Abstract
Several studies have revealed that traditional risk factors are less effective in predicting CVD risk in the elderly, suggesting the need to identify new biomarkers. Here, we evaluated the association between serum cholesterol efflux capacity (CEC), an atheroprotective property of HDL recently identified as a novel marker of CVD risk, and atherosclerotic burden in a cohort of very old, healthy individuals. Serum CEC values were not significantly correlated either with calcium score or with markers of vulnerable plaque, such as positive remodeling, hypodensity, spotty calcification, or napking-ring sign. In addition, no association was detected between CEC and telomere length, a marker of biological aging that has been linked to atherosclerosis extent. Interestingly, elderly subjects presented a remarkably higher CEC (+30.2%; P < 0.0001) compared with values obtained from a cohort of sex-matched, cardiovascular event-free, middle-aged individuals. In conclusion, serum CEC is not related to traditional risk factors in very old, cardiovascular event-free subjects, but has significantly higher values compared with a healthy, younger population. Whether this improved HDL functionality may represent a protective factor in CVD onset must be established in future studies.
Keywords: atherosclerosis, high density lipoprotein, heart, lipoproteins, vascular biology, aging
In the last decades, the worldwide population has exhibited an increasing life expectancy with a consequent rise in the elderly population (1). Aging is accompanied by a progressive deterioration of the physiological functions in several organs, including the cardiovascular apparatus (2). In particular, CVD is the leading cause of morbidity and mortality in individuals over the age of 65 and aging itself is one of the most powerful cardiovascular risk factors, as indicated by the scores published in recent guidelines (3).
Interestingly, traditional lipid risk factors might be less effective in predicting CVD risk in the elderly compared with middle-aged individuals. For instance, the Framingham Risk Score, which estimates individual cardiovascular disease risk at10 years and takes into account the contributions of all serum lipoproteins and additional risk factors, has been shown to have only a mild ability to identify CVD risk among old people with longevity potential (4). Focusing on plasma lipids, the positive association between total cholesterol (TC) and cardiovascular risk is strong in middle-aged adults, but it becomes weaker in those 80 years of age or older (5). In addition, we recently reported that high LDL cholesterol (LDL-C) levels are not associated to subclinical coronary artery disease in healthy octogenarians (6) and results of a recent meta-analysis showed either a lack or an inverse association between LDL-C and both all-cause and CVD mortality at late-life age (7).
Whether HDL cholesterol (HDL-C) level can be used to predict CVD in old people is still a matter of debate. Some findings suggest that plasma levels of these lipoproteins may be a more valuable predictor of cardiovascular events compared with TC, LDL-C, and nonHDL-C level in older age (8). This concept is supported by studies that observed an inverse association between HDL-C levels and the risk of coronary artery disease or ischemic stroke in the elderly (9, 10), including a recent work in which we demonstrated that low HDL-C is independently associated with subclinical coronary atherosclerosis in a cohort of healthy octogenarians (6). Consistently, a prospective study showed that persistently low HDL-C levels were a risk factor for the development of CVD events in the elderly (11). To the contrary, other data reported weaker or lack of association between HDL-C and CVD risk in old people (12, 13). Overall, this decline in the predictivity of the traditional risk factors with aging makes stratification in the elderly more difficult compared with younger individuals, suggesting an urgent need to identify new biomarkers able to provide a more precise estimate of cardiovascular risk in these subjects.
Among the novel and recently proposed cardiovascular risk biomarkers is HDL cholesterol efflux capacity (CEC), the most established HDL antiatherogenic property (14, 15), consisting in the capacity to promote the release of excess cholesterol from macrophages of the arterial wall (16). Growing evidence indicates an inverse relationship between HDL CEC and both the prevalence and the incidence of CVD (17–19). Interestingly, this association has been found to persist even after adjustment for traditional risk factors, including HDL-C plasma levels, suggesting that HDL function may represent a better predictor of CVD than the risk estimation based solely on the amount of particles present in the bloodstream. In support of this concept, HDL CEC has been shown to improve risk prediction over the traditional risk factors such as HDL-C plasma concentration (20).
Currently, little is known of the potential association between serum HDL CEC and atherosclerosis in the elderly. In an effort to fill this gap of knowledge, we carried out this study by measuring serum CEC in a cohort of healthy individuals over 80 years of age. In particular, we looked at the relationship between CEC and atherosclerotic burden in the coronary arteries, measured by coronary artery calcium (CAC) and features of plaque vulnerability. The former indicates the presence of coronary atherosclerosis, and it has been used to improve risk prediction for incident coronary heart disease (21). In addition, we explored the potential relationship between serum CEC and telomere length (TL) in old individuals, as shortening of the telomeric DNA, a marker of cell senescence occurring during aging, has been associated to age-related CVD risk (22, 23). Finally, we compared serum CEC values of old subjects with those of a cohort of younger individuals.
METHODS
Subjects
Healthy individuals aged 80 years or more (n = 59) were selected from the entire healthy cohort (n = 208) of the Brazilian Study on Healthy Aging (24). The study was approved by the Institutional Research Ethics Committee of the University of Brasilia, DF, Brazil. Serum CEC results of middle-aged subjects included in the present work belong to a previously published study (18) and were selected from the database of the Brisighella Heart Study (25), for which the approval of the Ethical Committee of the University of Bologna was obtained. All protocols were in accordance with the ethical standards of the Helsinki Declaration, with all participants having signed informed consent before enrollment. The demographic and clinical data of elderly and middle-aged subjects are reported in Tables 1 and 2, respectively.
TABLE 1.
