Abstract
Phytocannabinoids, such as Δ9-tetrahydrocannabinol (THC), bind and activate cannabinoid (CB) receptors, thereby “piggy-backing” on the same pathway’s endogenous endocannabinoids (ECs). The recent discovery that liver fatty acid binding protein-1 (FABP1) is the major cytosolic “chaperone” protein with high affinity for both Δ9-THC and ECs suggests that Δ9-THC may alter hepatic EC levels. Therefore, the impact of Δ9-THC or EC treatment on the levels of endogenous ECs, such as N-arachidonoylethanolamide (AEA) and 2-arachidonoylglycerol (2-AG), was examined in cultured primary mouse hepatocytes from WT and Fabp1 gene-ablated (LKO) mice. Δ9-THC alone or 2-AG alone significantly increased AEA and especially 2-AG levels in WT hepatocytes. LKO alone markedly increased AEA and 2-AG levels. However, LKO blocked/diminished the ability of Δ9-THC to further increase both AEA and 2-AG. In contrast, LKO potentiated the ability of exogenous 2-AG to increase the hepatocyte level of AEA and 2-AG. These and other data suggest that Δ9-THC increases hepatocyte EC levels, at least in part, by upregulating endogenous AEA and 2-AG levels. This may arise from Δ9-THC competing with AEA and 2-AG binding to FABP1, thereby decreasing targeting of bound AEA and 2-AG to the degradative enzymes, fatty acid amide hydrolase and monoacylglyceride lipase, to decrease hydrolysis within hepatocytes.
Keywords: fatty acid binding protein-1, binding, structure, metabolism, N-arachidonoylethanolamide, 2-arachidonoylglycerol
Since the discovery of key elements of the endocannabinoid (EC) system in liver and its roles in nonalcoholic fatty liver disease (NAFLD), much research has focused on development of agonists/antagonists of this system. Cannabinoid (CB) receptors, together with their endogenous EC ligands, i.e., N-arachidonoylethanolamide (AEA) and 2-arachidonoylglycerol (2-AG), constitute a novel system for modulating not only behavior, satiety, pain, and inflammation (1–7), but also hepatic fat accumulation (8–10). Δ9-Tetrahydrocannabinol (THC), the main psychotropic component of cannabis, binds and activates CB receptors, thereby piggy-backing on the endogenous EC pathway (11–13).
Several studies indicate that cannabis use or Δ9-THC treatment promotes steatosis and is associated with development of NAFLD (14, 15). Similarly, activation of the endogenous EC system to increase AEA, 2-AG, and CB receptor-1 (CB1) is linked to NAFLD (8–10). CB1 is upregulated in patients with NAFLD (9, 10). Finally, studies with gene-targeted mice reveal that CB1 activation is essential for NAFLD development (8, 9, 16). The mechanism whereby CB1 induces hepatic lipid accumulation involves downstream upregulation of SREBP1c, which in turn induces transcription of de novo lipogenic enzymes (8, 14). However, it is not known whether Δ9-THC elicits its steatotic effects in liver only by directly activating hepatic CB1 and/or also by indirectly upregulating the liver endogenous EC activators of CB1 (AEA, 2-AG). In support of the latter possibility, Δ9-THC treatment increases circulating levels and brain levels of ECs (AEA, 2-AG) in humans and rodents (17, 18). However, nothing is known regarding the impact of Δ9-THC on hepatic EC levels or the mechanism(s) whereby this may occur.
Recent novel discoveries suggest mechanistic involvement of liver fatty acid binding protein-1 (FABP1) in the hepatic EC system (19–21). FABP1 binds both phytocannabinoids (Δ9-THC) and ECs (AEA, 2-AG) with high affinity and is the major cytosolic chaperone protein for transport/targeting of these lipid ligands to intracellular metabolic and regulatory sites (19–21). Moreover, the level of hepatic FABP1 protein is markedly upregulated in animal models of NAFLD (22, 23) and human NAFLD (24), especially in human subjects expressing the highly prevalent FABP1 T94A variant (26–38% minor allele frequency; 8.3 ± 1.9% homozygous) [reviewed in (21)]. Although the human FABP1 T94A variant protein differs only modestly from its WT FABP counterpart in affinities for ECs (AEA, 2-AG) (25) as well as a variety of other ligands (26–29), the conformation of the FABP1 T94A variant is much less responsive to ligand-induced change (25, 26), which in turn diminishes its ability to enter nuclei and the interaction with and activation of PPARα therein (29).
To begin to address these issues, the effect of Δ9-THC treatment on ECs and proteins in the EC system was examined in cultured primary hepatocytes from male WT C57BL/6N mice and from Fabp1 gene-ablated (LKO) mice on the same background. Hepatic levels of ECs (AEA, 2-AG) as well as non-arachidonic acid N-acylethanolamides (NAEs) and 2-monoacylglycerols (2-MGs) were determined by LC-MS, while hepatic proteins in the EC system were quantitated by Western blotting. The results showed that Δ9-THC treatment induced hepatic accumulation of AEA and 2-AG, effects antagonized by loss of FABP1.
MATERIALS AND METHODS
Materials
AEA, oleoylethanolamide (OEA), palmitoylethanolamide (PEA), N-docosahexaenoylethanolamide (DHEA), N-eicosapentaenoylethanolamide (EPEA), 2-AG, 2-oleoylglycerol (2-OG), and 2-palmitoylglycerol (2-PG) were purchased from Cayman Chemical (Ann Arbor, MI). Deuterated AEA-d4, OEA-d2, PEA-d4, DHEA-d4, EPEA-d4, and 2-AG-d8 were also from Cayman Chemical. The phytocannabinoid, Δ9-THC (also called dronabinol), was also acquired from Cayman Chemical.
The following antibodies to liver proteins involved in the hepatic EC system were obtained commercially as follows: anti-fatty acid amide hydrolase (FAAH; sc-26427), anti-N-acylphosphatidylethanolamide phospholipase-D (NAPE-PLD; sc-163117), anti-fatty acid transport protein (FATP)4 (sc-5834), rabbit polyclonal anti-monoacylglyceride lipase (MAGL; sc-134789), anti-diacylglycerol lipase (DAGL)α (sc-133307), and FABP1 (L-FABP; sc-16064) were from Santa Cruz Biotechnology (Santa Cruz, CA); anti-FATP4 (sc-5834), polyclonal anti-FATP2 (ab83763), and specific monoclonal anti-mouse heat shock protein-70 (HSP70; ab2787) were from Abcam (Cambridge, MA). Mouse monoclonal anti-GAPDH (MAB374) was from Millipore, Inc. (Billerica, MA). Rabbit polyclonal anti-sterol carrier protein (SCP)-2 recognizing both Scp-2/Scp-x gene products (58 kDa SCP-x and 13.2 kDa SCP-2) was prepared as in (30). All solvents and reagents used were of the highest commercial grade available.
WT and Fabp1 gene-ablated mice
Male WT and Fabp1 gene-ablated (LKO) mice were obtained similarly as described earlier (31, 32). During maintenance of the mouse colony, all mice were fed a standard rodent chow mix [5% calories from fat, D8604 Teklad Rodent Diet; Teklad Diets (Madison, WI)] and were maintained in barrier cages on ventilated racks at 12 h light/dark cycle in a temperature-controlled facility (25°C) with ad libitum food and water until study initiation. Mice were sentinel monitored quarterly and confirmed free of all known rodent pathogens. Experimental protocols for animal use were approved by the Institutional Animal Care and Use Committee at Texas A&M University.
Isolation and culture of primary mouse hepatocytes
Male WT and LKO mice 5–7 months old were maintained, housed, and fed as described above and primary hepatocytes isolated as described earlier (32–34). Briefly, mice were euthanized by CO2 asphyxiation, livers excised, and perfused with buffer A [10 mM HEPES (pH 7.4) in calcium/magnesium-free HBSS, gentamycin sulfate (1 mg/ml medium), and 0.5 mM EGTA]. To release the hepatocytes, the livers were then perfused with buffer B [buffer A without EGTA, supplemented with 5 mM CaCl2 and collagenase type IV (Sigma) at a concentration of 100 units/ml] and 5% FBS. To facilitate hepatocyte release, the liver capsule was gently palpated during perfusion. Primary hepatocytes were then washed twice in cold DMEM with 5% FBS, purified with Percoll gradient (33), plated on collagen-coated dishes at 3 × 103 cells/100 mm culture dish, and cultured overnight at 37°C in a CO2 incubator as described in the cited papers (32–34).
Treatment of cultured primary mouse hepatocytes with phytocannabinoid (Δ9-THC) and endogenous (2-AG) agonists of CB1
A time course and dose response treatment was performed as follows. Primary mouse hepatocytes were isolated from WT mice and cultured overnight as described above. The hepatocytes were washed with PBS and further incubated with 2 μM THC/0.015% BSA, 20 μM THC/0.15% BSA, and 40 μM THC/0.3% BSA in Puck’s buffer for 0–2 h time, as indicated in the figure legends, in a 37°C incubator, as described (32–34). These concentrations were chosen to be in the range of those used previously with a variety of hepatoma and other cell lines (35–37). The concentrations of BSA were chosen based upon an approximate 1:1 binding stoichiometry at the micromolar concentrations of Δ9-THC and 2-AG (34–36). The control was incubated in Puck’s buffer with the same amount of methanol vehicle and 0.15% BSA (without Δ9-ΤHC). The 20 μM Δ9-THC concentration was chosen as used previously (37). Multiple culture dishes of primary mouse hepatocytes were incubated at 20 μM THC/0.15% BSA in Puck’s buffer for 1 h in a 37°C incubator, as described (32–34). The control was incubated in Puck’s buffer with the same amount of methanol vehicle and 0.15% BSA (without Δ9-THC).
For treatment with endogenous CB1 agonist, hepatocytes were incubated similarly as above except without or with 2-AG (1 μM), a low concentration that does not produce adverse effects in cultured primary hepatocytes (38, 39).
Lipid extraction from cultured primary mouse hepatocytes
Hepatocytes were removed from the culture plate and stored in sealed microtubes at −80°C as described earlier (32, 40, 41). For lipid analysis, the hepatocytes in each microtube were thawed on ice, vortexed, and the contents subsequently transferred to an ice-cold Dounce homogenizer. The microtubes were then rinsed with 1 ml of ice-cold KPD [20 mM potassium phosphate (pH 7.4) and 1 mM DTT] to remove any residual hepatocytes. This solution was also vortexed and combined with the previous hepatocyte/buffer mixture into the Dounce homogenizer. This hepatocyte/buffer mixture was homogenized using 10 up/down strokes of the Dounce homogenizer. For protein quantification, an aliquot (200 μl) of the homogenate was removed and placed in a separate microtube as described previously (19, 40). The following internal standard mixture was added: 4,000 pg each of AEA-d4, OEA-d2, PEA-d4, and EPEA-d4, and 40,000 pg of 2-AG-d5 to the remaining homogenate in the Dounce homogenizer. Extraction of the lipids from the hepatocyte homogenate was performed as described previously (40). The final lipid extract was dried under N2 and then dissolved in 60 μl of acetonitrile with the addition of 60 μl of water. Each of the samples was then purged with N2 gas and stored at −80°C.
