Abstract
α-Chlorofatty aldehydes (α-ClFALDs) and α-bromofatty aldehydes (α-BrFALDs) are produced in activated neutrophils and eosinophils. This study investigated the ability of α-BrFALD and α-ClFALD to react with the thiols of GSH and protein cysteinyl residues. Initial studies showed that 2-bromohexadecanal (2-BrHDA) and 2-chlorohexadecanal (2-ClHDA) react with GSH producing the same fatty aldehyde-GSH adduct (FALD-GSH). In both synthetic and cellular reactions, FALD-GSH production was more robust with 2-BrHDA compared with 2-ClHDA as precursor. NaBr-supplemented phorbol myristate acetate (PMA)-activated neutrophils formed more α-BrFALD and FALD-GSH compared with non-NaBr-supplemented neutrophils. Primary human eosinophils, which preferentially produce hypobromous acid and α-BrFALD, accumulated FALD-GSH following PMA stimulation. Mice exposed to Br2 gas had increased levels of both α-BrFALD and FALD-GSH in the lungs, as well as elevated systemic plasma levels of FALD-GSH in comparison to mice exposed to air. Similar relative reactivity of α-ClFALD and α-BrFALD with protein thiols was shown using click analogs of these aldehydes. Collectively, these data demonstrate that GSH and protein adduct formation are much greater as a result of nucleophilic attack of cysteinyl residues on α-BrFALD compared with α-ClFALD, which was observed in both primary leukocytes and in mice exposed to bromine gas.
Keywords: fatty aldehydes, glutathione, plasmalogen, hypochlorous acid, hypobromous acid, thiols
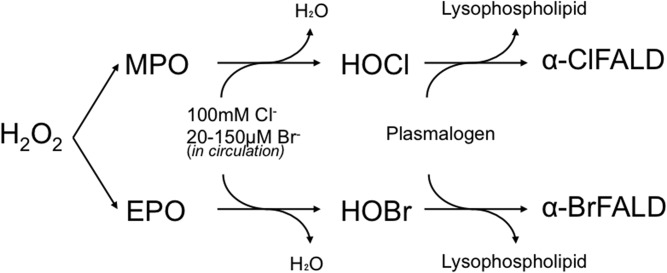
α-Halofatty aldehydes are produced by hypohalous acid attack of the vinyl ether bond of plasmalogen (1, 2). The most well-characterized α-halofatty aldehyde is α-chlorofatty aldehyde (α-ClFALD), produced by reactions between hypochlorous acid (HOCl), derived from neutrophils, monocytes, and some tissue macrophages (3, 4), and plasmalogen (Fig. 1). Production of α-ClFALD has been shown in human atherosclerotic plaques (5) and infarcted rat myocardium (6). Myeloperoxidase (MPO)-derived α-ClFALD has also been implicated in endothelial dysfunction (7), neuronal apoptosis (8), activating the nuclear factor κB pathway, eNOS inhibition (9), neutrophil chemotaxis (3), and myocardial contractile dysfunction (6). Recently, α-ClFALD was also found in the lungs of mice and rats exposed to chlorine gas (Cl2) along with its GSH adduct [fatty aldehyde-GSH adduct (FALD-GSH)], and it was found to contribute to increased lung inflammation and permeability as well as systemic loss of eNOS-dependent vasodilation (10).
Fig. 1.
MPO selectively produces HOCl and EPO selectively produces HOBr. MPO found in neutrophils, monocytes, and macrophages utilize Cl− ions and H2O2 to produce HOCl following a respiratory burst and degranulation. However, some Br- ions are used by MPO to produce HOBr (not depicted). EPO, found in eosinophils, selectively utilizes Br- ions and H2O2 to produce HOBr even though Br- ion concentrations are 1,000 times lower than the Cl− concentrations physiologically. Both HOCl and HOBr can attack the vinyl either bond of plasmalogen to release lysosphospholipid and α-ClFALD or α-BrFALD, respectively.
The metabolism and physiologic relevance of α-bromofatty aldehyde (α-BrFALD) remains to be elucidated. Eosinophils contain eosinophil peroxidase that selectively produces hypobromous acid (HOBr), which can target plasmalogens yielding α-BrFALD (11) (Fig. 1). Although it has been shown that α-ClFALD reacts with cellular GSH (12), an analogous pathway for α-BrFALD has not been reported. The reaction between α-ClFALD and GSH is a nucleophilic substitution reaction of the GSH thiol at the α-chlorinated carbon coupled with ejection of the chlorine as a leaving group (12). Because bromine is a better leaving group in nucleophilic substitution reactions, it follows that α-BrFALD will be very reactive with GSH.
The mechanisms through which α-ClFALD and α-BrFALD elicit cellular dysfunction and signaling are unknown, but studies have shown that α-ClFALD can react with cellular proteins through unspecified mechanisms (13), which may account for signaling alterations. In this study, we show that compared with α-ClFALD, α-BrFALD is more potent in reacting with thiols. Importantly, this selectivity of thiol reactivity is also observed in the presence of endogenously produced α-ClFALD and α-BrFALD in eosinophils, neutrophils, and mice exposed to bromine gas. Furthermore, using click-chemistry analogs of α-ClFALD and α-BrFALD, we demonstrate the robust modifications of cellular proteins by α-BrFALD in comparison to α-ClFALD.
MATERIALS AND METHODS
Materials
HPLC grade methanol, acetonitrile, and isopropanol were purchased from (Fisher Scientific). TLC plates (20 × 20 cm, 40 Å silica gel) were obtained from EMB Millipore. All other chemicals were purchased from Sigma-Aldrich or Fisher Scientific.
Instrumentation
MS was performed using a Thermo Fisher TSQ Quantum Ultra mass spectrometer (Thermo Fisher, Waltham, MA). For experiments requiring LC-MS, a Thermo Fisher Surveyor LC system was coupled to the Quantum Ultra. LC-MS data analysis was performed using XCalibur software (Thermo Fisher).
GSH in vitro reactions with halogenated lipids and TLC purification of reaction product
Unless otherwise indicated, all reactions were carried out in PBS (pH 7.4) and ethanol at a ratio of 30:70. Briefly, GSH (1 μmol) was first suspended in 60 μl of PBS (pH 7.4) in a borosilicate reaction vessel. One micromole of lipid [hexadecanal (HDA), 2-chlorohexadecanal (2-ClHDA), 2-chlorohexadecanol (2-ClHOH), 2-chlorohexadecanoic acid (2-ClHA), 2-bromohexadecanal (2-BrHDA), or 2-bromohexadecanoic acid (2-BrHA)] was added to the GSH in 140 μl ethanol and the reaction mixture was vortexed, capped, and incubated at 37°C for 4 h. Reaction aliquots (20 μl) were subjected to TLC using 40 Å silica gel TLC plates as stationary phase and a mobile phase comprised of chloroform/acetone/methanol/water/acetic acid (6/8/2/2/1, v/v/v/v/v). Reaction products were visualized following 2,4-dinitrophenylhydrazine staining. In some cases, TLC plates were not stained because TLC was used for purification of reaction products [e.g., 2-halohexadecanal adduct with GSH (HDA-GSH)] and silica associated with reaction products was scraped from plates and extracted using acetonitrile/water (7/3, v/v) supplemented with 0.25% formic acid. Silica was subsequently separated from solvent by centrifugation (2,000 g maximum) for 5 min. The supernatant extract was subsequently dried under nitrogen and suspended in 150 μl acetonitrile/methanol/water (2/1/1, v/v/v) supplemented with 0.1% formic acid for ESI-MS/MS analysis.
2-BrHDA and GSH in vitro product characterization
TLC-purified HDA-GSH was diluted 2,000 times with acetonitrile/methanol/water (2/1/1, v/v/v) supplemented with 0.1% formic acid, and was analyzed by ESI-MS/MS by direct infusion at a flow rate of 5 μl/min. For ESI-MS/MS, the ionization energy and temperature were set at 3,900 V and 270°C in positive ion mode. A collision energy of 18 eV and collision gas of 1.0 Torr argon were used for MS/MS analyses in positive ion mode.