Clinical characteristics and laboratory parameters of elderly subjects (n = 59)
| Variable | Elderly Subjects |
| Age (years) | 86 ± 5 |
| Male gender, n (%) | 14 (24) |
| BMI (kg/m2) | 25.9 ± 4.5 |
| Systolic blood pressure (mmHg) | 142 ± 18 |
| Diastolic blood pressure (mmHg) | 75 ± 11 |
| Current smoking, n (%) | 1 (1.6) |
| Physically active according to WHO, n (%) | 18 (30) |
| Hypertension, n (%) | 42 (70) |
| Type 2 diabetes, n (%) | 16 (27) |
| Lipid lowering treatment, n (%) | 17 (29) |
| Coronary calcium score, Agatston | 99 (0−3856) |
| Subjects with CAC = 0, n (%) | 19 (32.2) |
| Positive remodeling plaques, n (%) | 23 (39) |
| Hypodense plaques, n (%) | 29 (49) |
| Spotty calcified plaques, n (%) | 26 (44) |
| Napkin-ring sign | 10 (17) |
| FMD (%) | 3.60 (1.07−7.40) |
| mean IMT (mm) | 0.86 ± 0.16 |
| TC (mg/dl) | 192 ± 43 |
| VLDL-C (mg/dl) | 29 ± 13 |
| LDL-C (mg/dl) | 108 ± 38 |
| ApoB (mg/dl) | 81 ± 24 |
| HDL-C (mg/dl) | 54 ± 17 |
| ApoA-I (mg/dl) | 147 ± 25 |
| TG (mg/dl) | 131 ± 66 |
| CRP (mg/L) | 2.37 ± 1.78 |
| Fibrinogen (mg/dl) | 357 ± 63 |
| Glucose (mg/dl) | 103 ± 34 |
| Uric acid (mg/dl) | 5.25 ± 1.56 |
| Creatinine (mg/dl) | 0.95 ± 0.28 |
Normally distributed data are presented as mean ± SD and nonnormally distributed data are presented in the form of median (interquartile range). FMD, flow-mediated dilation; IMT, intima-media thickness; WHO, World Health Organization.
TABLE 2.
Demographic and clinical data of middle-aged individuals (n = 140)
| Variable | Middle-aged Subjects | P Compared with Elderly |
| N | 140 | |
| Age | 56 ± 11 | <0.0001 |
| Male gender, n (%) | 33 (24) | 0.98 |
| BMI (kg/m2) | 25.7 ± 4.8 | 0.81 |
| Systolic blood pressure (mmHg) | 112 ± 13 | 0.0001 |
| Diastolic blood pressure (mmHg) | 90 ± 9 | 0.0001 |
| TC (mg/dl) | 210 ± 30 | 0.003 |
| LDL-C (mg/dl) | 139 ± 28 | 0.0001 |
| ApoB (mg/dl) | 99 ± 24 | 0.0001 |
| HDL-C (mg/dl) | 51 ± 10 | 0.31 |
| ApoA-I (mg/dl) | 167 ± 31 | 0.0001 |
| TG (mg/dl) | 97 ± 38 | 0.0001 |
| Glucose (mg/dl) | 97 ± 10 | 0.17 |
| Creatinine (mg/dl) | 0.88 ± 0.16 | 0.0001 |
Statistical significance of middle-aged subject parameters is calculated versus the corresponding value reported in Table 1 for elderly subjects by nonparametric Mann-Whitney unpaired t-test.
Biochemical analyses
After 12 h of overnight fasting, the study participants underwent blood sampling. Samples were centrifuged at 1,600 g at 5°C for 15 min to separate plasma and to make the following measurements: glucose (Glucose GOD-PAP, Roche Diagnostics, Mannheim, Germany), total cholesterol (CHOD-PAP, Roche Diagnostics), triglycerides (GPO-PAP, Roche Diagnostics), HDL-C (HDL cholesterol without pretreatment, Roche Diagnostics), C-reactive protein (highly sensitive CRP, CardioPhase, Dade Behring, Marburg, Germany), creatinine (GLDH, Hitachi, Tokyo, Japan), uric acid (Uricase-Peroxidase method, Hitachi 747; Hitachi), apoA-I and apoB (Behring Nephelometer BNII, Dade Behring), and fibrinogen (Sysmex CA 1500, Siemens, Munich, Germany). LDL-C and VLDL-C were calculated using the Friedewald formula. All samples were evaluated immediately. EDTA plasma samples were also frozen at −80°C for CEC measurements.
CEC
Serum CEC was quantified by measuring the efflux of radioactive cholesterol as previously described (26). Briefly, murine macrophage J774 cells were grown in DMEM (Lonza Verviers Sprl, Verviers, Belgium) containing 10% FBS (Sigma-Aldrich, Milano, Italy) and 1% penicillin/streptomycin in 5% carbon dioxide at 37°C. Cells were plated onto 24-well plates (250,000 cells/well) and labeled with 1 μCi/ml [1,2-3H] cholesterol (Perkin Elmer) in medium with 1% FBS, in the presence of 2 µg/ml of an inhibitor of the enzyme esterifying cholesterol, ACAT (Sandoz 58035; Sigma-Aldrich), to prevent the accumulation of cholesteryl esters. After 24 h, cholesterol label was removed, cells were washed twice with PBS (Lonza) and successively stimulated by incubation with a cAMP analog, cpt-cAMP (Sigma Aldrich), at 0.3 mM in DMEM with 0.2% free fatty acid BSA (Sigma Aldrich) for 18 h to upregulate the membrane cholesterol transporter ABCA1 (27). Cells were finally exposed to 2% (v/v) of subjects’ whole serum for 4 h. Serum samples were obtained at admission into the study and stored at −80°C until use. Before the addition to cells, sera were gently thawed in ice. After the efflux period, the medium was removed from each well, filtered to remove cellular debris, and 3H cholesterol quantified by liquid scintillation counting. Percentage efflux was determined as the radioactivity detected in the media divided by the total radioactivity incorporated by cells. All samples were run in triplicate. To check for adequate cAMP-induced ABCA1 expression, the specific ABCA1 cholesterol acceptor lipid-free human apoA-I was tested together with serum samples. Values were normalized by dividing the efflux capacity of individual subjects by the efflux capacity of a standard serum run in each assay (19). The direct comparison between octogenarians and middle-aged subjects in terms of CEC was feasible because the same serum standard was used for normalization in all the assays.