LC-MS analysis of NAEs and 2-MGs in cultured primary mouse hepatocytes
The lipid extracts (above) were resolved to determine NAE and 2-MG levels as quantitated by LC-MS analysis in the Protein Chemistry Laboratory (directed by Dr. Larry Dangott at Texas A&M University) as described (19, 42). An external standard bracket to determine linear range was performed of 0.05, 0.10, 0.25, 0.50, 1.00, 2.50, 5.00, 10.0, 25.0, or 50.0 ng of AEA, OEA, PEA, DHEA, EPEA, 2-AG, 2-OG, and 2-PG, respectively. Individual NAE or 2-MG values were expressed based on hepatocyte homogenate milligrams of protein.
Impact of Δ9-THC treatment on protein levels in the EC system of cultured primary hepatocytes from livers of WT and LKO mice
To determine whether the altered EC levels in WT and/or LKO hepatocytes treated with Δ9-THC resulted from altered expression of proteins in the EC system, hepatocytes were homogenized, protein determined, 2–10 μg protein loaded/resolved by SDS-PAGE, bands of interest identified by Western blotting, and quantitated as described (32, 40, 41). Hepatocyte levels of the following groups of proteins involved in the liver EC system were determined by Western blotting: i) synthetic enzymes: NAPE-PLD and DAGLα ii) degradative enzymes: FAAH, NAE-hydrolyzing acid amidase, and MAGL; iii) CB receptors: CB1; iv) cytosolic transport/chaperone proteins: FABP1, HSP70, and SCP-2 (recognizes 58 kDa SCP-x as well as 13.2 kDa SCP-2); and v) membrane fatty acid translocases involved in uptake of arachidonic acid (ARA) (the precursor from which ECs are derived): FATP2 and FATP4. GAPDH was used as internal loading control. Individual protein bands on Western blots were quantitated by densitometric analysis by ImageJ software (National Institutes of Health, Bethesda, MD) and normalized to GAPDH as in (42). Representative Western blots were then cropped and inserted (separated by a white line/space) into figure panels as in earlier publications.
Statistical analysis
Values represent the mean ± SEM. Statistical analysis was performed by one-way ANOVA followed with the Newman-Keuls post hoc analysis using Sigma Plot software (Systat, San Jose, CA). P < 0.05 was considered statistically significant and was denoted by a # (FABP1 KO vs. WT) or * (Δ9-THC treated vs. untreated of the same genotype).
RESULTS
Impact of Δ9-THC treatment on hepatocyte level of endogenous CB1 agonists AEA and 2-AG: time and Δ9-THC concentration dependence
Hepatocytes primarily express CB1 (43), which is activated by both phytocannabinoids (e.g., Δ9-THC) and ECs (e.g., AEA, 2-AG) (12, 44). Likewise, the major liver cytosolic lipid binding/chaperone protein (i.e., FABP1) has high affinity for both Δ9-THC and ECs (AEA, 2-AG) (19–21). Therefore, the possibility that Δ9-THC treatment might indirectly impact CB1 activation by altering the level of the AEA and 2-AG was examined.
WT cultured primary mouse hepatocytes were incubated with increasing Δ9-THC (2–40 μM) and time (up to 2 h), and the hepatocyte AEA and 2-AG levels were determined as in the Materials and Methods. Δ9-THC treatment elicited a concentration-dependent increase in AEA level at all time points examined (Fig. 1A). Likewise, increasing Δ9-THC generally increased the hepatocyte 2-AG level (Fig. 1B). Based on these findings, a 20 μM Δ9-THC concentration and 1 h incubation time were chosen as the set of nonsaturating conditions to determine statistical significance of the impact of Δ9-THC on AEA and 2-AG levels, as described in the following sections.
Fig. 1.
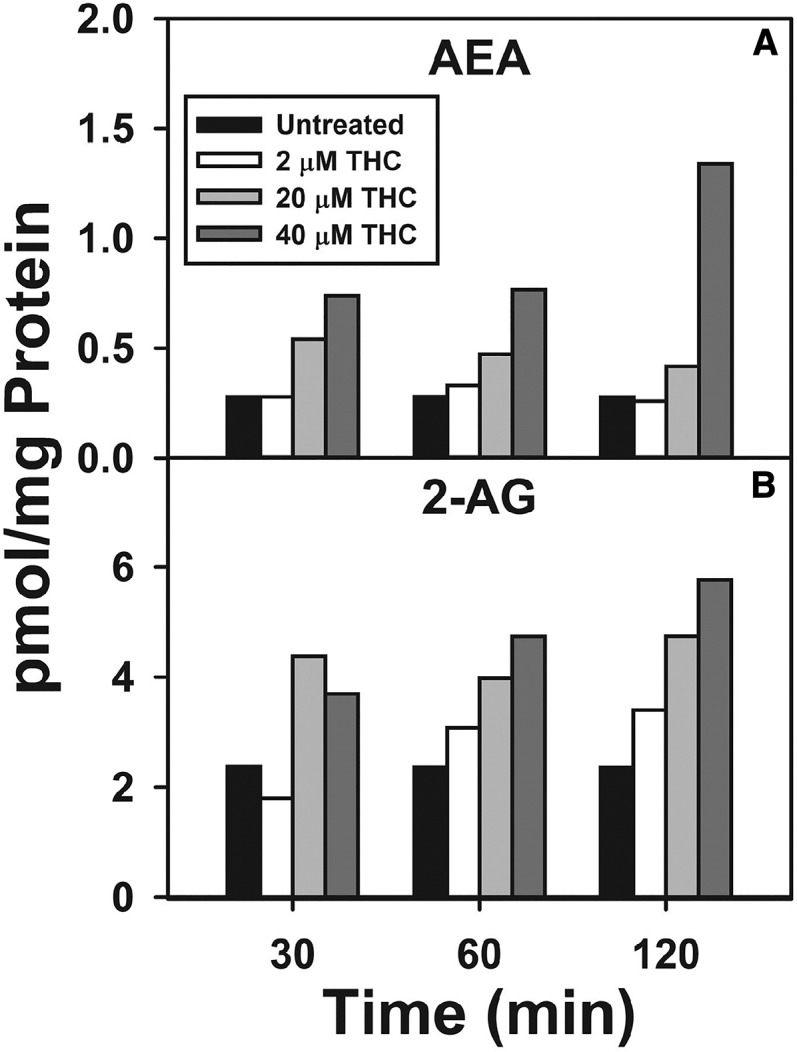
Time and concentration dependence of Δ9-THC impact on hepatocyte levels of AEA and 2-AG. Cultured primary hepatocytes were isolated from WT mice, plated on culture dishes, and incubated with increasing Δ9-THC (2–40 μM) for increasing time (0–2 h), as described in the Materials and Methods. Lipids were then extracted followed by analysis and quantitation by LC-MS as described in the Materials and Methods. AEA (A) and 2-AG (B) (n = 1).
Δ9-THC treatment increases cultured primary hepatocyte level of AEA: impact of Fabp1 gene ablation
Because FABP1 has high affinity for ECs as well as Δ9-THC (19–21), the possibility that loss of FABP1 protein (i.e., Fabp1 gene ablation, LKO) might alter the ability of Δ9-THC to increase levels of AEA, an endogenous CB1 agonist, was examined in cultured primary hepatocytes from WT and LKO mice.
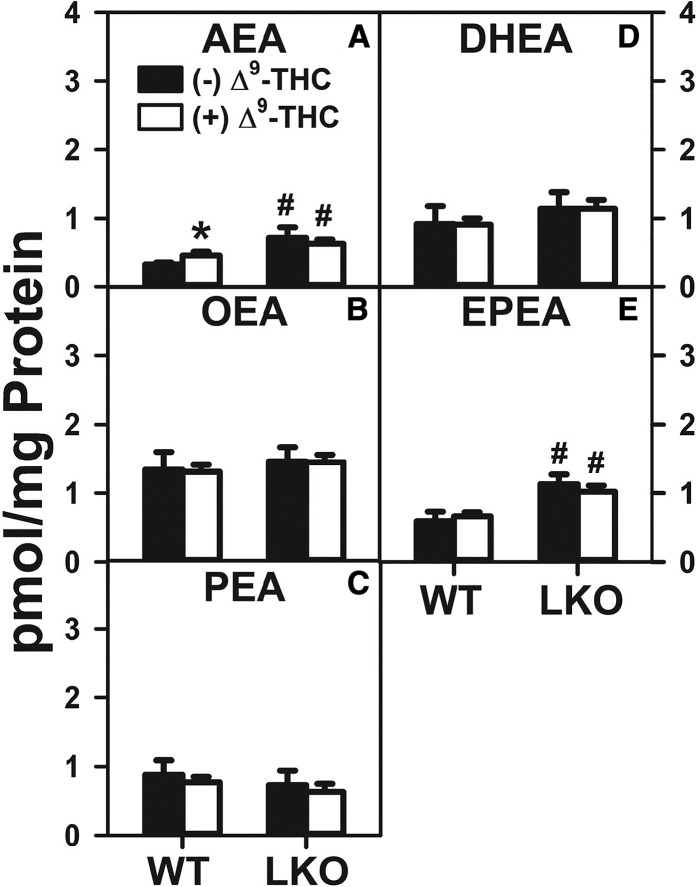
Treatment of WT hepatocytes with Δ9-THC alone significantly increased AEA nearly 30% (Fig. 2A). LKO alone (in the absence of Δ9-THC) increased WT hepatocyte AEA by nearly 2-fold (Fig. 2A). However, Δ9-THC treatment of LKO hepatocytes did not significantly increase the AEA level (Fig. 2A).
Fig. 2.
Effect of Δ9-THC and Fabp1 gene ablation on hepatocyte levels of AEA and non-ARA-containing NAEs. Cultured primary hepatocytes were isolated from WT or LKO mice, plated on culture dishes, and incubated with Δ9-THC (20 μM) for 1 h, as described in the Materials and Methods. Lipids were then extracted followed by AEA and NAE analysis and quantitation by LC-MS, also as described in the Materials and Methods. AEA (A), OEA (B), PEA (C), DHEA (D), and EPEA (E). Values represent the mean ± SEM, n = 4–6. #P < 0.05 versus WT in the same treatment groups; *P < 0.05 versus untreated of the same genotype.
Taken together, the above data indicated that both Δ9-THC alone and, even more so, LKO alone significantly increased the WT hepatocyte level of AEA. However, LKO blocked the ability of Δ9-THC to further increase AEA content in LKO hepatocytes.
Effect of Δ9-THC treatment on hepatocyte levels of non-ARA-containing NAEs (OEA, PEA, DHEA, and EPA)
Non-ARA-containing NAEs (PEA, OEA, DHEA, and EPEA) and 2-MGs (2-OG and 2-PG) do not directly bind/activate CB receptors. Nevertheless, such NAEs and 2-MGs can act as “entourage” molecules that enhance the effects of AEA and/or 2-AG by competing either with transporters or the enzymes mediating the inactivation of ECs or by enhancing binding/action of ECs, such as AEA and 2-AG, on CB receptors (45–53).
Δ9-THC treatment alone did not significantly affect NAE levels in cultured primary hepatocytes from WT mice (Fig. 2B–E). LKO alone increased the hepatocyte level of EPEA by 1.8-fold (Fig. 2E), but did not significantly alter that of OEA (Fig. 2B), PEA (Fig. 2C), or DHEA (Fig. 2D). LKO completely blocked the effect of Δ9-THC on increasing the hepatocyte level of EPEA (Fig. 2E).
