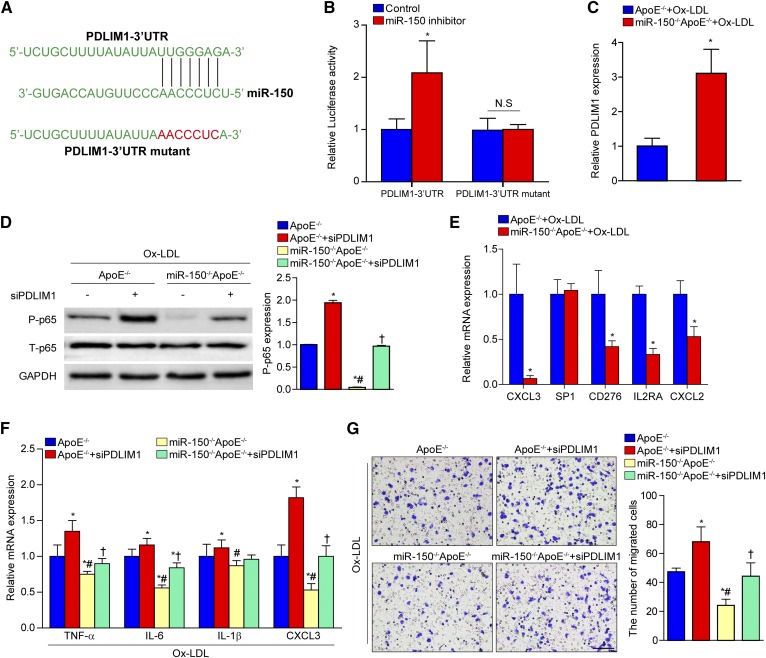
To elucidate the mechanism by which miR-150 affected macrophage activation, we performed bioinformatics analysis using the program TargetScan. The results indicated that PDLIM1 contained a putative binding site for miR-150 in the 3′UTR (Fig. 7A). Using luciferase reporter assays, we demonstrated that miR-150 inhibitor transfection promoted PDLIM1 luciferase activity and that this effect was blocked when the predicted binding sites within the PDLIM1 3′UTR were mutated (Fig. 7B). We also found that PDLIM1 expression was dramatically upregulated in the BMDMs of miR-150−/−ApoE−/− mice (Fig. 7C). A previous study suggested the PDLIM1 inhibited phosphorylated p65 and NF-κB activation, as well as pro-inflammatory cytokine/chemokine secretion (21). As expected, we observed that phosphorylated p65 expression was increased by PDLIM1 knockdown and that the decrease in p65 expression caused by miR-150 ablation was abolished by PDLIM1 deficiency (Fig. 7D). We also assessed the expression of several other potential targets of miR-150, including CXCL3, SP1, CD276, IL2RA, and CXCL2. These proteins are also important mediators of inflammatory response. Interestingly, we observed that CXCL3 and CXCL2 were significantly downregulated in the BMDMs of miR-150−/−ApoE−/− mice (Fig. 7E), indicating that these molecules were not direct targets for miR-150 here in BMDMs. We also assessed whether PDLIM1 was involved in the decreases in pro-inflammatory cytokine/chemokine secretion and macrophage infiltration caused by miR-150 deficiency. We found that PDLIM1 knockdown reversed the suppressed expression and macrophage infiltration caused by miR-150 ablation (Fig. 7F, G). Collectively, these findings suggested that the effects of miR-150 deficiency on atherogenesis were partially mediated by the upregulated expression of its molecular target PDLIM1.
Fig. 7.