Cardiac computed tomography
Cardiac computed tomography was performed to evaluate coronary calcium score on a 64-detector row scanner (Aquilion 64, Toshiba, Ottawara, Tokyo, Japan). Axial slices of 3-mm thickness were acquired in synchrony with an electrocardiographic tracing in 70% of the RR interval. Coronary calcifications were defined by at least three continuous pixels with a minimum of 130 Hounsfield units and were analyzed by a single certified radiologist. The Agatston method was used to express the values of coronary calcification. Prior to the scans, all patients with heart frequency above 60 beats per minute received a maximum dose of 25 mg endovenous cardioselective β-blocker metoprolol (Seloken®, Astra-Zeneca) and 5 mg Isosorbide dinitrate (Isordil®, EMS). For coronary computed tomography angiography analyses, the coronary arteries were grouped in four major arteries: left main trunk, left anterior descendent, left circumflex, and right coronary artery, including their major and minor branches. Coronary atherosclerotic plaques were defined as any tissue >1mm2 within or adjacent to the lumen that could be discriminated from surrounding pericardial tissue, epicardial fat, or lumen and that could be identified in at least 2 planes. Plaque morphology was visually described as 1) noncalcified plaque, if there was no signal of higher density in the plaque; 2) calcified plaque, if calcification larger than 3 mm was detected; 3) spotty calcified plaques, if calcification was present <3 mm in size on curved multiplane reformatting images and occupied only one side on sectional images (28); and 4) positive remodeling plaques, when the diameter in the vessel diameter at the plaque site was at least 10% larger than the segment reference set proximal to the lesion in a normal vessel segment. The presence or absence of vulnerable features in the coronary plaques was evaluated by using the Motoyama criteria (28). For each coronary artery (left main, right coronary, circumflex coronary, anterior descending coronary) one point was given if the characteristic was present. Hence, plaque remodeling was counted from 0 (absence) to 4 (presence in the four coronary branches). The same was done for hypodensity and presence of calcium spots. Napkin-ring sign was identified by the presence of a ring of high attenuation around the coronary artery plaque and ring attenuation presenting greater than those of the adjacent plaque and not greater than 130 Hounsfield units (29).
TL
TL was estimated according to a previously described method (30). Briefly, genomic DNA was extracted from leukocyte homogenate using Qiagen DNA extraction kit and TL determined by real-time RT-PCR. Amplification reaction was composed by 35 ng of DNA Sybr Mastermix (Invitrogen) and primers for telomere (forward 5′CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGG TTTGGGTT3 ’and backward 5′GGCTTGCCTTACCCTTACCCTTACCC TTACCCTTACCCT3′) and for housekeeping control, the 36b4, (forward 5′CAGCAAGTGGGAAGGTGTAATCC3 ’and backward 5′CCCATTCTATCATCAACGGGTACAA3′). Fluorescence detection was performed with Rotor-Gene 6000 (Corbett Research). For each DNA sample, we performed three quantitative PCRs for telomere repeat sequence (T) and three for the reference single copy gene (S), that is, the 36b4 gene. The TL was estimated by the average of the three T measurements divided by the average of the three S measurements. TL values were available for 51 of 59 subjects.
Statistical analysis
Data distribution was assessed for normality with the use of both histograms and the Shapiro Wilk test. Normally distributed data are presented as mean ± SD and nonnormally distributed data are presented in the form of median (interquartile range). Gaussian curves were created by nonlinear regression of the frequency distribution. Proportional differences between groups were evaluated using chi-square. Mean differences between groups were evaluated with one-way ANOVA and ANCOVA where there was need for adjustments. Nonparametric data were compared by using the Mann-Whitney unpaired t-test. To keep the comparison between middle-aged and elderly adults balanced and unsaturated, a propensity score was generated using age, sex, and variables that showed significant or marginal differences in the unadjusted comparison between these two groups: mean blood pressure, presence of diabetes, apoB, apoA-I, creatinine, triglycerides, and glucose. Hence, CEC values were compared between these two groups by ANCOVA adjusting for the propensity score. Statistical analyses were conducted using SPSS software version 21.0 or Graph Pad Prism software (version 6.01).
RESULTS
The baseline demographic and clinical characteristics of elderly participants are summarized in Table 1. In agreement with the overall demographics of this age group, most octogenarians in the study were women. To rule out comorbidities that could indirectly interfere with markers of atherosclerotic risk, such as cancer or inflammatory diseases, we used strict selection criteria. Thus, as well as the absence of these diseases, lipid profile, glucose, uric acid, fibrinogen, CRP, and creatinine were within normal reference ranges. Calcium supplement plus bisphosphonate was used by eleven elderly subjects, whose individual characteristics are reported in supplemental Table S1. Calcitriol was not used by any enrolled patient. The use of calcium plus bisphosphonate was not associated with CAC (P = 0.48). Over 30% of the population had CAC = 0. The coronary calcium score distribution in male and female subjects is shown in supplemental Fig. S1. Table 1 also shows the presence or absence of remodeling, hypodensity, spotty calcification, and napkin-ring sign, markers of unstable plaques in the arteries of analyzed subjects.
Overall, 39% of subjects (n = 23) had positive remodeling plaques, 49% (n = 29) had hypodense plaques, 44% (n = 26) presented spotty calcified plaques, and 17% (n = 10) had napkin-ring sign. Nine subjects (15%) had all four vulnerable plaque markers.
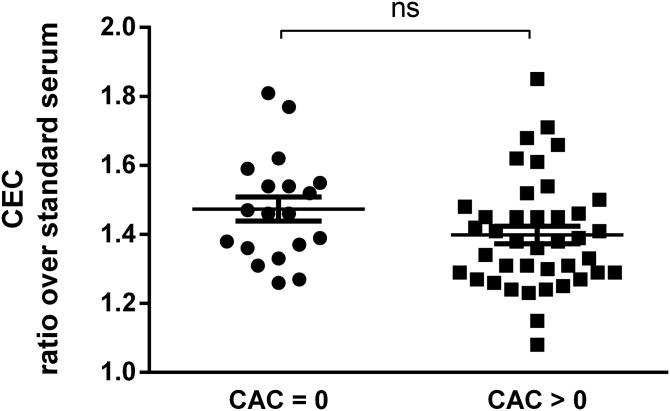
To explore the potential relationship between serum CEC and CAC in the elderly subjects, we stratified CEC values between subjects with CAC = 0 and with CAC > 0 (31). As can be seen in Fig. 1, no significant difference was observed between the two groups (average CEC ratio over standard serum was 1.47 ± 0.15 for CAC = 0, compared with 1.40 ± 0.16 for CAC > 0; P = 0.09). We further stratified CEC values according to tertiles of CAC. We observed that CEC values at the first CAC tertile were higher compared with the second (P = 0.017) but not to the third tertile (supplemental Fig. S2).
Fig. 1.