RAW 264.7 cell treatments with 2-BrHDA and 2-ClHDA
RAW 264.7 cells were grown to confluence in 6-well plates (9.5 cm2 growth area) and were treated with either 0, 10, 25, or 50 μM 2-BrHDA or 2-ClHDA in DMEM supplemented with 2% FBS for 4 h. The medium was then removed at 4 h after the start of the treatment and cells washed with PBS. The cells were scraped in 1.25 ml PBS containing 10 mM N-ethylmaleimide (NEM) and immediately frozen prior to analysis of FALD-GSH. In other experiments, RAW 264.7 cells were treated with either 0, 10, 25, or 50 μM 2-BrHDA, 2-ClHDA, or HDA in DMEM supplemented with 2% FBS for 4 h. The medium was then removed at 0.5, 1, 2, 3, and 4 h after the start of the treatment and cells washed with PBS. The cells were scraped in 1.25 ml PBS containing 10 mM NEM and immediately frozen along with the medium prior to analysis of FALD-GSH
Human neutrophil studies
Whole blood (50 ml) was taken from healthy volunteers and anticoagulated with EDTA (final concentration 5.4 mM) prior to the isolation of neutrophils using a Ficoll-Hypaque gradient as previously described (12). Neutrophil and eosinophil studies were approved and authorized by the Saint Louis University Institutional Review Board Protocol 9952. Informed consent was obtained from the human subjects. Neutrophils were diluted to 1 million neutrophils per milliliter in HBS ± 1 mM NaBr. Phorbol myristate acetate (PMA) (100 nM) in ethanol (0.1%) was then added as indicated and then the neutrophils were incubated for 30 min at 37°C. At the end of each reaction, 10 mM of NEM were added and the neutrophils were immediately frozen prior to FALD-GSH analysis.
Human eosinophil studies
Primary human eosinophils were prepared as previously described (11). Whole blood was taken from hypersensitive volunteers, and eosinophils were isolated using a Percoll gradient followed by a CD16 immunomagnetic bead sorting system on a Miltenyi MACS column. Purified eosinophils were resuspended in Hanks’ balanced salt solution (pH 7.3) supplemented with 100 μM NaBr, as is found physiologically (14–16). Eosinophils (1 × 106 cells/ml) were treated with or without (control) 200 nM PMA for 1 h at 37°C. Reactions were terminated by snap-freezing in liquid nitrogen and FALD-GSH was analyzed.
Mouse exposure to Br2 gas
All animal procedures were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee. Whole body exposures of male mice to Br2 were performed as previously described (17, 18). Exposures were performed with two mice in the same chamber at any one time, and all exposures were performed between 8:00 AM and 12:00 PM. Exposure conditions were 400 or 600 ppm Br2 in air for 30 min. Flow meters were used to control flow rates of Br2 and room air to achieve the chamber Br2 target concentrations. A bubble flow meter was used to validate their performance on a weekly basis. In each case, immediately following exposure, mice were returned to room air. Food and water were provided ad libitum.
Extraction and quantification of FALD-GSH
FALD-GSH was extracted from cell extracts and quantitated as previously described (12). Briefly, 90 fmol of [d4]HDA-GSH were added to 100 μl of the RAW 264.7 cell homogenate and 45 fmol of [d4]HDA-GSH were added to 1 ml of neutrophil or eosinophil suspension. One volume of methanol/acetonitrile (1/1, v/v) was added to each mixture and then each mixture was vortexed for 30 s, sonicated in a sonication bath for 15 s, and vortexed for another 30 s. The resulting solution was centrifuged at 1,000 g for 5 min, and the supernatant was carefully removed from the cell debris pellet. The supernatant was loaded on a Strata-X column (60 mg bed weight) that was preconditioned with 1.2 ml methanol followed by 1.2 ml water/methanol (4/1, v/v). Columns were washed two times with 0.6 ml water/methanol (4/1, v/v), and then 2-ClHDA conjugates of GSH were eluted with 1.2 ml of methanol/acetonitrile (3/1, v/v) containing 0.25% formic acid. The eluted adduct was dried under nitrogen and suspended in 100 μl of 6/4/5 acetonitrile/isopropanol/water containing 0.15% formic acid for analysis by LC-MS/MS.
Lung and plasma of Br2-exposed mice were analyzed for FALD-GSH as previously described (10). Briefly, 25 µl of plasma were spiked with 110 fmol of [d4]HDA-GSH and10 mg of pulverized lung tissue were spiked with 1,100 fmol [d4]HDA-GSH. The aqueous layer of a Bligh and Dyer lipid of extraction, as used for most halogenated lipid extractions (19), was isolated. The remaining organic layer was reextracted with 1 vol methanol:water (1:1, v:v) and combined with the first aqueous extraction. The combined aqueous layers were extracted on a Strata-X SPE extraction column after dilution with 1/3 vol of water and then quantitated by ESI-LC-MS/MS quantitation.
LC was performed using a Phenomenex Onyx C-18 column (50 × 2.0 mm) with ESI-MS/MS detection using selected reaction monitoring. Two mobile phases were used for LC. Solvent A was water containing 0.15% formic acid and solvent B was acetonitrile/isopropanol (3/2, v/v) containing 0.15% formic acid. Initial column conditions were 65/35 (A/B) at a flow rate of 200 μl/min, which was held for 2 min following the injection of 25 μl of the Strata-X-purified solutions onto the column. Subsequently, GSH adducts of α-halofatty aldehydes were eluted by a 3 min linear gradient to 100% solvent B.
Extraction and quantification of α-BrFALD
α-BrFALD was extracted from Br2-exposed mice, as was previously described for α-ClFALD extraction from Cl2-exposed mice (10). Briefly, 10 mg of frozen pulverized lung tissue were extracted by a Bligh and Dyer lipid extraction in the presence of 20 pmol [d4]ClHDA. Lipid extracts were then derivatized with pentafluorobenzyl hydroxylamine and quantitated by GC-MS as previously described (11).
Synthesis of HDyA, ClHDyA, and BrHDyA alkyne
The alkyne analogs of HDA [hexadec-15-ynal (HDyA)], 2-ClHDA [2-chlorohexadec-15-ynal (2-ClHDyA)], and 2-BrHDA [2-bromohexadec-15-ynal (2-BrHDyA)] were synthesized as previously described, with variations (20). Briefly, hexadec-7-ynol was converted to hexadec-15-ynol using sodium hydride and diaminopropane. The alcohol of hexadec-15-ynol was oxidized to an aldehyde in a solution of 2-iodoxybenzoic acid in DMSO, which resulted in HDyA. This aldehyde was chlorinated using N-chlorosuccinimide and proline for 1 h or brominated with N-bromosuccinimide and proline for 1 h. A portion of HDyA was not halogenated for use as a control. After each step, the desired product was purified by flash chromatography using 30 g silica gel (high purity grade, pore size 60Å Sigma-Aldrich). Samples were loaded and eluted with three column volumes of 30:10:60:5 (ethyl acetate:hexane:toluene:acetic acid). Fractions (1 ml each) were collected, analyzed by TLC (see below), and the desired products were combined.
THP-1 cell experiments
THP-1 human leukemic monocyte cells were grown in RPMI + 10% FBS. Five million THP-1 cells were centrifuged and suspended in 4 ml of RPMI with no FBS. Cells were pretreated with or without 1 mM NEM, or 5 mM N-acetylcysteine (NAC) for 30 min at 37°C. Then THP-1 cells were treated with either 5 or 10 μM HDyA, 2-ClHDyA, or 2-BrHDyA for 30 min. Then cells were pelleted by centrifugation at 1,500 g for 5 min. The supernatant was removed and the pellet was suspended in 4 ml ice-cold PBS followed by centrifugation for 5 min at 1,500 g. The supernatant was removed and 300 µl of ice-cold RIPA buffer with HALT protease inhibitor were added, mixed by pipetting, and then left to sit on ice for 20 min. The extracted THP-1 cells were then centrifuged at 14,000 g for 15 min. The protein-containing supernatant was extracted and the protein content was quantitated using a Bradford assay, and then frozen at −20°C until click chemistry visualization.