The upregulation of PDLIM1 expression by miR-150 deficiency. A: Potential target sites for miR-150 in the 3′UTR of murine PDLIM1 mRNA (blue). The sequence in the binding region that is highlighted in red was mutated. B: Luciferase reporter assays in HEK293 cells treated with a miR-150 inhibitor or negative control using a pEZX-MT01 vector containing the PDLIM1-3′UTR or the PDLIM1-3′UTR with mutations in the predicted miR-150 binding site. *P < 0.05 versus control group. C: qPCR analysis of PDLIM1 expression in BMDMs from miR-150−/−ApoE−/− or ApoE−/− mice stimulated with Ox-LDL. *P < 0.05 versus the ApoE−/− group. D: Western blot analysis of phosphorylated p65 (P-p65) expression levels in BMDMs from miR-150−/−ApoE−/− or ApoE−/− mice transfected with control or PDLIM1-specific siRNAs before Ox-LDL treatment. *P < 0.05 versus the ApoE−/− group; #P < 0.05 versus the ApoE−/− group treated with PDLIM1-specific siRNA; †P < 0.05 versus the miR-150−/−ApoE−/− group. E: qPCR analysis of the expression of potential target genes in BMDMs from miR-150−/−ApoE−/− or ApoE−/− mice stimulated with Ox-LDL. *P < 0.05 versus the ApoE−/− group. F: Inflammatory cytokine mRNA expression in BMDMs from miR-150−/−ApoE−/− or ApoE−/− mice pretreated with control or PDLIM1-specific siRNA before Ox-LDL treatment. *P < 0.05 versus the ApoE−/− group; #P < 0.05 versus the ApoE−/− group treated with PDLIM1-specific siRNA; †P < 0.05 versus the miR-150−/−ApoE−/− group. G: Macrophage infiltration ability was tested with transwell assays and crystal violet staining. *P < 0.05 versus the ApoE−/− group; #P < 0.05 versus the ApoE−/− group treated with PDLIM1-specific siRNA; †P < 0.05 versus the miR-150−/−ApoE−/− group. Scale bar = 100 μm.
DISCUSSION
In the current study, we first demonstrated that miR-150 deficiency exhibited anti-atherosclerotic effects, which were accompanied by ameliorated inflammation and plaque vulnerability. Furthermore, we also verified that the effects of miR-150 ablation on atherogenesis were largely attributed to its effects on macrophages, particularly its effects on the attenuation of the inflammatory response and macrophage infiltration. The absence of miR-150 attenuated increases in phosphorylated p65 expression, inflammatory cytokine secretion and macrophage infiltration by directly upregulating PDLIM1 expression.
During the past decade, multiple studies have demonstrated that various microRNAs are widely involved in numerous human disorders (7, 22–24), and researchers believe that most of the human genome is under microRNA regulation (25). Atherosclerosis is recognized as a complex multifactorial pathological process, and microRNAs have emerged as important regulators of atherosclerosis development (26). miR-150 is an emerged microRNA of 22 nucleotides in length that is known to be highly expressed in lymph nodes, the spleen, mature lymphocytes, regulatory T cells, and B cells (9). However, several published articles have denoted that miR-150 is also abnormally expressed in human CVDs. For example, microRNA expression is upregulated in patients with acute ST-segment elevation myocardial infarction and unstable angina (27, 28); however, miR-150 expression is downregulated in patients with heart failure (29). Additionally, microbubbles isolated from the serum of patients with atherosclerosis that contain more miR-150 can promote atherogenesis (12). In our study, we showed that miR-150 expression was significantly increased in patients with CHD. We obtained similar results in the experiments involving HFD-fed mice. These data suggest that miR-150 is closely related to cardiovascular diseases. More importantly, previous studies have confirmed that miR-150 plays important physiological roles in cell immunity (5) and hematopoiesis (30) and also plays key regulatory roles in monocyte migration, pro-inflammatory cell production, and cardioprotection after acute myocardial infarction (31). Using global-knockout mice, we determined that miR-150 deficiency ameliorated atherosclerosis development by attenuating inflammatory cytokine secretion. Furthermore, we noted that miR-150 expression increased significantly in BMDMs upon Ox-LDL stimulation and that the effects of miR-150 ablation on atherogenesis were largely dependent on its effects on macrophages. Specifically, miR-150 ablation attenuated the inflammatory response and macrophage infiltration. Therefore, our study suggested that the upregulation of miR-150 expression was involved in the development of atherosclerosis and that miR-150 mediates atherosclerosis development through its effects on macrophages. Notably, previous studies have demonstrated that miR-150 mimics ameliorate neovascularization in atherosclerotic conditions and angiogenesis, as well as endothelial cell migration. Additionally, miR-150 can attenuate Ox-LDL-mediated lipoprotein accumulation in cultured macrophages in vitro. This evidence suggests that miR-150 may regulate atherogenesis by engaging distinct downstream pathways in different cell types. Nevertheless, our present study first utilized double-knockout mice to explore the role of miR-150 deficiency on the development of atherosclerosis in vivo. The results indicated that the effects of miR-150 deficiency on atherogenesis were largely dependent on its function on inflammatory response in macrophages.