Stratification of CEC values with CAC = 0 or CAC > 0 in elderly subjects. Each point represents the mean of CEC values obtained from an evaluation in triplicate of each serum sample. CEC values are expressed as a ratio over a standard serum as described in Methods section. Statistical significance was calculated by nonparametric Mann-Whitney unpaired t-test. n = 19 subjects with CAC = 0 and n = 40 with CAC > 0. ns, not significant.
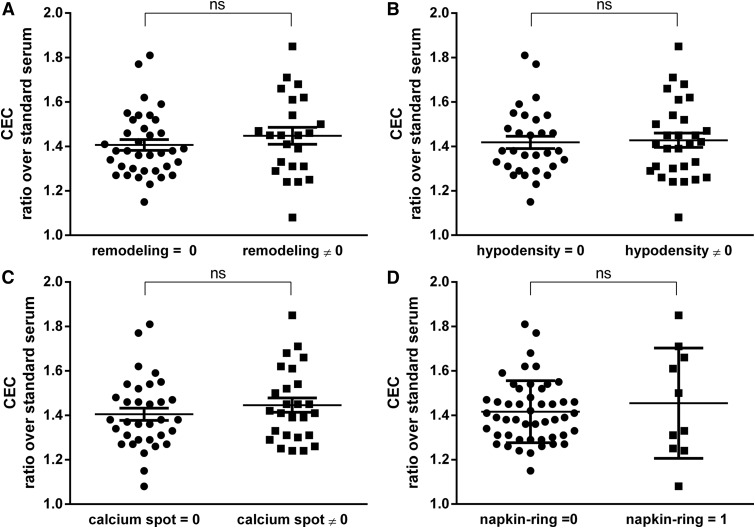
We subsequently evaluated the relationship between serum CEC and the presence of atherosclerotic plaques with positive remodeling, hypodensity, spotty calcification, or napkin-ring sign. Individuals were categorized depending on the absence or presence of vulnerable plaques markers and their serum CEC values were compared. As shown in Fig. 2, comparable CEC values were displayed by subjects negative or positive for arterial remodeling (average CEC ratio over standard serum was 1.41 ± 0.14 compared with 1.45 ± 0.18, not significant, Fig. 2A), hypodensity (average CEC ratio over standard serum was 1.42 ± 0.15 compared with 1.43 ± 0.17, not significant, Fig. 2B) calcium spot (average CEC ratio over standard serum was 1.41 ± 0.16 compared with 1.45 ± 0.16, not significant, Fig. 2C), and napkin-ring sign (average CEC ratio over standard serum was 1.42 ± 0.14 compared with 1.45 ± 0.25, not significant, Fig. 2D).
Fig. 2.
Stratification of CEC values according to the absence (0) or the presence (≠ 0) of vulnerability plaque features in elderly subjects. Each point represents the mean of the values obtained from an evaluation in triplicate of each serum sample. CEC values are expressed as a ratio over a standard serum as described in Methods section. Statistical significance was calculated by nonparametric Mann-Whitney unpaired t-test. (A: remodeling, B: hypodensity, C: spotty calcification, and D: napking-ring sign). n = 36 subjects with remodeling = 0 and n = 23 with remodeling ≠ 0; n = 30 subjects with hypodensity = 0 and n = 29 with hypodensity ≠ 0; n = 33 subjects with calcium spot = 0 and n = 26 with calcium spot ≠ 0; n = 49 subjects with napkin-ring sign = 0 and n = 10 with napking-ring sign = 1. ns, not significant.
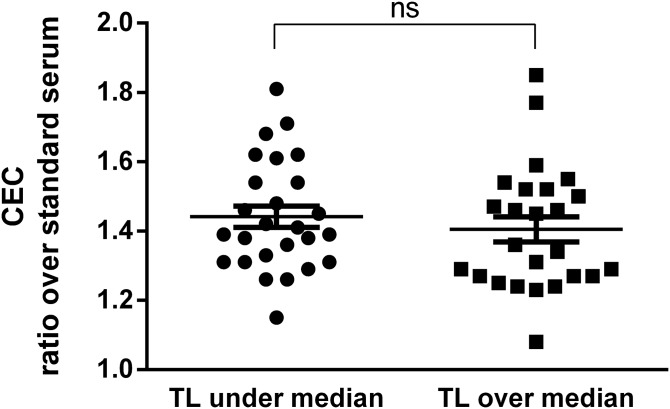
We also evaluated whether serum CEC is associated with TL in our population, considering that telomere shortening, occurring with aging, has previously been related to early markers of cardiovascular disease and subclinical atherosclerosis (32, 33). Similar to what was observed for the parameters considered above, individuals with TL values under and over the median were not different in terms of serum CEC; average CEC ratio over standard serum was 1.44 ± 0.16 and 1.40 ± 0.18 in subjects with TL values under and over the median, respectively (not significant, Fig. 3). No differences were detected by further stratifying CEC values by tertiles of TL (ANOVA, P = 0.968, supplemental Fig. S3). In addition, TL did not associate either with the measured markers of subclinical atherosclerosis (flow-mediated dilation and intima-media thickness) or with CAC (data not shown).
Fig. 3.
Stratification of CEC values according to telomere length (TL) values under and over the median in elderly subjects. Each point represents the mean of the values obtained from an evaluation in triplicate of each serum sample. CEC values are expressed as a ratio over a standard serum as described in Methods section. Statistical significance was calculated by nonparametric Mann-Whitney unpaired t-test. Median TL value = 79.16%. n = 26 subjects with TL values under the median and n = 25 with TL values over the median. ns, not significant.
Finally, we compared serum CEC values of elderly subjects with those obtained from a cohort of sex-matched, cardiovascular event-free, middle-aged individuals (n = 140) that we analyzed in a previous study (18), whose demographic and clinical characteristics are reported in Table 2.