Thus, Δ9-THC alone did not significantly alter WT hepatocyte levels of NAEs. In contrast, LKO alone selectively increased the hepatocyte level of EPEA regardless of the presence or absence of Δ9-THC.
The 2-AG treatment increases hepatocyte levels of AEA: impact of Fabp1 gene ablation
To determine whether the Δ9-THC-induced increase in hepatocyte AEA was specific to this exogenous phytocannabinoid CB1 activator, the effect of the endogenous CB1 agonist, 2-AG, was examined. FABP1 has high affinity for the endogenous CB1 receptor agonist, 2-AG (19–21). Therefore, the impact of 2-AG and/or FABP1 on the hepatocyte level of AEA was examined in cultured primary hepatocytes from WT and Fabp1 gene-ablated (LKO) mice.
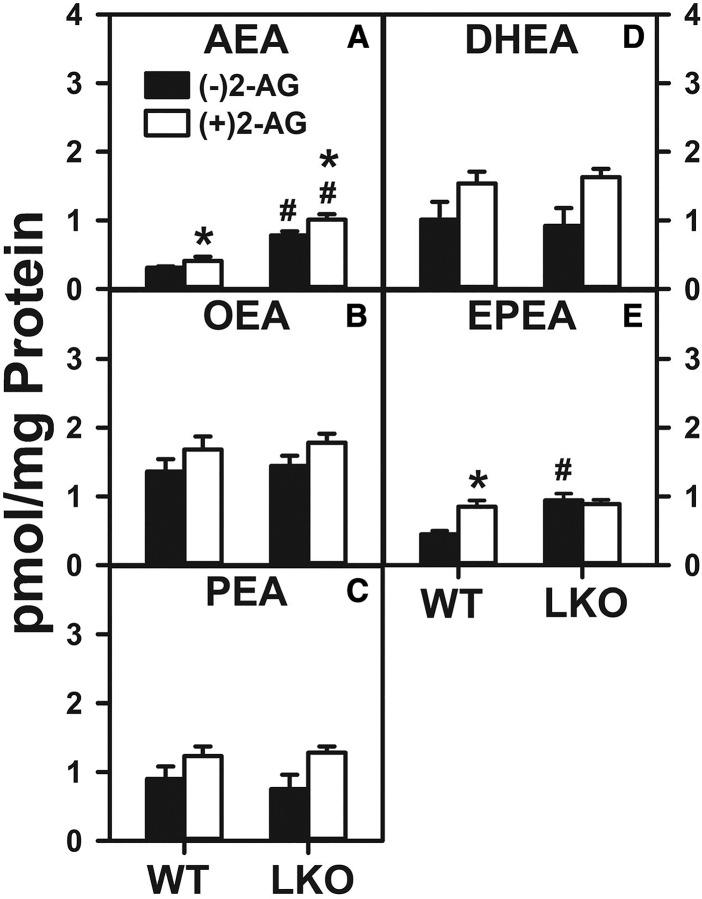
Treatment of WT hepatocytes with 2-AG significantly increased the level of AEA (Fig. 3A). In contrast, LKO alone (in the absence of 2-AG) increased AEA by 2.2-fold (Fig. 3A). Furthermore, LKO conferred on 2-AG the ability to increase WT hepatocyte AEA level (Fig. 3A).
Fig. 3.
Effect of 2-AG and Fabp1 gene ablation on hepatocyte levels of AEA and non-ARA-containing NAEs. Hepatocytes were isolated from WT or LKO mice, plated on culture dishes, and incubated with 2-AG (1 μM) for 1 h. Lipids were then extracted and analyzed by LC-MS to determine AEA and NAE levels, as described in the Materials and Methods. AEA (A), OEA (B), PEA (C), DHEA (D), and EPEA (E). Values represent the mean ± SEM, n = 4–6. #P < 0.05 versus WT in the same treatment groups; *P < 0.05 versus untreated of the same genotype.
With regard to the non-ARA-containing NAEs, 2-AG treatment of WT hepatocytes increased the level of EPEA by 2-fold (Fig. 3E), while not changing that of OEA, PEA, or DHEA (Fig. 3B–D). LKO alone likewise increased the WT hepatocyte level of EPEA by 2-fold (Fig. 3E), but not that of OEA, PEA, or DHEA (Fig. 3B–D). Finally, LKO blocked the ability of 2-AG to increase the hepatocyte level of EPEA (Fig. 3E) and did not affect levels of OEA, PEA, or DHEA (Fig. 3B–D).
These data indicated that 2-AG treatment alone increased WT hepatocyte AEA level analogous to Δ9-THC-induced AEA increase (Fig. 2A). LKO alone significantly increased the hepatocyte level of AEA and, moreover, conferred on 2-AG the ability to increase hepatocyte AEA content. This was in marked contrast to LKO blocking of the ability of Δ9-THC to further increase AEA content therein (Fig. 2A).
Impact of Δ9-THC and Fabp1 gene ablation on hepatocyte levels of the EC, 2-AG, and non-ARA-containing 2-MGs (2-OG, 2-PG)
Because FABP1 exhibits high affinity for 2-AG as well as Δ9-THC (19, 20), the effect of Δ9-THC on cultured primary hepatocyte levels of the 2-MGs was examined, as described in the Materials and Methods.
Δ9-THC treatment significantly increased the WT hepatocyte level of 2-AG 1.8-fold (Fig. 4A). LKO alone increased the 2-AG level even more, i.e., by 3.3-fold (Fig. 4A). However, LKO impaired the ability of Δ9-THC to increase hepatocyte 2-AG by nearly half (Fig. 4A).
Fig. 4.
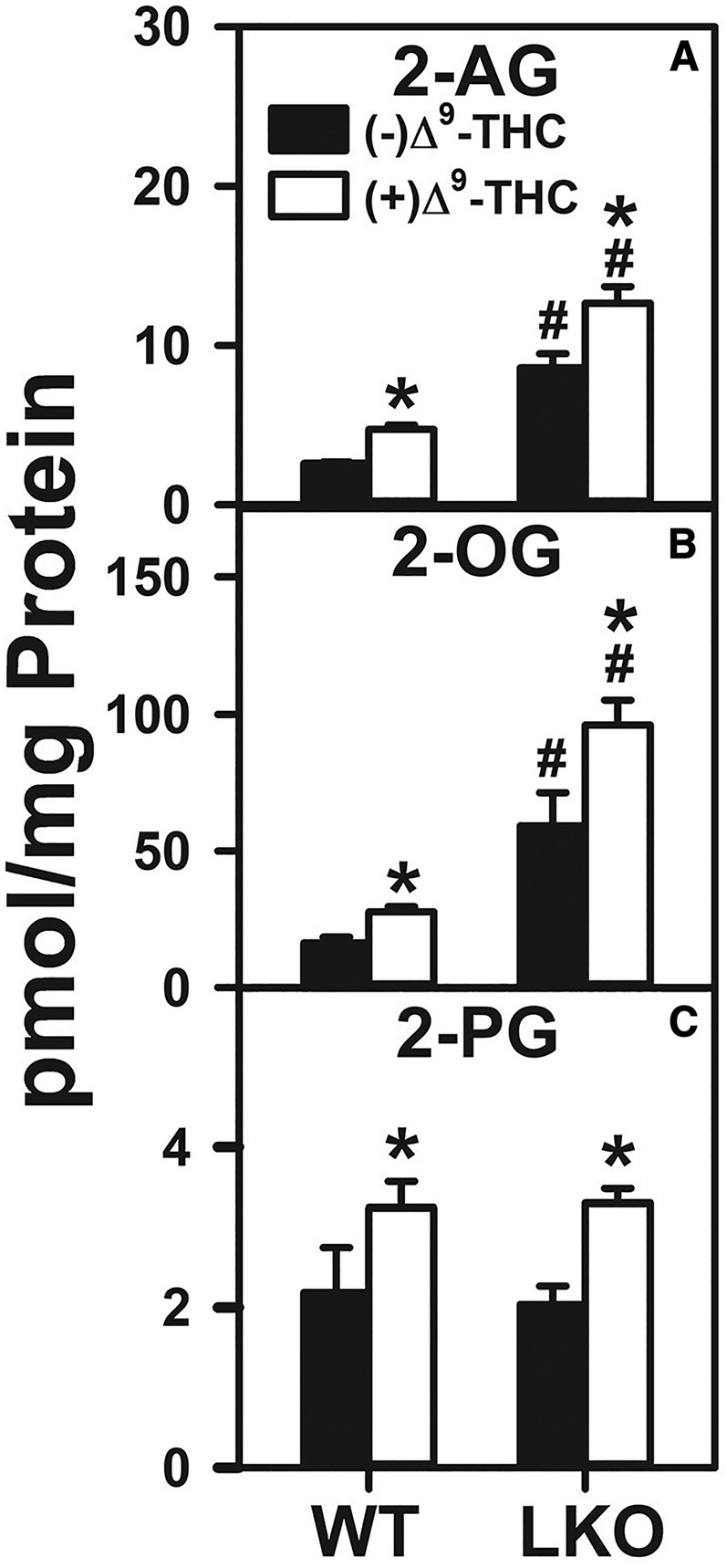
Effect of Δ9-THC and Fabp1 gene ablation on hepatocyte levels of 2-AG and non-ARA-containing 2-MGs. Hepatocytes were isolated from WT or LKO mice, plated on culture dishes, and incubated with Δ9-THC (20 μM) for 1 h, as described in the Materials and Methods. Lipids were then extracted followed by 2-AG and 2-MG analysis and quantitation by LC-MS, also as described in Materials and Methods. 2-AG (A), 2-OG (B), and 2-PG (C). Values represent the mean ± SEM, n = 4–6. #P < 0.05 versus WT in the same treatment groups; *P < 0.05 versus untreated of the same genotype.
With regard to the non-ARA-containing 2-MGs, Δ9-THC treatment significantly increased the WT hepatocyte level of 2-OG by 1.7-fold (Fig. 4B), while also increasing that of 2-PG by 1.5-fold (Fig. 4C). LKO alone increased the levels of 2-OG even more by 3.5-fold (Fig. 4B), but not the level of 2-PG (Fig. 4C). However, LKO did not further potentiate the ability of Δ9-THC to increase hepatocyte 2-OG (Fig. 4B) or 2-PG (Fig. 4C), as compared with their Δ9-THC-treated WT counterparts.
Thus, Δ9-THC treatment alone in WT hepatocytes and, even more so, LKO alone, markedly increased hepatocyte levels of 2-AG much more than those of AEA (Fig. 2A). Furthermore, both Δ9-THC treatment alone in WT hepatocytes and LKO alone markedly increased hepatocyte levels of 2-OG and 2-PG, in marked contrast to the effect of Δ9-THC treatment on the non-ARA-containing 2-MGs, which were not further increased by LKO (Fig. 4).