It is well-known that microRNAs are capable of regulating target gene expression by binding to the 3′UTRs of their target mRNAs and that microRNAs are involved in negatively regulating gene expression at the posttranscriptional level. microRNAs cause the degradation of and/or inhibit the translation of the target mRNAs and ultimately affect the pathophysiological regulation of the corresponding cells, tissues, and organs (22, 32, 33). miR-150 seems to play multiple roles in various pathophysiological processes by targeting different transcripts. In the immune system, miR-150 prevents early B cell development by targeting c-Myb (34), regulates T cell activation by targeting ARRB2 and enhances bone marrow-derived mononuclear cell mobilization and migration by targeting CXCR4 (35, 36). miR-150 deficiency attenuates endothelial cell apoptosis by targeting ELK1 (37), modulates endothelial cell differentiation by targeting ZEB1, reduces c-Myb expression, and enhances endothelial cell migration (12, 38). Additionally, miR-150 inhibits macrophage foam cell formation by targeting adiponectin receptor 2 (16). Using in silico target prediction analysis and luciferase reporter assays, we observed that PDLIM1 was the target gene most significantly upregulated in the BMDMs of miR-150−/−ApoE−/− mice and that PDLIM1 luciferase activity was enhanced by miR-150 inhibitor transfection; however, these changes were prevented by the mutation of the predicted binding sites within the PDLIM1 3′UTR. Moreover, we noted that CXCL3 and CXCL2, which play important roles in macrophage recruitment and plaque progression (39–41), were downregulated in the BMDMs of miR-150−/−ApoE−/− mice. In a previous study, PDLIM1 decreased phosphorylated p65 expression and attenuated pro-inflammatory cytokine/chemokine secretions (42). As expected, our results were consistent with those, as we noted that the decreases in phosphorylated p65 expression, inflammatory cytokine/chemokine excretion, and macrophage infiltration caused by miR-150 deficiency were largely reversed by PDLIM1 deficiency. Thus, we speculated that miR-150 deficiency protects against atherosclerosis in part by upregulating PDLIM1 expression, a change that leads to decreased pro-inflammatory cytokine and chemokine secretion and decreased macrophage infiltration.
Atherosclerotic plaque instability is the most important cause of acute clinical cardiovascular events. Macrophage engulfment and apoptosis play important roles in plaque formation and stability (43, 44). Insufficient foam cell apoptosis secondary to necrosis leads to the formation of necrotic cores in plaques (45, 46). SMCs in plaques increase the thickness of the fibrous cap; however, SMCs also promote macrophage apoptosis and thus reduce the size of the necrotic core (47). Recent studies have indicated that microRNA expression, namely, miR-146a (48) and miR-210 (49) expression, may be closely related to plaque stability. In our study, we observed that miR-150 deficiency dramatically increased plaque stability, a change characterized by increases in collagen and SMC content and decreases in macrophage infiltration and lipid accumulation. Moreover, miR-150 ablation effectively repressed the release of inflammatory factors, such as TNF-α, IL-6, IL-1β, and iNOS. Collectively, these data suggest that miR-150 deficiency is effective in maintaining atherosclerotic plaque stability.
In conclusion, we demonstrated that miR-150 deficiency inhibits atherosclerosis development and inflammatory factor secretion and promotes plaque stability. The athero-protective effect of miR-150 ablation was partially reversed by directly knocking down PDLIM1 expression. Our study suggests that miR-150 is an important novel target for the clinical prevention and treatment of atherosclerosis.
Footnotes
Abbreviations:
- BMDM
- bone marrow-derived macrophage
- CHD
- coronary heart disease
- HFD
- high-fat diet
- IL
- interleukin
- iNOS
- inducible nitric oxide synthase
- MCP-1
- monocyte chemotactic protein 1
- NC
- normal chow
- NF-κB
- nuclear transcription factor-κB
- Ox-LDL
- oxidized LDL
- PDLIM1
- PDZ and LIM domain 1
- qPCR
- quantitative PCR
- SMC
- smooth muscle cell
- VSMC
- vascular smooth muscle cell
This work was supported by National Science Fund for Distinguished Young Scholars Grant 81425005; National Natural Science Foundation of China Grants 81330005, 81370209, 81370365, 81270184, 31371481, and 91639304; and National Science and Technology Support Project Grants 2013YQ030923-05, 2014BAI02B01, 2015BAI08B01, and 2016YFF0101500. The authors declare no conflicts of interest.