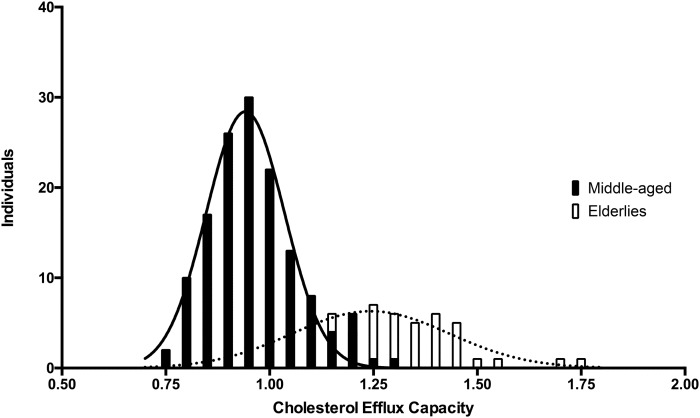
As expected according to the established age-dependent variations of blood pressure (34), middle-aged individuals presented lower values of systolic blood pressure and higher values of diastolic blood pressure. Plasma levels of TC, LDL-C, apoA-I, and apoB were significantly higher and TG was significantly lower in middle-aged subjects, whereas both populations displayed similar HDL-C values. CEC values were significantly different between middle-aged adults and octogenarians in the unadjusted analysis. Notably, elderly subjects showed a markedly higher serum CEC compared with middle-aged adult subjects (average CEC ratio over serum standard was 1.25 ± 0.19 compared with 0.96 ± 0.10; P < 0.0001; Fig. 4). This difference remained significant even after adjusting for the propensity score (P < 0.0001).
Fig. 4.
Histograms of CEC values with the Gaussian curves created by nonlinear regression of the frequency distribution. Elderly subjects (white bars and dashed lines) are interleaved with middle-aged subjects (black bars and continuous lines).
DISCUSSION
In the present study, we demonstrated that in healthy individuals aged 80 years or more (80–102 years), serum CEC does not associate either with atherosclerotic burden in the coronary arteries or with features of plaque vulnerability. In addition, no relationship was detected between serum CEC and TL. Also, as a secondary endpoint, we made comparisons with a cohort of middle-aged subjects, finding that octogenarians present higher CEC.
It is important to note that the elderly subjects enrolled in our study, as well as lack of CVD, showed good nutritional status and had no evidence of neoplastic disease. This thorough selection allowed for the avoidance of potential confounding factors, the impact of which on cardiovascular risk factors is well documented (35).
In our previous paper involving a larger group of octogenarian individuals, we demonstrated that HDL-C independently associates with severity of subclinical coronary artery disease, whereas LDL-C does not (6). The current study adds new information, indicating that in very old subjects, HDL function measured by CEC is not related to traditional cardiovascular risk factors, such as CAC or characteristics of vulnerable plaque. In addition, for the first time, we examined the association between serum CEC and TL, but found no relationship.
Serum CEC is a metric of HDL functionality that has been associated to atherosclerosis and cardiovascular diseases in both a cross-sectional and longitudinal manner, indicating that this parameter may be used as a valid cardiovascular risk biomarker independent of plasma HDL-C concentrations (36, 37). Notably, in most published CEC evaluations, apoB-depleted serum (HDL fraction) has been utilized, whereas in this study we used whole serum. This could be considered a limitation in the assessment of HDL functionality. Indeed, in sera with cholesterol levels in the normal range, which is the case of our study, previous observations indicate that cholesterol efflux from cAMP-treated macrophages to both whole and apoB-depleted serum normally leads to similar results (38, 39). In our cohort, we did not find differences in CEC values in subjects with a CAC score = 0 and with CAC > 0, although a nonsignificant tendency of lower serum CEC in subjects with a CAC score > 0 was revealed. A further stratification by CAC tertiles revealed that subjects in the lowest tertile of CAC exhibited the same CEC as those in the highest tertile of CAC. Surprisingly, individuals in the intermediate tertile of CAC showed lower CEC than those in the lowest and the highest tertiles. We believe this pattern may represent a play of chance due to the limited number of subjects in the study. Previous works examined the association between CEC and coronary calcium; one recent study reported a reduction of HDL CEC in subjects with prevalent CAC (>0) (20), whereas other authors did not find any significant association between the two parameters (17). In both studies the analyzed population consisted of middle-aged subjects; thus, the results may not reflect the situation in subjects over 80 years of age. Our data show in favor of the absence of a relationship between HDL functionality and CAC, which, for the first time, has been evaluated in individuals aged 80 years or more. However, because the posthoc power analysis (β=39%) was not sufficient to discard a potential mild difference between groups, this finding certainly deserves reevaluation in future, larger studies.
The lack of association between CAC and CEC could be explained by confounding factors differently affecting these parameters. Probably the main element to be considered is the divergence between the cumulative nature of the CAC and the dynamic nature of the CEC. Whereas the former corresponds to the entire 80 years or more of life of these individuals, CEC may reflect the function of HDL over a shorter time period. Lifestyle and medication use over a period of years may have had a stronger impact on atherosclerotic burden than HDL function, dampening a potential association between the two parameters. Statins, for example, are known to increase CAC (40) without affecting CEC (41, 42). Although in our cohort, statin therapy was not related either to CAC [median and interquartile range: 17 (0.0–763.5) and 119.5 (19.25–811.3) in users and nonusers respectively; P = 0.312] or to CEC (mean ± SD: 1.45 ± 0.15 and 1.41 ± 0.17 in users and nonusers respectively; P = 0.402), the present data is not sufficient to rule out this potential interaction.
The observation that octogenarians present significantly higher CEC compared with younger individuals is in contrast with a previous report from Berrougui et al. (43). These authors compared CEC of two small cohorts, demonstrating that aging promotes phenotypic changes of HDL and impairment of their capacity to promote ABCA1-mediated cholesterol efflux, particularly the one driven by the smaller HDL3 sub-population. In addition, Berrougui et al. showed that, differently from HDL3, the more mature particles HDL2 from elderly subjects did not reveal impaired function. We did not perform a structural characterization of HDL subpopulations in this present work, but based on the findings of Berrougui et al., we hypothesize that the HDL2 contribution to cholesterol efflux may compensate for HDL3 impairment, possibly justifying the improved CEC observed in our subjects. In addition, in the work of Berrougui et al., the isolation of HDL was performed with ultracentrifugation that caused the elimination of nascent preβ HDL particles, as the authors themselves state, particles that are well known to be efficient acceptors of cholesterol released through ABCA1 and ABCG1 (44, 45), both expressed in the cellular model that we adopted (18). Conversely, in our samples, it is conceivable that preβ HDL significantly contributes to increased CEC by these mechanisms.