Impact of 2-AG and Fabp1 gene ablation hepatocyte level of 2-AG and non-ARA-containing 2-MGs
It is not known whether the ability of Δ9-THC to markedly increase hepatocyte 2-AG and other 2-MGs noted above was unique to this exogenous phytocannabinoid CB1 agonist or also shared by the endogenous CB1 agonist, 2-AG. Therefore, the impact of exogenous 2-AG on hepatocyte levels of 2-AG and non-ARA-containing 2-MGs was examined in cultured primary hepatocytes from WT and LKO mice, as described in the Materials and Methods.
The 2-AG treatment alone significantly increased 2-AG levels by 16-fold in WT hepatocytes (Fig. 5A). LKO alone also increased the 2-AG level to the same extent, i.e., 15-fold (Fig. 5A). Further, LKO markedly potentiated the ability of exogenous 2-AG-increased hepatocyte 2-AG content to a level over 5-fold more than in 2-AG-treated WT hepatocytes (Fig. 5A).
Fig. 5.
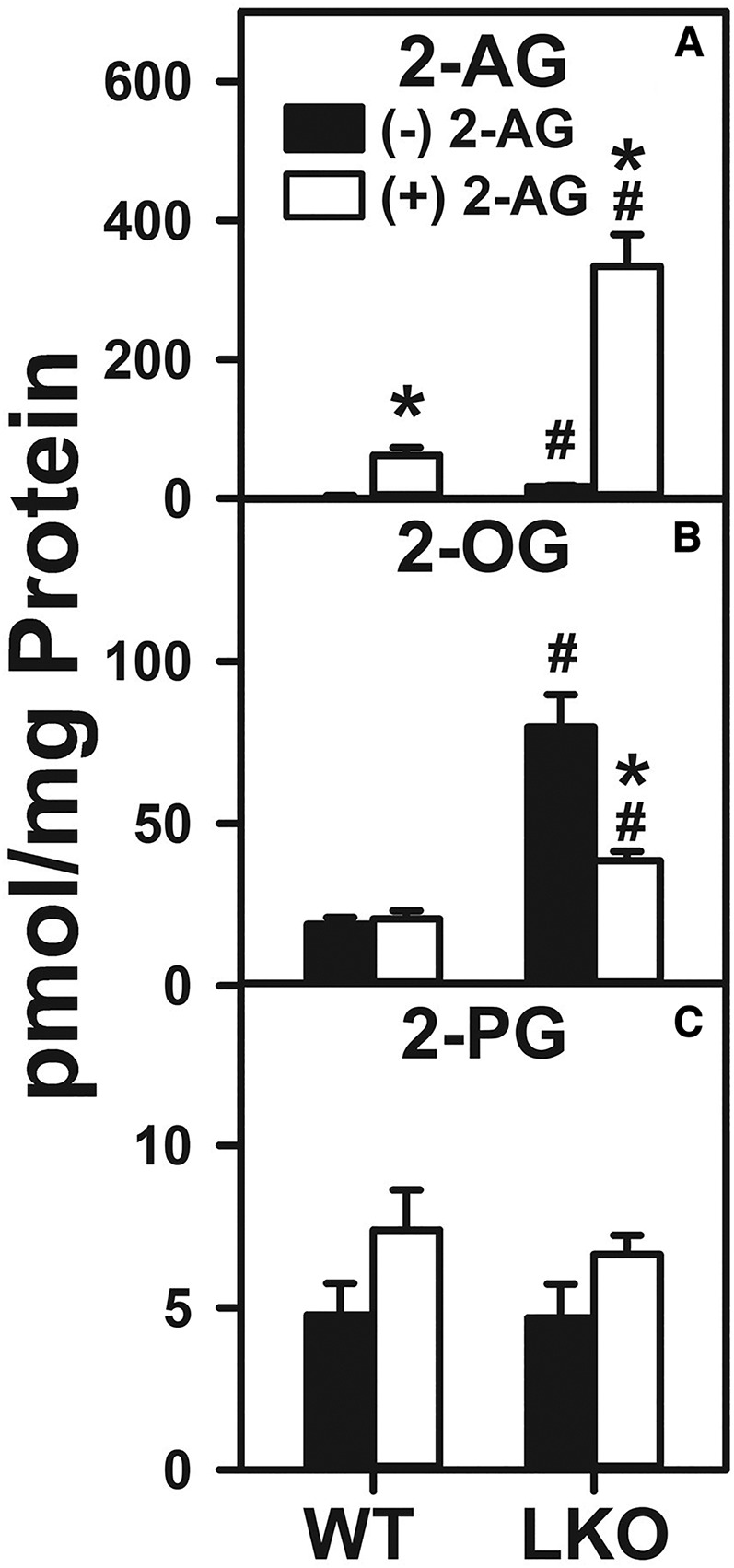
Effect of 2-AG and Fabp1 gene ablation on hepatocyte levels of 2-AG and non-ARA-containing 2-MGs. Hepatocytes were isolated from WT or LKO mice, plated on culture dishes, and incubated with 2-AG (1 μM) for 1 h, as described in the Materials and Methods. Lipids were then extracted followed by 2-AG and 2-MG analysis and quantitation by LC-MS, also as described in the Materials and Methods. 2-AG (A), 2-OG (B), and 2-PG (C). Values represent the mean ± SEM, n = 4–6. #P < 0.05 versus WT in the same treatment groups; *P < 0.05 versus untreated of the same genotype.
With regard to the non-ARA-containing 2-MGs, 2-AG treatment and/or LKO selectively impacted only the 2-OG level (Fig. 5B), but not the 2-PG level (Fig. 5C). While 2-AG alone had no effect on the WT hepatocyte 2-OG level, LKO alone increased the hepatocyte 2-OG level by 4-fold (Fig. 5B). In contrast, LKO diminished by 50% the ability of 2-AG to increase the 2-AG level in the hepatocytes (Fig. 5A). Neither 2-AG alone, LKO alone, nor both together significantly affected the 2-PG level in the hepatocytes (Fig. 5C).
Taken together, these data showed that 2-AG treatment markedly increased the hepatocyte levels of 2-AG several-fold in WT hepatocytes, in marked contrast to Δ9-THC, which induced 2-AG much less in WT hepatocytes (Fig. 4A). LKO exacerbated this 2-AG-induced increase by several-fold in the hepatocyte 2-AG level, again in marked contrast to LKO diminishing the ability of Δ9-THC to increase 2-AG (Fig. 4A). The simple explanation for this may be that the exogenous agent became sequestered in membranes, accounting for the significant 2-AG increase that would be unrelated to the presence of intracellular trafficking proteins. This possibility was addressed in a control experiment wherein cultured primary mouse hepatocytes were incubated without and with a solution of 900 nM 2-AG and 100 nM 2-AG-d8 similarly as described herein for unlabeled 2-AG (54). While the hepatocyte levels of both unlabeled 2-AG and 2-AG-d8 were increased similarly in WT hepatocytes, this effect was exacerbated by LKO.
Impact of Δ9-THC and Fabp1 gene ablation on hepatocyte protein levels of enzymes in EC synthesis and degradation
FABP1 is the major hepatic cytosol binding/chaperone protein for both Δ9-THC and ECs such as AEA and 2-AG (19, 21, 25, 55). Competition between these ligands may potentially impact hepatocyte protein levels of the enzymes in EC synthesis and degradation, or CB1 receptor. Therefore, cultured primary hepatocytes from WT and Fabp1-ablated (LKO) mice were treated with or without Δ9-THC and Western blotting of the respective proteins was performed as described in the Materials and Methods.
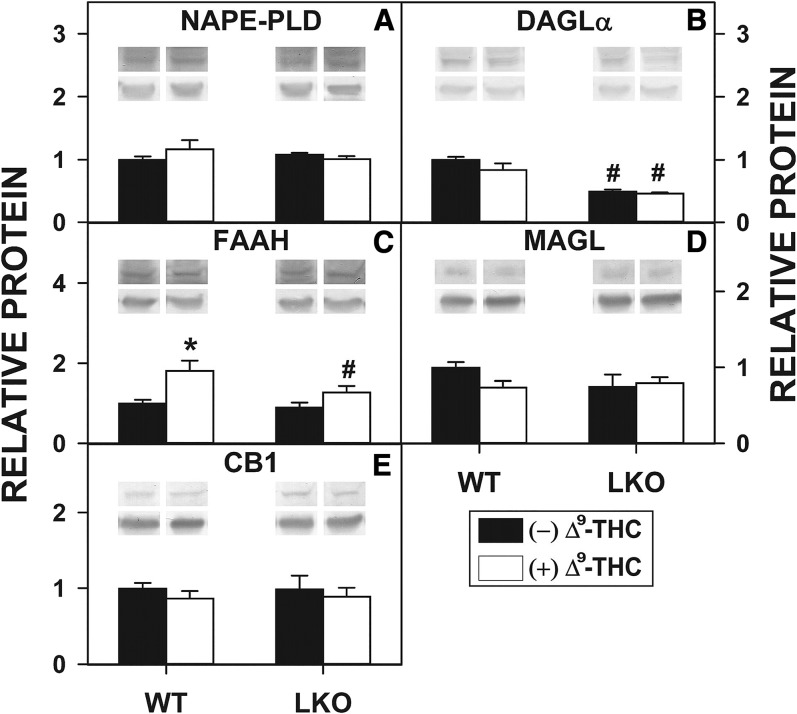
Δ9-THC differentially impacted hepatocyte protein levels of the AEA and 2-AG synthetic enzymes, NAPE-PLD and DAGL. Neither Δ9-THC nor LKO significantly altered the hepatocyte protein level of NAPE-PLD, the key enzyme in AEA and NAE synthesis (Fig. 6A). Δ9-THC alone did not alter the protein level of DAGL, the key enzyme in 2-AG and 2-MG synthesis, in WT hepatocytes (Fig. 6B). In contrast, the DAGL protein level in LKO hepatocytes was decreased regardless of the presence or absence of Δ9-THC (Fig. 6B).
Fig. 6.
Effect of Δ9-THC and Fabp1 gene ablation on hepatocyte levels of proteins involved in EC synthesis, degradation, and action. Cultured primary hepatocytes were isolated from WT or LKO mice, plated on culture dishes, and incubated with Δ9-THC (20 μM) for 1 h, as described in the Materials and Methods. Hepatocytes were then washed, homogenized, protein determined, and aliquots used for SDS-PAGE and Western blotting [as we described (79, 87)] to determine levels of the following proteins: 46 kDa NAPE-PLD (A), 120 kDa DAGLα (B), 63 kDa FAAH (C), 33 kDa MAGL (D), and 53 kDa CB1 (E). The insets show representative Western blots of the respective protein (upper blot) and the gel-loading control protein (37 kDa GAPDH, lower blot). Relative protein was normalized to internal control and WT was set to 1. Values represent the mean ± SEM, n = 4–6. #P < 0.05 versus WT in the same treatment groups; *P < 0.05 versus untreated of the same genotype.
Δ9-THC alone also differentially impacted hepatocyte protein levels of AEA and 2-AG degradative enzymes, FAAH and MAGL. Δ9-THC alone significantly increased the protein level of FAAH, the major AEA and NAE hydrolysis enzyme, in cultured primary hepatocytes from WT mice, an effect abolished by LKO (Fig. 6C). In contrast, neither Δ9-THC nor LKO significantly altered the protein level of MAGL (degrades 2-AG and 2-MGs) in hepatocytes (Fig. 6D).
Finally, neither Δ9-THC nor LKO altered the protein level of CB1 (major CB receptor) in hepatocytes (Fig. 6E).