Click chemistry visualization of THP-1 cell protein
Aliquots of extracted THP-1 cell protein (100 μg) were transferred into 1.5 ml Eppendorf tubes. One hundred microliters of 250 mM PBS were added to each sample and the volume was brought to 170 μl with diH2O. Twenty microliters of a mixture of 75 mM THPTA and 15 mM Cu(II)SO4 in water were then added and the sample was vortexed for 5 s. Four microliters of 2.5 mM TAMRA-azide in DMSO were added to each sample and then vortexed for 5 s. Ten microliters of 400 mM sodium ascorbate were then added to each reaction followed by 5 s of vortexing. The reaction mixture was protected from the light and incubated at room temperature for 30 min with gentle rocking. To purify the clicked protein, 600 μl of methanol, 150 μl of CHCl3, and 400 μl of water were added followed by 5 s of vortexing. Samples were then centrifuged for 5 min at 13,000–18,000 g. The upper layer was carefully removed to make sure not to disturb the interface. Four hundred and fifty microliters of methanol were then added to the remaining solution and vortexed for 5 s. The homogenous solution was centrifuged for 5 min at 13,000–18,000 g, and then the supernatant was removed while not disturbing the pellet. An additional 450 µl of methanol were added to the pellet, vortexed for 5 s, and centrifuged for 5 min at 13,000–18,000 g followed by careful removal of the supernatant. The pellet was then allowed to air dry for 30–60 min. Sample loading buffer was then added to the dry pellet at a concentration of 1–2 mg/ml. The samples were then vortexed for 10 min at room temperature followed by heating at 70°C for 10 min. After the samples had cooled to room temperature, they were centrifuged for 1 min at 1,500 g. Samples were then loaded onto at 12% Bis-Tris gel in the stated quantities. Gel electrophoresis was performed at 100 V in MOPS running buffer. Once completed, the gel was removed, briefly washed in water, and then immediately visualized at 532/580 nm (excitation/emission). After visualization, the gel was fixed and stained by Coomassie for total protein visualization.
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide assay
The metabolic activity of THP-1 cells was examined using the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. Cells were plated in a clear 96-well plate and grown to confluence. Cells were treated with the indicated concentrations of lipids in phenol red-free growth medium with 10% FBS for 2 h. MTT (Sigma-Aldrich, M2128; 1.2 mM in 100 μl serum-free phenol red-free medium) was added to cells for 4 h. Seventy-five microliters of medium were removed and 50 μl of DMSO were added for 10 min to lyse the cells. Absorbance was read at 540 nm on an Enspire multimode plate reader and corrected for background absorbance. Triton (0.1%) in PBS was used as a positive control. MTT reduction is expressed as percent MTT reduction (blank designated as 100%).
Statistical analyses
Statistical analysis for the presented data utilized ANOVA to compare three or more groups to a control condition followed by Dunnett’s post hoc test. When statistically appropriate, Tukey’s post hoc test was used. All data are presented as mean ± SEM unless otherwise indicated.
RESULTS
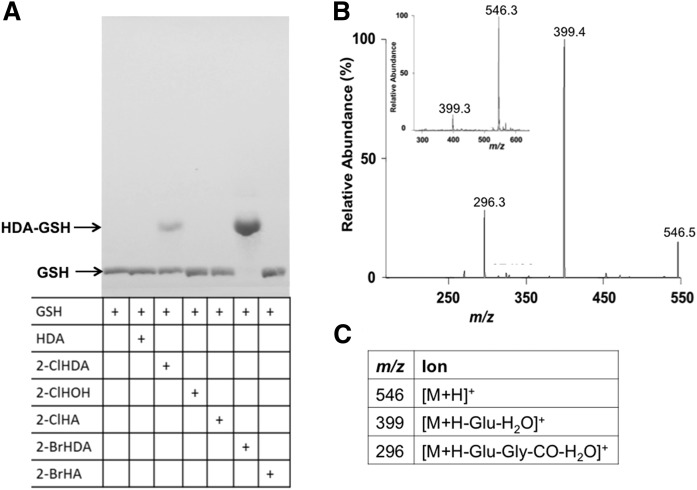
Previous studies have shown that α-BrFALD is produced in human neutrophils and eosinophils (2, 11). Because bromine is a better leaving group than chlorine, it was anticipated that α-BrFALD would react with GSH through a thiol-dependent mechanism similar to that of α-ClFALD with GSH (12). Initial studies shown in Fig. 2 compared the reaction of GSH with either the 16-carbon α-BrFALD or α-ClFALD, 2-BrHDA or 2-ClHDA, respectively. The band visualized from the reaction product of 2-BrHDA and GSH migrated to the same position on the TLC as that of 2-ClHDA and GSH. However, the reaction product of 2-BrHDA and GSH was much darker as visualized by TLC staining with phosphomolybdic acid, and GSH was completely consumed in reactions with 2-BrHDA compared with 2-ClHDA. In comparison to the reactivity of 2-ClHDA and 2-BrHDA with GSH after 4 h, TLC analyses did not reveal any reactions between GSH and either 2-ClHOH, 2-ClHA, 2-BrHA, or HDA (Fig. 2A). Importantly, the nonhalogenated fatty aldehyde, HDA, did not react with GSH, which confirms the reaction to be dependent on the presence of the α-halogenated carbon.
Fig. 2.
The 2-BrHDA reacts with GSH to form HDA-GSH. Reaction products of GSH (1 µmol) with 1 µmol of either HDA, 2-ClHDA, 2-ClHOH, 2-ClHA, 2-BrHDA, or 2-BrHA were visualized by phosphomolybdic acid staining following TLC chromatography as described in the Materials and Methods (A). TLC purified reaction products from 2-BrHDA and GSH were subsequently analyzed by ESI-MS/MS. Both the Q1+ parent molecular ion at m/z of 546.3 [(B) insert] and CAD analyses of m/z 546.3 (B) were the same as that previously reported for the reaction product of 2-ClHDA (12) and assignments for the depicted molecular ions in (B) are provided in (C).
Previously, the reaction product of 2-ClHDA with GSH was found to be a peptidofatty aldehyde (HDA-GSH) (12). To confirm the same product of the reaction of 2-BrHDA and GSH, the TLC-purified product was analyzed by ESI-MS/MS. The parent [M+H+] ion of m/z 546 (Fig. 2B insert) and its fragment ions m/z 399 and 296 (Fig. 2B) are identical to those molecular ions previously reported for HDA-GSH, which, in conjunction with comigration of reaction products by TLC, indicate that HDA-GSH is the product.