Given our results, we are tempted to speculate that improved CEC may represent a beneficial process, protecting elderly individuals from cardiovascular events, thus possibly explaining the lack of relationship with atherosclerosis in these subjects. However, a conclusive statement should be supported by a comparison of the CEC values of octogenarian subjects with cardiovascular disease as well with the CEC values of their descendants. We are well aware that a major limitation of the comparison of the two populations included in this study is the different ethnicity. Although most of the white elderly subjects in this study are first and second generation European descendants who migrated to Brazil in the last century, we cannot rule out that the observed difference is related, at least in part, to this. In this regard, little is known about the impact of race on serum HDL CEC at this time. A recent study specifically designed to compare HDL functionality in South Asians and Caucasians failed to demonstrate any difference, whereas Rohatgi et al. (17), by investigating the epidemiology of CEC in a large cohort from the Dallas Heart Study, observed that CEC was significantly lower in blacks than whites. In the future, properly designed studies are warranted to clarify this point.
In the present study we utilized CAC scoring as a risk-stratification tool because it is known to be useful in both middle-aged and elderly subjects. It has also been shown that in the elderly, elevated CAC is an independent predictor of cardiovascular events (46). Additionally, telomere shortening, considered a marker of biological aging, has been linked to age-related diseases such as atherosclerosis and identified as a predictor of its clinical outcomes (32). We did not find a correlation between TL and the markers of subclinical atherosclerosis flow-mediated dilation and intima-media thickness, in line with previous findings (32). In our population, we did not find an association between TL and coronary calcification either. This is in contrast with the literature, where a relationship between these two parameters has emerged (47, 48). However, this relationship has never been studied in the elderly; thus, it is possible that, similar to TC, the predictive power of TL is lost for those at very old age, further strengthening the concept that identifying risk factors in this specific population is difficult. For this reason, further studies are needed to more deeply investigate this aspect.
In our subjects, even the association between TL and outcomes of plaque remodeling is inconsistent. Whether this observation is related to the characteristics of enrolled subjects or reflects the absence of interrelationship among these outcomes is still to be clarified. The former hypothesis could be supported by the observation that aging drives the loss of well-established associations among cardiovascular outcomes (5). The latter could be investigated by evaluating CEC, TL, and remodeling indexes in different cohorts of patients. To the best of our knowledge, these associations have not been evaluated yet, either in middle-aged subjects or in elderly patients with chronic diseases.
In conclusion, similar to the report by Mutharasan et al (49), our study failed to associate the atheroprotective parameter of serum CEC with indexes of atherosclerosis in very old subjects. As also suggested by these authors, it is possible to speculate that CEC plays a more protective role in the initial stages of atherosclerosis, thus explaining why associations of CEC with subclinical atherosclerosis and cardiovascular events are more evident in younger populations. However, our original observation that people reaching old age in healthy condition seem to have significantly higher CEC compared with a healthy, middle-aged population offers a hint of great interest. Whether CEC represents the cause or is simply a marker of such healthy longevity needs to be further investigated.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Arrigo Cicero and Prof. Claudio Borghi (both from the University of Bologna, Italy) for providing the serum samples of the middle-aged subjects.
Footnotes
Abbreviations:
- CAC
- coronary artery calcium
- CEC
- cholesterol efflux capacity
- CRP
- C-reactive protein
- HDL-C
- HDL cholesterol
- LDL-C
- LDL cholesterol
- TC
- total cholesterol
- TL
- telomere length
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Mathers C. D., Stevens G. A., Boerma T., White R. A., and Tobias M. I.. 2015. Causes of international increases in older age life expectancy. Lancet. 385: 540–548. [DOI] [PubMed] [Google Scholar]
- 2.Camici G. G., Savarese G., Akhmedov A., and Luscher T. F.. 2015. Molecular mechanism of endothelial and vascular aging: implications for cardiovascular disease. Eur. Heart J. 36: 3392–3403. [DOI] [PubMed] [Google Scholar]
- 3.Windecker, S., P. Kolh, F. Alfonso, J. P. Collet, J. Cremer, V. Falk, G. Filippatos, C. Hamm, S. J. Head, P. Juni, A. et al. 2014. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 35: 2541–2619. [DOI] [PubMed] [Google Scholar]
- 4.Beekman M., Uh H. W., van Heemst D., Wuhrer M., Ruhaak L. R., Gonzalez-Covarrubias V., Hankemeier T., Houwing-Duistermaat J. J., and Slagboom P. E.. 2016. Classification for Longevity Potential: The Use of Novel Biomarkers. Front. Public Health. 4: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prospective Studies Collaboration, Lewington S., Whitlock G., Clarke R., Sherliker P., Emberson J., Halsey J., Qizilbash N., Peto R., and Collins R.. 2007. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 370: 1829–1839. [DOI] [PubMed] [Google Scholar]
- 6.Freitas W. M., Quaglia L. A., Santos S. N., de Paula R. C., Santos R. D., Blaha M., Rivera J. J., Cury R., Blumenthal R., Nadruz-Junior W., Agatston A., Figueiredo V. N., Nasir K., Sposito A. C., and the Brazilian Study on Healthy Aging. 2015. Low HDL cholesterol but not high LDL cholesterol is independently associated with subclinical coronary atherosclerosis in healthy octogenarians. Aging Clin. Exp. Res. 27: 61–67. [DOI] [PubMed] [Google Scholar]