Overall, these data indicated that the Δ9-THC-induced higher levels of AEA (Fig. 3) and 2-AG (Fig. 5) in WT hepatocytes were not associated with any upregulation of synthetic enzymes (NAPE-PLD, DAGL) or downregulation of degradative enzymes (FAAH, MAGL). On the contrary, Δ9-THC-induced upregulation of FAAH would have been expected to decrease rather than increase the AEA level. It appears that Δ9-THC enhanced hepatocyte levels (to varying degrees of significance) of all acylethanolamides in WT hepatocytes (Fig. 2). Perhaps this was due to modulation of other lipid metabolic systems by Δ9-THC, e.g., COX and LOX isoforms. Likewise, the LKO-induced higher levels of AEA (Fig. 2) and 2-AG (Fig. 4) were also not associated with any upregulation of synthetic enzymes (NAPE-PLD, DAGL) or downregulation of degradative enzymes (FAAH, MAGL). In fact, some downregulation of DAGL occurred in the LKO hepatocytes (both without and with Δ9-THC), possibly because of significant accumulation of 2-AG in the LKO hepatocytes as compared with the WT hepatocytes. Some evidence indicates that DAGL synthesis of 2-AG occurs “on demand” (56). While these possibilities contextualize the findings, it is beyond the scope of the present work to experimentally resolve all potential mechanisms.
Effect of Δ9-THC and Fabp1 gene ablation on hepatocyte protein levels of membrane transport/translocase proteins involved in uptake of ARA from which AEA and 2-AG are derived
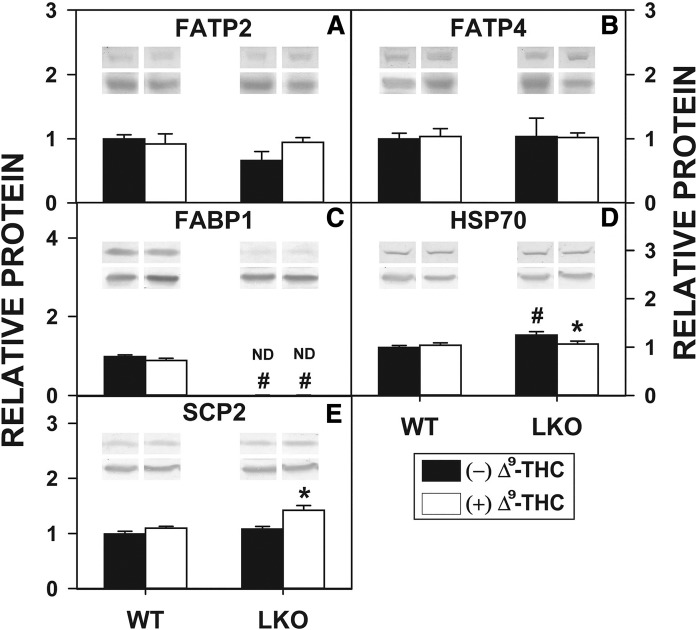
While the mechanism(s) whereby Δ9-THC, AEA, and 2-AG undergo uptake/reuptake across membranes is not yet clear, uptake of fatty acids such as ARA (precursor of AEA and 2-AG) occurs, at least in part, by membrane-associated FATPs (e.g., FATP2, FATP4) (57). Because Δ9-THC and LKO both increased hepatocyte levels of AEA and 2-AG, Western blotting was performed to determine the impact of Δ9-THC on protein levels of FATP2 and FATP4 therein. Neither Δ9-THC nor LKO significantly altered hepatocyte protein levels of FATP2 or FATP4. Thus, the Δ9-THC-induced increase in hepatocyte AEA (Fig. 2) and 2-AG (Fig. 4) was not associated with altered expression of membrane translocases involved in ARA uptake.
Effect of Δ9-THC and Fabp1 gene ablation on hepatocyte protein levels of cytosolic proteins that bind/chaperone Δ9-THC, AEA, 2-AG, and/or ARA
Several liver cytosolic proteins bind/chaperone lipidic ligands, such as ARA [FABP1 (58, 59)], AEA [FABP1 (19–21, 58), SCP-2 (60), HSP70 (61)], 2-AG [FABP1 (19–21), SCP-2 (60)], and Δ9-THC [FABP1 (19–21)]. Therefore, Western blotting was performed to determine the impact of Δ9-THC on protein levels of these cytosolic binding/chaperone proteins in cultured primary hepatocytes isolated from livers of WT and Fabp1-ablated (LKO) mice.
In cultured primary hepatocytes isolated from the livers of WT mice, Δ9-THC treatment alone did not significantly alter the protein level of the binding/chaperone proteins, FABP1 (Fig. 7C), HSP70 (Fig. 7D), or SCP-2 (Fig. 7E). In contrast, LKO alone modestly increased the hepatocyte protein level of HSP70 (Fig. 7D), but not SCP-2 (Fig. 7E). However, LKO differentially impacted the ability of Δ9-THC to alter the protein levels of these proteins in cultured primary hepatocytes, decreasing that of HSP70 (Fig. 7D), which was offset by an increase in SCP-2 (Fig. 7E). Δ9-THC has now been shown to bind not only to FABP1 (19, 21) but also to other members of the fatty acid binding protein (FABP) family (e.g., FABP3, FABP5, and FABP7) (37) with moderate affinities. Because liver also expresses the intestinal form of FABP, i.e., FABP2, it seems plausible that concomitant upregulation of FABP2 may also play some transport role for ECs and/or CBs in hepatocytes. However, despite a significant 35% increase in upregulation of the intestinal FABP (FABP2) in the LKO hepatocytes (data not shown), FABP2 represents a very small constituent in the liver, much lower than that of FABP1 (62). Furthermore, FABP2 has not been found to bind monoacylglycerols (63), although its binding affinities for ECs and Δ9-THC have not been directly ascertained.
Fig. 7.
Δ9-THC and Fabp1 gene ablation impact hepatocyte protein levels of membrane proteins and cytosolic proteins involved in uptake and cytosolic binding/chaperoning of bound Δ9-THC, AEA, 2-AG, and/or fatty acids such as ARA. All conditions were as in the legend to Fig. 6, except that Western blot analysis was performed to determine levels of FATP2 (A), FATP4 (B), FABP1 (C), HSP70 (D), and SCP-2 (E). The insets show representative Western blots of the respective protein (upper blot) and the gel-loading control protein (37 kDa GAPDH, lower blot). Relative protein was normalized to internal control and WT was set to 1. Values represent the mean ± SEM, n = 4–6. #P < 0.05 versus WT in the same treatment groups; *P < 0.05 versus untreated of the same genotype.
Thus, the Δ9-THC-induced higher levels of AEA (Fig. 2) and 2-AG (Fig. 4) in cultured primary hepatocytes from livers of WT mice did not appear to be associated with overall altered expression of cytosolic binding/chaperone proteins in hepatocytes from livers from either WT or LKO mice.
DISCUSSION
Since the discovery that key elements of the EC system are present in liver and are involved in NAFLD (9, 64), much research has focused on development of agonists/antagonists of this system. Δ9-THC, the main psychotropic component of cannabis, binds and activates CB receptors, thereby piggy-backing on the endogenous EC pathway (11–13). Although liver expresses both CB1 and CB2 receptor subtypes, their cellular distribution differs significantly, such that hepatocytes contain primarily CB1 (43). CB1 binds and is activated by both phytocannabinoids (e.g., Δ9-THC) and endogenous ECs (e.g., AEA, 2-AG (12, 44). However, it is not known whether phytocannabinoids, such as Δ9-THC, or ECs, such as 2-AG, themselves may indirectly impact CB1 by concomitantly altering levels of other endogenous ECs in liver hepatocytes. This possibility is suggested by recent findings that the most prevalent cytosolic lipidic ligand binding protein in hepatocytes, i.e., FABP1, has high affinity for both phytocannabinoid (e.g., Δ9-THC) and EC (e.g., AEA, 2-AG) agonists of CB1 (19–21, 65). Therefore, the impact of exogenous Δ9-THC and 2-AG treatment on hepatocyte AEA and 2-AG levels was examined in cultured primary mouse hepatocytes from WT and Fabp1-ablated (LKO) mice. The data provide the following new insights.
First, the phytocannabinoid CB1 agonist, Δ9-THC, significantly increased AEA and 2-AG levels in WT hepatocytes. Consistent with this finding, phytocannabinoids (i.e., Δ9-THC, cannabidiol) increase levels of AEA and 2-AG in the blood and brains of humans and rodents (17, 18, 37). Because CB1 has a similar affinity for AEA as for Δ9-THC (44), this suggests that Δ9-THC may, at least in part, exert its activating effect on CB1 by increasing the hepatocytes’ endogenous level of AEA. Δ9-THC even more dramatically increased the WT hepatocyte level of 2-AG by 2-fold more than AEA. Despite CB1’s weaker affinity for 2-AG than for either AEA or Δ9-THC (44), 2-AG is about 3-fold more potent than AEA at CB1 (8, 66, 67). While the 2-AG-induced increase in WT hepatocyte level of 2-AG may be attributable, at least in part, to increased 2-AG available for uptake, 2-AG had no effect on the non-ARA-containing 2-MGs, i.e., 2-OG and 2-PG, in WT hepatocytes. Taken together, these novel observations showed that exogenously added Δ9-THC, as well as 2-AG, increased the WT hepatocyte level of AEA and, even more so, 2-AG. Although the hepatocytes were incubated with about 20-fold higher concentration levels than typically observed in mouse serum after either intravenous injection of 3 mg/kg or inhalation of 20 mg of Δ9-THC (68), uptake did not appear saturated with respect to concentration.
Second, loss of FABP1 (i.e., Fabp1 gene ablation) alone increased AEA and 2-AG levels in cultured primary mouse hepatocytes by more than 2-fold. This finding is physiologically significant because LKO also significantly increased AEA and 2-AG in mouse liver, albeit to a smaller extent, near 30% (19). In addition, LKO concomitantly increased WT hepatocyte levels of EPEA and 2-OG by >2- and 4-fold, respectively. A similar effect, albeit also of smaller magnitude, was also observed in livers of LKO mice (19). The significance of LKO’s impact on the non-ARA-containing NAE (i.e., EPEA) and 2-MG (i.e., 2-OG) lies in their ability to indirectly alter the effectiveness of CB1 agonists. While non-ARA-containing NAEs (OEA, PEA) and 2-MGs (2-OG, 2-PG) do not directly bind/activate CB receptors, they represent entourage molecules that may enhance the effects of AEA by competing with either the transporters or the enzymes mediating the inactivation of ECs or by enhancing binding/action of ECs, such as AEA (45–52). In contrast, the EPA-derived EPEA displaces AEA and 2-AG from cell membranes to reduce AEA and 2-AG release by synthetic enzymes (53). In fact, EPA supplementation in humans and animals decreases 2-AG and AEA in brain and plasma (53). Because LKO elicits a several-fold larger increase in hepatocyte 2-OG than EPEA, this would suggest potential net potentiation of CB1 agonists.