HDA-GSH formation in RAW 264.7 cells treated with exogenous 2-BrHDA and 2-ClHDA
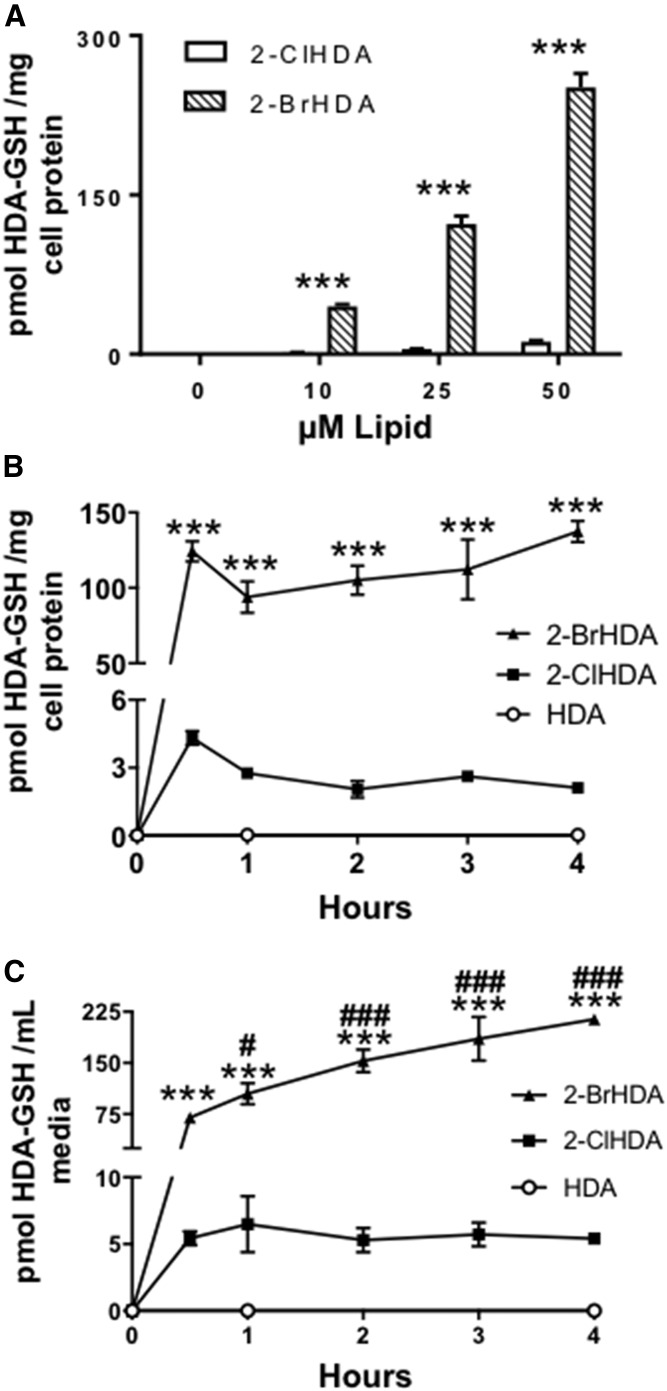
Further studies were performed to determine whether the same preferred reactivity of GSH with 2-BrHDA compared with 2-ClHDA occurred in RAW 264.7 cells. At all concentrations tested (10, 25, and 50 μM), 2-BrHDA conversion to HDA-GSH was approximately 25 times greater than that in cells treated with 2-ClHDA (Fig. 3A). RAW 264.7 production of HDA-GSH following treatments with either 2-ClHDA or 2-BrHDA (20 μM) treatment was rapid, occurring within the first 30 min of treatment with no further significant increases at later time points examined (Fig. 3B, C). At all time points analyzed, the conversion of 2-BrHDA to HDA-GSH was at least 25 times greater using 2-BrHDA compared with 2-ClHDA, suggesting that the kinetics of this reaction is over 25-fold greater using 2-BrHDA compared with 2-ClHDA. In both 2-ClHDA and 2-BrHDA media, export of HDA-GSH was observed at 30 min (Fig. 3C). However, while media levels in the 2-ClHDA treatment remained unchanged after 30 min, the media of cells treated with 2-BrHDA at 1, 2, 3, and 4 h had elevated HDA-GSH levels when compared with 30 min. The requirement for halogen at the α-position for the reaction with GSH was further confirmed in the cell culture studies because HDA-GSH was not detected in either the media or cells of HDA-treated RAW 264.7 cells (Fig. 3B, C).
Fig. 3.
HDA-GSH production in RAW 264.7 cells treated with exogenous 2-BrHDA and 2-ClHDA. RAW 264.7 cells were treated with 0, 1, 10, 25, or 50 μM 2-ClHDA or 2-BrHDA for 4 h (A) and HDA-GSH was quantified as described in the Materials and Methods. Data in panels B and C are RAW 264.7 cellular (C) and media (B) levels of HDA-GSH following treatments with either 20 μM HDA, 2-ClHDA, or 2-BrHDA for the indicated time intervals. ***P < 0.001 for HDA-GSH production in cells treated with 2-BrHDA compared with those treated with 2-ClHDA; n = 3 for each treatment. #P < 0.05 and ###P < 0.001 for comparisons of HDA-GSH in the media of 2-BrHDA treatments at 30 min compared with HDA-GSH in the media of 2-BrHDA treatments at other time points; n = 3 for each treatment.
FALD-GSH production in activated human neutrophils and eosinophils
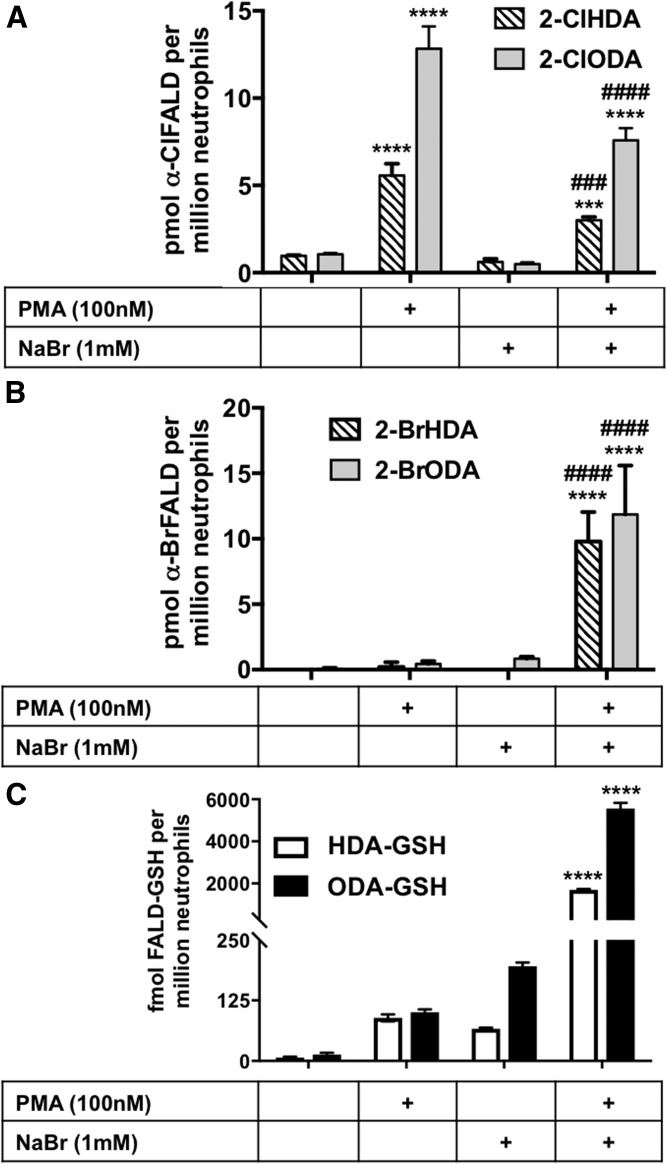
Primary human neutrophils were utilized to analyze the production of FALD-GSH as a result of HOBr or HOCl attack on plasmalogen and subsequent release of α-BrFALD or α-ClFALD. Activated human neutrophils contain MPO, which, under physiologic conditions, primarily produces HOCl and α-ClFALD (1). However, in the presence of 1 mM NaBr, neutrophils can produce HOBr and α-BrFALD (2, 11). Neutrophils stimulated with PMA in the presence of 1 mM NaBr produced significant amounts α-BrFALD compared with neutrophils that were not supplemented with 1 mM NaBr (Fig. 4A). Furthermore, the production of α-BrFALD in neutrophils incubated with NaBr led to over an order of magnitude greater accumulation of the FALD-GSH molecular species, HDA-GSH and 2-halooctadecanal adduct with GSH (ODA-GSH), compared with conditions with no NaBr supplementation.