- 7.Ravnskov U., Diamond D. M., Hama R., Hamazaki T., Hammarskjold B., Hynes N., Kendrick M., Langsjoen P. H., Malhotra A., Mascitelli L., et al. 2016. Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: a systematic review. BMJ Open. 6: e010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sniderman A. D., Islam S., McQueen M., Pencina M., Furberg C. D., Thanassoulis G., and Yusuf S.. 2016. Age and cardiovascular risk attributable to apolipoprotein B, low-density lipoprotein cholesterol or non-high-density lipoprotein cholesterol. J. Am. Heart Assoc. 5: e003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sacco R. L., Benson R. T., Kargman D. E., Boden-Albala B., Tuck C., Lin I. F., Cheng J. F., Paik M. C., Shea S., and Berglund L.. 2001. High-density lipoprotein cholesterol and ischemic stroke in the elderly: the Northern Manhattan Stroke Study. JAMA. 285: 2729–2735. [DOI] [PubMed] [Google Scholar]
- 10.Weverling-Rijnsburger A. W., Jonkers I. J., van Exel E., Gussekloo J., and Westendorp R. G.. 2003. High-density vs low-density lipoprotein cholesterol as the risk factor for coronary artery disease and stroke in old age. Arch. Intern. Med. 163: 1549–1554. [DOI] [PubMed] [Google Scholar]
- 11.de Freitas E. V., Brandao A. A., Pozzan R., Magalhaes M. E., Fonseca F., Pizzi O., Campana E., and Brandao A. P.. 2011. Importance of high-density lipoprotein-cholesterol (HDL-C) levels to the incidence of cardiovascular disease (CVD) in the elderly. Arch. Gerontol. Geriatr. 52: 217–222. [DOI] [PubMed] [Google Scholar]
- 12.Störk S., Feelders R. A., van den Beld A. W., Steyerberg E. W., Savelkoul H. F., Lamberts S. W., Grobbee D. E., and Bots M. L.. 2006. Prediction of mortality risk in the elderly. Am. J. Med. 119: 519–525. [DOI] [PubMed] [Google Scholar]
- 13.Araujo A. B., Chiu G. R., Christian J. B., Kim H. Y., Evans W. J., and Clark R. V.. 2014. Longitudinal changes in high-density lipoprotein cholesterol and cardiovascular events in older adults. Clin. Endocrinol. (Oxf.). 80: 662–670. [DOI] [PubMed] [Google Scholar]
- 14.Hutchins P. M., and Heinecke J. W.. 2015. Cholesterol efflux capacity, macrophage reverse cholesterol transport and cardioprotective HDL. Curr. Opin. Lipidol. 26: 388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos-Gallego C. G., Badimon J. J., and Rosenson R. S.. 2014. Beginning to understand high-density lipoproteins. Endocrinol. Metab. Clin. North Am. 43: 913–947. [DOI] [PubMed] [Google Scholar]
- 16.Favari E., Chroni A., Tietge U. J., Zanotti I., Escola-Gil J. C., and Bernini F.. 2015. Cholesterol efflux and reverse cholesterol transport. Handb. Exp. Pharmacol. 224: 181–206. [DOI] [PubMed] [Google Scholar]
- 17.Rohatgi A., Khera A., Berry J. D., Givens E. G., Ayers C. R., Wedin K. E., Neeland I. J., Yuhanna I. S., Rader D. R., de Lemos J. A., et al. 2014. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 371: 2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Favari E., Ronda N., Adorni M. P., Zimetti F., Salvi P., Manfredini M., Bernini F., Borghi C., and Cicero A. F.. 2013. ABCA1-dependent serum cholesterol efflux capacity inversely correlates with pulse wave velocity in healthy subjects. J. Lipid Res. 54: 238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khera A. V., Cuchel M., de la Llera-Moya M., Rodrigues A., Burke M. F., Jafri K., French B. C., Phillips J. A., Mucksavage M. L., Wilensky R. L., et al. 2011. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364: 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mody P., Joshi P. H., Khera A., Ayers C. R., and Rohatgi A.. 2016. Beyond coronary calcification, family history, and C-reactive protein: cholesterol efflux capacity and cardiovascular risk prediction. J. Am. Coll. Cardiol. 67: 2480–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Detrano R., Guerci A. D., Carr J. J., Bild D. E., Burke G., Folsom A. R., Liu K., Shea S., Szklo M., Bluemke D. A., et al. 2008. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N. Engl. J. Med. 358: 1336–1345. [DOI] [PubMed] [Google Scholar]
- 22.Spigoni V., Aldigeri R., Picconi A., Derlindati E., Franzini L., Haddoub S., Prampolini G., Vigna G. B., Zavaroni I., Bonadonna R. C., et al. 2016. Telomere length is independently associated with subclinical atherosclerosis in subjects with type 2 diabetes: a cross-sectional study. Acta Diabetol. 53: 661–667. [DOI] [PubMed] [Google Scholar]
- 23.O’Donnell C. J., Demissie S., Kimura M., Levy D., Gardner J. P., White C., D’Agostino R. B., Wolf P. A., Polak J., Cupples L. A., et al. 2008. Leukocyte telomere length and carotid artery intimal medial thickness: the Framingham Heart Study. Arterioscler. Thromb. Vasc. Biol. 28: 1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freitas W. M., Quaglia L. A., Santos S. N., Soares A. A., Japiassu A. V., Boaventura V., dos Santos Barros E., Cordova C., Nobrega O. T., and Sposito A. C.. 2011. Association of systemic inflammatory activity with coronary and carotid atherosclerosis in the very elderly. Atherosclerosis. 216: 212–216. [DOI] [PubMed] [Google Scholar]
- 25.Descovich G. C. 1990. The Brisighella Heart Study: an interim report. Eur. Heart J. 11 Suppl H: 32–37. [DOI] [PubMed] [Google Scholar]
- 26.Adorni M. P., Zimetti F., Puntoni M., Bigazzi F., Sbrana F., Minichilli F., Bernini F., Ronda N., Favari E., and Sampietro T.. 2012. Cellular cholesterol efflux and cholesterol loading capacity of serum: effects of LDL-apheresis. J. Lipid Res. 53: 984–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bortnick A. E., Rothblat G. H., Stoudt G., Hoppe K. L., Royer L. J., McNeish J., and Francone O. L.. 2000. The correlation of ATP-binding cassette 1 mRNA levels with cholesterol efflux from various cell lines. J. Biol. Chem. 275: 28634–28640. [DOI] [PubMed] [Google Scholar]
- 28.Motoyama S., Sarai M., Harigaya H., Anno H., Inoue K., Hara T., Naruse H., Ishii J., Hishida H., Wong N. D., et al. 2009. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J. Am. Coll. Cardiol. 54: 49–57. [DOI] [PubMed] [Google Scholar]