Third, LKO blocked/diminished the ability of Δ9-THC to increase both AEA and 2-AG, but, in contrast, potentiated the ability of 2-AG to increase the hepatocyte level of AEA and 2-AG. The reasons for the opposite effects of LKO on the ability of Δ9-THC and 2-AG to impact hepatocyte AEA and 2-AG are not completely clear. One possibility is based on differences in CB1’s and FABP1’s affinities for these ligands. For example, CB1 binds Δ9-THC with nearly 10-fold higher affinity than for 2-AG (44). On the other hand FABP1 binds 2-AG with 10-fold higher affinity than for Δ9-THC (19). An alternate possibility may relate to a mechanistic difference in uptake of Δ9-THC and 2-AG. Nearly 90% of oral CB undergoes first-pass removal by the liver (69–73) by an as yet poorly understood mechanism (72–76). Although the mechanism of EC (AEA, 2-AG) uptake across the plasma membrane is also not completely clear (74, 75), AEA uptake appears to be driven by intracellular degradative enzymes (37, 76). Much less is known about 2-AG uptake, except that it is saturable and blocking 2-AG hydrolysis does not alter the rate of 2-AG uptake (76, 77).
A potential mechanism whereby FABP1 may be involved in mediating the above effects of Δ9-THC and 2-AG on hepatocyte levels of AEA and 2-AG is shown in a proposed schematic model (Fig. 8). This model is based on the fact that Δ9-THC, AEA, and 2-AG are all highly lipophilic molecules that are highly associated with membranes, as shown in Fig. 8. Within the hepatocyte, the lipophilicity of Δ9-THC, AEA, and 2-AG requires soluble binding/chaperone proteins that facilitate cytosolic transport to intracellular target sites (e.g., release, reuptake, degradation). This is analogous to what has been demonstrated for FABP5 and FABP7 in cultured transfected cells (75, 78–82). Because FABP1 is the most prevalent high-affinity liver cytosolic binding protein for both Δ9-THC and ECs, such as AEA and 2-AG (19–21, 65), FABP1 may similarly function in this role in hepatocytes (see the middle portion of Fig. 8). In support of this possibility, FABP1 is known to enhance uptake, cytosolic transport, and targeting of other lipophilic ligands (e.g., fatty acids) to oxidative organelles in cultured primary mouse hepatocytes (31, 40) and transfected cells overexpressing FABP1 (83–86). The fact that Δ9-THC inhibits uptake of AEA (37) suggests that inhibition of AEA internalization (for subsequent degradation) by Δ9-THC treatment may account, at least in part, for the increased hepatocyte AEA level. Furthermore, once Δ9-THC translocates across the WT hepatocyte plasma membrane, the FABP1 would bind/facilitate Δ9-THC desorption into cytosol as well as trafficking to intracellular sites for metabolism (endoplasmic reticulum) or excretion (bile). It is important to note that Δ9-THC is itself not an inhibitor of AEA hydrolysis by FAAH (37). Although FABP1 binds 2-AG and AEA more strongly than Δ9-THC (19–21, 65), AEA and 2-AG levels in WT hepatocytes (shown herein) and WT liver [shown earlier (19)] are normally very low. Thus, once sufficient Δ9-THC is taken up, it would displace FABP1-bound AEA and 2-AG (see the left side of the schematic in Fig. 8). This, in turn, would decrease the quantity of AEA and 2-AG trafficking/targeting to intracellular degradative sites, thereby increasing the hepatocyte level of AEA and 2-AG, as was indeed observed herein. WT hepatocyte treatment with 2-AG significantly increased 2-AG, but did not statistically increase AEA. However, LKO markedly enhanced the impact of 2-AG by increasing both AEA and 2-AG.
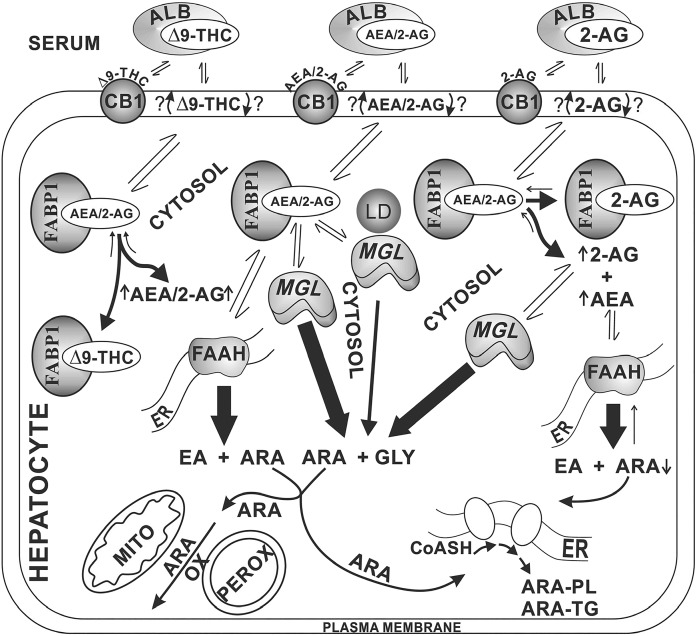
Fig. 8.
Proposed pathway regulating cultured primary mouse hepatocyte levels of ECs: impact of phytocannabinoids (Δ9-THC), 2-AG, and Fabp1 gene ablation. In serum, the highly lipophilic Δ9-THC, AEA, and 2-AG are bound by albumin (and lipoproteins) (34, 35, 61, 88, 89). After binding to hepatocyte CB1 and/or entering the plasma membrane (90), these ligands are translocated across the plasma membrane by an as yet unknown mechanism(s), with most evidence suggesting rapid spontaneous transbilayer migration (74, 75, 91). Due to their lipophilicity, these ligands require cytosolic binding/chaperone proteins for trafficking to intracellular sites of degradation/hydrolysis (21, 37, 79). In liver hepatocytes, the FABP1 is the most highly prevalent cytosolic lipophilic ligand binding/chaperone protein, recently shown to serve this function for phytocannabinoids, such as Δ9-THC, and ECs, such as AEA and 2-AG (19–21, 55). FABP1 then transports the bound AEA primarily to endoplasmic reticulum (ER) for hydrolysis by FAAH to yield ARA and ethanolamine (EA). Similarly, FABP1 also transports bound 2-AG for targeting to monoacylglycerol hydrolase (MGL), localized in cytosol and associated with lipid droplets (LD) to yield ARA and glycerol (GLY). The released ARA is then available for subsequent metabolism, i.e., oxidation (OX) [mitochondria (MITO), peroxisomes (PEROX)] or utilized for synthesis of ARA-containing phospholipids (PL) and triacylglycerols (TG). As shown on the left side of the figure, Δ9-THC competes with FABP1-bound AEA and 2-AG to displace them from the FABP1 binding site, thereby increasing hepatocyte AEA and 2-AG levels. As shown on the right side of the figure, 2-AG similarly competes with FABP1-bound AEA and 2-AG to displace them from the FABP1 binding site, thereby increasing hepatocyte AEA and 2-AG levels. Because 2-AG uptake is not driven by intracellular hydrolysis (77), continued uptake of 2-AG further exacerbates the hepatocyte level of 2-AG.
In summary, the studies presented herein show for the first time that phytocannabinoids such as Δ9-THC not only directly bind/activate CB1 receptors in WT hepatocytes, but also indirectly activate CB1 receptors by increasing WT hepatocyte levels of both major ECs (AEA, 2-AG). The latter effect was likely due to internalized Δ9-THC displacing these endogenous CB1 activators from FABP1 binding sites and thereby decreasing their hydrolysis by FAAH and MAGL. Fabp1 gene ablation LKO alone more markedly increased AEA and 2-AG levels because loss of this major AEA and 2-AG binding protein would be expected to decrease even further the targeting of these ECs for hydrolysis. In contrast, treatment of WT hepatocytes with the EC, 2-AG, did not increase AEA levels, but only increased that of 2-AG, likely due to the increased availability of 2-AG for uptake rather than any reduction in 2-AG transport to degradative sites. However, relatively little is known about the uptake of 2-AG (76, 77). Finally, LKO potentiated the ability of both Δ9-THC and 2-AG to increase the hepatocyte level of AEA and 2-AG. Taken together, these and other data suggested that Δ9-THC increases hepatocyte EC levels, at least in part, by competing for binding to FABP1, a cytosolic chaperone that facilitates targeting of bound EC to intracellular degradative enzymes.
Footnotes
Abbreviations:
- AEA
- N-arachidonoylethanolamide (anandamide)
- 2-AG
- 2-arachidonoylglycerol
- ARA
- arachidonic acid
- CB
- cannabinoid
- CB1
- cannabinoid receptor-1
- DAGLα
- diacylglycerol lipase α
- DHEA
- N-docosahexaenoylethanolamide
- EC
- endocannabinoid
- EPEA
- N-eicosapentaenoylethanolamide
- FAAH
- fatty acid amide hydrolase
- FABP
- fatty acid binding protein
- FABP1
- liver fatty acid binding protein-1
- FATP
- fatty acid transport protein
- HSP70
- heat shock protein-70
- LKO
- liver fatty acid binding protein-1 gene-ablated mouse on C57BL/6NCr background
- MAGL
- monoacylglyceride lipase
- 2-MG
- 2-monoacylglycerol
- NAE
- N-acylethanolamide
- NAFLD
- nonalcoholic fatty liver disease
- NAPE-PLD
- N-acylphosphatidylethanolamide phospholipase-D
- OEA
- oleoylethanolamide
- 2-OG
- 2-oleoylglycerol
- PEA
- palmitoylethanolamide
- 2-PG
- 2-palmitoylglycerol
- SCP
- sterol carrier protein
- THC
- tetrahydrocannabinol
This work was supported in part by Texas AgriLife Research stimulus funds (F.S.). The authors have no conflicts of interest with this article’s content, which is solely the responsibility of the authors and does not necessarily represent the official views of Texas AgriLife Research.