Fig. 4.
α-HaloFALD and FALD-GSH produced in NaBr-supplemented neutrophils. Primary human neutrophils were isolated and treated with either PMA or vehicle in the presence or absence of 1 mM NaBr. α-Halo-FALD and FALD-GSH were quantitated using GC-MS and ESI-MS/MS, respectively, as described in the Materials and Methods. *** and **** indicate P < 0.001 and P < 0.0001, respectively, for comparisons of each species with and without PMA present. ### and #### indicate P < 0.001 and P < 0.0001, respectively, for comparisons of each species with and without NaBr supplementation; n = 3 for each treatment.
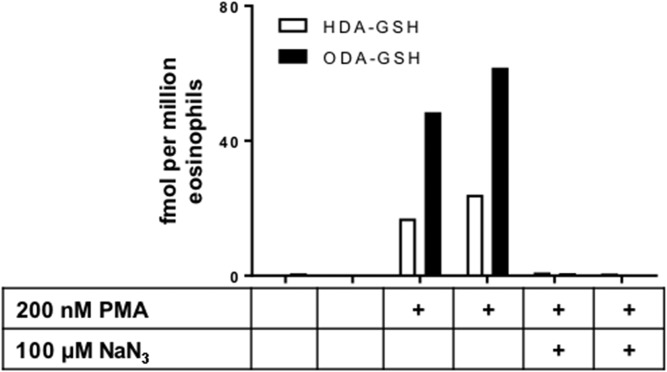
Unlike human neutrophil MPO, human eosinophil peroxidase selectively produces HOBr and α-BrFALD in comparison to HOCl and α-ClFALD, respectively, even with Cl− levels exceeding Br− levels by over three orders of magnitude. Accordingly, studies were designed to assess FALD-GSH production in PMA-stimulated primary human eosinophils. The data in Fig. 5 show that PMA-stimulated eosinophils produced the FALD-GSH molecular species, HDA-GSH and ODA-GSH. Additionally the production of FALD-GSH produced by stimulated eosinophils can also be inhibited by pretreatment with the heme enzyme inhibitor, sodium azide (NaN3) (21).
Fig. 5.
Eosinophils produce FALD-GSH. Primary human eosinophils were incubated in Hank’s balanced salt solution supplemented with 100 μM NaBr, and were subsequently activated with 200 nM PMA, as indicated in the presence or absence of 100 μM NaN3 in duplicate. PMA-stimulated eosinophils readily produce FALD-GSH and NaN3 blocks the production of the FALD-GSH. FALD-GSH product was determined as described in the Materials and Methods.
Production of α-BrFALD and FALD-GSH in Br2-exposed mice
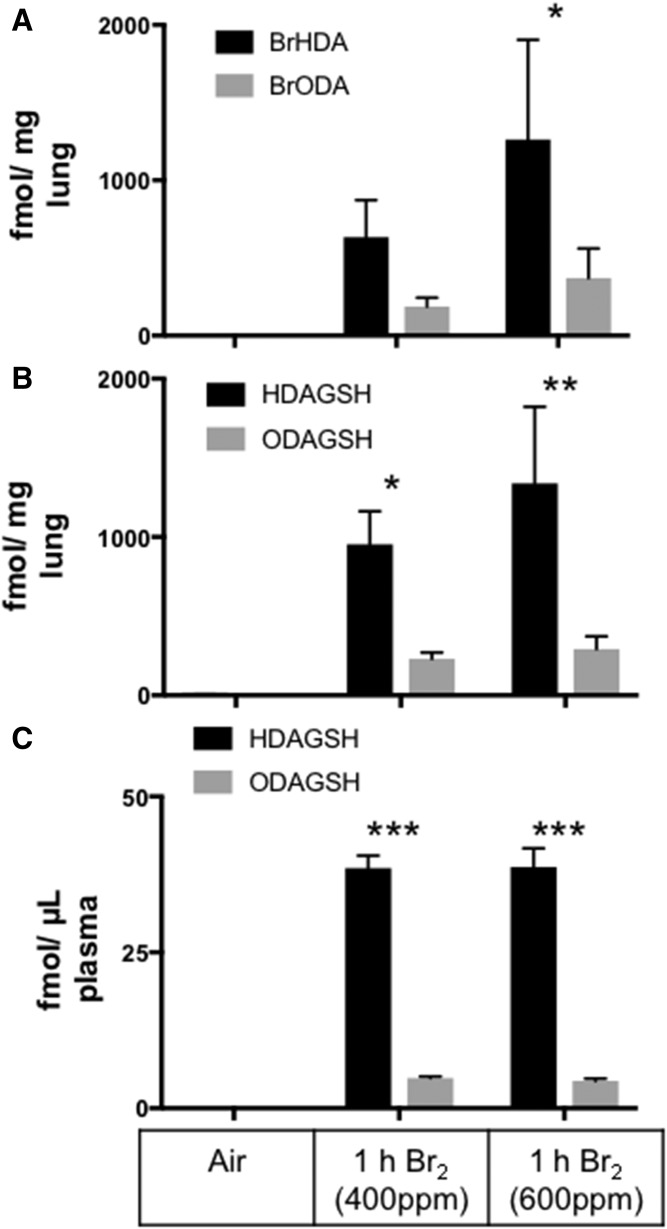
While most physiologic α-halofatty aldehyde production is likely due to hypohalous acid produced by peroxidases, a recent study demonstrated that α-ClFALD and FALD-GSH were produced in the lungs of chlorine gas (Cl2)-exposed mice and rats (10). Similar to those previous studies in chlorine gas-exposed rodents, the data in Fig. 6A show that α-BrFALD was elevated in the lungs of mice exposed to 30 min of 400 or 600 ppm Br2 and then returned to room air for 1 h. Lung (Fig. 6B) and plasma (Fig. 6C) levels of FALD-GSH levels were also elevated following Br2 exposure, indicating that α-BrFALD derived from either Br2 or HOBr-targeting plasmalogens is readily converted to GSH adducts in the lung and potentially the pulmonary circulation. Alternatively, systemic blood increases in FALD-GSH post Br2 exposure may be the result of washout of FALD-GSH from its production site, the lung.
Fig. 6.
The 2-BrFALD and FALD-GSH in mice exposed to bromine gas. C57bl/6 mice were exposed to Br2 gas (400 or 600 ppm, 30 min) and then brought back to room air. At 1 h following exposure, lungs and plasma were collected. The 2-BrFALD (A) and FALD-GSH (B) were then measured in the lung and FALD-GSH (C) was measured in the plasma. FALD-GSH was extracted from the lung tissue and plasma and quantitated using ESI-MS/MS selective reaction monitoring as described in the Materials and Methods. *P < 0.05, **P < 0.005, and ***P < 0.001 for production of FALD-GSH compared with air controls; n = 3 for each treatment.