REFERENCES
- 1.Benjamin E. J., Blaha M. J., Chiuve S. E., Cushman M., Das S. R., Deo R., de Ferranti S. D., Floyd J., Fornage M., Gillespie C., et al. . 2017. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 135: e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross R. 1999. Atherosclerosis–an inflammatory disease. N. Engl. J. Med. 340: 115–126. [DOI] [PubMed] [Google Scholar]
- 3.Kim C. H. J., Mitchell J. B., Bursill C. A., Sowers A. L., Thetford A., Cook J. A., van Reyk D. M., and Davies M. J.. 2015. The nitroxide radical TEMPOL prevents obesity, hyperlipidaemia, elevation of inflammatory cytokines, and modulates atherosclerotic plaque composition in apoE-/- mice. Atherosclerosis. 240: 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber C., and von Hundelshausen P.. 2017. CANTOS trial validates the inflammatory pathogenesis of atherosclerosis: setting the stage for a new chapter in therapeutic targeting. Circ. Res. 121: 1119–1121. [DOI] [PubMed] [Google Scholar]
- 5.Kluiver J. L., and Chen C. Z.. 2012. MicroRNAs regulate B-cell receptor signaling-induced apoptosis. Genes Immun. 13: 239–244. [DOI] [PubMed] [Google Scholar]
- 6.Lin Y. C., Kuo M. W., Yu J., Kuo H. H., Lin R. J., Lo W. L., and Yu A. L.. 2008. c-Myb is an evolutionary conserved miR-150 target and miR-150/c-Myb interaction is important for embryonic development. Mol. Biol. Evol. 25: 2189–2198. [DOI] [PubMed] [Google Scholar]
- 7.Haga H., Yan I., Takahashi K., Wood J., and Patel T.. 2014. Emerging insights into the role of microRNAs in the pathogenesis of cholangiocarcinoma. Gene Expr. 16: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W., Zhang Y., Wang D., Gao S., Wang X., Gao H., and Wang L.. 2016. Prognostic role of microRNA-150 in various carcinomas: a meta-analysis. Onco Targets Ther. 9: 1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mi Q-S., Xu Y-P., Qi R-Q., Shi Y-L., and Zhou L.. 2012. Lack of microRNA miR-150 reduces the capacity of epidermal Langerhans cell cross-presentation. Exp. Dermatol. 21: 876–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeller T., Keller T., Ojeda F., Reichlin T., Twerenbold R., Tzikas S., Wild P. S., Reiter M., Czyz E., Lackner K. J., et al. . 2014. Assessment of microRNAs in patients with unstable angina pectoris. Eur. Heart J. 35: 2106–2114. [DOI] [PubMed] [Google Scholar]
- 11.Huan T., Rong J., Tanriverdi K., Meng Q., Bhattacharya A., McManus D. D., Joehanes R., Assimes T. L., McPherson R., Samani N. J., et al. . 2015. Dissecting the roles of microRNAs in coronary heart disease via integrative genomic analyses. Arterioscler. Thromb. Vasc. Biol. 35: 1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y., Liu D., Chen X., Li J., Li L., Bian Z., Sun F., Lu J., Yin Y., Cai X., et al. . 2010. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol. Cell. 39: 133–144. [DOI] [PubMed] [Google Scholar]
- 13.Li J., Zhang Y., Liu Y., Dai X., Li W., Cai X., Yin Y., Wang Q., Xue Y., Wang C., et al. . 2013. Microvesicle-mediated transfer of microRNA-150 from monocytes to endothelial cells promotes angiogenesis. J. Biol. Chem. 288: 23586–23596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y., Li Y., Luo P., Gao Y., Yang J., Lao K-H., Wang G., Cockerill G., Hu Y., Xu Q., et al. . 2016. XBP1 splicing triggers miR-150 transfer from smooth muscle cells to endothelial cells via extracellular vesicles. Sci. Rep. 6: 28627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ying W., Tseng A., Chang R. C-A., Wang H., Lin Y-l., Kanameni S., Brehm T., Morin A., Jones B., Splawn T., et al. . 2016. miR-150 regulates obesity-associated insulin resistance by controlling B cell functions. Sci. Rep. 6: 20176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J., and Zhang S.. 2016. microRNA-150 inhibits the formation of macrophage foam cells through targeting adiponectin receptor 2. Biochem. Biophys. Res. Commun. 476: 218–224. [DOI] [PubMed] [Google Scholar]