- 29.Otsuka K., Fukuda S., Tanaka A., Nakanishi K., Taguchi H., Yoshikawa J., Shimada K., and Yoshiyama M.. 2013. Napkin-ring sign on coronary CT angiography for the prediction of acute coronary syndrome. JACC Cardiovasc. Imaging. 6: 448–457. [DOI] [PubMed] [Google Scholar]
- 30.Cawthon R. M. 2002. Telomere measurement by quantitative PCR. Nucleic Acids Res. 30: e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blaha M. J., Cainzos-Achirica M., Greenland P., McEvoy J. W., Blankstein R., Budoff M. J., Dardari Z., Sibley C. T., Burke G. L., Kronmal R. A., et al. 2016. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 133: 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammadah M., Al Mheid I., Wilmot K., Ramadan R., Abdelhadi N., Alkhoder A., Obideen M., Pimple P. M., Levantsevych O., Kelli H. M., et al. 2017. Telomere shortening, regenerative capacity, and cardiovascular outcomes. Circ. Res. 120: 1130–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rehkopf D. H., Needham B. L., Lin J., Blackburn E. H., Zota A. R., Wojcicki J. M., and Epel E. S.. 2016. Leukocyte telomere length in relation to 17 biomarkers of cardiovascular disease risk: a cross-sectional study of US adults. PLoS Med. 13: e1002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buford T. W. 2016. Hypertension and aging. Ageing Res. Rev. 26: 96–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iribarren C., Reed D. M., Burchfiel C. M., and Dwyer J. H.. 1995. Serum total cholesterol and mortality. confounding factors and risk modification in Japanese-American men. JAMA. 273: 1926–1932. [PubMed] [Google Scholar]
- 36.Anastasius M., Kockx M., Jessup W., Sullivan D., Rye K. A., and Kritharides L.. 2016. Cholesterol efflux capacity: An introduction for clinicians. Am. Heart J. 180: 54–63. [DOI] [PubMed] [Google Scholar]
- 37.Santos-Gallego C. G., Giannarelli C., and Badimon J. J.. 2011. Experimental models for the investigation of high-density lipoprotein-mediated cholesterol efflux. Curr. Atheroscler. Rep. 13: 266–276. [DOI] [PubMed] [Google Scholar]
- 38.Pisciotta L., Vitali C., Favari E., Fossa P., Adorni M. P., Leone D., Artom N., Fresa R., Calabresi L., Calandra S., et al. 2015. A complex phenotype in a child with familial HDL deficiency due to a novel frameshift mutation in APOA1 gene (apoA-IGuastalla). J. Clin. Lipidol. 9: 837–846. [DOI] [PubMed] [Google Scholar]
- 39.Pisciotta L., Favari E., Magnolo L., Simonelli S., Adorni M. P., Sallo R., Fancello T., Zavaroni I., Ardigo D., Bernini F., et al. 2012. Characterization of three kindreds with familial combined hypolipidemia caused by loss-of-function mutations of ANGPTL3. Circ Cardiovasc Genet. 5: 42–50. [DOI] [PubMed] [Google Scholar]
- 40.Puri R., Nicholls S. J., Shao M., Kataoka Y., Uno K., Kapadia S. R., Tuzcu E. M., and Nissen S. E.. 2015. Impact of statins on serial coronary calcification during atheroma progression and regression. J. Am. Coll. Cardiol. 65: 1273–1282. [DOI] [PubMed] [Google Scholar]
- 41.Khera A. V., Demler O. V., Adelman S. J., Collins H. L., Glynn R. J., Ridker P. M., Rader D. J., and Mora S.. 2017. Cholesterol efflux capacity, high-density lipoprotein particle number, and incident cardiovascular events: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). Circulation. 135: 2494–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franceschini G., Favari E., Calabresi L., Simonelli S., Bondioli A., Adorni M. P., Zimetti F., Gomaraschi M., Coutant K., Rossomanno S., et al. 2013. Differential effects of fenofibrate and extended-release niacin on high-density lipoprotein particle size distribution and cholesterol efflux capacity in dyslipidemic patients. J. Clin. Lipidol. 7: 414–422. [DOI] [PubMed] [Google Scholar]
- 43.Berrougui H., Isabelle M., Cloutier M., Grenier G., and Khalil A.. 2007. Age-related impairment of HDL-mediated cholesterol efflux. J. Lipid Res. 48: 328–336. [DOI] [PubMed] [Google Scholar]
- 44.Favari E., Gomaraschi M., Zanotti I., Bernini F., Lee-Rueckert M., Kovanen P. T., Sirtori C. R., Franceschini G., and Calabresi L.. 2007. A unique protease-sensitive high density lipoprotein particle containing the apolipoprotein A-I(Milano) dimer effectively promotes ATP-binding cassette A1-mediated cell cholesterol efflux. J. Biol. Chem. 282: 5125–5132. [DOI] [PubMed] [Google Scholar]
- 45.Favari E., Calabresi L., Adorni M. P., Jessup W., Simonelli S., Franceschini G., and Bernini F.. 2009. Small discoidal pre-beta1 HDL particles are efficient acceptors of cell cholesterol via ABCA1 and ABCG1. Biochemistry. 48: 11067–11074. [DOI] [PubMed] [Google Scholar]
- 46.Tota-Maharaj R., Blaha M. J., Blankstein R., Silverman M. G., Eng J., Shaw L. J., Blumenthal R. S., Budoff M. J., and Nasir K.. 2014. Association of coronary artery calcium and coronary heart disease events in young and elderly participants in the multi-ethnic study of atherosclerosis: a secondary analysis of a prospective, population-based cohort. Mayo Clin. Proc. 89: 1350–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mainous A. G. 3rd, Codd V., Diaz V. A., Schoepf U. J., Everett C. J., Player M. S., and Samani N. J.. 2010. Leukocyte telomere length and coronary artery calcification. Atherosclerosis. 210: 262–267. [DOI] [PubMed] [Google Scholar]
- 48.Diaz V. A., Mainous A. G. 3rd, Everett C. J., Schoepf U. J., Codd V., and Samani N. J.. 2010. Effect of healthy lifestyle behaviors on the association between leukocyte telomere length and coronary artery calcium. Am. J. Cardiol. 106: 659–663. [DOI] [PubMed] [Google Scholar]
- 49.Mutharasan R. K., Thaxton C. S., Berry J., Daviglus M. L., Yuan C., Sun J., Ayers C., Lloyd-Jones D. M., and Wilkins J. T.. 2017. HDL efflux capacity, HDL particle size, and high-risk carotid atherosclerosis in a cohort of asymptomatic older adults: the Chicago Healthy Aging Study. J. Lipid Res. 58: 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.