α-ClFALD and α-BrFALD modification of cellular protein thiols
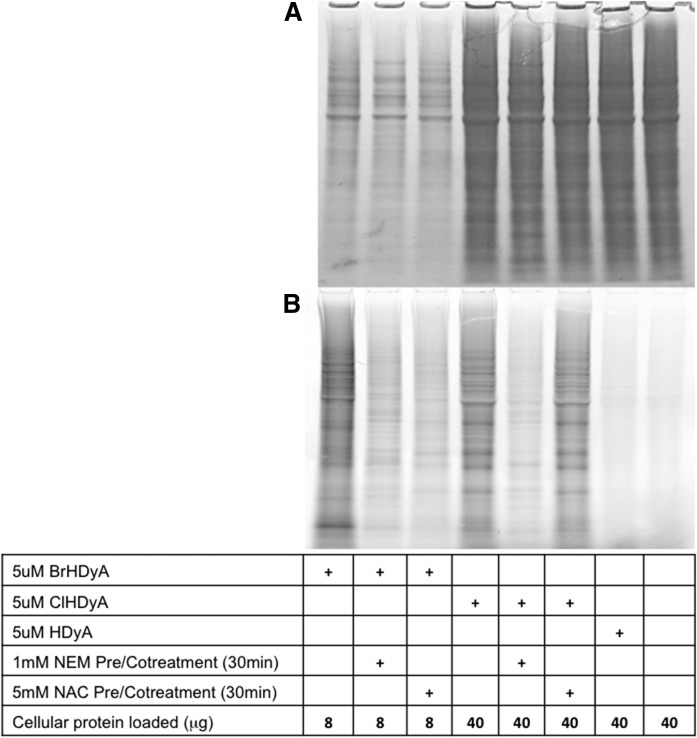
Previous studies have shown that α-ClFALD modifies proteins (13), but the mechanism for this modification was not examined, with the most likely mechanisms being through thiol modification or Schiff base adduct formation with amines. We investigated the role of thiol modification of α-BrFALD and α-ClFALD reactivity with protein using the clickable alkyne analogs, 2-BrHDyA, 2-ClHDyA, and HDyA. Figure 7B demonstrates that both 2-ClHDyA and 2-BrHDyA form protein adducts, while HDyA adducts are barely present. Because the 2-BrHDyA adducted protein to a greater extent than 2-ClHDyA, less total protein from 2-BrHDyA-treated cells was loaded to allow for simultaneous visualization of all conditions. The differential protein loading for these conditions can be visualized by the Coomassie staining in Fig. 7A.
Fig. 7.
NEM blocks 2-BrHDyA and 2-ClHDyA reaction with THP-1 cell protein. THP-1 cells were pretreated with or without 1 mM NEM or 5 mM NAC for 30 min at 37°C followed by treatment with 5 μM HDyA, 2-ClHDyA, or 2-BrHDyA for 30 min at 37°C. TAMRA-clicked THP-1 cell protein was visualized after gel electrophoresis. Panel A shows Coomassie blue total protein stain. NEM pretreatment blocked the fluorescence signal of 2-BrHDyA and 2-ClHDyA, but NAC pretreatment only significantly decreased the 2-BrHDyA signal (B).
The enhanced protein adduction of 2-BrHDyA compared with that of 2-ClHDyA suggested that thiol reactivity was the mechanism of protein binding, as studies with GSH showed that bromine was a better leaving group than chlorine in reactions with thiols. To confirm the importance of thiols to 2-ClHDyA and 2-BrHDyA protein binding, THP-1 cells were pretreated with the thiol-reactive compound, NEM. NEM pretreatment greatly decreased both 2-BrHDyA and 2-ClHDyA protein binding. In comparison, the thiol competitor bait, NAC, decreased 2-BrHDyA protein modification to a greater extent compared with 2-ClHDyA, which is consistent with data shown in Fig. 2 indicating that 2-BrHDA reacts with GSH to a greater extent compared with reactions with 2-ClHDA.
Metabolic activity of THP-1 cells following treatments with halogenated aldehydes
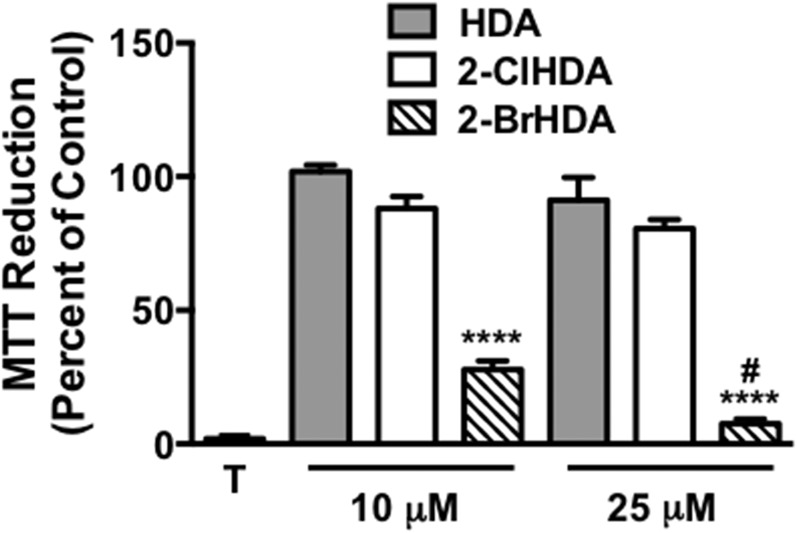
Based on the enhanced reactivity of 2-BrHDA with thiols compared with 2-ClHDA, further studies examined alterations in cell metabolic activity elicited by these halogenated aldehydes. Data shown in Fig. 8 demonstrate that treatments with 10 and 25 μM 2-BrHDA significantly decrease THP-1 cell metabolic activity, as ascertained by MTT assay, in comparison to changes elicited by those concentrations of 2-ClHDA and HDA, which did not significantly alter metabolic activity. Additionally, metabolic activity decreased as the 2-BrHDA concentration was increased from 10 to 25 μM.
Fig. 8.
Disparate effect of 2-BrHDA and 2-ClHDA on THP-1 cell metabolic activity. THP-1 cells were treated with the indicated concentrations of either HDA, 2-ClHDA, or 2-BrHDA for 2 h at 37°C. Metabolic activity of HCAEC was measured using an MTT assay following the manufacturer’s protocol. MTT reduction is expressed as percent of control MTT reduction (vehicle only, designed as 100%). Data are expressed as mean ± SEM; n = 4 for each treatment. T indicates treatment of THP-1 cells with Triton X-100. #P < 0.05 for comparisons between 2-BrHDA at the two concentrations examined. ****P < 0.0001 for comparisons between 2-BrHDA and either 2-ClHDA or HDA at the indicated concentrations.
DISCUSSION
α-ClFALD reacts with GSH to produce FALD-GSH (12). Because MPO and eosinophil peroxidase can produce HOBr and, subsequently, α-BrFALD, one of the goals of this study was to examine the reactivity of α-BrFALD compared with α-ClFALD with GSH and other thiol-containing target molecules. Initial experiments showed the reaction product of GSH with either 2-ClHDA and 2-BrHDA yielded the same reaction product (FALD-GSH) as ascertained by: 1) TLC analyses [each reaction product had an rf = 0.27 using 40 Å silica gel TLC plates and a mobile phase comprised of chloroform-acetone-methanol-water-acetic acid (6:8:2:2:1, v/v/v/v/v)]; and 2) ESI-MS/MS analyses of TLC-purified products having the same parent ion and product ions. These reactions also indicated the reaction of 2-BrHDA with GSH was much more robust than that with 2-ClHDA. Furthermore, while both 2-BrHDA and 2-ClHDA reacted with GSH, no reaction products were observed by TLC using nonhalogenated fatty aldehyde, HDA, or α-halogenated fatty acids and α-chlorofatty alcohol. The selective synthesis of FALD-GSH using 2-BrHDA (compared with that using 2-ClHDA) was supported by: 1) synthetic reactions of 2-BrHDA with GSH resulting in a more intense stained reaction product by TLC analyses, as well as near total consumption of GSH in comparisons to reactions of 2-ClHDA with GSH; 2) cellular production of FALD-GSH was 25 times greater in cells treated with 2-BrHDA compared with 2-ClHDA; and 3) enhancing α-BrFALD in comparison to α-ClFALD production in neutrophils by NaBr supplementation in the media led to an order of magnitude increase in FALD-GSH compared with neutrophils synthesizing only α-ClFALD (no NaBr supplementation). The finding that endogenous α-BrFALD in neutrophils is readily converted to FALD-GSH indicates that some α-BrFALD may be made under normal conditions (no NaBr supplementation), but may not be detected due to the rapid reaction with GSH. Interestingly, applying exogenous 2-BrHDA and 2-ClHDA to RAW 264.7 cells led to HDA-GSH production in cells as well as the appearance of this peptidoaldehyde in the cell culture media. The export of HDA-GSH from cells treated with either 2-ClHDA or 2-BrHDA suggests that FALD-GSH may be a stable metabolite of α-halofatty aldehydes that may be found in plasma during inflammation involving leukocyte activation, and FALD-GSH may be targeted for further metabolism by other organs or uptake for excretion.