REFERENCES
- 1.Sarchielli P., Pini L. A., Coppola F., Rossi C., Baldi A., Mancini M. L., and Calabresi P.. 2007. Endocannabinoids in chronic migraine: CSF findings suggest a system failure. Neuropsychopharmacology. 32: 1384–1390. [DOI] [PubMed] [Google Scholar]
- 2.Christie M. J., and Mallet C.. 2009. Endocannabinoids can open the pain gate. Sci. Signal. 2: pe57. [DOI] [PubMed] [Google Scholar]
- 3.Rani Sagar D., Burston J. J., Woodhams S. G., and Chapman V.. 2012. Dynamic changes to the endocannabinoid system in models of chronic pain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367: 3300–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fine P. G., and Rosenfeld M. J.. 2013. The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides Med. J. 4: e0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guasti L., Richardson D., Jhaveri M., Eldeeb K., Barrett D., Elphick M. R., Alexander S. P., Kendall D., Michael G. J., and Chapman V.. 2009. Minocycline treatment inhibits microglial activation and alters spinal levels of endocannabinoids in a rat model of neuropathic pain. Mol. Pain. 5: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Petrocellis L., Melck D., Bisogno T., and Di Marzo V.. 2000. Endocannabinoids and fatty acid amides in cancer, inflammation, and related disorders. Chem. Phys. Lipids. 108: 191–209. [DOI] [PubMed] [Google Scholar]
- 7.Luongo L., Malcangio M., Salvemini D., and Starowicz K.. 2015. Chronic pain: new insights in molecular and cellular mechanisms. BioMed Res. Int. 2015: 676725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alswat K. A. 2013. The role of endocannabinoid system in fatty liver disease and therapeutic potential. Saudi J. Gastroenterol. 19: 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tam J., Liu J., Mukhopadhyay B., Cinar R., Godlewski G., and Kunos G.. 2011. Endocannabinoids in liver disease. Hepatology. 53: 346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regnell S. E. 2013. Cannabinoid 1 receptor in fatty liver. Hepatol. Res. 43: 131–138. [DOI] [PubMed] [Google Scholar]
- 11.Pertwee R. G. 2006. The pharmacology of cannabinoid receptors and their ligands. Int. J. Obes. (Lond.). 30: S13–S18. [DOI] [PubMed] [Google Scholar]
- 12.Pertwee R. G. 2008. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta-9- tetrahydrocannabinol, cannabidiol, and delta-9-tetrahydrocannabivarin. Br. J. Pharmacol. 153: 199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grotenhermen F., and Muller-Vahl K.. 2012. The therapeutic potential of cannabis and cannabinoids. Dtsch. Arztebl. Int. 109: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purohit V., Rapaka R., and Shurtleff D.. 2010. Role of cannabinoids in the development of fatty liver (steatosis). AAPS J. 12: 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patsenker E., and Stickel F.. 2016. Cannabinoids and liver diseases. Clin. Liver Dis. (Hoboken). 7: 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osei-Hyiaman D., Liu J., Zhou L., Godlewski G., Harvey-White J., Jeong W., Batkai S., Marsicano G., Lutz B., Buettner C., et al. 2008. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J. Clin. Invest. 118: 3160–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter C., Ferreirós N., Bishay P., Geisslinger G., Tegeder I., and Lötsch J.. 2013. Exogenous delta9-tetrahydrocannabinol influences circulating endogenous cannabinoids in humans. J. Clin. Psychopharmacol. 33: 699–705. [DOI] [PubMed] [Google Scholar]
- 18.Ellgren M., Artmann A., Tkalych O., Gupta A., Hansen H. S., Hansen S. H., Devi L. A., and Hurd Y. L.. 2008. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur. Neuropsychopharmacol. 18: 826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang H., McIntosh A. L., Martin G. G., Landrock D., Chung S., Landrock K. K., Dangott L. J., Li S., Kier A. B., and Schroeder F.. 2016. FABP1: a novel hepatic endocannabinoid and cannabinoid binding protein. Biochemistry. 55: 5243–5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin G. G., Huang H., McIntosh A. L., Kier A. B., and Schroeder F.. 2017. Endocannabinoid interaction with human FABP1: Impact of T94A variant. Biochemistry. 56: 5147–5159. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder F., McIntosh A. L., Martin G. G., Huang H., Landrock D., Chung S., Landrock K. K., Dangott L. J., Li S., Kaczocha M., et al. 2016. Fatty acid binding protein-1 (FABP1) and the human FABP1 T94A variant: Roles in the endocannabinoid system and dyslipidemias. Lipids. 51: 655–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang S. Y., He X. Y., and Schulz H.. 1987. Fatty acid oxidation in rat brain is limited by the low activity of 3-ketoacyl-coenzyme A thiolase. J. Biol. Chem. 262: 13027–13032. [PubMed] [Google Scholar]
- 23.Baumgardner J. N., Shankar K., Hennings L., Badger T. M., and Ronis M. J. J.. 2008. A new model for nonalcoholic steatohepatitis in the rat utilizing total enteral nutrition to overfeed a high-polyunsaturated fat diet. Am. J. Physiol. Gastrointest. Liver Physiol. 294: G27–G38. [DOI] [PubMed] [Google Scholar]
- 24.Guzmán C., Benet M., Pisonero-Vaquero S., Moya M., García-Mediavilla M. V., Martínez-Chantar M. L., González-Gallego J., Castell J. V., Sánchez-Campos S., and Jover R.. 2013. The human liver fatty acid binding protein (FABP1) gene is activated by FOXA1 and PPARa; and repressed by C/EBPa: implicaiton in FABP1 down-regulation in nonalcoholic liver disease. Biochim. Biophys. Acta. 1831: 803–818. [DOI] [PubMed] [Google Scholar]
- 25.Martin G. G., Huang H., McIntosh A. L., Kier A. B., and Schroeder F.. 2017. Endocannabinoid interaction with human FABP1: impact of the T94A variant. Biochemistry. 56: 5147–5159. [DOI] [PubMed] [Google Scholar]
- 26.Huang H., McIntosh A. L., Martin G. G., Landrock K., Landrock D., Gupta S., Atshaves B. P., Kier A. B., and Schroeder F.. 2014. Structural and functional interaction of fatty acids with human liver fatty acid binding protein (L-FABP) T94A variant. FEBS J. 281: 2266–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang H., McIntosh A. L., Martin G. G., Landrock K. K., Landrock D., Storey S. M., Gupta S., Atshaves B. P., Kier A. B., and Schroeder F.. 2015. Human L-FABP T94A variant enhances cholesterol uptake. Biochim. Biophys. Acta. 1851: 946–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin G. G., McIntosh A. L., Huang H., Gupta S., Atshaves B. P., Kier A. B., and Schroeder F.. 2013. Human liver fatty acid binding protein (L-FABP) T94A variant alters structure, stability, and interaction with fibrates. Biochemistry. 52: 9347–9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McIntosh A. L., Huang H., Storey S. M., Landrock K., Landrock D., Petrescu A. D., Gupta S., Atshaves B. P., Kier A. B., and Schroeder F.. 2014. Human FABP1 T94A variant impacts fatty acid metabolism and PPARa activation in cultured human female hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 307: G164–G176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroeder F., Myers-Payne S. C., Billheimer J. T., and Wood W. G.. 1995. Probing the ligand binding sites of fatty acid and sterol carrier proteins: effects of ethanol. Biochemistry. 34: 11919–11927. [DOI] [PubMed] [Google Scholar]
- 31.McIntosh A. L., Atshaves B. P., Hostetler H. A., Huang H., Davis J., Lyuksyutova O. I., Landrock D., Kier A. B., and Schroeder F.. 2009. Liver type fatty acid binding protein (L-FABP) gene ablation reduces nuclear ligand distribution and peroxisome proliferator activated receptor-alpha activity in cultured primary hepatocytes. Arch. Biochem. Biophys. 485: 160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Storey S. M., McIntosh A. L., Huang H., Martin G. G., Landrock K. K., Landrock D., Payne H. R., Kier A. B., and Schroeder F.. 2012. Loss of intracellular lipid binding proteins differentially impacts saturated fatty acid uptake and nuclear targeting in mouse hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 303: G837–G850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreamer B. L., Staecker J. L., Sawada N., Sattler G. L., Hsia M. T. S., and Pitot H. C.. 1986. Use of a low-speed iso-density Percoll centrifugation method to increase the viability of isolated rat hepatocyte preparations. In Vitro Cell. Dev. Biol. 22: 201–211. [DOI] [PubMed] [Google Scholar]
- 34.Fanali G., Cao Y., Ascenzi P., Trezza V., Rubino T., Parolaro D., and Fasano M.. 2011. Binding of delta-9-tetrahydrocannabinol and diazepam to human serum albumin. IUBMB Life. 63: 446–451. [DOI] [PubMed] [Google Scholar]
- 35.Thumser A. E., Buckland A. G., and Wilton D. C.. 1998. Monoacylglycerol binding to human serum albumin: evidence that monooleoylglycerol binds at the dansylsarcosine site. J. Lipid Res. 39: 1033–1038. [PubMed] [Google Scholar]
- 36.Bojesen I. N., and Hansen H. S.. 2003. Binding of anandamide to bovine serum albumin. J. Lipid Res. 44: 1790–1794. [DOI] [PubMed] [Google Scholar]
- 37.Elmes M. W., Kaczocha M., Berger W. T., Leung K. N., Ralph B. P., Wang L., Sweeney J. M., Miyauchi J. T., Tsirka S. E., Ojima I., et al. 2015. Fatty acid binding proteins are intracellular carriers for delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J. Biol. Chem. 290: 8711–8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siegmund S. V., Qian T., de Minicis S., Harvey-White J., Kunos G., Vinod K. Y., Hungund B., and Schwabe R. F.. 2007. The endocannabinoid 2-arachidonoylglycer induces death of hepatic stellate cells via mitochondrial reactive oxygen species. FASEB J. 21: 2798–2806. [DOI] [PubMed] [Google Scholar]
- 39.Siegmund S. V., Wojtalla A., Schlosser M., Zimmer A., and Singer M. V.. 2013. Fatty acid amide hydrolase but not monoacyl glycerol lipase controls cell death induced by the endocannabinoid 2-arachidonoyl glycerol in hepatic cell populations. Biochem. Biophys. Res. Commun. 437: 48–54. [DOI] [PubMed] [Google Scholar]
- 40.Atshaves B. P., McIntosh A. L., Lyuksyutova O. I., Zipfel W. R., Webb W. W., and Schroeder F.. 2004. Liver fatty acid binding protein gene ablation inhibits branched-chain fatty acid metabolism in cultured primary hepatocytes. J. Biol. Chem. 279: 30954–30965. [DOI] [PubMed] [Google Scholar]
- 41.Petrescu A. D., McIntosh A. L., Storey S. M., Huang H., Martin G. G., Landrock D., Kier A. B., and Schroeder F.. 2013. High glucose potentiates liver fatty acid binding protein (L-FABP) mediated fibrate induction of PPARa in mouse hepatocytes. Biochim. Biophys. Acta. 1831: 1412–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin G. G., Chung S., Landrock D., Landrock K. K., Huang H., Dangott L. J., Peng X., Kaczocha M., Seeger D. R., Murphy E. J., et al. 2016. FABP1 gene ablation impacts brain endocannabinoid system in male mice. J. Neurochem. 138: 407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Auguet T., Berlanga A., Guiu-Jurado E., Terra X., Martinez S., Aguilar C., Filiu E., Alibalic A., Sabench F., Hernández M., et al. 2014. Endocannabinoid receptors gene experssion in morbidly obese women with NAFLD. BioMed Res. Int. 2014: 502542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McPartland J. M., Glass M., and Pertwee R. G.. 2007. Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. Br. J. Pharmacol. 152: 583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ho W. S., Barrett D. A., and Randall M. D.. 2008. ‘Entourage’ effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br. J. Pharmacol. 155: 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smart D., Jonsson K-O., Vanvoorde S., Lambert D. M., and Fowler C. J.. 2002. Entourage effects of N-acyl ethanolamines at human vanilloid receptors. Comparison of effects upon anandamide-induced vanilloid receptor activation and upon anandamide metabolism. Br. J. Pharmacol. 136: 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piomelli S., and Seaman C.. 1993. Mechanism of red blood cell aging: relationship of cell density and cell age. Am. J. Hematol. 42: 46–52. [DOI] [PubMed] [Google Scholar]
- 48.Franklin A., Parmentier-Batteur S., Walter L., Greenbert D. A., and Stella N.. 2003. Palmitoylethanolamide increases after focal cerebral ischemia and potentiates microglial cell motility. J. Neurosci. 23: 7767–7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ben-Shabat S., Fride E., Sheskin T., Tamiri T., Rhee M. H., Vogel Z., Bisogno T., De Petrocellis L., Di Marzo V., and Mechoulam R.. 1998. An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur. J. Pharmacol. 353: 23–31. [DOI] [PubMed] [Google Scholar]
- 50.Mechoulam R., Fride E., Hanus L., Sheskin T., Bisogno T., Di Marzo V., Bayewitch M., and Vogel Z.. 1997. Anandamide may mediate sleep induction. Nature. 389: 25–26. [DOI] [PubMed] [Google Scholar]
- 51.De Petrocellis L., Bisagno T., and Di Marzo V.. 2004. Endocannabinoids. In Neuroscience Intelligence Unit: Cannabinoids. V. Di Marzo, editor. Kluwer Academic/Plenum Publishers, New York. 98–130. [Google Scholar]
- 52.Izzo A. A., Muccioli G. G., Ruggieri M. R., and Schicho R.. 2004. Endocannabinoids and the digestive tract and bladder health and disease. In Endocannabinoids. R. G. Pertwee, editor. Springer International Publishers, A.G., Basel. 423–448. [Google Scholar]
- 53.Naughton S. S., Mathai M. L., Hryciw D. H., and McAinch A. J.. 2013. Fatty acid modulation of the endocannabinoid system and the effect on food intake and metabolism. Int. J. Endocrinol. 2013: 361895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McIntosh A. L., Huang H., Landrock D., Martin G., Li S., Kier A. B., and Schroeder F.. 2018. Impact of Fabp1 gene ablation on uptake and degradation of endocannabinoids in mouse hepatocytes. Lipids. In press. [DOI] [PubMed] [Google Scholar]
- 55.Atshaves B. P., Storey S., Huang H., and Schroeder F.. 2004. Liver fatty acid binding protein expression enhances branched-chain fatty acid metabolism. Mol. Cell. Biochem. 259: 115–129. [DOI] [PubMed] [Google Scholar]
- 56.Markwick R. L. L. 2018. Regulation of DAG Lipase Activity–Implications for ‘On-Demand’ Endocannabinoid Signaling. PhD Dissertation. King’s College, London, United Kingdom.