- 17.Liu H., Cheng W. L., Jiang X., Wang P. X., Fang C., Zhu X. Y., Huang Z., She Z. G., and Li H.. 2017. Ablation of interferon regulatory factor 3 protects against atherosclerosis in apolipoprotein E-deficient mice. Hypertension. 69: 510–520. [DOI] [PubMed] [Google Scholar]
- 18.Ni W., Egashira K., Kitamoto S., Kataoka C., Koyanagi M., Inoue S., Imaizumi K., Akiyama C., Nishida K. I., and Takeshita A.. 2001. New anti-monocyte chemoattractant protein-1 gene therapy attenuates atherosclerosis in apolipoprotein E-knockout mice. Circulation. 103: 2096–2101. [DOI] [PubMed] [Google Scholar]
- 19.Cheng W. L., Wang P. X., Wang T., Zhang Y., Du C., Li H., and Ji Y.. 2015. Regulator of G-protein signalling 5 protects against atherosclerosis in apolipoprotein E-deficient mice. Br. J. Pharmacol. 172: 5676–5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P. X., Ji Y. X., Zhang X. J., Zhao L. P., Yan Z. Z., Zhang P., Shen L. J., Yang X., Fang J., Tian S., et al. . 2017. Targeting CASP8 and FADD-like apoptosis regulator ameliorates nonalcoholic steatohepatitis in mice and nonhuman primates. Nat. Med. 23: 439–449. [DOI] [PubMed] [Google Scholar]
- 21.Ono R., Kaisho T., and Tanaka T.. 2015. PDLIM1 inhibits NF-kappaB-mediated inflammatory signaling by sequestering the p65 subunit of NF-kappaB in the cytoplasm. Sci. Rep. 5: 18327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laffont B., and Rayner K. J.. 2017. MicroRNAs in the pathobiology and therapy of atherosclerosis. Can. J. Cardiol. 33: 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reyes R. K., Motiwala T., and Jacob S. T.. 2014. Regulation of glucose metabolism in hepatocarcinogenesis by microRNAs. Gene Expr. 16: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teng K. Y., and Ghoshal K.. 2015. Role of noncoding RNAs as biomarker and therapeutic targets for liver fibrosis. Gene Expr. 16: 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartel D. P. 2009. MicroRNAs: target recognition and regulatory functions. Cell. 136: 215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartmann P., Schober A., and Weber C.. 2015. Chemokines and microRNAs in atherosclerosis. Cell. Mol. Life Sci. 72: 3253–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang R., Lan C., Pei H., Duan G., Huang L., and Li L.. 2015. Expression of circulating miR-486 and miR-150 in patients with acute myocardial infarction. BMC Cardiovasc. Disord. 15: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu A., Chen S-J., Chang Y-S., Chen H-C., and Chu P-H.. 2014. Systemic approach to identify serum microRNAs as potential biomarkers for acute myocardial infarction. BioMed Res. Int. 2014: 418628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scrutinio D., Conserva F., Passantino A., Iacoviello M., Lagioia R., and Gesualdo L.. 2017. Circulating microRNA-150-5p as a novel biomarker for advanced heart failure: a genome-wide prospective study. J. Heart Lung Transplant. 36: 616–624. [DOI] [PubMed] [Google Scholar]
- 30.Sun Z., Wang Y., Han X., Zhao X., Peng Y., Li Y., Peng M., Song J., Wu K., Sun S., et al. . 2015. miR-150 inhibits terminal erythroid proliferation and differentiation. Oncotarget. 6: 43033–43047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z., Ye P., Wang S., Wu J., Sun Y., Zhang A., Ren L., Cheng C., Huang X., Wang K., et al. . 2015. MicroRNA-150 protects the heart from injury by inhibiting monocyte accumulation in a mouse model of acute myocardial infarction. Circ. Cardiovasc. Genet. 8: 11–20. [DOI] [PubMed] [Google Scholar]
- 32.Bhat S. S., Jarmolowski A., and Szweykowska-Kulińska Z.. 2016. MicroRNA biogenesis: Epigenetic modifications as another layer of complexity in the microRNA expression regulation. Acta Biochim. Pol. 63: 717–723. [DOI] [PubMed] [Google Scholar]