α-BrFALD and α-ClFALD are produced by either HOBr or Br2, and either HOCl or Cl2, respectively, mediated oxidation of the vinyl ether bond at the sn-1 position of plasmalogens. HOBr-mediated oxidation of plasmalogens has not been investigated in detail. HOBr is produced by: 1) MPO in the presence of NaBr; 2) eosinophil peroxidase; and 3) the hydration of Br2 under conditions of Br2 exposure. Regarding MPO production of HOBr, activated neutrophils produce HOBr and 2-BrHDA when supplemented with buffers containing 100 μM NaBr (2). It should be appreciated that normal physiological concentrations of bromide in human plasma range from 20 to 150 μM (16), and thus it is likely that neutrophils do indeed produce HOBr under physiological concentrations. Additionally, bromination of host molecules by activated neutrophils has previously been reported (2, 22). In this study, we examined whether FALD-GSH is produced under conditions leading to endogenous α-BrFALD production. Accordingly, in addition to α-BrFALD reacting with GSH to yield FALD-GSH under synthetic conditions and cell culture conditions with exogenously supplied α-BrFALD, the data herein show FALD-GSH production under conditions of neutrophil activation leading to endogenous α-BrFALD production, conditions leading to eosinophil activation and α-BrFALD production; and in in vivo conditions with mice exposed to bromine with subsequent α-BrFALD production.
In these studies, we examined the in vivo production of lung α-BrFALD and FALD-GSH as well as plasma FALD-GSH in mice exposed to sublethal levels of bromine gas. Inhalation of chlorine and bromine gas has been shown to elicit severe systemic physiological effects, including cardiac dysfunction and vasoregulatory dysfunction (10, 23, 24). Bromine gas has many industrial applications, and is manufactured and transported world-wide. Accordingly, bromine as well as chlorine present a persistent hazardous material threat because their release can cause massive human casualties. In humans, inhalation of high concentrations of Br2 causes respiratory and myocardial injury, cardiac arrest, and circulatory collapse (25, 26). Following bromine exposure, bromide ions and brominated compounds formed on the pulmonary epithelial surface are stable and may persist in the circulation for days following exposure (27, 28). The discovery of biomarkers of bromine exposure thus is of great importance, and the data herein indicate that FALD-GSH is an excellent blood-borne biomarker of bromine exposure. Based on our findings of α-BrFALD and FALD-GSH, it is likely that the primary source of circulating FALD-GSH is from the lung. Further future studies will need to be performed to determine the temporal course that FALD-GSH remains elevated post bromine exposure.
After determining that the presence of a thiol was necessary for both 2-ClHDA and 2-BrHDA reactions with cysteine, the ability of α-ClFALD and α-BrFALD to react with THP-1 human monocyte cellular protein was examined using alkyne analogs of HDA (HDyA), 2-ClHDA (2-ClHDyA), and 2-BrHDA (2-BrHDyA). These experiments and analyses were performed without Schiff base reduction and, therefore, HDyA did not modify proteins (Fig. 7). The reactivity, however, of 2-ClHyDA and 2-BrHyDA with proteins in the absence of Schiff base reduction suggested the requirement of the α-halogen for reactivity with thiols for this covalent modification of proteins. Furthermore, 2-BrHDyA modification of protein was greater than that by 2-ClHDyA, similar to that with reactions of 2-BrHDA and 2-ClHDA with GSH that reflects bromine as a better leaving group for thiol nucleophilic attack. NEM greatly diminished protein binding in both the 2-ClHDyA- and the 2-BrHDyA-treated samples, supporting the mechanism of covalent modification being through protein thiols. NAC pretreatment was shown to block 2-BrHDyA protein binding, but not 2-ClHDyA. The discrepancy was likely due to the reduced reactivity of the thiol of NAC with 2-ClHDyA compared with 2-BrHDyA, which allowed 2-ClHDyA to enter the cell and bind protein. Collectively these studies demonstrate that α-BrFALD and α-ClFALD covalently modify cellular protein through thiol-dependent mechanisms. Interestingly, Nusshold et al. (13) demonstrated a number of endothelial cell proteins that react with 2-ClHDA. Proteomic analyses revealed that proteins associated with cytoskeletal structure, metabolic activity, and oxidative stress were modified by 2-ClHDA. Also, they showed decreased metabolic activity, determined by MTT assays, in endothelial cells treated with 25 and 50 μM 2-ClHDA. MTT activity was not statistically lowered in endothelial cells treated with 10 μM 2-ClHDA. The results herein are similar to those observed by Nusshold et al. (13), with no decreases in MTT activity observed at 10 μM 2-ClHDA in THP-1 cells and a slight, yet statistically insignificant, decrease in MTT activity observed at 25 μM 2-ClHDA. The present studies revealed that 2-BrHDA significantly decreased metabolic activity. It is possible that future proteomic studies may also reveal that proteins associated with metabolic activity will be modified by 2-BrHDA.
These studies support that FALD-GSH is a metabolite found endogenously as a result of the conjugation of α-BrFALD with GSH during neutrophil and eosinophil activation, as well as in mice following bromine exposure. It will be important in the future to determine whether FALD-GSH has biological activities in addition to its putative role as a biomarker. Interestingly, FALD-GSH can be compared structurally to LTC4, suggesting that the impact of FALD-GSH on LTC4 signaling pathways should be investigated. It will also be important to determine the metabolic clearance of FALD-GSH, including whether or not the aldehyde functional group can be oxidized or reduced yielding a GSH adduct containing a carboxylic acid or alcohol, respectively. Additionally, protein modification by thiol reactivity with α-BrFALD needs to be explored as a regulatory mechanism of protein function.