- 57.Watkins P. A. 2008. Very long chain acyl-CoA synthetases. J. Biol. Chem. 283: 1773–1777. [DOI] [PubMed] [Google Scholar]
- 58.Frolov A., Cho T. H., Murphy E. J., and Schroeder F.. 1997. Isoforms of rat liver fatty acid binding protein differ in structure and affinity for fatty acids and fatty acyl CoAs. Biochemistry. 36: 6545–6555. [DOI] [PubMed] [Google Scholar]
- 59.Richieri G. V., Ogata R. T., and Kleinfeld A. M.. 1994. Equilibrium constants for the binding of fatty acids with fatty acid binding proteins from adipocyte, intestine, heart, and liver measured with the fluorescent probe ADIFAB. J. Biol. Chem. 269: 23918–23930. [PubMed] [Google Scholar]
- 60.Frolov A., Cho T. H., Billheimer J. T., and Schroeder F.. 1996. Sterol carrier protein-2, a new fatty acyl coenzyme A-binding protein. J. Biol. Chem. 271: 31878–31884. [DOI] [PubMed] [Google Scholar]
- 61.Oddi S., Fezza F., Pasquariello N., D’Agostino A., Catanzaro G., De Simone C., Rapino C., Finazzi-Agrò A., and Maccarrone M.. 2009. Molecular identification of albumin and Hsp70 as cytosolic anandamide binding proteins. Chem. Biol. 16: 624–632. [DOI] [PubMed] [Google Scholar]
- 62.Bass N. M., and Manning J. A.. 1986. Tissue expression of three structurally different fatty acid binding proteins from rat heart muscle, liver, and intestine. Biochem. Biophys. Res. Commun. 137: 929–935. [DOI] [PubMed] [Google Scholar]
- 63.Storch J. 1993. Diversity of fatty acid-binding protein structure and function: studies with fluorescent ligands. Mol. Cell. Biochem. 123: 45–53. [DOI] [PubMed] [Google Scholar]
- 64.Kunos G., and Gao B.. 2008. Endocannabinoids, CB1 receptors, and liver disease: hitting more than one bird with the same stone. Gastroenterology. 134: 622–625. [DOI] [PubMed] [Google Scholar]
- 65.Martin G. G., Landrock D., Chung S., Dangott L. J., McIntosh A. L., Mackie J. T., Kier A. B., and Schroeder F.. 2017. Loss of fatty acid binding protein-1 alters the hepatic endocannabinoid system response to a high fat diet. J. Lipid Res. 58: 2114–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gonsiorek W., Lunn C., Fan X., Narula S., Lundell D., and Hipkin R. W.. 2000. Endocannabinoid 2-AG is a full agonist through human CB2: antagonism by anandamide. Mol. Pharmacol. 57: 1045–1050. [PubMed] [Google Scholar]
- 67.Magen I., Avraham Y., Berry Y., and Mechoulam R.. 2008. Endocannabinoids in liver disease and hepatic encephalopathy. Curr. Pharm. Des. 14: 2362–2369. [DOI] [PubMed] [Google Scholar]
- 68.Varvel S. A., Bridgen D. T., Tao Q., Thomas B. F., Martin B. R., and Lichtman A. H.. 2005. Delta-9-tetrahydrocannabinol accounts for teh aninociceptive, hypothermic, and cataleptic effects of marijuana in mice. J. Pharmacol. Exp. Ther. 314: 329–337. [DOI] [PubMed] [Google Scholar]
- 69.Huestis M. A. 2007. Human cannabinoid pharmacokinetics. Chem. Biodivers. 4: 1770–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mattes R. D., Shaw L. M., Edling-Owens J., Engelman K., and Elsohly M. A.. 1993. Bypassing the first-pass effect for the therapeutic use of cannabinoids. Pharmacol. Biochem. Behav. 44: 745–747. [DOI] [PubMed] [Google Scholar]
- 71.Trevaskis N. L., Shackleford D. M., Charman W. N., Edwards G. A., Gardin A., Appel-Dingemanse S., Kretz O., Galli B., and Porter C. J.. 2009. Intestinal lymphatic transport enhances the post-prandial oral bioavailability of a novel cannabinoid receptor agonist via avoidance of first-pass metabolism. Pharm. Res. 26: 1486–1495. [DOI] [PubMed] [Google Scholar]
- 72.Grotenhermen F. 2003. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 42: 327–360. [DOI] [PubMed] [Google Scholar]
- 73.Ashton C. H. 2001. Pharmacology and effects of cannabis: a brief review. Br. J. Psychiatry. 178: 101–106. [DOI] [PubMed] [Google Scholar]
- 74.Fowler C. J. 2013. Transport of endocannabinoids across plasma membrane and within the cell. FEBS J. 280: 1895–1904. [DOI] [PubMed] [Google Scholar]
- 75.Muccioli G. G. 2010. Endocannabinoid biosynthesis and inactivation, from simple to complex. Drug Discov. Today. 15: 474–483. [DOI] [PubMed] [Google Scholar]
- 76.Deutsch D. G., Glaser S. T., Howell J. M., Kunz J. S., Puffenbarger R. A., Hillard C. J., and Abumrad N.. 2001. The cellular uptake of anandamide is coupled to its breakdown by FAAH. J. Biol. Chem. 276: 6967–6973. [DOI] [PubMed] [Google Scholar]
- 77.Fowler C. J. 2012. Anandamide uptake explained. Trends Pharmacol. Sci. 33: 181–185. [DOI] [PubMed] [Google Scholar]
- 78.Berger W. T., Ralph B. P., Kaczocha M., Sun J., Balius T. E., Rizzo R. C., Haj-Dahmane S., Ojima I., and Deutsch D. G.. 2012. Targeting FABP anandamide transporters–a novel strategy for development of anti-inflammatory and anti-nociceptive drugs. PLoS One. 7: e50968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaczocha M., Glaser S. T., and Deutsch D. G.. 2009. Identification of intracellular carriers for the endocannabinoid anandamide. Proc. Natl. Acad. Sci. USA. 106: 6375–6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaczocha M., Vivieca S., Sun J., Glaser S. T., and Deutsch D. G.. 2012. Fatty acid binding proteins transport N-acylethanolamines to nuclear receptors and are targets of endocannabinoid transport inhibitors. J. Biol. Chem. 287: 3415–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaczocha M. 2009. Role of Fatty Acid Binding Proteins and FAAH-2 in Endocannabinoid Uptake and Inactivation. PhD Dissertation. Stony Brook University, Stony Brook, NY.
- 82.Kaczocha M., Rebecchi M. J., Ralph B. P., Teng Y-H. G., Berger W. T., Galbavy W., Elmes M. W., Glaser S. T., Wang L., Rizzo R. C., et al. 2014. Inhibition of fatty acid binding protein elevates brain anandamide levels and produces analgesia. PLoS One. 9: e94200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murphy E. J., Prows D. R., Jefferson J. R., and Schroeder F.. 1996. Liver fatty acid binding protein expression in transfected fibroblasts stimulates fatty acid uptake and metabolism. Biochim. Biophys. Acta. 1301: 191–198. [DOI] [PubMed] [Google Scholar]
- 84.Murphy E. J. 1998. L-FABP and I-FABP expression increase NBD-stearate uptake and cytoplasmic diffusion in L-cells. Am. J. Physiol. 275: G244–G249. [DOI] [PubMed] [Google Scholar]
- 85.McArthur M. J., Atshaves B. P., Frolov A., Foxworth W. D., Kier A. B., and Schroeder F.. 1999. Cellular uptake and intracellular trafficking of long chain fatty acids. J. Lipid Res. 40: 1371–1383. [PubMed] [Google Scholar]
- 86.Weisiger R. A. 2002. Cytosolic fatty acid binding proteins catalyze two distinct steps in intracellular transport of their ligands. Mol. Cell. Biochem. 239: 35–43. [PubMed] [Google Scholar]
- 87.Martin G. G., Atshaves B. P., Landrock K. K., Landrock D., Schroeder F., and Kier A. B.. 2015. Loss of L-FABP, SCP-2/SCP-x, or both induces hepatic lipid accumulation in female mice. Arch. Biochem. Biophys. 580: 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Capelle N., Treich I., Ayraut-Jarrier M., Hoellinger H., and Scherrmann J. M.. 1976. Binding of delta-8-thc to human serum proteins: binding as a function of lipoprotein composition. Eur. J. Toxicol. Environ. Hyg. 9: 5–9. [PubMed] [Google Scholar]
- 89.Khaliullina H., Bilgin M., Sampaio J. L., Shevchenko A., and Eaton S.. 2015. Endocannabinoids are conserved inhibitors of the Hedgehog pathway. Proc. Natl. Acad. Sci. USA. 112: 3415–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McFarland M. J., and Barker E. J.. 2005. Lipid rafts: A nexus for endocannabinoid signaling. Life Sci. 77: 1640–1650. [DOI] [PubMed] [Google Scholar]
- 91.Kaczocha M., Lin Q., Nelson L. D., London E., and Deutsch D. G.. 2012. Anandamide externally added to lipid vesicles containing trapped FAAH is readily hydrolyzed in a sterol modulated fashion. ACS Chem. Neurosci. 3: 364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]