- 33.Neudecker V., Yuan X., Bowser J. L., and Eltzschig H. K.. 2017. MicroRNAs in mucosal inflammation. J. Mol. Med. 95: 935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao C., Calado D. P., Galler G., Thai T-H., Patterson H. C., Wang J., Rajewsky N., Bender T. P., and Rajewsky K.. 2007. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell. 131: 146–159. [DOI] [PubMed] [Google Scholar]
- 35.Sang W., Wang Y., Zhang C., Zhang D., Sun C., Niu M., Zhang Z., Wei X., Pan B., Chen W., et al. . 2016. MiR-150 impairs inflammatory cytokine production by targeting ARRB-2 after blocking CD28/B7 costimulatory pathway. Immunol. Lett. 172: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tano N., Kim H. W., and Ashraf M.. 2011. microRNA-150 regulates mobilization and migration of bone marrow-derived mononuclear cells by targeting Cxcr4. PLoS One. 6: e23114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin B., Shu Y., Xiao L., Lu T., Lin Y., Yang H., and Lu Z.. 2017. MicroRNA-150 targets ELK1 and modulates the apoptosis induced by ox-LDL in endothelial cells. Mol. Cell. Biochem. 429: 45–58. [DOI] [PubMed] [Google Scholar]
- 38.Luo Z., Wen G., Wang G., Pu X., Ye S., Xu Q., Wang W., and Xiao Q.. 2013. MicroRNA-200C and -150 play an important role in endothelial cell differentiation and vasculogenesis by targeting transcription repressor ZEB1. Stem Cells. 31: 1749–1762. [DOI] [PubMed] [Google Scholar]
- 39.Altara R., Manca M., Brandao R. D., Zeidan A., Booz G. W., and Zouein F. A.. 2016. Emerging importance of chemokine receptor CXCR3 and its ligands in cardiovascular diseases. Clin. Sci. 130: 463–478. [DOI] [PubMed] [Google Scholar]
- 40.Combadière C., Potteaux S., Rodero M., Simon T., Pezard A., Esposito B., Merval R., Proudfoot A., Tedgui A., and Mallat Z.. 2008. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 117: 1649–1657. [DOI] [PubMed] [Google Scholar]
- 41.Tabas I. 2010. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 10: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ono R., Kaisho T., and Tanaka T.. 2015. PDLIM1 inhibits NF-κB-mediated inflammatory signaling by sequestering the p65 subunit of NF-κB in the cytoplasm. Sci. Rep. 5: 18327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rébé C., Raveneau M., Chevriaux A., Lakomy D., Sberna A. L., Costa A., Bessède G., Athias A., Steinmetz E., Lobaccaro J. M., et al. . 2009. Induction of transglutaminase 2 by a liver X receptor/retinoic acid receptor alpha pathway increases the clearance of apoptotic cells by human macrophages. Circ. Res. 105: 393–401. [DOI] [PubMed] [Google Scholar]
- 44.Tabas I., García-Cardeña G., and Owens G. K.. 2015. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 209: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez L., and Trigatti B. L.. 2017. Macrophage apoptosis and necrotic core development in atherosclerosis: a rapidly advancing field with clinical relevance to imaging and therapy. Can. J. Cardiol. 33: 303–312. [DOI] [PubMed] [Google Scholar]
- 46.Thorp E., Cui D., Schrijvers D. M., Kuriakose G., and Tabas I.. 2008. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe-/- mice. Arterioscler. Thromb. Vasc. Biol. 28: 1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai Q. 2004. Interaction of monocytes with vascular smooth muscle cells regulates monocyte survival and differentiation through distinct pathways. Arterioscler. Thromb. Vasc. Biol. 24: 2263–2270. [DOI] [PubMed] [Google Scholar]
- 48.Hulsmans M., Van Dooren E., Mathieu C., and Holvoet P.. 2012. Decrease of miR-146b-5p in monocytes during obesity is associated with loss of the anti-inflammatory but not insulin signaling action of adiponectin. PLoS One. 7: e32794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eken S. M., Jin H., Chernogubova E., Li Y., Simon N., Sun C., Korzunowicz G., Busch A., Backlund A., Osterholm C., et al. . 2017. MicroRNA-210 enhances fibrous cap stability in advanced atherosclerotic lesions. Circ. Res. 120: 633–644. [DOI] [PubMed] [Google Scholar]