Footnotes
Abbreviations:
- α-BrFALD
- α-bromofatty aldehyde
- α-ClFALD
- α-chlorofatty aldehyde
- 2-BrHA
- 2-bromohexadecanoic acid
- 2-BrHDA
- 2-bromohexadecanal
- 2-BrHDyA
- 2-bromohexadec-15-ynal
- 2-ClHA
- 2-chlorohexadecanoic acid
- 2-ClHDA
- 2-chlorohexadecanal
- 2-ClHDyA
- 2-chlorohexadec-15-ynal
- 2-ClHOH
- 2-chlorohexadecanol
- FALD-GSH
- fatty aldehyde-GSH adduct
- HDA
- hexadecanal
- HDA-GSH
- 2-halohexadecanal adduct with GSH
- HDyA
- hexadec-15-ynal
- HOBr
- hypobromous acid
- HOCl
- hypochlorous acid
- MPO
- myeloperoxidase
- MTT
- 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
- NAC
- N-acetylcysteine
- NEM
- N-ethylmaleimide
- ODA-GSH
- 2-halooctadecanal adduct with GSH
- PMA
- phorbol myristate acetate
This work was supported by National Institutes of Health Grant R01GM115552 (D.A.F.) and National Institute of Environmental Health Sciences Grants U01ES023759 (R.P.P), U01ES026458 (S.M.), and U01ES027697 (S.M.). Additional support was provided through subcontracts to D.A.F. from National Institute of Environmental Health Sciences Grants U01ES023759, U01ES026458, and U01ES027697. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Albert C. J., Crowley J. R., Hsu F. F., Thukkani A. K., and Ford D. A.. 2001. Reactive chlorinating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: identification of 2-chlorohexadecanal. J. Biol. Chem. 276: 23733–23741. [DOI] [PubMed] [Google Scholar]
- 2.Albert C. J., Crowley J. R., Hsu F. F., Thukkani A. K., and Ford D. A.. 2002. Reactive brominating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: disparate utilization of sodium halides in the production of alpha-halo fatty aldehydes. J. Biol. Chem. 277: 4694–4703. [DOI] [PubMed] [Google Scholar]
- 3.Thukkani A. K., Hsu F-F. F., Crowley J. R., Wysolmerski R. B., Albert C. J., and Ford D. a.. 2002. Reactive chlorinating species produced during neutrophil activation target tissue plasmalogens: production of the chemoattractant, 2-chlorohexadecanal. J. Biol. Chem. 277: 3842–3849. [DOI] [PubMed] [Google Scholar]
- 4.Thukkani A. K., Albert C. J., Wildsmith K. R., Messner M. C., Martinson B. D., Hsu F. F., and Ford D. A.. 2003. Myeloperoxidase-derived reactive chlorinating species from human monocytes target plasmalogens in low density lipoprotein. J. Biol. Chem. 278: 36365–36372. [DOI] [PubMed] [Google Scholar]
- 5.Thukkani A. K., McHowat J., Hsu F. F., Brennan M. L., Hazen S. L., and Ford D. A.. 2003. Identification of alpha-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic lesions. Circulation. 108: 3128–3133. [DOI] [PubMed] [Google Scholar]
- 6.Thukkani A. K., Martinson B. D., Albert C. J., Vogler G. A., and Ford D. A.. 2005. Neutrophil-mediated accumulation of 2-ClHDA during myocardial infarction: 2-ClHDA-mediated myocardial injury. Am. J. Physiol. Heart Circ. Physiol. 288: H2955–H2964. [DOI] [PubMed] [Google Scholar]
- 7.Ullen A., Fauler G., Bernhart E., Nusshold C., Reicher H., Leis H-J., Malle E., and Sattler W.. 2012. Phloretin ameliorates 2-chlorohexadecanal-mediated brain microvascular endothelial cell dysfunction in vitro. Free Radic. Biol. Med. 53: 1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nusshold C., Kollroser M., Köfeler H., Rechberger G., Reicher H., Ullen A., Bernhart E., Waltl S., Kratzer I., Hermetter A., et al. . 2010. Hypochlorite modification of sphingomyelin generates chlorinated lipid species that induce apoptosis and proteome alterations in dopaminergic pc12 neurons in vitro. Free Radic. Biol. Med. 48: 1588–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsche G., Heller R., Fauler G., Kovacevic A., Nuszkowski A., Graier W., Sattler W., and Malle E.. 2004. 2-chlorohexadecanal derived from hypochlorite-modified high-density lipoprotein-associated plasmalogen is a natural inhibitor of endothelial nitric oxide biosynthesis. Arterioscler. Thromb. Vasc. Biol. 24: 2302–2306. [DOI] [PubMed] [Google Scholar]
- 10.Ford D. A., Honavar J., Albert C. J., Duerr M. A., Oh J. Y., Doran S., Matalon S., and Patel R. P.. 2016. Formation of chlorinated lipids post-chlorine gas exposure. J. Lipid Res. 57: 1529–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albert C. J., Thukkani A. K., Heuertz R. M., Slungaard A., Hazen S. L., and Ford D. A.. 2003. Eosinophil peroxidase-derived reactive brominating species target the vinyl ether bond of plasmalogens generating a novel chemoattractant, alpha-bromo fatty aldehyde. J. Biol. Chem. 278: 8942–8950. [DOI] [PubMed] [Google Scholar]
- 12.Duerr M. A., Aurora R., and Ford D. A.. 2015. Identification of glutathione adducts of alpha-chlorofatty aldehydes produced in activated neutrophils. J. Lipid Res. 56: 1014–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nusshold C., Ullen A., Kogelnik N., Bernhart E., Reicher H., Plastira I., Glasnov T., Zangger K., Rechberger G., Kollroser M., et al. . 2016. Assessment of electrophile damage in a human brain endothelial cell line utilizing a clickable alkyne analog of 2-chlorohexadecanal. Free Radic. Biol. Med. 90: 59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Dalen C. J., and Kettle A. J.. 2001. Substrates and products of eosinophil peroxidase. Biochem. J. 358: 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss S. J., Test S. T., Eckmann C. M., Roos D., and Regiani S.. 1986. Brominating oxidants generated by human eosinophils. Science. 234: 200–203. [DOI] [PubMed] [Google Scholar]
- 16.Tietz N. W. 1999. Tietz Textbook of Clinical Chemistry. C. A. Burtis, E. R. Ashwood, and N.W. Tietz, editors. W.B. Saunders, Philadelphia, PA. [Google Scholar]
- 17.Leustik M., Doran S., Bracher A., Williams S., Squadrito G. L., Schoeb T. R., Postlethwait E., and Matalon S.. 2008. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am. J. Physiol. Lung Cell. Mol. Physiol. 295: L733–L743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aggarwal S., Lam A., Bolisetty S., Carlisle M. A., Traylor A., Agarwal A., and Matalon S.. 2016. Heme attenuation ameliorates irritant gas inhalation-induced acute lung injury. Antioxid. Redox Signal. 24: 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wacker B. K., Albert C. J., Ford B. A., and Ford D. A.. 2013. Strategies for the analysis of chlorinated lipids in biological systems. Free Radic. Biol. Med. 59: 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng B., DeRan M., Li X., Liao X., Fukata M., and Wu X.. 2013. 2-bromopalmitate analogues as activity-based probes to explore palmitoyl acyltransferases. J. Am. Chem. Soc. 135: 7082–7085. [DOI] [PubMed] [Google Scholar]
- 21.Kukreja R. C., Weaver A. B., and Hess M. L.. 1989. Stimulated human neutrophils damage cardiac sarcoplasmic reticulum function by generation of oxidants. Biochim. Biophys. Acta. 990: 198–205. [DOI] [PubMed] [Google Scholar]
- 22.Henderson J. P., Byun J., Williams M. V., Mueller D. M., McCormick M. L., and Heinecke J. W.. 2001. Production of brominating intermediates by myeloperoxidase. A transhalogenation pathway for generating mutagenic nucleobases during inflammation. J. Biol. Chem. 276: 7867–7875. [DOI] [PubMed] [Google Scholar]
- 23.Zaky A., Ahmad A., Dell’Italia L. J., Jahromi L., Riesenberg L. A., Matalon S., and Ahmad S.. 2015. Inhaled matters of the heart. Cardiovasc. Regen. Med. 2: e997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert J. A., Carlisle M. A., Lam A., Aggarwal S., Doran S., Ren C., Bradley W. E., Dell’Italia L., Ambalavanan N., Ford D. A., et al. . 2017. Mechanisms and treatment of halogen inhalation-induced pulmonary and systemic injuries in pregnant mice. Hypertension. 70: 390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liubchenko P. N., and Alekseeva G. A.. 1991. Acute poisoning with bromine vapors of a pharmaceutical plant operator. [Article in Russian] Gig. Tr. Prof. Zabol. 9: 32–34. [PubMed] [Google Scholar]
- 26.Makarovsky I., Markel G., Hoffman A., Schein O., Brosh-Nissimov T. M., Finkelstien A., Tashma Z., Dushnitsky T., and Eisenkraft A.. 2007. Bromine–the red cloud approaching. Isr. Med. Assoc. J. 9: 677–679. [PubMed] [Google Scholar]
- 27.Hustinx W. N., van de Laar R. T., van Huffelen A. C., Verwey J. C., Meulenbelt J., and Savelkoul T. J.. 1993. Systemic effects of inhalational methyl bromide poisoning: a study of nine cases occupationally exposed due to inadvertent spread during fumigation. Br. J. Ind. Med. 50: 155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soremark R. 1960. Distribution and kinetics of bromide ions in the mallalian body: Some experimental investigations using br80m and br82. Acta Radiol. Suppl. 190: 1–114. [PubMed] [Google Scholar]